Abstract
Heart failure and cardiovascular disorders represent the leading cause of death in diabetic patients. Here we present a systematic review of the main mechanisms underlying the development of diabetic cardiomyopathy. We also provide an excursus on the relative contribution of cardiomyocytes, fibroblasts, endothelial and smooth muscle cells to the pathophysiology of heart failure in diabetes. After having described the preclinical tools currently available to dissect the mechanisms of this complex disease, we conclude with a section on the most recent updates of the literature on clinical management.
Keywords: Heart failure, Diabetic cardiomyopathy, Bioenergetics, Diabetes mellitus, Cardiovascular endocrinology, T2DM, T1DM, Oxidative stress, Fibroblasts, HFpEF, Senescence, Mitochondria, VSMC, Cardiomyocytes, ROS, Fibrosis, Aging, Diastolic dysfunction, Endothelium, NADH, BHB, FOXO1, Adrenergic receptors
1. Introduction
The heart represents an organ with a high metabolic activity, consuming >400 kcal/kg/day at rest. The processes of excitation-contraction coupling within cardiomyocytes heavily rely on ATP-dependent reactions: approximately 60–70% of ATP hydrolysis in cardiomyocytes is spent on the contractile shortening and most of the remaining ATP fuels the functioning of sarco/endoplasmic reticulum Ca2+-ATPase 2 (SERCA) and other ion pumps [1]. A thorough evaluation of ATP, ADP and Pi concentrations in vivo revealed that their levels remain mostly unchanged throughout the cardiac cycle and during physiological intensifications in cardiac work and oxygen consumption [2], suggesting that an increase in ATP production is the main mechanism responsible for fulfilling cardiac energy demands. Nearly 95% of ATP in cardiomyocytes is produced by oxidative phosphorylation in mitochondria. This process is fueled by NADH and to a lesser extent by succinate, both produced in the tricarboxylic acid cycle (TCA, also known as citric acid cycle or Krebs cycle) with acetyl coenzyme A (acetyl-CoA) being indispensable for keeping the TCA cycle running [3,4]. Three main sources of acetyl-CoA production exist in the cell: β-oxidation of fatty acids, decarboxylation of pyruvate provided by glycolysis, and ketone bodies oxidation [5,6].
A fundamental feature of cardiac metabolism is its flexibility; indeed, the heart is able to switch between different substrates to fulfill its energetic needs with the nutrients that are more available at the moment [7]. The adult heart under non-ischemic conditions produces 60% to 90% of acetyl-CoA by means of fatty acid oxidation and only 10–40% by pyruvate carboxylation [8]. Production of ketone bodies (e.g. β-hydroxybutyrate, BHB) is quite low in normal conditions and their contribution to myocardial bioenergetics has been estimated to be <10% [9-11]. It is important to note that the heart ability to store energy substrates in form of glycogen and triglycerides is limited, hence the increase in heart work relies on its ability to increase the uptake and utilization of glycogen or fatty acids [12-14].
2. Diabetes mellitus (DM) impairs cardiac metabolic flexibility
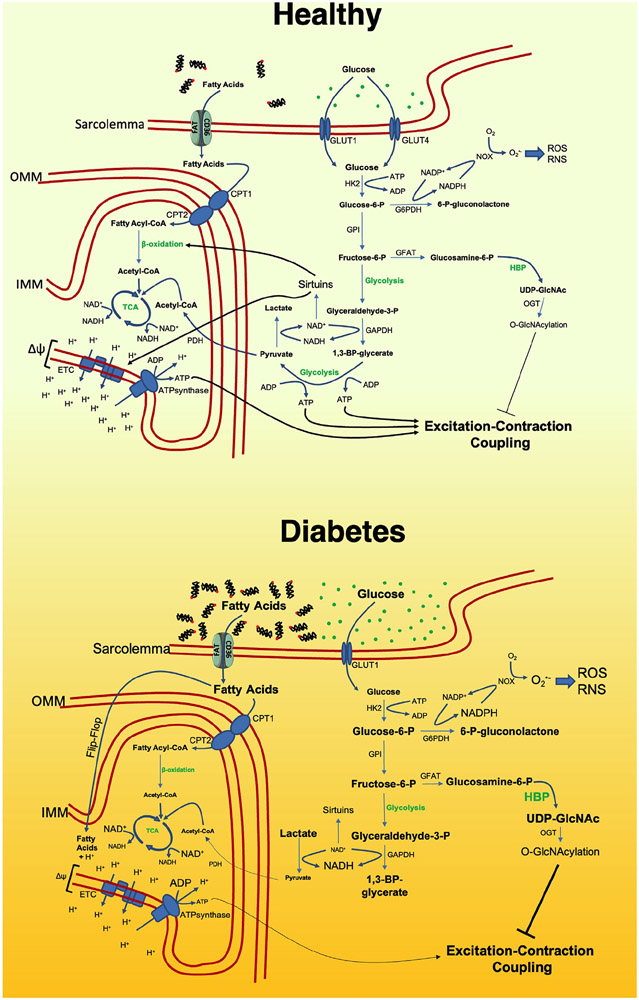
Heart disease remains the leading cause of death for both Type 1 and Type 2 diabetes mellitus (T1DM and T2DM) patients [15]. In diabetic patients, the availability and the usage of substrates by the heart is drastically disturbed (Fig. 1). Impaired insulin signaling causes both hyperglycemia and hyperlipidemia due to the decreased glucose uptake by the skeletal muscle and increased free FA secretion by the adipose tissue [16-22]. Despite the excessive supply of both types of energy substrates, the overall contribution of glycolysis to cardiac ATP production is drastically decreased and heart reliance on fatty acid oxidation becomes higher [23-28]. The mechanisms underlying this shift are complex and multifaceted [7].
Fig. 1. Myocardial bioenergetics in the healthy and in the diabetic heart.
6-P-gluconolactone: 6-phospho-gluconolactone; Acetyl-CoA: acetyl coenzyme A; ADP: Adenosine diphosphate; ATP: Adenosine triphosphate; CD36/FAT: Cluster of differentiation 36/fatty acid translocase; CPT1: Carnitine palmitoyl transferase I; CPT2: Carnitine palmitoyl transferase II; ETC: Electron transport chain; Fructose-6-P: fructose-6-phosphate; G6PDH: Glucose-6-phosphate dehydrogenase; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; GFAT: Glutamine fructose-6-phosphate aminotransferase; Glucosamine-6-P: glucosamine-6-phosphate; Glucose-6-P: glucose-6-phosphate; GLUT1: Glucose transporter type 1; GLUT4: Glucose transporter type 4; GPI: Glucose-6-phosphate isomerase; HBP: hexosamine biosynthetic pathway; HK2: hexokinase 2; IMM: inner mitochondrial membrane; NAD: Nicotinamide adenine dinucleotide; NOX: NADPH oxidase; O-GlcNAcylation: O-linked-N-acetylglucosaminylation; OGT: O-linked N-acetylglucosaminyltransferase; OMM: outer mitochondrial membrane; PDH: pyruvate dehydrogenase; RNS: reactive nitrogen species; ROS: reactive oxygen species; TCA: tricarboxylic acid cycle; UDP-GlcNAc: Uridine diphosphate N-acetylglucosamine.
In T1DM, the main mechanisms plausibly relate to the vanishing of insulin signaling. Insulin promotes cardiac glucose uptake inducing glucose transporter 4 (GLUT4) expression and translocation to cell membrane [29-32], and GLUT4 abundance is markedly diminished in T1DM [33]. Insulin signaling prevents degradation of phosphofructokinase 2 (PFK2), an enzyme that leads to the generation of fructose-2,6-biphosphate (F-2,6-BP), a potent activator of glycolysis [34-36]. Insulin signaling also removes inhibition of pyruvate dehydrogenase (PDH), which converts the pyruvate into the TCA substrate Acetyl-CoA [25,37]. So, insulin signaling has a complex role in regulation of glycolysis and its loss diminishes glucose uptake, glucose conversion into pyruvate, and pyruvate incorporation into TCA. Moreover, insulin regulates cardiomyocyte usage of fatty acid. Insulin-dependent downregulation of Forkhead box protein O1 (FOXO1) dampens fatty acid cellular and mitochondrial uptake [38,39], while insulin-dependent phosphorylation of protein kinase B (AKT) and protein kinase C δ (PKCδ) downregulates the oxidation of fatty acids [25,37,40,41]. All these data indicate that insulin plays an imperative role in determining the substrate choice in the heart.
The exact mechanisms underlying the substrate switch in T2DM remain mostly unclear, as the development of cardiac insulin resistance in T2DM is quite elusive [42]. A minimal cardiac responsiveness to insulin has been shown in numerous in vivo assays [28,43-45] and a diminished cardiac glucose uptake in T2DM patients was reported in a clinical trial [46]. Insulin treatment was shown to result in reductions in fatty oxidation, measured using positron emission tomography (PET), in both control subjects and T2DM patients but the achieved rates of myocardial fatty oxidation remained elevated in T2DM compared with controls [24]. Another clinical study demonstrated that insulin effectively improved heart bioenergetics in T2DM patients [47].
The metabolic switch observed in T2DM may be explained by the inhibitory action of fatty acids accumulation on glycolysis. As depicted in Fig. 1, cardiomyocytes become heavily overloaded with fatty acids in T2DM [48-52]. Animal studies point on peroxisome proliferator-activated receptors (PPAR) α and γ as culprits of the metabolic switch [51-55]. PPARα and PPARγ are directly activated by fatty acids and then upregulate the expression of pyruvate dehydrogenase kinase 4 (PDK4), which inhibits PDH, thus preventing the oxidizing of pyruvate [52,55]. Since data from T2DM human hearts confirmed the upregulation of PDK4 but argued against PPAR activation [56], further research is required in this direction. An alternative mechanism of fatty acid dependent downregulation of glycolysis could be the hyperacetylation of its key enzymes [26,57].
2.1. Interplay between dysregulated glycolysis and accessory metabolic pathways
Despite the downregulation of glucose transporters in the diabetic heart, glucose uptake is not fully abrogated; besides, the multistep process of glucose conversion into pyruvate is retarded. This situation eventually results in the accumulation of intermediate products of glycolysis feeding accessory metabolic pathways (Fig. 1).
One example of these processes is the activation of the hexosamine biosynthetic pathway (HBP) in the diabetic heart [58], which most likely is triggered by the loss of insulin-dependent stimulation of PFK1 activity and the subsequent retarded conversion of fructose-6-phospahte (F-6-P) into F-1,6-BP. F-6-P also serves as precursor for glucosamine synthesis, an initial step of HBP [59]. In the HBP, uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) is produced, which is further involved in intracellular signaling as a substrate for O-linked N-acetylglucosamine transferases (OGTs). OGTs are responsible for O-GlcNAc posttranslational modifications on a number of proteins involved in cardiac function [58]. For instance the massive protein hyper-O-GlcNAcylation was observed in experimental models of DM [60,61].
Another consequence of dysbalanced glycolysis is the decreased NAD+/NADH ratio, which may be attributable to an excessive availability of glyceraldehyde-3-phosphate, converted into 1,3-bisphophoglycerate with concomitant reduction of NAD+ to NADH [62]. In normal conditions, NAD+ pull is replenished by the activity of lactate dehydrogenase making lactate from pyruvate; however, under conditions of lactic acidosis, the oversupply of lactate slows down this reaction [63]. NAD+ levels are of great importance as NAD+ serves as cofactor for a class of lysin deacetylases called sirtuins [64]. The decrease in NAD+ dampens their activity resulting in decreased activity of sirtuins [50,65-67] and subsequent augmentation of protein acetylation [28,68].
Glycolysis retardation may also result in glucose-6-phosphate (G-6-P) accumulation [69]. Besides being utilized in glycolysis, G-6-P is also converted into 6-phosphogluconolactone (6-PGL) by glucose-6-phosphate dehydrogenase (G6PDH) to be further metabolized in the pentose phosphate pathway [70-73]. Unlike glycolysis and glucose aerobic oxidation, the pentose phosphate pathway does not lead to the production of adenosine 5′-triphosphate (ATP) but supplies NADPH and ribose 5-phosphate (R5P). R5P is a building block for nucleic acid synthesis. NADPH provides the reducing power required for the synthesis of fatty acids, sterols, nucleotides and non-essential amino acids. NADPH also serves as substrate for NADPH-oxidases (NOXs), enzymes involved in the production of superoxide from oxygen, initiating a cascade of reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation [74-79]. DM has been also shown to alter the esterified lipid biosynthesis (the glycerolipid biosynthetic pathway, a.k.a. Kennedy pathway [80]), a mechanism that can further contribute to diabetic cardiomyopathy [81-84].
2.2. Metabolic inefficiency of the diabetic heart
The shift in cardiac metabolism profoundly affects the function of the heart. Mounting evidence suggests that the contractile performance of the heart at a given level of oxygen consumption is greater when the heart is oxidizing more glucose and lactate, and less fatty acids [85-88]. A plausible explanation for this phenomenon is that fatty acids can mediate the uncoupling of mitochondria [89]; the mechanisms of fatty acids mediated uncoupling are based on the ability of fatty acids to cross the mitochondrial membrane not only via carnitine-palmitoyl-transferase, but also by a ‘flip-flop’ mechanism. In the mitochondrial matrix, fatty acids become deprotonated, thus ‘smuggling’ protons across the inner mitochondrial membrane and diminishing the proton gradient across it. Importantly, these ‘stowaway’ fatty acids cannot be utilized in β-oxidation, as they enter mitochondria in their unesterified form and do not contribute to energy production. Moreover they are exported out of mitochondria by uncoupling protein 3 (UCP3) and then can again enter mitochondria by a ‘flip-flop’ mechanism, creating an uncoupling circuit [90,91]. As a result, a significant portion of protons pumped across the mitochondrial membrane by electron transport chain enzymes is ‘wasted’ instead of being used for ATP production.
Taken together, these data indicate that cardiac efficacy of the diabetic myocardium is drastically decreased, meaning that for accomplishing the same work the diabetic heart requires much more oxygen compared to the healthy one [61]. Indeed, clinical studies demonstrated ATP deficit and poor oxygenation of the heart in T2DM patients subjected to exercise [92].
An exacerbation of the diabetic heart metabolic inefficiency is caused by protein hyperacetylation due to decreased NAD+/NADH ratio and subsequent deactivation of sirtuins, as mentioned above. Lysin acetylation plays essential roles in modulating the activity of a number of enzymes involved in fatty acid metabolism [93,94]. Indeed, mitochondria from diabetic hearts exhibit a worsened ability to utilize fatty acids for respiration compared to mitochondria from healthy hearts both in animal and human studies [68,95,96]. Restoration of NAD+/NADH ratio via dietary NAD+ supplementation or pharmacological activation of sirtuins improved both mitochondrial function and cardiac performance in animal models of DM [65,96].
2.3. Role of dysregulated metabolism in the functional changes observed in the diabetic heart
Experimental evidence indicates that glycolytic enzymes can form a subdomain adjacent to the endo/sarcoplasmic reticulum and plasma membrane [97,98]. Furthermore, ATP produced by glycolysis is primarily consumed by SERCA2 and plasmalemmal ion pumps removing Ca2+ from the cytosol [1]. Indeed, glycolysis inhibition does not affect the overall concentration of ATP in the cell, neither cardiac contraction [99-102]; nevertheless, left ventricular end-diastolic pressure was shown to be increased alongside an augmentation in Ca2+ and K+ concentrations [102,103]. Transgenic mice with PFK2 deficiency exhibited prolonged time of myocardial relaxation [35]. All these alterations compromise cardiomyocyte relaxation and provide mechanistical explanations for some hallmarks of diabetic cardiomyopathy, namely increased myocardial stiffness, impaired relaxation, and decreased left ventricular filling [104].
Metabolic dysregulation in diabetic heart may also contribute to impaired relaxation via fatty acid mediated uncoupling. In this sense, mitochondrial uncoupling following UCP2 overexpression was shown to inhibit Ca2+ uptake by mitochondria and to prolong cytosolic Ca2+ clearance [105]. Plausibly, fatty acid mediated uncoupling may have similar effects on mitochondrial Ca2+ handling.
The excessive O-GlcNAcylation of proteins within the cardiomyocyte, resulting from upregulated HBP, as discussed above, also contributes to the impaired myocardial function. Ergo, O-GlcNAcylation of Ca2+/calmodulin-dependent protein kinase II (CaMKII), phospholamban (PLN), L-type Ca2+ channels (Cav), and transcriptional factor specificity protein 1 (Sp1), was found in animal models of diabetic cardiomyopathy as well as in diabetic patients [60,106,107]. Supporting this view, a downregulation of HBP, providing a substrate for O-GlcNAcylation or ablation of OGTs genes, specifically responsible for this posttranslational modification, resulted in partial functional recovery of the heart [60,107].
3. Cellular and molecular impact of DM on the cardiovascular system
DM has profound effects on signaling pathways in different cell types of the cardiovascular system. For instance, insulin resistance is known to predispose patients to inflammation, increase mitochondrial dysfunction, and cause the activation of the renin-angiotensin-aldosterone system. Insulin resistance in diabetic patients reduces glucose uptake in peripheral tissues [108]. Dysregulation of cardiac insulin signaling has been shown to impair phosphoinositide-3-kinase (PI3K)-protein kinase B (Akt) pathway and to increase mTOR/S6K1 signaling. Impairment of PI3K-Akt signaling can suppress GLUT4 receptor, reducing glucose uptake in cardiomyocytes. Inactivation of PI3K-Akt decreases nitric oxide (NO) production; such a reduction in NO levels leads to the inhibition of cyclic guanosine monophosphate (cGMP), protein kinase G (PKG), and eventually cell death and cardiac dysfunction. All of these processes have a detrimental impact on myocardial energetics and eventually promote cardiac hypertrophy and interstitial fibrosis [109].
In the next paragraphs of this section, we will examine in detail the impact of DM on adrenergic signaling and the specific effects on cardiac fibroblasts cardiomyocytes, endothelial cells and vascular smooth muscle cells (VSMCs).
3.1. Effects of DM on cardiac adrenergic signaling
DM has been shown to affect both the expression and the responsiveness of the subtypes of beta adrenergic receptors (β-ARs), crucial in cardiac contractility [110]. A seminal study had demonstrated that the number of β-AR was decreased by 28% in the ventricular rat tissue 8 weeks after streptozotocin (STZ) injection [111]; such decrease in β-AR density in the heart was taken into account to explain the depressed contractile responsiveness to adrenergic stimuli in diabetic cardiomyopathy [112].
However, these findings were not confirmed by other investigators [113-117]; controversial results were also reported when using other animal models, like rabbits [118,119] and pigs [120,121]. These discrepancies could be attributed to the different expression of the three β-AR subtypes, namely β1, β2, and β3. Indeed, subsequent investigations have demonstrated a reduction of β1-AR [122-125] and an increase of β3-AR [122,123,126,127], whereas results on β2-AR remained controversial [122,124,127,128], most likely because of the different methods used to determine their expression (immunoblots, radioligand binding assays, RT-qPCR).
When exploring the β-AR signaling pathway instead of the mere β-AR expression, isoproterenol-stimulated PKA activity was shown to be decreased in T2DM mice and T1DM rats [129,130], although phosphorylation of PKA was increased when the duration of DM reached 12 weeks [131]. Alterations in PKA-dependent phosphorylation of proteins involved in excitation-contraction coupling have been evidenced in diabetic hearts, with increased phosphorylation of RyR expression at both Ser2814 and Ser2808, which are known to enhance pathologic intracellular Ca2+ leak [132], in T1DM rats [133], and decreased phosphorylation of phospholamban in both T1DM and T2DM animal models [125,129,131,134]. Levels of GRK2, essential in cardiac β-AR desensitization [135-139], were found to be significantly increased in the left ventricle of 12-week-old db/db mice compared to age-matched non-diabetic littermates as well as in peripheral blood mononuclear cells (PBMCs) of T2DM patients compared to non-diabetic controls [140]. Consistent with these findings, inhibiting GRK2 was shown to have anti-inflammatory effects and to reduce cardiac fibrosis and oxidative stress [141,142].
3.2. Effects of DM on cardiomyocytes and cardiac fibroblasts
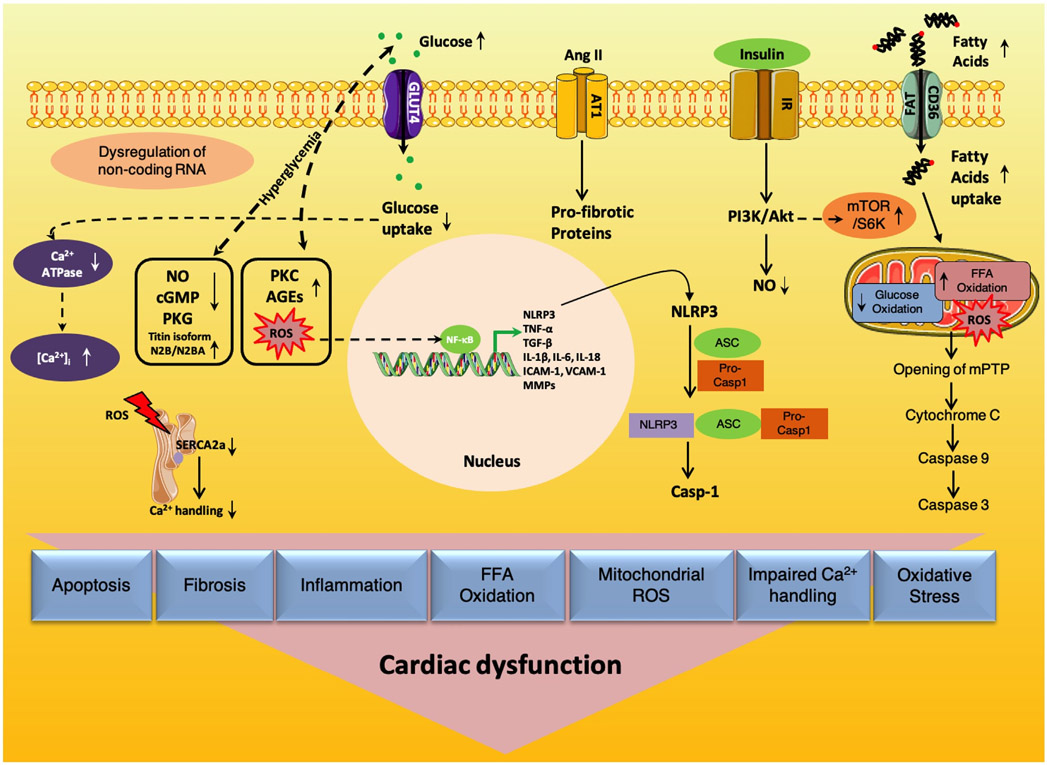
Hyperglycemia is one of the main mechanisms that can trigger cardiomyocyte apoptosis through means of the activation of the cytochrome c stimulated caspase-3 pathway [143]. Besides, hyperglycemia causes mitochondrial dysfunction, prompting an enhanced production of ROS, and restricts the ability of cardiomyocytes to use glucose as an energy source (Fig. 2), leading to increased FFA oxidation, whose uptake occurs by the cluster of differentiation 36 - fatty acid translocase (CD36/FAT) [144-147].
Fig. 2. Main molecular mechanisms underlying cardiac dysfunction in diabetes.
AGEs: Advanced glycation end products; Ang II: Angiotensin II; ASC: Apoptosis-associated speck-like protein containing a caspase-recruitment domain; AT1: Angiotensin II type 1 receptor; ATPase: Adenosine triphosphatase; [Ca2+]i: Intracellular calcium; Casp-1: Caspase-1; CD36/FAT: Cluster of differentiation 36/fatty acid translocase; cGMP: Cyclic guanosine monophosphate; FFA: Free fatty acid; GLUT4: Glucose transporter type 4; ICAM-1: Intercellular adhesion molecule; IL-18: Interleukin 18; IL-1β: Interleukin 1 beta; IL-6: Interleukin 6; IR: Insulin receptor; MMPs: Matrix metalloproteinases; mTOR/S6K: Mechanistic target of rapamycin (mTOR)-ribosomal S6 kinase (S6K) pathway; NF-kB: Nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: Nucleotide-binding oligomerization domain like receptor (NLR) pyrin domain containing 3; NO: Nitric oxide; PI3K/Akt: Phosphoinositide-3-kinase (PI3K)-protein kinase B (Akt); PKC: Protein kinase C; PKG: Protein kinase G; Pro-casp1: Pro-caspase-1; PTP: Permeability transition pore; ROS: Reactive oxygen species; SERCA2a: Sarcoplasmic reticulum Ca2+-ATPase 2a; TGF-β: Transforming Growth Factor beta; TNF-α: Tumor Necrosis Factor alpha; VCAM-1: Vascular cell adhesion molecule 1.
Fibrosis, defined as the excessive and/or inappropriate deposition of extracellular matrix proteins, is a typical characteristic of diabetic cardiomyopathy. ROS generation and oxidative stress have been specifically linked with the pathogenesis of cardiac fibrosis and cardiomyopathy [148,149] and several investigators have reported elevated oxidative stress in experimental models of T2DM [150,151]. Whereas during normal conditions a little amount of oxygen is transformed in ROS, in a diabetic status an excessive amount of ROS is generated. ROS can activate transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which can in turn regulate the transcription of multiple pro-inflammatory genes including Tumor Necrosis Factor α (TNF-α), Transforming Growth Factor β1 (TGF-β1), and Interleukins (IL-1β, IL-6, IL-18) that are known to be involved in the pathogenesis of DM-associated heart failure (HF) [152]. TNF-α stimulation stimulates collagen synthesis while IL-1β promotes the pro-inflammatory fibroblast phenotype [153].
Numerous studies have shown that oxidative stress induces cardiac fibrosis by stimulating TGF-β1 expression [154,155]. TGF-β1 acts as a central mediator in the pathogenesis of tissue fibrosis [148]. Hyperglycemia and hyperinsulinemia activate angiotensin II, TGF-β1/SMAD signaling, and elevate protein kinase C (PKC) activity in fibroblasts [156,157]. These processes in turn elicit interstitial collagen deposition and fibrosis, which is associated with increased expression of TGF-β1. TGF-β1 mediates myofibroblast trans-differentiation and promotes matrix preservation [158-160]. Oxidative stress is also triggered by chronic angiotensin II, which is linked to a pro-fibrogenic phenotype in the heart, and causes modifications in the extracellular matrix by reducing matrix metalloproteinases (MMPs) [161]. Additionally, NF-κB increases the expression of intercellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) [145].
NLRP3 inflammasome is a relatively novel molecular marker in diabetic cardiomyopathy [162]. Some of the factors that activate the NLRP3 inflammasome are high FFA levels, impaired insulin metabolic signaling and hyperglycemia. The NLRP3 inflammasome stimulates myocardial dysfunction through the activation of IL-1β. Activated caspase-1 cleaves IL-1β and IL-18 precursors and promotes multiple proinflammatory pathways involving NF-κB, chemokines, and ROS [163]. Furthermore, NF-κB increases NLRP3 inflammasome assembly and activates pro-caspase-1 [164,165].
In diabetic cardiomyopathy, reduced SERCA2 is associated with the decrease of recaptured Ca2+ and lowers the overall Ca2+ content in the sarcoplasmic reticulum [166]. Since cardiac contractility is controlled by changes in intracellular Ca2+ concentrations [1,167,168], reduced Ca2+ for the next contraction leads to an impaired cardiomyocyte contractility. In fact, some studies have shown that diastolic dysfunction is linked to an impaired myocardial Ca2+ handling [169,170].
Mitochondrial dysfunction is another strategic player in the development of diabetic cardiomyopathy and HF [75,171-174]. Generally, ~90% of intracellular ATP production in cardiomyocytes is generated by mitochondrial oxidative phosphorylation. However, in T2DM, mitochondria substitute glucose with FFA oxidation for ATP production which in turn causes increased production of mitochondrial ROS and reduced oxidative phosphorylation [7,145]. Altered mitochondrial Ca2+ handling further induces mitochondrial respiratory dysfunction and ultimately causes cell death [172,175].
3.3. Effects of DM on the endothelium
Both endothelial cells and VSMCs play key roles in controlling the myogenic tone of the vessels as well as blood pressure, fundamental aspects in the pathogenesis of HF [176,177].
Three decades ago, Torsten Deckert of the Steno Memorial Hospital of Copenhagen formulated the “Steno hypothesis”, the revolutionary idea, for that time, ascribing to endothelial dysfunction most of the clinical manifestations of DM [178]. Years later, the pivotal role of vascular endothelium in the development of microalbuminuria and hypertension associated to DM was confirmed [179,180]. It is clear now that if macrovascular complications in DM are similar to the processes occurring during atherosclerosis of non-diabetic origin, the microvascular alterations with a predominant participation of the endothelium represent a DM signature [181]. In fact, ultrastructural alterations of the glomerular endothelium have been detected in patients with T2DM before the development of microalbuminuria [182].
One of the main factors altered in the diabetic endothelium is permeability, and different mechanisms have been suggested as responsible for the increased endothelial permeability in DM [183-192]. VEGF signaling could be involved, as this factor is one of the main agents able to induce endothelial permeabilization [193]. Several studies focused on the assessment of VEGF expression in DM, however the results were controversial; data were ambiguous not only for the results on the overexpression or downregulation of VEGF in diabetic patients, but also concerning the efficacy of VEGF targeting as therapeutic strategy [194].
An additional mechanism suggested as responsible for the increased endothelial permeability is inflammation. Macrophages are able to secrete cathepsin-S, activating protease-activated receptor-2 (PAR2) on endothelial cells [195]. The interference with PAR-2 pathway determines modifications of endothelial barrier integrity, with increased microvascular permeability [196].
The impaired ability to induce vasorelaxation is another hallmark of the diabetic endothelium [197]. The relaxation response to endothelium-dependent vasodilators, including ACh and BK, is impaired in both the early phase and in the late stage of DM, and the responses to ACh are significantly improved following pre-treatment with indomethacin [197].
Mechanistically, the impaired endothelial dependent vasodilatation occurs in DM as a consequence of 1) decreased NO availability, 2) oxidative stress, 3) increased of harmful metabolites, and 4) inflammation.
Brodsky and colleagues demonstrated the relationship between high glucose levels and reduced NO bioavailability. Specifically, they verified that NO-dependent responses of endothelium to bradykinin were negatively affected by high glucose levels [198]. Mass spectrometry studies identified the formation of a covalent unitary addition of NO to glucose, so that glucose itself could act as scavenger for NO [199]. Moreover, hyperglycemia induces uncoupling of mitochondrial NOS (mtNOS) in endothelial cells, reducing the production of NO and favoring hydrogen peroxide generation [200]. Consistent with these findings, patients with T2DM and nephropathy, show decreased intravascular NO synthesis from L-arginine [201].
This switch from NO to superoxide production, alongside with the metabolic abnormalities of DM, may cause mitochondrial superoxide overproduction and oxidative stress in endothelial cells [202]. In turn, oxidative stress exacerbates the metabolic alterations and NO production, generating a vicious cycle. The use of a mitochondrial-targeted superoxide dismutase mimetic was able to correct the metabolic disarrangement of endothelial cells in diabetic rats, supporting the central role of oxidative stress in the development and progression of the disease [203].
The eNOS uncoupling alongside with superoxide overproduction can generate RNS, such as peroxynitrite (ONOO−), a toxic metabolite derived from NO, and superoxide anion (O2*−) [204]. The mechanisms by which RNS can modify vascular function are poorly understood. However, it is known that endothelial peroxynitrite accumulation induces nitration and inactivation of prostacyclin synthase (PGIS), which inhibits vasodilator, antithrombotic, and antiadhesive effects of prostacyclin (PGI2), increasing the release of a potent vasoconstrictor and prothrombotic factor like thromboxane A2 (TxA2) [205]. The functional contribution of peroxynitrite in the clinical manifestations in DM is supported by a study using diabetic rats with signs of peroxynitrite formation; the use of ebselen, a peroxynitrite scavenger, was able to prevent endothelial damage and nephropathy [203].
Another mechanism of endothelial damage in DM is mediated by Advanced Glycation End-Products (AGEs), which have been shown to induce premature endothelial senescence by activating the receptor for advanced glycation end products (RAGE) [206,207]. AGEs are derived from proteins or lipids that become glycated after prolonged exposure to aldose sugars [202,208,209]. They represent a sort of record of the cumulative prevalence and duration of the hyperglycemic status. An accelerated buildup of AGEs in both the intracellular and extracellular space mediates inflammation and cardiac fibrosis through multiple pathways. AGEs can trigger upregulation of the RAGE and increase oxidative stress [181]. AGE-RAGE interaction increases the production of matrix protein and connective tissue through the activation of Janus Kinase (JAK) and mitogen-activated protein kinase (MAPK) pathways. Additionally, activation of RAGE involves NF-αB and causes reduction of myocardial contractility during HF. Some of the actions of AGEs may be also mediated by angiotensin II [210] or by the inhibition of NO activity in the endothelium [145,211].
The effects of specific AGEs, such as glycosylated-collagen, on premature senescence of the endothelium could involve the sirtuins SIRT1, one of the most important factors in regulating cell survival and longevity [212]. SIRT1 expression was downregulated in diabetic mice [207]; glycosylated-collagen mediated cell apoptosis and senescence were attenuated by SIRT1 overexpression, while further SIRT1-downregulation enhanced endothelial cell death [213]. Furthermore, the exposure of endothelial cells to glycosylated-collagen induces lysosomal permeabilization [214-216]. This evidence can explain the mechanism of SIRT1 regulation by AGEs. Indeed, by promoting lysosomal permeabilization, glycosylated-collagen can induce cathepsins release from the organelle, as observed by the Goligorsky's research group [217]. Interestingly, cathepsins are able to cleave SIRT1, so this mechanism could be responsible for SIRT1 degradation in diabetic endothelium with increased senescence [207].
Endothelin (ET-1) is a potent vasoconstrictor secreted by vascular endothelial cells in response to specific cytokines, growth factors, thrombin, or hypoxia. ET-1 has also complex effects on the renin-angiotensin system. In diabetic mice, ET-1 is decisive in DM-associated vascular remodeling and promotes cardiac hypertrophy, which is also due to overstimulation of the IGF-1 receptor on cardiomyocytes [211,218,219].
3.4. Effects of DM on VSMCs
DM is an independent risk factor for atherosclerosis [220]. Indeed, in diabetic patients the phenomenon of macrovascular atherosclerosis is very frequent making them the most common subjects undergoing peripheral vascular and coronary revascularization procedures [221,222]. This association is mainly attributable to the direct impact of DM on the vasculature, affecting different compartments, from VSMCs to the endothelium. VSMCs play strategic roles in the pathophysiology of atherosclerosis. Specifically, a phenotypic switch of these cells is crucial for atheroma formation and progression [223]. In fact, the switch from contractile (differentiated, quiescent, non-migratory) to synthetic (dedifferentiated, proliferative, migratory) phenotypes typically supports plaque deposition and progression. The proatherogenic phenotype of VSMCs seems to be more aggressive in diabetic patients, as suggested by their increased intimal hyperplastic activity [224]. An elegant study by Faries and colleagues reported intrinsic differences in VSMCs obtained from diabetic patients compared to healthy control cells; specifically, VSMCs of diabetic origin displayed abnormal morphology and increased proliferation, migration, and adhesion rates [225]. These features are consistent with the increased rate of atherosclerotic events and the augmented risk of restenosis reported in diabetic patients [226] and, more recently, in non-diabetic patients with hyperglycemia [227]. Interestingly, those phenotypes were observed in vitro, using an isolated system without other cofactors that could have affected the vascular behavior in vivo [225]. Therefore, the intrinsic alterations of VSMC in diabetic patients should be considered also in designing new therapies.
The exact factors promoting the atherogenic transformation of diabetic VSMCs and the underling mechanisms are still mostlyunknown. Among these factors, microRNAs (miRNAs) produced by diabetic VSMCs have been recently proposed; miRNAs are a class of non-coding RNAs that can play vital roles in gene expression as well as cardiac remodeling. Increased oxidative stress downregulates some miRNAs including miR-1, miR-499, miR-133a, and miR-133b [228]. The up-regulation of miR-221 and miR-212 has been reported in hypertrophy and autophagic responses [145]. In a recent study, a high throughput RNA sequencing identified >100 miRNAs upregulated in VSMCs from db/db mice [229], an established animal model of DM (see below the section on preclinical models). In particular, miR-504 was highly expressed and new partners regulated by miR-504 in diabetic VSMCs were identified, like Egr2 and Grb10, molecules involved in gene transcription regulation and pro-proliferative pathways [229]. Conversely, miR-132 was significantly decreased in VSMCs from diabetic rats or after hyperglycemia exposure in vitro. Accordingly, its direct target E2F5 was upregulated, determining an increased migration and proliferation capacity of diabetic VSMCs [230]. Other miRNAs have been linked to the dysfunction of VSMCs behavior during disease development. miR-24 is able to regulate VSMCs phenotyping switch induced by high-glucose conditions [231]; the inhibitory effect of miR-24 on VSMCs proliferation and migration is due to its ability to target HMGB1-box, reducing NF-κB nuclear translocation and DNA binding and blocking proinflammatory signals like TNF-α and IL-6 production. Despite the extensive progress in understanding the molecular mechanisms that regulate VSMCs phenotypic plasticity, most of the story is still unknown. Lately, the involvement of epigenetic mechanisms has emerged in the reactivation of embryonic and stem-cell related genes [232].
One of the major factors regulating epigenetic phenomena during VSMCs phenotypic switch is KLF4, which interacts with phosphorylated Elk-1 (pElk-1) and histone deacetylase 2 (HDAC2) to repress the expression of contractile and cytoskeletal proteins [233,234]. In a similar way, KLF4 is able to induce the activation of pluripotency related genes like Sox2 and Oct4. Hyperglycemia has been shown to cause the down-regulation of KLF4 in VSMCs in vitro, and reduced KLF4 levels have been detected in arteries from diabetic patients and mice [235]. Interestingly, KLF4 downregulation seems to be mediated by miR-29c, whose expression is induced by Foxa2.
Alterations of Ca2+ signaling in VSMCs are elemental in the development and progression of HF and other vascular complications in DM [236]. A defective expression/activity of several partners belonging to the VSMC-Ca2+ signalosome, including Ca2+ channels, pumps, and regulators has been reported in DM [236]. For instance, both expression and distribution of the intracellular Ca2+ release channel ryanodine receptor (RyR) change in response to high glucose [237]. The different subcellular localization and clusterization of RyR may affect the entity of Ca2+ sparks which normally generate spontaneous transient outward currents (STOCs) by activating the big conductance Ca2+ sensitive K+ channels (BK) [238,239]. Ca2+ sparks and STOCs in VSMCs constitute the functional unit that regulates arterial tone; indeed, the generated STOCs reduce the membrane potential limiting Ca2+ influx through VDCCs, consequently diminishing cytosolic Ca2+ concentration and vasoconstriction. Losing the appropriate STOCs generation in DM due to redistribution of RyR can be responsible for a decline in vasorelaxation [237]. Consistent with this scenario, several experimental models of DM show reduced expression of RyR in VSMCs [240,241] and Ca2+ spark amplitude and duration were reduced in VSMCs freshly isolated from cerebral arteries of male db/db mice [241]. Interestingly, this behavior was reported in male mice but not in diabetic females, which exhibited an unchanged amplitude of spontaneous Ca2+ sparks [236]. These data are in line with the clinical observation of a higher occurrence of microvascular alterations in diabetic men [242]. Alongside the reduction in RyR expression, levels of IP3R, the other main intracellular Ca2+ release channel, are reduced as well in diabetic VSMCs [240] and in VSMCs chronically exposed to hyperglycemia [237]. IP3R Ca2+ signaling in DM seems to be also regulated at the protein-protein interaction level; specifically, the interaction of the receptor with anti-apoptotic proteins like Bcl-2 has been shown to have an instrumental role [243,244]. Indeed, IP3-induced Ca2+ transients were shown to be significantly larger in diabetic VSMCs due to enhanced IP3R excitability via Bcl2-IP3R protein interactions [245]. This phenomenon could have important implications in the diabetic VSMCs phenotype, especially in terms of proliferation/apoptosis balance.
Another aspect of vascular remodeling in diabetic patients is the perturbation of the balance between cell proliferation and cell death. VSMCs isolated from the internal mammary arteries of diabetic patients show resistance to apoptosis induced by several stimuli [246]. This phenomenon could be attributed to the over-expression of Bcl-2 in diabetic VSMCs, as its downregulation by RNA-silencing restored the sensitivity to pro-apoptotic signals. The different apoptotic/proliferative rate is exacerbated by high-glucose conditions, inducing activation of protein kinase C (PKC) [247]. Other evidence supports the impairment of the Fas/Fas-ligand pathway in DM, contributing to the down-regulation of apoptosis [248]. The altered balance between proliferation and apoptosis is considered decisive in determining the intima/media thickening in DM, with particular clinical relevance for T2DM patients [227,249-251].
Another point to take in consideration regarding VSMCs behavior in DM is the perturbation of insulin signaling. Physiologically, insulin can maintain VSMC quiescence and, on the other hand, can promote VSMC migration [252]. Indeed, insulin keeps VSMCs quiescent by activating PI3K pathway, while it promotes their migration via the activation of the MAPK pathway [252]. Impaired PI3 kinase signaling and intact MAPK signaling characterize insulin-resistance; therefore, in diabetic conditions insulin seems to lose its ability to maintain VSMCs quiescent, pathologically promoting their migration.
4. Animal models of diabetic cardiomyopathy
Due to the complex pathophysiology of the disease, research in DM relies on the use of in vivo models of the disease. Both genetically induced spontaneous and experimentally induced non-spontaneous DM models are commonly used as animal models in preclinical research on DM [253,254]. Various rodent models of DM that are useful for studying mechanisms and pharmacological therapies are currently available (Fig. 3). Undoubtedly, research on animal models has significantly expanded our knowledge of the molecular mechanisms responsible for diabetic cardiomyopathy.
Fig. 3. Animal models of diabetic cardiomyopathy.
4.1. High Fat Diet/Streptozotocin (HFD/STZ)-induced rodents
The high-fat diet/STZ (HFD/STZ)-treated rodents represent a widely used experimentally induced diabetic model [255,256]. The HFD/STZ model mimics the early or late stages of T2DM [256,257]. To develop the corresponding animal pathology model, high fat diet (HFD) is often fed to the mice to induce insulin resistance, followed by low-dose STZ injection to cause β-cell impairment without completely compromising insulin secretion, thereby mimicking the characteristics of T2DM [257-259]. Generally, HFD induces metabolic changes that are commonly observed in T2DM patients, such as development of obesity and decrease in insulin sensitivity [260,261]. Several studies have been conducted to establish the best HFD feeding time and the ideal STZ doses to induce the T2DM models [256,259,262]. The diabetic condition is assessed by routine tests such as food and water intake, body weight gain, glucose tolerance test (GTT), and insulin tolerance test (ITT) among others. The interaction of AGEs with RAGE causes progression of diabetic complications and renal injuries [263,264]. Primarily, HFD/STZ-induced model can be beneficial in evaluating the effectiveness of anti-diabetic and anti-obesity drugs in T2DM. More recently, the combination of HFD and sucrose (known as HFS) has been shown to be a reliable model to induce DM in small rodents [265-267].
4.2. OVE26 mouse
The OVE26 mouse was generated by Epstein and collaborators in 1989 on a FVB background [268,269]. These mice have lower insulin levels and elevated serum triglyceride levels. They exhibit severe early-onset T1DM and can survive for up to 2 years without insulin administration [270]. They develop severe hyperglycemia after they reach 2–3 weeks due to overexpression of calmodulin in pancreatic β cells [271-273].
Increased cardiac mitochondrial biogenesis and oxidative stress have been reported in these mice [274]. However, one of the studies performed on these rodents revealed that the contractility of cardiomyocytes isolated from diabetic OVE26 mice remained stable during culture and that OVE26 hearts in young animals have quite normal values of ejection fraction and fractional shortening [274]. More recent experimental assays have shown that the development of diabetic cardiomyopathy in these mice is both age and sex dependent and is mainly attributable to fibrosis, ROS, and inflammation [275].
This model is one of the simplest ones for studying DM-associated complications as it exerts direct effects on pancreatic β cells, displays early onset DM, which can last for up to 2 years without any insulin treatment [270].
4.3. The ob/ob mouse
The ob/ob mouse has a recessive mutation in leptin (Lepob) [276-279] and the severity of DM is dependent on the strain's genetic background. They are most used to model DM and obesity. The ob/ob mice exhibit mild DM with marked obesity, hyperphagia, hyperinsulinemia, and transient hyperglycemia [280,281].
Hyperglycemia develops in these mice between 8 and 15 weeks. The maximum blood glucose levels have been recorded when these mice reach 3–5 months; then, blood glucose starts decreasing and normalizes as they grow older. On the BL/6 inbred background, ob/ob mice develop cardiac hypertrophy and hyperplasia [281]. There is only mild or no impairment in systemic dysfunction in ob/ob mice, however, they have diastolic dysfunction, most likely due to cardiac lipid accumulation, which may trigger inflammation and oxidative stress, as also validated in clinical studies [282]; ob/ob mice exhibit increased left ventricular (LV) systolic pressure (LVSP) and LV end-diastolic pressure (LVEDP) as they get old; dP/dt is elevated in ob/ob mice at 4 weeks of age whereas it decreases when the mice are 8-weeks old [283].
Isolated perfused hearts from ob/ob mice exhibited decreased LV function [284]. Cardiac metabolism studies have shown that there is a reduction in glucose oxidation whereas the rates of fatty acid oxidation [285] and myocardial triglyceride storage [286] increase. Moreover, mitochondrial dysfunction and oxidative stress have been reported in ob/ob hearts [287].
4.4. The db/db mouse
The genetically diabetic-obese (db/db) mice are homozygous for a mutation in the leptin receptor (Leprdb) [276] and are widely used to model T2DM and obesity. In this type of mice, leptin action is impaired due to the defect of the leptin receptor. In these mice, obesity starts at three to four weeks of age [288]. On the BL/Ks background, these rodents exhibit elevated levels of blood sugar, transient high plasma insulin levels, atrophy of insulin-producing pancreatic β-cells, peripheral neuropathy, and myocardial disease [280]. Hyperglycemia develops in db/db mice between 4 and 8 weeks of age [284].
Elevated serum concentrations of triglycerides in db/db mice have been described by various investigators [289,290]. The levels of triglycerides were shown to be ~2.2-times greater in db/db mice of 15 weeks old versus ob/ob mice of the same age [284]. Isolated perfused hearts from 10 to 14 weeks old db/db mice exhibited the characteristics of diabetic cardiomyopathy, including a decrease in contractile performance and alterations in cardiac metabolism [291,292]. A thorough evaluation by cardiac ultrasound evidenced an age-dependent diastolic dysfunction, recapitulating features of human HF with preserved ejection fraction (HFpEF), more pronounced in female mice [293]. Invasive assessment of LV function showed an increased LVSP, increased LVEDP as early as 4-weeks of age and an increase in dP/dt over time. The rates of fatty acid oxidation and myocardial lipid storage are increased in db/db hearts [284–286,294] alongside a reduction of glucose uptake and oxidation and an impaired mitochondrial function [295].
4.5. The Zucker fatty rat and Zucker diabetic fatty rat
Zucker fatty rats have a homozygous missense mutation (fatty, Fa) in the gene encoding for the rat leptin receptor (Ob-R). The mutation occurs in a simple autosomal recessive gene, Fa, that is common to all leptin receptor isoforms [296,297]. Zucker fatty rats are obese, hyperphagic, hyperinsulinemic, but are not often hyperglycemic. Henceforth, they are an excellent model for studying the long-term effects of obesity. They have increased serum levels of triglycerides, fatty acids, and insulin. These rats start becoming obese at 3–5 weeks and their body is composed of >40% lipid by the time they reach 14 weeks [298].
The Zucker diabetic fatty (ZFD) rat was derived by selective breeding of the Zucker fatty rats with high serum glucose levels to obtain a model with a DM trait. These rats exhibit obesity with DM and are most commonly used for T2DM research. Nonfunctioning leptin receptors is believed to be the main cause of their obesity [299]. ZDF rats are obese, hyperinsulinemic, hyperglycemic, hyperleptinemic, and have high levels of serum fatty acids and triglycerides [300,301]. Until they are 6 weeks old, ZDF rats are insulin resistant but euglycemic; they start to develop hyperglycemia at around 6 weeks of age and they usually develop stable hyperglycemia at 10–12 weeks, displaying reduced insulin levels as a result of pancreatic β-cell deficiency [302].
Oltman and colleagues [303] reported that the development of vascular and neural dysfunction was slow in obese Zucker rats as compared to the ZDF T2DM rat model; however, since ZDF rats are inbred, it is complicated to directly compare the obese and diabetic Zucker rats because of the differences in the genetic background [302]. Other studies demonstrated that there were negligible changes in substrate oxidation and irregularities in cardiac contractility in Zucker fatty rats, as compared to diabetic ZDF rats; Zucker fatty rats develop oxidative stress, cardiac hypertrophy, and interstitial fibrosis [304,305]. Various studies conducted in Zucker fatty rats have shown that there is a decrease in cardiac function in isolated hearts whereas there is an increased rate pressure-product [306,307]. However, cardiac efficiency remains mostly unchanged [294,307]. Young and collaborators demonstrated that there is a decrease in carbohydrate oxidation whereas oleate oxidation and expression of genes involved in fatty acid oxidation do not rise in Zucker fatty rats [307]. Studies carried out by Luiken and colleagues revealed that palmitate uptake and myocardial lipid content are increased in cardiomyocytes isolated from Zucker fatty rats [308].
ZFD rats are not as much obese than Zucker fatty rats but are more insulin resistant [309]. The male ZDF rat rapidly progresses to T2DM because of the inability to compensate adequately for insulin resistance. According to the data obtained from echocardiography, ZDF rats have a more depressed LV fractional shortening in comparison to ZF rats [310]. A reduced LV insulin-mediated glucose utilization in ZDF rats has also been reported [310]. ZDF rats exhibit left ventricular hypertrophy and increased myocardial lipid storage [311-313]. Moreover, in ZDF rats there is an increase in the rates of fatty acids oxidation and expression of genes involved in this process, whereas carbohydrate oxidation and Glut4 expression are both decreased [312-314].
5. The heart in DM: clinical insights
For years, population-based studies have evidenced a significantly increased risk of HF in diabetic patients unexplained by traditional risk factors (obesity, hypertension, age, coronary artery disease, dyslipidemia, and significant valvular disease) [315-322]. In fact, even after adjustment for these factors, diabetic patients have up to a two-times relative risk of developing HF [323,324]. This ventricular dysfunction is deemed diabetic cardiomyopathy and is most commonly associated with diastolic dysfunction and HFpEF — through left ventricular hypertrophy, AGE deposition, and interstitial fibrosis early on in its development. Of note, ~30%–40% of individuals with HFpEF have DM [325,326]. Albeit several parameters of diastolic function were reported to be similar in diabetic and non-diabetic HFpEF patients, a trend toward higher left atrial filling pressures was noted in HFpEF patients with DM [327]. Furthermore, increased cardiac fibrosis, cardiomyocyte hypertrophy, endothelial dysfunction, and AGE deposition, have been reported to be major components of HFpEF in DM patients [328,329]. Coronary microvascular inflammation with endothelial dysfunction can be considered a common denominator among HFpEF, DM, and obesity, that may explain the pathophysiology of HFpEF and its synergistic relationship with DM and obesity [320,330].
A principal involvement of endothelial cells in the cardiac remodeling process could represent the core mechanism underlying HFpEF, opposed to a main involvement of cardiomyocytes in HFrEF [330-333]
Diabetic cardiomyopathy is often asymptomatic in its early stages [334]. However, left atrial enlargement and higher end-diastolic pressure will eventually result from the thickened left ventricle and impaired diastolic filling. The prevalence of diastolic dysfunction is high in diabetic patients, reported to be 23%–75% in various studies, and increases markedly with age [335-337]. As the disease progresses, systolic dysfunction may arise in the form of worsening ventricular dilation through eccentric hypertrophy and HF with reduced ejection fraction (HFrEF, ejection fraction ≤ 40%) [338]. At this point, the classic signs of congestive HF should be observed, including pulmonary rales, orthopnea, fatigue, and nocturia. The mechanisms underlying the change in phenotype are not fully understood but, as mentioned above in this review, most likely involve impaired Ca2+ handling in the mitochondria and sarcoplasmic reticulum, insulin resistance, autonomic neuropathy, and endothelial/microvascular dysfunction — influenced in part by the patient's glycemic control [334,339]. It is interesting to note that data from the Framingham Heart Study revealed that diabetic patients develop greater age-associated increases in left ventricular wall thickness [340]; markers of insulin resistance, including augmented serum insulin levels, have been also shown to be associated with greater left ventricular mass and concentric remodeling [341,342].
Unfortunately, there are no established clinical signs or histological markers that can definitively identify diabetic cardiomyopathy [318,323]. This disease has a long subclinical phase in which major histological and functional changes may occur, but clinical manifestations have not developed. Therefore, identifying cases within asymptomatic individuals with DM could be particularly useful. A study conducted in 101 patients with diabetic cardiomyopathy showed that screening using stress echocardiography and Doppler is useful in identifying asymptomatic DM patients with left ventricular hypertrophy, whereas measuring hypertrophic markers like brain natriuretic peptide (BNP) was not sufficiently sensitive to diagnose subclinical disease [343]. 2D/3D-Speckle Tracking Echocardiography is a relatively new technique that shows promise in detecting mechanical deformation and can definitely help stratify diabetic patients [344,345]. Other investigators have demonstrated that both magnetic resonance and nuclear imaging — including PET and single-positron emission computed tomography (SPECT) — are powerful tools in predicting cardiovascular mortality in asymptomatic diabetic patients [346]. Yet, the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study, a trial of 1123 diabetic patients, revealed a non-significant decrease in cardiac events with screening via adenosine-stress radionuclide myocardial perfusion imaging (MPI) — bringing into question the utility of these screening methods in the clinic [347].
More recently, non-coding RNAs have been sought out as potential diagnostic criteria for their role in the pathogenesis of diabetic cardiomyopathy (via hypertrophy, fibrosis, and oxidative stress) [348-350]. If properly validated, anti-miRNA and miRNA mimic therapies could be an exciting new avenue for treatment, since miRNAs represent druggable therapeutic targets. A recent study demonstrated that decreased levels of miR-21 could be used as a reliable diagnostic biomarker of diabetic cardiomyopathy; the same study revealed that overexpressing miR-21 could improve mitochondrial biogenesis (including increased expression of PGC-1α), a process that was impaired in db/db mice [351]. Cardiomyocyte-derived exosomes could also serve as a specific biomarker for diabetic cardiomyopathy. Exosomes are membrane-derived nanovesicles that serve a role in extracellular communication between different cells and tissues through shuttling cellular components like DNA, non-coding RNA, and heat-shock proteins [352,353]. A 2014 preclinical study demonstrated that cardiomyocyte-derived exosomes containing miR-320 help mediate myocardial vascular deficiency present in diabetic cardiomyopathy [354]. However, this fascinating cross-talk between cardiomyocytes and endothelial cells has not been studied extensively as a diagnostic criterion or therapy in the clinical scenario.
5.1. Sex differences in diabetic cardiomyopathy
Diabetic cardiomyopathy, defined as ventricular dysfunction occurring in diabetic patients independent of a coronary heart disease or hypertension [355], has been shown to be more prevalent in diabetic women than in diabetic men [356]. Similarly, the Framingham Heart Study reported a two-fold increase in HFpEF in females vs males [357]. These findings are intriguing, because HFpEF has been shown to have a strong link with metabolic syndrome [358], which includes insulin resistance, lipid disorders, obesity, and hypertension.
The mechanisms underlying these differences are mostly unknown, but can include disparities in Ca2+ handling and other ion channels in cardiomyocytes [359-365], a sexually dimorphic function of contractile proteins [366,367], a dissimilar response to adrenergic stimuli [368,369], as well as a diverse remodeling response [370,371].
Some data from clinical studies can be very helpful in better understanding the relationship linking gender, HFpEF, and diabetic cardiomyopathy, although further dedicated studies are necessary: for instance, a functional role for estrogen in regulating insulin sensitivity has emerged in the Women's Health Initiative Hormone Trial [372], and metformin has been shown to decrease fatty acid clearance in men but not in women [373].
5.2. Notes on clinical management
As previously mentioned, glycemic control is a powerful way to reduce the risk of HF in patients with both T1DM and T2DM. Notably, a 2017 prospective study found a significant increase in mortality with higher admission blood glucose (ABG) in HF patients with both pre-existing DM (>200 mg/dL versus <110 mg/dL, HR = 1.20, p = 0.032) and those without DM (HR = 1.04 per 18 mg/dL increase in ABG, p = 0.014) over a follow-up period of 10 years [374]. Therefore, a constant glycemic therapy — including metformin, sulfonylureas, insulin, and glucagon-like peptide 1 (GLP-1) receptor agonists — is of utmost importance and has been shown in several prospective studies to ameliorate both systolic and diastolic dysfunction [375,376]. Diastolic dysfunction is a common characteristic in both T1DM and T2DM patients with diabetic cardiomyopathy [377], However, studies report fewer clinical symptoms of HF in T1DM patients compared to T2DM, possibly owing to T1DM patients being of younger age and having glycemia well-controlled with insulin [378,379].
SGLT2 inhibitors (SGLT2Is) are a promising new-line treatment that not only reduces hyperglycemia but also seems to have direct beneficial effects on cardiomyocytes — independent of glucose levels — including improved Ca2+ handling and myocardial contractility [380]. In patients with T2DM, treatment with empagliflozin decreased adverse cardiac remodeling [381,382]. Although there are limited reports regarding if SGLT2Is are beneficial in treating existing HF inpatients with DM, promising data are showing their effectiveness in preventing HF development across a broad spectrum of cardiovascular risk [383,384]. Additional putative benefits of SGLT2Is include improved cardiovascular energetics, reduced vascular tone and blood pressure [380], decreased renal dysfunction, increased circulating levels of ketone bodies [385], and overall reduced systemic inflammation [109].
Like SGLT2Is, GLP-1 agonists have also been shown to have cardioprotective properties, independent of their glucose-lowering effects, in patients with T2DM. However, the results of the 2016 Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial suggest that the benefits of GLP-1 agonists may be more closely tied to the mitigation of atherosclerosis rather than the hemodynamic benefits in the previously mentioned empagliflozin studies [334,386-388].
6. Conclusions and perspectives
At this moment, there are no proven therapies for reducing mortality in HFpEF [389-392], probably due to its heterogeneous nature. For example, as shown by the CHARM-Preserved (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity in HFpEF] study, treatment using the angiotensin II receptor blocker (ARB) candesartan failed to reach statistical significance in mortality reduction (unadjusted HR 0.89 [95% Cl 0.77–1.03], p = 0.118) [393]. Instead, addressing any co-morbidities is the mainstay of HFpEF clinical management. Once symptoms of HFrEF begin to arise, patients should be treated with proven therapies such as angiotensin-converting enzyme (ACE) inhibitors, ARBs, and beta-blockers. In either case, compounds that promote diuresis, including the angiotensin receptor neprilysin inhibitor (ARNI), should be considered to relieve symptoms of volume overload with caution in avoiding hypotension [1,394]. For patients with arrhythmia, pharmacologic control of heart rate should be used; implantable cardiac defibrillators (ICDs) may be considered even in HF patients without arrhythmia as secondary prevention for sudden death [395,396].
Lifestyle changes are very effective in improving the quality of life in patients with asymptomatic diastolic dysfunction and HFpEF [397]; specific modifications that should be considered to reduce the burden of HFpEF include: stopping smoking, losing weight, getting active, eating better, managing blood pressure, controlling cholesterol, and reducing blood sugar [157,320,397]. Exercise training, in those who are able, has been shown to yield improvements in cardiorespiratory fitness and quality of life surveys, with mild, yet non-significant, changes in left ventricular diastolic function [22,398,399].
Understanding the molecular mechanisms underlying diabetic cardiomyopathyand HFpEF and finding new therapeutic strategies represent some of the main future challenges in cardiovascular endocrinology [400].
Funding
The Santulli's Lab is supported in part by the National Institutes of Health (NIH: R01-DK123259, R01-HL146691, R01-DK033823, R01-HL159062, R56-AG066431, T32-HL144456, and R00-DK107895, to G.S.), by the Irma T. Hirschl and Monique Weill-Caulier Trusts (to G.S.), by the Diabetes Action Research and Education Foundation (to G. S.), and by the American Heart Association (AHA-21POST836407 to S.S.J. and AHA-20POST35211151 to J.G.).
Footnotes
CRediT authorship contribution statement
Stanislovas S. Jankauskas: Writing–original draft, Writing–review & editing. Urna Kansakar: Writing – original draft, Writing – review & editing. Fahimeh Varzideh: Writing – original draft, Writing – review & editing. Scott Wilson: Writing – original draft. Pasquale Mone: Writing – original draft. Angela Lombardi: Writing – original draft. Jessica Gambardella: Writing – original draft. Gaetano Santulli: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
Nothing to declare.
References
- [1].Kansakar U, Varzideh F, Jankauskas SS, Gambardella J, Trimarco B, Santulli G. Advances in the understanding of excitation-contraction coupling: the pulsing quest for drugs against heart failure and arrhythmias. Eur Heart J Cardiovasc Pharmacother. 2021:pvab069 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Balaban RS, Kantor HL, Katz LA, Briggs RW. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986;232:1121–3. [DOI] [PubMed] [Google Scholar]
- [3].Zhao Q, Sun Q, Zhou L, Liu K, Jiao K. Complex regulation of mitochondrial function during cardiac development. J Am Heart Assoc. 2019;8:e012731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang Y, Christopher BA, Wilson KA, Muoio D, McGarrah RW, Brunengraber H, et al. Propionate-induced changes in cardiac metabolism, notably CoA trapping, are not altered by l-carnitine. Am J Physiol Endocrinol Metab. 2018;315 (E622–E33). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang Y, Taufalele PV, Cochran JD, Robillard-Frayne I, Marx JM, Soto J, et al. Mitochondrial pyruvate carriers are required for myocardial stress adaptation. Nat Metab. 2020;2:1248–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Turer A, Altamirano F, Schiattarella GG, May H, Gillette TG, Malloy CR, et al. Remodeling of substrate consumption in the murine sTAC model of heart failure. J Mol Cell Cardiol. 2019;134:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gambardella J, Lombardi A, Santulli G. Metabolic flexibility of mitochondria plays a key role in balancing glucose and fatty acid metabolism in the diabetic heart. Diabetes. 2020;69:2054–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129. [DOI] [PubMed] [Google Scholar]
- [9].Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med. 1954;16:504–15. [DOI] [PubMed] [Google Scholar]
- [10].Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lopaschuk GD, Karwi QG, Ho KL, Pherwani S, Ketema EB. Ketone metabolism in the failing heart. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158813. [DOI] [PubMed] [Google Scholar]
- [12].Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Opie LH, Mansford KR, Owen P. Effects of increased heart work on glycolysis and adenine nucleotides in the perfused heart of normal and diabetic rats. Biochem J. 1971;124:475–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids, Studies with 14C-labeled substrates in humans. J Clin Invest. 1987;79:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang J, Song Y, Wang Q, Kralik PM, Epstein PN. Causes and characteristics of diabetic cardiomyopathy. Rev Diabet Stud. 2006;3:108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63:1469–79. [DOI] [PubMed] [Google Scholar]
- [17].Cipolletta E, Del Giudice C, Santulli G, Trimarco B, Iaccarino G. Opposite effects of beta2-adrenoceptor gene deletion on insulin signaling in liver and skeletal muscle. Nutr Metab Cardiovasc Dis. 2017;27:615–23. [DOI] [PubMed] [Google Scholar]
- [18].Sardu C, Marfella R, Santulli G. Impact of diabetes mellitus on the clinical response to cardiac resynchronization therapy in elderly people. J Cardiovasc Transl Res. 2014;7:362–8. [DOI] [PubMed] [Google Scholar]
- [19].Lembo G, Napoli R, Capaldo B, Rendina V, Iaccarino G, Volpe M, et al. Abnormal sympathetic overactivity evoked by insulin in the skeletal muscle of patients with essential hypertension. J Clin Invest. 1992;90:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sorriento D, Rusciano MR, Visco V, Fiordelisi A, Cerasuolo FA, Poggio P, et al. The metabolic role of GRK2 in insulin resistance and associated conditions. Cells. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ciccarelli M, Chuprun JK, Rengo G, Gao E, Wei Z, Peroutka RJ, et al. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation. 2011;123:1953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Iaccarino G, Franco D, Sorriento D, Strisciuglio T, Barbato E, Morisco C. Modulation of insulin sensitivity by exercise training: implications for cardiovascular prevention. J Cardiovasc Transl Res. 2021;14:256–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Herrero P, Peterson LR, McGill JB, Matthew S, Lesniak D, Dence C, et al. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol. 2006;47:598–604. [DOI] [PubMed] [Google Scholar]
- [24].Mather KJ, Hutchins GD, Perry K, Territo W, Chisholm R, Acton A, et al. Assessment of myocardial metabolic flexibility and work efficiency in human type 2 diabetes using 16-[18F]fluoro-4-thiapalmitate, a novel PET fatty acid tracer. Am J Physiol Endocrinol Metab. 2016;310:E452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yan D, Cai Y, Luo J, Liu J, Li X, Ying F, et al. FOXO1 contributes to diabetic cardiomyopathy via inducing imbalanced oxidative metabolism in type 1 diabetes. J Cell Mol Med. 2020;24:7850–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thapa D, Xie B, Zhang M, Stoner MW, Manning JR, Huckestein BR, et al. Adropin treatment restores cardiac glucose oxidation in pre-diabetic obese mice. J Mol Cell Cardiol. 2019;129:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sardu C, De Lucia C, Wallner M, Santulli G. Diabetes mellitus and its cardiovascular complications: new insights into an old disease. J Diabetes Res. 2019;2019:1905194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, et al. Empagliflozin increases cardiac energy production in Diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci. 2018;3:575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fazakerley DJ, Lawrence SP, Lizunov VA, Cushman SW, Holman GD. A common trafficking route for GLUT4 in cardiomyocytes in response to insulin, contraction and energy-status signalling. J Cell Sci. 2009;122:727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Petersen S, Bahr M, Eckel J. Insulin-dependent regulation of Glut4 gene expression in ventricular cardiomyocytes: evidence for a direct effect on Glut4 transcription. Biochem Biophys Res Commun. 1995;213:533–40. [DOI] [PubMed] [Google Scholar]
- [31].Becker C, Sevilla L, Tomas E, Palacin M, Zorzano A, Fischer Y. The endosomal compartment is an insulin-sensitive recruitment site for GLUT4 and GLUT1 glucose transporters in cardiac myocytes. Endocrinology. 2001;142:5267–76. [DOI] [PubMed] [Google Scholar]
- [32].Fischer Y, Thomas J, Sevilla L, Munoz P, Becker C, Holman G, et al. Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes. Evidence of the existence of different intracellular GLUT4 vesicle populations. J Biol Chem. 1997;272:7085–92. [DOI] [PubMed] [Google Scholar]
- [33].Maria Z, Campolo AR, Lacombe VA. Diabetes alters the expression and translocation of the insulin-sensitive glucose transporters 4 and 8 in the Atria. PLoS One. 2015;10:e0146033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bockus LB, Matsuzaki S, Vadvalkar SS, Young ZT, Giorgione JR, Newhardt MF, et al. Cardiac insulin signaling regulates glycolysis through phosphofructokinase 2 content and activity. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Donthi RV, Ye G, Wu C, McClain DA, Lange AJ, Epstein PN. Cardiac expression of kinase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase inhibits glycolysis, promotes hypertrophy, impairs myocyte function, and reduces insulin sensitivity. J Biol Chem. 2004;279:48085–90. [DOI] [PubMed] [Google Scholar]
- [36].Depre C, Rider MH, Veitch K, Hue L. Role of fructose 2,6-bisphosphate in the control of heart glycolysis. J Biol Chem. 1993;268:13274–9. [PubMed] [Google Scholar]
- [37].Karwi QG, Wagg CS, Altamimi TR, Uddin GM, Ho KL, Darwesh AM, et al. Insulin directly stimulates mitochondrial glucose oxidation in the heart. Cardiovasc Diabetol. 2020;19:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim-Muller JY, Kim YJ, Fan J, Zhao S, Banks AS, Prentki M, et al. FoxO1 deacetylation decreases fatty acid oxidation in beta-cells and sustains insulin secretion in Diabetes. J Biol Chem. 2016;291:10162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sakaguchi M, Cai W, Wang CH, Cederquist CT, Damasio M, Homan EP, et al. FoxK1 and FoxK2 in insulin regulation of cellular and mitochondrial metabolism. Nat Commun. 2019;10:1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Weeks KL, Tham YK, Yildiz SG, Alexander Y, Donner DG, Kiriazis H, et al. FoxO1 is required for physiological cardiac hypertrophy induced by exercise but not by constitutively active PI3K. Am J Physiol Heart Circ Physiol. 2021;320 (H1470–H85). [DOI] [PubMed] [Google Scholar]
- [41].Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23:804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fillmore N, Wagg CS, Zhang L, Fukushima A, Lopaschuk GD. Cardiac branched-chain amino acid oxidation is reduced during insulin resistance in the heart. Am J Physiol Endocrinol Metab. 2018;315 (E1046–E52). [DOI] [PubMed] [Google Scholar]
- [44].Desrois M, Sidell RJ, Gauguier D, King LM, Radda GK, Clarke K. Initial steps of insulin signaling and glucose transport are defective in the type 2 diabetic rat heart. Cardiovasc Res. 2004;61:288–96. [DOI] [PubMed] [Google Scholar]
- [45].Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, et al. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hu L, Qiu C, Wang X, Xu M, Shao X, Wang Y. The association between diabetes mellitus and reduction in myocardial glucose uptake: a population-based (18)F-FDG PET/CT study. BMC Cardiovasc Disord. 2018;18:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Guazzi M, Tumminello G, Matturri M, Guazzi MD. Insulin ameliorates exercise ventilatory efficiency and oxygen uptake in patients with heart failure-type 2 diabetes comorbidity. J Am Coll Cardiol. 2003;42:1044–50. [DOI] [PubMed] [Google Scholar]
- [48].Tong M, Saito T, Zhai P, Oka SI, Mizushima W, Nakamura M, et al. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res. 2019;124:1360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Son NH, Basu D, Samovski D, Pietka TA, Peche VS, Willecke F, et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J Clin Invest. 2018;128:4329–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lou PH, Lucchinetti E, Scott KY, Huang Y, Gandhi M, Hersberger M, et al. Alterations in fatty acid metabolism and sirtuin signaling characterize early type-2 diabetic hearts of fructose-fed rats. Phys Rep. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li X, Wu Y, Zhao J, Wang H, Tan J, Yang M, et al. Distinct cardiac energy metabolism and oxidative stress adaptations between obese and non-obese type 2 diabetes mellitus. Theranostics. 2020;10:2675–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nakamura M, Liu T, Husain S, Zhai P, Warren JS, Hsu CP, et al. Glycogen synthase kinase-3alpha promotes fatty acid uptake and Lipotoxic cardiomyopathy. Cell Metab. 2019;29(1119–34):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yin Z, Zhao Y, He M, Li H, Fan J, Nie X, et al. MiR-30c/PGC-1beta protects against diabetic cardiomyopathy via PPARalpha. Cardiovasc Diabetol. 2019;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sikder K, Shukla SK, Patel N, Singh H, Rafiq K. High fat diet upregulates fatty acid oxidation and Ketogenesis via intervention of PPAR-gamma. Cell Physiol Biochem. 2018;48:1317–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pettersen IKN, Tusubira D, Ashrafi H, Dyrstad SE, Hansen L, Liu XZ, et al. Upregulated PDK4 expression is a sensitive marker of increased fatty acid oxidation. Mitochondrion. 2019;49:97–110. [DOI] [PubMed] [Google Scholar]
- [56].Razeghi P, Young ME, Cockrill TC, Frazier OH, Taegtmeyer H. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation. 2002;106:407–11. [DOI] [PubMed] [Google Scholar]
- [57].Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, et al. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res. 2014;103:485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ducheix S, Magre J, Cariou B, Prieur X. Chronic O-GlcNAcylation and diabetic cardiomyopathy: the bitterness of glucose. Front Endocrinol. 2018;9:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107:171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Qin L, Wang J, Zhao R, Zhang X, Mei Y. Ginsenoside-Rb1 improved diabetic cardiomyopathy through regulating calcium signaling by alleviating protein O-GlcNAcylation. J Agric Food Chem. 2019;67:14074–85. [DOI] [PubMed] [Google Scholar]
- [61].Wende AR, Schell JC, Ha CM, Pepin ME, Khalimonchuk O, Schwertz H, et al. Maintaining myocardial glucose utilization in diabetic cardiomyopathy accelerates mitochondrial dysfunction. Diabetes. 2020;69:2094–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Xiao W, Loscalzo J. Metabolic responses to reductive stress. Antioxid Redox Signal. 2020;32:1330–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Acharya AP, Rafi M, Woods EC, Gardner AB, Murthy N. Metabolic engineering of lactate dehydrogenase rescues mice from acidosis. Sci Rep. 2014;4:5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].North BJ, Verdin E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 2004;5:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Paramesha B, Anwar MS, Meghwani H, Maulik SK, Arava SK, Banerjee SK. Sirt1 and Sirt3 activation improved cardiac function of diabetic rats via modulation of mitochondrial function. Antioxidants. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kanwal A, Pillai VB, Samant S, Gupta M, Gupta MP. The nuclear and mitochondrial sirtuins, Sirt6 and Sirt3, regulate each other’s activity and protect the heart from developing obesity-mediated diabetic cardiomyopathy. FASEB J. 2019;33:10872–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang AJ, Wang S, Wang BJ, Xiao M, Guo Y, Tang Y, et al. Epigenetic regulation associated with Sirtuin 1 in complications of Diabetes mellitus. Front Endocrinol. 2020;11:598012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vazquez EJ, Berthiaume JM, Kamath V, Achike O, Buchanan E, Montano MM, et al. Mitochondrial complex I defect and increased fatty acid oxidation enhance protein lysine acetylation in the diabetic heart. Cardiovasc Res. 2015;107:453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].von Wilamowitz-Moellendorff A, Hunter RW, Garcia-Rocha M, Kang L, Lopez-Soldado I, Lantier L, et al. Glucose-6-phosphate-mediated activation of liver glycogen synthase plays a key role in hepatic glycogen synthesis. Diabetes. 2013;62:4070–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ge T, Yang J, Zhou S, Wang Y, Li Y, Tong X. The role of the pentose phosphate pathway in diabetes and cancer. Front Endocrinol. 2020;11:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Katare R, Oikawa A, Cesselli D, Beltrami AP, Avolio E, Muthukrishnan D, et al. Boosting the pentose phosphate pathway restores cardiac progenitor cell availability in diabetes. Cardiovasc Res. 2013;97:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tran DH, Wang ZV. Glucose metabolism in cardiac hypertrophy and heart failure. J Am Heart Assoc. 2019;8:e012673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Byrne NJ, Rajasekaran NS, Abel ED, Bugger H. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radic Biol Med. 2021;169:317–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lin C, Guo Y, Xia Y, Li C, Xu X, Qi T, et al. FNDC5/Irisin attenuates diabetic cardiomyopathy in a type 2 diabetes mouse model by activation of integrin alphaV/beta5-AKT signaling and reduction of oxidative/nitrosative stress. J Mol Cell Cardiol. 2021;160:27–41. [DOI] [PubMed] [Google Scholar]
- [76].Ulusu NN, Gok M, Sayin Sakul AA, Ari N, Stefek M, Karasu C, et al. Antioxidant SMe1EC2 modulates pentose phosphate pathway and glutathione-dependent enzyme activities in tissues of aged diabetic rats. Interdiscip Toxicol. 2017;10:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Santulli G. Cardiovascular disease and diabetes. Front Endocrinol. 2019;10:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Matsushima S, Kinugawa S, Yokota T, Inoue N, Ohta Y, Hamaguchi S, et al. Increased myocardial NAD(P)H oxidase-derived superoxide causes the exacerbation of postinfarct heart failure in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2009;297:H409–16. [DOI] [PubMed] [Google Scholar]
- [79].Zhang Y, Tocchetti CG, Krieg T, Moens AL. Oxidative and nitrosative stress in the maintenance of myocardial function. Free Radic Biol Med. 2012;53:1531–40. [DOI] [PubMed] [Google Scholar]
- [80].Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- [81].Prentki M, Madiraju SR. Glycerolipid metabolism and signaling in health and disease. Endocr Rev. 2008;29:647–76. [DOI] [PubMed] [Google Scholar]
- [82].Salabei JK, Lorkiewicz PK, Mehra P, Gibb AA, Haberzettl P, Hong KU, et al. Type 2 Diabetes dysregulates glucose metabolism in cardiac progenitor cells. J Biol Chem. 2016;291:13634–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Yu J, Loh K, Song ZY, Yang HQ, Zhang Y, Lin S. Update on glycerol-3-phosphate acyltransferases: the roles in the development of insulin resistance. Nutr Diabetes. 2018;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Guan HP, Li Y, Jensen MV, Newgard CB, Steppan CM, Lazar MA. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat Med. 2002;8:1122–8. [DOI] [PubMed] [Google Scholar]
- [85].Burkhoff D, Weiss RG, Schulman SP, Kalil-Filho R, Wannenburg T, Gerstenblith G. Influence of metabolic substrate on rat heart function and metabolism at different coronary flows. Am J Phys. 1991;261:H741–50. [DOI] [PubMed] [Google Scholar]
- [86].Hutter JF, Schweickhardt C, Piper HM, Spieckermann PG. Inhibition of fatty acid oxidation and decrease of oxygen consumption of working rat heart by 4-bromocrotonic acid. J Mol Cell Cardiol. 1984;16:105–8. [DOI] [PubMed] [Google Scholar]
- [87].Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1345–51. [DOI] [PubMed] [Google Scholar]
- [88].Boardman NT, Larsen TS, Severson DL, Essop MF, Aasum E. Chronic and acute exposure of mouse hearts to fatty acids increases oxygen cost of excitation-contraction coupling. Am J Physiol Heart Circ Physiol. 2011;300:H1631–6. [DOI] [PubMed] [Google Scholar]
- [89].Skulachev VP. Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 1991;294:158–62. [DOI] [PubMed] [Google Scholar]
- [90].Schrauwen P, Hoeks J, Schaart G, Kornips E, Binas B, Van De Vusse GJ, et al. Uncoupling protein 3 as a mitochondrial fatty acid anion exporter. FASEB J. 2003;17:2272–4. [DOI] [PubMed] [Google Scholar]
- [91].Aon MA, Bhatt N, Cortassa SC. Mitochondrial and cellular mechanisms for managing lipid excess. Front Physiol. 2014;5:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040–6. [DOI] [PubMed] [Google Scholar]
- [93].Fukushima A, Lopaschuk GD. Acetylation control of cardiac fatty acid beta-oxidation and energy metabolism in obesity, diabetes, and heart failure. Biochim Biophys Acta. 2016;1862:2211–20. [DOI] [PubMed] [Google Scholar]
- [94].Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ljubkovic M, Gressette M, Bulat C, Cavar M, Bakovic D, Fabijanic D, et al. Disturbed fatty acid oxidation, endoplasmic reticulum stress, and apoptosis in left ventricle of patients with type 2 Diabetes. Diabetes. 2019;68:1924–33. [DOI] [PubMed] [Google Scholar]
- [96].Tong D, Schiattarella GG, Jiang N, Altamirano F, Szweda PA, Elnwasany A, et al. NAD (+) repletion reverses heart failure with preserved ejection fraction. Circ Res. 2021;128:1629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Entman ML, Bornet EP, Van Winkle WB, Goldstein MA, Schwartz A. Association of glycoge no lysis with cardiac sarcoplasmic reticulum: II, Effect of glycogen depletion, deoxycholate solubilization and cardiac ischemia: evidence for a phorphorylase kinase membrane complex. J Mol Cell Cardiol. 1977;9:515–28. [DOI] [PubMed] [Google Scholar]
- [98].Zhou L, Salem JE, Saidel GM, Stanley WC, Cabrera ME. Mechanistic model of cardiac energy metabolism predicts localization of glycolysis to cytosolic subdomain during ischemia. Am J Physiol Heart Circ Physiol. 2005;288:H2400–11. [DOI] [PubMed] [Google Scholar]
- [99].Belke DD, Swanson E, Suarez J, Scott BT, Stenbit AE, Dillmann WH. Increased expression of SERCA in the hearts of transgenic mice results in increased oxidation of glucose. Am J Physiol Heart Circ Physiol. 2007;292:H1755–63. [DOI] [PubMed] [Google Scholar]
- [100].Xu KY, Zweier JL, Becker LC. Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ Res. 1995;77:88–97. [DOI] [PubMed] [Google Scholar]
- [101].Sardu C, Santulli G, Guerra G, Trotta MC, Santamaria M, Sacra C, et al. Modulation of SERCA in patients with persistent atrial fibrillation treated by Epicardial Thoracoscopic ablation: the CAMAF study. J Clin Med. 2020;9(2):544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Weiss J, Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest. 1985;75:436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Jeremy RW, Koretsune Y, Marban E, Becker LC. Relation between glycolysis and calcium homeostasis in postischemic myocardium. Circ Res. 1992;70:1180–90. [DOI] [PubMed] [Google Scholar]
- [104].Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122:624–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Turner JD, Gaspers LD, Wang G, Thomas AP. Uncoupling protein-2 modulates myocardial excitation-contraction coupling. Circ Res. 2010;106:730–8. [DOI] [PubMed] [Google Scholar]
- [106].Hegyi B, Fasoli A, Ko CY, Van BW, Alim CC, Shen EY, et al. CaMKII serine 280 O-GlcNAcylation links diabetic hyperglycemia to Proarrhythmia. Circ Res. 2021;129:98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ednie AR, Bennett ES. Intracellular O-linked glycosylation directly regulates cardiomyocyte L-type Ca(2+) channel activity and excitation-contraction coupling. Basic Res Cardiol. 2020;115:59. [DOI] [PubMed] [Google Scholar]
- [108].Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Shah KS, Fang JC. Sodium-glucose cotransporter 2 inhibitors in heart failure. Annu Rev Pharmacol Toxicol. 2021. In press. [DOI] [PubMed] [Google Scholar]
- [110].Ciccarelli M, Sorriento D, Coscioni E, Iaccarino G, Santulli G, Adrenergic Receptors. In: Schisler JC, Lang CH, Willis MS, editors. “Endocrinology of the heart in health and disease” - doi: 10.1016/B978-0-12-803111-7.00011-7. Elsevier; 2016. p. 285–315. [DOI] [Google Scholar]
- [111].Savarese JJ, Berkowitz BA. Beta-adrenergic receptor decrease in diabetic rat hearts. Life Sci. 1979;25:2075–8. [DOI] [PubMed] [Google Scholar]
- [112].Heyliger CE, Pierce GN, Singal PK, Beamish RE, Dhalla NS. Cardiac alpha- and beta-adrenergic receptor alterations in diabetic cardiomyopathy. Basic Res Cardiol. 1982;77:610–8. [DOI] [PubMed] [Google Scholar]
- [113].Gotzsche O. The adrenergic beta-receptor adenylate cyclase system in heart and lymphocytes from streptozotocin-diabetic rats. In vivo and in vitro evidence for a desensitized myocardial beta-receptor. Diabetes. 1983;32:1110–6. [DOI] [PubMed] [Google Scholar]
- [114].Austin CE, Chess-Williams R. Diabetes-induced changes in cardiac beta-adrenoceptor responsiveness: effects of aldose reductase inhibition with ponalrestat. Br J Pharmacol. 1991;102:478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Myers RB, Fomovsky GM, Lee S, Tan M, Wang BF, Patwari P, et al. Deletion of thioredoxin-interacting protein improves cardiac inotropic reserve in the streptozotocin-induced diabetic heart. Am J Physiol Heart Circ Physiol. 2016;310:H1748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Garris DR. The effects of estradiol and progesterone on reproductive tract atrophy and tissue adrenergic indices in diabetic C57BL/KsJ mice. Proc Soc Exp Biol Med. 1990;193:39–45. [DOI] [PubMed] [Google Scholar]
- [117].Schaffer SW, Allo S, Punna S, White T. Defective response to cAMP-dependent protein kinase in non-insulin-dependent diabetic heart. Am J Phys. 1991;261:E369–76. [DOI] [PubMed] [Google Scholar]
- [118].Zola BE, Miller B, Stiles GL, Rao PS, Sonnenblick EH, Fein FS. Heart rate control in diabetic rabbits: blunted response to isoproterenol. Am J Phys. 1988;255:E636–41. [DOI] [PubMed] [Google Scholar]
- [119].Lee JR, Zhang XJ, Lin BK, Reigel CE, Tenner TE Jr. Altered inotropic reactivity in diabetic rabbit right ventricular myocardium. Can J Physiol Pharmacol. 2004;82:903–10. [DOI] [PubMed] [Google Scholar]
- [120].Roth DA, White CD, Hamilton CD, Hall JL, Stanley WC. Adrenergic desensitization in left ventricle from streptozotocin diabetic swine. J Mol Cell Cardiol. 1995;27:2315–25. [DOI] [PubMed] [Google Scholar]
- [121].Stanley WC, Dore JJ, Hall JL, Hamilton CD, Pizzurro RD, Roth DA. Diabetes reduces right atrial beta-adrenergic signaling but not agonist stimulation of heart rate in swine. Can J Physiol Pharmacol. 2001;79:346–51. [PubMed] [Google Scholar]
- [122].Dincer UD, Bidasee KR, Guner S, Tay A, Ozcelikay AT, Altan VM. The effect of diabetes on expression of beta1-, beta2-, and beta3-adrenoreceptors in rat hearts. Diabetes. 2001;50:455–61. [DOI] [PubMed] [Google Scholar]
- [123].Amour J, Loyer X, Le Guen M, Mabrouk N, David JS, Camors E, et al. Altered contractile response due to increased beta3-adrenoceptor stimulation in diabetic cardiomyopathy: the role of nitric oxide synthase 1-derived nitric oxide. Anesthesiology. 2007;107:452–60. [DOI] [PubMed] [Google Scholar]
- [124].Bidasee KR, Zheng H, Shao CH, Parbhu SK, Rozanski GJ, Patel KP. Exercise training initiated after the onset of diabetes preserves myocardial function: effects on expression of beta-adrenoceptors. J Appl Physiol. 1985;2008(105):907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Erdogan BR, Michel MC, Arioglu-Inan E. Expression and signaling of beta-adrenoceptor subtypes in the diabetic heart. Cells. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Le Douairon Lahaye S, Rebillard A, Zguira MS, Malarde L, Saiag B, Gratas-Delamarche A, et al. Effects of exercise training combined with insulin treatment on cardiac NOS1 signaling pathways in type 1 diabetic rats. Mol Cell Biochem. 2011;347:53–62. [DOI] [PubMed] [Google Scholar]
- [127].Sharma V, Parsons H, Allard MF, McNeill JH. Metoprolol increases the expression of beta(3)-adrenoceptors in the diabetic heart: effects on nitric oxide signaling and forkhead transcription factor-3. Eur J Pharmacol. 2008;595:44–51. [DOI] [PubMed] [Google Scholar]
- [128].Mishra PK, Givvimani S, Metreveli N, Tyagi SC. Attenuation of beta2-adrenergic receptors and homocysteine metabolic enzymes cause diabetic cardiomyopathy. Biochem Biophys Res Commun. 2010;401:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].West TM, Wang Q, Deng B, Zhang Y, Barbagallo F, Reddy GR, et al. Phosphodiesterase 5 associates with beta2 adrenergic receptor to modulate cardiac function in type 2 diabetic hearts. J Am Heart Assoc. 2019;8:e012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Yu Z, Quamme GA, McNeill JH. Depressed [Ca2+]i responses to isoproterenol and cAMP in isolated cardiomyocytes from experimental diabetic rats. Am J Phys. 1994;266:H2334–42. [DOI] [PubMed] [Google Scholar]
- [131].Tuncay E, Okatan EN, Vassort G, Turan B. Beta-blocker timolol prevents arrhythmogenic Ca(2)(+) release and normalizes Ca(2)(+) and Zn(2)(+) dyshomeostasis in hyperglycemic rat heart. PLoS One. 2013;8:e71014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Santulli G, Lewis D, des Georges A, Marks AR, Frank J.. Ryanodine receptor structure and function in health and disease. Subcell Biochem. 2018;87:329–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Shao CH, Wehrens XH, Wyatt TA, Parbhu S, Rozanski GJ, Patel KP, et al. Exercise training during diabetes attenuates cardiac ryanodine receptor dysregulation. J Appl Physiol. 1985;2009(106):1280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wang Q, Liu Y, Fu Q, Xu B, Zhang Y, Kim S, et al. Inhibiting insulin-mediated beta2-adrenergic receptor activation prevents Diabetes-associated cardiac dysfunction. Circulation. 2017;135:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sorriento D, Santulli G, Franco A, Cipolletta E, Napolitano L, Gambardella J, et al. Integrating GRK2 and NFkappaB in the pathophysiology of cardiac hypertrophy. J Cardiovasc Transl Res. 2015;8:493–502. [DOI] [PubMed] [Google Scholar]
- [136].Murga C, Arcones AC, Cruces-Sande M, Briones AM, Salaices M, Mayor F Jr G protein-coupled receptor kinase 2 (GRK2) as a potential therapeutic target in cardiovascular and metabolic diseases. Front Pharmacol. 2019;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Salazar NC, Vallejos X, Siryk A, Rengo G, Cannavo A, Liccardo D, et al. GRK2 blockade with betaARKct is essential for cardiac beta2-adrenergic receptor signaling towards increased contractility. Cell Commun Signal. 2013;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Santulli G, Campanile A, Spinelli L, Assante di Panzillo E, Ciccarelli M, Trimarco B, et al. G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am J Cardiol. 2011;107:1125–30. [DOI] [PubMed] [Google Scholar]
- [139].Cipolletta E, Campanile A, Santulli G, Sanzari E, Leosco D, Campiglia P, et al. The G protein coupled receptor kinase 2 plays an essential role in beta-adrenergic receptor-induced insulin resistance. Cardiovasc Res. 2009;84:407–15. [DOI] [PubMed] [Google Scholar]
- [140].Lai S, Fu X, Yang S, Zhang S, Lin Q, Zhang M, et al. G protein-coupled receptor kinase-2: a potential biomarker for early diabetic cardiomyopathy. J Diabetes. 2020;12:247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Cipolletta E, Gambardella J, Fiordelisi A, Del Giudice C, Di Vaia E, Ciccarelli M, et al. Antidiabetic and Cardioprotective effects of pharmacological inhibition of GRK2 in db/db mice. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Woodall MC, Woodall BP, Gao E, Yuan A, Koch WJ. Cardiac fibroblast GRK2 deletion enhances contractility and remodeling following ischemia/reperfusion injury. Circ Res. 2016;119:1116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–48. [DOI] [PubMed] [Google Scholar]
- [144].Schwenk RW, Luiken JJ, Bonen A, Glatz JFC. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res. 2008;79(2):249–58. [DOI] [PubMed] [Google Scholar]
- [145].Evangelista I, Nuti R, Picchioni T, Dotta F, Palazzuoli A. Molecular dysfunction and phenotypic derangement in diabetic cardiomyopathy. Int J Mol Sci. 2019;20(13):3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].De Rosa M, Gambardella J, Shu J, Santulli G. Dietary fat is a key determinant in balancing mitochondrial dynamics in heart failure: a novel mechanism underlying the obesity paradox. Cardiovasc Res. 2018;114:925–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Guo Y, Wang Z, Qin X, Xu J, Hou Z, Yang H, et al. Enhancing fatty acid utilization ameliorates mitochondrial fragmentation and cardiac dysfunction via rebalancing optic atrophy 1 processing in the failing heart. Cardiovasc Res. 2018;114:979–91. [DOI] [PubMed] [Google Scholar]
- [148].Tuleta I, Frangogiannis NG. Diabetic fibrosis. Biochim Biophys Acta Mol basis Dis. 2021;1867:166044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Bell DS. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949–51. [DOI] [PubMed] [Google Scholar]
- [150].Ding W, Chang WG, Guo XC, Liu Y, Xiao DD, Ding D, et al. Exenatide protects against cardiac dysfunction by attenuating oxidative stress in the diabetic mouse heart. Front Endocrinol. 2019;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat Inflamm. 2010;2010:453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Chen F, Lai J, Zhu Y, He M, Hou H, Wang J, et al. Cardioprotective effect of decorin in type 2 diabetes. Front Endocrinol. 2020;11:479258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–65. [DOI] [PubMed] [Google Scholar]
- [154].Aragno M, Mastrocola R, Alloatti G, Vercellinatto I, Bardini P, Geuna S, et al. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology. 2008;149:380–8. [DOI] [PubMed] [Google Scholar]
- [155].Purnomo Y, Piccart Y, Coenen T, Prihadi JS, Lijnen PJ. Oxidative stress and transforming growth factor-beta1-induced cardiac fibrosis. Cardiovasc Hematol Disord Drug Targets. 2013;13:165–72. [DOI] [PubMed] [Google Scholar]
- [156].Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Crisafulli A, Pagliaro P, Roberto S, Cugusi L, Mercuro G, Lazou A, et al. Diabetic cardiomyopathy and ischemic heart disease: prevention and therapy by exercise and conditioning. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Purnomo Y, Piccart Y, Coenen T, Prihadi JS, Lijnen PJCJ, Targets HD-D. Oxidative stress and transforming growth factor-β1-induced cardiac fibrosis. . 2013;13:165–72. [DOI] [PubMed] [Google Scholar]
- [159].Morelli MB, Shu J, Sardu C, Matarese A, Santulli G. Cardiosomal microRNAs are essential in post-infarction myofibroblast phenoconversion. Int J Mol Sci. 2019;21(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Gibb AA, Lazaropoulos MP, Elrod JW. Myofibroblasts and fibrosis: mitochondrial and metabolic control of cellular differentiation. Circ Res. 2020;127:427–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Westermann D, Rutschow S, Jäger S, Linderer A, Anker S, Riad A, et al. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. 2007;56(3):641–6. [DOI] [PubMed] [Google Scholar]
- [162].Sharma A, Tate M, Mathew G, Vince JE, Ritchie RH, de Haan JB. Oxidative stress and NLRP3-Inflammasome activity as significant drivers of diabetic cardiovascular complications: therapeutic implications. Front Physiol. 2018;9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Luo B, Huang F, Liu Y, Liang Y, Wei Z, Ke H, et al. NLRP3 inflammasome as a molecular marker in diabetic cardiomyopathy. Front Physiol. 2017;8:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Zheng D, Liwinski T, Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH. Overexpression of the sarcoplasmic reticulum Ca2+ ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes. 2002;51:1166–71. [DOI] [PubMed] [Google Scholar]
- [167].Santulli G, Lewis DR, Marks AR. Physiology and pathophysiology of excitation-contraction coupling: the functional role of ryanodine receptor. J Muscle Res Cell Motil. 2017;38:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Gambardella J, Trimarco B, Iaccarino G, Santulli G. New insights in cardiac calcium handling and excitation-contraction coupling. Adv Exp Med Biol. 2018;1067:373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Starčević JN, Janić M, Šabovič M. Molecular mechanisms responsible for diastolic dysfunction in diabetes mellitus patients. Int J Mol Sci. 2019;20(5):1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Lacombe VA, Viatchenko-Karpinski S, Terentyev D, Sridhar A, Emani S, Bonagura JD, et al. Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes. Am J Phys Regul Integr Comp Phys. 2007;293:R1787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Hu L, Ding M, Tang D, Gao E, Li C, Wang K, et al. Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics. 2019;9:3687–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015;112:11389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Morciano G, Vitto VAM, Bouhamida E, Giorgi C, Pinton P. Mitochondrial bioenergetics and dynamism in the failing heart. Life. 2021;11(5):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Morales PE, Arias-Duran C, Avalos-Guajardo Y, Aedo G, Verdejo HE, Parra V, et al. Emerging role of mitophagy in cardiovascular physiology and pathology. Mol Asp Med. 2020;71:100822. [DOI] [PubMed] [Google Scholar]
- [175].Xu HX, Cui SM, Zhang YM, Ren J. Mitochondrial ca(2+) regulation in the etiology of heart failure: physiological and pathophysiological implications. Acta Pharmacol Sin. 2020;41:1301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Strey CH, Young JM, Molyneux SL, George PM, Florkowski CM, Scott RS, et al. Endothelium-ameliorating effects of statin therapy and coenzyme Q10 reductions in chronic heart failure. Atherosclerosis. 2005;179:201–6. [DOI] [PubMed] [Google Scholar]
- [177].Ragot H, Monfort A, Baudet M, Azibani F, Fazal L, Merval R, et al. Loss of Notch3 signaling in vascular smooth muscle cells promotes severe heart failure upon hypertension. Hypertension. 2016;68:392–400. [DOI] [PubMed] [Google Scholar]
- [178].Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. Steno Hypothesis Diabetol. 1989;32:219–26. [DOI] [PubMed] [Google Scholar]
- [179].Volpe M. Microalbuminuria screening in patients with hypertension: recommendations for clinical practice. Int J Clin Pract. 2008;62:97–108. [DOI] [PubMed] [Google Scholar]
- [180].Mogensen CE. Microalbuminuria, blood pressure and diabetic renal disease: origin and development of ideas. Diabetologia. 1999;42:263–85. [DOI] [PubMed] [Google Scholar]
- [181].Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–60. [DOI] [PubMed] [Google Scholar]
- [183].Cunha-Vaz J, Faria de Abreu JR, Campos AJ. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975;59:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Parving HH, Noer I, Deckert T, Evrin PE, Nielsen SL, Lyngsoe J, et al. The effect of metabolic regulation on microvascular permeability to small and large molecules in short-term juvenile diabetics. Diabetologia. 1976;12:161–6. [DOI] [PubMed] [Google Scholar]
- [185].Parving HH, Nielsen FS, Bang LE, Smidt UM, Svendsen TL, Chen JW, et al. Macro-microangiopathy and endothelial dysfunction in NIDDM patients with and without diabetic nephropathy. Diabetologia. 1996;39:1590–7. [DOI] [PubMed] [Google Scholar]
- [186].Bollinger A, Frey J, Jager K, Furrer J, Seglias J, Siegenthaler W. Patterns of diffusion through skin capillaries in patients with long-term diabetes. N Engl J Med. 1982;307:1305–10. [DOI] [PubMed] [Google Scholar]
- [187].Chen S, Apostolova MD, Cherian MG, Chakrabarti S. Interaction of endothelin-1 with vasoactive factors in mediating glucose-induced increased permeability in endothelial cells. Lab Investig. 2000;80:1311–21. [DOI] [PubMed] [Google Scholar]
- [188].Knudsen ST, Bek T, Poulsen PL, Hove MN, Rehling M, Mogensen CE. Macular edema reflects generalized vascular hyperpermeability in type 2 diabetic patients with retinopathy. Diabetes Care. 2002;25:2328–34. [DOI] [PubMed] [Google Scholar]
- [189].Perrin RM, Harper SJ, Bates DO. A role for the endothelial glycocalyx in regulating microvascular permeability in diabetes mellitus. Cell Biochem Biophys. 2007;49:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Vervoort G, Lutterman JA, Smits P, Berden JH, Wetzels JF. Transcapillary escape rate of albumin is increased and related to haemodynamic changes in normo-albuminuric type 1 diabetic patients. J Hypertens. 1999;17:1911–6. [DOI] [PubMed] [Google Scholar]
- [191].Williamson JR, Chang K, Tilton RG, Prater C, Jeffrey JR, Weigel C, et al. Increased vascular permeability in spontaneously diabetic BB/W rats and in rats with mild versus severe streptozocin-induced diabetes. Prevention by aldose reductase inhibitors and castration. Diabetes. 1987;36:813–21. [DOI] [PubMed] [Google Scholar]
- [192].Yuan SY, Ustinova EE, Wu MH, Tinsley JH, Xu W, Korompai FL, et al. Protein kinase C activation contributes to microvascular barrier dysfunction in the heart at early stages of diabetes. Circ Res. 2000;87:412–7. [DOI] [PubMed] [Google Scholar]
- [193].Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Maezawa Y, Takemoto M, Yokote K. Cell biology of diabetic nephropathy: roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Investig. 2015;6:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Kumar Vr S, Darisipudi MN, Steiger S, Devarapu SK, Tato M, Kukarni OP, et al. Cathepsin S cleavage of protease-activated Receptor-2 on endothelial cells promotes microvascular Diabetes complications. J Am Soc Nephrol. 2016;27:1635–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Feistritzer C, Lenta R, Riewald M. Protease-activated receptors-1 and -2 can mediate endothelial barrier protection: role in factor Xa signaling. J Thromb Haemost. 2005;3:2798–805. [DOI] [PubMed] [Google Scholar]
- [197].Heygate KM, Lawrence IG, Bennett MA, Thurston H. Impaired endothelium-dependent relaxation in isolated resistance arteries of spontaneously diabetic rats. Br J Pharmacol. 1995;116:3251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Brodsky SV, Morrishow AM, Dharia N, Gross SS, Goligorsky MS. Glucose scavenging of nitric oxide. Am J Physiol Ren Physiol. 2001;280:F480–6. [DOI] [PubMed] [Google Scholar]
- [199].Goligorsky MS, Chen J, Brodsky S. Workshop: endothelial cell dysfunction leading to diabetic nephropathy : focus on nitric oxide. Hypertension. 2001;37:744–8. [DOI] [PubMed] [Google Scholar]
- [200].Brodsky SV, Gao S, Li H, Goligorsky MS. Hyperglycemic switch from mitochondrial nitric oxide to superoxide production in endothelial cells. Am J Physiol Heart Circ Physiol. 2002;283:H2130–9. [DOI] [PubMed] [Google Scholar]
- [201].Tessari P, Cecchet D, Cosma A, Vettore M, Coracina A, Millioni R, et al. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes. 2010;59:2152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Chander PN, Gealekman O, Brodsky SV, Elitok S, Tojo A, Crabtree M, et al. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen. J Am Soc Nephrol. 2004;15:2391–403. [DOI] [PubMed] [Google Scholar]
- [204].Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. [DOI] [PubMed] [Google Scholar]
- [205].Zou MH. Peroxynitrite and protein tyrosine nitration of prostacyclin synthase. Prostaglandins Other Lipid Mediat. 2007;82:119–27. [DOI] [PubMed] [Google Scholar]
- [206].Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Goligorsky MS. Vascular endothelium in diabetes. Am J Physiol Ren Physiol. 2017;312:F266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Cassese A, Esposito I, Fiory F, Barbagallo AP, Paturzo F, Mirra P, et al. In skeletal muscle advanced glycation end products (AGEs) inhibit insulin action and induce the formation of multimolecular complexes including the receptor for AGEs. J Biol Chem. 2008;283:36088–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Jannapureddy S, Sharma M, Yepuri G, Schmidt AM, Ramasamy R. Aldose reductase: an emerging target for development of interventions for diabetic cardiovascular complications. Front Endocrinol. 2021;12:636267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Levick SP, Widiapradja A. The diabetic cardiac fibroblast: mechanisms underlying phenotype and function. Int J Mol Sci. 2020;21(3):970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Salminen A, Kaarniranta K. SIRT1: regulation of longevity via autophagy. Cell Signal. 2009;21:1356–60. [DOI] [PubMed] [Google Scholar]
- [213].Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Patschan S, Goligorsky MS. Autophagy: the missing link between non-enzymatically glycated proteins inducing apoptosis and premature senescence of endothelial cells? Autophagy. 2008;4:521–3. [DOI] [PubMed] [Google Scholar]
- [215].Patschan S, Chen J, Gealekman O, Krupincza K, Wang M, Shu L, et al. Mapping mechanisms and charting the time course of premature cell senescence and apoptosis: lysosomal dysfunction and ganglioside accumulation in endothelial cells. Am J Physiol Ren Physiol. 2008;294:F100–9. [DOI] [PubMed] [Google Scholar]
- [216].Patschan S, Chen J, Polotskaia A, Mendelev N, Cheng J, Patschan D, et al. Lipid mediators of autophagy in stress-induced premature senescence of endothelial cells. Am J Physiol Heart Circ Physiol. 2008;294(3):H1119–29. [DOI] [PubMed] [Google Scholar]
- [217].Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, et al. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol. 2012;180:973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Gallagher PE, Ferrario CM, Tallant EA JAJoP-H. Physiology C Regulation of ACE2 in cardiac myocytes and fibroblasts, 295; 2008. (H2373–H9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Ock S, Lee WS, Ahn J, Kim HM, Kang H, Kim HS, et al. Deletion of IGF-1 receptors in cardiomyocytes attenuates cardiac aging in male mice. Endocrinology. 2016;157:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Stokes J 3rd, Kannel WB, Wolf PA, Cupples LA, D’Agostino RB. The relative importance of selected risk factors for various manifestations of cardiovascular disease among men and women from 35 to 64 years old: 30 years of follow-up in the Framingham study. Circulation. 1987;75:V65–73. [PubMed] [Google Scholar]
- [221].Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. [DOI] [PubMed] [Google Scholar]
- [222].LoGerfo FW, Coffman JD. Current concepts. vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med. 1984;311:1615–9. [DOI] [PubMed] [Google Scholar]
- [223].Kansakar U, Jankauskas SS, Gambardella J, Santulli G. Targeting the phenotypic switch of vascular smooth muscle cells to tackle atherosclerosis. Atherosclerosis. 2021;324:117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Jaminon A, Reesink K, Kroon A, Schurgers L. The role of vascular smooth muscle cells in arterial remodeling: focus on calcification-related processes. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Faries PL, Rohan DI, Takahara H, Wyers MC, Contreras MA, Quist WC, et al. Human vascular smooth muscle cells of diabetic origin exhibit increased proliferation, adhesion, and migration. J Vasc Surg. 2001;33:601–7. [DOI] [PubMed] [Google Scholar]
- [226].De Luca G, Dirksen MT, Spaulding C, Kelbaek H, Schalij M, Thuesen L, et al. Impact of diabetes on long-term outcome after primary angioplasty: insights from the DESERT cooperation. Diabetes Care. 2013;36:1020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Mone P, Gambardella J, Minicucci F, Lombardi A, Mauro C, Santulli G. Hyperglycemia drives stent restenosis in STEMI patients. Diabetes Care. 2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Yildirim SS, Akman D, Catalucci D, Turan B. Relationship between downregulation of miRNAs and increase of oxidative stress in the development of diabetic cardiac dysfunction: junctin as a target protein of miR-1. Cell Biochem Biophys. 2013;67(3):1397–408. [DOI] [PubMed] [Google Scholar]
- [229].Reddy MA, Das S, Zhuo C, Jin W, Wang M, Lanting L, et al. Regulation of vascular smooth muscle cell dysfunction under diabetic conditions by miR-504. Arterioscler Thromb Vasc Biol. 2016;36:864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Xu Q, Liang Y, Liu X, Zhang C, Liu X, Li H, et al. miR132 inhibits high glucoseinduced vascular smooth muscle cell proliferation and migration by targeting E2F5. Mol Med Rep. 2019;20:2012–20. [DOI] [PubMed] [Google Scholar]
- [231].Yang J, Chen L, Ding J, Fan Z, Li S, Wu H, et al. MicroRNA-24 inhibits high glucose-induced vascular smooth muscle cell proliferation and migration by targeting HMGB1. Gene. 2016;586:268–73. [DOI] [PubMed] [Google Scholar]
- [232].Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. [DOI] [PubMed] [Google Scholar]
- [233].Yoshida T, Gan Q, Owens GK. Kruppel-like factor 4, Elk-1, and histone deacetylases cooperatively suppress smooth muscle cell differentiation markers in response to oxidized phospholipids. Am J Phys Cell Phys. 2008;295:C1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Hien TT, Garcia-Vaz E, Stenkula KG, Sjogren J, Nilsson J, Gomez MF, et al. MicroRNA-dependent regulation of KLF4 by glucose in vascular smooth muscle. J Cell Physiol. 2018;233:7195–205. [DOI] [PubMed] [Google Scholar]
- [236].Fernandez-Velasco M, Ruiz-Hurtado G, Gomez AM, Rueda A. Ca(2+) handling alterations and vascular dysfunction in diabetes. Cell Calcium. 2014;56:397–407. [DOI] [PubMed] [Google Scholar]
- [237].Searls YM, Loganathan R, Smirnova IV, Stehno-Bittel L. Intracellular Ca2+ regulating proteins in vascular smooth muscle cells are altered with type 1 diabetes due to the direct effects of hyperglycemia. Cardiovasc Diabetol. 2010;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–7. [DOI] [PubMed] [Google Scholar]
- [239].Essin K, Gollasch M. Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle. J Biomed Biotechnol. 2009;2009:135249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Ma L, Zhu B, Chen X, Liu J, Guan Y, Ren J. Abnormalities of sarcoplasmic reticulum Ca2+ mobilization in aortic smooth muscle cells from streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol. 2008;35:568–73. [DOI] [PubMed] [Google Scholar]
- [241].Rueda A, Fernandez-Velasco M, Benitah JP, Gomez AM. Abnormal Ca2+ spark/STOC coupling in cerebral artery smooth muscle cells of obese type 2 diabetic mice. PLoS One. 2013;8:e53321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Abbate R, Mannucci E, Cioni G, Fatini C, Marcucci R. Diabetes and sex: from pathophysiology to personalized medicine. Intern Emerg Med. 2012;7(Suppl. 3):S215–9. [DOI] [PubMed] [Google Scholar]
- [243].Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci U S A. 2007;104:12565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Velmurugan GV, White C. Calcium homeostasis in vascular smooth muscle cells is altered in type 2 diabetes by Bcl-2 protein modulation of InsPSR calcium release channels. Am J Physiol Heart Circ Physiol. 2012;302:H124–34. [DOI] [PubMed] [Google Scholar]
- [245].White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Ruiz E, Gordillo-Moscoso A, Padilla E, Redondo S, Rodriguez E, Reguillo F, et al. Human vascular smooth muscle cells from diabetic patients are resistant to induced apoptosis due to high Bcl-2 expression. Diabetes. 2006;55:1243–51. [DOI] [PubMed] [Google Scholar]
- [247].Hall JL, Matter CM, Wang X, Gibbons GH. Hyperglycemia inhibits vascular smooth muscle cell apoptosis through a protein kinase C-dependent pathway. Circ Res. 2000;87:574–80. [DOI] [PubMed] [Google Scholar]
- [248].Cosson E, Bringuier AF, Paries J, Guillot R, Vaysse J, Attali JR, et al. Fas/Fas-ligand pathway is impaired in patients with type 2 diabetes. Influence of hypertension and insulin resistance. Diabetes Metab. 2005;31:47–54. [DOI] [PubMed] [Google Scholar]
- [249].Henry RM, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Kamp O, et al. Carotid arterial remodeling: a maladaptive phenomenon in type 2 diabetes but not in impaired glucose metabolism: the Hoorn study. Stroke. 2004;35:671–6. [DOI] [PubMed] [Google Scholar]
- [250].Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest. 1996;97:1767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–66. [DOI] [PubMed] [Google Scholar]
- [252].Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes. 2003;52:2562–9. [DOI] [PubMed] [Google Scholar]
- [253].Wang YW, Sun GD, Sun J, Liu SJ, Wang J, Xu XH, et al. Spontaneous type 2 diabetic rodent models. J Diabetes Res. 2013;2013:401723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Islam MS, Wilson RD. Experimentally induced rodent models of type 2 diabetes. Methods Mol Biol. 2012;933:161–74. [DOI] [PubMed] [Google Scholar]
- [255].Skovso S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014;5:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Mansor LS, Gonzalez ER, Cole MA, Tyler DJ, Beeson JH, Clarke K, et al. Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin. Cardiovasc Diabetol. 2013;12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Vatandoust N, Rami F, Salehi AR, Khosravi S, Dashti G, Eslami G, et al. Novel high-fat diet formulation and Streptozotocin treatment for induction of prediabetes and type 2 Diabetes in rats. Adv Biomed Res. 2018;7:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Guo XX, Wang Y, Wang K, Ji BP, Zhou F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J Zhejiang Univ Sci B. 2018;19:559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–20. [DOI] [PubMed] [Google Scholar]
- [260].Watts LM, Manchem VP, Leedom TA, Rivard AL, McKay RA, Bao D, et al. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes. 2005;54:1846–53. [DOI] [PubMed] [Google Scholar]
- [261].Sahin K, Onderci M, Tuzcu M, Ustundag B, Cikim G, Ozercan IH, et al. Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: the fat-fed, streptozotocin-treated rat. Metabolism. 2007;56:1233–40. [DOI] [PubMed] [Google Scholar]
- [262].Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, et al. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49:1390–4. [DOI] [PubMed] [Google Scholar]
- [263].Zhang MH, Feng L, Zhu MM, Gu JF, Jiang J, Cheng XD, et al. The anti-inflammation effect of Moutan cortex on advanced glycation end products-induced rat mesangial cells dysfunction and high-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats. J Ethnopharmacol. 2014;151:591–600. [DOI] [PubMed] [Google Scholar]
- [264].Glastras SJ, Chen H, Teh R, McGrath RT, Chen J, Pollock CA, et al. Mouse models of diabetes, obesity and related kidney disease. PLoS One. 2016;11:e0162131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Chanseaume E, Giraudet C, Gryson C, Walrand S, Rousset P, Boirie Y, et al. Enhanced muscle mixed and mitochondrial protein synthesis rates after a high-fat or high-sucrose diet. Obesity (Silver Spring). 2007;15:853–9. [DOI] [PubMed] [Google Scholar]
- [266].Rasool S, Geetha T, Broderick TL, Babu JR. High fat with high sucrose diet leads to obesity and induces Myodegeneration. Front Physiol. 2018;9:1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Yasutake Y, Mizokami A, Kawakubo-Yasukochi T, Chishaki S, Takahashi I, Takeuchi H, et al. Long-term oral administration of osteocalcin induces insulin resistance in male mice fed a high-fat, high-sucrose diet. Am J Physiol Endocrinol Metab. 2016;310 (E662–E75). [DOI] [PubMed] [Google Scholar]
- [268].Thibodeau JF, Holterman CE, Burger D, Read NC, Reudelhuber TL, Kennedy CR. A novel mouse model of advanced diabetic kidney disease. PLoS One. 2014;9:e113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Teiken JM, Audettey JL, Laturnus DI, Zheng S, Epstein PN, Carlson EC. Podocyte loss in aging OVE26 diabetic mice. Anat Rec. 2008;291:114–21. [DOI] [PubMed] [Google Scholar]
- [270].Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, et al. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes. 2004;53:3248–57. [DOI] [PubMed] [Google Scholar]
- [271].Dadi PK, Vierra NC, Ustione A, Piston DW, Colbran RJ, Jacobson DA. Inhibition of pancreatic beta-cell Ca2+/calmodulin-dependent protein kinase II reduces glucose-stimulated calcium influx and insulin secretion, impairing glucose tolerance. J Biol Chem. 2014;289:12435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Epstein PN, Overbeek PA, Means AR. Calmodulin-induced early-onset diabetes in transgenic mice. Cell. 1989;58:1067–73. [DOI] [PubMed] [Google Scholar]
- [273].Epstein PN, Ribar TJ, Decker GL, Yaney G, Means AR. Elevated beta-cell calmodulin produces a unique insulin secretory defect in transgenic mice. Endocrinology. 1992;130:1387–93. [DOI] [PubMed] [Google Scholar]
- [274].Song Y, Du Y, Prabhu SD, Epstein PN. Diabetic cardiomyopathy in OVE26 mice shows mitochondrial ROS production and divergence between in vivo and in vitro contractility. Rev Diabet Stud. 2007;4:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Tang X, Jiang S, Zhang J, Zhou S, Zheng Y. Sex differences in progression of diabetic cardiomyopathy in OVE26 type 1 diabetic mice. Oxidative Med Cell Longev. 2020;2020:6961348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10:131–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Skowronski AA, Ravussin Y, Leibel RL, LeDuc CA. Energy homeostasis in leptin deficient Lepob/Ob mice. PLoS One. 2017;12:e0189784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Green ED, Maffei M, Braden VV, Proenca R, DeSilva U, Zhang Y, et al. The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7. Genome Res. 1995;5:5–12. [DOI] [PubMed] [Google Scholar]
- [279].Leibel RL, Chung WK, Chua SC Jr. The molecular genetics of rodent single gene obesities. J Biol Chem. 1997;272:31937–40. [DOI] [PubMed] [Google Scholar]
- [280].Ross SA, Gulve EA, Wang M. Chemistry and biochemistry of type 2 diabetes. Chem Rev. 2004;104:1255–82. [DOI] [PubMed] [Google Scholar]
- [281].Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–8. [DOI] [PubMed] [Google Scholar]
- [282].Marfella R, Amarelli C, Cacciatore F, Balestrieri ML, Mansueto G, D’Onofrio N, et al. Lipid accumulation in hearts transplanted From nondiabetic donors to diabetic recipients. J Am Coll Cardiol. 2020;75:1249–62. [DOI] [PubMed] [Google Scholar]
- [283].Wichi R, Malfitano C, Rosa K, De Souza SB, Salemi V, Mostarda C, et al. Noninvasive and invasive evaluation of cardiac dysfunction in experimental diabetes in rodents. Cardiovasc Diabetol. 2007;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–9. [DOI] [PubMed] [Google Scholar]
- [285].Sankaralingam S, Abo Alrob O, Zhang L, Jaswal JS, Wagg CS, Fukushima A, et al. Lowering body weight in obese mice with diastolic heart failure improves cardiac insulin sensitivity and function: implications for the obesity paradox. Diabetes. 2015;64:1643–57. [DOI] [PubMed] [Google Scholar]
- [286].Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant Ob/Ob mouse hearts. Diabetes. 2004;53:2366–74. [DOI] [PubMed] [Google Scholar]
- [287].Wang X, West JA, Murray AJ, Griffin JL. Comprehensive metabolic profiling of age-related mitochondrial dysfunction in the high-fat-fed Ob/Ob mouse heart. J Proteome Res. 2015;14:2849–62. [DOI] [PubMed] [Google Scholar]
- [288].Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60:2935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Nishina PM, Lowe S, Wang J, Paigen B. Characterization of plasma lipids in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism. 1994;43:549–53. [DOI] [PubMed] [Google Scholar]
- [290].Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000;49:22–31. [DOI] [PubMed] [Google Scholar]
- [291].Belke DD, Severson DL. Diabetes in mice with monogenic obesity: the db/db mouse and its use in the study of cardiac consequences. Methods Mol Biol. 2012;933:47–57. [DOI] [PubMed] [Google Scholar]
- [292].Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002;283:H976–82. [DOI] [PubMed] [Google Scholar]
- [293].Alex L, Russo I, Holoborodko V, Frangogiannis NG. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2018;315 (H934–H49). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Riehle C, Bauersachs J. Of mice and men: models and mechanisms of diabetic cardiomyopathy. Basic Res Cardiol. 2018;114:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Substitution at codon 269 (glutamine –> proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun. 1996;224:597–604. [DOI] [PubMed] [Google Scholar]
- [297].Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18–9. [DOI] [PubMed] [Google Scholar]
- [298].Yorek MA. Alternatives to the Streptozotocin-diabetic rodent. Int Rev Neurobiol. 2016;127:89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Yokoi N, Hoshino M, Hidaka S, Yoshida E, Beppu M, Hoshikawa R, et al. A novel rat model of type 2 Diabetes: the Zucker fatty Diabetes mellitus ZFDM rat. J Diabetes Res. 2013;2013:103731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Clark JB, Palmer CJ, Shaw WN. The diabetic Zucker fatty rat. Proc Soc Exp Biol Med. 1983;173:68–75. [DOI] [PubMed] [Google Scholar]
- [301].Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2005;288:H2102–10. [DOI] [PubMed] [Google Scholar]
- [302].Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009;2:454–66. [DOI] [PubMed] [Google Scholar]
- [303].Oltman CL, Coppey LJ, Gellett JS, Davidson EP, Lund DD, Yorek MA. Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty and Zucker rats. Am J Physiol Endocrinol Metab. 2005;289:E113–22. [DOI] [PubMed] [Google Scholar]
- [304].Luiken JJ, Arumugam Y, Dyck DJ, Bell RC, Pelsers MM, Turcotte LP, et al. Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem. 2001;276:40567–73. [DOI] [PubMed] [Google Scholar]
- [305].Conti M, Renaud IM, Poirier B, Michel O, Belair MF, Mandet C, et al. High levels of myocardial antioxidant defense in aging nondiabetic normotensive Zucker obese rats. Am J Phys Regul Integr Comp Phys. 2004;286:R793–800. [DOI] [PubMed] [Google Scholar]
- [306].Vincent HK, Powers SK, Dirks AJ, Scarpace PJ. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord. 2001;25:378–88. [DOI] [PubMed] [Google Scholar]
- [307].Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, et al. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–95. [DOI] [PubMed] [Google Scholar]
- [308].Luiken JJ, Willems J, van der Vusse GJ, Glatz JF. Electrostimulation enhances FAT/CD36-mediated long-chain fatty acid uptake by isolated rat cardiac myocytes. Am J Physiol Endocrinol Metab. 2001;281:E704–12. [DOI] [PubMed] [Google Scholar]
- [309].Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–64. [DOI] [PubMed] [Google Scholar]
- [310].van den Brom CE, Bosmans JW, Vlasblom R, Handoko LM, Huisman MC, Lubberink M, et al. Diabetic cardiomyopathy in Zucker diabetic fatty rats: the forgotten right ventricle. Cardiovasc Diabetol. 2010;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–700. [DOI] [PubMed] [Google Scholar]
- [313].Golfman LS, Wilson CR, Sharma S, Burgmaier M, Young ME, Guthrie PH, et al. Activation of PPARgamma enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol Endocrinol Metab. 2005;289:E328–36. [DOI] [PubMed] [Google Scholar]
- [314].Chatham JC, Seymour AM. Cardiac carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc Res. 2002;55:104–12. [DOI] [PubMed] [Google Scholar]
- [315].Lundbaek K. Diabetic angiopathy: a specific vascular disease. Lancet. 1954;266:377–9. [DOI] [PubMed] [Google Scholar]
- [316].Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. [DOI] [PubMed] [Google Scholar]
- [317].Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil J-G. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes. Importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24:5–10. [DOI] [PubMed] [Google Scholar]
- [318].Gulsin GS, Athithan L, McCann GP. Diabetic cardiomyopathy: prevalence, determinants and potential treatments. Ther Adv Endocrinol Metab. 2019;10 (2042018819834869). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Shu J, Matarese A, Santulli G. Diabetes, body fat, skeletal muscle, and hypertension: the ominous chiasmus? J Clin Hypertens. 2019;21:239–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Altara R, Giordano M, Norden ES, Cataliotti A, Kurdi M, Bajestani SN, et al. Targeting obesity and Diabetes to treat heart failure with preserved ejection fraction. Front Endocrinol. 2017;8:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–67. [DOI] [PubMed] [Google Scholar]
- [322].Maisch B, Alter P, Pankuweit S. Diabetic cardiomyopathy–fact or fiction? Herz. 2011;36:102–15. [DOI] [PubMed] [Google Scholar]
- [323].Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy. Circ Res. 2018;122:624–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88. [DOI] [PubMed] [Google Scholar]
- [325].Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, et al. EuroHeart failure survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–36. [DOI] [PubMed] [Google Scholar]
- [326].Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- [327].Lindman BR, Davila-Roman VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol. 2014;64:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. [DOI] [PubMed] [Google Scholar]
- [329].Meagher P, Adam M, Civitarese R, Bugyei-Twum A, Connelly KA. Heart failure with preserved ejection fraction in Diabetes: mechanisms and management. Can J Cardiol. 2018;34:632–43. [DOI] [PubMed] [Google Scholar]
- [330].Zeng H, Chen JX. Microvascular rarefaction and heart failure with preserved ejection fraction. Front Cardiovasc Med. 2019;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- [332].Seferovic PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36:1718–27 (27a-27c). [DOI] [PubMed] [Google Scholar]
- [333].Yap J, Tay WT, Teng TK, Anand I, Richards AM, Ling LH, et al. Association of Diabetes Mellitus on cardiac remodeling, quality of life, and clinical outcomes in heart failure with reduced and preserved ejection fraction. J Am Heart Assoc. 2019;8:e013114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Borghetti G, von Lewinski D, Eaton DM, Sourij H, Houser SR, Wallner M. Diabetic cardiomyopathy: current and future therapies. Beyond Glycemic Control Front Physiol. 2018;9:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Brooks BA, Franjic B, Ban CR, Swaraj K, Yue DK, Celermajer DS, et al. Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes Obes Metab. 2008;10:739–46. [DOI] [PubMed] [Google Scholar]
- [336].Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–5. [DOI] [PubMed] [Google Scholar]
- [337].From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Fonarow GC, Srikanthan P. Diabetic cardiomyopathy. Endocrinol Metab Clin N Am. 2006;35(575–99):ix. [DOI] [PubMed] [Google Scholar]
- [339].Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol. 2006;48:1548–51. [DOI] [PubMed] [Google Scholar]
- [340].Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, et al. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham heart study. Circulation. 2010;122:570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Uusitupa M, Siitonen O, Pyorala K, Mustonen J, Voutilainen E, Hersio K, et al. Relationship of blood pressure and left ventricular mass to serum insulin levels in newly diagnosed non-insulin-dependent (type 2) diabetic patients and in non-diabetic subjects. Diabetes Res. 1987;4:19–25. [PubMed] [Google Scholar]
- [342].Shah RV, Abbasi SA, Heydari B, Rickers C, Jacobs DR Jr, Wang L, et al. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2013;61:1698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Fang ZY, Schull-Meade R, Leano R, Mottram PM, Prins JB, Marwick TH. Screening for heart disease in diabetic subjects. Am Heart J. 2005;149:349–54. [DOI] [PubMed] [Google Scholar]
- [344].Lorenzo-Almorós A, Tuñón J, Orejas M, Cortés M, Egido J, Lorenzo Ó. Diagnostic aproaches for diabetic cardiomyopathy. Cardiovasc Diabetol. 2017;16:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600. [DOI] [PubMed] [Google Scholar]
- [346].Mordi IR. Non-invasive imaging in diabetic cardiomyopathy. J Cardiovasc Dev Dis. 2019;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Young LH, Wackers FJT, Chyun DA, Davey JA, Barrett EJ, Taillefer R, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 Diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Guo R, Nair S. Role of microRNA in diabetic cardiomyopathy: from mechanism to intervention. Biochim Biophys Acta Mol basis Dis. 2017;1863:2070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Raut SK, Khullar M. The big entity of new RNA world: long non-coding RNAs in microvascular complications of Diabetes. Front Endocrinol. 2018;9:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Jankauskas SS, Gambardella J, Sardu C, Lombardi A, Santulli G. Functional role of miR-155 in the pathogenesis of Diabetes mellitus and its complications. Noncoding RNA. 2021;7(3):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Tao L, Huang X, Xu M, Qin Z, Zhang F, Hua F, et al. Value of circulating miRNA-21 in the diagnosis of subclinical diabetic cardiomyopathy. Mol Cell Endocrinol. 2020;518:110944. [DOI] [PubMed] [Google Scholar]
- [352].Tao L, Shi J, Yang X, Yang L, Hua F. The exosome: a new player in diabetic cardiomyopathy. J Cardiovasc Transl Res. 2019;12:62–7. [DOI] [PubMed] [Google Scholar]
- [353].Salem ESB, Fan GC. Pathological effects of exosomes in mediating diabetic cardiomyopathy. Adv Exp Med Biol. 2017;998:113–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, et al. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. [DOI] [PubMed] [Google Scholar]
- [356].Norhammar A, Schenck-Gustafsson K. Type 2 diabetes and cardiovascular disease in women. Diabetologia. 2013;56:1–9. [DOI] [PubMed] [Google Scholar]
- [357].Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17–22. [DOI] [PubMed] [Google Scholar]
- [359].Curl CL, Wendt IR, Kotsanas G. Effects of gender on intracellular. Pflugers Arch. 2001;441:709–16. [DOI] [PubMed] [Google Scholar]
- [360].Gaborit N, Varro A, Le Bouter S, Szuts V, Escande D, Nattel S, et al. Gender-related differences in ion-channel and transporter subunit expression in non-diseased human hearts. J Mol Cell Cardiol. 2010;49:639–46. [DOI] [PubMed] [Google Scholar]
- [361].Farrell SR, Ross JL, Howlett SE. Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2010;299:H36–45. [DOI] [PubMed] [Google Scholar]
- [362].Iacobas DA, Iacobas S, Thomas N, Spray DC. Sex-dependent gene regulatory networks of the heart rhythm. Funct Integr Genomics. 2010;10:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Hoeker GS, Hood AR, Katra RP, Poelzing S, Pogwizd SM. Sex differences in beta-adrenergic responsiveness of action potentials and intracellular calcium handling in isolated rabbit hearts. PLoS One. 2014;9:e111411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Parks RJ, Howlett SE. Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflugers Arch. 2013;465:747–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Tran QK. Reciprocality between estrogen biology and calcium signaling in the cardiovascular system. Front Endocrinol. 2020;11:568203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Kararigas G, Bito V, Tinel H, Becher E, Baczko I, Knosalla C, et al. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J Am Coll Cardiol. 2012;59:410–7. [DOI] [PubMed] [Google Scholar]
- [367].McKee LA, Chen H, Regan JA, Behunin SM, Walker JW, Walker JS, et al. Sexually dimorphic myofilament function and cardiac troponin I phosphospecies distribution in hypertrophic cardiomyopathy mice. Arch Biochem Biophys. 2013;535:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Bell JR, Curl CL, Harding TW, Vila Petroff M, Harrap SB, Delbridge LMD. Male and female hypertrophic rat cardiac myocyte functional responses to ischemic stress and beta-adrenergic challenge are different. Biol Sex Differ. 2016;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].de Jong M, Peters SAE, de Ritter R, van der Kallen CJH, Sep SJS, Woodward M, et al. Sex disparities in cardiovascular risk factor assessment and screening for Diabetes-related complications in individuals with Diabetes: a systematic review. Front Endocrinol. 2021;12:617902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32:1118–25. [DOI] [PubMed] [Google Scholar]
- [371].Schuster I, Mahmoodzadeh S, Dworatzek E, Jaisser F, Messaoudi S, Morano I, et al. Cardiomyocyte-specific overexpression of oestrogen receptor beta improves survival and cardiac function after myocardial infarction in female and male mice. Clin Sci (Lond). 2016;130:365–76. [DOI] [PubMed] [Google Scholar]
- [372].Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative hormone trial. Diabetologia. 2004;47:1175–87. [DOI] [PubMed] [Google Scholar]
- [373].Lyons MR, Peterson LR, McGill JB, Herrero P, Coggan AR, Saeed IM, et al. Impact of sex on the heart’s metabolic and functional responses to diabetic therapies. Am J Physiol Heart Circ Physiol. 2013;305:H1584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Itzhaki Ben Zadok O, Kornowski R, Goldenberg I, Klempfner R, Toledano Y, Biton Y, et al. Admission blood glucose and 10-year mortality among patients with or without pre-existing diabetes mellitus hospitalized with heart failure. Cardiovasc Diabetol. 2017;16:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Dziubak A, Wójcicka G, Wojtak A, Bełtowski J. Metabolic effects of metformin in the failing heart. Int J Mol Sci. 2018;19:2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Leung M, Wong VW, Hudson M, Leung DY. Impact of improved glycemic control on cardiac function in type 2 Diabetes mellitus. Circ Cardiovasc Imag. 2016;9:e003643. [DOI] [PubMed] [Google Scholar]
- [377].Marfella R, Sardu C, Mansueto G, Napoli C, Paolisso G. Evidence for human diabetic cardiomyopathy. Acta Diabetol. 2021;58:983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Kenny HC, Abel ED. Heart failure in type 2 Diabetes mellitus. Circ Res. 2019;124:121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37:2843–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [380].Varzideh F, Kansakar U, Santulli G. SGLT2 inhibitors in cardiovascular medicine. Eur Heart J Cardiovasc Pharmacother. 2021;7(4):e67 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Oka S, Kai T, Hoshino K, Watanabe K, Nakamura J, Abe M, et al. Effects of empagliflozin in different phases of diabetes mellitus-related cardiomyopathy: a prospective observational study. BMC Cardiovasc Disord. 2021;21:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of Empagliflozin on left ventricular mass in patients with type 2 Diabetes mellitus and coronary artery disease. Circulation. 2019;140:1693–702. [DOI] [PubMed] [Google Scholar]
- [383].Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bühm M, et al. EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection. N Engl J Med. 2021;385(16):1451–61. [DOI] [PubMed] [Google Scholar]
- [384].Zhang A, Luo X, Meng H, Kang J, Qin G, Chen Y, et al. Sodium glucose cotransporter 2 inhibitors reduce the risk of heart failure hospitalization in patients with type 2 Diabetes mellitus: A systematic review and Meta-analysis of randomized controlled trials. Front Endocrinol. 2020;11:604250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [385].Prattichizzo F, De Nigris V, Micheloni S, La Sala L, Ceriello A. Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: is low-grade inflammation the neglected component? Diabetes Obes Metab. 2018;20:2515–22. [DOI] [PubMed] [Google Scholar]
- [386].Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Giacco F, Du X, Carratu A, Gerfen GJ, D’Apolito M, Giardino I, et al. GLP-1 cleavage product reverses persistent ROS generation after transient hyperglycemia by disrupting an ROS-generating feedback loop. Diabetes. 2015;64:3273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Matarese A, Gambardella J, Lombardi A, Wang X, Santulli G. miR-7 regulates GLP-1-mediated insulin release by targeting beta-Arrestin 1. Cells. 2020;9(7):1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Roh J, Houstis N, Rosenzweig A. Why Don’t we have proven treatments for HFpEF? Circ Res. 2017;120:1243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Kjeldsen SE, von Lueder TG, Smiseth OA, Wachtell K, Mistry N, Westheim AS, et al. Medical therapies for heart failure with preserved ejection fraction. Hypertension. 2020;75:23–32. [DOI] [PubMed] [Google Scholar]
- [391].Toth PP, Gauthier D. Heart failure with preserved ejection fraction: strategies for disease management and emerging therapeutic approaches. Postgrad Med. 2021;133:125–39. [DOI] [PubMed] [Google Scholar]
- [392].Wintrich J, Kindermann I, Ukena C, Selejan S, Werner C, Maack C, et al. Therapeutic approaches in heart failure with preserved ejection fraction: past, present, and future. Clin Res Cardiol. 2020;109:1079–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [393].Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777–81. [DOI] [PubMed] [Google Scholar]
- [394].Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [395].Breitenstein A, Steffel J. Devices in heart failure patients-who benefits from ICD and CRT? Front Cardiovasc Med. 2019;6:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [396].Borne RT, Katz D, Betz J, Peterson PN, Masoudi FA. Implantable cardioverter-defibrillators for secondary prevention of sudden cardiac death: a review. J Am Heart Assoc. 2017:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [397].Cilia L, Saeed A, Ganga HV, Wu WC. Heart failure with preserved ejection fraction: prevention and management. Am J Lifestyle Med. 2019;13:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [398].Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, et al. Exercise training in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2015;8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [399].Zheng J, Cheng J, Zheng S, Zhang L, Guo X, Zhang J, et al. Physical exercise and its protective effects on diabetic cardiomyopathy: what is the evidence? Front Endocrinol. 2018;9:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Varzideh F, Kansakar U, Jankauskas SS, Gambardella J, Santulli G. Cardiovascular endocrinology: evolving concepts and updated epidemiology of relevant diseases. Front Endocrinol. 2021:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]