ABSTRACT
Avian leukosis virus subgroup J (ALV-J) induces myelocytomas, which can metastasize to multiple organs in diseased chickens. Although metastasis is the primary cause of death in such cases, the mechanism for it remains unclear. Here, we found that interaction between ALV-J surface protein (SU) and doublecortin-like kinase 1 (DCLK1) promotes epithelial-mesenchymal transition (EMT) and cell proliferation. We found that ALV-J can activate EMT in infected cells. Subsequently, proteomics analysis revealed that DCLK1, a well-established putative tumor stem cell marker, which is highly expressed in ALV-J-infected DF-1 cells and chickens, might be a potential factor mediating EMT. Furthermore, using immunofluorescence and immunoprecipitation, we verified that SU interacts with DCLK1. Functional studies suggested that overexpression of DCLK1 increased viral replication and promoted cell proliferation by accelerating the progression of cells from the G0/G1 phase to the S phase of cell cycle, whereas RNA interference of DCLK1 reduced viral replication and arrested cell proliferation by retarding cell cycle progression from the late G1 phase into the S phase in ALV-J-infected cells. Moreover, we demonstrate that the increased accumulation of DCLK1 promotes EMT by increasing the expression of N-cadherin, vimentin, MMP2, and transcription factor Snail1 and decreasing the expression of epithelial marker E-cadherin. These results suggest that ALV-J SU interacts with DCLK1, and accelerates cell proliferation, leading to increased viral replication and ultimately activating EMT, which paves the way for tumor metastasis.
IMPORTANCE Tumor metastasis is a major challenge in cancer research, because of its systemic nature and the resistance of disseminated tumor cells to existing therapeutic agents. It is estimated that >90% of mortality from cancer is attributable to metastases. We found that ALV-J can activate EMT, which plays a critical role in cancer metastasis. Subsequently, we identified a tumor stem cell marker, DCLK1, in ALV-J infected cells, which interacts with surface protein (SU) of ALV-J to promote virus replication, activate EMT, and accelerate cell proliferation enabling ALV-J to obtain metastatic ability. Understanding the process of participation of ALV-J in EMT and the route of metastasis will help elucidate the mechanism of virus-induced tumor metastasis and help identify promising molecular targets and key obstacles for ALV-J control and clinical technology development.
KEYWORDS: avian leukosis virus subgroup J, surface protein, doublecortin-like kinase 1, epithelial-mesenchymal transition, cell proliferation, tumor metastasis
INTRODUCTION
Avian leukosis virus subgroup J (ALV-J) is an oncogenic avian retrovirus (1). Its complete genome contains gag, pol, and env genes, which encode viral structural proteins, RNA-dependent DNA polymerase, and envelope glycoprotein, respectively (2). First reported in 1991 in the United Kingdom (3), ALV-J is the most pathogenic and infectious among all subgroups of ALV and causes the most clinical cases in chickens, accounting for more than 90% of avian leukemia (AL) cases (4). In the absence of any effective vaccine against ALV-J, eradication remains the major strategy against this virus (1). ALV-J mainly induces myelocytomas by targeting the cells of the myeloid lineage (5, 6). In the late phase of ALV-J infection, in addition to myelocytomas, several tumors, including histiocytic sarcomas, nephromas, hemangiomas, and erythroblastosis, metastasize to multiple organs, leading to higher mortality (7–9). Previous research on ALV-J mainly focused on the pathogenesis, including promoter insertion, viral oncogene, and enhancer activation (10). However, the mechanism underlying the metastasis of ALV-J-induced tumors is not clear.
The epithelial-mesenchymal transition (EMT) is considered necessary for tumor metastasis (11). Decreased E-cadherin expression and induction of N-cadherin, vimentin, Zeb1, and MMP2 is characteristic of EMT (12, 13). EMT triggers cellular mobility and induces tumor cell invasion (14), which is a key step toward tumor metastasis (11). Cancer death is mainly caused by tumor metastasis, including local invasion, internal invasion, circulation, extravasation, micrometastasis formation, and metastasis colonization (15). Currently, oncoviruses, including human papillomaviruses (HPVs) (16), Epstein-Barr virus (EBV) (17), and hepatitis B and C viruses (HBVs and HCVs) (18, 19), are considered to play a key role in cancer metastasis, particularly in EMT (20). The circ-Vav3/gga-miR-375/YAP1 axis has been shown to induce EMT and promote ALV-J-induced tumorigenesis (21). There may be other modulating factors that participate in EMT. Doublecortin-like kinase 1 (DCLK1) has been reported to be involved in EMT during tumor development (22). However, little is known about the relationship between EMT and ALV-J-induced metastasis.
DCLK1 is a member of the doublecortin family and the protein kinase superfamily, which is involved in the regulation of tumorigenesis and EMT in cancers, such as colorectal cancer (CRC), renal clear cell carcinoma (RCC), pancreatic cancer, and hepatocellular carcinoma (HCC) (23). In many solid tumors, DCLK1 is considered a master regulator of cancer cell pluripotency factors and EMT transcription factors, including E-cadherin, Slug, N-cadherin, and Zeb1, in metastasis and survival (22, 24). DCLK1 expression is important for cancer growth, EMT, and metastasis. Although several studies have focused on the relationship between DCLK1 and EMT, whether DCLK1 regulates EMT in ALV-J-induced tumorigenesis remains unclear.
In this study, we investigated the role of DCLK1 in ALV-J-induced tumorigenesis. We found that ALV-J activates DCLK1 and EMT in infected cells. Functional studies revealed that DCLK1 interacts with ALV-J surface protein (SU), increases viral replication, promotes cell proliferation, and promotes EMT by affecting the expression of mesenchymal markers, N-cadherin, vimentin, MMP2, transcription factor Snail1, and the epithelial marker E-cadherin. These findings provide new insights into ALV-J-induced tumor metastasis.
RESULTS
ALV-J promotes EMT.
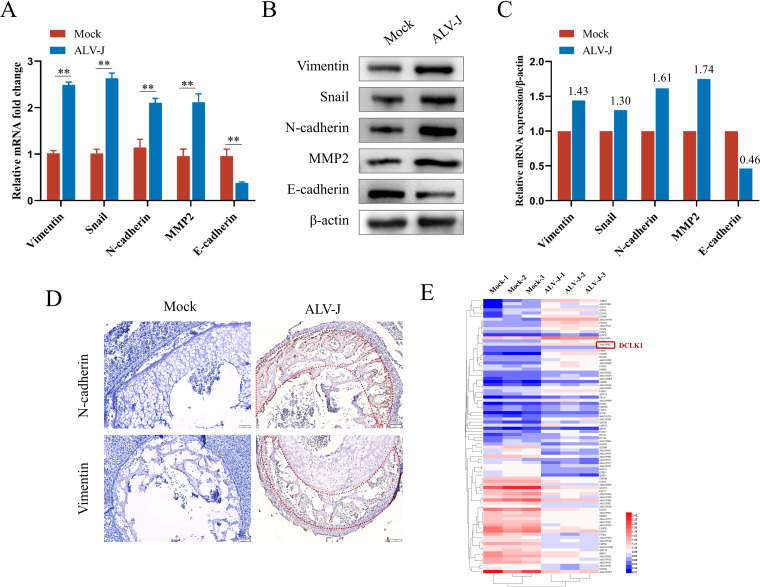
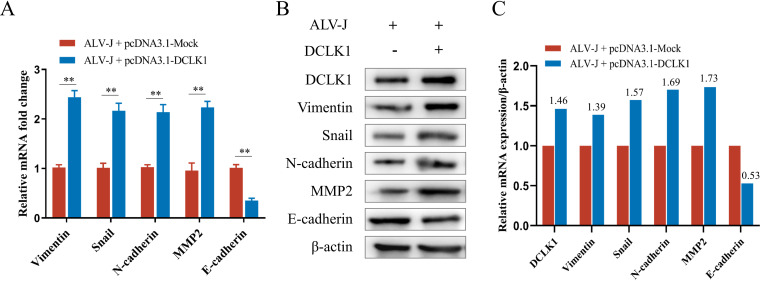
To determine whether ALV-J is involved in EMT for metastasis, the expression of EMT-related markers was detected in ALV-J-infected DF-1 cells. The expression of E-cadherin, an epithelial marker, decreased in ALV-J-infected cells, whereas the expression of the mesenchymal markers N-cadherin, vimentin, and MMP2 and the transcription factor Snail1 increased (Fig. 1A and B). As ALV-J induces the transformation of bone marrow-derived myeloid cells into myelocytomas and metastasis (25–27), the expression levels of the main EMT markers in embryonic bone marrow were detected by immunohistochemistry (IHC). IHC results confirmed that the expression of N-cadherin and vimentin in ALV-J-infected bone marrow was significantly higher than in control bone marrow, and positive staining signals were mainly distributed in the regions of endochondral ossification (Fig. 1D). Overexpression of vimentin and N-cadherin contributes to increased cell motility. As a result, these cells easily spread to distant or surrounding tissues favoring metastasis (28, 29). To identify the potential factors involved in EMT induced by ALV-J, we employed tandem mass tag (TMT) quantitative proteomic analysis. A total of 88 significantly different proteins were identified, which included 46 significantly upregulated proteins and 42 significantly downregulated proteins. The heat map for the differential expression of proteins (Fig. 1E) shows the upregulation of the tumor stem cell marker DCLK1 in ALV-J-infected cells, indicating that it might be a potential factor regulating EMT in ALV-J-induced tumorigenesis.
FIG 1.
ALV-J promoted the epithelial-mesenchymal transition (EMT) in vitro and in vivo. (A) ALV-J enhances the mRNA expression of EMT markers. The transcriptional changes in EMT markers in DF-1 cells infected with ALV-J were detected by qPCR at 48 hpi. (B) ALV-J promoted the protein expression of EMT markers. The changes in the levels of EMT marker proteins in the above-mentioned cells were detected by Western blot analysis. (C) Levels of vimentin, Snail, N-cadherin, MMP2, and E-cadherin proteins in panel B are shown as fold change after normalization to β-actin levels. (D) N-cadherin and vimentin were highly expressed in endochondral ossification of embryonic chicken bone marrow infected with ALV-J as determined using immunohistochemistry. Images show embryonic chicken bone marrow stained for N-cadherin and vimentin at a magnification of ×200. (E) DCLK1 was upregulated in ALV-J-infected cells. The differential protein clustering heat map shows significant differences between uninfected DF-1 cells and DF-1 cells infected with ALV-J. The horizontal level represents the clustering of samples longitudinally and the horizontal protein clustering; the shorter the clustering branch, the higher the similarity. Data are means and SD from at least three independent experiments. **, P ≤ 0.01.
ALV-J facilitates DCLK1 expression in vitro and in vivo.
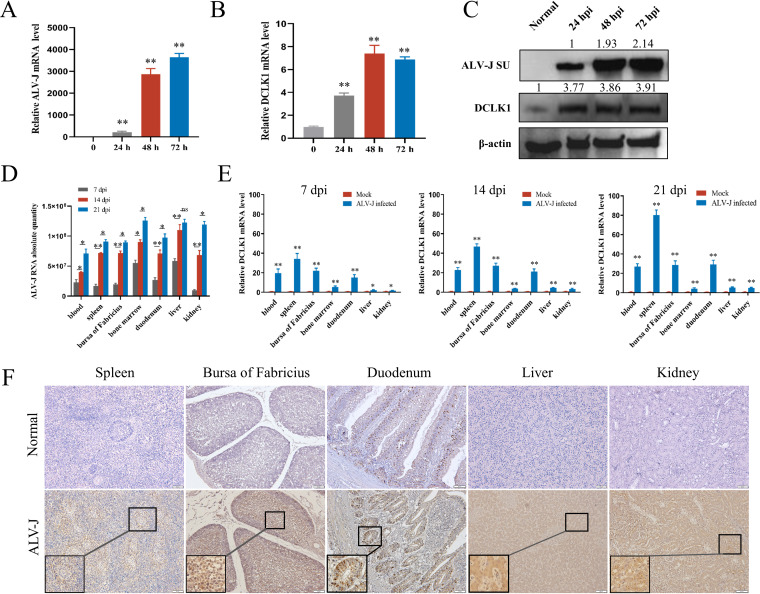
To confirm whether ALV-J activates DCLK1 expression in vitro, DF-1 cells were infected with ALV-J, and the dynamic expression of ALV-J and DCLK1 was detected using quantitative real-time PCR (qPCR) and Western blot analysis. Upon ALV-J infection, the mRNA and protein levels of DCLK1 continuously increased from 24 to 72 h postinfection (hpi) (P < 0.01) (Fig. 2A to C). These results suggest that ALV-J activates DCLK1 expression in vitro. To further confirm whether the expression of DCLK1 is activated by ALV-J in vivo, a 9-day-old-embryo model of congenital ALV-J infection was established. After hatching, DCLK1 mRNA and protein levels were detected using qPCR and immunohistochemistry at the age of 7, 14, and 21 days. With the increase in ALV-J viral load (Fig. 2D), the relative levels of DCLK1 mRNA were increased in the blood, spleen, bursa of Fabricius, bone marrow, duodenum, liver, and kidney (Fig. 2E). The IHC results showed that both the number of DCLK1-positive cells and DCLK1 levels in the above-mentioned tissues of ALV-J-infected chickens were substantially higher than those in uninfected chickens (Fig. 2F). The DCLK1-positive signal in the duodenum was regularly distributed in the intestinal crypts and villi.
FIG 2.
ALV-J enhanced DCLK1 expression in vitro and in vivo. (A and B) DCLK1 was upregulated in ALV-J-infected cells. DF-1 cells were infected with ALV-J. Total RNA was extracted from ALV-J-infected and uninfected cells as described in Materials and Methods. The transcriptional changes in ALV-J env gene (A) and DCLK1 (B) were detected using qPCR at 24, 48, and 72 h after ALV-J infection. These results demonstrate that the ALV-J-infected DF-1 cell system was successfully constructed, and the transcript level of DCLK1 showed an increasing trend with the increasing transcript level of the ALV-J env gene. (C) ALV-J promoted the expression of DCLK1 protein. The protein levels of ALV-J SU and DCLK1 in ALV-J-infected and uninfected DF-1 cells were detected by Western blot analysis. With the increase in ALV-J SU protein levels, the levels of DCLK1 also showed an increasing trend. (D) Dynamic changes in the viral load in ALV-J-infected tissues. Chicks were infected with ALV-J (103.8 TCID50). The copy number of the ALV-J genome in the tissues was detected using qPCR. (E) ALV-J promoted the mRNA expression of DCLK1 in vivo. RNA was extracted from tissues at 7, 14, and 2 days postinfection (dpi). The copy number of DCLK1 mRNA in the tissues was determined using qPCR. (F) DCLK1 protein expression was elevated in ALV-J-infected tissues. DCLK1 expression in ALV-J-infected and uninfected tissues was detected immunohistochemically. DCLK1-positive cells are shown in brown. Nuclei are counterstained in blue and stained with hematoxylin. Data are means and SD from at least three independent experiments. *, P ≤ 0.05; **, P ≤ 0.01; ns, no significance.
SU of ALV-J interacts with DCLK1.
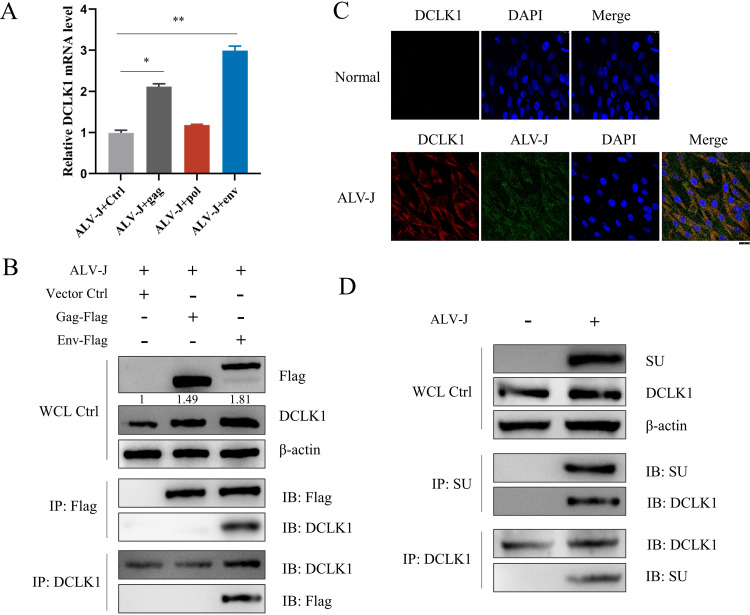
To determine which structural protein of ALV-J activates DCLK1 expression, the gag, pol, and env genes were transfected into ALV-J-infected DF-1 cells, and the mRNA levels of DCLK1 were detected. It was observed that gag and env, but not pol, activated DCLK1; in particular, env activated DCLK1 expression significantly (Fig. 3A). The protein expression of DCLK1 was upregulated 1.49-fold after transfection with gag and 1.81-fold after transfection with env (Fig. 3B). To explore how gag and env of ALV-J activate the expression of DCLK1, we investigated the interaction between gag or env and DCLK1 by coimmunoprecipitation (Co-IP). It was observed that env interacted with DCLK1 (Fig. 3B). To further clarify whether ALV-J recruits DCLK1 through SU, confocal laser scanning microscopy (CLSM) and Co-IP analyses were performed. CLSM results further confirmed that ALV-J could induce the expression of DCLK1, and the merged yellow fluorescence of ALV-J and DCLK1 in the cytoplasm indicated the interaction between SU and DCLK1 (Fig. 3C). Next, a direct interaction between SU and DCLK1 was confirmed in Co-IP experiments (Fig. 3D).
FIG 3.
DCLK1 interacted with SU of ALV-J. (A) DCLK1 mRNA level was significantly increased after transfection with env gene element. gag, pol, and env were transfected into ALV-J-infected DF-1 cells, and the mRNA expression level of DCLK1 was detected by qPCR. (B) DCLK1 interacted with env gene element. DF-1 cells were transduced with vector control (Vector Ctrl), gag-Flag, pol-Flag, or env-Flag for 12 h and then stimulated with ALV-J for another 2 h. Whole-cell lysates were immunoprecipitated with anti-Flag or anti-DCLK1 antibodies. The immunoprecipitants and cell lysate control samples were analyzed by immunoblotting using anti-Flag or anti-DCLK1 antibodies. Protein expression in whole-cell lysates was normalized to β-actin. (C) The expression of ALV-J and DCLK1 was detected in the cytoplasm of DF-1 cells. At 48 hpi, the expression of DCLK1 protein in ALV-J-infected cells was significantly higher than that in uninfected cells. The combined yellow fluorescence shows that DCLK1 interacts with ALV-J SU. (D) DCLK1 interacted with SU. DF-1 cells were collected at 48 hpi with ALV-J. Whole-cell lysates were immunoprecipitated with anti-SU or anti-DCLK1 antibodies. The immunoprecipitants and cell lysate control samples were analyzed by immunoblotting using anti-SU or anti-DCLK1 antibodies. Data are means and SD from at least three independent experiments. *, P ≤ 0.05; **, P ≤ 0.01.
DCLK1 efficiently promotes replication of ALV-J.
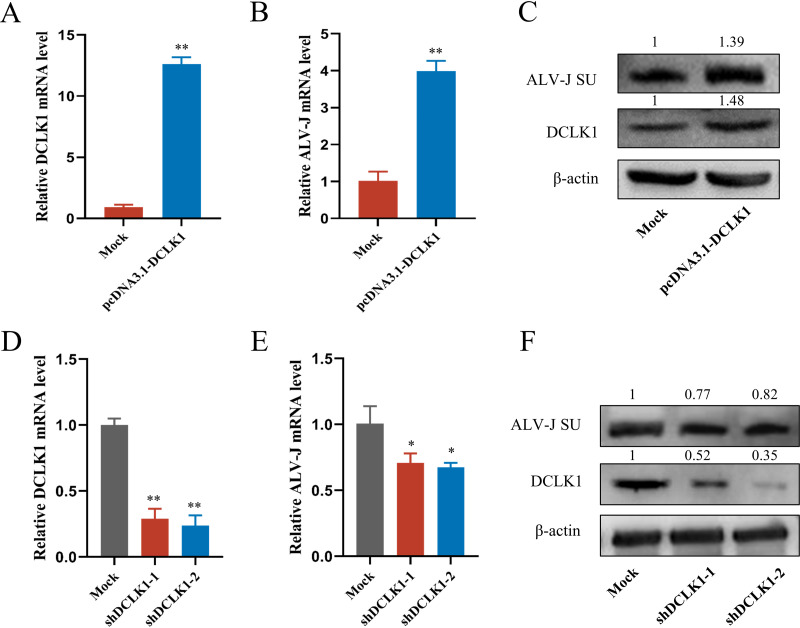
To evaluate the biological importance of DCLK1 in ALV-J replication, recombinant pcDNA3.1-DCLK1 plasmid and short hairpin RNA (shRNA) targeting DCLK1 were constructed and transfected into DF-1 cells, which were then infected with ALV-J. The cells were harvested to detect DCLK1 mRNA levels at 72 hpi. The overexpression of DCLK1 (Fig. 4A) significantly enhanced ALV-J replication at the mRNA and protein levels (Fig. 4B and C). Correspondingly, the knockdown of DCLK1 (Fig. 4D) inhibited ALV-J replication at mRNA and protein levels (Fig. 4E and F). All these results demonstrate that DCLK1 can efficiently promote the replication of ALV-J.
FIG 4.
DCLK1 promoted ALV-J replication. (A and B) Overexpression of DCLK1 promoted the transcription level of ALV-J. DF-1 cells were transfected with the control plasmid (pcDNA3.1-Mock) and pcDNA3.1-DCLK1 and then infected with ALV-J, and total RNA was extracted from cells. DCLK1 (A) and ALV-J env (B) genes were measured by qPCR. (C) Overexpression of DCLK1 promoted the expression of ALV-J protein. Total protein was extracted from the transfected cells, and then DCLK1 and ALV-J SU protein levels were detected by Western blotting. (D and E) Interference with DCLK1 reduced ALV-J mRNA level. DF-1 cells were transfected with shRNAs (shDCLK1-1, shDCLK1-2, or shNC) and then infected with ALV-J, and total RNA was extracted from the transfected cells. DCLK1 (D) and ALV-J env (E) genes were measured by qPCR. (F) Interference with DCLK1 reduced ALV-J protein level. Total protein was extracted from the transfected cells, and then DCLK1 and SU protein levels were detected by Western blotting. Data are means and SD from at least three independent experiments. *, P ≤ 0.05; **, P ≤ 0.01.
DCLK1 promotes EMT in ALV-J-infected cells.
The activation of the EMT promotes tumor cell metastasis. To evaluate the effect of DCLK1 on EMT, we transfected the pcDNA3.1-DCLK1 plasmid into DF-1 cells infected with ALV-J and examined the expression of EMT biomarker proteins. The mRNA and protein levels of E-cadherin were downregulated, whereas those of N-cadherin, vimentin, MMP2, and Snail1 were upregulated, after pcDNA3.1-DCLK1 transfection (Fig. 5A to C). These findings support the role of DCLK1 in promoting the regulation of EMT metastasis during ALV-J infection.
FIG 5.
DCLK1 promoted EMT in ALV-J infected cells. (A) DCLK1 promoted the mRNA expression of EMT markers in ALV-J-infected cells. Transcript levels of EMT markers were detected using qPCR after 48 h of DCLK1 overexpression in ALV-J-infected DF-1 cells. (B) DCLK1 promoted the expression of EMT marker proteins in ALV-J-infected cells. The changes in the levels of DCLK1 and EMT markers in the above-mentioned cells were detected by Western blot analysis. (C) Levels of DCLK1 and EMT marker proteins in panel B are shown as fold change after normalization to β-actin levels. Data are means and SD from at least three independent experiments. **, P ≤ 0.01.
DCLK1 promotes cell proliferation by accelerating G1/S cell cycle progression.
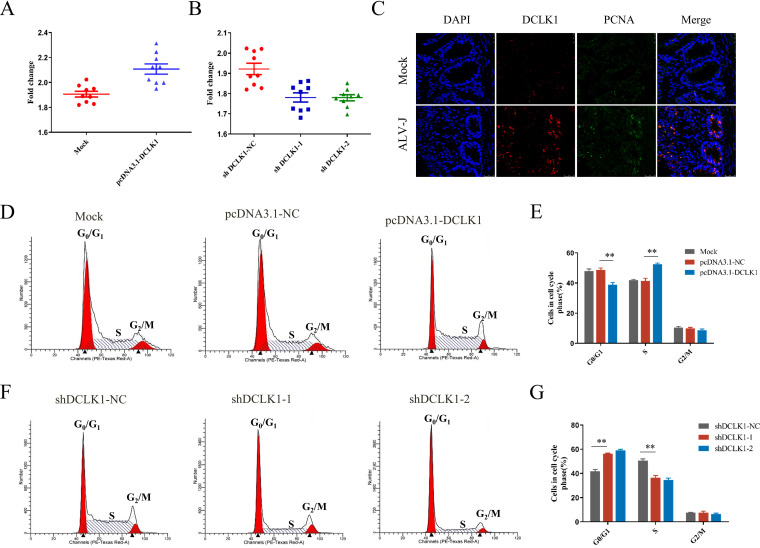
To investigate whether DCLK1 expression affects cell proliferation, recombinant pcDNA3.1-DCLK1 plasmid and short hairpin RNA (shRNA) targeting DCLK1 were transfected into ALV-J-infected DF-1 cells. The results showed that overexpression of DCLK1 significantly increased (Fig. 6A) and its knockdown significantly reduced (Fig. 6B) the proliferation of DF-1 cells. These results illustrate that DCLK1 promotes the proliferation of ALV-J-infected cells. Furthermore, we explored whether DCLK1 promoted cell proliferation in vivo. As shown in Fig. 2F, DCLK1-positive cells showed higher expression of the protein in the duodenum of ALV-J-infected chickens. To investigate whether DCLK1 expression affects cell proliferation in vivo, double-immunofluorescence labeling for DCLK1 and proliferating-cell nuclear antigen (PCNA), a marker for proliferation, was performed using duodenum (30). The expression of DCLK1 and PCNA highly overlapped in the intestinal crypts of chickens infected with ALV-J as evident in the confocal imaging of immunofluorescence (Fig. 6C). We confirmed that DCLK1 accelerated the proliferation of cells in ALV-J-infected tissues, which is consistent with previous findings that DCLK1 regulates self-renewal of intestinal tumor cells (31).
FIG 6.
DCLK1 promoted cell proliferation by accelerating cell cycle progression. (A and B) Overexpression of DCLK1 accelerated cell proliferation, whereas its knockdown slowed proliferation. The CCK-8 assay was used to measure cell viability upon overexpression (A) and knockdown (B) of DCLK1. (C) DCLK1 and PCNA were coexpressed in the duodenum infected with ALV-J. Costaining of PCNA and DCLK1 in the duodenum by immunofluorescence assay and expression of PCNA (FITC) and DCLK1 (Cy3) in ALV-J-infected (3-week-old) chickens are shown. (D) DCLK1 overexpression induced cell cycle progression from G1 to S phase. Flow cytometry was used to examine the cell cycle of cells infected with ALV-J and transfected with pcDNA3.1-Mock and pcDNA3.1-DCLK1 plasmids. (E) Statistical representation of cell cycle distribution of the cells described for panel D. (F) RNA interference of DCLK1-retarded cell cycle progression from the G1 phase to the S phase in ALV-J-infected cells. The distribution of cell cycle after DCLK1 knockdown in ALV-J-infected cells is shown. (G) Statistical representation of cell cycle distribution of the cells described for panel F.
Cell cycle is a key event in EMT (32). Flow cytometry of cells transfected for overexpression or knockdown of DCLK1 was performed after staining with propidium iodide to investigate the effect of DCLK1 on cell cycle, and all tests were carried out using the ALV-J infection system. A lower proportion of pcDNA3.1-DCLK1 cells was found to be in the G1 phase (38.92%) than the pcDNA3.1-Mock (48.66%) and ALV-J (47.84%) cells (Fig. 6D and E). Moreover, a higher percentage of DCLK1-overexpressing cells accumulated at the S phase. A higher percentage of shDCLK1 cells was in the G1 phase (58.96%) than the shDCLK1-NC cells (41.78%). Compared with the control, shDCLK1 led to a significant accumulation of cells in the G1 phase and an obvious decrease in the S-phase cells (Fig. 6F and G). These results show that DCLK1 promotes G1/S cell cycle progression.
DISCUSSION
Accumulating evidence indicates that oncoviruses and their encoded proteins significantly affect metastasis, especially the EMT process (20, 33). Early in metastasis, oncoviruses typically accelerate the EMT process by encoding oncoproteins (20). Identification of the roles of oncoviruses in the progress of EMT may, therefore, help clarify the mechanisms of tumor metastasis. ALV-J is a typical oncovirus, which mainly causes myelocytomas that metastasize to multiple organs. The mortality of diseased chickens is mainly due to metastasis and resultant organ damage (34). However, the molecular mechanisms driving metastasis remain unclear. In this study, we found downregulation of the epithelial marker E-cadherin and upregulation of the mesenchymal markers N-cadherin, vimentin, and MMP2, and the transcription factor Snail in ALV-J-infected DF-1 cells, indicating that ALV-J can activate EMT in infected cells. The association of vimentin and N-cadherin with the risk of metastasis has been assessed for several cancers, including breast, prostate, and colorectal cancers (35–37). In this study, the expression of N-cadherin and vimentin was upregulated in ALV-J-infected chicken embryo bone marrow slices, which may be related to the metastasis of myeloma caused by ALV-J.
One of the prerequisites for metastasis is the tumor-initiating capacity (38), which relies on a small proportion of malignant cells, especially cancer stem cells with indefinite self-renewal capacity (38, 39). DCLK1 is a well-known tumor stem cell marker (40, 41), involved in the regulation of tumorigenesis, which regulates the EMT phenotype and facilitates tumor metastasis (42–45). Based on our observation that DCLK1 is highly expressed in ALV-J-infected DF-1 cells and chickens, we speculated that DCLK1 may be a potential factor mediating EMT in ALV-J infection. We noticed an interesting phenomenon: the relative levels of DCLK1 mRNA were increased in blood with the increase in ALV-J load. This might be due to the fact that during the growth of primary or metastatic tumors, tumor cells may leave the tumor site, invade blood vessels or lymphatic vessels, and spread across the body via the blood circulation (46, 47).
The virus-host interaction occurs during multiple stages of infection, during which the virus recruits host factors and utilizes them to its advantage (48). The env element of ALV-J has been reported to be closely related to tumorigenicity, and the main antigen, antigenic determinant, and neutralizing reaction site are located in the SU region of the env gene (49, 50). We show that the env element of ALV-J significantly activates the expression of DCLK1, which interacts with the SU protein of ALV-J. Furthermore, we show that ALV-J facilitates viral replication through interaction between DCLK1 and SU, which is in agreement with the conclusion in previous studies that host factors essential for enterovirus replication are recruited through an interaction between a host factor and a viral protein and facilitate its progression (51).
Cells undergoing EMT acquire stem cell-like properties that increase tumorigenic and proliferative potential (52). Functional links between EMT and DCLK1 are correlated with metastasis (22); however, it remains unclear whether DCLK1 could promote EMT in ALV-J-infected cells. Our findings suggest that this conjecture might be true. In DF-1 cells infected with ALV-J, overexpression of DCLK1 promotes upregulation of the mesenchymal markers N-cadherin, vimentin, and MMP2 and the transcription factor Snail and downregulation of the epithelial marker E-cadherin to promote EMT. EMT is associated with cancer progression and metastasis (53, 54). The induction of EMT is facilitated by genomic alterations acquired by cancer cells to become metastatic (55), and it generates cells with invasive properties, allowing them to enter the bloodstream and spread to other organs (53). This study illustrates the role of DCLK1 in the regulation of EMT and promotion of metastasis during ALV-J infection. However, the more detailed mechanisms still need to be further explored.
Rapid cell cycle progression and proliferation ability are critical factors contributing to cancer metastasis (56). EMT promotes cellular proliferation, which is a crucial initial step in metastasis (57). We found that DCLK1 promotes cell proliferation in ALV-J-infected cells, and the expression of DCLK1-positive cells in the duodenum crypt is particularly obvious in ALV-J-infected tissues, whereas intestinal epithelial cell proliferation and differentiation are driven by active stem cells located at the base of crypts (58). The results of previous studies suggest that DCLK1 plays an important role in advancing intestinal tumorigenesis (31). We speculated that after ALV-J infection, high expression of DCLK1 in the intestinal crypt accelerates the proliferation of intestinal epithelial cells. We observed a high degree of overlap in in the expression of DCLK1 and PCNA in the intestinal crypt in ALV-J-infected chickens. Intestinal crypts contain stem cells that can proliferate and differentiate (59, 60). This suggests that the proliferation potential of DCLK1-positive cells accelerates the cell proliferation in ALV-J-infected tissues. This is consistent with previous reports that DCLK1 expression is enhanced in intestinal tumors, that there is elevated tumor stemness and survival through the regulation of the prosurvival signaling pathways (31), and that cancer stem cells proliferate faster and are more likely to induce cancer metastasis (61). Moreover, cell proliferation depends on an orderly progression of the cell cycle (62). We found that DCLK1 promotes cell proliferation by accelerating G1/S progression (63). The deinhibition of G1/S transcription allows cells to enter the S phase in an unrestricted manner, a hallmark of cancer (64). This finding may provide a strategy to combat ALV-J tumorigenesis by altering the cell cycle processes. Given that no ALV-J vaccine is currently available, targeting DCLK1-positive cells could be a new strategy for ALV-J control.
In conclusion, this study demonstrated that DCLK1 plays a significant effect in ALV-J-induced tumorigenesis. DCLK1 interacts with SU of ALV-J to promote virus replication, activate EMT, and accelerate cell proliferation, paving the way for tumor metastasis.
MATERIALS AND METHODS
Virus, cells, and embryos.
The NX0101 strain of ALV-J was maintained in our laboratory. The 50% tissue culture infective dose (TCID50) of the NX0101 strain was determined using DF-1 cells by limiting dilution. DF-1 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (GIBCO, USA) at 37°C in a 5% CO2 atmosphere. Specific-pathogen-free (SPF) chicken embryos were purchased from Jinan Spafas Animal, Inc. (Spafas, Jinan, China), and hatched chicks were maintained under SPF conditions. Animal experiments were carried out in accordance with the guidelines of the Shandong Agricultural University Animal Care and Use Committee (permit no. SDAU 18–096; 7 July 2018).
Proteomics analysis.
ALV-J-infected and uninfected DF-1 cells (n = 3) were used to extract proteins for analysis. The cells were lysed in a lysis buffer (8 M urea, 1% protease inhibitor cocktail) by sonicating three times on ice using a high-intensity ultrasonic processor (Scientz). The debris was removed by centrifugation, and the supernatant was collected. The concentration of proteins in the supernatant was determined with a bicinchoninic acid (BCA) assay kit. The protein extract was reduced with 5 mM dithiothreitol and alkylated with 11 mM iodoacetamide. Finally, trypsin was added at a trypsin-to-protein mass ratio of 1:100 for digestion. The peptides were desalted using a Strata X C18 SPE column (Phenomenex) and vacuum dried. The peptide was reconstituted in 0.5 M 80% and processed according to the manufacturer’s protocol for the TMT kit/iTRAQ kit, followed by high-performance liquid chromatography (HPLC) fractionation and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. The tryptic peptides were dissolved in 0.1% formic acid (solvent A), directly loaded onto a homemade reversed-phase analytical column (15-cm length, 75-μm inside diameter [i.d.]), and separated at a constant flow rate of 400 nL/min on an EASY-nLC 1000 ultrahigh-performance LC (UPLC) system. The peptides were subjected to a nanospray ionization (NSI) source followed by MS/MS in Q Exactive Plus (Thermo) coupled online to the UPLC. The resulting MS/MS data were processed using the MaxQuant search engine. To identify differentially expressed proteins, Bonferroni P values were adjusted (P < 10−16) and paired t tests were used. Only proteins with corrected P values of <0.05 and a fold change of >1.3 were considered differentially expressed.
Reverse transcription and qPCR.
Total RNA was extracted from cells and tissues and reverse-transcribed to cDNA using a kit from Accurate Biology (Hunan, China) according to the manufacturer’s protocol. The PCR mixture containing 10 ng cDNA, SYBR green sequence detection reagents (Accurate Biology, Hunan, China), and gene-specific primers was assayed on an ABI 7500 sequence detection system (Applied Biosystems). GAPDH was used as an internal control. Before the actual sample was run, an assay was performed to verify that the concentration that would lead to optimal DNA amplification. To quantify the viral load in vivo, the ALV-J viral env mRNA level was determined by absolute quantification real-time PCR. The env gene was cloned in the pMD18-T vector (TaKaRa, Japan), and the recombinant vector was termed pMD-env. The pMD-env was diluted in a 10-fold gradient multiplet, and qPCR amplification was performed. The standard curve and regression equation were calculated from the cycle threshold (CT) values. The viral load of each tissue in vivo was calculated. The reactions were run using the following program: 1 cycle at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and at 60°C for 34 s; a melting curve was generated at 95°C for 10 s, 65°C for 60 s, and 97°C for 1 s. Primer sequences are presented in Table 1. All assays were done in triplicate. The 2−ΔΔCT method was used to analyze the data (65).
TABLE 1.
Primers used for quantitative reverse transcription-PCR
| Gene target | Primer sequence | Fragment size (bp) |
|---|---|---|
| DCLK1 | Forward: TCAAGAAGCTGGAGTACACGAAGAATG | 90 |
| Reverse: CAAGAGACGGCACTGAACGAGAAG | ||
| ALV-J | Forward: TGCGTGCGTGGTTATTATTTC | 144 |
| Reverse: AATGGTGAGGTCGCTGACTGT | ||
| Vimentin | Forward: GACCGCTTCGCCAACTACATCG | 133 |
| Reverse: CCCGCATCTCCTCCTCGTACAG | ||
| ALV-J gag | Forward: GCAGCGAGATGCGAAGAT | 158 |
| Reverse: CCGCCAGGGAAGGATACA | ||
| ALV-J pol | Forward: AGACCTGCCCGCATTGTA | 183 |
| Reverse: CACGGCCATGCTGAGTTA | ||
| ALV-J env | Forward: GACTGGGCACCCTGGAAA | 131 |
| Reverse: CCTGGGACAACGGAAATA | ||
| Snail | Forward: TGGTCTGCTCTCCAGCCTCTTC | 117 |
| Reverse: CTCTTGCCCTCATCCTCCTCACTAG | ||
| N-cadherin | Forward: TGGATGAAGCGCCGTGATAA | 177 |
| Reverse: AGGTTTGATGGCGTCTGGTT | ||
| MMP2 | Forward: CAACAGAAGGCAGGACAGATGGATAC | 150 |
| Reverse: GGAAGATGAAGGGGAATACACAAGGAG | ||
| E-cadherin | Forward: GAAGACAGCCAAGGGCCTG | 224 |
| Reverse: GGGCCGTGTAGGATGTAACC | ||
| GAPDH | Forward: GAACATCATCCCAGCGTCCA | 132 |
| Reverse: CGGCAGGTCAGGTCAACAAC |
Western blot analysis.
Total protein lysates were prepared from treated DF-1 cells using a lysis buffer. The protein concentration was measured using a BCA protein assay kit (Beyotime, Shanghai, China). Twenty micrograms of protein was separated on a 10% SDS polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA). The membranes were blocked in 5% Difco skim milk (Solarbio) at 37°C for 2 h and then incubated with diluted primary antibody at 4°C overnight. The primary antibodies used were rabbit polyclonal anti-DCLK1 (1:800; Abcam, Cambridge, UK) and an in-house mouse monoclonal anti-ALV-J gp85 antibody (1D4) and rabbit monoclonal anti-N-cadherin antibody (1:1,000; Abcam, Cambridge, UK), rabbit monoclonal anti-E-cadherin antibody (1:1,000; Abcam, Cambridge, UK), and rabbit monoclonal antivimentin antibody (1:1,000; Abcam, Cambridge, UK). The membranes were then incubated at 37°C for 1 h with a horseradish peroxidase-conjugated secondary antibody (Abbkine, Beijing, China). The proteins were detected using enhanced chemiluminescence (ECL) Western blot detection reagent (Beyotime, Shanghai, China). β-Actin (1:2,000; Engibody, Milwaukee, USA) was used as a loading control.
DCLK1 overexpression and RNA interference.
The DCLK1 overexpression vector (pcDNA3.1-DCLK1) and its negative-control (NC) vector were constructed by GenePharma (Shanghai, China). The pGPU6/GFP/Neo-DCLK1 and empty pGPU6/GFP/Neo plasmids were also constructed by GenePharma (Shanghai, China). Target shRNA sequences for DCLK1 were 5′-GGCCTAAACTAGTCACTATCA-3′ (shDCLK1-1) and 5′-GAGTCAAGCATCCCAATATTG-3′ (shDCLK1-2). To clarify the effect of DCLK1 on ALV-J infection, DF-1 cells were seeded on six-well plates for 12 h and transfected with pcDNA3.1-DCLK1, shDCLK1-1, shDCLK1-2, and shNC (150 nM) using X-tremeGENE HP DNA transfection reagent (Roche) according to manufacturer’s instructions. The mRNA and protein levels of DCLK1 and ALV-J were determined by qPCR and Western blot analysis, respectively, at 24 h, 48 h, and 72 h after infection of ALV-J.
IHC.
To determine the localization of DCLK1 in tissues and cells, IHC was performed according to the following procedure. Tissue sections were dewaxed in xylene and rehydrated in a graded series of ethanol. The slides were subjected to microwave treatment four times in 10 mM citrate buffer (pH 6) for heat-induced antigen retrieval. After cooling, the slides were incubated in 5% bovine serum albumin (BSA) blocking solution at 37°C for 20 min, followed by incubation with the anti-DCLK1 antibody, anti-N-cadherin antibody, and antivimentin antibody (1:200; Abcam, Cambridge, UK) overnight at 4°C. After washing with phosphate-buffered saline (PBS) three times, the sections were stained with corresponding biotinylated goat secondary antibody for 30 min at room temperature. The sections were again washed with PBS three times and then incubated with streptavidin peroxidase and 3,3N-diaminobenzidine tertrahydrochloride (DAB) substrate. The slides were then counterstained with hematoxylin, subjected to stepwise dehydration with alcohol, and sealed with coverslips. All images were taken under a Nikon Eclipse TE2000-S microscope using cellSens imaging software.
CLSM.
Df-1 cells were subcultured, inoculated into a petri dish for confocal imaging, and maintained in DMEM containing 10% FBS. When the cells were 80% confluent, 200 μL of ALV-J strain NX0101 with a virulence of 103.8 TCID50 was inoculated in each plate. The cells were immobilized at room temperature for 10 min with a fixative solution (1 mL solution contained 600 μL acetone and 400 μL alcohol), washed with PBS three times, and incubated overnight with anti-DCLK1 (1:100) or anti-SU (1:200) antibody at 4°C. Thereafter, the cells were washed with PBS three times and incubated with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (heavy plus light chain [H+L]) (1:500; Abbkine, Beijing, China) and Cy3-labeled goat anti-rabbit IgG (H+L) (1:500; Beyotime, Shanghai, China) at 37°C for 1 h. The nuclei were stained with DAPI. Finally, the slides were mounted with 50% glycerol and examined by laser confocal microscopy (Leica SP8; Leica, Berlin, Germany).
Coimmunoprecipitation assay.
DF-1 cells were seeded in 12-well plates. The cells were infected with ALV-J (103.8 TCID50) after being transfected with the pcDNA3.1-DCLK1 plasmid. A pellet consisting of 4 × 106 cells was resuspended in 600 μL lysis/equilibration buffer containing 6 μL protease inhibitor cocktail. The recommended amount of antibody was incubated with clarified cell lysates for 1 h at 4°C. The immunoprecipitate was washed with Tris-buffered saline containing Tween-20 (TBS-T) three times. The samples were analyzed using SDS-PAGE and Western blotting.
Immunofluorescence.
Frozen sections of tissues were incubated with anti-DCLK1 polyclonal antibody (1:100) or anti-PCNA monoclonal antibody (1:200) at 4°C overnight. The sections were then washed three times with PBS, and incubated with FITC-labeled goat anti-rabbit IgG (H+L) (1:500) and Cy3-labeled goat anti-rabbit IgG (H+L) (1:500) at 37°C for 1 h. The nuclei of all the infected cells were stained with 4′,6-diamidino-2-phenylindole (DAPI). Finally, the sections were examined under a laser confocal microscope (Leica SP8; Leica, Berlin, Germany).
Cell proliferation assay.
DF-1 cells were inoculated in 96-well plates at a density of 4 × 103 cells per well, and recombinant pcDNA3.1-DCLK1 plasmid and shRNA targeting DCLK1 were transfected into ALV-J-infected DF-1 cells. Cell Counting Kit-8 (CCK-8; Dojindo, Tokyo, Japan) was used to detect the effect of DCLK1 expression on the cell proliferation of ALV-J infected cells. The cells were treated at 37°C for 2 h, and 10 μL CCK-8 reagent was added. Absorbance rates were measured at 450 nm using a microplate reader (Bio-Rad). Each experimental group had at least 9 replicates. The experiment was repeated three times independently. Cell proliferation was calculated by subtracting the absorbance value of the sample from that of the medium (background level). Relative cell proliferation was normalized to the respective control.
Cell cycling analysis.
The cells were washed twice in cold PBS and fixed overnight with 70% ethanol at 4°C. The cells were digested with 200 μL PBS containing 50 μg/mL RNase A for 20 min at room temperature and then incubated with 20 μg/mL propidium iodide (PI; 300 μL) for 10 min. Flow cytometry was performed on a flow cytometer (BD Biosciences) to analyze cell cycle distribution. All tests were carried out in triplicate.
Statistical analysis.
Statistical analysis was carried out using the SPSS software. The qPCR experiment was repeated at least three times. Comparisons of more than two groups of data were analyzed using univariate or multivariate analysis of variance (ANOVA), and finally, multiple comparison correction was performed. All data are expressed as means and standard deviations (SD).
ACKNOWLEDGMENTS
The work was supported by grants from the National Natural Science Foundation of China (32072816, 31902233), the Shandong Modern Agricultural Technology and Industry System (no. SDAIT-11-04), and the Shandong Science and Technology Development Project (no. 2019JZZY010735).
Z.C. and J.Z. designed experiments; J.Z. performed experiments, analyzed data, and wrote the manuscript; Z.C. conceived the study, wrote the manuscript, and secured funding; D.Z., X.D., J.X., G.W., and J.Y. provided technical assistance. All authors read and approved the final version.
We declare that we have no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Ziqiang Cheng, Email: czqsd@126.com.
Viviana Simon, Icahn School of Medicine at Mount Sinai.
REFERENCES
- 1.Payne LN, Nair V. 2012. The long view: 40 years of avian leukosis research. Avian Pathol 41:11–19. 10.1080/03079457.2011.646237. [DOI] [PubMed] [Google Scholar]
- 2.Meng QW, Zhang ZP, Wang W, Tian J, Xiao ZG. 2011. Enhanced inhibition of avian leukosis virus subgroup J replication by multi-target miRNAs. Virol J 8:556. 10.1186/1743-422X-8-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne LN, Brown SR, Bumstead N, Howes K, Frazier JA, Thouless ME. 1991. A novel subgroup of exogenous avian leukosis virus in chickens. J Gen Virol 72:801–807. 10.1099/0022-1317-72-4-801. [DOI] [PubMed] [Google Scholar]
- 4.Deng Q, Li M, He C, Lu Q, Gao Y, Li Q, Shi M, Wang P, Wei P. 2021. Genetic diversity of avian leukosis virus subgroup J (ALV-J): toward a unified phylogenetic classification and nomenclature system. Virus Evol 7:veab037. 10.1093/ve/veab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne LN, Gillespie AM, Howes K. 1992. Myeloid leukaemogenicity and transmission of the HPRS-103 strain of avian leukosis virus. Leukemia 6:1167–1176. [PubMed] [Google Scholar]
- 6.Cheng Z, Liu J, Cui Z, Zhang L. 2010. Tumors associated with avian leukosis virus subgroup J in layer hens during 2007 to 2009 in China. J Vet Med Sci 72:1027–1033. 10.1292/jvms.09-0564. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Zhao GL, Wang XM, Du XS, Su S, Li CG, Nair V, Yao YX, Cheng ZQ. 2018. Synergistic viral replication of Marek's disease virus and avian leukosis virus subgroup J is responsible for the enhanced pathogenicity in the superinfection of chickens. Viruses 10:271. 10.3390/v10050271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stedman NL, Brown TP. 1999. Body weight suppression in broilers naturally infected with avian leukosis virus subgroup J. Avian Dis 43:604–610. 10.2307/1592664. [DOI] [PubMed] [Google Scholar]
- 9.Sironi G, Manarolla G, Pisoni G, Recordati C, Rampin T. 2006. Myotropic avian leukosis virus subgroup J infection in a chicken. J Vet Med B Infect Dis Vet Public Health 53:347–349. 10.1111/j.1439-0450.2006.00961.x. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Xu H, Yan C, Zhu S, Lan X, Lu Y, He Q, Yin H, Zhu Q, Zhao X, Li D, Liu Y, Wang Y. 2020. gga-miR-148a-5p-Targeting PDPK1 inhibits proliferation and cell cycle progression of avain leukosis virus subgroup J (ALV-J)-infected cells. Front Cell Dev Biol 8:587889. 10.3389/fcell.2020.587889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieto MA, Huang RY, Jackson RA, Thiery JP. 2016. EMT: 2016. Cell 166:21–45. 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Weinberg RA. 2008. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14:818–829. 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D'Alessio AC, Young RA, Weinberg RA. 2013. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell 154:61–74. 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibue T, Weinberg RA. 2017. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 14:611–629. 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong SY, Hynes RO. 2006. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle 5:812–817. 10.4161/cc.5.8.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng YM, Chou CY, Hsu YC, Chen MJ, Wing LY. 2012. The role of human papillomavirus type 16 E6/E7 oncoproteins in cervical epithelial-mesenchymal transition and carcinogenesis. Oncol Lett 3:667–671. 10.3892/ol.2011.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaur N, Gandhi J, Robertson ES, Verma SC, Kaul R. 2015. Epstein-Barr virus latent antigens EBNA3C and EBNA1 modulate epithelial to mesenchymal transition of cancer cells associated with tumor metastasis. Tumour Biol 36:3051–3060. 10.1007/s13277-014-2941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Dong Z, Liang J, Cao C, Sun J, Ding Y, Wu D. 2014. As an independent prognostic factor, FAT10 promotes hepatitis B virus-related hepatocellular carcinoma progression via Akt/GSK3β pathway. Oncogene 33:909–920. 10.1038/onc.2013.236. [DOI] [PubMed] [Google Scholar]
- 19.Akkari L, Grégoire D, Floc'h N, Moreau M, Hernandez C, Simonin Y, Rosenberg AR, Lassus P, Hibner U. 2012. Hepatitis C viral protein NS5A induces EMT and participates in oncogenic transformation of primary hepatocyte precursors. J Hepatol 57:1021–1028. 10.1016/j.jhep.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Bode AM, Dong Z, Cao Y. 2016. The epithelial-mesenchymal transition (EMT) is regulated by oncoviruses in cancer. FASEB J 30:3001–3010. 10.1096/fj.201600388R. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Yan Y, Lin W, Li A, Zhang H, Lei X, Dai Z, Li X, Li H, Chen W, Chen F, Ma J, Xie Q. 2019. Circular RNA Vav3 sponges gga-miR-375 to promote epithelial-mesenchymal transition. RNA Biol 16:118–132. 10.1080/15476286.2018.1564462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandrakesan P, Panneerselvam J, Qu D, Weygant N, May R, Bronze MS, Houchen CW. 2016. Regulatory roles of dclk1 in epithelial mesenchymal transition and cancer stem cells. J Carcinog Mutagen 7:257. 10.4172/2157-2518.1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panneerselvam J, Mohandoss P, Patel R, Gillan H, Li M, Kumar K, Nguyen D, Weygant N, Qu D, Pitts K, Lightfoot S, Rao C, Houchen C, Bronze M, Chandrakesan P. 2020. DCLK1 regulates tumor stemness and cisplatin resistance in non-small cell lung cancer via ABCD-member-4. Mol Ther Oncolytics 18:24–36. 10.1016/j.omto.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sureban SM, May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG, Houchen CW. 2013. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS One 8:e73940. 10.1371/journal.pone.0073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. 2001. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7:1194–1201. 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 26.Autiero M, Luttun A, Tjwa M, Carmeliet P. 2003. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost 1:1356–1370. 10.1046/j.1538-7836.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 27.Neeson PJ, Thurlow PJ, Jamieson GP, Bradley C. 2003. Lymphocyte-facilitated tumour cell adhesion to endothelial cells: the role of high affinity leucocyte integrins. Pathology 35:50–55. [PubMed] [Google Scholar]
- 28.Loh CY, Chai JY, Tang TF, Wong WF, Sethi G, Shanmugam MK, Chong PP, Looi CY. 2019. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells 8:1118. 10.3390/cells8101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai KH, Ahmed J. 2019. A correlative study of N-cadherin expression with different grades of oral squamous cell carcinoma projecting as a marker of epithelial to mesenchymal transition in tumor progression. Asian Pac J Cancer Prev 20:2327–2332. 10.31557/APJCP.2019.20.8.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong W, Peng J, He H, Wu D, Han Z, Bi X, Dai Q. 2008. Ki-67 and PCNA expression in prostate cancer and benign prostatic hyperplasia. Clin Invest Med 31:E8–E15. 10.25011/cim.v31i1.3136. [DOI] [PubMed] [Google Scholar]
- 31.Chandrakesan P, Yao J, Qu D, May R, Weygant N, Ge Y, Ali N, Sureban SM, Gude M, Vega K, Bannerman-Menson E, Xia L, Bronze M, An G, Houchen CW. 2017. Dclk1, a tumor stem cell marker, regulates pro-survival signaling and self-renewal of intestinal tumor cells. Mol Cancer 16:30. 10.1186/s12943-017-0594-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Tian HY, Zhang KH, Gao X, Lei WW, Zhang L, Yu ML, Song JG, Zhao FK. 2009. Comparative proteomic analysis of cell cycle-dependent apoptosis induced by transforming growth factor-beta. Biochim Biophys Acta 1794:1387–1397. 10.1016/j.bbapap.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Jha HC, Sun Z, Upadhyay SK, El-Naccache DW, Singh RK, Sahu SK, Robertson ES. 2016. KSHV-mediated regulation of Par3 and SNAIL contributes to B-cell proliferation. PLoS Pathog 12:e1005801. 10.1371/journal.ppat.1005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steeg PS. 2006. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12:895–904. 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 35.Quan Z, Li T, Xia Y, Liu J, Du Z, Luo C, He Y, Wu X. 2020. PLCɛ maintains the functionality of AR signaling in prostate cancer via an autophagy-dependent mechanism. Cell Death Dis 11:716. 10.1038/s41419-020-02917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agnoletto C, Corrà F, Minotti L, Baldassari F, Crudele F, Cook W, Di Leva G, d’Adamo A, Gasparini P, Volinia S. 2019. Heterogeneity in circulating tumor cells: the relevance of the stem-cell subset. Cancers (Basel) 11:483. 10.3390/cancers11040483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ, Garcia-Blanco MA. 2011. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res 9:997–1007. 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta GP, Massagué J. 2006. Cancer metastasis: building a framework. Cell 127:679–695. 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Pardal R, Clarke MF, Morrison SJ. 2003. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer 3:895–902. 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 40.Mirzaei A, Tavoosidana G, Modarressi MH, Rad AA, Fazeli MS, Shirkoohi R, Tavakoli-Yaraki M, Madjd Z. 2015. Upregulation of circulating cancer stem cell marker, DCLK1 but not Lgr5, in chemoradiotherapy-treated colorectal cancer patients. Tumour Biol 36:4801–4810. 10.1007/s13277-015-3132-9. [DOI] [PubMed] [Google Scholar]
- 41.Mohammadi Y, Tavangar SM, Saidijam M, Amini R, Etemadi K, Karimi Dermani F, Najafi R. 2018. DCLK1 plays an important role in colorectal cancer tumorgenesis through the regulation of miR-200c. Biomed Pharmacother 103:301–307. 10.1016/j.biopha.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 42.Zhao H, Duan Q, Zhang Z, Li H, Wu H, Shen Q, Wang C, Yin T. 2017. Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. J Cell Mol Med 21:2055–2067. 10.1111/jcmm.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sureban SM, May R, Lightfoot SA, Hoskins AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao CV, Wyche JH, Anant S, Houchen CW. 2011. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res 71:2328–2338. 10.1158/0008-5472.CAN-10-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito H, Tanaka S, Akiyama Y, Shimada S, Adikrisna R, Matsumura S, Aihara A, Mitsunori Y, Ban D, Ochiai T, Kudo A, Arii S, Yamaoka S, Tanabe M. 2016. Dominant expression of DCLK1 in human pancreatic cancer stem cells accelerates tumor invasion and metastasis. PLoS One 11:e0146564. 10.1371/journal.pone.0146564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandrakesan P, Roy B, Jakkula LU, Ahmed I, Ramamoorthy P, Tawfik O, Papineni R, Houchen C, Anant S, Umar S. 2014. Utility of a bacterial infection model to study epithelial-mesenchymal transition, mesenchymal-epithelial transition or tumorigenesis. Oncogene 33:2639–2654. 10.1038/onc.2013.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarin D. 1982. Investigations of the mechanisms of metastatic spread of naturally occurring neoplasms. Cancer Metastasis Rev 1:215–225. [6309367]. 10.1007/BF00046828. [DOI] [PubMed] [Google Scholar]
- 47.Diamantopoulou Z, Castro-Giner F, Aceto N. 2020. Circulating tumor cells: ready for translation? J Exp Med 217:e20200356. 10.1084/jem.20200356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Hu L, Chen T, Chang M, Deng F, Hu Z, Wang H, Wang M. 2020. Host AAA+ ATPase TER94 plays critical roles in building the baculovirus viral replication factory and virion morphogenesis. J Virol 94:e01674-19. 10.1128/JVI.01674-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li T, Yao X, Li C, Zhang J, Xie Q, Wang W, Lu H, Fu H, Li L, Xie J, Shao H, Gao W, Qin A, Ye J. 2020. Gp37 Regulates the pathogenesis of avian leukosis virus subgroup J via its C terminus. J Virol 94:e02180-19. 10.1128/JVI.02180-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bai J, Payne LN, Skinner MA. 1995. HPRS-103 (exogenous avian leukosis virus, subgroup J) has an env gene related to those of endogenous elements EAV-0 and E51 and an E element found previously only in sarcoma viruses. J Virol 69:779–784. [7815543]. 10.1128/jvi.69.2.779-784.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horova V, Lyoo H, Różycki B, Chalupska D, Smola M, Humpolickova J, Strating J, van Kuppeveld FJM, Boura E, Klima M. 2019. Convergent evolution in the mechanisms of ACBD3 recruitment to picornavirus replication sites. PLoS Pathog 15:e1007962. 10.1371/journal.ppat.1007962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Qi C, Liu X, Li C, Chen J, Shi M. 2018. Fibulin-3 knockdown inhibits cervical cancer cell growth and metastasis in vitro and in vivo. Sci Rep 8:10594. 10.1038/s41598-018-28906-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Kalluri R, Weinberg RA. 2009. The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428. 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryoo IG, Ha H, Kwak MK. 2014. Inhibitory role of the KEAP1-NRF2 pathway in TGFβ1-stimulated renal epithelial transition to fibroblastic cells: a modulatory effect on SMAD signaling. PLoS One 9:e93265. 10.1371/journal.pone.0093265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pallasch FB, Schumacher U. 2020. Angiotensin inhibition, TGF-β and EMT in cancer. Cancers (Basel) 12:2785. 10.3390/cancers12102785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malumbres M, Barbacid M. 2009. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9:153–166. 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 57.Samantarrai D, Sahu M, Roy J, Mohanty BB, Singh G, Bhushan C, Mallick B. 2015. Unraveling novel TF-miRNA regulatory crosstalk in metastasis of soft tissue sarcoma. Sci Rep 5:9742. 10.1038/srep09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fitzgerald SF, Beckett AE, Palarea-Albaladejo J, McAteer S, Shaaban S, Morgan J, Ahmad NI, Young R, Mabbott NA, Morrison L, Bono JL, Gally DL, McNeilly TN. 2019. Shiga toxin sub-type 2a increases the efficiency of Escherichia coli O157 transmission between animals and restricts epithelial regeneration in bovine enteroids. PLoS Pathog 15:e1008003. 10.1371/journal.ppat.1008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCarthy N, Manieri E, Storm EE, Saadatpour A, Luoma AM, Kapoor VN, Madha S, Gaynor LT, Cox C, Keerthivasan S, Wucherpfennig K, Yuan GC, de Sauvage FJ, Turley SJ, Shivdasani RA. 2020. Distinct mesenchymal cell populations generate the essential intestinal BMP signaling gradient. Cell Stem Cell 26:391–402.E5. 10.1016/j.stem.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Couesnon A, Molgó J, Connan C, Popoff MR. 2012. Preferential entry of botulinum neurotoxin A Hc domain through intestinal crypt cells and targeting to cholinergic neurons of the mouse intestine. PLoS Pathog 8:e1002583. 10.1371/journal.ppat.1002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhdanov VP. 2008. Stochastic model of the formation of cancer metastases via cancer stem cells. Eur Biophys J 37:1329–1334. 10.1007/s00249-008-0341-9. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, Wang Z, Xie L, Zhang Y, Deng T, Li J, Liu J, Xiong W, Zhang L, Zhang L, Peng B, He L, Ye M, Hu X, Tan W. 2018. Molecular recognition and in-vitro-targeted inhibition of renal cell carcinoma using a DNA aptamer. Mol Ther Nucleic Acids 12:758–768. 10.1016/j.omtn.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molinari M. 2000. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif 33:261–274. 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bertoli C, Skotheim JM, de Bruin RA. 2013. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 14:518–528. 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moroney JB, Vasudev A, Pertsemlidis A, Zan H, Casali P. 2020. Integrative transcriptome and chromatin landscape analysis reveals distinct epigenetic regulations in human memory B cells. Nat Commun 11:5435. 10.1038/s41467-020-19242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download jvi.01657-21-s0001.xls, XLS file, 3.3 MB (3.3MB, xls)