Abstract
Background
Posttraumatic stress disorder (PTSD) is a devastating psychological disorder. Patients with PTSD canonically demonstrate an increased risk for inflammatory diseases as well as increased sympathetic tone and norepinephrine outflow. Yet, the exact etiology and causal nature of these physiologic changes remain unclear. Previously, we demonstrated that exogenous norepinephrine alters mitochondrial superoxide in T lymphocytes to produce a proinflammatory T helper 17 phenotype and observed similar T helper 17 polarization in a preclinical model of PTSD. Therefore, we hypothesized sympathetic-driven neuroimmune interactions could mediate psychological trauma–induced T lymphocyte inflammation.
Methods
Repeated social defeat stress (RSDS) is a preclinical murine model that recapitulates the behavioral, autonomic, and inflammatory aspects of PTSD. Targeted splenic denervation was performed to deduce the contribution of splenic sympathetic nerves to RSDS-induced inflammation. Denervation or sham operation was performed in 85 C57BL/6J mice, followed by RSDS or control paradigms. Animals were assessed for behavioral, autonomic, inflammatory, and redox profiles.
Results
Denervation did not alter the antisocial or anxiety-like behavior induced by RSDS. In circulation, denervation/RSDS animals exhibited diminished levels of T lymphocyte–specific cytokines (interleukin 2 [IL-2], IL-17A, and IL-22) compared with intact animals, whereas other nonspecific inflammatory cytokines (e.g., IL-6, tumor necrosis factor α, and IL-10) were unaffected by denervation. Importantly, denervation specifically ameliorated the increases in RSDS-induced T lymphocyte mitochondrial superoxide, T helper 17 polarization, and proinflammatory gene expression with minimal impact to non–T lymphocyte immune populations.
Conclusions
Overall, our data suggest that sympathetic nerves regulate RSDS-induced splenic T lymphocyte inflammation but play less of a role in the behavioral and non–T lymphocyte inflammatory phenotypes induced by this psychological trauma paradigm.
Keywords: Cytokine, Norepinephrine, PTSD, Redox, Superoxide, Sympathetic
Posttraumatic stress disorder (PTSD) is a stress-related disorder that is characterized by intrusive re-experiences of trauma (e.g., flashbacks), avoidance of reminiscent stimuli, affective changes, hyperarousal, and significant functional impairment (1). Among patients with PTSD, well-controlled clinical studies have repeatedly demonstrated an increased risk for a variety of inflammation-driven diseases such as rheumatoid arthritis, systemic erythematosus lupus, and cardiovascular disease (2, 3, 4, 5, 6). Importantly, the relationship and directionality between PTSD and the altered immune milieu remains a crucial question to address the inflammation-driven diseases patients with PTSD experience [reviewed by Sumner et al. (7); (8)].
A wide range of studies has established that PTSD is associated with significantly increased activation of the sympathetic nervous system. This is evidenced by reported increases in urinary and cerebrospinal fluid norepinephrine (NE) content, baroreflex sensitivity, and muscle sympathetic nerve activity (9, 10, 11, 12), many of which have also been found to correlate with PTSD symptom severity (11,12). This association between sympathetic tone and PTSD is subject to the same causality dilemma as the increased incidence of inflammatory diseases in these patients. Interestingly, secondary lymphoid organs such as the spleen are exclusively innervated by sympathetic efferent nerves that terminate near T lymphocyte–rich areas (13, 14, 15, 16, 17), suggesting that NE release may play a role in immune regulation during states of sympathoexcitation. Indeed, work from our laboratory has established that exogenous NE enhances ex vivo T lymphocyte production of interleukin 6 (IL-6) and IL-17A. During investigations probing the intracellular mechanisms responsible, we demonstrated that the NE-induced increase in T lymphocyte production of IL-6 and IL-17A could be partially reversed by scavenging of mitochondrial superoxide in these T lymphocytes (18). Importantly, IL-6 and IL-17A have been heavily implicated in the pathogenesis of a number of inflammation-driven diseases (19, 20, 21), with human studies demonstrating their increase in patients with PTSD [summarized exceptionally well by Wang et al. (8)]. Moreover, in a preclinical mouse model of PTSD (i.e., repeated social defeat stress [RSDS]), we elucidated a tight association between splenic T lymphocyte NE content, mitochondrial superoxide, and inflammatory cytokine expression (22). Therefore, we sought to delineate the potential causal relationship between increased splenic sympathetic tone and T lymphocyte inflammation in the context of RSDS.
To mechanistically investigate the role of RSDS-induced splenic sympathoexcitation on T lymphocyte function, we performed selective sympathetic nerve ablation specifically to the spleen. Denervation of targeted sympathetic nerve beds has recently shown significant clinical utility in the cardiovascular arena by its ability to reduce blood pressure, improve cardiac and renal function, and lower blood glucose in the long term with minimal to no adverse effects (23, 24, 25, 26, 27, 28, 29). Specifically, splenic denervation has also shown promise in a large animal model of inflammation (30) and is currently being considered for clinical trials of inflammatory conditions. However, to our knowledge, this approach has not been reported in any preclinical or clinical study of PTSD. In this study, we show that splenic denervation has a significant impact on systemic T lymphocyte–derived inflammation. These results demonstrate the importance of direct neuroimmune interactions in RSDS and put forth a novel translational finding that may attenuate psychological trauma–induced inflammation.
Methods and Materials
Mice
All experimental mice were wild-type male mice on a C57BL/6J background (The Jackson Laboratory). All experimental mice were bred in-house to attenuate the introduction of confounding stressors or flora related to shipping or differential facilities. Retired breeder male CD-1 mice of approximately 4 to 8 months of age were purchased from Charles River Laboratories, Inc., and prescreened for aggression as previously described (22,31,32). Experimental mice were group housed along with same-sex littermates until the time of the experiment. Mice were housed with corncob bedding and paper nesting materials and given ad libitum access to water and standard chow (Teklad laboratory diet; Harlan Laboratories, Inc.). All experimental mice were euthanized by intraperitoneal injection of 150 mg/kg pentobarbital (Fatal-Plus; Vortech Pharmaceuticals, Ltd.). Euthanasia occurred between 7 am and 9 am to attenuate described effects of circadian rhythm on inflammation. All procedures were reviewed and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee.
Splenic Denervation
To selectively ablate the splenic nerve, mice were first anesthetized with 2.5% nebulized isoflurane supplemented with oxygen until appropriate depth of anesthesia was achieved. Mice were then shaved along the left lateral side, and the site was cleaned with surgical grade Betadine (Avrio Health L.P.) followed by 70% ethanol. A small incision was made along the left side caudal to the ribs through the skin and peritoneum to visualize the spleen. Under magnification, the splenic artery was carefully dissected away from surrounding adipose and pancreas tissue. A cotton applicator soaked in 10% phenol in ethanol was gently applied to the splenic artery for 5 to 10 seconds until visual vasodilation was evident, with care taken to avoid contact with the spleen or surrounding tissue. Sham-operated mice were subjected to identical conditions with saline applied to the splenic artery. Following the operation, the muscular and peritoneal layers were sutured closed using 4-0 absorbable Vicryl sutures (Ethicon, Inc.), followed by skin and external fascia closure with 6-0 Prolene sutures (Ethicon, Inc.). Efficacy of denervation was assessed by measurement of splenic NE (discussed below) after euthanasia. Following denervation or sham operation, mice recovered for 7 days before entering RSDS or control protocols (described below).
Repeated Social Defeat Stress
RSDS was conducted as we have previously described (22,31). Standardized RSDS uses aggressive CD-1 mice, which precludes the inclusion of female mice, thus preventing the investigation of potential sex differences in this study. Briefly, aggressive CD-1 mice were singly housed for 3 days followed by introduction of experimental mice into the CD-1 home cage for social defeat for 5 minutes daily. During social defeat bouts, CD-1 and experimental mice were monitored for appropriate aggressive and socially defeated behaviors, respectively, to ensure adequate stress induction. Following social defeat, CD-1 and experimental mice were co-housed for the remaining 24 hours separated by a clear, perforated acrylic barrier to prevent physical interaction but allow for visual, auditory, and olfactory stimulation. Social defeat was repeated for 10 consecutive days with experimental mice rotating to a new CD-1 home cage daily. Control mice were pair housed with one another (no exposure to CD-1 mice) across an acrylic barrier to prevent physical interaction for 10 consecutive days. Following the 10-day RSDS or control paradigms, mice underwent behavioral testing on day 11, and biological substances were harvested on day 12. All mice were assessed for visually apparent wounding (>1 cm) or lameness, and affected mice were excluded (4 mice were excluded in this study owing to these parameters; these mice were not compiled into the final animal count).
Behavioral Testing
Behavioral testing was conducted as we have previously reported (22,31). Briefly, all experimental mice were assessed first by social interaction test followed by elevated zero maze between 7 am and 2 pm. The social interaction test was performed using a 40 × 40 cm open field maze (Noldus Information Technology) with a transparent enclosure with 6.5 × 10 cm wire mesh caging. Experimental or control mice were placed in the open field with an empty enclosure devoid of prior mice contact, followed by analogous testing with an identical enclosure housing a novel CD-1 mouse. Ratios of time spent in proximity to the enclosure (interaction zone) or in the distant corners of the maze (corner zones) between trials with or without CD-1 mice were calculated to produce social interaction and corner zone ratios, respectively (31,32). Each trial lasted 2.5 minutes, with all sessions recorded and digitally analyzed by accompanying EthoVision XT 13 software (Noldus Information Technology). The elevated zero maze was performed using a standard elevated circular track, 50 cm diameter × 5 cm track width (Noldus Information Technology) with 50% enclosed (20 cm wall height) and 50% open. Each trial was 5 minutes, with all sessions recorded and digitally analyzed by accompanying EthoVision XT 13 software.
T Lymphocyte Immunophenotyping
Splenocytes were incubated at 37 °C for 4 hours in Roswell Park Memorial Institute Medium (RPMI) media supplemented with phorbol 12-myristate 13-acetate (10 ng/mL), ionomycin (0.5 μg/mL), and BD GolgiPlug Protein Transport Inhibitor (containing brefeldin A; 1 μL/mL; BD Biosciences). Cells were then washed, resuspended in cold phosphate-buffered saline, and stained for viability for 30 minutes at 4 °C using LIVE/DEAD Fixable Blue Dead Cell Stain Kit for ultraviolet excitation (1:1000; Thermo Fisher Scientific). Subsequently, cells were washed and resuspended in RPMI supplemented with the following fluorescently tagged antibodies targeting extracellular T lymphocyte proteins for 30 minutes: CD3ε PE-Cy7 (clone 145-2C11; eBioscience, San Diego, CA), CD4 Alexa Fluor 488 (clone GK1.5; eBioscience), CD8a APC (clone 53-6.7; BD Biosciences), and CD25 BV605 (clone PC61; BD Biosciences). Following this, samples were washed, fixed, and permeabilized using the FOXP3 fixation and permeabilization kit (eBioscience) per the manufacturer’s instructions. Per protocol, cells were washed again and resuspended in permeabilization buffer with the following fluorescently tagged antibodies targeting intracellular T lymphocyte proteins for 30 minutes: IL-4 BV 421 (clone 11B11; BD Biosciences), interferon gamma APC-Cy7 (clone XMG1.2; BD Biosciences), IL-17A PE (clone TC11-18H10; BD Biosciences), and FOXP3 PE-Cy5 (clone FJK-16s; eBioscience). Cells were washed and resuspended in phosphate-buffered saline, and data were acquired using a customized BD LSR II flow cytometer (BD Biosciences). All flow cytometry experiments were conducted with accompanying single-color and fluorescence-minus-one control tubes. Data were analyzed using FlowJo software (BD Biosciences).
Simultaneous Splenocyte Immunophenotype and Mitochondrial Redox Analysis
Freshly isolated live splenocytes were incubated in RPMI supplemented with the following fluorescently tagged antibodies targeting extracellular proteins: CD3ε PE-Cy7, CD19 APC-Cy7 (clone 6D5; BioLegend), CD11b SB-436 (clone M1/70; eBioscience), CD11c APC (clone N418; eBioscience), and NK1.1 SB-600 (clone PK136; eBioscience). Concurrently, 1 μM MitoSOX Red mitochondrial superoxide indicator (Thermo Fisher Scientific) was added, and cells were incubated for 30 minutes at 37 °C. Cells were washed and resuspended in phosphate-buffered saline, and data were acquired using a customized BD LSR II flow cytometer. MitoSOX Red mean fluorescence intensities were normalized to intraexperiment sham-operated control samples. All flow cytometry experiments were conducted with accompanying single-color and fluorescence-minus-one control tubes. Data were analyzed using FlowJo software.
Splenocyte Isolation
Splenocytes were isolated as previously described (33); see Supplemental Methods for further details.
T Lymphocyte Isolation
Splenic T lymphocyte isolation was performed as previously described (22); see Supplemental Methods for further details.
RNA Extraction, Complementary DNA Production, and Real-Time Reverse Transcriptase Quantitative Polymerase Chain Reaction
Reverse transcriptase quantitative polymerase chain reaction was performed as previously described (22); see Supplemental Methods for further details.
Immunoblotting
Immunoblotting to quantify relative protein content was performed as previously described (34); see Supplemental Methods for further details.
Catecholamine Assessment
Catecholamines were assessed as previously described (22); see Supplemental Methods for further details.
Circulating Cytokine Analysis
Circulating cytokines were measured as previously described (31); see Supplemental Methods for further details.
Statistical Analyses
A total of 85 animals (38 control mice, 47 RSDS mice) were used in these studies. All mice were randomly assigned to 1 of the 4 cohorts (sham-control, sham-RSDS, denervation-control, denervation-RSDS), with all efforts made to ensure experimenters were blinded during biological assay, data acquisition, and data analysis. At least 3 independent experimental repeats were conducted, with animals across each cohort included. Not all biological parameters were able to be run in a single animal; thus, figures are individually labeled with N values and statistical information used for a specific set of experiments. Individual data are presented along with mean ± SEM. All data were assessed for parametric distribution to determine the appropriate statistical analysis. Differences were considered significant at p < .05.
Results
Splenic Denervation Attenuates RSDS-Induced Elevations in Splenic Sympathetic Tone
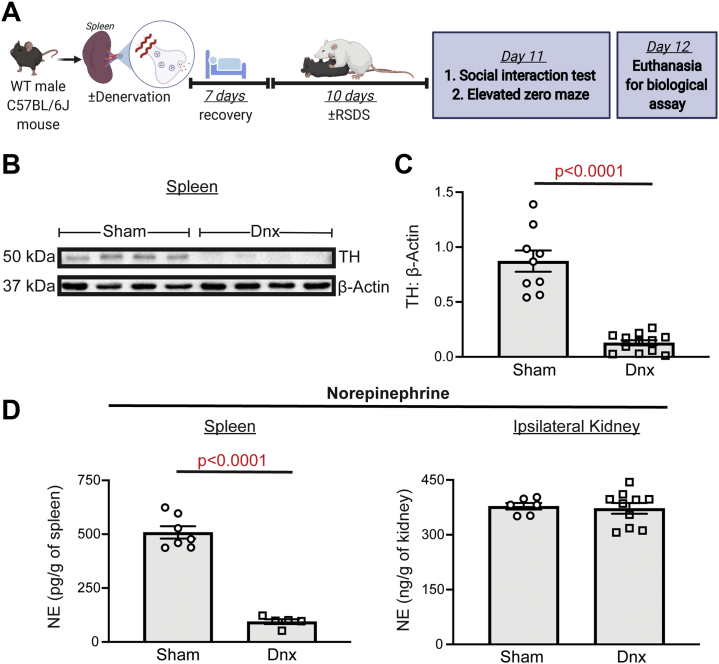
To effectively evaluate the role of splenic innervation in RSDS-induced inflammation, targeted splenic denervation was performed before the RSDS paradigm (Figure 1A). Before RSDS induction, denervation was able to significantly reduce levels of tyrosine hydroxylase (the rate-limiting enzyme of catecholamine synthesis) in splenic lysate compared with sham animals (Figure 1B, C). Direct measurement of NE content within the spleen showed a 79% reduction in splenic NE compared with sham operation (Figure 1D), with no effect on ipsilateral kidney NE content (Figure 1D). To ensure that splenic blood flow was not affected by the denervation procedure, pulsed-wave echo Doppler ultrasound was performed to demonstrate no significant differences in peak velocity or velocity time integral between sham or denervation in blood flow of the splenic artery (Figure S1).
Figure 1.
Splenic denervation is a robust and specific method to reduce splenic NE. WT C57BL/6J mice underwent sham operation or denervation, with biologicals assessed after 7 days before introduction to RSDS. (A) Overall experimental schematic. (B) Representative Western blot analysis of splenic TH in sham and Dnx mice. (C) Quantification of splenic TH normalized to β-actin protein. (D) NE content in tissue lysate by enzyme-linked immunosorbent assay. (Left panel) NE content in spleen normalized to tissue weight. (Right panel) NE content in ipsilateral (left) kidney normalized to tissue weight. Comparisons between sham and Dnx by Mann-Whitney U test. Dnx, denervation; NE, norepinephrine; RSDS, repeated social defeat stress; TH, tyrosine hydroxylase; WT, wild-type.
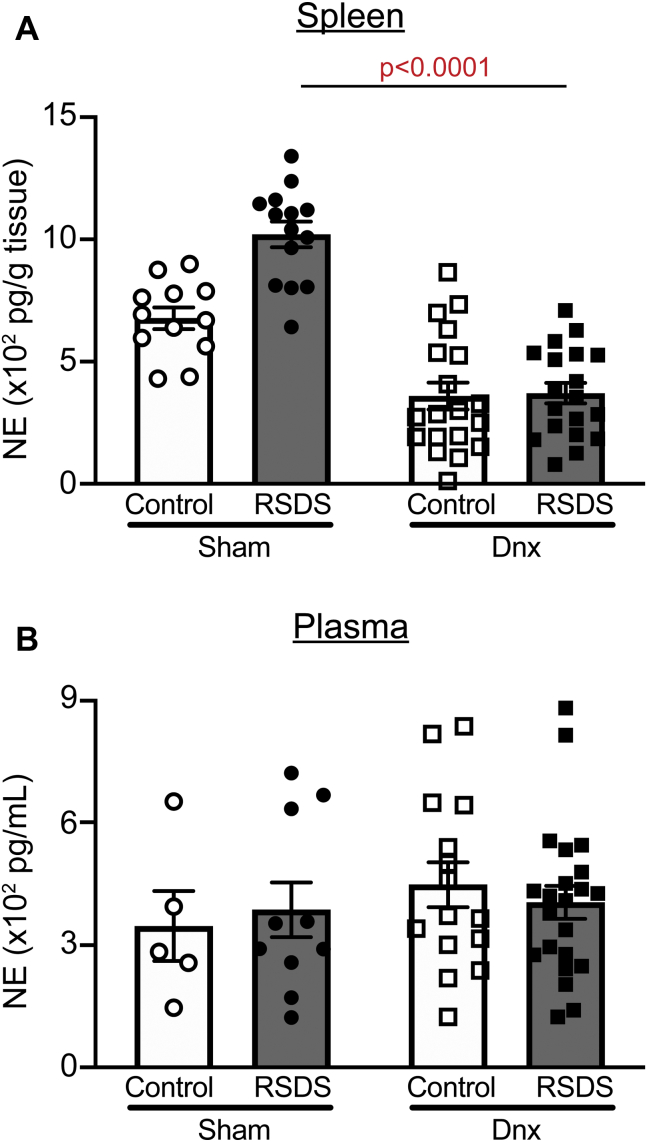
After 7 days of recovery, sham and denervation mice were assigned to control or RSDS paradigms. Splenic NE was elevated in RSDS mice compared with control mice in the sham group, though the difference did not reach statistical significance (Figure 2A). In contrast, denervation significantly attenuated splenic NE in both control and RSDS mice compared with mice in respective sham groups (p = .0197 and p < .0001, respectively) (Figure 2A). Within the plasma, there were no significant differences in NE concentration across all groups (Figure 2B). Together, these data demonstrate that denervation is a feasible, robust, and specific method of attenuating RSDS-induced sympathetic tone.
Figure 2.
Denervation abrogates RSDS alterations in splenic NE, with no effect on plasma NE concentration. Mice were randomly assigned to sham or Dnx conditions, then control or RSDS cohorts, and NE was assessed in tissue or plasma by enzyme-linked immunosorbent assay at the completion of the stress paradigm. (A) NE content in spleen normalized to tissue weight (two-way analysis of variance group results; stress p = .0010, Dnx p < .0001, interaction p = .0055). (B) Plasma NE concentration (two-way analysis of variance group results; stress p = .981, Dnx p = .3592, interaction p = .5241). Statistical analyses by two-way analysis of variance with Tukey post hoc correction; post hoc p value of interest listed on figure where respective group effect was found to be significant. Dnx, denervation; NE, norepinephrine; RSDS, repeated social defeat stress.
Denervation Does Not Alter Antisocial or Anxiety-like Behavior Seen in RSDS
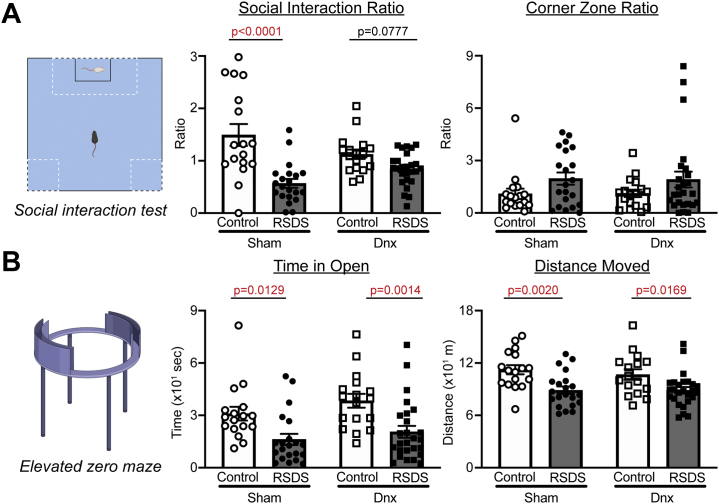
As we and others have previously reported (22,31,32), RSDS resulted in significantly decreased sociability by social interaction test in sham-operated mice (Figure 3A). Interestingly, denervation mice exposed to RSDS also showed decreased sociability, but the variability did not allow for corrected statistical significance compared with sham RSDS (p = .0777) (Figure 3A). Similar to what we have seen and reported previously (31), corner zone ratio did not reliably display significant differences between any groups (Figure 3A).
Figure 3.
Denervation does not affect RSDS-induced antisocial or anxiety-like behavior. Mice were tested for prosocial and anxiety-like behavior after exposure to sham or Dnx conditions, followed by control or RSDS protocols. (A) (Left panel) Representation of social interaction test for social behavior. (Middle panel) Social interaction zone ratio (two-way ANOVA group results; stress p < .0001, Dnx p = .6714, interaction p = .0059). (Right panel) Corner zone ratio (two-way ANOVA group results; stress p = .0304, Dnx p = .9669, interaction p = .9218). (B) (Left panel) Representation of elevated zero maze for anxiety-like behavior. (Middle panel) Time spent in open arm of maze (two-way ANOVA group results; stress p < .0001, Dnx p = .1113, interaction p = .6793). (Right panel) Total distance moved (two-way ANOVA group results; stress p < .0001, Dnx p = .5647, interaction p = .5719). Statistical analyses by two-way ANOVA with Tukey post hoc correction; post hoc p values of interest listed on each figure if respective group effects were found to be significant. ANOVA, analysis of variance; Dnx, denervation; RSDS, repeated social defeat stress.
To assess anxiety-like behavior, we performed elevated zero maze tests. Time spent in the open arms of the maze was significantly decreased in RSDS mice compared with control mice in both sham-operated and denervation groups (Figure 3B). Additionally, distance moved during the elevated zero maze test showed similar decreases in RSDS mice with no statistical differences between RSDS-exposed sham or denervation groups (Figure 3B). Overall, these data suggest that splenic denervation does not greatly affect antisocial or anxiety-like behavior induced by RSDS.
Circulating Inflammation Owing to RSDS Is Partially Ameliorated by Splenic Denervation
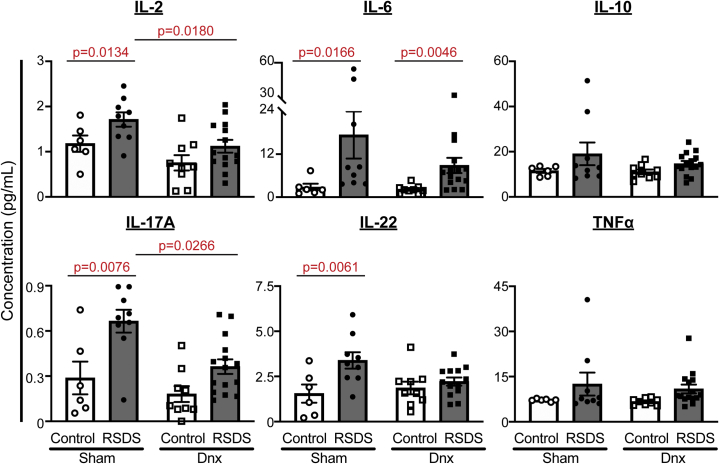
We recently reported that several cytokines are significantly elevated in circulation after RSDS (31), including IL-2, IL-6, IL-10, IL-17A, IL-22, and tumor necrosis factor α (TNF-α). As expected, RSDS increased circulating levels of these cytokines in sham-operated animals (Figure 4), with IL-10 and TNF-α not reaching statistical significance in this cohort (the current study has significantly fewer animals than our previous report). Denervation was able to significantly attenuate RSDS-induced increases in circulating IL-2, IL-17A, and IL-22, whereas IL-6, IL-10, and TNF-α were largely unaffected by denervation (Figure 4). Importantly, IL-2, IL-17A, and IL-22 are produced almost exclusively by T lymphocytes, whereas the others are produced by a variety of cell types. These data demonstrate that denervation is able to attenuate RSDS-induced increases in specific circulating cytokines, which may be linked to T lymphocyte subtypes.
Figure 4.
Denervation attenuates circulating levels of select cytokines increased by RSDS. Circulating inflammatory proteins were assessed at the completion of the stress paradigm in plasma of sham or Dnx mice, followed by control or RSDS mice by Meso Scale Discovery V-PLEX assay. Two-way analysis of variance group results: IL-2 stress p = .0012, Dnx p = .0348, interaction p = .1169; IL-6 stress p = .0063, Dnx p = .2208, interaction p = .2858; IL-10 stress p = .0488, Dnx p = .3795, interaction p = .4651; IL-17A stress p = .0001, Dnx p = .0128, interaction p = .2741; IL-22 stress p = .0059, Dnx p = .2544, interaction p = .0546; TNF-α stress p = .0400, Dnx p = .3776, interaction p = .5316. Statistical analyses by two-way analysis of variance with Tukey post hoc correction; post hoc p values of interest listed on each figure if respective group effects were found to be significant. Dnx, denervation; IL, interleukin; RSDS, repeated social defeat stress; TNF-α, tumor necrosis factor α.
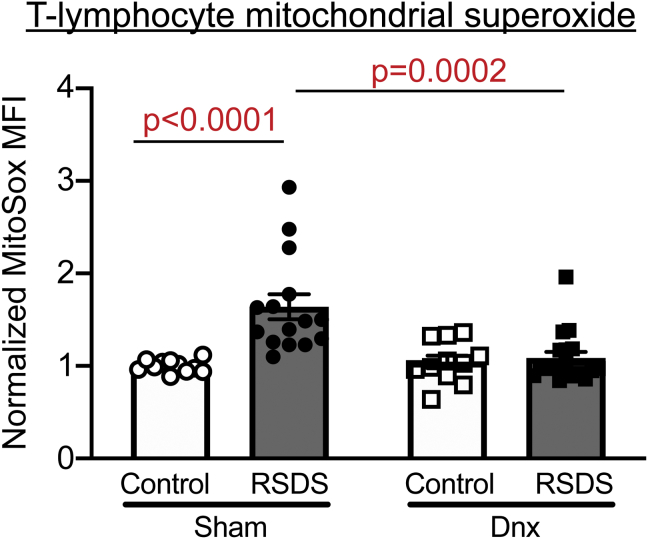
Denervation Attenuates RSDS-Induced Splenic T Lymphocyte Mitochondrial Superoxide
We have previously demonstrated that both exogenous NE and RSDS result in an increase in mitochondrial superoxide within T lymphocytes that is linked to a proinflammatory phenotype (18,22,33). As before, RSDS induced an increase in T lymphocyte mitochondrial superoxide in sham mice compared with control mice (Figure 5). Strikingly, denervation completely attenuated this RSDS-induced increase in mitochondrial superoxide (Figure 5). Additionally, natural killer cells in the spleen also routinely demonstrate increases in mitochondrial superoxide after RSDS (Figure S2). However, natural killer cell mitochondrial superoxide was not significantly attenuated by denervation (compared with the respective control mice) (Figure S2). In examining the effects on immune cell populations, RSDS and denervation did not show clear effects on T lymphocytes (CD3) (Figure S4), despite previous literature demonstrating small but significant decreases in the number of T lymphocytes in stress paradigms (22,35). Moreover, no other cell type in the spleen demonstrated any population alterations with denervation (Figure S2). Overall, these data suggest that splenic innervation and RSDS have significant and robust effects on mitochondrial superoxide within T lymphocytes, with minimal impact on cell population distribution.
Figure 5.
Denervation ameliorates RSDS-induced increase in mitochondrial superoxide in T lymphocytes. Mice were randomly assigned to sham or Dnx conditions, then control or RSDS cohorts followed by live splenocyte isolation and assessment by flow cytometry. Quantification of MitoSOX Red MFI in CD3+ splenocytes, normalized to intraexperiment sham control-housed animals (two-way analysis of variance group results; stress p = .0005, Dnx p = .0079, interaction p = .0021). Statistical analyses by two-way analysis of variance with Tukey post hoc correction; post hoc p values of interest listed on each figure if respective group effects were found to be significant. Dnx, denervation; MFI, mean fluorescence intensity; RSDS, repeated social defeat stress.
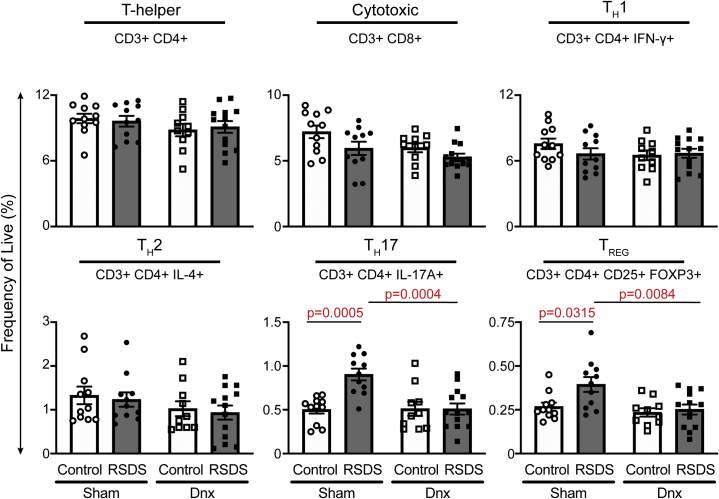
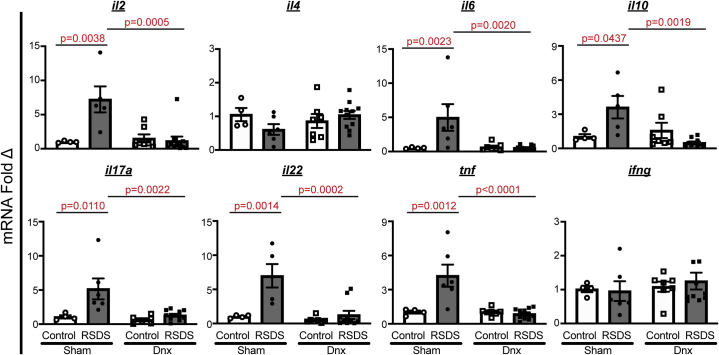
Denervation Reduces RSDS-Induced Splenic T Helper 17 Lymphocytes and Proinflammatory Gene Expression
Using a hierarchical gating strategy, we found that CD4+ helper and CD8+ cytotoxic T lymphocyte populations were not significantly altered by RSDS or denervation (Figure 6). Within CD4+ subtypes, T helper 1 (TH1) and TH2 were unchanged, whereas TH17 and T regulatory cell populations were increased by RSDS in sham mice (Figure 6). Importantly, denervation tempered this RSDS effect in both TH17 and T regulatory cell subtypes (Figure 6). We further investigated inflammatory differences by examining overall T lymphocyte gene expression. Within purified splenic T lymphocytes of sham mice, RSDS produced increased expression of IL-2, IL-6, IL-10, IL-17A, IL-22, and TNF-α (Figure 7). Within the denervation group, RSDS attenuated these 6 cytokines within splenic T lymphocytes (Figure 7), whereas cytokines such as interferon gamma and IL-4 showed no differences across any groups. These data provide further evidence for the role of splenic innervation in RSDS-induced T lymphocyte regulation.
Figure 6.
Denervation reverses RSDS increases in T lymphocyte subtypes TH17 and TREG, while other subtypes remain unaffected. Mice were randomly assigned to sham or Dnx conditions, then control or RSDS cohorts followed by immunophenotyping of splenic T lymphocyte populations by flow cytometry. All values represent frequency of live cells by fixable viability stain, with marker positivity listed below each subtype assay. Two-way analysis of variance group results: CD4+ stress p = .9659, Dnx p = .1315, interaction p = .6076; CD8+ stress p = .0195, Dnx p = .0256, interaction p = .5089; TH1 stress p = .4305, Dnx p = .2904, interaction p = .2417; TH2 stress p = .6024, Dnx p = .0953, interaction p = .9874; TH17 stress p = .0039, Dnx p = .0051, interaction p = .0036; TREG stress p = .0258, Dnx p = .0069, interaction p = .0836. Statistical analyses by two-way analysis of variance with Tukey post hoc correction; post hoc p values of interest listed on each figure if respective group effects were found to be significant. Dnx, denervation; IFN, interferon; IL, interleukin; RSDS, repeated social defeat stress; TH, T helper; TREG, T regulatory.
Figure 7.
Denervation alters RSDS-induced changes in gene expression within purified splenic T lymphocytes. Splenic T lymphocytes were purified by negative selection, followed by RNA extraction and complementary DNA conversion for quantitative real-time reverse transcriptase polymerase chain reaction. Data shown are fold change normalized to sham-operated control-housed animals, with all ΔCT values normalized to 18s ribosomal RNA loading control. Two-way analysis of variance group results: IL-2 stress p = .0067, Dnx p = .0097, interaction p = .0028; IL-4 stress p = .4622, Dnx p = .5022, interaction p = .0892; IL-6 stress p = .0038, Dnx p = .0047, interaction p = .0021; IL-10 stress p = .0309, Dnx p = .0037, interaction p = .1969; IL-17A stress p = .0045, Dnx p = .0093, interaction p = .0333; IL-22 stress p = .0007, Dnx p = .0014, interaction p = .0057; TNF-α stress p = .0032, Dnx p = .0014, interaction p = .0013; IFN-γ stress p = .8341, Dnx p = .4799, interaction p = .6654. Statistical analyses by two-way analysis of variance with Tukey post hoc correction; post hoc p values of interest listed on each figure if respective group effects were found significant. Dnx, denervation; IFN, interferon; IL, interleukin; mRNA, messenger RNA; RSDS, repeated social defeat stress; TNF-α, tumor necrosis factor α.
Discussion
In our previous work (22), we demonstrated an association between RSDS-induced splenic catecholamines, T lymphocyte inflammatory gene signatures, and T lymphocyte mitochondrial superoxide. Herein, we have demonstrated a causal role for neural-derived NE in the regulation of distinct facets of T lymphocyte–driven inflammation during RSDS. By using a method of splenic denervation that effectively reduced splenic tyrosine hydroxylase and NE, we deduced the role of this neuroimmune connection in vivo on the behavioral, inflammatory, and redox phenotypes of RSDS. Denervation did not affect RSDS-induced antisocial or anxiety-like behavior, but it did attenuate RSDS-induced increases in circulating cytokines, T lymphocyte mitochondrial superoxide, TH17 and regulatory T cell populations, and T lymphocyte gene signatures. Together, these data provide valuable insight into splenic neuroimmune interactions that are responsible for RSDS changes in T lymphocyte–driven inflammation.
PTSD is a complex behavioral disorder with important physiological manifestations. To perform mechanistic investigations, current studies rely on one of many preclinical mouse models (36,37). Among the various PTSD models that have arisen, RSDS has been lauded for its ability to recapitulate inflammatory characteristics of PTSD (36,37). Additionally, many works employing RSDS have used the social interaction test to divide mice into susceptible and resilient groups based on the social interaction ratio (32,38). However, we recently demonstrated that this divide does not associate with another commonly used behavioral test of anxiety, the elevated zero maze, or with circulating inflammatory proteins that increase during RSDS (31). In the investigations herein, splenic denervation did not have a profound effect on the behavioral parameters measured, but it did significantly impact T lymphocyte–derived inflammation. Importantly, denervation did not robustly affect other aspects of inflammation, such as circulating IL-6 and other splenocyte populations. Additionally, the changes to circulating inflammation demonstrated an attenuation of the RSDS-induced increases, not a complete ablation, which belies the known existence of neuroimmune connections within other lymphoid organs. Moreover, splenic denervation likely has no effect on central noradrenergic tone, which may be responsible for the resultant changes in behavior. Overall, the complex relationship between behavior and inflammation is likely systemic, involves numerous immune cell types, and has both neural and hormonal contributions. The connections between behavior and inflammation remain a significant area of interest that requires more nuanced approaches that must rely on nonbinary outputs of behavior or peripheral inflammation (39,40). Additionally, future studies examining RSDS and peripheral inflammation would benefit from the inclusion of other behavioral paradigms outside of the social interaction test and elevated zero maze, such as startle responses, and hypervigilance.
Herein, we similarly examined alterations to peripheral inflammation. We demonstrated that denervation ameliorates characteristic increases in some circulating cytokines after RSDS (likely not completely owing to the targeting of only one secondary lymphoid organ), with a partiality for cytokines more canonically associated with T lymphocytes (IL-2, IL-17A, and IL-22), while other cytokines (IL-6, TNF-α, and IL-10) were grossly unaffected. Works by pioneers of RSDS such as Sheridan and his group have previously examined the effects of RSDS on splenocyte populations, primarily monocytes (35). They described that RSDS induces the amount of glucocorticoid-insensitive monocytes in the spleen and circulation, which contribute to the RSDS-induced increase in circulating cytokines such as IL-6, CCL2, and TNF-α [summarized by Reader et al. (41)]. Our data confirm and extend these findings by showing that splenic innervation appears to primarily affect T lymphocyte populations, while the innate immune system response to RSDS is preserved even in the absence of splenic innervation. Combined with this current work, these data provide further evidence for the specificity—anatomical, cellular, and hormonal—of immune responses to RSDS.
At the anatomical level, this work is built on an important premise that the spleen receives exclusively sympathetic innervation, and thus splenic denervation allows for effective study of this single variable. This topic has been reviewed exhaustively elsewhere (42), with a breadth of data demonstrating a lack of parasympathetic innervation to the spleen. Conversely, classic and recent functional studies have elucidated the role of splenic sympathetic efferent nerves in various immune responses (14,30,43, 44, 45, 46, 47). In recent works examining the anti-inflammatory reflex, NE released from the splenic nerve has been shown to signal to choline acetyltransferase–positive T lymphocytes through their β2-adrenergic receptors. Subsequently, these choline acetyltransferase–positive T lymphocytes produce acetylcholine, which signals through the alpha-7 nicotinic receptor on macrophages to suppress the release of inflammatory cytokines into circulation (48,49). However, the nature of pathways upstream from the splenic nerve in this pathway is a matter of controversy. Work by Tracey’s group has provided evidence that this is mediated upstream by the vagal efferent, which synapses at the celiac ganglion to modulate the splenic nerve, while McAllen’s group has shown that these splenic nerve fibers originate from sympathetic splanchnic nerves (46,50, 51, 52, 53). Overall, the data presented herein provide further evidence for the influence of splenic NE on T lymphocytes (primarily proinflammatory) in the important context of psychological trauma. The relationship between our findings and various facets of the anti-inflammatory reflex remains unresolved but is a potential area for future investigations.
At the cellular and molecular levels, this investigation provides new insights into how psychological trauma affects the complex ability of the sympathetic nervous system to modulate splenic T lymphocyte–driven inflammation. It has been well established that splenic T lymphocytes are exposed to high concentrations of NE released by sympathetic nerve terminals (14,54,55). However, the response of T lymphocytes to this NE and other catecholamines has been shown to be largely dependent on experimental details, such as type of immune challenge, timing of sympathoexcitation, activation state of T lymphocytes, and murine strain (56). While a significant body of work has pointed to the β2-adrenergic receptor playing a primary role in NE effects in T lymphocytes (57, 58, 59), other work (including our own) has also demonstrated α-adrenergic receptors in the redox and inflammatory response of T lymphocytes (18,60). Similar to many systems, it is highly probable that adrenergic receptors (and other neurotransmitter receptors) on T lymphocytes do not work autonomously, but rather work together in a system-dependent fashion to respond to the neurotransmitter milieu. Identification of these signaling cascades is indeed important to understand regulation of inflammation via autonomic signaling, but given the numerous intercommunicating cell types, dozens of neurotransmitters, and multitudes of receptors, this is a highly complex and challenging aspect of neuroimmune communication investigations.
Importantly, our previous work has strongly demonstrated that NE-induced increases in specific cytokines, such as IL-6 and IL-17A, is partially mediated by increases in mitochondrial superoxide, as evidenced by an attenuation of these cytokines after treatment with MitoTEMPOL, a selective mitochondrial superoxide scavenger (18). Additionally, in a genetic MnSOD (manganese superoxide dismutase) knockout model with increased mitochondrial superoxide, ex vivo IL-17A production was distinctly increased (61). Importantly, these changes in the mitochondrial redox environment cannot be divorced from the overall metabolic status of these T lymphocytes (62). It is possible that the increase in mitochondrial superoxide we have observed in T lymphocytes after RSDS may be due to alterations in cellular metabolism or vice versa. Metabolism has been recently shown to be a primary driver of T lymphocyte activation and differentiation (63,64), but this has not been fully explored in the context of psychological stress disorders. Untangling the cellular mechanism by which altered mitochondrial redox and metabolism are able to ultimately effect T lymphocyte function during psychological trauma is an important future direction for this research. In furthering this work, we hope to find new therapeutic targets to ameliorate the deleterious proinflammatory shifts that are linked to PTSD, with a keen interest in understanding the directionality of PTSD and inflammation.
This study has important implications but is not without limitations. An important concept of note is the ability of immune cells to produce catecholamines, which could function in an autocrine or paracrine fashion to exert similar effects (56,65, 66, 67, 68). This has been repeatedly demonstrated and requires further investigation to understand the potential role for these immune cell–derived neurotransmitters in the context of health and disease. Additionally, RSDS is a preclinical model of PTSD that recapitulates only certain aspects of the human condition. Our interest in the autonomic and inflammatory relationship prompted our use of this model, but the findings herein could be further validated by using other psychological trauma animal models to probe the role of this neuroimmune connection. Notably, RSDS as a model of PTSD has limitations. Physical interaction allows for wounding, which is a potential confounding factor when investigating inflammation and immune cell populations, although other studies have demonstrated similar immune changes in witnessed social defeat paradigms (69). Additionally, RSDS does not display certain characteristic features of PTSD, such as decreases in fear extinction or altered hypothalamic-pituitary-adrenal feedback (36,37). Last, the standardized model of RSDS precludes examination of these effects in females, which is important given their higher risk for PTSD. New adaptations of RSDS that include females do exist but often rely on differential action of females versus males, which prevents direct comparison between the sexes. This further warrants examination of these findings in additional preclinical models of PTSD as well as in human subjects.
Overall, T lymphocyte–driven inflammation—specifically signatures of TH17-driven inflammation—has been implicated in a variety of inflammation-driven and autoimmune disorders (20,70,71) as well as having been shown to be increased in patients with PTSD (6,72,73). In the current study, we have presented a clear role for increased sympathetic tone—a known hallmark of PTSD—in being responsible for this TH17 phenotype shift. Splenic denervation as a therapeutic technique has already been translated to larger animals, which enhances the clinical relevance of our preclinical PTSD findings described herein. By furthering our mechanistic understanding of how splenic neuroimmune connections in lymphoid organs could be responsible for the increased morbidity and mortality from trauma-induced inflammatory diseases, we can provide novel treatment strategies for patients with PTSD and other trauma-related disorders.
Acknowledgments and Disclosures
This work was supported by the National Institutes of Health (Grant No. R00HL123471 [to AJC] and Grant Nos. T32NS105594 and F30HL154535 [to SKE]) and American Heart Association (Grant No. 20PRE35080059 [to SKE]). The Small Animal Ultrasound Core is supported in part by funding from the Centers of Biomedical Research Excellence Nebraska Center for Nanomedicine National Institute of General Medical Science Grant (Grant No. 5P30 GM127200-03) and National Heart, Lung, and Blood Institute Program Project Grant (Grant No. 5P01HL062222-20).
SKE, KPP, and AJC conceptualized the overall investigation. SKE, CMM, GFW, KK, and AJC designed all research methods and experimental studies. SKE, CMM, GFW, and ADS conducted experiments and analyzed data. SKE and AJC wrote the manuscript. All authors reviewed, edited, and approved the manuscript. AJC provided primary experimental oversight.
We thank Dr. Bryan Hackfort for his expert technical assistance with ultrasound imaging.
A previous version of this article was published as a preprint on bioRxiv: https://doi.org/10.1101/2021.01.16.426952.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.05.004.
Supplementary Material
References
- 1.American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Bisson J.I. Stress related disorders and physical health. BMJ. 2019;367:l6036. doi: 10.1136/bmj.l6036. [DOI] [PubMed] [Google Scholar]
- 3.Edmondson D., von Kanel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4:320–329. doi: 10.1016/S2215-0366(16)30377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donovan A., Cohen B.E., Seal K.H., Bertenthal D., Margaretten M., Nishimi K., et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remch M., Laskaris Z., Flory J., Mora-McLaughlin C., Morabia A. Post-traumatic stress disorder and cardiovascular diseases: A cohort study of men and women involved in cleaning the debris of the World Trade Center complex. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.117.004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maloley P.M., England B.R., Sayles H., Thiele G.M., Michaud K., Sokolove J., et al. Post-traumatic stress disorder and serum cytokine and chemokine concentrations in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2019;49:229–235. doi: 10.1016/j.semarthrit.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumner J.A., Nishimi K.M., Koenen K.C., Roberts A.L., Kubzansky L.D. Posttraumatic stress disorder and inflammation: Untangling issues of bidirectionality. Biol Psychiatry. 2020;87:885–897. doi: 10.1016/j.biopsych.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z., Caughron B., Young M.R.I. Posttraumatic stress disorder: An immunological disorder? Front Psychiatry. 2017;8:222. doi: 10.3389/fpsyt.2017.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wingenfeld K., Whooley M.A., Neylan T.C., Otte C., Cohen B.E. Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: Results from the Mind Your Heart Study. Psychoneuroendocrinology. 2015;52:83–91. doi: 10.1016/j.psyneuen.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J., Marvar P.J., Liao P., Kankam M.L., Norrholm S.D., Downey R.M., et al. Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. J Physiol. 2017;595:4893–4908. doi: 10.1113/JP274269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geracioti T.D., Jr., Baker D.G., Ekhator N.N., West S.A., Hill K.K., Bruce A.B., et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- 12.Fonkoue I.T., Marvar P.J., Norrholm S., Li Y., Kankam M.L., Jones T.N., et al. Symptom severity impacts sympathetic dysregulation and inflammation in post-traumatic stress disorder (PTSD) Brain Behav Immun. 2020;83:260–269. doi: 10.1016/j.bbi.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellinger D.L., Lorton D., Hamill R.W., Felten S.Y., Felten D.L. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: Lack of evidence for cholinergic innervation. Brain Behav Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- 14.Felten S.Y., Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18:37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- 15.Felten D.L., Felten S.Y., Carlson S.L., Olschowka J.A., Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- 16.Bratton B.O., Martelli D., McKinley M.J., Trevaks D., Anderson C.R., McAllen R.M. Neural regulation of inflammation: No neural connection from the vagus to splenic sympathetic neurons. Exp Physiol. 2012;97:1180–1185. doi: 10.1113/expphysiol.2011.061531. [DOI] [PubMed] [Google Scholar]
- 17.Verlinden T.J.M., van Dijk P., Hikspoors J., Herrler A., Lamers W.H., Köhler S.E. Innervation of the human spleen: A complete hilum-embedding approach. Brain Behav Immun. 2019;77:92–100. doi: 10.1016/j.bbi.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Case A.J., Roessner C.T., Tian J., Zimmerman M.C. Mitochondrial superoxide signaling contributes to norepinephrine-mediated T-lymphocyte cytokine profiles. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwabara T., Ishikawa F., Kondo M., Kakiuchi T. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm. 2017;2017:3908061. doi: 10.1155/2017/3908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miossec P., Kolls J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moshfegh C.M., Elkhatib S.K., Collins C.W., Kohl A.J., Case A.J. Autonomic and redox imbalance correlates with T-lymphocyte inflammation in a model of chronic social defeat stress. Front Behav Neurosci. 2019;13:103. doi: 10.3389/fnbeh.2019.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foss J.D., Fink G.D., Osborn J.W. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension. 2013;61:806–811. doi: 10.1161/HYPERTENSIONAHA.111.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao L., Kirabo A., Wu J., Saleh M.A., Zhu L., Wang F., et al. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ Res. 2015;117:547–557. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asirvatham-Jeyaraj N., Fiege J.K., Han R., Foss J., Banek C.T., Burbach B.J., et al. Renal denervation normalizes arterial pressure with no effect on glucose metabolism or renal inflammation in obese hypertensive mice. Hypertension. 2016;68:929–936. doi: 10.1161/HYPERTENSIONAHA.116.07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osborn J.W., Banek C.T. Catheter-based renal nerve ablation as a novel hypertension therapy: Lost, and then found, in translation. Hypertension. 2018;71:383–388. doi: 10.1161/HYPERTENSIONAHA.117.08928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esler M.D., Krum H., Sobotka P.A., Schlaich M.P., Schmieder R.E., Böhm M., et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): A randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 28.Krum H., Schlaich M.P., Sobotka P.A., Böhm M., Mahfoud F., Rocha-Singh K., et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: Final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 29.Bhatt D.L., Kandzari D.E., O’Neill W.W., D’Agostino R., Flack J.M., Katzen B.T., et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 30.Albaghdadi M., Garcia-Polite F., Zani B., Keating J., Melidone R., Spognardi A., et al. Splenic artery denervation: Target micro-anatomy, feasibility, and early preclinical experience. Transl Res. 2019;213:100–111. doi: 10.1016/j.trsl.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Elkhatib S.K., Moshfegh C.M., Watson G.F., Case A.J. Peripheral inflammation is strongly linked to elevated zero maze behavior in repeated social defeat stress. Brain Behav Immun. 2020;90:279–285. doi: 10.1016/j.bbi.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golden S.A., Covington H.E., 3rd, Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Case A.J., Zimmerman M.C. Redox-regulated suppression of splenic T-lymphocyte activation in a model of sympathoexcitation. Hypertension. 2015;65:916–923. doi: 10.1161/HYPERTENSIONAHA.114.05075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Case A.J., Li S., Basu U., Tian J., Zimmerman M.C. Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am J Physiol Heart Circ Physiol. 2013;305:H19–H28. doi: 10.1152/ajpheart.00974.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avitsur R., Stark J.L., Dhabhar F.S., Sheridan J.F. Social stress alters splenocyte phenotype and function. J Neuroimmunol. 2002;132:66–71. doi: 10.1016/s0165-5728(02)00310-7. [DOI] [PubMed] [Google Scholar]
- 36.Aspesi D., Pinna G. Animal models of post-traumatic stress disorder and novel treatment targets. Behav Pharmacol. 2019;30(2 and 3-Spec Issue):130–150. doi: 10.1097/FBP.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 37.Deslauriers J., Toth M., Der-Avakian A., Risbrough V.B. Current status of animal models of posttraumatic stress disorder: Behavioral and biological phenotypes, and future challenges in improving translation. Biol Psychiatry. 2018;83:895–907. doi: 10.1016/j.biopsych.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodes G.E., Pfau M.L., Leboeuf M., Golden S.A., Christoffel D.J., Bregman D., et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Insel T.R. The NIMH Research Domain Criteria (RDoC) Project: Precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt U., Vermetten E. Integrating NIMH Research Domain Criteria (RDoC) into PTSD research. Curr Top Behav Neurosci. 2018;38:69–91. doi: 10.1007/7854_2017_1. [DOI] [PubMed] [Google Scholar]
- 41.Reader B.F., Jarrett B.L., McKim D.B., Wohleb E.S., Godbout J.P., Sheridan J.F. Peripheral and central effects of repeated social defeat stress: Monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015;289:429–442. doi: 10.1016/j.neuroscience.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nance D.M., Sanders V.M. Autonomic innervation and regulation of the immune system (1987-2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madden K.S., Felten S.Y., Felten D.L., Sundaresan P.R., Livnat S. Sympathetic neural modulation of the immune system. I. Depression of T cell immunity in vivo and vitro following chemical sympathectomy. Brain Behav Immun. 1989;3:72–89. doi: 10.1016/0889-1591(89)90007-x. [DOI] [PubMed] [Google Scholar]
- 44.Madden K.S., Moynihan J.A., Brenner G.J., Felten S.Y., Felten D.L., Livnat S. Sympathetic nervous system modulation of the immune system. III. Alterations in T and B cell proliferation and differentiation in vitro following chemical sympathectomy. J Neuroimmunol. 1994;49:77–87. doi: 10.1016/0165-5728(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 45.Mark A.L. Sympathetic neural contribution to salt-induced hypertension in Dahl rats. Hypertension. 1991;17(1 Suppl):I86–I90. doi: 10.1161/01.hyp.17.1_suppl.i86. [DOI] [PubMed] [Google Scholar]
- 46.Martelli D., Yao S.T., McKinley M.J., McAllen R.M. Reflex control of inflammation by sympathetic nerves, not the vagus. J Physiol. 2014;592:1677–1686. doi: 10.1113/jphysiol.2013.268573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grebe K.M., Takeda K., Hickman H.D., Bailey A.L., Bailey A.M., Embry A.C., et al. Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J Immunol. 2010;184:540–544. doi: 10.4049/jimmunol.0903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reardon C., Duncan G.S., Brüstle A., Brenner D., Tusche M.W., Olofsson P.S., et al. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc Natl Acad Sci U S A. 2013;110:1410–1415. doi: 10.1073/pnas.1221655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosas-Ballina M., Olofsson P.S., Ochani M., Valdes-Ferrer S.I., Levine Y.A., Reardon C., et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martelli D., McKinley M.J., McAllen R.M. The cholinergic anti-inflammatory pathway: A critical review. Auton Neurosci. 2014;182:65–69. doi: 10.1016/j.autneu.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 51.Martelli D., Farmer D.G., Yao S.T. The splanchnic anti-inflammatory pathway: Could it be the efferent arm of the inflammatory reflex? Exp Physiol. 2016;101:1245–1252. doi: 10.1113/EP085559. [DOI] [PubMed] [Google Scholar]
- 52.Pavlov V.A., Tracey K.J. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pavlov V.A., Chavan S.S., Tracey K.J. Molecular and functional neuroscience in immunity. Annu Rev Immunol. 2018;36:783–812. doi: 10.1146/annurev-immunol-042617-053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Felten D.L., Felten S.Y., Bellinger D.L., Carlson S.L., Ackerman K.D., Madden K.S., et al. Noradrenergic sympathetic neural interactions with the immune system: Structure and function. Immunol Rev. 1987;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 55.Livnat S., Madden K.S., Felten D.L., Felten S.Y. Regulation of the immune system by sympathetic neural mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:145–152. doi: 10.1016/0278-5846(87)90052-2. [DOI] [PubMed] [Google Scholar]
- 56.Elkhatib S.K., Case A.J. Autonomic regulation of T-lymphocytes: Implications in cardiovascular disease. Pharmacol Res. 2019;146:104293. doi: 10.1016/j.phrs.2019.104293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kin N.W., Sanders V.M. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–1104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- 58.Kohm A.P., Sanders V.M. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- 59.McAlees J.W., Smith L.T., Erbe R.S., Jarjoura D., Ponzio N.M., Sanders V.M. Epigenetic regulation of beta2-adrenergic receptor expression in T(H)1 and T(H)2 cells. Brain Behav Immun. 2011;25:408–415. doi: 10.1016/j.bbi.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grisanti L.A., Perez D.M., Porter J.E. Modulation of immune cell function by α(1)-adrenergic receptor activation. Curr Top Membr. 2011;67:113–138. doi: 10.1016/B978-0-12-384921-2.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moshfegh C.M., Collins C.W., Gunda V., Vasanthakumar A., Cao J.Z., Singh P.K., et al. Mitochondrial superoxide disrupts the metabolic and epigenetic landscape of CD4+ and CD8+ T-lymphocytes. Redox Biol. 2019;27:101141. doi: 10.1016/j.redox.2019.101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moshfegh C.M., Case A.J. The redox-metabolic couple of T-lymphocytes: Potential consequences for hypertension. Antioxid Redox Signal. 2021;34:915–935. doi: 10.1089/ars.2020.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buck M.D., O’Sullivan D., Pearce E.L. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Sullivan D., Pearce E.L. Immunology. Expanding the role of metabolism in T cells. Science. 2015;348:976–977. doi: 10.1126/science.aac4997. [DOI] [PubMed] [Google Scholar]
- 65.Cosentino M., Fietta A.M., Ferrari M., Rasini E., Bombelli R., Carcano E., et al. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109:632–642. doi: 10.1182/blood-2006-01-028423. [DOI] [PubMed] [Google Scholar]
- 66.Qiu Y.H., Cheng C., Dai L., Peng Y.P. Effect of endogenous catecholamines in lymphocytes on lymphocyte function. J Neuroimmunol. 2005;167:45–52. doi: 10.1016/j.jneuroim.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Huang Y., Wang X.Q., Peng Y.P., Qiu Y.H. Effect of tyrosine hydroxylase gene silencing in CD4+ T lymphocytes on differentiation and function of helper T cells. Neuro Endocrinol Lett. 2012;33:643–650. [PubMed] [Google Scholar]
- 68.Qiu Y.H., Peng Y.P., Jiang J.M., Wang J.J. Expression of tyrosine hydroxylase in lymphocytes and effect of endogenous catecholamines on lymphocyte function. Neuroimmunomodulation. 2004;11:75–83. doi: 10.1159/000075316. [DOI] [PubMed] [Google Scholar]
- 69.Finnell J.E., Lombard C.M., Padi A.R., Moffitt C.M., Wilson L.B., Wood C.S., et al. Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Littman D.R., Rudensky A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 71.Zambrano-Zaragoza J.F., Romo-Martinez E.J., Duran-Avelar Mde J., Garcia-Magallanes N., Vibanco-Perez N. Th17 cells in autoimmune and infectious diseases. Int J Inflam. 2014;2014:651503. doi: 10.1155/2014/651503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z., Mandel H., Levingston C.A., Young M.R.I. An exploratory approach demonstrating immune skewing and a loss of coordination among cytokines in plasma and saliva of veterans with combat-related PTSD. Hum Immunol. 2016;77:652–657. doi: 10.1016/j.humimm.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou J., Nagarkatti P., Zhong Y., Ginsberg J.P., Singh N.P., Zhang J., et al. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.