Abstract
Six fluoroquinolones presently in clinical use and four investigational tetracyclic fluoroquinolones were tested for in vitro activity against bloodstream-form Trypanosoma brucei brucei. All compounds had measurable activity, but the tetracyclic analogs were most potent, with 50% effective concentrations in the low micromolar range. In general, trypanosomes were more susceptible than L1210 leukemia cells. Consistent with the notion that they target type II topoisomerase in trypanosomes, the fluoroquinolones promote the formation of protein-DNA covalent complexes.
African trypanosomes are the parasitic protozoa that cause sleeping sickness, a disease that has undergone a dramatic and devastating resurgence in recent years (28). Untreated, sleeping sickness is fatal, and the treatment options presently available are increasingly limited (14, 19, 21). This unfortunate situation also pertains to diseases caused by closely related pathogens, the American trypanosome and Leishmania spp. The need for new molecular targets on which to base future treatment strategies is clear and immediate.
Promising targets for new broad-spectrum antitrypanosomal drugs are the DNA topoisomerases (3, 7). Poisoning of these enzymes is the molecular mechanism of action for clinically useful antitumor and antibacterial agents (9, 11). These compounds act by stabilizing intracellular DNA-topoisomerase complexes. Upon the addition of alkali or strong denaturants, the enzyme may be recovered covalently attached to its DNA substrate (18). Inhibitor-induced covalent complexes have been used to demonstrate the existence of both mitochondrial and nuclear topoisomerases in trypanosomes (25, 27). A number of investigators have reported that fluoroquinolones (which target type II topoisomerases in prokaryotes) have activity in vitro or in vivo against trypanosomes or Leishmania (1, 10, 12, 22–24, 32). However, none of these studies provides experimental evidence that the observed antiparasitic effect is a result of topoisomerase poisoning. We evaluated an array of fluoroquinolones against Trypanosoma brucei brucei in vitro to determine whether they are cytotoxic and whether they promote the formation of protein-DNA complexes.
Fluoroquinolones.
Norfloxacin was obtained from Merck Sharp & Dohme (West Point, Pa.), enoxacin from Parke-Davis, Pharmaceutical Research Division, Warner-Lambert Company (Ann Arbor, Mich.), ciprofloxacin from Miles, Inc. (West Haven, Conn.), pefloxacin from Rhone-Poulenc Rorer, (Mexico City, Mexico), fleroxacin from Hoffmann-La Roche Inc. (Nutley, N.J.), and ofloxacin from R.W. Johnson Pharmaceutical Research Institute (Raritan, N.J.). KB-5246, KB-5290, KB-6600, and KB-6625 were supplied by Kanebo, Ltd. (Osaka, Japan) (16, 17, 30). VM26 was a kind gift from Leroy Lui (Robert Wood Johnson Medical School, Piscataway, N.J.). Stock solutions were prepared as follows: ciprofloxacin, pefloxacin, fleroxacin, and KB-5246 were dissolved in sterile water; norfloxacin, enoxacin, ofloxacin, KB-5290, KB-6600, and KB-6625 in 100 mM NaOH; and VM26 in dimethyl sulfoxide.
Assays.
Bloodstream-form T. brucei (MiTat 1.2, strain 427) organisms were grown axenically (8) in phenol red-free medium, as we described previously (2). L1210 (ATCC CCL-219) mouse leukemia cells were maintained in phenol red-free RPMI 1640 supplemented with 15% heat-inactivated fetal bovine serum (Life Technologies). Ten concentrations of each fluoroquinolone were assayed in quadruplicate. Exponentially growing cells were incubated with or without fluoroquinolone for 20 h, then lysed and incubated for 3 to 6 h with p-nitrophenyl phosphate. Acid phosphatase activity was determined, and 50% effective concentrations (EC50) were obtained (2, 4). Covalent protein-DNA complexes were assayed by the potassium sodium dodecyl sulfate (KSDS) method as described previously (3, 25), with the modification that cultured trypanosomes were labeled with [methyl-3H]thymidine (NET027E; 20 Ci/mmol; New England Nuclear, Boston, Mass.) at 8.3 μM in the medium.
Antitrypanosomal activities of fluoroquinolones.
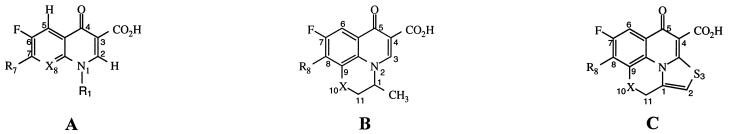
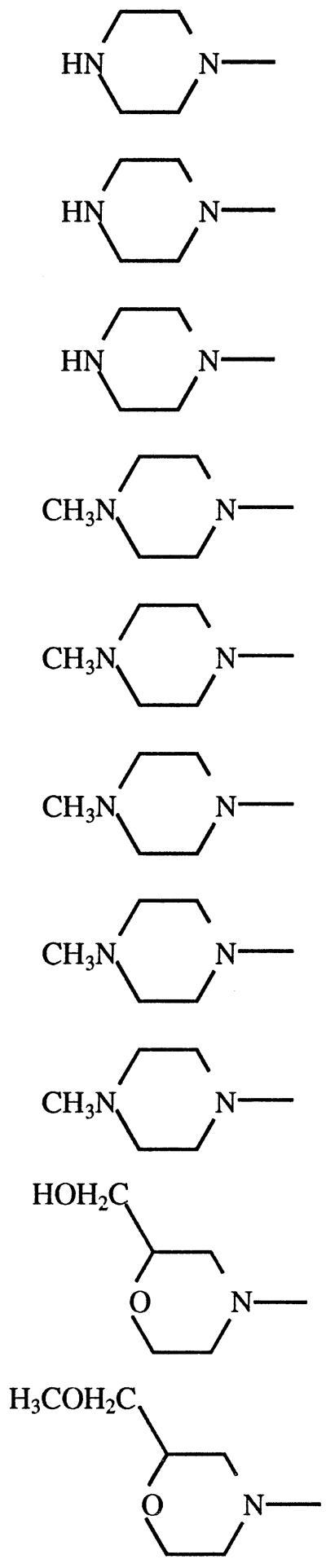
We tested six commercially available fluoroquinolones and four experimental tetracyclic fluoroquinolones for antitrypanosomal activity against axenically cultured bloodstream-form T. brucei (Table 1). Each of these compounds was cytotoxic, not cytostatic, in the assay. All ten had measurable activity, with EC50 that varied some 100-fold. Against trypanosomes, norfloxacin had an EC50 in the micromolar range, which is somewhat improved by N substitution at X8 (enoxacin) or by substituting a cyclopropyl group at R1 (ciprofloxacin). Conversely, activity was somewhat reduced by addition of an N-methyl group to the R7 piperazine side group (pefloxacin) or, more markedly, by addition of fluorine to the R1 ethyl and X8 sites (fleroxacin). Activity was similarly diminished by bridging the X8 and R1 sites to form a tricyclic compound (ofloxacin).
TABLE 1.
Antitrypanosomal activities of tested fluoroquinolones
| Compound | Structure
|
EC50a
|
|||||
|---|---|---|---|---|---|---|---|
| Nucleus | R1 | R7 (nucleus A) or R8 (nucleus B or C) | X8 (nucleus A) or X10 (nucleus B or C) | Against T. brucei (μM) | Against L1210 cells (μM) | Ratiob | |
| Norfloxacin | A | CH3CH2- | CH | 70 | >100 | >1.4 | |
| Enoxacin | A | CH3CH2- | N | 51 | 60 | 1.2 | |
| Ciprofloxacin | A | c-C3H5 | CH | 52 | 73 | 1.4 | |
| Pefloxacin | A | CH3CH2- | CH | 97 | 68 | 0.7 | |
| Fleroxacin | A | CH2FCH2- | CF | >100 | >100 | 1 | |
| Ofloxacin | B | O | >100 | >100 | 1 | ||
| KB-5246 | C | O | 1.7 | 3.7 | 2.2 | ||
| KB-5290 | C | N-CH3 | 3.7 | 9.5 | 2.6 | ||
| KB-6600 | C | N-CH3 | 11 | 29 | 2.6 | ||
| KB-6625 | C | N-CH3 | 14 | 21 | 1.5 | ||
Average of two determinations. EC50 of >100 μM indicate that curves could not be adequately fitted due to limited solubility of the fluoroquinolone. Differences between the average and individual EC50 never exceeded 30%. The r2 value obtained for each concentration-cell killing curve always exceeded 0.96 and averaged 0.99 (standard deviation, ±0.01); the coefficient of variation for quadruplicate determinations in the assay was always less than 18% and averaged 4.6% (≤18%).
Ratio of the EC50 with L1210 cells to that with T. brucei.
Most dramatic was the effect of generating a tetracyclic structure (KB-5246). This compound was >50-fold more cytotoxic to T. brucei than ofloxacin, from which it differs only by the addition of a thiazole ring. Further structural modifications in KB-5246, including a CH3N substitution at X10 (KB-5290) and addition of various morpholino groups at R8 (KB-6600 or KB-6625) resulted in reduced antitrypanosomal activity. In KB-5246 the methyl group at the optically active C-1 of the ofloxacin nucleus is tethered and held in the planar thiazole ring. The thiazole ring also extends the aromaticity of the fluoroquinolone nucleus and provides a sulfur atom as a potential H-bond acceptor. Either (or both) of these features may provide the dramatic increase in antitrypanosomal potency. The low micromolar EC50 of the tetracyclic congeners are comparable to those we found previously for some of the clinically useful antitrypanosomal agents (2, 3).
Selective toxicity.
For all compounds but pefloxacin, toxicity was as much as 2.6-fold greater for trypanosomes than for L1210 mammalian cells (Table 1). Though modest, this margin of difference is encouraging and may be even greater between parasites and nonmalignant mammalian cells. L1210 cells are nonadherent and have a rapid doubling time of about 14 h, ideal characteristics for our assay system. However, the topoisomerase II content of such cells is usually higher than that of nonmalignant cells (6, 15). Because the degree of toxicity is related to the number of complexes that are formed (20, 29), use of topoisomerase II-rich L1210 cells likely leads to overestimation of the toxicity to normal host tissues.
The tetracyclic fluoroquinolones exhibited twofold-greater toxicity toward T. brucei than toward L1210 cells. Variants of the piperazine or pyrrolidine group at R8 (nucleus C [Table 1]) may further improve this selective toxicity. For example, in a series of sparfloxacin analogs, trans-3,5-dimethylpiperazine at R7 (nucleus A [Table 1]) was more than 50 times more active than its cis isomer at inducing mammalian topoisomerase II-mediated DNA breaks (13). However, the two isomers were equally active in stimulating gyrase-mediated cleavage. Screening of tetracyclic fluoroquinolones with 3,5-dimethylpiperazine or other piperazinyl or pyrrolidinyl moieties at R8 may allow discovery of an analog that is similarly selective between mammalian and trypanosome topoisomerase II.
Protein-DNA complexes.
Fluoroquinolone-promoted protein-DNA complexes are detectable in African trypanosomes (Table 2), and there is a tendency for complex formation to correlate with antiparasitic activity. The tetracyclic compounds, which are most cytotoxic, trapped 4 to 11% of labeled DNA, whereas the less potent fluoroquinolones trapped only 1 to 3% of the label, effects that are dose related (data not shown). A striking exception is KB-5246, which has unexpectedly low complex formation given its antiparasitic potency (Table 2). Although this finding may be explained in a variety of ways (e.g., compound penetration into cells), one intriguing possibility is that the cytotoxicity of KB-5246 is attributable, at least in part, to protein-DNA complex formation with the topologically highly complex mitochondrial DNA (kDNA) of these organisms (26). The KSDS assay primarily reports complex formation that is nuclear in origin, since approximately 96% of the DNA in T. brucei is nuclear (5). Supporting evidence exists for the possibility of distinct nuclear and mitochondrial topoisomerase II forms in kinetoplastids and other parasites, and these enzymes may be distinguished from one another by differing drug susceptibilities (25, 31). Perhaps the bicyclic fluoroquinolones we tested target a mitochondrial topoisomerase II in T. brucei, whereas the tetracyclic fluoroquinolones target both mitochondrial and nuclear enzymes.
TABLE 2.
Fluoroquinolone-promoted intracellular protein-DNA complex formation in T. brucei
| Compounda | Protein-DNA complexesb (% of total incorporated radioactivity) |
|---|---|
| Ofloxacin | 1.2 ± 0.4 |
| Pefloxacin | 2.5 ± 0.3 |
| Ciprofloxacin | 0.3 ± 1 |
| KB-6625 | 3.6 ± 1.1 |
| KB-5290 | 11 ± 1 |
| KB-5246 | 4.4 ± 0.6 |
| VM-26c | 56 ± 4 |
Listed in order of increasing antitrypanosomal activity.
Each compound was evaluated, at 100 μM in the KSDS assay, for its ability to promote covalent protein-DNA complex formation. The value for control cells with no inhibitor (20 ± 1%) has been subtracted from the data and is a measure of naturally occurring complexes. Values are means ± standard deviations from two independent experiments.
Though clearly not of immediate clinical utility, these results provide evidence that fluoroquinolone inhibition of type II DNA topoisomerases may afford a suitable approach for the development of much-needed new antitrypanosomal drugs. The findings also indicate that further structure-activity investigation, particularly of tetracyclic fluoroquinolones, is warranted.
Acknowledgments
We thank Goro Tsukamotomo of Kanebo Ltd. New Drug Research Laboratories for his interest in this work and for providing the tetracyclic fluoroquinolones. Lourdes Juan Lopez of Rhone-Poulenc Rorer, S.A. de C.V., kindly and effectively expedited our request for pefloxacin. We thank Annette Bodley, Donna Klinedinst, and Suji Xie for helpful scientific discussions and technical assistance.
This work was supported by Public Health Service grant AI28855 from the National Institutes of Health, by the Burroughs Wellcome Fund, by the Swiss National Research Foundation (C.B.), and by the Janggen-Poehn Foundation (C.B.).
REFERENCES
- 1.Betbeder D, Hutchinson D W, Baltz T, Cros S. Trypanocidal and antitumor activities of nalidixic and oxolinic acid derivatives. Med Sci Res. 1988;16:141–142. [Google Scholar]
- 2.Bodley A L, McGarry M W, Shapiro T A. Drug cytotoxicity assay for African trypanosomes and Leishmania species. J Infect Dis. 1995;172:1157–1159. doi: 10.1093/infdis/172.4.1157. [DOI] [PubMed] [Google Scholar]
- 3.Bodley A L, Shapiro T A. Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor, on trypanosomes and Leishmania. Proc Natl Acad Sci USA. 1995;92:3726–3730. doi: 10.1073/pnas.92.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodley A L, Wani M C, Wall M E, Shapiro T A. Antitrypanosomal activity of camptothecin analogs: structure-activity correlations. Biochem Pharmacol. 1995;50:937–942. doi: 10.1016/0006-2952(95)00215-l. [DOI] [PubMed] [Google Scholar]
- 5.Borst P, van der Ploeg M, van Hoek J F M, Tas J, James J. On the DNA content and ploidy of trypanosomes. Mol Biochem Parasitol. 1982;6:13–23. doi: 10.1016/0166-6851(82)90049-4. [DOI] [PubMed] [Google Scholar]
- 6.Burden D A, Osheroff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta. 1998;1400:139–154. doi: 10.1016/s0167-4781(98)00132-8. [DOI] [PubMed] [Google Scholar]
- 7.Burri C, Bodley A L, Shapiro T A. Topoisomerases in kinetoplastids. Parasitol Today. 1996;12:226–231. doi: 10.1016/0169-4758(96)10017-x. [DOI] [PubMed] [Google Scholar]
- 8.Carruthers V B, Cross G A M. High efficiency clonal growth of bloodstream- and insect-form Trypanosoma brucei on agarose plates. Proc Natl Acad Sci USA. 1992;89:8818–8821. doi: 10.1073/pnas.89.18.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen A Y, Lui L F. DNA topoisomerases: essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol. 1994;34:191–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- 10.Croft S L, Hogg J. Limited activity of bacterial DNA topoisomerase II inhibitors against Leishmania donovani and Trypanosoma cruzi amastigotes in vitro. Trans R Soc Trop Med Hyg. 1988;82:856. doi: 10.1016/0035-9203(88)90017-x. [DOI] [PubMed] [Google Scholar]
- 11.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzales-Perdomo M, Lisboa de Castro S, Meirelles M N S L, Goldenberg S. Trypanosoma cruzi proliferation and differentiation are blocked by topoisomerase II inhibitors. Antimicrob Agents Chemother. 1990;34:1707–1714. doi: 10.1128/aac.34.9.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gootz T D, McGuirk P R, Moynihan M S, Haskell S L. Placement of alkyl substituents on the C-7 piperazine ring of fluoroquinolones: dramatic differential effects on mammalian topoisomerase II and DNA gyrase. Antimicrob Agents Chemother. 1994;38:130–133. doi: 10.1128/aac.38.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajduk S L, Englund P T, Smith D H. African trypanosomiasis. In: Warren K S, Mahmoud A A F, editors. Tropical and geographical medicine. 2nd ed. New York, N.Y: McGraw-Hill; 1990. pp. 268–281. [Google Scholar]
- 15.Heck M M S, Earnshaw W C. Topoisomerase II: a specific marker for cell proliferation. J Cell Biol. 1986;103:2569–2581. doi: 10.1083/jcb.103.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jinbo Y, Kondo H, Inoue Y, Taguchi M, Tsujishita H, Kotera Y, Sakamoto F, Tsukamoto G. Synthesis and antibacterial activity of a new series of tetracyclic pyridone carboxylic acids. J Med Chem. 1993;36:2621–2626. doi: 10.1021/jm00070a005. [DOI] [PubMed] [Google Scholar]
- 17.Jinbo Y, Kondo H, Taguchi M, Inoue Y, Sakamoto F, Tsukamoto G. Synthesis and antibacterial activity of thiazolopyrazine-incorporated tetracyclic quinolone antibacterial agents. J Med Chem. 1994;37:2791–2796. doi: 10.1021/jm00043a018. [DOI] [PubMed] [Google Scholar]
- 18.Liu L F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- 19.Mäser P, Kaminsky R. The mechanisms of drug resistance in Trypanosoma brucei spp. Recent Res. Dev. Antimicrob Agents Chemother. 1997;2:113–125. [Google Scholar]
- 20.Nitiss J L, Liu Y-A, Harbury P, Jannatipour M, Wasserman R, Wang J C. Amsacrine and etoposide hypersensitivity of yeast cells overexpressing DNA topoisomerase II. Cancer Res. 1992;52:4467–4472. [PubMed] [Google Scholar]
- 21.Pépin J, Milord F, Khonde A, Niyonsenga T, Loko L, Mpia B. Gambiense trypanosomiasis: frequency of, and risk factors for, failure of melarsoprol therapy. Trans R Soc Trop Med Hyg. 1994;88:447–452. doi: 10.1016/0035-9203(94)90430-8. [DOI] [PubMed] [Google Scholar]
- 22.Raether W, Seidenath H, Hofmann J. Potent antibacterial fluoroquinolones with marked activity against Leishmania donovani in vivo. Parasitol Res. 1989;75:412–413. doi: 10.1007/BF00931138. [DOI] [PubMed] [Google Scholar]
- 23.Sanguigni S, Marangi M, Gramiccia M, Orsini S, Paparo B S, Nicodemo G, Gradoni L. Ciprofloxacin in the treatment of leishmaniasis. G Mal Infett Parassit. 1993;45:447–449. [Google Scholar]
- 24.Savoia D, Biglino S, Cestaro A, Zucca M. In vitro and in vivo activity of some fluoroquinolones on two Leishmania species. Eur Bull Drug Res. 1993;2:135–138. [Google Scholar]
- 25.Shapiro T A, Englund P T. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc Natl Acad Sci USA. 1990;87:950–954. doi: 10.1073/pnas.87.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapiro T A, Englund P T. The structure and replication of kinetoplast DNA. Annu Rev Microbiol. 1995;49:117–143. doi: 10.1146/annurev.mi.49.100195.001001. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro T A, Klein V A, Englund P T. Drug-promoted cleavage of kinetoplast DNA minicircles: evidence for type II topoisomerase activity in trypanosome mitochondria. J Biol Chem. 1989;264:4173–4178. [PubMed] [Google Scholar]
- 28.Smith D H, Pepin J, Stich A H R. Human African trypanosomiasis: an emerging public health crisis. Br Med Bull. 1998;54:341–355. doi: 10.1093/oxfordjournals.bmb.a011692. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan D M, Latham M D, Ross W E. Proliferation-dependent topoisomerase II content as a determinant of antineoplastic drug action in human mouse and Chinese hamster ovary cells. Cancer Res. 1987;47:3973–3979. [PubMed] [Google Scholar]
- 30.Taguchi M, Kondo H, Inoue Y, Kawahata Y, Jinbo Y, Sakamoto F, Tsukamoto G. Synthesis and antibacterial activity of new tetracyclic quinolone antibacterials. J Med Chem. 1992;35:94–99. doi: 10.1021/jm00079a011. [DOI] [PubMed] [Google Scholar]
- 31.Weissig V, Vetro-Widenhouse T S, Rowe T C. Topoisomerase II inhibitors induce cleavage of nuclear and 35-kb plastid DNAs in the malarial parasite Plasmodium falciparum. DNA Cell Biol. 1997;16:1483–1492. doi: 10.1089/dna.1997.16.1483. [DOI] [PubMed] [Google Scholar]
- 32.Zucca M, Millesimo M, Giovarelli M, Diverio D, Musso T, Savoia D. Protective role of the pefloxacin-IFN-gamma association in Leishmania major-infected mice. New Microbiol. 1996;19:39–46. [PubMed] [Google Scholar]