Abstract
Immunoengineering continues to revolutionize healthcare, generating new approaches for treating previously intractable diseases, particularly in regard to cancer immunotherapy. In joint diseases, such as osteoarthritis (OA) and rheumatoid arthritis (RA), biomaterials and anti-cytokine treatments have previously been at that forefront of therapeutic innovation. However, while many of the existing anti-cytokine treatments are successful for a subset of patients, these treatments can also pose severe risks, adverse events and off-target effects due to continuous delivery at high dosages or a lack of disease-specific targets. The inadequacy of these current treatments has motivated the development of new immunoengineering strategies that offer a safer and more efficacious alternative therapies through the precise and controlled targeting of specific upstream immune responses, including direct and mechanistically-driven immunoengineering approaches. Advances in the understanding of the immunomodulatory pathways involved in musculoskeletal disease, in combination with the growing emphasis on personalized medicine, stress the need for carefully considering the delivery strategies and therapeutic targets when designing therapeutics to better treat RA and OA. Here, we focus on recent advances in biomaterial and cell-based immunomodulation, in combination with genetic engineering, for therapeutic applications in joint diseases. The application of immunoengineering principles to the study of joint disease will not only help to elucidate the mechanisms of disease pathogenesis but will also generate novel disease-specific therapeutics by harnessing cellular and biomaterial responses.
Keywords: cartilage, synovium, drug delivery, scaffolds, cytokine, gene therapy, stem cell therapy, CRISPR-Cas9, orthobiologics
1. Introduction
Mounting evidence throughout the past decade suggests the immune system plays a critical role in the initiation and progression of numerous chronic diseases. An improved understanding of immune mediators involved in these processes offers clues toward treating historically intractable musculoskeletal diseases, such as osteoarthritis, that have been traditionally attributed to mechanical injuries or overuse. The application of recent technological advances (e.g., Next-Generation Sequencing) to link immunological processes to musculoskeletal pathologies has enabled detailed investigation into the molecular pathways and targets that regulate the immune response in disease pathogenesis. Importantly, this advanced understanding of signaling pathways involved in musculoskeletal disease will contribute to the development of more precise and sophisticated immunomodulatory technologies for treating these chronic diseases. In this review, we focus on the development of recent advances in biomaterial and cell-based immunomodulation for the design of specialized therapeutics to treat precise molecular targets involved in joint diseases. In this regard, the future of arthritis therapy may lie in the application of immunoengineering strategies – that is the targeted modulation of key pathways in immune cell activation – to harness cellular responses as novel disease-specific therapies.
Arthritis affects around 23% of adults, or 54 million people, and is the leading cause of work disability in the United States [1]. Osteoarthritis (OA) and rheumatoid arthritis (RA), two of the most common forms of arthritis, are chronic musculoskeletal disorders of diarthrodial joints and are both associated with chronic pain and reduced joint mobility [2]. In OA and RA, age, sex, and genetic predisposition are understood to be major risk factors for disease development [3, 4]. Environmental, biomechanical, and biochemical factors are also associated with OA development [4, 5]. While OA and RA both affect the synovial joint, their etiologies and pathogeneses are distinct. OA is traditionally characterized by cartilage breakdown as well as pathologic changes affecting the entire joint organ system. These changes include inflammation of the synovial membrane, structural changes in the meniscus and ligaments, subchondral bone remodeling and osteophyte formation due to cell proliferation and enhanced matrix remodeling, and increased expression of catabolic factors driving loss of extracellular matrix components as well as chondrocyte hypertrophy and terminal differentiation [6–8]. Increasing evidence highlights the role of low-grade inflammation as a key factor in the initiation and progression of OA pathogenesis in conjunction with biomechanical alterations within the joint [9]. Furthermore, pro-inflammatory mediators and cytokines in synovial fluid of OA joints stimulate degradation of cartilage and inhibit matrix synthesis [10]. Taken together, OA is a complex disease of multifactorial etiopathogenesis, involving both local and systemic factors that must be taken into consideration in the development of new therapeutic approaches. RA primarily involves morphological changes of the synovial membrane, bone tissue destruction, and subsequently, cartilage damage. However, unlike OA, RA is a chronic autoimmune disease characterized by dynamic, episodic flares of systemic inflammation, often affecting the synovial membrane in multiple joints [11–14].
The morphology and cellular composition of the synovium are often used as biomarkers of arthritis progression; indeed, while early stages may present with little to no histological effects, many late-stage OA and RA patients possess significant synovitis, which has led to the importance of the synovium as a potential immunological target [15]. In a healthy joint, the synovium is made up of a variety of cells grouped into two layers: (1) an intimal lining only one to two cells thick comprised of macrophages, fibroblast stromal cells, and synoviocytes, and (2) a distinct synovial sublining consisting of various connective tissue and blood vessels [16]. With joint inflammation, the intimal lining layer becomes hypertrophic, infiltrated with T cells, dendritic cells, macrophages, fibroblasts, mast cells, neutrophils, and B cells, increasing the cellular density of the lining 5–10 times [17]. These infiltrating cells alter the synovial fluid composition by promoting the aberrant production of pro-inflammatory cytokines and catabolic products, a prominent source of these pro-inflammatory mediators in OA [14]. The composition of inflammatory cells in OA, differing considerably from that observed in RA synovial tissues, possesses a lower abundance of macrophages and T cells but a higher percentage of mast cells, creating unique cytokine expression profiles between the two pathologies [18–20]. Therefore, using immunoengineering strategies to target the distinct cells and signaling cascades that drive disease-specific inflammatory progression is critical to understand disease pathologies and design effective and specialized arthritis treatment approaches for OA and RA.
2. Immune regulation in arthritis
Pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1α, IL-1β), IL-6, and IL-17 have all been implicated in the pathogenesis of OA and RA (Figure 1) [18, 21–24]. Secretion of these factors is initiated by resident cells such as chondrocytes, synoviocytes, and immune cells and acts to amplify the inflammatory environment of arthritis. This secretion can be stimulated by inflammatory triggers (e.g., cartilage damage (OA) or flares (RA)) in which affected cells release initiating signals (e.g., damage-associated molecular patterns) that stimulate these resident cell populations to release various chemokines and cytokines (e.g., IL-8, IL-17, and monocyte chemoattractant protein-1/CCL2), initiating an early influx of activated neutrophils and monocytes that home to the joint [25, 26]. Recruited immune cells then act to secrete pro-inflammatory mediators (e.g., TNF-α, IL-1α, IL-1β, and IL-6). These cytokines not only facilitate the recruitment of further immune cells (e.g., additional macrophages and effector T cells) into the joint, but also increase the release of inflammatory cytokines and matrix metalloproteinases (MMP) [27]. This damaging cycle, in which cells residing within the joint produce cytokines and proteolytic enzymes that further induce inflammation and immune cell infiltration, ultimately increases the duration and severity of disease.
Figure 1.
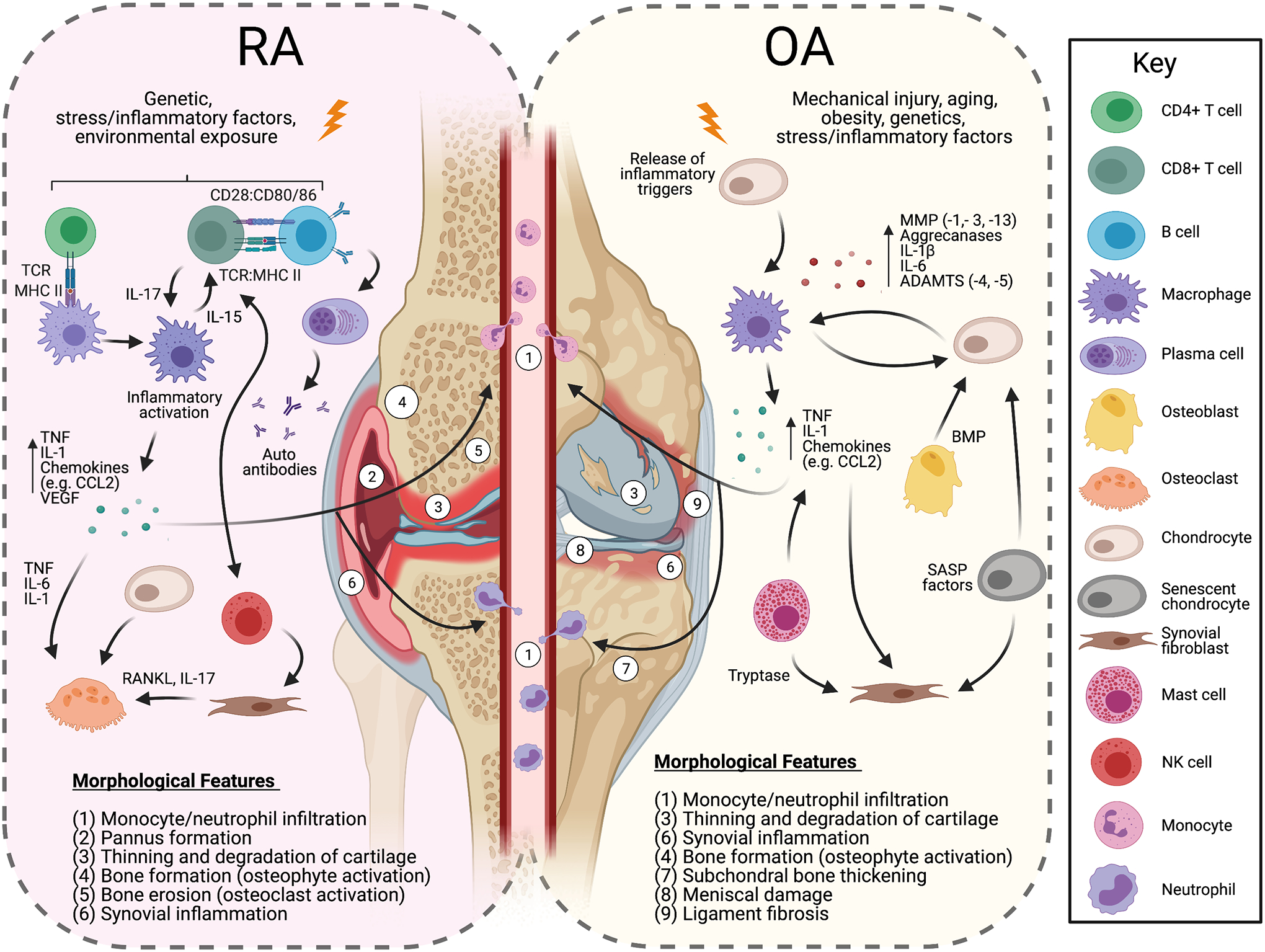
Initiation of OA and RA cellular pathways in early-stage disease. Immunological signals propagate as the disease progresses to all components of the joint organ system. The key cytokine pathways targeted in current OA and RA therapies primarily include TNF, IL-1, and IL-6, although other cytokines (e.g., IL-17) are now being targeted as well. Abbreviations: TCR: Toll-like receptor; MHC: Major Histocompatibility Complex; MMP: Matrix metallopeptidase; ADAMTS: A disintegrin and metalloproteinase with a thrombospondin type; TNF: Tumor necrosis factor; CCL2: C-C Motif Chemokine Ligand 2; VEGF: Vascular endothelial growth factor; BMP: Bone morphogenetic proteins; SASP: senescence-associated secretory phenotype; RANKL: Receptor activator of nuclear factor kappa-B ligand
While many pro-inflammatory mediators contribute to OA and RA, each disease has distinct factors associated with specific pathogenic events. In OA, TNF-α and IL-1 generated by resident chondrocytes and synovial cells are believed to be the prominent cytokines that contribute to disease propagation. IL-1 signaling stimulates chondrocyte production of MMPs (e.g., MMP-1, -3, and -13) and aggrecanases (e.g., disintegrin and metalloproteinase with thrombospondin motifs; ADAMTS-4 and -5) [28, 29]. IL-1 also acts to suppresses proteoglycan and type II collagen synthesis while promoting increased production of reactive oxygen species factors like nitric oxide [28]. TNF-α has been shown to have a similar and synergistic effect as IL-1 and increases the macrophage production of IL-6 and IL-8 as well as CCL2 and other various chemokines [30]. In contrast to OA, RA initiation involves signaling by further inflammatory cytokines in addition to IL-1 and TNF-α, including IL-6, IL-17, IL-23. In RA, IL-6 has numerous roles including stimulating pannus formation through increased vascular endothelial growth factor expression and altering the differentiation of monocytes to macrophages through the upregulation of the macrophage colony-stimulating factor (M-CSF) receptor [31]. TNF-α, IL-6, and IL-1 act to amplify osteoclast functions, while IL-23 induces the expansion and activation of T helper 17 (Th17) cells that act on macrophages to maintain their pro-inflammatory state and perpetuate inflammation [17, 32]. IL-17 also acts on fibroblast-like synoviocytes and other cells to increase the production and activity of other pro-inflammatory cytokines [23]. Though OA and RA inflammatory signaling involve many similar pathways and factors, OA-related inflammation is distinct from RA-related inflammation, as illustrated by the differences in cytokine profiles described above. This distinction is further underscored by the failure of current RA treatments to modulate OA disease progression as well as key differences observed between OA and RA in genome-wide association studies, in vitro studies, and ex-vivo tissue analysis [33]. Recent and continuing research to investigate the immunological mediators specific to each disease will likely prove necessary for developing effective and disease-specific treatments [9, 34].
3. Current anti-inflammatory/anti-cytokine therapies
Over the last decade, the refined identification of immune regulation in OA and RA has stimulated interest towards designing therapies to focus on modulating the immune response to treat these diseases. Current immunomodulatory therapies have focused on reducing pain and inflammation through either broad anti-inflammatory suppression (e.g., nonsteroidal anti-inflammatory drugs (NSAIDs)) or disease-modifying antirheumatic drugs (DMARDs), such as methotrexate. However, these treatments have offered limited efficacy for disease-specific immunoregulation and long-term clinical application significantly increases risks for a wide range of complications (e.g., infection, lupus, interstitial lung disease), as well as impeding tissue regeneration and repair [34–38]. In arthritis, broadly acting non-specific therapeutics aid in symptom management for patients: anti-inflammatory pharmaceuticals, such as NSAIDs, cyclooxygenase (COX)-2 inhibitors, and corticosteroids, are among the most widely prescribed and recommended classes of therapeutics for addressing symptomatic arthritis [5, 39–41]. For example, Celecoxib, a selective COX-2 inhibitor, is widely used in OA treatment and affects all intra-articular tissues of OA pathogenesis: cartilage, bone, and synovium [40, 42, 43]. DMARDs can help slow the progression of arthritis, but often need to be given in conjunction with broader anti-inflammatory drugs for symptom management in RA [44]. Within the joint, these anti-inflammatory drugs non-specifically interact with various cells, including chondrocytes, pro-inflammatory macrophages, and activated synoviocytes, thereby mitigating joint inflammation. However, the widespread distribution of these drugs by systemic administration in combination with short therapeutic half-life, results in the need for high and frequent dosing schedules which are necessary to maintain a sufficient therapeutic concentration at the site of action within the joint. This dosing approach can result in deleterious side effects (e.g., immunosuppressive, renal, hepatic, gastrointestinal, cardiovascular) as well as high treatment cost [45]. In attempts to reduce the risk of long-term use and lack of specificity of systemic treatments, shorter-lived intra-articular therapies such as hyaluronans, corticosteroids, such as prednisolone, and glucocorticoids, such as triamcinolone hexacetonide and methylprednisolone acetate, have been administered to reduce joint pain and swelling, and restore joint function [2, 46, 47]. While these treatments effectively reduce inflammation and symptoms, complications also arise due to the high risk of infection, joint disability, intolerance in patients, and the need for frequent injections which puts financial burden on the patient. Furthermore, many of these treatments do not take into consideration the synergistic roles that different pathways (RA) and mechanical and inflammatory factors (OA) play in disease propagation.
In contrast to medications for generalized inflammatory suppression, the recent development of biologic DMARDs has allowed the more specific targeting of precise cytokines. A number of therapies targeting IL-1 and TNF-α signaling have been explored for RA patients, including the delivery of protein-based cytokine antagonists. TNF-α targeting therapies have been used successfully to treat patients with RA for many years and include anti-TNF monoclonal antibodies (e.g., infliximab, adalimumab, certolizumab, and golimumab) and soluble TNF receptor fusion proteins (e.g., sTNFR1 and etanercept) [48–50]. These various anti-TNF treatments act to: neutralize extracellular-, transmembrane-, and receptor-bound TNF, induce lymphocyte/monocyte apoptosis, reduce infiltrating granulocytes and macrophages, lower inflammation through the downregulation of chemokines and cytokines (e.g., IL-1-α, IL-1β, IL-6, and CCL2) in the synovium, and upregulate transforming growth factor-beta (TGF-β) which stimulates regulatory T cells to suppress pro-inflammatory macrophage [51, 52]. Similarly, protein-based anti-IL-1 therapies have also been developed, such as IL-1 receptor antagonist (IL-1Ra, anakinra) and human monoclonal antibodies (e.g., canakinumab/ILARIS that targets IL-1β) [53–55]. However, systemic inhibition of IL-1 signaling with anti-IL-1 antibodies or IL-1Ra provides only modest anti-inflammatory effects at the joint level and has generally failed to show disease modification [56]. Both TNF-α and IL-1 biologics have some efficacy in patients with RA and have been shown to relieve pain and reduce synovitis [57, 58]. However, as with previously mentioned anti-inflammatory therapies, these biologics are administered at highly immunosuppressive doses in order to obtain therapeutic levels at the pathological site, further suggesting that immunoengineering approaches to deliver therapeutic agents in an on-demand manner may prove beneficial for effectively treating RA with fewer systemic side-effects.
Despite limitations to current clinical therapies, the potential for IL-1 and TNF-α antagonists to block their targets and successfully resolve inflammation in RA are well appreciated, and many RA patients demonstrate positive clinical outcomes; unfortunately, the therapeutic efficacy of these same protein cytokine blockers in OA has been minimal owing to the distinct immunological profiles of the two diseases, as well as difficulties in sustained drug delivery [50, 59]. In particular, while both diseases may involve many of the same cytokines and pathways, their etiologies and effecter cells are unique. Indeed, next generation sequencing continues to identify distinctive biomarkers and molecular targets, including initiating factors between RA and OA. Numerous studies by our lab and others have shown that targeting immune signals important in disease initiation (e.g., adipokines and mechanically sensitive ion channels (e.g., TRPV4) in OA; fibroblast-like synoviocytes and specific macrophage and T cell subsets in RA) can help ameliorate disease. These observations, combined with the lack of precision afforded by systemic drug delivery and adverse events present with current therapies, have motivated research examining suitable targets and methods for prolonged and targeted drug delivery to treat OA and RA as discrete diseases [10, 54, 60, 61].
4. Immunoengineering strategies for arthritis
To develop novel treatments targeting the specific mechanisms involved in OA and RA inflammation, investigators have evaluated cellular and biomaterial delivery strategies as a means to deliver therapeutics in recent years (Figure 2). While the ultimate goal, to design selective and effective anti-inflammatory therapies, remains the same for OA and RA, the differences in disease initiation and inflammatory propagation are essential factors to consider when assessing a biomaterial, cellular, or genetic engineering-based approach. Indeed, as we develop a better understanding of how the pathophysiology diverges in different types of arthritis, therapeutic strategies have begun to combine these approaches for deploying targeted and long-lasting treatments, a strategy likely to continue with advances in biomaterials and genetic engineering technologies. Below, we highlight a selection of promising current and emerging biomaterial, cellular, and genetic immunoengineering strategies for arthritis treatment.
Figure 2.
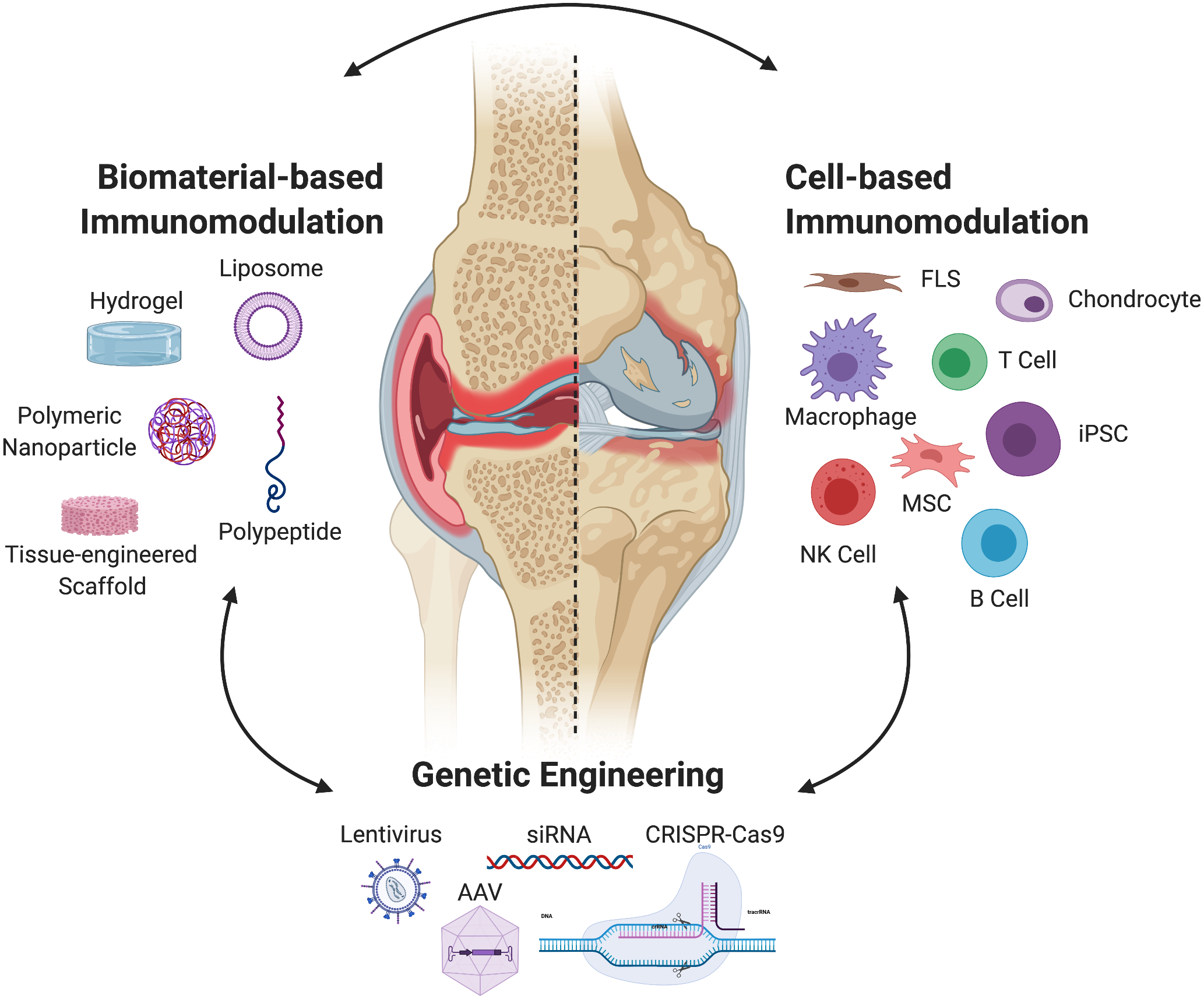
Current immunomodulatory strategies rely on three types of approaches: (1) the exogenous delivery of autologous or allogenic cells, (2) genetic engineering or gene therapy to alter resident and exogenous cell populations, or (3) biomaterial-based systems that act by themselves as immunomodulators as well as aid in the delivery of cells or material for genetic engineering of resident cell populations. While each of these strategies may be used on their own, many immunoengineering approaches combine their use for the design of more controlled and precise treatment. Abbreviations: FLS: Fibroblast-like synoviocytes; MSC: Mesenchymal stem cells; iPSC: Induced pluripotent stem cell; NK: Natural killer cell; CRISPR-Cas9: Clustered regularly interspaced short palindromic repeats, (CRISPR)-associated protein 9; siRNA; small interfering RNA; AAV: adeno-associated virus.
4.1. Biomaterial-based systems
Biomaterial-based immunomodulation offers a wide range of therapeutic potential. These strategies are able not only to limit adverse side effects by more efficiently and specifically delivering drugs, but also can actively target and immunomodulate resident cell populations to achieve disease-specific treatment. In this section, we will discuss promising biomaterial-based systems (Figure 2) for immunomodulation that have been examined for use in OA and RA therapy using synthetic and natural materials in combination with genetic engineering strategies (Table 1).
Table 1.
Highlights of emerging biomaterial-based strategies for immunoengineering in arthritis.
| Reference | |||||
|---|---|---|---|---|---|
| Synthetic | |||||
| Poly (lactic-co-glycolic acid) | Microsphere; triamcinolone acetonide | Macrophage | OA, clinical | [62] | |
| (Poly)glycolic (PEG) | Nanoparticle; release of TNF-α or IL-1 blocking antibodies | Fibroblast-like synoviocytes | RA, in vitro | [63] | |
| Nanoparticle, paclitaxel | No specific target | In vitro, rats | [64] | ||
| Scaffold; delivery of bound IL-4 | Macrophage | RA, mouse | [65] | ||
| Hydrogel; dexamethasone | Cartilage, osteophyte | PTOA, mouse | [66] | ||
| PEG-PCL | Nanoparticle; release of Methotrexate | Synovial macrophages | RA, rats | [67] | |
| Natural | |||||
| Chitosan | Scaffold | Neutrophils and macrophages | OA, rabbit; OA, clinical | [68, 69] | |
| Nanosphere; kartogenin and diclofenac | Chondrocytes and macrophage | OA, rats | [70] | ||
| Elastin-like polypeptides | Biopolymer; non-aggregating and aggregating ELP | Whole joint | Knee (OA), rat | [71] | |
| Biopolymer, sTNFRII or -IL1Ra | Fibrochondrocytes, thymocytes, and lymphocytes | Intervertebral disc, in vitro | [72] | ||
| Biopolymer; loaded with IL-1Ra or sTNFRII | Whole joint | PTA (articular fracture), mouse | [37] | ||
| Type II collagen | Scaffold; squid type II collagen | Macrophage | OA, rat | [73] | |
|
Albumin/
melittin |
Nanoparticle; delivery of siRNA for NF-κB inhibition | Innate and adaptive immune cells | RA and PTOA, in vivo | [74–77] | |
| Lipids | Nanoparticle; delivery of siRNA for Indian Hedgehog | Whole joint | OA, rat | [78] | |
| Agarose | Hydrogel | Monocyte | OA | [79] | |
| Silk fibroin | Scaffold | MSC/T cell | OA, rabbit | [80] | |
| Hyaluronic acid/silk fibroin | Hydrogel; vanillic acid or epimedin C | Chondrocytes | Cartilage engineering | [81] | |
| Combined | |||||
| Heparan/PEG | Microparticles; tumor necrosis factor-alpha stimulated gene-6 | Leukocytes, neutrophils, macrophages, MSCs | In vitro; OA, rat | [82] | |
| Gellan gum/silk fibroin | Hydrogel; betamethasone | Chondrocyte | RA, in vitro | [83] |
4.1.1. Biomaterial-based drug delivery for immunomodulation
Biomaterial-based strategies for sustained drug release and local drug delivery have been developed for many years to overcome poor drug targeting and subsequent low drug availability within arthritic joints [12, 84]. Many synthetic and natural polymers have been formulated to provide sustained drug release, including (poly)glycolic or (poly)lactic acid, chitosan, heparan, silk fibroin, and elastin-like polypeptides [84]. Whether prepared as an injectable drug depot with a conjugated or entrapped drug, or as injectable micro- or nano-particle formulations, these materials sequester drugs as a means to provide local and targeted therapeutic delivery over a long-lived duration, obviating the deleterious systemic side effects of clinical anti-inflammatory application (e.g., immunosuppression). These vehicles can be developed according to therapeutically relevant specifications (e.g., surface structure, charge, protein adhesion, and pH modifiers) to target cells relevant to OA or RA specific pathways as well as generally enhance the delivery potential of more limited therapeutics [84, 85].
For example, L-carnitine-conjugated nanoparticles with a polyethylene glycol (PEG) linker enhance oral delivery and increase cellular uptake [64]. In several studies, these delivery methods have been used to aid in the delivery and release of TNF-α or IL-1 inhibitors as well as other drugs. One promising therapeutic approach for delivering etanercept has been in a copolymer-microsphere material [63]. These microspheres delivered etanercept for upwards of 90 days, suggesting a long-lived therapy that may avoid the systemic side-effects associated with the clinical anti-TNF-α injections. Culturing microspheres with fibroblast-like synoviocytes led to a moderate reduction in cytokine and MMP production, demonstrating etanercept bio-functionality after release from the biomaterial [63]. Another study illustrated the role PEG-poly lactic-co-glycolic acid-folic acid tagged nanocarriers which encapsulated the RA drug Methotrexate to target synovial macrophages in RA. In rats with adjuvant-induced arthritis, these nanocarriers exhibited superior cellular uptake and cytotoxicity in these macrophages and led to a significant decrease in paw thickness and clinical score in comparison to those treated with free methotrexate [67]. Optimizing these formulations may provide further increases in anti-inflammatory potential while limiting overall systemic effects.
Depots or microparticles based on protein biomaterials have also been developed that allow functionalization or entrapment of protein drug without the use of harsh solvents or conditions needed for microparticle encapsulation. For example, elastin-like polypeptides (ELP) have been developed as injectable drug depots for the local and sustained release of protein drugs in body cavities, including the joint space for arthritis treatment [71]. ELPs are thermally responsive pentapeptide sequences from native human elastin with biocompatible and non-immunogenic properties and have been used successfully in the delivery of recombinant protein drugs such as ELP-sTNFRII or ELP-IL1Ra [72]. In pre-clinical work, this approach has been shown to provide prolonged drug bioactivity at the targeted site to treat neuroinflammation and joint post-traumatic arthritis [37]. This work illustrates the potential for protein-based drug carriers to support the delivery of large molecule drugs and may also reduce the high costs and high drug doses required for systemic administration.
In other approaches, biomaterial-based drug delivery has been combined with the specificity offered by genetic targeting to inhibit the catabolic activity within inflamed joints. Recent studies have applied a nanoparticle-based system for the delivery of targeted small interfering RNA (siRNA) to hinder OA and RA progression [75–78]. siRNAs specifically target messenger RNA (mRNA) transcripts, inducing the degradation of the targeted mRNA, and reducing protein translation. Combined delivery of siRNA using nanoparticles to specifically knock down the p65 subunit of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) complex successfully inhibited the onset of joint-wide inflammation in both RA and post-traumatic OA (PTOA) murine models of arthritis [76]. Further studies in the mitigation of obesity-related inflammation demonstrated the successful disruption of the gene of interest in neutrophils, which are known to antagonize OA onset and progression [86]. Together, these studies provide promise that long-lived targeting of specific molecular pathways in the initiation and progression of arthritis may be clinically targetable in the near future using advanced biomaterial delivery approaches [7, 87].
4.1.2. Immunomodulatory properties of biomaterial scaffolds
Treatment of the arthritic joint through intrinsic properties of biomaterial scaffolds is another attractive avenue for immunomodulation due to the inflammation-mediated tissue degradation present in arthritis, particularly in OA which is characterized by degradation of articular cartilage. As cartilage possesses a limited capacity for regeneration due to its low cellularity and lack of vasculature, biomaterial scaffold and tissue engineering strategies have been studied to replace degenerative cartilage with biomimetics that recapitulate the functional properties of native tissue [4, 88]. Strategies employing immunomodulation have capitalized on this approach by engineering biomaterial scaffolds to promote a pro-regenerative or anti-inflammatory environment within the joint. In this manner, investigations on how scaffolds remediate the arthritic joint now frequently assess the immunologic characteristics distinct to OA or RA that are impacted by the scaffolds. Type II collagen, the predominant extracellular matrix protein of articular cartilage, has been widely researched as a promising biomimetic scaffold [89]. New research found that isolating type II collagen from the squid provides an immunomodulatory scaffold, supportive of pro-regenerative anti-inflammatory macrophages [73]. Additionally, in vitro and in vivo, scaffold-polarized anti-inflammatory macrophages upregulate the expression of TGF-β, suggesting a mechanism to enhance matrix regeneration by chondrocytes within the joint [90].
Scaffolds can also serve to program immune cells as they migrate into and within the joint. Clinically, a technique called microfracture is commonly used a method to enhance cartilage repair [68]. In microfracture, damaged cartilage tissue is removed and holes are created in the subchondral bone, allowing blood from the bone marrow to pool and clot within the cartilage defect. While effectively filling in the cartilage defect after a repair process, microfracture repairs are less resilient to mechanical loads and differ in biochemical composition from native articular cartilage. To improve this microfracture repair tissue, a chitosan-glycerol phosphate scaffold was used to support the microfracture legion repair in a rabbit OA model [68]. The scaffold-reinforced defects, with the chemoattractive properties of chitosan, resulted in an increased recruitment of neutrophils and alternatively activated pro-regenerative macrophages. Clinical trials based on these scaffolds mixed with whole blood were able to create a stable and quickly solidifying implant for aiding in the microfracture repair processes [69]. This represents an interesting paradigm for incorporating treatment of both mechanical and inflammation factors of OA within the confines of standard clinical management.
In addition to biomaterial drug delivery and therapeutic-scaffold delivery, recent developments have highlighted the role of biomaterials as dynamic regulators for immunomodulation. As the pharmacokinetic features of anti-inflammatory cytokines have often led to problems of administration, one approach involved the development murine IL-4 that was bound to a PEG or PEG-folate conjugate. These conjugates were able to not only successfully travel to the joint, but also induce primary murine macrophage polarization and increase IL-4 terminal half-life in an antigen-induced arthritis mouse model [65]. In an effort to develop an activatable immunomodulatory biomaterial, a different group utilized a protease-cleavable PEG-based biomaterial with the potential to deliver dexamethasone corticosteroid therapeutics over extended durations and at high doses, using a “mechanical pillow” biomaterial. The mechanical pillow decreased mechanical load within the joint to reduce symptoms of PTOA in a mouse model, highlighting the role biomaterial scaffolds may play in OA immunomodulation through maintaining homeostatic loads within the joint [66]. Indeed, in similar mechanical loading environments, monocytes encapsulated in an agarose biomaterial were less responsive to soluble inflammatory mediators than unloaded controls, demonstrating that biomaterials may serve a key role in programming immune cells in OA [79]. Similarly, recent results on macrophage polarization depict a clear role of mechanical factors and constraint in the modulation of macrophage phenotype towards a pro-regenerative phenotype [91]. Together, these results suggest a potential direction of biomaterial development for the specific immunoengineering of synergistic mechanical and anti-inflammatory approaches for OA treatment.
4.2. Cell-based systems
While biomaterial-based strategies can address some limitations in current therapies, they are highly reliant on resident cell populations. In OA, which increased in prevalence with aging, or in PTOA or RA, where the resident cell populations may be altered by the traumatic/inflammatory nature of the disease, tissue engineering and gene therapy approaches can be utilized to create cellular-immunomodulators capable of replacing damaged tissue while delivering therapeutic drugs to diseased joints. However, while cell-based therapies are now being tested clinically, both in combination with biomaterials and on their own, delivery and success in the area of treatment are still ongoing challenges due to a variety of factors, including age, diet, sex, infection, comorbidities [92].
As the joint is a source of a heterogeneous group of cells, potential directions of OA- and RA-specific anti-inflammatory components have been studied in many different cell types, including mesenchymal stem cells, macrophages, chondrocytes, fibroblast-like synoviocytes, T cells, natural killer cells, B cells, and induced pluripotent stem cells (iPSCs) (Figure 2 and Table 2).
Table 2.
Highlights of emerging cell-based strategies for immunoengineering in arthritis.
| Reference | |||||
|---|---|---|---|---|---|
| MSCs | |||||
| Human bone marrow | Lentiviral; doxycycline-inducible IL-1Ra | In vitro | OA | [93] | |
| Equine bone marrow | Lentiviral; NF-κB-responsive IL-1Ra | In vitro | OA | [94, Gabner, 2018 #103, 95] | |
| Human bone marrow | Lentiviral; doxycycline-inducible GRASLND; CRISPR-dCas9, GRASLND | In vitro | OA, musculoskeletal disease | [96] | |
| Rat bone marrow | Lentivirus; heat shock protein 27 | In vitro; direct injection | Myocardial infarction | [97] | |
| Human bone marrow | Lipofectamine; mcTNFR2 | In vitro; intraperitoneal | CIA | [98] | |
| Human bone marrow | Adenovirus; IFN-β | In vitro; Intramuscularly (mice) | Cancer | [99] | |
| Baboon bone marrow | Erythropoietin | Intramuscularly (mice); implanted device (baboons) | N/A | [100] | |
| Macrophage | |||||
| Resident | Clodronate liposomes | Tail, back | CIA | [32] | |
| Resident | MaFIA transgenic mouse; AP20187 molecule | Water | OA | [101] | |
| Resident | Nanoparticle; IL-10 plasmid DNA | Intraperitoneal | RA | [102] | |
| Mouse/human bone marrow | Drug-loaded nanoparticles | - | OA | [103, 104] | |
| RAW 264.7, THP1, IC21 cell lines | siRNA (Luc, GFP, calcium integrin binding protein-1) | In vitro | Cancer | [105] | |
| Murine bone marrow | IFN-γ loaded backpack | In vitro; intra-tumoral | Cancer | [106] | |
| Mouse adipose tissue | Knock down, Receptor Interacting Protein 140 | Intraperitoneal | Obesity | [107] | |
| FLS and fibroblasts | |||||
| Immortalized embryonic DBA/1 fibroblasts | Retroviral; IFN-β and AAV5; TNF-α | Intraperitoneal | CIA | [108] | |
| Human synovial biopsies | AAV5; chimeric human TNF soluble receptor I | Intra-articular | RA | [109] | |
| Joint tissue; human, rhesus monkeys, rodents and rabbits | AAV5; NF-κB driven IFN-β | In vitro; intra-articular | RA | [110] | |
| Human and equine capsular tissue | AAV (2,5, and 8); IL-1Ra | In vitro | OA | [111] | |
| Chondrocyte | |||||
| Pigs femur | PTGS2 responsive IL-1 | In vitro | OA | [112] | |
| Human cartilage | AAV; IL-1Ra | In vitro | OA | [113] | |
| AAV (2,5, and 8); IL-1Ra | In vitro | OA | [111] | ||
| B cells | |||||
| Human primary blood | Nanoparticles; CRISPR; gB220 | In vitro | RA | [114] | |
| Human peripheral blood | CRISPR; CD19 | In vitro | N/A | [115] | |
| Mouse primary | CRISPR; many candidates | In vitro | N/A | [116] | |
| T cells | |||||
| Human T cells | CAR T; urokinase-type plasminogen activator receptor | Intravenous | Lung adenocarcinoma and liver fibrosis | [117] | |
| Human T cells | CAR T; citrullinated peptide epitopes | In vitro | RA | [118] | |
| iPSC | |||||
| Mouse | CRISPR; NF-κB driven IL-1Ra | In vitro | OA | [119] | |
| Mouse | CRISPR; CCL2 driven IL-1Ra or sTNFR1 | In vitro; implant | OA and RA | [74, 120] | |
| Mouse | Retroviral; FoxP3 | In vitro; tail vein | RA | [121] |
4.2.1. Mesenchymal stem cells (MSCs)
Mesenchymal stem cells (MSCs), multipotent progenitor cells that originate from the mesoderm, are found in many musculoskeletal tissues including bone marrow, adipose tissue, synovium, and infrapatellar fat pad. With their capacity to differentiate into cartilage, bone, or muscle, MSCs have been extensively studied in immunoregulation, as well as in organ regeneration, anti-tumor effects, and neuroprotection [122]. The extensive display of MSC immunological properties enhances their therapeutic potential in tissue regeneration [123]. MSCs have been shown to exhibit immunomodulatory effects via a number of secreted factors (e.g., TGF-β, prostaglandin E2, HLA-G, IDO, IL-10, CCL2) [124]. Additionally, MSCs serve as promising candidates for immunomodulation and tissue regeneration due to their high proliferation rates and homing abilities illustrated by proven multi-tissue migration after intravenous injection [125, 126]. However, the factors driving MSC homing and engraftment, as well as the underlying mechanisms behind MSCs immunomodulatory effects, are not yet completely understood.
Despite their potential as immunological regulators, current clinical use of MSCs has been limited, with few phase 1 dose-determining clinical trials progressing to phase 3 [127]. Studies suggest this systemic failure might be due in part to mixed proliferation and survival rates as well as the lack of standardization of evaluation between trials in the clinic [97, 128]. Current techniques utilizing genetic engineering have been proposed to overcome those limitations. MSCs are commonly used in gene therapy via approaches including physical (electroporation) and chemical (liposomes) non-viral gene delivery methods, viral-based vectors (e.g., retrovirus, lentivirus, adenovirus, or adeno-associated virus) and CRISPR-Cas9 genome engineering [129, 130]. In this regard, several methods of improving MSC immunomodulatory abilities have recently been proposed.
For example, scaffolds seeded with MSCs transduced with lentivirus to express IL-1Ra in the presence of the exogenous doxycycline supplementation for resurfacing degenerated articular cartilage were resistant to severe inflammation at concentrations up to 1 ng/mL IL-1α administered in vitro for up to 27 days, suggesting the strong immunomodulatory potential of MSCs when combined with biomaterial and engineered cellular approaches [93]. In other studies, equine bone marrow derived MSCs lentivirally-transduced with an expression cassette that uses NF-κB signaling to drive IL-1Ra expression, respond to pro-inflammatory TNF-α stimulation by producing the anti-inflammatory drug IL-1Ra [94, 95]. Engineered MSCs in those studies were able to produce biologically active IL‐1Ra protein and mitigate in an in vitro model of OA. Other methods proposed improving MSC immunomodulation of disease pathogenesis using adeno-associated viruses (AAV) or CRISPR-Cas9 genome engineering, which have proven to be beneficial in improving MSC chondrogenic potential and matrix production [96]. Finally, MSCs transfected with recombinant minicircles encoding etanercept were able to serve as an alternative method for the delivery of biologics [98]. While not currently used to treat OA or RA, engineered MSCs have been shown to mediate inflammation in a wide range of studies through the use of systems including mRNA transfection, delivery vehicles, and immunomodulators [99, 100, 124, 131]. With the proven capacity of MSCs for both effectiveness in immunomodulation and response to precise genetic manipulation, immunoengineering of MSCs offers great potential to improving the treatment of OA and RA.
4.2.2. Macrophages
Macrophages are highly plastic immune cells that act as potent cytokine and chemokine producers in the inflamed joint. Macrophages are strongly regulated by local environmental cues such as the presence of damaged cells, microbial products, pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6, immunomodulatory cytokines such as IL-4 and IL-10, or mechanical forces [132, 133]. Through these cues macrophages polarize into specifically characterized phenotypes (e.g., pro-inflammatory or immunomodulatory) [134–138]. Macrophages have long been used as immunomodulators or as targets for immunotherapies focused on altering selective macrophage functions or polarization. Studies have examined the removal of macrophages from the joint as they are one of the key modulators of aberrant inflammation. Intra-articular injection of clodronate liposomes has been a commonly applied method to ablate macrophages; its use to target synovial macrophages has shown to decrease expression of MMP-3 and MMP-9 as well as adhesion molecules, ICAM-1 and VCAM-1 [32, 139]. However, systemic depletion of macrophages two weeks prior to destabilization of the medial meniscus in obese Fas-induced apoptosis mice was not sufficient to attenuate OA, and resulted in the migration of CD3+ T cells and neutrophils into the injured joint [101]. Taken together, these studies suggest targeting macrophages has the potential to ameliorate arthritis, but therapeutic efficacy of this approach will require the targeting of precise macrophage populations within the joint. To this end, groups have focused on genetic engineering resident macrophage populations; non-condensing alginate nanoparticles containing IL-10 plasmid DNA were shown to localize to the paws of arthritic rats, successfully reprogram synovial macrophage to an anti-inflammatory phenotype (i.e. reduced pro-inflammatory cytokine expression), and prevent joint damage [102]. Through each of these studies, altering resident macrophage presence as well as activation in OA and RA has met with promising results; as more becomes known about the polarization states and origins of macrophage populations in arthritis, better targets can be designed and examined for therapeutic benefit.
Capitalizing on the intrinsic homing ability of macrophages to migrate to injury or inflammatory sites, including arthritic joints, macrophages are now being used as drug delivery vehicles for prolonged and localized drug delivery. Typical methods include delivery of macrophage possessing a biomaterial encapsulated drug either within the cell or “backpacked” on its exterior. Nanoparticle-encapsulated drugs inside autologous pro-inflammatory macrophages have been shown to stimulate transient phagosome maturation arrest [104]. Additionally, macrophages with attached “backpacks” or biomaterial cytokine reservoirs have been delivered successfully to induce antitumor cell phenotypes in vitro and in vivo up to five days [106]. Also encouraging is a recent study demonstrating the ability of macrophages to horizontally transfer loaded siRNAs to cancer cells, a promising advance towards direct genome targeting therapeutics to diseased tissues of the joint through macrophage homing [105]. Injecting engineered macrophages is also becoming a promising therapy for use in arthritis, one group showing that local injection of anti-inflammatory macrophages induced white adipose tissue browning and other protective effects on low-grade inflammation caused by obesity in OA [107]. These studies all point to the importance and therapeutic potential of macrophage-based immunoengineering for OA and RA treatments.
4.2.3. Immunoengineering of other resident or transplanted cells
As a more precise method of RA and OA treatment, cell populations important in disease-specific propagation have been identified and targeted for immunoengineering. Fibroblast-like synoviocytes (FLS) have been proposed as an important cell type for immunomodulating the pathogenesis of RA, as they are responsible for secreting a wide range of cytokines and matrix factors that recruit other immune cells and lead to cartilage and bone deterioration [140]. Earlier studies investigating viral-based methods of gene delivery targeting FLS including retroviral-delivered IFN-β or AAV5-delivered TNF-α blocking agents were proposed as successful ways of more precisely mitigating RA in mice [108, 109]. A more recent study also examined the use of recombinant AAV5 encoding IFN-β under NF-κB as a treatment for RA both in vitro, in transduced FLS, and in vivo, upon intra-articular injection in rats. A promising finding of this study was that local expression of the engineered AAV construct was present for 7 weeks following injection [110]. Chondrocytes, while not classically thought to serve in a major immunologic capacity within the joint, may also be suitable candidates for developing immunomodulatory therapies. For example, engineered cartilage tissues made from chondrocytes were designed capable of producing immunomodulatory IL-4 in response to activation of the PTGS2 gene via transiently transfected circuits, introducing a novel approach for immunomodulatory regulation by repurposing downstream targets of inflammation [112]. Alternatively, for OA treatment, the use of AAV gene editing to produce IL-1Ra in human-derived chondrocytes resulted in the protection of cartilage damage in an explant culture model [113]. Additionally, in a more recent approach, equine synovial fibroblasts and chondrocytes were transduced with several serotypes of AAV (2,5, and 8) which enabled them to produce IL-1Ra at a biologically relevant levels in the horse joint and are postulated to serve as potential cell sources in future genetic engineering for OA [111].
Many other immune cells, part of both of the innate or adaptive immune system, have also been proposed as immunoengineering targets for precise treatment of arthritis-related inflammation. For example, T cell adoptive therapy approaches have gained attention because of their success in controlling autoimmunity. T cells have been highlighted as a major player in RA pathogenesis and observed to a lesser extent in OA. CD4+ T cells (T helper cell/Th1 cell) have been identified in the peripheral blood of both RA and OA patients. Th1 cells induce inflammation in the early stages of OA through C-X-C chemokine receptor type 5, inducible co-stimulator, and programmed cell death [29, 141]. Targeting the ratio of Th1 (inflammatory)/Th2 (anti-inflammatory) subsets have been proposed as promising immunotherapy candidates for OA and RA [142]. Through the depletion of inflammation inducing T cells, antibody-based protocols have been successful in correcting T cell ratio; yet this leads to lymphopenia while memory T cells act to preserve pro-arthritogenic T cells after depletion [142, 143]. Regulatory T cells (Treg cells) act as an immunoregulator for the secretion of anti-inflammatory cytokines and expression of cytokine receptors [121]. In that respect, depletion of Treg cells has been shown to help induce the onset of OA and RA while delivery of Treg cells acts to alleviate RA symptoms [142]. However, typically large numbers of Tregs are necessary to restore an appropriate Th cell ratio, requiring ex vivo expansion [21]. Genetic engineering using CRISPR-Cas9 mediated Treg therapy could rectify limitations surrounding their therapeutic delivery, but the in vivo longevity, delivery, and plasticity of these cells is not well understood. Chimeric antigen receptor (CAR) T cells, which revolutionized therapies for malignancies, arthritis. CAR T cells can be modified to target antigens specific to a pathological cell’s surface based on synthetic receptors (CAR) that alter T cell specificity and function [143]. In this regard, engineering CAR T cells against urokinase-type plasminogen activator receptor, which is associated with senescence, successfully eliminated senescent cells in multiple models outside of arthritis (e.g., lung adenocarcinoma, liver fibrosis) [117]. This could be a promising therapy to treat arthritis as the removal of senescent cells from the joint has been shown to reduce and protect against the development of PTOA and OA [144]. Further methods have been designed to decrease aberrant immune signaling in RA; for example, CAR T cells have been developed to target immunodominant peptides on autoreactive B cells for RA autoimmunity [118, 143].
Natural Killer (NK) cells, an additional therapeutic cell target, demonstrate high activity in advanced active RA [145]. Thymus-derived invariant NK T cells improved joint swelling in the α-galactosylceramide RA model [22]. Levels of IL-4 and IL-10 were increased in response to the delivery of invariant NK T cells, and serum TNF-α, IFN-ɣ, and IL-6 were decreased [22]. These cells also acted to correct the Th cell ratio and mitigated disease progression. B cells also have a known role in the onset and progression of pro-inflammatory diseases, like RA and OA [29]. Recently, nanocarriers have been used to deliver CRISPR-Cas9 to edit B cells in vivo. This system has been applied in proof-of-principle studies to disrupt B220 expression, a protein tyrosine phosphatase that plays a critical role in cytokine receptor signaling within the B cell, and control B cell activity [114]. Editing B cells is an attractive therapeutic opportunity as B cells can generate and secrete large amounts of protein, a local source of regulated and tunable antibody production, and can also be used in vivo as a screening system adaptable to other immune cells [115, 116]. Immunoengineering specific resident and exogenous cell populations in OA and RA offers increased specificity over general anti-inflammatory therapies.
4.2.4. Induced pluripotent stem cells (iPSCs)
As an alternative to primary cells, iPSCs are an attractive cell source for immunoengineering as they can be easily genetically engineered; they possess a highly plastic phenotype and expanded longevity for therapeutic delivery; they can be sourced in large numbers of precisely defined, patient-matched cells; and they are capable of being differentiated into immunologically relevant cell types within the joint. Both gene therapy and cellular engineering approaches, such as CRISPR-Cas9, have been executed in iPSCs to improve disease-specific immunomodulation of the joint [146, 147]. In one study, a synthetic gene promoter system was developed based on consensus motifs for the NF-κB dimers, which was lentivirally delivered to murine iPSCs [148]. This system was used to amplify and drive expression of anti-cytokine drugs such as IL-1Ra in response to IL-1 signaling in chondrogenically differentiated iPSCs as a targeted approach to treat aberrant signaling in OA. Murine iPSCs have also been CRISPR-Cas9 edited to harbor a functional deletion of the IL-1 receptor I (IL1R1) [119]. These cells were capable of differentiating and creating a cartilaginous matrix that was protected from IL-1 mediated tissue degradation. Additionally, CRISPR-Cas9 was used in murine iPSCs to create engineered cells that have targeted gene addition of IL-1Ra (Il1rn) or soluble TNFR1 (Tnfrsf1a) genes downstream of the Ccl2 promoter [120]. This synthetic gene circuit in the edited iPSCs created a dynamic negative feedback loop where, upon stimulation with IL-1 or TNF-α, the cells synthesize anti-cytokine therapies to effectively inhibit inflammation in a self-regulating manner. These edited iPSCs were engineered to create implantable self-regulating tissue constructs that have promising efficacy in early studies in an RA model [74]. Additionally, iPSCs can be differentiated into iPSC-Tregs with the use of retroviral transduction of FoxP3 and an antigen specific T cell receptor. iPSC-Tregs delivered to the joint in antigen induced arthritis reduced joint inflammation, swelling, and prevention of bone loss [121]. Similarly, another study tested these Tregs in a collagen induced arthritis model of RA in mice and observed that the cells significantly suppressed the host immune response and reduced disease severity [149]. Advances in iPSCs and genome engineering continue to allow researchers to immunomodulate previously difficult cell and mechanistic targets and will be increasingly important tools as more becomes know about the distinct pathways driving OA and RA.
5. Future Directions, Applications, and Conclusions
The rapid development of immunoengineering strategies has expanded the potential for personalized arthritis treatments beyond general anti-inflammatory and biomaterial-based applications. The inability of current immunomodulatory approaches to effectively target and inhibit precise inflammatory propagators in musculoskeletal disease pathogenesis poses an ongoing challenge.
There is a vast opportunity to apply new advances in biomaterial and cell-based immunoengineering to joint diseases to meet the challenge of disease-specific OA and RA treatment (Figure 3). For example, in the context of RA, our laboratory has applied the aforementioned genome-engineered murine iPSCs to form a bioartificial implant to deliver anti-cytokine therapy in an autoregulated manner in vivo, which mitigated structural damage, bone erosions, and pain in a murine model of inflammatory RA [74, 119, 120]. Coupling synthetic biology with tissue engineering in this manner can be applied to generate designer cells and biomaterial delivery systems that hijack endogenous disease-specific signaling pathways to trigger specific immunomodulatory responses to the pathology of interest. Though we highlighted potential immunoengineering techniques for both OA and RA in this review, it is clear that further study into designing both mechanically and immunomodulatory relevant therapeutics for OA is necessary to fill the large gap between anti-inflammatory drugs and joint replacements available to OA patients. In this regard, we have employed mechanical loading to deliver a pre-programmed biologic drug via the mechanically-sensitive ion channel transient receptor potential vanilloid-4 (TRPV4) to autonomously produce IL-1Ra in response to physiologically-relevant loading [112]. These emerging immunoengineering solutions can serve as the platform for the development of treatments that not only possess a more precise and synergistic control over the immune and mechano-biologic response, but also leverage the joint’s heterogenous cell population for a wider range of therapeutic application both within and external to the joint organ system.
Figure 3.
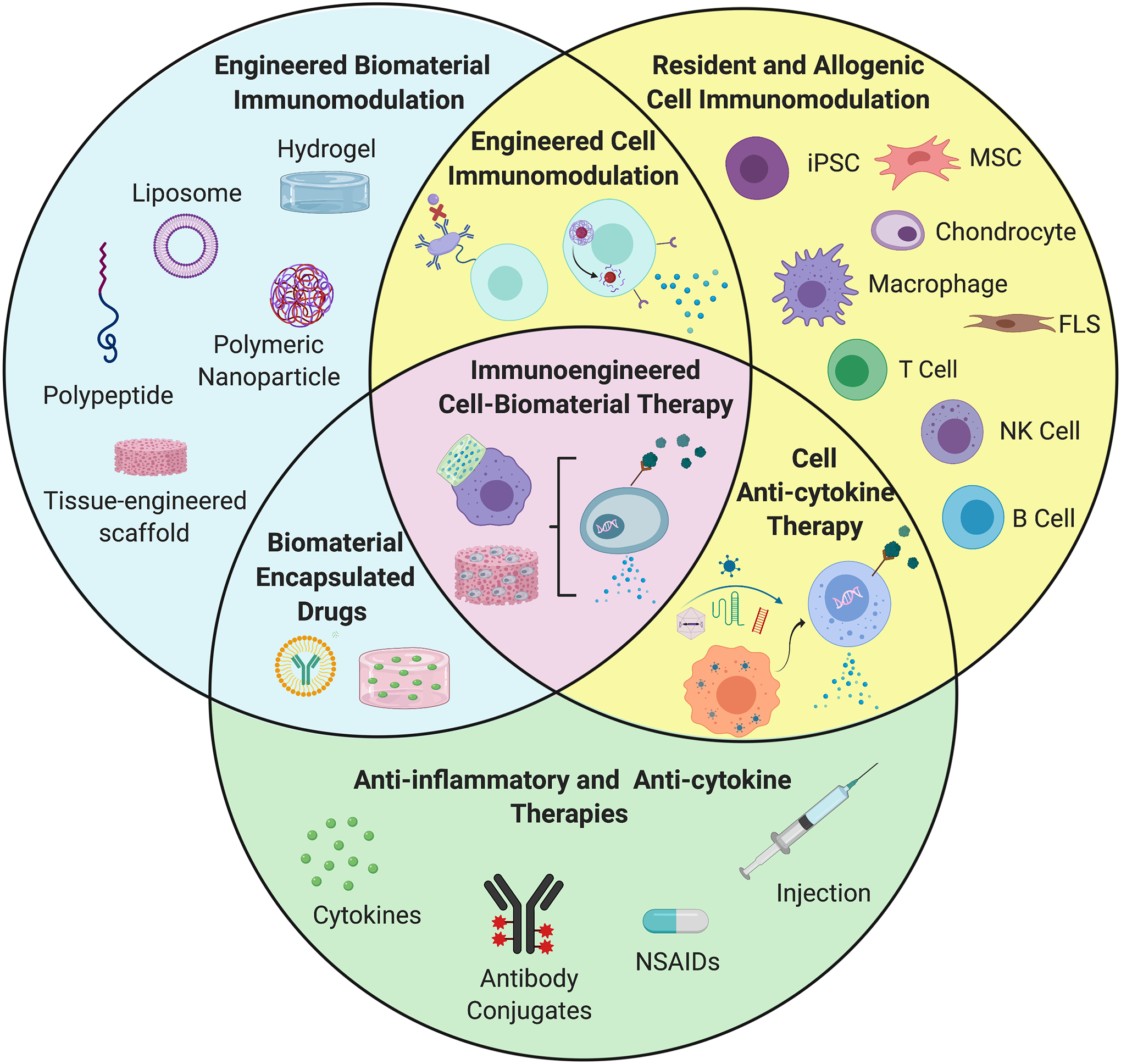
The future of OA and RA immunoengineering will ultimately harness the combined strength of cell and biomaterial immunoengineering strategies to improve upon the use of current anti-inflammatory and anti-cytokine therapies. Furthermore, the inclusion of genetic engineering in both cell and biomaterial systems to alter resident or exogenous cell behavior offers additional potential to these therapies, while the use of iPSCs provides an ever-expanding platform with which to develop personalized and disease-specific treatments. Abbreviations: FLS: Fibroblast-like synoviocytes; MSC: Mesenchymal stem cells; iPSC: Induced pluripotent stem cell; NK: Natural killer cell; NSAIDS: Non-steroidal anti-inflammatory drugs.
Continued improvement upon biomaterial and cell-instructed immunomodulatory therapies will be dependent upon increasing the understanding of a comprehensive list of immunomodulatory factors including cytokine profiles, signaling pathways, and cell–biomaterial interactions, as well as innovating genetic engineering tool sets. We can seek guidance from numerous examples in other fields, like cancer immunotherapy and therapeutic nanotechnology, that can be readily applied to musculoskeletal problems to design novel solutions using immunoengineering strategies. For example, macrophages with engineered biomaterial cytokine reservoirs can be employed in the context of OA and RA as a precise tool for cell-specific biomaterial-based mediation of inflammation [106]. Application of current drugs (e.g., anti-inflammatories and biologics) along with new studies testing the ability of engineered cells (e.g., resident and exogenous) as well as biomaterials (e.g., scaffolds, nanomaterials, gels, and polypeptides) to guide and proactively influence immune response to drug delivery and genetically engineered systems will not only improve upon current immunomodulatory strategies but will increase our collective understanding of immune cell involvement in musculoskeletal disease pathogenesis (Figure 3). Development of a more precise and disease-specific set of immunomodulatory solutions to meet the challenge of creating precision arthritis therapies is crucial for designing effective interventions. We believe that the advent of immunoengineering, in particular strategies that combine multiple methods of immunomodulation, and its application to the development of novel treatment strategies will revolutionize the manner in which musculoskeletal diseases are understood, managed, and ultimately prevented.
Statement of Significance.
It is now apparent that joint diseases such as osteoarthritis and rheumatoid arthritis involve the immune system at both local (i.e., within the joint) and systemic levels. In this regard, targeting the immune system using both biomaterial-based or cellular approaches may generate new joint-specific treatment strategies that are well-controlled, safe, and efficacious. In this review, we focus on recent advances in immunoengineering that leverage biomaterials and/or genetically engineered cells for therapeutic applications in joint diseases. The application of such approaches, especially synergistic strategies that target multiple immunoregulatory pathways, has the potential to revolutionize our understanding, treatment, and prevention of joint diseases.
Acknowledgments
All figures were created with Biorender.com. This work was supported by the Shriners Hospitals for Children, the National Institutes of Health (AR76665, AG46927, AG15768, AR74240, AR072999, AR073752, AR074992, AR070975, AR069588, AR077678, T32DK007120, T32DK108742), the Nancy Taylor Foundation, the Arthritis Foundation, and the Philip and Sima Needleman Fellowship from the Washington University Center of Regenerative Medicine.
Footnotes
Declaration of Interests
FG is an employee and shareholder in Cytex Therapeutics, Inc.
References
- [1].Hootman JM, Murphy LB, Omura JD, Brady TJ, Boring M, Barbour KE, Helmick CG, Health Care Provider Counseling for Physical Activity or Exercise Among Adults with Arthritis - United States, 2002 and 2014, MMWR Morb Mortal Wkly Rep 66(51–52) (2018) 1398–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Saccomano SJ, Osteoarthritis treatment: Decreasing pain, improving mobility, Nurse Pract 43(9) (2018) 49–55. [DOI] [PubMed] [Google Scholar]
- [3].Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM, Genetic and environmental risk factors for rheumatoid arthritis, Best Pract Res Clin Rheumatol 31(1) (2017) 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A, Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression, Int J Mol Sci 16(3) (2015) 6093–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].He Y, Li Z, Alexander PG, Ocasio-Nieves BD, Yocum L, Lin H, Tuan RS, Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models, Biology (Basel) 9(8) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, Zheng MH, Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes, Arthritis Res Ther 15(6) (2013) 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sondag GR, Haqqi TM, The Role of MicroRNAs and Their Targets in Osteoarthritis, Curr Rheumatol Rep 18(8) (2016) 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Loeser RF, Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix, Osteoarthritis Cartilage 17(8) (2009) 971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Woodell-May JE, Sommerfeld SD, Role of Inflammation and the Immune System in the Progression of Osteoarthritis, J Orthop Res 38(2) (2020) 253–257. [DOI] [PubMed] [Google Scholar]
- [10].Goldring MB, Berenbaum F, Emerging targets in osteoarthritis therapy, Curr Opin Pharmacol 22 (2015) 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Otero M, Goldring MB, Cells of the synovium in rheumatoid arthritis. Chondrocytes, Arthritis Res Ther 9(5) (2007) 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kesharwani D, Paliwal R, Satapathy T, Das Paul S, Rheumatiod Arthritis: An Updated Overview of Latest Therapy and Drug Delivery, J Pharmacopuncture 22(4) (2019) 210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schett G, Firestein GS, Mr Outside and Mr Inside: classic and alternative views on the pathogenesis of rheumatoid arthritis, Ann Rheum Dis 69(5) (2010) 787–9. [DOI] [PubMed] [Google Scholar]
- [14].Falconer J, Murphy AN, Young SP, Clark AR, Tiziani S, Guma M, Buckley CD, Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis, Arthritis Rheumatol 70(7) (2018) 984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wenham CY, Conaghan PG, The role of synovitis in osteoarthritis, Ther Adv Musculoskelet Dis 2(6) (2010) 349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Firestein GS, McInnes IB, Immunopathogenesis of Rheumatoid Arthritis, Immunity 46(2) (2017) 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Culemann S, Gruneboom A, Nicolas-Avila JA, Weidner D, Lammle KF, Rothe T, Quintana JA, Kirchner P, Krljanac B, Eberhardt M, Ferrazzi F, Kretzschmar E, Schicht M, Fischer K, Gelse K, Faas M, Pfeifle R, Ackermann JA, Pachowsky M, Renner N, Simon D, Haseloff RF, Ekici AB, Bauerle T, Blasig IE, Vera J, Voehringer D, Kleyer A, Paulsen F, Schett G, Hidalgo A, Kronke G, Locally renewing resident synovial macrophages provide a protective barrier for the joint, Nature 572(7771) (2019) 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J, Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis, Nat Rev Rheumatol 12(10) (2016) 580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xie J, Huang Z, Yu X, Zhou L, Pei F, Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee, Cytokine Growth Factor Rev 46 (2019) 36–44. [DOI] [PubMed] [Google Scholar]
- [20].de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, Zuurmond AM, Schoones J, Toes RE, Huizinga TW, Kloppenburg M, Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review, Osteoarthritis Cartilage 20(12) (2012) 1484–99. [DOI] [PubMed] [Google Scholar]
- [21].Roeleveld DM, Koenders MI, The role of the Th17 cytokines IL-17 and IL-22 in Rheumatoid Arthritis pathogenesis and developments in cytokine immunotherapy, Cytokine 74(1) (2015) 101–7. [DOI] [PubMed] [Google Scholar]
- [22].Chen D, Liu H, Wang Y, Chen S, Liu J, Li W, Dou H, Hou W, Meng M, Study of the adoptive immunotherapy on rheumatoid arthritis with Thymus-derived invariant natural killer T cells, Int Immunopharmacol 67 (2019) 427–440. [DOI] [PubMed] [Google Scholar]
- [23].Miller RE, Miller RJ, Malfait AM, Osteoarthritis joint pain: the cytokine connection, Cytokine 70(2) (2014) 185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Srenathan U, Steel K, Taams LS, IL-17+ CD8+ T cells: Differentiation, phenotype and role in inflammatory disease, Immunol Lett 178 (2016) 20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].van der Kraan PM, The Interaction between Joint Inflammation and Cartilage Repair, Tissue Eng Regen Med 16(4) (2019) 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alivernini S, Tolusso B, Ferraccioli G, Gremese E, Kurowska-Stolarska M, McInnes IB, Driving chronicity in rheumatoid arthritis: perpetuating role of myeloid cells, Clin Exp Immunol 193(1) (2018) 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Firestein GS, Zvaifler NJ, How important are T cells in chronic rheumatoid synovitis?, Arthritis Rheum 33(6) (1990) 768–73. [DOI] [PubMed] [Google Scholar]
- [28].Yang CY, Chanalaris A, Troeberg L, ADAMTS and ADAM metalloproteinases in osteoarthritis - looking beyond the ‘usual suspects’, Osteoarthritis Cartilage 25(7) (2017) 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Haseeb A, Haqqi TM, Immunopathogenesis of osteoarthritis, Clin Immunol 146(3) (2013) 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H, Role of proinflammatory cytokines in the pathophysiology of osteoarthritis, Nat Rev Rheumatol 7(1) (2011) 33–42. [DOI] [PubMed] [Google Scholar]
- [31].Gschwandtner M, Derler R, Midwood KS, More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis, Front Immunol 10 (2019) 2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tu J, Hong W, Guo Y, Zhang P, Fang Y, Wang X, Chen X, Lu S, Wei W, Ontogeny of Synovial Macrophages and the Roles of Synovial Macrophages From Different Origins in Arthritis, Front Immunol 10 (2019) 1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vincent TL, Of mice and men: converging on a common molecular understanding of osteoarthritis, Lancet Rheumatol 2(10) (2020) e633–e645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gierut A, Perlman H, Pope RM, Innate immunity and rheumatoid arthritis, Rheum Dis Clin North Am 36(2) (2010) 271–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ramos-Casals M, Brito-Zeron P, Munoz S, Soria N, Galiana D, Bertolaccini L, Cuadrado MJ, Khamashta MA, Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases, Medicine (Baltimore) 86(4) (2007) 242–51. [DOI] [PubMed] [Google Scholar]
- [36].Gopinath SD, Rando TA, Stem cell review series: aging of the skeletal muscle stem cell niche, Aging Cell 7(4) (2008) 590–8. [DOI] [PubMed] [Google Scholar]
- [37].Kimmerling KA, Furman BD, Mangiapani DS, Moverman MA, Sinclair SM, Huebner JL, Chilkoti A, Kraus VB, Setton LA, Guilak F, Olson SA, Sustained intra-articular delivery of IL-1RA from a thermally-responsive elastin-like polypeptide as a therapy for post-traumatic arthritis, Eur Cell Mater 29 (2015) 124–39; discussion 139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mozzetta C, Minetti G, Puri PL, Regenerative pharmacology in the treatment of genetic diseases: the paradigm of muscular dystrophy, Int J Biochem Cell Biol 41(4) (2009) 701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu-Bryan R, Terkeltaub R, Emerging regulators of the inflammatory process in osteoarthritis, Nat Rev Rheumatol 11(1) (2015) 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nakata K, Hanai T, Take Y, Osada T, Tsuchiya T, Shima D, Fujimoto Y, Disease-modifying effects of COX-2 selective inhibitors and non-selective NSAIDs in osteoarthritis: a systematic review, Osteoarthritis Cartilage 26(10) (2018) 1263–1273. [DOI] [PubMed] [Google Scholar]
- [41].Dennison EM, Cooper C, Corticosteroids in rheumatoid arthritis, BMJ 316(7134) (1998) 789–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Petit A, Redout EM, van de Lest CH, de Grauw JC, Muller B, Meyboom R, van Midwoud P, Vermonden T, Hennink WE, Rene van Weeren P, Sustained intra-articular release of celecoxib from in situ forming gels made of acetyl-capped PCLA-PEG-PCLA triblock copolymers in horses, Biomaterials 53 (2015) 426–36. [DOI] [PubMed] [Google Scholar]
- [43].Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, Setton LA, Weinberg JB, The role of biomechanics and inflammation in cartilage injury and repair, Clin Orthop Relat Res (423) (2004) 17–26. [DOI] [PubMed] [Google Scholar]
- [44].Quan LD, Thiele GM, Tian J, Wang D, The Development of Novel Therapies for Rheumatoid Arthritis, Expert Opin Ther Pat 18(7) (2008) 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lin YJ, Anzaghe M, Schulke S, Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis, Cells 9(4) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wehling P, Evans C, Wehling J, Maixner W, Effectiveness of intra-articular therapies in osteoarthritis: a literature review, Ther Adv Musculoskelet Dis 9(8) (2017) 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nicholls MA, Fierlinger A, Niazi F, Bhandari M, The Disease-Modifying Effects of Hyaluronan in the Osteoarthritic Disease State, Clin Med Insights Arthritis Musculoskelet Disord 10 (2017) 1179544117723611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Choy EH, Kavanaugh AF, Jones SA, The problem of choice: current biologic agents and future prospects in RA, Nat Rev Rheumatol 9(3) (2013) 154–63. [DOI] [PubMed] [Google Scholar]
- [49].Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH, Huebner JL, Kraus VB, Guilak F, Olson SA, Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis, Arthritis Res Ther 16(3) (2014) R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, Loeuille D, Kivitz AJ, Silver D, Appleton BE, Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study, Arthritis Rheum 61(3) (2009) 344–52. [DOI] [PubMed] [Google Scholar]
- [51].Alvarez MM, Liu JC, Trujillo-de Santiago G, Cha BH, Vishwakarma A, Ghaemmaghami AM, Khademhosseini A, Delivery strategies to control inflammatory response: Modulating M1-M2 polarization in tissue engineering applications, J Control Release 240 (2016) 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Emami J, Ansarypour Z, Receptor targeting drug delivery strategies and prospects in the treatment of rheumatoid arthritis, Res Pharm Sci 14(6) (2019) 471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wehling P, Reinecke J, Baltzer AW, Granrath M, Schulitz KP, Schultz C, Krauspe R, Whiteside TW, Elder E, Ghivizzani SC, Robbins PD, Evans CH, Clinical responses to gene therapy in joints of two subjects with rheumatoid arthritis, Hum Gene Ther 20(2) (2009) 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Evans CH, Ghivizzani SC, Robbins PD, Gene Delivery to Joints by Intra-Articular Injection, Hum Gene Ther 29(1) (2018) 2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Evans CH, Robbins PD, Ghivizzani SC, Wasko MC, Tomaino MM, Kang R, Muzzonigro TA, Vogt M, Elder EM, Whiteside TL, Watkins SC, Herndon JH, Gene transfer to human joints: progress toward a gene therapy of arthritis, Proc Natl Acad Sci U S A 102(24) (2005) 8698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ramirez J, Canete JD, Anakinra for the treatment of rheumatoid arthritis: a safety evaluation, Expert Opin Drug Saf 17(7) (2018) 727–732. [DOI] [PubMed] [Google Scholar]
- [57].Grunke M, Schulze-Koops H, Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade, Ann Rheum Dis 65(4) (2006) 555–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fioravanti A, Fabbroni M, Cerase A, Galeazzi M, Treatment of erosive osteoarthritis of the hands by intra-articular infliximab injections: a pilot study, Rheumatol Int 29(8) (2009) 961–5. [DOI] [PubMed] [Google Scholar]
- [59].Kloppenburg M, Ramonda R, Bobacz K, Kwok WY, Elewaut D, Huizinga TWJ, Kroon FPB, Punzi L, Smolen JS, Vander Cruyssen B, Wolterbeek R, Verbruggen G, Wittoek R, Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial, Ann Rheum Dis 77(12) (2018) 1757–1764. [DOI] [PubMed] [Google Scholar]
- [60].Gottenberg JE, Brocq O, Perdriger A, Lassoued S, Berthelot JM, Wendling D, Euller-Ziegler L, Soubrier M, Richez C, Fautrel B, Constantin AL, Mariette X, Morel J, Gilson M, Cormier G, Salmon JH, Rist S, Liote F, Marotte H, Bonnet C, Marcelli C, Sellam J, Meyer O, Solau-Gervais E, Guis S, Ziza JM, Zarnitsky C, Chary-Valckenaere I, Vittecoq O, Saraux A, Pers YM, Gayraud M, Bolla G, Claudepierre P, Ardizzone M, Dernis E, Breban MA, Fain O, Balblanc JC, Aberkane O, Vazel M, Back C, Candon S, Chatenoud L, Perrodeau E, Sibilia J, Ravaud P, Non-TNF-Targeted Biologic vs a Second Anti-TNF Drug to Treat Rheumatoid Arthritis in Patients With Insufficient Response to a First Anti-TNF Drug: A Randomized Clinical Trial, JAMA 316(11) (2016) 1172–1180. [DOI] [PubMed] [Google Scholar]
- [61].Matthews GL, Hunter DJ, Emerging drugs for osteoarthritis, Expert Opin Emerg Drugs 16(3) (2011) 479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Conaghan PG, Hunter DJ, Cohen SB, Kraus VB, Berenbaum F, Lieberman JR, Jones DG, Spitzer AI, Jevsevar DS, Katz NP, Burgess DJ, Lufkin J, Johnson JR, Bodick N, Investigators FXP, Effects of a Single Intra-Articular Injection of a Microsphere Formulation of Triamcinolone Acetonide on Knee Osteoarthritis Pain: A Double-Blinded, Randomized, Placebo-Controlled, Multinational Study, J Bone Joint Surg Am 100(8) (2018) 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Erdemli O, Ozen S, Keskin D, Usanmaz A, Batu ED, Atilla B, Tezcaner A, In vitro evaluation of effects of sustained anti-TNF release from MPEG-PCL-MPEG and PCL microspheres on human rheumatoid arthritis synoviocytes, J Biomater Appl 29(4) (2014) 524–42. [DOI] [PubMed] [Google Scholar]
- [64].Kou L, Sun R, Xiao S, Cui X, Sun J, Ganapathy V, Yao Q, Chen R, OCTN2-targeted nanoparticles for oral delivery of paclitaxel: differential impact of the polyethylene glycol linker size on drug delivery in vitro, in situ, and in vivo, Drug Deliv 27(1) (2020) 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Spieler V, Ludwig MG, Dawson J, Tigani B, Littlewood-Evans A, Safina C, Ebersbach H, Seuwen K, Raschig M, Ter Mors B, Muller TD, Meinel L, Luhmann T, Targeting interleukin-4 to the arthritic joint, J Control Release 326 (2020) 172–180. [DOI] [PubMed] [Google Scholar]
- [66].Holyoak DT, Wheeler TA, van der Meulen MCH, Singh A, Injectable mechanical pillows for attenuation of load-induced post-traumatic osteoarthritis, Regen Biomater 6(4) (2019) 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhao J, Zhao M, Yu C, Zhang X, Liu J, Cheng X, Lee RJ, Sun F, Teng L, Li Y, Multifunctional folate receptor-targeting and pH-responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis, Int J Nanomedicine 12 (2017) 6735–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hoemann CD, Chen G, Marchand C, Tran-Khanh N, Thibault M, Chevrier A, Sun J, Shive MS, Fernandes MJ, Poubelle PE, Centola M, El-Gabalawy H, Scaffold-guided subchondral bone repair: implication of neutrophils and alternatively activated arginase-1+ macrophages, Am J Sports Med 38(9) (2010) 1845–56. [DOI] [PubMed] [Google Scholar]
- [69].Sofu H, Camurcu Y, Ucpunar H, Ozcan S, Yurten H, Sahin V, Clinical and radiographic outcomes of chitosan-glycerol phosphate/blood implant are similar with hyaluronic acid-based cell-free scaffold in the treatment of focal osteochondral lesions of the knee joint, Knee Surg Sports Traumatol Arthrosc 27(3) (2019) 773–781. [DOI] [PubMed] [Google Scholar]
- [70].Kang ML, Kim JE, Im GI, Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis, Acta Biomater 39 (2016) 65–78. [DOI] [PubMed] [Google Scholar]
- [71].Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA, A thermally responsive biopolymer for intra-articular drug delivery, J Control Release 115(2) (2006) 175–82. [DOI] [PubMed] [Google Scholar]
- [72].Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, Masuda K, Setton LA, Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: sustained release of a local antiinflammatory therapeutic, Arthritis Rheum 56(11) (2007) 3650–61. [DOI] [PubMed] [Google Scholar]
- [73].Dai M, Sui B, Xue Y, Liu X, Sun J, Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes, Biomaterials 180 (2018) 91–103. [DOI] [PubMed] [Google Scholar]
- [74].Choi Y-R, Collins KH, Springer LE, Pferdehirt L, Ross AK, Wu C-L, Moutos FT, Harasymowicz NS, Brunger JM, Pham CTN, Guilak F, A Genome-engineered Bioartificial Implant for Autoregulated Anti-Cytokine Drug Delivery, bioRxiv (2019) 535609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Rai MF, Pan H, Yan H, Sandell LJ, Pham CTN, Wickline SA, Applications of RNA interference in the treatment of arthritis, Transl Res 214 (2019) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yan H, Duan X, Pan H, Holguin N, Rai MF, Akk A, Springer LE, Wickline SA, Sandell LJ, Pham CT, Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury, Proc Natl Acad Sci U S A 113(41) (2016) E6199–E6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhou HF, Yan H, Pan H, Hou KK, Akk A, Springer LE, Hu Y, Allen JS, Wickline SA, Pham CT, Peptide-siRNA nanocomplexes targeting NF-kappaB subunit p65 suppress nascent experimental arthritis, J Clin Invest 124(10) (2014) 4363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang S, Wei X, Sun X, Chen C, Zhou J, Zhang G, Wu H, Guo B, Wei L, A novel therapeutic strategy for cartilage diseases based on lipid nanoparticle-RNAi delivery system, Int J Nanomedicine 13 (2018) 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fahy N, Menzel U, Alini M, Stoddart MJ, Shear and Dynamic Compression Modulates the Inflammatory Phenotype of Human Monocytes in vitro, Front Immunol 10 (2019) 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Li F, Chen YZ, Miao ZN, Zheng SY, Jin J, Human placenta-derived mesenchymal stem cells with silk fibroin biomaterial in the repair of articular cartilage defects, Cell Reprogram 14(4) (2012) 334–41. [DOI] [PubMed] [Google Scholar]
- [81].Ziadlou R, Rotman S, Teuschl A, Salzer E, Barbero A, Martin I, Alini M, Eglin D, Grad S, Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs, Mater Sci Eng C Mater Biol Appl 120 (2021) 111701. [DOI] [PubMed] [Google Scholar]
- [82].Tellier LE, Trevino EA, Brimeyer AL, Reece DS, Willett NJ, Guldberg RE, Temenoff JS, Intra-articular TSG-6 delivery from heparin-based microparticles reduces cartilage damage in a rat model of osteoarthritis, Biomater Sci 6(5) (2018) 1159–1167. [DOI] [PubMed] [Google Scholar]
- [83].Oliveira IM, Goncalves C, Shin ME, Lee S, Reis RL, Khang G, Oliveira JM, Anti-Inflammatory Properties of Injectable Betamethasone-Loaded Tyramine-Modified Gellan Gum/Silk Fibroin Hydrogels, Biomolecules 10(10) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Patel JM, Saleh KS, Burdick JA, Mauck RL, Bioactive factors for cartilage repair and regeneration: Improving delivery, retention, and activity, Acta Biomater 93 (2019) 222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Burt HM, Tsallas A, Gilchrist S, Liang LS, Intra-articular drug delivery systems: Overcoming the shortcomings of joint disease therapy, Expert Opin Drug Deliv 6(1) (2009) 17–26. [DOI] [PubMed] [Google Scholar]
- [86].Mohammadinejad R, Ashrafizadeh M, Pardakhty A, Uzieliene I, Denkovskij J, Bernotiene E, Janssen L, Lorite GS, Saarakkala S, Mobasheri A, Nanotechnological Strategies for Osteoarthritis Diagnosis, Monitoring, Clinical Management, and Regenerative Medicine: Recent Advances and Future Opportunities, Curr Rheumatol Rep 22(4) (2020) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wu Y, Lu X, Shen B, Zeng Y, The Therapeutic Potential and Role of miRNA, lncRNA, and circRNA in Osteoarthritis, Curr Gene Ther 19(4) (2019) 255–263. [DOI] [PubMed] [Google Scholar]
- [88].Taraballi F, Bauza G, McCulloch P, Harris J, Tasciotti E, Concise Review: Biomimetic Functionalization of Biomaterials to Stimulate the Endogenous Healing Process of Cartilage and Bone Tissue, Stem Cells Transl Med 6(12) (2017) 2186–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Irawan V, Sung TC, Higuchi A, Ikoma T, Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development, Tissue Eng Regen Med 15(6) (2018) 673–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schultz C, Targeting the extracellular matrix for delivery of bioactive molecules to sites of arthritis, Br J Pharmacol 176(1) (2019) 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jain N, Vogel V, Spatial confinement downsizes the inflammatory response of macrophages, Nat Mater 17(12) (2018) 1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Moore EM, Maestas DR Jr., Comeau HY, Elisseeff JH, The Immune System and Its Contribution to Variability in Regenerative Medicine, Tissue Eng Part B Rev (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Glass KA, Link JM, Brunger JM, Moutos FT, Gersbach CA, Guilak F, Tissue-engineered cartilage with inducible and tunable immunomodulatory properties, Biomaterials 35(22) (2014) 5921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gabner S, Hlavaty J, Velde K, Renner M, Jenner F, Egerbacher M, Inflammation-induced transgene expression in genetically engineered equine mesenchymal stem cells, J Gene Med 18(8) (2016) 154–64. [DOI] [PubMed] [Google Scholar]
- [95].Gabner S, Ertl R, Velde K, Renner M, Jenner F, Egerbacher M, Hlavaty J, Cytokine-induced interleukin-1 receptor antagonist protein expression in genetically engineered equine mesenchymal stem cells for osteoarthritis treatment, J Gene Med 20(5) (2018) e3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Huynh NP, Gloss CC, Lorentz J, Tang R, Brunger JM, McAlinden A, Zhang B, Guilak F, Long non-coding RNA GRASLND enhances chondrogenesis via suppression of the interferon type II signaling pathway, Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].McGinley LM, McMahon J, Stocca A, Duffy A, Flynn A, O’Toole D, O’Brien T, Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression, Hum Gene Ther 24(10) (2013) 840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Park N, Rim YA, Jung H, Kim J, Yi H, Kim Y, Jang Y, Jung SM, Lee J, Kwok SK, Park SH, Ju JH, Etanercept-Synthesising Mesenchymal Stem Cells Efficiently Ameliorate Collagen-Induced Arthritis, Sci Rep 7 (2017) 39593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M, Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors, Cancer Res 62(13) (2002) 3603–8. [PubMed] [Google Scholar]
- [100].Bartholomew A, Patil S, Mackay A, Nelson M, Buyaner D, Hardy W, Mosca J, Sturgeon C, Siatskas M, Mahmud N, Ferrer K, Deans R, Moseley A, Hoffman R, Devine SM, Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo, Hum Gene Ther 12(12) (2001) 1527–41. [DOI] [PubMed] [Google Scholar]
- [101].Wu CL, McNeill J, Goon K, Little D, Kimmerling K, Huebner J, Kraus V, Guilak F, Conditional Macrophage Depletion Increases Inflammation and Does Not Inhibit the Development of Osteoarthritis in Obese Macrophage Fas-Induced Apoptosis-Transgenic Mice, Arthritis Rheumatol 69(9) (2017) 1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Jain S, Tran TH, Amiji M, Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis, Biomaterials 61 (2015) 162–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hu G, Guo M, Xu J, Wu F, Fan J, Huang Q, Yang G, Lv Z, Wang X, Jin Y, Nanoparticles Targeting Macrophages as Potential Clinical Therapeutic Agents Against Cancer and Inflammation, Front Immunol 10 (2019) 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Visser JG, Van Staden ADP, Smith C, Harnessing Macrophages for Controlled-Release Drug Delivery: Lessons From Microbes, Front Pharmacol 10 (2019) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wayne EC, Long C, Haney MJ, Batrakova EV, Leisner TM, Parise LV, Kabanov AV, Targeted Delivery of siRNA Lipoplexes to Cancer Cells Using Macrophage Transient Horizontal Gene Transfer, Adv Sci (Weinh) 6(21) (2019) 1900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Shields C.W.t., Evans MA, Wang LL, Baugh N, Iyer S, Wu D, Zhao Z, Pusuluri A, Ukidve A, Pan DC, Mitragotri S, Cellular backpacks for macrophage immunotherapy, Sci Adv 6(18) (2020) eaaz6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Liu PS, Lin YW, Burton FH, Wei LN, Injecting engineered anti-inflammatory macrophages therapeutically induces white adipose tissue browning and improves diet-induced insulin resistance, Adipocyte 4(2) (2015) 123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Triantaphyllopoulos KA, Williams RO, Tailor H, Chernajovsky Y, Amelioration of collagen-induced arthritis and suppression of interferon-gamma, interleukin-12, and tumor necrosis factor alpha production by interferon-beta gene therapy, Arthritis Rheum 42(1) (1999) 90–9. [DOI] [PubMed] [Google Scholar]
- [109].Adriaansen J, Khoury M, de Cortie CJ, Fallaux FJ, Bigey P, Scherman D, Gould DJ, Chernajovsky Y, Apparailly F, Jorgensen C, Vervoordeldonk MJ, Tak PP, Reduction of arthritis following intra-articular administration of an adeno-associated virus serotype 5 expressing a disease-inducible TNF-blocking agent, Ann Rheum Dis 66(9) (2007) 1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Aalbers CJ, Bevaart L, Loiler S, de Cortie K, Wright JF, Mingozzi F, Tak PP, Vervoordeldonk MJ, Preclinical Potency and Biodistribution Studies of an AAV 5 Vector Expressing Human Interferon-beta (ART-I02) for Local Treatment of Patients with Rheumatoid Arthritis, PLoS One 10(6) (2015) e0130612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Watson RS, Broome TA, Levings PP, Rice BL, Kay JD, Smith AD, Gouze E, Gouze JN, Dacanay EA, Hauswirth WW, Nickerson DM, Dark MJ, Colahan PT, Ghivizzani SC, scAAV-mediated gene transfer of interleukin-1-receptor antagonist to synovium and articular cartilage in large mammalian joints, Gene Ther 20(6) (2013) 670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Nims RJ, Pferdehirt L, Ho NB, Savadipour A, Lorentz J, Sohi S, Kassab J, Ross AK, O’Conor CJ, Liedtke WB, Zhang B, McNulty AL, Guilak F, A synthetic mechanogenetic gene circuit for autonomous drug delivery in engineered tissues, Sci Adv 7(5) (2021) eabd9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Baragi VM, Renkiewicz RR, Jordan H, Bonadio J, Hartman JW, Roessler BJ, Transplantation of transduced chondrocytes protects articular cartilage from interleukin 1-induced extracellular matrix degradation, J Clin Invest 96(5) (1995) 2454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Li M, Fan Y-N, Chen Z-Y, Luo Y-L, Wang Y-C, Lian Z-X, Xu C-F, Wang J, Optimized nanoparticle-mediated delivery of CRISPR-Cas9 system for B cell intervention, Nano Research 11(12) (2018) 6270–6282. [Google Scholar]
- [115].Johnson MJ, Laoharawee K, Lahr WS, Webber BR, Moriarity BS, Engineering of Primary Human B cells with CRISPR/Cas9 Targeted Nuclease, Sci Rep 8(1) (2018) 12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chu VT, Graf R, Wirtz T, Weber T, Favret J, Li X, Petsch K, Tran NT, Sieweke MH, Berek C, Kuhn R, Rajewsky K, Efficient CRISPR-mediated mutagenesis in primary immune cells using CrispRGold and a C57BL/6 Cas9 transgenic mouse line, Proc Natl Acad Sci U S A 113(44) (2016) 12514–12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, Mansilla-Soto J, Boyer JA, Li X, Giavridis T, Kulick A, Houlihan S, Peerschke E, Friedman SL, Ponomarev V, Piersigilli A, Sadelain M, Lowe SW, Senolytic CAR T cells reverse senescence-associated pathologies, Nature 583(7814) (2020) 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhang B, Wang Y, Yuan Y, Sun J, Liu L, Huang D, Hu J, Wang M, Li S, Song W, Chen H, Zhou D, Zhang X, In vitro elimination of autoreactive B cells from rheumatoid arthritis patients by universal chimeric antigen receptor T cells, Annals of the Rheumatic Diseases 80(2) (2021) 176–184. [DOI] [PubMed] [Google Scholar]
- [119].Brunger JM, Zutshi A, Willard VP, Gersbach CA, Guilak F, CRISPR/Cas9 Editing of Murine Induced Pluripotent Stem Cells for Engineering Inflammation-Resistant Tissues, Arthritis Rheumatol 69(5) (2017) 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Brunger JM, Zutshi A, Willard VP, Gersbach CA, Guilak F, Genome Engineering of Stem Cells for Autonomously Regulated, Closed-Loop Delivery of Biologic Drugs, Stem Cell Reports 8(5) (2017) 1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Haque M, Fino K, Sandhu P, Song J, Development of Stem Cell-derived Antigen-specific Regulatory T Cells Against Autoimmunity, J Vis Exp (117) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, Alturki M, Fallatah M, Almalik A, Alhasan AH, Shah K, Karp JM, Shattering barriers toward clinically meaningful MSC therapies, Sci Adv 6(30) (2020) eaba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hu MS, Walmsley GG, Barnes LA, Weiskopf K, Rennert RC, Duscher D, Januszyk M, Maan ZN, Hong WX, Cheung AT, Leavitt T, Marshall CD, Ransom RC, Malhotra S, Moore AL, Rajadas J, Lorenz HP, Weissman IL, Gurtner GC, Longaker MT, Delivery of monocyte lineage cells in a biomimetic scaffold enhances tissue repair, JCI Insight 2(19) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q, Mesenchymal stem cells and immunomodulation: current status and future prospects, Cell Death Dis 7 (2016) e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Vasilev G, Ivanova M, Ivanova-Todorova E, Tumangelova-Yuzeir K, Krasimirova E, Stoilov R, Kyurkchiev D, Secretory factors produced by adipose mesenchymal stem cells downregulate Th17 and increase Treg cells in peripheral blood mononuclear cells from rheumatoid arthritis patients, Rheumatol Int 39(5) (2019) 819–826. [DOI] [PubMed] [Google Scholar]
- [126].Rustad KC, Gurtner GC, Mesenchymal Stem Cells Home to Sites of Injury and Inflammation, Adv Wound Care (New Rochelle) 1(4) (2012) 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Lopez-Santalla M, Fernandez-Perez R, Garin MI, Mesenchymal Stem/Stromal Cells for Rheumatoid Arthritis Treatment: An Update on Clinical Applications, Cells 9(8) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Barry F, MSC Therapy for Osteoarthritis: An Unfinished Story, J Orthop Res 37(6) (2019) 1229–1235. [DOI] [PubMed] [Google Scholar]
- [129].Gerace D, Martiniello-Wilks R, Nassif NT, Lal S, Steptoe R, Simpson AM, CRISPR-targeted genome editing of mesenchymal stem cell-derived therapies for type 1 diabetes: a path to clinical success?, Stem Cell Res Ther 8(1) (2017) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Pawitan JA, Bui TA, Mubarok W, Antarianto RD, Nurhayati RW, Dilogo IH, Oceandy D, Enhancement of the Therapeutic Capacity of Mesenchymal Stem Cells by Genetic Modification: A Systematic Review, Front Cell Dev Biol 8 (2020) 587776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Saeedi P, Halabian R, Imani Fooladi AA, A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies, Stem Cell Investig 6 (2019) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wang N, Liang H, Zen K, Molecular mechanisms that influence the macrophage m1-m2 polarization balance, Front Immunol 5 (2014) 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Dutta B, Arya RK, Goswami R, Alharbi MO, Sharma S, Rahaman SO, Role of macrophage TRPV4 in inflammation, Lab Invest 100(2) (2020) 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Liu B, Zhang M, Zhao J, Zheng M, Yang H, Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis, Exp Ther Med 16(6) (2018) 5009–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Graney PL, Ben-Shaul S, Landau S, Bajpai A, Singh B, Eager J, Cohen A, Levenberg S, Spiller KL, Macrophages of diverse phenotypes drive vascularization of engineered tissues, Sci Adv 6(18) (2020) eaay6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Watanabe S, Alexander M, Misharin AV, Budinger GRS, The role of macrophages in the resolution of inflammation, J Clin Invest 129(7) (2019) 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Hesketh M, Sahin KB, West ZE, Murray RZ, Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing, Int J Mol Sci 18(7) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Guilliams M, Scott CL, Does niche competition determine the origin of tissue-resident macrophages?, Nat Rev Immunol 17(7) (2017) 451–460. [DOI] [PubMed] [Google Scholar]
- [139].Tu J, Wang X, Gong X, Hong W, Han D, Fang Y, Guo Y, Wei W, Synovial Macrophages in Rheumatoid Arthritis: The Past, Present, and Future, Mediators Inflamm 2020 (2020) 1583647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Huang W, Zhang L, Cheng C, Shan W, Ma R, Yin Z, Zhu C, Parallel comparison of fibroblast-like synoviocytes from the surgically removed hyperplastic synovial tissues of rheumatoid arthritis and osteoarthritis patients, BMC Musculoskelet Disord 20(1) (2019) 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Rao DA, T Cells That Help B Cells in Chronically Inflamed Tissues, Front Immunol 9 (2018) 1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Komatsu N, Takayanagi H, Regulatory T cells in Arthritis, Prog Mol Biol Transl Sci 136 (2015) 207–15. [DOI] [PubMed] [Google Scholar]
- [143].Safari F, Farajnia S, Arya M, Zarredar H, Nasrolahi A, CRISPR and personalized Treg therapy: new insights into the treatment of rheumatoid arthritis, Immunopharmacol Immunotoxicol 40(3) (2018) 201–211. [DOI] [PubMed] [Google Scholar]
- [144].Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH, Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment, Nat Med 23(6) (2017) 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Yamin R, Berhani O, Peleg H, Aamar S, Stein N, Gamliel M, Hindi I, Scheiman-Elazary A, Gur C, High percentages and activity of synovial fluid NK cells present in patients with advanced stage active Rheumatoid Arthritis, Sci Rep 9(1) (2019) 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, Guilak F, Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells, Proc Natl Acad Sci U S A 109(47) (2012) 19172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Willard VP, Diekman BO, Sanchez-Adams J, Christoforou N, Leong KW, Guilak F, Use of cartilage derived from murine induced pluripotent stem cells for osteoarthritis drug screening, Arthritis Rheumatol 66(11) (2014) 3062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Lara Pferdehirt AKR, Jonathan M Brunger, and Farshid Guilak., A Synthetic Gene Circuit for Self-Regulating Delivery of Biologic Drugs in Engineered Tissues, Tissue Engineering Part A 25 (May 2019) 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Haque R, Lei F, Xiong X, Bian Y, Zhao B, Wu Y, Song J, Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity, J Immunol 189(3) (2012) 1228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


