ABSTRACT
In this work we have determined that heat shock protein 90 (Hsp90) is essential for avian reovirus (ARV) replication by chaperoning the ARV p17 protein. p17 modulates the formation of the Hsp90/Cdc37 complex by phosphorylation of Cdc37, and this chaperone machinery protects p17 from ubiquitin-proteasome degradation. Inhibition of the Hsp90/Cdc37 complex by inhibitors (17-N-allylamino-17-demethoxygeldanamycin 17-AGG, and celastrol) or short hairpin RNAs (shRNAs) significantly reduced expression levels of viral proteins and virus yield, suggesting that the Hsp90/Cdc37 chaperone complex functions in virus replication. The expression levels of p17 were decreased at the examined time points (2 to 7 h and 7 to 16 h) in 17-AAG-treated cells in a dose-dependent manner while the expression levels of viral proteins σA, σC, and σNS were decreased at the examined time point (7 to 16 h). Interestingly, the expression levels of σC, σA, and σNS proteins increased along with coexpression of p17 protein. p17 together with the Hsp90/Cdc37 complex does not increase viral genome replication but enhances viral protein stability, maturation, and virus production. Virus factories of ARV are composed of nonstructural proteins σNS and μNS. We found that the Hsp90/Cdc37 chaperone complex plays an important role in accumulation of the outer-capsid protein σC, inner core protein σA, and nonstructural protein σNS of ARV in viral factories. Depletion of Hsp90 inhibited σA, σC, and p17 proteins colocalized with σNS in viral factories. This study provides novel insights into p17-modulated formation of the Hsp90/Cdc37 chaperone complex governing virus replication via stabilization and maturation of viral proteins and accumulation of viral proteins in viral factories for virus assembly.
IMPORTANCE Molecular mechanisms that control stabilization of ARV proteins and the intermolecular interactions among inclusion components remain largely unknown. Here, we show that the ARV p17 is an Hsp90 client protein. The Hsp90/Cdc37 chaperone complex is essential for ARV replication by protecting p17 chaperone from ubiquitin-proteasome degradation. p17 modulates the formation of Hsp90/Cdc37 complex by phosphorylation of Cdc37, and this chaperone machinery protects p17 from ubiquitin-proteasome degradation, suggesting a feedback loop between p17 and the Hsp90/Cdc37 chaperone complex. p17 together with the Hsp90/Cdc37 complex does not increase viral genome replication but enhances viral protein stability and virus production. Depletion of Hsp90 prevented viral proteins σA, σC, and p17 from colocalizing with σNS in viral factories. Our findings elucidate that the Hsp90/Cdc37 complex chaperones p17, which, in turn, promotes the synthesis of viral proteins σA, σC, and σNS and facilitates accumulation of the outer-capsid protein σC and inner core protein σA in viral factories for virus assembly.
KEYWORDS: avian reovirus, nonstructural protein p17, Hsp90/Cdc37 chaperone machinery, outer-capsid protein σC, inner core protein σA, nonstructural protein σNS, viral factory
INTRODUCTION
The chaperones of eukaryotic cells have been classified into two types, heat shock protein (Hsp) and T-complex protein-1 ring complex (TRiC). The molecular chaperone Hsp90 forms a dimer to be functional and can be divided into three regions according to the structure, including N-terminal domain (NTD), middle domain (MD), and C-terminal domain (CTD) (1). The ATP-binding site is crucial for Hsp90 ATPase activity. The Hsp90 conformation is modulated by the binding of ATP to its N-terminal binding domain (NBD). After ATP binding, the NBD of Hsp90 switches to the “closed” state, which allows Hsp90 to clamp onto the target protein, thereby assisting target proteins in acquiring their native active conformation to exert its function (2, 3). The ATPase activity of Hsp90 cleaves ATP into ADP and Pi, which results in Hsp90 in the “open” state, thereby releasing the target protein from Hsp90 (2, 3). Hsp90 activity requires phosphorylation in molecular chaperone region where the N-terminal domain and middle domain are connected (4). The molecular chaperone Hsp90 together with its cochaperone proteins is involved in the regulation of protein folding, maturation, and activation to maintain cellular homeostasis and survival (5, 6). Cdc37 is a cochaperone of Hsp90 that serves as an adaptor to target Hsp90 to cellular kinases and assists Hsp90 to stabilize and activate target proteins (7). Cdc37 interacts with the NBD of Hsp90, thereby inhibiting the Hsp90 ATPase cycle and permitting the loading of target proteins (8). Therefore, the interaction of Cdc37 with Hsp90 has been thought essential to chaperone target proteins. Another molecular chaperone, TRiC, also called CCT, has a double ring structure with each ring composed of 8 subunits (CCT1 to -8) (9, 10). TRiC has a central cavity that binds to unfolded polypeptides and folds the protein, requiring ATP hydrolysis. Cellular actin and tubulin are also folded by TRiC (9, 11).
Avian reovirus (ARV) is an oncolytic virus (12–14) that has been applied in anti-liver-cancer research (12, 14). Our previous study suggested that ARV enters the cell following attachment to membrane-bound receptors and caveolin-1-mediated and dynamin-2-dependent endocytosis (15). The particle is uncoated by cysteine proteases, leading to delivery of the transcriptionally active core into the cytoplasm. ARV replicates in cytoplasmic inclusion bodies, also known as virus factories or viroplasms (16). Eariler studies suggested that virus factories of ARV are composed of nonstructural proteins σNS and μNS (17–19). μNS has been demonstrated to recruit σNS and λA proteins to these structures but not inner core protein σA or outer-capsid protein σC, suggesting that the recruitment of viral proteins into ARV factories has specificity (17, 18). Our recent reports have demonstrated that the nonstructural protein p17 of ARV interacts with several cellular proteins such as nucleoporin Tpr, vimentin, lamin A/C, hnRNP A1, and cyclin-dependent kinase (CDK), suggesting that p17 plays an important role in regulating cellular signal pathways which modulate autophagy, cell cycle and, virus replication (20–22). To date, molecular mechanisms that control stabilization of ARV proteins, the genesis of inclusions, the intermolecular interactions among inclusion components, and specific roles that inclusions play in morphogenesis of progeny virions remain largely unknown. The aim of this study is to investigate stabilization of p17, viral protein synthesis and maturation, and accumulation of inner core protein σA and outer-capsid protein σC required in the viral factory for virus assembly. In this study, we conducted two-dimensional liquid chromatography tandem mass spectrometry (2D-LC-MS/MS) analysis, coimmunoprecipitation assays, glutathione S-transferase (GST) pulldown assays, and immunostaining to identify Hsp90 interaction with p17, to reveal p17-modulated formation of Hsp90/Cdc37 chaperone complex, and to study Hsp90-mediated accumulation of σA, σC, and σNS proteins of ARV in viral factories. In this work, we found that the ARV p17 is an Hsp90 client protein. The ARV p17 protein modulates the formation of the Hsp90/Cdc37 chaperone machinery, thereby enhancing its stabilization and promoting the synthesis of other viral proteins. The current study provides novel insights into p17-modulated Hsp90/Cdc37 chaperone machinery governing virus replication through protecting p17 from ubiquitin-proteasome degradation and accumulating inner core protein σA and outer-capsid protein σC in viral factories for virus assembly.
RESULTS
p17 interacts with Hsp90.
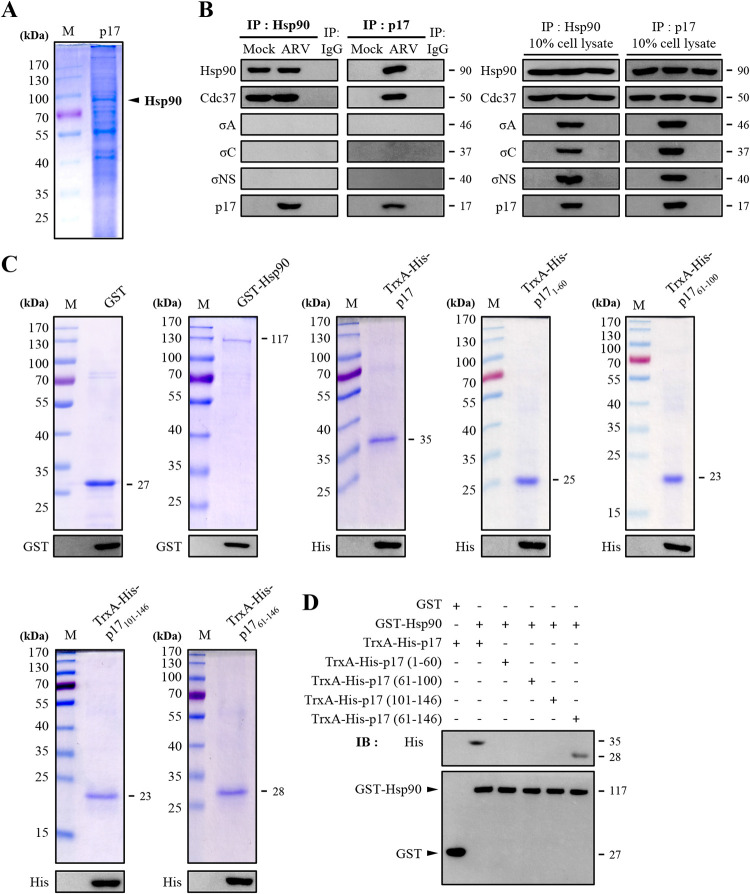
We have demonstrated previously that the ARV p17 protein interacts with several cellular proteins (20–22). To investigate whether host factors can interact with p17 to regulate the stability of p17, Vero cells were transfected with the pcDNA3.1-p17-Flag plasmid for 24 h followed by a coimmunoprecipitation assay with the Flag antibody. To obtain large amounts of p17-coimmunoprecipitated proteins, Vero cells in 15 6-cm cell culture dishes were harvested (20). Specific bands migrating at 90 kDa of p17-coimmunoprecipitated proteins revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 1A) were excised for 2D-LC-MS/MS analysis. Chaperone Hsp90 was analyzed to determine whether it can interact with viral proteins. In the present study, a reciprocal coimmunoprecipitation assay was performed with Hsp90 and p17 antibodies in ARV-infected Vero cells. The results reveal that Hsp90 and Cdc37 coimmunoprecipitated with p17 but not with other viral proteins σA, σC, and σNS (Fig. 1B, left panel). Both Hsp90 and Cdc37 coimmunoprecipitated with p17 while ARV σA, σC, and σNS proteins did not (Fig. 1B, right panel).
FIG 1.
Identification of potential cellular factors that interact with p17. (A) Cellular proteins coimmunoprecipitated by the p17 antibody were analyzed by SDS-PAGE. Vero cells were transfected with pcDNA3.1-p17 plasmid for 24 h, followed by a coimmunoprecipitation assay with p17 antibody. Cellular proteins coimmunoprecipitated by the p17 antibody were analyzed by SDS-PAGE. (B) Coimmunoprecipitation assays in ARV-infected Vero cells were performed with the Hsp90 and p17 antibodies, respectively. Following immunoblotting, Hsp90, Cdc37, σA, σC, σNS, and p17 were detected by the respective antibodies. (C) To define the region within p17 that was involved in Hsp90 binding, a series of truncated versions of His-tagged p17 constructs were established. The purified protein TrxA-His-p17, TrxA-His-p17 mutants, and GST-Hsp90 fusion proteins were analyzed by SDS-PAGE. The expected sizes of purified fusion proteins are indicated. The expressed fusion proteins were confirmed by Western blotting assays with the anti-GST or anti-His antibodies. (D) An in vitro GST pulldown assay was carried out with purified His- or GST-tagged fusion proteins (2 μg). Elution fractions were examined by Western blotting with the indicated antibodies (GST and His). The representative data are from three independent experiments. The predicted size of each protein was labeled at the right of gels and blots in KDa in each figure.
As the above coimmunoprecipitation experiments cannot rule out the possibility of indirect interactions due to the presence of other proteins, we also performed glutathione S-transferase (GST) pulldown analysis. To perform this assay, constructs expressing TrxA-His-p17, TrxA-His-p17 mutants, and GST-Hsp90 fusion proteins were expressed and purified. The expression levels were analyzed by SDS-PAGE and confirmed by Western blotting (Fig. 1C). The GST pulldown assay showed efficient precipitation of TrxA-His-p17 by GST-Hsp90 (Fig. 1D). We subsequently mapped the region of p17 protein involved in Hsp90 binding using a series of TrxA-His-tagged p17 deletion mutants. Deletion of the amino or carboxyl terminus of p17 in p171–60, p1761–100, and p17101–146 abolished p17 binding to Hsp90 (Fig. 1D) while the p1761–146 protein has the ability to bind Hsp90 (Fig. 1D).
The Hsp90/Cdc37 chaperone complex stabilizes the ARV p17 protein, which enhances the expression levels of σA, σC, and σNS of ARV.
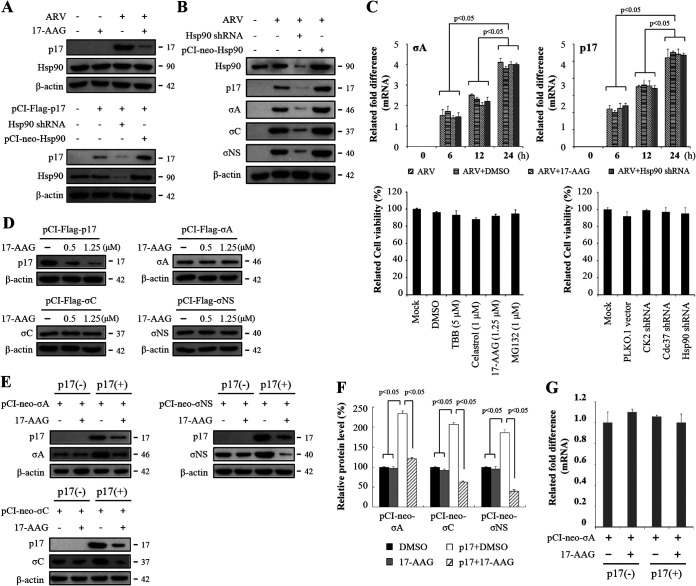
Hsp90 is a chaperone protein, and its function mainly protects the newly generated polypeptide from being folded and transported (23). The identification of Hsp90/p17 interaction led us to hypothesize that Hsp90 functions in ARV replication. Thus, the role of Hsp90 for the p17 stabilization and virus replication was further studied. 17-AAG blocks Hsp90 chaperone by binding to its ATP binding pocket, thereby locking the conformation of Hsp90 in the “open” state, leading to subsequent target protein misfolding and degradation (24–26). Thus, 17-AAG was used to inhibit Hsp90. Inhibition of Hsp90 by 17-AAG reduced the expression levels of the ARV p17 protein in ARV-infected Vero cells (Fig. 2A). To mitigate the possibility of off-target effects or that the inhibitor concentrations used were insufficient to affect ARV, we undertook short hairpin RNA (shRNA) knockdown experiments targeting Hsp90. The results revealed that the expression level of p17 decreased in Hsp90-knockdown Vero cells while overexpression of Hsp90 increased the expression levels of p17 (Fig. 2A). As shown in Fig. 1B and D, Hsp90 interacts with p17 but not with other viral proteins (σA, σC, or σNS). To study whether Hsp90 chaperones p17, σA, σC, and σNS proteins of ARV, the expression levels of these proteins were analyzed by Western blotting in Vero cells treated with 17-AAG, overexpressing Hsp90, and having Hsp90 knocked down, respectively. Interestingly, expression levels of ARV p17, σA, σC, and σNS proteins were reduced in Hsp90-knockdown Vero cells while their expression levels were increased in Vero cells overexpressing Hsp90 (Fig. 2B). To study whether Hsp90 transcriptionally regulates viral genes, the mRNA levels of σA-encoding and p17-encoding genes of ARV in Vero cells treated with 17-AAG (1.25 μM) or Hsp90 shRNA were quantified by quantitative real‐time reverse transcription-PCR (qRT‐PCR), respectively. As shown in Fig. 2C, upper panel, the mRNA levels of σA and p17 genes of ARV were not changed in 17-AAG-treated cells or Hsp90-knockdown cells, suggesting that Hsp90 does not transcriptionally regulate these viral genes. To investigate whether inhibitors of Hsp90 have deleterious effects on the cell, the viability of the cells was assessed by a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Cell viability in 17-AAG-treated and Hsp90-knockdown cells was only slightly reduced compared to the mock-treatment cells (Fig. 2C, lower panel). Collectively, our data suggest that Hsp90 is required for stability of the p17 protein of ARV.
FIG 2.
The Hsp90/Cdc37 chaperone complex protects p17 from ubiquitin-proteasome-mediated degradation, and p17 increases the expression levels of σA, σC, and σNS proteins of ARV. (A) To study whether Hsp90 stabilizes p17, inhibition of Hsp90 by 17-AAG (1.25 μM) in ARV-infected cells as well as depletion of Hsp90 with an shRNA or overexpression of Hsp90 in p17-transfected Vero cells was carried out. Vero cells were infected with an MOI of 10 for 24 h. The expression levels of p17 and Hsp90 were examined by Western blot analysis with the indicated antibodies. β-Actin was used as an internal control. (B) To examine whether Hsp90 affects other structural proteins, inhibition of Hsp90 with an shRNA or overexpression of Hsp90 in ARV-infected cells was carried out. The expression levels of viral proteins and Hsp90 were examined by Western blot analysis with the indicated antibodies. β-Actin was used as an internal control. (C) To further study whether Hsp90 transcriptionally regulates the σA and p17 genes of ARV, mRNA levels of the σA-encoding and p17-encoding genes of ARV in Vero cells treated with 17-AAG and Hsp90 shRNA were analyzed. Vero cells were infected at different time points as indicated. The mRNA levels of σA-encoding and p17-encoding genes of ARV in Vero cells treated with 17-AAG (1.25 μM) and Hsp90 shRNA were measured by qRT‐PCR, respectively. Vero cells were transfected with the Hsp90 shRNA plasmid for 6 h, followed by infection with ARV at an MOI of 10. To examine whether the compounds or shRNAs had deleterious effects on cells, cell viability was determined using the MTT assay. Each value represents mean ± SE from three independent experiments. DMSO, dimethyl sulfoxide. (D) 17-AAG was also used to inhibit Hsp90 in the indicated plasmid-transfected Vero cells. The expression levels of viral proteins p17, σA, σC, and σNS were examined by Western blotting with the indicated antibodies. β-Actin was used as an internal control. All experiments were conducted in three replicate experiments. (E) Expression plasmids (pCI-Flag-σA, pCI-Flag-σC, and pCI-Flag-σNS) were expressed in Vero cells with or without the p17 protein. The medium was replaced with fresh medium containing 1.25 μM 17-AAG at 6 h posttransfection. At 24 h posttransfection, the cell lysates were prepared and subjected to immunoblotting with the indicated antibodies. (F) Densitometry analysis results for Western blotting are expressed as percentages representing p17, σA, σC, and σNS protein levels, normalized to β-actin. The results were calculated from the data shown in panel D. Mock cells were considered 100%. All data shown represent the means ± standard deviations (SD) calculated from three independent experiments. (G) To investigate whether p17 affected the mRNA levels of the σA-encoding gene of ARV, the mRNA levels of the σA-encoding gene of ARV were quantified by real-time qRT-PCR in p17-transfected cells in the presence or absence of the indicated drugs. Each value represents the mean ± SE from three independent experiments. Signals were quantified using ImageJ software.
To further determine whether Hsp90 chaperones viral proteins p17, σA, σC, and σNS, inhibition of Hsp90 by 17-AAG in plasmid-transfected cells was carried out. We found that inhibition of Hsp90 reduced the expression levels of p17 in p17-transfected Vero cells while the expression levels of σA, σC, and σNS proteins were not affected in the same cells (Fig. 2D). As seen from our previous report suggesting that exogenous expression of p17 in ARV-infected cells significantly enhances virus yield (13), we next wanted to investigate whether the decreased levels of σA, σC, and σNS are due to the decreased level of p17 induced by Hsp90 inhibition. To study whether p17 protein regulates the expression levels of σA, σC, and σNS proteins of ARV, plasmids (pCI-Flag-σA, pCI-Flag-σC, and pCI-Flag-σNS) encoding σA, σC, and σNS proteins were expressed in Vero cells with or without the p17 protein. In the presence of 17-AAG, the levels of viral proteins σA, σC, and σNS were not altered when expressed alone (Fig. 2E). Interestingly, increased levels of these viral proteins were seen when coexpressed with the p17 protein (Fig. 2E). Inhibition of Hsp90 by 17-AAG decreased the expression levels of p17 with a concomitant decrease in the expression levels of σA, σC, and σNS proteins. Densitometry quantification confirmed a significant increase in all examined viral protein levels in p17-transfected cells (Fig. 2F). Taken together, our results revealed that the Hsp90/Cdc37 chaperone complex stabilizes the ARV p17 protein, which enhances the expression levels of σA, σC, and σNS proteins. The findings are consistent with our previous study suggesting that p17 enhances viral proteins and virus yield (13). To further study whether p17 transcriptionally regulates viral genes, we examined the mRNA levels of the σA-encoding gene of ARV in the presence of 17-AAG (1.25 μM) or cotransfection with the plasmid pCI-Flag-p17. The result reveals that neither Hsp90 nor p17 transcriptionally regulates the σA-encoding gene of ARV (Fig. 2G).
The ARV p17 protein enhances the formation of Hsp90/Cdc37 chaperone machinery, which protects p17 from ubiquitin-proteasome-mediated degradation.
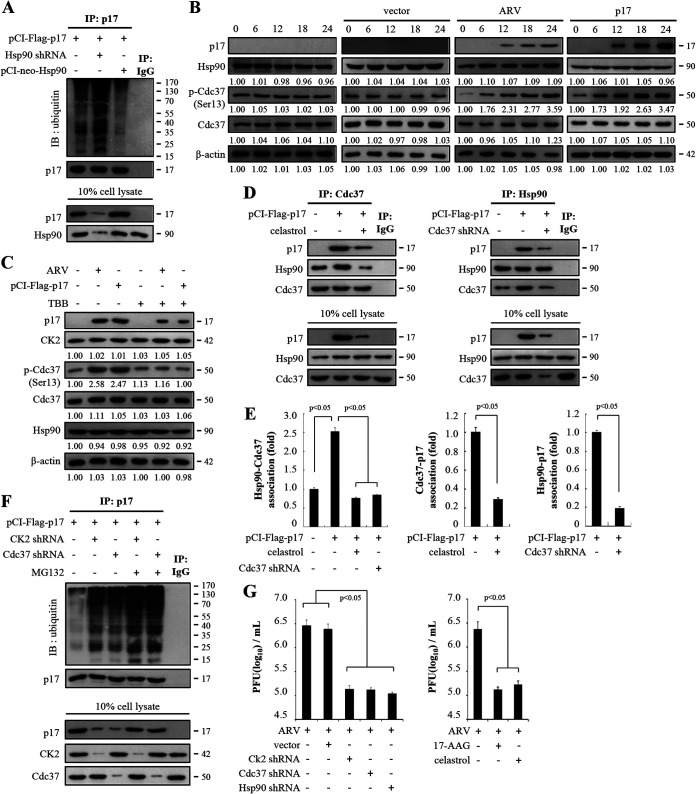
To further investigate how Hsp90 is required for stability of the ARV p17 protein, we examined whether Hsp90 protects the p17 protein from ubiquitin-proteasome-mediated degradation. Analysis of the role of Hsp90 in p17 stability showed the levels of polyubiquitinated p17 increased in Hsp90-knockdown Vero cells while overexpression of Hsp90 reduced the amounts of ubiquitin attached to p17 (Fig. 3A). These results suggest that Hsp90 plays a critical role in protecting p17 from ubiquitination and degradation. The molecular chaperone Hsp90 is regulated by cochaperones such as Cdc37. Functionally, Cdc37 acts as an adaptor or scaffold molecule to facilitate the interaction of clients with Hsp90 (27). Cdc37 function is regulated through phosphorylation of Ser13 by casein kinase 2 (CK2) (28). The phosphorylation of Cdc37 by CK2 is crucial for the recruitment of Hsp90 to the protein kinase-Cdc37 complexes (28). Our previous study has demonstrated that CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) blunts p17-modulated phosphorylation of PTEN by CK2 (20). We next wanted to examine whether the p17 protein modulates phosphorylation of Cdc37. Interestingly, the phosphorylated form of Cdc37 increased in ARV-infected and pCI-Flag-p17-transfected Vero cells (Fig. 3B) and the elevated phosphorylated form of Cdc37 was reversed in TBB-treated cells (Fig. 3C). In the presence of CK2 inhibitor TBB, the expression levels of p17 protein were also decreased in ARV-infected and pCI-Flag-p17-transfected Vero cells (Fig. 3C). The expression levels of CK2 and Hsp90 were not altered in ARV-infected and pCI-Flag-p17-transfected cells (Fig. 3B and C). Our results suggest that p17 mediates phosphorylation of Cdc37 through CK2. To investigate whether p17 modulates the formation of the Hsp90/Cdc37 complex and whether p17 associates with Hsp90 and Cdc37, celastrol, an allosteric inhibitor that binds to the Hsp90 C-terminal domain (29), was used. Coimmunoprecipitation data revealed that p17 elevates the amounts of the Hsp90/Cdc37 chaperone complex while the association of p17/Cdc37, p17/Hsp90, and Hsp90/Cdc37 was reduced in celastrol-treated cells or in Cdc37-knockdown cells (Fig. 3D and E). The results showed that p17 modulated the formation of the Hsp90/Cdc37 chaperone complex. Based on these findings, we next examined whether CK2 and Cdc37 also affect polyubiquitylation of p17. As shown in Fig. 3F, increased amounts of p17 polyubiquitylation were seen in both CK2- and Cdc37-knockdown cells compared to untreated cells. The amounts of p17 polyubiquitylation were increased in the presence of MG132, suggesting that CK2 and Cdc37 are involved in protecting p17 from ubiquitin-proteasome degradation. Taken together, our findings suggest that the chaperoning of the ARV p17 protein requires the formation of the Hsp90/Cdc37 chaperone complex.
FIG 3.
The ARV p17 protein enhances the formation of Hsp90/Cdc37 chaperone machinery. (A) To investigate whether Hsp90 protects p17 protein from ubiquitin-proteasome-mediated degradation, the signals of polyubiquitinated p17 were examined in Hsp90 knockdown and Hsp90 overexpression in p17-transfected Vero cells. Rabbit IgG served as negative control. (B) To investigate whether the p17 protein modulates phosphorylation of Cdc37, levels of Hsp90 and p-Cdc37 in ARV-infected and p17-transfected Vero cells were analyzed. The expression levels of p17, Hsp90, Cdc37, and p-Cdc37 in Vero cells infected with ARV at an MOI of 10 or transfected with the pCI-Flag-p17 plasmid were examined by Western blotting assays. Vero cells were ARV infected and pCI-p17 transfected at the indicated time points (hours). The levels of the indicated proteins in the mock-control group (0 h) were considered 1-fold. The fold changes indicated below each lane were normalized against values for the mock-control group. Protein levels were normalized to those for β‐actin. Signals in all Western blots were quantified with ImageJ software. All experiments were conducted in three independent experiments. (C) To study whether the p17 protein modulates phosphorylation of Cdc37, the effect of CK2 inhibitor TBB on p17-mediated phosphorylation of Cdc37 was assayed. The expression levels of p17, CK2, p-Cdc37, Cdc37, and Hsp90 were examined in p17-transfected and ARV-infected Vero cells. Vero cells were transfected with the pCI-Flag-p17 plasmid for 6 h, followed by treatment with CK2 inhibitor TBB (5 μM) for 24 h. In the ARV-infected group, Vero cells were simultaneously treated with CK2 inhibitor TBB and infected with ARV at an MOI of 10 for 24 h. The levels of indicated proteins in the mock-control group were considered 1-fold. The fold changes indicated below each lane were normalized against values for the mock-control group. Protein levels were normalized to those for β‐actin. Signals in Western blots were quantified with ImageJ software. All experiments were conducted in three independent experiments. (D) To investigate whether p17 modulates the formation of the Hsp90/Cdc37 complex and whether p17 associates with Hsp90 and Cdc37, the celastrol inhibitor and Cdc37 shRNA were used in p17-transfected Vero cells. p17 and cellular proteins coimmunoprecipitated by Hsp90 or Cdc37 antibodies were analyzed. Vero cells were transfected with the pCI-Flag-p17 plasmid for 6 h, followed by treatment with celastrol for 24 h. Furthermore, Vero cells were simultaneously transfected with the pCI-Flag-p17 and Cdc37 shRNA plasmid DNA. The amounts of p17/Hsp90, p17/Cdc37, and Hsp90/Cdc37 association were examined in the indicated treatments. The protein levels were detected by the indicated antibodies. (E) Densitometry analysis results for Western blotting are expressed as percentages representing the amount of protein association in panel D. Mock cells were considered 1-fold. Signals for all blots were quantified using ImageJ software. All experiments were conducted in three independent experiments. (F) To assess whether CK2 and Cdc37 are involved in p17 stabilization, Vero cells were transfected with the respective pCI-Flag-p17 or cotransfected with pCI-Flag-p17 and shRNA plasmid DNA for 6 h, followed by treatment with MG132 (1 μM). The amounts of p17 polyubiquitylation were examined by Western blotting assays. Similar results were obtained in three independent experiments. (G) To examine whether Hsp90/Cdc37 chaperone machinery plays a critical role in virus propagation, cells treated with celastrol (1 μM) or knockdown of CK2, Cdc37, and Hsp90 were analyzed. Vero cells were transfected with the respective Hsp90 shRNA plasmid for 6 h, followed by infection with ARV at an MOI of 10 for 24 h. In drug-treated groups, Vero cells were simultaneously treated with celastrol and infected with ARV at an MOI of 10 for 24 h. Cell lysates were collected 24 h postinfection for determination of virus titers by plaque assays. Each value represents the mean ± SE from three independent experiments.
Furthermore, we examined whether this Hsp90/Cdc37 chaperone machinery plays a critical role in virus propagation. As shown in Fig. 3G, virus yield was significantly reduced under the same treatment conditions, suggesting that the Hsp90/Cdc37 chaperone complex controls virus replication. In the present study, inhibitors and knockdown of CK2, Cdc37, and Hsp90 have no obvious effects on cell viability (Fig. 2C). Our results suggest a mechanism whereby p17 plays an important role in modulation of the formation of the Hsp90/Cdc37 chaperone complex and this chaperone machinery plays a critical role in protecting the ARV p17 protein from ubiquitin-proteasome degradation, thereby benefiting virus replication.
Hsp90/Cdc37 chaperone machinery is required for ARV replication.
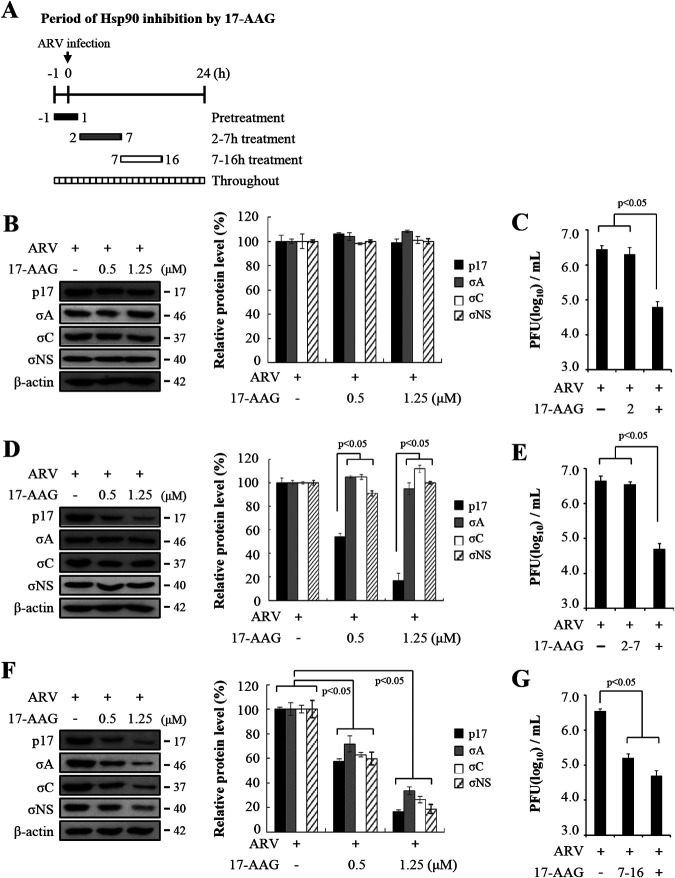
In order to obtain direct evidence that Hsp90 is involved during the virus life cycle, we used 17-AAG, which binds the N-terminal ATP/ADP binding domain of Hsp90 and interferes with its ability to stabilize client proteins (24–26). We examined viral genome replication and protein synthesis alongside viral particle assembly during ARV infection. Vero cells were treated with 17-AAG during different time windows before and after ARV infection at a multiplicity of infection (MOI) of 10 for 24 h. The cells were washed to remove the drug and further incubated until 24 h postinfection. All drug-treated and untreated-cells were collected 24 h postinfection for Western blotting and determination of virus titers. Figure 4A represents the experimental design for 17-AAG treatment of ARV-infected cells at different time points in the viral life cycle. At 1h prior to infection, 17-AAG treatment of ARV-infected cells were harvested at 24 h postinfection for Western blotting using the indicated antibodies. All assayed viral protein levels in treated cells were similar to those of the untreated group (Fig. 4B). The virus titers of the group treated before infection were similar to those of the untreated group (Fig. 4C). This signified that Hsp90 may not have played a role during virus entry. In this work, the expression levels of p17 were decreased at the time window of 2 to 7 h in 17-AAG-treated cells in a dose-dependent manner while the expression levels of viral proteins σA, σC, and σNS were not altered at the same time points (Fig. 4D). Densitometry quantification confirmed a decrease in p17 protein levels of 80% at an inhibitor concentration of 1.25 μM (Fig. 4D). Figure 4E reveals that the virus titer of the groups treated for the time window of 2 to 7 h was similar to that of the untreated group. Interestingly, the expression levels of viral proteins σA, σC, and σNS were reduced at time windows of 7 to 16 h (Fig. 4F). Densitometry quantification confirmed a significant decrease in all examined viral protein levels of between 70 and 85% in the presence of 17-AAG (1.25 μM) (Fig. 4F). The virus titer of the groups treated for the time window of 7 to 16 h was 1.5 log units lower than that of the untreated sample (Fig. 4G). Furthermore, virus titers of cells treated with 17-AAG for 24 h were 2 log units lower than those of the untreated samples as shown in Fig. 4C, E, and G. The kinetic study suggested that the Hsp90/Cdc37 chaperone complex is involved likely at the middle to late stages in the virus life cycle, since the inhibitory effect of 17-AAG was seen primarily in the 7- to 16-h treatment group (Fig. 4F and G).
FIG 4.
The Hsp90/Cdc37 chaperone machinery is required for ARV replication. (A) Experimental design for pulse treatment with 17-AAG in ARV-infected cells at different time points in the viral life cycle is shown. To elucidate inhibition of Hsp90 in the presence of different concentrations of 17-AAG, Vero cells were treated with 17-AAG during different time windows before and after ARV infection at an MOI of 10. After treatment with 17-AAG on different time windows, cells were washed to remove the drug. (B to G) All 17-AAG-treated and untreated cell lysates were collected at 24 h postinfection for Western blotting (B, D, and F) and determination of virus titers (C, E, and G). The expression levels of viral proteins p17, σA, σC, and σNS were analyzed by Western blotting assays. Densitometry analysis results for Western blotting are expressed as percentages representing p17, σA, σC, and σNS protein levels, normalized to β-actin. Untreated cells were considered 100%. Signals for all blots were quantified using ImageJ software. All experiments were conducted in three independent experiments. (C, E, and G) The effect of Hsp90 on ARV replication was also analyzed by determining the virus titers of the groups treated for different time points. Vero cells were infected with ARV at an MOI of 10 for 24 h and treated with 17-AAG (1.25 μM) for different time windows. All 17-AAG-treated and untreated cell lysates were collected at 24 h postinfection for determination of virus titers. Each value represents the mean ± SE from three independent experiments.
The nonstructural protein p17 of ARV codistributes with σNS in cytoplasmic viral inclusions.
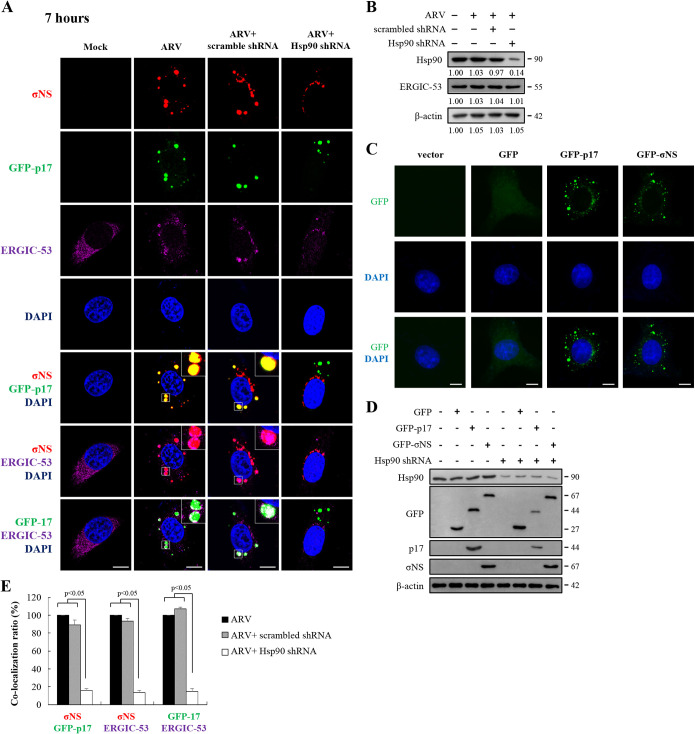
Earlier studies suggested that viral factories of ARV are composed of nonstructural proteins σNS and μNS (17–19). μNS recruits σNS and λA to these structures, but not inner core protein σA or outer-capsid protein σC (17, 18). To study whether Hsp90 modulates inner core protein σA and outer-capsid protein σC accumulated in ARV factories, Vero cells were transfected with an Hsp90 shRNA for 6 h, followed by infection with ARV with an MOI of 10. Vero cells were immunostained for p17, σNS, σA, σC, and ERGIC-53 with the respective antibodies. Since viral factories of ARV are composed of nonstructural proteins σNS and μNS (17–19), viral factories were identified by σNS staining. Since p17, σNS, σA, σC, and ERGIC-53 antibodies were produced in mice, we could not use our mouse anti-p17 and anti-σNS antibodies to visualize p17 and σNS in ARV-infected cells. To overcome this problem, we decided to express p17 and σNS as fusion proteins attached to the C terminus of the green fluorescent protein (GFP-p17 and GFP-σNS), which could thus be detected without using antibodies. For transient expression of GFP-p17 and GFP-σNS, the p17- and σNS-encoding genes of ARV were inserted into the pcDNA3.1 vector to create the recombinant plasmids pcDNA3.1-GFP-p17 and pcDNA3.1-GFP-σNS, respectively. ERGIC-53 is a 53-kDa nonglycosylated type I single-spanning transmembrane protein that has been identified as a marker protein for ERGIC (30). An earlier study suggested that ERGIC-53 and mammalian reovirus (MRV) double-stranded RNAs (dsRNAs) colocalized with the MRV σNS protein and ERGIC-53 were found inside inclusions (31). Thus, we also examined whether p17 and Hsp90 affect accumulation of ERGIC-53 and ARV dsRNA in viral factories. As shown in Fig. 5A, GFP-p17 codistributes with σNS and endoplasmic reticulum (ER)-Golgi intermediate compartment-53 (ERGIC-53). Colocalization of σNS and ERGIC-53 was also observed (Fig. 5A). The expression levels of Hsp90 and ERGIC-53 were also examined by Western blotting assays. The results revealed that ARV does not affect the expression levels of ERGIC-53 and Hsp90 and knockdown of Hsp90 did not influence the expression levels of ERGIC-53 (Fig. 5B). Furthermore, immunofluorescence results of GFP, GFP-p17, and GFP-σNS reveal that GFP fusion to p17 or σNS does not affect distribution of p17 and σNS in mock-treated Vero cells (Fig. 5C). To examine whether the GFP-p17 and GFP-σNS fusion proteins affect stabilization of p17 and σNS, the expression levels of GFP, GFP-p17, GFP-σNS, and Hsp90 in Hsp90-depleted cells were assayed by Western blotting. As shown in Fig. 5D, the expression levels of GFP-p17 were decreased while the expression levels of GFP-σNS and GFP alone were not altered, suggesting that GFP does not mask the chaperone effect. These data are consistent with the data shown in Fig. 2D. Furthermore, in colocalization analysis, the colocalization ratios of GFP-p17/σNS, σNS/ERGIC-53, and GFP-p17/ERGIC-53 were decreased in Hsp90-depleted cells (Fig. 5E).
FIG 5.
Distribution of GFP-p17, σNS, and ERGIC-53 in cytoplasmic viral inclusions and Hsp90 promotion of accumulation of p17 in viral inclusions. (A) Confocal immunofluorescence images of ARV-infected Vero cells (MOI of 10, 7 h postinfection) stained with DAPI (blue) and antibodies specific for σNS (red) and ERGIC-53 (purple). For transient expression of GFP-p17, the recombinant plasmid pcDNA3.1-GFP-p17 was used. The subcellular localization of GFP-p17, σNS, and ERGIC-53 was visualized by confocal microscopy. To examine whether Hsp90 enhances accumulation of p17 and σNS in viral inclusions and ERGIC, the subcellular localization of p17, σNS, and ERGIC-53 was visualized in Hsp90-knockdown Vero cells. Vero cells were transfected with the Hsp90 shRNA plasmid for 6 h, followed by infection with ARV at an MOI of 10. Viral inclusions are identifiable by σNS. ERGIC-53 is a marker protein for ERGIC. Enlarged images correspond to the region indicated by the white box in the merged image. The representative images are from three independent experiments. Scale bars, 20 μm. (B) The expression levels of Hsp90 and ERGIC-53 were examined by Western blotting. To study whether knockdown of Hsp90 influences the expression levels of ERGIC-53, Vero cells were transfected with the Hsp90 shRNA plasmid for 6 h, followed by infection with ARV at an MOI of 10. The levels of the indicated proteins in the mock-control group were considered 1-fold. The fold changes indicated below each lane were normalized against values for the mock-control group. Protein levels were normalized to those for β‐actin. Signals in all Western blots were quantified with ImageJ software. Experiments were conducted in three independent experiments. (C) Immunofluorescence images of GFP, GFP-p17, and GFP-σNS in Vero cells were observed at 7 h posttransfection. Vero cells were stained with DAPI (blue). (D) To examine whether the GFP-p17 and GFP-σNS fusion proteins affect stabilization of p17 and σNS, the expression levels of GFP, GFP-p17, GFP-σNS, and Hsp90 in Hsp90-depleted cells were assayed by Western blotting. (E) Quantification ratios of the colocalization of GFP-p17/σNS, σNS/ERGIC-53, and GFP-p17/ERGIC-53 in ARV-infected, scrambled shRNA-transfected, and Hsp90 shRNA-transfected Vero cells (A). ARV-infected cells were considered 100%. At least 10 fields of 1 to 2 cells per field were taken for each sample per experiment while the exposure settings were unchanged during acquisition of various samples. The colocalization was quantified with ImageJ software. Each experiment was performed at least three times. Statistical analysis was performed using Student’s t test.
The inner core protein σA and outer-capsid protein σC of ARV codistribute with p17, σNS, and ERGIC-53 in cytoplasmic viral inclusions.
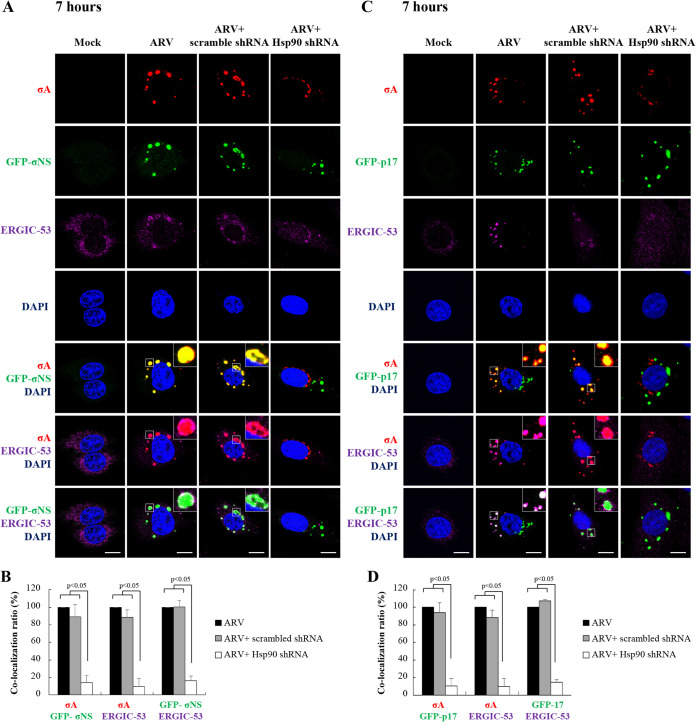
In this work, colocalization of GFP-σNS/σA, GFP-p17/σA, σA/ERGIC-53, GFP-σNS/σC, GFP-p17/σC, and σC/ERGIC-53 in ARV-infected Vero cells was observed at 7 h postinfection (Fig. 6A and C and Fig. 7A and C). Interestingly, colocalization was disrupted in Hsp90-depleted cells. In colocalization analysis, the colocalization ratios of GFP-σNS/σA, GFP-p17/σA, σA/ERGIC-53, GFP-σNS/σC, GFP-p17/σC, and σC/ERGIC-53 were significantly decreased in Hsp90-depleted cells (Fig. 6B and D and Fig. 7B and D). Taken together, intracellular distribution and accumulation of viral proteins σA, σC, σNS, and p17 in viral factories were altered in Hsp90-depleted cells, suggesting that Hsp90 plays an important role in accumulation of viral proteins p17, σNS, σA, and σC in viral factories. Furthermore, we observed colocalization of σA/ERGIC-53, σA/dsRNA, and dsRNA/ERGIC-53 at 12 h postinfection (Fig. 8A), but this colocalization was also disrupted in Hsp90-depleted cells. The colocalization ratios of σA/ERGIC-53, σA/dsRNA, and dsRNA/ERGIC-53 were decreased in Hsp90-depleted cells (Fig. 8A and B). Colocalization of σA and dsRNA at 12 h postinfection was observed by confocal microscopy, consistent with previous studies suggesting that σA and σNS proteins of ARV possess dsRNA and single-stranded RNA (ssRNA) binding activity, respectively (32, 33). Taken together, our results indicated that viral proteins σA and σC are colocalized with σNS and ERGIC-53. Immunofluorescence confocal microscopy analysis demonstrated that ERGIC-53 codistributes with viral proteins (σA, σC, p17, σNS) and dsRNA in viral factories during infection (Fig. 5A, Fig. 6A and B, Fig. 7A and B, and Fig. 8A and B).
FIG 6.
Distribution of inner core protein σA, GFP-p17, and GFP-σNS in cytoplasmic viral inclusions and Hsp90 promotion of accumulation of inner core protein σA in viral inclusions. (A and C) Confocal immunofluorescence images of ARV-infected Vero cells (MOI of 10, 7 h postinfection) stained with DAPI (blue) and antibodies specific for inner core protein σA (red). For transient expression of GFP-p17 and GFP-σNS, the recombinant plasmids pcDNA3.1-GFP-p17 and pcDNA3.1-GFP-σNS were used. The subcellular localization of σA with GFP-σNS (A) or GFP-p17 (C) was visualized by confocal microscopy. To examine whether Hsp90 enhances accumulation of σA, p17, and σNS proteins in viral inclusions and ERGIC, the subcellular localization of these proteins was visualized in Hsp90-knockdown Vero cells. Viral inclusions are identifiable by σNS. Enlarged images correspond to the region indicated by the white box in the merged image. The representative images are from three independent experiments. Scale bars, 20 μm. (B and D) Quantification ratios of the colocalization of GFP-σNS/σA, σA/ERGIC-53, and GFP-σNS/ERGIC-53 (B) as well as the colocalization of GFP-p17/σA, σA/ERGIC-53, and GFP-p17/ERGIC-53 (D) in ARV-infected, scrambled shRNA-transfected, and Hsp90 shRNA-transfected Vero cells. Quantification of the colocalization was done with ImageJ software. ARV-infected cells were considered 100%. At least 10 fields of 1 to 2 cells per field were taken for each sample per experiment. All experiments were conducted in three independent experiments. Statistical analysis was performed using Student’s t test.
FIG 7.
Distribution of outer-capsid protein σC, GFP-p17, and GFP-σNS in cytoplasmic viral inclusions and Hsp90 promotion of accumulation of outer-capsid protein σC in viral inclusions. Confocal immunofluorescence images of ARV-infected Vero cells (MOI of 10, 7 h postinfection) stained with DAPI (blue) and antibodies specific for outer-capsid protein σC (red). For transient expression of GFP-p17 and GFP-σNS, the recombinant plasmids pcDNA3.1-GFP-p17 and pcDNA3.1-GFP-σNS were used. (A and C) The subcellular localization of σC with GFP-σNS (A) or GFP-p17 (C) was visualized by confocal microscopy. To examine whether Hsp90 enhances accumulation of viral proteins σC, p17, and σNS in viral inclusions, the subcellular localization of outer-capsid protein σC, GFP-σNS, and GFP-p17 was visualized in Hsp90-knockdown Vero cells. Viral inclusions are identifiable by σNS. Enlarged images correspond to the region indicated by the white box in the merged image. The representative images are from three independent experiments. Scale bars, 20 μm. (B and D) Quantification ratios of the colocalization of GFP-σNS/σC, σC/ERGIC-53, and GFP-σNS/ERGIC-53 (B) as well as the colocalization of GFP-p17/σC, σC/ERGIC-53, and GFP-p17/ERGIC-53 (D) in ARV-infected, scrambled shRNA-transfected, and Hsp90 shRNA-transfected Vero cells. The colocalization was quantified with ImageJ software. ARV-infected cells were considered 100%. At least 10 fields of 1 to 2 cells per field were taken for each sample per experiment. All experiments were conducted in three independent experiments. Statistical analysis was performed using Student’s t test.
FIG 8.
Distribution of inner core protein σA, dsRNA, and ERGIC-53 in cytoplasmic viral inclusions and Hsp90 promotion of accumulation of inner core protein σA in viral inclusions. (A) Confocal immunofluorescence images of ARV-infected Vero cells (MOI of 10, 12 h postinfection) stained with DAPI (blue) and antibodies specific for σA (red), dsRNA (green), and ERGIC-53 (purple). The subcellular localization of inner core protein σA (red), dsRNA (green), and ERGIC-53 was visualized by confocal microscopy. To examine whether Hsp90 enhances accumulation of inner core protein σA, dsRNA, and ERGIC in viral factories, the subcellular localization of inner core protein σA and dsRNA was also visualized in Hsp90-knockdown Vero cells. Enlarged images correspond to the region indicated by the white box in the merged image. The representative images are from three independent experiments. Scale bars, 20 μm. (B) Quantification ratios of the colocalization of σA/dsRNA, σA/ERGIC-53, and ERGIC-53/dsRNA. Quantification of the colocalization was done with ImageJ software. ARV-infected cells were considered 100%. At least 10 fields of 1 to 2 cells per field were taken for each sample per experiment. Each experiment was performed at least three times. Statistical analysis was performed using Student’s t test.
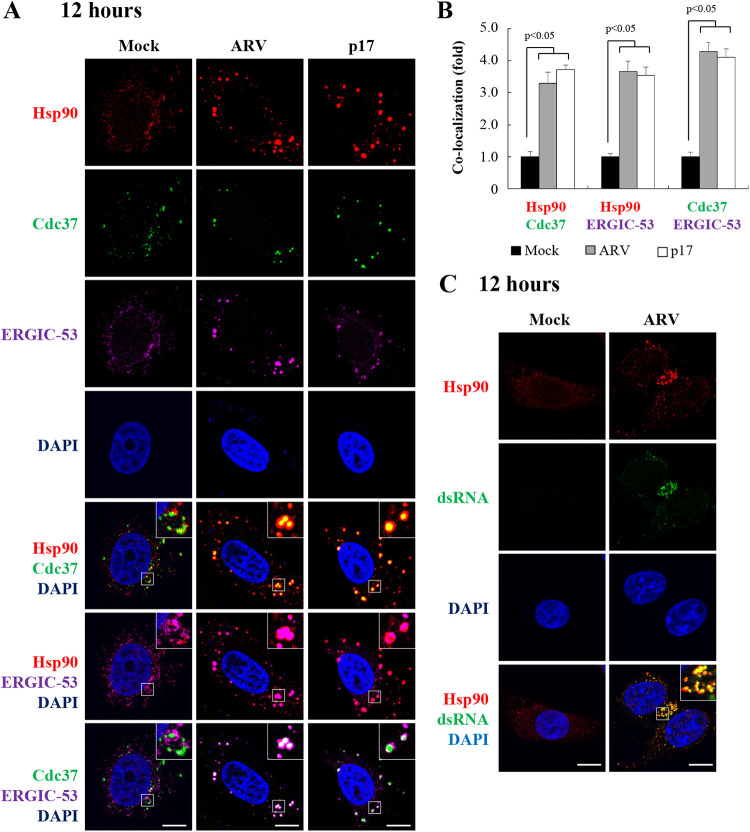
Hsp90 colocalized with Cdc37 and ERGIC-53.
Having shown that Hsp90 enhances accumulation of viral proteins p17, σNS, σA, and σC in ARV factories, we next examined the distribution of Hsp90/Cdc37, Hsp90/ERGIC-53, Cdc37/ERGIC-53, and Hsp90/dsRNA in ARV-infected and p17-transfected cells at 12 h postinfection. Colocalization of Hsp90/Cdc37, Hsp90/ERGIC-53, and Cdc37/ERGIC-53 in ARV-infected and p17-transfected Vero cells was observed (Fig. 9A) compared to the mock group. Importantly, we found that ARV infection and p17 transfection promote colocalization of Hsp90/Cdc37, Hsp90/ERGIC-53, and Cdc37/ERGIC-53 (Fig. 9A). The colocalization of Hsp90/Cdc37, Hsp90/ERGIC-53, and Cdc37/ERGIC-53 in ARV-infected and p17-transfected Vero cells is shown in Fig. 9B. Our data reveal that ARV infection or p17 transfection increases colocalization of Hsp90/Cdc37, Hsp90/ERGIC-53, and Cdc37/ERGIC-53. Furthermore, colocalization of Hsp90 and dsRNA at 12 h postinfection was observed by confocal microscopy (Fig. 9C).
FIG 9.
Colocalization of Hsp90 and Cdc37 in cytoplasmic viral inclusions. (A) Confocal immunofluorescence images of ARV-infected Vero cells (MOI of 10, 12 h postinfection) stained with DAPI (blue) and antibodies specific for Hsp90 (red), Cdc37 (green), and ERGIC-53 (purple). Viral inclusions are identifiable by ERGIC-53. Enlarged images correspond to the region indicated by the white box in the merged image. The representative images are from three independent experiments. (B) Quantification of the colocalization of Hsp90/Cdc37, Hsp90/ERGIC-53, and Cdc37/ERGIC-53 in mock- or ARV-infected or p17-transfected cells was analyzed with ImageJ software. Mock cells were considered 1-fold. At least 10 fields of 1 to 2 cells per field were taken for each sample per experiment. All experiments were conducted in three independent experiments. Statistical analysis was performed using Student’s t test. (C) Colocalization of Hsp90 and dsRNA in ARV-infected Vero cells (MOI of 10, 12 h postinfection) stained with DAPI (blue) and antibodies specific for Hsp90 (red) or dsRNA (green). All experiments were conducted in three independent experiments.
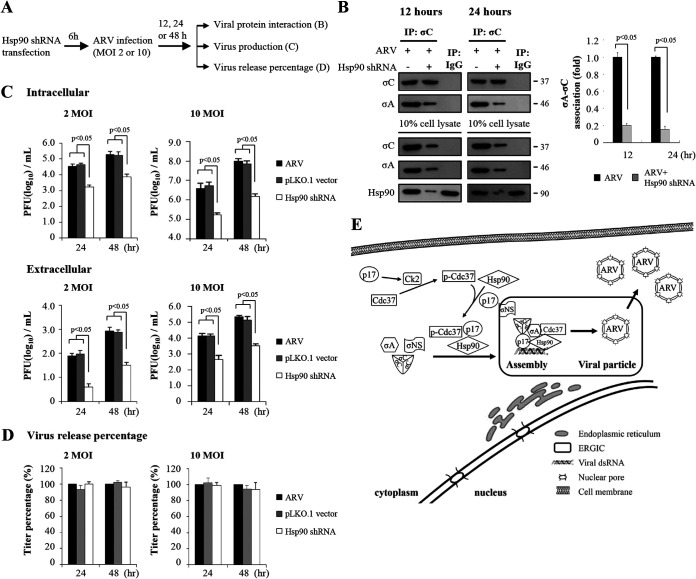
Hsp90 plays a critical role in virion biogenesis.
In the present study, we examined whether Hsp90 is required for the assembly of viral particles. The accumulation of intracellular and extracellular infectious virus was examined in Hsp90-knockdown cells. Since knockdown of Hsp90 inhibited recruitment of viral proteins σNS, σA, and σC to ARV factories, we investigated whether Hsp90 affects association of σA and σC. Fig. 10A is a schematic of the experimental procedures showing the times of protein collection, Hsp90 inhibition by shRNA, and collection of cell lysates. The results showed that knockdown of Hsp90 reduced the amounts of σA and σC association (Fig. 10B). As shown in Fig. 4G, inhibition of Hsp90 by 17-AAG reduced virus yields in groups treated for 7 to 16 h and 24 h. We further examined whether the virus yield was also reduced in Hsp90-knockdown Vero cells. The results revealed that virus yields were reduced at 24 and 48 h postinfection compared to untreated cells (Fig. 10C). Virus titers of Vero cells with Hsp90 knockdown and infected with ARV at MOIs of 2 and 10 at different time points (24 h and 48 h) were 1.5 and 2 log units lower than those of the untreated samples (Fig. 10C). We next examined whether Hsp90 affects virus release. Virus release percentage was calculated according to a formula described in Materials and Methods. We found that that extracellular virus accumulation was not reduced compared to untreated cells (Fig. 10D). Collectively, the results reveal that Hsp90 plays a critical role in virion assembly and production but is not involved in virus release. A model of p17-modulated formation of Hsp90/Cdc37 chaperone complex by phosphorylation of Cdc37 and stabilization of p17 and recruitment of viral proteins to viral factories for virus assembly by molecular chaperone Hsp90/Cdc37 is shown in Fig. 10E.
FIG 10.
Hsp90/Cdc37 chaperone machinery is required for recruiting inner core protein σA and outer-capsid protein σC to viral inclusions and virion production. (A) Schematic of the experimental procedures showing the times of protein collection and Hsp90 inhibition by shRNA and collection of cell lysates. (B) The amounts of σA and σC association were examined by coimmunoprecipitation assays in ARV-infected and Hsp90-knockdown Vero cells. Vero cells were infected with ARV at an MOI of 10 for 12 and 24 h, respectively. The protein levels were detected by the indicated antibodies. Densitometry analysis results for Western blotting are expressed as percentages representing the amount of σA and association as shown in the upper panel. Signals for all blots were quantified using ImageJ software. Mock cells were considered 1-fold. Each experiment was performed at least three times. (C) Intracellular and extracellular virus production was examined in Hsp90-depleted cells. Vero cells were infected with an MOI of 2 and 10 for 24 or 48 h, respectively. (D) Virus release percentage was calculated according to the formula described in Materials and Methods. (E) A model showing how the p17-modulated Hsp90/Cdc37 chaperone complex accumulates viral proteins in viral factories for virus assembly. All experiments were conducted in three independent experiments. Statistical analysis was performed using Student’s t test.
DISCUSSION
RNA viruses rely on the host machinery to support their life cycle, providing a window into fundamental cellular processes. During the infectious cycle, viral and host factors interact to advance the multistep process required to generate progeny virions. Although substantial progress has been made in understanding virus-host protein interaction, much remains largely unknown, especially the host factors that function during infection. Here, we have identified that the molecular chaperone Hsp90/Cdc37 is essential for protecting the ARV p17 protein from ubiquitin-proteasome degradation and promotes accumulation of outer-capsid protein σC, inner core protein σA, nonstructural protein σNS, viral dsRNA, and ERGIC-53 in viral factories. By 2D-LC-MS/MS analysis, coimmunoprecipitation analysis, and GST pulldown assays, we demonstrated that p17 is an Hsp90 client protein. Coimmunoprecipitation experiments showed that the Hsp90/Cdc37 chaperone complex interacts with p17 but not with the σC, σA, and σNS proteins of ARV. Our data suggest that Hsp90/Cdc37 serves as a chaperone of p17 by preventing its degradation. Inhibition of the Hsp90/Cdc37 chaperone complex by 17-AAG and celastrol or shRNAs significantly reduced intracellular virus production but not viral RNA synthesis. During the Hsp90 inhibition experiments with 17-AAG or shRNA, we found that Hsp90 is not involved in virus entry and virus release. In this work, our findings provide evidence suggesting that the Hsp90/Cdc37 chaperone machinery controls ARV replication by protecting the ARV p17 protein from ubiquitin-proteasome degradation and accumulating viral proteins in viral factories for virus assembly.
Although antiviral effects of Hsp90 inhibition have been demonstrated, a detailed mechanism of the Hsp90/Cdc37 chaperone complex in stabilization of viral protein and virus replication has been elucidated in few viruses. Hsp90 serves as a host chaperone to facilitate transcription and cell survival, cellular protein folding, the formation of replication complex, and virus replication (34–46). Cdc37 is a universal kinase-specific cochaperone that interacts with kinases but not with transcription factors or E3 ligases (46). Cdc37 together with Hsp90 modulates maturation of numerous protein kinases (8, 46). During chaperoning of the kinase targets, phosphorylation of Cdc37 at Ser13 is important for kinase binding and maturation (28, 47). The mature kinases can regulate the chaperone activity of Cdc37 through phosphorylation of Cdc37 on Ser13 (28). To date, only a few studies suggest that kinases or kinase-like proteins of viruses interact with the Hsp90/Cdc37 chaperone complex. An earlier report by Wang et al. suggested that the Hsp90/Cdc37 chaperone complex interacts with the reverse transcriptase of hepadnavirus, a kinase c-Raf-like protein, to promote virus assembly and replication (48). It was reported that the Hsp90/Cdc37 complex associates with the protein kinase of the Epstein-Barr virus (49). The ARV p17 protein is a multifunctional and nonkinase protein that is involved in induction of autophagy (50), cell cycle retardation (13, 21), upregulation of p53 and PTEN (20), and downregulation of the phosphatidylinositol 3-kinase (PI3K)/Akt/mTORC1 pathway and mTORC2 (20, 51). Coimmunoprecipitation assays and GST pulldown assays revealed that p17 associates with Hsp90 and Cdc37 (Fig. 1A and C). p17 promotes phosphorylation of Cdc37 by CK2, leading the p17 protein to be loaded onto the Hsp90 chaperone machinery followed by Cdc37 binding to Hsp90. Cdc37 acts as a bridge to direct Hsp90 to target p17 and may facilitate Hsp90-mediated p17 protein stabilization and maturation. Therefore, CK2 and Cdc37 may constitute a positive feedback loop to coordinate nucleotide-mediated conformational switching of Hsp90. Our results suggest a mechanism whereby p17 plays an important role in modulating the formation of the Hsp90/Cdc37 chaperone complex, and in turn, this chaperone machinery protects the p17 protein from ubiquitin-proteasome degradation. The chaperoning of the ARV p17 protein requires phosphorylation of Cdc37 to facilitate the formation of the Hsp90/Cdc37 chaperone complex. The study provides a novel mechanism elucidating a feedback loop between p17 and the Hsp90/Cdc37 chaperone complex. To the best of our knowledge, this is the first report to show that viral nonkinase proteins can be clients for the Hsp90/Cdc37 chaperone complex. Our study highlights a novel mechanism whereby the Hsp90/Cdc37complex chaperones a viral nonkinase target. This work complements and extends the current model for the mechanism of Cdc37 action in coordinating with Hsp90 in chaperoning nonkinase p17 protein.
A previous report suggested that TRiC governs of mammalian reovirus (MRV) replication through folding of the major outer-capsid protein σ3 into a form capable of assembling onto virus particles (52). The MRV core proteins λ1, λ2, and σ2 as well as outer capsid protein σ3 can be recruited to σNS and μNS viral factories in transfected cells (53). Recently, TRiC was also found to chaperone the σC, σA, and σNS proteins of ARV, thereby protecting these viral proteins from ubiquitin-proteasome degradation (54). Viroplasms of ARV are composed of nonstructural proteins σNS and μNS (17–19). μNS protein recruits viral proteins σNS and λA to viral factories, but not σA or σC proteins (17). In this study, we found that knockdown of Hsp90 changed intracellular distributions of inner core protein σA and outer-capsid protein σC of ARV. This work further provides a mechanism suggesting that p17 and Hsp90, in combination with the Cdc37 cochaperone, play an important role in accumulation of inner core protein σA and outer-capsid protein σC of ARV in viral factories. This study suggests that ARV and MRV may use different strategies or host factors for recruiting core proteins or outer-capsid proteins to viral factories. Our results reveal that accumulation of viral proteins into ARV factories is a selective and controlled process through different nonstructural proteins.
The σC protein of ARV forms a homotrimeric complex that decorates the viral capsid and mediates binding to host cell receptors (15, 55). The ARV sigma NS protein has been demonstrated to accelerate RNA folding, thus acting as an RNA chaperone, consistent with its role in genome segment precursor selection by facilitating specific RNA-RNA interactions in viroplasms (56). More recently, MRV nonstructural protein σNS has also been demonstrated to be an RNA stability factor promoting viral genome replication (57). In this study, we found that another nonstructural protein, p17 of ARV, plays a key role in modulating the formation of the Hsp90/Cdc37 chaperone complex to accumulate σA, σC, and σNS in viral factories. Interestingly, p17 increases levels of viral proteins σA, σC, and σNS when coexpressed with the p17 protein (Fig. 2E and F). Our previous study suggests that exogenous expression of p17 in ARV-infected cells significantly increases virus production (13). Our findings reveal that Hsp90 inhibition disrupts accumulation of inner core protein σA and outer-capsid protein σC of ARV in viral factories, reduces the levels of inner core protein σA and outer-capsid protein σC at the late stage of virus replication, and impedes σA and σC interaction. Importantly, the nonstructural protein p17 of ARV together with the Hsp90/Cdc37 chaperone complex does not increase viral genome replication but enhances viral protein stability, maturation, and virus yield. This study provides evidence suggesting that p17 and the Hsp90/Cdc37 chaperone machinery control virus replication through a mechanism that involves accumulating viral core protein σA and outer-capsid protein σC in ARV factories, potentially directing their assembly into mature particles.
Our data define a critical function for p17 and the Hsp90/Cdc37 chaperone complex in maturation of σA, σC, and σNS proteins in the middle and late stages of the viral life cycle and accumulation of inner core protein σA and outer-capsid protein σC of ARV in viral factories for virus assembly. Administration of Hsp90 inhibitors to infected animals was shown to reduce the replication of different viruses, with little toxicity to the infected host (58, 59). Thus, inhibition of Hsp90 may be a promising antiviral approach for prevention of ARV diseases. A model is proposed to illustrate how p17-modulated molecular chaperone Hsp90/Cdc37 controls virus replication in maturation and accumulation of viral proteins σA, σC, and σNS in viral factories for virus assembly (Fig. 10E).
MATERIALS AND METHODS
Cells and virus.
The S1133 strain of ARV was used in this study. African green monkey kidney (Vero) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 10 mM HEPES (pH 7. 2) at 37°C in a 5% CO2 incubator.
Reagents and antibodies.
The protein kinase CK2 inhibitor TBB, and Hsp90 inhibitor 17-AAG were purchased from Calbiochem Co. (San Diego, CA, USA). Celastrol was purchased from Cayman Chemical (Ann Arbor, MI, USA). The p17 polyclonal antibodies were produced by our laboratory (13). Monoclonal antibodies against the σA, σC, and σNS proteins of ARV were prepared by our laboratory (60–62). Anti-mouse IgG(H+L) and anti-rabbit IgG(H+L) antibodies were purchased from KPL (Washington, DC, USA). The catalog numbers and dilution factors of the respective antibodies used in this study are shown in Table 1.
TABLE 1.
Catalog numbers and dilution factors of the respective antibodies used in this study
| Antibody | Catalog no. | Clone name | Dilution factor | Manufacturer |
|---|---|---|---|---|
| *Mouse anti-p17a | 2,000 | Our laboratory | ||
| Mouse anti-σA | 4,000 | Our laboratory | ||
| Mouse anti-σC | 4,000 | Our laboratory | ||
| *Mouse anti-σNS | 4,000 | Our laboratory | ||
| Mouse anti-GST | 2624 | 26H1 | 3,000 | Cell Signaling |
| Rabbit anti-Hsp90 | 4877 | C45G5 | 2,000 | Cell Signaling |
| Rabbit anti-p-Cdc37 (S13) | 13248 | D8P8F | 2,000 | Cell Signaling |
| Mouse anti-Flag | F3165 | M2 | 2,000 | Merck |
| *Rabbit anti-GFP | ab290 | 3,000 | Abcam | |
| Mouse anti-Cdc37 | sc-17758 | C-11 | 2,000 | Santa Cruz |
| Mouse antiubiquitin | sc-8017 | P4D1 | 1,000 | Santa Cruz |
| Mouse anti-casein kinase IIα | sc-12738 | 1AD9 | 1,000 | Santa Cruz |
| Mouse anti-ERGIC-53 | sc-398777 | F-3 | 1,000 | Santa Cruz |
| ERGIC-53 antibody Alexa Fluor 647 | sc-365158 AF647 | C6 | 200 | Santa Cruz |
| Rabbit anti-dsRNA | J2 | 500 | Scicons | |
| Mouse anti-β-actin | MAB1501 | C4 | 10,000 | Millipore |
| Goat anti-mouse IgG(H+L) HRPb | 5220-0341 | 5,000 | SeraCare | |
| Goat anti-rabbit IgG(H+L) HRP | 5220-0336 | 5,000 | SeraCare | |
| Goat anti-mouse IgG(H+L) FITC-labeled, Alexa Fluor 488 | 5230-0307 | 500 | SeraCare | |
| Goat anti-mouse IgG(H+L) TRITC-labeled, Alexa Fluor 546 | 5230-0336 | 500 | SeraCare | |
| Goat anti-rabbit IgG(H+L) TRITC-labeled, Alexa Fluor 546 | 5230-0336 | 500 | SeraCare |
Antibodies marked with an asterisk are polyclonal antibodies.
HRP, horseradish peroxidase.
shRNAs used in this study.
The pLKO-AS1-puro plasmid-encoding shRNAs were obtained from the National RNAi Core Facility, Academia Sinica, Taiwan. The target sequences for Hsp90, CK2, and Cdc37 are as follows: 5′-TATGGCATGACAACTACTTTA-3′ (TRCN0000315007), 5′-CGTAAACAACACAGACTTCAA-3′ (TRCN0000000607), and 5′-CCGGCAGTTCTTCACTAAGAT-3′ (TRCN0000116634), respectively. Vero cells were transfected with the respective shRNA for 6 h, followed by infection with ARV at an MOI of 10 for 24 h, respectively. Whole-cell lysates were collected for Western blotting.
Plasmid construction.
To study the interaction region of p17/Hsp90, a series of His-tagged p17-deletion fragments were constructed in the pET32a vector. The pcDNA3.1-FLAG-p17, pET32a-p17-(1–146), and pET32a-p17-(1–60) constructs were generated previously (13). For the construction of pCI-neo-Hsp90, pGEX4T-1-Hsp90, pcDNA3.1-GFP-p17, and pcDNA3.1-GFP-σNS plasmids, Hsp90, p17, and σNS gene fragments were amplified by PCR using specific primers shown in Table 2.
TABLE 2.
Primers used in this study for amplification of the respective targeted genes
| Gene | Accession no. | Sequence (5′–3′)a | Location | Expected size (bp) | |
|---|---|---|---|---|---|
| Generation of pET32a-p17 and pGEX-4T1-Hsp90 for in vitro binding assay | |||||
| Hsp90 | NM_001195667 | F: AACTCGAGATGCCTGAGGAAACGCAGACCCAGG (XhoI) | 60–84 | 2,202 | |
| R: GCGGCCGCTTAGTCTACTTCTTCCATGCGTGATG (NotI) | 2261–2236 | ||||
| p17 (full) | AF330703 | F: CAGAATTCATGCAATGGCTCCGCCATACGA (EcoRI) | 293–314 | 441 | |
| R: CCGCTCGAGTCATAGATCGGCGTCAAATCGC (XhoI) | 733–712 | ||||
| p17 (1–60) | AF330703 | F: CAGAATTCATGCAATGGCTCCGCCATACGA (EcoRI) | 293–314 | 180 | |
| R: GTCTCGAGTCAAGATAACAGAGTAGG (XhoI) | 472–458 | ||||
| p17 (61–100) | AF330703 | F: AAGAATTCCAACCTTGTAGGGTCCACGTGCGGCTGA (EcoRI) | 473–500 | 120 | |
| R: GACTCGAGTCAAGGCAATGGAACGATAGCGTGTGG (XhoI) | 592–569 | ||||
| p17 (101–146) | AF330703 | F: CGGAATTCGCATCCGATCGGCGGTCTTGTCTTATAG (EcoRI) | 593–620 | 141 | |
| R: CCGCTCGAGTCATAGATCGGCGTCAAATCGC (XhoI) | 733–712 | ||||
| p17 (61–146) | AF330703 | F: AAGAATTCCAACCTTGTAGGGTCCACGTGCGGCTGA (EcoRI) | 473–500 | 261 | |
| R: CCGCTCGAGTCATAGATCGGCGTCAAATCGC (XhoI) | 733–712 | ||||
| Generation of pCI-Flag-p17, σA, σC, and σNS plasmids for transfection | |||||
| p17 (full) | AF330703 | F: CGGAATTCATGCAATGGCTCCGCCATACGA (EcoRI) | 293–314 | 441 | |
| R: GCTCTAGATCATAGATCGGCGTCAAATCGC (XbaI) | 733–712 | ||||
| σA | KF741763.1 | F: TCTAGAACGATGGCGCGTGCCATATACGACTT (XbaI) | 16–38 | 1,251 | |
| R: GTCGACCCTAGGCGGTAAAAGTGGC (SalI) | 1266–1249 | ||||
| σC | AF330703 | F: CAGAATTCATGGCGGGTCTCAATCCA (EcoRI) | 630–647 | 981 | |
| R: GCGGCCGCTTAGGTGTCGATGCCGGTACG (NotI) | 1610–1590 | ||||
| σNS | KF741765.1 | F: GAGAATTCATGGACAACACCGTGCGTGTTG (EcoRI) | 23–45 | 1,104 | |
| R: GCATTCTAGATTACGCCATCCTAGCTGGAG (XbaI) | 1126–1108 | ||||
| Generation of pcDNA3.1-GFP-p17 and σNS for immunofluorescence staining | |||||
| p17 (full) | AF330703 | F: CGGAATTCATGCAATGGCTCCGCCATACGA (EcoRI) | 293–314 | 441 | |
| R: GCTCTAGATCATAGATCGGCGTCAAATCGC (XhoI) | 733–712 | ||||
| σNS | KF741765.1 | F: GAGAATTCATGGACAACACCGTGCGTGTTG (EcoRI) | 23–45 | 1,104 | |
| R: GCATCTCGAGTTACGCCATCCTAGCTGGAG (XhoI) | 1126–1108 | ||||
| Primers for quantitative real-time RT-PCR | |||||
| p17 | AF330703 | F: ATGCAATGGCTCCGCCATACGA | 293–314 | 441 | |
| R: TCATAGATCGGCGTCAAATCGC | 733–712 | ||||
| σA | KF741763.1 | F: TTAACCAAGCGCAGAACCGAC | 938–958 | 328 | |
| R: CTAGGCGGTAAAAGTGGCTAG | 1266–1246 | ||||
| GAPDH | NM_002046 | F: CACCACCATGGAGAAGGCTGGGGCTCA | 480–506 | 454 | |
| R: GGCAGGTTTCTCCAGACGGCAGGTCAG | 933–907 | ||||
Underlines indicate the respective restriction sites.
To study whether p17 or Hsp90 regulates stabilization of viral proteins, pCI-Flag-σC, pCI-Flag-σA, pCI-Flag-σNS, and pCI-Flag-p17 constructs were generated. To prepare cDNA for the ARV p17, σA, σC, and σNS genes, total RNA was extracted from ARV-infected Vero cells using TRIzol solution (Thermo Fisher Scientific Inc.) according to the manufacturer’s protocol. Three microliters of total RNA was used as the templates for reverse transcription (RT) with Moloney murine leukemia virus (M-MLV) reverse transcriptase using the respective primers shown in Table 2 (Promega Co., Madison, WI, USA). Reverse transcription was carried out at 42°C for 15 min and 72°C for 15 min. RT-PCR primers are shown in Table 2. PCR was performed with 1 μL of cDNA, 1 μL of each primer, 2 μL of PCR mix, and 15 μL of double-distilled water (ddH2O) in a total volume of 20 μL. The PCR conditions for amplification were 95°C for 5 min and 35 cycles of 95°C for 30 s, 55°C for 30 s, and extension at 72°C for 30 s (p17) or 2 min (σA, σC, and σNS), followed by 72°C for 10 min for a final extension. The products were electrophoresed in a 1% agarose gel. PCR products were purified using an agarose gel DNA extraction kit (Bio kit, Taiwan). Purified PCR products were digested with the respective enzymes, followed by ligation into the same restriction sites of the pCI-neo-Flag plasmid to generate pCI-Flag-p17, σA, σC, and σNS plasmids, respectively. All constructs were confirmed by DNA sequencing.
Analysis of expression levels of TrxA-His-p17, TrxA-His-p17 mutants, and GST-Hsp90 fusion proteins by SDS-PAGE and Coomassie brilliant blue staining.
The integrity of the purified TrxA-His-p17, TrxA-His-p17 mutants, and GST-Hsp90 fusion proteins was analyzed by SDS-PAGE and Coomassie brilliant blue staining. The expected sizes of expressed fusion proteins are indicated. Purified His- or GST-tagged fusion proteins were used in in vitro GST pulldown assays. To obtain soluble forms of GST-tagged proteins, cell pellets were treated with 0.5% sodium lauroyl sarcosinate. The purified proteins were confirmed by SDS-PAGE and Western blotting with the indicated antibodies.
Analysis of p17-modulated formation of the Hsp90/Cdc37 chaperone complex and stabilization of the p17 protein.
To examine whether Hsp90/Cdc37 chaperone is capable of stabilizing p17 protein, inhibition of Hsp90 by 17-AAG (1.25 μM) in ARV-infected cells was carried out. The expression levels of the ARV p17 protein and Hsp90 were examined by Western blotting assays with the indicated antibodies. To mitigate the possibility of off-target effects, Hsp90 knockdown with an shRNA and overexpression of Hsp90 in p17-transfected Vero cells were carried out, followed by Western blot analysis. To study whether the p17 protein modulates phosphorylation of Cdc37, the effect of CK2 inhibitor TBB on p17-mediated phosphorylation of Cdc37 was assayed. The expression levels of p17, CK2, p-Cdc37, Cdc37, and Hsp90 were examined in p17-transfected and ARV-infected Vero cells. Vero cells were transfected with the pCI-Flag-p17 plasmid for 6 h, followed by treatment with CK2 inhibitor TBB (5 μM) for 24 h. In the ARV-infected group, Vero cells were simultaneously treated with CK2 inhibitor TBB and infected with ARV at an MOI of 10 for 24 h. To explore whether Hsp90 protects p17 protein from ubiquitin-proteasome-mediated degradation, the signals of polyubiquitinated p17 were examined in Hsp90 knockdown and Hsp90 overexpression in p17-transfected Vero cells. To investigate whether CK2 and Cdc37 are involved in p17 stabilization, Vero cells were transfected with the pCI-Flag-p17 or shRNA plasmid for 6 h, followed by treatment with MG132 (1 μM). The amounts of p17 polyubiquitylation were examined by Western blotting assays. To investigate whether the formation of the Hsp90/Cdc37 complex is required for stabilizing the ARV p17 protein, the celastrol inhibitor and Cdc37 shRNA were used in p17-transfected Vero cells. Cellular proteins coimmunoprecipitated by Hsp90 or Cdc37 antibodies were used to analyze the levels of p17, Hsp90, and Cdc37, respectively. To further examine whether Hsp90 affects other structural proteins, 17-AAG was used to inhibit Hsp90 in the indicated plasmid-transfected Vero cells. The expression levels of viral proteins were examined by Western blot analysis. The effect of Hsp90/Cdc37 molecular chaperone machinery on virus replication was also analyzed. Vero cells treated with celastrol (1 μM) and depleted by CK2, Cdc37, and Hsp90 shRNAs were analyzed. Virus titer was determined by a viral proteins plaque assay.
Coimmunoprecipitation assays.
To investigate the interaction among viral proteins (p17, σA, σC, σNS) and Hsp90, immunoprecipitation was performed using the Catch and Release kit (Upstate Biotechnology) based on the manufacturer’s protocol. Vero cells were seeded in 6-cm cell culture dishes with DMEM containing 5% FBS and incubated at 37°C with 5% CO2 until cell confluence reached about 75%. Cells were infected with ARV and collected 24 h posttransfection. Cells were washed twice with 1× phosphate-buffered saline (PBS) and lysed in 300 μL of 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) lysis buffer (40 mM HEPES [pH 7.5], 1 mM EDTA, 10 mM glycerophosphate, 120 mM NaCl, 50 mM NaF, 10 mM pyrophosphate, and 0.3% CHAPS). One thousand micrograms of total proteins collected from each sample was incubated with 2 μg of the p17, Cdc37, Hsp90, σA, σC, and σNS antibodies, or rabbit IgG (negative control) at 4°C for 24 h. The immunoprecipitated proteins were separated by SDS-PAGE followed by Western blotting with the respective antibodies.
GST pulldown assays.
To examine whether p17 interacts directly with Hsp90, an in vitro GST pulldown assay was carried out. SP Sepharose beads were purchased from GE Healthcare (Uppsala, Sweden). In interaction experiments, 2 μg purified GST protein or GST-tagged Hsp90 was coupled to the glutathione-Sepharose 4B beads and incubated at 4°C overnight with 100 ng purified TrxA-His-p17 or p17-deletion proteins in binding buffer (20 mM Tris-HCl, 25 mM NaCl, 10% glycerol, 1 mM dithiothreitol [DTT], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 10 μg/mL cocktail protease inhibitor). The protein-bound glutathione beads were then washed five times with binding buffer and eluted with elution buffer (50 mM Tris-HCl, pH 8.0, 10 mM reduced glutathione). Elution fractions were boiled and examined by Western blotting.
qRT-PCR.
To investigate whether Hsp90 regulates the transcription of the p17-encoding and σA-encoding genes of ARV, ARV-infected Vero cells were treated with drug (17-AAG) or Hsp90 shRNA. Vero cells were infected with ARV at an MOI of 10. All cultures were collected and lysed at different time points. Total RNA was isolated from drug-treated or Hsp90 shRNA-transfected cells using TRIzol and the RNeasy minikit (Qiagen) according to the manufacturer’s protocols. Total RNAs were subjected to real-time qRT-PCR as described previously (63). To obtain cDNAs from the RNA samples, RT was carried out at 42°C for 60 min with 2 μg of total RNA, 4 μL of 2.5 mM deoxynucleoside triphosphate (dNTP), 500 ng of oligo(dT), 5 μL of 5× RT buffer and 1 μL of M‐MLV reverse transcriptase (200 U/μL) (Promega, Fitchburg, WI, USA), and nuclease‐free water in a total volume of 25 μL. Target cDNAs were further amplified with iQ SYBR green Supermix (Bio-Rad, Hercules, CA, USA) with the primers listed in Table 2. The reaction mixtures contained 0.25 μg total cDNA, 0.5 μL forward and reverse primers (0.5 μM) each, 10 μL of SYBR green Supermix, and double-distilled water (ddH2O) to a final volume of 20 μL. The PCR amplification program was 95°C for 3 min followed by 35 cycles of 95°C for 15 s and 56°C for 1 min. Relative quantitation results were analyzed using the CFX connect model of the real-time PCR detection system (Bio-Rad). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal control for normalization.
Electrophoresis and Western blotting assays.
Cells were seeded in 6-well cell culture dishes 1 day before infection with virus or transfection with the plasmid DNA. Collected cells were washed twice with 1× PBS and lysed with lysis buffer (Cell Signaling). The concentrations of solubilized protein in the cell lysates were determined with the Bio-Rad protein assay (Bio-Rad Laboratories, USA) based on the manufacturer’s protocol. Equal amounts of samples were mixed with 2.5× Laemmli loading buffer and boiled for 10 min in a water bath. The samples were electrophoresed in a 10% or 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare Life Sciences, Chicago, IL, USA). The expression levels of protein were examined using the appropriate primary antibody and horseradish peroxidase secondary antibody conjugate. The results were detected on X-ray films (Kodak, Rochester, NY, USA) after membrane incubation with the enhanced chemiluminescence (ECL plus) reagent (Amersham Biosciences, Little Chalfont, England).
Immunofluorescence staining.
Vero cells were seeded on 18- by 18-mm coverslips and transfected with shRNA of Hsp90, followed by infection with ARV at an MOI of 10. The cells were washed twice with 1× PBS and then fixed at the indicated times with 4% paraformaldehyde (Alfa Aesar, Haverhill, MA, USA) for 20 min at room temperature. Next, fixed cells were incubated in PBS with 0.1% Triton X-100 for 10 min. The cells were washed twice with 1× PBS and blocked with Superblock T20 solution (Thermo Scientific, Bellefonte, PA, USA) at room temperature for 1 h. After two washes with 1× PBS, cells were incubated with the indicated primary antibodies at 4°C overnight. The solution was removed, and cells were washed three times in PBS, 5 min for each wash. Cells were incubated with fluorescein isothiocyanate (FITC) (Alexa Fluor 488, green)-, tetramethyl rhodamine isocyanate (TRITC) (Alexa Fluor 546, red)-, or ERGIC-53 (Alexa Fluor 647, purple)-conjugated secondary antibody at 4°C overnight (in the dark). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min. After washing the cells three times with 1× PBS, the cells were observed under the confocal microscope (Olympus FV1000, Tokyo, Japan).
Quantification of colocalization.
For quantifying the colocalization of viral proteins (σA, σC, σNS, and p17), ERGIC, Hsp90, Cdc37, and dsRNA, at least 10 fields of 1 to 2 cells per field were taken for each sample per experiment. Each experiment was performed at least three times while the exposure settings were unchanged during acquisition of various samples. Quantification of the colocalization was performed with ImageJ software.
Determination of virus titer and virus release percentage.
To determine the effect of Hsp90 on ARV replication, shRNAs were used to knock down Hsp90 in ARV-infected cells. Vero cells were transfected with the respective shRNAs for 6 h followed infection with ARV at different MOIs (2 and 10) for 24 or 48 h, respectively. Collected supernatants (extracellular proportion) were stored at −80°C for later viral titration. After collecting the extracellular supernatants, the same volume of DMEM was added to each well and the plate was frozen and thawed three times to disrupt cell membranes. The supernatants were centrifuged at 12,000 × g for 10 min at 4°C to collect the intracellular portion and stored at −80°C for further viral titration. Virus titer was determined as described previously (16). Virus release percentage was calculated. After ARV infection, the values of extracellular virus numbers divided by total virus numbers served as 100% (virus release percentage [%] = extracellular virus numbers/total virus numbers).
Statistical analysis.
All data obtained in this study were evaluated for statistical significance using the Student t test. The data were expressed as averages from three independent experiments. P values of less than 0.05 were considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Science and Technology in Taiwan (109-2313-B-005-006-MY3), The iEGG and Animal Biotechnology Center from The Feature Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (110S0023A), Taichung Veterans General Hospital (TCVGH-NCHU-1097602 and TCVGH-NCHU-1107613), and National Chung Hsing University (TCVGH-NCHU-1097602 and TCVGH-NCHU-1107613).
All authors made substantive intellectual contributions to the present study and approved the final manuscript. H.-J.L. conceived of the study and generated the original hypothesis, wrote the paper, and supervised the project; W.-R.H. performed most of the experiments. W.-R.H., J.-Y.L., Y.-Y.W., T.-L.L., B.L.N., and H.-J.L. analyzed data; H.-J.L. and B.L.N. revised and edited the manuscript.
We declare that we have no competing interests.
Contributor Information
Hung-Jen Liu, Email: hjliu5257@nchu.edu.tw.
Susana López, Instituto de Biotecnologia/UNAM.
REFERENCES
- 1.Didenko T, Duarte AM, Karagoz GE, Rudiger SG. 2012. Hsp90 structure and function studied by NMR spectroscopy. Biochim Biophys Acta 1823:636–647. 10.1016/j.bbamcr.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Prodromou C, Panaretou B, Chohan S, Siligardi G, O’Brien R, Ladbury JE, Roe SM, Piper PW, Pearl LH. 2000. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J 19:4383–4392. 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young JC, Moarefi I, Hartl FU. 2001. Hsp90: a specialized but essential protein-folding tool. J Cell Sci 154:267–273. 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park M, Yong Kang C, Krishna P. 1998. Brassica napus Hsp90 can autophosphorylate and phosphorylate other protein substrates. Mol Cell Biochem 185:33–38. 10.1023/A:1006884306169. [DOI] [PubMed] [Google Scholar]
- 5.Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. 1989. Hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol 9:3919–3930. 10.1128/mcb.9.9.3919-3930.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taipale M, Jarosz DF, Lindquist S. 2010. Hsp90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11:515–528. 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 7.Stepanova L, Leng X, Parker SB, Harper JW. 1996. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev 10:1491–1502. 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- 8.Roe SM, Ali MM, Meyer P, Vaughan CK, Panaretou B, Piper PW, Prodromou C, Pearl LH. 2004. The mechanism of Hsp90 regulation by the protein kinase-specific cochaperone p50(cdc37). Cell 116:87–98. 10.1016/S0092-8674(03)01027-4. [DOI] [PubMed] [Google Scholar]
- 9.Lewis V, Hynes G, Zheng D, Saibil H, Willison K. 1992. T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature 358:249–252. 10.1038/358249a0. [DOI] [PubMed] [Google Scholar]
- 10.Kubota H, Hynes G, Carne A, Ashworth A, Willison K. 1994. Identification of six Tcp-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin. Curr Biol 4:89–99. 10.1016/S0960-9822(94)00024-2. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Thomas JO, Chow RL, Lee GH, Cowan NJ. 1992. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell 69:1043–1050. 10.1016/0092-8674(92)90622-J. [DOI] [PubMed] [Google Scholar]
- 12.Kozak RA, Hattin L, Biondi MJ, Corredor JC, Walsh S, Xue-Zhong M, Manuel J, McGilvray ID, Morgenstern J, Lusty E, Cherepanov V, McBey BA, Leishman D, Feld JJ, Bridle B, Nagy É. 2017. Replication and oncolytic activity of an avian orthoreovirus in human hepatocellular carcinoma cells. Viruses 9:90. 10.3390/v9040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu HC, Huang WR, Liao TL, Chi PI, Nielsen BL, Liu JH, Liu HJ. 2018. Mechanistic insights into avian reovirus p17-modulated suppression of cell cycle CDK-cyclin complexes and enhancement of p53 and cyclin H interaction. J Biol Chem 293:12542–12562. 10.1074/jbc.RA118.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai R, Meng G, Li Y, Wang W, Diao Y, Zhao S, Feng Q, Tang Y. 2019. The oncolytic efficacy and safety of avian reovirus and its dynamic distribution in infected mice. Exp Biol Med (Maywood) 244:983–991. 10.1177/1535370219861928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang WR, Wang YC, Chi PI, Wang L, Wang CY, Lin CH, Liu HJ. 2011. Cell entry of avian reovirus follows a caveolin-1-mediated and dynamin-2-dependent endocytic pathway that requires activation of p38 mitogen-activated protein kinase (MAPK) and Src signaling pathways as well as microtubules and small GTPase Rab5 protein. J Biol Chem 286:30780–30794. 10.1074/jbc.M111.257154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patton JT, Silvestri LS, Tortorici MA, Vasquez-Del Carpio R, Taraporewala ZF. 2006. Rotavirus genome replication and morphogenesis: role of the viroplasm. Curr Top Microbiol Immunol 309:169–187. 10.1007/3-540-30773-7_6. [DOI] [PubMed] [Google Scholar]
- 17.Tourís-Otero F, Cortez-San Martín M, Martínez-Costas J, Benavente J. 2004. Avian reovirus morphogenesis occurs within viral factories and begins with the selective recruitment of sigmaNS and lambdaA to microNS inclusions. J Mol Biol 341:361–374. 10.1016/j.jmb.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 18.Touris-Otero F, Martínez-Costas J, Vakharia VN, Benavente J. 2004. Avian reovirus nonstructural protein microNS forms viroplasm-like inclusions and recruits protein sigmaNS to these structures. Virology 319:94–106. 10.1016/j.virol.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Benavente J, Martínez-Costas J. 2006. Early steps in avian reovirus morphogenesis. Curr Top Microbiol Immunol 309:67–85. 10.1007/3-540-30773-7_3. [DOI] [PubMed] [Google Scholar]
- 20.Huang WR, Chiu HC, Liao TL, Chuang KP, Shih WL, Liu HJ. 2015. Avian reovirus protein p17 functions as a nucleoporin Tpr suppressor leading to activation of p53, p21 and PTEN and inactivation of PI3K/AKT/mTOR and ERK signaling pathways. PLoS One 10:e0133699. 10.1371/journal.pone.0133699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu HC, Huang WR, Liao TL, Wu HY, Munir M, Shih WL, Liu HJ. 2016. Suppression of vimentin phosphorylation by the avian reovirus p17 through inhibition of CDK1 and Plk1 impacting the G2/M phase of the cell cycle. PLoS One 11:e0162356. 10.1371/journal.pone.0162356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu HC, Huang WR, Wang YY, Li JY, Liao TL, Nielsen BL, Liu HJ. 2019. Heterogeneous nuclear ribonucleoprotein A1 and lamin A/C modulate nucleocytoplasmic shuttling of avian reovirus p17. J Virol 93:e00851-19. 10.1128/JVI.00851-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frydman J. 2001. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem 70:603–647. 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 24.Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, Krutzsch H, Ochel HJ, Schulte TW, Sausville E, Neckers LM, Toft DO. 1997. The amino-terminal domain of heat shock protein 90 (Hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates Hsp90 conformation. J Biol Chem 272:23843–23850. 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 25.Prodromou C, Roe S, O’Brien R, Ladbury J, Piper P, Pearl L. 1997. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90:65–75. 10.1016/S0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 26.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. 1997. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 89:239–250. 10.1016/S0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 27.Verma S, Singh A, Mishra A. 2012. Dual inhibition of chaperoning process by taxifolin: molecular dynamics simulation study. J Mol Graph Model 37:27–38. 10.1016/j.jmgm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Miyata Y, Nishida E. 2005. CK binds, phosphorylates, and regulates its pivotal substrate Cdc37, an Hsp90-cochaperone. Mol Cell Biochem 274:171–179. 10.1007/s11010-005-2949-8. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Li Y, Yu Y, Zou P, Jiang Y, Sun D. 2009. Characterization of celastrol to inhibit Hsp90 and cdc37 interaction. J Biol Chem 284:35381–35389. 10.1074/jbc.M109.051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YC, Zhou Y, Yang CZ, Xiong DS. 2009. A review of ERGIC-53: its structure, functions, regulation and relations with diseases. Histol Histopathol 24:1193–1204. 10.14670/HH-24.1193. [DOI] [PubMed] [Google Scholar]
- 31.de Castro IF, Zamora PF, Ooms L, Fernández JJ, Lai CMH, Mainou BA, Dermody TS, Risco C. 2014. Reovirus forms neo-organelles for progeny particle assembly within reorganized cell membranes. mBio 5:e00931-13. 10.1128/mBio.00931-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin HS, Lee LH. 1998. Identification and characterization of RNA-binding activities of avian reovirus non-structural protein sigma NS. J Gen Virol 79:1411–1413. 10.1099/0022-1317-79-6-1411. [DOI] [PubMed] [Google Scholar]
- 33.Yin HS, Shien JH, Lee LH. 2000. Synthesis in Escherichia coli of avian reovirus core protein ςA and its dsRNA-binding activity. Virology 266:33–41. 10.1006/viro.1999.0020. [DOI] [PubMed] [Google Scholar]
- 34.Burch AD, Weller SK. 2005. Herpes simplex virus type 1 DNA polymerase requires the mammalian chaperone Hsp90 for proper localization to the nucleus. J Virol 79:10740–10749. 10.1128/JVI.79.16.10740-10749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naito T, Momose F, Kawaguchi A, Nagata K. 2007. Involvement of Hsp90 in assembly and nuclear import of influenza virus RNA polymerase subunits. J Virol 81:1339–1349. 10.1128/JVI.01917-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geller R, Taguwa S, Frydman J. 2012. Broad action of Hsp90 as a host chaperone required for viral replication. Biochim Biophys Acta 1823:698–706. 10.1016/j.bbamcr.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartl FU. 1996. Molecular chaperones in cellular protein folding. Nature 381:571–579. 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 38.Schopf FH, Biebl MM, Buchner J. 2017. The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 18:345–360. 10.1038/nrm.2017.20. [DOI] [PubMed] [Google Scholar]
- 39.Hung JJ, Chung CS, Chang W. 2002. Molecular chaperone Hsp90 is important for vaccinia virus growth in cells. J Virol 76:1379–1390. 10.1128/JVI.76.3.1379-1390.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connor JH, McKenzie MO, Parks GD, Lyles DS. 2007. Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology 362:109–119. 10.1016/j.virol.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taguwa S, Kambara H, Omori H, Tani H, Abe T, Mori Y, Suzuki T, Yoshimori T, Moriishi K, Matsuura Y. 2009. Cochaperone activity of human butyrate-induced transcript 1 facilitates hepatitis C virus replication through an Hsp90-dependent pathway. J Virol 83:10427–10436. 10.1128/JVI.01035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vozzolo L, Loh B, Gane PJ, Tribak M, Zhou L, Anderson I, Nyakatura E, Jenner RG, Selwood D, Fassati A. 2010. Gyrase B inhibitor impairs HIV-1 replication by targeting Hsp90 and the capsid protein. J Biol Chem 285:39314–39328. 10.1074/jbc.M110.155275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith DR, McCarthy S, Chrovian A, Olinger G, Stossel A, Geisbert TW, Hensley LE, Connor JH. 2010. Inhibition of heat-shock protein 90 reduces Ebola virus replication. Antiviral Res 87:187–194. 10.1016/j.antiviral.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das I, Basantray I, Mamidi P, Nayak TK, Pratheek BM, Chattopadhyay S, Chattopadhyay S. 2014. Heat shock protein 90 positively regulates Chikungunya virus replication by stabilizing viral non-structural protein nsP2 during infection. PLoS One 9:e100531. 10.1371/journal.pone.0100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katoh H, Kubota T, Nakatsu Y, Tahara M, Kidokoro M, Takeda M. 2017. Heat shock protein 90 ensures efficient mumps virus replication by assisting with viral polymerase complex formation. J Virol 91:e02220-16. 10.1128/JVI.02220-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohl BP, Roy P. 2019. Hsp90 chaperones bluetongue virus proteins and prevents proteasomal degradation. J Virol 93:e00898-19. 10.1128/JVI.00898-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao J, Prince T, Hartson SD, Matts RL. 2003. Phosphorylation of serine 13 is required for the proper function of the Hsp90 co-chaperone, Cdc37. J Biol Chem 278:38117–38120. 10.1074/jbc.C300330200. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Grammatikakis N, Hu J. 2002. Role of p50/CDC37 in hepadnavirus assembly and replication. J Biol Chem 277:24361–24367. 10.1074/jbc.M202198200. [DOI] [PubMed] [Google Scholar]
- 49.Sun X, Bristol JA, Iwahori S, Hagemeier SR, Meng Q, Barlow EA, Fingeroth JD, Tarakanova VL, Kalejta RF, Kenney SC. 2013. Hsp90 inhibitor 17-DMAG decreases expression of conserved herpesvirus protein kinases and reduces virus production in Epstein-Barr virus-infected cells. J Virol 87:10126–10138. 10.1128/JVI.01671-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chi PI, Huang WR, Lai IH, Cheng CY, Liu HJ. 2013. The p17 nonstructural protein of avian reovirus triggers autophagy enhancing virus replication via activation of phosphatase and tensin deleted on chromosome 10 (PTEN) and AMP-activated protein kinase (AMPK), as well as dsRNA-dependent protein kinase (PKR)/eIF2α signaling pathways. J Biol Chem 288:3571–3584. 10.1074/jbc.M112.390245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang WR, Chi PI, Chiu HC, Hsu JL, Nielsen BL, Liao TL, Liu HJ. 2017. Avian reovirus p17 and sigma A act cooperatively to downregulate Akt by suppressing mTORC2 and CDK2/cyclin A2 and upregulating proteasome PSMB6. Sci Rep 7:5226. 10.1038/s41598-017-05510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knowlton JJ, Fernandez C, Ashbrook AW, Gestaut DR, Zamora PF, Bauer JA, Forrest JC, Frydman J, Risco C, Dermody TS. 2018. The TRiC chaperonin controls reovirus replication through outer-capsid folding. Nat Microbiol 3:481–493. 10.1038/s41564-018-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tenorio R, Fernández de Castro I, Knowlton JJ, Zamora PF, Lee CH, Mainou BA, Dermody TS, Risco C. 2018. Reovirus σNS and μNS proteins remodel the endoplasmic reticulum to build replication neo-organelles. mBio 9:e01253-18. 10.1128/mBio.01253-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang WR, Li JY, Liao TL, Yeh CM, Wang CY, Wen HW, Hu NJ, Wu YY, Hsu CY, Chang YK, Chang CD, Nielsen BL, Liu HJ. 2022. Molecular chaperone TRiC governs avian reovirus replication by preventing outer-capsid protein σC and core protein σA and non-structural protein σNS from ubiquitin-proteasome degradation. Vet Microbiol 264:109277. 10.1016/j.vetmic.2021.109277. [DOI] [PubMed] [Google Scholar]
- 55.Guardado Calvo P, Fox GC, Hermo Parrado XL, Llamas-Saiz AL, Costas C, Martínez-Costas J, Benavente J, van Raaij MJ. 2005. Structure of the carboxy-terminal receptor-binding domain of avian reovirus fibre sigmaC. J Mol Biol 354:137–149. 10.1016/j.jmb.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 56.Borodavka A, Ault J, Stockley PG, Tuma R. 2015. Evidence that avian reovirus σNS is an RNA chaperone: implications for genome segment assortment. Nucleic Acids Res 43:7044–7057. 10.1093/nar/gkv639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zamora PF, Hu L, Knowlton JJ, Lahr RM, Moreno RA, Berman AJ, Prasad B, Dermody TS. 2018. Reovirus nonstructural protein σNS acts as an RNA stability factor promoting viral genome replication. J Virol 92:e00563-18. 10.1128/JVI.00563-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geller R, Vignuzzi M, Andino R, Frydman J. 2007. Evolutionary constrains on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev 21:195–205. 10.1101/gad.1505307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakagawa S, Umehara T, Matsuda C, Kuge S, Sudoh M, Kohara M. 2007. Hsp90 inhibitors suppress HCV replication in replicon cells and humanized liver mice. Biochem Biophys Res Commun 353:882–888. 10.1016/j.bbrc.2006.12.117. [DOI] [PubMed] [Google Scholar]
- 60.Huang PH, Li YJ, Su YP, Lee LH, Liu HJ. 2005. Epitope mapping and functional analysis of sigma A and sigma NS proteins of avian reovirus. Virology 332:584–595. 10.1016/j.virol.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Hsu CJ, Wang CY, Lee LH, Shih WL, Chang CI, Cheng HL, Chulu JLC, Ji WT, Liu HJ. 2006. Development and characterization of monoclonal antibodies against avian reovirus sigma C protein and their application in detection of avian reovirus isolates. Avian Pathol 35:320–326. 10.1080/03079450600823386. [DOI] [PubMed] [Google Scholar]
- 62.Chi PI, Huang WR, Chiu HC, Li JY, Nielsen BL, Liu HJ. 2018. Avian reovirus σA-modulated suppression of lactate dehydrogenase and upregulation of glutaminolysis and the mTOC1/eIF4E/HIF-1α pathway to enhance glycolysis and the TCA cycle for virus replication. Cell Microbiol 20:e12946. 10.1111/cmi.12946. [DOI] [PubMed] [Google Scholar]
- 63.Tseng HH, Huang WR, Cheng CY, Chiu HC, Liao TL, Nielsen BL, Liu HJ. 2020. Aspirin and 5-aminoimidazole-4-carboxamide riboside attenuate bovine ephemeral fever virus replication by inhibiting BEFV-induced autophagy. Front Immunol 11:556838. 10.3389/fimmu.2020.556838. [DOI] [PMC free article] [PubMed] [Google Scholar]