Abstract
Tacrolimus is a calcineurin inhibitor (CNI), an immunosuppressive agent used to prevent graft versus host disease following allogeneic hematopoietic cell transplantation (HCT). Side-effects of tacrolimus treatment include neuropsychiatric symptoms, for example, affective disturbances, psychosis, and akinetic mutism. The onset of side-effects is independent of tacrolimus blood concentration and can occur years after treatment initiation. To our knowledge, case-reports describing tacrolimus-induced neuropsychiatric symptoms following HCT are sparse. This article reports the case of a 60-year-old woman with T-cell prolymphocytic leukemia, who developed memory loss, affective disturbances, and delusions, 1-year after HCT, and tacrolimus treatmentinitiation. Upon hospital admission, she was motionless and mute, albeit easily roused. The routine physical examination was without pathological findings. Blood work and microbiological analyses of blood and cerebrospinal fluid were normal. The neuroimaging showed chronic structural changes without relation to the debut of neuropsychiatric symptoms. Tacrolimus was discontinued on suspicion of tacrolimus-induced neuropsychiatric symptoms. The patient recovered within 48 hours of discontinuation. She was switch to prednisone treatment, and there has been no reemergence of neuropsychiatric symptoms since.
Keywords: Hematopoietic stem cell transplantation, Immunosuppressants, Adverse effects, Psychiatric disorder
Introduction
Calcineurin inhibitors (CNIs) are used to ensure engraftment and prevent Graft versus host disease (GVHD) following allogeneic hematopoietic cell transplantation. CNIs include tacrolimus and cyclosporine, immunosuppressive agents that inhibit T-cell proliferation and differentiation. 1
Side effects comprise neuropsychiatric symptoms, for example, disturbed sleep/wake cycle and affective disturbances, and in rare cases memory deficits, and akinetic mutism. 2 CNIs are also associated with Posterior Reversible Leukoencephalopathy Syndrome (PRES), characterized by hypertension, altered mental status, and brain edema. 3 The underlying mechanisms resulting in CNIs related side effects are not fully understood, but damage to the blood-brain barrier, cerebral vasospasm, mitochondrial dysfunction, and subcortical edema has been suggested. 2 Blood concentrations above therapeutic levels may increase the risk for side effects. 4 Cytochrome P450 enzyme deficiency, and the co-administration of CYP3A4 inhibitors, for example, clarithromycin, can cause concentration spikes. However, side effects have also occurred in patients with therapeutic blood concentrations, even years after treatment initiation,5,6 making the clinical diagnoses a challenge.
The discontinuation of CNIs is not without complications; however, patients with severe psychiatric symptoms have reduced medical compliance and, consequently, increased risk of complications, for example, GVHD.
To our knowledge, there are no guidelines for the management of CNIs-related neuropsychiatric symptoms. The literature is sparse, especially the literature describing side effects following hematopoietic stem cell transplantation.
This article reports the case of a patient who, 1-year post-hematopoietic stem cell transplantation, was admitted to the hospital with tacrolimus-related neuropsychiatric symptoms.
Case Report
A 60-year-old woman with T-cell prolymphocytic leukemia (T-PLL) and chronic Graft versus host disease (GVHD) in the skin and lungs was admitted to the hospital with neuropsychiatric symptoms.
The patient was in stable treatment with photopheresis, tacrolimus (0.25 mg/daily), and steroids (7.5 mg/daily). She was recovering from pneumonia and had been treated with clarithromycin (1000 mg/daily) for 3 days.
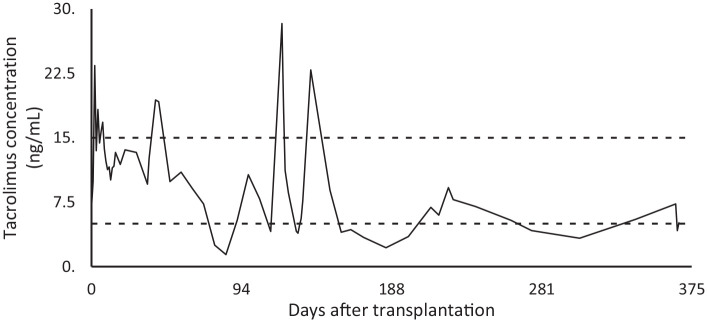
The patient received her last chemotherapy treatment in July 2019, followed by radiation therapy (3 Gy total body irradiation at 6 to 7 cGy/min from a linear accelerator.) in November 2019. Subsequently, she underwent allogeneic hematopoietic cell transplantation (HCT) with a matched related donor. Post-transplantation, prophylaxis against GVHD was initiated, consisting of tacrolimus 0.06 mg/kg. Tacrolimus blood trough levels were targeted between 10 and 15 ng/mL for the first 28 days and thereafter, between 7.5 and 15 ng/mL. On several occasions, blood tacrolimus concentrations had exceeded upper therapeutic levels (Figure 1). Consequently, doses had been reduced and supplemented with steroids.
Figure 1.
Tacrolimus blood concentration measurements.
Despite her illness, the patient was doing well and had a thriving family and social life. She had no history of substance abuse or mental illness but was predisposed to bipolar disorder (BD) through her mother.
The husband reported that the patient had debuted with an acute onset of short-term memory deficit 4 weeks before admission. Symptoms had progressed to involve affective disturbances, dismissive and aggressive behavior.
Later, she developed delusions of persecution, accusing her husband of mal intentions—specifically that he was responsibly for her illness without her being able to go into any details on how. According to the husband, the patient had maintained her regular sleeping pattern, going to bed at 9 PM and getting up at 7 AM. Her medication was self-administered, and the level of compliance was unknown.
The patient was voluntarily admitted; She cooperated with blood test and neuroimaging but could not comply with the physical examination. She was mainly unresponsive to verbal stimuli but moved in response to pain stimuli. She appeared to be awake, but her eyes were closed. Mostly, she was thronged up in the same position, motionless and mute but easily roused. On occasions, she became physically aggressive toward the health care professionals; other times, she urinated on the floor, remaining motionless on the floor, gazing into the room. However, sometimes she would respond to repeated commands and carry them out in an insufficient manner.
Passive movements of the neck and extremities were normal with no hypertonia. Pupillary light reflex, cornea reflex, and oculocephalic reflex were also normal, as were the plantar reflexes.
She was hypertensive (180/91) with tachycardia (97); other vital signs were normal. Treatment with acyclovir and solumedrol was initiated on suspicion of viral encephalopathy.
Blood tests showed low hemoglobin (6.1 mmol/L), elevated thrombocytes (119 E 9 /L), and lactate dehydrogenase levels (361 E 9 /L). C-reactive protein (5 mg/L), leukocytes (4.3 E 9 /L), creatinine (71 μmol/L) and eGFR (79 mL/min) measurements were normal.
Analyses of the cerebrospinal fluid (CSF): glucose (2.8 mmol/L), lactate (1.3 mmol/l), protein 0.41 (g/L), erythrocytes (<300 E 6 /L), and polymorphonuclear leukocytes (<3 E 6 /L), were also normal. Microbiological analyses were equally without pathological findings.
Magnetic resonance imaging (MR) of the cerebrum showed white matter hyperintensities, probable of ischemic microvascular origin. There were no indications of large vessel occlusions, hemorrhage, tumors, or posterior leukoencephalopathy (PRES).
Antipsychotic treatment with haloperidol pro-necessitates was initiated. The patient received 0.5 mg intravenously on the first day of admission and 1 mg on the second. On admission day three, she was switched to peroral haloperidol treatment, 2.5 mg/daily, and tacrolimus was discontinued on suspicion of tacrolimus-induced neuropsychiatric symptoms.
Thirty-six hours after discontinuation, the patient showed signs of rapid recovery. She began to speak, presenting with elusive delusions of persecution and suicidal thoughts. After 48 hours, she was without psychotic symptoms and suicidal thoughts and had regained her habitual functioning level. She remained admitted for an additional 2 days for observation during the phasing out haloperidol. In total, she received 9 mg haloperidol during the 4-day admission. Tacrolimus treatment was never reinitiated but replaced with steroid treatment 37.5 mg/day. By February 2021, there had been no recurrence of psychotic symptoms.
Discussion
A 60-year-old woman with T-PLL and chronic GVHD was admitted to the hospital with dismissive, motionless, and mute behavior. The symptoms could indicate several conditions, for example, delirium, schizophrenia, progressed affective disorder, catatonic syndrome, and akinetic mutism.
Delirium
The patient was awake, responsive to pain stimuli but mostly unresponsive to verbal stimuli. Consequently, orientation could not be tested. On occasions, she had left the bed and urinated on the floor. Her deviant behavior tended to be fluctuating and more pronounced in the evenings, compatible with delirium. 7 However, the patient slept 5 to 7 hours most nights during the hospital stay, which is not normally observed in patients with delirium7,8 (Table 1). Paraclinical analyses excluded inflammations and infections. Vitamin concentrations were not measured. Although cobalamin and folate deficiency could theoretically have caused a delirious state, symptoms remitted without vitamin supplements. Likewise, thyroid-stimulating hormone (TSH) was not measured upon admission but had previously been within normal range and symptoms remitted
Table 1.
Clinical presentation.
| Delirium DSM-5 diagnostic criteria |
Findings |
|---|---|
| Disturbance in attention: that is, reduced ability to direct, focus, sustain and shift attention, and reduced orientation to the environment. | ✓ |
| The disturbance develops over hours to a few days, and tends to fluctuate in severity. | |
| Addtional disturbance in cognition: for example, memory deficit, disorientation, or perception. | ✓ |
| The disturbances in Criteria A and C are not better explained by a pre-existing, established or evolving neurocognitive disorder. | ✓ |
| The disturbance is a direct physiological consequence of another medical condition, substance intoxication or withdrawal. | |
| Catatonia DSM-5 diagnostic criteria ( ⩾3) | |
| Catalepsy | |
| Stupor | |
| Agitation | |
| Abnormal posturing | |
| Negativism | ✓ |
| Mutism | ✓ |
| Flixibilitas cerea | |
| Mannerisms | |
| Stereotypy | |
| Grimacing | |
| Echolalia | |
| Echopraxia | |
| Akinetic mutism Main clinical features | |
| Excessive sleep | |
| Easely roused | ✓ |
| Akinesis, or movements only in response to pain stimulus | ✓ |
| Mutism, or expression of few words. | ✓ |
| Attention to movements of objects, or reaction to sounds | ✓ |
| Oft-repeated commands might be carried out | ✓ |
Catatonia
Catatonia is a psychomotor symptom-complex disorder characterized by behavioral changes, abnormal movements, and immobility. Catatonia can be observed in several conditions, for example, schizophrenia and affective disorders, neurological and other medical conditions.7,9,10
The patient presented with delusions of persecution, although not of a bizarre character. She had no visual or auditory hallucinations or first-rank symptoms, for example, thought insertion or withdrawal, thought broadcasting, delusions of control. Also, the psychotic symptoms had lasted less than a month; hence, she did not fulfill the ICD-10 criteria for schizophrenia. 7
Catatonic symptoms are most frequently observed in patients with mood disorders. 9 The patient was predisposed to bipolar disorder (BD) through her mother; It has been suggested that predisposition is associated with an early onset of symptoms. 11 Albeit the patient was 60-year of age when she debuted with catatonic symptoms. Furthermore, she had no history of depressive or maniform episodes or suicidal behavior. As a Danish citizen, the patient is registered in the national Danish Civil Registration System including the patient’s complete medical history. Consulting the database showed no prior admission to any Danish hospital with psychiatric symptoms.
Neurological and other medical conditions
The Diagnostic and Statistical Manual of Mental Disorders (DSM–5) is equivalent to the ICD-10.7,9 The DSM-5 catatonic syndrome involves (at least) 3 of 12 psychomotor symptoms.10,12 In the present case, the patient was mute and exhibited negativism, lack of response to verbal stimuli and instructions. She was motionless but without signs of rigidity, hence, symptoms did not fulfill the diagnostic criteria for the catatonic syndrome 10 (Table 1).
Akinetic mutism syndrome
Akinetic mutism syndrome is a rare neuropsychiatric side effect of tacrolimus treatment. 13 Patients with akinetic mutism have impaired voluntary muscle movement and speech to various extents. The patients can be unresponsive, respond solely to pain stimuli, but episodes of intentional movements have also been reported, and patients can be easily roused.2,14,15
The description matches the clinical presentation in the present case; the patient was mute and motionless but moved in response to pain stimuli and sometimes repeated requests. She had elusive episodes of intentional movements and was easily roused (Table 1).
Dementia
Neuroimaging showed small non-specific microvascular changes in the subcortical white matter. Small vessel dementia was considered; however, the cognitive deterioration is often slow, with stepwise functional decline. 16 In contrast, the patient suffered a sudden onset of globally impaired cognition. The neuroimaging was evaluated by a board-certified cognitive neurologist, who concluded that the radiological changes were not the origin of symptoms. Large vessel occlusions, hemorrhage, and tumors were not found.
Encephalopathy
Diagnostic workup to exclude encephalopathy was performed; Leukocytes and CRP levels were within the normal range, and the CSF and microbiology were negative for pathogens. In rare cases, treatment with CNIs can cause PRES, involving hypertension, altered mental status, and usually structural changes on neuroimaging; PRES has various radiographic presentations but often includes frontal and occipital edema, although, in rare cases, the imaging is normal. 17 The patient was hypertensive but without radiographic signs of PRES. The diagnosis seemed less plausible but could not be excluded.
Transplant-associated thrombotic microangiopathy (TA-TMA)
TA-TMA is a severe complication of HSCT that can manifest as acute anemia and posterior leukoencephalopathy syndrome (PRESS) 17 Although the patient was anemic (6.1 mmol/mL) upon admission, her hemoglobin levels had fluctuated since she underwent HSCT.
She also presented with hypertension (180/91 mmHg) which raised the suspicion of PRES. The radiographic manifestation of PRESS most often includes frontal and occipital edema. 17 Albeit, in this case, there were no signs of brain edemas on the CTC.
Drug interactions
The medical record was scrutinized for potential drug interactions, 1 problematic co-administration of tacrolimus and clarithromycin, was identified. The liver enzyme CYP3A4 metabolizes tacrolimus. Since clarithromycin is a CYP3A4 inhibitor 18 the co-administration might have caused spikes in blood tacrolimus levels.
The most reported side effect to co-administration is renal failure; However, eGFR and creatinine levels were normal. Also, the presentation of symptoms occurred weeks before the clarithromycin regimen was initiated.
Tacrolimus blood concentrations
The patient had a history of mal controlled blood tacrolimus, exceeding upper therapeutic levels of 15 ng/mL (Figure 1). Whether the concentration spikes are due to blood sampling before the daily tacrolimus administration is uncertain. During a day, normal blood concentrations can rise to 20 ng/mL.19,20 In addition, CNIs-induced neuropsychiatric symptoms have been described in patients with therapeutic blood concentrations.21-23
Conclusion
The patient suffered a sudden onset of memory deficits. Over 4 weeks the symptoms developed to involve delusions, dismissive and catatonic-like behavior. The symptomatology was mimicking delirium and catatonia, but mostly akinetic mutism (Table 1). Paraclinical were normal, except for habitual fluctuations in hemoglobin and thrombocytes. Radiographic findings could not explain the patient’s symptoms and excluded acute pathology. Symptoms remitted after tacrolimus discontinuation and haloperidol administration. The patient has remained asymptomatic after tacrolimus replacement with steroids and phasing out of haloperidol.
Tacrolimus-induced neuropsychiatric symptoms cannot be verified by biomarkers or neuroimaging, and symptoms can occur even years after treatment initiation. The diagnosis is characterized by rapid recovery and complete remission in symptoms in response to tacrolimus discontinuation, and changes in the treatment regimen, for example, to mycophenolate and prednisone.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Linda W. Loehde: Conceptualization, methodology, investigation, writing, original draft preparation, editing, and visualization.
Adrian Bentzon: Conceptualization, methodology, investigation, writing, original draft preparation, editing, and visualization.
Brian T Kornblit and Peter Ross: Review and editing, Supervision.
Anders Fink-Jensen: Review and editing, supervision, and project administration.
Data Availability Statement: Due to the nature of this research, supporting data is not available.
Ethics Statement: There is no reviewed board information to share.
Informed Consent: The patient has given her informed consent oral and in writing.
ORCID iD: Adrian Bentzon  https://orcid.org/0000-0001-5438-1513
https://orcid.org/0000-0001-5438-1513
References
- 1. Crabtree GR. Calcium, calcineurin, and the control of transcription. J Biol Chem. 2001;276:2313-2316. [DOI] [PubMed] [Google Scholar]
- 2. Anghel D, Tanasescu R, Campeanu A, Lupescu I, Podda G, Bajenaru O. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica (Buchar). 2013;8:170-175. http://www.ncbi.nlm.nih.gov/pubmed/24371481%0A; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3865126 [PMC free article] [PubMed] [Google Scholar]
- 3. Burnett MM, Hess CP, Roberts JP, Bass NM, Douglas VC, Josephson SA. Presentation of reversible posterior leukoencephalopathy syndrome in patients on calcineurin inhibitors. Clin Neurol Neurosurg. 2010;112:886-891. http://www.sciencedirect.com/science/article/pii/S0303846710002374 [DOI] [PubMed] [Google Scholar]
- 4. Varghese J, Reddy MS, Venugopal K, et al. Tacrolimus-related adverse effects in liver transplant recipients: its association with trough concentrations. Indian J Gastroenterol. 2014;33:219-225. [DOI] [PubMed] [Google Scholar]
- 5. Chegounchi M, Hanna MG, Neild GH. Progressive neurological disease induced by tacrolimus in a renal transplant recipient: case presentation. BMC Nephrol. 2006;7:7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sikavi D, McMahon J, Fromson JA. Catatonia due to tacrolimus toxicity 16 years after renal transplantation: case report and literature review. J Psychiatr Pract. 2019;25:481-484. [DOI] [PubMed] [Google Scholar]
- 7. ICD-10 Version: 2019. Accessed January 4, 2021. https://icd.who.int/browse10/2019/en
- 8. Wilson JE, Mart MF, Cunningham C, et al. Delirium. Nat Rev Dis Primers. 2020;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. J Psychiatry. 2009;54:437-445. [DOI] [PubMed] [Google Scholar]
- 10. DSM-5. Accessed Febuary 5, 2021 https://www.psychiatry.org/psychiatrists/practice/dsm
- 11. Post RM, Leverich GS, Kupka R, et al. Increased parental history of bipolar disorder in the United States: association with early age of onset. Acta Psychiatr Scand. 2014;129:375-382. [DOI] [PubMed] [Google Scholar]
- 12. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150:26-30. [DOI] [PubMed] [Google Scholar]
- 13. Najera JE, Alousi A, De Lima M, Ciurea SO. Akinetic mutism-a serious complication to tacrolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2013;48:157-158. [DOI] [PubMed] [Google Scholar]
- 14. Jang SH, Kim SH, Lee HD. Akinetic mutism following prefrontal injury by an electrical grinder a case report: A diffusion tensor tractography study. Medicine. 2018;97:e9845. https://journals.lww.com/md-journal/Fulltext/2018/02090/Akinetic_mutism_following_prefrontal_injury_by_an.35.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wijdicks EFM, Cranford RE. Clinical diagnosis of prolonged states of impaired consciousness in adults. Mayo Clin Proc. 2005;80:1037-1046. http://www.sciencedirect.com/science/article/pii/S0025619611615863 [DOI] [PubMed] [Google Scholar]
- 16. van der Flier WM, Skoog I, Schneider JA, et al. Vascular cognitive impairment. Nat Rev Dis Prim [Internet]. 2018;4:1-16. [DOI] [PubMed] [Google Scholar]
- 17. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914-925. [DOI] [PubMed] [Google Scholar]
- 18. Christians U, Jacobsen W, Benet LZ, Lampen A. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet. 2002;41:813-851. [DOI] [PubMed] [Google Scholar]
- 19. Staatz CE, Tett SE. Clinical pharmacokinetics of once-daily tacrolimus in solid-organ transplant patients. Clin Pharmacokinet. 2015;54:993-1025. [DOI] [PubMed] [Google Scholar]
- 20. Yano S, Mori S, Saito T, et al. Pharmacokinetics for once-daily modified release formulation of tacrolimus hydrate in unrelated hematopoietic stem cell transplantation. Ann Hematol. 2015;94:491-496. [DOI] [PubMed] [Google Scholar]
- 21. Wu G, Weng FL, Balaraman V. Tacrolimus-induced encephalopathy and polyneuropathy in a renal transplant recipient. BMJ Case Rep. 2013;2013:bcr2013201099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kusztal M, Piotrowski P, Mazanowska O, et al. Catatonic episode after kidney transplantation. Gen Hosp Psychiatry. 2014;36:360.e3-360.e5. [DOI] [PubMed] [Google Scholar]
- 23. Chopra A, Das P, Rai A, et al. Catatonia as a manifestation of tacrolimus-induced neurotoxicity in organ transplant patients: a case series. Gen Hosp Psychiatry. 2012;34:209.e9-209.e11. [DOI] [PubMed] [Google Scholar]