Abstract
Background
Chronic kidney disease (CKD) is a significant risk factor for premature cardiovascular disease and death. Increased oxidative stress in people with CKD has been implicated as a potential causative factor for some cardiovascular diseases. Antioxidant therapy may reduce cardiovascular mortality and morbidity in people with CKD.
Objectives
To examine the benefits and harms of antioxidant therapy on mortality and cardiovascular events in people with CKD stages 3 to 5; dialysis, and kidney transplantation patients.
Search methods
We searched the Cochrane Renal Group's specialised register (July 2011), CENTRAL (Issue 6, 2011), MEDLINE (from 1966) and EMBASE (from 1980).
Selection criteria
We included all randomised controlled trials (RCTs) investigating the use of antioxidants for people with CKD, or subsets of RCTs reporting outcomes for participants with CKD.
Data collection and analysis
Titles and abstracts were screened independently by two authors who also performed data extraction using standardised forms. Results were pooled using the random effects model and expressed as either risk ratios (RR) or mean difference (MD) with 95% confidence intervals (CI).
Main results
We identified 10 studies (1979 participants) that assessed antioxidant therapy in haemodialysis patients (two studies); kidney transplant recipients (four studies); dialysis and non‐dialysis CKD patients (one study); and patients requiring surgery (one study). Two additional studies reported the effect of an oral antioxidant inflammation modulator in patients with CKD (estimated glomerular filtration rate (eGFR) 20 to 45 mL/min/1.73 m²), and post‐hoc findings from a subgroup of people with mild‐to‐moderate renal insufficiency (serum creatinine ≥125 μmol/L) respectively. Interventions included different doses of vitamin E (two studies); multiple antioxidant therapy (three studies); co‐enzyme Q (one study); acetylcysteine (one study); bardoxolone methyl (one study); and human recombinant superoxide dismutase (two studies).
Compared with placebo, antioxidant therapy showed no clear overall effect on cardiovascular mortality (RR 0.95, 95% CI 0.70 to 1.27; P = 0.71); all‐cause mortality (RR 0.93, 95% CI 0.76 to 1.14; P = 0.48); cardiovascular disease (RR 0.78, 95% CI 0.52 to 1.18; P = 0.24); coronary heart disease (RR 0.71, 95% CI 0.42 to 1.23; P = 0.22); cerebrovascular disease (RR 0.91, 95% CI 0.63 to 1.32; P = 0.63); or peripheral vascular disease (RR 0.54, 95% CI 0.26 to 1.12; P = 0.10). Subgroup analyses found no evidence of significant heterogeneity based on proportions of males (P = 0.99) or diabetes (P = 0.87) for cardiovascular disease. There was significant heterogeneity for cardiovascular disease when studies were analysed by CKD stage (P = 0.003). Significant benefit was conferred by antioxidant therapy for cardiovascular disease prevention in dialysis patients (RR 0.57, 95% CI 0.41 to 0.80; P = 0.001), although no effect was observed in CKD patients (RR 1.06, 95% CI 0.84 to 1.32; P = 0.63).
Antioxidant therapy was found to significantly reduce development of end‐stage of kidney disease (ESKD) (RR 0.50, 95% CI 0.25 to 1.00; P = 0.05); lowered serum creatinine levels (MD 1.10 mg/dL, 95% CI 0.39 to 1.81; P = 0.003); and improved creatinine clearance (MD 14.53 mL/min, 95% CI 1.20 to 27.86; P = 0.03). Serious adverse events were not significantly increased by antioxidants (RR 2.26, 95% CI 0.74 to 6.95; P = 0.15).
Risk of bias was assessed for all studies. Studies that were classified as unclear for random sequence generation or allocation concealment reported significant benefits from antioxidant therapy (RR 0.57, 95% CI 0.41 to 0.80; P = 0.001) compared with studies at low risk of bias (RR 1.06, 95% CI 0.84 to 1.32; P = 0.63).
Authors' conclusions
Although antioxidant therapy does not reduce the risk of cardiovascular and all‐cause death or major cardiovascular events in people with CKD, it is possible that some benefit may be present, particularly in those on dialysis. However, the small size and generally suboptimal quality of the included studies highlighted the need for sufficiently powered studies to confirm this possibility. Current evidence suggests that antioxidant therapy in predialysis CKD patients may prevent progression to ESKD; this finding was however based on a very small number of events. Further studies with longer follow‐up are needed for confirmation. Appropriately powered studies are needed to reliably assess the effects of antioxidant therapy in people with CKD.
Plain language summary
Is antioxidant therapy beneficial for people with chronic kidney disease?
People with chronic kidney disease (CKD) have high risk of developing heart disease and dying prematurely. Although heart disease has many causes, damage caused by poor oxygen exchange in the body's cells (oxidative stress) is thought to be a major problem. People with CKD often have evidence of oxidative stress and this is positively associated with the rate of kidney disease progression. We assessed current evidence to evaluate how antioxidant therapy influenced outcomes for patients with CKD. Overall, we found that antioxidant therapy did not reduce the risk of heart disease or death in people with CKD, but that this could vary depending on CKD stage. There was some evidence to suggest that people on dialysis may benefit from antioxidant treatment, and that these therapies could reduce the risk of kidney disease becoming worse. However, these results are based on very limited evidence and further studies are needed to confirm if antioxidant therapy could be of benefit for people with CKD.
Background
Description of the condition
People with chronic kidney disease (CKD) are at high risk of premature cardiovascular disease and death (Sarnak 2003). This risk applies to people at all stages of CKD, ranging from a relatively modest magnitude of increase among those with microalbuminuria (de Zeeuw 2006), to more than 20‐fold increased risk in people with end‐stage kidney disease (ESKD) (ANZOD 2004; Lowrie 1990). Although not age‐specific overall, risk is markedly higher in younger patients, among whom ESKD is associated with a several hundred‐fold increase in the risk of cardiovascular mortality (Lowrie 1990).
The aetiology of cardiovascular disease in CKD is complex and remains relatively poorly understood. Heightened cardiovascular disease risk may result from both an increased prevalence of traditional cardiovascular risk factors among people with CKD (Landray 2001), as well as the so‐called non‐traditional risk factors that are unique to CKD (Himmelfarb 2000; Stenvinkel 2001; Zoccali 2000; Zoccali 2002) which may augment cardiovascular risk. Studies of strategies to reduce cardiovascular risk in people with ESKD have been inconclusive. Studies of lipid lowering therapies (Wanner 2005), dialysis technique modification (Eknoyan 2002), anaemia normalisation (Phrommintikul 2007), and phosphate lowering therapies (St Peter 2008) have yet to demonstrate overall benefits. New strategies to reduce risk are therefore urgently required.
Description of the intervention
Increased oxidative stress has been implicated as a potential causative factor in various types of cardiovascular diseases, including: atherosclerosis (Bachem 1999; Galle 1999), cardiomyopathy (Giugliano 1995; Lee 1991; Singal 1981; Singal 1982; Singal 1983; Wohaieb 1987), and heart failure (Dhalla 1996). Increased oxidative stress in both cardiac and vascular myocytes may be a consequence of either an increase in the formation of reactive oxygen species (Kukreja 1992; Kukreja 1994; Singal 1998), and a decrease in the antioxidant reserve (Halliwell 1994), or both.
Oxidative stress is increased in people with CKD (Himmelfarb 2000). A range of naturally occurring vitamins (such as vitamins A, C, E) and other substances (N‐acetyl cysteine) have demonstrated antioxidant activity in vitro and in vivo (Brunet 1995; Frei 1989; Islam 2000; Meydani 1994). However, the effects of clinical interventions to reduce oxidative stress in people with CKD remain uncertain.
How the intervention might work
Studies assessing the effects of antioxidant therapy in the general population have not identified any cardiovascular benefit (Bjelakovic 2008). It is possible however, that these studies may not have been able to identify important benefits of antioxidant therapy in selected groups, particularly among those who may stand to benefit most from these agents. Increased oxidative stress is common in people with kidney dysfunction, particularly among those with ESKD (Himmelfarb 2000; Himmelfarb 2002). Elevated oxidative stress has long been implicated as a factor in the development of cardiovascular disease (Giugliano 1995; Singal 1981), and because oxidative stress levels are often higher among people with CKD, antioxidant therapy may potentially offer benefits for these patients.
Why it is important to do this review
Strategies to reduce morbidity and mortality in CKD are required urgently. Despite numerous in vitro and animal studies supporting the role of antioxidants in preventing cardiovascular morbidity and mortality (Jolly 1984; Kaul 1995; Peng 1995; Prasad 1997), large‐scale human clinical studies conducted in the general population have failed to demonstrate any such benefits, and indeed, have suggested potential harm (Bjelakovic 2008). A strong scientific rationale exists for the potential value of antioxidants in the CKD population, among whom oxidative stress is substantially increased. The aim of this review was to provide a comprehensive overview of the available evidence regarding the effect of antioxidant therapy in people with CKD.
Objectives
The aim of this review was to investigate the benefits and harms associated with antioxidant therapy (vitamins A, C, E, beta carotene, N‐acetyl cysteine) in patients with CKD stages 3 to 5; dialysis, and transplantation patients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) assessing the use of antioxidants in people with CKD, or subsets of broader RCTs reporting outcomes for CKD. A minimum of 100 patient‐years per treatment arm was planned to reduce the risk of reporting or publication bias; however, this criterion was subsequently abandoned because of the small number of trials identified. Studies with sequential or cross‐over designs were excluded.
Types of participants
Adults (over 18 years of age) on dialysis, who were kidney transplantation recipients, or with CKD stages 3 to 5 as defined by the KDOQI guidelines (KDOQI 2002) or by the author.
Types of interventions
Studies randomising patients to antioxidants compared with placebo, standard, or no treatment. Studies of agents in which a major mechanism of action was not thought to be antioxidant were excluded, such as statins.
Comparisons investigated were:
Vitamin A versus placebo, standard, or no treatment
Vitamin C versus placebo, standard, or no treatment
Vitamin E versus placebo, standard, or no treatment
N‐acetyl cysteine versus placebo, standard, or no treatment
Beta carotene versus placebo, standard, or no treatment
Flavonoids versus placebo, standard, or no treatment
Any antioxidant versus placebo, standard, or no treatment
Any combination of antioxidants versus placebo, standard, or no treatment.
Studies were included where any of the above comparisons were delivered via any schedule of dosing and any route of administration.
Types of outcome measures
Primary outcomes
Cardiovascular mortality.
Secondary outcomes
All‐cause mortality
Cardiovascular disease defined as a composite of the following outcomes: fatal and non‐fatal myocardial infarction (MI), fatal and non‐fatal stroke and cardiovascular death, or as defined by study authors
Coronary heart disease consisting of fatal and non‐fatal MI as well as coronary revascularisation, or as defined by authors
Cerebrovascular disease including stroke (overall and by subtype), transient ischaemic attacks, and cerebrovascular revascularisation
Peripheral vascular disease consisting of lower limb revascularisation or amputation
Kidney‐specific outcomes consisting of ESKD in CKD participants as defined by the requirement for renal replacement therapy (RRT) or death due to kidney disease, mean annual change in estimated glomerular filtration rate (eGFR), thrombosis of vascular access
-
Adverse events from antioxidant therapy:
Malignancy
Haematological events (such as cerebral or other haemorrhage)
Gastrointestinal events
Infection
Any self‐reported adverse events
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register (6 July 2011) through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
The Cochrane Renal Group’s Specialised Register contains studies identified from:
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of nephrology textbooks, review articles and relevant studies.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies relevant to this review. Titles and abstracts were screened independently by two authors (MJ, VV), who discarded studies that were not applicable; however, studies and reviews that might include relevant data or information on studies were retained. Two authors (MJ, VV) independently assessed and retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria. Disagreements were resolved in discussion with a third author (VP).
Data extraction and management
Data extraction was carried out independently by three authors (MJ, VV, BC) using standard data extraction forms. Publication in non‐English language was not an exclusion criterion. Where more than one publication of one study existed, only the publication with the most complete data for each individual outcome was included in that analysis. Any discrepancy between published versions was highlighted.
Assessment of risk of bias in included studies
The following items were assessed using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (all‐cause mortality, MI, coronary revascularisation, cardiovascular death, stroke, cerebrovascular revascularisation, peripheral vascular disease, and ESKD‐specific outcomes) results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (such as serum creatinine), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were used.
Unit of analysis issues
Analyses were performed using the intention‐to‐treat principle wherever possible.
Dealing with missing data
Where outcomes were reported in insufficient detail to enable meta‐analysis, and further information was not forthcoming from investigators, these outcomes were tabulated and assessed using descriptive techniques; and where possible, risk difference (RD) with 95% CI were calculated.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on n‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
Although it was planned that risk of small study bias which would be investigated by creating and analysing funnel plots, insufficient data were identified to enable assessment. Attrition bias was assessed using the loss/event ratio. Although we had planned to assess the presence of publication and other reporting biases by interpreting funnel plots and using statistical tests (Beggs’ test), there were insufficient data to do so.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Meta‐regression (for the outcome of major cardiovascular events) and subgroup analysis was performed to explore possible sources of heterogeneity (e.g. participants, interventions and study quality). Meta‐regression analyses were performed using STATA version 9.2 (STATA 9.2). Adverse effects were tabulated and assessed with descriptive techniques, as they were described and defined differently across the included studies. Where possible, the RD with 95% CI was calculated for each adverse effect, either compared to no treatment or to another agent.
Heterogeneity was analysed using the Cochran Q test on N‐1 degrees of freedom, with P < 0.05 used to denote statistical significance, and the I² test (with uncertainty intervals).
Subgroup analysis was conducted according to the following characteristics:
gender
history of cardiac disease or diabetes mellitus
prior vitamin supplementation
concurrent vitamin supplementation
concomitant medications (e.g. statins)
KDOQI stage of CKD
study quality
Plausible explanations for variations in treatment effect were explored using subgroup analyses based on study quality and length of follow‐up.
Sensitivity analysis
Sensitivity analyses were undertaken to assess the impact of individual studies on the overall results if significant evidence of heterogeneity was observed.
Results
Description of studies
Results of the search
The combined search of MEDLINE, EMBASE, the Cochrane Renal Group’s specialised register and CENTRAL identified 1080 reports of which 672 were excluded (Figure 1). The major reasons for exclusion were search overlap and reporting outcomes that were not relevant to this review.
1.
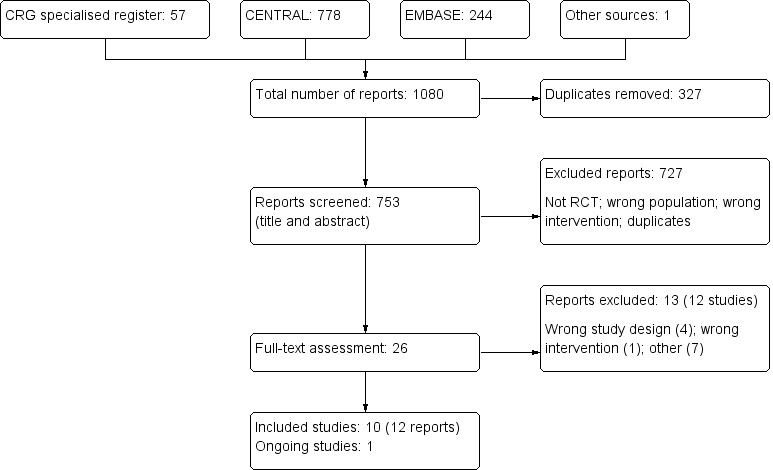
Flowchart indicating the process of study identification and selection for this review
We assessed 25 full‐text articles (and information from the authors of abstracts of scientific proceedings). Our initial restriction of only including studies with at least 100 patient‐years/treatment arm was not applied due to the very limited number of relevant studies available. After assessment, we identified 10 completed studies (BEAM Study 2011; HOPE 2000; Land 1994; Pollak 1993; Rabl 1993; Shoskes 2005; Singh 2000; SPACE Study 2000; Tepel 2003; Wijnen 2002); of these, three had at least 100 patient‐years of follow‐up/treatment arm (HOPE 2000; Land 1994; SPACE Study 2000). One on‐going study was identified and will be analysed in a future update (BEACON Study).
Included studies
We included 10 studies (1979 participants) in the final analysis. SPACE Study 2000 and Tepel 2003 assessed effects of antioxidant therapies in haemodialysis patients; Singh 2000 included both dialysis and non‐dialysis CKD patients; Land 1994, Pollak 1993, Rabl 1993 and Shoskes 2005 included kidney transplant recipients; BEAM Study 2011 assessed the effects of an oral antioxidant inflammation modulator in CKD patients (defined as eGFR 20 to 45 mL/min/1.73 m²); HOPE 2000 reported post‐hoc findings of a subgroup from a large study of participants with mild‐to‐moderate renal insufficiency (defined as serum creatinine ≥125 μmol/L); and Wijnen 2002 included patients undergoing elective surgery for infrarenal abdominal aneurysm. Follow‐up duration ranged from 6 days to 4.5 years.
Excluded studies
We excluded 13 studies after assessment of the full‐text articles. Of these, seven did not report outcomes relevant to this review (Abendroth 1992; Agarwal 2004a; Mune 1999; Noel 2000; Perkins 2009; Schneeberger 1989; Schramm 2002); one investigated an agent whose major mechanism of action was not antioxidation (Wlodarczyk 2000); and four used either sequential or cross‐over designs (ATIC Study 2005; Blackhall 2005; Panzetta 1995; Yukawa 1995).
Risk of bias in included studies
Study quality varied, but overall, there was insufficient reported information regarding randomisation procedures and allocation concealment among the included studies (Figure 2; Figure 3).
2.
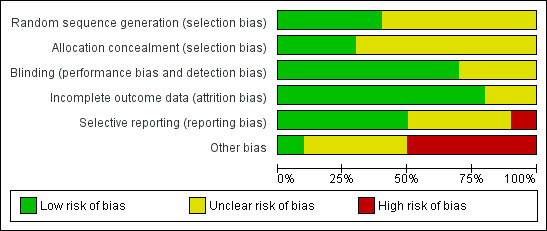
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
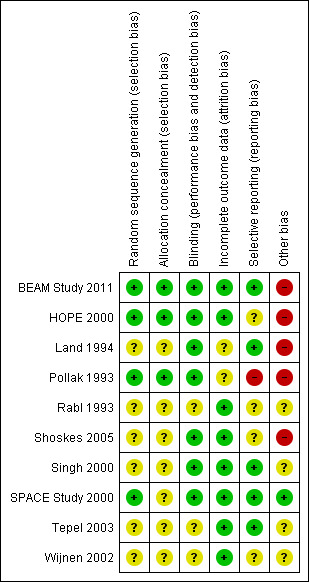
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed eight included studies as either uncertain risk or high risk of selection bias that stemmed from lack of allocation concealment.
Blinding
Among the included studies, six were reported as being double‐blinded (BEAM Study 2011; HOPE 2000; Land 1994; Pollak 1993; Singh 2000; Tepel 2003), but of these, only three explicitly reported double‐blinding methodologies (HOPE 2000; Land 1994; Singh 2000). Tepel 2003 specifically reported no blinding. The Heart Outcomes Prevention Evaluation (HOPE) Study, which provided the subgroup data in this review, reported that the study outcome assessors were blinded (HOPE 2000). No studies reported blinding of data assessors. Three studies reported no information regarding blinding (Rabl 1993; Shoskes 2005; Wijnen 2002).
Incomplete outcome data
In a report of the HOPE study, it was reported that 89% of participants were still taking vitamin E at the final visit (Yusuf 2000); and HOPE 2000, which reported subgroup data for this study, stated that only data from the original intention‐to‐treat analysis were used. Tepel 2003 did not report withdrawals but stated that all analyses were based on an intention‐to‐treat basis. Land 1994 reported complete follow‐up, but there was no reporting of analyses being conducted on an intention‐to‐treat basis. SPACE Study 2000 did not report withdrawals or exclusion of participant data from the analysis. Neither SPACE Study 2000 nor Pollak 1993 reported withdrawal or adherence rates.
Selective reporting
Publication bias could not be assessed due to inconsistency of data reporting in the included studies.
Other potential sources of bias
Randomisation methodology was not clearly described in five studies (Land 1994; Rabl 1993; Shoskes 2005; Tepel 2003; Wijnen 2002).
Effects of interventions
Cardiovascular mortality
There was no significant difference in cardiovascular mortality between antioxidant and placebo groups (Analysis 1.1 (3 studies, 1323 participants): RR 0.95, 95% CI 0.70 to 1.27; P = 0.71). There was no significant heterogeneity (Chi² = 1.60; P = 0.45, I² = 0%).
1.1. Analysis.
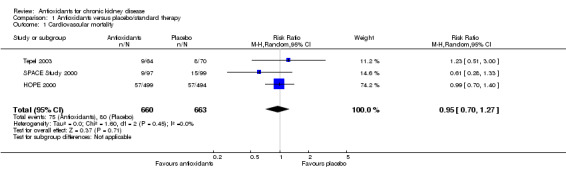
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 1 Cardiovascular mortality.
All‐cause mortality
There was no significant difference in all‐cause mortality between antioxidant and placebo groups (Analysis 1.2 (5 studies, 1727 participants): RR 0.93, 95% CI 0.76 to 1.14; P = 0.48). There was no significant heterogeneity (Chi² = 2.25; P = 0.69, I² = 0%).
1.2. Analysis.
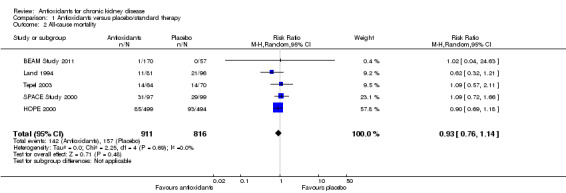
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 2 All‐cause mortality.
Cardiovascular disease
There was no clear evidence of protection against cardiovascular disease among people treated with antioxidants (Analysis 1.3 (4 studies, 1550 participants): RR 0.78, 95% CI 0.52 to 1.18; P = 0.24). There was significant heterogeneity (Chi² = 9.13, P = 0.03, I² = 67%). This was further investigated in the subgroup analyses reported below.
1.3. Analysis.
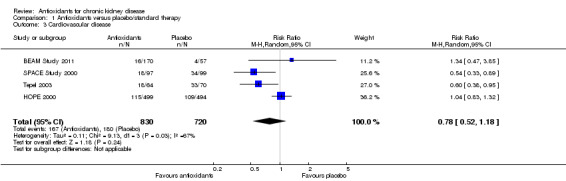
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 3 Cardiovascular disease.
Coronary heart disease
There was no significant difference in coronary heart disease between antioxidant and placebo groups (Analysis 1.4 (4 studies, 1550 participants): RR 0.71, 95% CI 0.42 to 1.23; P = 0.22).There was a moderate level of heterogeneity (Chi² = 5.78; P = 0.12, I² = 48%) primarily attributed to the SPACE Study 2000. Sensitivity analysis excluding this study removed the heterogeneity (Chi² = 0.69; P = 0.71, I² = 0%) without affecting the direction of the summary estimate (RR 0.94, 95% CI 0.72 to 1.21; P = 0.62).
1.4. Analysis.
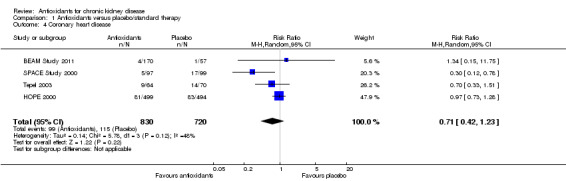
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 4 Coronary heart disease.
Cerebrovascular disease
There was no significant difference in cerebrovascular disease between antioxidant and placebo groups (Analysis 1.5 (3 studies, 1323 participants): RR 0.91, 95% CI 0.63 to 1.32; P = 0.63). There was no significant heterogeneity (Chi² = 2.09; P = 0.35, I² = 5%).
1.5. Analysis.
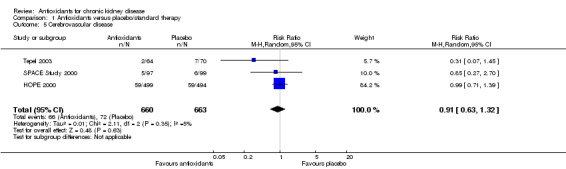
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 5 Cerebrovascular disease.
Peripheral vascular disease
There was no significant difference in peripheral vascular disease between antioxidant and placebos (Analysis 1.6 (2 studies, 330 participants): RR 0.54, 95% CI 0.26 to 1.12; P = 0.10). There was no significant heterogeneity (Chi² = 0.41; P = 0.52, I² = 0%).
1.6. Analysis.
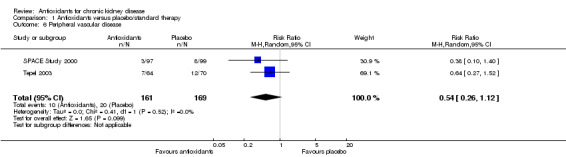
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 6 Peripheral vascular disease.
Serum creatinine
Antioxidant therapy significantly reduced serum creatinine levels (Analysis 1.7 (5 studies, 234 participants): mean reduction 1.10 mg/dL, 95% CI 0.39 to 1.81 reduction; P = 0.003). There was significant heterogeneity (Chi² = 55.80; P < 0.00001, I² = 93%). BEAM Study 2011 reported change in serum creatinine from baseline rather than end of study values, and thus, these data could not be pooled. BEAM Study 2011 reported that serum creatinine was significantly reduced among antioxidant group participants compared with placebo group participants (‐0.1 ± 1.3 versus 0.1 ± 0.8; P = 0.015).
1.7. Analysis.
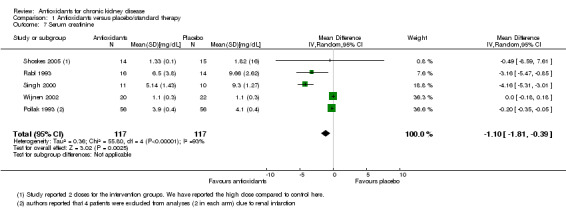
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 7 Serum creatinine.
Mean change in eGFR
Antioxidant therapy significantly improved kidney function (defined as creatinine clearance) (Analysis 1.8 (4 studies, 195 participants): improvement of 14.53 mL/min, 95% CI 1.20 to 27.86 increase; P = 0.03). BEAM Study 2011 reported that patients receiving bardoxolone methyl had significantly increased mean eGFR levels, compared with placebo at 24 weeks and maintained at 52 weeks. When compared with placebo, the between‐group difference in eGFR (mL/min/1.73 m²) was 8.2 ± 1.5 in the 25 mg group, 11.4 ± 1.5 in the 75 mg group, and 10.4 ± 1.5 in the 150 mg group.
1.8. Analysis.
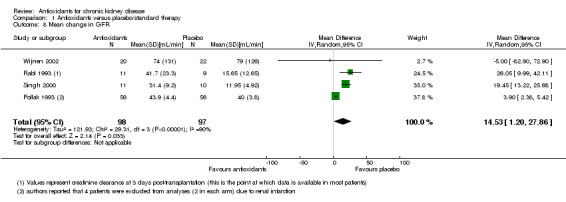
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 8 Mean change in GFR.
End‐stage kidney disease (ESKD)
Antioxidant therapy significantly reduced the risk of ESKD development (Analysis 1.9 (2 studies, 404 participants): RR 0.50, 95% CI 0.25 to 1.00; P = 0.05). There was no significant heterogeneity (Chi² = 0.19; P = 0.66, I² = 0%).
1.9. Analysis.
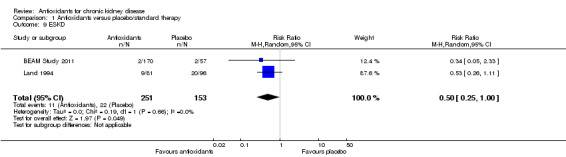
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 9 ESKD.
Adverse effects
SPACE Study 2000 reported no difference in adverse events (defined as drug‐related side effects); five occurred in the antioxidant therapy arm and three in the placebo arm. There were also three gastrointestinal distress events and two of itching (pruritus) reported in participants who received antioxidant therapy. Tepel 2003 reported five events of gastrointestinal discomfort in antioxidant therapy arm participants. BEAM Study 2011 reported that in the intervention arms 50/170 participants experienced at least one treatment‐related severe adverse event, compared with 14/57 participants in the placebo arm. Overall, total severe adverse events (as defined by authors) were not significantly increased by antioxidants (Analysis 1.10 (3 studies, 557 participants): RR 2.26, 95% CI 0.74 to 6.95; P = 0.15).
1.10. Analysis.
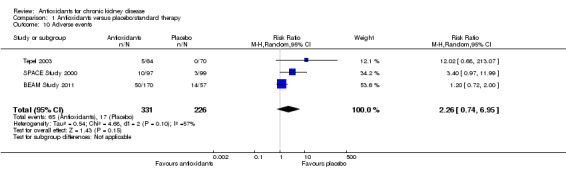
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 10 Adverse events.
Cancer
Two studies reported cancer (BEAM Study 2011; SPACE Study 2000). There was no significant difference in the reported incidence of cancer between the antioxidant and placebo groups (Analysis 1.11 (2 studies, 423 participants): RR 1.03, 95% CI 0.07 to 15.39; P 0.96).
1.11. Analysis.
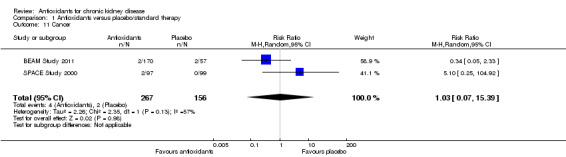
Comparison 1 Antioxidants versus placebo/standard therapy, Outcome 11 Cancer.
Meta‐regression and subgroup analyses
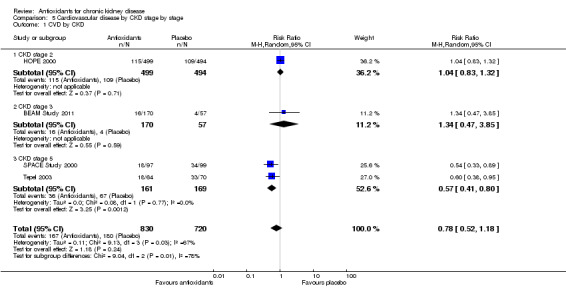
Univariate meta‐regression of major cardiovascular events suggested that a range of factors could explain the observed heterogeneity, including: mean age (P = 0.006); proportion of males (P = 0.02); and study size (P = 0.009) (Table 1). Subgroup analyses were performed according to prespecified characteristics. There was no evidence of significant heterogeneity among subgroups based on the proportion of males (P = 0.99) and the proportion participants with diabetes (P = 0.87) for major cardiovascular events. In subgroup analyses, there was evidence of significant heterogeneity for the outcome of cardiovascular disease when studies were analysed based on CKD stage (P = 0.003). In studies of dialysis patients (SPACE Study 2000; Tepel 2003), there was significant benefit conferred by antioxidant therapy against development of cardiovascular disease (RR 0.57; 95% CI 0.41 to 0.80; P = 0.001), but no effect was observed in a study of CKD patients (HOPE 2000; BEAM Study 2011; RR 1.06; 95% CI 0.84 to1.32; P = 0.63).
1. Meta‐regression for major cardiovascular outcome.
| Source of heterogeneity | Scale | RR | 95% CI | P value | |
| Age | Every 1 year | 1.12 | 1.04 | 1.20 | 0.006 |
| Male % | Every 10% increase | 1.19 | 1.04 | 1.35 | 0.023 |
| Diabetes % | Every 10% increase | 0.80 | ‐0.02 | 1.63 | 0.605 |
| Study size | Every 50 participants | 1.03 | 1.01 | 1.06 | 0.009 |
| Number of cardiovascular events | Every 10 events | 1.07 | 1.01 | 1.14 | 0.022 |
CI ‐ confidence interval; RR ‐ risk ratio
In terms of study quality, those assessed at unclear or high risk of bias for random sequence generation and allocation concealment reported significant benefits from antioxidant therapy (SPACE Study 2000; Tepel 2003; RR 0.57, 95% CI 0.41 to 0.80; P = 0.001) compared with studies at low risk of bias (BEAM Study 2011; HOPE 2000; RR 1.06, 95% CI 0.84 to 1.32; P = 0.63).
The limited number of studies identified prevented conclusive subgroup analyses being conducted. Subgroup analyses for history of cardiac disease, prior vitamin supplementation, and concomitant medications were not possible due to limited data reporting.
Discussion
Summary of main results
This review found no evidence to indicate that antioxidant therapy could reduce death or cardiovascular disease among people with CKD. These findings are consistent with a meta‐analysis of large studies in the general population (Bjelakovic 2008). However, there is evidence to suggest that antioxidant therapy reduces serum creatinine levels and improves kidney function which may contribute to the observed reduced risk of progression to ESKD. Subgroup analyses raised the possibility of cardiovascular and coronary benefit from antioxidant therapy in the dialysis population but not in the CKD subgroup. There was no evidence that antioxidants caused harm among people with CKD.
Overall completeness and applicability of evidence
This review assessed available data regarding the effects of antioxidants in people with CKD. We identified 10 relevant studies with a combined total of almost 2000 participants that reported 347 major cardiovascular events, 214 coronary events, and 298 deaths. Overall, results showed that antioxidant therapy did not reduce the risk of death or cardiovascular disease among people with CKD. This outcome is largely consistent with a previous Cochrane review of antioxidant therapy in the general population (Bjelakovic 2008). However, beneficial effects of antioxidant therapy on short‐term renal outcomes, including reduction of serum creatinine levels and improvements in kidney function, may suggest possibility for long‐term renal and cardiovascular benefits, particularly among people on dialysis.
Two studies undertaken in populations of participants undergoing haemodialysis found that cardiovascular mortality, cardiovascular events and peripheral vascular disease were substantially reduced in antioxidant therapy arm participants compared with those who received placebo. This finding contrasts with larger studies conducted in the general population (GISSI 1999; Heart Protection Study 2002). It has been reported previously that oxidative stress is markedly increased in people on dialysis who have cardiovascular disease compared with other patient populations (Toborek 1992).
Oxidative stress may play a causative role in elevated inflammation from the toxic effects of reactive oxygen species which may contribute to the pathogenesis of renal damage (Rodriguez‐Iturbe 2001). Given that oxidative stress and inflammation are important factors in the progression of kidney disease, antioxidant therapy to address these factors, particularly in those among whom these are more pronounced, remains a promising option. In support, this review showed that antioxidant therapy assists in reducing serum creatinine levels and improving creatinine clearance in relatively short periods. Hence, there remains possibility that benefits may be conferred for dialysis patients, a hypothesis which needs to be tested in sufficiently powered studies specifically involving this population.
An important observation was that antioxidant therapy significantly reduced the risk of ESKD. However, it is acknowledged that the number of studies providing such information was limited, and in those that did so, the overall number of events was exceedingly low. Thus, interpretations of such results must be made with caution. However, promising results from smaller trials that assessed the effects of antioxidant therapy on short‐term creatinine levels and kidney function suggested that it may be likely that antioxidant therapy confers long‐term renal benefits. Recently, the 52‐week Bardoxolone Methyl Treatment: Renal Function in CKD and Type 2 Diabetes (BEAM) trial (BEAM Study 2011), currently the largest study assessing the effects of antioxidant therapy on the progression of CKD, reported that antioxidant therapy for 52 weeks in people with diabetic kidney disease significantly increased eGFR compared with placebo. Improvements in GFR are clinically significant because this may potentially prevent progression to ESKD or delay dialysis initiation because it has been shown that for some patients dialysis can be delayed until GFR decreases below 7.0 mL/min/1.73 m² (Cooper 2010). Such promising results highlight the importance of studies assessing the benefits and harms of antioxidant therapy on major outcomes in CKD, such as the ongoing Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes (BEACON) trial (BEACON Study).
This review benefits from the comprehensive nature of the search strategy and the inclusion of studies assessing a broad range of CKD patients including kidney transplant recipients, people with early stage CKD, pre‐dialysis CKD patients, and dialysis patients. There are, however, limitations to this review, mainly related to the limited available evidence of antioxidant therapy in patients with CKD. Relevant identified studies were small in size with relatively short follow‐up, ranging from six days to 4.5 years, and the availability of reported data was inconsistent. As such, the power of these studies to demonstrate clinically important benefits is limited. Most identified studies were of suboptimal quality. There was no clear evidence of harm observed among the studies of antioxidants in CKD; however, assessment was again limited by lack of statistical power resulting from the modest amount of data available. The underlying mechanism for the significant reduction in serum creatinine and improvement of kidney function with antioxidant therapy is poorly understood. This provides a strong rationale for new sufficiently powered studies to be conducted in CKD patients, particularly since this review has identified the possibility for specific benefit in this population.
Quality of the evidence
Study quality varied, but was generally suboptimal. It was noted that based on the quality assessment of the included studies, two that reported beneficial effects of antioxidant therapy in terms of cardiovascular outcomes, were small in size and methodologically flawed; one study was not blinded. It is accepted that adequate reporting of randomisation methodology is a validated measure of study conduct quality and strongly related to internal validity of RCTs (Huwiler‐Muntener 2002). Therefore, it is uncertain that the reported benefits of these studies were robust, or that reported outcomes were attributable to chance or suboptimal study methodology.
Potential biases in the review process
The search results were screened individually by two independent investigators and any disagreement was resolved with a third author. This minimised the possibility of any potential bias in the review process. This review benefits from the comprehensive search of the literature of randomised trials assessing the effects of antioxidant therapy in people with CKD. Our review does, however, have limitations, primarily as a consequence of the limited availability of trials in this area. The majority of the trials identified were small and lacked power to detect clinically important effects of antioxidant therapy. We performed analyses based on published summary level data which limited the capacity to fully explore the effects of antioxidant therapy in people with CKD.
Agreements and disagreements with other studies or reviews
The overall findings are consistent with studies in the general population; these studies have clearly demonstrated that antioxidants are not protective against cardiovascular events. A recent systematic review in the general population has suggested the possibility of an increased risk of adverse outcomes associated with antioxidant therapy (Bjelakovic 2008). Our review found no such associations. Subgroup analyses suggest potential for benefit in the dialysis population, but the small size and generally suboptimal quality of the included studies meant that the reliability of this result remains open to question. However, it is important to note that this review was limited in that relevant studies identified regarding the effects of antioxidant therapy in patients with CKD were insufficiently powered, and thus, clinically important benefits or harms of antioxidant therapy were unlikely to have been demonstrated.
Authors' conclusions
Implications for practice.
The current evidence does not support the routine use of antioxidants in the management of people with CKD. Although it remains possible that some benefit may be present, particularly in people receiving dialysis, this has yet to be conclusively determined. Current evidence suggests that antioxidant therapy in pre‐dialysis CKD patients may prevent progression to end‐stage kidney disease; however, this is based on a very small number of events. Further studies with longer‐term follow‐up are needed to confirm this potential benefit. Further evidence from sufficiently powered studies is required to determine the role of antioxidant therapy as a preventative treatment option in patients with CKD.
Implications for research.
The limited data currently available, and the benefit observed in subgroup analyses based on CKD stage for the outcome of cardiovascular disease, suggest that a larger outcomes trial should be undertaken to define the effects of antioxidants in the dialysis population. Such a trial would be potentially extremely beneficial because the imperative in clinical practice is to reduce the substantial burden of excess morbidity and mortality in this high‐risk patient population.
What's new
| Date | Event | Description |
|---|---|---|
| 7 May 2014 | Amended | Minor copy edit made to study names |
Acknowledgements
We would like to thank the referees for their editorial advice during the preparation of the review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Antioxidants versus placebo/standard therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular mortality | 3 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.27] |
| 2 All‐cause mortality | 5 | 1727 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.14] |
| 3 Cardiovascular disease | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.18] |
| 4 Coronary heart disease | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.42, 1.23] |
| 5 Cerebrovascular disease | 3 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.32] |
| 6 Peripheral vascular disease | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.12] |
| 7 Serum creatinine | 5 | 234 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.81, ‐0.39] |
| 8 Mean change in GFR | 4 | 195 | Mean Difference (IV, Random, 95% CI) | 14.53 [1.20, 27.86] |
| 9 ESKD | 2 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.25, 1.00] |
| 10 Adverse events | 3 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.74, 6.95] |
| 11 Cancer | 2 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.07, 15.39] |
Comparison 2. Subgroup analysis: Gender and outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular disease by gender | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.18] |
| 1.1 ≤ 60% male | 2 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.37, 1.66] |
| 1.2 > 60% male | 2 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.48] |
| 2 Cardiovascular death by gender | 3 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.27] |
| 2.1 ≤ 60% male | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.51, 3.00] |
| 2.2 > 60% male | 2 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.60, 1.32] |
| 3 All‐cause mortality by gender | 4 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.14] |
| 3.1 < 64% male | 2 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.47, 1.44] |
| 3.2 ≥ 64% male | 2 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.20] |
2.1. Analysis.
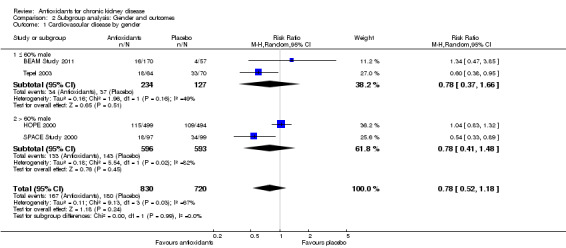
Comparison 2 Subgroup analysis: Gender and outcomes, Outcome 1 Cardiovascular disease by gender.
2.2. Analysis.
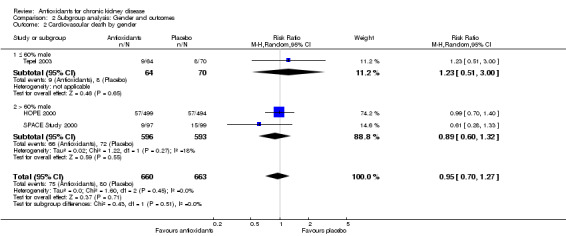
Comparison 2 Subgroup analysis: Gender and outcomes, Outcome 2 Cardiovascular death by gender.
2.3. Analysis.
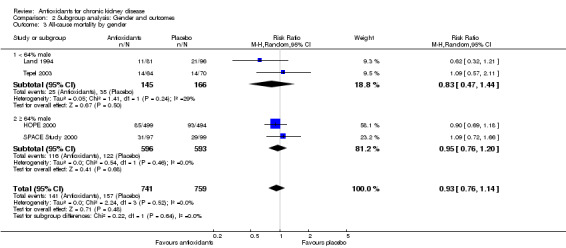
Comparison 2 Subgroup analysis: Gender and outcomes, Outcome 3 All‐cause mortality by gender.
Comparison 3. Subgroup analysis: Clinical outcomes by diabetes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular disease by diabetes | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.18] |
| 1.1 ≤ 35% diabetes | 2 | 1127 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.48, 1.41] |
| 1.2 > 35% diabetes | 2 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.79] |
| 2 Cardiovascular death by diabetes | 3 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.27] |
| 2.1 ≤ 30% diabetes | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.51, 3.00] |
| 2.2 > 30% diabetes | 2 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.60, 1.32] |
3.1. Analysis.
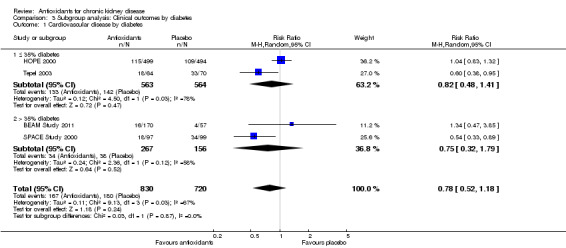
Comparison 3 Subgroup analysis: Clinical outcomes by diabetes, Outcome 1 Cardiovascular disease by diabetes.
3.2. Analysis.
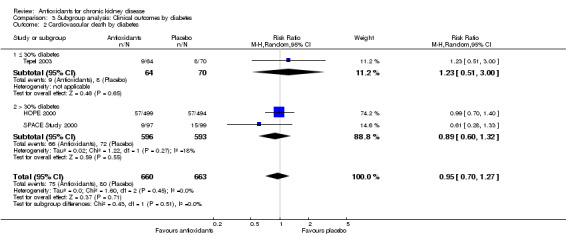
Comparison 3 Subgroup analysis: Clinical outcomes by diabetes, Outcome 2 Cardiovascular death by diabetes.
Comparison 4. Subgroup analysis: Clinical outcomes by CKD stage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular disease by CKD stage | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.18] |
| 1.1 CKD | 2 | 1220 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.84, 1.32] |
| 1.2 Dialysis | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.41, 0.80] |
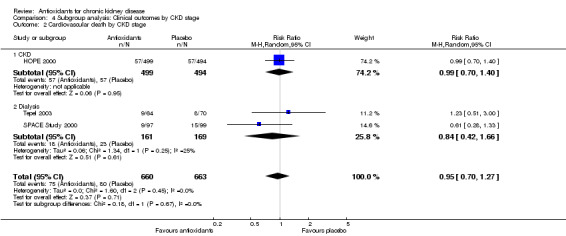
| 2 Cardiovascular death by CKD stage | 3 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.27] |
| 2.1 CKD | 1 | 993 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.70, 1.40] |
| 2.2 Dialysis | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.42, 1.66] |
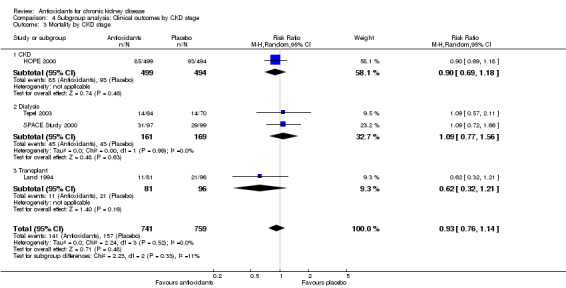
| 3 Mortality by CKD stage | 4 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.14] |
| 3.1 CKD | 1 | 993 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.69, 1.18] |
| 3.2 Dialysis | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.77, 1.56] |
| 3.3 Transplant | 1 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.32, 1.21] |
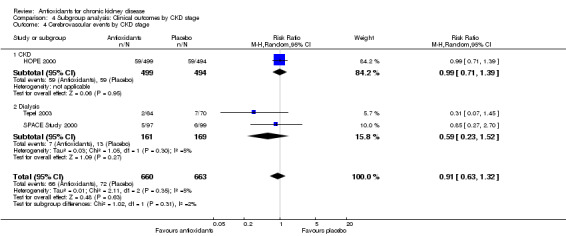
| 4 Cerebrovascular events by CKD stage | 3 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.32] |
| 4.1 CKD | 1 | 993 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.71, 1.39] |
| 4.2 Dialysis | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.23, 1.52] |
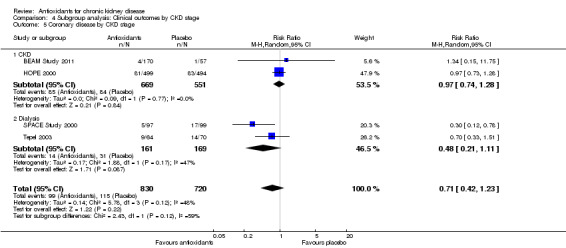
| 5 Coronary disease by CKD stage | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.42, 1.23] |
| 5.1 CKD | 2 | 1220 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.74, 1.28] |
| 5.2 Dialysis | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.21, 1.11] |
4.1. Analysis.
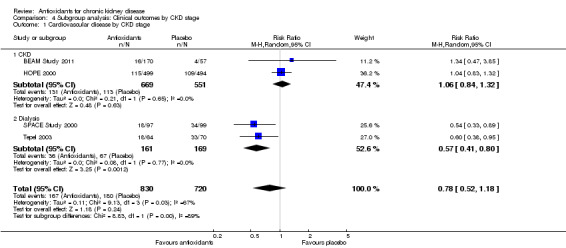
Comparison 4 Subgroup analysis: Clinical outcomes by CKD stage, Outcome 1 Cardiovascular disease by CKD stage.
4.2. Analysis.
Comparison 4 Subgroup analysis: Clinical outcomes by CKD stage, Outcome 2 Cardiovascular death by CKD stage.
4.3. Analysis.
Comparison 4 Subgroup analysis: Clinical outcomes by CKD stage, Outcome 3 Mortality by CKD stage.
4.4. Analysis.
Comparison 4 Subgroup analysis: Clinical outcomes by CKD stage, Outcome 4 Cerebrovascular events by CKD stage.
4.5. Analysis.
Comparison 4 Subgroup analysis: Clinical outcomes by CKD stage, Outcome 5 Coronary disease by CKD stage.
Comparison 5. Cardiovascular disease by CKD stage by stage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CVD by CKD | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.18] |
| 1.1 CKD stage 2 | 1 | 993 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.83, 1.32] |
| 1.2 CKD stage 3 | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.47, 3.85] |
| 1.3 CKD stage 5 | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.41, 0.80] |
5.1. Analysis.
Comparison 5 Cardiovascular disease by CKD stage by stage, Outcome 1 CVD by CKD.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
BEAM Study 2011.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation (according to eGFR, urinary albumin‐to‐creatinine ratio, glycated haemoglobin level) |
| Allocation concealment (selection bias) | Low risk | Central randomisation; procedure described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study has stated that it was double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study provided a detailed flow chart specifying the flow of patients (supplementary appendix). All patients initially randomised have been included in the analyses implying the use of intention‐to‐treat principles in the analyses |
| Selective reporting (reporting bias) | Low risk | Study protocol was published online and all of the study's pre‐specified (primary and secondary) outcomes outlined in the protocol were reported in the main publication |
| Other bias | High risk | Funding: This study was sponsored by Reata Pharmaceuticals and was designed by the first author and representatives of the sponsor. Study investigators and coordinators jointly managed the study with the sponsor. |
HOPE 2000.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Post‐hoc analysis of an international, multicentre RCT‐ Heart Outcomes Prevention Evaluation (HOPE) Study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation using a 4 digit code, performed in blocks of 8 and stratified per centre |
| Allocation concealment (selection bias) | Low risk | Study protocol has been published and has reported the use of central randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study has reported the use of double blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Compliance reported, all analyses have been reported to be conducted based on the intention‐to‐treat principle |
| Selective reporting (reporting bias) | Unclear risk | Study protocol has been published and has specified study design. All pre‐specified outcomes have been published |
| Other bias | High risk | Funding: This study was funded by the Medical Research Council of Canada (Grants MT12790 and UI12362); Hoechst‐Marion Roussel; AstraZeneca; King Pharmaceuticals; Natural Source Vitamin E Association; NEGMA and the Heart and Stroke Foundation of Ontario. Salim Yusuf was supported by a Senior Scientist award of the Medical Research Council of Canada, and a Heart and Stroke Foundation of Ontario research chair. |
Land 1994.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Retrospective analysis of data collected in prospective RCT (double‐blinded and placebo controlled) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study reports randomisation of patients, however, procedure was not described |
| Allocation concealment (selection bias) | Unclear risk | One of the authors (HS) of the study (although not involved in the patients' care) received the "randomisation code". This author was responsible for collecting all information about study. Study reports that the physicians in charge and the patients were not informed about the initial treatment. No information was provided regarding randomisation methodology |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study reported the use of double blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported complete follow‐up, no information regarding the conduct of analyses based on the intention‐to‐treat principle |
| Selective reporting (reporting bias) | Low risk | The study protocol is not separately available but the published report includes all expected outcomes |
| Other bias | High risk | Funding: Two authors employees of 1) Shaman Pharmaceuticals (San Carlos, CA) and 2) The Lipsome Company Inc (Princeton, NJ) |
Pollak 1993.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors report use of computer‐generated assignment schedule |
| Allocation concealment (selection bias) | Low risk | Study reported that the only randomisation master code was held by the Bristol‐Myers Company. No investigator had access to randomisation codes until study termination |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study reported double blinding. "No investigator had access to the code until the study was terminated." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information regarding completeness of follow‐up reported, no information regarding the conduct of analyses based on the intention‐to‐treat principle |
| Selective reporting (reporting bias) | High risk | Study reported in the methods that routine "serum chemistry, cyclosporine blood levels, and hematology" were collected, however, these were not reported in the results |
| Other bias | High risk | Study reported early termination by the monitor citing that it was "unlikely that a benefit would be shown for rh‐SOD by the addition of 84 extra subjects" Bristol‐Myers Squibb Company supported the study and provided the recombinant SOD. Bristol‐Myers Company held the only master code. |
Rabl 1993.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors report "random selection" but no further details |
| Allocation concealment (selection bias) | Unclear risk | No information provided regarding the concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided regarding blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on all 30 patients provided |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not separately available but it appears all expected outcomes have been reported |
| Other bias | Unclear risk | Funding: Omnibionta concentrate for infusion was from Merck', however information concerning its provision was not stated. |
Shoskes 2005.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Follow‐up: 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | No information provided regarding the concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Patients were randomized in a blinded fashion to receive either the Oxy‐Q or placebo". No additional information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals reported, intention‐to‐treat and per‐protocol analyses reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not separately available, however, the published report includes all expected outcomes |
| Other bias | High risk | Control group had a higher proportion of men (71%) compared with the 2 intervention groups (50%). Funding: not stated. Bioflavonoid preparation Oxy‐Q (Farr Labs, Santa Monica, CA) |
Singh 2000.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Follow‐up: 28 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation system used in this study may be insufficient, it was not stated how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation achieved through the selection of sealed envelopes. Patients were asked to select one of two cards (A or B) which were enclosed in sealed envelopes. Study does not specify whether these were opaque |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study reported the use of double blinding and stated that the physicians examining the patients and technicians analysing the blood were blinded to treatment allocation. Study reports the use of placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all patients |
| Selective reporting (reporting bias) | Low risk | Study protocol is not separately available, however, all expected outcomes were included |
| Other bias | Unclear risk | Funding: financial support from the Centre of Nutrition Tishcon Corporation, Westbury, NY provided coenzyme Q10 |
SPACE Study 2000.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Mean duration of follow‐up: 1.4 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated coin toss, each participating centre randomised separately |
| Allocation concealment (selection bias) | Unclear risk | No information regarding methods to prevent allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study reported the used of double blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all initially randomised patients were reported (although the use of intention‐to‐treat analyses was not explicitly stated in the methods) |
| Selective reporting (reporting bias) | Low risk | Study protocol is not separately available, however, it appears all expected outcomes, including those that were pre‐specified, have been reported |
| Other bias | Low risk | Funding: This research was funded by grant 4204 from the Chief Scientist’s Office, Ministry of Health, Israel Vitamin E provided by Solgar, Inc, New York, USA, during the first year and Henkel Corp, La Grange, IL, USA, during the second year. |
Tepel 2003.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Mean duration of follow‐up: 1.2 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on randomisation procedures |
| Allocation concealment (selection bias) | Unclear risk | No information provided on randomisation procedures |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study reported that it was placebo‐controlled, however, no information was provided regarding the methods involved in the blinding of study investigators or those involved with the analyses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes have been reported on all initially randomised patients. Analysis was performed based on the intention‐to‐treat principle |
| Selective reporting (reporting bias) | Low risk | Study protocol was not published separately, however, all expected outcomes, including those which were pre‐specified, were included |
| Other bias | Unclear risk | Funding: not stated |
Wijnen 2002.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Follow‐up: 7 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided regarding randomisation procedures |
| Allocation concealment (selection bias) | Unclear risk | No information provided regarding allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided regarding blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study reported outcomes for all patients initially randomised patients |
| Selective reporting (reporting bias) | Unclear risk | The methods section of the study reported what was performed; however, there were pre‐specified outcomes |
| Other bias | Unclear risk | Funding: not stated |
AKI ‐ acute kidney injury; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; eGFR ‐ estimated glomerular filtration rate; GFR ‐ glomerular filtration rate; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abendroth 1992 | No relevant outcomes |
| Agarwal 2004a | No relevant outcomes |
| ATIC Study 2005 | Not RCT |
| Blackhall 2005 | Cross‐over study design |
| Mune 1999 | No relevant outcomes |
| Noel 2000 | No relevant outcomes |
| Panzetta 1995 | Not RCT |
| Perkins 2009 | No relevant outcomes |
| Schneeberger 1989 | No relevant outcomes |
| Schramm 2002 | No relevant outcomes |
| Wlodarczyk 2000 | The main mechanism of action of the intervention used was not antioxidation |
| Yukawa 1995 | Not RCT |
RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
BEACON Study.
| Trial name or title | Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes: the Occurrence of Renal Events (BEACON) |
| Methods | Allocation: Randomized Endpoint Classification: Efficacy Study Intervention Model: Parallel Assignment Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor) Primary Purpose: Treatment |
| Participants |
|
| Interventions | Drug: Bardoxolone Methyl: 20 mg, oral, once daily. Other Name: RTA‐402 Drug: Placebo Oral, once daily |
| Outcomes | Primary Outcome Measures
Secondary Outcome Measures
|
| Starting date | June 2011 |
| Contact information | Reata Pharmaceuticals, Inc. |
| Notes |
Differences between protocol and review
In the protocol for this review, we indicated that it was our aim to restrict inclusion of studies to those with at least 100 patient years per treatment arm. This parameter was not applied due to the very limited number of relevant studies identified. We identified only three studies that met this criterion (HOPE 2000; Land 1994; SPACE Study 2000). Publication bias could not be assessed due to inconsistency of data reporting by identified studies. Furthermore, the limited number of studies identified prevented conclusive subgroup analyses being conducted. Subgroup analyses for history of cardiac disease, prior vitamin supplementation, and concomitant medications were not possible due to limited data reporting.
Contributions of authors
Draft the protocol: VV, BC, MJ, SZ, AW, VP
Study selection: MJ, VV
Extract data from studies: MJ, VV, BC
Enter data into RevMan: MJ, VV, BC
Carry out the analysis: MJ, VV
Interpret the analysis: MJ, VV, MR, BC, TN, SZ, AW, VP
Draft the final review: MJ, VV, VP
Disagreement resolution: VP
Update the review: MJ, VV, VP
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Health and Medical Research Council (NHMRC), Australia.
Program Grant (Study ID: 571281)
Declarations of interest
Min Jun: none known
Vinod Venkataraman: none known
Mona Razavian: none known
Bruce Cooper: none known
Sophia Zoungas has previously received speaker honoria from Servier, MSD, Novartis, Novo Nordisk, Sanofi Aventis, Boehringer Ingelheim, GSK, Pfizer, and Astra Zeneca/BMS and has previously served on external advisory boards for MSD, Novo Nordisk, Boehringer Ingleheim, Sanofi Aventis and Astra Zeneca/BMS.
Toshiharu Ninomiya: none known
Angela C Webster: none known
Vlado Perkovic is supported by a fellowship from the Heart Foundation of Australia and a various grants from the Australian National Health and Medical Research Council. He has received speakers fees from Roche, Servier and Astra Zeneca, funding for a clinical trial from Baxter, and serves on Steering Committees for trials funded by Johnson and Johnson, Boehringer Ingelheim, Vitae and Abbott. His employer conducts clinical trials funded by Servier, Johnson and Johnson, Roche and Merck.
Edited (no change to conclusions)
References
References to studies included in this review
BEAM Study 2011 {published data only}
- Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. New England Journal of Medicine 2011;365(4):327‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HOPE 2000 {published data only}
- Mann JF, Lonn EM, Yi Q, Gerstein HC, Hoogwerf BJ, Pogue J, et al. Effects of vitamin E on cardiovascular outcomes in people with mild‐to‐moderate renal insufficiency: results of the HOPE study. Kidney International 2004;65(4):1375‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McQueen MJ, Lonn E, Gerstein HC, Bosch J, Yusuf S. The HOPE (Heart Outcomes Prevention Evaluation) Study and its consequences. Scandinavian Journal of Clinical & Laboratory Investigation Supplement 2005;240:143‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Land 1994 {published data only}
- Land W, Schneeberger H, Schleibner S, Illner WD, Abendroth D, Rutili G, et al. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation 1994;57(2):211‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pollak 1993 {published data only}
- Pollak R, Andrisevic JH, Maddux MS, Gruber SA, Paller MS. A randomized double‐blind trial of the use of human recombinant superoxide dismutase in renal transplantation. Transplantation 1993;55(1):57‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rabl 1993 {published data only}
- Rabl H, Khoschsorur G, Colombo T, Petritsch P, Rauchenwald M, Költringer P, et al. A multivitamin infusion prevents lipid peroxidation and improves transplantation performance. Kidney International 1993;43(4):912‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shoskes 2005 {published data only}
- Shoskes D, Lapierre C, Cruz‐Correa M, Muruve N, Rosario R, Fromkin B, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation 2005;80(11):1556‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singh 2000 {published data only}
- Singh RB, Khanna HK, Niaz MA. Randomized, double‐blind placebo‐controlled trial of coenzyme Q10 in chronic renal failure: Discovery of a new role. Journal of Nutritional & Environmental Medicine 2000;10(4):281‐8. [EMBASE: 2001029318] [Google Scholar]
SPACE Study 2000 {published data only}
- Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo‐controlled trial. Lancet 2000;356(9237):1213‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Boaz M, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, et al. Secondary prevention using antioxidants of cardiovascular disease in endstage renal disease:SPACE [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A11. [DOI] [PubMed] [Google Scholar]
Tepel 2003 {published data only}
- Tepel M, Giet M, Statz M, Jankowski J, Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end‐stage renal failure: a randomized, controlled trial. Circulation 2003;107(7):992‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wijnen 2002 {published data only}
- Wijnen MH, Vader HL, Wall Bake AW, Roumen RM. Can renal dysfunction after infra‐renal aortic aneurysm repair be modified by multi‐antioxidant supplementation?. Journal of Cardiovascular Surgery 2002;43(4):483‐8. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Abendroth 1992 {published data only}
- Abendroth D, Schneeberger H, Schleibner S, Illner WD, Land W. Status of treatment with free radical scavengers following kidney and pancreas transplantation. Zentralblatt für Chirurgie 1992;117(9):502‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Agarwal 2004a {published data only}
- Agarwal R, Vasavada N, Sachs NG, Chase S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney International 2004;65(6):2279‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ATIC Study 2005 {published data only}
- Nanayakkara PW, Guldener C, ter Wee PM, Scheffer PG, Ittersum FJ, Twisk JW, et al. Effect of a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on carotid intima‐media thickness, endothelial function, and renal function in patients with mild to moderate chronic kidney disease: results from the Anti‐Oxidant Therapy in Chronic Renal Insufficiency (ATIC) Study. Archives of Internal Medicine 2007;167(12):1262‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nanayakkara PWB, Guldener C, Ittersum F, Stehouwer CDA, ter Wee PM. Effect of an oxidative‐stress‐reducing strategy on carotid intima‐media thickness, endothelial function and renal function in patients with mild‐to‐moderate chronic kidney disease [abstract]. Journal of the American Society of Nephrology 2006;17(Abstracts):367A. [Google Scholar]
Blackhall 2005 {published data only}
- Blackhall ML, Fassett RG, Sharman JE, Geraghty DP, Coombes JS. Effects of antioxidant supplementation on blood cyclosporin A and glomerular filtration rate in renal transplant recipients. Nephrology Dialysis Transplantation 2005;20(9):1970‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mune 1999 {published data only}
- Mune M, Yukawa S, Kishino M, Otani H, Kimura K, Nishikawa O, et al. Effect of vitamin E on lipid metabolism and atherosclerosis in ESRD patients. Kidney International ‐ Supplement 1999;71:S126‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Noel 2000 {published data only}
- Noel C, Copin MC, Hazzan M, Labalette M, Susen S, Lelievre G, et al. Immunomodulatory effect of pentoxifylline during human allograft rejection: involvement of tumor necrosis factor‐alpha and adhesion molecules. Transplantation 2000;69(6):1102‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Panzetta 1995 {published data only}
- Panzetta O, Cominacini L, Garbin U, Fratta Pasini A, Gammaro L, Bianco F, et al. Increased susceptibility of LDL to in vitro oxidation in patients on maintenance hemodialysis: effects of fish oil and vitamin E administration. Clinical Nephrology 1995;44(5):303‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Perkins 2009 {published data only}
- Perkins RM, Aboudara MC, Uy AL, Olson SW, Cushner HM, Yuan CM. Effect of pentoxifylline on GFR decline in CKD: a pilot, double‐blind, randomized, placebo‐controlled trial. American Journal of Kidney Diseases 2009;53(4):606‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schneeberger 1989 {published data only}
- Schneeberger H, Illner WD, Abendroth D, Bulkley G, Rutili F, Williams M, et al. First clinical experiences with superoxide dismutase in kidney transplantation‐‐results of a double‐blind randomized study. Transplantation Proceedings 1989;21(1 Pt 2):1245‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Schramm 2002 {published data only}
- Schramm L, La M, Heidbreder E, Hecker M, Beckman J S, Lopau K, et al. L‐arginine deficiency and supplementation in experimental acute renal failure and in human kidney transplantation. Kidney International 2002;61(4):1423‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wlodarczyk 2000 {published data only}
- Wlodarczyk Z, Oko A, Glyda M, Karczewski M, Idasiak‐Piechocka I, Czekalski S. Effects of pentoxifylline treatment on delayed graft function in cadaveric kidney recipients. Transplantation Proceedings 2000;32(6):1382‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yukawa 1995 {published data only}
- Yukawa S, Hibino A, Maeda T, Mimura K, Yukawa A, Maeda A, et al. Effect of alpha‐tocopherol on in vitro and in vivo metabolism of low‐density lipoproteins in haemodialysis patients. Nephrology Dialysis Transplantation 1995;10 Suppl 3:1‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
BEACON Study {published data only}
- BEACON Study. Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes (BEACON). http://www.clinicaltrials.gov/show/NCT01351675 (accessed August 2012).
Additional references
ANZOD 2004
- Excell L, Russ GR, Wride P (editors). Australia and New Zealand Organ Donation Registry. ANZOD 2004 Annual Report. http://www.anzdata.org.au/anzod/anzodreport/2004/2004Contents.pdf (accessed August 2012).
Bachem 1999
- Bachem MG, Wendelin D, Schneiderhan W, Haug C, Zorn U, Gross HJ, et al. Depending on their concentration oxidized low density lipoproteins stimulate extracellular matrix synthesis or induce apoptosis in human coronary artery smooth muscle cells. Clinical Chemistry & Laboratory Medicine 1999;37(3):319‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bjelakovic 2008
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD007176] [DOI] [PubMed] [Google Scholar]
Brunet 1995
- Brunet J, Boily MJ, Cordeau S, Rosiers C. Effects of N‐acetylcysteine in the rat heart reperfused after low‐flow ischemia: evidence for a direct scavenging of hydroxyl radicals and a nitric oxide‐dependent increase in coronary flow. Free Radical Biology & Medicine 1995;19(5):627–38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cooper 2010
- Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. A randomized, controlled trial of early versus late initiation of dialysis. New England Journal of Medicine 2010;363(7):609‐19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Zeeuw 2006
- Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. Journal of the American Society of Nephrology 2006;17(8):2100‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dhalla 1996
- Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. Journal of the American College of Cardiology 1996;28(2):506‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eknoyan 2002
- Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. New England Journal of Medicine 2002;347(25):2010‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frei 1989
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proceedings of the National Academy of Sciences of United States of America 1989;86(16):6377–81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galle 1999
- Galle J, Heermeier K, Wanner C. Atherogenic lipoproteins, oxidative stress and cell death. Kidney International ‐ Supplement 1999;71:S62‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GISSI 1999
- Anonymous. Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 1999;354(9177):447‐55. [MEDLINE: ] [PubMed] [Google Scholar]
Giugliano 1995
- Giugliano D, Ceriello A, Paolisso G. Diabetes mellitus, hypertension and cardiovascular disease: which role for oxidative stress?. Metabolism: Clinical & Experimental 1995;44(3):363‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Halliwell 1994
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence?. Lancet 1994;344(8924):721‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heart Protection Study 2002
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 2002;360(9326):23‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Himmelfarb 2000
- Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney International 2000;58(6):2571‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Himmelfarb 2002
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney International 2002;62(5):1524‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huwiler‐Muntener 2002
- Huwiler‐Muntener K, Juni P, Junker C, Egger M. Quality of reporting of randomized trials as a measure of methodologic quality. JAMA 2002;287(21):2801‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Islam 2000
- Islam KN, O'Byrne D, Devaraj S, Palmer B, Grundy SM, Jialal I. Alpha‐tocopherol supplementation decreases the oxidative susceptibility of LDL in renal failure patients on dialysis therapy. Atherosclerosis 2000;150(1):217‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jolly 1984
- Jolly SR, Kane WJ, Bailie MB, Abrams GD, Lucchesi BR. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circulation Research 1984;54(3):277‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kaul 1995
- Kaul N, Siveski‐Iliskovic N, Thomas TP, Hill M, Khaper N, Singal PK. Probucol improves antioxidant activity and modulates development of diabetic cardiomyopathy. Nutrition 1995;11(5 Suppl):551‐4. [MEDLINE: ] [PubMed] [Google Scholar]
KDOQI 2002
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Part 4. Definition and classification of stages of chronic kidney disease. American Journal of Kidney Diseases 2002;39(2 Suppl 1):S46‐75. [MEDLINE: ] [PubMed] [Google Scholar]
Kukreja 1992
- Kukreja RC, Hess ML. The oxygen free radical system: from equations through membrane‐protein interactions to cardiovascular injury and protection. Cardiovascular Research 1992;26(7):641‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kukreja 1994
- Kukreja RC Hess ML. Free radicals, cardiovascular dysfunction and protection strategies. Austin: RG Landes Co, 1994. [ISBN: 1570590125] [Google Scholar]
Landray 2001
- Landray MJ, Thambyrajah J, McGlynn FJ, Jones HJ, Baigent C, Kendall MJ, et al. Epidemiological evaluation of known and suspected cardiovascular risk factors in chronic renal impairment. American Journal of Kidney Diseases 2001;38(3):537‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 1991
- Lee V, Randhawa AK, Singal PK. Adriamycin‐induced myocardial dysfunction in vitro is mediated by free radicals. American Journal of Physiology 1991;261(4 Pt 2):H989‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lowrie 1990
- Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. American Journal of Kidney Diseases 1990;15(5):458‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meydani 1994
- Meydani M, Martin A, Ribaya‐Mercado JD, Gong J, Blumberg JB, Russell RM. Beta‐carotene supplementation increases antioxidant capacity of plasma in older women. Journal of Nutrition 1994;124(12):2397‐403. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peng 1995
- Peng M, Huang L, Xie ZJ, Huang WH, Askari A. Oxidant‐induced activations of nuclear factor‐kappa B and activator protein‐1 in cardiac myocytes. Cellular & Molecular Biology Research 1995;41(3):189‐97. [MEDLINE: ] [PubMed] [Google Scholar]
Phrommintikul 2007
- Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta‐analysis. Lancet 2007;369(9559):381‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prasad 1997
- Prasad K. Dietary flax seed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis 1997;132(1):69‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodriguez‐Iturbe 2001
- Rodríguez‐Iturbe B, Pons H, Herrera‐Acosta J, Johnson RJ. Role of immunocompetent cells in nonimmune renal diseases. Kidney International 2001;59(5):1626‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sarnak 2003
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108(17):2154‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singal 1981
- Singal PK, Yates JC, Beamish RE, Dhalla NS. Influence of reducing agents on adrenochrome‐induced changes in the heart. Archives of Pathology & Laboratory Medicine 1981;105(12):664‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Singal 1982
- Singal PK, Kapur N, Dhillon KS, Beamish RE, Dhalla NS. Role of free radicals in catecholamine‐induced cardiomyopathy. Canadian Journal of Physiology & Pharmacology 1982;60(11):1390‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singal 1983
- Singal PK, Beamish RE, Dhalla NS. Potential oxidative pathways of catecholamines in the formation of lipid peroxides and genesis of heart disease. Advances in Experimental Medicine & Biology 1983;161:391‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singal 1998
- Singal PK, Khaper N, Palace V, Kumar D. The role of oxidative stress in the genesis of heart disease. Cardiovascular Research 1998;40(3):426‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
St Peter 2008
- Peter WL, Liu J, Weinhandl E, Fan Q. A comparison of sevelamer and calcium‐based phosphate binders on mortality, hospitalization, and morbidity in hemodialysis: a secondary analysis of the Dialysis Clinical Outcomes Revisited (DCOR) randomized trial using claims data. American Journal of Kidney Diseases 2008;51(3):445‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
STATA 9.2
- StataCorp. 2005. Stata Statistical Software: Release 9 College Station, TX: StataCorp LP.
Stenvinkel 2001
- Stenvinkel P. Malnutrition and chronic inflammation as risk factors for cardiovascular disease in chronic renal failure. Blood Purification 2001;19(2):143‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Toborek 1992
- Toborek M, Wasik T, Drodz M, Klin M, Magner‐Wrobel K, Kopieczna‐Grzebieniak E. Effect of hemodialysis on lipid peroxidation and antioxidant system in patients with chronic renal failure. Metabolism: Clinical & Experimental 1992;41(11):1229‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wanner 2005
- Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. New England Journal of Medicine 2005;353(3):238‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wohaieb 1987
- Wohaieb SA, Godin DV. Alterations in free radical tissue‐defense mechanisms in streptozocin‐induced diabetes in rat. Effects of insulin treatment. Diabetes 1987;36(9):1014‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yusuf 2000
- Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New England Journal of Medicine 2000;342(3):154‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zoccali 2000
- Zoccali C, Mallamaci F, Tripepi G. AGEs and carbonyl stress: potential pathogenetic factors of long‐term uraemic complications. Nephrology Dialysis Transplantation 2000;15 Suppl 2:7‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zoccali 2002
- Zoccali C, Benedetto FA, Maas R, Mallamaci F, Tripepi G, Malatino LS, et al. Asymmetric dimethylarginine, C‐reactive protein, and carotid intima‐media thickness in end‐stage renal disease. Journal of the American Society of Nephrology 2002;13(2):490‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Perkovic 2009
- Perkovic V, Venkataraman V, Cooper B, Zoungas S, Webster AC. Antioxidants for chronic kidney disease. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008176] [DOI] [PMC free article] [PubMed] [Google Scholar]


