Abstract
Background
Many postmortem studies address the cardiovascular effects of COVID-19 and provide valuable information, but are limited by their small sample size.
Objectives
The aim of this systematic review is to better understand the various aspects of the cardiovascular complications of COVID-19 by pooling data from a large number of autopsy studies.
Data sources
We searched the online databases Ovid EBM Reviews, Ovid Embase, Ovid Medline, Scopus, and Web of Science for concepts of autopsy or histopathology combined with COVID-19, published between database inception and February 2021. We also searched for unpublished manuscripts using the medRxiv services operated by Cold Spring Harbor Laboratory.
Study eligibility criteria
Articles were considered eligible for inclusion if they reported human postmortem cardiovascular findings among individuals with a confirmed SARS coronavirus type 2 (CoV-2) infection.
Participants
Confirmed COVID-19 patients with post-mortem cardiovascular findings.
Interventions
None.
Methods
Studies were individually assessed for risk of selection, detection, and reporting biases. The median prevalence of different autopsy findings with associated interquartile ranges (IQRs).
Results
This review cohort contained 50 studies including 548 hearts. The median age of the deceased was 69 years. The most prevalent acute cardiovascular findings were myocardial necrosis (median: 100.0%; IQR, 20%–100%; number of studies = 9; number of patients = 64) and myocardial oedema (median: 55.5%; IQR, 19.5%–92.5%; number of studies = 4; number of patients = 46). The median reported prevalence of extensive, focal active, and multifocal myocarditis were all 0.0%. The most prevalent chronic changes were myocyte hypertrophy (median: 69.0%; IQR, 46.8%–92.1%) and fibrosis (median: 35.0%; IQR, 35.0%–90.5%). SARS-CoV-2 was detected in the myocardium with median prevalence of 60.8% (IQR 40.4-95.6%).
Conclusions
Our systematic review confirmed the high prevalence of acute and chronic cardiac pathologies in COVID-19 and SARS-CoV-2 cardiac tropism, as well as the low prevalence of myocarditis in COVID-19.
Keywords: Cardiac pathology, COVID-19, Myocarditis, Postmortem, SARS-CoV-2, Systematic review
Introduction
Preexisting cardiovascular comorbidities are prevalent among patients with COVID-19 and associated with a higher mortality rate [[1], [2], [3]]. For example, in the study reported by the Chinese Centre for Disease Control and Prevention describing the early experience with the epidemic in the Hubie province, patients with cardiovascular comorbidities had a case fatality rate of 10.5% compared with an overall cohort fatality rate of 2.3% [4]. There is also emerging robust evidence to suggest long-term cardiovascular sequalae after acute COVID-19 infection with an increased risk of incident conditions, including dysrhythmias, ischemic and nonischemic heart disease, myocarditis, and thromboembolic disease, among different COVID-19 disease severity groups compared with patients not infected with COVID-19 [5].
In addition, echocardiographic studies in populations infected with COVID-19 have demonstrated a high prevalence of ventricular dysfunction. In a prospective international study of 1216 patients with COVID-19, overall left and right ventricular dysfunction were reported in 39% and 33%, respectively [6]. Even in patients without preexisting cardiac disease, abnormal echocardiographic findings were evident in 46% of patients, with 13% manifesting severe abnormalities [6]. Acute myocardial injury manifesting as an elevation in cardiac troponins has been reported in 7% to 28% of patients with COVID-19 [[7], [8], [9], [10]]. Such acute cardiac injury was associated with higher overall mortality [10]. In a meta-analysis of 13 studies, the risk of death was high among patients with COVID-19 who had acute myocardial injury as defined by elevated serum troponins (risk ratio: 7.95; CI, 5.12–12.34; p <0.001; I2 = 65%) [11].
Several mechanisms have been proposed to explain acute myocardial injury and ventricular dysfunction in patients with COVID-19, including supply–demand mismatch secondary to hypoxemia and elevated cardiac demand, direct damage inflicted by inflammatory cytokines, microvascular dysfunction, myocarditis, coagulation abnormalities, and coronary artery plaque instability [12,13]. Other proposed mechanisms, such as vasospasm, microvascular thrombosis, and myocarditis, could be responsible for the ST-segment elevation [14].
A direct pathologic cardiovascular examination of decedents provides important information about the true frequency of cardiac complications among patients with COVID-19, and sheds light on possible pathologic mechanisms. Early on, small postmortem studies described evidence of myocardial inflammation associated with myocyte necrosis in patients with COVD-19 [15,16], as well as a possible direct SARS coronavirus type 2 (CoV-2) infection of the heart [17]. Moreover, nonspecific longstanding findings, such as cardiac hypertrophy and fibrosis, suggest underlying cardiovascular disease in a subset of these patients. Multiple subsequent studies have been published with varying sample sizes, methodologies, and findings. These studies provide valuable information about the nature of cardiac involvement in patients with COVID-19. However, their small sample sizes make deriving a clear picture of the true frequencies of cardiovascular complications in this novel disease challenging. In this international collaboration, we undertook a systematic review to better understand the pathologic cardiac findings in patients with COVID-19 at the time of postmortem evaluation.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. The protocol of this review was registered in PROSPERO (CRD42020223551).
Literature search and study selection
The literature was searched by a medical librarian for the concepts of autopsy or histopathology combined with COVID-19. The search strategies were created using keywords and standardized index terms (Doc. S1). Searches were run in February 2021 in Ovid EBM Reviews, Ovid Embase (1974+), Ovid Medline (1946+, including ePUB ahead of print, in-process, and other nonindexed citations), Scopus (1970+), and Web of Science (1975+). We also searched for unpublished manuscripts using the medRxiv services operated by Cold Spring Harbor Laboratory. In addition, we searched Google Scholar and the references of eligible studies and review articles.
Articles were considered eligible for inclusion if they were studies with human participants and reported cardiac autopsy findings among individuals with a SARS-CoV-2 infection. We included studies published in any language.
Identification of studies
Two reviewers (RA and SO) examined the titles and abstracts of articles using the studies selection criteria. Then, they examined the full texts to confirm that each article met the eligibility criteria.
Data collection
Data were extracted by two reviewers (R.A. and S.O.) and in duplicates into a prespecified data collection form. Disagreements were discussed with the senior reviewers (I.T. and T.K.). Data were collected on the following prespecified outcomes: 1) Study location, study type, number of cases, patient selection, selection bias, and autopsy type; 2) baseline characteristics, including age, sex, ethnicity, body mass index, cause of death, days to death, and presence of comorbidities; 3) laboratory test values, including maximum serum troponin levels, serum brain natriuretic peptide, serum ferritin, and D-dimer levels; 4) cardiac autopsy findings; and 5) ultrastructural studies, including immunohistochemistry and electron microscopy. The Cardiac Autopsy in COVID-19 Study Group collaborators completed a data collection form (Doc. S2).
One author assessed the studies for risk of selection, detection, and reporting biases. Specifically, studies were evaluated on whether consecutively deceased patients with COVID-19 underwent a cardiac autopsy to reduce selection bias.
Statistical analyses
The number and percentage of patients manifesting different findings during cardiac autopsies were extracted from each study and confirmed with the studies' authors. We initially planned to perform meta-analyses to obtain pooled estimates of the different findings' prevalences. However, this was not possible due to the limited number of studies that performed consecutive cardiac autopsies. We report the median prevalence of cardiac autopsy findings across studies with sample sizes ≥5, with associated interquartile ranges (IQRs) (see Table 1 ).
Table 1.
Summary of median prevalence of cardiac autopsy findings of studies with ≥5 patients
| Autopsy finding | Pathology classification | Number of studies | Total number of patients | Median, % | Quarter 1, % | Quarter 3, % |
|---|---|---|---|---|---|---|
| Viral presence | Virology | 10 | 116 | 60.8 | 40.4 | 95.6 |
| Extensive myocarditis | Myocarditis | 10 | 175 | 0.0 | 0.0 | 0.0 |
| Focal active myocarditis | Myocarditis | 13 | 235 | 0.0 | 0.0 | 13.4 |
| Multifocal myocarditis | Myocarditis | 9 | 131 | 0.0 | 0.0 | 2.1 |
| Infiltrates without myocyte injury | Myocarditis | 15 | 279 | 0.6 | 0.0 | 9.8 |
| Pulmonary embolism | Thromboembolic | 15 | 311 | 22.2 | 16.7 | 32.1 |
| Microvessel thrombi | Thromboembolic | 8 | 103 | 36.2 | 17.6 | 61.7 |
| Cardiac large vessel thrombi | Thromboembolic | 9 | 162 | 14.3 | 13.3 | 22.8 |
| Acute myocardial infarction | Thromboembolic | 7 | 104 | 11.8 | 7.9 | 13.8 |
| Small vessel vasculitis | Inflammatory | 3 | 86 | 28.6 | 16.0 | 32.5 |
| Epi-pericarditis | Inflammatory | 6 | 110 | 15.5 | 11.9 | 19.2 |
| Cardiac oedema | Gross pathology | 4 | 46 | 55.5 | 19.5 | 92.5 |
| Necrosis | Gross pathology | 9 | 64 | 100.0 | 20.0 | 100.0 |
| Fibrosis | Chronic | 13 | 183 | 42.9 | 35.0 | 90.5 |
| Amyloidosis | Chronic | 8 | 131 | 13.6 | 9.8 | 17.4 |
| Atherosclerotic coronary artery disease | Chronic | 14 | 250 | 46.2 | 21.6 | 80.1 |
| Hypertrophy | Chronic | 18 | 303 | 69.0 | 46.8 | 92.1 |
Results
Search results and studies characteristics
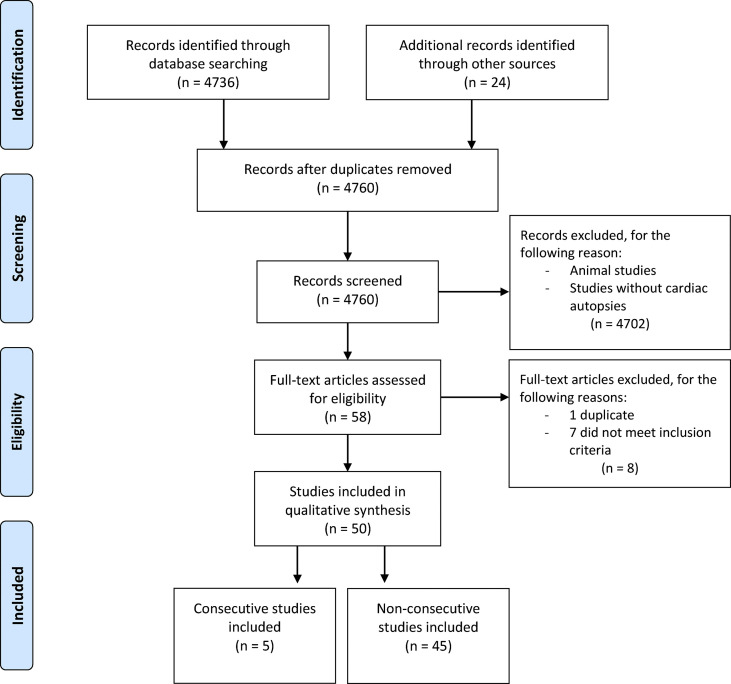
The search yielded 4760 results. We examined the entire text of 58 manuscripts after removing duplicates and screening the titles. However, eight studies were excluded, leaving 50 studies with 548 hearts in the final cohort (Fig. 1 ). Most studies were case reports (n = 13) or case series (n = 37; Doc. S3). Autopsy cases were acquired from manuscripts spanning experiences from 15 countries. The number of cases per study ranged from 1 to 80 (median: 4.5), and five cases were identified as reporting consecutive autopsies (encompassing 155 subjects) [[18], [19], [20], [21], [22]]. There were 42 minimally invasive autopsies, 102 partial autopsies, and 301 complete autopsies among the autopsies where completeness was stated or could be inferred.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses 2009 flow diagram.
Patient demographics, comorbidities, and cause of death
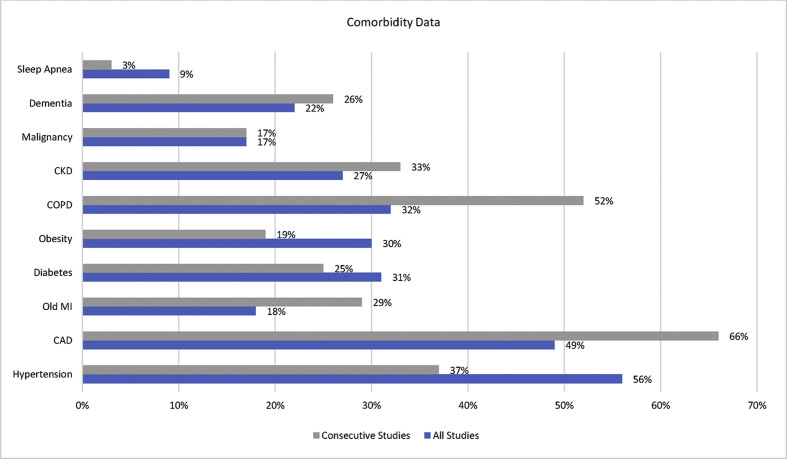
The median age of the deceased was 69 years (range, 22–97 years; n = 548), and 62% of cases were men (n = 338 of 548). The most common comorbidities were systemic hypertension (n = 298; 56%) and coronary artery disease (n = 252; 49%). Other less common comorbidities included chronic obstructive pulmonary disease, diabetes, obesity, chronic kidney disease, old myocardial infarction, dementia, malignancy, and sleep apnoea (Fig. 2 ). Elevated troponin was demonstrated in 55% of cases.
Fig. 2.
Bar chart showing reported comorbidities of deceased patients included in this cohort. CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction. Data labels show the prevalence of reported comorbidities (can overlap in a single patient).
The cause of death was reported in 479 cases, with the most reported being respiratory in origin. However, in 62 cases, cardiac involvement was identified as a key factor in mortality. The median time from the onset of symptoms to death was 9 days (range, 0–71 days; n = 401).
Cardiovascular autopsy findings
General findings
Cardiac abnormalities were found in gross pathology or histology test results in almost all cases. Heart weights were available for 276 hearts (51%), with a median weight of 465 g (range, 238–1070 g).
SARS coronavirus type to infection of the heart
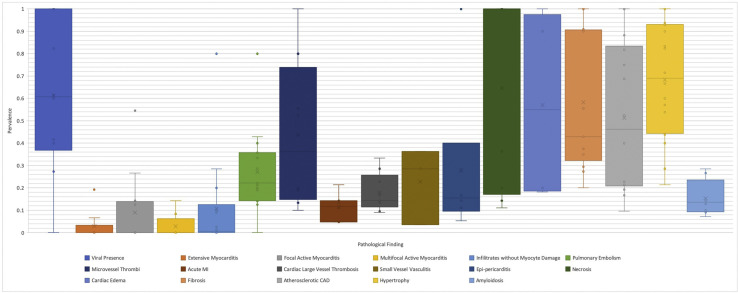
Nineteen studies [[17], [18], [19], [23], [24], [25], [26], [27], [28]] with 217 cases explored the presence and localization of SARS-CoV-2 infection in the heart using different modalities, including RT-PCR, immunohistochemistry, in situ hybridization, and electron microscopy. Ten studies [18,19,[26], [27], [28],30,32,33,35,37] with a total of 116 cases detected SARS-CoV-2 infection in the cardiac tissues in 70 cases with a median of 60.8% (IQR, 40.4%–95.6%; Fig. 3 ).
Fig. 3.
Box-and-whisker plot of cardiac autopsy findings of studies with ≥5 patients as median percentage prevalence and associated interquartile ranges. CAD, coronary artery disease.
Active replication of SARS-CoV-2 within the heart was determined using the RNA scope in situ hybridization technique looking for the presence of the negative strand of the SARS-CoV-2 viral RNA or through the identification of subgenomic RNA, both of which indicate active viral replication. Four investigators employed these techniques in 55 cases [18,27,28,33], and verified the presence of active SARS-CoV-2 viral replication in 15 hearts (27%).
Localization of SARS-CoV-2 within different cardiac cell compartments was studied by nine investigators [23,25,26,30,33,34,[36], [37], [38]] in 56 hearts from total of 95 cases using electron microscopy or immunohistochemistry. The presence of SARS-CoV-2 infection within the cardiomyocytes was reported in 11 hearts by four investigators [30,33,34,36]. SARS-CoV-2 infection was also detected in cardiac vascular endothelial cells in seven hearts and in cardiac fibroblasts in one heart [26,34]. On the other hand, other investigators [23,25,26,37,38] could not detect SARS-CoV-2 infection within any cell type in the heart.
Myocarditis
The majority of studies did not specify what definition of myocarditis was used. However, we inferred from the description of the histopathological findings that the Dallas criteria were used by most studies. Several investigators used immunohistochemical studies with different antibodies to identify subtypes of cellular infiltrates, but most did not use immunohistochemical criteria to diagnose myocarditis. In total, 36 cases had myocarditis and 16 had inflammatory infiltrates but no myocyte damage (Fig. 3).
Few cases reported extensive myocarditis, ranging from 0.0% to 19.3%, with a median of 0.0% across 10 studies [20,23,26,28,32,37,[39], [40], [41], [42]] with a total of 175 cases (Fig. 3). Grosse et al., who authored the only consecutive study to report a prevalence for this finding, did not find any cases of extensive myocarditis across 14 cases. Focal active myocarditis was reported by 13 studies [20,21,23,25,26,28,32,[37], [38], [39], [40],42,43], ranging from 0.0% to 55.5%. Nine studies [19,20,26,28,32,37,39,42,44] with total of 131 cases described multifocal myocarditis with a median prevalence of 0.0% (IQR, 0.0%–2.1%). Finally, 15 studies [18,[20], [21], [22],[26], [27], [28],32,35,37,39,40,42,44,45] with 279 cases reported infiltrates without myocyte damage with a median prevalence of 0.6% (range, 0.0%–28.9%; Fig. 3).
Other acute cardiac pathologic changes
Necrosis had the highest median reported prevalence across nine studies, of which none were considered consecutive studies, including 64 autopsies [28,29,31,34,40,43,[46], [47], [48]] with a median of 100% (n = 64; IQR, 20.0%–100%). This was followed by cardiac interstitial oedema (n = 46; median: 55%; IQR, 19.5%–92.5%) [22,27,30,42], with Duarte-Neto et al. reporting a prevalence of 90% across ten consecutive autopsies (Fig. 3).
Microvessel thrombi had the highest reported median prevalence across the category of thromboembolic disease among eight studies, reporting a similar prevalence across the studies [22,23,27,28,30,38,44,49] with 43 of 103 cases (median: 36.2%; IQR, 17.5%–61.7%). Alternatively, acute myocardial infarction had the lowest reported median prevalence in this category (median: 11.8%; IQR, 7.9%–13.8%; Fig. 3). Acute epi-pericarditis was reported with a median prevalence of 15.5% (IQR, 11.9%–19.2%) across six studies [28,30,33,38,39,41] in 29 of 110 cases, and small vessel vasculitis had a median reported prevalence of 28.6% (IQR, 16.0%–32.5%) across three studies [38,41,43] in 12 of 86 cases (Fig. 3). Other less frequently reported findings include single-cell ischemia in one of seven patients [35], myocyte ischemic degeneration with pyknosis in one case report [50], and contraction bands in one of three cases [51].
Chronic cardiac findings
Hypertrophy was the most common pathological finding with a median of 69.0% (IQR, 46.8%–92.1%) across 18 studies [[18], [19], [20],22,23,25,28,[31], [32], [33],35,38,39,[41], [42], [43],45,49] in 197 of 303 cases. Fibrosis was reported in 13 studies [20,22,25,27,28,[30], [31], [32],37,[42], [43], [44], [45]] with a median of 42.9% (IQR, 35.0%–90.5%) in 104 of 183 cases. Among these 13 studies, ten studies reported various details about the nature of fibrotic changes [20,25,27,28,30,31,37,42,44,45]. Two studies [42,44] reported on the severity of fibrosis with 32 cases (20 with mild and 8 with moderate fibrosis). Six studies [20,27,28,30,31,44] reported on the extent of fibrosis in a total of 98 cases. There was fibrosis in 46 of these cases, which was diffuse in 18 cases and focal or patchy in 28 cases. Replacement fibrosis was described by two authors [25,27] in eight cases. Eight studies [18,20,23,25,28,31,35,45,49] with 131 patients observed amyloidosis in 21 cases with a median prevalence of 13.6% (IQR, 9.8%–17.4%). The type of amyloidosis was reported in 14 of these cases, and determined to be transthyretin in 13 cases and amyloid P in one case, with patient age ranging between 71 and 96 years [20,23,28,31,35,45]. Other less-reported pathologies were chronic pericarditis, reported by three studies in 17 of 47 cases [28,33,43], and ischemic heart disease in 1 of 3 cases in one study [50].
Discussion
General autopsy findings and clinical correlation
This systematic review of pathology-derived cardiac changes in patients with COVID-19 included 50 studies with more than 500 cases, and was the effort of an international collaboration. The most prevalent chronic changes were myocardial hypertrophy, underlying coronary artery disease, and fibrosis (median: 69.0%, 46.2%, and 42.9%, respectively). The high prevalence of chronic cardiac pathologies among patients who died due to COVID-19 supports the findings from previously published epidemiologic studies [[1], [2], [3]].
Interestingly, another underlying cardiac disease, amyloidosis, was reported in a median of 13.6% of patients with COVID-19, with patient age ranging between 71 and 96 years. The overall prevalence of cardiac amyloidosis in an unselected, sequential autopsy population was reported at approximately 4% [23]. Conversely, a Finnish autopsy study of individuals age >85 years detected cardiac amyloidosis in 25% of cases [52]. Although cardiac amyloidosis prevalence almost certainly increases with patient age, cardiac amyloidosis is also likely underdiagnosed, particularly among patients with heart failure and preserved ejection fraction [[53], [54], [55]]. Nevertheless, the relatively high proportion of cardiac amyloidosis among decedents with COVID-19 compared with unselected autopsy rates suggests that this condition may render patients vulnerable to adverse outcomes from SARS-CoV-2 infection. This is further supported by the average age of patients with cardiac amyloidosis who died of COVID-19. Possible mechanisms for this complication have been proposed; however, decreased cardiac reserve innate to underlying cardiovascular disease, including amyloidosis, likely plays a significant role [56].
The prevalence of acute thromboembolic pathologies in descending frequency included microvessel thrombi (36.2%), pulmonary embolism (22.2%), cardiac large vessel thrombosis (14.3%), and acute myocardial infarction (11.8%). The increased cardiac and pulmonary vascular thrombi correlate strongly with the clinical evidence of increased thromboembolic phenomena in patients with COVID-19. Moreover, these thrombotic changes, along with the observed high prevalence of acute cardiac injuries (e.g. necrosis, oedema, and epi-pericarditis) are concordant with the clinically documented ventricular dysfunction [6] and serologic markers of cardiac injury, such as increased troponins [[7], [8], [9], [10]]. In fact, microvascular thrombosis has been cited as the causative agent of cardiac injury in most decedents with COVID-19 [57]. This finding dovetails with the lower prevalence of large vessel cardiac thrombosis, and is consistent with the coronary angiographic findings in patients with COVID-19, wherein a culprit lesion was not identified in more than 40% of patients with suspected acute myocardial infarction [14].
SARS-CoV-2 cardiac tropism
SARS-CoV-2 gains entry into the host cells through the binding of its spike protein to the angiotensin-converting enzyme 2 with the help of the host transmembrane protease serine 2 [58]. Both proteins have been shown to be expressed in the heart [23,[59], [60], [61]]. The predecessor of SARS-CoV-2 (SARS-CoV and its associated syndrome SARS) also uses the angiotensin-converting enzyme 2 protein for cell entry, and has been shown to infect the heart and induce inflammatory changes based on data from the first decade of the 21st century [61]. These findings, along with the clinical observations of acute cardiac injury among patients with COVID-19, prompted several investigators to address three important questions: 1) Is SARS-CoV-2 present in the hearts of decedents with COVID-19; 2) if so, which cell type(s) does SARS-CoV-2 infect; and 3) can SARS-CoV-2 replicate in heart tissues?
Twenty studies explored the presence of SARS-CoV-2 within the heart, the majority of which targeted the identification of SARS-CoV-2 RNA in heart tissues using RT-PCR or in situ hybridization. Other employed techniques included immunohistochemistry to identify SARS-CoV-2 proteins (e.g. spike or nucleocapsid protein), as well as electron microscopy. These investigators identified the presence of SARS-CoV-2 in almost 60% of the examined hearts. Additionally, a few investigators identified SARS-CoV-2 replication within heart tissues in several cases [18,27,28,33]. Furthermore, studies investigated cell-type localization of SARS-CoV-2 within the heart, and provided evidence of the presence of SARS-CoV-2 viral particles within the cardiomyocytes [30,33,34,36]. Bulfamante et al. observed degenerative changes in cardiomyocytes containing SARS-CoV-2 viral particles [33]. These findings are supported by a study that demonstrated SARS-CoV-2 infection and propagation in induced pluripotent stem cell-derived cardiomyocytes [62]. SARS-CoV-2 has also been found in vascular endothelial cells and cardiac fibroblasts [26,34]. These reports establish SARS-CoV-2 cardiac tropism, and present a possible link between SARS-CoV-2 and certain acute cardiac pathologies (e.g. myocarditis). However, although RT-PCR represents a time-efficient method to determine tissue positive for SARS-CoV-2, RT-PCR does not allow for tissue localization. Wong et al. suggested and attempted to validate a Fluorescence In Situ Hybridization (FISH) method using positive and negative controls by detecting endogenous human genes (POLR2A and PPIB) and a bacterial gene (dap gene of Bacillus subtilis) to allow for a tissue-specific analysis [63].
SARS-CoV-2-induced myocarditis
In this review, we subdivided the reported cardiac inflammatory processes in patients with COVID-19 into four categories based on the degree of myocardial involvement and the presence of associated myocyte damage. Overall, the prevalence of each category was low, with vast differences between individual studies that cannot be explained solely by the methodological differences of the studies, and likely indicate significant selection and reporting bias. The median reported prevalence of extensive myocarditis, multifocal active myocarditis, and focal active myocarditis were all 0.0%, and the median prevalence of inflammatory infiltrate without myocyte damage was 0.6%.
Regrettably, clinical correlation or pooled prevalence estimates in the included reported autopsy series were not possible due to the heterogenous results and paucity of clinical and imaging data provided. Nonetheless, reports of clinically diagnosed myocarditis with pathologic correlation have been reported among inpatients with COVID-19 [36,48,64]. Intriguingly, Gauchotte et al. demonstrated pathologic evidence of myocarditis without lung involvement, and further showed the presence of the SARS-CoV-2 genome in cardiomyocytes in this case [36]. This finding is concordant with other studies suggesting a greater degree of inflammation with viral presence in the heart [65].
The diagnosis of most cases of myocarditis included in this review were based on the Dallas criteria. This methodology, although widely accepted, is not without its inherent limitations. First and foremost, the Dallas criteria were developed to diagnose myocarditis by endomyocardial biopsy (EMB), not autopsy, wherein a more abundant amount of tissue is available for histologic evaluation. The generalization (and clinical significance) of small foci of myocyte damage within autopsy-derived cardiac tissue is challenging to ascertain. Other limitations of the Dallas criteria include significant interobserver variability and sampling errors [66,67].
Although less of an issue in autopsy-derived tissue, the focal nature of the disease leads to sampling errors that have been shown to compromise the sensitivity of the histopathological diagnosis of myocarditis by EMB [68,69]. Chow et al. had estimated that a mean of 17 samples per patient would be required to establish a diagnosis of myocarditis [69], which likely explains why examining an increased number of cardiac tissue blocks at the time of autopsy resulted in a greater likelihood of identifying focal myocarditis.
The overall low prevalence of myocarditis in patients with COVID-19 is of interest, particularly when placed in the greater context of the available literature. In a recent meta-analysis on the diagnosis of myocarditis by EMB (including 61 studies with 10,491 patients), the prevalence of myocarditis according to the Dallas criteria was 8.04% [70]. This diagnosis was made on the relatively limited amount of tissue provided by EMB. In contrast, this review shows a myocarditis prevalence of 8% in abundant available tissue, often comprising multiple blocks of myocardium with greater orders of magnitude in the amount of tissue to examine. The pretest factors among these data points differ, but underscores the overall low prevalence of myocarditis in COVID-19 deaths and is concordant with previous literature reviews on the topic [71].
Prior studies have shown the added sensitivity of immunohistochemistry in the diagnosis of myocarditis. Katzmann et al. showed that the sensitivity of the Dallas criteria in detecting myocarditis was much lower than when immunohistochemistry is utilized, with a detection rate of 50.8% (vs. 8.04% without immunohistochemistry) [70]. In another study of 84 cases of myocarditis based on the immunohistochemistry criteria, applying the Dallas criteria without immunohistochemistry would have categorized only 8% of these case as active myocarditis [72].
The true prevalence of myocarditis in COVID-19 remains very hard to determine from the current autopsy and imaging studies, the latter of which shows a discordantly high prevalence of myocarditis compared with postmortem examinations. A recent systematic review of cardiovascular magnetic resonance findings in COVID-19 including 199 patients showed that myocarditis was the most prevalent diagnosis (40.2%) [73]. Future studies should integrate clinical imaging and more rigorous and systematic autopsy studies to help resolve this issue. Such initiative should be conducted in the form of an international registry that uses a unified autopsy examination and imaging protocols in accordance with published guidelines [74,75].
Limitations
This systematic review included the largest number of studies and cases published to date on cardiac changes in fatal COVID-19 with both qualitative and quantitative analyses of different cardiac pathologies observed in COVID-19. However, our study has several limitations. First, the majority of the included studies were small. Second, these studies were heterogeneous in their methodologies and patient cultural origins, with very few studies performing consecutive autopsies, which makes meta-analyses unfeasible. Moreover, selection and reporting bias likely affected most included studies, as evidenced by the nonconsecutive nature of case recruitment and the very high differences between the studies in the perveances of reported pathologies.
Conclusions
Our systematic review confirmed the high prevalence of acute and chronic cardiac pathologies in the autopsy-derived hearts of decedents with COVID-19. These findings help explain observations from clinical epidemiologic studies, such as thromboembolic phenomena and acute myocardial injury. Our study also provides evidence for SARS-CoV-2 cardiac tropism, and confirmed the low prevalence of myocarditis in patients with COVID-19.
Transparency declaration
Elie Berbari reports royalties or licenses from UTD of <$5000 per year. Amy V. Rapkiewicz reports payment for expert testimony by Eric Hack, Esq. Bruno Märkl reports grants or contracts from the German Registry of COVID-19 Autopsies, funded by the Federal Ministry of Health and the Federal Ministry of Education and Research within the framework of the network of university medicine. Diana Lindner reports support for the present manuscript, grants, and contracts from the German Centre for Cardiovascular Research, Deutsche Herzstiftung. Dirk Westermann reports consulting fees and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Abiomed, Bayer, AstraZeneca, Novartis, and Medtronic. Klaus Hirschbühl reports grants or contracts from the German Registry of COVID-19 Autopsies, funded by the Federal Ministry of Health and the Federal Ministry of Education and Research within the framework of the network of university medicine. Luiz Fernando Ferraz da Silva reports grants or contracts, paid to their institution, from the Bill and Melinda Gates Foundation. Martin Lammens reports support for the present manuscript from the Belgian Fund for Scientific Research–Flanders. Michael Osborn reports grants or contracts from the North West London pathology research grant (£10,000), paid by their own institution, to set up a tissue bank and fund the procurement and use of tissue included in the current research, as well as payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Yale University ($300 for talking about COVID). He also reports a leadership or fiduciary role on a board, society, committee, or advocacy group as president of the Royal College of Pathologists, secretary of the BDIAP, president AAPT (all unpaid). Paulo Hilario Nascimento Saldiva reports support for the present manuscript from the Bill and Melinda Gates Foundation, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Fundação de Amparo à Pesquisa do Estado de São Paulo, and Hospital das Clinicas da Faculdade de Medicina da Universidade de Paulo–HC Convida. Tadaka Suzuki reports grants or contracts from the Japan Agency for Medical Research and Development and Japan Society for the Promotion of Science (grants in aid). There was no funding source for this study.
Author contributions
IMT designed the study. IMT, RA, SO, and MCB coordinated the study. DG designed and ran the literature search. RA, SO, OAO, RT, ZC, BZS, EB, and TK acquired the data, screened records, and extracted the data. IMT and OAO conducted the formal analyses. TK wrote the report with input from MCB and JJM. All authors provided critical conceptual input, analyzed and interpreted data, and critically revised the report.
Acknowledgments
The Cardiac Autopsy in COVID-19 Study Group consists of Alberto E. Paniz Mondolfi (Department of Pathology, Molecular and Cell-Based Medicine, New York, New York), Aloke V. Finn (CVPath Institute, Inc., Gaithersburg, Maryland; and University of Maryland, Baltimore, Maryland), Amaro Nunes Duarte-Neto (Departamento de Patologia, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil), Amy V. Rapkiewicz (NYU Winthrop Hospital, Department of Pathology, Long Island School of Medicine, Long Island, New York), Andrea Frustaci (Department of Clinical, Internal, Anesthesiologist and Cardiovascular Sciences, La Sapienza University, Rome, Italy; and Cellular and Molecular Cardiology Lab, IRCCS L. Spallanzani, Rome, Italy), Arthur-Atilla Keresztesi (Fogolyan Kristof Emergency County Hospital, Covasna County Institution of Forensic Medicine, Covasna, Romania), Brian Hanley (Department of Cellular Pathology, Northwest London Pathology, Imperial College London NHS Trust, London, UK; and Centre for Inflammatory Disease, Imperial College London, London, UK), Bruno Märkl (Institute of Pathology and Molecular Diagnostics, University Medical Center Augsburg, Augsburg, Germany), Christelle Lardi (University Center of Legal Medicine, Geneva University Hospital, Geneva, Switzerland), Clare Bryce (Icahn School of Medicine at Mount Sinai, New York, New York), Diana Lindner (Department of Cardiology, University Heart and Vascular Centre, Hamburg, Germany; and DZHK–German Center for Cardiovascular Research, Partner site, Hamburg/Kiel/Lübeck, Germany), Diego Aguiar (University Center of Legal Medicine, Geneva University Hospital, Geneva, Switzerland), Dirk Westermann (Department of Cardiology, University Heart and Vascular Centre, Hamburg, Germany; and DZHK–German Center for Cardiovascular Research, Partner site, Hamburg/Kiel/Lübeck, Germany), Edana Stroberg (Office of the Chief Medical Examiner, Oklahoma City, Oklahoma), Eric J. Duval (Office of the Chief Medical Examiner, Oklahoma City, Oklahoma), Esther Youd (Forensic Medicine and Science, University of Glasgow, Glasgow, UK), Gaetano Pietro Bulfamante (Unità di Anatomia Patologica, Dipartimento di Scienze della Salute, Università degli Studi di Milano, Milan, Italy; and Struttura Complessa di Anatomia Patologica e Genetica Medica, ASST Santi Paolo e Carlo, Milan, Italy), Isabelle Salmon (Department of Pathology, Erasme Hospital, Université Libre de Bruxelles (ULB), Brussels, Belgium; Centre Universitaire inter Régional d'expertise en Anatomie Pathologique Hospitalière, Jumet, Belgium; and DIAPath, Center for Microscopy and Molecular Imaging, ULB, Gosselies, Belgium), Johann Auer (Department of Cardiology and Intensive Care, St. Josef Hospital Braunau, Austria; and Department of Cardiology and Intensive Care, Kepler University of Medicine Linz, Austria), Joseph J. Maleszewski (Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota; and Department of Cardiovascular Medicine, Mayo Clinic, Rochester, Minnesota), Klaus Hirschbühl (Department of Hematology and Clinical Oncology, University Medical Center Augsburg, Augsburg, Germany), Lara Absil (Department of Pathology, Erasme Hospital, ULB, Brussels, Belgium), Lisa M. Barton (Office of the Chief Medical Examiner, Oklahoma City, Oklahoma), Luiz Fernando Ferraz da Silva (Departamento de Patologia, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; and Serviço de Verificação de Óbitos da Capital, Universidade de São Paulo, São Paulo, Brazil), Luiza Moore (Department of Histopathology, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; and Cancer, Ageing and Somatic Mutation, Wellcome Sanger Institute, Cambridge, UK), Marisa Dolhnikoff (Departamento de Patologia, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil), Martin Lammens (Department of Pathology, Antwerp University Hospital, University of Antwerp, Edegem, Belgium), Melanie C. Bois (Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota), Michael Osborn (Department of Cellular Pathology, Northwest London Pathology, Imperial College London NHS Trust, London, UK; Death Investigation Committee, Royal College of Pathologists, London, UK; and Nightingale NHS Hospital, London, UK), Myriam Remmelink (Department of Pathology, Erasme Hospital, ULB, Brussels, Belgium), Paulo Hilario Nascimento Saldiva (Departamento de Patologia, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil), Philippe G. Jorens (Infla-Med Research Consortium of Excellence, University of Antwerp, Antwerp, Belgium; Department of Medicine and Health Sciences, Laboratory of Experimental Medicine and Pediatrics, University of Antwerp, Antwerp, Belgium; and Department of Intensive Care Medicine, Antwerp University Hospital, University of Antwerp, Edegem, Belgium), Randall Craver (Children's Hospital of New Orleans, New Orleans, Louisiana; and Louisiana State University Health Sciences Center, New Orleans, Louisiana), Renata Aparecida de Almeida Monteiro (Departamento de Patologia, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil), Roberto Scendoni (Institute of Legal Medicine, Department of Law, University of Macerata, Macerata, Italy), Sanjay Mukhopadhyay (Department of Pathology, Cleveland Clinic, Cleveland, Ohio), Tadaki Suzuki (National Institute of Infectious Diseases, Tokyo, Japan), Thais Mauad (Departamento de Patologia, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil), Tony Fracasso (University Center of Legal Medicine, Geneva University Hospital, Geneva, Switzerland), and Zachary Grimes (Icahn School of Medicine at Mount Sinai, New York, New York).
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.03.021.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P., et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., et al. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 5.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dweck M.R., Bularga A., Hahn R.T., Bing R., Lee K.K., Chapman A.R., et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2021;44:352–357. doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang Y., Chen T., Mui D., Ferrari V., Jagasia D., Scherrer-Crosbie M., et al. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libby P. The heart in COVID-19: primary target or secondary bystander? JACC Basic Transl Sci. 2020;5:537–542. doi: 10.1016/j.jacbts.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanini G.G., Montorfano M., Trabattoni D., Andreini D., Ferrante G., Ancona M., et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C., Zhou Y., Wang D.W. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230–232. doi: 10.1007/s00059-020-04909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M., et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindner D., Fitzek A., Bräuninger H., Aleshcheva G., Edler C., Meissner K., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosse C., Grosse A., Salzer H.J.F., Dünser M.W., Motz R., Langer R. Analysis of cardiopulmonary findings in COVID-19 fatalities: high incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. 2020;49:107263. doi: 10.1016/j.carpath.2020.107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edler C., Schröder A.S., Aepfelbacher M., Fitzek A., Heinemann A., Heinrich F., et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Leg Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duarte-Neto A.N., Monteiro R.A.A., da Silva L.F.F., Malheiros D., de Oliveira E.P., Theodoro-Filho J., et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bois M.C., Boire N.A., Layman A.J., Aubry M.-C., Alexander M.P., Roden A.C., et al. COVID-19-associated nonocclusive fibrin microthrombi in the heart. Circulation. 2021;143:230–243. doi: 10.1161/CIRCULATIONAHA.120.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bösmüller H., Traxler S., Bitzer M., Häberle H., Raiser W., Nann D., et al. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Archiv. 2020;477:349–357. doi: 10.1007/s00428-020-02881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox S.E., Li G., Akmatbekov A., Harbert J.L., Lameira F.S., Brown J.Q., et al. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142:1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- 27.Brook O.R., Piper K.G., Mercado N.B., Gebre M.S., Barouch D.H., Busman-Sahay K., et al. Feasibility and safety of ultrasound-guided minimally invasive autopsy in COVID-19 patients. Abdom Radiol (NY) 2021;46:1263–1271. doi: 10.1007/s00261-020-02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs W., Lammens M., Kerckhofs A., Voets E., Van San E., Van Coillie S., et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020;7:3772–3781. doi: 10.1002/ehf2.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schurink B., Roos E., Radonic T., Barbe E., Bouman C.S.C., de Boer H.H., et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remmelink M., De Mendonça R., D’Haene N., De Clercq S., Verocq C., Lebrun L., et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24:495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulfamante G.P., Perrucci G.L., Falleni M., Sommariva E., Tosi D., Martinelli C., et al. Evidence of SARS-CoV-2 transcriptional activity in cardiomyocytes of COVID-19 patients without clinical signs of cardiac involvement. Biomedicines. 2020;8:626. doi: 10.3390/biomedicines8120626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolhnikoff M., Ferreira Ferranti J., de Almeida Monteiro R.A., Duarte-Neto A.N., Soares Gomes-Gouvêa M., Viu Degaspare N., et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4:790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Recalde-Zamacona B., García-Tobar L., Argueta A., Álvarez L., De Andrea C.E., Fernández Alonso M., et al. Histopathological findings in fatal COVID-19 severe acute respiratory syndrome: preliminary experience from a series of 10 Spanish patients. Thorax. 2020;75:1116–1118. doi: 10.1136/thoraxjnl-2020-215577. [DOI] [PubMed] [Google Scholar]
- 36.Gauchotte G., Venard V., Segondy M., Cadoz C., Esposito-Fava A., Barraud D., et al. SARS-Cov-2 fulminant myocarditis: an autopsy and histopathological case study. Int J Leg Med. 2021;135:577–581. doi: 10.1007/s00414-020-02500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martines R.B., Ritter J.M., Matkovic E., Gary J., Bollweg B.C., Bullock H., et al. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 2020;26:2005–2015. doi: 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapkiewicz A.V., Mai X., Carsons S.E., Pittaluga S., Kleiner D.E., Berger J.S., et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaller T., Hirschbühl K., Burkhardt K., Braun G., Trepel M., Märkl B., et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beigmohammadi M.T., Jahanbin B., Safaei M., Amoozadeh L., Khoshavi M., Mehrtash V., et al. Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Int J Surg Pathol. 2021;29:135–145. doi: 10.1177/1066896920935195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.del Nonno F., Frustaci A., Verardo R., Chimenti C., Nicastri E., Antinori A., et al. Virus-negative myopericarditis in human coronavirus infection. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Michele S., Sun Y., Yilmaz M.M., Katsyv I., Salvatore M., Dzierba A.L., et al. Forty postmortem examinations in COVID-19 patients. Am J Clin Pathol. 2020;154:748–760. doi: 10.1093/ajcp/aqaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falasca L., Nardacci R., Colombo D., Lalle E., Di Caro A., Nicastri E., et al. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. 2020;222:1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basso C., Leone O., Rizzo S., De Gaspari M., van der Wal A.C., Aubry M.C., et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guagliumi G., Sonzogni A., Pescetelli I., Pellegrini D., Finn A.V. Microthrombi and ST-segment–elevation myocardial infarction in COVID-19. Circulation. 2020;142:804–809. doi: 10.1161/CIRCULATIONAHA.120.049294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buja L.M., Wolf D.A., Zhao B., Akkanti B., McDonald M., Lelenwa L., et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Craver R., Huber S., Sandomirsky M., McKenna D., Schieffelin J., Finger L. Fatal eosinophilic myocarditis in a healthy 17-year-old male with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2c) Fetal Pediatr Pathol. 2020;39:263–268. doi: 10.1080/15513815.2020.1761491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keresztesi A.A., Perde F., Ghita-Nanu A., Radu C.C., Negrea M., Keresztesi G. Post-mortem diagnosis and autopsy findings in SARS-CoV-2 infection: Forensic case series. Diagnostics. 2020;10:1070. doi: 10.3390/diagnostics10121070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X.X., Shao C., Huang X.J., Sun L., Meng L.J., Liu H., et al. Histopathological features of multiorgan percutaneous tissue core biopsy in patients with COVID-19. J Clin Pathol. 2021;74:522. doi: 10.1136/jclinpath-2020-206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Youd E., Moore L. COVID-19 autopsy in people who died in community settings: the first series. J Clin Pathol. 2020;73:840. doi: 10.1136/jclinpath-2020-206710. [DOI] [PubMed] [Google Scholar]
- 52.Tanskanen M., Peuralinna T., Polvikoski T., Notkola I.L., Sulkava R., Hardy J., et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–239. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 53.González-López E., Gallego-Delgado M., Guzzo-Merello G., de Haro-del Moral F.J., Cobo-Marcos M., Robles C., et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 54.Bennani Smires Y., Victor G., Ribes D., Berry M., Cognet T., Méjean S., et al. Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Cardiovasc Imaging. 2016;32:1403–1413. doi: 10.1007/s10554-016-0915-z. [DOI] [PubMed] [Google Scholar]
- 55.Mohammed S.F., Mirzoyev S.A., Edwards W.D., Dogan A., Grogan D.R., Dunlay S.M., et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brannagan T.H., 3rd, Auer-Grumbach M., Berk J.L., Briani C., Bril V., Coelho T., et al. ATTR amyloidosis during the COVID-19 pandemic: insights from a global medical roundtable. Orphanet J Rare Dis. 2021;16:204. doi: 10.1186/s13023-021-01834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pellegrini D., Kawakami R., Guagliumi G., Sakamoto A., Kawai K., Gianatti A., et al. Microthrombi as a major cause of cardiac injury in COVID-19. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu H., Gai S., Wang X., Zeng J., Sun C., Zhao Y., et al. Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovasc Res. 2020;116:1733–1741. doi: 10.1093/cvr/cvaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong M., Zhang J., Ma X., Tan J., Chen L., Liu S., et al. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131:110678. doi: 10.1016/j.biopha.2020.110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pérez-Bermejo J.A., Kang S., Rockwood S.J., Simoneau C.R., Joy D.A., Ramadoss G.N., et al. SARS-CoV-2 infection of human iPSC-derived cardiac cells predicts novel cytopathic features in hearts of COVID-19 patients. bioRxiv. 2020 doi: 10.1126/scitranslmed.abf7872. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong D.W.L., Klinkhammer B.M., Djudjaj S., Villwock S., Timm M.C., Buhl E.M., et al. Multisystemic cellular tropism of SARS-CoV-2 in autopsies of COVID-19 patients. Cells. 2021;10:1900. doi: 10.3390/cells10081900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auer J., Neuhierl F., Hetzmann Z. COVID-19-related fatal myocarditis in a 42-year-old female patient. Cardiol J. 2020;27:642–643. doi: 10.5603/CJ.2020.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bearse M., Hung Y.P., Krauson A.J., Bonanno L., Boyraz B., Harris C.K., et al. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Mod Pathol. 2021;34:1345–1357. doi: 10.1038/s41379-021-00790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mason J.W., O'Connell J.B., Herskowitz A., Rose N.R., McManus B.M., Billingham M.E., et al. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 67.Shanes J.G., Ghali J., Billingham M.E., Ferrans V.J., Fenoglio J.J., Edwards W.D., et al. Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation. 1987;75:401–405. doi: 10.1161/01.cir.75.2.401. [DOI] [PubMed] [Google Scholar]
- 68.Hauck A.J., Kearney D.L., Edwards W.D. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64:1235–1245. doi: 10.1016/s0025-6196(12)61286-5. [DOI] [PubMed] [Google Scholar]
- 69.Chow L.H., Radio S.J., Sears T.D., McManus B.M. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915–920. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 70.Katzmann J.L., Schlattmann P., Rigopoulos A.G., Noutsias E., Bigalke B., Pauschinger M., et al. Meta-analysis on the immunohistological detection of inflammatory cardiomyopathy in endomyocardial biopsies. Heart Fail Rev. 2020;25:277–294. doi: 10.1007/s10741-019-09835-9. [DOI] [PubMed] [Google Scholar]
- 71.Halushka M.K., Vander Heide R.S. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wojnicz R., Nowalany-Kozielska E., Wojciechowska C., Glanowska G., Wilczewski P., Niklewski T., et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy. Circulation. 2001;104:39–45. doi: 10.1161/01.cir.104.1.39. [DOI] [PubMed] [Google Scholar]
- 73.Ojha V., Verma M., Pandey N.N., Mani A., Malhi A.S., Kumar S., et al. Cardiac magnetic resonance imaging in coronavirus disease 2019 (COVID-19): a systematic review of cardiac magnetic resonance imaging findings in 199 patients. J Thorac Imaging. 2021;36:73–83. doi: 10.1097/RTI.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 74.Basso C., Aguilera B., Banner J., Cohle S., d'Amati G., de Gouveia R.H., et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017;471:691–705. doi: 10.1007/s00428-017-2221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caforio A.L.P., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.