Abstract
Background
Perceived health is one of the strongest determinants of subjective well-being, but it has received little attention among survivors of ARDS.
Research question
How well do self-reported measures of physical, emotional, and social functioning predict perceived overall health (measured using the EQ-5D visual analog scale [EQ-5D-VAS]) among adult survivors of ARDS? Are demographic features, comorbidity, or severity of illness correlated with perceived health after controlling for self-reported functioning?
Study Design and Methods
We analyzed the ARDSNet Long Term Outcomes Study (ALTOS) and Improving Care of Acute Lung Injury Patients (ICAP) Study, two longitudinal cohorts with a total of 823 survivors from 44 US hospitals, which prospectively assessed survivors at 6 and 12 months after ARDS. Perceived health, evaluated using the EQ-5D-VAS, was predicted using ridge regression and self-reported measures of physical, emotional, and social functioning. The difference between observed and predicted perceived health was termed perspective deviation (PD). Correlations between PD and demographics, comorbidities, and severity of illness were explored.
Results
The correlation between observed and predicted EQ-5D-VAS scores ranged from 0.68 to 0.73 across the two cohorts and time points. PD ranged from –80 to +34 and was more than the minimum clinically important difference for 52% to 55% of survivors. Neither demographic features, comorbidity, nor severity of illness were correlated strongly with PD, with |r| < 0.25 for all continuous variables in both cohorts and time points. The correlation between PD at 6- and 12-month assessments was weak (ALTOS: r = 0.22, P < .001; ICAP: r = 0.20, P = .02).
Interpretation
About half of survivors of ARDS showed clinically important differences in actual perceived health vs predicted perceived health based on self-reported measures of functioning. Survivors of ARDS demographic features, comorbidities, and severity of illness were correlated only weakly with perceived health after controlling for measures of perceived functioning, highlighting the challenge of predicting how individual patients will respond psychologically to new impairments after critical illness.
Key Words: critical care outcomes, functional status, respiratory distress syndrome, survivorship
Abbreviations: ALTOS, ARDSNet Long Term Outcomes Study; EQ-5D-VAS, EQ-5D visual analog scale; ICAP, Improving Care of Acute Lung Injury Patients; PD, perspective deviation; PROMS, patient-reported outcome measures; SF-36v2, 36-item Short Form Health Survey version 2
Advances in critical care and aging populations have contributed to a growing number of survivors of ARDS.1, 2, 3, 4 Many survivors of ARDS experience new and persistent physical, cognitive, and mental health impairments.5, 6, 7, 8 In response, professional societies have identified improving long-term outcomes as a key research priority,9, 10, 11, 12, 13 and the number of peer-reviewed articles reporting outcomes after discharge of those who were treated in the ICU has risen dramatically.14 Models to predict physical, cognitive, and mental health impairments after ARDS now exist,15, 16, 17, 18 and physicians are encouraged to discuss the risk of long-term functional impairment routinely with patients in ICUs and their families.19 Critical care follow-up clinics also are proliferating to address these impairments20 and are struggling valiantly to improve life for survivors.21, 22, 23, 24, 25, 26, 27
Self-reported measures of participation, or the ability to perform common daily activities, are collected almost universally in research on patients treated in and discharged from the ICU. This is because the abilities to bathe oneself, prepare food, and climb a flight of stairs are good predictors of whether a person can live independently. At the same time, these measures are only moderately predictive of more personal, patient-important outcomes.28,29 Psychological and cultural factors also shape how survivors feel about their bodies, their health, and their lives relative to their value systems. When symptoms and impairments do not match how a survivor perceives their health, we infer the existence of trait resilience,30, 31, 32 expectations,33,34 and psychological adaptation to impairments (ie, response shift).34, 35, 36 However, it is extremely difficult to measure these factors and demonstrate their role in survivors’ lives.
Perceived health matters because it is one of the strongest determinants of subjective well-being, or happiness.37 If we can readily identify critical illness survivors at risk of negative health perceptions, regardless of recovered functional status (eg, ability to participate in daily activities), they may benefit from interventions to address mental health and self-care in survivorship.31,38 With this background and rationale, we evaluated data from two longitudinal, multisite cohort studies of survivors of ARDS to explore unmeasured determinants of health perception after critical illness, with three objectives: (1) to use self-reported measures of physical, emotional, and social functioning to predict perceived health during the first year after ARDS; (2) to identify survivors with clinically important positive or negative differences in predicted vs perceived health; and (3) to describe the baseline demographic and critical illness-related characteristics of survivors with strongly negative or positive views of their health relative to their self-reported functional status.
Methods
Conceptual Model and Definitions
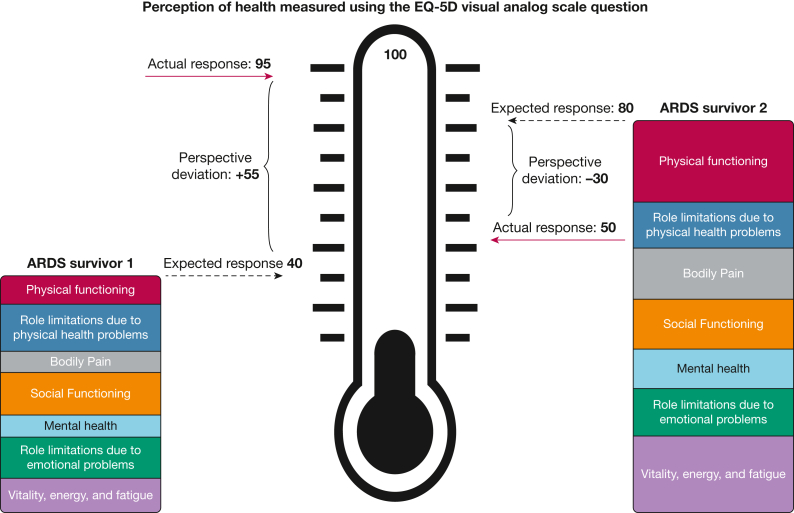
Within the conceptual model for this research (Fig 1), we recognized health perception as a subjective self-evaluation. We predicted how individual survivors of ARDS would perceive their health using data from other survivors of ARDS with similar self-reported physical, emotional, and social functioning and approximately the same number of months since ARDS development. This framework does not assume the existence of a “true” or correct health perception. No term exists to describe the difference between an individual’s perception of their health and the expected perception of health among peers with similar self-reported function. Therefore, to recognize this concept, we created the term perspective deviation (PD), defined as the difference between a person’s perception of their health, Hi, and the average or expected perception of health, E(Hi), given their self-reported measures of physical, emotional, and social functioning , after a period in a new health state (eg, 6 months after ARDS):
Figure 1.
Diagram showing conceptual model of two hypothetical survivors of ARDS. Each survivor’s perception of their health was ascertained using the EQ-5D visual analog scale. The wording of the question was: “To help you say how good or bad your state of health is, I’d like you to try to picture in your mind a scale that looks like a thermometer. The best state you can imagine is marked 100 at the top of the scale and the worst state you can imagine is marked zero at the bottom. I would like you to tell me the point on this scale where you would put your own state of health TODAY.” The survivor’s expected response is predicted using their responses to questions evaluating seven domains of the 36-item Short Form Health Survey version 2 depicted by the seven colored boxes. The red arrow indicates the survivor’s actual response. We defined perspective deviation as a survivor’s expected response minus their actual or observed response. ARDS survivor 1 exhibited positive perspective deviation, whereas ARDS survivor 2 exhibited negative perspective deviation.
Participants
Two longitudinal multisite cohort studies of adult survivors of ARDS provided data for this study: The National Heart, Lung, and Blood Institute ARDS Network (ARDSNet) Long Term Outcomes Study (ALTOS) and Improving Care of Acute Lung Injury Patients (ICAP) Study. ALTOS (ClinicalTrials.gov Identifier: NCT00719446) examined the outcomes for survivors of ARDS at 6 and 12 months after enrollment in four National Institutes of Health-funded ARDS Network clinical trials that recruited from 44 hospitals at 11 study sites across the United States.39, 40, 41, 42 Major exclusion criteria included pre-existing severe chronic lung, liver, or neuromuscular disease or restriction in the use of life support at eligibility. Evaluations of survivors used a battery of validated surveys, administered by phone. Follow-up for ALTOS was excellent, with 97% and 95% of enrolled survivors having an assessment at 6 and 12 months, respectively. The ICAP Study prospectively enrolled consecutive mechanically ventilated patients with ARDS, diagnosed in an identical manner to those in ALTOS, from 13 ICUs at four hospitals in Baltimore, Maryland (ClinicalTrials.gov Identifier: NCT00300248).43 Inclusion criteria for enrollment in the ICAP Study were less stringent than in ALTOS, resulting in a sicker study population on average. Self-reported physical and mental health outcomes in the ICAP Study were collected for 95% and 92% of enrolled survivors at 6 and 12 months, respectively. The Johns Hopkins School of Medicine Institutional Review Board approved this study (Identifier: IRB00162140).
Outcome Measures
In both prospective studies, all participants were asked about their perceived health at 6 and 12 months after ARDS onset using the EQ-5D visual analog scale (EQ-5D-VAS).44,45 The EQ-5D-VAS ranges from 0 to 100, with higher scores representing better perceived health.
Symptoms and self-reported function at 6 and 12 months were assessed using the five questions in the EQ-5D-3L, a standardized measure developed by the EuroQol Group to provide a simple, generic questionnaire for use in clinical and economic appraisal or population health status surveys (ie, mobility, self-care, usual activities, pain or discomfort, and anxiety or depression), and 30 questions from the 36-item Short Form Health Survey version 2 (SF-36v2; ie, physical functioning, role limitations resulting from physical health problems, bodily pain, social functioning, mental health, role limitations resulting from emotional problems, and vitality).46 Descriptions of this battery of 35 questions are included in e-Table 1. Both the EQ-5D-3L and SF-36v2 are recommended instruments for studies of patients treated in and discharged from the ICU.47
Additionally, the following data were collected for participants in both the ALTOS and ICAP Study: demographics (including age, sex, race, years of formal education, and median household income within the participant’s zip code) and severity of illness (Acute Physiology and Chronic Health Evaluation II score,48 ICU length of stay, hospital length of stay). For all ICAP Study participants and for ALTOS participants from five of the 11 study sites, comorbidity was evaluated via the Charlson Comorbidity Index49 and the Functional Comorbidity Index,50,51 in addition to any documented past medical history of depression. Participants also were asked to assess retrospectively their health status before ARDS using the EQ-5D-VAS.
Statistical Analysis
Continuous variables were described using medians and interquartile ranges, whereas categorical variables were presented using frequencies and percentages. Responses to five questions about health state from the EQ-5D-3L and 30 questions from the SF-36v2 at 6 and 12 months after enrollment were used to predict EQ-5D-VAS score during the same 6- and 12-month assessments. Importantly, the resulting prediction model was not intended for use in the clinical setting. To avoid overfitting a model with 35 predictors, we randomly divided the ALTOS cohort into a training set of 500 survivors and an internal validation set of 186 survivors using a random number generator (Fig 2). We considered six approaches to developing a prediction model, as detailed in e-Table 2. Trialed approaches included shrinkage methods (least absolute shrinkage and selection operator,52 ridge regression,53 and elastic net54), and tree-based ensemble methods (random forest55 and extreme gradient boosting56). Hyperparameters for each model were tuned using 10-fold cross-validation within the training set. Optimal combinations of parameters were chosen based on the lowest root mean square error. Each model was used to predict the EQ-5D-VAS for the 186 survivors in the ALTOS validation set at 6 and 12 months, with accuracy evaluated by mean squared error. The final model minimized mean squared error in the ALTOS validation set and then was used to predict the EQ-5D-VAS score at 6 and 12 months in the full ALTOS cohort (N = 686). Because the central tendency of EQ-5D-VAS scores in the ICAP Study cohort was substantially lower than in ALTOS, we fit a second ICAP-specific prediction model with hyperparameters tuned using 10-fold cross-validation.
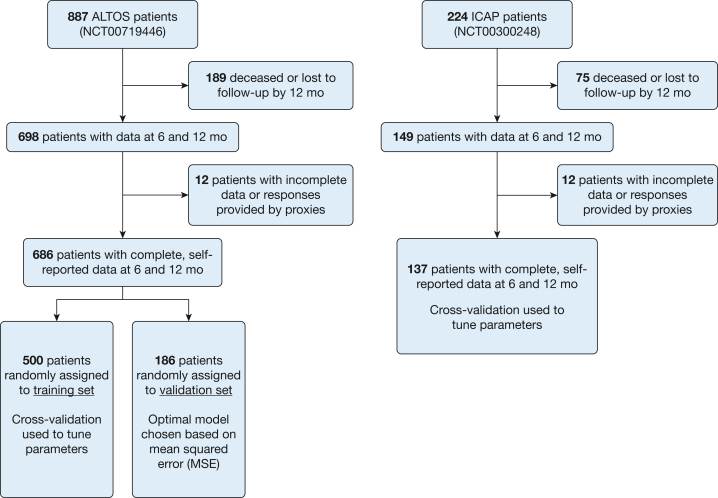
Figure 2.
Flow diagram of longitudinal cohort studies. The ALTOS examined patient outcomes 6 and 12 months after enrollment into four National Institutes of Health-funded ARDS Network Clinical Trials for patients with ARDS. Data on health-related outcomes were collected by phone via a battery of validated surveys for survivors from 41 hospitals at 11 study centers across the United States. Participants from five of 11 ARDS Network centers provided additional data on formal education, comorbidities before ARDS, and retrospective perception of their health before ARDS. The ICAP prospective cohort study evaluated the effects of lower tidal volume ventilation and other aspects of critical illness and ICU care on the long-term physical and mental health outcomes of participants at 13 ICUs at four hospitals in Baltimore, Maryland. Follow-up assessments were conducted at 6 and 12 months. ALTOS = ARDSNet Long Term Outcomes Study; ICAP = Improving Care of Acute Lung Injury Patients.
PD for survivors in both the ICAP Study and ALTOS was calculated as observed EQ-5D-VAS score minus predicted EQ-5D-VAS (ie, residuals) at 6 and 12 months. Thus, positive PD values occurred when a survivor perceived his or her health to be better than predicted using the 35 self-reported measures, and negative PD occurred when a survivor’s perceived health was worse than predicted. Histograms of PD stratified by study (ie, ICAP Study vs ALTOS) and time point (ie, 6 months vs 12 months) were plotted. The correlation between PD at 6 and 12 months was examined visually and summarized using Pearson correlation coefficient (r).
To explore correlations between patient characteristics and PD, we plotted PD against age, years of formal education, median household income in participant zip code, sex, race, Charlson Comorbidity Index, functional comorbidity index, past medical history of depression, and retrospective perception of health before ARDS assessed using the EQ-5D-VAS question. For age, years of education, and Acute Physiology and Chronic Health Evaluation II score, the Pearson correlation coefficient was reported, and Student t test was used to test the null hypothesis of no correlation. Spearman rank correlation coefficient was reported for continuous variables with skewed distributions and Spearman ρ test statistic was used to test the null hypothesis of no correlation. The association between race and PD was explored using a one-way analysis of variance model. We used a conservative minimal clinically important difference of 8 points on the EQ-5D-VAS to identify meaningful PD based on previous work.57,58 A P value of < .05 was considered statistically significant. All analyses were performed using R version 4.0.2 software (R Foundation for Statistical Computing).
Results
Participants in ALTOS and the ICAP Study generally were similar (Table 1), although a higher proportion of ICAP Study patients were Black and their median hospital and ICU lengths of stay were longer. Hyperparameters selected via cross-validation for each potential model are reported in e-Table 2 and e-Figure 1. Models using shrinkage methods predicted perceived health in the validation cohort most successfully (e-Table 3). Ridge regression with all 35 questions about self-reported physical, emotional, and social functioning from the EQ-5D-3L and SF-36v2 generated the most accurate (lowest mean square error) predictions in the validation set at both 6 and 12 months. Therefore, we used ridge regression models to predict perceived health (EQ-5D-VAS scores) at 6 and 12 months in the full ALTOS and ICAP Study cohorts.
Table 1.
ALTOS and ICAP Patient Cohorts
| Characteristic | ALTOS (n = 686) | ICAP (n = 137) |
|---|---|---|
| Age, y | 51 (41-59) | 47 (40-57) |
| Female sex | 356 (52) | 63 (46) |
| Race | ||
| White | 555 (81) | 80 (58) |
| Black | 99 (14) | 54 (39) |
| Other | 14 (2) | 2 (2) |
| Missing | 18 (3) | 1 (1) |
| Years of educationa | 13 (12-14) | 12 (11-14) |
| Median income of zip code (USD) | $47,300 ($37,900-$62,000) | $46,230 ($39,724-$65,744) |
| Charlson Comorbidity Indexb | 1 (0-1) | 1 (0-3) |
| Functional Comorbidity Indexb | 2 (1-3) | 1 (1-2) |
| Past medical history of depressionb | 67 (28)b | 27 (20) |
| APACHE IIc | 25 (20-32) | 23 (18-28) |
| LOS, d | ||
| Hospitald | 17 (12-26) | 23 (15-34) |
| ICUe | 10 (7-16) | 14 (8-21) |
| Retrospective perception of health before ARDSf | 80 (60-90) | 75 (50-90) |
Data are presented as No. (%) or median (interquartile range). Percentages do not total 100% because of rounding. ALTOS = ARDSNET Long Term Outcomes Study; APACHE = Acute Physiology and Chronic Health Evaluation; ICAP = Improving Care of Acute Lung Injury Patients study; LOS = length of stay; USD = US dollars.
Available for 307 ALTOS survivors and 130 ICAP survivors.
Available for all ICAP survivors and 236 ALTOS survivors from five of 11 study sites.
Missing for 20 ALTOS survivors.
Missing for five ALTOS survivors.
Missing for four ALTOS survivors.
Available for 109 ICAP survivors and 236 ALTOS survivors from five of 11 study sites.
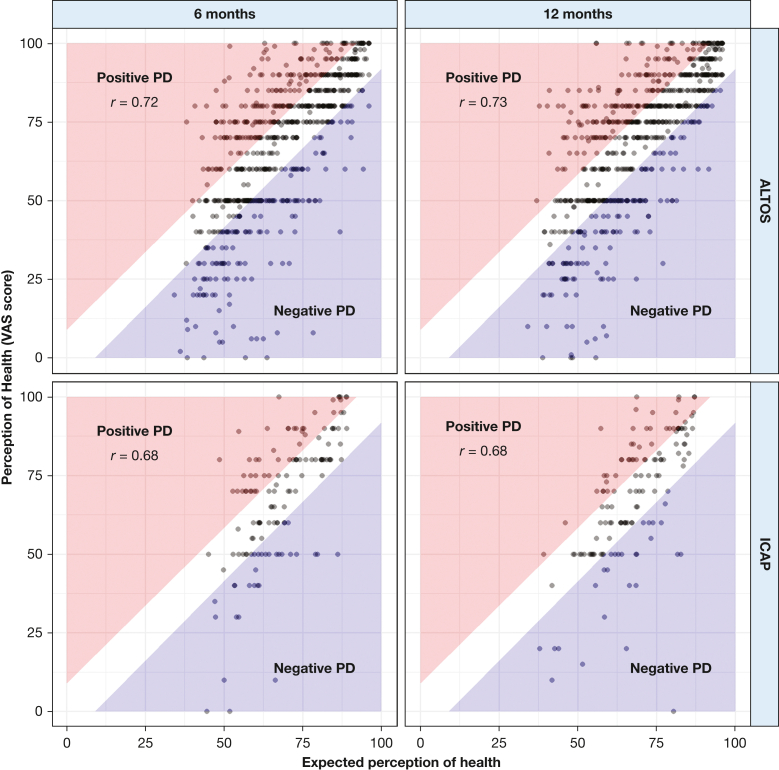
The relationship between predicted and reported perceived health was similar in both cohorts and at both follow-up assessments (ie, 6 and 12 months) after ARDS (Fig 3). The correlation between predicted and observed EQ-5D-VAS was 0.72 and 0.73 at the 6- and 12-month assessments, respectively, in the ALTOS cohort and 0.68 at both time points in the ICAP Study cohort. Residuals, which served as estimates of PD, appeared to be approximately normally distributed in both cohorts and at both time points (e-Fig 2). Within the ALTOS cohort, estimated PD ranged from –70 to +47 at 6 months and –56 to +44 at 12 months. PD for ICAP Study survivors ranged from –56 to +34 at 6 months and –80 to +32 at 12 months. The absolute value of PD was > 8 (minimal clinically important difference) for 375 patients in ALTOS at 6 months (55%), 358 patients in ALTOS at 12 months (52%), 76 patients in the ICAP Study at 6 months (55%), and 73 patients in the ICAP Study at 12 months (53%). The correlation between PD at the 6- and 12-month assessments was weak (ALTOS: r = 0.22, P < .001; ICAP: r = 0.20, P = 0.02) (e-Fig 3).
Figure 3.
Graphs showing expected vs observed perception of health at 6 and 12 months in the ALTOS and ICAP cohorts. Perception of health was evaluated using the EQ-5D-VAS questionnaire. Expected perceptions of health estimated using patient responses to 35 questions from the 36-item Short Form Health Survey version 2 and EQ-5D-VAS about physical functioning, social functioning, mental health, bodily pain, role limitations resulting from physical health or emotional problems, vitality, energy, and fatigue. Areas of the plot shaded red indicate that the survivor’s perception of his or her health was > 8 points better than predicted, corresponding to positive perception deviation. Areas of the plot shaded blue indicate that the survivor’s perception of his or her health was > 8 points worse than predicted, corresponding to negative perception deviation. Pearson correlation coefficients reported. P < .001 for the test of the null hypothesis, and r = 0 in both cohorts at both time points. ALTOS = ARDSNet Long Term Outcomes Study; ICAP = Improving Care of Acute Lung Injury Patients study; PD = perception deviation; VAS = visual analog scale.
In bivariate comparisons of PD and survivor demographics, correlations were < 0.25 for all continuous variables in both cohorts and at both time points (Table 2, e-Figs 4-7). Mean PD was significantly more positive among women compared with men in the ALTOS cohort at 6 and 12 months, but men showed a more positive mean PD than women in the ICAP Study cohort. Retrospective perception of health before ARDS was correlated weakly with PD at 6 months in both ALTOS (r = 0.17, P = .01) and the ICAP Study (r = 0.34, P = .0003) (e-Fig 8). This correlation also was present at 12 months, but was even weaker in the ICAP Study cohort (ALTOS: r = 0.18, P = .001; ICAP: r = 0.13, P = .19).
Table 2.
Correlations Between Patient Characteristics and PD
| Characteristic | ALTOS |
ICAP |
||||
|---|---|---|---|---|---|---|
| Correlationa | Test Statisticb | P Value | Correlationa | Test Statisticb | P Value | |
| 6 mo | ||||||
| Age | –0.02 | –0.51 | .61 | –0.03 | –0.3 | .77 |
| Sex | NA | –2.9 | .004 | NA | –1.1 | .27 |
| Race | NA | 0.47 | .70 | NA | 3.7 | .01 |
| Years of educationc | 0.07 | 1.3 | .21 | 0.04 | 0.39 | .69 |
| Median income of zip code (USD) | 0.06 | 5.1e + 07 | .12 | –0.09 | 4.7e + 05 | .29 |
| Charlson Comorbidity Indexd | –0.06 | 2.3e + 06 | .32 | –0.06 | 4.5e + 05 | .49 |
| Functional Comorbidity Indexd | –0.09 | 2.4e + 06 | .17 | 0.03 | 4.1e + 05 | .71 |
| Past medical history of depressiond | NA | –1.5 | .14 | NA | –0.9 | .37 |
| APACHE IIe | 0.09 | 2.3 | .02 | –0.21 | –2.5 | .01 |
| LOS, d | ||||||
| Hospitalf | 0.06 | 5.0e + 07 | .14 | –0.07 | 4.6e + 05 | .41 |
| ICUg | 0.003 | 5.3e + 07 | .93 | –0.004 | 4.3e + 05 | .96 |
| Retrospective perception of health before ARDSh | 0.17 | 1.8e + 06 | .01 | 0.34 | 1.4e + 05 | .0003 |
| 12 mo | ||||||
| Age | –0.03 | –0.82 | .41 | 0.08 | 0.9 | .37 |
| Sex | NA | –2.2 | .03 | NA | –1.1 | .26 |
| Race | NA | 0.53 | .66 | NA | 3.3 | .02 |
| Years of educationc | 0.04 | 0.64 | .52 | 0.2 | 2.3 | .03 |
| Median income of zip code (USD) | < 0.001 | 5.4e+07 | .99 | 0.18 | 3.5 e+05 | .03 |
| Charlson Comorbidity Indexd | –0.001 | 2.2e+06 | .99 | –0.15 | 4.9 e+05 | .08 |
| Functional Comorbidity Indexd | –0.05 | 2.3e+06 | .44 | –0.008 | 4.3 e+05 | .93 |
| Past medical history of depressiond | NA | –1.0 | .30 | NA | –0.9 | .36 |
| APACHE IIe | 0.007 | 0.17 | .86 | –0.15 | –1.8 | .08 |
| LOS, d | ||||||
| Hospitalf | 0.02 | 5.1e+07 | .51 | –0.08 | 4.6 e+05 | .37 |
| ICUg | –0.006 | 5.3e+07 | .87 | –0.02 | 4.4 e+05 | .84 |
| Retrospective perception of health before ARDSh | 0.18 | 1.8e+06 | .007 | 0.13 | 1.9 e+05 | .19 |
For sex and for normally distributed continuous variables (age, education, APACHE II), we report the parametric Student t test. For skewed continuous variables (income, Charlson Comorbidity Index, Functional Comorbidity Index, hospital LOS, ICU LOS, retrospective perception of health before ARDS), we report Spearman ρ statistic. The relationship between race and PD was explored using a one-way analysis of variance model. We report the F statistic. APACHE = Acute Physiology and Chronic Health Evaluation; ICAP = Improving Care of Acute Lung Injury Patients study; LOS = length of stay; NA = not applicable; PD = perspective deviation; USD = US dollars.
Correlation coefficients are reported for continuous variables. The Pearson correlation coefficient was reported for age, years of education, and APACHE II score. Spearman rank correlation coefficient was reported for the following characteristics with skewed distributions: median income of zip code, Charlson Comorbidity Index, hospital LOS, ICU LOS, and retrospective perception of health before ARDS. In both cases, correlations with absolute value of < 0.30 are weak, those of 0.3 to 0.7 are moderate, and those > 0.70 are high.
Reported test statistics.
Available for 307 ALTOS patients and 130 ICAP patients.
Available for all ICAP patients and 236 ALTOS patients from five of 11 study sites.
Missing for 20 ALTOS patients.
Missing for 5 ALTOS patients.
Missing for 4 ALTOS patients.
Available for 109 ICAP patients and 236 ALTOS patients from five of 11 study sites.
Discussion
We used 35 self-reported measures of physical, emotional, and social functioning to predict how > 800 adults treated in and discharged from the ICU from 44 US hospitals would perceive their health at 6 and 12 months after ARDS. A moderate correlation was found between predicted and actual health perception across both cohorts and time points, with about half of survivors reporting a perception that differed from predictions by more than the minimal clinically important difference for the EQ-5D-VAS. We termed the difference between predicted and actual survivor perceptions of health as perspective deviation. In exploratory analyses, the correlation between survivor demographics, illness severity, and comorbidity with PD was negligible. Estimated PD 6 months after ARDS was correlated weakly with PD 12 months after ARDS, suggesting that perceptions of health relative to self-reported functioning may change substantially over time during survivorship.
Our research builds on a robust literature describing patient-reported outcome measures (PROMS) after critical illness.59,60 Studying health perception is challenging because it requires using subjective self-evaluation PROMS like the EQ-5D-VAS question or the general health perceptions subscale of the 36-item Short Form Health Survey.61 Self-evaluation PROMS ask respondents to compare their experiences with personal, internal standards,62 and these standards or definitions can change, particularly when a person becomes ill. In response to this phenomenon, termed response shift,36 psychometricians have developed nearly a dozen methods63,64 to answer the question: How would this person have responded to this self-evaluation PROM if their internal standard or definition had not changed? In contrast, we attempted to answer a different question: How does this person’s perceived health compared with the perceived health of other people who also survived critical illness at a similar time and are experiencing similar levels of self-reported physical, emotional, and social functioning?
Our findings suggest that combined measures of physical, emotional, and social functioning are important predictors of perceived health after ARDS, but they are not determinative. One year after ARDS, survivors who gave similar answers to 35 well-validated questions covering a wide range of issues, including lifting and carrying groceries, feeling depressed, and feeling pain, sometimes gave vastly different answers when asked about their overall health status using the EQ-5D-VAS. Why might this be? One possibility is that the SF-36v2 and EQ-5D-3L lack questions about important determinants of perceived health. Some have argued that the experience of ICU survivorship is unique in ways that generic PROMS like the SF-36v2 and EQ-5D-3L fail to capture.65,66 For example, neither the SF-36v2 nor the EQ-5D-3L address sleep,67 and sleep disturbance is common in ICU survivorship.68 However, sleep disturbance is likely to affect fatigue, mental health, and productivity at work, and items in the SF-36v2 query each of these issues.
Variability in psychological resilience, or the ability to adapt and adjust to adversity and significant sources of stress,30 also may contribute to PD. How individuals approach and react to negative events and their beliefs about their ability to recover from these negative events, termed trait resilience,32 contribute to well-being and likely vary across survivors. A 2016 study of adult survivors of medical ICUs found resilience was correlated inversely with self-reported symptoms of anxiety, depression, posttraumatic stress, pain, and difficulty with self-care.31
Another possible factor influencing perceived health is social comparison. Decades of well-being research has demonstrated that people tend to answer questions about subjective states like health and happiness by comparing themselves to both internal (their past selves) and external (peer) reference groups.69, 70, 71 For example, a survivor with mild fatigue 6 months after ARDS who views healthy people of the same age as peers may describe their health as being only fair. However, if that same survivor views people with severe symptoms after critical COVID-19 as their peers, they instead may view their health as good. As clinics and support groups for those treated in ICUs proliferate, more such patients may believe that they are part of a community with its own norms and standards of health.
Finally, expectations may serve as a key comparator for survivors of ARDS reflecting on their health. Patient expectations about ARDS survivorship largely are unstudied, and many intensivists report reluctance to discuss functional outcomes after discharge from the ICU with patients and their families.72 A recent review of English-language media stories on ARDS found that few reports commented on disability among survivors of ARDS, and reports that mentioned survivorship largely reported no functional deficits.73 Appropriately, the Intensive Care Society identifies expectation management and improving public understanding of intensive care as a priority.74
Our results have important implications for clinicians caring for critically ill patients and those treated and discharged from ICUs. Patients and families who struggle to imagine survivorship can be reminded that how survivors feel about their health is not determined solely by what their bodies and minds can do. Similarly, clinicians should not make assumptions about how a patient will adapt psychologically to a new health state based on their demographics, severity of illness, comorbidity burden, or history of depression. The fact that 6- and 12-month PD values were correlated only weakly implies that survivorship is a process and that perceptions of health can change substantially throughout that process. The good news is that although functioning, demographics, and diagnoses are not strongly predictive, ascertaining perceived health is a straightforward procedure. It requires only that clinicians make time to ask and listen.
This investigation has limitations. First, we studied adult survivors of ARDS in the United States who may not be representative of survivors of ARDS in other countries or patients treated in and discharged from ICUs more broadly. Patients from ALTOS were also enrolled in ARDS Network clinical trials, and thus had fewer baseline comorbidities than the general population of patients with ARDS treated in the ICU. We also do not assume that these results are applicable to survivors of COVID-19, given the comparatively robust media coverage of long-COVID.75 Our prediction models are not designed to be broadly applicable to cohorts outside of our study. The fact that survivors in the ICAP cohort reported worse perceived health than survivors in the ALTOS cohort (after controlling for measures of function) implies there may be geographical or center effects in studies of perceived health after critical illness. We also lacked data on social support that could play a role in perceived health.
Finally, the EQ-5D-VAS is a single, imperfect measure of perceived health that requires respondents to conceive of a two-dimensional line as representative of a complex, abstract concept.76 Robust methodologies for assessing perceived health, such as ecological momentary assessment,77 which involves repeated real-time sampling of a person’s experience in their natural environment, could help to maximize the validity of perceived health measures. We also encourage replication of this investigation using other patient cohorts and other techniques to assess perceived health (eg, time tradeoff or the general health perceptions subscale of the 36-item Short Form Health Survey).
Interpretation
Perceived health is one of the strongest determinants of subjective well-being, but it is not determined solely by perceived physical, emotional, and social functioning among survivors of ARDS. For survivors who self-reported similar levels of functioning, we found that demographics, comorbidity burden, and severity of illness were correlated only weakly with perceived health. We hypothesize that resilience, psychological adaptation, social comparison, and expectations about survivorship could influence perceived health, and we encourage further study to test these hypotheses. In the meantime, clinicians in both the ICU and rehabilitation settings should refrain from making assumptions about how survivors of ARDS will adapt psychologically to new health states based on demographics and diagnoses.
Take-home Points.
Study Question: Among survivors of ARDS, is perspective deviation (PD; defined as the difference between observed vs predicted perceived health) associated with survivors’ demographics, comorbidity, or ICU severity of illness?
Results: Demographics, comorbidity, and ICU severity of illness were not associated strongly with PD in two large cohorts of survivors of ARDS assessed at 6 and 12 months after ARDS.
Interpretation: Clinicians should refrain from making assumptions based on commonly available medical data about how survivors of ARDS will perceive their health over the first year of recovery.
Acknowledgments
Author contributions: A. E. T. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A. E. T., A. W. W., and D. M. N. contributed to conception and design of the study. V. D. D., C. D. H., C. B. S., P. A. M.-T., and R. O. H. contributed to data collection. H. J. verified the statistical methods. A. E. T. performed data analyses and wrote the manuscript. All authors provided critical feedback, aided in interpreting the results, and commented on the manuscript.
Financial/nonfinancial disclosures: None declared.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This research was supported by the National Heart, Lung, and Blood Institute [Grant K01HL141637]. Role of the sponsors: This research was supported by the National Heart, Lung, and Blood Institute [Grant K01HL141637]. The ARDSNet Long Term Outcomes Study was supported by the ALTA, EDEN, OMEGA, and SAILS trials [National Heart, Lung, and Blood Institute contracts HHSN268200536165C to HHSN268200536176C and HHSN268200536179C] and the National Institutes of Health [Grants N01HR56170, R01HL091760, and R01HL091760-02S1]. The Improving Care of Acute Lung Injury Patients Study was supported by the National Institutes of Health [Grants P050HL73994, K24HL088551, and R01HL88045] and the Johns Hopkins Institute for Clinical and Translational Research [Grant UL1TR000424].
Supplementary Data
References
- 1.Wunsch H., Linde-Zwirble W.T., Angus D.C., Hartman M.E., Milbrandt E.B., Kahn J.M. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947–1953. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman J.E., Kramer A.A., Knaus W.A. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care. 2013;17(2):R81. doi: 10.1186/cc12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwashyna T.J., Cooke C.R., Wunsch H., Kahn J.M. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim Z.J., Subramaniam A., Ponnapa Reddy M., et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med. 2020;203(1):54–66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herridge M.S., Tansey C.M., Matté A., et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 6.Needham D.M., Dinglas V.D., Morris P.E., et al. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188(5):567–576. doi: 10.1164/rccm.201304-0651OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bienvenu O.J., Colantuoni E., Mendez-Tellez P.A., et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43(3):642–653. doi: 10.1097/CCM.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marra A, Pandharipande PP, Girard TD, et al. Cooccurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med. 2018;46(9):1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spragg R.G., Bernard G.R., Checkley W., et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181(10):1121–1127. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutschman C.S., Ahrens T., Cairns C.B., Sessler C.N., Parsons P.E. Critical Care Societies Collaborative/USCIITG Task Force on Critical Care Research. Multisociety task force for critical care research: key issues and recommendations. Am J Respir Crit Care Med. 2012;185(1):96–102. doi: 10.1164/rccm.201110-1848ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieu T.A., Au D., Krishnan J.A., et al. Comparative effectiveness research in lung diseases and sleep disorders: recommendations from the National Heart, Lung, and Blood Institute workshop. Am J Respir Crit Care Med. 2011;184(7):848–856. doi: 10.1164/rccm.201104-0634WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Needham D.M., Davidson J., Cohen H., et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 13.Semler M.W., Bernard G.R., Aaron S.D., et al. Identifying clinical research priorities in adult pulmonary and critical care. NHLBI Working Group Report. Am J Respir Crit Care Med. 2020;202(4):511–523. doi: 10.1164/rccm.201908-1595WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbull A.E., Rabiee A., Davis W.E., et al. Outcome measurement in ICU survivorship research from 1970 to 2013: a scoping review of 425 publications. Crit Care Med. 2016;44(7):1267–1277. doi: 10.1097/CCM.0000000000001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milton A., Schandl A., Soliman I.W., et al. Development of an ICU discharge instrument predicting psychological morbidity: a multinational study. Intensive Care Med. 2018;44(12):2038–2047. doi: 10.1007/s00134-018-5467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Detsky M.E., Harhay M.O., Bayard D.F., et al. Six-month morbidity and mortality among intensive care unit patients receiving life-sustaining therapy. A prospective cohort study. Annals ATS. 2017;14(10):1562–1570. doi: 10.1513/AnnalsATS.201611-875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schandl A., Bottai M., Holdar U., Hellgren E., Sackey P. Early prediction of new-onset physical disability after intensive care unit stay: a preliminary instrument. Critical Care. 2014;18(4):455. doi: 10.1186/s13054-014-0455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haines K.J., Hibbert E., McPeake J., et al. Prediction models for physical, cognitive, and mental health impairments after critical illness: a systematic review and critical appraisal. Crit Care Med. 2020;48(12):1871–1880. doi: 10.1097/CCM.0000000000004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kon A.A., Davidson J.E., Morrison W., Danis M., White D.B. Shared decision making in ICUs: an American College of Critical Care Medicine and American Thoracic Society policy statement. Crit Care Med. 2016;44(1):188–201. doi: 10.1097/CCM.0000000000001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasiter S., Oles S.K., Mundell J., London S., Khan B. Critical care follow-up clinics: a scoping review of interventions and outcomes. Clin Nurse Spec. 2016;30(4):227–237. doi: 10.1097/NUR.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuthbertson B.H., Rattray J., Campbell M.K., et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehlhorn J., Freytag A., Schmidt K., et al. Rehabilitation interventions for postintensive care syndrome: a systematic review∗. Crit Care Med. 2014;42(5):1263–1271. doi: 10.1097/CCM.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 23.Kross E.K., Hough C.L. Broken wings and resilience after critical illness. Annals ATS. 2016;13(8):1219–1220. doi: 10.1513/AnnalsATS.201605-327ED. [DOI] [PubMed] [Google Scholar]
- 24.Sevin C.M., Bloom S.L., Jackson J.C., Wang L., Ely E.W., Stollings J.L. Comprehensive care of ICU survivors: development and implementation of an ICU recovery center. J Crit Care. 2018;46:141–148. doi: 10.1016/j.jcrc.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geense W.W., van den Boogaard M., van der Hoeven J.G., Vermeulen H., Hannink G., Zegers M. Nonpharmacologic interventions to prevent or mitigate adverse long-term outcomes among ICU survivors: a systematic review and meta-analysis. Crit Care Med. 2019;47(11):1607–1618. doi: 10.1097/CCM.0000000000003974. [DOI] [PubMed] [Google Scholar]
- 26.Elliott D., McKinley S., Alison J., et al. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care. 2011;15(3):R142. doi: 10.1186/cc10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen J.F., Egerod I., Bestle M.H., et al. A recovery program to improve quality of life, sense of coherence and psychological health in ICU survivors: a multicenter randomized controlled trial, the RAPIT study. Intensive Care Med. 2016;42(11):1733–1743. doi: 10.1007/s00134-016-4522-1. [DOI] [PubMed] [Google Scholar]
- 28.Iwashyna T.J., Walsh T.S. Interplay of physiology, social, familial and behavioural adaptation in the long-term outcome of ARDS. Thorax. 2017;72(10):872–873. doi: 10.1136/thoraxjnl-2016-209859. [DOI] [PubMed] [Google Scholar]
- 29.Dinglas V.D., Faraone L.N., Needham D.M. Understanding patient-important outcomes after critical illness: a synthesis of recent qualitative, empirical, and consensus-related studies. Curr Opin Crit Care. 2018;24(5):401–409. doi: 10.1097/MCC.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charney D.S. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 31.Maley J.H., Brewster I., Mayoral I., et al. Resilience in survivors of critical illness in the context of the survivors’ experience and recovery. Annals ATS. 2016;13(8):1351–1360. doi: 10.1513/AnnalsATS.201511-782OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maltby J., Day L., Hall S. Refining trait resilience: identifying engineering, ecological, and adaptive facets from extant measures of resilience. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0131826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waljee J., McGlinn E.P., Sears E.D., Chung K.C. Patient expectations and patient-reported outcomes in surgery: a systematic review. Surgery. 2014;155(5):799–808. doi: 10.1016/j.surg.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turnbull A.E., Hurley M.S., Oppenheim I.M., Hosey M.M., Parker A.M. Curb your enthusiasm: definitions, adaptation, and expectations for quality of life in ICU survivorship. Annals ATS. 2020;17(4):406–411. doi: 10.1513/AnnalsATS.201910-772IP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprangers M.A., Schwartz C.E. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 36.Oort F.J., Visser M.R.M., Sprangers M.A.G. Formal definitions of measurement bias and explanation bias clarify measurement and conceptual perspectives on response shift. J Clin Epidemiol. 2009;62(11):1126–1137. doi: 10.1016/j.jclinepi.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Clark A.E., Oswald A.J. A simple statistical method for measuring how life events affect happiness. Int J Epidemiol. 2002;31(6):1139–1144. doi: 10.1093/ije/31.6.1139. [DOI] [PubMed] [Google Scholar]
- 38.Blumenthal J.A., Emery C.F., Smith P.J., et al. The effects of a telehealth coping skills intervention on outcomes in chronic obstructive pulmonary disease: primary results from the INSPIRE-II Study. Psychosom Med. 2014;76(8):581–592. doi: 10.1097/PSY.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Matthay M.A., Brower R.G., et al. Randomized, placebo-controlled clinical trial of an aerosolized β₂-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Rice T.W., Wheeler A.P., et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice T.W., Wheeler A.P., Thompson B., et al. Enteral omega-3 fatty acid, γ-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Truwit J.D., Bernard G.R., et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Needham D.M., Colantuoni E., Mendez-Tellez P.A., et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabin R., de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 45.Shaw J.W., Johnson J.A., Coons S.J. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Chrispin P.S., Scotton H., Rogers J., Lloyd D., Ridley S.A. Short Form 36 in the intensive care unit: assessment of acceptability, reliability and validity of the questionnaire. Anaesthesia. 1997;52(1):15–23. doi: 10.1111/j.1365-2044.1997.015-az014.x. [DOI] [PubMed] [Google Scholar]
- 47.Needham D.M., Sepulveda K.A., Dinglas V.D., et al. Core outcome measures for clinical research in acute respiratory failure survivors. An International Modified Delphi Consensus Study. Am J Respir Crit Care Med. 2017;196(9):1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 49.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 50.Groll D.L., To T., Bombardier C., Wright J.G. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Groll D.L., Heyland D.K., Caeser M., Wright J.G. Assessment of long-term physical function in acute respiratory distress syndrome (ARDS) patients: comparison of the Charlson Comorbidity Index and the Functional Comorbidity Index. Am J Phys Med Rehabil. 2006;85(7):574–581. doi: 10.1097/01.phm.0000223220.91914.61. [DOI] [PubMed] [Google Scholar]
- 52.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol. 1996;58(1):267–288. [Google Scholar]
- 53.Hoerl A.E., Kennard R.W. Ridge regression: biased estimation for nonorthogonal problems. Technometrics. 1970;12(1):55–67. [Google Scholar]
- 54.Zou H., Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol. 2005;67(2):301–320. [Google Scholar]
- 55.Breiman L. Random forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 56.Mason L, Baxter J, Bartlett P, Frean M. Boosting algorithms as gradient descent. In: Proceedings of the 12th International Conference on Neural Information Processing Systems. NIPS’99. MIT Press; 1999:512-518.
- 57.Zanini A., Aiello M., Adamo D., et al. Estimation of minimal clinically important difference in EQ-5D visual analog scale score after pulmonary rehabilitation in subjects with COPD. Respir Care. 2015;60(1):88–95. doi: 10.4187/respcare.03272. [DOI] [PubMed] [Google Scholar]
- 58.Nolan C.M., Longworth L., Lord J., et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax. 2016;71(6):493–500. doi: 10.1136/thoraxjnl-2015-207782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwashyna T.J., Netzer G. The burdens of survivorship: an approach to thinking about long-term outcomes after critical illness. Semin Respir Crit Care Med. 2012;33(4):327–338. doi: 10.1055/s-0032-1321982. [DOI] [PubMed] [Google Scholar]
- 60.Rousseau A.-F., Prescott H.C., Brett S.J., et al. Long-term outcomes after critical illness: recent insights. Critical Care. 2021;25(1):108. doi: 10.1186/s13054-021-03535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brummel N.E. Measuring outcomes after critical illness. Crit Care Clin. 2018;34(4):515–526. doi: 10.1016/j.ccc.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz C.E., Rapkin B.D. Reconsidering the psychometrics of quality of life assessment in light of response shift and appraisal. Health Qual Life Outcomes. 2004;2:16. doi: 10.1186/1477-7525-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sajobi T.T., Brahmbatt R., Lix L.M., Zumbo B.D., Sawatzky R. Scoping review of response shift methods: current reporting practices and recommendations. Qual Life Res. 2018;27(5):1133–1146. doi: 10.1007/s11136-017-1751-x. [DOI] [PubMed] [Google Scholar]
- 64.Sébille V, Lix LM, Ayilara OF, et al. Critical examination of current response shift methods and proposal for advancing new methods [published online ahead of print February 17, 2021]. Qual Life Res. https://doi.org/10.1007/s11136-020-02755-4. [DOI] [PMC free article] [PubMed]
- 65.Lim W.C., Black N., Lamping D., Rowan K., Mays N. Conceptualizing and measuring health-related quality of life in critical care. J Crit Care. 2016;31(1):183–193. doi: 10.1016/j.jcrc.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 66.Malmgren J., Waldenström A.-C., Rylander C., Johannesson E., Lundin S. Long-term health-related quality of life and burden of disease after intensive care: development of a patient-reported outcome measure. Critical Care. 2021;25(1):82. doi: 10.1186/s13054-021-03496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manocchia M., Keller S., Ware J.E. Sleep problems, health-related quality of life, work functioning and health care utilization among the chronically ill. Qual Life Res. 2001;10(4):331–345. doi: 10.1023/a:1012299519637. [DOI] [PubMed] [Google Scholar]
- 68.Altman M.T., Knauert M.P., Pisani M.A. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457–1468. doi: 10.1513/AnnalsATS.201702-148SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baron-Epel O., Kaplan G. General subjective health status or age-related subjective health status: does it make a difference? Soc Sci Med. 2001;53(10):1373–1381. doi: 10.1016/s0277-9536(00)00426-3. [DOI] [PubMed] [Google Scholar]
- 70.Powdthavee N. Ill-health as a household norm: evidence from other people’s health problems. Soc Sci Med. 2009;68(2):251–259. doi: 10.1016/j.socscimed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 71.Carrieri V. Social comparison and subjective well-being: does the health of others matter? Bull Econ Res. 2012;64(1):31–55. doi: 10.1111/j.1467-8586.2011.00393.x. [DOI] [PubMed] [Google Scholar]
- 72.Turnbull A.E., Davis W.E., Needham D.M., White D.B., Eakin M.N. Intensivist-reported facilitators and barriers to discussing post-discharge outcomes with intensive care unit surrogates: a qualitative study. Ann Am Thorac Soc. 2016;13(9):1546–1552. doi: 10.1513/AnnalsATS.201603-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernando SM, Mathew R, Hodgson CL, Fan E, Brodie D. Media portrayals of the acute respiratory distress syndrome. Chest. 2021;160(3):965–968. doi: 10.1016/j.chest.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 74.Intensive Care Society Intensive care 2020 and beyond. Published 2020. Intensive Care Society website. https://www.ics.ac.uk/ICS/Guidelines/PDFs/Intensive_Care_2020_and_Beyond
- 75.Hosey M.M., Needham D.M. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6(1):1–2. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wewers M.E., Lowe N.K. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 77.Shiffman S., Stone A.A., Hufford M.R. Ecological momentary assessment. Ann Rev Clin Psychol. 2008;4(1):1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.