Abstract
The potential for N-nitrosamine impurities in pharmaceutical products presents a challenge for the quality management of medicinal products. N-Nitrosamines are considered cohort-of-concern compounds due to the potent carcinogenicity of many of the structurally simple chemicals within this structural class. In the past 2 years, a number of drug products containing certain active pharmaceutical ingredients have been withdrawn or recalled from the market due to the presence of carcinogenic low-molecular-weight N,N-dialkylnitrosamine impurities. Regulatory authorities have issued guidance to market authorization holders to review all commercial drug substances/products for the potential risk of N-nitrosamine impurities, and in cases where a significant risk of N-nitrosamine impurity is identified, analytical confirmatory testing is required. A key factor to consider prior to analytical testing is the estimation of the daily acceptable intake (AI) of the N-nitrosamine impurity. A significant proportion of N-nitrosamine drug product impurities are unique/complex structures for which the development of low-level analytical methods is challenging. Moreover, these unique/complex impurities may be less potent carcinogens compared to simple nitrosamines. In the present work, our objective was to derive AIs for a large number of complex N-nitrosamines without carcinogenicity data that were identified as potential low-level impurities. The impurities were first cataloged and grouped according to common structural features, with a total of 13 groups defined with distinct structural features. Subsequently, carcinogenicity data were reviewed for structurally related N-nitrosamines relevant to each of the 13 structural groups and group AIs were derived conservatively based on the most potent N-nitrosamine within each group. The 13 structural group AIs were used as the basis for assigning AIs to each of the structurally related complex N-nitrosamine impurities. The AIs of several N-nitrosamine groups were found to be considerably higher than those for the simple N,N-dialkylnitrosamines, which translates to commensurately higher analytical method detection limits.
Introduction
N-Nitrosamines are categorized as “cohort-of-concern (CoC)” compounds due to the high carcinogenic potency of some of the chemicals within this structural class.1,2 The ICH M7(R1) guideline,3 which provides a framework for safety assessments and control of mutagenic impurities in pharmaceuticals, identifies N-nitrosamines as one of several structural classes for which daily acceptable intakes (AIs) are likely to be significantly lower than the exposure limits or thresholds of toxicological concern (TTCs) for non-CoC carcinogens. However, not all N-nitrosamines are highly potent carcinogens. For instance, animal carcinogenicity data compiled for 228 low-molecular-weight N-nitrosamine derivatives revealed that 18% of the N-nitrosamines were noncarcinogenic.4 Moreover, for carcinogenic N-nitrosamines, the log TD50 values spanned approximately 4 orders of magnitude, overlapping with non-N-nitrosamine carcinogens including some that are not in the CoC category.4
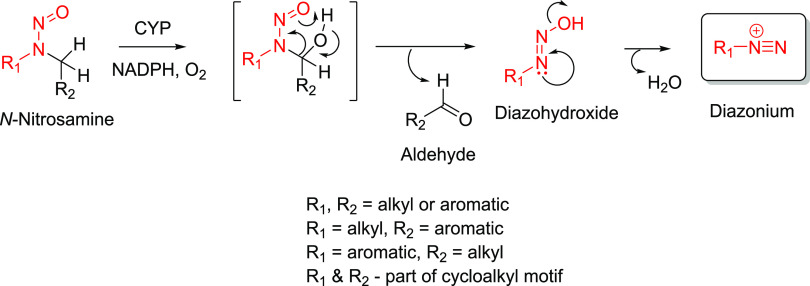
Cumulative evidence gathered over the last few decades suggests that the rate-limiting step in the carcinogenicity of simple N-nitrosamines (e.g., N,N-dialkyl, cycloalkyl, and N-alkyl-N-aromatic) involves their oxidative metabolism (bioactivation) by cytochrome P450 (CYP) enzymes (Figure 1).5−8 CYP-mediated hydroxylation at the carbon atom α to the N-nitroso moiety results in the formation of a N-nitrosocarbinolamine species, which spontaneously decomposes to the corresponding aldehyde and diazohydroxide intermediates. Further decomposition of the N-substituted-diazohydroxide yields an electrophilic N-alkyl- or N-aryl-diazonium species capable of covalently adducting to DNA. Contribution of detoxication pathways (e.g., enzymatic denitrosation of the N-nitrosamine,9−11 reaction of the diazonium species with water, and/or reduced glutathione to form innocuous metabolites such as a primary alcohol5,12 and/or glutathione conjugate13) is expected to govern the ultimate reactivity of the N-alkyl- or N-aryl diazonium with DNA base(s). Based on the established bioactivation mechanism, however, it can be concluded that the presence of an oxidizable carbon center α to the N-nitroso functionality appears to be a critical requirement for the metabolism-dependent carcinogenicity of N-nitrosamines.
Figure 1.
CYP-mediated bioactivation of N-nitrosamines to electrophilic diazonium intermediates capable of reacting with DNA.
Within the past 2 years, a considerable number of drug product lots containing active pharmaceutical ingredients (APIs)—valsartan, irbesartan, losartan, ranitidine, nizatidine, and metformin—have been withdrawn or recalled from the market due to the presence of carcinogenic N-nitrosamine impurities (e.g., N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), and N-nitroso-N-methyl-4-aminobutyric acid (NMBA)).14−16 Regulatory guidance has been issued where market authorization holders have been asked to review all chemically synthesized commercial drug substances and drug products for the presence of N-nitrosamine impurities,17,18 and a similar evaluation has been requested for biologics in some regions.18 In cases where N-nitrosamine impurities are anticipated to be present above the daily AI in the drug substance/product, analytical testing to confirm or refute the presence of the impurity is expected.17−20 If the potential risk is confirmed, then effective risk control and/or mitigating measures need to be implemented. Given the volume of N-nitrosamine impurity risk assessments currently underway across the pharmaceutical industry, and the structural diversity of complex N-nitrosamines that can potentially arise from the reaction between a nitrosating agent (e.g., nitrous acid, nitrite salts, nitrogen oxides, etc.)21,22 and a vulnerable secondary and/or tertiary amine in synthetic intermediates, complex amine-containing reagents, API-related impurities, or degradants and/or the API itself,23 a practical and science-based approach was needed for identifying AIs for complex N-nitrosamines in Pfizer-marketed commercial drug products.
A key factor to consider when assessing whether a particular drug substance or drug product N-nitrosamine impurity risk warrants analytical testing is the AI limit that corresponds to a theoretical excess cancer risk of 1 in 100 000 patients consistent with the principles defined in ICH M7(R1).3 For example, if the AI is substantially higher than the predicted amount of N-nitrosamine impurity that could be formed,24 then analytical testing may not be required. For simple N,N-dialkyl-N-nitrosamines (e.g., NDMA and NDEA), which were the initial focus of the investigations with the sartan class of antihypertensive drugs, extensive rodent carcinogenicity study data are available to support the derivation of specific AIs. However, in the case of N-nitrosamine impurities that are derived from complex APIs, animal carcinogenicity data on the specific impurity are unlikely to be available. In such cases, the European Medicines Agency (EMA) has recommended two approaches, one being the application of a conservative class limit TTC of 18 ng/day, which is based on the most potent N-nitrosamines.25 Alternatively, with appropriate scientific justification, a suitable AI may be derived by a read-across assessment, where available animal carcinogenicity data for structurally related N-nitrosamine derivatives are used to estimate the TD50 (defined as the dose required to induce a 50% tumor incidence with lifetime exposure) for an N-nitrosamine impurity with unknown carcinogenic potential. The latter approach is consistent with the recommendation in ICH M7(R1),3 which suggests a case-by-case approach using available carcinogenicity data for structurally analogous compounds to derive/justify AIs for CoC chemicals. The use of read-across methodology is of great interest given that not all N-nitrosamines are exceptionally potent carcinogens, which warrant control to the proposed class limit of 18 ng/day.
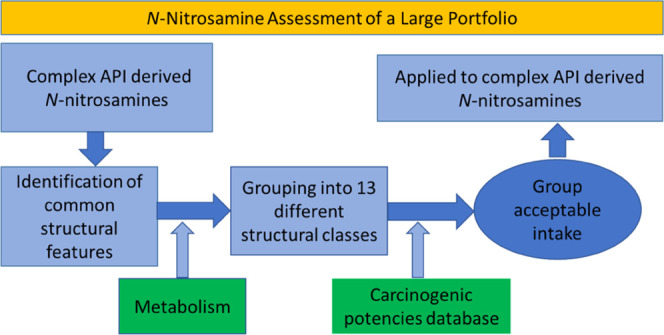
In the present work, a practical science-based and conservative approach was applied in the derivation of AIs for complex N-nitrosamines of APIs. The impurities were first cataloged and grouped according to common structural features, with a total of 13 groups defined with distinct structural features. Subsequently, carcinogenicity data were reviewed for structurally related N-nitrosamines relevant to each of the 13 structural groups and group AIs were derived conservatively based on the most potent N-nitrosamine within each group. The 13 structural group AIs were used as the basis for assigning AIs to each of the structurally related complex N-nitrosamine impurities. The AIs of several N-nitrosamine groups were found to be considerably higher than those for the simple N,N-dialkylnitrosamines, which translated to commensurately higher analytical method detection limits.
Materials and Methods
N-Nitrosamine Carcinogenicity Explorer/TD50 Calculator
N-Nitrosamines with carcinogenicity data summarized in the Lhasa Carcinogenicity Database (LCDB)26 and the Lhasa Vitic database were merged into a single internal database (henceforth referred to as the Pfizer N-Nitroso Carcinogenicity Explorer). LCDB is a structure-searchable repository of carcinogenicity data that is freely available online (Lhasa Carcinogenicity Database (lhasalimited.org)). The database contains all of the data from the original Carcinogenic Potency Database (CPDB) developed by Gold et al.27 as well as additional data curated by Lhasa, with over 1700 chemicals represented.26 The Pfizer N-Nitroso Carcinogenicity Explorer database was housed in a MySQL relational database with 970 studies from 248 N-nitrosamines with 1435 treatment groups and 2612 end points, i.e., tissue- and tumor-specific TD50 values (mg/kg/day).
Structural Grouping of Complex N-Nitrosamines
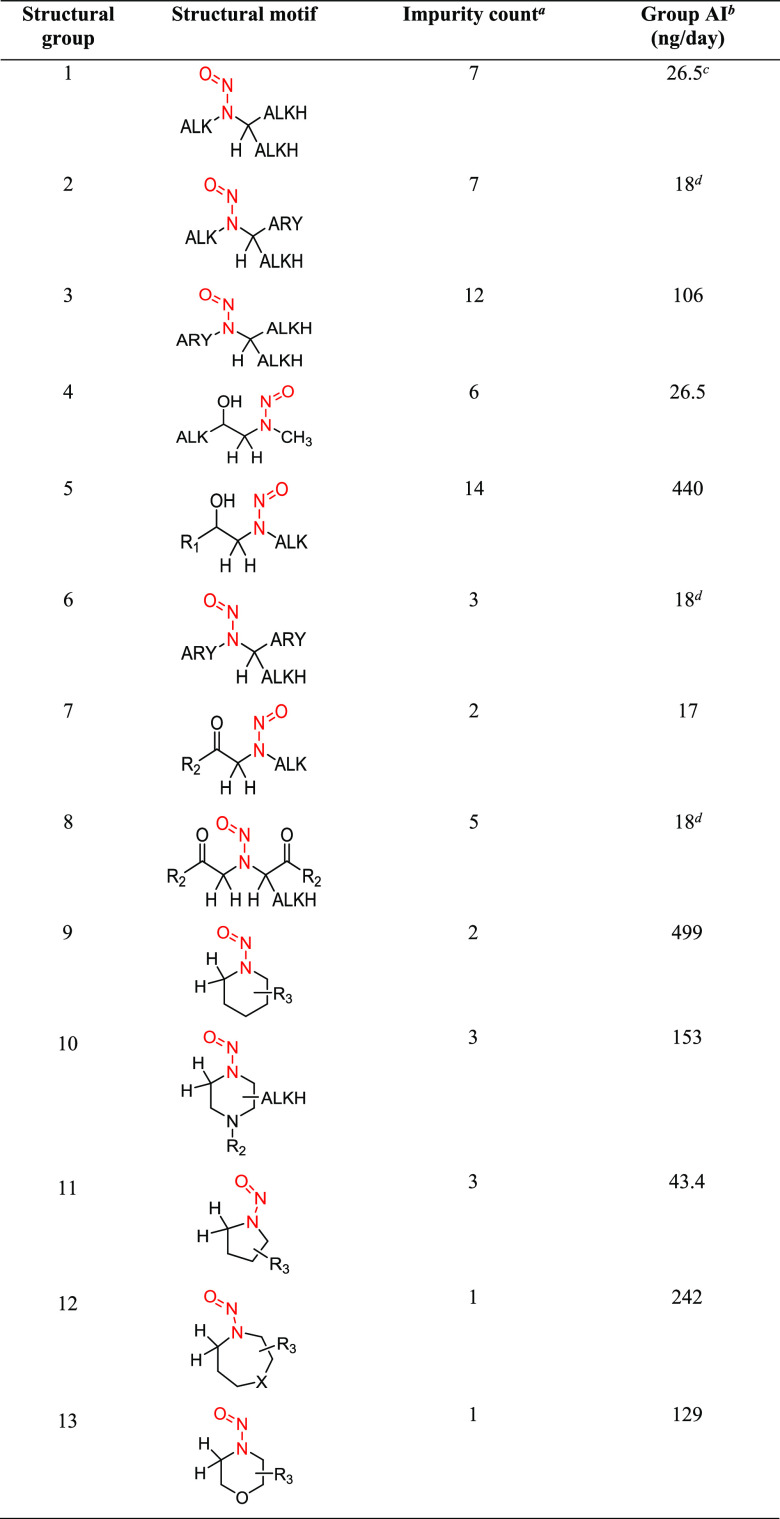
As part of the assessment of Pfizer’s portfolio, a considerable number of drug product N-nitrosamines required estimates of daily AIs to determine which ones needed analytical testing. These structurally complex N-nitrosamines could be potentially formed via reaction between a nitrosating agent and secondary amine precursors present in the API itself or as an impurity in the API and/or drug product degradant. Because the estimation of individual AIs for a large number of impurities using a read-across approach was a labor-intensive activity, we assigned the structurally complex N-nitrosamines to 13 structural groups (Table 1) based on the commonality of structural features in the immediate vicinity of the N-nitrosamine substituent, given the prior knowledge around structure–activity relationships for carcinogenic potencies of low-molecular-weight N-nitrosamines. The selection of read-across substrates for each structural group was then performed by independent visual inspections of the compounds in the Pfizer N-Nitroso Carcinogenicity Explorer to select appropriate N-nitrosamine chemicals, which were structurally analogous to members of the various structural groups in terms of electronic and steric environments. Daily AIs for each of the 13 structural groups were then determined using the read-across methodology (vide infra). A single worst-case AI was derived for each group, which could be applied to any related complex N-nitrosamine assigned to the group on the basis of structural similarities.
Table 1. Common Structural Motifs Observed among Complex Nitrosamine Impuritiese.
Number of complex N-nitrosamines that are categorized into each structural group.
AI assigned to each group based on the lowest TD50 for a structurally similar N-nitrosamine from the Pfizer Carcinogenicity Explorer Database. See Tables 3–11 and S1–S9 for details regarding the data and references, which underpin the AIs.
As NDEA belongs to this structural group, the EMA limit for NDEA (26.5 ng/day) is applied to this group.25
No N-nitrosamines with adequate carcinogenicity data were available to support an assessment of this structural group. EMA precautionary default limit of 18 ng/day is applied.25
ALK, alkyl chain that can be substituted and can contain heteroatoms and/or unsaturations; ALKH, alkyl chain that can contain heteroatoms and/or unsaturations or hydrogen; ARY, substituted or unsubstituted mono or poly aryl or heteroaryl rings; R1, hydrogen or any substituent (can be a cyclic structure); R2, any substituent apart from hydrogen; R3, any substituent (including hydrogen) or multiple substituents; and X, nitrosamine (N-NO) or carbon with any substituent(s) (including hydrogen).
Evaluation of the Carcinogenic Potency Data
LCDB was the primary source used to identify carcinogenic potency data (i.e., TD50 values). Within the LCDB, the original TD50 values calculated by Gold et al.27 are utilized (herein referred to as Gold TD50). In addition, Lhasa Limited independently calculated TD50 values (herein referred to as Lhasa TD50) based on the same data used for the Gold TD50 and using Lhasa’s published methodology.28 The LCDB was searched for available TD50 values for carcinogenic N-nitrosamines of interest and the most robust available study identified according to criteria noted in ICH M7(R1).3 Briefly, ideal studies for AI derivation contained 3 dose groups, at least 50 animals per group, with animals treated for a lifetime, with dosing at least 5 days per week; however, studies that did not meet all of these criteria were also considered for AI derivation in our evaluation, particularly when there was a robust tumor response. When both Lhasa and Gold TD50 values existed for a given N-nitrosamine, Lhasa values were utilized since the Lhasa database is current and calculations of TD50 values are transparent.28 Additionally, the studies with Lhasa TD50 values are generally more robust than those with only Gold TD50 values since Lhasa requires more than one treatment group for the derivation of a TD50. In instances where a compound was not reported in the LCDB, tumor incidence data were collected from literature references and a TD50 calculated using R code was adapted from Lhasa.28 In short, a model, based on binomial likelihood and optimized using the Broyden–Fletcher–Goldfarb–Shanno algorithm within the optim function used a linear model to capture the effect of dose on the probability of tumor occurrence and to ultimately estimate the TD50. For every N-nitrosamine evaluated, the TD50 value selected to represent its carcinogenic potency was taken (in a conservative approach) from the most robust study (if more than one study was reported) and, within that study, the most sensitive species, sex, and target organ. The harmonic mean TD50 value was not used.
In a few of the original study publications where evidence of carcinogenicity was reported, the description of the carcinogenicity study design and/or tumor incidence data was insufficient to calculate a TD50 value. In addition, there were numerous N-nitrosamines for which there was no evidence of carcinogenicity (either in LCDB or in peer-reviewed publications) at the doses administered in the carcinogenicity studies. In such cases, it was assumed that the nitrosamines have little or no carcinogenic potential and the highest dose tested rather than TD50 value is reported in the summary and supplemental data tables. Finally, in cases where all of the treated groups had a 100% tumor incidence, the TD50 was not calculated and/or used to establish the group AI, as the output may provide reasonable upper-bound estimates of the carcinogenic potency but provides little to no confidence in the lower-bound estimate of carcinogenic potency.
Derivation of AI for Individual Structural Groups
For each structural group, the TD50 values selected or calculated for representative N-nitrosamine carcinogens were summarized, and the group AI was derived based on the carcinogenic N-nitrosamine with the lowest TD50 value. The calculation of the AI relied on linear extrapolation from the TD50 value (dose associated with a tumor incidence of 1 in 2) to a dose that is associated with an excess cancer risk of 1 in 100 000. This was accomplished using the below equation as per ICH M7(R1)3
Results
Illustrations of each of the 13 structural groups, relevant to complex N-nitrosamine impurities, and the corresponding AI derived for each structural group are summarized in Table 1. The number of complex N-nitrosamine impurities that were categorized within each of the structural groupings ranged from 1 (groups 12 and 13) to 14 (group 5). When considering all of the N-nitrosamine impurities, 76% (50/66) were assigned to a group with an AI higher than the precautionary default limit of 18 ng/day.
Group 1 N-nitrosamines, comprised of N,N-dialkylnitrosamines, are structurally analogous to the highly carcinogenic, simple symmetrical N,N-dialkylnitrosamines such as NDMA and NDEA for which AIs have been widely published by several regulatory agencies.17,25 Consequently, the AI for structural group 1 was assigned as 26.5 ng/day, which is the lowest AI recommended for NDEA.17,25 Likewise, the EMA default limit of 18 ng/day was applied for structural groups 2, 6, and 8 since relevant structural analogues with adequate carcinogenicity data were not identified to support AI calculations. The very conservative limits for structural groups 2, 6, and 8 may be subject to change as more knowledge regarding structure–activity relationships (with respect to carcinogenicity) becomes available. For the remaining nine structural groups (3, 4, 5, 7, 9–13), N-nitrosamine analogues with suitable carcinogenicity data were available (Table 2). An AI for each structural group was established based on the lowest published or calculated TD50 value in the group, which resulted in AIs ranging from 17 ng/day (group 7) to 499 ng/day (group 9). Seven structural groups had at least one N-nitrosamine with a TD50 similar to or higher than 1.5 mg/kg/day, which translates to an AI similar to or higher than the default lifetime TTC (1.5 μg/day) defined in ICH M7(R1),3 and five structural groups had at least one N-nitrosamine that was reported as noncarcinogenic, supporting the observation that not all N-nitrosamines are CoC carcinogens.
Table 2. Summary of Carcinogenic Potencies of N-Nitrosamines.
| structural | no. of | range of TD50 values | no. of noncarcin. | group AI |
|---|---|---|---|---|
| group | N-nitrosaminesa | (mg/kg/day)b | N-nitrosamines | (ng/day)b |
| 3 | 12 | 0.106–18.1 | 4 | 106 |
| 4 | 2 | 0.020c–0.646 | 0 | 26.5 |
| 5 | 10 | 0.440d–8.11 | 2 | 440 |
| 7 | 8 | 0.017–1.1 | 0 | 17 |
| 9 | 14 | 0.499–49.4 | 5 | 499 |
| 10 | 8 | 0.140–34.6 | 0 | 153 |
| 11 | 9 | 0.0434c–31.3 | 3 | 43.4 |
| 12 | 2 | 0.242–0.313 | 0 | 242 |
| 13 | 4 | 0.129–1.22 | 2 | 129 |
Number of N-nitrosamines with published carcinogenicity data.
See Tables 3–11 and S1–S9 for details regarding the data and references, which underpin the range of TD50 values and the AIs.
Represents the 99% lower confidence interval (CI) of the most conservative TD50 value.
Represents the 95% lower CI of the most conservative TD50 value.
Tables 3–11 depict the structures of N-nitrosamines with carcinogenicity data and TD50 values (from the most sensitive species/sex/organ site from the study deemed most robust), which were classified using the structural criteria defined in Table 1. The N-nitrosamines are presented from the lowest to the highest TD50 value. For all N-nitrosamines included in our evaluation, details of the carcinogenicity studies that supported the derivation of the TD50 values are summarized in the Supporting Tables accompanying this manuscript.
Table 3. Structural Group 3: Summary of Carcinogenic Potencies for Group AI Determination26,31,32,h.
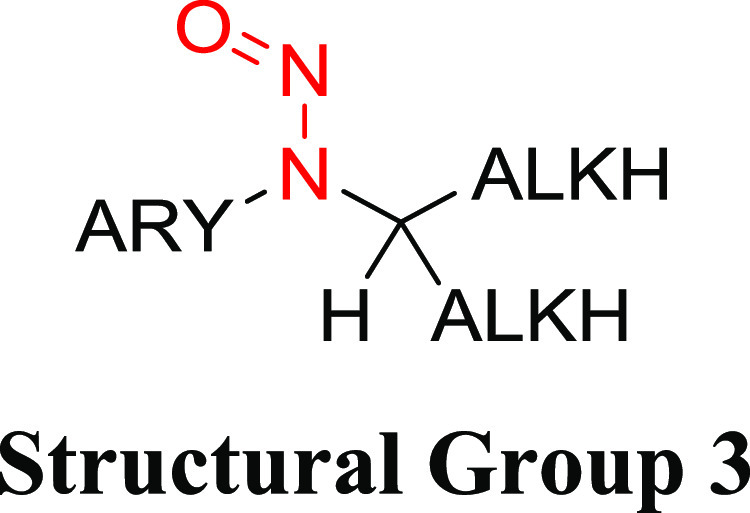
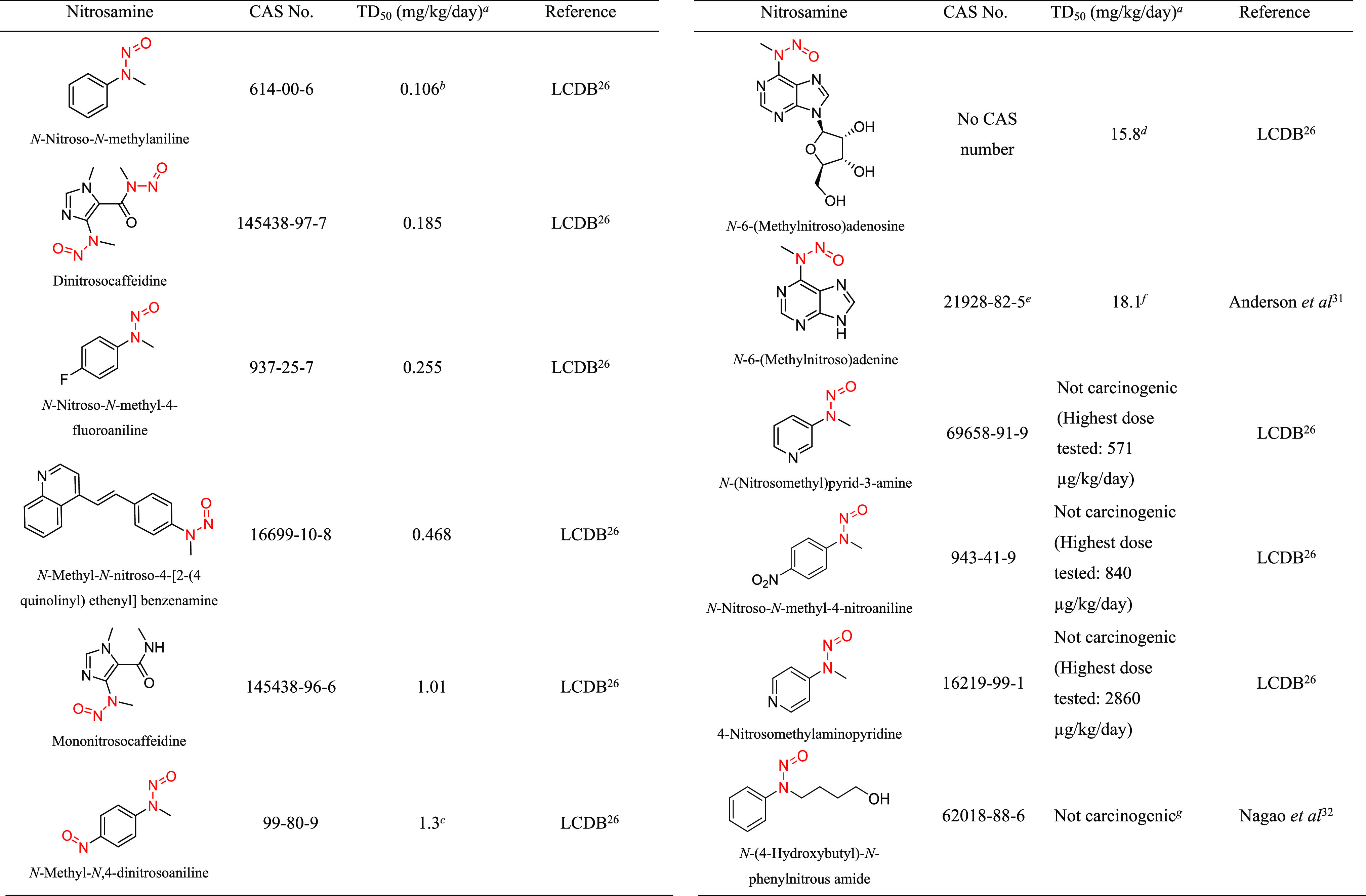
As reported in LCDB unless otherwise noted; the TD50 from the most sensitive organ site from the study deemed most robust is reported.
The AI for structural group 3 was calculated based on the worst-case TD50 from N-nitroso-N-methylaniline.
Gold TD50 reported in LCDB, but results are not statistically significant.
When a user enters CAS number 21928-82-5 into LCDB, it will pull back a record associated with N6-methyladenosine. It should be noted that the CAS number provided in CPDB and LCDB corresponds to the structure for N6-(methylnitroso)adenine in CAS (though CAS does list both names). There is no unique CAS number provided for N6-(methylnitroso)adenosine. The data presented in LCDB do correspond to that for N6-(methylnitroso)adenosine from Anderson et al.31
When a user enters CAS number 21928-82-5 into LCDB, it will pull back a record of carcinogenicity data associated with N6-methyladenosine. However, this CAS number is associated with N6-(methylnitroso)adenine in CAS and one must refer to the source document31 to find the relevant carcinogenicity data for N6-(methylnitroso)adenine.
TD50 calculated.
No data reported in the LCDB or the CPDB.
LCDB, Lhasa Carcinogenicity Database; TD50, dose producing a 50% tumor incidence.
Table 11. Structural Group 13: Summary of Carcinogenic Potencies for Group AI Determination26,53,54,d.
As reported in LCDB unless otherwise noted; the TD50 from the most sensitive organ site from the study deemed the most robust study is reported.
The AI for structural group 13 was calculated based on the worst-case TD50 from 4-nitrosomorpholine.
TD50 not calculated due to limitations of the mouse study.54
LCDB, Lhasa Carcinogenicity Database; TD50, dose resulting in tumors in 50% of animals.
Structural Group 3 AI Determination
Structural group 3 is comprised of N-alkyl-N-arylnitrosamines (Table 3). Of the 12 relevant N-nitrosamines, 4 were not considered carcinogenic based on the available data. The TD50 values for the other eight N-nitrosamines ranged from 0.106 to 18.1 mg/kg/day (Tables 3 and S1). Using the lowest TD50, which is associated with N-nitrosomethylaniline, an AI of 106 ng/day was derived for structural group 3. It should be noted that the U.S. Food and Drug Administration (FDA) has published an AI for N-nitrosomethylaniline of 26.5 ng/day, which may be based on read-across from NDEA (rationale for limit was not discussed);17 however, in our review of the available carcinogenicity data for N-nitrosomethylaniline, it was considered suitable to support the derivation of an AI. The EMA has also published an AI for N-nitrosomethylaniline of 34.3 ng/day, which is based on the same carcinogenicity study and tumor end point that we used to derive an AI of 106 ng/day.29 The reason for this difference is that the EMA selected the TD50 value that was calculated/published in CPDB, whereas we based the AI on the TD50 value calculated by Lhasa. As stated in the Materials and Methods section, when both Lhasa and Gold TD50 values existed for a given N-nitrosamine, Lhasa values were utilized since the Lhasa database is current and calculations of TD50 values are transparent.28
N-Nitrosomethylaniline was tested for the induction of tumors in rats, in studies of varying robustness, with the esophagus being identified as the most sensitive site.26 The TD50 of 0.106 mg/kg/day was chosen for the calculation of an AI for N-nitrosomethylaniline, as the associated study was considered the most robust.30 In the study, N-nitrosomethylaniline was administered for a lifetime to Sprague–Dawley rats (48/group) in drinking water at doses of 0.3 or 1.5 mg/kg (total doses of 61 or 232 mg/kg, respectively).30 The adjusted doses for the drinking water study are reported as 83.3 and 319 μg/kg/day in the LCDB. Following daily administration in drinking water, 80 and 87% of animals (irrespective of sex) developed esophageal tumors with a mean induction time of 290 and 200 days, respectively. Esophageal tumor incidence data are reported as mixed sex with 0/48 in controls, 39/48 at 83.8 μg/kg/day and 42/48 at 319 μg/kg/day, with a resulting TD50 of 0.106 mg/kg/day in the LCDB.
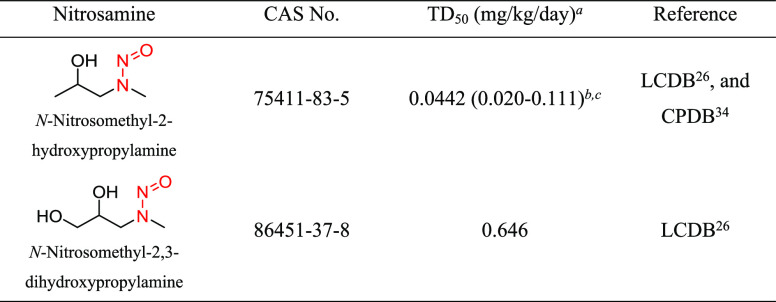
Structural Group 4 AI Determination
Structural group 4 is comprised of N-methyl-N-alkan-2-ol-based N-nitrosamine structures (Table 4). Two relevant N-nitrosamines with TD50 values of 0.0442 and 0.646 mg/kg/day were considered, and N-nitrosomethyl-2-hydroxypropylamine having the lower TD50 was selected for AI derivation (Tables 4 and S2).
Table 4. Structural Group 4: Summary of Carcinogenic Potencies for Group AI Determination26,34,d.
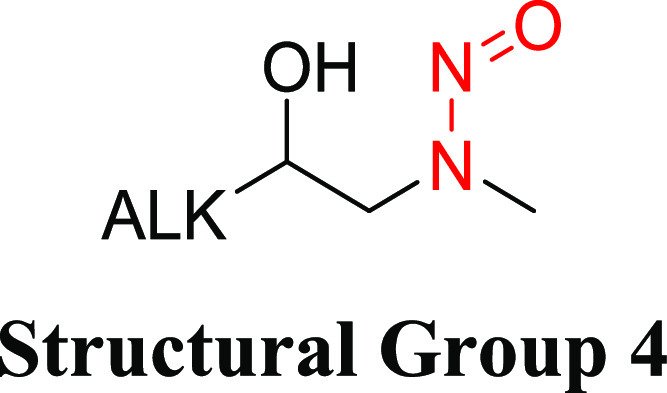
As reported in LCDB unless otherwise noted; the TD50 from the most sensitive organ site from the study deemed the most robust study is reported.
The AI for structural group 4 considered the worst-case TD50 from N-nitrosomethyl-2-hydroxypropylamine as well as the associated TD50 99% lower confidence interval.34
Confidence interval (CI) in parentheses.
LCDB, Lhasa Carcinogenicity Database; TD50, dose producing a 50% tumor incidence.
The carcinogenic potential of N-nitrosomethyl-2-hydroxypropylamine (total cumulative dose = 75 mg) was examined in Fischer rats (group of 20/sex) following daily (5 days/week) administration in drinking water for a period of 30 weeks.33 The longest surviving animals were examined at 75 weeks of age. The corrected doses of the nitrosamine in male and female rats were 0.286 and 0.408 mg/kg/day, respectively, as reported in the LCDB.26 The predominant tumor types induced in treated animals (relative to a matching group of 20 control animals/sex) were papillomas and carcinomas of the esophagus and squamous cell papillomas and carcinomas of the nasal cavity. The lowest TD50 (0.0442 mg/kg/day) for N-nitrosomethyl-2-hydroxypropylamine is based on multiple tumor types in the nasal cavity of male rats,26 with a 99% confidence interval (CI) of 0.020–0.111 mg/kg/ day.34 Since there was only one treated group and a control group with low animal numbers, the study design was not considered robust. Therefore, the lower bound of the 99% CI of the TD50 was considered for the derivation of the AI since this is the lowest likely TD50 value. Given the AI for N-nitrosomethyl-2-hydroxypropylamine using the TD50 value (44.2 ng/day) or the lower CI of the TD50 value (20 ng/day) is comparable to the AI for NDEA (26.5 ng/day), a limit of 26.5 ng/day was considered appropriate for structural group 4.
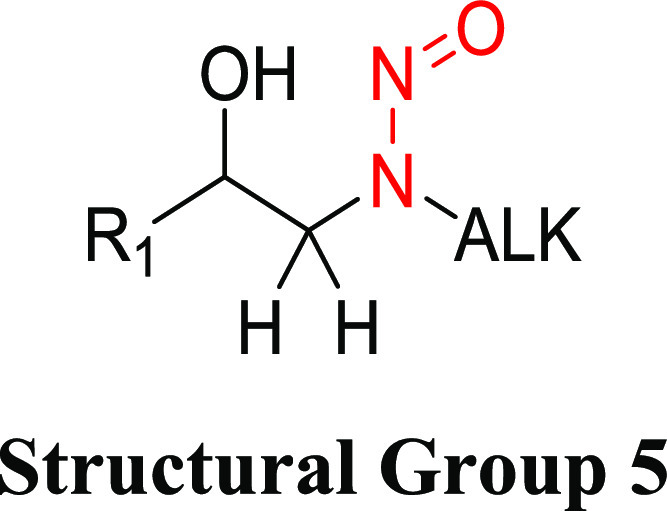
Structural Group 5 AI Determination
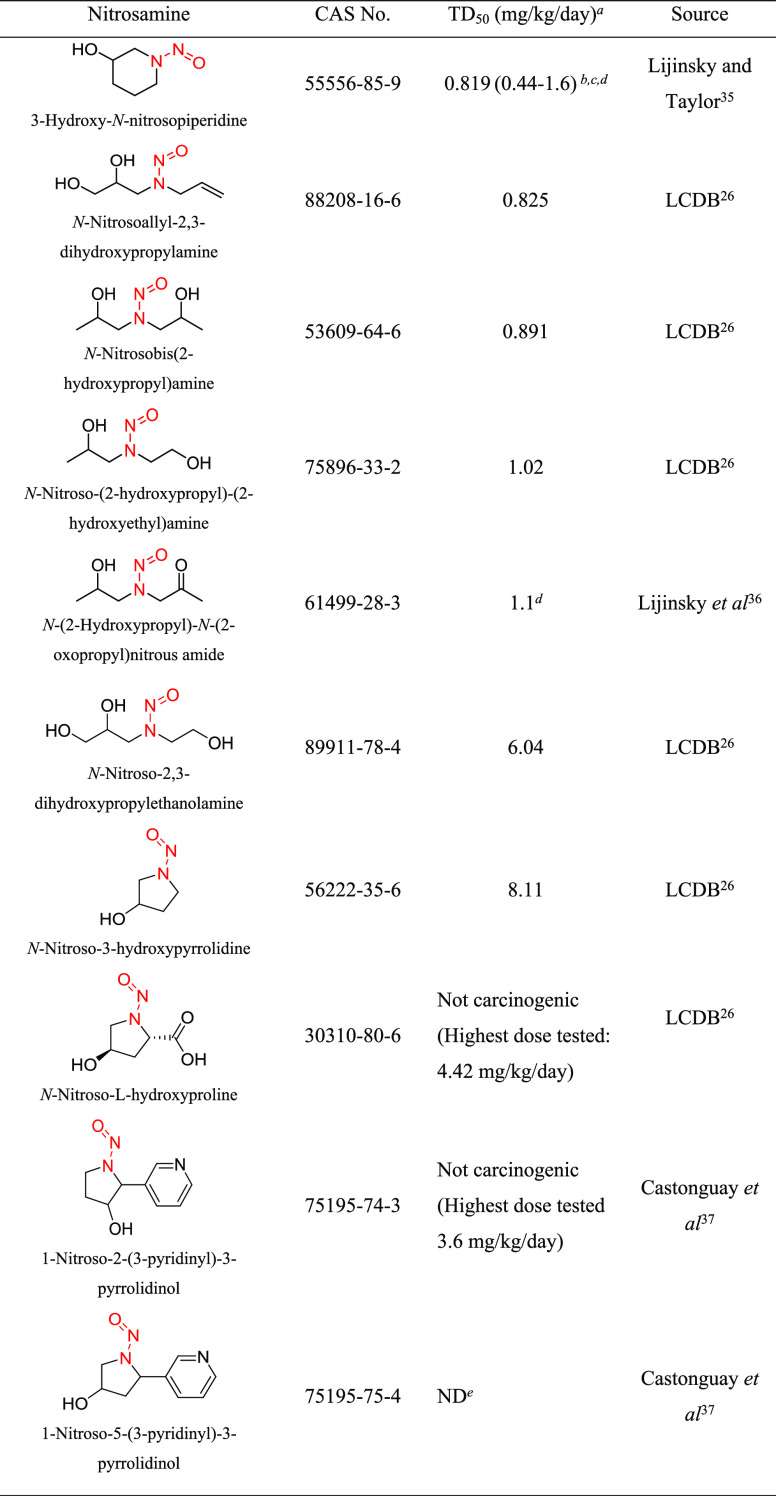
Structural group 5 is similar to structural group 4 except the N-methyl group is substituted by a larger N-alkyl substituent (Table 5). This structural group contained the largest number (14) of complex N-nitrosamine impurities requiring a read-across AI (Table 1). Ten relevant N-nitrosamines with carcinogenicity data were reviewed, of which two were not considered carcinogenic based on the available data. For another (1-nitroso-5-(3-pyridinyl)-3-pyrrolidinol), the study design did not allow for a reliable calculation of a TD50 value. However, 1-nitroso-5-(3-pyridinyl)-3-pyrrolidinol is structurally quite similar to the two noncarcinogens and therefore would not be expected to be among the most potent N-nitrosamines in this group. The TD50 values for the seven other N-nitrosamines ranged from 0.819 to 8.11 mg/kg/day (Tables 5 and S3).
Table 5. Structural Group 5: Summary of Carcinogenic Potencies for Group AI Determination26,35−37,f.
As reported in LCDB unless otherwise noted; the TD50 from the most sensitive organ site from the study deemed the most robust study is reported.
The AI for structural group 5 considered the worst-case TD50 from 3-hydroxy-N-nitrosopiperidine as well as the associated TD50 95% lower confidence interval.
CI in parentheses.
TD50, as well as upper and lower 95% confidence intervals, was calculated.
Study design does not allow for a reliable estimate of TD50. Dosing was limited to 3× per week for 7.3 weeks
LCDB, Lhasa Carcinogenicity Database; TD50, dose producing a 50% tumor incidence; ND, not determined.
3-Hydroxy-N-nitrosopiperidine (CAS 55556-85-9) had the lowest TD50 of the N-nitrosamines in structural group 5 and was selected for establishing the AI for this group. The carcinogenicity of 3-hydroxy-N-nitrosopiperidine was evaluated in rats (15 males, 14 females) after administration via drinking water for 36 weeks (total study duration 50 weeks) resulting in adjusted daily doses of 2.38 (males) and 3.40 mg/kg/day (females).35 A TD50 of 0.819 mg/kg/day (CI 0.44–1.6) was calculated for the predominant tumor site (nasal cavity [12/15 males and 10/14 females]) using the more sensitive sex. An untreated control group was not included in the study; therefore, taking a conservative approach, a background tumor incidence of 0 in 15 was assumed for purposes of supporting the calculation. Given that the study is not robust, the TD50 lower-bound 95% CI (0.44 mg/kg/day) was used to derive the group AI of 440 ng/day.
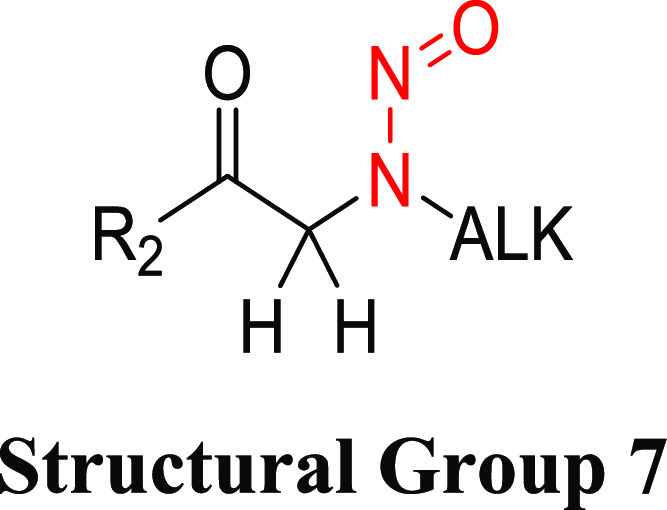
Structural Group 7 AI Determination
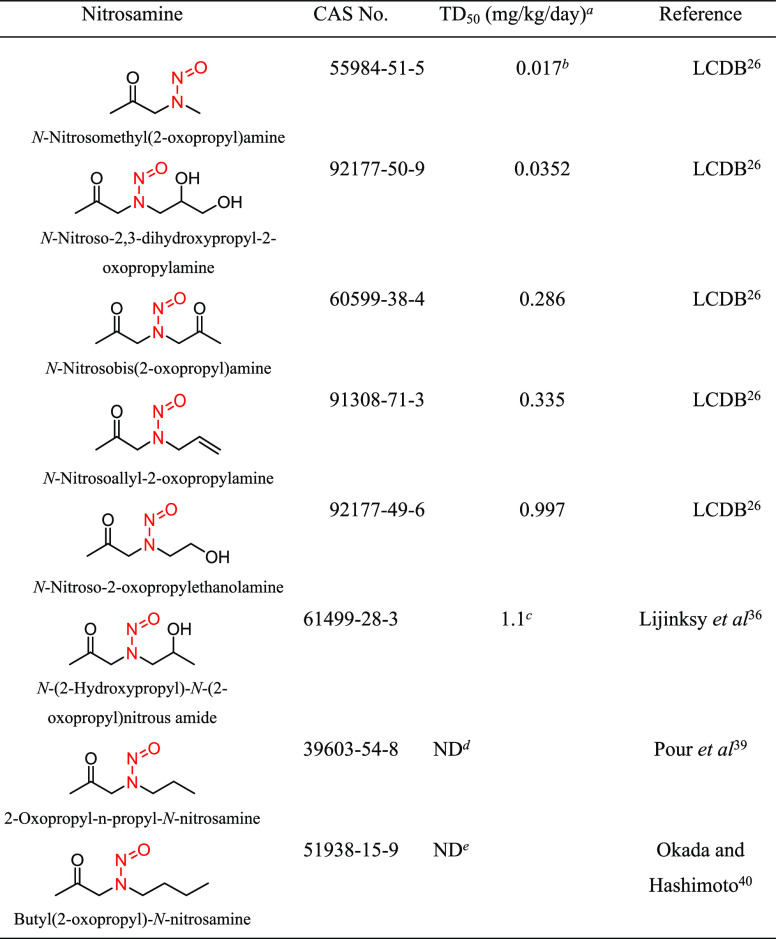
Structural group 7 is comprised of N-alkyl-N-alkan-2-one-type N-nitrosamine structures (Table 6). Of the eight relevant N-nitrosamines, two had insufficient data to calculate TD50 values. The TD50 values for the other six nitrosamines ranged from 0.017 to 1.11 mg/kg/day (Tables 6 and S4). Using the lowest TD50 associated with N-nitrosomethyl-(2-oxopropyl)amine (CAS 55984-51-5), an AI of 17 ng/day was derived for structural group 7.
Table 6. Structural Group 7: Summary of Carcinogenic Potencies for Group AI Determination26,36,39,40,f.
As reported in LCDB unless otherwise noted; the TD50 from the most sensitive organ site from the study deemed the most robust study is reported.
The AI for structural group 7 was calculated based on the worst-case TD50 from N-nitrosomethyl(2-oxopropyl)amine.
TD50 calculated.
Study design does not allow for a reliable estimate of TD50. Dosing was limited to 1× per week for 52 weeks.
Study design does not allow for a reliable estimate of TD50. Dosing was limited to 4–17 weeks
LCDB, Lhasa Carcinogenicity Database; TD50, dose producing a 50% tumor incidence; ND, not determined.
There was one carcinogenicity study wherein outbred MRC-Wistar rats (15 animals/sex/group) were orally administered N-nitrosomethyl-(2-oxopropyl)amine (0.9, 1.8, or 3.5 mg/kg/day)38 for 67 weeks, with adjusted doses of 129, 257, or 500 μg/kg/day reported in the LCDB. The naval cavity was the most sensitive tissue for neoplasm development, and female rats were deemed more sensitive. The incidence of tumors in the nasal cavity in female rats was 93% (14/15), 100% (15/15), and 64% (9/14) at 129, 257, or 500 μg/kg/day, respectively, with no tumors detected in vehicle-treated animals. The TD50 reported in the LCBD for N-nitrosomethyl-(2-oxopropyl)amine was 0.017 mg/kg/day.
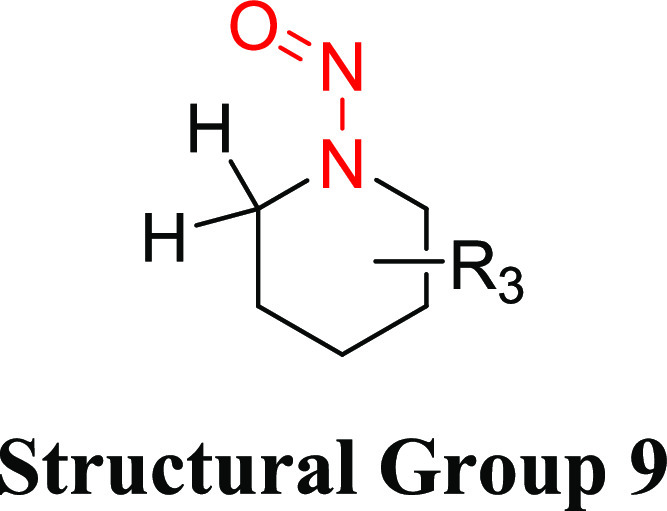
Structural Group 9 AI Determination
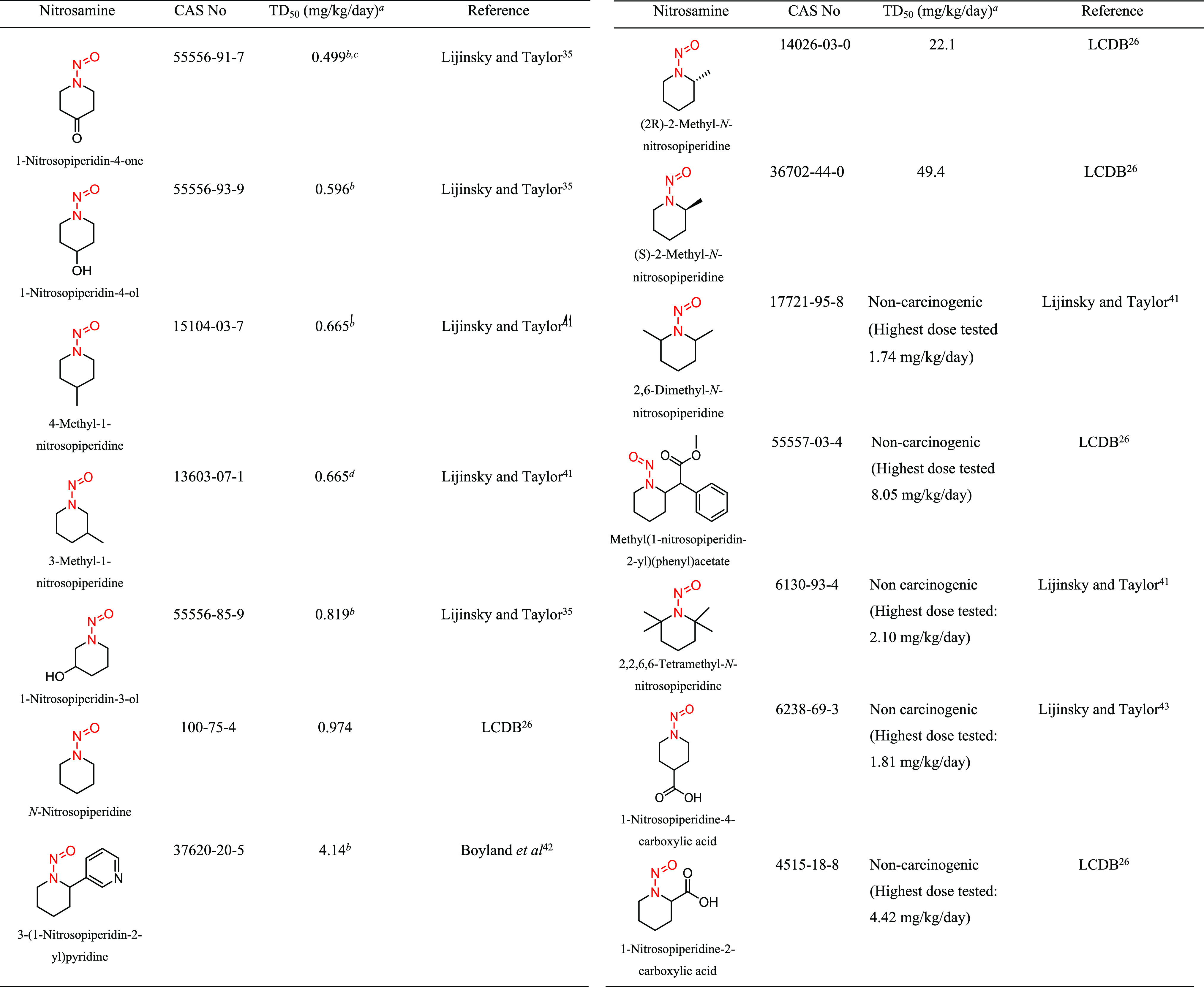
Structural group 9 is comprised of N-nitrosopiperidine (NPIP) derivatives (Table 7). Of the 14 N-nitrosopiperidines, 5 were not considered carcinogenic based on the available data. The TD50 values for the other nine N-nitrosopiperidines, ranged from 0.499 to 49.4 mg/kg/day (Tables 7 and S5). Using the lowest TD50 associated with 1-nitrosopiperidin-4-one (CAS 55556-91-7), an AI of 499 ng/day was derived for structural group 9.
Table 7. Structural Group 9: Summary of Carcinogenic Potencies for Group AI Determination26,35,41−43,e.
As reported in LCDB unless otherwise noted; the TD50 from the most sensitive organ site from the study deemed most robust is reported.
TD50 calculated.
The AI for structural group 9 was calculated based on the worst-case TD50 from 1-nitrosopiperidin-4-one.
As all animals treated with 3-methylnitrosopiperidine had gastrointestinal tumors, it is not possible to calculate a reliable TD50. Given the structural similarity to 4-methylnitrosopiperidine (whereby the N-nitroso group is not hindered by the methyl substitution) and that the overall tumor incidence reveals a pattern similar to that reported for 4-methylnitrosopiperidine, the TD50 of 3-methylnitrosopiperidine is expected to be similar.
LCDB, Lhasa Carcinogenicity Database; TD50, dose producing a 50% tumor incidence.
There was one carcinogenicity study reported in the literature for 1-nitrosopiperidine-4-one, where the N-nitrosamine was administered daily (5 days/week) in drinking water to Sprague–Dawley rats for 36 weeks with a total study duration of 60 weeks.35 The total cumulative administered dose of 1-nitrosopiperidin-4-one was 3.2 mmol, with corresponding adjusted daily doses in male and female rats of 1.95 and 2.80 mg/kg/day, respectively (Table S5). Both male (14/15) and female (14/15) rats developed adenocarcinomas of the nasal cavity, which was the most sensitive tumor site. Because no concurrent control animals were included in the study, a conservative estimate of 0 tumors/15 control animals was assumed, which led to the calculated TD50 values of 0.499 and 0.717 mg/kg/day for male and female rats, respectively.
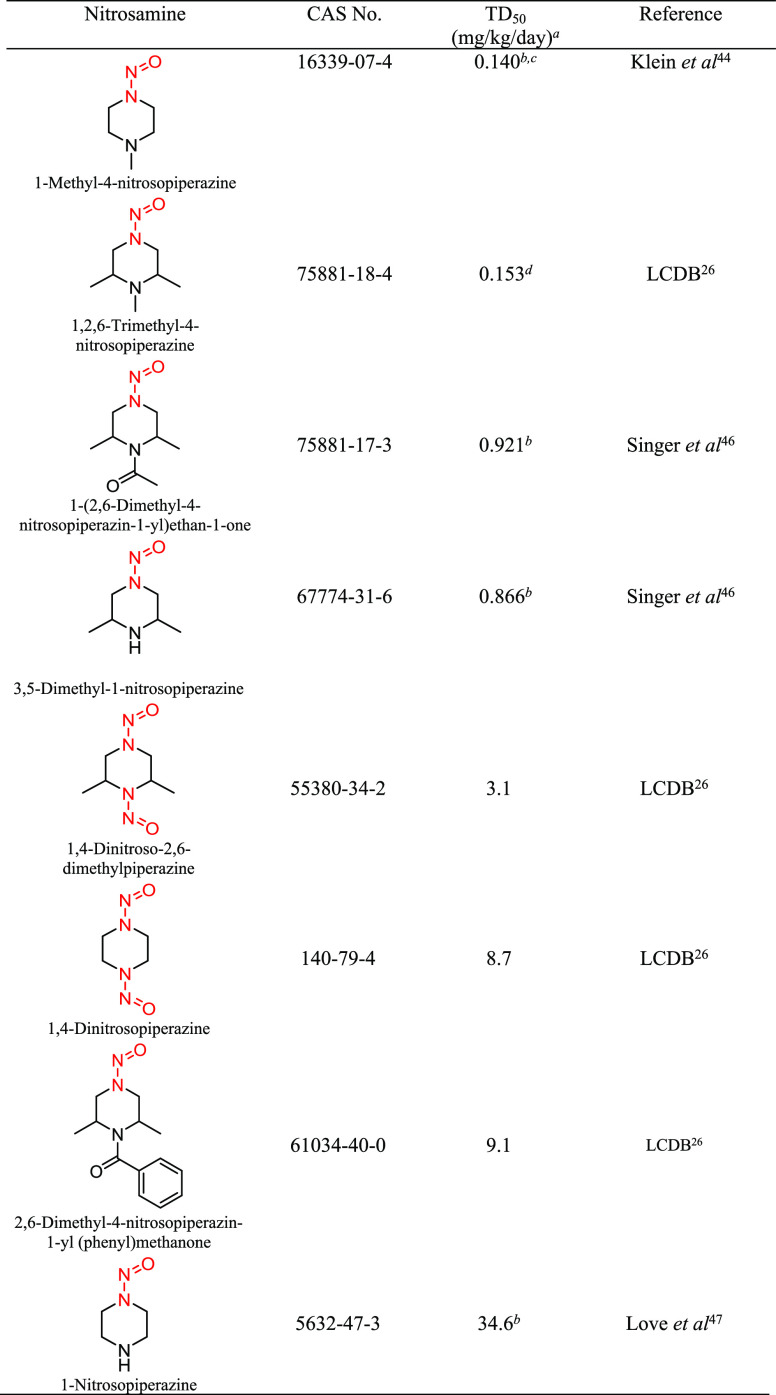
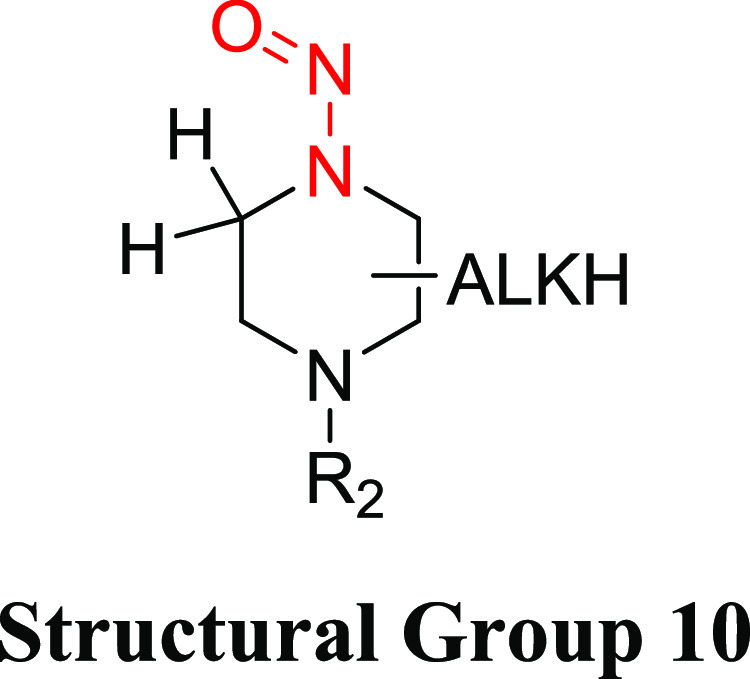
Structural Group 10 AI Determination
Structural group 10 is comprised of N-nitrosopiperazines (Table 8). The TD50 values of the eight relevant N-nitrosopiperazines ranged from 0.140 to 34.6 mg/kg/day (Tables 8 and S6). The lowest robust TD50 value (0.153 mg/kg/day) associated with 1,2,6-trimethyl-4-nitrosopiperazine (CAS 75881-18-4)26 was selected for the derivation of the AI (153 ng/day) for structural group 10. It was considered that EMA has published an AI of 26.5 ng/day for 1-methyl-4-nitrosopiperazine based on read-across to NDEA;29 however, there is no rationale provided for the appropriateness of using NDEA to read across to 1-methyl-4-nitrosopiperazine specifically or N-nitrosopiperazines in general. In addition, in our review of available carcinogenicity data, a slightly lower TD50 value (140 ng/day) was calculated for 1-methyl-4-nitrosopiperazine (CAS 16339-07-4). However, this TD50 value was not used to establish the AI for group 10 because the TD50 was derived from a single-dose study in which there was a 100% incidence of olfactory carcinomas (Table S6).44 While the TD50 value from the single-dose study could provide a reasonable upper-bound estimate of potency, there is no reliable indication of the lower-bound estimate of carcinogenic potency. Consequently, with a similar and more reliable TD50 calculated using a higher-quality study design, the TD50 generated from the study by Klein et al.44 was excluded.
Table 8. Structural Group 10: Summary of Carcinogenic Potencies for Group AI Determination26,44,46,47,e.
As reported in LCDB unless otherwise noted; the TD50 from the most sensitive organ site from the study deemed the most robust study is reported.
Calculated TD50.
A 100% tumor incidence observed in the only treatment group included in the study therefore does not result in a reliable estimate of TD50. This TD50 value was not considered in the derivation of the AI.
The AI for structural group 10 was calculated based on the lowest most robust TD50 from 1,2,6-trimethyl-4-nitrosopiperazine.
LCDB, Lhasa Carcinogenicity Database; TD50, dose producing a 50% tumor incidence.
The carcinogenicity of 1,2,6-trimethyl-4-nitrosopiperazine has been investigated in rats and hamsters.45 The study in rats was considered more robust given the existence of two treatment groups and a control group, relative to the study in hamsters, which included one treatment group and a control group. The daily (5 days/week) administration of 1,2,6-trimethyl-4-nitrosopiperazine in drinking water (adjusted doses of 259 or 980 μg/kg/day) to female Fischer rats (20/group) for 30 weeks resulted in no animals surviving 60 weeks following treatment at 980 μg/kg/day and 90 weeks post treatment at 259 μg/kg/day. The predominant tumor type was olfactory carcinomas of the nasal cavity in 13/20 and 18/20 rats at 259 and 980 μg/kg/day, respectively. Based on the olfactory carcinomas, the most sensitive TD50 of 0.153 mg/kg/day (153 μg/kg/day) is reported in LCDB.26
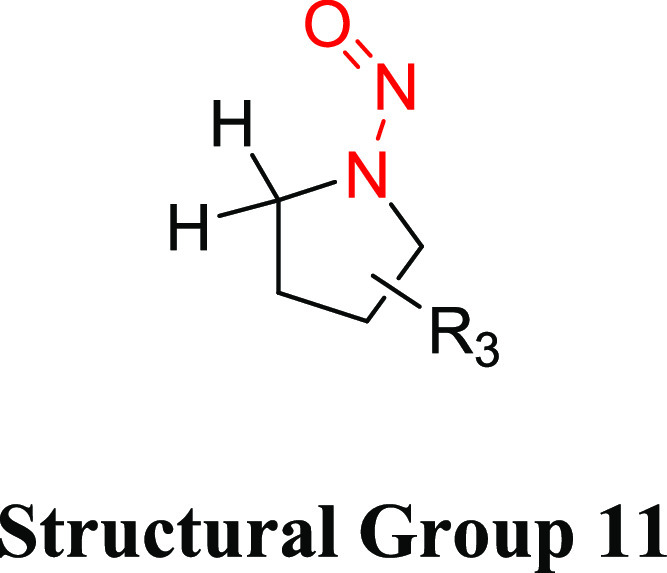
Structural Group 11 AI Determination
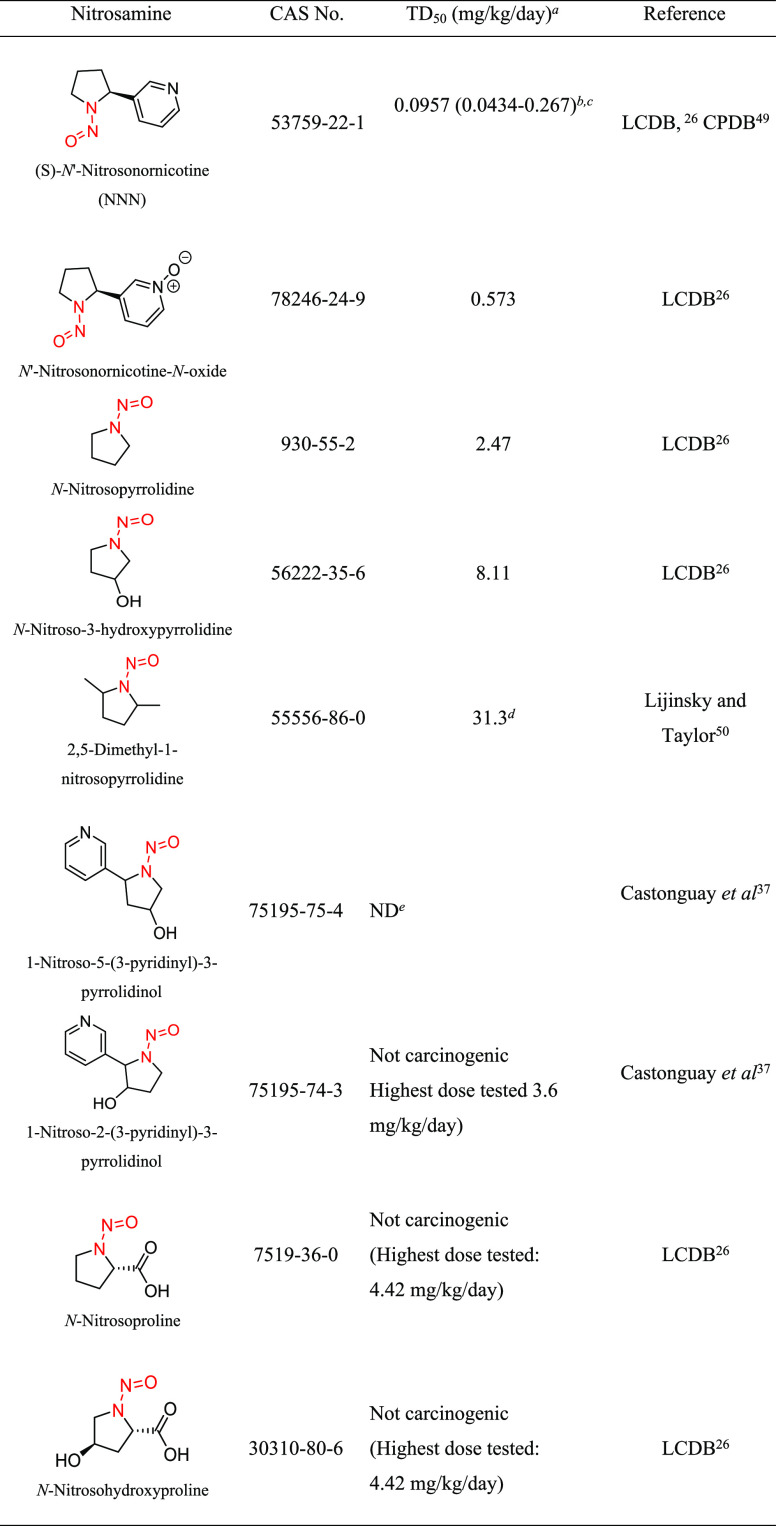
Structural group 11 is comprised of N-nitrosopyrrolidine (NNP) derivatives (Table 9). Of the nine relevant NNPs, three were concluded to be noncarcinogenic under the study conditions, and the carcinogenicity study design for one compound (1-nitroso-5-(3-pyridinyl)-3-pyrrolidinol) was not considered sufficiently robust to calculate a TD50. As stated previously, this N-nitrosamine is structurally similar to those that were not carcinogenic and therefore would not be expected to be a potent carcinogen. For the remaining five NNPs, the TD50 values ranged from 0.0957 to 31.3 mg/kg/day (Tables 9 and S7). (S)-N′-Nitrosonornicotine (NNN; CAS 53759-22-1) had the lowest TD50 and therefore was selected for the derivation of the AI for structural group 11.
Table 9. Structural Group 11: Summary of Carcinogenic Potencies for Group AI Determination26,37,49,50,f.
TD50, calculated dose resulting in tumor in 50% of animals as reported in LCDB unless otherwise noted; the TD50 from the most sensitive organ site from the study deemed the most robust study is reported.
CI in parentheses.
The AI for structural group 11 considered the worst-case TD50 from NNN as well as the associated TD50 99% lower confidence interval.49
Calculated TD50.
Study design does not allow for a reliable estimate of TD50. Dosing was limited to 3× per week for 7.3 weeks.
CPDB, Carcinogenic Potency Database; LCDB, Lhasa Carcinogenicity Database; ND, not determined.
Two carcinogenicity investigations with NNN are reported in LCDB: one in rat and the other in hamster. Both studies included a single treatment group and a control group with small numbers of animals. The duration of exposure in rats and hamsters was 87 weeks (experimental duration of 87 weeks) and 31 weeks (experimental duration of 96 weeks), respectively. Since these studies were of similar quality, the rat study was selected for the derivation of the AI because the lowest TD50 value was observed in this species. In the rat carcinogenicity study, NNN was administered to male F344 rats (20/control, 40/treated) in drinking water at an average daily dose of 250 μg/kg/day.26,48 The TD50 was derived based on the incidence of esophageal tumors (0 in control animals and 10/14 in treated). The TD50 calculated by Gold et al.27 was 0.0957 (99% CI 0.0434–0.267) mg/kg/day.49 Given that the TD50 is based on a study with a single treatment group and a limited number of animals, the lower CI of the TD50 was used to derive an AI of 43.4 ng/day.
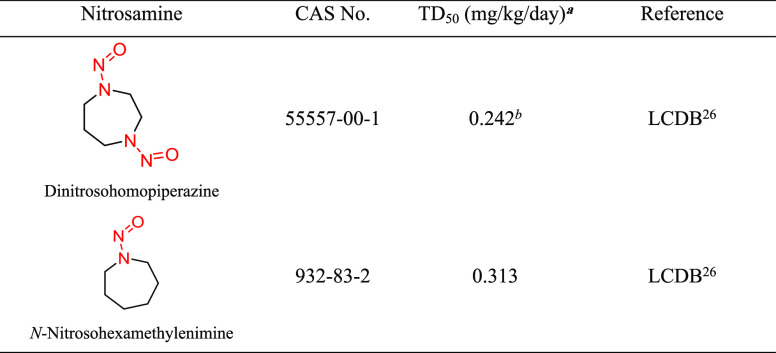
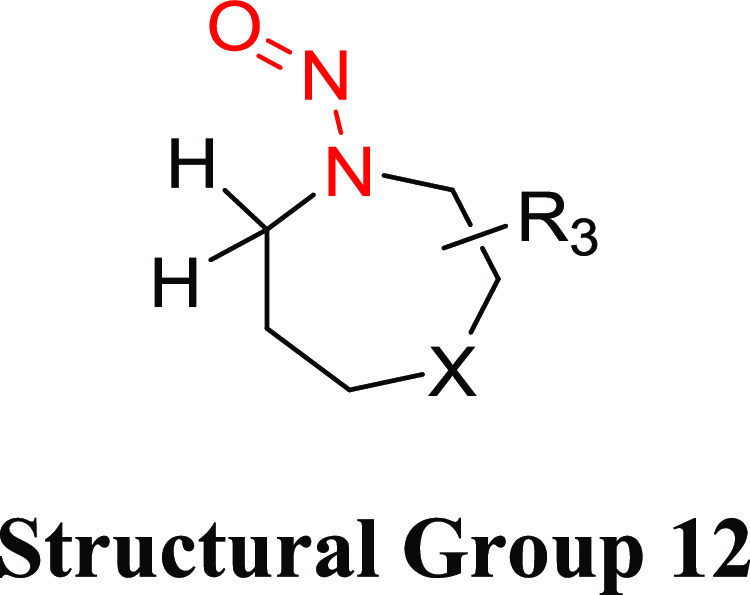
Structural Group 12 AI Determination
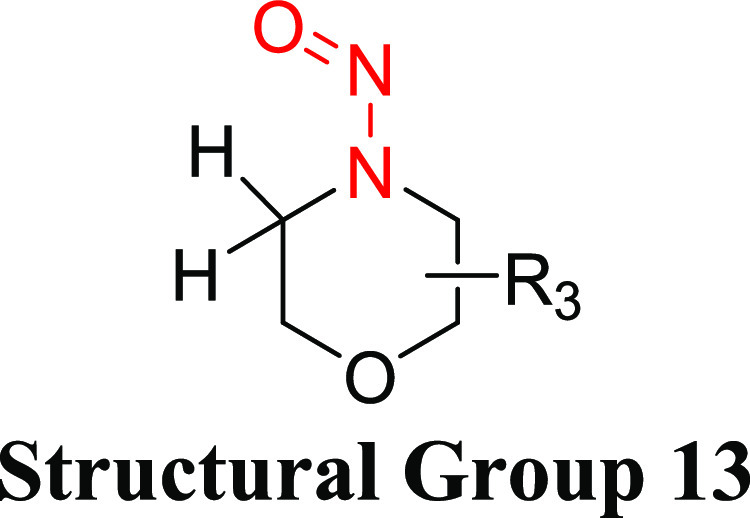
Structural group 12 is comprised of N-nitrosohomopiperazine and N-nitrosohomopiperidine derivatives. The TD50s of two relevant nitrosamines are quite similar (0.313 and 0.242 mg/kg/day) (Tables 10 and S8). Taking a conservative approach, the lower TD50 of the two nitrosamines in group 12, which was calculated for dinitrosohomopiperazine (CAS 55557-00-1), was selected to derive a group AI of 242 ng/day.
Table 10. Structural Group 12: Summary of Carcinogenic Potencies for Group AI Determination26,c.
As reported in LCDB unless otherwise noted; the TD50 from the most sensitive organ site from the study deemed the most robust study is reported.
The AI for structural group 12 was calculated based on the worst-case TD50 from dinitrohomopiperazine.
LCDB, Lhasa Carcinogenicity Database; TD50, dose producing tumors in 50% of animals.
Dinitrosohomopiperazine was tested for carcinogenicity in female Fischer 344 rats using several study designs.26,51 In the most robust of these studies (6 treatment groups, 20 animals per group, oral exposure for 30 weeks), the daily corrected doses of dinitrosohomopiperazine ranged from 10.1 to 2930 μg/kg/day.26 The incidence of the predominant tumor type, carcinomas of the upper gastrointestinal tract, ranged from 1/20 to 14/20 across the treatment groups. The associated TD50, 0.242 mg/kg/day (242 μg/kg/day), was used to establish the group AI.
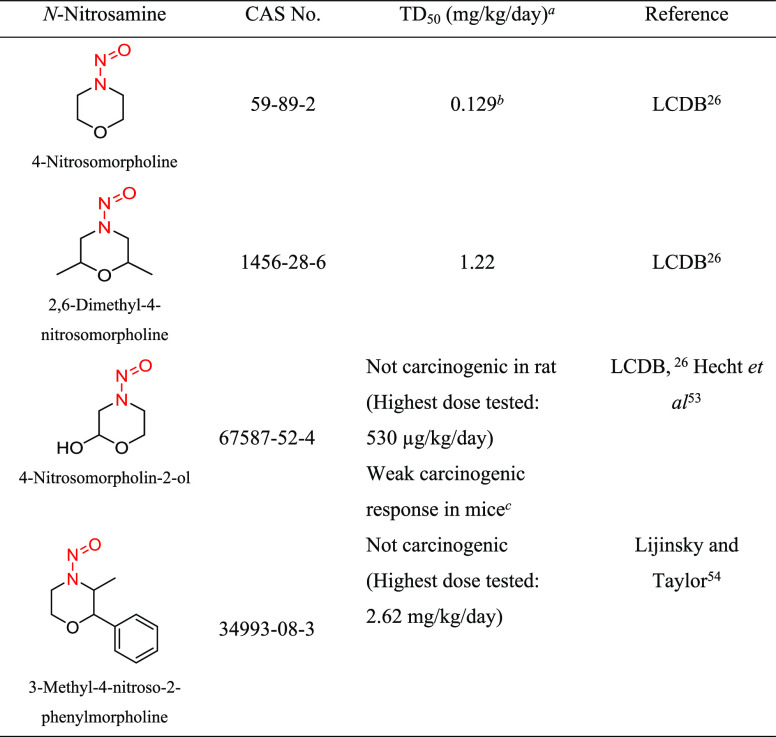
Structural Group 13 AI Determination
Structural group 13 is comprised of N-nitrosomorpholine derivatives. Of four relevant N-nitrosomorpholines, one was not carcinogenic and one was reported as weakly carcinogenic in mice. The other two N-nitrosomorpholines had TD50 values (0.129 and 1.22 mg/kg/day) that were considered for the AI calculation (Tables 11 and S8). As N-nitrosomorpholine (CAS 59-89-2) had the lower TD50, it was used to derive the AI (129 ng/day) for group 13. (Note: EMA recently published an AI for N-nitrosomorpholine of 127 ng/day.)29
N-Nitrosomorpholine was tested for the induction of tumors in rats and Syrian hamsters, with TD50 values from the most sensitive site for each study ranging from 0.0805 to 11.4 mg/kg/day.26 The most robust study was conducted in female Fischer 344 rats, with N-nitrosomorpholine administered orally for 100 weeks and examined for tumor incidence at the end of life.52 Daily corrected doses of N-nitrosomorpholine of 0, 2.27, 5.83, 14.6, 35.6, 84.2, or 249 μg/kg/day were administered to groups of 80, 100, 99, 47, 48, 48, or 24 rats, respectively. The liver was the most sensitive organ, with the lowest TD50 of 0.129 mg/kg/day for multiple tumor types,26 which was used as the basis for the group 13 AI.
Discussion
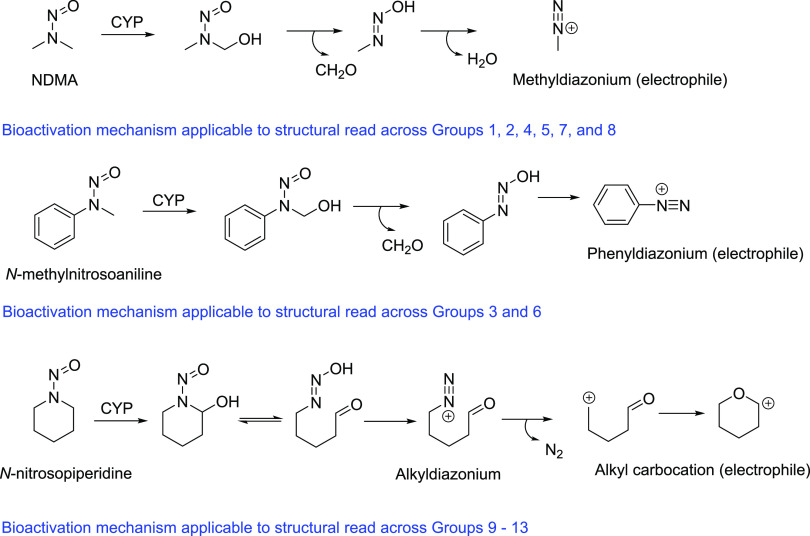
To address regulatory guidance on the risk assessment of N-nitrosamine impurities in all marketed or approved drugs,17,18,25 a science-based and practical endeavor involving the determination of AI limits for various structural groups of potential (theoretical) N-nitrosamine impurities was initiated. The exercise herein reported was limited to secondary amine-based APIs (and corresponding secondary amine impurities present within the API) that could potentially form N-nitrosamines upon reaction with a nitrosating source.23 The secondary amine-based APIs were structurally diverse with a broad range of physicochemical properties (e.g., molecular weight, topological polar surface area, lipophilicity, etc.), which is not surprising given their use in the treatment of a range of diseases (e.g., secondary amine-containing-fluoroquinolone antibiotics, -thiazide loop diuretics, -ethanolamine antihypertensives, etc.). However, since the secondary amine (and the corresponding theoretical N-nitrosamine) moiety was a common structural feature in all of the APIs, the N-nitrosamine impurities were classified into structural groups (Table 1) based on the substituents (e.g., N,N-dialkyl, N-alkyl-N-aryl, cycloalkyl [5-, 6-, 7-membered] amines) attached to the secondary amine nitrogen. Furthermore, all acyclic and cycloalkyl N-nitrosamine derivatives in the structural groups 1–13 contained at least one oxidizable center (α-C–H bond) that could be potentially bioactivated by CYP enzymes to the obligatory alkyl or aryl diazonium intermediate (Figure 2), as previously demonstrated with several low-molecular-weight acyclic or cycloalkyl N-nitrosamine carcinogens.5−8,55,56
Figure 2.
Bioactivation pathways for acyclic (N,N-dialkyl and N-alkyl-N-aryl) and cyclic N-nitrosamines from the structural groups 1–13.
Against this backdrop, it is noteworthy to point out that the propensity of the complex N-nitrosamines (from structural groups 1–13) toward CYP-catalyzed bioactivation to the electrophilic diazonium (alky or aryl) intermediate is presently not known. Examination of the metabolic pathways from available human mass balance/metabolism studies on several secondary amine-based APIs in humans revealed no evidence of N-dealkylated metabolites that could be derived from α-carbon hydroxylation adjacent to the amine nitrogen (unpublished observations). Whether the corresponding N-nitrosamine derivatives share an identical metabolic fate as their parent precursors remains unclear at the present time. A side-by-side comparison of the in vitro metabolism (e.g., reduced nicotinamide adenine dinucleotide phosphate (NADPH)-supplemented liver microsomes or S-9 fractions from animals and humans) for structurally diverse parent amines and corresponding N-nitrosamine derivatives, along with a comparison of their mutagenic responses in the bacterial reverse mutation assay (Ames test) (±metabolic activation), would be a useful next step to establish an understanding of the relationship between mutagenicity and metabolism/bioactivation (or lack thereof) of the parent amine and the corresponding N-nitrosamine impurities.
Carcinogenicity databases were mined for the identification of N-nitrosamines, which met the structural criteria defined in Table 1 for each of the groups (1–13). Published and/or calculated TD50 values were collected, and the lowest animal TD50 value within each group of N-nitrosamines was used to establish group AIs to use for read-across for the complex N-nitrosamine impurities with unknown carcinogenic potential. Overall, the strategy used here is aligned with ICH M7(R1) guidance,3 which recommends that an AI can be established for a chemically defined class based on carcinogenic potency data and applied to structurally similar mutagenic impurities without carcinogenicity data.
An exhaustive review of the carcinogenicity data for numerous structurally diverse N-nitrosamines revealed a broad range of carcinogenic potencies, with TD50 values in the μg/kg range to no evidence of carcinogenicity when tested at doses into the mg/kg range. For instance, considering the totality of N-nitrosamine carcinogenicity data included in our review, a 2900-fold difference between the lowest and highest TD50 values (0.017–49.4 mg/kg/day) was observed, which equates to an ∼29-fold difference when comparing the lowest (17 ng/day, group 7) and highest (499 ng/day, group 8) structural group AIs (Table 1). It is also useful to consider the range of TD50 values observed within each N-nitrosamine structural group relative to the TTC value of 1.5 μg/day, which is considered an acceptable lifetime daily intake for mutagenic impurities not classified as CoC.3 A TD50 value ≥1.5 mg/kg/day correlates to an AI that is equal to or greater than the default TTC. The majority of N-nitrosamine structural groups (Table 2) had examples of N-nitrosamines, with TD50s close to (groups 7 and 13) or exceeding (groups 3, 5, 9, 10, and 11) 1.5 mg/kg/day, and 5 N-nitrosamine structural groups (groups 3, 5, 9, 11, and 13; Table 2) had examples of N-nitrosamines that produced no evidence of carcinogenicity. It is entirely possible that the steric and electronic properties of the complex API-derived N-nitrosamines will not be optimal for CYP-mediated bioactivation leading to the electrophilic diazonium species, and therefore, it is reasonable to expect that some complex N-nitrosamine impurities will be less potent carcinogens (i.e., not possessing CoC potency). This is supported by a recent analysis of the impact of specific substitution patterns on the carcinogenic potency of simple N-nitrosamines, which demonstrated that steric hindrance at the α-carbon and electron-withdrawing groups at the β-carbon were associated with a decrease in carcinogenic potency.57
In the majority of cases, the most carcinogenic N-nitrosamine in each group was a simple chemical with no steric hindrance around the N-nitroso group. This is an expected observation and supports the conclusion that the AIs established for each structural group are conservative for read-across to complex N-nitrosamine impurities. The exception was group 11 where the sterically hindered N-nitrosopyrrolidines (NNN) and N′-nitrosonornicotine-N-oxide (NNNO), which would be expected to be less potent carcinogens, possessed TD50 values 26- and 4-fold lower than the simplest derivative N-nitrosopyrrolidine (NNP).26 It is possible that NNN and NNNO are in fact not more potent carcinogens relative to NNP but rather that the experimentally derived TD50 values were influenced by differences in the carcinogenicity study design, with NNP having a much more robust study relative to those conducted for NNN and NNNO (see Table S7 for details of these studies).
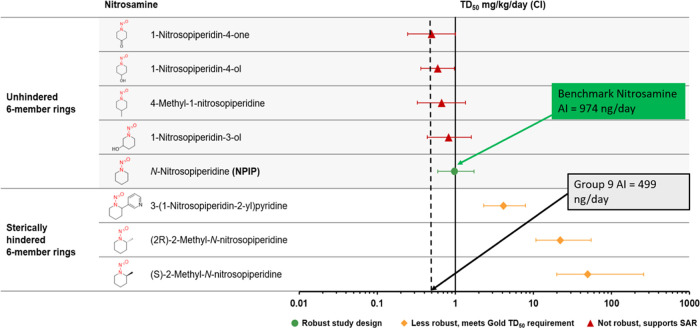
In addition to the LCDB, a more comprehensive data set was included in our review by considering all of the N-nitrosamine carcinogenicity studies that Lhasa has curated into the Vitic database. Using this approach, additional relevant N-nitrosamines with carcinogenicity data were identified, and whenever possible, TD50 values were calculated. Although not all of the carcinogenicity studies are optimal for deriving TD50 values, the totality of the data from these investigations provides weight of evidence for the potency of a group of structurally related N-nitrosamines, as well as informs the impact of structural features on potency. This is exemplified by the N-nitrosopiperidine (NPIP) structural group, which has a robust carcinogenicity study available for NPIP (TD50 = 0.974 mg/kg/day), the potency of which can be considered as a benchmark for comparison to the other structurally related NPIPs, which have been examined in less robust carcinogenicity studies (Figure 3). Direct comparison of NPIP carcinogenic potency to that of NPIPs with various substitutions was investigated by administering equal molar doses of NPIP and substituted NPIPs to small groups of rats.35,41 From these studies, it was concluded that methyl substitution at the 3- and 4-positions of the piperidine ring has little to no impact on the carcinogenic potency relative to NPIP;41 also, there was little difference in the carcinogenic potency of NPIP when compared to 1-nitrosopiperidine-4-one, 1-nitrosopiperidine-4-ol, and 1-nitrosopiperidin-3-ol.35 The similarity in carcinogenic potencies is reflected by overlapping CIs in Figure 3. It was also reported that substitutions at the 2- and 6-positions have the effect of reducing or eliminating carcinogenicity relative to NPIP,41 which was attributed to steric hindrance and/or lack of abstractable hydrogen atoms at carbon atoms α to the N-nitroso group. The hindered NPIPs that maintain some carcinogenic activity are distinctly less potent than NPIP (Figure 3).
Figure 3.
Distribution of TD50 values and upper and lower confidence intervals for N-nitrosopiperidines (group 9). TD50 values and 99% upper and lower confidence intervals are published in LCDB for N-nitrosopiperidine, (2R)-2-methyl-N-nitrosopiperidine, and (S)-2-methyl-N-nitrosopiperidine. For the remainder, TD50 values and 95% upper and lower confidence intervals were calculated.
In the case of structural group 1, which is comprised of N,N-dialkylnitrosamine impurities, a default AI of 26.5 ng/day was assigned based on the corresponding value proposed for the carcinogen NDEA.17,25 An AI of 26.5 ng/day for NDEA, as defined by the EMA25 and FDA,17 is based on the harmonic mean TD50 derived from the CPDB. Use of the harmonic mean is in contrast to the ICH M7(R1) recommendation3 that AIs should be calculated from the most robust animal study using the most sensitive organ site, species, and sex. Following the ICH guidance, an AI of 49.8 ng/day is derived for NDEA based on a lifetime drinking water study in male and female rats, with 15 treated groups of 50 or more animals/group/sex and 240 control animals/sex where the most sensitive end point was liver neoplasms with a TD50 of 0.0498 mg/kg/day.58 It is also important to note that among N,N-dialkylnitrosamines, there are also examples of weak carcinogens (e.g., N-nitrosodiisopropylamine) and noncarcinogens (e.g., N-nitroso-di-sec-butylamine).59
An important consideration in the application of the AIs presented here is the duration of treatment for the medication. Many pharmaceuticals are indicated for acute and/or intermittent administration, whereas the structural group AIs determined in this study assumed chronic daily exposure for a lifetime. The ICH M7(R1) guidance3 provides a framework for adjusting AIs according to the product’s duration of use, and empirical data from rodent carcinogenicity studies for NDEA support the application of the less than lifetime adjustment factors to nitrosamines.60
Establishing AIs for 13 different N-nitrosamine structural groups represented a useful and science-based approach to evaluate and establish AIs for a large number of N-nitrosamine impurities. The structural group AIs are conservative and based on the most potent N-nitrosamine in each structural group and assume chronic daily exposure for a lifetime. Overall, the comprehensive evaluation of available N-nitrosamine carcinogenicity data enabled the rapid differentiation of drug substance/products that did (or did not) require additional confirmatory testing, including the development of a suitably sensitive analytical methodology to monitor the presence of N-nitrosamine impurities. It is important to note that the work presented here should be viewed as a conservative approach for defining the AI for some (not all) N-nitrosamine impurities since the N-nitrosamine structural groups studied herein were of interest to Pfizer. Given that the structural group AIs are based on the most potent carcinogenic N-nitrosamine in each group, a useful extension of this study would be a more detailed analysis of structure–activity relationships for metabolism (bioactivation) and carcinogenic potency to enable compound-specific read-across assessments, which can potentially increase the daily AIs for individual compounds within the structural group framework.
Acknowledgments
The authors would like to thank Lhasa Limited for sharing the N-nitrosamine carcinogenicity data that they collated from the literature prior to releasing the information in the Lhasa Vitic database. Also, the authors appreciate Marianna Saporito who provided project management support during the data gathering and evaluation phase of this project, Simon Davies for his input in defining the N-nitrosamine structural groups, and Ron Ogilvie for his critical review and guidance throughout the conduct of this work. This work was supported by Pfizer, Inc.
Glossary
Abbreviations
- CoC
cohort of concern
- CYP
cytochrome P450
- TTC
threshold of therapeutic concern
- AI
acceptable intake
- APIs
active pharmaceutical ingredients
- ICH
International Council for Harmonization
- NDMA
N-nitrosodimethylamine
- NDEA
N-nitrosodiethylamine
- NMBA
N-nitroso-N-methyl-4-aminobutyric acid
- NNN
(S)-N′-nitrosonornicotine
- NNNO
N′-nitrosonornicotine-N-oxide
- NNP
N-nitrosopyrrolidine
- NPIP
N-nitrosopiperidine
- TD50
dose required to induce a 50% tumor incidence with lifetime exposure
- EMA
European Medicines Agency
- LCDB
Lhasa carcinogenicity database
- CPBD
carcinogenic potency database
- CI
confidence interval
- U.S. FDA
United States Food and Drug Administration
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemrestox.1c00369.
Details of carcinogenicity studies from which TD50 values were derived for N-nitrosamine derivatives from structural groups 3, 4, 5, 7, and 9–13 (Tables S1–S9) (PDF)
This work was funded by Pfizer. During this work, some authors were employees and held stock in Pfizer Inc.
The authors declare no competing financial interest.
Supplementary Material
References
- Cheeseman M. A.; Machuga E. J.; Bailey A. B. A tiered approach to threshold of regulation. Food Chem. Toxicol. 1999, 37, 387–412. 10.1016/S0278-6915(99)00024-1. [DOI] [PubMed] [Google Scholar]
- Kroes R.; Renwick A. G.; Cheeseman M.; Kleiner J.; Mangelsdorf I.; Piersma A.; Schilter B.; Schlatter J.; van Schothorst F.; Vos J. G.; Wurtzen G. European branch of the International Life Sciences Institute. Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food Chem. Toxicol. 2004, 42, 65–83. 10.1016/j.fct.2003.08.006. [DOI] [PubMed] [Google Scholar]
- International Council for Harmonisation of Technical Requirements forPharmaceuticals for Human Use (ICH) . Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk, M7(R1), Step 4; ICH, 2017.
- Thresher A.; Foster R.; Ponting D. J.; Stalford S. A.; Tennant R. E.; Thomas R. Are all nitrosamines concerning? A review of mutagenicity and carcinogenicity data. Regul. Toxicol. Pharmacol. 2020, 116, 104749 10.1016/j.yrtph.2020.104749. [DOI] [PubMed] [Google Scholar]
- Archer M. C. Mechanisms of action of N-nitroso compounds. Cancer Surv. 1989, 8, 241–250. [PubMed] [Google Scholar]
- Hecht S. S. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998, 11, 559–603. 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Loeppky R. N.; Sukhtankar S.; Gu F.; Park M. The carcinogenic significance of reactive intermediates derived from 3-acetoxy- and 5-acetoxy-2-hydroxy-N-nitrosomorpholine. Chem. Res. Toxicol. 2005, 18, 1955–1966. 10.1021/tx0502037. [DOI] [PubMed] [Google Scholar]
- Young-Sciame R.; Wang M.; Chung F. L.; Hecht S. S. Reactions of alpha-acetoxy-N-nitrosopyrrolidine and alpha-acetoxy-N-nitrosopiperidine with deoxyguanosine: formation of N2-tetrahydrofuranyl and N2-tetrahydropyranyl adducts. Chem. Res. Toxicol. 1995, 8, 607–616. 10.1021/tx00046a016. [DOI] [PubMed] [Google Scholar]
- Lee V. M.; Keefer L. K.; Archer M. C. An evaluation of the roles of metabolic denitrosation and alpha-hydroxylation in the hepatotoxicity of N-nitrosodimethylamine. Chem. Res. Toxicol. 1996, 9, 1319–1324. 10.1021/tx960077u. [DOI] [PubMed] [Google Scholar]
- Wade D.; Yang C. S.; Metral C. J.; Roman J. M.; Hrabie J. A.; Riggs C. W.; Anjo T.; Keefer L. K.; Mico B. A. Deuterium isotope effect on denitrosation and demethylation of N-nitrosodimethylamine by rat liver microsomes. Cancer Res. 1987, 47, 3373–3377. [PubMed] [Google Scholar]
- Appel K. E.; Görsdorf S.; Scheper T.; Spiegelhalder B.; Wiessler M.; Schoepke M.; Engeholm C.; Kramer R. Metabolic denitrosation of N-nitrosamines: mechanism and biological consequences. IARC Sci. Publ. 1991, 105, 351–357. [PubMed] [Google Scholar]
- Magee P. N.; Barnes J. M. Carcinogenic nitroso compounds. Adv. Cancer Res. 1967, 10, 163–246. [DOI] [PubMed] [Google Scholar]
- Sheweita S. A.; Mostafa M. H. N-Nitrosamines and their effects on the level of glutathione, glutathione reductase and glutathione S-transferase activities in the liver of male mice. Cancer Lett. 1996, 99, 29–34. 10.1016/0304-3835(95)04034-X. [DOI] [PubMed] [Google Scholar]
- Elder D. P.; Johnson G. E.; Snodin D. J. Tolerability of risk: A commentary on the nitrosamine contamination issue. J. Pharm. Sci. 2021, 110, 2311–2328. 10.1016/j.xphs.2021.02.028. [DOI] [PubMed] [Google Scholar]
- Bharate S. S. Critical analysis of drug product recalls due to nitrosamine impurities. J. Med. Chem. 2021, 64, 2923–2936. 10.1021/acs.jmedchem.0c02120. [DOI] [PubMed] [Google Scholar]
- Erskine D.; Wood D. What is the significance of nitrosamine contamination in medicines?. Drug Ther. Bull. 2021, 59, 39–42. 10.1136/dtb.2020.000036. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) . Control of Nitrosamine Impurities in Human Drugs. Guidance for Industry; FDA, 2021. [Google Scholar]
- European Medicines Regulatory Network Approach for the Implementation of the CHMP Opinion Pursuant to Article 5(3) of Regulation (EC) No 726/2004 for Nitrosamine Impurities in Human Medicines, 2021. Implementation Process_Article 5(3) Nitrosamine (europa.eu).
- Tuesuwan B.; Vongsutilers V. Nitrosamine contamination in pharmaceuticals: threat, impact, and control. J. Pharm. Sci. 2021, 110, 3118–3128. 10.1016/j.xphs.2021.04.021. [DOI] [PubMed] [Google Scholar]
- Maundrell N. Nitrosamine impurities: From raw materials to final drug product. Bioanalysis 2021, 63–66. 10.4155/bio-2021-0238. [DOI] [PubMed] [Google Scholar]
- Mirvish S. S. Formation of N-nitroso compounds: chemistry, kinetics, and in vivo occurrence. Toxicol. Appl. Pharmacol. 1975, 31, 325–351. 10.1016/0041-008X(75)90255-0. [DOI] [PubMed] [Google Scholar]
- Ashworth I. W.; Dirat O.; Teasdale A.; Whiting M. Potential for the formation of N-Nitrosamines during the manufacture of active pharmaceutical ingredients: an assessment of the risk posed by trace nitrite in water. Org. Process Res. Dev. 2020, 24, 1629–1646. 10.1021/acs.oprd.0c00224. [DOI] [Google Scholar]
- López-Rodríguez R.; McManus J. A.; Murphy N. S.; Ott M. A.; Burns M. J. Pathways for N-nitroso compound formation. Secondary amines and beyond. Org. Process Res. Dev. 2020, 24, 1558–1585. 10.1021/acs.oprd.0c00323. [DOI] [Google Scholar]
- Workflows for Quality Risk Management of Nitrosamine Risks in Medicines; EFPIA, Dec 2020. https://www.efpia.eu/media/580594/workflows-for-quality-risk-management-of-nitrosamine-risks-in-medicines.pdf PowerPoint Presentation (efpia.eu).
- European Medicines Agency. Assessment Report. Procedure under Article 5(3) Regulation EC (No) 726/2004. Nitrosamines Impurities In Human Medicinal Products. Procedure Number: EMEA/H/A-5(3)/1490; European Medicines Agency, 2020.
- Lhasa Limited Carcinogenicity Database. Lhasa Carcinogenicity Database. https://carcdb.lhasalimited.org/.
- Gold L. S.; Slone T. H.; Manley N. B.; Garfinkel G. B.; Hudes E. S.; Rohrbach L.; Ames B. N. The carcinogenicity potency database: analyses of 4000 chronic animal cancer experiments published in the general literature and by the U.S. National Cancer Institute/National Toxicology Program. Environ. Health Perspect. 1991, 96, 11–15. 10.1289/ehp.919611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thresher A.; Gosling J. P.; Williams R. Generation of TD50 values for carcinogenicity study data. Toxicol. Res. 2019, 8, 696–703. 10.1039/c9tx00118b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Questions and answers for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal products. EMA/409815/2020 Rev.6, Oct 14, 2021.
- Schmähl D. Investigations on esophageal carcinogenicity by methyl-phenyl-nitrosamine and ethyl alcohol in rats. Cancer Lett. 1975, 1, 215–218. 10.1016/S0304-3835(75)96970-0. [DOI] [PubMed] [Google Scholar]
- Anderson L. M.; Giner-Sorolla A.; Greenbaum J. H.; Last-Barney K.; Budinger J. M. Induction of reproductive system tumors in mice by N6-(methylnitroso)-adenosine and a tumorigenic effect of its combined precursors. Int. J. Cancer 1979, 24, 319–322. 10.1002/ijc.2910240308. [DOI] [PubMed] [Google Scholar]
- Nagao M.; Suzuki E.; Yasuo K.; Yahagi T.; Seino Y. Mutagenicity of N-butyl-N-(4-hydroxybutyl) nitrosamine, a bladder carcinogen, and related compounds. Cancer Res. 1977, 37, 399–407. [PubMed] [Google Scholar]
- Lijinsky W.; Reuber M. D. Carcinogenesis in rats by nitrosodimethylamine and other nitrosomethylalkylamines at low doses. Cancer Lett. 1984, 22, 83–88. 10.1016/0304-3835(84)90047-8. [DOI] [PubMed] [Google Scholar]
- Carcinogenic Potency Database (CPDB). Accessed using ToxPlanet (subscription required) at: https://files.toxplanet.com/cpdb/chempages/N-NITROSOMETHYL-2-HYDROXYPROPYLAMINE.html (last updated Oct 3, 2007). Download Carcinogenic Potency Database (CPDB) Data (nih.gov).
- Lijinsky W.; Taylor H. W. Tumorigenesis by oxygenated nitrosopiperidines in rats. J. Natl. Cancer Inst. 1975, 55, 705–708. 10.1093/jnci/55.3.705. [DOI] [PubMed] [Google Scholar]
- Lijinsky W.; Saavedra J. E.; Reuber M. D. Carcinogenesis in F-344 rats by nitrosobis(2-oxopropyl)amine and related compounds administered in drinking water. J. Cancer Res. Clin. Oncol. 1984, 107, 178–182. 10.1007/BF01032604. [DOI] [PubMed] [Google Scholar]
- Castonguay A.; Lin D.; Stoner G. D.; Radok P.; Furuya K.; Hecht S. S.; Schut H. A.; Klaunig J. E. Comparative carcinogenicity in A/J mice and metabolism by cultured mouse peripheral lung of N′-nitrosonornicotine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, and their analogues. Cancer Res. 1983, 43, 1223–1229. [PubMed] [Google Scholar]
- Pour P. M.; Stepan K. Comparative carcinogenesis of N-nitrosobis(2-oxopropyl)-amine and N-nitrosomethyl(2-oxopropyl)amine following subcutaneous or oral administration to rats. Cancer Lett. 1989, 45, 49–57. 10.1016/0304-3835(89)90035-9. [DOI] [PubMed] [Google Scholar]
- Pour P.; Althoff J.; Cardesa A.; Krüger F. W.; Mohr U. Effect of beta-oxidized nitrosamines on Syrian golden hamsters. II. 2-Oxopropyl-n-propylnitrosamine. J. Natl. Cancer Inst. 1974, 52, 1869–1874. 10.1093/jnci/52.6.1869. [DOI] [PubMed] [Google Scholar]
- Okada M.; Hashimoto Y. Carcinogenic effect of N-nitrosamines related to butyl(4-hydroxybutyl)nitrosamine in ACI/N rats, with special reference to induction of urinary bladder tumors. Gan 1974, 65, 13–19. [PubMed] [Google Scholar]
- Lijinsky W.; Taylor H. W. Carcinogenicity of methylated nitrosopiperidines. Int. J Cancer 1975, 16, 318–322. 10.1002/ijc.2910160215. [DOI] [PubMed] [Google Scholar]
- Boyland E.; Roe F. J.; Gorrod J. W.; Mitchley B. C. The carcinogenicity of nitrosoanabasine, a possible constituent of tobacco smoke. Br. J. Cancer 1964, 18, 265–270. 10.1038/bjc.1964.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijinsky W.; Taylor H. W. Carcinogenesis tests of nitroso-N-methylpiperazine, 2,3,5,6-tetramethyldinitrosopiperazine, nitrosoisonipecotic acid and nitrosomethoxymethylamine in rats. Z. Krebforsch. Klin. Onkol. Cancer Res. Clin. Oncol. 1977, 89, 31–36. 10.1007/BF02571686. [DOI] [PubMed] [Google Scholar]
- Klein R. G.; Schmezer P.; Hermann R.; Waas P.; Spiegelhalder B.; Bartsch H. Strong nasal carcinogenicity and genotoxicity of 1-nitroso-4-methylpiperazine after low dose inhalation in rats. Carcinogenesis 1999, 20, 1629–1631. 10.1093/carcin/20.8.1629. [DOI] [PubMed] [Google Scholar]
- Lijinsky W.; Knutsen G. M.; Reuber M. D. Carcinogenicity of methylated nitrosopiperazines in rats and hamsters. Carcinogenesis 1983, 4, 1165–1167. 10.1093/carcin/4.9.1165. [DOI] [PubMed] [Google Scholar]
- Singer S. S.; Singer G. M.; Saavedra J. E.; Reuber M. D.; Lijinsky W. Carcinogenesis by derivatives of 1-nitroso-3,5-dimethylpiperazine in rats. Cancer Res. 1981, 41, 1034–1038. [PubMed] [Google Scholar]
- Love L. A.; Lijinsky W.; Keefer L. K.; Garcia H. Chronic oral administratin of 1-nitrosopiperazine at high doses to MRC rats. Z. Krebforsch. Klin. Onkol. Cancer Res. Clin. Oncol. 1977, 89, 69–73. 10.1007/BF02571691. [DOI] [PubMed] [Google Scholar]
- Stoner G. D.; Adams C.; Kresty L. A.; Amin S. G.; Desai D.; Hecht S. S.; Murphy S. A.; Morse M. A. Inhibition of N-nitrosonornicotine-induced esophageal tumorigenesis by 3-phenylpropylisothiocyanate. Carcinogenesis 1998, 19, 2139–2143. 10.1093/carcin/19.12.2139. [DOI] [PubMed] [Google Scholar]
- Carcinogenic Potency Database. Accessed using ToxPlanet (subscription required) at: https://files.toxplanet.com/cpdb/chempages/N’-NITROSONORNICOTINE.html (last updated Oct 3, 2007). Download Carcinogenic Potency Database (CPDB) Data (nih.gov).
- Lijinsky W.; Taylor H. W. The Effect of substituents on the carcinogenicity of N-nitrosopyrrolidine in Sprague-Dawley rats. Cancer Res. 1976, 36, 1988–1990. [PubMed] [Google Scholar]
- Lijinsky W.; Reuber M. D.; Davies T. C.; Riggs C. W. Dose-response studies with nitroso-1,2,3,6-tetrahydropyridine and dinitrosohomopiperazine in F344 rats. Ecotoxicol. Environ. Saf. 1982, 6, 513–527. 10.1016/0147-6513(82)90033-1. [DOI] [PubMed] [Google Scholar]
- Lijinsky W.; Kovatch R. M.; Riggs C. W.; Walter P. T. Dose response study with N-nitrosomorpholine in drinking water of F-344 Rats. Cancer Res. 1988, 48, 2089–2095. [PubMed] [Google Scholar]
- Hecht S. S.; Lijinsky W.; Kovatch R. M.; Chung F. L.; Saavedra J. E. Comparative tumorigenicity of N-nitroso-2-hydroxymorpholine, N-nitrosodiethanolamine and N-nitrosomorpholine in A/J mice and F344 rats. Carcinogenesis 1989, 10, 1475–1477. 10.1093/carcin/10.8.1475. [DOI] [PubMed] [Google Scholar]
- Lijinsky W.; Taylor W. H. Carcinogenicity tests of N-nitroso derivatives of two drugs, phenmetrazine and methylphenidate. Cancer Lett. 1976, 1, 359–363. 10.1016/S0304-3835(75)98645-0. [DOI] [Google Scholar]
- Stiborova M.; Asfaw B.; Frei E.; Schmeiser H. H.; Wiessler M. Benzenediazonium ion derived from sudan I forms an 8-(phenylazo)guanine adduct in DNA. Chem. Res. Toxicol. 1995, 8, 489–498. 10.1021/tx00046a002. [DOI] [PubMed] [Google Scholar]
- Wong H.; Murphy S. E.; Wang M.; Hecht S. S. Comparative metabolism of N-nitrosopiperidine and N-nitrosopyrrolidine by rat liver and esophageal microsomes and cytochrome P4502A3. Carcinogenesis 2003, 24, 291–300. 10.1093/carcin/24.2.291. [DOI] [PubMed] [Google Scholar]
- Cross K. P.; Ponting D. J. Developing structure-activity relationships for N-nitrosamine activity. Comput. Toxicol. 2021, 20, 100186 10.1016/j.comtox.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R.; Gray R.; Brantom P.; Grasso P. Effects on 4080 rats of chronic ingestion of N-nitrosodiethylamine or N-nitrosodimethylamine: a detailed dose-response study. Cancer Res. 1991, 51, 6415–6551. [PubMed] [Google Scholar]
- Lijinsky W.; Taylor H. W. Carcinogenicity of methylated derivatives of N-nitrosodiethylamine and related compounds in Sprague-Dawley rats. J. Natl. Cancer Inst. 1979, 62, 407–410. [PubMed] [Google Scholar]
- Bercu J. P.; Masuda-Herrera M.; Johnson G.; Czich A.; Glowienke S.; Kenyon M.; Thomas R.; Ponting D. J.; White A.; Cross K.; Waechter F.; Rodrigues M. A. C. Use of less-than-lifetime (LTL) durational limits for nitrosamines: case study of N-nitrosodiethylamine (NDEA). Regul. Toxicol. Pharmacol. 2021, 123, 104926 10.1016/j.yrtph.2021.104926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.