Abstract
Ovulation is the fundamental biological process during which an oocyte is expelled from the ovary, and it is an essential step toward establishing pregnancy. Understanding regulatory mechanisms governing the ovulation process is essential for diagnosing and treating causes of infertility, identifying contraceptive targets, and developing novel contraception methods. EDN2 is a 21-amino acid long peptide that is transiently synthesized by granulosa cells of the ovulatory follicle prior to ovulation and plays an essential role in ovulation via promoting contraction in the myofibroblast cells of the theca layer of the follicle. This review describes the organization of the endothelin system, summarizes recent findings on the expression and synthesis of the endothelin system in the ovary, illustrates the roles that EDN2 plays in regulating ovulation, and discusses EDN2 as a potential target of contraception.
Ovulation and its regulators
In the ovary, once a group of small primordial follicles are recruited to grow, they sequentially develop to primary, secondary, and mature follicles ready to respond to an ovulatory luteinizing hormone (LH) surge. Upon stimulation by an LH surge, a mature follicle undergoes a series of ovulatory processes, eventually expelling an oocyte along with its nursing cells (cumulus cells) (Starup & Visfeldt 1974, Wandji et al. 1996, Oktay et al. 1998, Matousek et al. 2001, Christian & Moenter 2010, Duffy et al. 2019). Prior to the LH surge, the cumulus oocyte complex is immersed in antral fluid and is loosely connected to mural granulosa cells that make up a layer adjacent to the basal lamina of the follicle. Whereas, the outer layer of the follicle, the theca cell layer, consists of steroidogenic theca cells, myofibroblasts, arteries, veins, capillaries, and resident immune cells (Espey 1967, Reviewed by Duffy et al. 2019). The roles of these cells and tissues in relation to the ovulatory process are discussed in detail in the following sections, with particular attention given to myofibroblasts in the theca layer that surround the follicle (Choi et al. 2011, Migone et al. 2016). The myofibroblasts provide structural integrity to the follicle and are the primary targets of endothelin-2 (EDN2) action in triggering follicle rupture and regulating ovulation (Ko et al. 2006).
Overview of EDN2 system and signaling
EDN2 was first isolated from porcine endothelial cell culture media and is a 21 amino acid peptide (Yanagisawa et al. 1988, Inoue et al. 1989). Its two isoforms, EDN1 and EDN3, are also 21-amino acids in length and largely structurally similar to EDN2. Because of their contraction-inducing activity, EDNs were thought to regulate systemic blood pressure and blood flow (Yanagisawa et al. 1988). However, this was just the beginning of the subsequent discoveries of a myriad of roles that EDNs play in a variety of organs and physiological conditions. In brief, EDNs are now known to be expressed in a wide range of tissues throughout the body that include, but not limited to, nervous, cardiovascular, respiratory, urinary, gastrointestinal and reproductive systems. EDNs play essential physiological roles and aberrant expression results in a pathological condition in an organ- or tissue-specific manner. Until recently, the focus of studies on EDNs has largely been on EDN1.
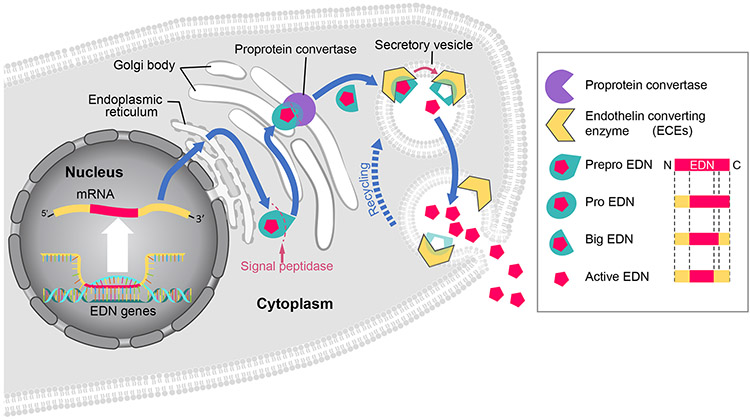
Molecularly, EDN2 and its isoforms are translated as inactive forms, referred to as prepro-EDNs. The N-terminal sequences of the prepro-EDNs are cleaved by peptidases in the endoplasmic reticulum resulting in pro-EDNs (Itoh et al. 1988, Bloch et al. 1989, Bloch et al. 1991). These pro-EDNs are then transferred to Golgi body where the C-terminal sequence is cleaved by proprotein convertases, producing big-EDNs that are further shortened into 21 amino acid-long bioactive forms by endothelin converting enzymes (ECEs) which are membrane-bound metalloproteases (Xu et al. 1994, Yuzugulen et al. 2017) (Fig. 1). EDNs elicit functions through three different endothelin receptors (Braasch et al. 2009). Endothelin receptors are G-Protein coupled receptors (GPCRs) that mediate different functions in a tissue-specific manner. In blood vessels, binding of endothelin receptor A (EDNRA, also known as ETA) results in vasoconstriction; conversely, binding to endothelin receptor B (EDNRB, also known as ETB) causes vasodilation (Maguire et al. 1994, Seo et al. 1994, Bacon & Davenport 1996, Bogoni et al. 1996, Verhaar et al. 1998, Mamluk et al. 1999, Braasch et al. 2009, Ohkita et al. 2012, Ramirez 2013). Endothelin receptor C was discovered later, and its precise function has yet to be elucidated (Karne et al. 1993, Unic et al. 2011). EDN1 and EDN2 bind to both EDNRA and EDNRB with an equal affinity. EDNRA has higher binding affinity to EDN1 and EDN2 than EDN3, while EDNRB binds to all three EDNs with equal affinity (Yanagisawa et al. 1988, Inoue et al. 1989, Bremnes et al. 2000, Maguire et al. 2012). Although EDN1 and EDN2 have structural and functional similarities, their roles and distribution in the ovary are distinctive.
Figure 1. Synthesis and release of endothelin (EDN).
EDN mRNA is translated into the preproEDN form located in the endoplasmic reticulum, where the N-terminal signal sequence is cleaved by a signal peptidase into proEDN. The proEDN is transferred to Golgi body and cleaved into bigEDN by a proprotein convertase. The bigEDN is then encapsulated by secretory vesicles where it is cleaved into a bioactive form by endothelin converting enzyme (ECE) that is located on the membranes of secretory vesicles. As secretory vesicles fuse with the plasma membrane, EDN is released to the extracellular space. Although each EDN isoform is translated into early proteins of different sizes, all three follow the same synthesis process and become 21-amino acid peptides in their bioactive form.
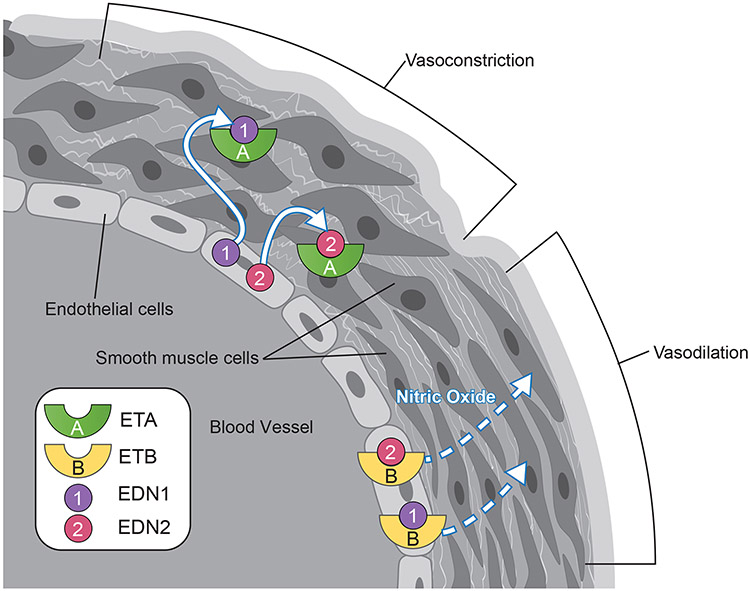
EDN1 is well studied in the ovary and other organs and provides an initial basis for understanding EDN2 in the ovary (Supplementary Table 1) (Usuki et al. 1991, Kamada et al. 1993a, Kamada et al. 1993b, Furger et al. 1995, Girsh et al. 1996a, Girsh et al. 1996b, Levin 1996, Magini et al. 1996, Mancina et al. 1997, Apa et al. 1998b, Flores et al. 1999, Denkova et al. 2000, Flores & Sasway 2000, Hinckley & Milvae 2001, Meidan & Levy 2007, Shimizu et al. 2007). EDN1 primarily acts as a vasoconstrictor through receptors on smooth muscle cells (or called as myofibroblast cells in some organs) and as a vasodilator through receptors on endothelial cells; EDN2 may exert similar functions given its similar receptor affinity to EDNRA and EDNRB. Tissue-differential action occurs because EDNRA and EDNRB are generally not expressed in the same cell, with EDNRA expressed in smooth muscle and EDNRB in endothelial cells (Fig. 2). Throughout the systemic vasculature, EDN1 increases blood pressure and decreases blood flow through vasoconstriction mediated via EDNRA (Agapitov & Haynes 2002). Within the ovary, EDN1 plays several non-contractile roles, regulating steroidogenesis, vascular homeostasis and cell proliferation; some of these roles may be redundant with EDN2 (Supplementary Table 1). For example, in luteal cells EDN1 inhibits progesterone synthesis via EDNRA (Kamada et al. 1993a, Girsh et al. 1996a, Girsh et al. 1996b, Levin 1996, Apa et al. 1998b, Flores & Sasway 2000, Hinckley & Milvae 2001, Girsh & Dekel 2002, Meidan & Levy 2007, Shimizu et al. 2007). Additionally, rising prostaglandin F2α (PGF2α) increases EDN1 expression in luteal cells (Hinckley & Milvae 2001, Girsh & Dekel 2002), and late stage luteal cells exhibit increased expression of ECE and EDNRA (Zorrilla et al. 2010), indicating that PGF2α-induced luteolysis is mediated at least in part by EDN1.
Figure 2. Endothelial cell-derived endothelins regulate vascular contractility.
The binding of EDN1 or END2 to EDNRA located in smooth muscle cells (myofibril cells) triggers contraction causing vasoconstriction. Conversely, the binding of EDN1 or END2 to EDNRB located in the endothelial cells induces nitric oxide (NO) production, which triggers smooth muscle cells to relax (vasodilation).
EDN2 is a key regulator of ovulation
Since its discovery, expression of Edn2 has been reported in a variety of organs and tissues including the ovary. It was nearly two decades after its discovery that EDN2 drew attention of reproductive biologists: two manuscripts published in 2006 showed almost identical ‘temporal and spatial’ expression patterns of Edn2 in rat and mouse ovaries, and both studies concluded that EDN2 is the trigger of follicle rupture (Ko et al. 2006, Palanisamy et al. 2006). Edn2 was shown to be transiently expressed 1-2 hours prior to follicle rupture, specifically in the granulosa cells of ovulatory follicles. Later, this unique expression pattern was confirmed by other independently performed studies (Jo et al. 2004, Kim et al. 2008, Bridges et al. 2010, Cacioppo et al. 2015, Cacioppo et al. 2017). The two initial studies, however, offered very different mechanisms for EDN2 action in triggering follicle rupture. Ko et al. proposed that EDN2 triggers follicle rupture by inducing follicle contraction via an EDNRA-mediated pathway (Ko et al. 2006). In contrast, Palanisamy et al. suggested an EDNRB-mediated increase of follicular pressure as a mechanism for inducing follicle rupture (Palanisamy et al. 2006). Since then, these two propositions had been an area of contention regarding the mechanism of EDN2 action during follicle rupture.
Differences in study design can best explain discrepancies in the initial discovery that EDN2 is essentially for ovulation. Both Ko et al. and Palanisamy et al. induced rodent ovulation through exogenous gonadotropins in these two initial studies. Ko et al. reported significantly fewer oocytes when mice were co-treated with the EDNRA antagonist BQ123, but no such impact was seen in animals co-treated with BQ788 (EDNRB antagonist). An EDNRA-mediated pathway as the primary stimulus of ovulation was supported by localized EDNRA expression in myofibroblast cells of the theca externa layer of the preovulatory follicles. Further, they showed that EDN2 induced a contractile response by an EDNRA-mediated pathway when ovaries were treated ex vivo. The contracted ovarian tissue became relaxed upon treatment with tezosentan, a pan-endothelin receptor antagonist, indicating that the constriction was mediated by an endothelin receptor(s). In contrast, Palanisamy et al. reported nearly an opposite ovulatory outcome under the influence of BQ123 and BQ788 in rats; fewer oocytes ovulated with systemic treatment of BQ788 (EDNRB antagonist). Additionally, EDNRB expression was detected in the endothelial cells around the follicles; thus a mechanism involving an EDNRB/NO-mediated pathway was proposed. A later study performed by Cho et al. supports an EDNRA-mediated pathway. They used mutant mice that lacked Ednrb expression; conclusively, the mice did not exhibit defective ovulation (Cho et al. 2012). Further, this mouse study not only showed that EDNRB-deficient female mice were fertile but that their litter sizes were larger than wild type littermates. Additionally, the ovaries of Ednrb-deficient mice had more corpora lutea than control animals. While involvement of EDNRB in regulating ovulation cannot be completely dismissed, results of Cho et al (Choi et al. 2011), indicate that any role of EDNRB would decrease or prevent, rather than cause ovulation, and also confers data to support mouse and rat ovulation being regulated by EDN2 in an EDNRA-dependent manner.
Given the above, it was hypothesized that EDNRA causes contractile activity of smooth muscle cells (Migone et al. 2016) surrounding the periovulatory follicles. In support, Bridges et al. (2010) demonstrated that EDN2 induces ovarian contraction via EDNRA and not EDNRB by using endothelin receptor antagonists. There was no effect observed when ovaries were treated with an EDNRB antagonist, but ovaries showed reduced contraction when treated with an EDNRA antagonist (Bridges et al. 2010). Therefore, the binding of EDN2 to EDNRA elicits contraction of smooth muscle cells, while its binding to EDNRB may help to maintain homeostasis of follicular contractility; thus, such an arrangement would allow contraction to last only momentarily at the time of ovulation. As EDNRA is expressed in granulosa cells and smooth muscle cells surrounding follicles (Ko et al. 2006) and EDN2 is expressed only in granulosa cells, this separation of ligand and receptor may prevent any episodic contractions before the preovulatory follicles become ready to ovulate. Having EDN2 and EDNRA separated by the basal lamina in a preovulatory follicle may allow the smooth muscle layer to contract only after the basal lamina is weakened.
The essential functional role that EDN2 plays in ovulation was later confirmed using another mouse model that was globally deficient in Edn2 expression (Cacioppo et al. 2017). Although mice lacking EDN2 do not survive to adulthood because loss of EDN2 is a lethal mutation (Chang et al. 2013), knockout mutants survived up to 3 weeks of ages. Histological analysis of mutant juvenile ovaries indicated that lack of EDN2 does not impact folliculogenesis or early-stage follicle growth. For longitudinal studies, Cacioppo et al. transplanted Edn2-knockout ovaries of Edn2-deficient neonatal mice to a kidney capsule of a prepubertal wild-type (WT) mouse. When a recipient mouse of pubertal age had ovulation induced, histological examination of transplanted ovaries showed that both WT and Edn2-deficient mutant had corpora lutea (CL). However, mutant Edn2-deficient ovaries had CL with entrapped oocytes that had not ovulated unlike WT controls, indicating ovulatory failure in EDN2-deficient ovaries.
The production of EDN2 specifically in granulosa cells as the cause of ovulation was further supported by studies using tissue-selective knockout mice. Cacioppo et al. utilized a mouse model where Edn2 expression was selectively ablated in the granulosa cell lineage by crossing a floxed-Edn2 mouse with transgenic mice that express Cre recombinase under the promoter of the Esr2 gene. Mice lacking EDN2 production by granulosa cells ovulated 75% fewer oocytes and had 30% smaller litters, although pregnancies per pairing and hormones within the reproductive axis remained intact (Cacioppo et al. 2017). A second mouse model where Ednra expression was lost in granulosa cells was also studied (floxed-Ednra mice crossed with Cre recombinase under the Esr2 promoter). Mice lacking EDNRA expression in granulosa cells ovulated 50% fewer oocytes but did not have smaller litters or fewer pregnancies per pairing (Cacioppo et al. 2017). These data indicate EDNRA plays a role in granulosa cells, but its lack does not prevent ovulation and successful pregnancy in the mouse. The propensity for an induced mouse to ovulate many oocytes, more than can successfully mature through pregnancy, is both an advantage and disadvantage in the in vivo study of the ovarian endothelin system.
Interplay between EDN2 and key molecules in ovulation
EDN2 is expressed only temporarily and immediately prior to the time of follicle rupture (Sudik et al. 1996, Ko et al. 2006, Palanisamy et al. 2006, Klipper et al. 2010, Cacioppo et al. 2017, Shrestha et al. 2018), after which expression subsides rapidly (Palanisamy et al. 2006, Klipper et al. 2010). EDN2 is triggered through the LH surge and subsequent progesterone receptor activity. While the tight transcriptional regulation of Edn2 expression is a subject of future investigation, hypoxia via Hypoxia-inducible factor 1-alpha (HIF1α) may drive Edn2 transcription following progesterone receptor activation (Na et al. 2008, Kim et al. 2009, Wang et al. 2015, Yalu et al. 2015). In support of this hypothesis, the promoter region of the Edn2 gene contains a hypoxia response element (HRE), and granulosa cells of preovulatory follicles experience hypoxia as the follicle rapidly grows after the LH surge, without an accompanying increase in oxygen from blood vessels located outside of the follicle (Klipper et al. 2010). Of note, the antrum of a growing follicle is not vascularized, causing the follicle to experience hypoxia as it experiences rapid growth in a short time (Suzuki et al. 1998, Zhang et al. 2019, Lim et al. 2021, Tang et al. 2021).
To further elucidate the role of hypoxia and possibly HIF-1α signaling in triggering Edn2 expression, a pilot study using WT and progesterone receptor-deficient mice in hypoxic chambers was performed. Although progesterone receptor-deficient mice had a 90-fold reduction in Edn2 expression at the time of ovulation, acute systemic hypoxemia did not cause a difference in Edn2 expression in ovaries, kidneys, or lungs (Cacioppo et al. 2015). It should also be noted HIF-1α expression increases throughout theca cells but not granulosa cells during the first six hours of the rat progesterone surge (Jo et al. 2004), and a more complex interaction may be occurring than 1:1 promoter signaling. A promoter analysis of the murine Edn2 gene was cross-referenced with an ovarian microarray database (Cacioppo et al. 2015), and the hypothesis that Edn2 expression is regulated by RUNX1 within the ovary during ovulation, with possible regulation through BCL6 expression, was generated. However, this hypothesis remains as a testing aim for future molecular work. It is also likely that tissue-specific epigenetic modifications also play a role in EDN2 regulation. A recent study showed that inhibition of MiR-210 decreased EDN2 expression in human primary and immortalized granulosa-lutein cells (Shrestha et al. 2018), indicating the involvement of microRNA in regulating EDN2 expression.
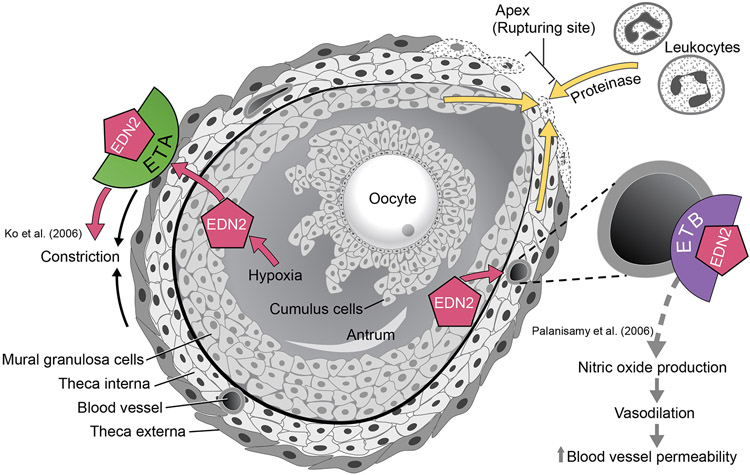
Increased intrafollicular pressure increase alone is not sufficient for ovulation. For rupture to occur, first the integrity of the basal lamina of the follicle needs to be weakened (LeMaire 1989, Colgin & Murdoch 1997, Oakley et al. 2010) by increased proteolytic activity of matrix metalloproteinases (MMPs) and the plasminogen system (Ny et al. 1999) (Fig. 3). Both ovarian cells and local leukocytes, which enter through circulation, secrete proteases within the ovary. MMP2 and MMP9 have been previously implicated in degradation of the follicle wall during ovulation in mouse models (Robker et al. 2000). Additionally, a decrease in activity of metallopeptidase inhibitors (TIMPs), specifically of TIMP1, has also been correlated with ovulation (Stilley & Sharpe-Timms 2012, Peng et al. 2015). Ovarian modulation of TIMPs and MMPs may occur through leukocytes, which double in quantity in the ovary prior to ovulation in the rodent, while decreasing by a similar amount in the spleen (Oakley et al. 2011). Various leukocytes, including macrophages, dendritic cells, and lymphocytes, are all localized to the mature follicle during ovulation, and dendritic cells are shown necessary for normal ovulation (Oakley et al. 2010, Cohen-Fredarow et al. 2014). Importantly, studies show that EDN2 acts as a chemoattractant for immune cells in various organs including the intestine, where EDN2 promotes macrophage accumulation in the lamina propria (Takahashi-Iwanaga et al. 1999, Grimshaw et al. 2002b, Takizawa et al. 2005). In a breast tumor cell model, EDN2 via EDNRB attracts and activates macrophages, where EDN2 binding to EDNRB activates the MAPK signaling pathway in the macrophage and triggers higher EDN2 concentrations. Reportedly, when breast tumor cells experience hypoxia, they express EDN2 which in turn attracts macrophages and stimulates the production of cytokines responsible for angiogenesis and tumor growth (Grimshaw et al. 2002a, Grimshaw et al. 2002b, Grimshaw et al. 2004), and this may explain why some tumor cells can grow under hypoxic conditions (Grimshaw et al. 2002a, Grimshaw et al. 2002b, Grimshaw et al. 2004, Olender et al. 2016). Overall, degradation of the follicular basal lamina and recruitment of leukocytes are necessary for follicular rupture as well, and the hypoxia generated through these processes may stimulate EDN2 expression as well as propagate their own actions.
Figure 3. EDN2 triggers contraction of mature follicles in the ovary.
Prior to follicular rupture, a follicle rapidly grows without support of an accompanying vascular oxygen supply. Granulosa cells of mature preovulatory follicles experiences hypoxia, triggering EDN2 expression. As the follicle grows, proteinases secreted by granulosa cells, theca cells and leukocytes weaken the follicular wall. EDN2 triggers the theca externa that is made of contractile myofibroblast cells to contract, leading to a rupture of the follicle at the apex from which the oocyte is released. EDN2 may bind to EDNRB on vascular endothelial cells and induce nitric oxide (NO) production, thereby causing vasodilation and increasing the vascular permeability; this change may influence follicular rupture but is likely unnecessary for a successful ovulation.
In summary, EDN2 is necessary for ovulation and is translated exclusively at the time of follicle rupture; it is produced in granulosa cells and acts through EDNRA, largely through contraction of smooth muscle cells in the theca cell layer of mature follicles (Fig. 3). This has now been well confirmed through multiple genetically manipulated mouse model studies and previous medication studies in rodents.
Endothelin in the human ovary
The presence of individual endothelin components in the human and primate ovary has been reported previously (Haq et al. 1996, Mancina et al. 1997, Apa et al. 1998b, Karam et al. 1999, Korth et al. 1999, Imbar et al. 2012), and functional studies revealed their role in the regulation of ovulation, steroidogenesis in the follicle and corpus luteum (Apa et al. 1998b, Denkova et al. 2000, Meidan & Levy 2007), and blood flow (Mancina et al. 1997, Meidan & Levy 2007). In the human ovary, presence of endothelin peptides was detected in follicular fluid which was collected at the time of oocyte aspiration from women undergoing ovulation induction for in vitro fertilization (Kamada et al. 1993b, Haq et al. 1996). Both EDN1 and EDN2 are detected in follicular fluid, but only EDN2 was reportedly showed an increased concentration associated with oocyte maturity (Haq et al. 1996). The mean concentration of EDN2 was significantly higher in the fluid of oocytes that could be fertilized and cleaved than in those with oocytes that did not fertilize or cleave (Sudik et al. 1996). Also of note, there was no significant difference in the average level of EDN1 in the fluid from small to large follicles, whereas a significantly higher level of EDN2 was present in large follicles, suggesting a critical intra-ovarian role for EDN2 in ovulation. While these reports are interesting, a caution has to be made in taking their findings because to-date no commercially available antibody has been shown to differentiate between the two endothelin subtypes. Nonetheless, as the amino acid sequences of the endothelin 1, 2 and 3 are highly conserved through all vertebrates (Braasch & Schartl 2014), their functional roles in follicular rupture may be highly conserved, along with the role of EDNs during development (Square et al. 2020).
In situ hybridization and Northern blot experiments revealed that EDN1 is expressed in granulosa cells of secondary-Graafian follicle stages and endothelial cells in the human ovary, but not in the primordial-primary follicles and stromal cells (Kamada et al. 1995, Magini et al. 1996). This granulosa cell-derived EDN1 regulates the proliferation and steroidogenesis of granulosa cells and reduces both basal and hCG-induced progesterone production of luteal cells in the human ovary (Kamada et al. 1995, Apa et al. 1998a, Apa et al. 1998b). EDN2 is the predominant EDN isoform in the aspirated preovulatory human granulosa cells (GC), where its mRNA expression level was 15-fold higher when compared to that of EDN1 (Choi et al. 2011). Interestingly, cumulus cells showed higher EDN2 expression than GC in the same experiment. While EDN1 and EDN2 are abundantly expressed in GC and cumulus cells, there was no significant expression of EDN3 in human preovulatory follicles (Choi et al. 2011). Indeed, EDN3 is not involved in inhibiting steroidogenesis and does not stimulate the proliferation of human granulosa cells (Ervin et al. 2017). Instead, the expression of EDN3 increased in the oviduct with treatment of gonadotropins and showed highest expression around the time of ovulation (Jeoung et al. 2010). On the other hand, EDN1 and EDN2 trigger a concentration-dependent contraction of the human isthmic segment of the oviduct during the luteal phase. (Jankovic et al. 2010) In general, it is thought that endothelins have a conserved role in the human oviduct during ovulation and oocyte transport as discussed below.
Mancina et al. labeled EDN1, EDN3, and selective analogs with I125 and showed that the density of the I125-labeled sites for EDNRA was 100-fold higher than that of EDNRB in the human ovarian sections obtained from women who underwent hysterectomy (Mancina et al. 1997). The study found no positive I125 signal in granulosa cells, indicating no or very low presence of bound-EDNRA or EDNRB in granulosa cells. Instead, they observed expression for EDNRA but not EDNRB in the blood vessels and theca interna (Mancina et al. 1997). Taken together, the authors concluded that EDNRA and not EDNRB is likely a primary receptor for EDNs in the human ovary. However, other studies report the expression of mRNA for both EDNRA and EDNRB in granulosa and cumulus cells of human ovulatory follicle (Gentili et al. 2001, Choi et al. 2011), thus requiring further investigation into the expression pattern of endothelin receptors in the human ovary. In doing so, the stages of the reproductive cycle and pathophysiological conditions should be considered because such differences likely impact the expression of endothelin receptors.
Systemic mutations in the EDN system generally result in embryonic death, while errors in endothelin expression in specific tissues cause clinically significant disease processes. One individual with an EDNRA mutation (c.907G>A) showed Johnson-McMillin syndrome (JMS) which is characterized by alopecia, hyposmia or anosmia, hearing loss, ear malformation and hypogonadotropic hypogonadism (Cushman et al. 2005, Gordon et al. 2015). Within reproductive studies, there is impaired EDN2 expression in granulosa cells of PCOS ovaries: EDN2 and MicroRNA-210 (miR-210, hypoxiamiR) are reduced in the granulosa-lutein cells (GLCs) from PCOS women, which may be part of a hypoxic response (Szymanska et al. 2021). Types of diet may impact EDN2 expression by altering ovarian steroidogenesis. Mice on a high fat diet were shown to display ovulatory dysregulation of key ovarian steroidogenic genes and low Edn2 expression, but that estradiol treatment increased Edn2 expression (Hohos et al. 2021), suggesting that impaired steroidogenesis while on a high fat diet as a model for obesity appears to be mediated through dysregulation of ovarian Edn2. Obesity and high fat diet are also linked to PCOS, and this is likely one of the important future directions of EDN research as the obesity epidemic continues.
Conclusion and future directions
Abundant experimental and observational evidence clearly show that EDN2 plays a key role in triggering follicle rupture and, therefore, successful ovulation and fertility. EDN2 is expressed in granulosa cells of the mature follicle at the time of ovulation. EDNRA-mediated follicular contraction is likely the mechanistic role played by EDN2 in ovulation, while EDNRB may have a nonessential homeostatic function involving perifollicular vasodilation, leukocyte cytokine signaling, or preventing ovulation until a specific time point, as contraction is not the only necessary mediator for ovulation of mature oocytes. Further clarification of the EDN2 signaling cascade, in model species and humans, both in and out of the ovary, would provide a better understanding of disease processes it may mediate. This is also true for EDNs in the oviduct. Lastly, video observation and M-mode ultrasonic measurement of human ovulation fluid ejection speeds could also help determine final human follicular pressure at the time of ovulation, and how this is influenced in various disease states.
Can EDN2 or endothelin receptors be a target for contraception in humans or in production animals? The answer will depend on whether targeting can be specific to the ovary. It is most likely that an endothelin aberration anywhere in the gonad-pituitary-reproductive axis can cause significant reproductive dysfunction, though EDNs remain necessary in embryonic neural crest cell migration, and development of the pharyngeal arches. Systemic contraceptive methods that inhibit the ovarian endothelin system would likely be far more detrimental than beneficial, as the endothelin system plays an important role in other organs and in regulating a variety of physiological regulatory systems at a more continuous and lower ligand level than in the ovary. Systemic disruption of homeostatic regulation of blood flow may impact heart, lung, brain or kidney functions and, therefore, cause potentially serious side effects. For this reason, macitentan (Opsumit®), an EDNRA inhibitor, is used to treat pulmonary arterial hypertension (Khadka et al. 2015), but is not promoted as a contraceptive. If EDN2 or EDNRA inhibition could be limited to the ovary alone, it may be a highly efficiency contraceptive target that bypasses common conventional hormonal side effects. The advent of epigenetic-targeting molecules (Cheng et al. 2019, Patnaik & Anupriya 2019) may be specific to ovarian EDN2 alone. EDNRA-antagonists may be coupled with steroid-specific cytochrome antibodies to target only steroidogenic organs. Possibly in vivo CRISPR technology or similar gene therapy (Ablain & Zon 2016, Cheng et al. 2020) will remove EDN2 from within the ovary alone with high fidelity. Alternatively, since the endothelin system plays a key role in luteal function, further research may elucidate causes of infertility in humans and animals tied to endothelin deficiency. Outside of reproduction, the endothelin system may be modified to allow greater revascularization during tissue transplantation or microsurgery. The future of the endothelin field remains promising for further investigation.
Supplementary Material
Funding
This work was supported by NIH grants HD071875 and HD094296 (CK).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
References
- Ablain J & Zon LI 2016. Tissue-specific gene targeting using CRISPR/Cas9. Methods in Cell Biology 135 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agapitov AV & Haynes WG 2002. Role of endothelin in cardiovascular disease. Journal of the Renin-Angiotensin-Aldosterone System 3 1–15. [DOI] [PubMed] [Google Scholar]
- Apa R, Miceli F, de Feo D, Mastrandrea ML, Mancuso S, Napolitano M & Lanzone A 1998a. Endothelin-1 inhibits basal and human chorionic gonadotrophin-stimulated progesterone production. Human Reproduction 13 2425–2429. [DOI] [PubMed] [Google Scholar]
- Apa R, Miceli F, de Feo D, Pierro E, Ayala G, Mancuso S, Napolitano M & Lanzone A 1998b. Endothelin-1: expression and role in human corpus luteum. American Journal of Reproductive Immunology 40 370–376. [DOI] [PubMed] [Google Scholar]
- Bacon CR & Davenport AP 1996. Endothelin receptors in human coronary artery and aorta. British Journal of Pharmacology 117 986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch KD, Eddy RL, Shows TB & Quertermous T 1989. cDNA cloning and chromosomal assignment of the gene encoding endothelin 3. Journal of Biological Chemistry 264 18156–18161. [PubMed] [Google Scholar]
- Bloch KD, Hong CC, Eddy RL, Shows TB & Quertermous T 1991. cDNA cloning and chromosomal assignment of the endothelin 2 gene: vasoactive intestinal contractor peptide is rat endothelin 2. Genomics 10 236–242. [DOI] [PubMed] [Google Scholar]
- Bogoni G, Rizzi A, Calo G, Campobasso C, D'Orleans-Juste P & Regoli D 1996. Characterization of endothelin receptors in the human umbilical artery and vein. British Journal of Pharmacology 119 1600–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch I & Schartl M 2014. Evolution of endothelin receptors in vertebrates. General and Comparative Endocrinology 209 21–34. [DOI] [PubMed] [Google Scholar]
- Braasch I, Volff JN & Schartl M 2009. The endothelin system: evolution of vertebrate-specific ligand-receptor interactions by three rounds of genome duplication. Molecular Biology and Evolution 26 783–799. [DOI] [PubMed] [Google Scholar]
- Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B & Attramadal H 2000. Regulation and intracellular trafficking pathways of the endothelin receptors. Journal of Biological Chemistry 275 17596–17604. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Jo M, Al Alem L, Na G, Su W, Gong MC, Jeoung M & Ko C 2010. Production and binding of endothelin-2 (EDN2) in the rat ovary: endothelin receptor subtype A (EDNRA)-mediated contraction. Reproduction, Fertility, and Development 22 780–787. [DOI] [PubMed] [Google Scholar]
- Cacioppo JA, Koo Y, Lin PC, Gal A & Ko C 2015. Generation and characterization of an endothelin-2 iCre mouse. Genesis 53 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JA, Lin PP, Hannon PR, McDougle DR, Gal A & Ko C 2017. Granulosa cell endothelin-2 expression is fundamental for ovulatory follicle rupture. Scientific Reports 7 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang I, Bramall AN, Baynash AG, Rattner A, Rakheja D, Post M, Joza S, McKerlie C, Stewart DJ, McInnes RR et al. 2013. Endothelin-2 deficiency causes growth retardation, hypothermia, and emphysema in mice. Journal of Clinical Investigation 123 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA & Siegwart DJ 2020. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol 15 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, Han J & Wei X 2019. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther 4 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Kim H, Kang DW, Yanagisawa M & Ko C 2012. Endothelin B receptor is not required but necessary for finite regulation of ovulation. Life Sciences 91 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DH, Kim EK, Kim KH, Lee KA, Kang DW, Kim HY, Bridges P & Ko C 2011. Expression pattern of endothelin system components and localization of smooth muscle cells in the human pre-ovulatory follicle. Human Reproduction 26 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA & Moenter SM 2010. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocrine Reviews 31 544–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Fredarow A, Tadmor A, Raz T, Meterani N, Addadi Y, Nevo N, Solomonov I, Sagi I, Mor G, Neeman M et al. 2014. Ovarian dendritic cells act as a double-edged pro-ovulatory and anti-inflammatory sword. Molecular Endocrinology 28 1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin DC & Murdoch WJ 1997. Evidence for a role of the ovarian surface epithelium in the ovulatory mechanism of the sheep: secretion of urokinase-type plasminogen activator. Anim Reprod Sci 47 197–204. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Torres-Martinez W & Weaver DD 2005. Johnson-McMillin syndrome: report of a new case with novel features. Birth Defects Research. Part A: Clinical and Molecular Teratology 73 638–641. [DOI] [PubMed] [Google Scholar]
- Denkova R, Bourneva V, Baleva K, Yaneva E, Nikolov B, Christov I & Ivanov I 2000. Modulation of steroidogenesis in human ovarian granulosa cells during aging. Endocrine Regulations 34 157–160. [PubMed] [Google Scholar]
- Duffy DM, Ko C, Jo M, Brannstrom M & Curry TE 2019. Ovulation: Parallels With Inflammatory Processes. Endocr Rev 40 369–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin JM, Schutz LF & Spicer LJ 2017. Current status of the role of endothelins in regulating ovarian follicular function: A review. Animal Reproduction Science 186 1–10. [DOI] [PubMed] [Google Scholar]
- Espey LL 1967. Ultrastructure of the apex of the rabbit graafian follicle during the ovulatory process. Endocrinology 81 267–276. [DOI] [PubMed] [Google Scholar]
- Flores JA, Garmey JC, Lahav M & Veldhuis JD 1999. Mechanisms underlying endothelin's inhibition of FSH-stimulated progesterone production by ovarian granulosa cells. Molecular and Cellular Endocrinology 156 169–178. [DOI] [PubMed] [Google Scholar]
- Flores JA & Sasway HM 2000. Gene expression of endothelin-1 in the porcine ovary: follicular development. Biology of Reproduction 63 1377–1382. [DOI] [PubMed] [Google Scholar]
- Furger C, Zorn JR & Ferre F 1995. Endothelins inhibit FSH-mediated function via ETA receptors in cultured human granulosa-lutein cells. Early Pregnancy 1 188–195. [PubMed] [Google Scholar]
- Gentili M, Obermuller N, Schleich HG, Melchert F & Weigel M 2001. Distinct expression of endothelin receptor subtypes A and B in luteinized human granulosa cells. Hormone and Metabolic Research 33 573–576. [DOI] [PubMed] [Google Scholar]
- Girsh E & Dekel N 2002. Involvement of endothelin-1 and its receptors in PGF2alpha-induced luteolysis in the rat. Molecular Reproduction and Development 63 71–78. [DOI] [PubMed] [Google Scholar]
- Girsh E, Milvae RA, Wang W & Meidan R 1996a. Effect of endothelin-1 on bovine luteal cell function: role in prostaglandin F2alpha-induced antisteroidogenic action. Endocrinology 137 1306–1312. [DOI] [PubMed] [Google Scholar]
- Girsh E, Wang W, Mamluk R, Arditi F, Friedman A, Milvae RA & Meidan R 1996b. Regulation of endothelin-1 expression in the bovine corpus luteum: elevation by prostaglandin F 2 alpha. Endocrinology 137 5191–5196. [DOI] [PubMed] [Google Scholar]
- Gordon CT, Weaver KN, Zechi-Ceide RM, Madsen EC, Tavares AL, Oufadem M, Kurihara Y, Adameyko I, Picard A, Breton S et al. 2015. Mutations in the endothelin receptor type A cause mandibulofacial dysostosis with alopecia. American Journal of Human Genetics 96 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw MJ, Hagemann T, Ayhan A, Gillett CE, Binder C & Balkwill FR 2004. A role for endothelin-2 and its receptors in breast tumor cell invasion. Cancer Research 64 2461–2468. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ, Naylor S & Balkwill FR 2002a. Endothelin-2 is a hypoxia-induced autocrine survival factor for breast tumor cells. Molecular Cancer Therapeutics 1 1273–1281. [PubMed] [Google Scholar]
- Grimshaw MJ, Wilson JL & Balkwill FR 2002b. Endothelin-2 is a macrophage chemoattractant: implications for macrophage distribution in tumors. European Journal of Immunology 32 2393–2400. [DOI] [PubMed] [Google Scholar]
- Haq A, Kayali M, Hammami MM, Jaroudi K & al-Sedairy ST 1996. Immunoreactive endothelin-1, endothelin-2 and big endothelin-1 in follicular fluids of women undergoing ovulation induction for in-vitro fertilization. Human Reproduction 11 269–273. [DOI] [PubMed] [Google Scholar]
- Hinckley ST & Milvae RA 2001. Endothelin-1 mediates prostaglandin F(2alpha)-induced luteal regression in the ewe. Biology of Reproduction 64 1619–1623. [DOI] [PubMed] [Google Scholar]
- Hohos NM, Elliott EM, Giornazi A, Silva E, Rice JD & Skaznik-Wikiel ME 2021. High-fat diet induces an ovulatory defect associated with dysregulated endothelin-2 in mice. Reproduction 161 307–317. [DOI] [PubMed] [Google Scholar]
- Imbar T, Klipper E, Greenfield C, Hurwitz A, Haimov-Kochman R & Meidan R 2012. Altered endothelin expression in granulosa-lutein cells of women with polycystic ovary syndrome. Life Sciences 91 703–709. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K & Masaki T 1989. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proceedings of the National Academy of Sciences of the United States of America 86 2863–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Yanagisawa M, Ohkubo S, Kimura C, Kosaka T, Inoue A, Ishida N, Mitsui Y, Onda H, Fujino M et al. 1988. Cloning and sequence analysis of cDNA encoding the precursor of a human endothelium-derived vasoconstrictor peptide, endothelin: identity of human and porcine endothelin. FEBS Letters 231 440–444. [DOI] [PubMed] [Google Scholar]
- Jankovic SM, Jankovic SV, Lukic G, Canovic D & Folic M 2010. The contractile effects of endothelins on isolated isthmic segment of human oviduct at the luteal phase of the menstrual cycle. Methods and Findings in Experimental and Clinical Pharmacology 32 91–95. [DOI] [PubMed] [Google Scholar]
- Jeoung M, Lee S, Hawng HK, Cheon YP, Jeong YK, Gye MC, Iglarz M, Ko C & Bridges PJ 2010. Identification of a novel role for endothelins within the oviduct. Endocrinology 151 2858–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo M, Gieske MC, Payne CE, Wheeler-Price SE, Gieske JB, Ignatius IV, Curry TE Jr. & Ko C 2004. Development and application of a rat ovarian gene expression database. Endocrinology 145 5384–5396. [DOI] [PubMed] [Google Scholar]
- Kamada S, Blackmore PF, Kubota T, Oehninger S, Asada Y, Gordon K, Hodgen GD & Aso T 1995. The role of endothelin-1 in regulating human granulosa cell proliferation and steroidogenesis in vitro. Journal of Clinical Endocrinology and Metabolism 80 3708–3714. [DOI] [PubMed] [Google Scholar]
- Kamada S, Kubota T, Hirata Y, Imai T, Ohta K, Taguchi M, Marumo F & Aso T 1993a. Endothelin-1 is an autocrine/paracrine regulator of porcine granulosa cells. Journal of Endocrinological Investigation 16 425–431. [DOI] [PubMed] [Google Scholar]
- Kamada S, Kubota T, Taguchi M & Aso T 1993b. High levels of immunoreactive endothelin-1 in human follicular fluids. Human Reproduction 8 674–677. [DOI] [PubMed] [Google Scholar]
- Karam H, Valdenaire O, Belair MF, Prigent-Sassy C, Rakotosalama A, Clozel M, Itskovitz J & Bruneval P 1999. The endothelin system in human and monkey ovaries: in situ gene expression of the different components. Cell and Tissue Research 295 101–109. [DOI] [PubMed] [Google Scholar]
- Karne S, Jayawickreme CK & Lerner MR 1993. Cloning and characterization of an endothelin-3 specific receptor (ETC receptor) from Xenopus laevis dermal melanophores. Journal of Biological Chemistry 268 19126–19133. [PubMed] [Google Scholar]
- Khadka A, Singh Brashier DB, Tejus A & Sharma AK 2015. Macitentan: An important addition to the treatment of pulmonary arterial hypertension. Journal of Pharmacology & Pharmacotherapeutics 6 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Bagchi IC & Bagchi MK 2009. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 150 3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC & Bagchi MK 2008. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Molecular and Cellular Biology 28 1770–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipper E, Levit A, Mastich Y, Berisha B, Schams D & Meidan R 2010. Induction of endothelin-2 expression by luteinizing hormone and hypoxia: possible role in bovine corpus luteum formation. Endocrinology 151 1914–1922. [DOI] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M & Koo Y 2006. Endothelin-2 in ovarian follicle rupture. Endocrinology 147 1770–1779. [DOI] [PubMed] [Google Scholar]
- Korth P, Bohle RM, Corvol P & Pinet F 1999. Cellular distribution of endothelin-converting enzyme-1 in human tissues. Journal of Histochemistry and Cytochemistry 47 447–462. [DOI] [PubMed] [Google Scholar]
- LeMaire WJ 1989. Mechanism of mammalian ovulation. Steroids 54 455–469. [DOI] [PubMed] [Google Scholar]
- Levin ER 1996. Editorial: Endothelin-1, prostaglandin F 2 alpha, and the corpus luteum--the crisis of lysis. Endocrinology 137 5189–5190. [DOI] [PubMed] [Google Scholar]
- Lim M, Thompson JG & Dunning KR 2021. HYPOXIA AND REPRODUCTIVE HEALTH: Hypoxia and ovarian function: follicle development, ovulation, oocyte maturation. Reproduction 161 F33–F40. [DOI] [PubMed] [Google Scholar]
- Magini A, Granchi S, Orlando C, Vannelli GB, Pellegrini S, Milani S, Grappone C, De Franco R, Susini T, Forti G et al. 1996. Expression of endothelin-1 gene and protein in human granulosa cells. Journal of Clinical Endocrinology and Metabolism 81 1428–1433. [DOI] [PubMed] [Google Scholar]
- Maguire JJ, Kuc RE & Davenport AP 2012. Radioligand binding assays and their analysis. Methods in Molecular Biology 897 31–77. [DOI] [PubMed] [Google Scholar]
- Maguire JJ, Kuc RE, O'Reilly G & Davenport AP 1994. Vasoconstrictor endothelin receptors characterized in human renal artery and vein in vitro. British Journal of Pharmacology 113 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamluk R, Levy N, Rueda B, Davis JS & Meidan R 1999. Characterization and regulation of type A endothelin receptor gene expression in bovine luteal cell types. Endocrinology 140 2110–2116. [DOI] [PubMed] [Google Scholar]
- Mancina R, Barni T, Calogero AE, Filippi S, Amerini S, Peri A, Susini T, Vannelli GB, Burrello N, Forti G et al. 1997. Identification, characterization, and biological activity of endothelin receptors in human ovary. Journal of Clinical Endocrinology and Metabolism 82 4122–4129. [DOI] [PubMed] [Google Scholar]
- Matousek M, Carati C, Gannon B & Brannstrom M 2001. Novel method for intrafollicular pressure measurements in the rat ovary: increased intrafollicular pressure after hCG stimulation. Reproduction 121 307–314. [DOI] [PubMed] [Google Scholar]
- Meidan R & Levy N 2007. The ovarian endothelin network: an evolving story. Trends in Endocrinology and Metabolism 18 379–385. [DOI] [PubMed] [Google Scholar]
- Migone FF, Cowan RG, Williams RM, Gorse KJ, Zipfel WR & Quirk SM 2016. In vivo imaging reveals an essential role of vasoconstriction in rupture of the ovarian follicle at ovulation. Proceedings of the National Academy of Sciences of the United States of America 113 2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na G, Bridges PJ, Koo Y & Ko C 2008. Role of hypoxia in the regulation of periovulatory EDN2 expression in the mouse. Canadian Journal of Physiology and Pharmacology 86 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny A, Leonardsson G, Hagglund AC, Hagglof P, Ploplis VA, Carmeliet P & Ny T 1999. Ovulation in plasminogen-deficient mice. Endocrinology 140 5030–5035. [DOI] [PubMed] [Google Scholar]
- Oakley OR, Frazer ML & Ko C 2011. Pituitary-ovary-spleen axis in ovulation. Trends Endocrinol Metab 22 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley OR, Kim H, El-Amouri I, Lin PC, Cho J, Bani-Ahmad M & Ko C 2010. Periovulatory leukocyte infiltration in the rat ovary. Endocrinology 151 4551–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkita M, Tawa M, Kitada K & Matsumura Y 2012. Pathophysiological roles of endothelin receptors in cardiovascular diseases. Journal of Pharmacological Sciences 119 302–313. [DOI] [PubMed] [Google Scholar]
- Oktay K, Newton H, Mullan J & Gosden RG 1998. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Human Reproduction 13 1133–1138. [DOI] [PubMed] [Google Scholar]
- Olender J, Nowakowska-Zajdel E, Walkiewicz K & Muc-Wierzgon M 2016. Endothelins and carcinogenesis. Postepy Hig Med Dosw (Online) 70 872–880. [DOI] [PubMed] [Google Scholar]
- Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK & Bagchi IC 2006. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Molecular Endocrinology 20 2784–2795. [DOI] [PubMed] [Google Scholar]
- Patnaik S & Anupriya 2019. Drugs Targeting Epigenetic Modifications and Plausible Therapeutic Strategies Against Colorectal Cancer. Frontiers in Pharmacology 10 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JY, Han P, Xin HY, Ji SY, Gao KX, An XP & Cao BY 2015. Molecular characterization and hormonal regulation of tissue inhibitor of metalloproteinase 1 in goat ovarian granulosa cells. Domestic Animal Endocrinology 52 1–10. [DOI] [PubMed] [Google Scholar]
- Ramirez GA 2013. Endothelin ETB1 receptor agonism as a new therapeutic strategy in pulmonary arterial hypertension and chronic heart failure. Medical Hypotheses 81 896–897. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW & Richards JS 2000. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proceedings of the National Academy of Sciences of the United States of America 97 4689–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo B, Oemar BS, Siebenmann R, von Segesser L & Luscher TF 1994. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation 89 1203–1208. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Berisha B, Schams D & Miyamoto A 2007. Changes in the messenger RNA expressions of the endothelin-1 and angiotensin systems in mature follicles of the superovulated bovine ovary. J Reprod Dev 53 655–662. [DOI] [PubMed] [Google Scholar]
- Shrestha K, Onasanya AE, Eisenberg I, Wigoda N, Yagel S, Yalu R, Meidan R & Imbar T 2018. miR-210 and GPD1L regulate EDN2 in primary and immortalized human granulosa-lutein cells. Reproduction 155 197–205. [DOI] [PubMed] [Google Scholar]
- Square TA, Jandzik D, Massey JL, Romasek M, Stein HP, Hansen AW, Purkayastha A, Cattell MV & Medeiros DM 2020. Evolution of the endothelin pathway drove neural crest cell diversification. Nature 585 563–568. [DOI] [PubMed] [Google Scholar]
- Starup J & Visfeldt J 1974. Ovarian morphology and pituitary gonadotrophins in serum during and after long-term treatment with oral contraceptives. Acta Obstetricia et Gynecologica Scandinavica 53 161–167. [DOI] [PubMed] [Google Scholar]
- Stilley JA & Sharpe-Timms KL 2012. TIMP1 contributes to ovarian anomalies in both an MMP-dependent and -independent manner in a rat model. Biology of Reproduction 86 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudik R, Chari S, Pascher E & Sturm G 1996. Human follicular fluid levels of endothelins in relation to oocyte maturity status. Experimental and Clinical Endocrinology and Diabetes 104 78–84. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takaya R, Fukaya T, Yajima A & Nagura H 1998. Cyclic changes of vasculature and vascular phenotypes in normal human ovaries. Human Reproduction 13 953–959. [DOI] [PubMed] [Google Scholar]
- Szymanska M, Shrestha K, Girsh E, Harlev A, Eisenberg I, Imbar T & Meidan R 2021. Reduced Endothelin-2 and Hypoxic Signaling Pathways in Granulosa-Lutein Cells of PCOS Women. International Journal of Molecular Sciences 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi-Iwanaga H, Iwanaga T & Isayama H 1999. Porosity of the epithelial basement membrane as an indicator of macrophage-enterocyte interaction in the intestinal mucosa. Archives of Histology and Cytology 62 471–481. [DOI] [PubMed] [Google Scholar]
- Takizawa S, Uchide T, Adur J, Kozakai T, Kotake-Nara E, Quan J & Saida K 2005. Differential expression of endothelin-2 along the mouse intestinal tract. Journal of Molecular Endocrinology 35 201–209. [DOI] [PubMed] [Google Scholar]
- Tang Z, Xu R, Zhang Z, Shi C, Zhang Y, Yang H, Lin Q, Liu Y, Lin F, Geng B et al. 2021. HIF-1alpha Protects Granulosa Cells From Hypoxia-Induced Apoptosis During Follicular Development by Inducing Autophagy. Front Cell Dev Biol 9 631016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unic A, Derek L, Hodak N, Marijancevic D, Ceprnja M, Serdar T, Krhac M & Romic Z 2011. Endothelins -- clinical perspectives. Biochemia Medica: Casopis Hrvatskoga Drustva Medicinskih Biokemicara 21 231–242. [DOI] [PubMed] [Google Scholar]
- Usuki S, Saitoh T, Suzuki N, Kitada C, Goto K & Masaki T 1991. Endothelin-1 and endothelin-3 stimulate ovarian steroidogenesis. Journal of Cardiovascular Pharmacology 17 Suppl 7 S256–259. [DOI] [PubMed] [Google Scholar]
- Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ & Webb DJ 1998. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation 97 752–756. [DOI] [PubMed] [Google Scholar]
- Wandji SA, Srsen V, Voss AK, Eppig JJ & Fortune JE 1996. Initiation in vitro of growth of bovine primordial follicles. Biology of Reproduction 55 942–948. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhang Z, Wang Z, Xiao K, Wang Q, Su J & Wang Z 2015. Expression and clinical significance of the HIF-1a/ET-2 signaling pathway during the development and treatment of polycystic ovary syndrome. Journal of Molecular Histology 46 173–181. [DOI] [PubMed] [Google Scholar]
- Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D & Yanagisawa M 1994. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell 78 473–485. [DOI] [PubMed] [Google Scholar]
- Yalu R, Oyesiji AE, Eisenberg I, Imbar T & Meidan R 2015. HIF1A-dependent increase in endothelin 2 levels in granulosa cells: role of hypoxia, LH/cAMP, and reactive oxygen species. Reproduction 149 11–20. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K & Masaki T 1988. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332 411–415. [DOI] [PubMed] [Google Scholar]
- Yuzugulen J, Douthwaite JA, Wood EG, Villar IC, Patel NSA, Jegard J, Gaertner H, Rossitto-Borlat I, Rose K, Hartley O et al. 2017. Characterisation of preproendothelin-1 derived peptides identifies Endothelin-Like Domain Peptide as a modulator of Endothelin-1. Scientific Reports 7 4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang F & Zhang Y 2019. Expression and Contribution of NLRP3 Inflammasome During the Follicular Development Induced by PMSG. Front Cell Dev Biol 7 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla LM, Sriperumbudur R & Gadsby JE 2010. Endothelin-1, endothelin converting enzyme-1 and endothelin receptors in the porcine corpus luteum. Domestic Animal Endocrinology 38 75–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.