Abstract
Background
Experimental data have shown that the developing brain is especially vulnerable to exogenous noxious substances. The potential effects of anesthetic drugs on brain growth and development are a matter of concern. Clinical studies of children who underwent general anesthesia in their earliest years can make a major contribution to our understanding of the effects of anesthetic drugs on infants and toddlers (i.e., children under age 5).
Methods
Children born at term during the years 2007–2011 who were exposed to general anesthesia before their third birthday were included in the study. Data on general anesthesia were retrospectively evaluated, and the overall intelligence quotient (IQ) was determined prospectively as the primary target parameter. Children who had not been exposed to general anesthesia were recruited as a control group. The non-inferiority threshold was set at a difference of 5 IQ points out of a consideration of clinical relevance.
Results
430 complete data sets were available from exposed children and 67 from members of the control group. The exposed group achieved a mean IQ score of 108.2, with a 95% confidence interval of [107; 109.4]; the corresponding values in the control group were 113 [110; 116.1]. Both groups achieved a mean score that was higher than the expected 100 points. After adjustment for age, socioeconomic status, and sex, the difference between the two groups was 2.9 points [0.2; 5.6], indicating a significantly better outcome in the control group than in the exposed group. The non-inferiority threshold of 5 IQ points was within the confidence interval; thus, non-inferiority was not demonstrated.
Conclusion
The fact that both groups achieved a higher IQ score than the expected 100 points may be attributable, at least in part, to the restriction of the study to children born at term. The results indicate that general anesthesia in early childhood is not associated with markedly reduced intelligence in later years, although non-inferiority could not be demonstrated.
In Germany, anesthesia for therapeutic procedures or diagnostic examinations in children within the first five years of life accounts for an estimated 400 000 anesthetic procedures, or approximately 2% of the anesthetic procedures performed annually (1).
Since the developing brain is highly vulnerable to exogenous noxious substances, especially in early childhood, given the essential developmental processes during this period (including cell migration and differentiation, synaptogenesis), research on the potential neurotoxic effects of anesthetics is highly relevant (2). In recent years, discussions about the possible effects of anesthesia in the first three years of life have been the focus of particular attention (3).
Several studies have already been conducted to investigate the effects on cognitive performance by anesthetic-induced neurotoxicity. However, their different designs limited any comparability of the results. Animal studies on rodents (4– 6) and non-human primates (7, 8) did not provide a clear picture. For example, neuroinflammatory responses with decreased protective glial cell-derived neurotrophic factors and reduced neurogenesis in the hippocampus were observed after a two-hour exposure of neonatal rats to 3% sevoflurane. These were functionally associated with limitations in behavioral tests in terms of cognitive dysfunction (9). Given the interaction of synaptogenesis with diverse neurotransmitter systems (including N-methyl-D-aspartate [NMDA] and gamma-aminobutyric acid [GABA] receptors), it appears likely from an animal experimental perspective that synaptogenesis is functionally modulated, and proapoptotic effects are induced, by anesthetics. However, results from animal studies have limited applicability to humans regarding pharmacokinetics, duration of exposure, and the level of maturation of the central nervous system at the time of exposure, amongst other things. Epidemiological studies are therefore indispensable.
Retrospective observational studies indicate that early exposure to anesthetics may increase the likelihood of developing learning deficits and mental disorders, among other things (10– 16). Other studies found no association between administration of anesthetics in early childhood and decreased cognitive performance (17, 18). The results of the few available prospective studies convey a similar picture (19– 21). No significant difference in full-scale intelligence quotient (IQ) was found between children under four years of age with and without exposure to anesthetics in the PANDA study (22, 23) and the MASK study (24, 25).
Given that anesthesia is usually indispensable for surgical procedures, controlled interventional studies on this are only possible to a limited extent. The GAS study (26, 27) therefore examined the effect of general anesthesia on neurodevelopmental outcome as compared with regional anesthesia. At the measurement time points (participants’ ages: two and five years), there were no significant differences in terms of full-scale IQ scores between the groups.
The present study, ANFOLKI-36 (“Anesthetic Consequences After Pediatric Anesthesia from Birth to Three Years of Age”), aimed to provide convincing results on the question of whether anesthesia affects long-term cognitive abilities in children under the age of three years. Children with well-documented exposure to anesthetics within the first three years of life were prospectively given a standardized test of their cognitive performance and compared with a control group without exposure to anesthetics.
Materials and methods
Inclusion criteria and recruitment
The present controlled cross-sectional study recruited children born between 2007 and 2011 who had undergone at least one anesthetic procedure at Erlangen University Hospital (UKER) before reaching the age of three years and who had not shown signs of impaired cognitive development before exposure to anesthetics (“International Statistical Classification of Diseases and Related Health Problems” [ICD]-10 F70–F73). Patients with severe malformations or underlying conditions (including metabolic diseases, cerebral palsy, dystrophy) diagnosed before exposure to anesthetics were not recruited, nor were patients who had undergone surgery of the central nervous system (Operation and Procedure Code [OPS] 5–01 – 5–02) or the heart (OPS 5–35 – 5–37). Custodial parents and children aged seven years and older gave their written informed consent. The control group included equivalent children not exposed to anesthesia before the age of three years.
Subsequently, recruited patients with ICD codes associated with potentially decreased intelligence were identified by a specialist in anesthesiology (G04.9, G08, G25.9, G81.1, G93.0, I46.0, P21.0, Q04.0, Q90.9), and affected patients were excluded. A specialist in anesthesiology determined whether surgery or diagnostic testing had been performed using the German procedure classification codes for the procedures performed on the day of anesthesia.
Potential subjects were identified using the patient data management system (PDMS) Narco Data (IMESO) of the Department of Anesthesiology. After having been contacted by mail and their willingness to participate had been expressed by the parents, they were then contacted by telephone. On that occasion, a medical member of the study team conducted the information and informed consent interview. Once consent had been provided, the study documents were sent out. The control group was recruited via the outpatient clinic of the pediatric department of the UKER as well as through pediatricians in private practice in the region.
Target parameters and measuring tools
The primary outcome parameter of the study was the full-scale IQ score. Secondary outcome parameters were the subtest scores on which the full-scale score was based and developmental neurological characteristics.
All anesthesia-related data (including procedure times and medications) had been documented in the electronic anesthesia record during anesthesia and were evaluated retrospectively for the study.
All other study-relevant data were collected prospectively between 2017 and 2019. The full-scale IQ score was assessed in the then 7 to 11-year-olds at the time of the study using a standardized test procedure: the Kaufman Assessment Battery for Children—Second Edition (KABC-II, German version), which was normed for the age range relevant to the study (28). The Wechsler test (29, 30) was planned as an alternative in case the KABC-II had already been completed but was not used. The psychological tests took place at the UKER and were performed by experienced neuropsychologists. Additional data (including demographic information, socioeconomic status, developmental milestones) were collected using a questionnaire.
Ethics committee and registration
The study received a positive ethics opinion from the Friedrich-Alexander University Erlangen-Nürnberg (reference number 226_16B). In addition, the study was registered on ClinicalTrials.gov (NCT number: NCT03034889).
Statistical methods
The reference values of the test procedure used were normally distributed with a mean of 100 and a standard deviation of 15 (28). For the primary outcome parameter, the study protocol planned an interval inclusion test for non-inferiority at the 5% significance level with regard to the difference in means, with a maximum tolerable deviation of five IQ points. The number of cases (2 : 1) for a power of 90% was 420 (270 exposed subjects, 150 subjects in the control group). Of relevance to the analysis were subjects with a complete data set consisting of a completed questionnaire and an IQ test administered by study investigators.
Socioeconomic status was defined as low (no school-leaving certificate/elementary school/secondary general school), middle (intermediate secondary school), or high (high school diploma/entrance qualification for university of applied sciences), based on the information provided in the questionnaire on the mother’s school education. The analysis was restricted to full-term infants. Classification as full-term was based on the duration of gestation from 37 weeks or, in the absence of information to this effect, from a birth weight of 2500 g.
A linear regression model adjusted for socioeconomic status, age, and sex was created.
Non-inferiority of the primary outcome criterion to the clinically relevant margin of five was examined using the 90% confidence interval (90% CI) centered around the estimator of the mean difference. The 95% confidence interval (95% CI) was also provided.
Apart from the subtests of the standardized testing procedure, no other secondary target parameters were included in the evaluation of the present manuscript.
The software environment R (version 3.5.3) was used for the statistical analysis (31).
Results
Subject characteristics
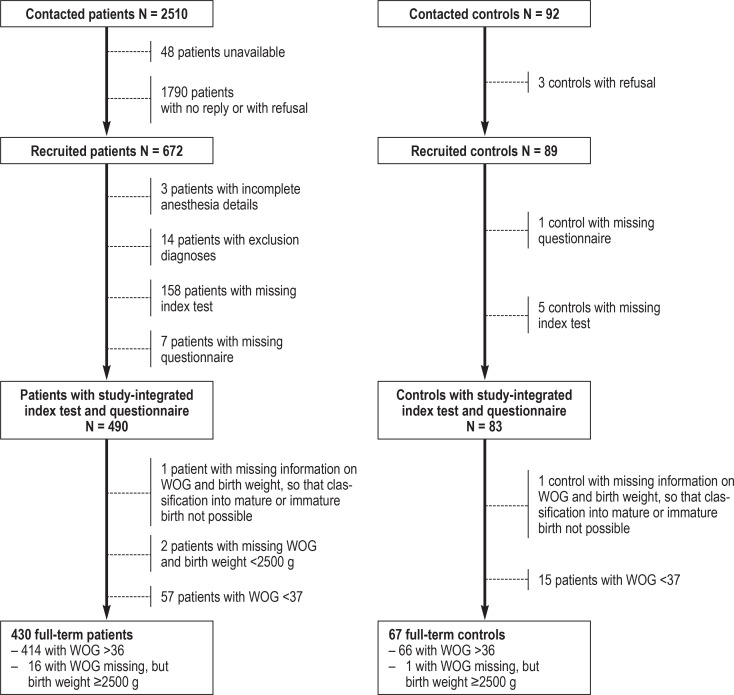
Out of a total study population of 2510 patients, 490 subjects with complete data sets were included. Eighty-three subjects were recruited for the control group. After exclusion of all preterm children, 430 and 67 subjects, respectively, remained for analysis (Figure).
Figure 1.
Flowchart for the selection of the final study population with respect to the exposed group and the control group, based on the potentially eligible total population.
WOG, week of gestation.
The average age at testing was 9.2 years among the exposed subjects and 9.9 years in the control group. Sixty-four percent of the exposed children were male compared with 44.8% in the control group. The breakdown of both groups according to socioeconomic status is shown in Table 1.
Table 1. Break-down of the full-term, exposed and non-exposed subjects.
| Parameter | Exposed subjects (n = 430) | Non-exposed subjects (n = 67) |
| Sex | ||
| – male | 277 (64.4%) | 30 (44.8%) |
| – female | 153 (35.6%) | 37 (55.2%) |
| Socioeconomic status | ||
| – low | 65 (15.1%) | 7 (10.4%) |
| – middle | 160 (37.2%) | 16 (23.9%) |
| – high | 205 (47.7%) | 44 (65.7%) |
| Age at index test in years; mean ±SD | 9,2 ± 1,5 | 9.9 ± 1.4 |
SD, standard deviation
Evaluation of anesthesia-related data
Evaluation of the anesthesia-related data of the 430 patients produced the following results (table 2): The mean age at the time of first anesthesia was 463.7 days. The majority of patients had undergone surgery once (293 of 430; 68.1%), while 93 children (21.6%) had had at least two operations. 10.2% (44 of 430) had received anesthesia for an examination. The average duration of anesthesia for the entire study cohort was 110 minutes. Types of anesthesia, subdivided into total intravenous anesthesia (TIVA) and inhalational anesthesia, are listed per patient with summary information for multiple anesthetics. One (or more) TIVAs had been performed in 264 subjects (61.4%), of whom 81 subjects had also received regional anesthesia during the procedure. In 113 children (26.3%), anesthesia had been maintained with inhalation anesthetics. In this group, regional anesthesia had been performed in 28 children. In the remaining 53 patients (12.3%), multiple anesthetic procedures had been performed, and both procedures had been used. Table 3 presents the distribution of patients with respect to the number of anesthetic procedures received before and after three years of age.
Table 2. Results of anesthesia-related analysis of subjects who received anesthesia between the ages of 0 and 36 months, limited to the first three years of life.
| Parameter | Exposed subjects (n = 430) | ||
| Age at first exposure to anesthetics in days; mean ± SD (mean, [IQR]) | 463.7 ± 321.1 (462. [158.8; 705.0]) | ||
| Number of operations (%) | |||
| 1 OP | 293 (68.1%) | ||
| 2 OPs | 51 (11.9%) | ||
| 3 OPs | 28 (6.5%) | ||
| 4 OPs | 8 (1.9%) | ||
| more than 4 OPs | 6 (1.4%) | ||
| no OP | 44 (10.2%) | ||
| Duration of anesthesia per anesthesia in minutes; mean ± SD (median, [IQR]) | 110 ± 74.8 (90. [61.0; 134.0]) | ||
| Type of anesthesia per patient | Regional anesthesia | Total | |
| yes*1 | no*2 | ||
| Inhalational anesthesia*3 | 28 | 85 | 113 |
| TIVA*4 | 81 | 183 | 264 |
| Inhalational anesthesia and TIVA*5 | 30 | 23 | 53 |
IQR, interquartile range; OP, operation; SD, standard deviation; TIVA, total intravenous anesthesia;
*1 in the event of multiple anesthetic procedures, at least one regional anesthesia;
*2 in the event of multiple anesthetic procedures, no regional anesthesia;
*3 in the event of multiple anesthetic procedures, always inhalational anesthesia;
*4 in the event of multiple anesthetic procedures, always TIVA;
*5 in the event of multiple anesthetic procedures, at least one TIVA and one inhalational anesthesia
Table 3. Distribution of patients (n = 430) in terms of the number of anesthetic procedures received before and after 3 years of age.
| Number of anesthetic procedures in the first 3 years of life | ||||||
| 1 | 2 | 3 | 4 | 5 + | ||
| Number of anesthetic procedures received after 3 years of age up until the IQ test | 0 | 285 | 50 | 18 | 9 | 4 |
| 1 | 26 | 8 | 3 | 3 | 3 | |
| 2 | 10 | 2 | 3 | 1 | 0 | |
| 3 + | 2 | 0 | 0 | 1 | 2 | |
A total of 694 anesthetic procedures had taken place in the study population, 166 of which had involved the addition of an inhalation anesthetic. The injection anesthetics used were propofol (593 of 694; 85.4%), esketamine (323 of 694; 46.5%), midazolam (398 of 694; 57.4%), and thiopenthal (53 of 694; 7.6%). Several anesthetics may have been used in combination. Sevoflurane was the main agent used for inhalational anesthesia.
Evaluation of the standardized test
The mean IQ score of the index test for full-term infants was 108.2 (95% CI: [107.0; 109.4]) for the exposed subjects and 113.0 (95% CI: [110.0; 116.1]) for the control group. The difference is 4.9, with a 90% CI of [2.2; 7.6] and a 95% CI of [1.7; 8.1]. The clinically relevant non-inferiority threshold of five IQ points was within the confidence interval; thus, non-inferiority was not demonstrated. Neither adjustment for socioeconomic status (difference of 3.6 with a 90% CI of [0.9; 6.2] and a 95% CI of [0.4; 6.7]) nor adjustment for socioeconomic status, age, and sex (difference of 2.9 with a 90% CI of [0.2; 5.6] and a 95% CI of [-0.3; 6.1]) had any effect on this result. The evaluation of the subtests confirmed the result of the full-scale IQ analysis (table 4).
Table 4. Evaluation of the standardized index test, including subtests, of exposed patients and the control group, adjusted for socioeconomic status as well as for socioeconomic status, age, and sex.
|
Controls
mean [95% CI] |
Patients
mean [95% CI] |
Difference
[90% CI] |
Difference
adjusted according to socioeconomic status [90% CI] |
Difference adjusted
according to socioeconomic status, ag,e and sex [90% CI] |
|
| Index test | 113.0 [110.0; 116.1] | 108.2 [107.0; 109.4] | 4.9 [2.2; 7.6] | 3.6 [0.9; 6.2] | 2.9 [0.2; 5.6] |
| IT subtest: sequential | 109.6 [106.3; 112.9] | 105.1 [103.7; 106.4] | 4.5 [1.5; 7.5] | 3.6 [0.5; 6.7] | 2.4 [−0.8; 5.5] |
| IT subtest: simultaneous | 110.4 [107.3; 113.6] | 107.5 [106.3; 108.7] | 3.0 [0.1; 5.8] | 1.9 [−0.8; 4.6] | 2.6 [−0.1; 5.3] |
| IT subtest: learning | 110.1 [107; 113.2] | 106.5 [105.2; 107.7] | 3.7 [0.9; 6.5] | 2.7 [0; 5.5] | 2.4 [−0.4; 5.3] |
| IT subtest: planing | 107.9 [104.6; 111.3] | 104.5 [103.2; 105.9] | 3.4 [0.4; 6.4] | 2.7 [−0.4; 5.8] | 1.2 [−2; 4.4] |
| IT subtest: knowledge | 114.1 [110.8; 117.5] | 109.6 [108.3; 110.9] | 4.5 [1.5; 7.5] | 2.9 [0.1; 5.7] | 3.1 [0.2; 5.9] |
IT, Index test; 90% CI, 90 % confidence interval
Subgroup analysis
Sensitivity analyses were performed for selected subgroups. In the subgroup based on number of anesthetic procedures within the first three years of life, the mean IQ score was comparable to the score of 108.2 for all full-term infants (patients with one anesthesia: 108.3; 95% CI: [106.9; 109.8], with two anesthetic procedures: 108.8; 95% CI: [106.0; 111.7], with three anesthetic procedures: 107.8; 95% CI: [103.2; 112.4]). This was equally true for the number of operations (patients with no surgery: 107.0; 95% CI: [103.8; 111.9], with one procedure: 108.2; 95% CI: [106.6; 109.8], with two procedures: 108.3 95% CI: [105.7; 110.9], with three procedures: 111.1; 95% CI: [107.3; 115.0]). The group of 323 patients with only one anesthesia within the first three years of life was examined with regard to anesthesia duration. Duration of anesthesia did not correlate with IQ test score (r = –0.029; 95% CI: [-0.138; 0.080]). In a linear model adjusted for socioeconomic status, sex, and age at testing, the effect of anesthesia duration was not significant (p = 0.394; estimated effect per minute: –0.010; 95% CI: [-0.0340; 0.01345]).
Discussion
The results of the present study showed that the non-inferiority hypothesis defined in advance for a group of patients with exposure to anesthetics in early childhood compared with a control group was not statistically supported. The group with anesthetic exposure had a significant difference of 2.9 IQ points adjusted for socioeconomic status, age, and sex in direct comparison with the control group. However, the full-scale IQ of the patients tested was 108.2, which was significantly higher than the normalized mean of 100 for the test procedure used. This relation suggests that, on average, anesthesia in early childhood is not associated with decreased intelligence in later life.
Discussion of the study cohort
After data collection, patients were first compared with the control group with respect to characteristics potentially affecting IQ, without taking the test results into consideration. For the primary patient cohort, the groups of patients and controls were confined to full-term infants because the control group contained proportionally very few formerly preterm infants.
The control group had a higher socioeconomic status and was on average one year older. The patient group contained significantly more male participants than female participants, as expected from the study protocol. Therefore, an additional linear regression model was used to adjust for socioeconomic status, age, and gender.
Socioeconomic statuses in the patient group (table 1) were similarly distributed as in the sample (13.2% low, 37.2% middle, 49.6% high) with the help of which the KABC-II was validated (28).
Discussion of the results
Studies looking at similar questions have arrived at comparatively different results. For example, in their PANDA study of 105 pairs of siblings, Sun et al. found no statistically significant difference in full-scale IQ between exposed and unexposed siblings. The average score at testing (8 to 15-year-olds) was even slightly higher at 111 points (22, 23) than in the present study. It should be noted that the inclusion criteria of Sun et al. involved children with a single exposure to elective hernia surgery within the first three years of life and no more than mild general illnesses. A similar selection with respect to indication for surgery was made by McCann et al, who compared the effects of regional with general anesthesia in 447 children (postmenstrual age <60 weeks), also after hernia surgery. This study also found no statistically significant difference in IQ scores between the two groups when tested at five years of age (regional anesthesia: 99.08, general anesthesia: 98.97) (27). The study by Warner et al., looking at the effects on school performance in 8 to 12 or 15 to 20-year-olds after single versus multiple exposures, also did not indicate any negative effects (24). In our study, we deliberately did not focus on a specific indication for anesthesia in order to obtain as comprehensive a picture as possible. Patients are well characterized in terms of number and duration of operations or anesthesia, anesthetic procedures, and diagnoses. In contrast to the numerous animal and retrospective studies that have observed cognitive deficits under laboratory conditions or with outdated anesthesia procedures (4– 6, 10, 13, 32), the present study reflects anesthesic procedures currently in use. We presume, however, that relatively complex cases are represented in the study population, given the wide range of disorders. Secondary effects associated with these disorders could account for the observed significance between the exposed patient group and the control group. The fact that the population was limited to full-term infants may have contributed to the expected value of IQ score of 100 being exceeded in both groups, because the validation sample of the KABC-II was not restricted to full-term infants. At the same time, severely ill preterm infants tend to have intelligence deficits and partial disorders more frequently. On the other hand, it cannot be assumed that the group of severely ill preterm infants was represented strongly enough in the validation sample of the KABC-II to explain such an effect.
Limitations
Some limitations must be borne in mind when interpreting the results. One weakness of the retrospective study design is that it was not possible to compare the time points before and after IQ testing. This would have been impossible to implement even in a purely prospectively planned study because of the young age of the subjects at the time of anesthesia. The anesthesia-related data were already available before the start of the study, so that no influence could be applied to the selection of the data to be collected or the measurement and documentation techniques used for this purpose.
However, the PDMS used guaranteed comprehensive and highly standardized documentation for all study-related parameters. Furthermore, instead of the intended 150 subjects, only 67 were included in the control group. The comparison is valid, however, because of the even distribution across the observed age groups and the larger enrollment in the patient group. Ultimately, studies looking at the effects of anesthetic-induced neurotoxicity are always limited in their conclusions, because anesthesia is never given as an end in itself. Anesthesia or sedation is always associated with a diagnostic or therapeutic procedure, which may have its indication in a condition that may per se have an impact on cognitive development.
CME credit for this unit can be obtained via cme.aerzteblatt.de until 9 December 2022. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which blood hormone increases iron absorption in ß-thalassemia?
Insulin
hemojuvelin
transferrin
erythroferrone
interleukin-6
Question 2
Hepcidin is produced in the liver and circulates in the blood. What is the name of the receptor to which hepcidin binds?
Transferrin receptor
Insulin receptor
TMPRSS6
Iron exporter ferroportin
NMDA receptor
Question 3
What is believed to be the function of hepcidin in the regulation of iron availability?
It increases the absorption of iron from food.
It inhibits iron absorption.
It enhances iron recycling by macrophages.
It increases glucagon expression.
It releases iron from macrophages.
Question 4
What is the most common cause of hypochromic–microcytic anemia?
Vitamin B12 deficiency
Renal anemia
Anemia of chronic disease
Iron deficiency
Folic acid deficiency
Question 5
Transferrin saturation is an important biomarker of iron availability. Which are the saturation levels that show iron deficiency and indicate iron overload?
Iron deficiency: <20%, iron overload: >40%
Iron deficiency: <30%, iron overload: >60%
Iron deficiency: <40%, iron overload: >70%
Iron deficiency: <50%, iron overload: >80%
Iron deficiency: <60%, iron overload: >90%
Question 6
What percentage of children in Europe are affected by iron deficiency?
0.05%–0.5%
0.1%–1%
2%–6%
8%–12%
14%–18%
Question 7
A 72-year-old patient with myelodysplastic syndrome becomes permanently dependent on transfusion due to progressive anemia. After the administration of how many units of packed red cells (PRC) can serum ferritin be expected to rise to approximately 1000 µµg/L?
About 5
about 10
about 20
about 40
about 60
Question 8
Which laboratory parameters should be measured when investigating iron deficiency?
Fasting blood sugar and glutamate oxaloacetate transaminase (aspartate aminotransferase)
Total LDL and C-reactive protein
HbA1c and creatinine
Alkaline phosphatase and urea
Ferritin and zinc protoporphyrin
Question 9
In older patients, the consequences of iron overload may be heavily overlaid by age-related medical problems. Which of the following diseases can be a result of systemic iron overload?
Ulcerative colitis
heart failure
glaucoma
Dupuytren‘s disease
morbid obesity
Question 10
What can mask iron deficiency?
High thyroid values
Immunosuppressive drugs
Pregnancy
Inflammatory disease
LDL cholesterol level > 180 mg/dL
Acknowledgments
Translated from the original German by Dr. Grahame Larkin, MD
Footnotes
Conflict of interest statement
The authors declare that no conflicts of interest exists.
Funding
This study was funded by the Doktor Robert Pfleger-Stiftung, Bamberg, Germany.
Acknowledgments
Our thanks go to Kathrin Girschick and Alexander Falk, who assisted with the recruitment of study subjects and data collection, to the psychologists Hannah Pflüger, Andrea Popper, Frauke Wirtz, Rebekka Lederer as well as Sarah Steinvorth, who conducted the standardized testing, to the staff of the “IT for Research and Management” department Thomas Ganslandt and Katharina Diesch as well as Andreas Ackermann, who were responsible for providing the data, as well as Stephanie Newe and Barbara Ullrich, who made the technical collection of the data possible, and especially Gabriele Göhring-Waldeck, whose involvement made a significant contribution to the success of the study.
Data sharing
The authors state that the individual patient data on which the results of this article are based cannot be shared because the data privacy statement signed by the patients does not cover this.
References
- 1.Destatis. Gesundheit - Fallpauschalenbezogene Krankenhausstatistik (DRG-Statistik) Operationen und Prozeduren der vollstationären Patientinnen und Patienten in Krankenhäusern (4-Steller) www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankenhaeuser/_inhalt.html (last accessed on 22 March 2021) [Google Scholar]
- 2.Sinner B, Becke K, Engelhard K. General anaesthetics and the developing brain: an overview. Anaesthesia. 2014;69:1009–1022. doi: 10.1111/anae.12637. [DOI] [PubMed] [Google Scholar]
- 3.Becke K, Höhne C, Eich C, Engelhardt T, Hansen T, Weiss M. Kinderanästhesie: Was wirklich wichtig ist. Dtsch Arztebl. 2017;114 A-166-9. [Google Scholar]
- 4.Huang J, Jing S, Chen X, et al. Propofol administration during early postnatal life suppresses hippocampal neurogenesis. Mol Neurobiol. 2016;53:1031–1044. doi: 10.1007/s12035-014-9052-7. [DOI] [PubMed] [Google Scholar]
- 5.Li G, Xue Q, Luo Y, Hu X, Yu B. S6 inhibition contributes to isoflurane neurotoxicity in the developing brain. Toxicol Lett. 2015;233:102–113. doi: 10.1016/j.toxlet.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Qiu L, Zhu C, Bodogan T, et al. Acute and long-term effects of brief sevoflurane anesthesia during the early postnatal period in rats. Tox Sci. 2016;149:121–133. doi: 10.1093/toxsci/kfv219. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, Wang Z, Zhou H, et al. Neonatal exposure to sevoflurane may not cause learning and memory deficits and behavioral abnormality in the childhood of Cynomolgus monkeys. Sci Rep. 2015;5 doi: 10.1038/srep11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twaroski D, Bosnjak ZJ, Bai X. MicroRNAs: new players in anesthetic-induced developmental neurotoxicity. Pharm Anal Acta. 2015;6:357–376. doi: 10.4172/2153-2435.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gui L, Lei X, Zuo Z. Decrease of glial cell-derived neurotrophic factor contributes to anesthesia- and surgery-induced learning and memory dysfunction in neonatal rats. J Mol Med. 2017;95:369–379. doi: 10.1007/s00109-017-1521-9. [DOI] [PubMed] [Google Scholar]
- 10.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2019;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–291. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiMaggio C, Sun L, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalkman CJ, Peelen L, Moons KG, et al. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;13:805–812. doi: 10.1097/ALN.0b013e31819c7124. [DOI] [PubMed] [Google Scholar]
- 14.Sprung J, Flick RP, Katusic SL, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakri MH, Ismail EA, Ali MS, Elsedfy GO, Sayed TA, Ibrahim A. Behavioral and emotional effects of repeated general anesthesia in young children. Saudi J Anaesth. 2015;9:161–166. doi: 10.4103/1658-354X.152843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raper J, Alvarado MC, Murphy KL, Baxter MG. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084–1092. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taghon T, Masunga AN, Small RH, Kashou NH. A comparison of functional magnetic resonance imaging findings in children with and without a history of early exposure to general anesthesia. Paediatr Anaesth. 2015;25:239–246. doi: 10.1111/pan.12606. [DOI] [PubMed] [Google Scholar]
- 18.Hansen TG, Pedersen JC, Henneberg SW, et al. Academic performance in adolescence after inguinal hernia repair in infancy A nationwide cohort study. Anesthesiology. 2011;114:1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 19.Yan J, Li Y, Zhang Y, Lu Y, Jiang H. Repeated exposure to anesthetic ketamine can negatively impact neurodevelopment in infants: a prospective preliminary clinical study. J Child Neurol. 2014;29:1333–1338. doi: 10.1177/0883073813517508. [DOI] [PubMed] [Google Scholar]
- 20.Bhutta AT, Schmitz ML, Swearingen C, et al. Ketamin as a neuroprotective and anti-inflammatory agent in children undergoing surgery on cardiopulmonary bypass: a pilot randomized, double-blind, placebo-controlled trial. Pediatr Crit Care Med. 2012;13:328–337. doi: 10.1097/PCC.0b013e31822f18f9. [DOI] [PubMed] [Google Scholar]
- 21.Naguib AN, Winch PD, Tobias JD, et al. Neurodevelopmental outcome after cardiac surgery utilizing cardiopulmonary bypass in children. Saudi J Anaesth. 2015;9:12–18. doi: 10.4103/1658-354X.146255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun LS, Papper EM, Li G, et al. Feasibility and pilot study of the Pediatric Anesthesia NeuroDevelopment Assessment (PANDA) project. J Neurosurg Anesthesiol. 2012;24:382–388. doi: 10.1097/ANA.0b013e31826a0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warner DO, Zaccariello MJ, Katusic SK, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology. 2018;129:89–105. doi: 10.1097/ALN.0000000000002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warner DO, Chelonis JJ, Paule MG, et al. Performance on the operant test battery in young children exposed to procedures requiring general anaesthesia: the MASK study. Br J Anaesth. 2019;122:470–479. doi: 10.1016/j.bja.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson AJ, Disma N, de Graaff JC, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCann ME, de Graaff JC, Dorris L, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 2019;393:664–677. doi: 10.1016/S0140-6736(18)32485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melchers P, Melchers M. Pearson Assessment. Frankfurt/Main: 2016. Kaufman Assessment Battery for Children-II Handbuch der deutschsprachigen Fassung. [Google Scholar]
- 29.Petermann F, Lipsius M, editors. Pearson Assessment. Frankfurt/Main: 2009. Wechsler Preschool and Primary Scale of Intelligence - III (deutsche Version) [Google Scholar]
- 30.Petermann F. Wechsler Intelligence Scale for Children - Fourth Edition (deutsche Version) In: Petermann U, editor. Pearson Assessment. Frankfurt/Main: 2011. [Google Scholar]
- 31.The R Foundation for Statistical Computing. www.R-project.org/ (last accessed on 22 March 2021) Vienna, Austria: The R project for statistical computing. [Google Scholar]
- 32.Backeljauw B, Holland SK, Altaye M, Loepke AW. Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics. 2015;136:e1–e12. doi: 10.1542/peds.2014-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]