Abstract
Background:
Many procedures to reconstruct osteochondral defects of the elbow radiocapitellar (RC) joint lack versatility or durability or do not directly address the subchondral bone structure and function.
Purpose/Hypothesis:
To biomechanically characterize the RC joint contact area, force, pressure, and peak pressure before and after reconstruction of osteochondral defects using a novel hybrid reconstructive procedure. It was hypothesized that the procedure would restore the contact characteristics to the intact condition.
Study Design:
Controlled laboratory study.
Methods:
A total of 10 cadaveric elbows (mean age 67 ± 2.7 years) were dissected to isolate the humerus and radial head. RC contact area, contact force, mean contact pressure, and peak contact pressure were measured with the elbow at 45° of flexion and neutral forearm rotation at compressive loads of 25, 50, and 75 N. Osteochondral defects 8 and 11 mm in diameter were created at the center of the capitellum; the defects were then reconstructed with a titanium fenestrated threaded implant, countersunk in the subchondral bone, with an acellular dermal matrix allograft sutured in place on top of the implant. Five conditions (intact, 8-mm defect, 8-mm repair, 11-mm defect, and 11-mm repair) were tested and results were compared using repeated-measures analysis of variance.
Results:
Both 8- and 11-mm defects significantly increased RC mean contact pressure at all compressive loads (P ≤ .008) and significantly increased peak contact pressure at compressive loads of 50 and 75 N (P < .002) compared with the intact condition. Repair of the 8-mm defect significantly decreased RC mean contact pressure at 25- and 50-N loads (P ≤ .009) and significantly decreased peak contact pressure at 50- and 75-N loads (P ≤ .035) compared with the defect condition. Repair of the 11-mm defect decreased mean contact pressure significantly at all compressive loads (P ≤ .001) and peak contact pressure at 50- and 75-N loads (P < .044) compared with the defect condition.
Conclusion:
RC joint contact pressure was restored to intact conditions while avoiding increased peak contact pressure or edge loading after repairing osteochondral defects related to osteochondrosis with a novel hybrid reconstruction technique.
Clinical Relevance:
This hybrid procedure that addresses the entire osteochondral unit may provide a new treatment option for osteochondral defects.
Keywords: radiocapitellar, osteochondritis dissecans, osteochondral defect, contact pressure, osteochondrosis, subchondral bone
Osteochondral defects, osteochondritis dissecans, or the general term osteochondrosis may occur in the knee, 7,9,20 elbow, 4,6,11,13,14,17 –19,21 and ankle. 1,5,20 Osteochondral defects and osteochondrosis involve structural and vascular compromise of the subchondral bone and progressive damage of the osteochondral unit via bone hypertrophy or cystic changes. 2,8,15 Etiology includes repetitive microtrauma from abnormal or excessive joint loading or traumatic injuries such as elbow dislocation. 4,6,11,18,19 Questions remain regarding a reliable treatment algorithm, and operative techniques are still in need of assessment of long-term outcomes and refinement. 4,11 A versatile surgical procedure that addresses the mechanical deficiencies of the bone, preserves marrow communication, and allows a biologic reconstruction that restores normal surface biomechanics is needed.
Humeral capitellar osteochondrosis and related elbow radiocapitellar (RC) joint osteochondral defects are prevalent in individuals who have played baseball or performed gymnastics actively since childhood. 4,18,19,21 Repetitive and excessive compressive force at the RC joint from either excessive valgus or axial loading have been suggested as the key contributors to elbow RC defects. 11,12 Previous clinical and biomechanical studies have shown that the size and location of the osteochondral defect affect prognosis in throwing athletes with capitellar osteochondritis dissecans with larger defects resulting in poorer clinical outcomes. 12,19 Current operative management options include debridement and microfracture as well as numerous cartilage restoration techniques. 11 Cartilage restoration procedures include osteochondral autograft transfer, 6,14 osteochondral allograft transplantation, 13 and autologous chondrocyte implantation. 17 Joint surface restoration and osteochondral defect reconstruction in the elbow are still in early development, and further studies with long-term clinical follow-up are necessary to establish the indications and effectiveness of these procedures. 11 These existing procedures lack versatility or durability or do not directly address the subchondral bone structure and function.
In this study, we introduce a hybrid reconstruction of humeral RC osteochondral defects utilizing titanium fenestrated threaded implants in the subchondral bone and human dermal allograft. The fenestrated threaded implant in the subchondral bone is designed to restore structural stability, preserve marrow communication, and serve as a fixation platform for osteochondral graft reconstruction. It was hypothesized that reconstruction of capitellum osteochondral defects would reduce RC contact pressure toward that of the intact RC joint.
Methods
Specimen Preparation
A total of 10 fresh-frozen cadaveric elbows were acquired for this study from Science Care. Institutional review board approval was waived by our institution, as this was a cadaveric basic science study. Elbows were transected 2 cm below the deltoid insertion and at the midulnar and midradial shaft and were dissected, removing all skin and subcutaneous tissue. The mean donor age was 67 ± 2.7 years old (range, 46-73 years) and consisted of 5 males and 5 females. None of the donor elbows showed any evidence of gross abnormalities.
The disarticulated humerus and radius were fixed in PVC pipes with plaster of Paris and wood screws. The humerus and radius were then mounted to a custom elbow testing system, with the humeral epicondylar axis parallel to the floor with the radial head above and directed toward the capitellum of the humerus (Figure 1). The radius was positioned in neutral forearm rotation, defined by the radial tuberosity directed medially in line with the humeral epicondylar axis.
Figure 1.

Photograph of the testing setup.
Biomechanical Testing
A Tekscan pressure sensor (Model 4000; maximum saturation pressure 10.3 MPa; Tekscan) was placed between the position of contact between the radial head and capitellum to measure the RC contact force, contact area, contact pressure, and peak contact pressure at 45° of elbow flexion based on the humeral and radial shaft. The Tekscan sensor’s sensitivity was set to 35 and calibrated using a 2-point calibration protocol with an applied force of 40 N and 80 N using an Instron 4111 load cell (Instron). The average saturation pressure after calibration was 1565 ± 64 kPa.
Specimens were preloaded in compression with 10 N followed by cyclic ramp loading in compression from 10 to 25 N for 5 cycles, 10 to 50 N for 5 cycles, and 10 to 75 N for 5 cycles using an Instron 3365. Two trials of 5 cycles at each load were recorded to ensure repeatability (maximum RC contact force <10% difference).
Five conditions were tested: intact, 8-mm defect, 8-mm repair, 11-mm defect, and 11-mm repair. The defect sizes were chosen to simulate clinically relevant osteochondral defects in the capitellum. Central capitellar osteochondral defects located 45° anteriorly to the shaft of the humerus, centered midway between the medial and lateral edges of the capitellum, were created using an 8-mm and 11-mm drill by drilling to a depth of 5 mm. 12 The depths of the defects were assessed with a caliper. For the repair conditions, 1 complete trial of cyclic loading from 10 to 25 N for 5 cycles, 10 to 50 N for 5 cycles, and 10 to 75 N for 5 cycles was performed before measurement to minimize the effect of the graft viscoelasticity.
Reconstruction of Capitellar Osteochondral Defects
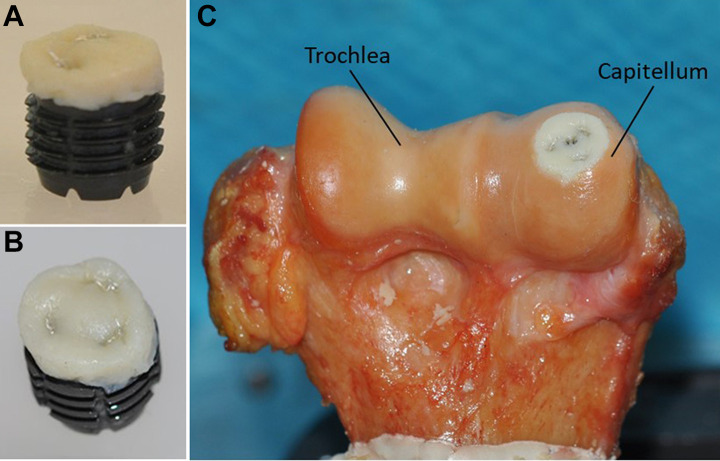
The S-CORE HA Implant (Subchondral Solutions) (Figure 2) is a titanium, hydroxyapatite-coated, fenestrated, cannulated fracture screw specifically designed for osteochondral fractures, osteochondral fracture defect reconstruction, and osteochondritis dissecans. The implant was secured into the subchondral bone defect, creating a fixation platform for securing human dermal allografts via suture, as well as a hybrid reconstruction. Human dermal allografts with an area of 4 × 6 cm and thickness of 3 mm (Matrix HD; RTI Surgical) were soaked in 0.9% saline and prepared at room temperature. Circular 8-mm and 11-mm diameter human dermal allograft discs were punched and sutured with 3-0 nylon sutures onto the 7-mm and 10-mm diameter titanium implants, respectively (mean thickness as measured with area micrometer was 1.82 ± 0.01 mm for 8-mm repairs and 1.81 ± 0.01 mm for 11-mm repairs). The defects were reconstructed with the hybrid construct, placing the upper portion of the allograft at the level of the surrounding cartilage.
Figure 2.
(A) Photograph of a 10-mm implant prepared with an 11-mm dermal allograft. (B) Superior view of dermal allograft. (C) Photograph of a right elbow following osteochondral repair of an 11-mm capitellar defect.
Statistical Analysis
The contact force, area, pressure, and peak pressure at the peak force of the cyclic loading was averaged across the 5 cycles for trial 1 and then for trial 2. The 2 trials were then averaged together for analysis. Mean values for the testing conditions were compared using 2-way repeated-measures analysis of variance followed by a post hoc test with Bonferroni correction for multiple comparisons (IBM SPSS Statistics 25.0). Statistical significance was defined as P <.05.
A sample size calculation was performed using the difference in contact pressure between the 11-mm defect and 11-mm repair at 50 N. Based on the mean and standard deviation of 3 specimens (mean difference: 0.23 kPa - 0.17 kPa = 0.06 kPa; standard deviation of the difference = 0.046 kPa), a total of 10 specimens were determined to be needed for α = 0.05 and power (1 - β) of 0.80.
RESULTS
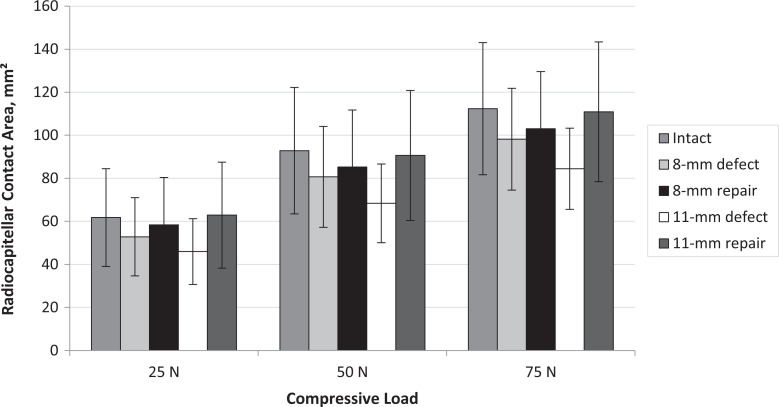
There were no statistically significant differences in RC contact area between any of the testing conditions (P > .502 for all comparisons) (Figures 3 and 4).
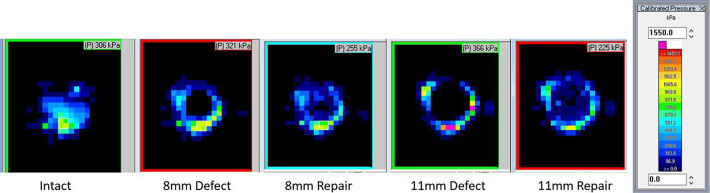
Figure 3.
Representative radiocapitellar contact from the Tekscan images from 1 specimen for each testing condition with calibrated pressure scale showing saturation pressure of 1550 kPa.
Figure 4.
Radiocapitellar contact area for each testing condition and compressive load. Data presented as mean with standard error bars.
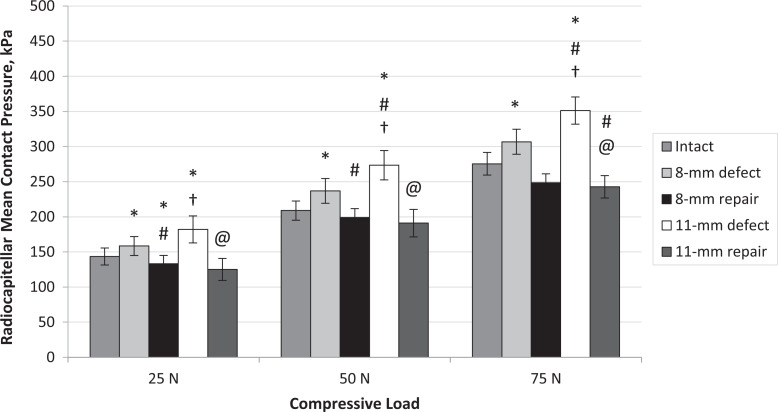
The 8-mm RC defect increased RC mean contact pressure significantly compared with the intact condition at all compressive loads (25 N, P = .019; 50 N, P = .039; 75 N, P = .020) (Figures 3 and 5). Repair of the 8-mm defect decreased RC mean contact pressure significantly for 25 N and 50 N compressive loads compared with the defect condition (25 N, P < .001; 50 N, P = .009) (Figure 5).
Figure 5.
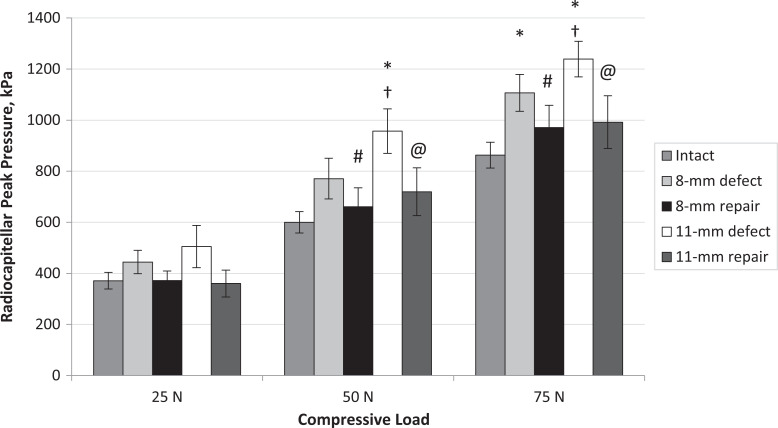
Radiocapitellar mean contact pressure for each testing condition and each compressive load. Error bars represent standard error. Statistically significant difference (P < .05) versus *intact, #8-mm defect, †8-mm repair, and @11-mm defect.
The 11-mm defect increased mean contact pressure significantly for all compressive loads compared with intact (25 N, P = .008; 50 N, P = .001; 75 N, P < .001) and compared with the 8-mm defect condition at 50 N and 75 N compressive loads (50 N, P = .014; 75 N, P = .009). Repair of the 11-mm defect decreased mean contact pressure significantly compared with the 11-mm defect for all compressive loads (25 N, P = .001; 50 N, P < .001; 75 N, P < .001) (Figure 5).
The 8-mm RC defect significantly increased RC peak contact pressure compared with the intact condition at 75 N compressive loads (P = .010) (Figure 6). Repair of the 8-mm defect significantly decreased peak pressure compared with the defect condition at 50 and 75 N (50 N, P = .009; 75 N, P = .035). The 11-mm defect increased RC peak contact pressure significantly at 50 and 75 N compared with intact (50 N, P = .002; 75 N, P = .001). Repair of the 11-mm defect significantly decreased peak pressure compared with 11-mm defect at 50 and 75 N (50 N, P = .006; 75 N, P = .044).
Figure 6.
Radiocapitellar peak contact pressure for each testing condition and compressive load. Error bars represent standard error. Statistically significant difference (P < .05) versus *intact, #8-mm defect, †8-mm repair, and @11-mm defect.
Discussion
Capitellum osteochondral defect repair using specialized titanium implants and human dermal allograft restored RC joint mean and peak contact pressure to intact levels. There was increased mean RC contact pressure on average across all loading conditions of 11.7% from intact for 8-mm defects (P < .039 for all loads) and 28.4% from intact for 11-mm defects (P < .008 for all loads). RC peak contact pressure increased an average of 25.5% and 46.3% from intact for 8-mm (P = .010 for 75 N load) and 11-mm defects (P < .002 for 50 and 75 N loads), respectively. Both mean and peak contact pressures were restored to intact levels following repair. There were no significant differences in the contact area for the defect or repair conditions. Joint incongruity may lend some explanation to the variation in the values for average contact area. These findings suggest that osteochondral defect repair of the capitellum with titanium implants and human dermal allograft can be a feasible option in treating RC joint osteochondral defects.
Literature on surface restoration of the elbow joint is limited, making validation or comparison of our work difficult. Osteochondral autograft transplantations, osteochondral allograft transfers, and autologous chondrocyte implantations have been well studied in the knee. 7,9 Kock et al 9 demonstrated that there were no significant differences in contact stresses with osteochondral autograft transplantation whether the implant is bottomed or unbottomed out with the procedure, resulting in 135% of the intact border contact pressure of human cadaver knees. Harris et al 7 demonstrated that, with osteochondral autograft transplantation and synthetic plugs, the peak contact pressure was significantly higher when placed in a proud position with the osteochondral autograft plug compared with the synthetic plug in human cadaver knees. In the studies with implants, there are concerns of increased contact pressure, leading to long-term complications such as graft subsidence and failure. In the current study, mean contact pressure after repairing defects was 88.1% to 95.3% of the intact state, with peak pressure no greater than 119.9% of the intact state in the elbow RC joint, with no significant differences of peak contact pressure compared with intact conditions. This would suggest that RC joint osteochondral defect repair with human dermal allograft was able to restore mean contact pressure while avoiding an increase in peak contact pressure or edge loading.
Joint congruity after repair is a significant factor, which can be a limitation. The intact joint has an uninterrupted and continuous cartilage surface, but a cartilage defect or repaired defect may have an interrupted surface or step off, which can lead to edge loading. This is akin to the findings of significantly increased contact pressure with slightly elevated or sunken osteochondral transplantation plugs during a study performed in femoral condyles of porcine knees. 10 Similarly, Bobrowitsch et al 3 performed a biomechanical study on joint contact pressure after osteochondral graft transplantation in an ovine carpometacarpal joint model, demonstrating significantly higher contact pressure with the high-implanted graft. Despite the variation in anatomic congruity of the articular surfaces in the elbow joint, the trend of contact characteristics in our study was clear and persistent in all specimens; mean and peak contact pressure were reduced when comparing the repair conditions with the respective defect conditions. This could be due to the versatile structure of the dermal allograft lining the defective surface after repair, which seems to flatten and adjust to the shape of the articular surface with mechanical compressive conditioning while its intrinsic viscoelastic property also provides a cushioning effect for the restored articular surface.
Delivery of scaffolds for joint resurfacing or defect reconstruction continues to be a challenge, especially in cases that involve compromise of the subchondral bone, osteochondrosis. Bone sequelae include bone mismatch in osteochondral transplant procedures, intralesional bone hypertrophy after microfracture, trabecular architectural changes, and cystic changes. As defects progress in size, edge loading with surrounding osteochondral tissue occurs; trabecular microcracks, cystic changes, vascular congestion, and bone edema ensue, leading to pain and further compromise of the subchondral bone. Therefore, a reconstruction of an osteochondral defect needs a hybrid reconstruction strategy to restore the subchondral bone’s structural integrity, permit healing by marrow communication as well as restoring the articulating surface congruency of the joint.
Limitations
The limitations of this biomechanical study include the removal in our cadaveric testing of all soft tissue, which can contribute to joint contact characteristics. It is worth noting that Sabo et al 16 demonstrated that osteochondral lesions of the capitellum do not affect elbow kinematics and stability with intact collateral ligaments. Therefore, further studies could verify this and may focus more on elucidating joint contact characteristics, possibly with different flexion angles. A second limitation is the inclusion of a Tekscan sensor between the articular surface may also affect contact characteristics but should not affect the comparisons between the testing conditions. A third limitation is that only unidirectional loading was applied. Other directions of loading, such as valgus loading or rotational loading, can occur at the RC joint, which were not accounted for in this study. A fourth limitation is the inability to account for healing biology in our cadaveric testing; the results of this study can represent only the normalization effects at time zero. The use of human dermal allograft for humeral RC joint osteochondral defect repair requires further clinical evaluation and long-term studies.
Conclusion
RC joint contact pressure was restored to intact conditions while avoiding increased peak contact pressure or edge loading after repairing osteochondral defects related to osteochondrosis with a novel hybrid reconstruction using specialized titanium implants and human dermal allograft. This hybrid reconstruction that addresses the entire osteochondral unit may provide a new treatment option for osteochondral defects.
Footnotes
Final revision submitted September 28, 2021; accepted December 15, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: The implants and instruments used in the study were donated by Subchondral Solutions, and the human dermal graft material was donated by RTI Surgical. Partial funding was provided by Congress Medical Foundation. D.T.D., M.H.M., and T.Q.L. have stock/stock options in Subchondral Solutions. D.T.D. has received hospitality payments from Globus Medical and Linvatec and has patents relevant to the content of this study. T.Q.L. has received research support and consulting fees from Arthrex, royalties from ConMed Linvatec, Smith & Nephew, and Stryker and has stock/stock options in Coracoid Solutions. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Bauer KL, Polousky JD. Management of osteochondritis dissecans lesions of the knee, elbow and ankle. Clin Sports Med. 2017;36(3):469–487. doi:10.1016/j.csm.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 2. Bobrowitsch E, Lorenz A, Jörg J, et al. Alterations of the subchondral bone in osteochondral repair - translational data and clinical evidence. Am J Sports Med. 2004;25(5):461–468. doi:10.1177/036354658401200503. [DOI] [PubMed] [Google Scholar]
- 3. Bobrowitsch E, Lorenz A, Jörg J, Leichtle UG, Wülker N, Walter C. Changes in dissipated energy and contact pressure after osteochondral graft transplantation. Med Eng Phys. 2014;36(9):1156–1161. doi:10.1016/j.medengphy.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 4. Bradley JP, Petrie RS. Osteochondritis dissecans of the humeral capitellum: diagnosis and treatment. Clin Sports Med. 2001;20(3):565–590. doi:10.1016/S0278-5919(05)70270-2. [DOI] [PubMed] [Google Scholar]
- 5. Bruns J, Habermann C, Werner M. Osteochondral lesions of the talus: a review on talus osteochondral injuries, including osteochondritis dissecans. Cartilage. 2021;13(1_suppl): 1380S–1401S. doi:10.1177/1947603520985182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gancarczyk SM, Makhni EC, Lombardi JM, Popkin CA, Ahmad CS. Arthroscopic articular reconstruction of capitellar osteochondral defects. Am J Sports Med. 2015;43(10):2452–2458. doi:10.1177/0363546515594448. [DOI] [PubMed] [Google Scholar]
- 7. Harris JD, Solak KK, Siston RA, Litsky A, Richards J, Flanigan DC. Contact pressure comparison of proud osteochondral autograft plugs versus proud synthetic plugs. Orthopedics. 2011;34(2):97. doi:10.3928/01477447-20101221-06. [DOI] [PubMed] [Google Scholar]
- 8. Imhof H, Sulzbacher I, Grampp S, Czerny C, Youssefzadeh S, Kainberger F. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol. 2000;35(10):581–588. doi:10.1097/00004424-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 9. Kock NB, Smolders JMH, Van Susante JLC, Buma P, Van Kampen A, Verdonschot N. A cadaveric analysis of contact stress restoration after osteochondral transplantation of a cylindrical cartilage defect. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):461–468. doi:10.1007/s00167-008-0494 -1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koh JL, Wirsing K, Lautenschlager E, Zhang LO. The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am J Sports Med. 2004;32(2):317–320. doi:10.1177/0363546503261730. [DOI] [PubMed] [Google Scholar]
- 11. Logli AL, Bernard CD, O’Driscoll SW, et al. Osteochondritis dissecans lesions of the capitellum in overhead athletes: a review of current evidence and proposed treatment algorithm. Curr Rev Musculoskelet Med. 2019;12(1):1–12. doi:10.1007/s12178-019-09528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mihata T, Quigley R, Robicheaux G, McGarry MH, Neo M, Lee TQ. Biomechanical characteristics of osteochondral defects of the humeral capitellum. Am J Sports Med. 2013;41(8):1909–1914. doi:10.1177/0363546513490652. [DOI] [PubMed] [Google Scholar]
- 13. Mirzayan R, Lim MJ. Fresh osteochondral allograft transplantation for osteochondritis dissecans of the capitellum in baseball players. J Shoulder Elbow Surg. 2016;25(11):1839–1847. doi:10.1016/j.jse.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 14. Oka Y, Ikeda M. Treatment of severe osteochondritis dissecans of the elbow using osteochondral grafts from a rib. J Bone Joint Surg Br. 2001;83(5):738–739. doi:10.1302/0301-620X.83B5.11767. [DOI] [PubMed] [Google Scholar]
- 15. Orth P, Cucchiarini M, Kohn D, Madry H. Alterations of the subchondral bone in osteochondral repair - translational data and clinical evidence. Eur Cells Mater. 2012;25:299–316. doi:10.22203/ecm.v025a21. [DOI] [PubMed] [Google Scholar]
- 16. Sabo MT, McDonald CP, Ferreira LM, Johnson JA, King GJ. Osteochondral lesions of the capitellum do not affect elbow kinematics and stability with intact collateral ligaments: an in vitro biomechanical study. J Hand Surg Am. 2011;36(1):74–80. doi:10.1016/j.jhsa.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 17. Sato M, Ochi M, Uchio Y, Agung M, Baba H. Transplantation of tissue-engineered cartilage for excessive osteochondritis dissecans of the elbow. J Shoulder Elbow Surg. 2004;13(2):221–225. doi:10.1016/j.jse.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 18. Singer KM, Roy SP. Osteochondrosis of the humeral capitellum. Am J Sports Med. 1984;12(5):351–360. doi:10.1177/036354658401200503. [DOI] [PubMed] [Google Scholar]
- 19. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2008;90(SUPPL. 2 PART 1):47–62. doi:10.2106/JBJS.G.01135. [DOI] [PubMed] [Google Scholar]
- 20. Weiss JM, Shea KG, Jacobs JC, et al. Incidence of osteochondritis dissecans in adults. Am J Sports Med. 2018;46(7):1592–1595. doi:10.1177/0363546518764676. [DOI] [PubMed] [Google Scholar]
- 21. Westermann RW, Hancock KJ, Buckwalter JA, Kopp B, Glass N, Wolf BR. Return to sport after operative management of osteochondritis dissecans of the capitellum: a systematic review and meta-analysis. Orthop J Sport Med. 2016;4(6):2325967116654651. doi:10.1177/2325967116654651. [DOI] [PMC free article] [PubMed] [Google Scholar]