Abstract
The current obesity epidemic is calling for action in the determination of contributing factors. Although social and life-style factors have been traditionally associated with metabolic disruption, a subset of endocrine-disrupting chemicals (EDCs), called obesogens are garnering increasing attention for their ability to promote adipose tissue differentiation and accumulation. For some chemicals, such as tributyltin, there is conclusive evidence regarding their ability to promote adipogenesis and their mechanism of action. In recent years, the list of chemicals that exert obesogenic potential is increasing. In this chapter, we review current knowledge of the most recent developments in the field of emerging obesogens with a specific focus on food additives, surfactants, sunscreens for many of which the mechanism of action remains unclear. We also review new evidence relative to the obesogenic potential of environmentally relevant chemical mixtures and point to potential therapeutic approaches to minimize the detrimental effects of obesogens. We conclude by discussing the available tools to investigate new obesogenic chemicals, strategies to maximize reproducibility in adipogenic studies, and future directions that will help propel the field forward.
Keywords: adipose tissue, adipogenesis, endocrine disrupting chemicals, emerging obesogens, food additives
1. Introduction
Obesity, defined as body mass index (BMI) > 30 kg/m2, continues to be among the most prevalent chronic co-morbidities in the US. In the past 2 decades, obesity prevalence has increased from 30.5% in 1999–2000 to 42.4% in 2017–2018 (Hales et al. 2020). Prevalence of severe obesity (BMI > 40 kg/m2) has also continued to increase from 4.7 to 9.2%, with women being disproportionately more affected by severe obesity than men (11.5 vs. 6.9%, respectively; (Hales et al. 2020). In 2016, obesity prevalence in U.S. children was 18.5%, representing 13 million children age 2 to 19, which is concerning since childhood obesity is strongly associated with obesity later in life (Hales et al. 2017). Because monogenic obesity, which is caused by a single gene mutation is relatively rare and obesity is more often considered to be syndromic (associated with other phenotypes, such as neurodevelopmental abnormalities) or polygenic (caused by a combination of genes and the environment) (Thaker 2017), the increasing obesity trends can only be explained by the latter. Notably, obesity prevalence disproportionately affects certain groups, with non-Hispanic African Americans (49.6%) being more affected by obesity compared to age-matched non-Hispanic Whites (44.8%) or Asians (17.4%) (Hales et al. 2020). Other socio-economic factors such as lower education and income have also been associated with a higher obesity prevalence (Ogden et al. 2017). The disparity in prevalence among socioeconomic groups points to non-caloric/non-genetic factors contributing to the increased obesity prevalence. Nutritional and lifestyle habits are thus considered major drivers of obesity. However, growing evidence also supports a contributory role of environmental factors at certain developmental stages to the obesity epidemic. Pioneered in the late 1980s by Barker et al. (Barker et al. 1989), studies in the field of developmental origins of health and disease (DOHaD) have demonstrated that pre-conceptional and peri-gestational factors, such as maternal nutrition (Davenport and Cabrero 2009; Strohmaier et al. 2020), maternal glycemic status (Tam et al. 2017), maternal stress (Leppert et al. 2018; Matvienko-Sikar et al. 2020), and/or paternal adiposity (Sharp and Lawlor 2019) can modulate the obesity risk of the progeny. Other environmental factors, such as chemical exposures can also modulate the risk to develop obesity during prenatal, as well as postnatal life (Egusquiza and Blumberg 2020; Heindel et al. 2017; Veiga-Lopez et al. 2018).
2. Adipose tissue: an overview
Obesity is generally described as excessive accumulation of white adipose tissue (WAT) in the body (Rosen and Spiegelman 2006) and it is a risk factor for comorbidities such as cardiovascular disease, type 2 diabetes, and cancer (Meigs et al. 2006). WAT depots differ not only in location, but also in function and developmental stage at which they appear for the first time (Chau et al. 2014). Retroperitoneal, perigonadal and mesenteric WATs are visceral depots, and their dysregulation or misfunction has been associated with adverse health outcomes (Chau et al. 2014), while interscapular and inguinal WAT depots are located subcutaneously, are associated with body thermoregulation, and have lower risks for development of other comorbidities.
The fact that the location of the WAT plays a role on the severity of obesity implies that each obesity case should be evaluated carefully. Not all obese individuals develop further metabolic complications such as high glucose levels or altered lipid panel, which are often linked to obesity-associated diseases (McLaughlin et al. 2007). The concept of metabolically healthy obesity was first coined in the 1950s and it is based in clinical observations of a cohort of obese patients with reduced predisposition to type 2 diabetes and atherosclerosis compared to metabolically unhealthy obese patients (Vague 1956). Although there is not a clear definition for metabolically healthy obesity, there is some consensus on the fact that it has been associated with the lack of ectopic fat storage, such as in the visceral cavity, liver or muscle tissue, and lack of insulin resistance, hypertension, and/or cardiovascular risk (Bluher 2020). However, obese metabolically healthy individuals are still at a higher risk of developing adverse comorbidities than lean individuals (Bluher 2020). Before discussing how environmental factors may contribute to the development of unhealthy adipose tissue and obesity, it is necessary to understand adipose tissue development, types, and functions.
2a. White adipose tissue development
WAT contains an heterogeneous cell population that includes mature adipocytes, which only constitutes ~50% of the white adipose tissue mass, and stromal cells (e.g., endothelial, blood cells, and adipocyte precursors) (Rodeheffer et al. 2008). One of the functions of WAT is to act as a nutrient sensor. Thus, in periods of high nutrient availability, adipocytes synthesize and store triglycerides that can be mobilized as free fatty acids in periods of high energy demand (Rosen and Spiegelman 2006). In addition to maintaining nutrient homeostasis, the adipose tissue also plays an important role as an endocrine organ, as it secretes adipokines (e.g., adiponectin and leptin) and enzymes that participate in steroid hormone metabolism (e.g., aromatase) (Kershaw and Flier 2004).
WAT precursors rise during prenatal development in two different forms: precursors that will contribute to the development of adipose tissue, and precursors involved in its homeostasis during adulthood (Jiang et al. 2014). One key characteristic of healthy WAT is its plasticity to adapt depending on environmental cues, such as nutrient availability, energy expenditure or temperature (Pellegrinelli et al. 2016). WAT can expand following two different pathways: hypertrophy (i.e., enlargement of cell size due to increased lipid storage) or hyperplasia (i.e., increased in the number of adipocytes due to proliferation of precursor cells). Hypertrophic adipose tissue is positively associated with chronic inflammation, fibrosis and insulin resistance (Vishvanath and Gupta 2019). In contrast, hyperplasia has been associated with a more metabolically healthy state (Vishvanath and Gupta 2019). Both processes are often accompanied by the remodeling of the extracellular matrix, which provides the tissue with mechanical flexibility and support to grow healthy when necessary, based on environmental stimuli. Unhealthy adipose tissue develops if the excessive growth of the tissue is accompanied by fibrosis, oxidative stress due to impaired angiogenesis, and recruitment of inflammatory cells such as macrophages and lymphocytes (Crewe et al. 2017).
2b. Other types of adipose tissue
Another type of adipose tissue found in mammals is the brown adipose tissue (BAT). In contrast to the detrimental effects of excessive accumulation of WAT, increased BAT accumulation introduces metabolic benefits has traditionally been described as a potential therapeutic target to treat obesity (Harms and Seale 2013). BAT is very vascularized and it is involved in body control of temperature, or thermoregulation, by burning out body fat (Harms and Seale 2013). In newborns, BAT constitutes ~5% of total fat content, reaching the highest percentages during puberty and going down to minimal presence in adulthood (Drubach et al. 2011). The amount of BAT in adults largely depends on each individual’s lifestyle, as it has been described that exercise and exposure to low temperatures, increases BAT development (Drubach et al. 2011).
If the different structure, function, and location of WAT and BAT was not complex enough, white adipocytes can acquire brown adipocyte behaviors upon exposure to certain environmental stimuli, such as low temperature (Vitali et al. 2012), and become beige adipocytes (Harms and Seale 2013).
The difference between adipose tissue type (WAT, BAT, and beige), its location in the body (subcutaneous vs. visceral), and whether it is healthy or dysfunctional can dramatically impact the health of the individual and contributes to whether the obese subject is metabolically healthy or unhealthy. Environmental factors have been shown to influence the accumulation and function of both WAT and BAT, and the capacity of WAT to become beige cells (Symonds et al. 2021; Xiao et al. 2020). As such, environmental factors play a critical role not only in the increasing prevalence of obesity worldwide, but also in its association with other disease such as type 2 diabetes and cardiovascular disease.
3. Obesogens, environmental factors that interfere with adipose tissue metabolism
Since the industrial revolution in the 1950s, an increasing number of chemicals have been introduced into the manufacturing chain of consumer products, with over 40,000 of them currently in commerce (EPA 2020). Their production volume also continues to increase with production of plastics worldwide from 1950 to 2018 increasing up to 6,300 metric tons / year (Geyer et al. 2017). Notably, some of these chemicals can interfere with normal endocrine function and are known as endocrine disrupting chemicals (EDCs) (Gore et al. 2015). Baillie-Hamilton proposed in 2002 evidence that certain chemicals can alter adipose tissue deposition contributing to obesity, which would later be known as obesogens (Baillie-Hamilton 2002; Grun et al. 2006). Bona fide obesogens are those that induce white adipose tissue storage in vivo, while potential obesogens are those that have only been shown to promote adipogenesis in vitro (Egusquiza and Blumberg 2020). Currently, ~20 chemicals are recognized as bona fide obesogens and include organotins, polychlorinated biphenyls, bisphenols, phthalates, parabens, and non-steroidal estrogens, among others (Andrews et al. 2020). These chemicals are components of common consumer products, such as plastic bottles, toys, vinyl flooring, food preservatives, disinfectants, and medical equipment, but also of building materials (paints, resins, solvents) and agrochemicals (Ren et al. 2020; Veiga-Lopez et al. 2018). Hence, exposure to these chemicals is ubiquitous and thus, human exposure virtually unavoidable. Since exposures to these chemicals occur throughout an individual’s lifetime (Hendryx and Luo 2018; Shim et al. 2017; Woodruff et al. 2011; Zota et al. 2014), recognizing the major events that shape an individual’s adipose tissue development is key in understanding the major windows of susceptibility to obesogenic chemical exposures.
For some obesogenic chemicals, there is conclusive evidence regarding their ability to modulate adipogenesis, as they activate the peroxisome proliferator-activated receptor gamma (PPARγ) or its heterodimeric partner, the retinoid X receptor alpha (RXRα) (Egusquiza and Blumberg 2020; Heindel et al. 2015; Veiga-Lopez et al. 2018). Other chemicals increase adipogenic differentiation and adipose tissue accumulation by modulating other receptors such liver X receptor (LXR), thyroid receptor-beta (TRβ), glucocorticoid receptor (GR), estrogen receptor (ER), androgen receptor (AR), and others (Chappell et al. 2018; Kassotis et al. 2019; Niemelä et al. 2008).
PPARγ is considered the “master” regulator of adipogenesis as it is involved in the initial steps of adipocyte differentiation (Tontonoz and Spiegelman 2008), while RXRα has been shown to play a critical role in the commitment of stem cell precursors into the adipogenic pathway (Shoucri et al. 2017). One example of bona fide obesogen that activates both PPARγ and RXRα is tributyltin (TBT) (Grun et al. 2006; Yanik et al. 2011), which increases adipogenic commitment and differentiation in vitro (Kirchner et al. 2010; Li et al. 2011). In vivo, TBT can induce adipose tissue accumulation upon postnatal exposure in mammals and fish (Penza et al. 2011; Tingaud-Sequeira et al. 2011) and lipid accumulation in non-adipose tissues, such as liver (Zuo et al. 2011), brain (Lyssimachou et al. 2015), and ovary (de Araujo et al. 2018). More importantly, prenatal TBT exposures can reprogram adipose tissue development and these traits are transmitted transgenerationally through epigenetic mechanisms (Chamorro-Garcia et al. 2017; Chamorro-Garcia et al. 2013; Diaz-Castillo et al. 2019; Kirchner et al. 2010). Other organotins, such as triphenyltin and dibutyltin have also shown to exert obesogenic effects (Chamorro-Garcia et al. 2018; Lutfi et al. 2017; Milton et al. 2017).
Although relatively underexplored, certain obesogens induce development of dysfunctional adipocytes in vitro by altering glucose uptake, reduction of antidiabetic adipokine adiponectin, and reduction in the cell ability to inhibit expression of proinflammatory and profibrotic markers (Ariemma et al. 2016; Regnier et al. 2015; Shoucri et al. 2018). One example of obesogen that promotes development of dysfunctional adipocytes is TBT which has been attributed to its capability of activating RXRα (Shoucri et al. 2018). The mechanistic evidence is less definitive for other chemicals, including polychlorinated biphenyls, bisphenols, phthalates, parabens, and non-steroidal estrogens, which has been reviewed elsewhere (Egusquiza and Blumberg 2020; Ren et al. 2020; Veiga-Lopez et al. 2018). To note, other tissues, such as the liver are also targets of obesogenic chemicals and have been linked to chronic metabolic diseases such as non-alcoholic fatty liver disease (Foulds et al. 2017). In this review, we have focused on chemicals that have been recently reported to display obesogenic potential with a central focus on the adipose tissue, but for which mechanism of action remain unclear (See Table 1 and Figure 1).
Table 1.
Emerging obesogens and chemical mixtures
| Short name | Use | Cell model | Mechanism | Animal model | |
|---|---|---|---|---|---|
| 1. Food additives | |||||
| Dioctyl sodium sulfosuccinate | DOSS | Thickener Solubilizer Emulsifier Flavoring |
3T3-L1 | PPARγ | Mice |
| Sorbitan monooleate | Span 80 | Emulsifier | 3T3-L1 | PPARγ RXRα |
— |
| Polyoxyethylene (20) sorbitan monooleate | Tween 80 Polysorbate 80 |
Emulsifier | 3T3-L1 | RXRα | — |
| 3-tertbutyl-4-hydroxyanisole | 3-BHA | Antioxidant | 3T3-L1 | PPARγ activators | Mice |
| 2. Alkylphenols | |||||
| Ethoxylated alkylphenols | EOAP | Non-ionic surfactant | 3T3-L1 | TRβ | — |
| 4-hexylphenol | 4-HP | Surfactant | 3T3-L1 HepG2 | PPARγ | — |
| 3. Sunscreens | |||||
| Avobenzone | AVB | UVA filter | hMSCs | PPARγ | — |
| Benzonphenone 3 and 8 | BP-3 BP-8 |
UV filter | hMSCs | PPARγ | — |
| 4. EDC mixtures | |||||
|
Unconventional oil and gas chemicals
Acrylamide, Alkylphenols, Benzenes, Bronopol, Diethanolamine, Ethanols, Ethylene glycol, Propylene glycol, Styrene, Toluene, Xylens |
UOG | — | 3T3-L1 | PPARγ independent |
— |
|
Oil sands process-affected water See Headly et al., 2013 and Pereira et al., 2013 and 2015 for chemical mixture |
OSPW | — | 3T3-L1 | PPARγ | — |
|
EDC mixture #1
Phthalates, Triclosan, Perflorinated substances |
— | — | — | — | Zebrafish |
|
EDC mixture #2
Di(2-ethylhexyl) phthalate, Bisphenol A, 2,3,7,8-tetrachlorodibenzo-dioxine (TCDD), polychlorinated biphenyl 153 (PCB153) |
— | — | — | — | Mice |
Figure 1. Effect of emerging chemicals on adipogenesis and adipose tissue accumulation.
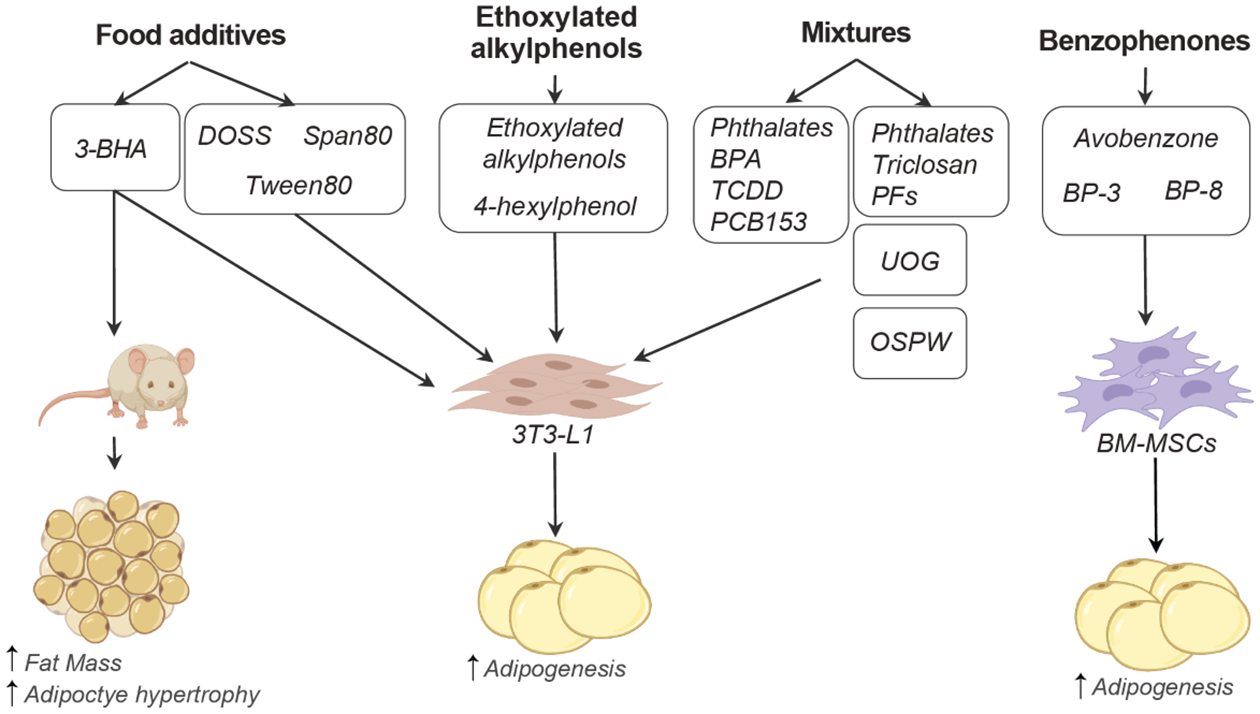
Emerging obesogenic chemicals are classified as food additives, ethoxylated alkylphenols, benzophenones, or chemical mixtures (top). Testing models that have been used so far for these emerging obesogens include cell-based models, such as the preadipocyte cell line 3T3-L1 and bone marrow mesenchymal stem cells (BM-MSCs) and mice. Additional in vivo studies to validate in vitro observations are needed. 3-BHA: 3-tertbutyl-4-hydroxyanisole, BP: benzophenone, BPA: bisphenol A, DOSS: dioctyl sodium sulfosuccinate, TCDD: 2,3,7,8-tetrachlorodibenzo-pdioxine, PCB: polychlorinated biphenyl, PF: perfluorinated chemicals, and UOG: unconventional oil and gas chemicals.
4. Emerging obesogens: the new kids on the block
Here, we discuss current knowledge on emerging obesogens defined as chemicals or mixture of chemicals for which there is preliminary evidence for their role to disturb adipose tissue development, but whose mechanisms of action remain unclear.
4a. Food additives
Food additives are compounds that help preserve food or change its qualities. Over 3,000 food additives are databased in the US Food and Drug Administration (FDA) and are classified into numerous categories including stabilizers, preservatives, coloring agents, flavor enhancers, antioxidants, and emulsifiers among others. While some food additives are natural products, such as vinegar or salt, others are not, but are considered by the US FDA as safe compounds. Here, we have focused on food additives, such as surfactants, emulsifiers, antioxidants, and flavor enhancers, that have been reported to enhance adipogenic differentiation and result in intracellular lipid accumulation.
Originally evaluated as a component of the oil dispersant COREXIT used in the clean-up of the Deepwater Horizon spill (USEPA 2010), dioctyl sodium sulfosuccinate (DOSS) is used as stabilizer, thickener, wetting agent, solubilizing agent, emulsifier, and flavoring agent in numerous food products, such as cream cheese, salad dressings, and milk (e-CFR 2020). Also known as docusate, DOSS is also approved for use as a stool softener. DOSS is rapidly absorbed and metabolized in the body making, which difficult its detection in human fluids (WHO 1975). DOSS has been reported to exert a dose-response increase in lipid accumulation in 3T3-L1 preadipocytes, with 118.6 μM of DOSS resulting in the same adipogenic differentiation as 1 μM of rosiglitazone (Temkin et al. 2016). DOSS’s adipogenic induction occurs through PPARγ (Bowers et al. 2016), but not RXRα activation (Bowers et al. 2016). Because of the rapid rate at which DOSS is metabolized in humans and other animals tested, it would be necessary to determine whether the results observed in vitro are indeed caused by DOSS or any of its metabolites. Sorbitan monooleate, also known as Span80, is another emulsifier part of COREXIT that can also enhance adipogenic differentiation of 3T3-L1 preadipocytes in a dose-dependent manner through transactivation of PPARγ and RXRα (Bowers et al. 2016). Polyoxyethylene (20) sorbitan monooleate, another emulsifier commonly known as Tween 80 or Polysorbate 80, can also enhance 3T3-L1 preadipocyte differentiation through RXRα activation (Bowers et al. 2016). Both, Span 80 and Tween 80 are derived from oleic acid, which has the ability to enhance adipogenic differentiation especially in the presence of insulin and dexamethasone through PPARγ (Madsen et al. 2005). Other surfactants, such as polyoxyethylene stearates (8 and 40), and polyethene sorbitans (monolaurate, monoleate, monopalmitate, monostearate, and tristearate), also containing saturated and monounsaturated fatty acids backbones, which have the ability to stimulate adipogenic differentiation (Madsen et al. 2005), have not been evaluated in the context of adipogenesis. Notably, mixture of these surfactants, such as DOSS and Span80, leads to significant increases adipocyte differentiation above that of each individual exposures (Bowers et al. 2016). Among all surfactants studied to date, only DOSS has been tested in vivo. A gestational DOSS exposure with an oral dose representing the use of stool softener (5μg/g BW) resulted in male, but not female mice offspring with increased body and visceral adipose tissue mass and accompanying glucose intolerance (Temkin et al. 2019). Additional studies should investigate whether surfactant mixtures with fatty acids backbones can lead to adipose tissue accumulation in vivo.
Introduced in the food industry in the 1950’s, 3-tertbutyl-4-hydroxyanisole (3-BHA) has been used as an antioxidant to prevent fat rancidification of foods. 3-BHA is also used in cholesterol lowering medications, such as lovastatin, and simvastatin and it is considered an EDC for its estrogenic and anti-estrogenic activities (Kang et al. 2005; Pop et al. 2013). Present not only in foodstuffs, but also in the environment (Liu et al. 2015; Wang et al. 2016; Wang and Kannan 2018), human exposure burden to synthetic phenolic antioxidants (SPA) is widespread; with BHA displaying lower percentage exposure and concentration compared to other SPAs (median: 0.014 ng/ml) (Liu and Mabury 2018). 3-BHA, but not 2-tert-butyl-4-hydroxyanisole (2-BHA) has been reported to enhance adipogenesis in 3T3-L1 preadipocytes (Sun et al. 2019). This effect however was driven by activation of upstream PPARγ modulators, cAMP-response element binding protein (CREB), upregulation of CAAT/enhancer-binding proteins β (C/EBPβ), but not direct PPARγ binding (Sun et al. 2019). Chronic (18 weeks) exposure to 3-BHA in male C57BL/6J mice did not affect body weight or insulin sensitivity, but increased percent inguinal subcutaneous adipose tissue, adipocyte size, and total cholesterol at 1 mg/kg BW, a dose well above human exposure ranges (Sun et al. 2020b). Whether these effects are reproducible at lower exposure ranges or can induce programming effects during gestational exposures has yet to be evaluated.
4b. Alkylphenols
Alkyphenols are organic compounds that belong to a class of synthetic-nonionic surfactants widely employed in the manufacture of detergents, adhesives, cosmetics, emulsifiers and household cleaners (Acir and Guenther 2018). Their global consumption of over 600 kilotonnes per year (Zgola-Grzeskowiak et al. 2015) makes them ubiquitous in the environment and human tissues. Human urine levels of alkylphenols have been estimated in ~12 ng/ml (You et al. 2011) Alkylphenols are considered xenoestrogens (Soto et al. 1991) and their effects on the nervous and immune systems have been widely studied (Acir and Guenther 2018). Because alkylphenols accumulate in human adipose tissue (Lopez-Espinosa et al. 2009; Muller et al. 1998) non-ethoxylated alkylphenols, such as 4-nonyphenol and octylphenol have been studied in the context of adipogenesis. For over a decade, we have known that both, 4-nonyphenol and octylphenol can lead to pro-adipogenic effects in vitro (Lee et al. 2008; Masuno et al. 2003; Miyawaki et al. 2008) and in vivo (Hao et al. 2012; Kim et al. 2015; Zhang et al. 2014). However, the effects of ethoxylated-alkylphenol on adipogenesis have only been explored recently. A study evaluating several alcohol- and ethoxylated-alkylphenols with chain lengths between 11 and 16 base carbons, reported that ethoxylated alkylphenols promote triglyceride accumulation with longer ethoxylate chains length resulting in a greater effect (Kassotis et al. 2018a). This effect was shown as low as 0.1 μM (0.1 ng/ml) and to be independent of PPARγ activation but partially driven by the TRβ antagonism in 3T3-L1 cells (Kassotis et al. 2018a). More recently, the alkylphenol 4-hexylphenol was also reported to enhance adipogenesis in 3T3-L1 cells and lipid accumulation in HepG2 cells (Sun et al. 2020a). However, in vivo exposure studies have not been reported to date.
4c. Sunscreens
Other chemicals that have recently been reported to enhance adipogenesis include those used as organic ingredients of sunscreen, such as benzophenones (Kim and Choi 2014). Bezonphenone 3 (BP-3) is present in human urine at 22.9 μg/l, while avobenzone is present in human plasma at 3.3–7.1 ng/ml for up to 21 days after the original exposure (Matta et al. 2020; Zamoiski et al. 2015). Considered EDCs for their ability to bind to the pregnane X receptor (Mikamo et al. 2003), benzophenones can block ultraviolet (UV) light and are thus also used to protect products, such as perfumes and soaps, from UV damage. Stemming from a microarray study, avobenzone, a dibenzoylmethane derivative used in sunscreens to absorb UV-A rays, was discovered to increase PPARγ transcription (Ahn et al. 2019). Using human bone marrow mesenchymal stem cells (hBM-MSCs), avobenzone was observed to promote adipogenesis (at 10 μM), but independently of the PPARγ pathway (Ahn et al. 2019). Using the same in vitro approach, benzonphenone (BP)-3 and BP-8 (at 30 μM) promoted adipogenic differentiation through PPARγ binding, with BP-3 being a full and BP-8 a partial PPARγ agonist (Shin et al. 2020). In vivo studies have yet to be conducted to validate these results at human exposure doses.
4d. Chemical mixtures
The complexity of studying chemical mixtures (Martin et al. 2020) have resulted in only a handful of studies that have attempted to evaluate the effect of a combination of chemicals on adipogenic differentiation. Using the 3T3-L1 cell model, Kassotis et al., 2018 (Kassotis et al. 2018b) tested a mixture of 23 commonly used unconventional oil and gas chemicals (UOG), including acrylamide, alkylphenols, benzenes, bronopol, diethanolamine, ethanols, ethylene glycol, propylene glycol, styrene, toluene, and xylens. This mixture resulted in an increase in tryglyceride accumulation and preadipocyte proliferation at 10 μM and 1 μM, respectively (Kassotis et al. 2018b). This effect was observed in both, laboratory mixtures as well as samples from UOG-impacted samples (Kassotis et al. 2018b). Notably, some UOG-impacted waters promoted PPARγ, but not the laboratory UOG mixture, demonstrating that UOG-induced triglyceride activation is PPARγ independent. These findings further demonstrate that chemicals that can independently promote adipogenesis, such as acrylamide and alkylphenols (Kassotis et al. 2018a; Lee and Pyo 2019) can act as obesogens in environmentally collected samples containing a complex chemical mixture. However, developmental exposure to a similar UOG mixture altered body weight and energy expenditure, but not body composition in C57BL/6 mice (Balise et al. 2019a; Balise et al. 2019b), which highlights the need to validate in vitro findings using animal models.
Other developmental exposures (prenatal and postnatal until day 140 of life) to complex mixtures, such as those found in bisphenol A- and nonylphenol-containing wastewaters have reported an increased adipose deposition and weight gain during adulthood in male mice (Biasiotto et al. 2016). Other chemical mixtures that have been tested for adipogenesis include those present in oil sands process-affected water (OSPW). This complex mixture that includes thousands of chemicals (Headley et al. 2013; Pereira et al. 2013; Pereira and Martin 2015) was found to have PPARγ activity (Peng et al. 2016). However, the pro-adipogenic effect of OSPW was restricted to the polar fractions at environmentally relevant concentrations (Peng et al. 2016). While these studies provide with a proof of principle regarding real-life exposures, the high complexity of the mixtures does not allow to make in-depth conclusions regarding the specific chemical(s) that drive the adipogenic effect. Less complex mixtures have also been evaluated. Using an EDC mixture design based on serum concentrations reported in pregnant women of a Swedish mother-child cohort (EDC mixture #1 in Table 1, the developmental effects of a mixture (4 phthalates, triclosan, and 2 perfluorinated chemicals; dose ranges: 3 – 28 nM per chemical) on adipogenesis and lipid storage have been evaluated in zebrafish (Mentor et al. 2020). With an exposure from 3 h until 17 days post-fertilization, the EDC mixture increased adipocyte number and visceral adipose tissue, but mild effects on adipocyte-related genes (Mentor et al. 2020). While these findings further support previous studies linking phthalates to adipogenic outcomes, not all studies are in support of the obesogenic nature of phthalates (Wassenaar et al. 2017). Another EDC mixture consisting of di(2-ethylhexyl) phthalate, bisphenol A, 2,3,7,8-tetrachlorodibenzo-pdioxine (TCDD), and polychlorinated biphenyl 153 (PCB153) below the NOAEL was used to expose adult male mice for 15 weeks (EDC mixture #2 in Table 1). This EDC mixture did not increase adipose tissue under standard diet conditions but resulted in lower plasma free fatty acids and triglycerides and mild expression changes in subcutaneous adipose tissue related to adipogenic differentiation (Naville et al. 2019). Whether these EDC mixtures exert sex-specific differences in adipose tissue depots of mammalian species or different developmental windows result in obesogenic outcomes, such as those seen in individual chemical exposures (Egusquiza and Blumberg 2020; Veiga-Lopez et al. 2018), warrants further research.
5. Approaches and Reproducibility
Chemicals described in this chapter represent only a subset of chemicals that have been recently evaluated for their potential role as obesogens, which suggests the existence of many other obesogens in the environment that have not been described yet. To determine whether a chemical is considered an obesogen, rigorous approaches to improve reproducibility should be followed considering all potential limitations. In vitro models allow for a first approximation to identify potential obesogens, that could subsequently be tested in animal models. Although there are significant efforts focused on standardizing the methodologies to determine the role environmental factors in human obesity (Trasande et al. 2016), differences across laboratories affect not only replication of results, but also data interpretation. One key example is the use of 3T3-L1 cells as a model of adipogenesis differentiation. Kassotis et al., made the observation that the source of the cell line, the plastic used to culture the cells (i.e., whether or not it is chemically treated by the manufacturer), and the protocol used for their differentiation, play a critical role in the detection of new potential obesogens in vitro (Kassotis et al. 2017). Determining the limiting factors and setting up strategies to implement reproducibility standards will be the ideal path forward to inform in vivo experimental approaches to confirm bona fide obesogens. Moving the field forward also requires high throughput strategies that allows screening of chemicals for not only receptor binding potential using classic binding assays (Foley et al. 2017; Hartman et al. 2018) or molecular docking-based methods (Jaladanki et al. 2021) but also for adipogenic potential (Graham et al. 2020). Importantly, these high throughput tools need to be coupled with efficient and automated analytical capabilities (Adomshick et al. 2020; Yuan et al. 2019).
Most of the studies reviewed here use the 3T3-L1 preadipocyte cell line as model to study adipogenesis. The limitation of 3T3-L1 cells is that they are committed to differentiate into adipocytes. Thus, they cannot be used to study the potential of chemicals to induce adipogenic commitment or their effects over other potential differentiation pathways precursors might follow prior to commitment. An alternative is the use of mesenchymal stem cells (MSCs). Derived from various adult tissues in mammals (e.g., adipose tissue, bone marrow, umbilical cord), these primary cells are commercially available from different species, and they are able to differentiate into various cell types, including adipocytes, depending on the specific in vitro stimuli (Ullah et al. 2015). MSCs are, therefore, and excellent tool to study commitment effects. Although further analyses are needed, Shoucri et al. showed that RXRα activators are potential inducers of adipogenic commitment (Shoucri et al. 2017). The food additives Span 80 and Tween 80 have been shown to induce final adipocyte differentiation in 3T3-L1 cells and to activate RXRα. These two food ingredients are potential candidates to be tested for their adipocyte commitment capacity in MSCs. Although some chemicals such as TBT and bisphenols have been demonstrated to have similar effects across species in vitro and in vivo (Gingrich et al. 2021; Pu et al. 2019; Veiga-Lopez et al. 2015), a potential limitation is that 3T3-L1 are murine cells, which limits the capability of drawing conclusions about mechanisms of action in humans. MSCs can be isolated from multiple species, including humans, which would broaden the capacity to evaluate interspecies effect of potential obesogens. Moreover, the use of a single cell line, stem cells isolated from a single individual, or representing only one sex, does not capture human inter-individual genetic and environmental exposure differences. Thus, to enhance study robustness and reproducibility, and whenever possible, multiple systems (cell lines, primary cultured cells from different individuals, and/or both sexes) should be included within the same study.
6. Conclusions & Future directions
Alteration of adipose tissue size and homeostasis play an important role not only in obesity, but also in the development of other metabolic co-morbidities such as type 2 diabetes and cardiovascular diseases (Bluher 2020). To note, obesity also induces wide-reaching systemic effects on other systems, such as the reproductive and the immune system (Francisco et al. 2018; Leisegang et al. 2021; Snider and Wood 2019). Thus, determining factors contributing to obesity, critical windows of susceptibility and mechanisms of action is instrumental to generate strategies for future prevention and treatment of a broad range of metabolic and reproductive disorders.
Epidemiological studies have shown the association between exposure to environmental factors and metabolic disruption (Kahn et al. 2020). These studies are extremely valuable to understand the impact of these factors on human health. However, due to the ethical and logistic limitations of the use of human tissues at certain stages in life, performing further analyses to confirm these associations becomes challenging. As mentioned in this chapter, adipose tissue structure is very dynamic throughout life in response to environmental cues (Pellegrinelli et al. 2016). Since the development of the adipose tissue starts prenatally (Jiang et al. 2014), environmental exposures during in utero development may contribute to lasting metabolic disruptions compromising health during adulthood (Figure 2). Animal models, from flies and worms, to fish, rodents, and large mammals and non-human primates, allow the scientific community to narrow down mechanisms of action during all stages in life that would not be possible to determine in humans (for review see Kleinert et al. 2018).
Figure 2. Model representing the effect of developmental exposure to emerging obesogens in obesity later in life.
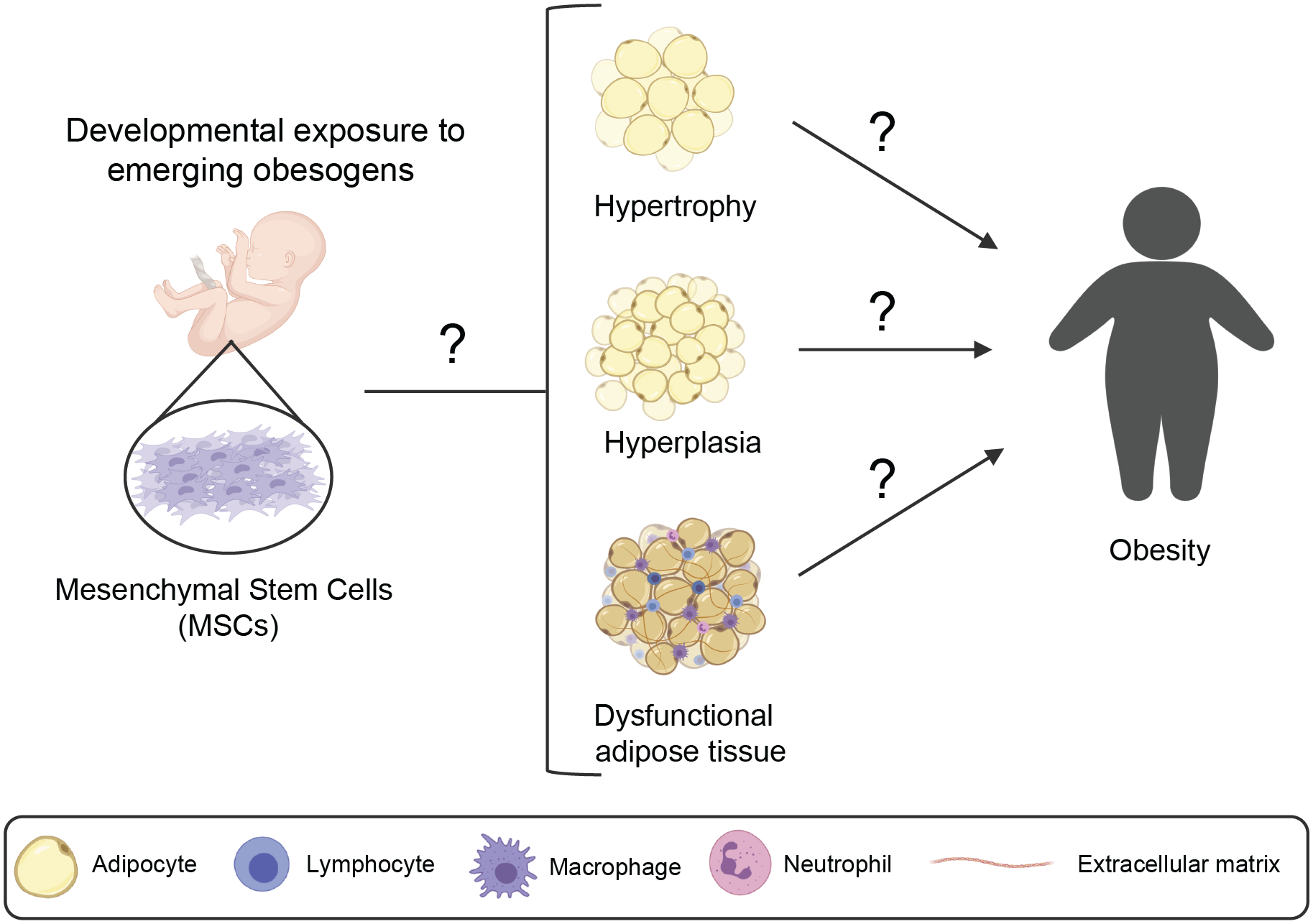
Emerging obesogens could reprogram mesenchymal stem cells (MSCs) during early stages of development to favor their differentiation into larger adipocytes (hypertrophy), more adipocytes (hyperplasia) or dysfunctional adipocytes. These alterations would lead to obesity and other metabolic disorders during adulthood. Question marks point to gaps in knowledge. These gaps include: 1) understanding if emerging obesogens alter adipose tissue development leading to adult obesity (left) and 2) the underlying mechanisms by which emerging obesogens can lead to adipose tissue hypertrophy, hyperplasia, and/or dysfunctional adipose tissue and ultimately obesity.
Potential therapeutic approaches to counteract the detrimental effects of obesogens would be the introduction of other dietary supplements that have been shown to promote beneficial metabolic outcomes. For example, there are food additives such as curcumin and capsaicin, that induce the development of BAT and mitochondrial biogenesis and that could therefore be used as therapeutics to counteract obesogen exposure. Curcumin and capsaicin, the natural polyphenol resveratrol (Burns et al. 2002) and polyunsaturated fatty acids (PUFAs) stimulate brown adipogenesis via the PPARγ/PRDM16 complex (for review see El Hadi et al. 2018). Green tea and menthol have also shown to modulate mitochondrial biogenesis (a characteristic of BAT development) via phosphodiesterase inhibition or the transient receptor potential cation channel melastatin (TRPM8) (El Hadi et al. 2018). Activators of other anti-adipogenic pathways such as the AMP-activated Protein Kinase (AMPK) (Ahmad et al. 2020), could be potential therapeutic targets for the treatment of obesity.
While the work performed using in vitro models contribute to a better understanding of mechanisms of action of emerging obesogens, multiple strategies to increase reproducibility, such as the use of multiple in vitro model systems, multiple species, and in vivo validation is highly warranted. This coupled with high throughput screenings to identify emerging obesogens will help fast-track the field and establish the contribution that obesogens have in the current obesity epidemic.
Acknowledgments:
Research reported in this publication was supported the National Institute of Environmental Health Sciences of the National Institute of Health (R01ES027863 to A.V-L) and the P30 UCSF EaRTH Center (P30-ES030284 to R.C-G). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources.
Non-standard Abbreviations
- 2-BHA
2-tertbutyl-4-hydroxyanisole
- 3-BHA
3-tertbutyl-4-hydroxyanisole
- AMPK
AMP-activated protein kinase
- AR
Androgen receptor
- BAT
Brown adipose tissue
- BMI
Body mass index
- BW
Body weight
- C/EBPb
CAAT/enhancer-binding proteins β
- CREB
cAMP-response element binding protein
- DOSS
Dioctyl sodium sulfosuccinate
- EDCs
Endocrine disrupting chemicals
- ER
Estrogen receptor
- FDA
Food and Drug Administration
- GR
Glucocorticoid receptor
- hBM-MSCs
human bone marrow mesenchymal stem cells
- LXR
Liver X Receptor
- NOAEL
Non-observed adverse effect level
- PCB153
Polychlorinated biphenyl 153
- PPARγ
Peroxisome proliferator-activator receptor gamma
- OSPW
Oil sands process-affected water
- PUFAs
Polyunsaturated fatty acids
- RXRα
Retinoid X receptor alpha
- SPA
Synthetic phenolic antioxidants
- TBT
Tributyltin
- TCDD
2,3,7,8-tetrachlorodibenzo-pdioxine
- TRb
Thyroid receptor-beta
- TRPM8
Transient receptor potential cation channel melastatin
- UOG
Unconventional oil and gas chemicals
- WAT
White adipose tissue
Footnotes
Conflict of interest: Authors have nothing to disclose
References
- Acir IH, Guenther K (2018) Endocrine-disrupting metabolites of alkylphenol ethoxylates - A critical review of analytical methods, environmental occurrences, toxicity, and regulation. Sci Total Environ 635:1530–1546 doi: 10.1016/j.scitotenv.2018.04.079 [DOI] [PubMed] [Google Scholar]
- Adomshick V, Pu Y, Veiga-Lopez A (2020) Automated lipid droplet quantification system for phenotypic analysis of adipocytes using CellProfiler. Toxicol Mech Methods 30(5):378–387 doi: 10.1080/15376516.2020.1747124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad B, Serpell CJ, Fong IL, Wong EH (2020) Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front Mol Biosci 7:76 doi: 10.3389/fmolb.2020.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, An S, Lee M, et al. (2019) A long-wave UVA filter avobenzone induces obesogenic phenotypes in normal human epidermal keratinocytes and mesenchymal stem cells. Arch Toxicol 93(7):1903–1915 doi: 10.1007/s00204-019-02462-1 [DOI] [PubMed] [Google Scholar]
- Andrews FV, Kim SM, Edwards L, Schlezinger JJ (2020) Identifying adipogenic chemicals: Disparate effects in 3T3-L1, OP9 and primary mesenchymal multipotent cell models. Toxicol In Vitro 67:104904 doi: 10.1016/j.tiv.2020.104904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariemma F, D’Esposito V, Liguoro D, et al. (2016) Low-Dose Bisphenol-A Impairs Adipogenesis and Generates Dysfunctional 3T3-L1 Adipocytes. PLoS One 11(3):e0150762 doi: 10.1371/journal.pone.0150762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie-Hamilton PF (2002) Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med 8(2):185–92 doi: 10.1089/107555302317371479 [DOI] [PubMed] [Google Scholar]
- Balise VD, Cornelius-Green JN, Kassotis CD, Rector RS, Thyfault JP, Nagel SC (2019a) Preconceptional, Gestational, and Lactational Exposure to an Unconventional Oil and Gas Chemical Mixture Alters Energy Expenditure in Adult Female Mice. Front Endocrinol (Lausanne) 10:323 doi: 10.3389/fendo.2019.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balise VD, Cornelius-Green JN, Parmenter B, et al. (2019b) Developmental Exposure to a Mixture of Unconventional Oil and Gas Chemicals Increased Risk-Taking Behavior, Activity and Energy Expenditure in Aged Female Mice After a Metabolic Challenge. Front Endocrinol (Lausanne) 10:460 doi: 10.3389/fendo.2019.00460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME (1989) Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298(6673):564–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasiotto G, Zanella I, Masserdotti A, et al. (2016) Municipal wastewater affects adipose deposition in male mice and increases 3T3-L1 cell differentiation. Toxicol Appl Pharmacol 297:32–40 doi: 10.1016/j.taap.2016.02.023 [DOI] [PubMed] [Google Scholar]
- Bluher M (2020) Metabolically Healthy Obesity. Endocr Rev 41(3) doi: 10.1210/endrev/bnaa004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers RR, Temkin AM, Guillette LJ, Baatz JE, Spyropoulos DD (2016) The commonly used nonionic surfactant Span 80 has RXRalpha transactivation activity, which likely increases the obesogenic potential of oil dispersants and food emulsifiers. Gen Comp Endocrinol 238:61–68 doi: 10.1016/j.ygcen.2016.04.029 [DOI] [PubMed] [Google Scholar]
- Burns J, Yokota T, Ashihara H, Lean ME, Crozier A (2002) Plant foods and herbal sources of resveratrol. J Agric Food Chem 50(11):3337–40 doi: 10.1021/jf0112973 [DOI] [PubMed] [Google Scholar]
- Chamorro-Garcia R, Diaz-Castillo C, Shoucri BM, et al. (2017) Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat Commun 8(1):2012 doi: 10.1038/s41467-017-01944-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B (2013) Transgenerational Inheritance of Increased Fat Depot Size, Stem Cell Reprogramming, and Hepatic Steatosis Elicited by Prenatal Exposure to the Obesogen Tributyltin in Mice. Environ Health Persp 121(3):359–366 doi: 10.1289/ehp.1205701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro-Garcia R, Shoucri BM, Willner S, Kach H, Janesick A, Blumberg B (2018) Effects of Perinatal Exposure to Dibutyltin Chloride on Fat and Glucose Metabolism in Mice, and Molecular Mechanisms, in Vitro. Environ Health Perspect 126(5):057006 doi: 10.1289/EHP3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell VA, Janesick A, Blumberg B, Fenton SE (2018) Tetrabromobisphenol-A Promotes Early Adipogenesis and Lipogenesis in 3T3-L1 Cells. Toxicol Sci 166(2):332–344 doi: 10.1093/toxsci/kfy209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau YY, Bandiera R, Serrels A, et al. (2014) Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 16(4):367–75 doi: 10.1038/ncb2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewe C, An YA, Scherer PE (2017) The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest 127(1):74–82 doi: 10.1172/JCI88883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MH, Cabrero MR (2009) Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol 587(Pt 14):3423–4 doi: 10.1113/jphysiol.2009.174896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo JFP, Podratz PL, Sena GC, et al. (2018) The obesogen tributyltin induces abnormal ovarian adipogenesis in adult female rats. Toxicol Lett 295:99–114 doi: 10.1016/j.toxlet.2018.06.1068 [DOI] [PubMed] [Google Scholar]
- Diaz-Castillo C, Chamorro-Garcia R, Shioda T, Blumberg B (2019) Transgenerational Self-Reconstruction of Disrupted Chromatin Organization After Exposure To An Environmental Stressor in Mice. Sci Rep 9(1):13057 doi: 10.1038/s41598-019-49440-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubach LA, Palmer EL 3rd, Connolly LP, Baker A, Zurakowski D, Cypess AM (2011) Pediatric brown adipose tissue: detection, epidemiology, and differences from adults. J Pediatr 159(6):939–44 doi: 10.1016/j.jpeds.2011.06.028 [DOI] [PubMed] [Google Scholar]
- e-CFR (2020) Food additives permitted for direct addition to food for human consumption. In: Register OotF (ed). Electronic Code of Federal Regulations, [PubMed] [Google Scholar]
- Egusquiza RJ, Blumberg B (2020) Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology 161(3) doi: 10.1210/endocr/bqaa024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hadi H, Di Vincenzo A, Vettor R, Rossato M (2018) Food Ingredients Involved in White-to-Brown Adipose Tissue Conversion and in Calorie Burning. Front Physiol 9:1954 doi: 10.3389/fphys.2018.01954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA US (2020) TSCA Chemical Substance Inventory. In: Agency USEP (ed). [Google Scholar]
- Foley B, Doheny DL, Black MB, et al. (2017) Editor’s Highlight: Screening ToxCast Prioritized Chemicals for PPARG Function in a Human Adipose-Derived Stem Cell Model of Adipogenesis. Toxicol Sci 155(1):85–100 doi: 10.1093/toxsci/kfw186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds CE, Trevino LS, York B, Walker CL (2017) Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol 13(8):445–457 doi: 10.1038/nrendo.2017.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco V, Pino J, Campos-Cabaleiro V, et al. (2018) Obesity, Fat Mass and Immune System: Role for Leptin. Front Physiol 9:640 doi: 10.3389/fphys.2018.00640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3(7) doi:ARTN e1700782 10.1126/sciadv.1700782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich J, Pu Y, Upham BL, et al. (2021) Bisphenol S enhances gap junction intercellular communication in ovarian theca cells. Chemosphere 263:128304 doi: 10.1016/j.chemosphere.2020.128304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, et al. (2015) Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36(6):593–602 doi: 10.1210/er.2015-1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AD, Pandey R, Tsancheva VS, et al. (2020) The development of a high throughput drug-responsive model of white adipose tissue comprising adipogenic 3T3-L1 cells in a 3D matrix. Biofabrication 12(1) doi:ARTN 015018 10.1088/1758-5090/ab56fe [DOI] [PubMed] [Google Scholar]
- Grun F, Watanabe H, Zamanian Z, et al. (2006) Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol 20(9):2141–2155 doi: 10.1210/me.2005-0367 [DOI] [PubMed] [Google Scholar]
- Hales C, Carroll M, Fryar C, Ogden C (2017) Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. In: National Center for Health Statistics CfDCaP (ed). NCHS Data Brief p1–8 [PubMed] [Google Scholar]
- Hales C, Carroll M, Fryar C, Ogden C (2020) Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. In: National Center for Health Statistics CfDCaP (ed). NCHS Data Brief p1–8 [PubMed] [Google Scholar]
- Hao CJ, Cheng XJ, Xia HF, Ma X (2012) The endocrine disruptor 4-nonylphenol promotes adipocyte differentiation and induces obesity in mice. Cell Physiol Biochem 30(2):382–94 doi: 10.1159/000339032 [DOI] [PubMed] [Google Scholar]
- Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19(10):1252–63 doi: 10.1038/nm.3361 [DOI] [PubMed] [Google Scholar]
- Hartman JK, Beames T, Parks B, et al. (2018) An in vitro approach for prioritization and evaluation of chemical effects on glucocorticoid receptor mediated adipogenesis. Toxicol Appl Pharmacol 355:112–126 doi: 10.1016/j.taap.2018.05.016 [DOI] [PubMed] [Google Scholar]
- Headley JV, Peru KM, Mohamed MH, et al. (2013) Chemical fingerprinting of naphthenic acids and oil sands process waters-A review of analytical methods for environmental samples. J Environ Sci Health A Tox Hazard Subst Environ Eng 48(10):1145–63 doi: 10.1080/10934529.2013.776332 [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, et al. (2017) Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 68:3–33 doi: 10.1016/j.reprotox.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Newbold R, Schug TT (2015) Endocrine disruptors and obesity. Nat Rev Endocrinol 11(11):653–61 doi: 10.1038/nrendo.2015.163 [DOI] [PubMed] [Google Scholar]
- Hendryx M, Luo J (2018) Children’s environmental chemical exposures in the USA, NHANES 2003–2012. Environ Sci Pollut Res Int 25(6):5336–5343 doi: 10.1007/s11356-017-0874-5 [DOI] [PubMed] [Google Scholar]
- Jaladanki CK, He Y, Zhao LN, et al. (2021) Virtual screening of potentially endocrine-disrupting chemicals against nuclear receptors and its application to identify PPARgamma-bound fatty acids. Arch Toxicol 95(1):355–374 doi: 10.1007/s00204-020-02897-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Berry DC, Tang W, Graff JM (2014) Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep 9(3):1007–22 doi: 10.1016/j.celrep.2014.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L (2020) Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol 8(8):703–718 doi: 10.1016/S2213-8587(20)30129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Jeong SH, Cho JH, Kim DG, Park JM, Cho MH (2005) Evaluation of estrogenic and androgenic activity of butylated hydroxyanisole in immature female and castrated rats. Toxicology 213(1–2):147–56 doi: 10.1016/j.tox.2005.05.027 [DOI] [PubMed] [Google Scholar]
- Kassotis CD, Kollitz EM, Ferguson PL, Stapleton HM (2018a) Nonionic Ethoxylated Surfactants Induce Adipogenesis in 3T3-L1 Cells. Toxicol Sci 162(1):124–136 doi: 10.1093/toxsci/kfx234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis CD, Kollitz EM, Hoffman K, Sosa JA, Stapleton HM (2019) Thyroid receptor antagonism as a contributory mechanism for adipogenesis induced by environmental mixtures in 3T3-L1 cells. Sci Total Environ 666:431–444 doi: 10.1016/j.scitotenv.2019.02.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis CD, Masse L, Kim S, Schlezinger JJ, Webster TF, Stapleton HM (2017) Characterization of Adipogenic Chemicals in Three Different Cell Culture Systems: Implications for Reproducibility Based on Cell Source and Handling. Sci Rep 7:42104 doi: 10.1038/srep42104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis CD, Nagel SC, Stapleton HM (2018b) Unconventional oil and gas chemicals and wastewater-impacted water samples promote adipogenesis via PPARgamma-dependent and independent mechanisms in 3T3-L1 cells. Sci Total Environ 640–641:1601–1610 doi: 10.1016/j.scitotenv.2018.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89(6):2548–56 doi: 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- Kim J, Kang EJ, Park MN, et al. (2015) The adverse effect of 4-tert-octylphenol on fat metabolism in pregnant rats via regulation of lipogenic proteins. Environ Toxicol Pharmacol 40(1):284–91 doi: 10.1016/j.etap.2015.06.020 [DOI] [PubMed] [Google Scholar]
- Kim S, Choi K (2014) Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: a mini-review. Environ Int 70:143–57 doi: 10.1016/j.envint.2014.05.015 [DOI] [PubMed] [Google Scholar]
- Kirchner S, Kieu T, Chow C, Casey S, Blumberg B (2010) Prenatal Exposure to the Environmental Obesogen Tributyltin Predisposes Multipotent Stem Cells to Become Adipocytes. Mol Endocrinol 24(3):526–539 doi: 10.1210/me.2009-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinert M, Clemmensen C, Hofmann SM, et al. (2018) Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol 14(3):140–162 doi: 10.1038/nrendo.2017.161 [DOI] [PubMed] [Google Scholar]
- Lee HW, Pyo S (2019) Acrylamide induces adipocyte differentiation and obesity in mice. Chem Biol Interact 298:24–34 doi: 10.1016/j.cbi.2018.10.021 [DOI] [PubMed] [Google Scholar]
- Lee MJ, Lin H, Liu CW, et al. (2008) Octylphenol stimulates resistin gene expression in 3T3-L1 adipocytes via the estrogen receptor and extracellular signal-regulated kinase pathways. Am J Physiol Cell Physiol 294(6):C1542–51 doi: 10.1152/ajpcell.00403.2007 [DOI] [PubMed] [Google Scholar]
- Leisegang K, Sengupta P, Agarwal A, Henkel R (2021) Obesity and male infertility: Mechanisms and management. Andrologia 53(1):e13617 doi: 10.1111/and.13617 [DOI] [PubMed] [Google Scholar]
- Leppert B, Junge KM, Roder S, et al. (2018) Early maternal perceived stress and children’s BMI: longitudinal impact and influencing factors. BMC Public Health 18(1):1211 doi: 10.1186/s12889-018-6110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ycaza J, Blumberg B (2011) The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem 127(1–2):9–15 doi: 10.1016/j.jsbmb.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Song S, Lin Y, Ruan T, Jiang G (2015) Occurrence of synthetic phenolic antioxidants and major metabolites in municipal sewage sludge in China. Environ Sci Technol 49(4):2073–80 doi: 10.1021/es505136k [DOI] [PubMed] [Google Scholar]
- Liu RZ, Mabury SA (2018) Synthetic Phenolic Antioxidants and Transformation Products in Human Sera from United States Donors. Environ Sci Tech Let 5(7):419–423 doi: 10.1021/acs.estlett.8b00223 [DOI] [Google Scholar]
- Lopez-Espinosa MJ, Freire C, Arrebola JP, et al. (2009) Nonylphenol and octylphenol in adipose tissue of women in Southern Spain. Chemosphere 76(6):847–52 doi: 10.1016/j.chemosphere.2009.03.063 [DOI] [PubMed] [Google Scholar]
- Lutfi E, Riera-Heredia N, Cordoba M, et al. (2017) Tributyltin and triphenyltin exposure promotes in vitro adipogenic differentiation but alters the adipocyte phenotype in rainbow trout. Aquat Toxicol 188:148–158 doi: 10.1016/j.aquatox.2017.05.001 [DOI] [PubMed] [Google Scholar]
- Lyssimachou A, Santos JG, Andre A, et al. (2015) The Mammalian “Obesogen” Tributyltin Targets Hepatic Triglyceride Accumulation and the Transcriptional Regulation of Lipid Metabolism in the Liver and Brain of Zebrafish. Plos One 10(12) doi:ARTN e0143911 10.1371/journal.pone.0143911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen L, Petersen RK, Kristiansen K (2005) Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim Biophys Acta 1740(2):266–86 doi: 10.1016/j.bbadis.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Martin O, Scholze M, Ermler S, et al. (2020) Ten years of research on synergisms and antagonisms in chemical mixtures: A systematic review and quantitative reappraisal of mixture studies. Environ Int 146:106206 doi: 10.1016/j.envint.2020.106206 [DOI] [PubMed] [Google Scholar]
- Masuno H, Okamoto S, Iwanami J, et al. (2003) Effect of 4-nonylphenol on cell proliferation and adipocyte formation in cultures of fully differentiated 3T3-L1 cells. Toxicol Sci 75(2):314–20 doi: 10.1093/toxsci/kfg203 [DOI] [PubMed] [Google Scholar]
- Matta MK, Florian J, Zusterzeel R, et al. (2020) Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA 323(3):256–267 doi: 10.1001/jama.2019.20747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matvienko-Sikar K, Cooney J, Flannery C, Murphy J, Khashan A, Huizink A (2020) Maternal stress in the first 1000 days and risk of childhood obesity: a systematic review. J Reprod Infant Psychol:1–25 doi: 10.1080/02646838.2020.1724917 [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Abbasi F, Lamendola C, Reaven G (2007) Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med 167(7):642–8 doi: 10.1001/archinte.167.7.642 [DOI] [PubMed] [Google Scholar]
- Meigs JB, Wilson PW, Fox CS, et al. (2006) Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 91(8):2906–12 doi: 10.1210/jc.2006-0594 [DOI] [PubMed] [Google Scholar]
- Mentor A, Brunstrom B, Mattsson A, Jonsson M (2020) Developmental exposure to a human relevant mixture of endocrine disruptors alters metabolism and adipogenesis in zebrafish (Danio rerio). Chemosphere 238:124584 doi: 10.1016/j.chemosphere.2019.124584 [DOI] [PubMed] [Google Scholar]
- Mikamo E, Harada S, Nishikawa J, Nishihara T (2003) Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicol Appl Pharmacol 193(1):66–72 doi: 10.1016/j.taap.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Milton FA, Lacerda MG, Sinoti SBP, et al. (2017) Dibutyltin Compounds Effects on PPAR gamma/RXR alpha Activity, Adipogenesis, and Inflammation in Mammalians Cells. Front Pharmacol 8 doi:ARTN 507 10.3389/fphar.2017.00507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki J, Kamei S, Sakayama K, Yamamoto H, Masuno H (2008) 4-tert-octylphenol regulates the differentiation of C3H10T1/2 cells into osteoblast and adipocyte lineages. Toxicol Sci 102(1):82–8 doi: 10.1093/toxsci/kfm296 [DOI] [PubMed] [Google Scholar]
- Muller S, Schmid P, Schlatter C (1998) Pharmacokinetic behavior of 4-nonylphenol in humans. Environ Toxicol Pharmacol 5(4):257–65 doi: 10.1016/s1382-6689(98)00009-x [DOI] [PubMed] [Google Scholar]
- Naville D, Gaillard G, Julien B, et al. (2019) Chronic exposure to a pollutant mixture at low doses led to tissue-specific metabolic alterations in male mice fed standard and high-fat high-sucrose diet. Chemosphere 220:1187–1199 doi: 10.1016/j.chemosphere.2018.12.177 [DOI] [PubMed] [Google Scholar]
- Niemelä S, Miettinen S, Sarkanen JR, Ashammakhi N (2008) Adipose Tissue and Adipocyte Differentiation: Molecular and Cellular Aspects and Tissue Engineering Applications Topics in Tissue Engineering. vol 4, p 1–26 [Google Scholar]
- Ogden C, Fakhouri T, Carroll M (2017) Prevalence of Obesity Among Adults, by Household Income and Education — United States, 2011–2014. In: National Center for Health Statistics CfDCaP (ed). MMWR Morb Mortal Wkly Rep p 1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrinelli V, Carobbio S, Vidal-Puig A (2016) Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia 59(6):1075–88 doi: 10.1007/s00125-016-3933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Sun J, Alharbi HA, Jones PD, Giesy JP, Wiseman S (2016) Peroxisome Proliferator-Activated Receptor gamma is a Sensitive Target for Oil Sands Process-Affected Water: Effects on Adipogenesis and Identification of Ligands. Environmental science & technology 50(14):7816–24 doi: 10.1021/acs.est.6b01890 [DOI] [PubMed] [Google Scholar]
- Penza M, Jeremic M, Marrazzo E, et al. (2011) The environmental chemical tributyltin chloride (TBT) shows both estrogenic and adipogenic activities in mice which might depend on the exposure dose. Toxicol Appl Pharmacol 255(1):65–75 doi:S0041–008X(11)00208–0 [pii] 10.1016/j.taap.2011.05.017 [DOI] [PubMed] [Google Scholar]
- Pereira AS, Bhattacharjee S, Martin JW (2013) Characterization of oil sands process-affected waters by liquid chromatography orbitrap mass spectrometry. Environmental science & technology 47(10):5504–13 doi: 10.1021/es401335t [DOI] [PubMed] [Google Scholar]
- Pereira AS, Martin JW (2015) Exploring the complexity of oil sands process-affected water by high efficiency supercritical fluid chromatography/orbitrap mass spectrometry. Rapid Commun Mass Spectrom 29(8):735–44 doi: 10.1002/rcm.7156 [DOI] [PubMed] [Google Scholar]
- Pop A, Kiss B, Loghin F (2013) Endocrine disrupting effects of butylated hydroxyanisole (BHA - E320). Clujul Med 86(1):16–20 [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Pearl S, Gingrich J, et al. (2019) Multispecies study: low-dose tributyltin impairs ovarian theca cell cholesterol homeostasis through the RXR pathway in five mammalian species including humans. Arch Toxicol 93(6):1665–1677 doi: 10.1007/s00204-019-02449-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier SM, El-Hashani E, Kamau W, Zhang X, Massad NL, Sargis RM (2015) Tributyltin differentially promotes development of a phenotypically distinct adipocyte. Obesity (Silver Spring) 23(9):1864–71 doi: 10.1002/oby.21174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XM, Kuo Y, Blumberg B (2020) Agrochemicals and obesity. Mol Cell Endocrinol 515:110926 doi: 10.1016/j.mce.2020.110926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM (2008) Identification of white adipocyte progenitor cells in vivo. Cell 135(2):240–9 doi: 10.1016/j.cell.2008.09.036 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444(7121):847–53 doi: 10.1038/nature05483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp GC, Lawlor DA (2019) Paternal impact on the life course development of obesity and type 2 diabetes in the offspring. Diabetologia 62(10):1802–1810 doi: 10.1007/s00125-019-4919-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim YK, Lewin MD, Ruiz P, Eichner JE, Mumtaz MM (2017) Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: The United States NHANES, 2007–2012. J Toxicol Environ Health A 80(9):502–512 doi: 10.1080/15287394.2017.1330581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JC, Lee E, An S, et al. (2020) Benzophenone-3 and benzophenone-8 exhibit obesogenic activity via peroxisome proliferator-activated receptor gamma pathway. Toxicol In Vitro 67:104886 doi: 10.1016/j.tiv.2020.104886 [DOI] [PubMed] [Google Scholar]
- Shoucri BM, Hung VT, Chamorro-Garcia R, Shioda T, Blumberg B (2018) Retinoid X Receptor Activation During Adipogenesis of Female Mesenchymal Stem Cells Programs a Dysfunctional Adipocyte. Endocrinology 159(8):2863–2883 doi: 10.1210/en.2018-00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoucri BM, Martinez ES, Abreo TJ, et al. (2017) Retinoid X Receptor Activation Alters the Chromatin Landscape To Commit Mesenchymal Stem Cells to the Adipose Lineage. Endocrinology 158(10):3109–3125 doi: 10.1210/en.2017-00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider AP, Wood JR (2019) Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction 158(3):R79–R90 doi: 10.1530/REP-18-0583 [DOI] [PubMed] [Google Scholar]
- Soto AM, Justicia H, Wray JW, Sonnenschein C (1991) p-Nonyl-phenol: an estrogenic xenobiotic released from “modified” polystyrene. Environ Health Perspect 92:167–73 doi: 10.1289/ehp.9192167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmaier S, Bogl LH, Eliassen AH, et al. (2020) Maternal healthful dietary patterns during peripregnancy and long-term overweight risk in their offspring. Eur J Epidemiol 35(3):283–293 doi: 10.1007/s10654-020-00621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Cao H, Liu QS, et al. (2020a) 4-Hexylphenol influences adipogenic differentiation and hepatic lipid accumulation in vitro. Environ Pollut 268(Pt A):115635 doi: 10.1016/j.envpol.2020.115635 [DOI] [PubMed] [Google Scholar]
- Sun Z, Tang Z, Yang X, et al. (2020b) Perturbation of 3-tert-butyl-4-hydroxyanisole in adipogenesis of male mice with normal and high fat diets. Sci Total Environ 703:135608 doi: 10.1016/j.scitotenv.2019.135608 [DOI] [PubMed] [Google Scholar]
- Sun Z, Yang X, Liu QS, et al. (2019) Butylated hydroxyanisole isomers induce distinct adipogenesis in 3T3-L1 cells. J Hazard Mater 379:120794 doi: 10.1016/j.jhazmat.2019.120794 [DOI] [PubMed] [Google Scholar]
- Symonds ME, Pope M, Bloor I, Law J, Alagal R, Budge H (2021) Adipose tissue growth and development: the modulating role of ambient temperature. J Endocrinol 248(1):R19–R28 doi: 10.1530/JOE-20-0075 [DOI] [PubMed] [Google Scholar]
- Tam WH, Ma RCW, Ozaki R, et al. (2017) In Utero Exposure to Maternal Hyperglycemia Increases Childhood Cardiometabolic Risk in Offspring. Diabetes Care 40(5):679–686 doi: 10.2337/dc16-2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin AM, Bowers RR, Magaletta ME, et al. (2016) Effects of Crude Oil/Dispersant Mixture and Dispersant Components on PPARgamma Activity in Vitro and in Vivo: Identification of Dioctyl Sodium Sulfosuccinate (DOSS; CAS #577–11-7) as a Probable Obesogen. Environ Health Perspect 124(1):112–9 doi: 10.1289/ehp.1409672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin AM, Bowers RR, Ulmer CZ, et al. (2019) Increased adiposity, inflammation, metabolic disruption and dyslipidemia in adult male offspring of DOSS treated C57BL/6 dams. Sci Rep 9(1):1530 doi: 10.1038/s41598-018-38383-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker VV (2017) Genetic and Epigenetic Causes of Obesity. Adolesc Med State Art Rev 28(2):379–405 [PMC free article] [PubMed] [Google Scholar]
- Tingaud-Sequeira A, Ouadah N, Babin PJ (2011) Zebrafish obesogenic test: a tool for screening molecules that target adiposity. J Lipid Res 52(9):1765–72 doi: 10.1194/jlr.D017012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 77:289–312 doi: 10.1146/annurev.biochem.77.061307.091829 [DOI] [PubMed] [Google Scholar]
- Trasande L, Vandenberg LN, Bourguignon JP, et al. (2016) Peer-reviewed and unbiased research, rather than ‘sound science’, should be used to evaluate endocrine-disrupting chemicals. J Epidemiol Community Health 70(11):1051–1056 doi: 10.1136/jech-2016-207841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells - current trends and future prospective. Biosci Rep 35(2) doi: 10.1042/BSR20150025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA (2010) US Environmental Protention Agency response to BP Spill in the Gulf of Mexico. Dispersant Monitoring and Assessment Directive for Subsurface Dispersant Application. 1–5 [Google Scholar]
- Vague J (1956) The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 4(1):20–34 doi: 10.1093/ajcn/4.1.20 [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Pennathur S, Kannan K, et al. (2015) Impact of Gestational Bisphenol A on Oxidative Stress and Free Fatty Acids: Human Association and Interspecies Animal Testing Studies. Endocrinology:en20141863 doi: 10.1210/en.2014-1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Pu Y, Gingrich J, Padmanabhan V (2018) Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps. Trends Endocrinol Metab 29(9):607–625 doi: 10.1016/j.tem.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L, Gupta RK (2019) Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest 129(10):4022–4031 doi: 10.1172/JCI129191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S (2012) The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res 53(4):619–29 doi: 10.1194/jlr.M018846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Asimakopoulos AG, Abualnaja KO, et al. (2016) Synthetic Phenolic Antioxidants and Their Metabolites in Indoor Dust from Homes and Microenvironments. Environmental Science & Technology 50(1):428–434 doi: 10.1021/acs.est.5b04826 [DOI] [PubMed] [Google Scholar]
- Wang W, Kannan K (2018) Inventory, loading and discharge of synthetic phenolic antioxidants and their metabolites in wastewater treatment plants. Water Res 129:413–418 doi: 10.1016/j.watres.2017.11.028 [DOI] [PubMed] [Google Scholar]
- Wassenaar PNH, Trasande L, Legler J (2017) Systematic Review and Meta-Analysis of Early-Life Exposure to Bisphenol A and Obesity-Related Outcomes in Rodents. Environ Health Perspect 125(10):106001 doi: 10.1289/EHP1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (1975) http://www.inchem.org/documents/jecfa/jecmono/v06je43.htm.
- Woodruff TJ, Zota AR, Schwartz JM (2011) Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119(6):878–85 doi: 10.1289/ehp.1002727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Liu D, Cline MA, Gilbert ER (2020) Chronic stress, epigenetics, and adipose tissue metabolism in the obese state. Nutr Metab (Lond) 17:88 doi: 10.1186/s12986-020-00513-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik SC, Baker AH, Mann KK, Schlezinger JJ (2011) Organotins Are Potent Activators of PPAR gamma and Adipocyte Differentiation in Bone Marrow Multipotent Mesenchymal Stromal Cells. Toxicol Sci 122(2):476–488 doi: 10.1093/toxsci/kfr140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L, Zhu X, Shrubsole MJ, et al. (2011) Renal function, bisphenol A, and alkylphenols: results from the National Health and Nutrition Examination Survey (NHANES 2003–2006). Environ Health Perspect 119(4):527–33 doi: 10.1289/ehp.1002572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Chakraborty S, Chitta KK, et al. (2019) Fast Adipogenesis Tracking System (FATS)-a robust, high-throughput, automation-ready adipogenesis quantification technique. Stem Cell Res Ther 10(1):38 doi: 10.1186/s13287-019-1141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamoiski RD, Cahoon EK, Michal Freedman D, Linet MS (2015) Self-reported sunscreen use and urinary benzophenone-3 concentrations in the United States: NHANES 2003–2006 and 2009–2012. Environ Res 142:563–7 doi: 10.1016/j.envres.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgola-Grzeskowiak A, Grzeskowiak T, Szymanski A (2015) Biodegradation of Nonylphenol Monopropoxyethoxylates. J Surfactants Deterg 18(2):355–364 doi: 10.1007/s11743-014-1652-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Xue WY, Li YY, et al. (2014) Perinatal exposure to 4-nonylphenol affects adipogenesis in first and second generation rats offspring. Toxicology letters 225(2):325–32 doi: 10.1016/j.toxlet.2013.12.011 [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ (2014) Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect 122(3):235–41 doi: 10.1289/ehp.1306681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo ZH, Chen SZ, Wu TA, et al. (2011) Tributyltin Causes Obesity and Hepatic Steatosis in Male Mice. Environ Toxicol 26(1):79–85 doi: 10.1002/tox.20531 [DOI] [PubMed] [Google Scholar]


