Abstract
Four species of members of the family Enterobacteriaceae harboring extended-spectrum β-lactamase (ESBL) were recovered in a single patient hospitalized in an intensive care unit. Among these isolates, we describe for the first time an ESBL-producing Providencia rettgeri strain. Bacteria from the same species were shown to be genetically related by pulsed-field gel electrophoresis analysis. These strains produced the same TEM derivative ESBL, characterized as TEM-24. This enzyme had the peculiarity of being encoded by a large conjugative plasmid of 180 kb, never previously described for such an ESBL.
Plasmid-mediated extended-spectrum β-lactamases (ESBLs) were first reported in a Klebsiella pneumoniae strain in 1983. Since then, infections caused by ESBL-producing members of the family Enterobacteriaceae rapidly increased (12, 15, 19). The isolates were characterized by the presence of β-lactamase mutants belonging to the TEM or SHV families (18). Most of them have been genetically characterized, and among the TEM derivative ESBLs, CAZ-6 was first described in 1988 (6). More and more species of Enterobacteriaceae are affected by this resistance (12, 15, 19), but bacteria from the genus Providencia are usually sensitive to extended-spectrum cephalosporins (9); however, a new TEM derivative enzyme, TEM-60, was recently described in Providencia stuartii (10).
In this study, we describe a multiplicity of TEM-24 ESBL-producing Enterobacteriaceae species recovered over a 4-month period in a single patient hospitalized in an intensive care unit (ICU) from Arnaud-de-Villeneuve hospital in Montpellier, France. Among these strains, a TEM-24-producing Providencia rettgeri strain was isolated from the respiratory tract. This report is the first description of ESBL production by P. rettgeri. The different enterobacterial ESBL-producing strains isolated from the patient were analyzed by pulsed-field gel electrophoresis (PFGE) in order to compare their restriction patterns. β-Lactamase characterization and plasmid content analysis were performed for each of the four ESBL-harboring Enterobacteriaceae species recovered in this patient.
Fourteen strains resistant to broad-spectrum cephalosporins were isolated from the same patient during his ICU stay: Enterobacter aerogenes (n = 3), Proteus mirabilis (n = 6), Klebsiella pneumoniae (n = 3), and P. rettgeri (n = 2) (Table 1). The patient developed several episodes of nosocomial pneumonia and urinary infections during his hospital care. The strains were isolated from urine, sputum, central veinous catheter, and feces samples. The patient was under cefotaxime treatment when the first ESBL-producing strain (E. aerogenes) was isolated in sputum. A broad-spectrum cephalosporin-susceptible K. pneumoniae strain was isolated at the same time in this sample. Antibiotic treatment including isepamicin, imipenem, and colimycin was administered during 11 days. One month later, a P. mirabilis ESBL-producing strain was isolated from urine and sputum. A combination of gentamicin, imipenem, and colimycin was prescribed. Three weeks later, two more ESBL-producing strains were then isolated from sputum samples: K. pneumoniae and P. rettgeri (Table 1). This episode of pneumonia was treated with amoxicillin-clavulanic acid during 1 week. A few days later, ESBL-harboring P. mirabilis was isolated from urine, and treatment including isepamicin and piperacillin-tazobactam was prescribed for 2 weeks. After this episode, no other antibiotic treatment was prescribed.
TABLE 1.
Multiresistant strains of members of the family Enterobacteriaceae isolated from the same patient over a 4-month period
| Straina | Species of ESBL-producing Enterobacteriaceae | Sample containing isolate | Length of stay in ICU in 1997 (days) | PFGE pattern after digestion by:
|
|
|---|---|---|---|---|---|
| XbaI | SmaI | ||||
| 1 | E. aerogenes | Sputum | 12 | NDb | ND |
| 1′ | K. pneumoniaec | Sputum | 12 | A | ND |
| 2 | E. aerogenes | Central veinous catheter | 18 | B | ND |
| 3 | P. mirabilis | Urine | 40 | C | D |
| 4 | P. mirabilis | Sputum | 43 | ND | ND |
| 5 | K. pneumoniae | Sputum | 62 | ND | ND |
| 6 | P. rettgeri | Sputum | 62 | E | F |
| 7 | P. mirabilis | Urine | 82 | C | D |
| 8 | P. rettgeri | Sputum | 83 | E | F |
| 9 | P. mirabilis | Urine | 102 | C | D |
| 10 | K. pneumoniae | Coproculture | 127 | A1 | ND |
| 11 | P. mirabilis | Coproculture | 127 | C | D1 |
| 12 | P. mirabilis | Urine | 128 | C | D |
| 13 | K. pneumoniae | Urine | 128 | A1 | ND |
| 14 | E. aerogenes | Central veinous catheter | 128 | B | ND |
Designated by chronology of isolation.
ND, not determined.
Non-ESBL-producing strain.
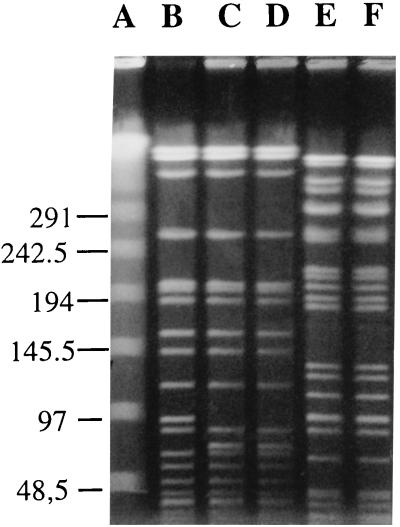
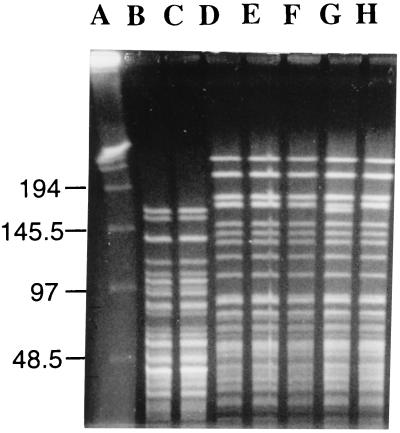
Among genomic subtyping methods, PFGE has been shown to be the most discriminatory for numerous bacterial species (7, 11, 21). In this study, we tried to determine by using PFGE the relationship between strains from the same species. PFGE analysis was performed with bacterial chromosomal DNA from 11 ESBL-producing strains and the first K. pneumoniae strain isolated (Table 1). A total of two E. aerogenes, five P. mirabilis, two P. rettgeri, and three K. pneumoniae strains were typed by PFGE. Genomic DNAs were prepared by a previously described method (11). Two restriction endonucleases were used to determine the most discriminatory comparison: XbaI and SmaI. K. pneumoniae and E. aerogenes DNAs were digested with the restriction enzyme XbaI, whereas P. mirabilis and P. rettgeri DNAs were digested with both XbaI and SmaI. The enzymes were obtained from New England Biolabs. Digestion was performed with 40 U of the endonuclease for 6 h at 25°C (SmaI) or 37°C (XbaI). PFGE was performed with a CHEF-DR III apparatus (Bio-Rad Laboratories) with 1% agarose gel in Tris-borate-EDTA buffer. For SmaI digests, the running parameters were as follows: initial pulse, 20 s; final pulse, 5 s; voltage, 4.5 V/cm. Electrophoresis was performed at 8°C for 40 h. XbaI digests were run for 40 h with pulses from 40 to 5 s. The gels were stained with ethidium bromide and photographed under UV light. A lambda ladder (Bio-Rad Laboratories) was used as a DNA molecular size marker and served as a control for the running parameters of the CHEF-DR III unit. PFGE strain patterns were compared by visual inspection and interpreted according to the criteria of Tenover et al. (22). Unrelated PFGE patterns were noted as type A, B, etc, whereas related patterns were named type A1, A2, etc. The results are summarized in Table 1. The same XbaI PFGE pattern was detected among the two E. aerogenes strains (Fig. 1). The five P. mirabilis strains were shown to be indistinguishable by PFGE after digestion by XbaI (data not shown), whereas after SmaI digestion, four strains were shown to be indistinguishable and the fifth strain was closely related to the others (Fig. 2). We noted that the SmaI digest gave the most useful restriction fragment patterns for P. mirabilis. The two P. rettgeri strains shared the same PFGE pattern after XbaI and after SmaI digestion (Fig. 2). The susceptible K. pneumoniae isolate was closely related to the two ESBL-producing K. pneumoniae strains (Fig. 1).
FIG. 1.
PFGE of total DNA from K. pneumoniae and ESBL-producing E. aerogenes cut by XbaI. Lanes: A, lambda ladder; B, K. pneumoniae with no ESBL (strain 1′); C and D, ESBL-producing K. pneumoniae (strains 10 and 13, respectively); E and F, E. aerogenes (strains 2 and 14, respectively). The size of the ladder is indicated in kilobases.
FIG. 2.
PFGE of total DNA from ESBL-producing P. rettgeri and P. mirabilis cut by SmaI. Lanes: A, lambda ladder; B and C, P. rettgeri (strains 6 and 8, respectively); D to H, P. mirabilis (strains 3, 7, 9, 11, and 12, respectively). Sizes are indicated in kilobases.
The first resistant strain isolated in each species was selected for further studies: susceptibility testing, conjugational transfer, analytical isoelectric focusing, plasmid content analysis, PCR amplification, and DNA sequencing. The plasmid-mediated β-lactamases produced by the different enterobacterial strains were transferred by conjugation into Escherichia coli HB101 cells resistant to rifampin. Transconjugants were selected on Mueller-Hinton agar containing rifampin (300 μg/ml) and ceftazidime (4 μg/ml) (14).
The susceptibility of strains was determined by the disk diffusion assay on Mueller-Hinton agar according to the recommendations of the Comité Français de l’Antibiogramme of the French Society for Microbiology (1). The double-disk synergy test was performed as previously described (5, 20). This test was positive for E. aerogenes and K. pneumoniae, with disks placed 20 mm apart. The best detection conditions for P. mirabilis and P. rettgeri were when expanded-spectrum cephalosporin disks were quartered to reduce their potency. The P. rettgeri isolate was highly resistant to amoxicillin and ticarcillin. The expanded-spectrum cephalosporins were active and showed large inhibition diameters (cefotaxime, 37 mm; cefpirome, 30 mm; cefepime, 30 mm; aztreonam, 40 mm). However, we noted that the strain was moderately susceptible to ceftazidime (diameter, 20 mm). A double-disk synergy test performed with quartered disks revealed a synergistic effect characteristic of an ESBL-producing strain. Aminoglycoside susceptibility patterns showed that the four ESBL-harboring strains were susceptible to gentamicin and resistant to amikacin, tobramycin, and netilmicin. The observation of this phenotype suggests the production of an AAC(6′)-I enzyme. β-Lactam and aminoglycoside resistances were transferred to E. coli HB101 transconjugants.
β-Lactam MICs were determined by a dilution method on Mueller-Hinton agar. Inocula of 105 to 106 CFU per ml were distributed with a multipoint inoculator (MIC 2000; Dynatech). The antimicrobial agents tested included ticarcillin, piperacillin, cephalothin, cefotaxime, ceftazidime, aztreonam, and cefpirome. Table 2 shows MIC results for the four ESBL-producing clinical strains and their E. coli HB101 transconjugants. All strains were resistant to ticarcillin with MICs of ≥128 μg/ml and showed various levels of susceptibility to piperacillin (MIC range, 2 to 128 μg/ml), cephalothin (MIC range, 16 to >512 μg/ml), cefotaxime (MIC range, ≤0.06 to 4 μg/ml), and aztreonam (MIC range, ≤0.06 to 16 μg/ml). We noted a greater hydrolytic activity against ceftazidime (MICs, 12- to 128-fold higher) than against cefotaxime, as previously described for TEM-24 (6). In P. mirabilis and P. rettgeri, the resistance level against expanded-spectrum cephalosporins and aztreonam were very low. For P. mirabilis, MICs of cefotaxime, aztreonam, and ceftazidime, were 0.5, 0.12, and 8 μg/ml, respectively. For P. rettgeri, MICs of cefotaxime and aztreonam were ≤0.06 μg/ml, and the MIC of ceftazidime was 4 μg/ml. A similar β-lactam level of resistance in the four transconjugants was observed, and we noted that β-lactam resistance was cotransferred with resistance to aminoglycosides (amikacin, kanamycin, netilmicin, and tobramycin), chloramphenicol, and sulfamides into E. coli HB101.
TABLE 2.
Antimicrobial susceptibilities of multiresistant clinical isolates and their transconjugants in E. coli HB101
| Straina | MIC (μg/ml)b
|
||||||
|---|---|---|---|---|---|---|---|
| TIC | PIP | CF | CTX | CAZ | ATM | FEP | |
| P. mirabilis | 1,024 | 128 | 32 | 0.5 | 8 | 0.12 | 0.5 |
| Tr P. mirabilis | 2,048 | 16 | 16 | 0.5 | 64 | 4 | 0.25 |
| P. rettgeri | 128 | 2 | 16 | ≤0.06 | 4 | ≤0.06 | 0.12 |
| Tr P. rettgeri | 2,048 | 16 | 16 | 0.5 | 64 | 4 | 0.25 |
| K. pneumoniae | >2,048 | 32 | 32 | 2 | 64 | 4 | 0.5 |
| Tr K. pneumoniae | 2,048 | 16 | 16 | 0.5 | 64 | 4 | 0.25 |
| E. aerogenes | >2,048 | 64 | >512 | 4 | 256 | 16 | 1 |
| Tr E. aerogenes | 2,048 | 8 | 16 | 0.5 | 64 | 4 | 0.25 |
Tr, transconjugant in E. coli HB101.
TIC, ticarcillin; PIP, piperacillin; CF, cephalothin; CTX, cefotaxime; CAZ, ceftazidime; ATM, aztreonam; FEP, cefepime.
Isoelectric focusing was performed as previously reported (6) with the LKB 2117 Multiphor II electrophoresis system (Pharmacia Biotech). The enzyme activities were located in the gels with nitrocefin. β-Lactamases with known pIs (TEM-1, pI 5.4; TEM-2, pI 5.6; TEM-3, pI 6.3; and TEM-24, pI 6.5) were used as standards. All clinical isolates and transconjugants harbored a β-lactamase with a pI of 6.5, consistent with that of a TEM-24 β-lactamase. The β-lactamase of pI 6.5 was the only β-lactamase detected in the P. rettgeri strain. This enzyme was produced in addition to the chromosomal enzymes SHV-1 (pI 7.7) and Amp C (pI 8.2) in the K. pneumoniae and the E. aerogenes strains, respectively. It was also produced with a TEM-2 (pI 5.6) in the P. mirabilis strain.
PCR was performed with crude bacterial extract from a P. rettgeri transconjugant with two amplification primers, A and B, specific for TEM genes (4), in a Perkin-Elmer Gene Amp PCR System 2004 DNA thermal cycler (Perkin-Elmer Cetus Instruments). Complete sequences of the gene and of its promoter region were determined on both strands directly on amplified product obtained as previously described (13) by the dideoxy chain termination procedure of Sanger et al. (17) on an ABI 1377 automatic sequencer by using the ABI Prism Dye Terminator Cycle Sequencing Ready Reaction Kit with Ampli Taq DNA polymerase FS (Perkin-Elmer/Applied Biosystems, Foster City, Calif.). The nucleotide sequence of the gene encoding the P. rettgeri enzyme was identical to that of the blaTEM-24 gene (4) and differed from that of the blaTEM-2 gene by four amino acid substitutions (4). According to Ambler nucleotide numbering (2), these amino acid substitutions are Glu→Lys 104, Arg→Ser 164, Ala→Thr 237, and Glu→Lys 240.
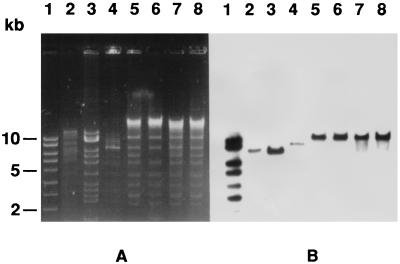
In order to characterize the plasmids encoding TEM-24, plasmid DNA of clinical isolates and transconjugants was extracted as described previously (16) by a protocol which is a modification of the method of Birnboim and Doly (3). DNA electrophoresis was performed in 0.7% agarose. A 180-kb plasmid was isolated from all clinical strains and transconjugants (data not shown). Plasmid DNA was digested with the restriction endonucleases EcoRI and SalI according to the recommendations of the manufacturer (Bethesda Research Laboratories, Inc.). Restriction fragments were visualized after electrophoresis in 0.8% agarose gels with a DNA ladder (Smartladder; Eurogentec). Similar restriction patterns were observed with the different plasmids (Fig. 3). A TEM-specific DNA probe was produced by PCR and was labeled with 32P as previously described (16). The specific amplification was achieved under standard conditions with the primers TEM-A and TEM-B (4). Hybridization and autoradiography were performed with DNA transferred and immobilized on Nytran filters (16). Plasmids pCFF04 (TEM-3 encoding an 85-kb plasmid), pCFF74 (TEM-24 encoding an 85-kb plasmid), and pCFF14 (TEM-5 encoding a 180-kb plasmid) were used for comparison. The same EcoRI-SalI fragment of >10 kb hybridized with the intragenic TEM-1-derived probe under low-stringency conditions (Fig. 3).
FIG. 3.
(A) Restriction fragments after gel electrophoresis of plasmid DNA digested with endonucleases EcoRI and SalI from transconjugants of P. mirabilis, P. rettgeri, K. pneumoniae, and E. aerogenes (lanes 5, 6, 7, and 8, respectively). Plasmids pCFF04 (TEM-3-encoding 85-kb plasmid), pCFF74 (TEM-24-encoding 85-kb plasmid), and pCFF14 (TEM-5-encoding 180-kb plasmid) were used for comparison (lanes 2, 3, and 4). Lane 1, Smartladder, Eurogentec. (B) Hybridization with TEM-specific DNA probe.
Dissemination of plasmid-mediated β-lactamases in gram-negative bacteria involves important problems in antibiotic treatment, especially in the ICUs. Among the multiple TEM derivative ESBLs, TEM-24 was first characterized as CAZ-6 in 1988 in a K. pneumoniae strain isolated from a sputum in a patient undergoing ceftazidime treatment (6). In 1988 to 1989, this enzyme was responsible for outbreaks in ICUs (9), and in 1994, TEM-24 was found in various species, with a predominance in E. aerogenes (5).
The present study describes persistent colonization and infection of a single patient by a multiplicity of TEM-24-producing strains of the family Enterobacteriaceae, including P. rettgeri. Strains from the same species shared concordant PFGE patterns, suggesting their clonal origin. The patient was first infected after 12 days of hospitalization with an ESBL-producing E. aerogenes strain already known in the ICU since 1993 as a nosocomial strain. This strain was probably selected by cefotaxime treatment. The susceptible and ESBL-producing K. pneumoniae strains were genetically closely related, suggesting a plasmid transfer to this species from the Enterobacter species. Characterization of ESBL showed that the four Enterobacteriaceae species harbored the same TEM-24 ESBL. TEM-24 is a plasmid-mediated β-lactamase conferring a higher level of resistance to ceftazidime than to cefotaxime, like all ceftazidimases. Detection of ESBL production was difficult in Providencia and Proteus species. We suspected that the P. mirabilis and P. rettgeri strains produce an ESBL because of the reduced diameters around the disk of ceftazidime. The suspicion was confirmed by a double-disk synergy test performed with quartered disks. The very weak expression of β-lactamase in Providencia and Proteus species assessed by determination of MICs could explain the difficulty of detection by disk diffusion susceptibility tests. In this report, plasmid analysis showed that in the four species, ESBLs were encoded by a large conjugative plasmid of 180 kb, never previously described for such an ESBL. Until our observation, TEM-24 was found to be encoded by an 85-kb plasmid.
Taken together, these results are strongly suggestive of an in vivo interspecies plasmid transfer. It appears that two different mechanisms could be involved in the dissemination of ESBL-producing strains. First there occurred dissemination of one strain of ESBL-producing E. aerogenes present in the ICU and then dissemination of the 180-kb plasmid encoding TEM-24 from E. aerogenes among various members of the family Enterobacteriaceae. The transfer of a TEM-24 β-lactamase from an E. aerogenes strain to E. coli and Citrobacter freundii strains had already been described (14), but never to a P. rettgeri isolate.
In conclusion, this study shows an example of in vivo multiresistance gene dissemination and is the first report of ESBL production by P. rettgeri. To our knowledge, this is the first observation of such a diversity of ESBL-harboring organisms in a single patient. The ceftazidimase TEM-24 usually encoded by an 85-kb plasmid was, in this study, encoded by a 180-kb plasmid. Dissemination of this 180-kb plasmid into four Enterobacteriaceae species, including P. mirabilis and P. rettgeri, respectively, rarely or never productive of ESBL, suggests a high capacity of conjugation of this plasmid (8). Further experiments are in progress in our laboratory in order to study the properties of this plasmid.
Acknowledgments
We thank Josiane Campos for PFGE analysis and Marlène Jan, Rolande Perroux, and Dominique Rubio for their technical assistance in ESBL characterization.
REFERENCES
- 1.Acar J, Carret G, Cavallo J D, Chardon H, Choutet P, Courvalin P, Dabernat H, Drugeon H, Dubreuil L, Goldstein F, Jarlier V, Leclercq R, Nicolas-Chanoine M H, Philippon A, Rouveix B, Sirot J, Soussy J C, Thabaut A. Antibiogram Committee of the French Society for Microbiology 1998 statement. Pathol Biol. 1998;46:I–XVI. [PubMed] [Google Scholar]
- 2.Ambler R P, Coulson A F W, Frere J-M, Ghuysen J-M, Joris B, Forsman M, Levesque R C, Tibary G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;376:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chanal C, Poupart M-C, Sirot D, Labia R, Sirot J, Cluzel R. Nucleotide sequences of CAZ-2, CAZ-6, and CAZ-7 β-lactamase genes. Antimicrob Agents Chemother. 1992;36:1817–1820. doi: 10.1128/aac.36.9.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanal C, Sirot D, Romaszko J P, Bret L, Sirot J. Survey of prevalence of extended spectrum β-lactamases among Enterobacteriaceae. J Antimicrob Chemother. 1996;38:127–132. doi: 10.1093/jac/38.1.127. [DOI] [PubMed] [Google Scholar]
- 6.Chanal C M, Sirot D L, Petit A, Labia R, Morand A, Sirot J L, Cluzel R A. Multiplicity of TEM-derived β-lactamases from Klebsiella pneumoniae strains isolated at the same hospital and relationships between the responsible plasmids. Antimicrob Agents Chemother. 1989;33:1915–1920. doi: 10.1128/aac.33.11.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chetoui H, Melin P, Struelens M J, Delhalle E, Nigo M M, De Ryck R, De Mol P. Comparison of biotyping, ribotyping, and pulsed-field gel electrophoresis for investigation of a common-source outbreak of Burkholderia pickettii bacteremia. J Clin Microbiol. 1997;35:1398–1403. doi: 10.1128/jcm.35.6.1398-1403.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darfeuille-Michaud A, Jallat C, Aubel D, Sirot D, Rich C, Sirot J, Joly B. R-plasmid-encoded adhesive factor in Klebsiella pneumoniae strains responsible for human nosocomial infections. Infect Immun. 1992;60:44–55. doi: 10.1128/iai.60.1.44-55.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Champs C, Sirot D, Chanal C, Poupart M C, Dumas M P, Sirot J. Concomitant dissemination of three extended-spectrum β-lactamases among different Enterobacteriaceae isolated in a French hospital. J Antimicrob Chemother. 1991;27:441–457. doi: 10.1093/jac/27.4.441. [DOI] [PubMed] [Google Scholar]
- 10.Franceschini N, Perilli M, Segatore B, Setacci D, Amicosante G, Mazzariol A, Cornaglia G. Ceftazidime and aztreonam resistance in Providencia stuartii: characterization of a natural TEM-derived extended-spectrum β-lactamase, TEM-60. Antimicrob Agents Chemother. 1998;42:1459–1462. doi: 10.1128/aac.42.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouby A, Neuwirth C, Bourg G, Bouzigues N, Carles-Nurit M J, Despaux E, Ramuz M. Epidemiological study by pulsed-field gel electrophoresis of an outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a geriatric hospital. J Clin Microbiol. 1994;32:301–305. doi: 10.1128/jcm.32.2.301-305.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacoby G A, Medeiros A A. More extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabilat C, Goussard S, Sougakoff W, Spencer R C, Courvalin P. Direct sequencing of the amplified structural gene and promoter for the extended broad-spectrum β-lactamase TEM-9 (RHH-1) of Klebsiella pneumoniae. Plasmid. 1990;23:27–34. doi: 10.1016/0147-619x(90)90041-a. [DOI] [PubMed] [Google Scholar]
- 14.Neuwirth C, Siebor E, Lopez J, Pechinot A, Kazmierczak A. Outbreak of TEM-24-producing Enterobacter aerogenes in an intensive care unit and dissemination of the extended-spectrum β-lactamase to other members of the family Enterobacteriaceae. J Clin Microbiol. 1996;34:76–79. doi: 10.1128/jcm.34.1.76-79.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philippon A, Labia R, Jacoby G. Extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1989;33:1131–1136. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 17.Sanger F, Nicklen S A, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirot D. Bêta-lactamases plasmidiques à spectre étendu. J Antimicrob Chemother. 1995;36(Suppl. A):19–34. doi: 10.1093/jac/36.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 19.Sirot D L, Goldstein F W, Soussy C J, Courtieu A L, Husson M O, Lemozy J, Meyran M, Morel C, Perez R, Quentin-Noury C, Reverdy M E, Scheftel J M, Rosembaum M, Rezvani Y. Resistance to cefotaxime and seven other β-lactams in members of the family Enterobacteriaceae: a 3-year survey in France. Antimicrob Agents Chemother. 1992;36:1677–1681. doi: 10.1128/aac.36.8.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirot J. Detection of extended-spectrum plasmid-mediated β-lactamases by disk diffusion. Clin Microbiol Infect. 1996;2(Suppl. 1):S35. doi: 10.1111/j.1469-0691.1996.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 21.Swaminathan B, Matar G M, Reeves M W, Graves L M, Ajello G, Bibb W F, Helsel L O, Morales M, Dronavalli H, El-Swify M, DeWitt W, Hunter S B. Molecular subtyping of Neisseria meningitidis serogroup B: comparison of five methods. J Clin Microbiol. 1996;34:1468–1473. doi: 10.1128/jcm.34.6.1468-1473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]