ABSTRACT
Bone marrow transplantation (BMT) recipients are at risk for substantial morbidity and mortality from human adenovirus infections, often in the setting of reactivation of persistent virus. Human adenovirus persistence in mucosal lymphocytes has been described, but specific cellular reservoirs of persistence and effects of persistence on host responses to unrelated stimuli are not completely understood. We used mouse adenovirus type 1 (MAV-1) to characterize persistence of an adenovirus in its natural host and test the hypothesis that persistence increases complications of BMT. Following intranasal infection of C57BL/6J mice, MAV-1 DNA was detected in lung, mediastinal lymph nodes, and liver during acute infection at 7 days postinfection (dpi), and at lower levels at 28 dpi that remained stable through 150 dpi. Expression of early and late viral transcripts was detected in those organs at 7 dpi but not at later time points. MAV-1 persistence was not affected by deficiency of IFN-γ. We detected no evidence of MAV-1 reactivation in vivo following allogeneic BMT of persistently infected mice. Persistent infection did not substantially affect mortality, weight loss, or pulmonary inflammation following BMT. However, T cell infiltration and increased expression of pro-inflammatory cytokines consistent with graft-versus-host disease (GVHD) were more pronounced in livers of persistently infected BMT mice than in uninfected BMT mice. These results suggest that MAV-1 persists in multiple sites without detectable evidence of ongoing replication. Our results indicate that MAV-1 persistence alters host responses to an unrelated challenge, even in the absence of detectable reactivation.
IMPORTANCE Long-term persistence in an infected host is an essential step in the life cycle of DNA viruses. Adenoviruses persist in their host following acute infection, but the nature of adenovirus persistence remains incompletely understood. Following intranasal infection of mice, we found that MAV-1 persists for a prolonged period in multiple organs, although we did not detect evidence of ongoing replication. Because BMT recipients are at risk for substantial morbidity and mortality from human adenovirus infections, often in the setting of reactivation of persistent virus in the recipient, we extended our findings using MAV-1 infection in a mouse model of BMT. MAV-1 persistence exacerbated GVHD-like inflammation following allogeneic BMT, even in the absence of virus reactivation. This novel finding suggests that adenovirus persistence has consequences, and it highlights the potential for a persistent adenovirus to influence host responses to unrelated challenges.
KEYWORDS: adenoviruses, bone marrow transplantation, graft-versus-host disease, persistence
INTRODUCTION
Acute human adenovirus (HAdV) infections cause many types of illnesses, including various forms of respiratory, eye, and gastrointestinal disease (1). Long-term persistence in an infected host following the resolution of acute infection is likely to be an important aspect of HAdV biology, as it is for the biology of many other DNA viruses. Studies in human lymphocyte and monocyte cell lines suggest that those cell types may support persistent HAdV infection (2–5). In vivo, HAdV persistence occurs in mucosal lymphoid tissue, predominantly in T lymphocytes (6, 7). HAdV DNA has also been detected in intestinal lymphocytes (8, 9) and peripheral blood lymphocytes (10, 11). HAdV may also persist in nonlymphoid cells, such as respiratory epithelial cells (12–15). It is unclear whether HAdVs persist due to ongoing low-level replication or establishment of a form of latent infection in which a limited set of viral genes are expressed without full replication. HAdV shedding has been documented in respiratory and fecal samples for extended periods of time (16, 17), and HAdV DNA can be detected in hearts without evidence of acute infection (18–20). Persistent HAdV infection has been associated with chronic heart disease (18, 19), chronic lung diseases such as asthma and chronic obstructive pulmonary disease (13–15, 21–23), and obesity (24).
Bone marrow transplantation (BMT) is an important therapy for a variety of malignant and nonmalignant diseases, but its use is limited by the incidence of complications such as infection (25) and graft-versus-host disease (GVHD), an immunological disorder based on genetic mismatch between donor and recipient that affects many organ systems (26). HAdVs cause substantial morbidity and mortality following BMT, with HAdV disease reported in up to 42% of BMT recipients (27, 28). There are many manifestations of HAdV infection in BMT patients, including pneumonitis, gastroenteritis, hemorrhagic cystitis, hepatitis, and disseminated disease (29, 30). HAdV-associated mortality is substantial in BMT patients, with rates up to 75% in patients with pneumonia or disseminated disease (31–35). HAdV disease in BMT patients may be caused by primary infection that occurs after transplantation. Reactivation of persistent HAdV infection is also a potential cause of disease. For instance, detection of HAdV identical to a strain isolated prior to immunosuppression suggested reactivation in the recipient as a cause of disease (27, 36). Potential effects of HAdV persistence in the absence of reactivation on BMT outcomes have not yet been clearly defined.
The strict species-specificity of the adenoviruses has limited detailed studies of HAdV pathogenesis in mouse models. To overcome this barrier, we use mouse adenovirus type 1 (MAV-1, also known as MAdV-1) to study the pathogenesis of an adenovirus in its natural host. MAV-1 replicates in lungs and hearts of C57BL/6J (B6) mice following intranasal (i.n.) inoculation, with virus-induced inflammation, organ dysfunction, and dissemination to other organs that is reminiscent of disease seen in humans infected with various HAdV types (37–40). Several studies have demonstrated long-term (up to at least 55 weeks) MAV-1 viruria (41–44). MAV-1 persists in brain, kidney, and spleen following intraperitoneal (i.p.) infection (42), and MAV-1 DNA is detected in hearts of mice at 9 weeks following i.n. infection (38). Allogeneic BMT prior to MAV-1 infection delays clearance of viral DNA from the lungs during acute primary MAV-1 infection (45). In this study, we used MAV-1 to further characterize in vivo the long-term persistence of an adenovirus following acute respiratory infection and to determine the extent to which MAV-1 persistence influenced outcomes of BMT. We found that MAV-1 persists in lungs and other target organs for extended periods of time without molecular signatures of ongoing replication. Even in the absence of detectable reactivation, MAV-1 persistence enhanced GVHD-like inflammatory responses following BMT.
RESULTS
MAV-1 persistence following acute respiratory infection.
Several studies have demonstrated long-term viruria in mice infected with MAV-1 (41–44, 46, 47). Using in situ hybridization in brain, kidney, and spleen and immunocapture PCR in urine, Smith et al. demonstrated MAV-1 persistence for at least 55 weeks following i.p. infection (42). We previously used nested PCR to demonstrate long-term persistence of MAV-1 DNA in hearts of mice infected as neonates (38). To further characterize long-term persistence of MAV-1 following acute respiratory infection, we infected adult B6 mice i.n. with MAV-1 and measured DNA viral loads during acute infection and extended time periods following the resolution of acute infection. Viral DNA was readily detected in the lung as early as 1 dpi, and viral loads increased substantially by 7 dpi (Fig. 1A). Viral loads remained detectable but had decreased by more than 3 log units to an amount at 28 dpi that remained essentially unchanged up to 150 dpi. To further characterize the state of MAV-1 persistence, we used RT-qPCR to detect and quantify representative early and late transcripts in the lungs of infected mice, including mRNA for early region 1A (E1A, Fig. 1D) and the MAV-1 tripartite leader, TPL, a three-exon untranslated sequence that is present in late viral gene mRNAs (Fig. 1G). During acute infection, E1A and TPL mRNA were detected in the lungs at 3 and 7 dpi. Viral gene expression was not detected in the lungs at 28 dpi. At 150 dpi, E1A mRNA was detected at very low levels above background in some lungs, but TPL mRNA was not detected.
FIG 1.
MAV-1 persistence following acute respiratory infection. C57BL/6J mice were infected intranasally with MAV-1 or mock-infected with conditioned media. Lungs, MLN, and livers were harvested at the indicated time points. (A to C) qPCR was used to quantify MAV-1 genome copies in the indicated organs. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. RT-qPCR was used to quantify (D to F) E1A and (G to I) TPL mRNA levels. mRNA levels are expressed in arbitrary units (A.U.) standardized to GAPDH. Individual symbols represent values for individual mice (n = 6 to 14 per group combined from four independent experiments), and horizontal bars represent geometric means (A to C) or means (D to I) for each group. Horizontal dotted lines in D to I correspond to background expression in mock-infected mice (lines fall very close to the x axis where not readily visible).
We detected similar patterns of viral loads and viral gene expression in the mediastinal lymph nodes (MLN) of infected mice. Viral loads in MLN were greatest at 7 dpi, although viral DNA was still detected in MLN of all mice at 28 dpi and several mice at 150 dpi (Fig. 1B). E1A (Fig. 1E) and TPL (Fig. 1H) mRNA was detected in MLN at early time points (3 and 7 dpi), but not at 28 or 150 dpi. We also detected viral DNA in livers at 7 dpi (Fig. 1C). Viral loads were detected at very low levels in a small number of livers at 28 and 150 dpi. E1A mRNA was also primarily detected at low levels in livers at 7 dpi, although it was detected in a small number of mice at very early (1 dpi) and late (28 and 150 dpi) time points (Fig. 1F). TPL mRNA was detected at very low levels in a small number of livers at 7, 28, and 150 dpi (Fig. 1I). Viral loads were lower in MLN and livers than in lungs at all time points. Likewise, E1A and TPL mRNA levels were lower in MLN and substantially lower in livers than in lungs. Collectively, these data indicate that MAV-1 persists for an extended period following acute respiratory infection in the primary target organ (the lung) and other sites, although without detectable evidence of ongoing replication.
MAV-1 persistence in lung T cells.
Next, we used MAV-1 to determine whether an adenovirus infected and persisted in T cells in the lungs, the primary target organ during acute respiratory infection and a site of long-term persistence following the resolution of acute infection (Fig. 1). We isolated T cells from lungs of infected mice during acute infection (7 dpi) and persistence (28 and 150 dpi) and quantified viral DNA and viral gene expression. Viral DNA was readily detected in T cells isolated from lungs at 7 dpi (Fig. 1A). Viral DNA was also detected in T cells isolated from lungs at 28 and 150 dpi, although viral loads were substantially lower at those time points than at 7 dpi. Similarly, we detected E1A (Fig. 2B) and TPL (Fig. 2C) mRNA in T cells isolated from lungs at 7 dpi but not at 28 or 150 dpi. Thus, while MAV-1 DNA persisted in lung T cells, there was no evidence of viral gene expression at late time points to suggest ongoing viral replication in T cells during long-term persistence.
FIG 2.
MAV-1 persistence in lung T cells. C57BL/6J mice were infected intranasally with MAV-1 or mock-infected with conditioned media. T cells were isolated from lungs harvested at the indicated time points. (A) qPCR was used to quantify MAV-1 genome copies. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. RT-qPCR was used to quantify (B) E1A and (C) TPL mRNA levels. mRNA levels are expressed in arbitrary units (A.U.) standardized to GAPDH. Individual symbols represent values for individual mice (n = 9 to 14 per group combined from four independent experiments), and horizontal bars represent geometric means (A) or means (B, C) for each group.
Effects of IFN-γ on MAV-1 persistence.
IFN-γ expression is substantially increased in lungs and other organs during acute MAV-1 infection (48–51). IFN-γ is not required for control of viral replication during acute infection and does not contribute to disease manifestations in adult B6 mice (45, 50), although it plays a more prominent pro-inflammatory role in MAV-1 myocarditis in neonatal B6 mice (38). In vitro, IFN-γ promotes HAdV persistence in immortalized human fibroblasts, but not in lymphocyte cell lines (52). To determine whether IFN-γ is essential for maintenance of long-term MAV-1 persistence in vivo, we infected B6 and IFN-γ−/− mice with MAV-1 and harvested organs at 150 dpi. Viral DNA was detected in the lungs of all B6 and IFN-γ−/− mice at 150 dpi, but there was no statistically significant difference between lung viral loads in the two groups (Fig. 3A). E1A mRNA was detected at low levels in lungs of B6 and IFN-γ−/− mice, but again there were no statistically significant differences between the two groups (Fig. 3D). TPL mRNA was not detected above background in the lungs of B6 or IFN-γ−/− mice (Fig. 3G). Viral DNA was present at low levels in MLN of most B6 and IFN-γ−/− mice at 150 dpi, but there were no statistically significant differences between viral loads in the two groups (Fig. 3B). We did not detect E1A or TPL mRNA in MLN of B6 or IFN-γ−/− mice (Fig. 3E and H). Viral loads were detected in livers of only 3 B6 mice but no IFN-γ−/− mice (Fig. 3C), while viral gene expression was not detected in livers of B6 or IFN-γ−/− mice at 150 dpi (Fig. 3F and I).
FIG 3.
Effects of IFN-γ on MAV-1 persistence. C57BL/6J and IFN-γ−/− mice were infected intranasally with MAV-1. Lungs, MLN, and livers were harvested at 150 dpi. (A to C) qPCR was used to quantify MAV-1 genome copies in the indicated organs. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. RT-qPCR was used to quantify (D to F) E1A and (G to I) TPL mRNA levels. mRNA levels are expressed in arbitrary units standardized to GAPDH. Individual symbols represent values for individual mice (n = 6 to 9 per group combined from two independent experiments), and horizontal bars represent geometric means (A to C) or means (D to I) for each group. Horizontal dotted lines in D-I correspond to background expression in mock-infected mice (lines fall very close to the x axis where not readily visible). There were no statistically significant differences between C57BL/6J and IFN-γ−/− mice in A to I. (J) Nested PCR was used to detect the presence of MAV-1 DNA and actin in the indicated organs. Bands in each lane correspond to organs from an individual mouse.
Because viral loads were very low or undetectable in MLN and livers at 150 dpi using qPCR, we used nested PCR as an additional approach to detect viral DNA in organs during long-term persistence (Fig. 3J). As expected, based on the magnitude of viral loads in the lungs during long-term persistence (Fig. 3A), we detected viral DNA in the lungs of all B6 and IFN-γ−/− mice at 150 dpi. Viral DNA was detected in MLN of all (9 out of 9) B6 mice, but only in half (3 out of 6) of IFN-γ−/− mice. Viral DNA was detected in livers of three B6 mice but in no IFN-γ−/− mice. Collectively, these data indicate that IFN-γ is not essential for long-term control of MAV-1 persistence in organs of infected mice, because viral loads were not greater and viral gene expression was not increased in the absence of IFN-γ. Viral DNA was detected in MLN and livers of fewer IFN-γ−/− mice than B6 mice, providing some support for the possibility that IFN-γ facilitates persistence. However, that was not a universal effect since viral DNA was detected in all B6 and IFN-γ−/− lungs.
Effects of BMT on MAV-1 persistence.
To determine whether BMT affected the state of MAV-1 persistence, we performed allogeneic BMT using uninfected BALB/cJ donors and infected B6 recipients. BMT was performed at a time point during long-term persistence, at 7 weeks postinfection. First, we confirmed persistence of MAV-1 in the majority of lungs (Fig. 4A) and livers (Fig. 4D) harvested from untransplanted B6 mice at that time point (day 0). Next, we used qPCR and RT-qPCR to detect and quantify MAV-1 viral loads and viral gene expression at 7 and 35 days post-BMT. Viral DNA was readily detectable at low levels in lungs (Fig. 4A) and livers (Fig. 4D) of mice at 7 days post-BMT (corresponding to 56 dpi), but there were no statistically significant differences between lung or liver viral loads in allogeneic BMT recipients (MAV-1/alloBMT mice) and untransplanted controls (MAV-1/control mice). Lung and liver viral loads increased slightly in both MAV-1/alloBMT and MAV-1/control mice by 35 days post-BMT (corresponding to 84 dpi), but again there were no statistically significant differences between the two groups at that time point. E1A mRNA was not detected in lungs (Fig. 4B) or livers (Fig. 4E) of any mice at 7 days post-BMT. TPL mRNA was detected at very low levels in the lungs of MAV-1/control mice and one MAV-1/alloBMT mouse (Fig. 4C) but not in the livers of MAV-1/control or MAV-1/alloBMT mice at 7 days post-BMT (Fig. 4F). At 35 days post-BMT, E1A mRNA was detected at very low levels in a portion of lungs and livers of both MAV-1/alloBMT and MAV-1/control mice. TPL mRNA was not detected in lungs or livers of MAV-1/control or MAV-1/alloBMT mice at 35 days post-BMT.
FIG 4.
Effects of BMT on MAV-1 persistence. C57BL/6J mice were infected intranasally with MAV-1. Allogeneic BMT (uninfected BALB/cJ donors) was performed at 7 weeks postinfection. Infected and mock-infected untransplanted C57BL/6 mice were included as controls. Organs and serum were harvested at 7 weeks postinfection before BMT and then at 7 and 35 days post-BMT (n = 4 to 10 per group combined from two independent experiments). (A, D) qPCR was used to quantify MAV-1 genome copies in the indicated organs. RT-qPCR was used to quantify (B, E) E1A and (C, F) TPL mRNA levels in the indicated organs. (G) qPCR was used to quantify MAV-1 genome copies in serum. (H) To validate the serum viral load assay, qPCR was used to quantify MAV-1 genome copies in PBS or in serum or whole blood obtained from uninfected mice after adding the indicated amounts of MAV-1. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA (A, D) or per mL (G, H). mRNA levels are expressed in arbitrary units standardized to GAPDH. Individual circles represent values for individual mice, and horizontal bars represent geometric means (A, D, G, H) or means (B, C, E, F) for each group. Horizontal dotted lines in D, C, E, and F correspond to background expression in mock-infected mice (lines fall very close to the x axis where not readily visible). There were no statistically significant differences between infected BMT mice and infected untransplanted controls in A to G.
We also used qPCR to determine whether viremia associated with reactivation of persistent MAV-1 occurred following allogeneic BMT. Viral DNA was not detected in the serum of MAV-1/alloBMT and MAV-1/control mice at 7 days post-BMT (Fig. 4G). We detected viral DNA in the serum of two of eight MAV-1/alloBMT mice but in no MAV-1/control mice at 35 days post-BMT. Thus, MAV-1 persisted in both alloBMT and untransplanted mice. We validated this approach by assaying samples in which increasing amounts of MAV-1 were added to PBS and to serum and whole blood obtained from uninfected mice. We successfully detected viral DNA in all samples over a wide range of input virus (Fig. 4H), suggesting that the assay was not inhibited by the presence of serum or whole blood. Detection of viral DNA in the serum of a small number of persistently infected mice following BMT suggests that MAV-1 is capable of reactivating in vivo. However, we did not detect increases in MAV-1 viral loads or substantial changes in viral gene expression consistent with BMT-induced reactivation of persistent virus in target organs.
Effects of MAV-1 persistence on survival and weight loss following BMT.
All mice survived up to 35 days following allogeneic BMT, regardless of infection status (Data not shown). Mock-infected and persistently infected untransplanted control (mock/control and MAV-1/control) mice continued to gain weight at similar rates over the course of the experiment (Fig. 5). Mock-infected and persistently infected (mock/alloBMT and MAV-1/alloBMT) mice lost weight to a comparable degree by 7 days post-BMT. Both mock/alloBMT and MAV-1/alloBMT mice began to regain weight between 14 and 21 days post-BMT. Although it appeared as though MAV-1/alloBMT mice regained weight more quickly than mock/alloBMT mice, there were no significant differences between the weights of mock/alloBMT and MAV-1/alloBMT mice at any time following BMT. Likewise, there were no significant differences between MAV-1/alloBMT mice and persistently infected B6 mice that underwent syngeneic BMT (MAV-1/synBMT mice). Thus, while both allogeneic and syngeneic BMT caused weight loss, MAV-1 persistence did not significantly affect BMT-induced weight loss.
FIG 5.
Effects of MAV-1 persistence on weight loss following BMT. C57BL/6J mice were infected intranasally with MAV-1 or mock-infected with conditioned media. Allogeneic BMT (uninfected BALB/cJ donors, alloBMT) or syngeneic BMT (uninfected C57BL/6J donors, synBMT) was performed at 7 weeks postinfection. Infected and mock-infected untransplanted C57BL/6 mice were included as controls. Body weights measured at the indicated time points are expressed as the percentage of weight on the day of BMT (mean ± S.E.M.; n = 9 to 13 per group combined from two independent experiments). Statistical comparisons were made using two-way ANOVA followed by Tukey’s multiple comparison tests. *, P < 0.05 and ***, P < 0.001 comparing mock/control and mock/alloBMT; #, P < 0.05, ##, P < 0.01, and ###, P < 0.001 comparing mock/control and MAV-1/alloBMT; †††P < 0.001 comparing mock/control and MAV-1/synBMT. There were no statistically significant differences between mock/control and MAV-1/control mice, between mock/alloBMT and MAV-1/alloBMT mice, or between MAV-1/alloBMT and MAV-1/synBMT mice at any time point.
Effects of MAV-1 persistence on BMT-induced lung inflammation.
Acute MAV-1 infection induces pulmonary inflammation characterized by leukocyte influx and upregulation of multiple proinflammatory cytokines and chemokines (39, 53). In mouse models, allogeneic BMT also induces pulmonary inflammation (54, 55). To determine whether MAV-1 persistence modulated effects of allogeneic BMT in the lungs, we assessed pulmonary inflammation in mock/control, MAV-1/control, mock/alloBMT, and MAV-1/alloBMT mice. At 35 days post-BMT, when marrow is fully engrafted (45), there were no qualitative differences in histological evidence of pulmonary inflammation between infected and mock-infected mice with or without BMT (Fig. 6A), and there were no statistically significant differences between groups in quantified lung pathology scores (Fig. 6B). Total leukocyte numbers in bronchoalveolar lavage fluid (BALF) were significantly greater in mock/alloBMT and MAV-1/alloBMT mice compared to mock/control mice (Fig. 6C). BALF total protein concentration, a marker of airway injury, was significantly greater in MAV-1/alloBMT mice than in mock/control and mock/alloBMT mice (Fig. 6D). mRNA levels of the pro-inflammatory cytokines IFN-γ, TNF, IL-1β, and IL-6 tended to be somewhat higher in lungs of alloBMT mice compared with untransplanted controls regardless of infection (Fig. 6E to H). IFN-γ mRNA levels were significantly greater in lungs of mock/alloBMT mice than in mock/control and MAV-1/control mice, but there were no statistically significant differences between groups for lung TNF, IL-1β, and IL-6 mRNA levels. Thus, MAV-1 persistence had minimal effect on BMT-induced lung injury.
FIG 6.
Effects of MAV-1 persistence on BMT-induced pulmonary inflammation. C57BL/6J mice were infected intranasally with MAV-1 or mock-infected with conditioned media. Allogeneic BMT (uninfected BALB/cJ donors) or syngeneic BMT (uninfected C57BL/6J donors) was performed at 7 weeks postinfection. Infected and mock-infected untransplanted C57BL/6 mice were included as controls. (A) Hematoxylin-and-eosin-stained sections were prepared from lungs harvested at 35 days post-BMT. Scale bars, 100 μm. (B) Pathology scores were generated to quantify cellular inflammation in the lungs of mock-infected and infected mice. (C) Total leukocyte counts and (D) total protein concentration were measured in BALF (n = 4 to 7 per group combined from two independent experiments). (E to H) RT-qPCR was used to quantify mRNA levels of the indicated cytokines in lungs (n = 5 to 7 per group combined from 2 independent experiments). mRNA levels are expressed in arbitrary units standardized to GAPDH. Individual symbols represent values for individual mice, and horizontal bars represent means for each group. Statistical comparisons were made using one-way ANOVA followed by Tukey’s multiple comparison tests. *, P < 0.05 and **, P < 0.01 for the indicated comparisons.
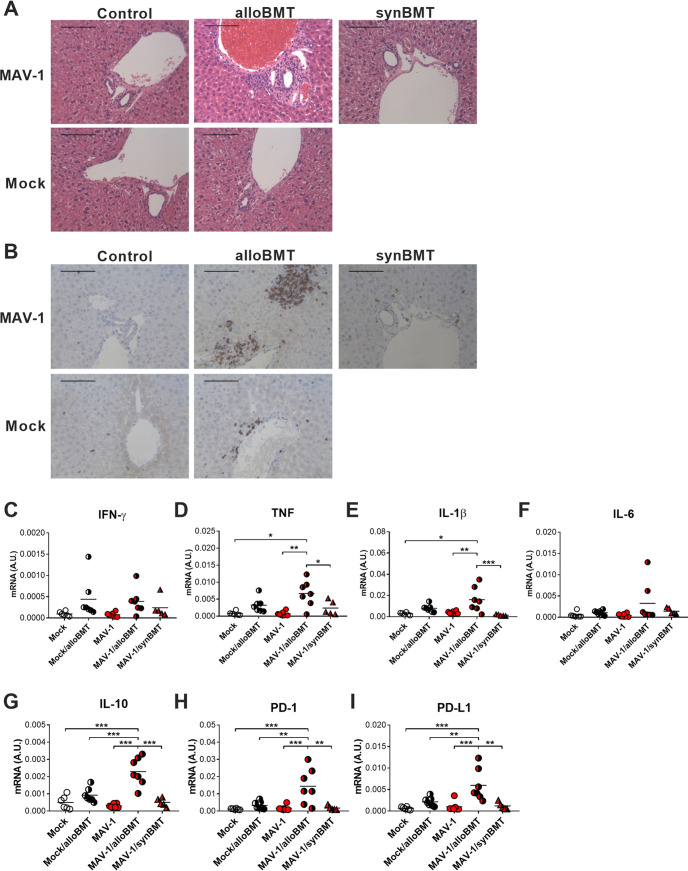
Effects of MAV-1 persistence on BMT-induced liver inflammation.
The liver is an additional target organ for acute GVHD in BMT patients, and liver inflammation resembling GVHD has been described in mouse models of BMT (reviewed in reference 56, 57). Because our data indicated that MAV-1 persists in the liver, we assessed inflammatory responses in the liver to determine whether MAV-1 persistence influenced allogeneic BMT-induced liver inflammation. At 35 days post-BMT, we observed histological evidence of injury that was more pronounced in livers of MAV-1/alloBMT mice than in mock/alloBMT mice but essentially absent in livers of mock/control and MAV-1/control mice (Fig. 7A). T cell infiltration into liver, which we assessed using immunohistochemistry to detect CD3+ cells, was also most pronounced in MAV-1/alloBMT mice (Fig. 7B). We also measured IFN-γ, TNF, IL-1β, and IL-6 mRNA levels in the liver. Expression of those pro-inflammatory cytokines was increased to variable degrees in both uninfected and persistently infected mice following allogeneic BMT (Fig. 7C to F). In particular, TNF and IL-1β mRNA levels were significantly greater in MAV-1/alloBMT mice than in mock/control and MAV-1/control mice, whereas levels in mock/alloBMT mice were not significantly different than in other groups.
FIG 7.
Effects of MAV-1 persistence on BMT-induced liver inflammation. C57BL/6J mice were infected intranasally with MAV-1 or mock-infected with conditioned media. Allogeneic BMT (uninfected BALB/cJ donors) or syngeneic BMT (uninfected C57BL/6J donors) was performed at 7 weeks postinfection. Infected and mock-infected untransplanted C57BL/6 mice were included as controls. (A) Hematoxylin-and-eosin-stained sections were prepared from livers harvested at 35 days post-BMT. (B) CD3-stained sections were prepared from paraffin-embedded sections. CD3-positive cells are stained dark brown. Scale bars in A and B, 100 μm. (C to I) RT-qPCR was used to quantify mRNA levels of the indicated genes in livers. mRNA levels are expressed in arbitrary units standardized to GAPDH. Individual symbols represent values for individual mice (n = 5 to 7 mice per group combined from two independent experiments), and horizontal bars represent means for each group. Statistical comparisons were made using one-way ANOVA followed by Tukey’s multiple comparison tests. *, P < 0.05, **, P < 0.01, and ***, P < 0.001 for the indicated comparisons.
In addition to the pro-inflammatory mediators described above, we used RT-qPCR to assess expression of immunomodulatory factors in the liver, including the cytokine IL-10, programmed death 1 (PD-1) protein, and PD-1 ligand (PD-L1). mRNA levels of IL-10, PD-1, and PD-L1 were significantly greater in livers of MAV-1/alloBMT mice than in all other groups (Fig. 7G to I). Otherwise, there were no significant differences between IL-10, PD-1, or PD-L1 mRNA levels in livers of mock/control, mock/alloBMT, or MAV-1/control mice.
Effects of genetic mismatch on BMT-induced inflammation in persistently infected mice.
The synergistic effects of persistent MAV-1 in alloBMT mice suggested the possibility that persistent MAV-1 enhanced GVHD-like inflammation following allogeneic BMT. To address this possibility, we compared liver inflammation in persistently infected mice 35 days after allogeneic or syngeneic BMT, reasoning that inflammation would be less in persistently infected syngeneic BMT mice that do not experience GVHD. BAL cell counts, BALF protein concentrations, and IFN-γ, TNF, and IL-1β mRNA levels tended to be lower in lungs of MAV-1/synBMT mice than in MAV-1/alloBMT mice, although the differences were not statistically significant (Fig. 6D to F). In the liver, TNF and IL-1β mRNA levels were significantly lower in MAV-1/synBMT mice than in MAV-1/alloBMT mice (Fig. 7D and E). We detected similar synergistic effects of persistent MAV-1 and BMT on IL-10, PD-1, and PD-L1 mRNA levels in the liver that were specific to allogeneic BMT (Fig. 7G to I). There were no differences in lung or liver viral loads in MAV-1/alloBMT and MAV-1/synBMT mice at 35 days post-transplant (Fig. 4A and D). MAV-1 DNA was detected in the serum of two MAV-1/synBMT mice, similar to findings in MAV-1/alloBMT mice (Fig. 4G). Collectively, these differences in inflammation following BMT in the absence of detectable differences in viral replication suggest that persistent MAV-1 influenced GVHD-associated inflammation.
DISCUSSION
Some HAdV types persist following acute infection, and persistent HAdV has been detected in mucosal lymphocytes isolated from healthy individuals (6, 7). Persistence is not unique to the HAdVs. Our laboratory and others have demonstrated that MAV-1 persists in infected mice after the resolution of acute infection (38, 41, 42, 46, 50). However, little is known about the range of cell types that harbor persistent adenoviruses and the state of adenovirus persistence (i.e., ongoing low-level replication or latency) in vivo. Associations have been made between HAdV persistence and a variety of diseases in humans, such as chronic lung disease (13–15, 22, 23), heart disease (18, 19), and obesity (24). In some cases, HAdV disease in BMT patients is likely to be associated with reactivation of persistent virus in the recipient (27, 56, 58). In general, however, long-term effects of HAdV persistence on human health and outcomes of other disease processes are not well defined. The strict species-specificity of the adenoviruses has precluded extensive in vivo studies of their persistence. To overcome this barrier, we used MAV-1 infection of mice, the natural host for MAV-1. Our data indicate that, following acute respiratory infection, MAV-1 persists for long periods of time in multiple organs without clear evidence of ongoing replication. Although we detected little evidence of MAV-1 reactivation following BMT, MAV-1 persistence enhanced GVHD in allogeneic BMT recipients.
MAV-1 persists in brain, kidney, and spleen following intraperitoneal (i.p.) inoculation (42), and several studies have demonstrated long-term viruria in infected mice (41–44, 46, 47). We previously reported detection of MAV-1 DNA at 9 weeks postinfection in hearts of mice infected i.n. as neonates (38). In this study, we characterized in greater detail the establishment of MAV-1 persistence in multiple organs following acute respiratory infection. We detected MAV-1 DNA as late as 150 dpi. The primary site of infection, in this case the lung, was the greatest reservoir of persistent virus, although viral DNA was also frequently detected in draining lymph nodes (MLN) and a distinct target organ (liver). We detected viral DNA in T cells isolated from lungs of infected mice at the same time points, suggesting that T cells serve as reservoirs for persistent MAV-1 similar to reports of HAdV persistence in lymphocytes isolated from asymptomatic humans. We focused on T cells in this study to draw parallels between MAV-1 and HAdV persistence. That approach does not exclude persistence of MAV-1 in other lymphocyte types or other hematopoietic or non-hematopoietic cell types. Representative early and late viral mRNAs were readily detected in organs and lung T cells at early time points during acute infection. However, during long-term persistence at 150 dpi, viral mRNAs were detected rarely and at very low levels in whole organs, and not at all in lung T cells. It is possible that the sensitivity of RT-qPCR was not sufficient to detect very small amounts of viral gene expression during long-term persistence. However, our results suggest that ongoing replication is not a predominant feature of MAV-1 persistence.
IFN-γ promotes HAdV persistence in vitro in immortalized human fibroblasts, but not lymphocytes, by suppressing expression of early viral genes (52). This action may contribute to virus evasion of host immune responses that would otherwise mediate virus clearance, ultimately facilitating persistence in an infected host. IFN-γ is upregulated in multiple organs during acute MAV-1 infection (37, 38, 50, 51). IFN-γ inhibits MAV-1 replication to some degree in a mouse fibroblast cell line (59), although IFN-γ is not essential for survival or for initial control of viral replication during acute MAV-1 respiratory infection of adult mice (45, 50). We used MAV-1 to determine whether IFN-γ is essential for establishment or maintenance of long-term viral persistence in vivo. Viral loads were comparable in persistently infected IFN-γ−/− mice and B6 controls at 150 dpi, and we did not detect increased viral gene expression in IFN-γ−/− mice compared with B6 controls, together indicating that IFN-γ is not essential for long-term control of MAV-1 during persistent infection. If IFN-γ were instead required for the maintenance of MAV-1 persistence in vivo, analogous to in vitro findings with HAdV, then evidence of persistent MAV-1 would likely be less in IFN-γ−/− mice than in controls. We detected viral DNA in MLN and livers of a smaller proportion of IFN-γ−/− mice than B6 mice, providing some support for the possibility that IFN-γ facilitated persistence. However, viral DNA was detected by nested PCR in all B6 and IFN-γ−/− lungs, with no quantitative differences in DNA viral loads. Cell type-specific differences in the effect of IFN-γ on MAV-1 persistence that were not detected in the assays of whole tissue used in our study may exist, as they do for HAdV in vitro (52). Ongoing work in our laboratory to define specific cellular reservoirs of MAV-1 persistence will be helpful in determining whether IFN-γ or other host factors facilitate MAV-1 persistence in individual cell types.
Our published work indicates that MAV-1 persistence is associated with long-term effects, including cardiac hypertrophy (38) and altered susceptibility to an unrelated infection (60). Unlike HAdV type 36 and a small number of other HAdV types that have been linked to obesity in humans (61), persistent MAV-1 is not associated with obesity (62). In this study, we assessed potential interactions between MAV-1 persistence and BMT, a different type of clinically relevant challenge. Despite the ablation of immune function by total body irradiation and then long-lasting immune dysfunction even after bone marrow reconstitution that we and others have demonstrated (45, 63), we detected only minimal evidence of MAV-1 reactivation following BMT. The addition of pharmacologic immunosuppression, similar to approaches used to prevent and treat GVHD following BMT in humans, may have increased the likelihood of MAV-1 reactivation in our model. If T cells or other hematopoietic cells serve as the primary reservoir for MAV-1 persistence, as our data suggest may be the case, then ablation of those cells by irradiation may have delayed evidence of reactivation from a small number of remaining persistently infected cells. However, DNA viral loads in organs of persistently infected BMT mice (Fig. 6 and 7) were comparable to those in persistently infected untransplanted controls (Fig. 1), making that possibility unlikely. Virus reactivation may have been more readily detected at later times following BMT, as it was for murine cytomegalovirus (MCMV) at 100 days post-BMT (55). MAV-1 disseminates to multiple organs following i.n. or i.p. infection, and data from this study and previously published work indicate that persistent virus is detected in multiple organs including lung, heart, liver, lymph nodes, spleen, brain, and kidneys (38, 64). We detected minimal evidence of reactivation in lung, liver, and blood, but it remains possible that increased MAV-1 replication occurred in organs that we did not assay. It is also possible that we could have detected increased shedding of virus in urine (42).
In addition to characterizing effects of BMT on MAV-1 persistence, we sought to determine whether persistent infection influenced outcomes of BMT. MAV-1 persistence did not increase mortality or exacerbate weight loss following BMT. However, we observed inflammation in mock/alloBMT mice that tended to be more pronounced in MAV-1/alloBMT mice but absent in mock/control or MAV-1/control mice. Effects in the lung were subtle, limited to increased BALF total protein content without increased pro-inflammatory cytokine expression or histological evidence of excess pathology. In the liver, significantly greater expression (compared to mock/control mice) of pro-inflammatory cytokines such as TNF and IL-1β was detected only in MAV-1/alloBMT mice. In addition, there was evidence of increased histological evidence of periportal inflammation and fibrosis in MAV-1/alloBMT mice compared to other groups. These changes are consistent with acute GVHD-like inflammation in the liver described in mice infected with MCMV before BMT (55). The absence of such increases in MAV-1/synBMT mice despite similar viral loads in MAV-1/alloBMT and MAV-1/synBMT mice also supports the likelihood that the findings were related to acute GVHD, which would not occur in recipients of syngeneic BMT. Evidence of GVHD may have been greater if we had examined a later time after BMT. Multiple factors influence severity of GVHD in mouse models (57, 65). We used MHC-disparate mouse strains in our study, with BALB/cJ (H2d) donors and B6 (H2b) recipients, but it is possible that a different combination of mouse strains would have led to different effects on GVHD.
GVHD is a risk factor for HAdV infection and disease (35, 58, 66–71), although it can be difficult to discriminate between risk due to GVHD itself and risk due to immunosuppression used to treat GVHD. Detection of HAdV and other viruses in BMT recipients may be a risk factor for the development of acute GVHD and other alloimmune disease (71, 72), although the substantial overlap in clinical presentations of infection and GVHD make it difficult to define specific effects of infections in the ontogeny of GVHD. One study using a mouse model of BMT to address interactions between MCMV persistence and BMT demonstrated increased evidence of lung and liver GVHD that was linked to reactivation of persistent MCMV (55). However, we detected synergistic effects of persistent MAV-1 on liver inflammation in MAV-1/alloBMT mice that were independent of MAV-1 reactivation. We also observed synergistic upregulation of IL-10, PD-1, and PD-L1 in MAV-1/alloBMT mice, suggesting that inhibition of those immunomodulatory factors by persistent MAV-1 was not a likely explanation for enhanced inflammation. Instead, it seems more likely that their upregulation was a compensatory response to increased inflammation.
Acute GVHD is an important toxicity of allogeneic BMT that involves damage to host tissue from conditioning regimens followed by activation of host antigen presenting cells (APCs), recruitment and priming of donor T cells to recognize host antigens, and inflammation and cell death mediated by effector T cells and other types of immune cells recruited to sites of tissue damage (73, 74). Data from our study do not permit us to define which of these processes are altered by MAV-1 to augment GVHD, but it is plausible that one or more of those components could be affected by MAV-1 infection and persistence. For instance, MAV-1 persistence may increase activation of recipient APCs or promote antigen presentation by non-professional APCs directly, if those cells serve as additional reservoirs for persistent virus, or indirectly, via long-term effects of cytokines induced during acute infection or ongoing cytokine responses to persistent infection. Residual tissue damage caused by acute or persistent infection may also augment APC presentation of host antigens. Although direct effects of MAV-1 persistence on donor T cell function seem unlikely, it may be that persistence has long-term effects on host factors such as chemokines or adhesion molecules that might influence donor T cell activation in secondary lymphoid organs or trafficking to target organs. Virus-mediated alterations in host cell surface proteins that are involved in T cell recognition and killing, such as MHC class I, Fas, or receptors for TNF or TNF-related apoptosis-inducing ligand (TRAIL), could also augment T cell-mediated damage. Proteins encoded by the HAdV E3 transcription unit are capable of interfering with many of those T cell effector functions (75) and could therefore potentially exert a protective effect if they were expressed during persistence. Of note, there are substantial differences between HAdV and MAV-1 E3 regions (76, 77), and similar activities of MAV-1 proteins have not been identified.
In summary, our data indicate that MAV-1 persists for extended periods of time in the lung, which is the target organ during acute respiratory infection, as well as MLN (the primary draining lymph nodes of the lung) and liver. MAV-1 persists in those sites and in lung T cells without substantial molecular evidence of ongoing replication in vivo. Despite previously published data suggesting that IFN-γ facilitates HAdV persistence in fibroblasts (52), we found no evidence indicating that IFN-γ was essential for maintaining MAV-1 persistence in vivo. Irradiation and BMT were not sufficient to induce increases in MAV-1 replication that would have suggested reactivation of persistent virus. Differences between MAV-1 and HAdV gene products, such as those encoded by the E3 region mentioned above (76, 77), along with differences between our mouse model of BMT and the many approaches to bone marrow and hematopoietic stem cell transplantation in humans, make it difficult to draw specific direct conclusions from our work with MAV-1 about HAdV persistence and reactivation in the context of BMT in humans. However, we detected an exacerbation of GVHD-like inflammation in persistently infected mice following allogeneic BMT, even in the absence of viral replication. This novel finding suggests that adenovirus persistence has consequences, and it highlights the potential for a persistent adenovirus to influence host responses to unrelated challenges.
MATERIALS AND METHODS
Mice.
All experiments were approved by the University of Michigan Institutional Animal Care and Use Committee. C57BL/6J (B6) and BALB/cJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Adult IFN-γ deficient mice (IFN-γ−/−, B6.129S7-Ifngtm1Ts/J) were purchased from The Jackson Laboratory. Male mice were used for all BMT experiments. Male and female mice were used for all other experiments. All mice were maintained under specific pathogen-free conditions and provided with food and water ad libitum.
Virus and infections.
MAV-1 was grown and passaged in mouse 3T6 fibroblasts maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 5% heat-inactivated calf serum, 2 mM l-glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin, and titers of viral stocks were determined by plaque assay on 3T6 cells as previously described (78). Adult mice were infected when they were 6 to 8 weeks old. Mice were anesthetized with ketamine and xylazine and then infected intranasally (i.n.) with 105 plaque-forming units (PFU) of MAV-1 in 40 μL of sterile phosphate-buffered saline (PBS). Control mice were mock-infected i.n. with conditioned medium at an equivalent dilution in sterile PBS. All mice were euthanized by ketamine/xylazine overdose at the indicated time points. Organs were harvested, snap-frozen in dry ice and stored at −80°C.
Bone marrow transplantation.
C57BL/6J (B6) mice were infected i.n. with 105 PFU MAV-1 or mock-infected with conditioned media as described above. Mice were allowed to recover until 7 weeks postinfection, when allogeneic BMT was performed as previously described (45, 79). B6 mice received 1,350 rad of total body irradiation using a 137Cs irradiator, delivered in two doses 3 h apart. Bone marrow cells (5 × 106) harvested from BALB/cJ donor mice were injected into the tail vein of irradiated recipient mice. In a subset of experiments, syngeneic BMT was performed using B6 donors and B6 recipients. Controls included mock-infected and infected B6 mice that did not undergo BMT. All mice were given acidified water (pH 3.3) for the first 3 weeks after BMT to reduce bacterial growth in water and reduce the risk for bacterial infection following BMT. Mice were euthanized by pentobarbital overdose at the indicated time points. Organs were harvested, snap-frozen in dry ice, and stored at −80°C until processed further.
Isolation of DNA and RNA from organs.
Portions of organs were homogenized using sterile glass beads in a Mini-Beadbeater (Biospec Products) for 30 s in 1 mL of TRIzol (Invitrogen). RNA and DNA were then isolated from homogenates according to the manufacturer’s protocol.
Isolation of lung T cells.
Leukocyte populations were isolated from freshly harvested lungs following collagenase digestion as previously described (53). T cells were isolated using antibody-coated magnetic beads (Mouse Pan T Cell isolation kit II; Miltenyi Biotec). DNA and RNA were isolated from isolated cells using TRIzol.
Analysis of viral and host gene expression.
Host gene expression was quantified using reverse transcriptase quantitative real-time PCR (RT-qPCR). RNA was reverse transcribed using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. In addition, 5 μL of cDNA were added to reactions containing Power SYBR green PCR Mix (Applied Biosystems) and forward and reverse primers, each at 200 nM final concentration in a 25 μL reaction volume. Primer used in these reactions are listed in Table 1. Separate reactions were prepared with primers for mouse GAPDH. RT-qPCR analysis consisted of 40 cycles of 15 s at 90°C and 60 s at 60°C. Quantification of target gene mRNA was normalized to GAPDH and expressed in arbitrary units as 2-ΔCt, where Ct is the threshold cycle and ΔCt = Ct(target) Ct(GAPDH).
TABLE 1.
Primers and probes used for real-time PCR analysis
| Target | Oligonucleotide | Sequence (5′ to 3′) |
|---|---|---|
| MAV-1 E1A genomic | Forward primer | GCACTCCATGGCAGGATTCT |
| Reverse primer | GGTCGAAGCAGACGGTTCTTC | |
| Probe | TACTGCCACTTCTGC | |
| MAV-1 E1A | Forward primer | AATGGGTTTTGCAGTCTGTGTTAC |
| Reverse primer | CGCCTGAGGCAGCAGATC | |
| MAV-1 TPL | Forward primer | CGAGTCGCCTCCTGTGATACT |
| Reverse primer | CAAGTCGATCTGTCGGAGCTT | |
| GAPDH | Forward primer | TGCACCACCAACTGCTTAG |
| Reverse primer | GGATGCAGGGATGATGTTC | |
| IFN-γ | Forward primer | AAAGAGATAATCTGGCTCTGC |
| Reverse primer | GCTCTGAGACAATGAACGCT | |
| TNF | Forward primer | CCACCACGCTCTTCTGTCTAC |
| Reverse primer | AGGGTCTGGGCCATAGAACT | |
| IL-1β | Forward primer | GCAACTGTTCCTGAACTCAACT |
| Reverse primer | ATCTTTTGGGGTCCGTCAACT | |
| IL-6 | Forward primer | CTGCAAGAGACTTCCATCCAG |
| Reverse primer | AGTGGTATAGACAGGTCTGTTGG | |
| IL-10 | Forward primer | GCCAAGCCTTATCGGAAATG |
| Reverse primer | CACCCAGGGAATTCAAATGC | |
| PD-1 | Forward primer | TTCAGGTTTACCACAAGCTGG |
| Reverse primer | TGACAATAGGAAACCGGGAA | |
| PD-L1 | Forward primer | GCTGAAGTCAATGCCCCATA |
| Reverse primer | TCCACGGAAATTCTCTGGTTG |
Analysis of viral loads in organs.
MAV-1 viral loads were measured in organs using quantitative real-time PCR (qPCR) as previously described (38, 39). Primers and probe used to detect a 59-bp region of the MAV-1 E1A gene are detailed in Table 1. Results were normalized to the nanogram (ng) amount of input DNA. For clarity in figures, viral loads lower than 1 copy of viral genome per 100 ng were graphed as 1.
Analysis of viral loads in serum.
DNA was extracted from 100 μL serum using DNeasy Blood & Tissue kits (Qiagen) according to the manufacturer’s instructions, except DNA was eluted from columns in 100 μL of HPLC-grade water. MAV-1 serum viral loads were measured using qPCR as above. Each sample was run in triplicate. A sample was considered undetectable if more than one threshold cycle value was greater than 38. Results were expressed as copies of viral genome per mL of serum. For clarity in figures, undetectable serum viral loads were graphed as 1 copy/mL. To validate this approach, we added dilutions of MAV-1 to PBS, serum, and anticoagulated whole blood obtained from uninfected mice. We then extracted DNA using DNeasy Blood & Tissue kits and used qPCR to detect and quantify viral DNA.
Nested PCR detection of viral DNA.
Nested PCR to detect a final 246-bp region of MAV-1 E1A was performed as previously described (38) with some modifications. Primers used in these reactions are listed in Table 2. Next, 500 ng of DNA were added to reaction mixtures containing 10x standard Taq buffer (New England BioLabs), 2 mM MgCl2, deoxynucleoside triphosphate mix (0.1 mM each dATP, dCTP, dGTP, and dTTP; Promega), 2.5 units of Taq polymerase (New England BioLabs), and 100 nM forward and reverse primers in a 100 μL reaction volume. The first PCR amplification was carried out with 1 cycle at 94°C for 10 min; 35 cycles of 94°C for 30 s, 47°C for 30 s; and 72°C for 30 s, and 1 cycle at 72°C for 7 min. After the initial round of PCR, 10 μL of the first-round PCR product was added to a fresh PCR mixture (without added MgCl2) and amplified in a second round of PCR. The second PCR round was conducted under the same cycling conditions with the exception of a 56°C annealing temperature and a total of 30 cycles. For controls, actin was detected in a single round of PCR in 50 μl reaction volumes without added MgCl2. Cycling conditions included a 56°C annealing temperature and a total of 35 cycles.
TABLE 2.
Primers and probes used for nested PCR analysis
| Target | Oligonucleotide | Sequence (5′ to 3′) |
|---|---|---|
| MAV-1 E1A Round 1 | Forward primer | ATGTCGCGGCTCCTACG |
| Reverse primer | CAACGAACCATAAAAAGACATCAT | |
| MAV-1 E1A Round 2 | Forward primer | ATGGGATGGTTCGCCTACTT |
| Reverse primer | CACCGCAGATCCATGTCCTCAA | |
| Actin | Forward primer | CCTAAGGCCAACCGTGAAAAGATG |
| Reverse primer | ACCGCTCGTTGCCAATAGTGATG |
Quantification of inflammatory cells and total protein in bronchoalveolar lavage fluid.
Following euthanasia, lungs were lavaged three times with the same aliquot of 1 mL sterile PBS containing protease inhibitor (complete, Mini, EDTA-free tablets; Roche Applied Science). Cells in BALF were counted using a hemocytometer. Total protein concentrations in BALF were determined using the Quick Start Bradford Protein Assay (Bio-Rad) according to the manufacturer’s instructions.
Histology.
Organs were harvested from a subset of mice and fixed in 10% formalin. Prior to fixation, lungs were inflated with PBS via the trachea to maintain lung architecture. After fixation, organs were embedded in paraffin and 5-μm sections were obtained for histopathology. Sections were stained with hematoxylin and eosin to evaluate cellular infiltration. Separate sections used for immunohistochemistry were stained with anti-CD3 antibody (Thermo Scientific) to identify CD3-positive cells. Sectioning and staining were performed by the University of Michigan Unit for Laboratory Animal Medicine Pathology Cores for Animal Research. Digital images were obtained with an EC3 digital imaging system (Leica Microsystems) using Leica Acquisition Software (Leica Microsystems). To quantify cellular inflammation in the lungs, slides were examined in a blinded fashion to determine a pathology index as previously described (45).
Statistics.
Statistical analysis was performed using Prism 9.2.0 (GraphPad Software, Inc.). Viral loads were log-transformed for statistical analysis. Differences between two groups were analyzed using the Mann-Whitney rank sum test. Differences among more than two groups at a single time point were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. Differences between groups at multiple time points were analyzed using two-way ANOVA followed by Tukey’s multiple comparison tests. P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We thank Kathy Spindler for helpful review of the manuscript. Expert technical assistance from Joel Whitfield in the University of Michigan Cancer Center Immunology Core is greatly appreciated. This research was supported by the National Institutes Health grant AI142073 and by a Charles Woodson Accelerator Award from the University of Michigan Department of Pediatrics (both to J.B.W.).
Contributor Information
Jason B. Weinberg, Email: jbwein@umich.edu.
Lawrence Banks, International Centre for Genetic Engineering and Biotechnology.
REFERENCES
- 1.Wold WSM, Isom MG. 2013. Adenoviruses, p 1732–1767. In Knipe DM, Howley PM (ed), Fields virology, 6 ed. Lippincott, Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Chu Y, Sperber K, Mayer L, Hsu MT. 1992. Persistent infection of human adenovirus type 5 in human monocyte cell lines. Virology 188:793–800. 10.1016/0042-6822(92)90534-v. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Huang W, Ornelles DA, Gooding LR. 2010. Modeling adenovirus latency in human lymphocyte cell lines. J Virol 84:8799–8810. 10.1128/JVI.00562-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silver L, Anderson CW. 1988. Interaction of human adenovirus serotype 2 with human lymphoid cells. Virology 165:377–387. 10.1016/0042-6822(88)90582-x. [DOI] [PubMed] [Google Scholar]
- 5.Wallace RE. 1969. Susceptibility of human lymphoblasts (RPMI 7466) to viral infections in vitro. Proc Soc Exp Biol Med 130:702–710. 10.3181/00379727-130-33638. [DOI] [PubMed] [Google Scholar]
- 6.Garnett CT, Erdman D, Xu W, Gooding LR. 2002. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol 76:10608–10616. 10.1128/jvi.76.21.10608-10616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnett CT, Talekar G, Mahr JA, Huang W, Zhang Y, Ornelles DA, Gooding LR. 2009. Latent species C adenoviruses in human tonsil tissues. J Virol 83:2417–2428. 10.1128/JVI.02392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy S, Calcedo R, Medina-Jaszek A, Keough M, Peng H, Wilson JM. 2011. Adenoviruses in lymphocytes of the human gastro-intestinal tract. PLoS One 6:e24859. 10.1371/journal.pone.0024859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosulin K, Geiger E, Vecsei A, Huber WD, Rauch M, Brenner E, Wrba F, Hammer K, Innerhofer A, Potschger U, Lawitschka A, Matthes-Leodolter S, Fritsch G, Lion T. 2016. Persistence and reactivation of human adenoviruses in the gastrointestinal tract. Clin Microbiol Infect 22:381 e381–388. [DOI] [PubMed] [Google Scholar]
- 10.Horvath J, Palkonyay L, Weber J. 1986. Group C adenovirus DNA sequences in human lymphoid cells. J Virol 59:189–192. 10.1128/JVI.59.1.189-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasz I, Kulcsar G, Dan P, Sallay K. 1971. A possible pathogenic role for virus-carrier lymphocytes. J Infect Dis 124:214–216. 10.1093/infdis/124.2.214. [DOI] [PubMed] [Google Scholar]
- 12.Eissa NT, Chu CS, Danel C, Crystal RG. 1994. Evaluation of the respiratory epithelium of normals and individuals with cystic fibrosis for the presence of adenovirus E1a sequences relevant to the use of E1a- adenovirus vectors for gene therapy for the respiratory manifestations of cystic fibrosis. Hum Gene Ther 5:1105–1114. 10.1089/hum.1994.5.9-1105. [DOI] [PubMed] [Google Scholar]
- 13.Elliott WM, Hayashi S, Hogg JC. 1995. Immunodetection of adenoviral E1A proteins in human lung tissue. Am J Respir Cell Mol Biol 12:642–648. 10.1165/ajrcmb.12.6.7766428. [DOI] [PubMed] [Google Scholar]
- 14.Matsuse T, Hayashi S, Kuwano K, Keunecke H, Jefferies WA, Hogg JC. 1992. Latent adenoviral infection in the pathogenesis of chronic airways obstruction. Am Rev Respir Dis 146:177–184. 10.1164/ajrccm/146.1.177. [DOI] [PubMed] [Google Scholar]
- 15.Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. 2001. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med 164:469–473. 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 16.Fox JP, Brandt CD, Wassermann FE, Hall CE, Spigland I, Kogon A, Elveback LR. 1969. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol 89:25–50. 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- 17.Fox JP, Hall CE, Cooney MK. 1977. The Seattle virus watch. VII. Observations of adenovirus infections. Am J Epidemiol 105:362–386. 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- 18.KüHl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss H-P. 2005. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112:1965–1970. 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 19.Pauschinger M, Bowles NE, Fuentes-Garcia FJ, Pham V, KüHl U, Schwimmbeck PL, Schultheiss H-P, Towbin JA. 1999. Detection of adenoviral genome in the myocardium of adult patients with idiopathic left ventricular dysfunction. Circulation 99:1348–1354. 10.1161/01.CIR.99.10.1348. [DOI] [PubMed] [Google Scholar]
- 20.Tatrai E, Hartyanszky I, Jr, Laszik A, Acsady G, Sotonyi P, Hubay M. 2011. The role of viral infections in the development of dilated cardiomyopathy. Pathol Oncol Res 17:229–235. 10.1007/s12253-010-9302-6. [DOI] [PubMed] [Google Scholar]
- 21.Vannella KM, Moore BB. 2008. Viruses as co-factors for the initiation or exacerbation of lung fibrosis. Fibrogenesis Tissue Repair 1:2. 10.1186/1755-1536-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macek V, Sorli J, Kopriva S, Marin J. 1994. Persistent adenoviral infection and chronic airway obstruction in children. Am J Respir Crit Care Med 150:7–10. 10.1164/ajrccm.150.1.8025775. [DOI] [PubMed] [Google Scholar]
- 23.Marin J, Jeler-Kacar D, Levstek V, Macek V. 2000. Persistence of viruses in upper respiratory tract of children with asthma. J Infect 41:69–72. 10.1053/jinf.2000.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marjani A, Khatami A, Saadati H, Asghari M, Razizadeh MH, Abbasi A, Zarei M, Beikzadeh L, Soleimani A. 2021. Association of adenovirus 36 infection and obesity; an updated meta-analysis of community-based studies. Rev Med Virol 32:e2255. [DOI] [PubMed] [Google Scholar]
- 25.Wingard JR, Hsu J, Hiemenz JW. 2010. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Infect Dis Clin North Am 24:257–272. 10.1016/j.idc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara JL, Levine JE, Reddy P, Holler E. 2009. Graft-versus-host disease. Lancet 373:1550–1561. 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Tol MJ, Claas EC, Heemskerk B, Veltrop-Duits LA, de Brouwer CS, van Vreeswijk T, Sombroek CC, Kroes AC, Beersma MF, de Klerk EP, Egeler RM, Lankester AC, Schilham MW. 2005. Adenovirus infection in children after allogeneic stem cell transplantation: diagnosis, treatment and immunity. Bone Marrow Transplant 35 Suppl 1:S73–S76. [DOI] [PubMed] [Google Scholar]
- 28.Walls T, Hawrami K, Ushiro-Lumb I, Shingadia D, Saha V, Shankar AG. 2005. Adenovirus infection after pediatric bone marrow transplantation: is treatment always necessary? Clin Infect Dis 40:1244–1249. 10.1086/429235. [DOI] [PubMed] [Google Scholar]
- 29.Kojaoghlanian T, Flomenberg P, Horwitz MS. 2003. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol 13:155–171. 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- 30.Lion T. 2014. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev 27:441–462. 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bordigoni P, Carret AS, Venard V, Witz F, Le Faou A. 2001. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 32:1290–1297. 10.1086/319984. [DOI] [PubMed] [Google Scholar]
- 32.Flomenberg P, Babbitt J, Drobyski WR, Ash RC, Carrigan DR, Sedmak GV, McAuliffe T, Camitta B, Horowitz MM, Bunin N, Casper JT. 1994. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J Infect Dis 169:775–781. 10.1093/infdis/169.4.775. [DOI] [PubMed] [Google Scholar]
- 33.Hale GA, Heslop HE, Krance RA, Brenner MA, Jayawardene D, Srivastava DK, Patrick CC. 1999. Adenovirus infection after pediatric bone marrow transplantation. Bone Marrow Transplant 23:277–282. 10.1038/sj.bmt.1701563. [DOI] [PubMed] [Google Scholar]
- 34.Howard DS, Phillips IG, Reece DE, Munn RK, Henslee-Downey J, Pittard M, Barker M, Pomeroy C. 1999. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 29:1494–1501. 10.1086/313514. [DOI] [PubMed] [Google Scholar]
- 35.La Rosa AM, Champlin RE, Mirza N, Gajewski J, Giralt S, Rolston KV, Raad I, Jacobson K, Kontoyiannis D, Elting L, Whimbey E. 2001. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis 32:871–876. 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 36.Veltrop-Duits LA, van Vreeswijk T, Heemskerk B, Thijssen JC, El Seady R, Jol-van der Zijde EM, Claas EC, Lankester AC, van Tol MJ, Schilham MW. 2011. High titers of pre-existing adenovirus serotype-specific neutralizing antibodies in the host predict viral reactivation after allogeneic stem cell transplantation in children. Clin Infect Dis 52:1405–1413. 10.1093/cid/cir231. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy MK, Malitz DH, Molloy CT, Procario MC, Greiner KE, Zhang L, Wang P, Day SM, Powell SR, Weinberg JB. 2016. Interferon-dependent immunoproteasome activity during mouse adenovirus type 1 infection. Virology 498:57–68. 10.1016/j.virol.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy MK, Procario MC, Twisselmann N, Wilkinson JE, Archambeau AJ, Michele DE, Day SM, Weinberg JB. 2015. Proinflammatory effects of interferon gamma in mouse adenovirus 1 myocarditis. J Virol 89:468–479. 10.1128/JVI.02077-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Procario MC, Levine RE, McCarthy MK, Kim E, Zhu L, Chang CH, Hershenson MB, Weinberg JB. 2012. Susceptibility to acute mouse adenovirus type 1 respiratory infection and establishment of protective immunity in neonatal mice. J Virol 86:4194–4203. 10.1128/JVI.06967-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg JB, Stempfle GS, Wilkinson JE, Younger JG, Spindler KR. 2005. Acute respiratory infection with mouse adenovirus type 1. Virology 340:245–254. 10.1016/j.virol.2005.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginder DR. 1964. Increased susceptibility of mice infected with mouse adenovirus to Escherichia Coli-induced pyelonephritis. J Exp Med 120:1117–1128. 10.1084/jem.120.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith K, Brown CC, Spindler KR. 1998. The role of mouse adenovirus type 1 early region 1A in acute and persistent infections in mice. J Virol 72:5699–5706. 10.1128/JVI.72.7.5699-5706.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowe WP, Hartley JW. 1962. A general review of the adenoviruses. Ann N Y Acad Sci 101:466–474. 10.1111/j.1749-6632.1962.tb18887.x. [DOI] [PubMed] [Google Scholar]
- 44.Wigand R. 1980. Age and susceptibility of Swiss mice for mouse adenovirus, strain FL. Arch Virol 64:349–357. 10.1007/BF01320620. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy MK, Procario MC, Wilke CA, Moore BB, Weinberg JB. 2015. Prostaglandin E2 production and T cell function in mouse adenovirus type 1 infection following allogeneic bone marrow transplantation. PLoS One 10:e0139235. 10.1371/journal.pone.0139235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Veen J, Mes A. 1973. Experimental infection with mouse adenovirus in adult mice. Arch Gesamte Virusforsch 42:235–241. 10.1007/BF01265648. [DOI] [PubMed] [Google Scholar]
- 47.Ball AO, Beard CW, Villegas P, Spindler KR. 1991. Early region 4 sequence and biological comparison of two isolates of mouse adenovirus type 1. Virology 180:257–265. 10.1016/0042-6822(91)90030-f. [DOI] [PubMed] [Google Scholar]
- 48.Adkins LJ, Molloy CT, Weinberg JB. 2018. Fas activity mediates airway inflammation during mouse adenovirus type 1 respiratory infection. Virology 521:129–137. 10.1016/j.virol.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Chandrasekaran A, Adkins LJ, Seltzer HM, Pant K, Tryban ST, Molloy CT, Weinberg JB. 2019. Age-dependent effects of immunoproteasome deficiency on mouse adenovirus type 1 pathogenesis. J Virol 93:e00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molloy CT, Andonian JS, Seltzer HM, Procario MC, Watson ME, Jr, Weinberg JB. 2017. Contributions of CD8 T cells to the pathogenesis of mouse adenovirus type 1 respiratory infection. Virology 507:64–74. 10.1016/j.virol.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pant K, Chandrasekaran A, Chang CJ, Vageesh A, Popkov AJ, Weinberg JB. 2020. Effects of tumor necrosis factor on viral replication and pulmonary inflammation during acute mouse adenovirus type 1 respiratory infection. Virology 547:12–19. 10.1016/j.virol.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Y, Stamminger T, Hearing P. 2016. E2F/Rb family proteins mediate interferon induced repression of adenovirus immediate early transcription to promote persistent viral infection. PLoS Pathog 12:e1005415. 10.1371/journal.ppat.1005415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarthy MK, Zhu L, Procario MC, Weinberg JB. 2014. IL-17 contributes to neutrophil recruitment but not to control of viral replication during acute mouse adenovirus type 1 respiratory infection. Virology 456–457:259–267. 10.1016/j.virol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooke KR, Krenger W, Hill G, Martin TR, Kobzik L, Brewer J, Simmons R, Crawford JM, van den Brink MR, Ferrara JL. 1998. Host reactive donor T cells are associated with lung injury after experimental allogeneic bone marrow transplantation. Blood 92:2571–2580. 10.1182/blood.V92.7.2571. [DOI] [PubMed] [Google Scholar]
- 55.Palaniyandi S, Radhakrishnan SV, Karlsson FJ, Stokes KY, Kittan N, Huber E, Hildebrandt GC. 2013. Murine cytomegalovirus immediate-early 1 gene expression correlates with increased GVHD after allogeneic hematopoietic cell transplantation in recipients reactivating from latent infection. PLoS One 8:e61841. 10.1371/journal.pone.0061841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boieri M, Shah P, Dressel R, Inngjerdingen M. 2016. The role of animal models in the study of hematopoietic stem cell transplantation and GvHD: a historical overview. Front Immunol 7:333. 10.3389/fimmu.2016.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reddy P, Ferrara JLM. 2008. Mouse models of graft-versus-host disease. StemBook, Cambridge (MA). 10.3824/stembook.1.36.1. [DOI] [PubMed] [Google Scholar]
- 58.Runde V, Ross S, Trenschel R, Lagemann E, Basu O, Renzing-Kohler K, Schaefer UW, Roggendorf M, Holler E. 2001. Adenoviral infection after allogeneic stem cell transplantation (SCT): report on 130 patients from a single SCT unit involved in a prospective multi center surveillance study. Bone Marrow Transplant 28:51–57. 10.1038/sj.bmt.1703083. [DOI] [PubMed] [Google Scholar]
- 59.Kajon AE, Spindler KR. 2000. Mouse adenovirus type 1 replication in vitro is resistant to interferon. Virology 274:213–219. 10.1006/viro.2000.0459. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen Y, McGuffie BA, Anderson VE, Weinberg JB. 2008. Gammaherpesvirus modulation of mouse adenovirus type 1 pathogenesis. Virology 380:182–190. 10.1016/j.virol.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voss JD, Atkinson RL, Dhurandhar NV. 2015. Role of adenoviruses in obesity. Rev Med Virol 25:379–387. 10.1002/rmv.1852. [DOI] [PubMed] [Google Scholar]
- 62.Molloy CT, Adkins LJ, Griffin C, Singer K, Weinberg JB. 2018. Mouse adenovirus type 1 infection of adipose tissue. Virus Res 244:90–98. 10.1016/j.virusres.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Gowdy KM, Martinu T, Nugent JL, Manzo ND, Zhang HL, Kelly FL, Holtzman MJ, Palmer SM. 2015. Impaired CD8(+) T cell immunity after allogeneic bone marrow transplantation leads to persistent and severe respiratory viral infection. Transpl Immunol 32:51–60. 10.1016/j.trim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kajon AE, Brown CC, Spindler KR. 1998. Distribution of mouse adenovirus type 1 in intraperitoneally and intranasally infected adult outbred mice. J Virol 72:1219–1223. 10.1128/JVI.72.2.1219-1223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schroeder MA, DiPersio JF. 2011. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech 4:318–333. 10.1242/dmm.006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shields AF, Hackman RC, Fife KH, Corey L, Meyers JD. 1985. Adenovirus infections in patients undergoing bone-marrow transplantation. N Engl J Med 312:529–533. 10.1056/NEJM198502283120901. [DOI] [PubMed] [Google Scholar]
- 67.Bruno B, Gooley T, Hackman RC, Davis C, Corey L, Boeckh M. 2003. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant 9:341–352. 10.1016/S1083-8791(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 68.Feghoul L, Chevret S, Cuinet A, Dalle JH, Ouachee M, Yacouben K, Fahd M, Guerin-El Khourouj V, Roupret-Serzec J, Sterkers G, Baruchel A, Simon F, LeGoff J. 2015. Adenovirus infection and disease in paediatric haematopoietic stem cell transplant patients: clues for antiviral pre-emptive treatment. Clin Microbiol Infect 21:701–709. 10.1016/j.cmi.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Lee YJ, Chung D, Xiao K, Papadopoulos EB, Barker JN, Small TN, Giralt SA, Zheng J, Jakubowski AA, Papanicolaou GA. 2013. Adenovirus viremia and disease: comparison of T cell-depleted and conventional hematopoietic stem cell transplantation recipients from a single institution. Biol Blood Marrow Transplant 19:387–392. 10.1016/j.bbmt.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Myers GD, Krance RA, Weiss H, Kuehnle I, Demmler G, Heslop HE, Bollard CM. 2005. Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath). Bone Marrow Transplant 36:1001–1008. 10.1038/sj.bmt.1705164. [DOI] [PubMed] [Google Scholar]
- 71.van Montfrans J, Schulz L, Versluys B, de Wildt A, Wolfs T, Bierings M, Gerhardt C, Lindemans C, Wensing A, Boelens JJ. 2015. Viral PCR positivity in stool before allogeneic hematopoietic cell transplantation is strongly associated with acute intestinal graft-versus-host disease. Biol Blood Marrow Transplant 21:772–774. 10.1016/j.bbmt.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 72.Versluys AB, Rossen JW, van Ewijk B, Schuurman R, Bierings MB, Boelens JJ. 2010. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant 16:782–791. 10.1016/j.bbmt.2009.12.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghimire S, Weber D, Mavin E, Wang XN, Dickinson AM, Holler E. 2017. Pathophysiology of GvHD and other HSCT-related major complications. Front Immunol 8:79. 10.3389/fimmu.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi SW, Levine JE, Ferrara JL. 2010. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am 30:75–101. 10.1016/j.iac.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lichtenstein DL, Toth K, Doronin K, Tollefson AE, Wold WS. 2004. Functions and mechanisms of action of the adenovirus E3 proteins. Int Rev Immunol 23:75–111. 10.1080/08830180490265556. [DOI] [PubMed] [Google Scholar]
- 76.Beard CW, Ball AO, Wooley EH, Spindler KR. 1990. Transcription mapping of mouse adenovirus type 1 early region 3. Virology 175:81–90. 10.1016/0042-6822(90)90188-w. [DOI] [PubMed] [Google Scholar]
- 77.Raviprakash KS, Grunhaus A, el Kholy MA, Horwitz MS. 1989. The mouse adenovirus type 1 contains an unusual E3 region. J Virol 63:5455–5458. 10.1128/JVI.63.12.5455-5458.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cauthen AN, Welton AR, Spindler KR. 2007. Construction of mouse adenovirus type 1 mutants. Methods Mol Med 130:41–59. 10.1385/1-59745-166-5:41. [DOI] [PubMed] [Google Scholar]
- 79.Coomes SM, Wilke CA, Moore TA, Moore BB. 2010. Induction of TGF-beta 1, not regulatory T cells, impairs antiviral immunity in the lung following bone marrow transplant. J Immunol 184:5130–5140. 10.4049/jimmunol.0901871. [DOI] [PMC free article] [PubMed] [Google Scholar]