Abstract
Temporal association between BNT162b2 mRNA COVID-19 vaccine and myocarditis (PCVM) has been reported. We herein present early and 6-month clinical follow-up and cardiac magnetic resonance imaging (CMR) of patients with PVCM. A retrospective collection of data from 15 patients with PCVM and abnormal CMR was performed. Clinical manifestation, laboratory data, hospitalizations, treatment protocols, and imaging studies were collected early (up to 2 months) and later. In nine patients, an additional CMR evaluation was performed 6 months after diagnosis. PCVM was diagnosed in 15 patients, mean age 17 ± 1 (median 17.2, range 14.9–19 years) years, predominantly in males. Mean time from vaccination to onset of symptoms was 4.4 ± 6.7 (median 3, range 0–28) days. All patients had CMR post diagnosis at 4 ± 3 (median 3, range 1–9) weeks, 4/5 patients had hyper enhancement on the T2 sequences representing edemaQuery, and 12 pathological Late glandolinium enhancement. A repeat scan performed after 5–6 months was positive for scar formation in 7/9 patients. PCVM is a rare complication, affecting predominantly males and appearing usually within the first week after administration of the second dose of the vaccine. It usually is a mild disease, with clinical resolution with anti-inflammatory treatment. Late CMR follow up demonstrated resolution of the edema in all patients, while some had evidence of residual myocardial scarring.
Keywords: Myocarditis, COVID-19, Vaccine, Cardiac magnetic resonance imaging (CMR), Follow-up
Introduction
The COVID-19 pandemic caused by the SARS COV virus has infected more than 240 million people worldwide and caused close to 4.9 million deaths. As of October 2021, over 2.7 million new cases and 46, 000 new deaths were being reported weekly worldwide [1]. Since its introduction, the The Pfzer–BioNTech mRNA BNT162b2 COVID-19 vaccine has been shown to significantly decrease rates of infection, severe disease, and death due to COVID-19 infection, while side effects of the vaccine have been mild and mostly self-limiting [2, 3].
While major mortality and morbidity have been mainly reported in the elderly and those with risk factors [4], children were affected in a milder way during the acute phase of the disease, however, some developed post-COVID-19 effects such as multisystem inflammatory syndrome (MIS-C) [5].
The Pfzer–BioNTech mRNA BNT162b2 COVID-19 vaccine, administered as two doses 21 days apart, was authorized for emergency use in Israel in December 2020. Since then more than 6 million citizens have been vaccinated with the first dose and 5.7 million with the second dose [6], covering more than 60% of the population. By May 2021, nearly 224,000 teenagers 16–19 years had received at least one dose, and approximately 200,000 had received two doses of the vaccine [7].
Initially sporadic cases of myocarditis following COVID-19 vaccination were reported [8]. By April 2021, there were increased reports by the Centers for Disease Control and Prevention (CDC) to the Vaccine Adverse Event Reporting System (VAERS) of cases of myocarditis and pericarditis occurring after mRNA COVID-19 vaccination (Pfizer-BioNTech and Moderna) in the United States [9]. The crude incidence of myocarditis was found to be approximately 1–2.13 per 100,000, with a 1.7 higher incidence following the second dose [7, 10, 11]. The highest incidence of myocarditis was found in male recipients 16–19 years [7]. Myocarditis following COVID-19 vaccination is usually mild and self- limiting, nevertheless rare cases of severe dysrhythmia and ventricular dysfunction have been reported [8].
Several recently published articles describe early cardiovascular magnetic resonance (CMR) findings in patients who were diagnosed with 19 post COVID-19 vaccination myocarditis (PCVM), mostly Late Glandolinium Enhancement (LGE) and myocardial edema [1, 12–17].
We recently performed a mid-term (5–6 months) CMR follow-up study in 9 of the 15 patients who presented at our institutions with PCVM. We herein report our findings.
Materials and Methods
Healthcare in Israel is universal and centralized. All citizens are entitled to medical care provided by government facilities and carried out by four main health maintenance organizations (HMO) [18]. This system allows for medical records and data to be readily available, as there is mandatory report of post-immunization adverse events to all HMO’s, by all hospitals in Israel. Since the initiation of the immunization campaign, more than 160,000 adolescents under 18 years of age have been immunized by Israel’s largest HMO, Clalit Health Services (insures > 50% of the population) [10, 19], and 15 patients with post-immunization myocardial involvement were identified in five major children’s medical centers in Israel. Myocarditis was defined clinically, based on the presence of two or more of the following: (1) signs and symptoms of acute myocardial involvement (e.g., chest pain, arrhythmia); (2) elevated troponin; (3) echocardiographic evidence of ventricular dysfunction without an alternative explanation; and (4) (ST-T) changes in the ECG. The study was approved by Schneider Children’s Medical Center of Israel’s institutional review board (Helsinki committee).
The cardiac MRI study was performed in three different sites up to 2 months after the initial diagnosis, and in nine patients during their 6-month follow-up. CMR scans were performed using 1.5T or 3T MR scanner (Ingenia 1.5T/3T; Philips Healthcare, Best, The Netherlands, Siemmens Magentum Sola). CMR imaging protocol was performed using multi-planar cine steady state free precession sequences. T2 weighted dark-blood TSE images were acquired in standard short- and long-axis views. T1 weighted dark blood TSE images post Gadolinium injection or T1 mapping protocol were not uniformly performed. Phase sensitive inversion recovery (PSIR) sequence was used to asses LGE. PSIR sequences were performed 10 min after intravenous administration of 0.2 milimol/kg of Gadolinium-based contrast agent.
Data analyses were performed on a dedicated CMR workstation (Intellispace Portal, version 11.0, Philips Co.) to determine cardiac volumes and functioning. Positive myocardial edema was diagnosed as either increased focal regional signal intensity, or increased signal intensity ratio over 1.9 as compared to striated muscle intensity on the same slice. Positive LGE was defined as an image intensity level of ≥ 2 standard deviations above the mean of the remote myocardium.
Results
After receiving the BNT162b2 mRNA COVID-19 vaccine, 15 patients were diagnosed with myocarditis based on clinical findings, elevated troponin levels, ECG, and echocardiography (Table 1). Only 4/15 (26%) patients met the full Lake Louise criteria for myocarditis. Mean age at presentation was 17 ± 1 (median 17.2, range 14.9–19) years. Post vaccine myocarditis occurred predominantly in males and almost exclusively after the second dose of the vaccine, with only one patient presenting after a third dose. Troponin levels were elevated in 14/15 patients, and mean time from vaccination to onset of symptoms was 4.4 ± 6.7 (median 3, range 0–28) days. Two patients presented with pericardial effusion, three with ventricular dysfunction, and 13 with nonspecific ST/T changes on ECG. All patients underwent serology for Anti S and Anti N COVID-19 protein and PCR testing for COVID-19, to exclude acute COVID-19 infection and confirm a serologic response to the vaccine. All patients underwent viral cultures, as are routinely done in children with myocarditis in Israel, and CMR on an average of 4 ± 3 (median 3, range 1–9) weeks after the initial diagnosis. Out of 15 patients, four had hyper enhancement on their T2 sequences (representing edema) and 14 evidence of abnormal late enhancement (representing inflammation and necrosis) (Fig. 1A, B, D, E). LGE location was typical for myocarditis of viral origin. The mid-myocardial subepicardial left ventricle was involved in all patients. There was no right ventricular involvement and sub-endocardium was unaffected in all patients (Table 2).
Table 1.
Demographics and clinical presenting symptoms of patients with PCVM
| Patient no. | Sex | Age (years) | Vaccine Dose | Days From Vaccine | Chief Complaint | Troponin-T (ng/L) | CRP (mg/dL) | ECG | Echo | Hospital Duration (d) | ICU | TX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 17.1 | 2nd | 0 | CP | 2037 | 5.6 | ST + PR changes | NE | 4 | No | Aspirin,Colchicie Cardiloc |
| 2 | M | 16.8 | 2nd | 2 | CP | 3,130 | 3.3 | ST changes | SF 28%, mild MR | 6 | Yes | NSAID |
| 3 | M | 16.3 | 2nd | 3 | CP | 1,649 | 5.6 | ST changes | NF, mild MR | 4 | Yes | NSAID |
| 4 | M | 16.2 | 2nd | 2 | CP fever | 2,205 | 4.6 | ST changes | NF | 6 | No | NSAID |
| 5 | M | 17.5 | 2nd | 1 | CP | 252 | 3.65 | Borderline ST changes | NF, effusion | 4 | No | NSAID |
| 6 | M | 16.7 | 1st | 6 | CP | 2,482 | 3.58 | ST changes | NF | 4 | Yes | None |
| 7 | M | 17.2 | 2nd | 3 | CP | 1,332 | 2.1 | ST changes | NF | 5 | No | NSAID |
| 8 | M | 17 | 2nd | 3 | CP | 9,856 | 2.21 | W-Wave inversion | NF | 4 | No | NSAID |
| 9 | M | 17.3 | 2nd | 4 | CP fever | 6,279 | 4.8 | ST changes | Mild LV dysf EF 45% | 7 | Yes | Colchicine Tritace |
| 10 | M | 17.7 | 2nd | 1 | CP | > 10,000 | ST changes | NF | ? | Yes | Aspirin | |
| 11 | M | 19 | 2nd | 6 | CP | 664 | 0.66 | Non-specific changes | NF | 3 | Yes | None |
| 12 | M | 14.9 | 2nd | 28 | CP fever | < 13 | 2.98 | Normal | NF, effusion | 2 + 7 | No | NSAID, Colchicine, Steroids |
| 13 | M | 17.2 | 2nd | 2 | CP fever | 697 | 6.5 | ST changes | Mild LV dysf FS 30% | 7 | Yes | Steroids, IVIG, NSAID |
| 14 | M | 17.6 | 2nd | 5 | CP | 344 | 0.34 | ST changes | NF | 7 | No | NSAID |
M male, F female, CP chest pain, NF normal function, PE pericardial effusion, CMR cardiac MRI, SF shortening fraction, NSAID non steroidal anti-inflamatory drugs
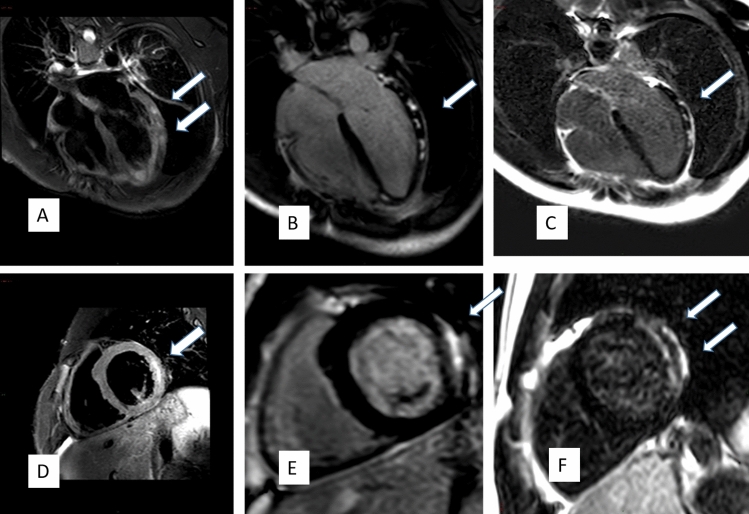
Fig. 1.
CMR Images of post COVID-19 Vaccination Myocarditis. 4CH T2 fat suppressed image showing increased signal indicating edema of the LV-free and lateral walls (A, D), patchy positive DLE (B, E) in the same distribution, and persistence of the DLE 5.5 months after patient presentation of the indicating scar formation (C, F)
Table 2.
Early and late ECG, echo and imaging studies of patients with PCVM
| ECG | ECHO | CMR | |||||
|---|---|---|---|---|---|---|---|
| Patient no. | 0 M | 6 M | 0 M | 6 M | 0 M | 6 M | 6 M STRESS TEST |
| 1 | ST changes | Not performed | NF | NF |
Positive LGE T2 negative EF 55% |
Positive LGE T2 Negative EF 57% |
|
| 2 | ST changes | Normal | SF 28% mild MR | NF, SF = 34%, mild MR |
Positive LGE T2 Positive EF 58% |
Positive LGE T2 Negative EF 57% |
Normal (12.2 min., 12.7 MET) |
| 3 | ST changes | Normal | NF, mild MR | NF, small effusion |
Positive LGE T2 Positive EF 55% |
Positive LGE T2 Negative EF 63% |
|
| 4 | ST changes | Biphasic T-wave II,III,V2 | NF | NF |
Positive LGE T2 positive Ef 66% |
Negative LGE T2 Negative EF 63% |
|
| 5 | Borderline ST changes | Normal | NF, effusion | NF |
Negative LGE T2 Negative EF 58% |
Negative LGE T2 Negative EF 63% |
Normal (6:23 min,13.5METS) |
| 6 | ST changes | Normal | NF | NF |
Positive LGE T2 Negative EF 64% |
Positive LGE T2 Negative EF 66% |
|
| 7 | ST changes | No follow-up | NF | No follow-up |
Positive LGE T2 Inconclusive EF 61% |
No follow-up | |
| 8 | ST changes | Biphasic T-wave II,III,AVF | NF | NF |
Positive LGE T2 Inconclusive EF 56% |
Positive LGE T2 Negative EF 61% |
|
| 9 | ST changes | Normal | Mild LV dysf EF 45% |
EF 50–54% |
Positive LGE T2 Inconclusive EF 61% |
Positive LGE T2 Negative EF 58% |
|
| 10 | ST changes | Normal | NF | NF |
Inconclusive LGE T2 Negative EF 45% |
Not performed | |
| 11 | Non specific | NF | NF |
Positive LGE T2 Inconclusive Ef52% |
Positive LGE T2 Negative EF 55% |
||
| 12 | Normal | Normal | NF, effusion | NF |
Positive LGE effusion T2 Inconclusive EF 66% |
Not performed | |
| 13 | ST changes | < = 1 mm ST elevation | Mild LV dysf FS 30% | no follow-up |
Inconclusive LGE T2 Negative EF 56% |
Not performed | |
| 14 | ST changes | Normal | NF | NF |
Positive LGE T2 Positive Ef 59% |
Not performed | |
| 15 | Normal | Normal | NF | NF |
Positive DG T2 Positive EF 56% |
Not performed | |
NF normal LV function, LGE late glandolinium enhancement, SF shortening fraction, MR mitral regurgitation, EF ejection fraction, LV left ventricle
After 6 months, clinical symptoms were resolved in all patients. Echocardiography demonstrated normal ventricular function, however, in one patient a small pericardial effusion remained. A repeat scan performed 5–6 months after the initial scan was positive for scar formation in 7/9 patients. Four patients had significant mid-myocardial and sub-epicardial patchy late enhancement, resembling the findings on the first CMR during the acute phase, and one patient had persistent mild myocardial dysfunction.
Discussion
Myocardial involvement as part of active COVID-19 infection has been reported in up to 17–20% of adult patients [20–22]. Silent myocardial involvement after COVID-19 infection is also prevalent; 2.3% of 1597 athletes testing positive for COVID-19 showed signs of myocardial involvement by MRI, while only 0.3% had symptoms—chest pain, dyspnea, and palpitations [23]. Milder symptoms have been found in children with COVID-19 who have a better prognosis than adults [24]; however, significant morbidity and even mortality have been reported [25].
Since the introduction of Pfzer–BioNTech mRNA BNT162b2 COVID-19 vaccines in Israel, close to 2,000,000 adolescents and young adults between the ages of 16–19 years were vaccinated with two doses of the vaccine. There were 30 cases of myocarditis reported in this patient population [7]. Recently, demographic data relating to post immunization myocarditis in adolescents aged 12–15 have been published. Out of 404,407 adolescents who received the first dose of the vaccine and 326,463 who received the second dose of the vaccine, 18 cases of myocarditis requiring hospitalization were recorded. The incidence of myocarditis after the second dose of the vaccine in this patient population was found to be 1 case per 12,361 in males and 1 case per 144,439 in females [26]. We have records of 15 of those patients who underwent CMR approximately 3 weeks after the initial diagnosis. In the majority of these patients, the disease was mild, resolving within days of anti-inflammatory treatment, though three patients presented with mild to moderate ventricular dysfunction and three with pericardial effusion.
Myocarditis after vaccination against viral diseases, such as influenza and smallpox, has been previously described [27]; its incidence is quite similar to myocarditis found after COVID-19 vaccination [28]. The characteristics of patients presenting with post COVID-19 vaccination myocarditis in our series are similar to those reported in the literature. Most patients who develop myocarditis following influenza and smallpox immunization developed symptoms 3 days after the administration of the second dose, some preceded by fever and myalgia. There was male predominance, and the patients usually presented with chest pain, elevated cardiac troponin, and ECG changes. Only a small percentage had ventricular dysfunction, and all had a pathologic CMR [11]. Only 26% of our patients met the Lake Louise criteria for myocarditis and had evidence of edema on CMR. This low rate may be due to the time that passed from the initial clinical presentation to CMR imaging (3 weeks). Positive myocardial edema is usually found between 7 and 14 days after the initial clinical presentation [29].
To the best of our knowledge, this is the first documentation of a 5–6 month CMR follow-up study of patients with proven post COVID-19 vaccination myocarditis. Of our 15 original patients, nine had repeat CMR studies, and in seven we found pathological findings demonstrating significant mid-myocardial and sub-epicardial scattered positive late enhancement (LGE) of the left ventricle, resembling the findings on the first CMR during the acute phase and suggesting residual scar. One patient had persistent mild myocardial dysfunction.
There are two myocardial pathological processes that need to be addressed clinically. The first is the acute inflammatory process. Acute inflammation and active myocarditis have been shown to be responsible for 2–8% of cardiac sudden deaths in athletes [30, 31]. There is no specific test that can establish complete resolution of the inflammatory process, as sudden cardiac death may occur despite normal LV function and regardless of the level of serum cardiac troponin. The general consensus is that patients with the diagnosis of myocarditis should be restricted from exercise programs for a period of 3–6 months according to the clinical duration and severity of the symptoms [32–34]. In our series, all patients made a full and rapid clinical recovery.
The second pathological process that has to be addressed is the resolution of the inflammatory process and presence of residual scar. CMR is an excellent tool in detecting myocarditis and edema during the acute phase [35, 36]. LGE during the acute phase of the disease is a strong independent predictor of events during follow-up [34].
In CMR studies performed 6 months after clinical myocarditis we found LGE in 7/9 patients who had an abnormal initial CMR scan, which is suggestive of residual myocardial scaring in this group, and only2 had resolution of CMR findings. The clinical significance of residual scarring is not known, although it has been demonstrated that myocardial scar is a potential substrate for myocardial arrhythmias [37]. Current recommendations for patients recovering from viral myocarditis are to resume physical activity if left ventricular function is preserved, and if the patient is free from ventricular and supraventricular arrhythmias during maximal exercise and on 24-h ECG monitoring. Furthermore, it is recommended that these patients should remain under annual clinical surveillance [32–34].
Conclusions
Myocarditis in adolescents following the Pfzer–BioNTech mRNA BNT162b2 COVID-19 vaccine is an extremely rare complication that affects predominantly males, and usually appears within the first week after administration of the second dose of the vaccine. It is usually mild and resolves with anti-inflammatory treatment. Late follow up demonstrated resolution of the acute inflammatory process in all patients, and a complete resolution of clinical symptoms. Some patients demonstrated evidence of residual myocardial scarring, though the significance of scarring remains unknown.
Abbreviations
- PCVM
Post-COVID vaccination myocarditis,
- MIS-C
Multisystem inflammatory syndrome
- CMR
Cardiovascular Magnetic resonance
- LGE
Delayed glandolinium enhancement
- M
Male
- F
Female
- CP
Chest pain
- NF
Normal function
- PE
Pericardial effusion
- CMR
Cardiac MRI
- SF
Shortening Fraction
- NSAID
Non-steroidal anti-inflamatory drugs
Funding
No funding was received for conducting this study.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO TEAM (2021) Weekly epidemiological update on COVID-19, World Health Organization, Emergency Response. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---19-october-2021. Accessed 9 Mar 2022
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021;49:15–28. doi: 10.1007/s15010-020-01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. 2021;180:2019–2034. doi: 10.1007/s00431-021-03993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Israel Ministry of Environment (2021) Status of vaccination in Israel. https://datadashboard.health.gov.il/COVID-19/general. Accessed Oct 2021. (In Hebrew)
- 7.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain SS, Steele JM, Fonseca B, et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148:e2021053427. doi: 10.1542/peds.2021-053427. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (2021) Myocarditis and pericarditis following mRNA COVID-19 vaccination. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html. Accessed 9 Mar 2022
- 10.Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw KE, Cavalcante JL, Han BK, Gössl M. Possible association between COVID-19 vaccine and myocarditis: clinical and CMR findings. JACC Cardiovasc Imaging. 2021;14:1856–1861. doi: 10.1016/j.jcmg.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickey JB, Albert E, Badr M, et al. A series of patients with myocarditis following SARS-CoV-2 vaccination with mRNA-1279 and BNT162b2. JACC Cardiovasc Imaging. 2021;14:1862–1863. doi: 10.1016/j.jcmg.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim RJ, Kim HW, Jenista ER, et al. Patients with acute myocarditis following mRNA covid-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson KF, Ammirati E, Adler ED, et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:506–508. doi: 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan M, Montgomery J, Engler R, et al. Myocarditis following immunization with mRNA covid-19 vaccines in members of the us military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosner CM, Genovese L, Tehrani BN, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rights of the Insured under the National Health Insurance Law. https://www.health.gov.il/English/Topics/RightsInsured/RightsUnderLaw/Pages/default.aspx. Accessed Mar 2022
- 19.Clalit Health Services HMO - Israel (2021) The number of patients 16–18 immunized by Clalit HMO. https://www.clalit.co.il/he/coronavirus/Pages/data.aspx. Accessed 9 Mar 2022
- 20.Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bavishi C, Bonow RO, Trivedi V, et al. Special article—Acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog Cardiovasc Dis. 2020;63:682–689. doi: 10.1016/j.pcad.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniels CJ, Rajpal S, Greenshields JT, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 cardiac registry. JAMA Cardiol. 2021;6:1078–1087. doi: 10.1001/jamacardio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Gonzalez M, Castellano-Martinez A, Cascales-Poyatos HM, Perez-Reviriego AA. Cardiovascular impact of COVID-19 with a focus on children: a systematic review. World J Clin Cases. 2020;8:5250–5283. doi: 10.12998/wjcc.v8.i21.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 vaccination in Israeli adolescents. N Engl J Med: NEJM. 2022 doi: 10.1056/NEJMc2116999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engler RJM, Nelson MR, Collins LC, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS ONE. 2015;10:e0118283. doi: 10.1371/journal.pone.0118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckart RE, Love SS, Atwood JE, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44:201–205. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Monney PA, Sekhri N, Burchell T, et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart. 2011;97:1312–1318. doi: 10.1136/hrt.2010.204818. [DOI] [PubMed] [Google Scholar]
- 30.Maron BJ, Doerer JJ, Haas TS, et al. Sudden deaths in young competitive athletes analysis of 1866 deaths in the united states, 1980–2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 31.Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic association athletes a decade in review. Circulation. 2015;132:10–19. doi: 10.1161/CIRCULATIONAHA.115.015431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelliccia A, Solberg EE, Papadakis M, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: Position statement of the sport cardiology section of the European Association of Preventive Cardiology (EAPC) Eur Heart J. 2019;40:19–33. doi: 10.1093/eurheartj/ehy730. [DOI] [PubMed] [Google Scholar]
- 33.Maron BJ, Udelson JE, Bonow RO, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 34.Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021;144:e123–e135. doi: 10.1161/CIR.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 35.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mewton N, Dernis A, Bresson D, et al. Myocardial biomarkers and delayed enhanced cardiac magnetic resonance relationship in clinically suspected myocarditis and insight on clinical outcome. J Cardiovasc Med. 2015;16:696–703. doi: 10.2459/JCM.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 37.Schnell F, Claessen G, La Gerche A, et al. Subepicardial delayed gadolinium enhancement in asymptomatic athletes: let sleeping dogs lie? Br J Sports Med. 2016;50:111–117. doi: 10.1136/bjsports-2014-094546. [DOI] [PubMed] [Google Scholar]