Abstract
Background
Iron deficiency anemia (IDA) and heavy menstrual bleeding are prevalent, interrelated issues impacting over 300 million premenopausal women worldwide. IDA is generally associated with increased platelet counts; however, the effects of IDA and its correction on platelet function in premenopausal women remain unknown.
Objectives
We sought to determine how IDA and intravenous iron affect platelet count and platelet function in premenopausal women.
Methods
Hematologic indices were assessed in a multicenter, retrospective cohort of 231 women repleted with intravenous iron. Pre‐ and postinfusion blood samples were then obtained from a prospective cohort of 13 women to analyze the effect of intravenous iron on hematologic parameters as well as platelet function with flow cytometry and platelet aggregation assays under physiologic shear.
Results
Following iron replacement, anemia improved, and mean platelet counts decreased by 26.5 and 16.0 K/mm3 in the retrospective and prospective cohorts, respectively. Replacement reduced baseline platelet surface P‐selectin levels while enhancing platelet secretory responses to agonists, including collagen‐related peptide and ADP. Platelet adhesion and aggregation on collagen under physiologic shear also significantly increased following repletion.
Conclusion
We find that intravenous iron improves anemia while restoring platelet counts and platelet secretory responses in premenopausal women with iron deficiency. Our results suggest that iron deficiency as well as iron replacement can have a range of effects on platelet production and function. Consequently, platelet reactivity profiles should be further examined in women and other groups with IDA where replacement offers a promising means to improve anemia as well as quality of life.
Keywords: blood platelet count, infusion, intravenous, iron deficiency anemia, platelet activation, women
Essentials.
The effects of iron deficiency on platelets in premenopausal women are poorly understood.
We evaluate platelet count and function iron deficient women receiving intravenous iron.
Intravenous iron decreased platelet count in women with iron deficiency.
Correction of iron deficiency in women enhanced ex vivo platelet reactivity.
1. INTRODUCTION
Iron deficiency is the leading cause of anemia and is especially prevalent in women of reproductive age, affecting the health and well‐being of one in five premenopausal women worldwide. 1 Blood loss from heavy menstrual bleeding (HMB) is commonly associated with iron deficiency anemia (IDA) in premenopausal women, 2 , 3 where deficiencies in hemostasis may synergize a continued cycle of bleeding and iron loss detrimental to health and quality of life. 4 Iron deficiency is associated with thrombocytosis, 5 or increased numbers of blood platelets, the cellular mediators of primary hemostasis, 6 suggesting that iron deficiency itself affects platelet production. Indeed, IDA is readily ameliorated with iron replacement therapy in a manner that also normalizes platelet counts as demonstrated in iron‐deficient blood donors, 7 subjects with anemia related to inflammatory bowel disease (IBD), 8 and patients with chronic kidney disease treated with low‐molecular‐weight iron dextran (LMWID). 9 Multiple studies have evaluated the effects of intravenous iron replacement therapy on anemia and general health in women; however, the effect of intravenous iron replacement on platelet counts in women remains poorly described.
While a number of studies demonstrate that iron replacement therapy improves anemia and reduces platelet counts in several different IDA contexts, the degree to which platelet function is affected by iron deficiency and its treatment with iron replacement therapy are not well understood. A mixed prospective cohort study of male and female adults and children suggested that collagen‐induced platelet aggregation is diminished in iron deficiency and improved with oral iron therapy. 10 In a cohort of patients with IBD and IDA, intravenous iron replacement therapy also decreased platelet counts but reduced platelet agonist reactivity. 11 A recent study of women with IDA demonstrated an increase in arachidonic acid–induced platelet aggregation with oral iron replacement. 12 Platelet agonist responses have also been noted to be diminished in a cohort of children with IDA. 13 Despite accumulating evidence that iron deficiency alters platelet physiology, the mechanisms and ubiquity of this finding remain unknown. Characterization of platelet function in adult premenopausal women undergoing intravenous iron replacement is especially lacking, and further investigation may inform our understanding of hemostatic factors contributing to HMB.
In this study, we aimed to determine whether iron replacement therapy affects platelet count and platelet function in women with IDA. We first examined the effects of iron replacement on anemia and platelet count in a large, retrospective cohort of 231 premenopausal woman with IDA. Then, we carried out a prospective cohort study to assess the effects of iron replacement on anemia and platelet count as well as platelet phenotype and reactivity in 12 women with IDA. Our results suggest that iron replacement improves anemia while lowering platelet counts, reversing a degranulated platelet phenotype to restore platelet reactivity in women with IDA.
2. METHODS
2.1. Retrospective cohort
This study was conducted with the approval of the Oregon Health & Science University (OHSU) Institutional Review Board (IRB; STUDY00019570) before initiation. Women between 18 and 40 years of age with an International Classification of Diseases, Tenth Revision, diagnosis of IDA who underwent intravenous infusion of ferumoxytol or LMWID between April 2018 and October 2020 were identified among OHSU and five affiliated regional outpatient community hematology practices. Women who achieved a state of iron repletion, meeting both criteria of preinfusion ferritin <20 µg/L and postinfusion ferritin ≥50 µg/L were included in our analysis. Preinfusion cell indices were documented from the last clinical lab draw occurring no more than 3 months in advance of the infusion date. Postinfusion cell indices were documented from clinical lab draws closest in time to 7 weeks. Thrombocytosis was defined as platelet counts ≥400 K/mm3. Patients who were pregnant or within 6 months postpartum, those with documented platelet disorders or hematologic malignancies, or those receiving antineoplastic chemotherapy were excluded from this cohort.
2.2. Prospective cohort
Women with levels of ferritin <50 µg/L undergoing iron replacement with a single dose of 1000 mg of LMWID (Infed) were invited to participate in a single‐center prospective study. Of the 17 women contacted between October 1, 2020, and May 31, 2021, 13 chose to participate and gave consent for the study. This study was approved by the OHSU IRB (STUDY00022104) before enrollment of any participants. Women who were pregnant, within 6 months postpartum, or taking antiplatelet therapies were excluded. All participants signed informed consent before initiation of any study procedures. Venous blood samples were collected from participants by venipuncture into 3.8% sodium citrate (1:9, v/v) on the date of infusion (preinfusion) and again at next follow‐up visit (median, 10 weeks; range, 6‐16 weeks). Standard‐of‐care monitoring of iron indices and complete blood counts were performed at both time points and extracted from the electronic health record. The minimum hemoglobin and peak platelet count in the 6 months preceding infusion were used in calculating rates of anemia or thrombocytosis during iron deficiency. Clinical and demographic information was also obtained from the electronic health record. The presumed cause of iron deficiency was acquired from the preinfusion hematology visit note or the documentation from the referring primary provider.
2.3. Flow cytometry analysis of platelet reactivity
Citrate‐anticoagulated blood was diluted 1:5 in modified N‐2‐hydroxyethylpiperazine‐N‐2‐ethane sulfonic acid (HEPES)/Tyrode buffer (HT; 129 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2; pH 7.3). Diluted whole‐blood samples were then aliquoted to a set of tubes preloaded with platelet agonists and fluorescently labeled antibodies. After 30‐minute incubation at 37°C, samples were fixed with 2% paraformaldehyde (PFA), diluted in phosphate buffered saline (PBS), and analyzed with a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA) as previously described for platelet α‐granule secretion and platelet integrin activation. 14 Data were analyzed using FlowJo software (Ashland, OR, USA) and presented as mean fluorescence intensity as previously described. 14
2.4. Platelet adhesion under flow
Ibidi µ‐slide VI chamber channels were coated with 100 µg/mL of fibrillar type I collagen (Chrono‐Log Corp, Havertown, PA, USA), washed with PBS, then blocked with denatured bovine serum albumin before connecting the chamber to a syringe pump. Anticoagulated whole‐blood samples were perfused through each channel at a shear rate of 300 s−1 for 5 minutes, followed by washing for 2 minutes with modified HT buffer at the same shear rate before being fixed in 4% PFA. Channels were imaged using differential interference contrast optics with a Zeiss 40× lens on a Axiovert 200 M microscope (Zeiss, Oberkochen, Germany) and SlideBook 6 software. To compute the surface area and number of platelet aggregates, aggregates were manually outlined and measured in each image using a Java plug‐in for ImageJ as described previously. 15
2.5. Statistical analysis
Pre‐ and postinfusion ferritin and cell counts were compared by two‐sided paired t tests. Rates of thrombocytosis at pre‐ and postinfusion time points were compared by chi‐square test. Fluorescence‐activated cell sorting and adhesion data were analyzed via two‐tailed paired sample t tests. Statistical significance was considered as P < .05. Statistical calculations were performed using Prism 9 (GraphPad Software, San Diego, CA, USA).
3. RESULTS
3.1. Iron replacement decreases platelet count in iron‐deficient women
We sought to evaluate the impact of iron repletion on IDA, platelet count, and other related hematologic indices in premenopausal women. First, we analyzed the medical records of a retrospective cohort of 231 iron‐deficient (ferritin <20 µg/L) women who underwent intravenous infusion with ferumoxytol or LMWID who had achieved iron repletion (ferritin >50 µg/L) at follow‐up. Clinical and demographic characteristics of the retrospective and prospective cohorts for this study are detailed in Table 1. The mean ferritin before infusion was 11.1 (±4.8; range, 2‐19) µg/L and increased to 133 (±80.9; range, 52‐531) µg/L after infusion. In the retrospective cohort, 143 women (62%) were anemic before infusion. Mean preinfusion hemoglobin was 12.1 (±1.5; range, 6.4‐15.5) g/dL and improved to 12.9 (±1.2; range, 4–15.3) g/dL after iron repletion (P < .0001; Figure 1A). Mean hematocrit improved from 37.4% (±4.0; range, 24.5‐47.8) to 39.2% (±3.1; range, 26.6‐47.5; P < .0001; Figure 1B) and red blood cell (RBC) count rose from 4.3 (±0.4; range, 3.2‐5.7) to 4.4 (±0.4; range, 3.1‐5.6) M/mm3 (P < .005) after intravenous iron therapy (Figure 1C). Mean preinfusion platelet count was 288 (±97; range, 46‐991) K/mm3 compared to 261 (±84; range, 43‐892) K/mm3 after infusion (P< .005; Figure 1D). The average change to platelet count with iron infusion was −26.5 K/mm3 (95% confidence interval [CI], −32.1 to −20.93). Thrombocytosis was observed in 18 (7.8%) patients before infusion, and 7 (3.0%) patients after infusion (P < .05). Mean platelet volume (MPV) (Figure 1E) and white blood cell (WBC) count (Figure 1F) were unchanged.
TABLE 1.
Patient demographic and clinical characteristics of the patients at baseline for the prospective cohort (n = 13) and retrospective cohort (n = 231) studies
| Prospective cohort | Retrospective cohort | |
|---|---|---|
| Number of women | 13 | 231 |
| Median age at infusion (IQR) | 36 (29‐47) | 33 (28‐37) |
| Median time to postinfusion labs, wks (IQR) | 10 (8‐12) | 9 (6‐14) |
| Race, n (%) | ||
| White | 12 (92) | 183 (79) |
| Black | … | 14 (6.0) |
| Asian | … | 3 (4) |
| Unknown, declined, or other | 1 (8) | 31 (13) |
| Ethnicity, n (%) | ||
| Non‐Hispanic | 11 (92) | 197 (85) |
| Hispanic | 2 (8) | 22 (10) |
| Unknown or declined | … | 12 (5) |
| Suspected cause of iron deficiency, n (%) | ||
| Heavy menstrual bleeding | 11 (85) | 116 (50) |
| Bariatric surgery | 2 (15) | 25 (11) |
| Insufficient dietary intake | … | 11 (5) |
| Inflammatory bowel disease | … | 13 (6) |
| Gastrointestinal blood loss | … | 10 (4) |
| Unknown etiology or other | … | 56 (24) |
| Preinfusion iron status and anemia | ||
| Iron deficiency without anemia, n (%) | 6 (46) | 88 (38) |
| Iron deficiency with anemia, n (%) | 7 (54) | 143 (62) |
| Iron deficiency with anemia and thrombocytosis, n (%) | 2/7 (15) | 14/143 (6) |
| Preinfusion ferritin, mean (±SD) ng/dL | 14.4 (±9.3) | 11.1 (±4.8) |
Abbreviations: IQR, interquartile range; SD, standard deviation.
FIGURE 1.

Intravenous iron replacement improves hemoglobin and red blood cell indices and reduces platelet count in a retrospective cohort of 231 women with IDA. Clinical laboratory indices for pre‐ and postinfusion time points are shown as bar graphs for (A) hemoglobin, (B) hematocrit, (C) red blood cell count, (D) platelet count (PLT), (E) mean platelet volume, and (F) white blood cell count. **P < .005; ****P < .0001
Given the effects of iron repletion on platelet count noted in the retrospective cohort above, we next prospectively enrolled a cohort of 13 premenopausal women who were iron deficient to examine hematologic indices and to also evaluate any alterations in platelet function that may occur after iron replacement. Eleven patients in this prospective cohort (85%) had iron deficiency attributed to HMB. As seen in Figure 2A, the mean ferritin before infusion was 14.4 (±9.2; range, 3‐37) µg/L and rose to 145 (±78; range 13–258) µg/L after infusion (P<.0001), where one patient failed to achieve iron repletion (>50 µg/L) after infusion and was not included in experiments going forward. The mean 6‐month hemoglobin nadir before infusion was 11.9 (±1.9; range, 8.2‐15.2) g/dL and rose to 13.3 (±1.1; range, 11.1‐15.2) g/dL after infusion (P < .005) (Figure 2B). The mean peak platelet count within 6 months before infusion was 309 K/mm3 (±89; range, 188‐450) compared to 274 (±64; range, 185‐414) K/mm3 after infusion (P < .05) (Figure 2E). The average change in platelet count after infusion was −35.2 K/mm3 (95% CI, −66.2 to −5.23). Like the retrospective cohort above, there was no significant change to MPV (Figure 2F) or WBC counts (Figure 2G) following iron replacement. Together with the findings from a retrospective cohort reported above (Figure 1), these results suggest that iron replacement therapy improves anemia in premenopausal women in a manner associated with a small but significant decrease in platelet count.
FIGURE 2.
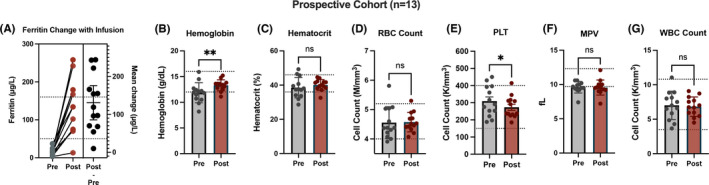
Iron replacement improves anemia and reduces platelet count in a prospective cohort of 13 women with IDA. (A) Change plot for ferritin concentration measured before and after infusion. Individual calculated ferritin change scores, mean change, and 95% confidence interval are provided. Bar graphs of clinical laboratory indices are shown for pre‐ and postinfusion time points for (B) hemoglobin, (C). hematocrit, (D) red blood cell (RBC) count, (E) platelet count (PLT), (F) mean platelet volume (MPV), and (G) white blood cell (WBC) count. *P < .05; **P < .005
3.2. Iron repletion enhances ex vivo platelet hemostatic responses
To determine how iron repletion affects platelet phenotype and reactivity, we used a flow cytometry workflow to analyze platelet α‐granule secretion and integrin αIIbβ3 activation in pre– and post–iron replacement therapy whole‐blood samples from 11 women in our prospective cohort above. Following collection into sodium citrate, whole blood was diluted into HT buffer (1:5) and aliquoted into a set of tubes preloaded with fluorophore‐conjugated antibodies (ie, PAC1‐FITC, CD62‐APC) and platelet agonists, including crosslinked collagen‐related peptide (CRP‐XL), 16 ADP, and thromboxane analog U46619. Previous studies from our group and others have established standardized protocols, where platelet α‐granule secretion and integrin αIIbβ3 activation increases in an agonist concentration–dependent manner. 17 , 18 , 19 , 20 At baseline, surface levels of platelet P‐selectin—a marker of platelet degranulation and α‐granule secretion—significantly decreased on unstimulated platelets in whole‐blood samples after iron repletion (Figure 3A,B). There was no significant change in platelet integrin activation in resting or agonist‐stimulated platelets following iron repletion. However, platelet secretory responses, measured as fold‐change surface P‐selectin, were enhanced in response to CRP‐XL and ADP but not U46619 for post–iron repletion samples. These results from flow cytometry experiments suggest that platelets in women with IDA circulate in a mildly degranulated state that corrects with iron repletion, restoring or enhancing platelet reactivity.
FIGURE 3.
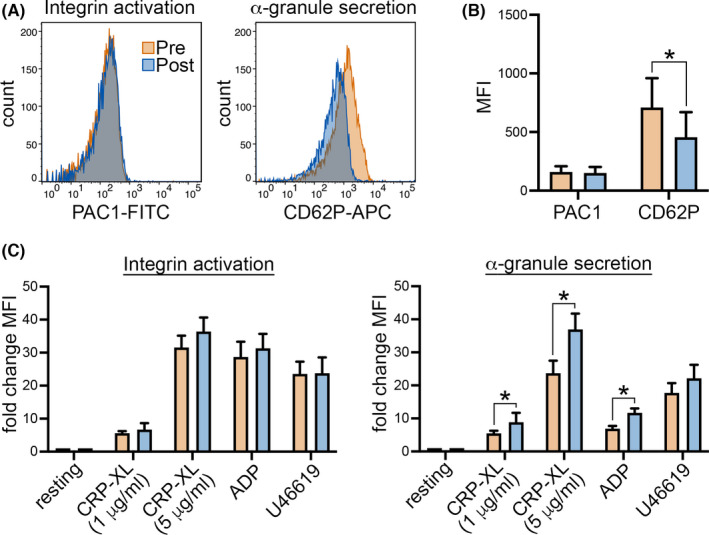
Iron replacement decreases P‐selectin on quiescent platelets from women with IDA while increasing platelet secretory reactivity. Pre‐ and postinfusion whole‐blood samples were collected from 11 women with IDA in the prospective cohort for flow cytometry analysis of platelet integrin activation and degranulation. (A) Representative histogram of (B) mean fluorescence intensity (MFI) of platelet PAC1 and CD62 expression as measured by flow cytometry for platelets in the absence of agonist stimulation, and (C) following 30‐minute incubation with crosslinked collagen‐related peptide (CRP‐XL), ADP, and the thromboxane analog U46619, before and after intravenous iron replacement. *P < .05
To assess the effects of IDA and iron replacement therapy on platelet function in a physiological context, we measured platelet adhesion and aggregation as whole blood was passed over type I collagen at venous shear (300 s−1 for 5 minutes), utilizing pre– and post–iron replacement blood samples from nine women in the prospective cohort. As seen in Figure 4, platelet adhesion and aggregate formation on collagen significantly increased in all paired blood samples after iron replacement therapy. The mean surface coverage was 18% before infusion versus 31% after infusion (P < .005) (Figure 4). Together with the flow cytometry experiments above, these results demonstrate that iron replacement therapy enhances aspects of platelet reactivity ex vivo in whole‐blood samples from women with IDA.
FIGURE 4.
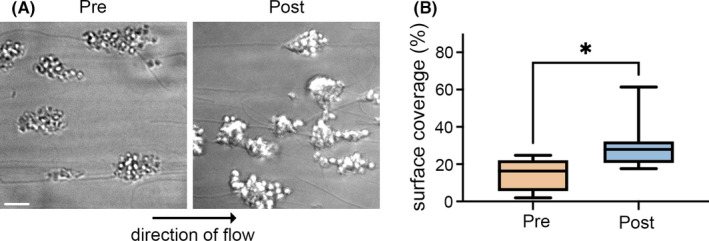
Iron replacement increases platelet aggregate formation on collagen in whole blood under shear. Whole‐blood samples from women (n = 9) before (Pre) and after (Post) intravenous iron were passed over type I collagen at venous shear rate (300 s−1, 5 minutes). (A) After fixation, platelet aggregates were imaged with differential interference contrast microscopy to determine (B) percentage of total surface covered by platelet aggregates before and after intravenous iron. Scale bar = 10 μm. *P = .02
4. DISCUSSION
In this study, we examined the contributions of IDA and its correction with iron replacement therapy to platelet count and platelet function in two cohorts of premenopausal women. In a large retrospective cohort of 231 premenopausal women with IDA, we found higher rates of thrombocytosis and noted a decrease in mean platelet counts with iron repletion. Similar findings were also apparent in a prospective cohort of 13 adult women with varying degrees of iron deficiency, where we measured a decrease in mean platelet count following iron replacement therapy. While it has previously been noted that iron replacement therapy can lower platelet counts in other contexts of IDA (ie, IBD, 8 , 11 chronic kidney disease 9 ), our study provides support for this phenomenon occurring in premenopausal women with iron deficiency, a condition strongly associated with HMB and diminished self‐reported quality of life. 21 In addition to decreasing platelet counts, iron repletion also decreased levels of P‐selectin on the surface of quiescent platelets from women with IDA while enhancing platelet degranulation upon exposure to CRP‐XL or ADP. Platelet adhesion to fibrillar collagen at venous shear rates also significantly increased after intravenous iron. Overall, our observations suggest that iron levels impact platelet count and may also have effects on platelet function in hemostasis.
Our study finds that iron replacement therapy decreases mean platelet counts in premenopausal women with IDA to a similar extent as other IDA patient groups treated with iron replacement therapies. For instance, in a retrospective cohort of adult blood donors, Eder et al 7 observed a decrease in platelet count (−19.8 K/mm3) in a subgroup analysis of female blood donors of any age with baseline ferritin <20 µg/L treated with oral iron. Recent studies have begun to investigate mechanistic underpinnings of these findings. Interestingly, megakaryocytes share a mutual precursor cell with erythrocytes, and recent studies have demonstrated that megakaryocytic/erythroid progenitor cells (MEPs) are sensitive to the body’s iron status. Using both genetic mouse models with homozygous deletions of the Tmprss6 gene (simulating the physiologic conditions of iron deficiency), and mouse models with acquired dietary iron deficiency, investigators have shown that MEPs preferentially differentiate toward the production of megakaryocytes in iron‐deficient states. 22 The mechanism regulating this differentiation during iron deficiency has not been demonstrated, though it appears to be independent of thrombopoietin. 23 On a more general level, it has been posited that thrombocytosis during IDA may be an adaptation to augment hemostasis in anemia related to blood loss. 24 An example of this phenomenon was recently demonstrated by Jimenez et al, 25 which observed elevated platelet counts in conjunction with augmented hemostatic responses to tail injury that reversed after iron repletion in a rat model of IDA.
A number of studies have noted increased platelet counts in IDA that decrease with iron replacement; however, the effects of IDA and its reversal with iron replacement therapy on platelet reactivity and function remain relatively unstudied. With flow cytometry analyses of whole blood, we found that iron repletion decreased P‐selectin levels on the surfaces of platelets from women with IDA (Figure 3). These findings suggest that platelets may circulate in a degranulated state in women with IDA in a manner similar to some chronic inflammatory states or trauma, 26 , 27 , 28 where exhausted platelets are refractory to hemostatic activation. Indeed, while iron repletion decreased levels of P‐selectin on quiescent platelets, iron repletion also enhanced platelet reactivity in response agonists—specifically, P‐selectin exposure (Figure 3). These may suggest that platelets degranulate in circulation and lose reactivity in a manner restored by iron repletion. Jiminez et al 25 similarly found that expression of P‐selectin was greater on platelets in iron deficiency than in nondeficient controls or iron‐deficient rodents following repletion. In agreement with iron replacement therapy restoring platelet secretory activities relevant to hemostasis in women with IDA, we also found a significant increase in platelet adhesion and aggregate formation when whole blood was flowed over collagen after iron repletion (Figure 4).
While our study offers some new insights into relationships between iron, platelets, and hemostasis in premenopausal women, the clinical implications of iron deficiency–associated thrombocytosis and alterations in platelet function remain unclear. For instance, in a large retrospective cohort study including 36 327 patients with IDA, thrombocytosis (defined in their study as a platelet count >450 × 109/L) was present in 32.6% of these cases, 29 and rates of thrombotic events were high and appeared to be associated with thrombocytosis. Other recent studies have also noted an upregulation of coagulation factor enzyme activities in IDA that can resolve following iron replacement therapy. 30 These and other similar findings suggest that IDA promotes thrombocytosis as well as thrombosis in some contexts, despite a general loss of platelet hemostatic function. In this study, we find that platelet reactivity increases following iron repletion; however, it is not clear if platelet reactivity is low in IDA or enhanced beyond normal levels following iron repletion. Overall, the clinical significance of iron deficiency–associated thrombocytosis and thrombotic risk in women who are iron deficient requires further investigation.
Our findings further suggest that specific molecular and cellular mechanisms may underlie platelet hyporesponsiveness in iron‐deficient states, especially for women with IDA. Our results are consistent with some prior studies showing diminished platelet aggregation in adult 10 , 31 and pediatric 32 , 33 patients with IDA, which improved after iron replacement therapy. These findings have not been consistent in the literature, however, as other analyses in certain settings such as pediatric patients with IDA 34 or patients with IBD and thrombocytosis 11 have reported the reverse effect. It is possible that platelet responses to iron deficiency differ based on the level of iron deficiency. In fact, there is evidence that iron induces platelet aggregation in a dose‐dependent manner. One such study investigated the role of iron in modulating platelet aggregation in response to collagen through free‐radical formation created in the Fenton reaction, where hemoglobin released from damaged RBCs is capable of inducing platelet aggregation, which is inhibited by radical scavengers. 35 In whole blood, platelet reactivity and aggregation was influenced by the availability of both endogenous and exogenous sources of Fe2+, which acted as a source of hydroxyl radicals that act on platelets through a protein kinase C–mediated mechanism. 36 This suggests that platelet function is impaired in the absence of iron. However, while platelet activation may be impaired in iron deficiency, there may also be elevated risk of thrombotic events in a manner related to, or independent of, platelet effects. The relevance of prothrombotic tendencies versus impaired platelet hemostatic activities in iron deficiency demands further evaluation in larger clinical and mechanistic studies.
4.1. Limitations
The strengths of our study include the use of mixed prospective and retrospective methods for evaluating changes to platelet count and other cell indices in women undergoing iron replacement. We applied multiple approaches to begin to explore a poorly understood relationship between iron deficiency and platelet function in premenopausal women. Limitations of our study stem from the small size of our prospective cohort, where it was not possible to draw robust conclusions about the clinical significance of these changes to platelet count and function. Moreover, due to the limited scope of our clinical study, only a small set of platelet indices were examined. For instance, clinical blood samples were not collected to assess intracellular signaling events or levels of phosphatidylserine exposure on prothrombotic platelets. Future studies will explore additional mechanistic questions in depth, aiming to uncover the biochemical pathways affected by iron levels in platelets with proteomics and systems biology tools 37 as well as microscopy 38 and other ex vivo methods.
5. CONCLUSION
In this study, we combined retrospective and prospective analyses to demonstrate that iron replacement therapy significantly reduces platelet count in premenopausal women with IDA while decreasing quiescent platelet P‐selectin surface expression, enhancing platelet degranulation upon exposure to agonists, and increasing platelet adhesion to fibrillar collagen at physiological sheer rates.
RELATIONSHIP DISCLOSURES
JJS receives consulting fees from Aronora, Inc. All other authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
BKE, TGD, JEA, and JJS conceived and designed this study. BKE, HHSL, ARM, KRJ, CY, DP, JEA, and JJS developed the methodology and collected and analyzed data. BKE, JEA, and JJS wrote the initial manuscript. BKE, KRJ, KLM, HSM, CL, TGD, and JJS procured and organized clinical data and samples. BKE, JOL, SRO, TGD, JEA, and JJS provided administrative, technical, and material support. All authors contributed to writing and editing the manuscript.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01HL151367 (to JJS) and R01HL146549 (to JEA)
Elstrott BK, Lakshmanan HHS, Melrose AR, et al. Platelet reactivity and platelet count in women with iron deficiency treated with intravenous iron. Res Pract Thromb Haemost. 2022;6:e12692. doi: 10.1002/rth2.12692
Joseph E. Aslan and Joseph J. Shatzel are senior authors and contributed equally to this work.
Handling Editor: Dr Neil Zakai
Funding information
This work was supported by the the National Heart, Lung, and Blood Institute/National Institutes of Health (R01HL146549 to JEA and R01HL151367 to JJS) and the American Society of Hematology (JEA).
Contributor Information
Joseph E. Aslan, @JoePlatelet.
Joseph J. Shatzel, Email: shatzel@ohsu.edu, @Clotmaster.
REFERENCES
- 1. Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615‐624. doi: 10.1182/blood-2013-06-508325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Camaschella C. Iron‐deficiency anemia. N Engl J Med. 2015;372(19):1832‐1843. doi: 10.1056/NEJMra1401038 [DOI] [PubMed] [Google Scholar]
- 3. Elstrott B, Khan L, Olson S, Raghunathan V, DeLoughery T, Shatzel JJ. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104(3):153‐161. doi: 10.1111/ejh.13345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurpad AV, Ghosh S, Thomas T, et al. Perspective: when the cure might become the malady: the layering of multiple interventions with mandatory micronutrient fortification of foods in India. Am J Clin Nutr. 2021;114(4):1261‐1266. doi: 10.1093/ajcn/nqab245 [DOI] [PubMed] [Google Scholar]
- 5. Park MJ, Park PW, Seo YH, et al. The relationship between iron parameters and platelet parameters in women with iron deficiency anemia and thrombocytosis. Platelets. 2013;24(5):348‐351. doi: 10.3109/09537104.2012.699641 [DOI] [PubMed] [Google Scholar]
- 6. Aslan JE. Platelet shape change. In: Gresele P, López J, Kleiman N, Page C, eds. Platelets in Thrombotic and Nonthrombotic Disorders. Springer; 2017:321–336. [Google Scholar]
- 7. Eder AF, Yau YY, West K. The effect of iron balance on platelet counts in blood donors. Transfusion. 2017;57(2):304‐312. doi: 10.1111/trf.13881 [DOI] [PubMed] [Google Scholar]
- 8. Kulnigg‐Dabsch S, Evstatiev R, Dejaco C, Gasche C. Effect of iron therapy on platelet counts in patients with inflammatory bowel disease–associated anemia. PLoS One. 2012;7(4):e34520. doi: 10.1371/journal.pone.0034520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yessayan L, Yee J, Zasuwa G, Frinak S, Besarab A. Iron repletion is associated with reduction in platelet counts in non‐dialysis chronic kidney disease patients independent of erythropoiesis‐stimulating agent use: a retrospective cohort study. BMC Nephrol. 2014;15(1):119. doi: 10.1186/1471-2369-15-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Çalişkan Ü, Öner AF, Kabakuş N, Koç H. Diminished platelet aggregation in patients with iron deficiency anemia. Clin Appl Thromb Hemost. 1999;5(3):161‐163. doi: 10.1177/107602969900500304 [DOI] [PubMed] [Google Scholar]
- 11. Kulnigg‐Dabsch S, Schmid W, Howaldt S, et al. Iron deficiency generates secondary thrombocytosis and platelet activation in IBD: the randomized, controlled thromboVIT trial. Inflamm Bowel Dis. 2013;19(8):1609‐1616. doi: 10.1097/MIB.0b013e318281f4db [DOI] [PubMed] [Google Scholar]
- 12. Akay OM, Akin E, Mutlu FS, Gulbas Z. Effect of iron therapy on platelet function among iron‐deficient women with unexplained menorrhagia. Pathophysiol Haemost Thromb. 2008;36(2):80‐83. doi: 10.1159/000173726 [DOI] [PubMed] [Google Scholar]
- 13. Yildirim ZK, Orhan MF, Buyukavci M. Platelet function alterations and their relation to P‐selectin (CD62P) expression in children with iron deficiency anemia. Blood Coagul Fibrinolysis. 2011;22(2):98‐101. doi: 10.1097/MBC.0b013e328342791b [DOI] [PubMed] [Google Scholar]
- 14. Babur O, Melrose AR, Cunliffe JM, et al. Phosphoproteomic quantitation and causal analysis reveal pathways in GPVI/ITAM‐mediated platelet activation programs. Blood. 2020;136(20):2346‐2358. doi: 10.1182/blood.2020005496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zilberman‐Rudenko J, Sylman JL, Lakshmanan HHS, McCarty OJT, Maddala J. Dynamics of blood flow and thrombus formation in a multi‐bypass microfluidic ladder network. Cell Mol Bioeng. 2017;10(1):16‐29. doi: 10.1007/s12195-016-0470-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma‐chain in collagen‐stimulated platelets. J Biol Chem. 1996;271(30):18095‐18099. doi: 10.1074/jbc.271.30.18095 [DOI] [PubMed] [Google Scholar]
- 17. Dunster JL, Bye AP, Kriek N, et al. Multiparameter phenotyping of platelet reactivity for stratification of human cohorts. Blood Adv. 2021;5(20):4017‐4030. doi: 10.1182/bloodadvances.2020003261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huskens D, Sang Y, Konings J, et al. Standardization and reference ranges for whole blood platelet function measurements using a flow cytometric platelet activation test. PLoS One. 2018;13(2):e0192079. doi: 10.1371/journal.pone.0192079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parra‐Izquierdo I, Lakshmanan HHS, Melrose AR, et al. The toll‐like receptor 2 ligand Pam2CSK4 activates platelet nuclear factor‐kappaB and Bruton's tyrosine kinase signaling to promote platelet‐endothelial cell interactions. Front Immunol. 2021;12:729951. doi: 10.3389/fimmu.2021.729951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel A, Kostyak J, Dangelmaier C, et al. ELMO1 deficiency enhances platelet function. Blood Adv. 2019;3(4):575‐587. doi: 10.1182/bloodadvances.2018016444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strauss WE, Auerbach M. Health‐related quality of life in patients with iron deficiency anemia: impact of treatment with intravenous iron. Patient Relat Outcome Meas. 2018;9:285‐298. doi: 10.2147/PROM.S169653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xavier‐Ferrucio J, Scanlon V, Li X, et al. Low iron promotes megakaryocytic commitment of megakaryocytic‐erythroid progenitors in humans and mice. Blood. 2019;134(18):1547‐1557. doi: 10.1182/blood.2019002039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evstatiev R, Bukaty A, Jimenez K, et al. Iron deficiency alters megakaryopoiesis and platelet phenotype independent of thrombopoietin. Am J Hematol. 2014;89(5):524‐529. doi: 10.1002/ajh.23682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brissot E, Troadec MB, Loreal O, Brissot P. Iron and platelets: a subtle, under‐recognized relationship. Am J Hematol. 2021;96(8):1008‐1016. doi: 10.1002/ajh.26189 [DOI] [PubMed] [Google Scholar]
- 25. Jimenez K, Leitner F, Leitner A, et al. Iron deficiency‐induced thrombocytosis increases thrombotic tendency in rats. Haematologica. 2021;106(3):782‐794. doi: 10.3324/haematol.2019.245092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pareti FI, Capitanio A, Mannucci L, Ponticelli C, Mannucci PM. Acquired dysfunction due to the circulation of "exhausted" platelets. Am J Med. 1980;69(2):235‐240. doi: 10.1016/0002-9343(80)90383-6 [DOI] [PubMed] [Google Scholar]
- 27. Kim OV, Nevzorova TA, Mordakhanova ER, et al. Fatal dysfunction and disintegration of thrombin‐stimulated platelets. Haematologica. 2019;104(9):1866‐1878. doi: 10.3324/haematol.2018.202309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baaten C, Ten Cate H, van der Meijden PEJ, Heemskerk JWM. Platelet populations and priming in hematological diseases. Blood Rev. 2017;31(6):389‐399. doi: 10.1016/j.blre.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 29. Song AB, Kuter DJ, Al‐Samkari H. Characterization of the rate, predictors, and thrombotic complications of thrombocytosis in iron deficiency anemia. Am J Hematol. 2020;95(10):1180‐1186. doi: 10.1002/ajh.25925 [DOI] [PubMed] [Google Scholar]
- 30. Nashashibi J, Avraham GR, Schwartz N, Awni Y, Elias M. Intravenous iron treatment reduces coagulability in patients with iron deficiency anaemia: a longitudinal study. Br J Haematol. 2019;185(1):93‐101. doi: 10.1111/bjh.15765 [DOI] [PubMed] [Google Scholar]
- 31. Pati HP, Bhargava VL, Saraya AK. Platelet hypoaggregation in iron deficiency anaemia: reversible with therapy. Platelets. 1990;1(2):85‐87. doi: 10.3109/09537109009005467 [DOI] [PubMed] [Google Scholar]
- 32. Kurekci AE, Atay AA, Sarici SU, Zeybek C, Koseoglu V, Ozcan O. Effect of iron therapy on the whole blood platelet aggregation in infants with iron deficiency anemia. Thromb Res. 2000;97(5):281‐285. doi: 10.1016/s0049-3848(99)00150-4 [DOI] [PubMed] [Google Scholar]
- 33. Mokhtar GM, Ibrahim WE, Kassim NA, Ragab IA, Saad AA, Abdel Raheem HG. Alterations of platelet functions in children and adolescents with iron‐deficiency anemia and response to therapy. Platelets. 2015;26(5):448‐452. doi: 10.3109/09537104.2014.931570 [DOI] [PubMed] [Google Scholar]
- 34. Kabakus N, Yilmaz B, Caliskan U. Investigation of platelet aggregation by impedance and optic methods in children with iron deficiency anaemia. Haematologia (Budap). 2000;30(2):107‐115. doi: 10.1163/15685590051130128 [DOI] [PubMed] [Google Scholar]
- 35. Iuliano L, Violi F, Pedersen JZ, Pratico D, Rotilio G, Balsano F. Free radical–mediated platelet activation by hemoglobin released from red blood cells. Arch Biochem Biophys. 1992;299(2):220‐224. doi: 10.1016/0003-9861(92)90267-z [DOI] [PubMed] [Google Scholar]
- 36. Pratico D, Pasin M, Barry OP, et al. Iron‐dependent human platelet activation and hydroxyl radical formation: involvement of protein kinase C. Circulation. 1999;99(24):3118‐3124. doi: 10.1161/01.cir.99.24.3118 [DOI] [PubMed] [Google Scholar]
- 37. Aslan JE. Platelet proteomes, pathways, and phenotypes as informants of vascular wellness and disease. Arterioscler Thromb Vasc Biol. 2021;41(3):999‐1011. doi: 10.1161/ATVBAHA.120.314647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aslan JE, Itakura A, Gertz JM, McCarty OJ. Platelet shape change and spreading. Methods Mol Biol. 2012;788:91‐100. doi: 10.1007/978-1-61779-307-3_7 [DOI] [PubMed] [Google Scholar]


