SUMMARY
Human T-lymphotropic virus type 1 (HTLV-1) is estimated to affect 5 to 10 million people globally and can cause severe and potentially fatal disease, including adult T-cell leukemia/lymphoma (ATL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). The burden of HTLV-1 infection appears to be geographically concentrated, with high prevalence in discrete regions and populations. While most high-income countries have introduced HTLV-1 screening of blood donations, few other public health measures have been implemented to prevent infection or its consequences. Recent advocacy from concerned researchers, clinicians, and community members has emphasized the potential for improved prevention and management of HTLV-1 infection. Despite all that has been learned in the 4 decades following the discovery of HTLV-1, gaps in knowledge across clinical and public health aspects persist, impeding optimal control and prevention, as well as the development of policies and guidelines. Awareness of HTLV-1 among health care providers, communities, and affected individuals remains limited, even in countries of endemicity. This review provides a comprehensive overview on HTLV-1 epidemiology and on clinical and public health and highlights key areas for further research and collaboration to advance the health of people with and at risk of HTLV-1 infection.
KEYWORDS: clinical methods, clinical therapeutics, diagnostics, epidemiology, human T-cell leukemia virus, oncogenic virus, pathogenesis, public health, sexually transmitted diseases, virology
INTRODUCTION
Human T-lymphotropic virus type 1 (HTLV-1) is estimated to affect 5 to 10 million people globally (1) and can cause severe and potentially fatal disease, including adult T-cell leukemia/lymphoma (ATL) (2–5) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (6). The burden of HTLV-1 infection is geographically concentrated, with high prevalence in discrete regions and populations. While most high-income countries have introduced HTLV-1 screening of blood donations, few other public health measures have been implemented to prevent infection or its consequences.
Gaps in knowledge and limited information about HTLV-1 have made it difficult for health care workers, researchers, policy makers, and people living with HTLV-1 infection to advance the clinical and public health response to HTLV-1. In recognition of this, the World Health Organization (WHO) held the first Global Consultation on HTLV-1 with member states and partners in November 2019, followed by the publication and launch of a series of documents, including a technical report (7), fact sheet, and meeting report (8) in March 2021. The recommendations outlined in these reports focused on the need to address HTLV-1 through a global public health approach. This review provides a comprehensive overview on HTLV-1 epidemiology and clinical and public health and highlights key areas for further research and collaboration to advance the health of people with and at risk for HTLV-1 infection.
GLOBAL OCCURRENCE OF HTLV-1
In 1977, researchers in Japan described a novel, rapidly progressing lymphoid malignancy, which became known as ATL (9). In addition to unique clinical and hematological features, the geographic distribution of patients by place of birth was notable, with almost all having been born on the southwestern island of Kyushu, a region now recognized as endemic for HTLV-1. The clustering of ATL cases led to the hypothesis that an oncogenic viral infection should be considered in disease causation (9). Shortly after, HTLV-1 was isolated from a patient with cutaneous T-cell lymphoma (10) and, following further serological and virological evidence, was determined to be the causative agent of ATL.
Once the association between ATL and HTLV-1 was established, epidemiological surveys of HTLV-1 prevalence were carried out across many countries and population groups, as well as investigations of transmission, disease progression, and associations with diseases other than ATL. The data on prevalence demonstrated a highly heterogeneous global distribution, with focal occurrence in various parts of the world. Even in areas where infection with HTLV-1 has been found, it occurs as endemic clusters, often within, or close to, populations with much lower prevalence. Given the substantial heterogeneity both within and between geographic areas, characterizing specific countries or even localities within countries as high versus low prevalence is not straightforward. The European Centre for Disease Prevention and Control proposed defining high prevalence as more than one in 10,000 positives among first-time blood donors, or 1% in the general population (11). Categorical thresholds such as these may not reliably define country or region level prevalence, but they are useful measures to inform policy decisions around cell and tissue donation screening practices. How HTLV-1 infection persists within discrete communities and geographies over time may be the result of a founder effect (the result of prolonged viral transmission within isolated groups), but this remains to be fully elucidated (1). A consistent finding is that HTLV-1 prevalence increases with age and, in most populations, is higher in adult females than adult males.
Genetically, HTLV-1 has been classified into seven subtypes, each with a characteristic geographic distribution explained by population migration. The cosmopolitan subtype A is the most widespread globally and classed further into subgroups (transcontinental, Japanese, West African, North African, Senegalese, and Afro-Peruvian) (1). Subtypes B, D, E, F, and G are found in specific parts of Africa, while subtype C is found in Australia and Oceania.
HTLV-1 likely originated from the genetically similar simian T-lymphotropic virus type 1 (STLV-1), a retrovirus endemic among many nonhuman primates in intertropical Africa. The greater genetic diversity of HTLV-1 subtypes in Africa may be the result of repeated zoonotic transmission events from human encounters with STLV-1 endemic nonhuman primates. Accordingly, higher prevalence of HTLV-1 is reported in people bitten by nonhuman primates with strikingly similar sequence homology to the STLV-1 sequences present in native primate species (12).
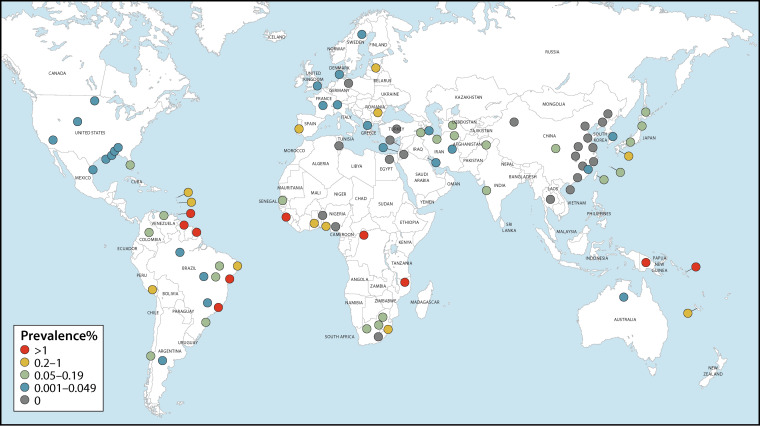
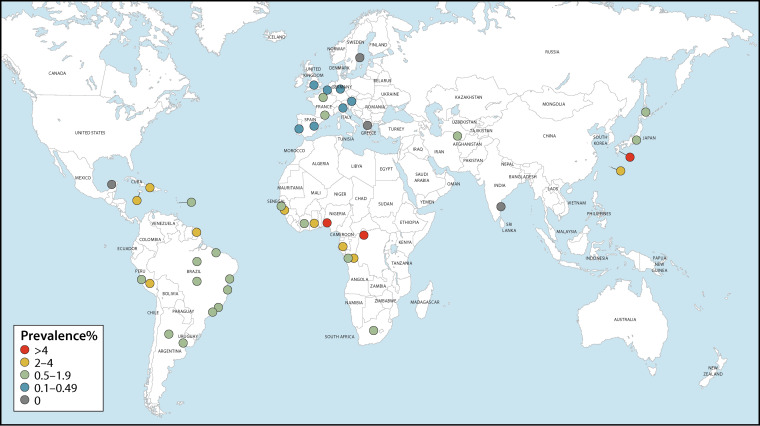
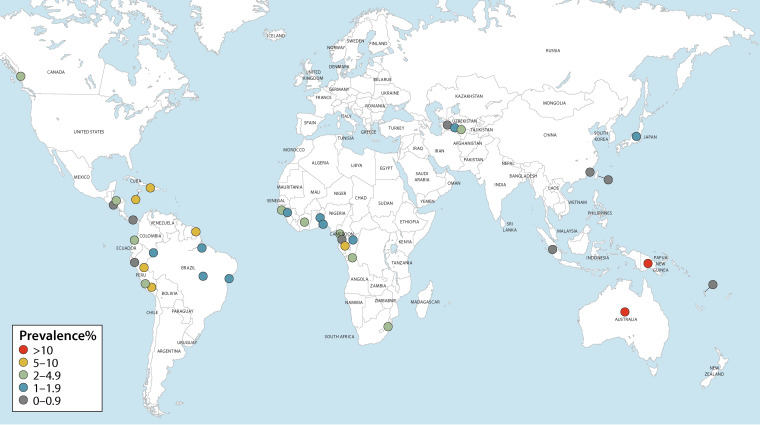
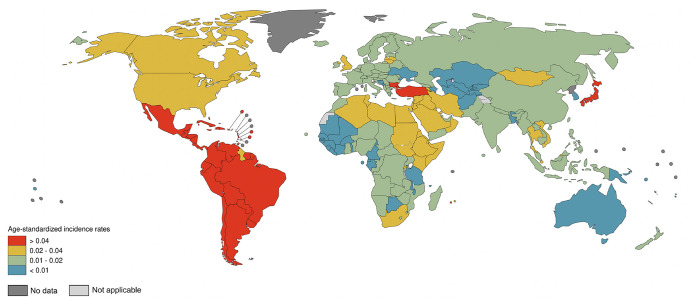
The number of people living with HTLV-1 worldwide was estimated in 2012 to be in the range of 5 to 10 million (1). Countries and regions broadly regarded as having focal areas where HTLV-1 is endemic include Japan (13–15), Iran (16–18), the Americas (19–27), the Caribbean (28–31), Melanesia (32–35), Central and West Africa (36–40), and Australia (41–43) (Fig. 1; see also Fig. 2 and 3). The paucity of reliable prevalence data from several highly populated countries, such as India and Nigeria, complicates calculations of global prevalence. Since estimations are based on and limited to known areas of endemicity, the available data are likely an underestimate of true global prevalence. The following overview on global occurrence of HTLV-1 reports prevalence data from studies (n = 186) whose methodological quality was critically appraised and where infection was validated by confirmatory testing. Data were excluded (n = 400) if no original prevalence data were reported, confirmatory testing was not carried out, or the sampling methodology was not representative of the general population.
FIG 1.
Global distribution of HTLV-1 seroprevalence among blood donors. These data were extracted from 65 critically appraised peer-reviewed publications reporting original prevalence data subject to confirmatory testing.
FIG 2.
Global distribution of HTLV-1 seroprevalence among pregnant women. These data were extracted from 37 critically appraised peer-reviewed publications reporting original prevalence data subject to confirmatory testing.
FIG 3.
Global distribution of HTLV-1 seroprevalence in general populations. These data were extracted from 37 critically appraised peer-reviewed publications reporting original prevalence data subject to confirmatory testing.
African Region
The WHO African region likely has the largest number of people living with HTLV-1 worldwide. High prevalence has been consistently detected in many populations and communities, with little change since the first studies conducted in the 1980s. Countries with well-defined areas of endemicity include Cameroon, Democratic Republic of the Congo, Gabon, Guinea, and Guinea-Bissau. In general, countries of central and west Africa are best characterized, with most prevalence data drawn from surveys in blood donors and pregnant women. A limited number of large community surveys in the general population have been carried out in countries of endemicity, notably Gabon and have been repeated to demonstrate the persistence of geographically localized infection over time. There remain large areas in north and east Africa with little reliable prevalence data.
For West Africa, prevalence data are available for Benin, Côte d’Ivoire, The Gambia, Ghana, Guinea, Guinea-Bissau, Nigeria, Senegal, and Togo, with prevalence ranges from 0 to 1.2% among blood donors, 1.2 to 2.2% among pregnant women, and 1 to 3.6% among the general population.
The highest prevalences are in Guinea-Bissau, demonstrated by three large general population studies reported prevalences ranging from 2.3 to 3.6%, with considerable variation by sex and age (37, 44, 45). In the capital city Bissau, seroprevalence increased with age from 0.5 and 1.1% in men and women aged 15 to 24 years, to 5.6 and 8.8% in those 55 years and above, respectively (37). Prevalence appears to have remained stable over time at 3.6% in 2000, 2.3% in 2009 (44), and 3% in 2018 (37, 44). A nationwide serosurvey among pregnant women found an overall prevalence of 2.2% (46). In contrast to many other countries where HTLV-1 is endemic, the distribution of infection in Guinea-Bissau appears to be relatively homogeneous (46).
The situation is similar in Guinea, with an overall prevalence of 1% in the general population (39) and a higher prevalence in the southern region of Samoé (2%). Seropositivity increased from 0.5% in children aged 10 years or younger to 5.7% in people aged 40 years or older. One blood donor study carried out in 1993 reported a prevalence of 1.2% (47). Although few studies report prevalence data from Cote d’Ivoire, one survey from the late 1980s found high levels in several population groups, including 1.9% among pregnant women, 2.2% among the general adult population, and 7.4% among female sex workers (48). Likewise, while there is some evidence of elevated prevalence in Nigeria, available studies are limited. Prevalence in blood donors ranged from 0% in the south-eastern city of Enugu to 0.73% in the capital of Lagos (49–51), while among pregnant women in the southwestern city of Ibadan a high prevalence of 5.5% was reported (52). In Benin, a national general population survey reported an overall prevalence of 1.5%, with substantial variation by geography, age, and sex (53).
In Accra, the capital of Ghana, antenatal screening detected a prevalence of 2.1% (54). Little reliable evidence is available for Senegal, with one study in 2006 reporting a seroprevalence of 0.14% among 4,900 blood donors (55), with five of the seven positive cases from the south Casamance region, indicating a potential region of endemicity. Among pregnant women from several regions across the Gambia, the prevalence was 1.2% (56). In Togo, HTLV-1 among outpatients the prevalence was 1.2% (57).
The prevalence in Central Africa countries is similarly high, ranging from 0.3 to 8.7% in the general population (36, 38, 58–63) and from 2 to 4.6% in pregnant women (64–67) in reports from Equatorial Guinea, Cameroon, Gabon, and the Democratic Republic of the Congo. The best-investigated country in Central Africa is Gabon, with four large population-based surveys carried out since the late 1980s (38, 58–60). Prevalences in the general population varied regionally, ranging from 2.2 to 12.5% (59). Higher positivity was associated with older age, exceeding 13% in people over 60 years (59). The prevalence in Gabon is generally high across all regions but is particularly high in the eastern provinces. A national survey of pregnant women across five provinces reported an overall prevalence of 2.1% (65) and, consistent with the general population studies, found clusters of higher prevalence in the eastern provinces (65).
The prevalence in the Democratic Republic of the Congo was similarly high. Among pregnant women, the HTLV-1 prevalence was 3.7 to 4.6% (64, 66) and 3.2 to 7.3% among female sex workers in Kinshasa (64, 68). The seroprevalence in blood donors from three regions (Basankusu, Gemena, and Dungu) was 4% (66).
For Cameroon, two studies reported on populations inhabiting rural areas in south and southeastern rainforest regions and found overall HTLV-1 seroprevalences of 2.6 and 2.9% in Pygmy populations, with particularly high rates in people over 60 years (11.3%) (61, 62). The HTLV-1 prevalence in Bantu populations was lower (1.3%) (61). The epidemiology of HTLV-1 in Equatorial Guinea has not been well described and is limited to one early study that reported a prevalence of 0.26% in the general population (69).
East African countries appear to have lower HTLV-1 prevalences than elsewhere in Africa. Data on HTLV-1 in Ethiopia comes from one study in 2012 that found no cases among 556 outpatients attending a rural hospital in a central part of the country (70). In Rwanda, one general population study reported prevalences of 0.2% in a rural setting and 0.3% in an urban sample (71).
In southern Africa, the prevalence among blood donors and outpatients in Mozambique were 0.9% and 1.9%, respectively (72, 73). For South Africa, the prevalence was generally lower at 0.1% among first-time blood donors, 0.2% among pregnant women, and 1.6% among asymptomatic hospital patients (66, 74, 75). Among South African blood donors, the prevalence varied by ethnic group, with higher prevalence of 0.16% among Black South Africans compared to 0 and 0.2% prevalence among White and Asian South Africans, respectively (75).
Region of the Americas
North America which is characterized by an overall low prevalence, while Central and South America have several regions and communities of endemicity.
In North America, seroprevalence has principally been derived from large studies among blood donors (76–79). Recent estimates among first-time blood donors in the United States found a very low prevalence of 0.005% (76), with a higher seroprevalence in people of African and Asian descent (76–78). Diagnoses of HTLV-1-associated diseases have largely occurred among people originating from endemic regions in the Caribbean basin (78). Similarly, in Canadian blood donors, HTLV-1 was generally detected among people who immigrated from known regions of endemicity (80). A survey in First Nation Canadians in coastal British Columbia found a high prevalence of 2.8% (23). In Mexico, HTLV-1 appears to be rare, with a zero prevalence reported from surveys among outpatients in Mexico City and pregnant women in the Yucatan peninsula (81, 82).
In Central and South America, prevalences have been reported in ranges of 0.01 to 1.3% among blood donors, 0 to 5.7% among pregnant women, and 0.2 to 6.7% in the general population. Regions of endemicity include Salvador in Brazil (21, 83) and Tumaco in Colombia (25). High prevalences have been found in particular ethnic groups, such as the Noir-Marron people in French Guiana (26, 84–86), the Shipibo-Conibo and Quechua peoples in Peru (19, 87, 88), and the nonmestizo population of Honduras (89).
In Brazil, there is high prevalence across several regions, particularly in the north and northeast of the country. The prevalences in first-time blood donors were reported to be 0.01% in Ribeiro Preto in the southeast and 0.6% in the northeastern state of Piau (90, 91). The city of Salvador has the highest overall prevalence of HTLV-1 in Brazil (21). Here, the prevalences in pregnant women, in general populations, and in blood donors were 0.85, 1.50, and 1.40%, respectively (92, 93). The prevalence among pregnant women ranged from 0.11% in Sau Paulo to 0.58% in Rio de Janeiro (94–98). Although men who have sex with men are infrequently studied with respect to HTLV-1, one survey reported a 0.70% prevalence (99).
Tumaco in Colombia’s southwest was found to have high rates of suspected HTLV-1-related neurological disease. Subsequent serological surveys reported prevalence of 2.8% in the general community, exceeding 14% in people older than 50 years. Outside of Tumaco, a prevalence of 0.05% in blood donors was found in the city of Medellin in Colombia’s central northwest (100).
In Peru studies have focused largely on indigenous communities among whom HTLV-1 is considered endemic, in particular the Shipibo-Conibo and Quechua people (19, 87, 88). Depending on geographic location, prevalence in Quechua communities varied considerably from 0 to 10% (88, 101, 102). Shipibo-Conibo communities within the Amazon region have similarly high levels of HTLV-1, with population-based studies reporting prevalences of 4.1 and 5.9% in Shipibo-Conibo women (19, 87). Consistent with other findings, high levels of HTLV-1 were found in female sex workers in Lima, with an overall prevalence of 9.6% increasing to 17.5% in women between 35 and 60 years old (103). The prevalence among pregnant women in French Guiana ranged from 3.8 to 5.7% (26, 84, 86). Importantly, women from the Noir-Marron population accounted for approximately 90% of all positive cases (85).
Population-based studies in Honduras showed clustering of HTLV-1 in cities along the Atlantic coast (89). Higher prevalence was reported in people of Afro-Caribbean descent (8%) than in people of American-European ancestry (0.5%) (89). In Georgetown Guyana, the prevalence in blood donors was 1.3%, with positivity associated with Guyanese-African and Guyanese-Indian ethnicity, older age, and female sex (27). In Panama, the overall seroprevalence in the general population was 0.6% (104).
In Argentina the prevalence ranged from 0.03% in blood donors to 0.1% in pregnant women (20, 105, 106). Data from Chile and Venezuela are derived from blood donor studies, with both reporting an overall prevalence of 0.1% (24, 107). For Chile, the prevalence in first-time blood donors was 0.1%, with a higher prevalence in Valparaiso in the north (0.17%) than in Concepcion (0.05%) in the south (24). For Venezuela, one study in Caracas found a 0.1% prevalence among blood donors (107).
The Caribbean has several high-prevalence regions. Prevalences ranged from 0.3 to 0.6% in blood donor studies (30, 108–110), 5.2 to 6% in general population studies (29, 111), and 1.2 to 3.8% in pregnant women studies (28, 112–116). In Guadeloupe, one blood donor survey reported an overall HTLV-1 prevalence of 0.33%, with geographic variation ranging from 0.21% in the southern regions of Basse Terre to 1.15% on the island of Marie Galante (31, 110). Among pregnant women in northern rural Haiti, 2.2% were found to be HTLV-1 positive (28). In addition, antenatal surveys carried out in French Guiana reported a prevalence of 4.2% in pregnant women of Haitian background (86). In Jamaica, a large population-based survey found a prevalence of 6% (29), while the prevalence in pregnant women ranged from 2 to 3.8% (113, 114, 116). HTLV-1 prevalence in the French overseas territory of Martinique was 2.4% among pregnant women (115), and 0.35% among first-time blood donors (108, 109). One blood donor study in Trinidad and Tobago reported an HTLV-1 prevalence of 1.5% (30).
South-East Asia Region
Although HTLV-1 in the WHO South-East Asia Region appears rare, most countries have substantial gaps in reliable prevalence data. Indonesia, Thailand, and India were the only countries with prevalence data where positive screening results were subject to confirmatory testing. No positive cases are reported in blood donors or population-based studies from Thailand and Indonesia (117–119). In India, one small blood donor study found prevalence of 0.14% (120). Nevertheless, case reports of ATL and HAM/TSP from India reflect the need for larger-scale studies to fully determine the extent and distribution of HTLV-1. Given the limited data and large population size, it is not possible to characterize the epidemiology of HTLV-1 in India.
Eastern Mediterranean Region
The overall prevalence of HTLV-1 in the WHO Eastern Mediterranean Region is generally low, with the Islamic Republic of Iran as the exception. In blood donor studies, zero prevalences are reported for Egypt, Saudi Arabia, Oman, Tunisia, Lebanon, and Jordan (121–128). Screening of blood donors in Kuwait found a prevalence of 0.07%, with the majority of positive cases of non-Kuwaiti origin (129). In Pakistan, one blood donor study in the northern city of Rawalpindi found an HTLV-1 prevalence of 0.2% (130). A prevalence of 0.06% was reported in a cohort of outpatients in northern Egypt (131).
Seroprevalence data in Iranian blood donors ranged from 0.05 to 0.7% and were associated with region and age (16, 17, 132–135). In Iran, HTLV-1 is described as a significant health issue particularly in the northeastern province of Razavi Khorasan. In Mashhad, the capital of Razavi Khorasan, a population-based survey reported an overall prevalence of 2% exceeding 12% in people older than 65 years (18). Two blood donor studies in Mashhad found prevalences of 0.7 and 0.18% (17, 132) and 1.5% among pregnant women (136).
Europe Region
The prevalence of HTLV-1 in the WHO Europe region is generally low. Blood donor studies range between 0 and 0.006% prevalence in Denmark, France, Germany, Greece, Sweden, Switzerland, the United Kingdom, Israel, and Turkey (137–146). Countries with higher prevalence include Portugal, Turkmenistan, Latvia, and Romania, with prevalences among blood donors ranging from 0.2 to 0.64% (147–150). In pregnant women, prevalence ranges from 0 to 0.02% in Belgium, Germany, Greece, Italy, Portugal, Slovenia, Spain, and Sweden, with higher prevalences reported in the United Kingdom (0.03%) and Paris, France (0.1%) (142, 151–154). One study examining prevalence among people who inject drugs found no cases of HTLV-1 (155).
Detection of positive cases in the United Kingdom (138, 156), France (140, 151, 157, 158), Portugal (148), Italy (151, 159), Spain (154, 160), The Netherlands (161), Greece (146), and Sweden (142) was generally associated with people from, or with sexual partners from, high-prevalence regions. Despite the role population migration plays in the detection of cases within these countries, there is little evidence of expansion beyond known regions of endemicity.
The notable exception in the Europe Region is Romania, which, for reasons unknown, is the only true country of endemicity in the Europe region. In Romania, the detection of ATL prompted a seroepidemiological survey that found a high prevalence of 0.64% among blood donors (149). The origin of HTLV-1 in Romania is unknown, and no reliable epidemiological surveys have been carried out since the mid-1990s. Nevertheless, evidence of higher prevalence in people of Romanian origin has been substantiated in part by the detection of higher rates of HTLV-1 in Romanian immigrants (145).
Western Pacific Region
HTLV-1 is found in several countries in the WHO Western Pacific Region, with multiple focal areas of high prevalence in Australia, Papua New Guinea, Solomon Islands, and southwest Japan. Prevalence ranged between 0.007 and 2% in blood donors (13, 15, 32, 33, 162–170) and between 0.5 and 39% in general population studies (34, 35, 41, 171–175). High levels of HTLV-1 infection have been found in communities in Papua New Guinea and Central Australian Aboriginal communities.
In Australia, HTLV-1 occurs at extremely high prevalences among Aboriginal people living in central Australia. Here, the first case of HTLV-1 was reported in 1988. Available evidence indicates that Aboriginal people in Central Australia have a prevalence of HTLV-1 infection 1,000 to 10,000 times higher than the Australian population as a whole, in which prevalence can only be inferred from routine testing in blood donors (41, 176). In 2016, a community-based survey in central Australia found an HTLV-1 prevalence of 33.3% (41). Between 2014 and 2018, a larger community-based survey in the same region found a similar HTLV-1 prevalence in adults of 39% and a lower prevalence of 6% in children aged 3 to 17 years (175). As with overseas studies, prevalence increased with age but, in contrast to most other populations affected by HTLV-1, was higher in older men than women (34).
For Papua New Guinea, studies reporting prevalence data are more than 20 years old. The available data indicate a high prevalence of 14.6% in the Madang province (173). Similarly, prevalence among Hagahai communities in Madang province was 14.2%, increasing to 35% in people older than 40 years (34). The overall prevalence among outpatients in the Solomon Islands was 3.5%, increasing to 7.4% in people aged 40 to 49 years (33). For blood donors in Honiara, the prevalence ranged from 0.7 to 2% (33, 170).
In China there was substantial regional variability, with clustering of elevated prevalence in the southeast (166, 172). Higher-prevalence regions include the province of Fujian and Kinmen County (164–166, 172). Prevalence was greater in Fujian born blood donors (0.02%) compared to non-Fujian born blood donors (0.003%) (166). In central China and Beijing, HTLV-1 is uncommon (164, 177).
For Taiwan, screening for HTLV-1 among blood donors between 1996 and 1999 reported an overall prevalence of 0.058%, with an annual decline in seropositivity from 0.13% in 1996 to 0.032% in 1999 (163). One community-based study reported an overall prevalence of 0.48% among people aged 18 years and older (174)
In Japan there was an estimated 10% decline between 1988 and 2012 in the number of people in Japan living with HTLV-1, from 1.2 million to 1.08 million (15, 178). The overall seroprevalence among first-time blood donors was 0.3%, with substantial regional variability by region from 0.06% in eastern Japan to 1.95% in the southwesternmost island of Kyushu (15). The prevalence among pregnant women from national antenatal screening data was 0.14% and, as in the case of blood donors, was highest in Kyushu (0.6%) (14).
Data on prevalence in South Korea are derived from blood donor studies (167–169), the most recent of which reported a low overall prevalence of 0.007% (168). For New Caledonia, the prevalence in adults aged 60 to 96 years was 0.06%, with all positive cases obtained from female donors (1.3%) (32). One large population-based study in Vanuatu reported an overall HTLV-1 prevalence of 0.62%, with regional variability ranging from 0% in the northernmost province of Torba to 1.93% in the northwesternmost province of Sanma (35).
Issues Related to Evaluation of HTLV-1 Prevalence and Burden of Infection
While a number of reports have evaluated HTLV-1 prevalence in various populations across the world, there remain many highly populated countries with ill-defined or undetermined HTLV-1 prevalence. Prevalence data have been collected across a broad time period from various populations and cohorts. During this time, screening and diagnostic methods have evolved considerably, constraining the validity of geographic and times-series analyses. With few prevalence surveys representative of general populations with reasonable sample sizes, the majority of prevalence data are derived from studies centered on routine screening practices such as those in blood donors and in pregnant women. Although blood donor screening is advantageous on the scale on which it can be carried out, this method favors selection of people at low risk of blood-borne viral infections, reducing the generalizability to the broader population. Similarly, screening of pregnant women provides a more accurate estimation of HTLV-1 in the general population but tells us little about the older cohort who likely have higher rates of infection.
In addition to the gaps in HTLV-1 prevalence data, systematically collected information on HTLV-1 related diseases is limited. In addition, there are knowledge gaps concerning the variation in HTLV-1 infection within countries of endemicity and within subpopulations that may be at higher risk, e.g., people who inject drugs and men who have sex with men. A standardized approach to monitoring both HTLV-1 and its associated diseases is needed in order to improve and inform public health decision-making and responses both nationally and globally.
HTLV-1 VIROLOGY AND PATHOGENESIS
HTLV-1 is a human type C retrovirus (family Retroviridae, genus Deltaretrovirus) which predominantly infects CD4+ T lymphocytes (179, 180). The sequence variability within the seven reported HTLV-1 subtypes (A to F) is low. These subtypes have been determined phylogenetically via analyses of the long terminal repeat (LTR) and env regions (181, 182), with the genetic divergence within subtype reported to reach 2.7% in the gp21-env gene (183). The HTLV-1 genome is encoded on two copies of positive single-stranded RNA. Single-stranded RNA is converted to double-stranded DNA and inserted into the human host cell DNA. When integrated into the host genome as provirus, persistent infection is established. In early infection, cell-to-cell transmission of HTLV-1 (through formation of a virological synapse and/or viral biofilm) is likely to predominate, resulting in polyclonal infection of CD4+ and CD8+ T lymphocytes. In established infections, HTLV-1 infection is maintained by clonal expansion of infected cells as described below, a process dependent on host cell DNA polymerase.
The HTLV-1 genome encodes structural proteins typical of a retrovirus (gag, pol, and env), along with proteins specific to HTLV-1, encoded by a unique region in its genome, named pX. Encoded in the pX region are nonstructural regulatory proteins, including p13, p12, p30, and p40 (Tax), and on the minus (complementary) strand of pX is HTLV-1 basic leucine zipper factor (HBZ). Tax and HBZ proteins are important for understanding HTLV-1 biology and pathogenesis.
As a retrovirus, HTLV-1 shares similarities with HIV-1 but differs very substantially in disease association and causation. Unlike HIV-1, HTLV-1 infection does not result in death of T lymphocytes; rather, it is thought to initially cause cell cycle arrest before undergoing further modification during the chronic phase of infection, leading to cell proliferation which can, in turn, lead to malignant transformation of these cells (184). HTLV-1 and its proteins (notably Tax and HBZ) interact with host cell proteins (often transcription factors) and alter their function. The net effect (of Tax and HBZ) in HTLV-1-infected cells is cellular proliferation, induction of genetic instability, and inhibition of apoptosis, leading to the persistent clonal expansion of infected cells and the promotion of oncogenesis (185).
Initially, the clonality of HTLV-1-infected T cells is heterogeneous and unstable, but it becomes stabilized over a long clinical latency, during which HBZ drives mitotic expansion of latently infected cells that are largely quiescent (186, 187). Recent data have indicated that Tax expression occurred intermittently in an ATL cell line, MT1, where it induced the expression of antiapoptotic factors via NF-κB to facilitate altruistic cell survival (188). In agreement with these results, Tax and HTLV-1 plus-strand mRNA transcripts have also been shown to be expressed in intense bursts in lymphocytes of infected individuals cultured ex vivo (189). These results—in combination with the known clastogenic and proinflammatory activities of Tax (190), likely delivered in small doses repeatedly over decades—may begin to explain the oncogenic and inflammatory effects associated with HTLV-1.
Both cellular and humoral responses are mounted by the host immune system against HTLV-1. Class 1-restricted CD8+ T lymphocytes (cytotoxic T cells) identify and destroy HTLV-1-infected-T cells. A balance between CD8+ T cell killing and HTLV-1 proviral replication through clonal expansion of host cells leads to a steady-state HTLV-1 proviral load. HTLV-1 also induces a proinflammatory, type 1 helper T cell (Th1) response in CCR4+ CD4+ T cells (191, 192), characterized by the production of interferon gamma, the induction of B cells, and the development of cell-mediated immunity. Antibodies to Gag proteins are produced within the first 2 months of infection, followed by antibodies specific for envelope proteins. Anti-Tax antibodies may develop later in the course of infection. While host immune responses appear to be relatively effective in controlling (but not eradicating) HTLV-1, the CD8+ and Th1 T cell responses generate inflammatory cytokines, which play a role in the pathogenesis of HAM/TSP and other inflammatory conditions (193). Tax also upregulates expression of other host genes which may be involved in HTLV-1 disease pathogenesis, including interleukin-2 (IL-2), granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor alpha (194).
HTLV-1 TESTING AND DIAGNOSIS
HTLV-1 Serology
The formation of HTLV-1-specific antibodies is considered to only take place in people who have acquired chronic infection. After acquisition of infection, seroconversion may take 40 to 65 days. In early reports, there were even longer times to seroconversion, with periods of several years reported in infants (195, 196) and in people who were immunocompromised (197, 198), probably due to the past use of lower-sensitivity assays.
HTLV-1 structural proteins are the common targets for the serological tests. These include Gag proteins (capsid [p24], nucleocapsid [p15], and matrix [p19]) and Envelope glycoproteins (gp46 [or its precursor gp61/68], and gp21). One of the regulatory proteins, Tax, is a less frequently used target. While it has been suggested that variability is greatest in the antigenic profile of the Envelope gp46 protein across HTLV-1 subtypes (a to g) (199), there does not appear to an association between HTLV-1 subtype and the sensitivity of current commercial serological tests (200). More recently, serological assays have used both gp46-I and gp46-II proteins to differentiate between HTLV-1 and HTLV-2 infection (201).
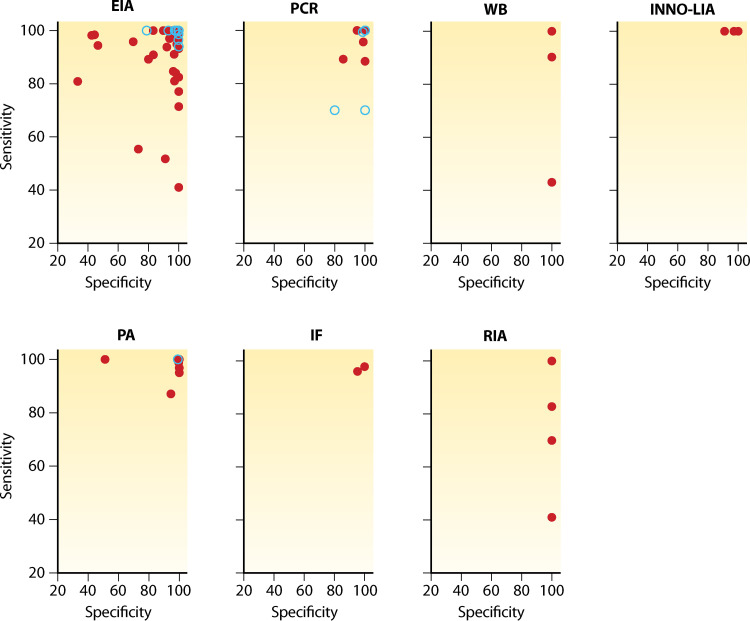
The most widely available serological tests are enzyme immunoassays (EIAs) (Table 1). Epidemiological surveys, and blood and transplant donor screening programs typically use EIAs because of their high sensitivity, simplicity, and throughput capacity. While first-generation EIAs had relatively low specificity, they have been improved with the addition of recombinant proteins, though specificity is still lower than the other serological assays, including Western blot and line immunoassays (INNO-LIA) (7, 202) (Fig. 4). False-positive results can occur as a result of cross-reactivity with antigens produced by other pathogens or autoantigens (203–205). Definitive diagnosis of HTLV-1 infection therefore requires confirmatory testing with either another serological assay or a molecular test.
TABLE 1.
Commercially available HTLV diagnostic immunoassays
| Assay type | Assay (manufacturer)a | Principle of assay |
|---|---|---|
| Enzyme immunoassay | Elecsys HTLV-I/II (Roche Diagnostics) | One-step double-antigen sandwich chemiluminescent immunoassay (ECLIA); detects antibodies to viral recombinant antigens gp21 and p24. |
| Enzyme immunoassay | Abbott Architect rHTLV-I/II (Abbott Laboratories) | Two-step chemiluminescent immunoassay (CLIA); detects antibodies to viral synthetic peptide gp46 and recombinant antigen gp21. |
| Enzyme immunoassay | Ortho Avioq HTLV-I/II Microelisa (Avioq, Inc.)* | Detects antibodies to purified viral lysate and recombinant HTLV-1 p21E antigen. |
| Enzyme immunoassay | Abbott Prism HTLV-I/HTLV-II (Abbott Laboratories)* | Three-step sandwich chemiluminescent immunoassay (CLIA); detects antibodies to HTLV-1 and HTLV-2 antigens. |
| Enzyme immunoassay | Murex HTLV I+II (Diasorin) | Detects antibodies to HTLV-1 and HTLV-2 antigens. |
| Enzyme immunoassay | Gold ELISA HTLV-1/2 (Rem Indústria e Comércio LTDA) | Uses recombinant antigens (gp46 and gp21) fixed on microplate; detects anti-HTLV-1 and HTLV-2 IgM, IgG and IgA antibodies in human serum or plasma by EIA. |
| Line immunoassay | Inno-LIA HTLV-I/II (Fujirebio, Inc.) | Purified recombinant proteins (rp19, rp24: I/II and rgp21: I/II) and synthetic peptides (gp46: I/II and p19: I) fixed on nylon membrane. |
| Particle agglutination | Serodia-HTLV-I PA (Fujirebio, Inc.) | Gelatin particle-coated cell culture derived HTLV-1 antigens are agglutinated in presence of antibodies to HTLV-1 in human serum/plasma. |
| Western blot | HTLV Blot 2.4 (MP Diagnostics)* | Seropositivity criteria for HTLV-1 includes reactivity to p19 Gag, with or without p24 Gag, and to GD21 with the presence of rgp46-I peptide, MTA-1. |
*, FDA licensed.
FIG 4.
Sensitivity and specificity of 165 HTLV-1 diagnostic assays. Data were extracted from 65 peer-reviewed publications. Gray dots indicate data extracted pre-2009 and black circles post-2009, highlighting improved sensitivity and specificity of more recent assays. EIA, enzyme immunoassay; IF, immunofluorescence assay; INNO-LIA, line immunoassay; PA, particle agglutination; RIA, radioimmunoassay; WB, Western blot.
The two main confirmatory serological tests are Western blotting, considered to be the “gold standard,” and INNO-LIA (201). The role of the Western blot as the gold standard, however, has been questioned with the arrival of molecular testing. Furthermore, its use has been hampered by the lack of a standard set of criteria for positivity and the consequent difficulties in interpretating results deemed “indeterminate” (61, 206). In the late 1980s, the U.S. Centers for Disease Control and Prevention (CDC) and the World Health Organization had similar definitions of Western blot positivity, requiring detection of anti-Gag (CDC, p24 only; WHO, p19 or p24) and anti-Envelope (CDC, gp46 or precursor gp61/68; WHO, gp46 and/or gp68) antibodies (207). In 1992, the CDC modified criteria to include reactivity to the anti-Gag (p24) and anti-Envelope (gp46) or the recombinant GD21 protein (a truncated recombinant portion of gp21) (208). In 1996, the HTLV European Research Network defined its own criteria which required the detection of at least one anti-Gag (p19 or p24) and two anti-Envelope (gp21 and gp46) antibodies, whether native or recombinant (209, 210). Based on these definitions, some samples considered positive according to WHO criteria would be classified as “indeterminate” based on CDC and European criteria. In addition to this complexity, commercial Western blot kits are also available with their own manufacturer-defined criteria for positivity.
Indeterminate Western blot results can pose a significant issue, particularly if other confirmatory assays are not available. The proportion of indeterminate Western blot results reported has ranged from 0 to 68%, with the highest proportion in studies from Central Africa and Zaire (61, 206). A number of factors have been identified as associated with “false” positivity, including (i) cross-reactivity with structural proteins from other HTLV types (211, 212); (ii) cross-reactivity with Plasmodium falciparum proteins in particular gp21 (203–205), a particular issue in areas where HTLV-1 and malaria are co-endemic (61, 206); and (iii) nonspecific cross-reactivity with host or other pathogen proteins (213). Given the limitations of Western blotting, it has been proposed that line immunoassay or molecular tests be considered for routine use as confirmatory assays (214). For the line immunoassay, INNO-LIA (Fujirebo), positivity is defined by reactivity to p19, p24, gp46, and gp21.
HTLV-1 Molecular Testing
Highly specific molecular diagnostic methods can detect HTLV-1 nucleic acid sequences by PCR in peripheral blood and tissue. Targeting the DNA provirus integrated into host cell genomes, this group of tests includes qualitative, quantitative real-time (Q) and droplet digital (dd) methods (215). Qualitative tests are often performed on whole blood or other tissues to confirm diagnosis.
Potential PCR targets include the pol, env, and tax regions. However, it is important to note that deletions in the HTLV-1 provirus can impact test sensitivity. While pol and env are prone to deletion (200, 216), pX (which includes tax) and 5′-LTR are highly conserved (215), suggesting that tax should be a focus for molecular diagnostic assays. Another factor which can affect the sensitivity of PCR testing is the proportion of potential target cells in a specimen with integrated HTLV-1 genomes. Another potential limitation for implementation of PCR testing, particularly in resource-limited settings, is the need to acquire and store cellular samples.
HTLV-1 proviral load can be quantified and is expressed as the number of HTLV-1 DNA copies detected per fixed number of peripheral blood mononuclear cells (PBMCs). It appears to remain relatively stable over time in an individual with HTLV-1 infection (usually <100 copies per 10,000 PBMCs [<1%] in asymptomatic infection) (217), as discussed in the transmission and health consequence sections of this review, is a strong predictor of both the risk of onward transmission and disease development (218–221). No HTLV-1 proviral load assay is produced commercially but would clearly valuable in public health and clinical applications.
HTLV-1 Testing and Diagnosis Algorithms
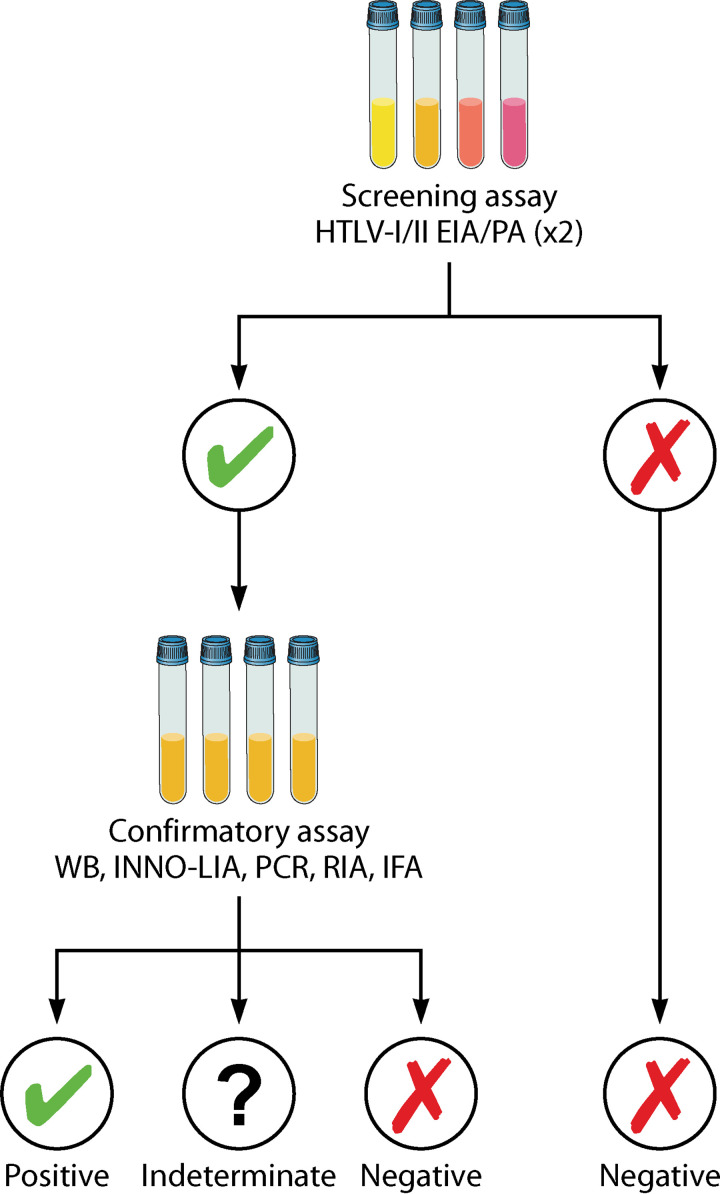
Current testing algorithms use a stepwise process to confirm HTLV infection, based on positive results on at least two different assays. A typical algorithm involves use of a sensitive serological test (usually an EIA) that, if positive will often be repeated, followed by a more specific confirmatory test (Western blot, INNO-LIA, or PCR) (Fig. 5). Most EIAs do not distinguish between HTLV types so the confirmatory tests are also important for this. In general, serum or plasma is used for immunoassays and PBMCs are used for PCR.
FIG 5.
HTLV-1 testing algorithm. Testing algorithms for HTLV-1 have generally required two or three assays. An EIA-based method is used for screening plasma or sera given high sensitivity, simplicity, and potential for high throughput. Positive samples are then retested, either with the same EIA or an alternative. Finally, repeatedly reactive plasma or serum samples are confirmed by another assay type, either Western blot, INNO-LIA, or PCR. EIA, enzyme immunoassay; IFA, immunofluorescence assay; IMMO-LIA, line immunoassay; PA, particle agglutination; RIA, radioimmunoassay; WB, Western blot.
At the WHO global consultation on HTLV-1 held in 2019, an expert group recommended that appropriate testing strategies should be selected on the basis of context and setting. For instance, it may be acceptable in low-prevalence settings to use a testing strategy that can produce a quick result, even if it does not have optimal sensitivity in situations such as organ donation, where timeliness is critical. However, more labor-intensive confirmatory testing is essential for individuals with suspected HTLV-1-associated disease.
Cost of Testing
Cost is a critical consideration when selecting the appropriate testing algorithm for large-scale, routine use (222, 223). In the context of serological screening of blood donors, Stigum et al. (224) calculated that at a prevalence of 1 in 100,000, the cost was $9.2 million per life saved through screening or $420,000 per quality adjusted life year (QALY). At a prevalence of 1 in 10,000, the cost was $0.9 million per life saved and $41,000 per QALY. In low-prevalence, high-income settings, measures have been taken to improve the cost-effectiveness, such as screening blood donors only at their first donation (e.g., Sweden) or using pooling of specimens (e.g., Wales and Scotland) (223).
HTLV-1 Testing: Whom To Test and When?
There are few published guidelines and strategies on who should be offered testing for HTLV-1 and when. Clinical recommendations are diverse and include testing pregnant women (i.e., Japan [225]), infants born to mother with HTLV-1 infection (i.e., Brazil [226, 227], Chile [228], and Japan [225]), sexual partners of people with HTLV-1 infection (i.e., Brazil [226, 227] and Chile [228]), people who inject drugs (i.e., Brazil [226, 227] and Chile [228]), people who engage in sex work (i.e., Brazil [226, 227] and Chile [228]), and people with occupational exposure to HTLV-1 infection (Australia [229]). There is little information on the implementation and uptake of these recommendations.
HTLV-1 TRANSMISSION: ROUTES AND RISK FACTORS
Transmission of HTLV-1 is generally understood to require direct contact between infected and uninfected cells in bodily fluid such as breastmilk, blood, and semen (230). From a public health perspective, the most important ways that transmission occurs are from mother to infant, via sexual contact, and through transfusion of cellular blood products (Table 2).
TABLE 2.
Routes and rates of HTLV-1 transmission
| Transmission route (references) | Detail (references) | Reported rates (%) |
|---|---|---|
| Maternal exposure (56, 116, 136, 196, 232, 234, 235, 239, 241, 246, 247, 249, 250, 456–460) | Through breastfeeding; limited evidence of intrauterine or peri-partum transmission | 3.2–46 |
| Sexual intercourse (87, 102, 254–256, 258–262, 461) | More frequent from male-to-female than female-to-male (252–255) | 2–65 |
| Therapeutic use of blood products and organ transplant (266, 268, 269, 462) | Primarily via products containing cellular components (i.e., whole blood, red blood cells, platelets) | 28–86 |
| Other blood contacta | Blood contact through nonsterile skin penetrating procedures; reported examples include injection of illicit drugs (79, 463–465) and traditional religious self-flagellation practices (466, 467) | NAb |
Injecting using nonsterile equipment is considered very high risk, and other forms of blood contact are not well documented.
NA, not applicable.
Mother-to-Child Transmission
Evidence of vertical transmission emerged soon after the discovery of HTLV-1, with the detection of infected lymphocytes in the breast milk of mothers with HTLV-1, and high rates of infection in children born to mothers with HTLV-1 (231, 232). Transmission estimates have ranged from 3.2 to 46%, with an average around 20%.
HTLV-1 has been detected in several specimen types, including breast milk (231), peripheral blood, and cord blood samples (233), while proviral DNA has been detected in placental tissue and breast milk (234) but not in cord blood (196, 232, 235). These findings, as well as the very low prevalence of infection in formula fed children born to HTLV-1-positive mothers, indicate that transmission in utero or during delivery is rare (236, 237) and that the major risk to infants is from breastfeeding (237, 238).
Furthermore, multiple epidemiological studies have shown that duration of breastfeeding is a strong predictor of mother-to-child transmission of HTLV-1. Breastfeeding for more than 6 months leads to transmission rates exceeding 30% (239, 240), while shorter duration conveys a much lower rate (116, 239–244). There is also a strong association between maternal proviral load in peripheral blood and breast milk and the risk of mother-to-child transmission (234, 245–250).
Sexual Transmission
HTLV-1 has been detected in both cervical secretions (237, 251) and seminal fluid (230), indicating the biological plausibility of sexual transmission. A wide range of transmission rates have been reported in epidemiological studies of heterosexual couples (Table 2). In general, male-to-female sexual transmission is more likely than from female to male, based on studies in which one member of a couple is positive and the other negative for HTLV-1 infection (252–255).
Several factors have been associated with an increased risk of heterosexual transmission of HTLV-1 (Table 3), including longer duration of a sexual relationship (253, 254) and older age of the male partner (253). A higher proviral load is a risk factor in both cross-sectional and longitudinal studies (253, 254, 256, 257). Cross-sectional surveys in populations considered to be at higher risk of acquiring sexually transmitted infections (102, 256, 258, 259), including sex workers, people attending STI clinics, and men who have sex with men), and investigations of sexual partners of people with HTLV-1 (253, 254, 257, 260) have identified a range of risk factors for sexual transmission. These include the number of sexual partners (261), a history of condomless sex (254), sex during menses, vaginal douching before and after sexual intercourse (102), earlier sexual debut (262), and other sexually transmitted infections, particularly ulcerative conditions such as syphilis or herpes simplex virus infection (102, 254, 258, 259, 261).
TABLE 3.
Risk factors for HTLV-1 transmission
| Transmission route | Risk factor (references) |
|---|---|
| Maternal exposure | Breastfeeding duration, particularly longer than 6 mo (116, 239–244) |
| Higher maternal HTLV-1 proviral load (234, 245–247, 249, 250) | |
| Sexual intercourse | Condomless sex (254) |
| Earlier age at first sex (262) | |
| No. of partners (261) | |
| Current or past sexually transmissible infections (102, 254, 258, 259, 261, 468)a | |
| Sex during menses and vaginal douching pre- and postintercourse (102) | |
| Higher HTLV-1 proviral load (253, 254, 256, 257) | |
| Duration of sexual relationship (253, 254) | |
| Therapeutic use of blood products | Shorter storage duration (266, 268, 269) |
It is difficult to distinguish sexually transmitted infections as a cofactor for HTLV-1 transmission from the alternative explanation that both HTLV-1 and other sexually transmitted infections are driven by sexual behavior as the common causal factor.
Such cross-sectional studies can generally not distinguish whether other sexually transmitted infections function as a cofactor facilitating the transmission of HTLV-1 or whether HTLV-1 and other sexually transmitted infections are driven by common causal factors related to sexual behaviors. It is also not clear whether the populations assessed as being at higher risk of sexually transmitted infection in fact had increased prevalence of HTLV-1 infection compared to the general populations in the settings from which they were drawn.
HTLV-1 Transmission through Blood and Blood Products
Evidence of transmission via blood transfusion emerged in the 1980s, when it was found that HTLV-1 prevalence in people who had received transfusions of cellular blood products was substantially higher than those with no history of blood transfusion or transfusion with acellular blood products (Table 2). Specific populations at higher risk were people with conditions—including thalassemia, (HTLV-1 prevalence, 6%), sickle cell anemia (4%), renal dialysis (20%), and neonatal complications (2%)—requiring therapeutic blood product transfusion (263–265). Transfusion of whole blood from a donor with HTLV-1 infection carries a risk of transmission exceeding 80% (266). A similar high risk applies in cases of a solid organ transplant from a donor with HTLV-1 infection (267).
A shorter duration (<14 days) of storage of blood has been associated with increased risk of transmission, likely because of the reduced lymphocyte viability in older blood units (266, 268, 269) and use of immunosuppressive therapy by recipients of positive blood products (Table 3) (268).
Other Routes of HTLV-1 Transmission
Investigations of household members have found higher than expected prevalence of HTLV-1 infection following a diagnosis in a household member but no evidence for routes of transmission other than those described above (270).
HEALTH CONSEQUENCES OF HTLV-1 INFECTION
Most people with HTLV-1 infection do not appear to develop health conditions that can be directly linked to the infection. However, there is a subgroup of people who experience severe complications (Table 4). The most well recognized are adult T-cell leukemia-lymphoma (ATL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), both of which can only be diagnosed in a person with HTLV-1 infection. Subsequently, HTLV-1-associated uveitis (HAU) and infective dermatitis were defined as disease entities that could only occur in a person with HTLV-1 infection.
TABLE 4.
Diseases associated with HTLV-1 infection
| Category | Disease | Lifetime riska |
|---|---|---|
| Diseases for which HTLV-1 infection is required for diagnosis | ||
| Cancer | Adult T-cell leukemia-lymphoma | 5% |
| Inflammatory conditions | HTLV-1-associated myelopathy/tropical spastic paraparesis | 2% |
| HTLV-1-associated uveitis | <1% | |
| Infective dermatitis | Unknown | |
| Diseases with epidemiological evidence of an association with HTLV-1b | ||
| Cancer | Lymphoma other than ATL | |
| Inflammatory conditions | HTLV-1-associated pulmonary disease (bronchiectasis, bronchitis, bronchiolitis) | |
| Rheumatoid arthritis, Sjogren’s syndrome | ||
| Seborrheic dermatitis (adults and children), eczema (children) | ||
| Other infectious diseases | Tuberculosis | |
| Disseminated strongyloidiasis and Strongyloides hyperinfection syndrome | ||
| Urinary tract infection | ||
| Dermatophyte infection | ||
| Community-acquired pneumonia |
Lifetime risk among people with HTLV-1.
Epidemiological evidence quality very limited to moderate. (Data from reference 271.)
Several other health conditions involving a range of organ systems, including lung disease and other infectious diseases, have also been assessed. A recent systematic review and meta-analysis of epidemiologic studies examined published associations between HTLV-1 infection and all-cause mortality, and a range of inflammatory, infective, and malignant conditions (271) (Table 4).
Adult T-Cell Leukemia-Lymphoma
In 1979, a novel virus (subsequently named HTLV-1) was isolated from a patient with a cutaneous T-cell malignancy in the United States (10), following initial reports of a similar clinical syndrome in Japan, which had been named ATL (9). The causal role of HTLV-1 in the pathogenesis of ATL was determined (2–5, 272–276), and HTLV-1 was declared carcinogenic to humans in 1996 by the International Agency for Research on Cancer (IARC) (277).
There are four recognized clinical subtypes of ATL: acute, lymphomatous, chronic, and smoldering (278–280) (Table 5). People with ATL may present with skin lesions (nodules, ulcers, and generalized papular rash), lytic bone lesions, lymphadenopathy, hepatosplenomegaly, hypercalcemia, and symptoms related to lung and other organ involvement. Lymphocytes with a characteristic appearance, referred to as “flower cells,” may be seen on blood film. Opportunistic infections may occur due to immunosuppression related to dysfunctional HTLV-1-infected T cells. Mortality is high for people diagnosed with aggressive forms of ATL (acute and lymphomatous), with a median survival less than 12 months (279, 280).
TABLE 5.
Diagnostic criteria and classification of clinical subtypes of ATL
| Criterion or classification | Diagnosisa |
|||
|---|---|---|---|---|
| Acute | Lymphomatous | Chronic | Smoldering | |
| Diagnostic criteria | ||||
| Anti-HTLV-1 antibody | Yes | Yes | Yes | Yes |
| Lymphocyte count (×109/L) | Any (often ↑↑↑) | <4 | ≥4 | <4 |
| Abnormal T lymphocytes | Yes* | ≤1% | Yes* | ≥5% |
| Flower cells | Yes | No | Occasional | Occasional |
| Lactate dehydrogenase | Any (often ↑) | Any | 1.5×–2× ULN | <1.5 ULN |
| Hypercalcaemia | Possible | Possible | No | No |
| Lymphadenopathy | Possible | Yes | Possible | No |
| Involvement of: | ||||
| Skin | Possible | Possible | Possible | Possible |
| Lung | Possible | Possible | Possible | Possible |
| Lymph node | Possible | Yes | Possible | No |
| Liver | Possible | Possible | Possible | No |
| Spleen | Possible | Possible | Possible | No |
| Central nervous system | Possible | Possible | No | No |
| Bone | Possible | Possible | No | No |
| Gastrointestinal tract | Possible | Possible | No | No |
| Clinical presentation: | ||||
| Ascites | Possible | Possible | No | No |
| Pleural effusion | Possible | Possible | No | No |
| Disease burdenb | ||||
| Proportion (%) of ATL cases | 50 | 26 | 13 | 11 |
| Median survival (mo) | 8.3 | 10.6 | 31.5 | 55.0 |
Knowledge of ATL occurrence, progression, and treatment largely come from Japan (280). In 2018, it was estimated by IARC that there were 3,600 cases of ATL worldwide, approximately one-third in Japan, which also had the highest reported incidence (281) (Fig. 6). Given underdiagnosis and underreporting of HTLV-1 and ATL in many parts of the world, the estimated global burden of ATL was likely to be very conservative. Of countries with cancer registries, few have coding systems that allow the routine analysis of ATL as a distinct entity.
FIG 6.
Age-standardized incidence rates of ATL worldwide per 100,000 population. Age-standardized incidence rates are reported for the general population and are not specific for people with HTLV-1 infection. (Adapted from references 7 and 281.)
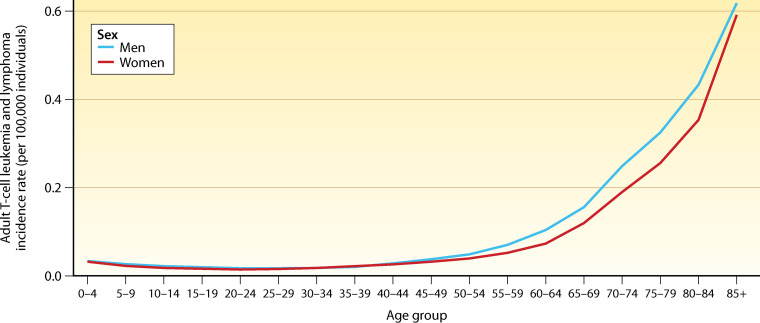
While ATL incidence generally increases with age (Fig. 7), age at diagnosis is markedly higher in Japan (median, 68 years; range, 34 to 100 years) (280) in comparison with Brazil (mean age, 44 years; range, 13 to 78 years) (282), and Jamaica (mean age, 43 years; range, 17 to 85 years) (283). Similarly, in a U.S. series, the median age at ATL diagnosis was 50 years (range, 22 to 82 years), with most cases (93%; 83/89) among immigrants to the United States from the Caribbean, Latin America, or Africa (284). In the 2018 IARC analysis, little difference was seen when stratified by gender, in contrast to previous cohort and population-based surveillance studies in which incidence was higher among men than women (Fig. 7) (285–288).
FIG 7.
ATL incidence by age and sex in 2018. (Adapted from references 7 and 281.)
The estimated lifetime risk of ATL among people with HTLV-1 infection is approximately 5% (276, 285–289). The incidence of ATL among people with HTLV-1 has ranged from 0.5 to 7.1 per 1,000 person-years in Japan (272–276, 290), and although data are limited, ATL incidence in regions outside Japan appears to be within a similar broad range (291–293).
Factors associated with diagnosis of ATL include older age, younger age at HTLV-1 infection, duration of HTLV-1 infection (>20 years), family history of ATL, and higher HTLV-1 proviral load (276, 281, 285, 287–291, 294–299). Among cohort studies of people with HTLV-1 infection, no cases of ATL have been diagnosed in those with a baseline proviral load of fewer than 400 copies per 10,000 PBMCs (276, 291). In addition, cross-sectional and case-control studies have suggested that HLA type, HTLV-1 antibody titer, soluble interleukin-2 receptor (sIL2R) level, and Strongyloides stercoralis coinfection may be associated with ATL (276, 291, 295–299). sIL2R and HTLV-1 antibody titer are correlated with HTLV-1 proviral load, while Strongyloides stercoralis coinfection has been associated with higher HTLV-1 proviral load and oligoclonal expansion of infected T cells (298), which may shorten the time between HTLV-1 infection and ATL development.
HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis
HAM/TSP is a chronic inflammatory disease of the central nervous system, characterized by progressive spastic weakness of the lower limbs, lower back pain, and bowel and bladder dysfunction (300, 301). An association between HTLV-1 and a chronic progressive myelopathy of previously unknown etiology, “tropical spastic paraparesis” (TSP) (6, 302, 303), was noted in 1985, followed by the description of a comparable syndrome, “HTLV-1-associated myelopathy” (HAM), in Japan in 1986 (304). Recognizing that these were the same clinical entity, the WHO proposed standardized diagnostic criteria for HAM/TSP in 1988 (301). Detection of HTLV-1 in blood or cerebrospinal fluid (CSF) by serological or molecular methods is required to make the diagnosis. The defining pathological features of HAM/TSP are an inflammatory phase involving perivascular lymphocytic infiltration of the spinal cord (with relative sparing of the posterior columns), followed by scarring, atrophy, and neurodegeneration (193).
HAM/TSP should be considered in people with HTLV-1 who present with gait abnormalities, lumbar pain, and bladder, bowel, or sexual dysfunction. Specific neurological manifestations of HAM/TSP include a spastic gait, lower limb weakness, hyperreflexia, and clonus, as well as an extensor plantar response. Weakness of the lower limbs tends to be more marked proximally. Bowel and bladder symptoms (urinary incontinence or retention [early feature], constipation [usually a late feature]), and sexual dysfunction (impotence or decreased libido) are common. Sensory symptoms (including paraesthesia) may be present, and vibration sense impaired, but there should not be a discrete sensory level. Onset of HAM/TSP symptomatology is usually insidious but may be sudden. While symptoms are typically slowly progressive, a spectrum exists, from static to rapidly progressive. Findings on examination of CSF in support of a diagnosis of HAM/TSP may include a mild pleocytosis, a mild to moderate increase in protein concentration, and positive antibody and/or molecular tests for HTLV-1. Notably, a definite diagnosis of HAM/TSP may be difficult, particularly in settings with limited health care.
The cumulative lifetime risk of HAM/TSP among people with HTLV-1 infection has been estimated to be approximately 2%, with higher risk in women than men (305–307). However, there are contrasting geographic findings, with different methods of cohort recruitment and case finding, probable underdiagnosis, and wide confidence intervals (CIs) signaling uncertainty in the reported estimates. In a Caribbean study based on registry data, the estimated lifetime risk was 1.7% (1.3% in men and 1.8% in women) (306), whereas in a Japanese study based on passive case reporting, the estimated lifetime risk was 0.23% in those who had acquired infection in infancy (0.18% in men and 0.26% in woman), with even lower estimates among those infected at an older age (305). In two cohorts among people with HTLV-1, HAM/TSP incidence was 5.3 per 1,000 person-years (95% CI = 0.3 to 10.9) in Brazil (308) and 1.8 per 1,000 person-years (95% CI = 0.2 to 6.7) in the United States (307).
A higher HTLV-1 proviral load has been associated with the development (305–307, 309–314) and progression (315) of HAM/TSP. In a cohort study conducted in Martinique, higher HTLV-1 proviral load (>1,000 per 10,000 PBMCs) was associated with shorter time from HAM/TSP onset to being wheelchair dependent (315). Other factors that influence HTLV-1 proviral load, including HTLV-1-specific cytotoxic T-cell responses (316) and HLA type (314, 317), also appear to moderate the risk of HAM/TSP.
Older age (315) and markers of CSF inflammation (318, 319) have also been associated with progression. Markers of CSF inflammation, namely, CXCL10 and neopterin, may be useful for documenting disease activity, the risk of neurological disease progression, and potentially the response to treatment (318, 319).
HAM/TSP can result in substantial disability and reduced quality of life. The mental health and social impact of HAM/TSP appear to be considerable (320) but have not been systematically evaluated. Unlike ATL, HAM/TSP is not life-threatening, but like many severe progressive neurological conditions it may indirectly contribute to reduced life expectancy, particularly in resource-limited settings (315).
Other forms of neurological disease, which do not fulfil diagnostic criteria for HAM, may be underrecognized among adults and children with HTLV-1 infection (321–323). A spectrum of predominantly urinary and motor abnormalities without overt myelopathy (including leg weakness, impaired gait, hyperreflexia, impaired vibration sense, and neurogenic bladder) have been reported and appear to occur more frequently among people with HTLV-1 compared to those who are uninfected (175, 321, 322), although there has been a marked variation in frequency across study cohorts (5 to 50%) (175, 321, 322). Case reports suggest that such presentations can be precursors of HAM/TSP, but they may also remain as isolated syndromes.
HTLV-1-Associated Uveitis
In 1989, an association between HTLV-1 infection and eye disease was suggested, with initial case reports stemming from a region of HTLV-1 endemicity in southern Japan (324). HTLV-1-associated uveitis (HAU) was subsequently recognized as a specific syndrome following clinical, epidemiological, and laboratory investigation (325–330). Ocular pathogenesis appears to be related to lymphocyte-driven inflammation mediated by HTLV-1-infected CD4+ T cells (328, 329).
HAU should be considered in people with HTLV-1 who present with uveitis, particularly “intermediate uveitis” (localized to the vitreous and peripheral retina), after other etiologies have been excluded. HAU may occur in adults and children, is more likely in women, and can involve one or both eyes. Blurred vision and “floaters” are common presenting symptoms. Many cases involve a single episode of mild to moderate uveitis with spontaneous or corticosteroid-induced (topical or systemic) resolution in weeks. Recurrence and even sight-threatening complications are uncommon but can include retinochoroidal degeneration, glaucoma, and corticosteroid-induced cataracts (331–333).
Based on limited data, HAU appears to occur in less than 1% of people with HTLV-1 infection (334, 335). It can occur as an isolated condition or in association with other HTLV-1-related diseases, including ATL and HAM/TSP, which may precede or follow it (331–333). In the Japanese HAM-net cohort, the incidence of uveitis among people with HAM/TSP was 6.5 (95% CI = 3.3 to 12.7) per 1,000 person-years (335). As with ATL and HAM/TSP, HAU appears to be associated with higher HTLV-1 proviral load (336).
Infective Dermatitis
Infective dermatitis was the first, and so far only, pediatric syndrome to be linked to HTLV-1 infection (337). In 1966, a severe exudative eczema was described in Jamaican children and labeled “infective dermatitis” (338). An association with HTLV-1 was suggested decades later (337, 339) and led to the publication of diagnostic criteria for a specific HTLV-1 syndrome in 1998 (339), with revisions suggested in 2012 (340). Despite being initially described in children, cases have subsequently been diagnosed in adults. Similar to HAM/TSP and HAU, the pathophysiology of infective dermatitis appears to be characterized by an exacerbated Th1 immune response (341).
Essential clinical features include chronic relapsing dermatitis, generally involving the scalp and retroauricular skin, with prompt response to antibiotic treatment, and recurrence on discontinuation (see Box 1). In a case-control study comparing the clinical, pathological, and immunological features of infective dermatitis (n = 50) and atopic dermatitis (n = 35), all (100%) people with infective dermatitis had antibodies to HTLV-1 compared to 14% of those with atopic dermatitis (339). Other features were similar between groups, with frequent growth of Staphylococcus aureus and beta-hemolytic streptococci from involved skin and no distinguishing skin biopsy findings. As such, clinical assessment and diagnosis of HTLV-1 infection are required to differentiate infective dermatitis from other skin conditions, including atopic and seborrheic dermatitis (342).
BOX 1.
Diagnostic criteria for infective dermatitis
Erythematous, scaly, exudative, and crusted lesions on scalp, retro-auricular areas, neck, axillae, groin, paranasal and perioral skin, ears, chest or abdomen, with at least 3 involved sites.
Crusting around the nostrils.
Chronic relapsing clinical course with prompt response to antibiotics but recurrence on discontinuation.
Diagnosis of HTLV-1 infection (by serology or molecular testing).
Note: Items indicated in boldface are mandatory for diagnosis. Published by La Grenade et al. (339) and modified by de Oliveira et al. (340).
Minimal data exist on the incidence and burden of infective dermatitis or on factors that predict occurrence among children with HTLV-1. In one study from Jamaica, the risk of infective dermatitis was 2% by age 4 (343). Infective dermatitis has been associated with higher HTLV-1 proviral load (341) and diagnosis of ATL and HAM/TSP later in life (340, 341, 344–350). In a case series from Brazil (n = 42), 20 (48%) people with infective dermatitis were diagnosed with HAM/TSP as a child or adolescent and one with ATL as an adult (340, 351).
As with other diseases linked to HTLV-1, there is marked geographic variation in reporting. Whereas case series of infectious dermatitis have been published from Africa (342, 352), the Caribbean (338), and South America (340, 351), few cases have been reported in Japan. The reasons for this apparent regional difference are unclear, but lack of recognition and diagnosis may be an issue.
HTLV-1-Associated Pulmonary Disease
HTLV-1 infection has been associated with spectrum of chronic lung disease, including bronchiectasis, bronchitis, and bronchiolitis, across varied populations, geographic regions, and HTLV-1 subtypes (271). Lung disease among people with HTLV-1 infection was first reported in 1987, with lymphocytic alveolitis diagnosed in five people with HAM/TSP in Japan (353). Subsequently, radiological assessment of people with HTLV-1a infection in Japan demonstrated a variety of abnormalities, with the most frequent being features consistent with bronchiolitis, bronchiectasis, and interstitial pneumonia (354, 355). Correlation of radiological and histological features on lung biopsy was also noted, with lymphocyte infiltration along bronchioles and into bronchovascular bundles and the interstitium (355).
Over the last decade, clinical research among Aboriginal people in central Australia has shown that HTLV-1 was associated with radiologically confirmed bronchiectasis, bronchitis, and bronchiolitis in community and hospital-based cohorts (175, 356, 357). Notably, higher HTLV-1 proviral load was associated with diagnosis of bronchiectasis (175) and death due to bronchiectasis (356) in this population. The prevalence of bronchiectasis has been found to be substantially higher among a clinic-based cohort of people with HTLV-1 infection (3.4%) in the United Kingdom compared to the general population (0.1%) (358). The proposed pathophysiology of lung disease in HTLV-1 infection has been likened to that of HAM/TSP, driven by a chronic inflammatory process involving the lungs (359).
Considering evolving evidence, diagnostic criteria for a new entity, HTLV-1-associated pulmonary disease, have been proposed (359), including HTLV-1 infection, along with radiological evidence of bronchiolitis, bronchiectasis, or interstitial pneumonia, and exclusion of other causes of lung disease. Other criteria could include an HTLV-1 proviral load greater than 1,000 copies per 10,000 mononuclear cells in induced sputum or bronchoalveolar lavage fluid and peribronchiolar or interstitial lymphocyte infiltrate on lung biopsy. While these require validation, the framework would support further work in the area, particularly if considering evaluating potential pharmacological and non-pharmacological interventions. For broad clinical use, “definite” and “probable” categories may be required in the proposed diagnostic criteria, with use of high-resolution CT imaging, bronchoscopy, and quantitative HTLV-1 PCR included as supplementary rather than obligatory, since they may not be feasible in many regions where HTLV-1 is endemic.
Tuberculosis
Several studies have reported associations between HTLV-1 and tuberculosis (360–366). The one cohort study reported a relative risk of incident tuberculosis of 2.30 (95% CI = 1.60 to 4.10) among people with HTLV-1 (360), and a meta-analysis found a pooled odds ratio of 2.25 for the association (95% CI = 1.48 to 3.43) (271).
Strongyloides stercoralis Infection
Regions of endemicity overlap for HTLV-1 and Strongyloides stercoralis, with the natural history of each apparently modified by coinfection with the other (367). Strongyloides stercoralis infection is associated with higher proviral load in people with HTLV-1 and is a predictor of subsequent ATL (298). Conversely, strongyloidiasis in people with HTLV-1 is associated with a higher parasite burden and greater risk of dissemination and hyperinfection syndrome than in people who do not have HTLV-1 (368). Given the importance of a robust Th2 response in dealing with parasitic infections, the hyperinfection syndrome among people with HTLV-1 appears to be mediated by an aberrant response with an increase in regulatory T cells, decreased production of IL-5, and a reduced eosinophil number, with resultant downregulation of the host defense against the parasite (369).
Strongyloides screening is routine in some centers caring for people with HTLV-1 infection. Limitations to accurate diagnosis of strongyloidiasis include low sensitivity of stool microscopy, variable specificity of serology, and limited access in endemic settings to molecular testing, now considered to be the most reliable method. Conflicting findings on the association between HTLV-1 and uncomplicated strongyloidiasis may be due to differing diagnostic methods (175, 370).
Effectiveness of standard treatment for S. stercoralis infection may also be reduced in the presence of HTLV-1 infection. A systematic review and meta-analysis favored ivermectin over albendazole for efficacy and over thiabendazole for safety (371). The study populations included people with and without HTLV-1 but did not draw separate conclusions. Published evidence and clinical experience support the use of ivermectin at 200 μg/kg with the duration depending on the severity of the strongyloidiasis presentation among people with HTLV-1.
HTLV-1 Infection and All-Cause Mortality
HTLV-1 infection has been associated with a 60% increased risk of death from any cause, based on a meta-analysis of eight longitudinal studies (271). Consistency of the finding was high across geographic areas (273, 274, 372–378). Despite its lethality, ATL cannot account for this substantial increase in risk of death among people with HTLV-1 infection, nor can any other disease or combination of diseases. The spectrum of pathology associated with HTLV-1 may be broader than generally recognized. Relatively few studies have investigated the role of HTLV-1 proviral load as a predictor of disease progression and mortality. Proviral load and HTLV-1 antibody titer (which is correlated with proviral load [379]) predicted mortality in African (374) and Japanese (273) studies, respectively.
MANAGEMENT OF HTLV-1 INFECTION AND HTLV-1-ASSOCIATED DISEASE
Asymptomatic HTLV-1 Infection
There is no direct evidence that screening asymptomatic people for HTLV-1 infection can affect the outcome of their infection, and there is no intervention known to prevent the development of HTLV-1-associated disease. Nevertheless, there will be people who for various reasons, such as blood donation or contact tracing, are diagnosed with HTLV-1 infection in the absence of symptoms, so it is reasonable to consider what additional investigation and management might be of value.
Management may include periodic clinical and laboratory assessment to (i) monitor for development of ATL, HAM/TSP, and other diseases known to be associated with HTLV-1; (ii) screen for relevant comorbidities and coinfection such as Strongyloides stercoralis and tuberculosis; and (iii) assess the risk of other infections known to be transmitted by the same pathways as HTLV-1, including HIV, HBV, HCV, syphilis, Chlamydia trachomatis, and Neisseria gonorrhoea, depending on local epidemiology and transmission risk (see Box 2). Education and counselling may reduce the risk of onward transmission and could include information regarding modes and efficiency of transmission, prevention, disease associations, and the probability of developing diseases due to HTLV-1. People diagnosed with HTLV-1 infection should be made aware that infection is lifelong and that they should not donate blood or other tissue. HTLV-1 proviral load is the only known prognostic indicator and may help in assessing risk of HTLV-1-associated disease and transmission, but the test is largely unavailable for routine clinical use outside a few high-income settings.
BOX 2.
Assessment of people diagnosed with HTLV-1 infection
Initial assessment*
History and examination
History: Address patient concerns about the infection; identify household and sexual contacts who might require further testing; assess risk factors for acquisition; assess host factors that may be associated with an increased risk of HTLV-1-associated disease.
Examination: Including assessment of gait and skin.
Full blood count (including differential) and blood film.
Electrolytes urea and creatinine.
HTLV-1 proviral load (if available).
*Consider screening for relevant coinfection, including Strongyloides stercoralis, tuberculosis, HIV, HBV, and HCV. Consider screening for other sexually transmitted infections, including syphilis, Chlamydia trachomatis, and Neisseria gonorrhoea.
Follow-up assessment†
History and examination
Full blood count (including differential) and blood film
Electrolytes urea and creatinine
†Consider yearly follow-up for people with asymptomatic HTLV-1 infection. Timing of follow-up assessment is based on expert opinion. More frequent review may be clinically indicated.
Note: Clinical recommendations are based on the expert opinion and publications from the UK (NHS) and Australia (ASHM): https://www.england.nhs.uk/wp-content/uploads/2013/06/b07-human-t-cell-lympho.pdf and https://hivmanagement.ashm.org.au/human-t-cell-leukemia-virus-coinfection/.
Adult T-Cell Leukemia/Lymphoma
There is substantial variation across ATL subtypes regarding short-term prognosis and management, with the aggressive subtypes (acute and lymphomatous) having the worst prognosis. Other negative prognostic factors include poor ECOG (Eastern Cooperative Oncology Group) performance status at diagnosis, age over 40 years, extensive disease, hypercalcemia, a high serum lactate dehydrogenase, and relapsed or refractory disease of any subtype (380). However, regardless of subtype and despite therapeutic advances, overall long-term survival for people diagnosed with ATL remains poor.
International recommendations for the management of ATL were first published in 2009 (380) and then updated in 2019 (381). Depending on subtype and treatment history, management options could include interferon-based antiviral therapy (382–386), chemotherapy (387–396), biologic agents (397–400), and hematopoietic stem cell transplantation (401–408). Management strategies are also dictated by local availability of specific agents and therapeutic modalities. As first-line therapy, guidelines state that interferon alpha and zidovudine should be considered for people with symptomatic smoldering ATL, favorable or unfavorable chronic ATL, and acute ATL, while intensive multi-agent chemotherapy be considered for people with lymphoma-type ATL (381). More recent attention has focused on the use of biological agents, with or without chemotherapy (397–400). Mogamulizumab, a first-in-class humanized anti-CCR4 monoclonal antibody, has been evaluated in patients that are CCR4+ chemotherapy naive (398) or who have relapsed or refractory ATL (397, 399), with subsequent regulatory approval in multiple jurisdictions. While allogeneic hematopoietic stem cell transplantation can improve survival in aggressive ATL (406), most patients are ineligible due to age, the availability of a stem cell source, and the lack of an adequate response to primary therapy.
HTLV-1-Associated Myelopathy
No curative treatment for HAM/TSP is available. Options for management include medications aimed at altering the disease course (“disease-modifying therapy”) or relieving symptoms. The International Retrovirology Association published consensus guidelines for the management of HAM/TSP in 2020, with a focus on agents that may be disease modifying (409). Due to a dearth of published evidence, most recommendations in the guidelines are based on expert opinion (409).
Corticosteroids (including pulsed methylprednisolone for induction and oral prednisolone for maintenance) have been the mainstay of disease-modifying therapy. Pilot trials and cohort studies suggest that any benefit is transient and limited to improvements in pain and spasticity (410–414). Retrospective observational studies have been cited in support of oral prednisolone (410, 413–415). In one of these studies, which compared people with HAM/TSP who were (n = 57) and who were not (n = 29) treated with low-dose oral prednisolone (mean follow-up, 3.4 years), the overall annual mean change in Osame motor disability score (OMDS [a higher score means greater disability]) was −0.13 in the corticosteroid group and +0.12 in the untreated group (415). The HAMLET-P randomized controlled trial comparing prednisolone with placebo among people with HAM/TSP is in progress (416).
As with ATL, biological agents have been used in HAM/TSP. In an uncontrolled phase I/IIa trial (n = 21), the safety and efficacy of mogamulizumab was assessed among people with glucocorticoid-refractory HAM/TSP (417). In the phase I study, dose-dependent declines in CCR4+ T cells, HTLV-1 proviral load, and CSF inflammatory markers (CXCL10, neopterin) were seen. In the phase IIa extension (n = 19), both HTLV-1 proviral load and CSF neopterin concentration had fallen by nearly 50% at week 24, and clinical measures improved, particularly in those with shorter duration of disease (<10 years) and mild disability (OMDS <5).
Given the low incidence of HAM/TSP and much of its occurrence being in resource-limited settings, there are substantial challenges in developing a robust evidence base to standardize clinical trial assessment and reporting, and guide management. The International Retrovirology Association has recommended that management of people with HAM/TSP be guided by speed of disease progression (409), highlighting the varied natural history of HAM/TSP. Given the uncertainties about treatment options, all patients with HAM/TSP should be considered for enrollment in a clinical trial, if available, regardless of severity and duration of disease.
EPIDEMIOLOGICAL AND CLINICAL COMPARISON OF HTLV-1 AND HTLV-2
Both HTLV-1 and HTLV-2 are retroviruses that integrate into T lymphocytes and cause lifelong infection. They share the same routes of transmission and increasing prevalence with age and have geographic distributions that are at once dispersed and focal, with some overlap. However, they differ in some key epidemiologic and clinical respects. HTLV-2 is endemic among a large number of Indigenous populations in North America and South America, and unlike HTLV-1, is prevalent in urban areas among people who inject drugs, with high prevalence detected in this population in several European, South-East Asian, and North American cities (418–422). High prevalence in people who inject drugs is likely the result of direct blood-to-blood contact via reuse of syringes and needles. It remains unclear why HTLV-1 is not also found at high prevalence among these populations despite common modes of transmission (423).
Unlike HTLV-1, HTLV-2 has not been shown to cause malignancy, but myelopathy and other neurological manifestations can occur, albeit less frequently than with HTLV-1 (1% versus 4% in a prospective cohort) (307, 321). The natural history of myelopathy may also differ, with HTLV-2 cases presenting with more mild, slowly progressive signs and symptoms (307). As with HTLV-1, a range of neurological symptoms related predominantly to lower limb motor and bladder function are more common among people with HTLV-2 than in the general population (321). HTLV-2 infection has been associated with an increase in all-cause mortality (373) and additional under-recognized health consequences (424), though the evidence is more limited than for HTLV-1 and is largely drawn from a blood donor cohort in the United States. There is limited evidence to support an association between HTLV-2 infection and acute bronchitis (425), and there are insufficient data to comment on an association with chronic lung disease (426).
PREVENTION OF HTLV-1 TRANSMISSION
At the WHO Global Consultation in 2019, a key recommendation was that policies for prevention of sexually transmitted infection and blood borne viral infections be adapted to incorporate HTLV-1. This recommendation effectively echoed the first WHO statement on HTLV-1 prevention in 1992, (427), which proposed consideration of HTLV-1 preventive measures related to breastfeeding, injecting drug use, and sex.
Evidence for HTLV-1 prevention measures comes principally from observational studies originally conducted to investigate specific transmission pathways. There has been very little prospective evaluation of preventive interventions at the population level. Development of a vaccine would be ideal and is considered biologically feasible given low antigenic variability. HTLV-1 proteins have been tested as vaccine immunogens, but preclinical evaluation is hampered by the selection of a representative animal model (428–432). No candidate vaccine has progressed to late-stage clinical trials (432).
Japan is the only country to have implemented a national policy on HTLV-1 prevention. Other countries have a policy on one or more of the interventions aimed at preventing maternal HTLV-1 transmission and transmission through donated blood or tissue. Many countries have policies related to preventing sexually transmitted and blood-borne infections that contain elements likely to reduce the risk of transmitting or acquiring HTLV-1 infection. Specifically, condom use is likely to reduce sexual transmission risk, and the use of sterile injecting equipment is likely to reduce the likelihood of transmission related to injected-drug use. There do not appear to be any policies or guidelines specifically aimed at the prevention of HTLV-1 infection through sexual exposure or injecting drug use.
Preventing Mother-to-Child Transmission
Duration of breast feeding.
Several investigations have demonstrated that shortening the duration of breastfeeding or eliminating it altogether substantially lowered the risk of mother-to-child transmission of HTLV-1 infection. These studies, predominantly from Japan, include a 20-year evaluation showing that the prevalence of HTLV-1 infection among formula-fed children was significantly lower than that in breastfed children (244) (Table 6). Among breastfed children, the prevalence was much lower among children who fed for less than 6 months than among those fed longer (≥6 months). These results conclusively confirmed findings from earlier evaluations (235, 240, 241, 243, 433).
TABLE 6.
Prevalence of HTLV-1 among children born to women with HTLV-1 infection, by feeding method
| Study | Country of research | Study design | Child age at survey (yrs) | No./total no. (%) |
OR (95% CI) | |
|---|---|---|---|---|---|---|
| Breast fed | Formula fed | |||||
| Ando et al., 1989 (433) | Japan | Prospective cohort | 2 | 24/31 (77.4) | 1/30 (3.3) | 4.3 (1.6–11.2) |
| Tsuji et al., 1990 (235) | Japan | Retrospective cohort | 1–13 | 17/44 (39) | 0/10 (0) | Unable to calculate |
| Takahashi et al., 1991 (241) | Japan | Retrospective and prospective cohort | 1–25 | 24/229 (10.0) | 9/158 (5.7) | 2.0 (0.9–5.1) |
| Hirata et al., 1992 (240) | Japan | Retrospective and prospective cohort | 0–19 (mean, 5.5) | 18/97 (18.6) | 10/78 (12.8) | 1.5 (0.6–4.0) |
| Takezaki et al., 1997 (243) | Japan | Prospective cohort | 0–3 | 15/115 (13.0) | 4/162 (2.5) | 5.9 (1.8–25.1) |
| Hino et al., 2011 (244) | Japan | Retrospective and prospective cohort | NAa | 89/567 (15.7) | 29/1152 (2.5) | 7.2 (4.6–11.5) |
NA; not applicable.
Any consideration of reduced duration of breastfeeding must take into account the numerous benefits of breastfeeding to both the infant and the mother (434). The balance must be weighed particularly carefully in contexts where safe alternatives to breastfeeding are not readily available, even in settings of high HTLV-1 prevalence.
Freeze-thaw method.
Freezing and thawing breastmilk disrupts the morphology of and results in a loss of function of cells contained in breastmilk, and has been demonstrated to impair the viability and infectivity of HTLV-1 antigen positive cells (435). Once thawed, the risk of HTLV-1 transmission from the breastmilk of seropositive mothers is substantially reduced. In a 12-month follow-up of 13 infants given exclusively frozen-thawed breast milk, none acquired HTLV-1 infection (436). This method relies on individual access to a high level of technology so not be feasible in low-resource settings.
Policies and guidelines: prevention of mother-to-child transmission.
Policies aimed at the prevention of mother-to-child HTLV-1 transmission involve screening women for HTLV-1 during pregnancy and then recommending a shortened duration of breastfeeding—or its avoidance altogether—in those found to have HTLV-1 infection. Japan is the only country that has implemented a nationwide program based on these principles (244, 437). All women are offered HTLV-1 testing in pregnancy, and those found to have infection are encouraged and supported to either formula feed their newborn, breastfeed for a shorter term (up to 3 months), or feed with thawed frozen milk.
National or local policies relevant to prevention of mother-to-child transmission have also been developed or are in development in other countries, including Australia, Brazil, Chile, France, and the United Kingdom (228, 438–440). Policies and recommendations differ, tailored to local epidemiology and infrastructure. Antenatal HTLV-1 screening is recommended in some regions of Brazil (438, 441). In France, screening is recommended for pregnant women and milk donors born in endemic regions (the Caribbean, Africa, Japan, South-East Asia), while the United Kingdom recommends screening of all breast milk donations. Universal antenatal screening has not been recommended outside Japan, and all available policies state that decisions regarding breastfeeding are in the hands of individual women, who should be provided with information tailored to local circumstances, including socioeconomic factors and health infrastructure at a community level and, at the individual level, HTLV-1-associated conditions, and HTLV-1 proviral load if available).
Preventing Transmission through Screening of Blood and Tissue
Serological assays for HTLV-1 screening became available in the middle to late 1980s and, by the mid-1990s, HTLV-1 screening of blood donors was implemented in many high-income countries and countries where HTLV-1 is endemic (223). Blood donor screening policies include testing of donations for the presence of HTLV-1, discarding those found positive; deferral or exclusion of people whose history provides evidence they are at higher risk of HTLV-1 acquisition; or technologies, such as leucoreduction, that remove infectious organisms, including viral particles in the process of manufacturing plasma-derived blood products. Studies from Japan and the United States in the late 1980s and early 1990s showed that universal antibody screening of blood donors reduced the rate of HTLV-1 seroconversion among whole-blood recipients (442–444) (Table 7).
TABLE 7.
Impact of screening on HTLV-1 transmission through blood products
| Study | Country of research | Study design | HTLV-1 screening | Prior to implementation of donor screening (%) | Postimplementation of donor screening (%) |
|---|---|---|---|---|---|
| Kamihira et al., 1987 (442) | Japan | Before and after comparison | PA | 53.6 | 0.9 (n = 1)a |
| Nelson et al., 1992 (443) | United States | Before and after comparison | ELISA and confirmatory WB/RIPA | 0.0039 (n = 2) | 0.0 |
| Inaba et al., 1999 (444) | Japan | Prospective cohort | Second-generation PA | 0.021 (n = 1)b |
One seroconversion occurred due to blood being used from a different donation center that did not screen.
Follow-up of patient failed to confirm whether or not seroconversion was due to transfusion.
In low-prevalence settings, there is ongoing debate regarding the cost-effectiveness of universal screening (222). Prinsze and colleagues note that there are substantial gaps in knowledge regarding screening strategies, as well as contradictory findings from the limited available research (445). A study from the United Kingdom used a lookback method to review outcomes in people receiving blood components from HTLV-positive donors (446) and found that filter-leukoreduced or buffy coat-reduced blood products effectively reduced the risk of HTLV infection. Buffy coat-reduced products made up the minority (14%) of the total, and should not be equated with filter reduction, since this approach is known to be less effective. Because HTLV-1 is almost always cell associated, leukoreduction may be as effective as blood donation screening (445).
POLICIES AND GUIDELINES: SCREENING OF BLOOD AND TISSUE DONATION
Mandatory antibody screening against HTLV-1 for all blood donations has been implemented in 23 countries (Table 8). The first were Japan and the United States, both in 1988 (447), followed by Canada, Caribbean nations (Dominican Republic, Haiti, and Jamaica), and French overseas departments (French Guiana, Guadeloupe, and Martinique) in 1989. For Argentina, China, Iran, and Venezuela, mandatory screening was implemented in areas designated endemic for HTLV-1. First-time donors are screened in Denmark, France, Guadeloupe, Martinique, Portugal, and Sweden, and while Norway adopted a similar policy in 2000, first-time donor screening was ceased due to the absence of positive cases in 2007 (448). In the African Region, Gabon appears to be the only country conducting routine HTLV-1 screening of all blood donations at the National Center for Blood Transfusion; however, there is no formal national screening policy or guideline.
TABLE 8.
Countries and territories with policies or guidelines on screening of blood donations for HTLV-1a
| WHO regionb | All donations | First-time donors only | Specific areas | Leucoreductionc |
|---|---|---|---|---|
| Africa region | Gabon (only at the National Centre for Blood Transfusion [no national formal policy]) | |||
| Eastern Mediterranean region | Saudi Arabia (469) | Iran (Khorasan) (470) | ||
| Europe regiond | French Guiana, Greece, Ireland, Israel, Netherlands, Romania, United Kingdom (222, 448, 471) | Denmark, Finland, France, Guadalupe, Martinique, Portugal, Sweden (27, 108, 448, 472) | Austria, Belgium, Czech Republic, Finland, France, Germany, Greece, Ireland, Italy, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain (222, 448, 471–474) | |
| Region of the Americas | Brazil, Canada, Chile, Colombia, Dominican Republic, Haiti, Jamaica, Peru, USA, Uruguay (208, 226, 228, 438, 475–482) | Argentina, Venezuela (438, 483) | ||
| Western Pacific region | Australia, China (Taiwan), Japan, New Zealand (163, 222, 447, 455, 484) | China (222, 484) |
Adapted from reference 7 (published under a CC BY-NC-SA 3.0 IGO license).
No information was available for the South-East Asia region.
All blood or cellular components, or on medical request.
Includes overseas departments of France: French Guiana, Guadalupe, Martinique.
For organ transplant and donation, few policies and guidelines for HTLV-1 screening exist, with no global consensus. In the United States, mandatory screening of deceased organ donors was eliminated in 2009 due to the presumed very high false-positive rate in this low-prevalence setting, leading to organ donor wastage (449, 450). Current guidance nevertheless recognizes that potential health issues are associated with HTLV-1 in organ donation (450). In 2012, a directive adopted by the European Commission required HTLV-1 testing of all donors of cells and tissues from high-prevalence areas for HTLV-1 infection or those whose sexual partners or parents come from such areas (451). The United Kingdom requires that all donors of tissues and cells be tested for HTLV-1 at the time of donation (452). In Spain, screening for HTLV-1 in organ and tissue donors from endemic countries is recommended, as well as for individuals with sex partners or parents from high-prevalence regions.
DISCUSSION AND FUTURE DIRECTIONS FOR RESEARCH AND PRACTICE
Recent advocacy from concerned researchers, clinicians and community members emphasized the potential for improved prevention and management of HTLV-1 infection (453). It is also clear that, despite all that has been learnt in the 4 decades following the discovery of HTLV-1, major gaps in knowledge across clinical and public health aspects of HTLV-1 need to be filled to ensure optimal control and prevention and inform policy and guideline development. The WHO consultation in late 2019 was the first global forum to consider recommendations on public health aspects of HTLV-1 and has already led to policy shifts in addressing this ancient human infectious disease (7, 8)
Robust surveillance data are needed to direct policy, service implementation, and health system investment. On the best available evidence, the geographic distribution of HTLV-1 infection remains highly focal, but prevalence in some highly populous countries has not been well characterized by surveys, making it difficult to estimate the global burden of infection beyond recognized areas of endemicity. Reports of ATL and HAM/TSP in heavily populated countries or regions with little to no HTLV-1 prevalence data highlights a requirement for wider tracking. In addition, knowledge gaps persist on prevalence variations within endemic regions and on prevalence among subpopulations that may be at higher risk, including men who have sex with men and people who inject drugs. Countries can build on existing surveillance networks (which may include making HTLV-1 nationally notifiable) or build new ones to gather reliable epidemiological data, allowing more accurate assessment of the burden of infection and related morbidity and mortality.
Cost-effective HTLV-1 testing approaches and strategies tailored to local infection patterns and settings, as well as the purpose of testing, are needed, particularly in low- and middle-income countries. Current commercially available serological assays have high sensitivity and specificity and are optimally used when combined for diagnosis. Molecular tests, with even better performance, are also available, but broad uptake is constrained by lack of commercial production and limited access outside high-income settings. Transitioning from the “gold standard” Western blot to the use of INNO-LIA or PCR for confirmatory testing is conceptually appealing but comes with cost and health system implications (214). Complementary to improving access to HTLV-1 testing is the need for clearer guidance on testing and for whom it should be offered. Populations who have been considered include (i) people attending sexual health clinics, (ii) donors and recipients of blood or tissue, (iii) both pregnant women and children born to women with HTLV-1, and (iv) people who inject drugs (453). Clinical consensus and guideline development focused on HTLV-1 testing, including who should be offered testing and how to communicate test results, will be of benefit to clinicians, policy makers and, most important, people and communities at risk of HTLV-1.
There is no biomedical prevention intervention currently available for HTLV-1 infection. A vaccine would be ideal but has never progressed beyond early preclinical development. Modes of HTLV-1 transmission are well established, but additional information is required to optimize prevention strategies. While breastfeeding duration has been associated with the risk of mother-to-child transmission, decisions regarding cessation or shorter duration of breastfeeding must be weighed against the numerous demonstrated benefits of breastfeeding to both the infant and mother, particularly in settings where safe alternatives are not readily available. The role of sexual contact in sustaining transmission in endemic settings is suggested by the clearly increasing HTLV-1 prevalence with age (87, 102, 252, 253, 258, 261, 262). More-detailed analyses of age-specific patterns of prevalence, starting in childhood and covering all adult age groups, will help to clarify the role of sexual transmission in different settings. At a biological level, higher proviral load is a well-established risk factor for transmission, but it is at present not known whether a person with a low or undetectable proviral load represents a transmission risk to sexual partners. HTLV-1-specific prevention strategies are at present limited to screening of blood donations, generally only in high-income countries and, in regions of endemicity in a small number of countries, offering testing to pregnant women followed by counselling to limit breastfeeding for those found to have HTLV-1 infection. Existing public health interventions related to the prevention of blood-borne viruses and sexually transmitted infections, including safe sex promotion and harm reduction strategies for drug injecting, are likely to be effective in reducing the risk of HTLV-1 transmission.
The burden of diseases attributed to HTLV-1 appears to be highly variable across affected populations. The reasons for apparent regional differences are unclear but lack of recognition, and diagnosis may be an issue, highlighting the need for education and testing in the appropriate clinical context. Beyond ATL and HAM/TSP, HTLV-1 infection may have other substantial negative health impacts that are either unknown or under acknowledged, as suggested by the substantial increase in all-cause mortality observed among people with HTLV-1 across three continents. There is a lack of systematically collected information on HTLV-1 infection and related diseases, with few examples of directed initiatives (454, 455). Although many countries have cancer registries, most coding systems do not distinguish ATL as a distinct entity. Japan has specific coding for ATL and HAM/TSP and reports the diseases in separate national registries. International collaboration will provide the basis for better understanding the apparent geographic differences in disease development and foster clinical research networks that can be used to investigate new therapeutic options.
Awareness of HTLV-1 among health care providers, communities, and affected individuals remains limited, even in countries of endemicity. Placing HLTV-1 firmly on the global health agenda and linking where appropriate to control strategies and research for other infectious diseases will advance the health and wellbeing of people living with and at risk for HTLV-1 infection.
ACKNOWLEDGMENTS
We thank Catherine de Martel for providing important ATL data and figures presented in this review.
We declare no conflicts of interest.
Biographies

Nicolas Legrand is a research epidemiologist and Ph.D. candidate at the Kirby Institute for Infection and Immunity in Society, UNSW Sydney. He holds a Bachelor of Arts and Sciences (Hons) from the University of Sydney, a Master of Public Health specializing in Infectious Disease Epidemiology and Control, and a Master of International Public Health from UNSW Sydney. Prior to joining the Kirby Institute in 2018, he worked in molecular biology research laboratories at the University of Sydney School of Medicine and completed an internship with the World Health Organization in the Philippines Country Office. He is an investigator on an Australian HTLV-1 surveillance study and on an HTLV-1 longitudinal cohort study among remote Aboriginal communities in Central Australia. His research interests include health equity and enhancing national surveillance of infectious diseases among priority populations.

Skye McGregor is an epidemiologist and Research Fellow at the Kirby Institute, UNSW Sydney. She holds a Bachelor of Science (2004), a Bachelor of Arts (2004), a Master of Arts (2008), and a Ph.D. in epidemiology (2015) from UNSW Sydney. Her research centers on the surveillance and prevention of sexually transmissible infections and blood borne viruses, with a particular focus on priority populations in the Australian context, including people living with HIV, gay and bisexual men, people from culturally and linguistically diverse backgrounds, and Aboriginal and Torres Strait Islander people.

Rowena Bull completed a Ph.D. in 2008 from University of New South Wales (UNSW) in Molecular Virology. A/Prof Bull was then awarded a NHMRC Early Career Fellowship at the School of Medical Sciences, UNSW, in 2010. A/Prof Bull currently holds a permanent academic position in the School of Medical Sciences at UNSW and a NHMRC Investigator award. A/Prof Bull has been investigating, since 2003, the viral and host factors that different RNA viruses use to generate diversity and maintain their survival in the population. She takes an interdisciplinary approach involving cutting edge virological, immunological, and bioinformatic techniques. Her contribution to virology ranges from the development of virological in vitro-based fitness assays, viral gene expression studies, molecular epidemiology, evolutionary analysis of adaptive immune responses against RNA viruses, and bioinformatics.

Sahar Bajis is Research Fellow at the Kirby Institute, UNSW Sydney. She completed her doctoral studies in medicine (clinical epidemiology) at the Kirby Institute. She holds a master’s degree in International Public Health from the University of Sydney and a Bachelor of Pharmacy degree from Curtin University, Australia. Her research interests in the field of infectious diseases epidemiology and public health include the areas of HTLV-1 infection, viral hepatitis, antimicrobial use and COVID-19. Dr. Bajis is an investigator on an Australia-first longitudinal cohort study aimed to evaluate the impact of HTLV-1 infection on morbidity and mortality and health service utilization among remote Aboriginal communities in Central Australia. She is also an investigator on a HTLV-1 surveillance study in Australia. She is a reviewer and writer of the WHO Human T-Lymphotropic Virus Type 1: Technical Report, 2020.

Braulio Mark Valencia, M.D., M.Sc., is an infectious diseases specialist and Ph.D. student majoring in human immunogenetics of infectious diseases and virology at the Kirby Institute, The University of New South Wales (UNSW). In 2018, he joined the Viral and Immunology Research Program (VISP) at the Kirby Institute as a high-degree researcher supported by the UNSW Scientia Ph.D. Scholarship Scheme. His research focuses on understanding the human genetic determinants of severe infections. His interest aims to translate cutting-edge technology into the understanding of the pathobiology, diagnosis, treatment, and prevention of neglected tropical diseases. He has published 24 peer-reviewed papers in diverse aspects of American tegumentary leishmaniasis and the severity of viral infections. He is an active peer reviewer of journals in genetics, immunology, parasitology, and infectious diseases and is passionate about medical education.

Amrita Ronnachit is an Infectious Diseases Physician who studied at the University of NSW, the University of Sydney, and the Gorgas Institute to complete her undergraduate medical training, as well as a Masters of Public Health, Masters of Infectious Diseases Intelligence, and Diploma of Tropical Medicine and Hygiene. She currently holds positions as a staff specialist at Royal Prince Alfred Hospital in Sydney Australia, as well as a senior technical advisor in health emergencies at The Burnet Institute in Melbourne, Australia. She has an interest in health equity and global public health.

Lloyd Einsiedel, B.M., B.S. (Honorary), Ph.D., FRACP, is a specialist in internal medicine and infectious diseases who studied at the Faculties of Medicine of Flinders University, Adelaide, and Monash University, Melbourne, Australia. Following early work on the neurovirology of HIV at the Burnet Institute, Melbourne, he moved to central Australia in 2005. He has extensive research experience in remote Australia where, as the executive director of Baker Heart and Diabetes Institute central Australia, he has been principal investigator of several multidisciplinary projects. His clinical experience in a region with the highest worldwide prevalence of HTLV-1 led to a particular interest in the epidemiology and clinical manifestations of HTLV-1. In collaboration with local and international researchers he continues to work to improve our understanding of the pathophysiological basis of HTLV-1-associated diseases.

Antoine Gessain, M.D., Ph.D., is a medical virologist who studied in Paris. In 1983 and 1984, he participated in field epidemiological studies on the human retrovirus HTLV-1 and associated diseases in the French West Indies, French Guyana, and western Africa. After his medical residency, he spent two years in the R. C. Gallo laboratory in Bethesda, MD. In 1991, he took up a position at the Institut Pasteur (G. de Thé, then L. Montagnier Units), where he is now Chief of the Epidemiology and Physiopathology of Oncogenic viruses unit. Most of his scientific achievements concern the clinical, epidemiological, and genetic variability features of HTLV-1 and HTLV-3 and their simian counterparts (STLV-1/STLV-3). Furthermore, he is recognized for works on HHV-8 and the associated tumors. Antoine Gessain has been the principal investigator of several long-term multidisciplinary projects including both laboratory studies and field work. He has published more than 400 publications (15,000 citations, H index of 62).

John Kaldor is a Scientia professor and an NHMRC senior principal research fellow at the Kirby Institute, University of New South Wales, Sydney. He has devoted more than 35 years to research into disease prevention and public health, with a primary focus on infectious diseases. After his doctoral studies in biostatistics (1982), he worked in cancer epidemiology at the International Agency for Research on Cancer in Lyon, France. In 1989, he returned to Australia and took up a research appointment at the Kirby Institute. He has been with Kirby Institute since then and is largely responsible for initiating and developing all areas of epidemiology and population health at the institute

Marianne Martinello is an infectious diseases physician at Prince of Wales Hospital and St Vincent’s Health Network Sydney and is a senior research fellow at the Kirby Institute, UNSW Sydney. She holds an Australian National Health and Medical Research Council fellowship. After completing a M.B.B.S. at the University of Adelaide (2006), she received her fellowship in adult medicine (infectious diseases) from the Royal Australia College of Physicians (2014) and Ph.D. in medicine from UNSW (2017). Her clinical and research interests have sought to optimize the diagnosis, management, and prevention of infectious diseases, particularly blood-borne viruses, among vulnerable populations, including people who inject drugs, gay and bisexual men, people who are incarcerated, and Aboriginal and Torres Strait Islander peoples.
REFERENCES
- 1.Gessain A, Cassar O. 2012. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 3:388. 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. 1984. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA 81:2534–2537. 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popovic M, Lange-Wantzin G, Sarin PS, Mann D, Gallo RC. 1983. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci USA 80:5402–5406. 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. 1981. Type C virus particles in a cord T-cell line derived by cocultivating normal human cord leukocytes and human leukemic T cells. Nature 294:770–771. 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 5.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, Shirakawa S, Miyoshi I. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA 78:6476–6480. 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de The G. 1985. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet 2:407–410. 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. 2021. Human T-lymphotropic virus type 1: technical report. License CC BY-NC-SA 3.0 IGO. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 8.World Health Organization. 2021. Public health impact and implications for future actions: WHO global consultation on the human T-lymphotropic virus type 1, Tokyo, Japan, 13–15 November 2019. https://apps.who.int/iris/handle/10665/339886. License CC BY-NC-SA 3.0 IGO. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 9.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481–492. 10.1182/blood.V50.3.481.481. [DOI] [PubMed] [Google Scholar]
- 10.Poiesz B, Ruscetti FW, Gazdar AF, Bunn PA, Jr, Minna JD, Gallo R. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA 77:7415–7419. 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control. 2015. Geographical distribution of areas with a high prevalence of HTLV-1 infection. ECDC, Stockholm, Sweden. [Google Scholar]
- 12.Filippone C, Betsem E, Tortevoye P, Cassar O, Bassot S, Froment A, Fontanet A, Gessain A. 2015. A severe bite from a nonhuman primate is a major risk factor for HTLV-1 infection in hunters from Central Africa. Clin Infect Dis 60:1667–1676. 10.1093/cid/civ145. [DOI] [PubMed] [Google Scholar]
- 13.Sagara Y, Iwanaga M, Morita M, Sagara Y, Nakamura H, Hirayama H, Irita K. 2018. Fine-scale geographic clustering pattern of human T-cell leukemia virus type 1 infection among blood donors in Kyushu-Okinawa, Japan. J Med Virol 90:1658–1665. 10.1002/jmv.25239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki S, Tanaka M, Matsuda H, Tsukahara Y, Kuribayashi Y, Gomibuchi H, Miyazaki R, Kamiya N, Nakai A, Kinoshita K, Japan Association of Gynecologists. 2014. Current status of HTLV-1 carrier in Japanese pregnant women. J Matern Fetal Neonatal Med 27:312–313. 10.3109/14767058.2013.814631. [DOI] [PubMed] [Google Scholar]
- 15.Satake M, Yamaguchi K, Tadokoro K. 2012. Current prevalence of HTLV-1 in Japan as determined by screening of blood donors. J Med Virol 84:327–335. 10.1002/jmv.23181. [DOI] [PubMed] [Google Scholar]
- 16.Karimi G, Zadsar M, Pourfathollah AA. 2017. Seroprevalence and geographical distribution of human T-lymphotropic virus type 1 among volunteer blood donors in endemic areas of Iran. Virol J 14:14. 10.1186/s12985-017-0693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safabakhsh H, Jalalian M, Karimi G. 2014. Seroepidemiology of human T-cell lymphotropic virus type-1 (HTLV-1) in Mashhad. Glob J Health Sci 6:99–104. 10.5539/gjhs.v6n5p99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafatpanah H, Hedayati-Moghaddam MR, Fathimoghadam F, Bidkhori HR, Shamsian SK, Ahmadi S, Sohgandi L, Azarpazhooh MR, Rezaee SA, Farid R, Bazarbachi A. 2011. High prevalence of HTLV-I infection in Mashhad, Northeast Iran: a population-based seroepidemiology survey. J Clin Virol 52:172–176. 10.1016/j.jcv.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Alva IE, Orellana ER, Blas MM, Bernabe-Ortiz A, Cotrina A, Chiappe M, Kochel TJ, Carcamo CP, Garcia PJ, Zunt JR, Buffardi AL, Montano SM. 2012. HTLV-1 and -2 infections among 10 indigenous groups in the Peruvian Amazon. Am J Trop Med Hyg 87:954–956. 10.4269/ajtmh.2012.12-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trenchi A, Gastaldello R, Balangero M, Irizar M, Cudolá A, Gallego S. 2007. Retrospective study of the prevalence of human T-cell lymphotropic virus-type 1/2, HIV, and HBV in pregnant women in Argentina. J Med Virol 79:1974–1978. 10.1002/jmv.21027. [DOI] [PubMed] [Google Scholar]
- 21.Nunes D, Boa-Sorte N, Grassi MFR, Pimentel K, Teixeira MG, Barreto ML, Dourado I, Galvao-Castro B. 2015. Evidence of a predominance of sexual transmission of HTLV-1 in Salvador, the city with the highest prevalence in Brazil. Retrovirology 12. 10.1186/1742-4690-12-S1-O3. [DOI] [Google Scholar]
- 22.Mello MAG, da Conceição AF, Sousa SMB, Alcântara LC, Marin LJ, Regina da Silva Raiol M, Boa-Sorte N, Santos LPS, de Almeida M, Galvão TC, Bastos RG, Lázaro N, Galvão-Castro B, Gadelha SR. 2014. HTLV-1 in pregnant women from the Southern Bahia, Brazil: a neglected condition despite the high prevalence. Virol J 11:28. 10.1186/1743-422X-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters AA, Coulthart MB, Oger JJ, Waters DJ, Crandall KA, Baumgartner AA, Ward RH, Dekaban GA. 2000. HTLV type I/II in British Columbia Amerindians: a seroprevalence study and sequence characterization of an HTLV type IIa isolate. AIDS Res Hum Retroviruses 16:883–892. 10.1089/08892220050042828. [DOI] [PubMed] [Google Scholar]
- 24.San Martin H, Balanda M, Vergara N, Valenzuela MA, Cartier L, Ayala S, Ramirez E. 2016. Human T-lymphotropic virus type 1 and 2 seroprevalence among first-time blood donors in Chile, 2011–2013. J Med Virol 88:1067–1075. 10.1002/jmv.24428. [DOI] [PubMed] [Google Scholar]
- 25.Trujillo JM, Concha M, Munoz A, Bergonzoli G, Mora C, Borrero I, Gibbs CJ, Jr, Arango C. 1992. Seroprevalence and cofactors of HTLV-I infection in Tumaco, Colombia. AIDS Res Hum Retroviruses 8:651–657. 10.1089/aid.1992.8.651. [DOI] [PubMed] [Google Scholar]
- 26.Tortevoye P, Tuppin P, Peneau C, Carles G, Gessain A. 2000. Decrease of human T-cell lymphotropic virus type I prevalence and low incidence among pregnant women from a high endemic ethnic group in French Guiana. Int J Cancer 87:534–538. . [DOI] [PubMed] [Google Scholar]
- 27.Pouliquen JF, Hardy L, Lavergne A, Kafiludine E, Kazanji M. 2004. High seroprevalence of human T-cell lymphotropic virus type 1 in blood donors in Guyana and molecular and phylogenetic analysis of new strains in the Guyana shelf (Guyana, Suriname, and French Guiana). J Clin Microbiol 42:2020–2026. 10.1128/JCM.42.5.2020-2026.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allain JP, Hodges W, Einstein MH, Geisler J, Neilly C, Delaney S, Hodges B, Lee H. 1992. Antibody to HIV-1, HTLV-I, and HCV in three populations of rural Haitians. J Acquir Immune Defic Syndr (1988) 5:1230–1236. [PubMed] [Google Scholar]
- 29.Murphy EL, Figueroa JP, Gibbs WN, Holding-Cobham M, Cranston B, Malley K, Bodner AJ, Alexander SS, Blattner WA. 1991. Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica. I. Demographic determinants. Am J Epidemiol 133:1114–1124. 10.1093/oxfordjournals.aje.a115824. [DOI] [PubMed] [Google Scholar]
- 30.Daisley H, Charles W, Landeau P, Jackman L, Batson M, Gomez-Adams K. 1991. Screening for HTLV-I in healthy blood donors in Trinidad and Tobago. Trop Med Parasitol 42:404–406. [PubMed] [Google Scholar]
- 31.Rouet F, Foucher C, Rabier M, Gawronski I, Taverne D, Chancerel B, Casman O, Strobel M. 1999. Human T-lymphotropic virus type I among blood donors from Guadeloupe: donation, demographic, and biologic characteristics. Transfusion 39:639–644. 10.1046/j.1537-2995.1999.39060639.x. [DOI] [PubMed] [Google Scholar]
- 32.Cassar O, Charavay F, Touzain F, Jeannin P, Grangeon JP, Laumond S, Chungue E, Martin PM, Gessain A. 2017. A novel human T-lymphotropic virus type 1c molecular variant in an indigenous individual from New Caledonia, Melanesia. PLoS Negl Trop Dis 11:e0005278. 10.1371/journal.pntd.0005278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furusyo N, Hayashi J, Kakuda K, Sawayama Y, Ariyama I, Eddie R, Kashiwagi S. 1999. Markedly high seroprevalence of hepatitis B virus infection in comparison to hepatitis C virus and human T lymphotropic virus type-1 infections in selected Solomon Islands populations. Am J Trop Med Hyg 61:85–91. 10.4269/ajtmh.1999.61.85. [DOI] [PubMed] [Google Scholar]
- 34.Yanagihara R, Jenkins CL, Alexander SS, Mora CA, Garruto RM. 1990. Human T lymphotropic virus type I infection in Papua New Guinea: high prevalence among the Hagahai confirmed by western analysis. J Infect Dis 162:649–654. 10.1093/infdis/162.3.649. [DOI] [PubMed] [Google Scholar]
- 35.Cassar O, Capuano C, Bassot S, Charavay F, Duprez R, Afonso PV, Abel M, Walter H, Mera W, Martin PM, Chungue E, Gessain A. 2007. Human T lymphotropic virus type 1 subtype C melanesian genetic variants of the Vanuatu Archipelago and Solomon Islands share a common ancestor. J Infect Dis 196:510–521. 10.1086/519167. [DOI] [PubMed] [Google Scholar]
- 36.Hogan CA, Iles J, Frost EH, Giroux G, Cassar O, Gessain A, Dion MJ, Ilunga V, Rambaut A, Yengo-Ki-Ngimbi AE, Behets F, Pybus OG, Pepin J. 2016. Epidemic history and iatrogenic transmission of blood-borne viruses in mid-20th century Kinshasa. J Infect Dis 214:353–360. 10.1093/infdis/jiw009. [DOI] [PubMed] [Google Scholar]
- 37.Kjerulff B, Hønge BL, Olesen JS, Jensen MM, da Silva ZJ, Erikstrup C, Christiansen M. 2018. Phylogeny of human T-lymphotropic virus-1 subtypes in Guinea-Bissau. Trans R Soc Trop Med Hyg 112:175–180. 10.1093/trstmh/try039. [DOI] [PubMed] [Google Scholar]
- 38.Djuicy DD, Mouinga-Ondeme A, Cassar O, Ramassamy JL, Idam Mamimandjiami A, Bikangui R, Fontanet A, Gessain A. 2018. Risk factors for HTLV-1 infection in Central Africa: a rural population-based survey in Gabon. PLoS Negl Trop Dis 12:e0006832. 10.1371/journal.pntd.0006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeannel D, Kourouma K, Fretz C, Zheng YM, Ureta VA, Drame L, Gessain A, Fournel JJ, de The G. 1995. Regional differences in human retroviral infections HIV-1, HIV-2, and HTLV-I/II in rural Guinea (West Africa). J Acquir Immune Defic Syndr Hum Retrovirol 8:315–318. 10.1097/00042560-199503010-00016. [DOI] [PubMed] [Google Scholar]
- 40.Ramassamy JL, Cassar O, Toumbiri M, Diane A, Idam Mamimandjiami A, Bengone C, Ntsame-Ndong JM, Mouinga-Ondeme A, Gessain A. 2020. High prevalence of human T-cell leukemia virus type-1b genotype among blood donors in Gabon. Central Africa Transfusion 60:1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Einsiedel LJ, Pham H, Woodman RJ, Pepperill C, Taylor KA. 2016. The prevalence and clinical associations of HTLV-1 infection in a remote Indigenous community. Med J Aust 205:305–309. 10.5694/mja16.00285. [DOI] [PubMed] [Google Scholar]
- 42.Bastian I, Hinuma Y, Doherty RR. 1993. HTLV-I among Northern Territory aborigines. Med J Aust 159:12–16. 10.5694/j.1326-5377.1993.tb137694.x. [DOI] [PubMed] [Google Scholar]
- 43.May JT, Stent G, Schnagl RD. 1988. Antibody to human T-cell lymphotropic virus type I in Australian aborigines. Med J Aust 149:104. 10.5694/j.1326-5377.1988.tb120516.x. [DOI] [PubMed] [Google Scholar]
- 44.da Silva ZJ, Nielsen J, Andersen A, Oliveira I, Dias F, Rodrigues A, Holmgren B, Andersson S, Aaby P. 2009. Decline in human T-cell lymphotropic virus-1 prevalence in urban areas of Bissau, Guinea-Bissau. AIDS 23:637–639. 10.1097/QAD.0b013e32832403e8. [DOI] [PubMed] [Google Scholar]
- 45.Larsen O, Andersson S, da Silva Z, Hedegaard K, Sandstrom A, Naucler A, Dias F, Melbye M, Aaby P. 2000. Prevalences of HTLV-1 infection and associated risk determinants in an urban population in Guinea-Bissau, West Africa. J Acquir Immune Defic Syndr 25:157–163. 10.1097/00126334-200010010-00010. [DOI] [PubMed] [Google Scholar]
- 46.Andersson S, Dias F, Mendez PJ, Rodrigues A, Biberfeld G. 1997. HTLV-I and -II infections in a nationwide survey of pregnant women in Guinea-Bissau, West Africa. J Acquir Immune Defic Syndr Hum Retrovirol 15:320–322. 10.1097/00042560-199708010-00014. [DOI] [PubMed] [Google Scholar]
- 47.Gessain A, Fretz C, Koulibaly M, Boudret ML, Bah A, Raphael M, de The G, Fournel JJ. 1993. Evidence of HTLV-II infection in Guinea, West Africa. J Acquir Immune Defic Syndr 6:324–325. [PubMed] [Google Scholar]
- 48.Verdier M, Denis F, Sangare A, Barin F, Gershy-Damet G, Rey J-L, Soro B, Leonard G, Mounier M, Hugon J. 1989. Prevalence of antibody to human T-cell leukemia virus type 1 (HTLV-1) in populations of Ivory Coast, West Africa. J Infect Dis 160:363–370. 10.1093/infdis/160.3.363. [DOI] [PubMed] [Google Scholar]
- 49.Okoye AE, Ibegbulam OG, Onoh RC, Ugwu NI, Anigbo CS, Nonyelu CE. 2015. Seroprevalence of human T-cell lymphoma/leukemia virus type-1 (HTLV-1) antibodies among blood donors at Enugu, Nigeria. J Blood Med 6:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Analo HI, Akanmu AS, Akinsete I, Njoku OS, Okany CC. 1998. Seroprevalence study of HTLV-1 and HIV infection in blood donors and patients with lymphoid malignancies in Lagos, Nigeria. Cent Afr J Med 44:130–134. [PubMed] [Google Scholar]
- 51.Durojaiye I, Akinbami A, Dosunmu A, Ajibola S, Adediran A, Uche E, Oshinaike O, Odesanya M, Dada A, Okunoye O. 2014. Seroprevalence of human T lymphotropic virus antibodies among healthy blood donors at a tertiary centre in Lagos, Nigeria. Pan Afr Med J 17. 10.11604/pamj.2014.17.301.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olaleye DO, Ekweozor CC, Sheng Z, Rasheed S. 1995. Evidence of serological cross-reactivities with human immunodeficiency virus types 1 and 2 and human T-lymphotropic virus types I and II in sera of pregnant women in Ibadan. Int J Epidemiol 24:198–203. 10.1093/ije/24.1.198. [DOI] [PubMed] [Google Scholar]
- 53.Dumas M, Houinato D, Verdier M, Zohoun T, Josse R, Bonis J, Zohoun I, Massougbodji A, Denis F. 1991. Seroepidemiology of human T-cell lymphotropic virus type I/II in Benin (West Africa). AIDS Res Hum Retroviruses 7:447–451. 10.1089/aid.1991.7.447. [DOI] [PubMed] [Google Scholar]
- 54.Armah HB, Narter-Olaga EG, Adjei AA, Asomaning K, Gyasi RK, Tettey Y. 2006. Seroprevalence of human T-cell lymphotropic virus type I among pregnant women in Accra, Ghana. J Med Microbiol 55:765–770. 10.1099/jmm.0.46426-0. [DOI] [PubMed] [Google Scholar]
- 55.Diop S, Calattini S, Abah-Dakou J, Thiam D, Diakhate L, Gessain A. 2006. Seroprevalence and molecular epidemiology of human T-Cell leukemia virus type 1 (HTLV-1) and HTLV-2 in blood donors from Dakar, Senegal. J Clin Microbiol 44:1550–1554. 10.1128/JCM.44.4.1550-1554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Del Mistro A, Chotard J, Hall AJ, Fortuin M, Whittle H, De Rossi A, Chieco-Bianchi L. 1994. HTLV-I/II seroprevalence in The Gambia: a study of mother-child pairs. AIDS Res Hum Retroviruses 10:617–620. 10.1089/aid.1994.10.617. [DOI] [PubMed] [Google Scholar]
- 57.Balogou AA, Grunitzky EK, Anani TK, Kowu A, Sadzo-Hetsu A, Nubukpo KA, Dumas M. 2000. Prevalence of HTLV-1 virus infection in Togo (Kozah prefecture and the University Hospital Center of Lome. Bull Soc Pathol Exot 93:3–5. [PubMed] [Google Scholar]
- 58.Bertherat E, Makuwa M, Renaut A, Nabias R, Georges-Courbot MC. 1998. HIV-1, HTLV-I, and HTLV-II in a semiurban population in East Gabon. J Acquir Immune Defic Syndr Hum Retrovirol 19:430–432. 10.1097/00042560-199812010-00018. [DOI] [PubMed] [Google Scholar]
- 59.Caron M, Besson G, Padilla C, Makuwa M, Nkoghe D, Leroy E, Kazanji M. 2018. Revisiting human T-cell lymphotropic virus types 1 and 2 infections among rural population in Gabon, central Africa thirty years after the first analysis. PLoS Negl Trop Dis 12:e0006833. 10.1371/journal.pntd.0006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delaporte E, Dupont A, Peeters M, Josse R, Merlin M, Schrijvers D, Hamono B, Bedjabaga L, Cheringou H, Boyer F, Brun-Vézinet F, Larouzé B. 1988. Epidemiology of HTLV-I in Gabon (Western Equatorial Africa). Int J Cancer 42:687–689. 10.1002/ijc.2910420509. [DOI] [PubMed] [Google Scholar]
- 61.Filippone C, Bassot S, Betsem E, Tortevoye P, Guillotte M, Mercereau-Puijalon O, Plancoulaine S, Calattini S, Gessain A. 2012. A new and frequent human T-cell leukemia virus indeterminate Western blot pattern: epidemiological determinants and PCR results in central African inhabitants. J Clin Microbiol 50:1663–1672. 10.1128/JCM.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mauclere P, Afonso PV, Meertens L, Plancoulaine S, Calattini S, Froment A, Van Beveren M, de The G, Quintana-Murci L, Mahieux R, Gessain A. 2011. HTLV-2B strains, similar to those found in several Amerindian tribes, are endemic in central African Bakola Pygmies. J Infect Dis 203:1316–1323. 10.1093/infdis/jir031. [DOI] [PubMed] [Google Scholar]
- 63.Vallejo A, Benito A, Varela JM, Casado C, Roche J, Alvar J, García-Sáiz A. 1994. Human T-cell leukaemia virus I/II infection in Equatorial Guinea. AIDS 8:1501–1513. [PubMed] [Google Scholar]
- 64.Delaporte E, Buve A, Nzila N, Goeman J, Dazza MC, Henzel D, Heyward W, St-Louis M, Piot P, Laga M. 1995. HTLV-I infection among prostitutes and pregnant women in Kinshasa, Zaire: how important is high-risk sexual behavior? J Acquir Immune Defic Syndr Hum Retrovirol 8:511–515. 10.1097/00042560-199504120-00012. [DOI] [PubMed] [Google Scholar]
- 65.Etenna SLD, Caron M, Besson G, Makuwa M, Gessain A, Mahe A, Kazanji M. 2008. New Insights into prevalence, genetic diversity, and proviral load of human T-cell leukemia virus types 1 and 2 in pregnant women in Gabon in equatorial Central Africa. J Clin Microbiol 46:3607–3614. 10.1128/JCM.01249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goubau P, Desmyter J, Swanson P, Reynders M, Shih J, Surmont I, Kazadi K, Lee H. 1993. Detection of HTLV-I and HTLV-II infection in Africans using type-specific envelope peptides. J Med Virol 39:28–32. 10.1002/jmv.1890390107. [DOI] [PubMed] [Google Scholar]
- 67.Tuppin P, Makuwa M, Guerma T, Bazabana MM, Loukaka JC, Jeannel D, M’Pele P, de The G. 1996. Low HTLV-I/II seroprevalence in pregnant women in Congo and a geographic cluster of an HTLV-like indeterminate Western blot pattern. J Acquir Immune Defic Syndr Hum Retrovirol 11:105–107. 10.1097/00042560-199601010-00014. [DOI] [PubMed] [Google Scholar]
- 68.Wiktor SZ, Piot P, Mann JM, Nzilambi N, Francis H, Vercauteren G, Blattner WA, Quinn TC. 1990. Human T-cell lymphotropic virus type I (HTLV-I) among female prostitutes in Kinshasa. J Infect Dis 161:1073–1077. 10.1093/infdis/161.6.1073. [DOI] [PubMed] [Google Scholar]
- 69.Vallejo A, Benito A, Varela JM, Casado C, Roche J, Alvar J, Garcia-Saiz A. 1994. Human T-cell leukemia virus-I/II infection in Equatorial Guinea. AIDS 8:1501–1503. [PubMed] [Google Scholar]
- 70.Ramos JM, Belda S, Reyes F, Rodriguez JC, Royo G, Gutierrez F. 2012. Prevalence of HIV, HBV, HCV, HTLV, and Treponema pallidum among patients attending a rural hospital in southern Ethiopia. J Clin Virol 53:268–269. 10.1016/j.jcv.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 71.Rwandan HIV Seroprevalence Study Group. 1989. Nationwide community-based serological survey of HIV-1 and other human retrovirus infections in a central African country Lancet i:941–943. [PubMed] [Google Scholar]
- 72.Gudo ES, Abreu CM, Mussa T, Augusto AD, Otsuki K, Chambo E, Amade N, Tanuri A, Ferreira OC, Jani IV, Grp HMS, HTLV in Mozambique Study Group. 2009. Serologic and molecular typing of human T-lymphotropic virus among blood donors in Maputo City. Mozambique Transfusion 49:1146–1150. [DOI] [PubMed] [Google Scholar]
- 73.Caterino-de-Araujo A, Magri MC, Costa EA, Manuel RC. 2010. Prevalence of human T-cell lymphotropic virus (HTLV-1/2) in individuals from public health centers in Mozambique. AIDS Res Hum Retroviruses 26:559–561. 10.1089/aid.2009.0269. [DOI] [PubMed] [Google Scholar]
- 74.van der Ryst E, Joubert G, Smith MS, Terblanche M, Mollentze F, Pretorius AM. 1996. HTLV-I infection in the Free State region of South Africa: a sero-epidemiologic study. Cent Afr J Med 42:65–68. [PubMed] [Google Scholar]
- 75.Vermeulen M, Sykes W, Coleman C, Custer B, Jacobs G, Jaza J, Kaidarova Z, Hlela C, Gessain A, Cassar O, Poole C, Ingram C, Murphy EL, Reddy R. 2019. The prevalence of human T-lymphotropic virus type 1 and 2 (HTLV-1/2) in South African blood donors. Vox Sang 114:451–458. 10.1111/vox.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang YB, Kaidarova Z, Hindes D, Bravo M, Kiely N, Kamel H, Dubay D, Hoose B, Murphy EL. 2014. Seroprevalence and demographic determinants of human T-lymphotropic virus type 1 and 2 infections among first-time blood donors—United States, 2000–2009. J Infect Dis 209:523–531. 10.1093/infdis/jit497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy EL, Watanabe K, Nass CC, Ownby H, Williams A, Nemo G. 1999. Evidence among blood donors for a 30-year-old epidemic of human T lymphotropic virus type II infection in the United States. J Infect Dis 180:1777–1783. 10.1086/315139. [DOI] [PubMed] [Google Scholar]
- 78.Parks WP, Lenes BA, Tomasulo PA, Schiff ER, Parks ES, Shaw GM, Lee H, Yan HQ, Lai SH, Hollingsworth CG, Nemo GJ, Mosley JW. 1991. Human T-cell lymphotropic virus infection among blood donors in south Florida. J Acquir Immune Defic Syndr Hum Retrovirol 4:89–96. [PubMed] [Google Scholar]
- 79.Lee HH, Swanson P, Rosenblatt JD, Chen IS, Sherwood WC, Smith DE, Tegtmeier GE, Fernando LP, Fang CT, Osame M, Kleinman SH. 1991. Relative prevalence and risk factors of HTLV-I and HTLV-II infection in US blood donors. Lancet 337:1435–1439. 10.1016/0140-6736(91)93126-T. [DOI] [PubMed] [Google Scholar]
- 80.O’Brien SF, Goldman M, Scalia V, Yi QL, Fan W, Xi G, Dines IR, Fearon MA. 2013. The epidemiology of human T-cell lymphotropic virus types I and II in Canadian blood donors. Transfus Med 23:358–366. [DOI] [PubMed] [Google Scholar]
- 81.Gongora-Biachi RA, Gonzalez-Martinez P, Castro-Sansores C, Vivas-Rosel ML, Gasque-Lopez F, Garrido-Hadad E. 1996. Lymphotropic viruses type I and II in pregnant women in Yucatan. Rev Invest Clin 48:383–384 [PubMed] [Google Scholar]
- 82.Islas S, Yamaguchi K, Silva R, Nishimura Y, Kawano F, Ponce M, Barra R, Takatsuki K. 1993. HTLV-I/II infection is absent among risk groups from Mexico City. Vox Sang 64:57–58. 10.1111/j.1423-0410.1993.tb02516.x. [DOI] [PubMed] [Google Scholar]
- 83.Nunes D, Boa-Sorte N, Grassi MF, Taylor GP, Teixeira MG, Barreto ML, Dourado I, Galvao-Castro B. 2017. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS One 12:e0171303. 10.1371/journal.pone.0171303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carles G, Tortevoye P, Tuppin P, Ureta-Vidal A, Peneau C, El Guindi W, Gessain A. 2004. HTLV1 infection and pregnancy. J Gynecol Obstet Biol Reprod 33:14–20. 10.1016/S0368-2315(04)96307-7. [DOI] [PubMed] [Google Scholar]
- 85.Plancoulaine S, Buigues RP, Murphy EL, van Beveren M, Pouliquen JF, Joubert M, Remy F, Tuppin P, Tortevoye P, de The G, Moreau JP, Gessain A. 1998. Demographic and familial characteristics of HTLV-1 infection among an isolated, highly endemic population of African origin in French Guiana. Int J Cancer 76:331–336. . [DOI] [PubMed] [Google Scholar]
- 86.Tortevoye P, Tuppin P, Carles G, Peneau C, Gessain A. 2005. Comparative trends of seroprevalence and seroincidence rates of human T cell lymphotropic virus type I and human immunodeficiency virus 1 in pregnant women of various ethnic groups sharing the same environment in French Guiana. Am J Trop Med Hyg 73:560–565. 10.4269/ajtmh.2005.73.560. [DOI] [PubMed] [Google Scholar]
- 87.Blas MM, Alva IE, Garcia PJ, Carcamo C, Montano SM, Mori N, Munante R, Zunt JR. 2013. High prevalence of human T-lymphotropic virus infection in indigenous women from the peruvian Amazon. PLoS One 8:e73978. 10.1371/journal.pone.0073978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ita F, Mayer EF, Verdonck K, Gonzalez E, Clark D, Gotuzzo E. 2014. Human T-lymphotropic virus type 1 infection is frequent in rural communities of the southern Andes of Peru. Int J Infect Dis 19:46–52. 10.1016/j.ijid.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 89.de Rivera IL, Amador L, Mourra S, Li Z, Rasheed S. 1995. Geographical clustering of human T-cell lymphotropic virus type 1 infection in Honduras. J Clin Microbiol 33:2999–3003. 10.1128/jcm.33.11.2999-3003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pinto MT, Rodrigues ES, Malta TM, Azevedo R, Takayanagui OM, Valente VB, Ubiali EM, Covas DT, Kashima S. 2012. HTLV-1/2 seroprevalence and coinfection rate in Brazilian first-time blood donors: an 11-year follow-up. Rev Inst Med Trop Sao Paulo 54:123–129. 10.1590/s0036-46652012000300002. [DOI] [PubMed] [Google Scholar]
- 91.Ribeiro IP, Kozlowski AG, Dias de Matos MA, da Costa ESAM, Dos Santos Carneiro MA, Vicente ACP, Martins RMB. 2018. HTLV-1 and -2 in a first-time blood donor population in Northeastern Brazil: prevalence, molecular characterization, and evidence of intrafamilial transmission. J Med Virol 90:1651–1657. 10.1002/jmv.25231. [DOI] [PubMed] [Google Scholar]
- 92.Bittencourt AL, Dourado I, Filho PB, Santos M, Valadão E, Alcantara LC, Galvão-Castro B. 2001. Human T-cell lymphotropic virus type 1 infection among pregnant women in northeastern Brazil. J Acquir Immune Defic Syndr 26:490–494. 10.1097/00126334-200104150-00016. [DOI] [PubMed] [Google Scholar]
- 93.Dourado I, Alcantara LC, Barreto ML, da Gloria Teixeira M, Galvao-Castro B. 2003. HTLV-I in the general population of Salvador, Brazil: a city with African ethnic and sociodemographic characteristics. J Acquir Immune Defic Syndr 34:527–531. 10.1097/00126334-200312150-00013. [DOI] [PubMed] [Google Scholar]
- 94.Monteiro DL, Taquette SR, Sodre Barmpas DB, Rodrigues NC, Teixeira SA, Villela LH, Boia MN, Trajano AJ. 2014. Prevalence of HTLV-1/2 in pregnant women living in the metropolitan area of Rio de Janeiro. PLoS Negl Trop Dis 8:e3146. 10.1371/journal.pntd.0003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sequeira CG, Tamegao-Lopes BP, Santos EJ, Ventura AM, Moraes-Pinto MI, Succi RC. 2012. Descriptive study of HTLV infection in a population of pregnant women from the state of Para, northern Brazil. Rev Soc Bras Med Trop 45:453–456. 10.1590/s0037-86822012005000007. [DOI] [PubMed] [Google Scholar]
- 96.de Souza VG, Martins ML, Carneiro-Proietti ABF, Januario JN, Ladeira RVP, Silva CMS, Pires C, Gomes SC, Martins CS, Mochel EG. 2012. High prevalence of HTLV-1 and -2 viruses in pregnant women in Sao Luis, State of Maranhao. Rev Soc Bras Med Trop 45:159–162. 10.1590/s0037-86822012000200004. [DOI] [PubMed] [Google Scholar]
- 97.Ydy RR, Ferreira D, Souto FJ, Fontes CJ. 2009. Prevalence of human T-cell lymphotropic virus (HTLV-1/2) infection among puerperae in Cuiaba, Mato Grosso, 2006. Rev Soc Bras Med Trop 42:28–32. 10.1590/s0037-86822009000100007. [DOI] [PubMed] [Google Scholar]
- 98.Olbrich NJ, Alves MD. 2004. Seroprevalence of HTLV-I/II, HIV, siphylis and toxoplasmosis among pregnant women seen at Botucatu, Sao Paulo, Brazil. Risk factors for HTLV-I/II infection. Revista da Sociedade Brasileira de Medicina Tropical 37:28–32. 10.1590/S0037-86822004000100008. [DOI] [PubMed] [Google Scholar]
- 99.Castro LS, Rezende GRD, Fernandes FRP, Bandeira LM, Puga MAM, Tanaka TSO, Weis-Torres SMDS, Vicente ACP, Otsuki K, Motta-Castro ARC. 2018. Human T cell lymphotropic virus type 1 infection among men who have sex with men in Central Brazil. Braz J Infect Dis 22:472–476. 10.1016/j.bjid.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Munoz M, Carvalho S, Donado JH, Barco GE, Jaramillo S. 2018. SHTLV-I/II seroprevalence in blood donors of Hospital Pablo Tobon Uribe Blood Bank during the period 2014–2015. Biomedica 38:37–41. 10.7705/biomedica.v38i0.3417. [DOI] [PubMed] [Google Scholar]
- 101.Sanchez-Palacios C, Gotuzzo E, Vandamme AM, Maldonado Y. 2003. Seroprevalence and risk factors for human T-cell lymphotropic virus (HTLV-I) infection among ethnically and geographically diverse Peruvian women. Int J Infect Dis 7:132–137. 10.1016/S1201-9712(03)90009-9. [DOI] [PubMed] [Google Scholar]
- 102.Zurita S, Costa C, Watts D, Indacochea S, Campos P, Sanchez J, Gotuzzo E. 1997. Prevalence of human retroviral infection in Quillabamba and Cuzco, Peru: a new endemic area for human T cell lymphotropic virus type 1. Am J Trop Med Hyg 56:561–565. 10.4269/ajtmh.1997.56.561. [DOI] [PubMed] [Google Scholar]
- 103.Stewart J, Heitzinger K, Pollett S, Calderon M, Alarcon J, Ton TG, Zunt JR. 2017. The changing epidemiology of human T-cell lymphotropic virus type 1 infection in Peruvian female sex workers, 1993–2010. Am J Trop Med Hyg 96:373–379. 10.4269/ajtmh.16-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reeves WC, Levine PH, Cuevas M, Quiroz E, Maloney E, Saxinger WC. 1990. Seroepidemiology of human T cell lymphotropic virus in the Republic of Panama. Am J Trop Med Hyg 42:374–379. 10.4269/ajtmh.1990.42.374. [DOI] [PubMed] [Google Scholar]
- 105.Berini CA, Delfino C, Torres O, Garcia G, Espejo R, Pianciola L, Juarez M, Arribere G, Nadal M, Eirin ME, Biglione MM. 2013. HTLV-1 cosmopolitan and HTLV-2 subtype b among pregnant women of non-endemic areas of Argentina. Sex Transm Infect 89:333–335. 10.1136/sextrans-2012-050594. [DOI] [PubMed] [Google Scholar]
- 106.Gutfraind Z, Blejer JL, Saguier MC, Gomez Carretero ML, Pirola DA, Carreras Vescio LA. 1994. Seroprevalence of HTLV-I/HTLV-II in blood donors in Buenos Aires (Argentina). Vox Sang 67:408–409. 10.1159/000462650. [DOI] [PubMed] [Google Scholar]
- 107.Leon G, Quiros AM, Lopez JL, Hung M, Diaz AM, Goncalves J, Da Costa O, Hernandez T, Chirinos M, Gomez R. 2003. Seropositivity for human T-lymphotropic virus types I and II among donors at the Municipal Blood Bank of Caracas and associated risk factors. Rev Panam Salud Publica 13:117–123. 10.1590/S1020-49892003000200012. [DOI] [PubMed] [Google Scholar]
- 108.Olindo S, Jeannin S, Saint-Vil M, Signate A, Edimonana-Kaptue M, Joux J, Merle H, Richard P, Granjeaud S, Cabre P, Smadja D, Cesaire R, Lezin A. 2018. Temporal trends in Human T-Lymphotropic virus 1 (HTLV-1) associated myelopathy/tropical spastic paraparesis (HAM/TSP) incidence in Martinique over 25 years (1986–2010). PLoS Negl Trop Dis 12:e0006304. 10.1371/journal.pntd.0006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cesaire R, Bera O, Maier H, Lezin A, Martial J, Ouka M, Kerob-Bauchet B, Ould Amar AK, Vernant JC. 1999. Seroindeterminate patterns and seroconversions to human T-lymphotropic virus type I positivity in blood donors from Martinique, French West Indies. Transfusion 39:1145–1149. 10.1046/j.1537-2995.1999.39101145.x. [DOI] [PubMed] [Google Scholar]
- 110.Rouet F, Rabier R, Foucher C, Chancerel B, Agis F, Strobel M. 1999. Geographical clustering of human T-cell lymphotropic virus type I in Guadeloupe, an endemic Caribbean area. Int J Cancer 81:330–334. . [DOI] [PubMed] [Google Scholar]
- 111.Schill H, Bruneau B, Lepage B, Humeau O, Grimault C, Tampreau V, Devaucouleurs AB, Buisson Y. 1989. A seroprevalence study of the anti-HIV antibodies in a Haitian rural population. Bull Soc Pathol Exotique 82:308–315. [PubMed] [Google Scholar]
- 112.Denis F, Verdier M, Chout R. 1988. Antibodies to HTLV-I in pregnant women from West Africa, Martinique, and of foreign origin living in France. Bull Acad Natl Med 172:717–722. [PubMed] [Google Scholar]
- 113.Dowe G, King SD, Smikle MF, Wynter HH, Chout R, Klaskala W. 1998. Prevalence of viral and bacterial sexually transmitted pathogens in Jamaican pregnant women. West Indian Med J 47:23–25. [PubMed] [Google Scholar]
- 114.Maloney EM, Yamano Y, Vanveldhuisen PC, Sawada T, Kim N, Cranston B, Hanchard B, Jacobson S, Hisada M. 2006. Natural history of viral markers in children infected with human T lymphotropic virus type I in Jamaica. J Infect Dis 194:552–560. 10.1086/506365. [DOI] [PubMed] [Google Scholar]
- 115.Mansuy JM, Schlegel L, Villeneuve L, Mengelle C, Magnaval JF. 1999. Seroprevalence of retroviral infections among pregnant women in Martinique (French West Indies). Am J Trop Med Hyg 61:598–599. 10.4269/ajtmh.1999.61.598. [DOI] [PubMed] [Google Scholar]
- 116.Wiktor SZ, Pate EJ, Murphy EL, Palker TJ, Champegnie E, Ramlal A, Cranston B, Hanchard B, Blattner WA. 1993. Mother-to-child transmission of human T-cell lymphotropic virus type I (HTLV-I) in Jamaica: association with antibodies to envelope glycoprotein (gp46) epitopes. J Acquir Immune Defic Syndr (1988) 6:1162–1167. [PubMed] [Google Scholar]
- 117.Oota SO. 2015. Prevalance of HTLV I/II infection in Thai blood donors. Vox Sang 1:245. [Google Scholar]
- 118.Tanggo Y, Gultom SP, Simanjuntak T, Sibuea WH, Matsuzaki H, Yamaguchi K. 2000. Human T lymphotropic virus I in Indonesia—very low seroprevalence in the Jakarta area: antibodies in healthy blood donors and in various nonhematological diseases. Intervirology 43:77–79. 10.1159/000025027. [DOI] [PubMed] [Google Scholar]
- 119.Urwijitaroon Y, Barusrux S, Puapairoj C, Romphruk A, Khampeera P. 1997. Seroepidemiology of HTLV-I infection in northeast Thailand: a four year surveillance. J Med Assoc Thai 80(Suppl 1):S102–S105. [PubMed] [Google Scholar]
- 120.Kumar H, Gupta PK. 2006. Is seroprevalence of HTLV-I/II among blood donors in India relevant? Indian J Pathol Microbiol 49:532–534. [PubMed] [Google Scholar]
- 121.Ali A. 2014. Human T lymphotropic virus-I (HTLV-I), the causative agent of acute T-cell leukaemia/lymphoma, is absent among blood donors in Aseer region, Saudi Arabia. J King Abdulaziz Univ Sci 26:35–52. [Google Scholar]
- 122.El-Hazmi MM. 2004. Prevalence of HBV, HCV, HIV-1, HIV-2, and HTLV-I/II infections among blood donors in a teaching hospital in the central region of Saudi Arabia. Saudi Med J 25:26–33. [PubMed] [Google Scholar]
- 123.Kawashti MI, Hindawi SI, Damanhouri GA, Rowehy NG, Bawazeer MM, Alshawa M. 2005. Serologial screening of human T cell lymphotropic virus I and II (HTLV I/II) in blood banks by immunoblotting and enzyme-immuno assays: to demand or to defeat? Egypt J Immunol 12:137–142. [PubMed] [Google Scholar]
- 124.Kilany M, Bin Dajem SM, Ibrahim YM, Alshehri A, Aljeamelani AA, Ibrahim EH. 2015. Seroprevalence of anti-Treponema pallidum antibodies (syphilis) in blood donors in the southern area of Saudi Arabia. Res J Pharm Biol Chem Sci 6:549–556. [Google Scholar]
- 125.Knox-Macaulay H, McDonald R, Al-Busaidy S, Ali N. 1997. HTLV-I seroprevalence in the Sultanate of Oman. Scand J Infect Dis 29:217–218. 10.3109/00365549709019030. [DOI] [PubMed] [Google Scholar]
- 126.Mojaat N, Kaabi H, Hmida S, Maamar M, Slama S, Boukef K. 1999. Seroprevalence of HTLV-I/II antibodies in blood donors and different groups at risk in Tunisia. J Acquir Immune Defic Syndr Hum Retrovirol 22:314–315. 10.1097/00126334-199911010-00018. [DOI] [PubMed] [Google Scholar]
- 127.Naman R, Klayme S, Naboulsi M, Mokhbat J, Jradi O, Ramia S. 2002. HTLV-I and HTLV-II infections in volunteer blood donors and high-risk groups in Lebanon. J Infect 45:29–31. 10.1053/jinf.2002.1006. [DOI] [PubMed] [Google Scholar]
- 128.Souan L, Tout F, Siag M, Sughayer MA. 2016. Seroprevalence rates of transfusion-transmitted infections among blood donors in Jordan. J Infect Dev Countries 10:377–383. 10.3855/jidc.8160. [DOI] [PubMed] [Google Scholar]
- 129.Al-Mufti S, Voevodin A, Ahmed S, Al Hamdan S, Al-Basheer AA. 1999. Seroprevalence of human T-cell leukemia/lymphoma virus type I and type II (HTLV-I/HTLV-II) infection among volunteer blood donors in Kuwait. Med Princ Pract 8:45–50. 10.1159/000026068. [DOI] [Google Scholar]
- 130.Niazi SK, Bhatti FA, Salamat N. 2015. Seroprevalence of human T-cell lymphotropic virus-1/2 in blood donors in northern Pakistan: implications for blood donor screening. J Coll Physicians Surgeons Pakistan 25. [PubMed] [Google Scholar]
- 131.el-Farrash MA, Badr MF, Hawas SA, el-Nashar NM, Imai J, Komoda H, Hinuma Y. 1988. Sporadic carriers of human T-lymphotropic virus type I in northern Egypt. Microbiol Immunol 32:981–984. 10.1111/j.1348-0421.1988.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 132.Abbaszadegan MR, Mehran G, Abbas T, Reza F, Massoud H, Abbaszadegan M. 2003. Prevalence of human T-lymphotropic virus type 1 among blood donors from Mashhad, Iran. J Clin Microbiol 41:2593–2595. 10.1128/JCM.41.6.2593-2595.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pirayeshfard L, Sharifi Z, Amini-Kafiabad S, Haghnazari Sadaghiani N. 2018. Phylogenetic analysis of HTLV-1 in Iranian blood donors, HIV-1 positive patients and patients with beta thalassemia. J Med Virol 90:1398–1405. 10.1002/jmv.25192. [DOI] [PubMed] [Google Scholar]
- 134.Yahyapour Y, Aghajanipour K, Mir SM, Khademian A, Sadeghi F. 2017. Human T-lymphotropic virus type 1 in blood donors from Babol County blood transfusion center: a pilot study from northern Iran. Jundishapur J Microbiol 10. 10.5812/jjm.13757. [DOI] [Google Scholar]
- 135.Maghsudlu M, Safabakhsh H, Jamili P. 2015. Seroepidemiology of human T-cell lymphotropic virus type-I in blood donors of northeastern Iran, Sabzevar. Asian J Transfus Sci 9:203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hamedi A, Akhlaghi F, Meshkat Z, Sezavar M, Nomani H, Meshkat M. 2012. The prevalence of human T-cell lymphotropic virus type 1 in pregnant women and their newborns. ISRN Obstet Gynecol 2012:975135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boni J, Bisset LR, Burckhardt JJ, Joller-Jemelka HI, Burgisser P, Perrin L, Gorgievski M, Erb P, Fierz W, Piffaretti JC, Schupbach J, Swiss HIV Cohort Study. 2004. Prevalence of human T-cell leukemia virus types I and II in Switzerland. J Med Virol 72:328–337. 10.1002/jmv.10541. [DOI] [PubMed] [Google Scholar]
- 138.Brennan M, Runganga J, Barbara JAJ, Contreras M, Tedder RS, Garson JA, Tuke PW, Mortimer PP, Mcalpine L, Tosswill JHC. 1993. Prevalence of antibodies to human T-cell leukemia-lymphoma virus in blood donors in north London. BMJ 307:1235–1239. 10.1136/bmj.307.6914.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Christiansen CB, Wantzin P, Dickmeiss E. 1995. Prevalence of antibodies to HTLV-I/II in high-risk patients and blood donors in Denmark. Vox Sang 68:63–64. 10.1111/j.1423-0410.1995.tb02550.x. [DOI] [PubMed] [Google Scholar]
- 140.Courouce AM, Pillonel J, Lemaire JM, Maniez M, Brunet JB. 1993. Seroepidemiology of HTLV-I/II in universal screening of blood donations in France. AIDS 7:841–847. 10.1097/00002030-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 141.Dickmeiss E, Christiansen AH, Smith E. 2001. Screening of donor blood for viral markers. Anti-HIV, HBsAg and anti-HTLV-I/II in Denmark 1990–1999. Ugeskr Laeger 163:2623–2628. (In Swedish.) [PubMed] [Google Scholar]
- 142.Malm K, Ekermo B, Hillgren K, Britton S, Fredlund H, Andersson S. 2012. Prevalence of human T-lymphotropic virus type 1 and 2 infections in Sweden. Scand J Infect Dis 44:852–859. 10.3109/00365548.2012.689847. [DOI] [PubMed] [Google Scholar]
- 143.Nubling M, Nubling CM, Seifried E, Weichert W, Lower J. 2001. Human T-cell lymphocytotrophic virus prevalence in German blood donors and “at-risk” groups. Vox Sang 81:204–206. 10.1046/j.1423-0410.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 144.Sertöz R, Turhan A, Bozkurt H, Samlıoğlu P, Değirmenci A, Aydınok Y, Erensoy S. 2010. Investigation of anti-HTLV I/II seroprevalence in healthy blood donors in Izmir region, Turkey. Mikrobiyol Bul 44:579–584. (In Turkish.) [PubMed] [Google Scholar]
- 145.Stienlauf S, Yahalom V, Schwartz E, Shinar E, Segal G, Sidi Y. 2009. Epidemiology of human T-cell lymphotropic virus type 1 infection in blood donors. Emerg Infect Dis 15:1116–1118. 10.3201/eid1507.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tseliou PM, Spanakis N, Spiliotakara A, Politis C, Legakis NJ, Tsakris A. 2003. HTLV-I and -II in southwestern Greece. Transfusion 43:1641–1642. 10.1046/j.1537-2995.2003.00569.x. [DOI] [PubMed] [Google Scholar]
- 147.Murovska M, Taguchi H, Iwahara Y, Sawada T, Kukaine R, Miyoshi I. 1991. Antibodies to HTLV-I among blood donors in Latvia, USSR. Int J Cancer 47:158–159. [DOI] [PubMed] [Google Scholar]
- 148.Cardoso EA, Robert-Guroff M, Franchini G, Gartner S, Moura-Nunes JF, Gallo RC, Terrinha AM. 1989. Seroprevalence of HTLV-I in Portugal and evidence of double retrovirus infection of a healthy donor. Int J Cancer 43:195–200. 10.1002/ijc.2910430204. [DOI] [PubMed] [Google Scholar]
- 149.Paun L, Ispas O, Del Mistro A, Chieco-Bianchi L. 1994. HTLV-I in Romania. Eur J Haematol 52:117–118. [DOI] [PubMed] [Google Scholar]
- 150.Senyuta N, Syrtsev A, Yamashita M, Stepina V, Susova O, Scherbak L, Pavlish O, Hayami M, Gurtsevitch V. 1998. Sero-epidemiologic and phylogenetic studies of HTLV-I infection in two countries of the Caspian Sea region. Int J Cancer 77:488–493. . [DOI] [PubMed] [Google Scholar]
- 151.Taylor GP, Bodeus M, Courtois F, Pauli G, Del Mistro A, Machuca A, Padua E, Andersson S, Goubau P, Chieco-Bianchi L, Soriano V, Coste J, Ades AE, Weber JN. 2005. The seroepidemiology of human T-lymphotropic viruses: types I and II in Europe: a prospective study of pregnant women. J Acquir Immune Defic Syndr 38:104–109. 10.1097/00126334-200501010-00018. [DOI] [PubMed] [Google Scholar]
- 152.Tseliou PM, Spanakis N, Spiliotakara A, Markogiannakis A, Legakis NJ, Tsakris A. 2006. Prevalence of infection by HTLV-I/II among pregnant women and high-risk groups in the Peloponnese peninsula, Greece. Int J STD AIDS 17:543–546. 10.1258/095646206778145541. [DOI] [PubMed] [Google Scholar]
- 153.Poljak M, Bednarik J, Rednak K, Seme K, Kristancic L, Celan-Lucu B. 1998. Seroprevalence of human T cell leukaemia/lymphoma virus type I (HTLV-I) in pregnant women, patients attending venereological outpatient services and intravenous drug users from Slovenia. Folia Biol 44:23–25. [PubMed] [Google Scholar]
- 154.Machuca A, Tuset C, Soriano V, Caballero E, Aguilera A, Ortiz de Lejarazu R, HTLV Spanish Study Group. 2000. Prevalence of HTLV infection in pregnant women in Spain. Sex Transm Infect 76:366–370. 10.1136/sti.76.5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jogeda EL, Avi R, Pauskar M, Kallas E, Karki T, Des Jarlais D, Uuskula A, Lutsar I, Huik K. 2016. Human T-lymphotropic virus types 1 and 2 are rare among intravenous drug users in Eastern Europe. Infect Genet Evol 43:83–85. 10.1016/j.meegid.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ades AE, Parker S, Walker J, Edginton M, Taylor GP, Weber JN. 2000. Human T cell leukemia/lymphoma virus infection in pregnant women in the United Kingdom: population study. BMJ 320:1497–1501. 10.1136/bmj.320.7248.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vignoli C, De Lamballerie X, Zandotti C, De Chesse R, Tamalet C, De Micco P. 1995. High prevalence of human T-lymphotropic virus (HTLV) infection in pregnant women in southern France. Eur J Epidemiol 11:93–94. 10.1007/BF01719953. [DOI] [PubMed] [Google Scholar]
- 158.Courtois F, Barin F, Larsen M, Brossard Y, Masselin A, Engelman P. 1990. HTLV-I/II infection in pregnant women in Paris. Lancet 335:1103–1103. 10.1016/0140-6736(90)92681-7. [DOI] [PubMed] [Google Scholar]
- 159.Alessio L, Minichini C, Starace M, Occhiello L, Caroprese M, Di Caprio G, Sagnelli C, Gualdieri L, Pisaturo M, Onorato L, Scotto G, Macera M, De Pascalis S, Sagnelli E, Coppola N. 2018. Low prevalence of HTLV1/2 infection in a population of immigrants living in southern Italy. PLoS Negl Trop Dis 12:e0006601. 10.1371/journal.pntd.0006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Trevino A, Aguilera A, Caballero E, Benito R, Parra P, Eiros JM, Hernandez A, Calderon E, Rodriguez M, Torres A, Garcia J, Ramos JM, Roc L, Marcaida G, Rodriguez C, Trigo M, Gomez C, de Lejarazu RO, de Mendoza C, Soriano V, HTLV Spanish Study Group. 2012. Trends in the prevalence and distribution of HTLV-1 and HTLV-2 infections in Spain. Virol J 9:71. 10.1186/1743-422X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van Doornum GJ, Hooykaas C, Huisman JG, van der Linden MM, Coutinho RA. 1990. Prevalence of human T-cell leukemia virus antibody among heterosexuals living in Amsterdam, The Netherlands. J Med Virol 32:183–188. 10.1002/jmv.1890320309. [DOI] [PubMed] [Google Scholar]
- 162.Bastian I, Dent J, Mcfarlane R, Karopoulos A, Way B. 1993. HTLV-I among Northern-Territory blood donors. Med J Aust 159:7–12. 10.5694/j.1326-5377.1993.tb137693.x. [DOI] [PubMed] [Google Scholar]
- 163.Lu SC, Kao CL, Chin LT, Chen JW, Yang CM, Chang JH, Hsu SC, Chang ACH, Chen BH. 2001. Seroprevalence and demographic characteristics of HTLV-I among blood donors in Taiwan: 1996–1999. Int J Hematol 74:333–337. 10.1007/BF02982070. [DOI] [PubMed] [Google Scholar]
- 164.Ma Y, Zheng S, Wang N, Duan Y, Sun X, Jin J, Zang W, Li M, Wang Y, Zhao G. 2013. Epidemiological analysis of HTLV-1 and HTLV-2 infection among different population in Central China. PLoS One 8:e66795. 10.1371/journal.pone.0066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Y, Li X, Song A, Zhang C, Chen Y, Chen C, Lin Y, Shun L, Li L, Liu Y, Yang J, Yang B, Tang Q, Harrison TJ. 2005. Prevalence and partial sequence analysis of human T cell lymphotropic virus type I in China. J Med Virol 76:613–618. 10.1002/jmv.20405. [DOI] [PubMed] [Google Scholar]
- 166.Xie J, Ge S, Zhang Y, Lin Y, Ni H, Zhang J, Chen C. 2015. The prevalence of human T-lymphotropic virus infection among blood donors in southeast China, 2004–2013. PLoS Negl Trop Dis 9:e0003685. 10.1371/journal.pntd.0003685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim JM, Song YG, Oho YC, Park HC, Kwon KH, Kim E, Lee SH, Kim KH. 1999. Antibodies to human T-cell lymphotropic virus type I (HTLV-I) by particle agglutination (PA) test in Korean blood donors. Yonsei Med J 40:173–177. 10.3349/ymj.1999.40.2.173. [DOI] [PubMed] [Google Scholar]
- 168.Kwon SY, Lim AH, Park JY, Han SH, Cho NS. 2008. Seroprevalence of human T-lymphotropic virus type 1 and 2 in Korean blood donors. J Med Virol 80:1864–1867. 10.1002/jmv.21260. [DOI] [PubMed] [Google Scholar]
- 169.Lee SY, Yamaguchi K, Takatsuki K, Kim BK, Park S, Lee M. 1986. Seroepidemiology of human T-cell leukemia virus type I in the Republic of Korea. Jpn J Cancer Res 77:250–254. [PubMed] [Google Scholar]
- 170.Yanagihara R, Hefner C, Ajdukiewicz AB. 1992. Human T-lymphotropic virus type I infection among blood donors in the Solomon Islands. Transfusion 32:89. 10.1046/j.1537-2995.1992.32192116444.x. [DOI] [PubMed] [Google Scholar]
- 171.Aoki T, Miyakoshi H, Koide H, Yoshida T, Ishikawa H, Sugisaki Y, Mizukoshi M, Tamura K, Misawa H, Hamada C, Ting RC, Robert-Guroff M, Gallo RC. 1985. Seroepidemiology of human T-lymphotropic retrovirus type I (HTLV-I) in residents of Niigata Prefecture, Japan: comparative studies by indirect immunofluorescence microscopy and enzyme-linked immunosorbent assay. Int J Cancer 35:301–306. 10.1002/ijc.2910350304. [DOI] [PubMed] [Google Scholar]
- 172.Chen YM, Lin HC, Chou P. 1996. A population-based epidemiological study of human T-cell leukemia virus type I infection in Kin-Hu, Kinmen. Int J Cancer 65:569–573. . [DOI] [PubMed] [Google Scholar]
- 173.Takao S, Ishida T, Bhatia KK, Saha N, Soemantri A, Kayame OW. 2000. Seroprevalence of human T-lymphotropic virus type 1 in Papua New Guinea and Irian Jaya measured using different western blot criteria. J Clin Virol 16:129–133. 10.1016/S1386-6532(99)00087-6. [DOI] [PubMed] [Google Scholar]
- 174.Wang CH, Chen CJ, Hu CY, You SL, Chu CT, Chou MJ, Essex M, Blattner WA, Liu CH, Yang CS. 1988. Seroepidemiology of human T-cell lymphotropic virus type I infection in Taiwan. Cancer Res. [PubMed] [Google Scholar]
- 175.Einsiedel L, Pham H, Talukder MRR, Liddle J, Taylor K, Wilson K, Jersmann H, Gessain A, Woodman R, Kaldor J. 2020. Pulmonary disease is associated with human T-cell leukaemia virus type 1c infection: a cross-sectional survey in remote Aboriginal Australian communities. Clin Infect Dis 73:e1498–e1506. 10.1093/cid/ciaa1401. [DOI] [PubMed] [Google Scholar]
- 176.Einsiedel L, Woodman RJ, Flynn M, Wilson K, Cassar O, Gessain A. 2016. Human T-lymphotropic virus type 1 infection in an indigenous Australian population: epidemiological insights from a hospital-based cohort study. BMC Public Health 16:787. 10.1186/s12889-016-3366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang W, Guo J, Li T, Zhang W, Guo C, Song Y, Che J, Zhang Y, Li M. 2015. Investigation of human cytomegalovirus and human T lymphotropic virus infection of voluntary blood donors in the Beijing area. J Modern Lab Med 30:35–38. [Google Scholar]
- 178.Tajima K, T- and B-Cell Malignancy Study Group. 1990. The 4th nationwide study of adult T-cell leukemia/lymphoma (ATL) in Japan: estimates of risk of ATL and its geographical and clinical features. Int J Cancer 45:237–243. 10.1002/ijc.2910450206. [DOI] [PubMed] [Google Scholar]
- 179.Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. 2007. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis 7:266–281. 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- 180.Furuta R, Yasunaga JI, Miura M, Sugata K, Saito A, Akari H, Ueno T, Takenouchi N, Fujisawa JI, Koh KR, Higuchi Y, Mahgoub M, Shimizu M, Matsuda F, Melamed A, Bangham CR, Matsuoka M. 2017. Human T-cell leukemia virus type 1 infects multiple lineage hematopoietic cells in vivo. PLoS Pathog 13:e1006722. 10.1371/journal.ppat.1006722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Miura T, Fukunaga T, Igarashi T, Yamashita M, Ido E, Funahashi S, Ishida T, Washio K, Ueda S, Hashimoto K. 1994. Phylogenetic subtypes of human T-lymphotropic virus type I and their relations to the anthropological background. Proc Natl Acad Sci USA 91:1124–1127. 10.1073/pnas.91.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gessain A, Boeri E, Yanagihara R, Gallo RC, Franchini G. 1993. Complete nucleotide sequence of a highly divergent human T-cell leukemia (lymphotropic) virus type I (HTLV-I) variant from melanesia: genetic and phylogenetic relationship to HTLV-I strains from other geographical regions. J Virol 67:1015–1023. 10.1128/jvi.67.2.1015-1023.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mahieux R, Ibrahim F, Mauclere P, Herve V, Michel P, Tekaia F, Chappey C, Garin B, Van Der Ryst E, Guillemain B, Ledru E, Delaporte E, de The G, Gessain A. 1997. Molecular epidemiology of 58 new African human T-cell leukemia virus type 1 (HTLV-1) strains: identification of a new and distinct HTLV-1 molecular subtype in Central Africa and in Pygmies. J Virol 71:1317–1333. 10.1128/jvi.71.2.1317-1333.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu M, Yang L, Zhang L, Liu B, Merling R, Xia Z, Giam CZ. 2008. Human T-cell leukemia virus type 1 infection leads to arrest in the G1 phase of the cell cycle. J Virol 82:8442–8455. 10.1128/JVI.00091-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ma G, Yasunaga J, Matsuoka M. 2016. Multifaceted functions and roles of HBZ in HTLV-1 pathogenesis. Retrovirology 13:16. 10.1186/s12977-016-0249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Umeki K, Hisada M, Maloney EM, Hanchard B, Okayama A. 2009. Proviral loads and clonal expansion of HTLV-1-infected cells following vertical transmission: a 10-year follow-up of children in Jamaica. Intervirology 52:115–122. 10.1159/000219384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tanaka G, Okayama A, Watanabe T, Aizawa S, Stuver S, Mueller N, Hsieh CC, Tsubouchi H. 2005. The clonal expansion of human T lymphotropic virus type 1-infected T cells: a comparison between seroconverters and long-term carriers. J Infect Dis 191:1140–1147. 10.1086/428625. [DOI] [PubMed] [Google Scholar]
- 188.Mahgoub M, Yasunaga JI, Iwami S, Nakaoka S, Koizumi Y, Shimura K, Matsuoka M. 2018. Sporadic on/off switching of HTLV-1 Tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc Natl Acad Sci USA 115:E1269–E1278. 10.1073/pnas.1715724115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Miura M, Dey S, Ramanayake S, Singh A, Rueda DS, Bangham CRM. 2019. Kinetics of HTLV-1 reactivation from latency quantified by single-molecule RNA FISH and stochastic modeling. PLoS Pathog 15:e1008164. 10.1371/journal.ppat.1008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Giam CZ, Semmes OJ. 2016. HTLV-1 infection and adult T-cell leukemia/lymphoma—a tale of two proteins: Tax and HBZ. Viruses 8:161. 10.3390/v8060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Araya N, Sato T, Ando H, Tomaru U, Yoshida M, Coler-Reilly A, Yagishita N, Yamauchi J, Hasegawa A, Kannagi M, Hasegawa Y, Takahashi K, Kunitomo Y, Tanaka Y, Nakajima T, Nishioka K, Utsunomiya A, Jacobson S, Yamano Y. 2014. HTLV-1 induces a Th1-like state in CD4+ CCR4+ T cells. J Clin Invest 124:3431–3442. 10.1172/JCI75250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamano Y, Coler-Reilly A. 2017. HTLV-1 induces a Th1-like state in CD4+ CCR4+ T cells that produces an inflammatory positive feedback loop via astrocytes in HAM/TSP. J Neuroimmunol 304:51–55. 10.1016/j.jneuroim.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 193.Bangham CR, Araujo A, Yamano Y, Taylor GP. 2015. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat Rev Dis Primers 1:15012. 10.1038/nrdp.2015.12. [DOI] [PubMed] [Google Scholar]
- 194.Hirons A, Khoury G, Purcell DFJ. 2021. Human T-cell lymphotropic virus type-1: a lifelong persistent infection, yet never truly silent. Lancet Infect Dis 21:e2–e10. 10.1016/S1473-3099(20)30328-5. [DOI] [PubMed] [Google Scholar]
- 195.Kusuhara K, Sonoda S, Takahashi K, Tokugawa K, Fukushige J, Ueda K. 1987. Mother-to-child transmission of human T-cell leukemia virus type I (HTLV-I): a fifteen-year follow-up study in Okinawa. Int J Cancer 40:755–757. 10.1002/ijc.2910400607. [DOI] [PubMed] [Google Scholar]
- 196.Nyambi PN, Ville Y, Louwagie J, Bedjabaga I, Glowaczower E, Peeters M, Kerouedan D, Dazza M, Larouze B, van der Groen G, Delaporte E. 1996. Mother-to-child transmission of human T-cell lymphotropic virus types I and II (HTLV-I/II) in Gabon: a prospective follow-up of 4 years. J Acquir Immune Defic Syndr Hum Retrovirol 12:187–192. 10.1097/00042560-199606010-00013. [DOI] [PubMed] [Google Scholar]
- 197.Jacob F, Santos-Fortuna E, Azevedo RS, Caterino-de-Araujo A. 2008. Serological patterns and temporal trends of HTLV-1/2 infection in high-risk populations attending public health units in São Paulo. Brazil J Clinical Virology 42:149–155. 10.1016/j.jcv.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 198.Suetake N, Takeuchi Y, Shimizu N, Uesato H, Kuroume T, Hoshino H. 1989. Frequent lack of antibodies against human T-cell leukemia virus type I gag proteins p24 or p19 in sera of patients with adult T-cell leukemia. J Cancer Res Clin Oncol 115:279–284. 10.1007/BF00391703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lipka JJ, Miyoshi I, Hadlock KG, Reyes GR, Chow TP, Blattner WA, Shaw GM, Hanson CV, Gallo D, Chan L, Foung SKH. 1992. Segregation of human T cell lymphotropic virus type I and II infections by antibody reactivity to unique viral epitopes. J Infectious Diseases 165:268–272. 10.1093/infdis/165.2.268. [DOI] [PubMed] [Google Scholar]
- 200.Kuramitsu M, Sekizuka T, Yamochi T, Firouzi S, Sato T, Umeki K, Sasaki D, Hasegawa H, Kubota R, Sobata R, Matsumoto C, Kaneko N, Momose H, Araki K, Saito M, Nosaka K, Utsunomiya A, Koh KR, Ogata M, Uchimaru K, Iwanaga M, Sagara Y, Yamano Y, Okayama A, Miura K, Satake M, Saito S, Itabashi K, Yamaguchi K, Kuroda M, Watanabe T, Okuma K, Hamaguchi I. 2017. Proviral features of human T cell leukemia virus type 1 in carriers with indeterminate Western blot analysis results. J Clin Microbiol 55:2838–2849. 10.1128/JCM.00659-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cassar O, Gessain A. 2017. Serological and molecular methods to study epidemiological aspects of human T-cell lymphotropic virus type 1 infection. Methods Mol Biol 1582:3–24. 10.1007/978-1-4939-6872-5_1. [DOI] [PubMed] [Google Scholar]
- 202.Jacob F, De Los Santos-Fortuna E, Azevedo RS, Caterino-De-Araujo A. 2007. Performances of HTLV serological tests in diagnosing HTLV infection in high-risk population of Sao Paulo. Rev Inst Med Trop Sao Paulo 49:361–364. 10.1590/S0036-46652007000600005. [DOI] [PubMed] [Google Scholar]
- 203.Hayes CG, Burans JR, Oberst RB. 1991. Antibodies to human T lymphotropic virus type I in a population from the Philippines: evidence for cross-reactivity with Plasmodium falciparum. J Infect Dis 163:257–262. 10.1093/infdis/163.2.257. [DOI] [PubMed] [Google Scholar]
- 204.Mahieux R, Horal P, Mauclère P, Mercereau-Puijalon O, Guillotte M, Meertens L, Murphy E, Gessain A. 2000. Human T-cell lymphotropic virus type 1 Gag indeterminate western blot patterns in Central Africa: relationship to Plasmodium falciparum infection. J Clin Microbiol 38:4049–4057. 10.1128/JCM.38.11.4049-4057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Porter KR, Liang L, Long GW, Bangs MJ, Anthony R, Andersen EM, Hayes CG. 1994. Evidence for anti-Plasmodium falciparum antibodies that cross-react with human T-lymphotropic virus type I proteins in a population in Irian Jaya, Indonesia. Clin Diagn Lab Immunol 1:11–15. 10.1128/cdli.1.1.11-15.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Garin B, Gosselin S, de Thé G, Gessain A. 1994. HTLV‐I/II infection in a high viral endemic area of Zaire, Central Africa: comparative evaluation of serology, PCR, and significance of indeterminate Western blot pattern. J Med Virol 44:104–109. 10.1002/jmv.1890440119. [DOI] [PubMed] [Google Scholar]
- 207.Centers for Disease Control. 1988. Licensure of screening tests for antibody to human T-lymphotropic virus type I. MMWR Morb Mortal Wkly Rep 37:736–740. [PubMed] [Google Scholar]
- 208.Centers for Disease Control and Prevention/U.S. Public Health Service Working Group. 1993. Recommendations for counseling persons infected with human T-lymphotrophic virus, types I and II. MMWR Recomm Rep 42:1–13. [PubMed] [Google Scholar]
- 209.Zaaijer HL, Cuypers HT, Dudok de Wit C, Lelie PN. 1994. Results of 1-year screening of donors in The Netherlands for human T-lymphotropic virus (HTLV) type I: significance of Western blot patterns for confirmation of HTLV infection. Transfusion 34:877–880. [DOI] [PubMed] [Google Scholar]
- 210.HTLV European Research Network. 1996. Seroepidemiology of the human T-cell leukaemia/lymphoma viruses in Europe. J Acquir Immune Defic Syndr Hum Retrovirol 13:68–77. [DOI] [PubMed] [Google Scholar]
- 211.Calattini S, Betsem E, Bassot S, Chevalier SA, Mahieux R, Froment A, Gessain A. 2009. New strain of human T lymphotropic virus (HTLV) type 3 in a Pygmy from Cameroon with peculiar HTLV serologic results. J Infect Dis 199:561–564. 10.1086/596206. [DOI] [PubMed] [Google Scholar]
- 212.Calattini S, Chevalier SA, Duprez R, Bassot S, Froment A, Mahieux R, Gessain A. 2005. Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology 2:30. 10.1186/1742-4690-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nishimura Y, Fukuoka N, Fukuyoshi Y, Matsuo Y, Yamaguchi K, Takatsuki K. 1990. Evaluation of specificity of EIA kit (ED-007). Rinsho Byori 38:1363–1367. [PubMed] [Google Scholar]
- 214.Costa EAS, Magri MC, Caterino-de-Araujo A. 2011. The best algorithm to confirm the diagnosis of HTLV-1 and HTLV-2 in at-risk individuals from Sao Paulo, Brazil. J Virol Methods 173:280–286. 10.1016/j.jviromet.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 215.Yurick D, Khoury G, Clemens B, Loh L, Pham H, Kedzierska K, Einsiedel L, Purcell DFJ. 2018. A multiplex droplet digital PCR assay for quantification of HTLV-1c DNA proviral load and T cells from blood and respiratory exudates sampled in a remote setting. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kamihira S, Sugahara K, Tsuruda K, Minami S, Uemura A, Akamatsu N, Nagai H, Murata K, Hasegawa H, Hirakata Y, Takasaki Y, Tsukasaki K, Yamada Y. 2005. Proviral status of HTLV-1 integrated into the host genomic DNA of adult T-cell leukemia cells. Clin Lab Haematol 27:235–241. 10.1111/j.1365-2257.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 217.Kwaan N, Lee TH, Chafets DM, Nass C, Newman B, Smith J, Garratty G, Murphy EL, HTLV Outcomes Study (HOST) Investigators. 2006. Long-term variations in human T lymphotropic virus (HTLV)-I and HTLV-II proviral loads and association with clinical data. J Infect Dis 194:1557–1564. 10.1086/508899. [DOI] [PubMed] [Google Scholar]
- 218.Furtado M, Andrade RG, Romanelli LCF, Ribeiro MA, Ribas JG, Torres EB, Barbosa-Stancioli EF, Proietti A, Martins ML. 2012. Monitoring the HTLV-1 proviral load in the peripheral blood of asymptomatic carriers and patients with HTLV-associated myelopathy/tropical spastic paraparesis from a Brazilian cohort: ROC curve analysis to establish the threshold for risk disease. J Med Virol 84:664–671. 10.1002/jmv.23227. [DOI] [PubMed] [Google Scholar]
- 219.Grassi MFR, Olavarria VN, Kruschewsky RA, Mascarenhas RE, Dourado I, Correia LCL, Castro-Costa CMd, Galvao-Castro B. 2011. Human T cell lymphotropic virus type 1 (HTLV-1) proviral load of HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients according to new diagnostic criteria of HAM/TSP. J Med Virol 83:1269–1274. 10.1002/jmv.22087. [DOI] [PubMed] [Google Scholar]
- 220.Hashimoto K, Higuchi I, Osame M, Izumo S. 1998. Quantitative in situ PCR assay of HTLV-1-infected cells in peripheral blood lymphocytes of patients with ATL, HAM/TSP, and asymptomatic carriers. J Neurol Sci 159:67–72. 10.1016/S0022-510X(98)00138-5. [DOI] [PubMed] [Google Scholar]
- 221.Waters A, Oliveira ALA, Coughlan S, de Venecia C, Schor D, Leite AC, Araujo AQC, Hall WW. 2011. Multiplex real-time PCR for the detection and quantitation of HTLV-1 and HTLV-2 proviral load: addressing the issue of indeterminate HTLV results. J Clin Virol 52:38–44. 10.1016/j.jcv.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 222.Murphy EL. 2016. Infection with human T-lymphotropic virus types-1 and -2 (HTLV-1 and -2): implications for blood transfusion safety. Transfus Clin Biol 23:13–19. 10.1016/j.tracli.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Goncalves DU, Proietti FA, Ribas JG, Araujo MG, Pinheiro SR, Guedes AC, Carneiro-Proietti AB. 2010. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev 23:577–589. 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stigum H, Magnus P, Samdal HH, Nord E. 2000. Human T-cell lymphotropic virus testing of blood donors in Norway: a cost-effect model. Int J Epidemiol 29:1076–1084. 10.1093/ije/29.6.1076. [DOI] [PubMed] [Google Scholar]
- 225.Itabashi K. 2016. HTLV-1 Boshikansen Yobo Taisaku Manyuaru-HTLV-1. https://www.mhlw.go.jp/bunya/kodomo/boshi-hoken16/dl/06.pdf.
- 226.Ministry of Health Brazil. 2013. Guia De Manejo Clinico Da Infeccao Pelo HTLV. http://www.sierj.org.br/artigos/htlv_manual_final_pdf_25082.pdf.
- 227.Ministry of Health Brazil. 2013. Manejo clinico HTLV [Clinical management of HTLV]. https://prefeitura.pbh.gov.br/sites/default/files/estrutura-de-governo/saude/2018/publicacaoes-da-vigilancia-em-saude/manejo_clinico_HTLV.PDF. Accessed 3 November 2021.
- 228.Ministry of Health Chile. 2018. Patient care protocol with HTLV-1. https://diprece.minsal.cl/programas-de-salud/programas-enfermedades-transmisibles/virus-linfotropico-humano-de-celulas-t-tipo-1-htlv-1/. Accessed 3 November 2021.
- 229.Hewagama S, Krishnaswamy S, King L, Davis J, Baird R. 2014. Human T-cell lymphotropic virus type 1 exposures following blood-borne virus incidents in central Australia, 2002–2012. Clin Infect Dis 59:85–87. 10.1093/cid/ciu227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Iwahara Y, Takehara N, Kataoka R, Sawada T, Ohtsuki Y, Nakachi H, Maehama T, Okayama T, Miyoshi I. 1990. Transmission of HTLV-I to rabbits via semen and breast milk from seropositive healthy persons. Int J Cancer 45:980–983. 10.1002/ijc.2910450534. [DOI] [PubMed] [Google Scholar]
- 231.Kinoshita K, Hino S, Amagaski T, Ikeda S, Yamada Y, Suzuyama J, Momita S, Toriya K, Kamihira S, Ichimaru M. 1984. Demonstration of adult T-cell leukemia virus antigen in milk from three sero-positive mothers. Gann 75:103–105. [PubMed] [Google Scholar]
- 232.Hino S, Yamaguchi K, Katamine S, Sugiyama H, Amagasaki T, Kinoshita K, Yoshida Y, Doi H, Tsuji Y, Miyamoto T. 1985. Mother-to-child transmission of human T-cell leukemia virus type I. Jpn J Cancer Res 76:474–480. [PubMed] [Google Scholar]
- 233.Satow Y, Hashido M, Honda H, Mizuno M, Kawana T, Ishikawa K, Hayami M. 1991. Detection of HTLV-I antigen in peripheral and cord blood lymphocytes from carrier mothers. Lancet 338:915–916. 10.1016/0140-6736(91)91775-P. [DOI] [PubMed] [Google Scholar]
- 234.Li HC, Biggar RJ, Miley WJ, Maloney EM, Cranston B, Hanchard B, Hisada M. 2004. Provirus load in breast milk and risk of mother-to-child transmission of human T lymphotropic virus type I. J Infect Dis 190:1275–1278. 10.1086/423941. [DOI] [PubMed] [Google Scholar]
- 235.Tsuji Y, Doi H, Yamabe T, Ishimaru T, Miyamoto T, Hino S. 1990. Prevention of mother-to-child transmission of human T-lymphotropic virus type-I. Pediatrics 86:11–17. 10.1542/peds.86.1.11. [DOI] [PubMed] [Google Scholar]
- 236.Komuro A, Hayami M, Fujii H, Miyahara S, Hirayama M. 1983. Vertical transmission of adult T-cell leukemia virus. Lancet i:240. [DOI] [PubMed] [Google Scholar]
- 237.Belec L, Georges-Courbot MC, Georges A, Mohamed AS, Londos-Gagliardi D, Hallouin MC, Hocini H, Guillemain B. 1996. Cervicovaginal synthesis of IgG antibodies to the immunodominant 175–199 domain of the surface glycoprotein gp46 of human T-cell leukemia virus type I. J Med Virol 50:42–49. . [DOI] [PubMed] [Google Scholar]
- 238.Carneiro-Proietti AB, Amaranto-Damasio MS, Leal-Horiguchi CF, Bastos RH, Seabra-Freitas G, Borowiak DR, Ribeiro MA, Proietti FA, Ferreira AS, Martins ML. 2014. Mother-to-child transmission of human T-cell lymphotropic viruses 1/2: what we know, and what are the gaps in understanding and preventing this route of infection. J Pediatr Infect Dis Soc 3(Suppl 1):S24–S29. 10.1093/jpids/piu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wiktor SZ, Pate EJ, Rosenberg PS, Barnett M, Palmer P, Medeiros D, Maloney EM, Blattner WA. 1997. Mother-to-child transmission of human T-cell lymphotropic virus type I associated with prolonged breast-feeding. J Hum Virol 1:37–44. [PubMed] [Google Scholar]
- 240.Hirata M, Hayashi J, Noguchi A, Nakashima K, Kajiyama W, Kashiwagi S, Sawada T. 1992. The effects of breastfeeding and presence of antibody to p40(tax) protein of human T cell lymphotropic virus type I on mother to child transmission. Int J Epidemiol 21:989–994. 10.1093/ije/21.5.989. [DOI] [PubMed] [Google Scholar]
- 241.Takahashi K, Takezaki T, Oki T, Kawakami K, Yashiki S, Fujiyoshi T, Usuku K, Mueller N, Osame M, Miyata K, Nagata Y, Sonoda S. , Mother-to-Child Transmission Study Group. 1991. Inhibitory effect of maternal antibody on mother-to-child transmission of human T-lymphotropic virus type I. Int J Cancer 49:673–677. 10.1002/ijc.2910490508. [DOI] [PubMed] [Google Scholar]
- 242.Oki T, Yoshinaga M, Otsuka H, Miyata K, Sonoda S, Nagata Y. 2010. A sero-epidemiological study on mother-to-child transmission of HTLV-I in southern Kyushu, Japan. Asia Oceania J Obstet Gynaecol 18:371–377. 10.1111/j.1447-0756.1992.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 243.Takezaki T, Tajima K, Ito M, Ito SI, Kinoshita KI, Tachibana K, Matsushita Y. 1997. Short-term breast-feeding may reduce the risk of vertical transmission of HTLV-I. Leukemia 11:60–62. [PubMed] [Google Scholar]
- 244.Hino S. 2011. Establishment of the milk-borne transmission as a key factor for the peculiar endemicity of human T-lymphotropic virus type 1 (HTLV-1): the ATL Prevention Program Nagasaki. Proc Jpn Acad Ser B 87:152–166. 10.2183/pjab.87.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Carles G, Tortevoye P, Tuppin P, Ureta-Vidal A, Peneau C, El Guindi W, Gessain A. 2004. HTLV1 infection and pregnancy (infection par le rétrovirus HTLV-1 et grossess). J Gynecol Obstet Biol Reprod 33:14–20. 10.1016/S0368-2315(04)96307-7. [DOI] [PubMed] [Google Scholar]
- 246.Van Tienen C, McConkey SJ, De Silva TI, Cotten M, Kaye S, Sarge-Njie R, Da Costa C, Goncalves N, Parker J, Vincent T, Jaye A, Aaby P, Whittle H, Van Der Loeff MS. 2011. Maternal proviral load and vertical transmission of human T cell lymphotropic virus type 1 in Guinea-Bissau. Retrovirology 8:A72. 10.1186/1742-4690-8-S1-A72. [DOI] [PubMed] [Google Scholar]
- 247.Ureta-Vidal A, Angelin-Duclos C, Tortevoye P, Murphy E, Lep-Re J-F, Buigues RP, Jolly N, Joubert M, Carles G, Pouliquen J-F, The GD, Moreau J-P, Gessain A. 1999. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: implication of high antiviral antibody titer and high proviral load in carrier mothers. Int J Cancer 82:832–836. . [DOI] [PubMed] [Google Scholar]
- 248.Paiva AM, Assone T, Haziot MEJ, Smid J, Fonseca LAM, Luiz ODC, de Oliveira ACP, Casseb J. 2018. Risk factors associated with HTLV-1 vertical transmission in Brazil: longer breastfeeding, higher maternal proviral load and previous HTLV-1-infected offspring. Sci Rep 8:7742. 10.1038/s41598-018-25939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Biggar RJ, Ng J, Kim N, Hisada M, Li HC, Cranston B, Hanchard B, Maloney EM. 2006. Human leukocyte antigen concordance and the transmission risk via breast-feeding of human T cell lymphotropic virus type I. J Infect Dis 193:277–282. 10.1086/498910. [DOI] [PubMed] [Google Scholar]
- 250.Hisada M, Maloney EM, Sawada T, Miley WJ, Palmer P, Hanchard B, Goedert JJ, Manns A. 2002. Virus markers associated with vertical transmission of human T lymphotropic virus type 1 in Jamaica. Clin Infect Dis 34:1551–1557. 10.1086/340537. [DOI] [PubMed] [Google Scholar]
- 251.Zunt JR, Dezzutti CS, Montano SM, Thomas KK, Alarcon JO, Quijano E, Courtois BN, Sanchez JL, Campos P, Gotuzzo E, Guenthner PC, Lal RB, Holmes KK. 2002. Cervical shedding of human T cell lymphotropic virus type I is associated with cervicitis. J Infect Dis 186:1669–1672. 10.1086/345364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kajiyama W, Kashiwagi S, Ikematsu H, Hayashi J, Nomura H, Okochi K. 1986. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis 154:851–857. 10.1093/infdis/154.5.851. [DOI] [PubMed] [Google Scholar]
- 253.Stuver SO, Tachibana N, Okayama A, Shioiri S, Tsunetoshi Y, Tsuda K, Mueller NE. 1993. Heterosexual transmission of human T cell leukemia/lymphoma virus type I among married couples in southwestern Japan: an initial report from the Miyazaki Cohort Study. J Infect Dis 167:57–65. 10.1093/infdis/167.1.57. [DOI] [PubMed] [Google Scholar]
- 254.Kaplan JE, Khabbaz RF, Murphy EL, Hermansen S, Roberts C, Lal R, Heneine W, Wright D, Matijas L, Thomson R, Rudolph D, Switzer WM, Kleinman S, Busch M, Schreiber GB. 1996. Male-to-female transmission of human T-cell lymphotropic virus types I and II: association with viral load. J Acquir Immune Defic Syndr Hum Retrovirol 12:193–201. 10.1097/00042560-199606010-00014. [DOI] [PubMed] [Google Scholar]
- 255.Diaz TH, Nibot SC, Cruz SO, Blanco de Armas M, Sanchez RJ, Lubian CA. 2009. Seroepidemiological follow-up of sexual partners of HTLV-I seropositive individuals in Cuba. Seguimiento Seroepidemiologico de Contactos Sexuales de Individuos Seropositivos al HTLV-I en Cuba 61:269–274. [Google Scholar]
- 256.Bellei NC, Granato CF, Tomyiama H, Castelo A, Ferreira O. 1996. HTLV infection in a group of prostitutes and their male sexual clients in Brazil: seroprevalence and risk factors. Trans R Soc Trop Med Hyg 90:122–125. 10.1016/S0035-9203(96)90107-8. [DOI] [PubMed] [Google Scholar]
- 257.Roucoux DF, Wang B, Smith D, Nass CC, Smith J, Hutching ST, Newman B, Lee TH, Chafets DM, Murphy EL, Investigators HOS, HTLV Outcomes Study Investigators. 2005. A prospective study of sexual transmission of human T lymphotropic virus (HTLV)-I and HTLV-II. J Infect Dis 191:1490–1497. 10.1086/429410. [DOI] [PubMed] [Google Scholar]
- 258.Wignall FS, Hyams KC, Phillips IA, Escamilla J, Tejada A, Li O, Lopez F, Chauca G, Sanchez S, Roberts CR. 1992. Sexual transmission of human T-lymphotropic virus type I in Peruvian prostitutes. J Med Virol 38:44–48. 10.1002/jmv.1890380110. [DOI] [PubMed] [Google Scholar]
- 259.Nakashima K, Kashiwagi S, Kajiyama W, Hirata M, Hayashi J, Noguchi A, Urabe K, Minami K, Maeda Y. 1995. Sexual transmission of human T-lymphotropic virus type I among female prostitutes and among patients with sexually transmitted diseases in Fukuoka, Kyushu, Japan. Am J Epidemiol 141:305–311. 10.1093/aje/141.4.305. [DOI] [PubMed] [Google Scholar]
- 260.Sullivan MT, Williams AE, Fang CT, Notari EP, Poiesz BJ, Ehrlich GD, American Red Cross HTLV-I/II Collaborative Study Group. 1993. Human T-lymphotropic virus (HTLV) types I and II infection in sexual contacts and family members of blood donors who are seropositive for HTLV type I or II. Transfusion 33:585–590. 10.1046/j.1537-2995.1993.33793325055.x. [DOI] [PubMed] [Google Scholar]
- 261.Murphy E, Figueroa JP, Gibbs WN, Brathwaite A, Holding-Cobham M, Waters D, Cranston B, Hanchard B, Blattner W. 1989. Sexual transmission of human T-lymphotropic virus type I (HTLV-I). Ann Intern Med 111:555–560. 10.7326/0003-4819-111-7-555. [DOI] [PubMed] [Google Scholar]
- 262.Alarcon JO, Friedman HB, Montano SM, Zunt JR, Holmes KK, Quinnan GV, Jr.. 2006. High endemicity of human T-cell lymphotropic virus type 1 among pregnant women in Peru. J Acquir Immune Defic Syndr 42:604–609. 10.1097/01.qai.0000221680.52563.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lee SY, Mastushita K, Machida J, Tajiri M, Yamaguchi K, Takatsuki K. 1987. Human T-cell leukemia virus type I infection in hemodialysis patients. Cancer 60:1474–1478. . [DOI] [PubMed] [Google Scholar]
- 264.Jason JM, McDougal JS, Cabradilla C, Kalyanaraman VS, Evatt BL. 1985. Human T-cell leukemia virus (HTLV-I) p24 antibody in New York City blood product recipients. Am J Hematol 20:129–137. 10.1002/ajh.2830200205. [DOI] [PubMed] [Google Scholar]
- 265.Hara T, Takahashi Y, Sonoda S, Kusuhara K, Ueda K. 1987. Human T-cell lymphotropic virus type I infection in neonates. Am J Dis Child 141:764–765. [DOI] [PubMed] [Google Scholar]
- 266.Donegan E, Lee H, Operskalski EA, Shaw GM, Kleinman SH, Busch MP, Stevens CE, Schiff ER, Nowicki MJ, Hollingsworth CG. 1994. Transfusion transmission of retroviruses: human T-lymphotropic virus types I and II compared with human immunodeficiency virus type 1. Transfusion 34:478–483. 10.1046/j.1537-2995.1994.34694295061.x. [DOI] [PubMed] [Google Scholar]
- 267.Yamauchi J, Yamano Y, Yuzawa K. 2019. Risk of human T-cell leukemia virus type 1 infection in kidney transplantation. N Engl J Med 380:296–298. 10.1056/NEJMc1809779. [DOI] [PubMed] [Google Scholar]
- 268.Manns A, Wilks RJ, Murphy EL, Haynes G, Figueroa JP, Barnett M, Hanchard B, Blattner WA. 1992. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int J Cancer 51:886–891. 10.1002/ijc.2910510609. [DOI] [PubMed] [Google Scholar]
- 269.Kleinman S, Swanson P, Allain JP, Lee H. 1993. Transfusion transmission of human T-lymphotropic virus type I and type II: serologic and polymerase chain-reaction results in recipients identified through look-back investigations. Transfusion 33:14–18. 10.1046/j.1537-2995.1993.33193142303.x. [DOI] [PubMed] [Google Scholar]
- 270.Bandeira LM, Uehara SNO, Puga MAM, Rezende GR, Vicente ACP, Domingos JA, do Lago BV, Niel C, Motta-Castro ARC. 2018. HTLV-1 intrafamilial transmission among Japanese immigrants in Brazil. J Med Virol 90:351–357. 10.1002/jmv.24938. [DOI] [PubMed] [Google Scholar]
- 271.Schierhout G, McGregor S, Gessain A, Einsiedel L, Martinello M, Kaldor J. 2019. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis 20:133–143. 10.1016/S1473-3099(19)30402-5. [DOI] [PubMed] [Google Scholar]
- 272.Kamihira S, Yamada Y, Ikeda S, Momita S, Atogami S, Sohda H, Tomonaga M, Tokudome S. 1992. Risk of adult T-cell leukemia developing in individuals with HTLV-I infection. Leuk Lymphoma 6:437–439. 10.3109/10428199209053580. [DOI] [Google Scholar]
- 273.Arisawa K, Soda M, Akahoshi M, Matsuo T, Nakashima E, Tomonaga M, Saito H. 1998. Human T-lymphotropic virus type-I infection, antibody titers and cause-specific mortality among atomic-bomb survivors. Jpn J Cancer Res 89:797–805. 10.1111/j.1349-7006.1998.tb00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Arisawa K, Sobue T, Yoshimi I, Soda M, Shirahama S, Doi H, Katamine S, Saito H, Urata M. M. 2003. Human T-lymphotropic virus type-I infection, survival and cancer risk in southwestern Japan: a prospective cohort study. Cancer Causes Control 14:889–896. 10.1023/B:CACO.0000003853.82298.96. [DOI] [PubMed] [Google Scholar]
- 275.Arisawa K, Soda M, Akahoshi M, Fujiwara S, Uemura H, Hiyoshi M, Takeda H, Kashino W, Suyama A. 2006. Human T-cell lymphotropic virus type 1 infection and risk of cancer: 15.4-year longitudinal study among atomic bomb survivors in Nagasaki. Cancer Sci 97:535–539. 10.1111/j.1349-7006.2006.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Iwanaga M, Watanabe T, Utsunomiya A, Okayama A, Uchimaru K, Koh KR, Ogata M, Kikuchi H, Sagara Y, Uozumi K, Mochizuki M, Tsukasaki K, Saburi Y, Yamamura M, Tanaka J, Moriuchi Y, Hino S, Kamihira S, Yamaguchi K, for the Joint Study on Predisposing Factors of ATL Development investigators. 2010. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood 116:1211–1219. 10.1182/blood-2009-12-257410. [DOI] [PubMed] [Google Scholar]
- 277.IARC Working Group. 1996. IARC monographs on the evaluation of carcinogenic risks to humans: human immunodeficiency viruses and human T-cell lymphotropic viruses. World Health Organization, London, United Kingdom. [PMC free article] [PubMed] [Google Scholar]
- 278.Shimoyama M, Lymphoma Study Group (1984–87). 1991. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: a report from the Lymphoma Study Group (1984–87). Br J Haematol 79:428–437. 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 279.Katsuya H, Ishitsuka K, Utsunomiya A, Hanada S, Eto T, Moriuchi Y, Saburi Y, Miyahara M, Sueoka E, Uike N, Yoshida S, Yamashita K, Tsukasaki K, Suzushima H, Ohno Y, Matsuoka H, Jo T, Amano M, Hino R, Shimokawa M, Kawai K, Suzumiya J, Tamura K, ATL-Prognostic Index Project. 2015. Treatment and survival among 1594 patients with ATL. Blood 126:2570–2577. 10.1182/blood-2015-03-632489. [DOI] [PubMed] [Google Scholar]
- 280.Nosaka K, Iwanaga M, Imaizumi Y, Ishitsuka K, Ishizawa K, Ishida Y, Amano M, Ishida T, Uike N, Utsunomiya A, Ohshima K, Kawai K, Tanaka J, Tokura Y, Tobinai K, Watanabe T, Uchimaru K, Tsukasaki K. 2017. Epidemiological and clinical features of adult T-cell leukemia-lymphoma in Japan, 2010–2011: a nationwide survey. Cancer Sci 108:2478–2486. 10.1111/cas.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. 2019. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 8:e180–e190. 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 282.Pombo De Oliveira MS, Loureiro P, Bittencourt A, Chiattone C, Borducchi D, De Carvalho SM, Barbosa HS, Rios M, Sill A, Cleghorn F, Blattner W, The Brazilian ATLL Study Group. 1999. Geographic diversity of adult T-cell leukemia/lymphoma in Brazil. Int J Cancer 83:291–298. . [DOI] [PubMed] [Google Scholar]
- 283.Hanchard B. 1996. Adult T-cell leukemia/lymphoma in Jamaica: 1986–1995. J Acquir Immune Defic Syndr Hum Retrovirol 13(Suppl 1):S20–S25. 10.1097/00042560-199600001-00005. [DOI] [PubMed] [Google Scholar]
- 284.Phillips AA, Shapira I, Willim RD, Sanmugarajah J, Solomon WB, Horwitz SM, Savage DG, Bhagat G, Soff G, Zain JM, Alobeid B, Seshan VE, O’Connor OA. 2010. A critical analysis of prognostic factors in North American patients with human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a multicenter clinicopathologic experience and new prognostic score. Cancer 116:3438–3446. 10.1002/cncr.25147. [DOI] [PubMed] [Google Scholar]
- 285.Arisawa K, Soda M, Endo S, Kurokawa K, Katamine S, Shimokawa I, Koba T, Takahashi T, Saito H, Doi H, Shirahama S. 2000. Evaluation of adult T-cell leukemia/lymphoma incidence and its impact on non-Hodgkin lymphoma incidence in southwestern Japan. Int J Cancer 85:319–324. . [DOI] [PubMed] [Google Scholar]
- 286.Satake M, Yamada Y, Atogami S, Yamaguchi K. 2015. The incidence of adult T-cell leukemia/lymphoma among human T-lymphotropic virus type 1 carriers in Japan. Leuk Lymphoma 56:1806–1812. 10.3109/10428194.2014.964700. [DOI] [PubMed] [Google Scholar]
- 287.Kondo T, Kono H, Nonaka H, Miyamoto N, Yoshida R, Bando F, Inoue H, Miyoshi I, Hinuma Y, Hanaoka M. 1987. Risk of adult T-cell leukemia/lymphoma in HTLV-I carriers. Lancet 2:159. [DOI] [PubMed] [Google Scholar]
- 288.Koga Y, Iwanaga M, Soda M, Inokuchi N, Sasaki D, Hasegawa H, Yanagihara K, Yamaguchi K, Kamihira S, Yamada Y. 2010. Trends in HTLV-1 prevalence and incidence of adult T-cell leukemia/lymphoma in Nagasaki, Japan. J Med Virol 82:668–674. 10.1002/jmv.21738. [DOI] [PubMed] [Google Scholar]
- 289.Murphy EL, Hanchard B, Figueroa JP, Gibbs WN, Lofters WS, Campbell M, Goedert JJ, Blattner WA. 1989. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int J Cancer 43:250–253. 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- 290.Kondo H, Soda M, Sawada N, Inoue M, Imaizumi Y, Miyazaki Y, Iwanaga M, Tanaka Y, Mizokami M, Tsugane S. 2016. Smoking is a risk factor for development of adult T-cell leukemia/lymphoma in Japanese human T-cell leukemia virus type-1 carriers. Cancer Causes Control 27:1059–1066. 10.1007/s10552-016-0784-8. [DOI] [PubMed] [Google Scholar]
- 291.Hodson A, Laydon DJ, Bain BJ, Fields PA, Taylor GP. 2013. Pre-morbid human T-lymphotropic virus type I proviral load, rather than percentage of abnormal lymphocytes, is associated with an increased risk of aggressive adult T-cell leukemia/lymphoma. Haematologica 98:385–388. 10.3324/haematol.2012.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Stienlauf S, Yahalom V, Shinar E, Sidi Y, Segal G, Schwartz E. 2013. Malignant diseases and mortality in blood donors infected with human T-lymphotropic virus type 1 in Israel. Int J Infect Dis 17:e1022–e1024. 10.1016/j.ijid.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 293.Ramassamy J-L, Tortevoye P, Ntab B, Seve B, Carles G, Gaquière D, Madec Y, Fontanet A, Gessain A. 2020. Adult T-cell leukemia/lymphoma incidence rate in French Guiana: a prospective cohort of women infected with HTLV-1. Blood Adv 4:2044–2048. 10.1182/bloodadvances.2020001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Tokudome S, Tokunaga O, Shimamoto Y, Miyamoto Y, Sumida I, Kikuchi M, Takeshita M, Ikeda T, Fujiwara K, Yoshihara M. 1989. Incidence of adult T-cell leukemia/lymphoma among human T-lymphotropic virus type I carriers in Saga. Cancer Res 49:226–228. [PubMed] [Google Scholar]
- 295.Manns A, Miley WJ, Wilks RJ, Morgan OS, Hanchard B, Wharfe G, Cranston B, Maloney E, Welles SL, Blattner WA, Waters D. 1999. Quantitative proviral DNA and antibody levels in the natural history of HTLV-I infection. J Infect Dis 180:1487–1493. 10.1086/315088. [DOI] [PubMed] [Google Scholar]
- 296.Arisaw K, Katamine S, Kamihira S, Kurokawa K, Sawada T, Soda M, Doi H, Saito H, Shirahama S. S. 2002. A nested case-control study of risk factors for adult T-cell leukemia/lymphoma among human T-cell lymphotropic virus type-I carriers in Japan. Cancer Causes Control 13:657–663. 10.1023/A:1019511224501. [DOI] [PubMed] [Google Scholar]
- 297.Hisada M, Okayama A, Shioiri S, Spiegelman DL, Stuver SO, Mueller NE. 1998. Risk factors for adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Blood 92:3557–3561. 10.1182/blood.V92.10.3557.422k09_3557_3561. [DOI] [PubMed] [Google Scholar]
- 298.Gabet A-S, Mortreux F, Talarmin A, Plumelle Y, Leclercq I, Leroy A, Gessain A, Clity E, Joubert M, Wattel E. 2000. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene 19:4954–4960. 10.1038/sj.onc.1203870. [DOI] [PubMed] [Google Scholar]
- 299.Yashiki S, Fujiyoshi T, Arima N, Osame M, Yoshinaga M, Nagata Y, Tara M, Nomura K, Utsunomiya A, Hanada S, Tajima K, Sonoda S. 2001. HLA-A*26, HLA-B*4002, HLA-B*4006, and HLA-B*4801 alleles predispose to adult T cell leukemia: the limited recognition of HTLV type 1 Tax peptide anchor motifs and epitopes to generate anti-HTLV type 1 tax CD8+ cytotoxic T lymphocytes. AIDS Res Hum Retroviruses 17:1047–1061. 10.1089/088922201300343735. [DOI] [PubMed] [Google Scholar]
- 300.De Castro-Costa CM, Araujo AQ, Barreto MM, Takayanagui OM, Sohler MP, da Silva EL, de Paula SM, Ishak R, Ribas JG, Rovirosa LC, Carton H, Gotuzzo E, Hall WW, Montano S, Murphy EL, Oger J, Remondegui C, Taylor GP. 2006. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res Hum Retroviruses 22:931–935. 10.1089/aid.2006.22.931. [DOI] [PubMed] [Google Scholar]
- 301.World Health Organization/Regional Office for the Western Pacific. 1988. Scientific Group on HTLV-I Infections and Associated Diseases, Kagoshima, Japan, 10–15 December 1988: report. WHO Regional Office for the Western Pacific, Manila, Philippines. [Google Scholar]
- 302.Cruickshank EK. 1956. A neuropathic syndrome of uncertain origin; review of 100 cases. West Indian Med J 5:147–158. [PubMed] [Google Scholar]
- 303.Montgomery RD, Cruickshank EK, Robertson WB, McMenemey WH. 1964. Clinical and pathological observations on Jamaican neuropathy; a report on 206 cases. Brain 87:425–462. 10.1093/brain/87.3.425. [DOI] [PubMed] [Google Scholar]
- 304.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet 1:1031–1032. [DOI] [PubMed] [Google Scholar]
- 305.Kaplan JE, Osame M, Kubota H, Igata A, Nishitani H, Maeda Y, Khabbaz RF, Janssen RS. 1990. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J Acquir Immune Defic Syndr 3:1096–1101. [PubMed] [Google Scholar]
- 306.Maloney EM, Cleghorn FR, Morgan OS, Rodgers-Johnson P, Cranston B, Jack N, Blattner WA, Bartholomew C, Manns A. 1998. Incidence of HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Jamaica and Trinidad. J Acquir Immune Defic Syndr Hum Retrovirol 17:167–170. 10.1097/00042560-199802010-00011. [DOI] [PubMed] [Google Scholar]
- 307.Orland JR, Engstrom J, Fridey J, Sacher RA, Smith JW, Nass C, Garratty G, Newman B, Smith D, Wang B, Loughlin K, Murphy EL, Study HO, HTLV Outcomes Study. 2003. Prevalence and clinical features of HTLV neurologic disease in the HTLV Outcomes Study. Neurology 61:1588–1594. 10.1212/01.WNL.0000096011.92542.DA. [DOI] [PubMed] [Google Scholar]
- 308.Romanelli LCF, Caramelli P, Martins ML, Goncalves DU, Proietti FA, Ribas JGR, Araujo MG, Carneiro-Proietti ABDF. 2013. Incidence of human T cell lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis in a long-term prospective cohort study of initially asymptomatic individuals in Brazil. AIDS Res Hum Retroviruses 29:1199–1202. 10.1089/aid.2013.0086. [DOI] [PubMed] [Google Scholar]
- 309.Primo J, Siqueira I, Nascimento MC, Oliveira MF, Farre L, Carvalho EM, Bittencourt AL. 2009. High HTLV-1 proviral load, a marker for HTLV-1 associated myelopathy/tropical spastic paraparesis, is also detected in patients with infective dermatitis associated with HTLV-1. Braz J Med Biol Res 42:761–764. 10.1590/S0100-879X2009005000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Olindo S, Lezin A, Cabre P, Merle H, Saint-Vil M, Edimonana Kaptue M, Signate A, Cesaire R, Smadja D. 2005. HTLV-1 proviral load in peripheral blood mononuclear cells quantified in 100 HAM/TSP patients: a marker of disease progression. J Neurol Sci 237:53–59. 10.1016/j.jns.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 311.Matsuzaki T, Nakagawa M, Nagai M, Usuku K, Higuchi I, Arimura K, Kubota H, Izumo S, Akiba S, Osame M. 2001. HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients, including 64 patients followed up for 10 years. J Neurovirol 7:228–234. [DOI] [PubMed] [Google Scholar]
- 312.Kira J, Koyanagi Y, Yamada T, Itoyama Y, Goto I, Yamamoto N, Sasaki H, Sakaki Y. 1991. Increased HTLV-I proviral DNA in HTLV-I-associated myelopathy: a quantitative polymerase chain reaction study. Ann Neurol 29:194–201. 10.1002/ana.410290214. [DOI] [PubMed] [Google Scholar]
- 313.Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, Ichinose M, Bangham CR, Izumo S, Osame M. 1998. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol 4:586–593. 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 314.Treviño A, Vicario JL, Lopez M, Parra P, Benito R, Ortiz De Lejarazu R, Ramos JM, Romero J, Mendoza C, Soriano V. 2013. Association between HLA alleles and HAM/TSP in individuals infected with HTLV-1. J Neurol 260:2551–2555. 10.1007/s00415-013-7014-z. [DOI] [PubMed] [Google Scholar]
- 315.Olindo S, Cabre P, Lezin A, Merle H, Saint-Vil M, Signate A, Bonnan M, Chalon A, Magnani L, Cesaire R, Smadja D. 2006. Natural history of human T-lymphotropic virus 1-associated myelopathy: a 14-year follow-up study. Arch Neurol 63:1560–1566. 10.1001/archneur.63.11.1560. [DOI] [PubMed] [Google Scholar]
- 316.Bangham CR, Hall SE, Jeffery KJ, Vine AM, Witkover A, Nowak MA, Wodarz D, Usuku K, Osame M. 1999. Genetic control and dynamics of the cellular immune response to the human T-cell leukaemia virus, HTLV-I. Philos Trans R Soc Lond B 354:691–700. 10.1098/rstb.1999.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Jeffery KJ, Usuku K, Hall SE, Matsumoto W, Taylor GP, Procter J, Bunce M, Ogg GS, Welsh KI, Weber JN, Lloyd AL, Nowak MA, Nagai M, Kodama D, Izumo S, Osame M, Bangham CR. 1999. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci USA 96:3848–3853. 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sato T, Yagishita N, Tamaki K, Inoue E, Hasegawa D, Nagasaka M, Suzuki H, Araya N, Coler-Reilly A, Hasegawa Y, Tsuboi Y, Takata A, Yamano Y. 2018. Proposal of classification criteria for HTLV-1-associated myelopathy/tropical spastic paraparesis disease activity. Front Microbiol 9:1651. 10.3389/fmicb.2018.01651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yamauchi J, Sato T, Yagishita N, Araya N, Hasegawa D, Tsutsumi S, Nagasaka M, Coler-Reilly A, Inoue E, Takata A, Hasegawa Y, Yamano Y. 2019. Use of cerebrospinal fluid CXCL10 and neopterin as biomarkers in HTLV-1-associated myelopathy/tropical spastic paraparesis treated with steroids. J Neurol Neurosurg Psychiatry 91:321–323. 10.1136/jnnp-2019-321955. [DOI] [PubMed] [Google Scholar]
- 320.Gascon MR, Capitao CG, Casseb J, Nogueira-Martins MC, Smid J, Oliveira AC. 2011. Prevalence of anxiety, depression and quality of life in HTLV-1-infected patients. Braz J Infect Dis 15:578–582. 10.1590/S1413-86702011000600013. [DOI] [PubMed] [Google Scholar]
- 321.Biswas HH, Engstrom JW, Kaidarova Z, Garratty G, Gibble JW, Newman BH, Smith JW, Ziman A, Fridey JL, Sacher RA, Murphy EL, Host HOS. 2009. Neurologic abnormalities in HTLV-I- and HTLV-II-infected individuals without overt myelopathy. Neurology 73:781–789. 10.1212/WNL.0b013e3181b6bba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Tanajura D, Castro N, Oliveira P, Neto A, Muniz A, Carvalho NB, Orge G, Santos S, Glesby MJ, Carvalho EM. 2015. Neurological manifestations in human T-cell lymphotropic virus type 1 (HTLV-1)-infected individuals without HTLV-1-associated myelopathy/tropical spastic paraparesis: a longitudinal cohort study. Clin Infect Dis 61:49–56. 10.1093/cid/civ229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kendall EA, González E, Espinoza I, Tipismana M, Verdonck K, Clark D, Vermund SH, Gotuzzo E. 2009. Early neurologic abnormalities associated with human T-cell lymphotropic virus type 1 infection in a cohort of Peruvian children. J Pediatr 155:700–706. 10.1016/j.jpeds.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ohba N, Matsumoto M, Sameshima M, Kabayama Y, Nakao K, Unoki K, Uehara F, Kawano K, Maruyama I, Osame M. 1989. Ocular manifestations in patients infected with human T-lymphotropic virus type I. Jpn J Ophthalmol 33:1–12. [PubMed] [Google Scholar]
- 325.Mochizuki M, Watanabe T, Yamaguchi K, Takatsuki K, Yoshimura K, Shirao M, Nakashima S, Mori S, Araki S, Miyata N. 1992. HTLV-I uveitis: a distinct clinical entity caused by HTLV-I. Jpn J Cancer Res 83:236–239. 10.1111/j.1349-7006.1992.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ohba N, Nakao K, Isashiki Y, Osame M, Sonoda S, Yashiki S, Yamaguchi K, Tajima K, Study Group for HTLV-I Associated Ocular Diseases. 1994. A multicenter case-control study of HTLV-I associated uveitis. Jpn J Ophthalmol 38:162–167. [PubMed] [Google Scholar]
- 327.Mochizuki M, Watanabe T, Yamaguchi K, Tajima K, Yoshimura K, Nakashima S, Shirao M, Araki S, Miyata N, Mori S. 1992. Uveitis associated with human T lymphotropic virus type I: seroepidemiologic, clinical, and virologic studies. J Infect Dis 166:943–944. 10.1093/infdis/166.4.943. [DOI] [PubMed] [Google Scholar]
- 328.Sagawa K, Mochizuki M, Masuoka K, Katagiri K, Katayama T, Maeda T, Tanimoto A, Sugita S, Watanabe T, Itoh K. 1995. Immunopathological mechanisms of human T cell lymphotropic virus type 1 (HTLV-I) uveitis: detection of HTLV-I-infected T cells in the eye and their constitutive cytokine production. J Clin Invest 95:852–858. 10.1172/JCI117735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ono A, Mochizuki M, Yamaguchi K, Miyata N, Watanabe T. 1997. Immunologic and virologic characterization of the primary infiltrating cells in the aqueous humor of human T-cell leukemia virus type-1 uveitis: accumulation of the human T-cell leukemia virus type-1-infected cells and constitutive expression of viral and interleukin-6 messenger ribonucleic acids. Invest Ophthalmol Vis Sci 38:676–689. [PubMed] [Google Scholar]
- 330.Nakao K, Ohba N, Matsumoto M. 1989. Noninfectious anterior uveitis in patients infected with human T-lymphotropic virus type I. Jpn J Ophthalmol 33:472–481. [PubMed] [Google Scholar]
- 331.Ohba N, Nakao K, Isashiki Y, Kaminagayoshi T, Sonoda S, Yashiki S, Osame M, Study Group for HTLV-I Associated Ocular Diseases. 1994. Clinical features of HTLV-I associated uveitis determined in multicenter collaborative study. Jpn J Ophthalmol 38:168–174. [PubMed] [Google Scholar]
- 332.Takahashi T, Takase H, Urano T, Sugita S, Miyata K, Miyata N, Mochizuki M. 2000. Clinical features of human T-lymphotropic virus type 1 uveitis: a long-term follow-up. Ocul Immunol Inflamm 8:235–241. 10.1076/ocii.8.4.235.6454. [DOI] [PubMed] [Google Scholar]
- 333.Nakao K, Ohba N, Nakagawa M, Osame M. 1999. Clinical course of HTLV-I-associated uveitis. Jpn J Ophthalmol 43:404–409. 10.1016/S0021-5155(99)00099-4. [DOI] [PubMed] [Google Scholar]
- 334.Ohguro N, Sonoda K-H, Takeuchi M, Matsumura M, Mochizuki M. 2012. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol 56:432–435. 10.1007/s10384-012-0158-z. [DOI] [PubMed] [Google Scholar]
- 335.Tsutsumi S, Sato T, Yagishita N, Yamauchi J, Araya N, Hasegawa D, Nagasaka M, Coler-Reilly ALG, Inoue E, Takata A, Yamano Y. 2019. Real-world clinical course of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Japan. Orphanet J Rare Dis 14:227. 10.1186/s13023-019-1212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ono A, Mochizuki M, Yamaguchi K, Miyata N, Watanabe T. 1995. Increased number of circulating HTLV-1-infected cells in peripheral blood mononuclear cells of HTLV-1 uveitis patients: a quantitative polymerase chain reaction study. Br J Ophthalmol 79:270–276. 10.1136/bjo.79.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.La Grenade L, Hanchard B, Fletcher V, Cranston B, Blattner W. 1990. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet 336:1345–1347. [DOI] [PubMed] [Google Scholar]
- 338.Sweet RD. 1966. A pattern of eczema in Jamaica. Br J Dermatol 78:93–100. 10.1111/j.1365-2133.1966.tb12181.x. [DOI] [PubMed] [Google Scholar]
- 339.La Grenade L, Manns A, Fletcher V, Derm D, Carberry C, Hanchard B, Maloney EM, Cranston B, Williams NP, Wilks R, Kang EC, Blattner WA. 1998. Clinical, pathologic, and immunologic features of human T-lymphotrophic virus type I-associated infective dermatitis in children. Arch Dermatol 134:439–444. 10.1001/archderm.134.4.439. [DOI] [PubMed] [Google Scholar]
- 340.De Oliveira M, Fatal PL, Primo JRL, Da Silva JLS, Batista EDS, Farre L, Bittencourt AL. 2012. Infective dermatitis associated with human T-cell lymphotropic virus type 1: evaluation of 42 cases observed in Bahia, Brazil. Clin Infect Dis 54:1714–1719. 10.1093/cid/cis273. [DOI] [PubMed] [Google Scholar]
- 341.Nascimento MC, Primo J, Bittencourt A, Siqueira I, de Fatima Oliveira M, Meyer R, Schriefer A, Santos SB, Carvalho EM. 2009. Infective dermatitis has similar immunological features to human T lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis. Clin Exp Immunol 156:455–462. 10.1111/j.1365-2249.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Hlela C, Graham N, Bhigjee AI, Taylor GP, Khumalo NP, Mosam A. 2013. Human T cell lymphotropic virus type 1- associated infective dermatitis in KwaZulu Natal. BMC Dermatol 13:11–11. 10.1186/1471-5945-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Maloney EM, Hisada M, Palmer P, Brooks K, Pate E, Wiktor SZ, Lagrenade L, Manns A. 2000. Human T cell lymphotropic virus type I-associated infective dermatitis in Jamaica: a case report of clinical and biologic correlates. Pediatr Infect Dis J 19:560–565. 10.1097/00006454-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 344.Hanchard B, LaGrenade L, Carberry C, Fletcher V, Williams E, Cranston B, Blattner WA, Manns A. 1991. Childhood infective dermatitis evolving into adult T-cell leukaemia after 17 years. Lancet 338:1593–1594. 10.1016/0140-6736(91)92413-V. [DOI] [PubMed] [Google Scholar]
- 345.LaGrenade L, Morgan C, Carberry C, Hanchard B, Fletcher V, Gray R, Cranston B, Rodgers-Johnson P, Manns A. 1995. Tropical spastic paraparesis occurring in HTLV-1 associated infective dermatitis: report of two cases. West Indian Med J 44:34–35. [PubMed] [Google Scholar]
- 346.Primo JRL, Brites C, de Oliveira M. d F S P, Moreno-Carvalho O, Machado M, Bittencourt AL. 2005. Infective dermatitis and human T cell lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis in childhood and adolescence. Clin Infect Dis 41:535–541. 10.1086/432058. [DOI] [PubMed] [Google Scholar]
- 347.Tsukasaki K, Yamada Y, Ikeda S, Tomonaga M. 1994. Infective dermatitis among patients with ATL in Japan. Int J Cancer 57:293. 10.1002/ijc.2910570227. [DOI] [PubMed] [Google Scholar]
- 348.Farre L, Paim de Oliveira MDF, Primo J, Vandamme A-M, Van Weyenbergh J, Bittencourt AL. 2008. Early sequential development of infective dermatitis, human T cell lymphotropic virus type 1-associated myelopathy, and adult T cell leukemia/lymphoma. Clin Infect Dis 46:440–442. 10.1086/524695. [DOI] [PubMed] [Google Scholar]
- 349.Maloney EM, Wiktor SZ, Palmer P, Cranston B, Pate EJ, Cohn S, Kim N, Miley W, Thomas TL, Blattner WA, Hanchard B. 2003. A cohort study of health effects of human T-cell lymphotropic virus type I infection in Jamaican children. Pediatrics 112:e136-42–e142. 10.1542/peds.112.2.e136. [DOI] [PubMed] [Google Scholar]
- 350.Oliveira PD, Kachimarek AC, Bittencourt AL. 2018. Early onset of HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) and Adult T-Cell Leukemia/Lymphoma (ATL): systematic search and review. J Trop Pediatr 64:151–161. 10.1093/tropej/fmx039. [DOI] [PubMed] [Google Scholar]
- 351.Varandas CMN, Da Silva JLS, Primo JRL, De Oliveira M, Moreno-Carvalho O, Farre L, Bittencourt AL. 2018. Early juvenile human T-cell lymphotropic virus type-1–associated myelopathy/tropical spastic paraparesis: study of 25 patients. Clin Infect Dis 67:1427–1433. 10.1093/cid/ciy289. [DOI] [PubMed] [Google Scholar]
- 352.Mahé A, Meertens L, Ly F, Sow PS, Diop CT, Samb ND, Diop OM, Valensi F, Gessain A. 2004. Human T-cell leukemia/lymphoma virus type 1-associated infective dermatitis in Africa: a report of five cases from Senegal. Br J Dermatol 150:958–965. 10.1111/j.1365-2133.2004.05834.x. [DOI] [PubMed] [Google Scholar]
- 353.Sugimoto M, Nakashima H, Watanabe S, Uyama E, Tanaka F, Ando M, Araki S, Kawasaki S. 1987. T-lymphocyte alveolitis in HTLV-I-associated myelopathy. Lancet 2:1220. [DOI] [PubMed] [Google Scholar]
- 354.Yamashiro T, Kamiya H, Miyara T, Gibo S, Ogawa K, Akamine T, Moromizato H, Yara S, Murayama S. 2012. CT scans of the chest in carriers of human T-cell lymphotropic virus type 1: presence of interstitial pneumonia. Acad Radiol 19:952–957. 10.1016/j.acra.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 355.Okada F, Ando Y, Yoshitake S, Yotsumoto S, Matsumoto S, Wakisaka M, Maeda T, Mori H. 2006. Pulmonary CT findings in 320 carriers of human T-lymphotropic virus type 1. Radiology 240:559–564. 10.1148/radiol.2402050886. [DOI] [PubMed] [Google Scholar]
- 356.Einsiedel L, Pham H, Wilson K, Walley R, Turpin J, Bangham C, Gessain A, Woodman RJ. 2018. Human T-Lymphotropic Virus type 1c subtype proviral loads, chronic lung disease and survival in a prospective cohort of Indigenous Australians. PLoS Negl Trop Dis 12:e0006281. 10.1371/journal.pntd.0006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Einsiedel L, Cassar O, Goeman E, Spelman T, Au V, Hatami S, Joseph S, Gessain A. 2014. Higher human T-lymphotropic virus type 1 subtype C proviral loads are associated with bronchiectasis in indigenous Australians: results of a case-control study. Open Forum Infect Dis 1:ofu023. 10.1093/ofid/ofu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Honarbakhsh S, Taylor GP. 2015. High prevalence of bronchiectasis is linked to HTLV-1-associated inflammatory disease. BMC Infect Dis 15:258–258. 10.1186/s12879-015-1002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Einsiedel L, Chiong F, Jersmann H, Taylor GP. 2021. Human T-cell leukemia virus type 1 associated pulmonary disease: clinical and pathological features of an under-recognized complication of HTLV-1 infection. Retrovirology 18:5. 10.1186/s12977-021-00549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Grassi MFR, dos Santos NP, Lírio M, Kritski AL, Chagas Almeida MC, Santana LP, Lázaro N, Dias J, Netto EM, Galvão-Castro B. 2016. Tuberculosis incidence in a cohort of individuals infected with human T-lymphotropic virus type 1 (HTLV-1) in Salvador, Brazil. BMC Infect Dis 16:491. 10.1186/s12879-016-1428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Murphy EL, Glynn SA, Fride J, Sacher RA, Smith JW, Wright DJ, Newman B, Gibble JW, Ameti DI, Newman B, Gibble JW, Ameti DI, Nemo GJ, for the Retrovirus Epidemiology Donor Study Study G. 1997. Increased prevalence of infectious diseases and other adverse outcomes in human T lymphotropic virus types I- and II-infected blood donors. J Infect Dis 176:1468–1475. 10.1086/514143. [DOI] [PubMed] [Google Scholar]
- 362.Stuver SO, Tachibana N, Okayama A, Mueller NE. 1996. Evaluation of morbidity among human T lymphotropic virus type I carriers in Miyazaki. J Infect Dis 173:584–591. 10.1093/infdis/173.3.584. [DOI] [PubMed] [Google Scholar]
- 363.Matsuzaki T, Otose H, Hashimoto K, Shibata Y, Arimura K, Osame M. 1993. Diseases among men living in human T-lymphotropic virus type I endemic areas in Japan. Intern Med 32:623–628. 10.2169/internalmedicine.32.623. [DOI] [PubMed] [Google Scholar]
- 364.Marinho J, Galvao-Castro B, Rodrigues LC, Barreto ML. 2005. Increased risk of tuberculosis with human T-lymphotropic virus-1 infection: a case-control study. J Acquir Immune Defic Syndr 40:625–628. 10.1097/01.qai.0000174252.73516.7a. [DOI] [PubMed] [Google Scholar]
- 365.Norrgren HR, Bamba S, Larsen O, Da Silva Z, Aaby P, Koivula T, Andersson S. 2008. Increased prevalence of HTLV-1 in patients with pulmonary tuberculosis coinfected with HIV, but not in HIV-negative patients with tuberculosis. J Acquir Immune Defic Syndr 48:607–610. 10.1097/QAI.0b013e31817efb83. [DOI] [PubMed] [Google Scholar]
- 366.Bastos M.dL, Santos SB, Souza A, Finkmoore B, Bispo O, Barreto T, Cardoso I, Bispo I, Bastos F, Pereira D, Riley L, Carvalho EM. 2012. Influence of HTLV-1 on the clinical, microbiologic and immunologic presentation of tuberculosis. BMC Infect Dis 12:199. 10.1186/1471-2334-12-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Keiser PB, Nutman TB. 2004. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev 17:208–217. 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Gotuzzo E, Terashima A, Alvarez H, Tello R, Infante R, Watts DM, Freedman DO. 1999. Strongyloides stercoralis hyperinfection associated with human T cell lymphotropic virus type-1 infection in Peru. Am J Trop Med Hyg 60:146–149. 10.4269/ajtmh.1999.60.146. [DOI] [PubMed] [Google Scholar]
- 369.Montes M, Sanchez C, Verdonck K, Lake JE, Gonzalez E, Lopez G, Terashima A, Nolan T, Lewis DE, Gotuzzo E, White AC, Jr.. 2009. Regulatory T cell expansion in HTLV-1 and strongyloidiasis coinfection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Negl Trop Dis 3:e456. 10.1371/journal.pntd.0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Carvalho EM, Da Fonseca Porto A. 2004. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunol 26:487–497. 10.1111/j.0141-9838.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 371.Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Jr, Terashima A, Samalvides F, Pérez-Molina JA, Plana MN. 2016. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev 2016:CD007745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Orland JR, Wang B, Wright DJ, Nass CC, Garratty G, Smith JW, Newman B, Smith DM, Murphy EL, HOST Investigators. 2004. Increased mortality associated with HTLV-II infection in blood donors: a prospective cohort study. Retrovirology 1:4–4. 10.1186/1742-4690-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Biswas HH, Kaidarova Z, Garratty G, Gibble JW, Newman BH, Smith JW, Ziman A, Fridey JL, Sacher RA, Murphy EL, Study HO, HTLV Outcomes Study. 2010. Increased all-cause and cancer mortality in HTLV-II infection. J Acquir Immune Defic Syndr 54:290–296. 10.1097/QAI.0b013e3181cc5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ariyoshi K, Berry N, Cham F, Jaffar S, Schim van der Loeff M, Jobe O, N’Gom PT, Larsen O, Andersson S, Aaby P, Whittle H. 2003. Quantification of human T-lymphotropic virus type I (HTLV-I) provirus load in a rural West African population: no enhancement of human immunodeficiency virus type 2 pathogenesis, but HTLV-I provirus load relates to mortality. J Infect Dis 188:1648–1651. 10.1086/379780. [DOI] [PubMed] [Google Scholar]
- 375.Iwata K, Ito S, Saito H, Ito M, Nagatomo M, Yamasaki T, Yoshida S, Suto H, Tajima K. 1994. Mortality among inhabitants of an HTLV-I endemic area in Japan. Jpn J Cancer Res 85:231–237. 10.1111/j.1349-7006.1994.tb02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Holmgren B, da Silva Z, Vastrup P, Larsen O, Andersson S, Ravn H, Aaby P. 2007. Mortality associated with HIV-1, HIV-2, and HTLV-I single and dual infections in a middle-aged and older population in Guinea-Bissau. Retrovirology 4:85. 10.1186/1742-4690-4-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.van Tienen C, Schim van der Loeff M, Peterson I, Cotten M, Andersson S, Holmgren B, Vincent T, de Silva T, Rowland-Jones S, Aaby P, Whittle H. 2011. HTLV-1 and HIV-2 infection are associated with increased mortality in a rural West African community. PLoS One 6:e29026. 10.1371/journal.pone.0029026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Melbye M, Poulsen AG, Gallo D, Pedersen JB, Biggar RJ, Larsen O, Dias F, Aaby P. 1998. HTLV-1 infection in a population-based cohort of older persons in Guinea-Bissau, West Africa: risk factors and impact on survival. Int J Cancer 76:293–298. . [DOI] [PubMed] [Google Scholar]
- 379.Kira J, Nakamura M, Sawada T, Koyanagi Y, Ohori N, Itoyama Y, Yamamoto N, Sakaki Y, Goto I. 1992. Antibody titers to HTLV-I-p40tax protein and gag-env hybrid protein in HTLV-I-associated myelopathy/tropical spastic paraparesis: correlation with increased HTLV-I proviral DNA load. J Neurol Sci 107:98–104. 10.1016/0022-510X(92)90215-7. [DOI] [PubMed] [Google Scholar]
- 380.Tsukasaki K, Hermine O, Bazarbachi A, Ratner L, Ramos JC, Harrington W, O’Mahony D, Janik JE, Bittencourt AL, Taylor GP, Yamaguchi K, Utsunomiya A, Tobinai K, Watanabe T. 2009. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol 27:453–459. 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Cook LB, Fuji S, Hermine O, Bazarbachi A, Ramos JC, Ratner L, Horwitz S, Fields P, Tanase A, Bumbea H, Cwynarski K, Taylor G, Waldmann TA, Bittencourt A, Marcais A, Suarez F, Sibon D, Phillips A, Lunning M, Farid R, Imaizumi Y, Choi I, Ishida T, Ishitsuka K, Fukushima T, Uchimaru K, Takaori-Kondo A, Tokura Y, Utsunomiya A, Matsuoka M, Tsukasaki K, Watanabe T. 2019. Revised adult T-cell leukemia-lymphoma international consensus meeting report. J Clin Oncol 37:677–687. 10.1200/JCO.18.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Gill PS, Harrington W, Jr, Kaplan MH, Ribeiro RC, Bennett JM, Liebman HA, Bernstein-Singer M, Espina BM, Cabral L, Allen S. 1995. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med 332:1744–1748. 10.1056/NEJM199506293322603. [DOI] [PubMed] [Google Scholar]
- 383.White JD, Wharfe G, Stewart DM, Maher VE, Eicher D, Herring B, Derby M, Jackson-Booth PG, Marshall M, Lucy D, Jain A, Cranston B, Hanchard B, Lee CC, Top LE, Fleisher TA, Nelson DL, Waldmann TA. 2001. The combination of zidovudine and interferon alpha-2B in the treatment of adult T-cell leukemia/lymphoma. Leuk Lymphoma 40:287–294. 10.3109/10428190109057927. [DOI] [PubMed] [Google Scholar]
- 384.Hermine O, Allard I, Levy V, Arnulf B, Gessain A, Bazarbachi A. 2003. A prospective phase II clinical trial with the use of zidovudine and iterferon-alpha in the acute and lymphoma forms of adult T-cell leukemia/lymphoma. Haematol J 3:276–282. 10.1038/sj.thj.6200195. [DOI] [PubMed] [Google Scholar]
- 385.Bazarbachi A, Plumelle Y, Ramos JC, Tortevoye P, Otrock Z, Taylor G, Gessain A, Harrington W, Panelatti G, Hermine O. 2010. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol 28:4177–4183. 10.1200/JCO.2010.28.0669. [DOI] [PubMed] [Google Scholar]
- 386.Hodson A, Crichton S, Montoto S, Mir N, Matutes E, Cwynarski K, Kumaran T, Ardeshna KM, Pagliuca A, Taylor GP, Fields PA. 2011. Use of zidovudine and interferon alfa with chemotherapy improves survival in both acute and lymphoma subtypes of adult T-cell leukemia/lymphoma. J Clin Oncol 29:4696–4701. 10.1200/JCO.2011.35.5578. [DOI] [PubMed] [Google Scholar]
- 387.Ohno R, Masaoka T, Shirakawa S, Sakamoto S, Hirano M, Hanada S, Yasunaga K, Yokomaku S, Mitomo Y, Nagai K, Yamada K, Furue H, MST-16 Study Group. 1993. Treatment of adult T‐cell leukemia/lymphoma with MST‐16, a new oral antitumor drug and a derivative of bis(2,6‐dioxopiperazine. Cancer 71:2217–2221. . [DOI] [PubMed] [Google Scholar]
- 388.Tsuda H, Takatsuki K, Ohno R, Masaoka T, Okada K, Shirakawa S, Ohashi Y, Ota K. 1994. Treatment of adult T-cell leukemia-lymphoma with irinotecan hydrochloride (CPT-11). Br J Cancer 70:771–774. 10.1038/bjc.1994.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Uozumi K, Hanada S, Ohno N, Ishitsuka K, Shimotakahara S, Otsuka M, Chyuman Y, Nakahara K, Takeshita T, Kuwazuru Y. 1995. Combination chemotherapy (RCM protocol: response-oriented cyclic multidrug protocol) for the acute or lymphoma type adult T-cell leukemia. Leuk Lymphoma 18:317–‐323. 10.3109/10428199509059624. [DOI] [PubMed] [Google Scholar]
- 390.Waldmann TA, White JD, Carrasquillo JA, Reynolds JC, Paik CH, Gansow OA, Brechbiel MW, Jaffe ES, Fleisher TA, Goldman CK, Top LE, Bamford R, Zaknoen E, Roessler E, Kasten-Sportes C, England R, Litou H, Johnson JA, Jackson-White T, Manns A, Hanchard B, Junghans RP, Nelson DL. 1995. Radioimmunotherapy of interleukin-2R alpha-expressing adult T-cell leukemia with Yttrium-90-labeled anti-Tac. Blood 86:4063–4075. 10.1182/blood.V86.11.4063.bloodjournal86114063. [DOI] [PubMed] [Google Scholar]
- 391.Taguchi T, Ohta K, Hotta T, Shirakawa S, Masaoka T, Kimura I. 1997. Menogaril (TUT-7) late phase II study for malignant lymphoma, adult T-cell leukemia and lymphoma (ATLL). Gan to Kagaku Ryoho 24:1263–1271. [PubMed] [Google Scholar]
- 392.Yamada Y, Tomonaga M, Fukuda H, Hanada S, Utsunomiya A, Tara M, Sano M, Ikeda S, Takatsuki K, Kozuru M, Araki K, Kawano F, Niimi M, Tobinai K, Hotta T, Shimoyama M. 2001. A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol 113:375–382. 10.1046/j.1365-2141.2001.02737.x. [DOI] [PubMed] [Google Scholar]
- 393.Tsukasaki K, Tobinai K, Shimoyama M, Kozuru M, Uikc N, Yamada Y, Tomonaga M, Araki K, Kasai M, Takatsuki K, Tara M, Mikuni C, Hotta T, Members of the Lymphoma Study Group (LSG) of the Japan Clinical Oncology Group (JCOG). 2003. Deoxycoformycin-containing combination chemotherapy for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study (JCOG9109). Int J Hematol 77:164–170. 10.1007/BF02983215. [DOI] [PubMed] [Google Scholar]
- 394.Tsukasaki K, Utsunomiya A, Fukuda H, Shibata T, Fukushima T, Takatsuka Y, Ikeda S, Masuda M, Nagoshi H, Ueda R, Tamura K, Sano M, Momita S, Yamaguchi K, Kawano F, Hanada S, Tobinai K, Shimoyama M, Hotta T, Tomonaga M. 2007. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 25:5458–‐5464. 10.1200/JCO.2007.11.9958. [DOI] [PubMed] [Google Scholar]
- 395.Takamatsu Y, Suzumiya J, Utsunomiya A, Maeda K, Matsuoka H, Suzushima H, Tsukada J, Shibata K, Tamura K, Kyushu Hematology Organization for Treatment Study Group (K-HOT). 2010. THP-COP regimen for the treatment of peripheral T-cell lymphoma and adult T-cell leukemia/lymphoma: a multicenter phase II study. Eur J Haematol 84:391–‐397. 10.1111/j.1600-0609.2010.01411.x. [DOI] [PubMed] [Google Scholar]
- 396.Ceesay MM, Matutes E, Taylor GP, Fields P, Cavenagh J, Simpson S, Ho A, Devereux S, Mufti GJ, Pagliuca A. 2012. Phase II study on combination therapy with CHOP-Zenapax for HTLV-I-associated adult T-cell leukaemia/lymphoma (ATLL). Leuk Res 36:857–861. 10.1016/j.leukres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 397.Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, Saburi Y, Miyamoto T, Takemoto S, Suzushima H, Tsukasaki K, Nosaka K, Fujiwara H, Ishitsuka K, Inagaki H, Ogura M, Akinaga S, Tomonaga M, Tobinai K, Ueda R. 2012. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol 30:837–842. 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- 398.Ishida T, Jo T, Takemoto S, Suzushima H, Uozumi K, Yamamoto K, Uike N, Saburi Y, Nosaka K, Utsunomiya A, Tobinai K, Fujiwara H, Ishitsuka K, Yoshida S, Taira N, Moriuchi Y, Imada K, Miyamoto T, Akinaga S, Tomonaga M, Ueda R. 2015. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study. Br J Haematol 169:672–‐682. 10.1111/bjh.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Phillips AA, Fields PA, Hermine O, Ramos JC, Beltran BE, Pereira J, Wandroo F, Feldman T, Taylor GP, Sawas A, Humphrey J, Kurman M, Moriya J, Dwyer K, Leoni M, Conlon K, Cook L, Gonsky J, Horwitz SM, 0761-009 Study Group. 2019. Mogamulizumab versus investigator’s choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica 104:993–1003. 10.3324/haematol.2018.205096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Ishida T, Fujiwara H, Nosaka K, Taira N, Abe Y, Imaizumi Y, Moriuchi Y, Jo T, Ishizawa K, Tobinai K, Tsukasaki K, Ito S, Yoshimitsu M, Otsuka M, Ogura M, Midorikawa S, Ruiz W, Ohtsu T. 2016. Multicenter phase II study of lenalidomide in relapsed or recurrent adult T-cell leukemia/lymphoma: ATLL-002. J Clin Oncol 34:4086–4093. 10.1200/JCO.2016.67.7732. [DOI] [PubMed] [Google Scholar]
- 401.Utsunomiya A, Miyazaki Y, Takatsuka Y, Hanada S, Uozumi K, Yashiki S, Tara M, Kawano F, Saburi Y, Kikuchi H, Hara M, Sao H, Morishima Y, Kodera Y, Sonoda S, Tomonaga M. 2001. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 27:15–20. 10.1038/sj.bmt.1702731. [DOI] [PubMed] [Google Scholar]
- 402.Okamura J, Uike N, Utsunomiya A, Tanosaki R. 2007. Allogeneic stem cell transplantation for adult T-cell leukemia/lymphoma. Int J Hematol 86:118–125. 10.1532/IJH97.07070. [DOI] [PubMed] [Google Scholar]
- 403.Tanosaki R, Uike N, Utsunomiya A, Saburi Y, Masuda M, Tomonaga M, Eto T, Hidaka M, Harada M, Choi I, Yamanaka T, Kannagi M, Matsuoka M, Okamura J. 2008. Allogeneic hematopoietic stem cell transplantation using reduced-intensity conditioning for adult T cell leukemia/lymphoma: impact of antithymocyte globulin on clinical outcome. Biol Blood Marrow Transplant 14:702–708. 10.1016/j.bbmt.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 404.Choi I, Tanosaki R, Uike N, Utsunomiya A, Tomonaga M, Harada M, Yamanaka T, Kannagi M, Okamura J, ATLL allo-HSCT Study Group. 2011. Long-term outcomes after hematopoietic SCT for adult T-cell leukemia/lymphoma: results of prospective trials. Bone Marrow Transplant 46:116–118. 10.1038/bmt.2010.92. [DOI] [PubMed] [Google Scholar]
- 405.Kawada H, Yoshimitsu M, Nakamura D, Arai A, Hayashida M, Kamada Y, Maekawa K, Fujino S, Arima M, Arima N, Tabuchi T, Inoue H, Hamda H, Suzuki S, Matsushita K, Arima N. 2015. A retrospective analysis of treatment outcomes in adult T cell leukemia/lymphoma patients with aggressive disease treated with or without allogeneic stem cell transplantation: a single-center experience. Biol Blood Marrow Transplant 21:696–700. 10.1016/j.bbmt.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 406.Fuji S, Inoue Y, Utsunomiya A, Moriuchi Y, Uchimaru K, Hanada S, Miyagi T, Taguchi J, Choi I, Otsuka E, Tomoyose T, Yamamoto H, Kurosawa S, Yamaguchi T, Fukuda T. 2016. Nationwide survey of aggressive adult T-Cell leukemia/lymphoma to compare the clinical outcome between non-transplanted and transplanted patients. Biol Blood Marrow Transplant 22:S39. 10.1016/j.bbmt.2015.11.318. [DOI] [Google Scholar]
- 407.Phillips EH, Hodson A, Hermine O, Bazarbachi A, Cwynarski K. 2016. Striving to cure adult T-cell leukemia/lymphoma: a role for allogeneic stem cell transplant. Bone Marrow Transplant 51:1549–1555. 10.1038/bmt.2016.154. [DOI] [PubMed] [Google Scholar]
- 408.Ishida T, Jo T, Takemoto S, Suzushima H, Suehiro Y, Yoshimitsu M, Saburi Y, Nosaka K, Utsunomiya A, et al. 2018. Follow-up of a randomized phase II study of chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukemia-lymphoma: impact on allogeneic hematopoietic stem cell transplantation. Br J Haematol 184:479–483. 10.1111/bjh.15123. [DOI] [PubMed] [Google Scholar]
- 409.Araujo A, Bangham CRM, Casseb J, Gotuzzo E, Jacobson S, Martin F, Penalva de Oliveira A, Puccioni-Sohler M, Taylor GP, Yamano Y2. 2019. Management of HAM/TSP: systematic review and consensus-based recommendations 2019 Neurol Clin Pract 11:49–56. 10.1212/CPJ.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Duncan J, Rudge P. 1990. Methylprednisolone therapy in tropical spastic paraparesis. J Neurol Neurosurg Psychiatry 53:173–174. 10.1136/jnnp.53.2.173-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Araujo AQ, Afonso CR, Leite AC, Dultra SV. 1993. Intravenous methylprednisolone in HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP). Arq Neuropsiquiatr 51:325–328. 10.1590/S0004-282X1993000300005. [DOI] [PubMed] [Google Scholar]
- 412.Nakagawa M, Nakahara K, Maruyama Y, Kawabata M, Higuchi I, Kubota H, Izumo S, Arimura K, Osame M. 1996. Therapeutic trials in 200 patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. J Neurovirol 2:345–355. 10.3109/13550289609146899. [DOI] [PubMed] [Google Scholar]
- 413.Croda MG, de Oliveira ACP, Vergara MPP, Bonasser F, Smid J, Duarte AJdS, Casseb J. 2008. Corticosteroid therapy in TSP/HAM patients: the results from a 10-year open cohort. J Neurol Sci 269:133–137. 10.1016/j.jns.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 414.Buell KG, Puri A, Demontis MA, Short CL, Adonis A, Haddow J, Martin F, Dhasmana D, Taylor GP. 2016. Effect of pulsed methylprednisolone on pain in patients with HTLV-1-associated myelopathy. PLoS One 11:e0152557. 10.1371/journal.pone.0152557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Coler-Reilly ALG, Sato T, Matsuzaki T, Nakagawa M, Niino M, Nagai M, Nakamura T, Takenouchi N, Araya N, Yagishita N, Inoue E, Yamano Y. 2017. Effectiveness of daily prednisolone to slow progression of human T-lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis: a multicenter retrospective cohort study. Neurotherapeutics 14:1084–1094. 10.1007/s13311-017-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Martin F, Inoue E, Grassi MFR, de Almeida Kruschewsky R, Cortese I, Silva MT, Jacobson S, Galvão-Castro B, Yamano Y, Taylor G, Bland JM. 2014. Change in timed walk as primary outcome measure of treatment response in HAMLET-P: HAM/TSP MuLticentre Efficacy Trial-Prednisolone. Retrovirology 11:P30. 10.1186/1742-4690-11-S1-P30. [DOI] [Google Scholar]
- 417.Sato T, Coler-Reilly ALG, Yagishita N, Araya N, Inoue E, Furuta R, Watanabe T, Uchimaru K, Matsuoka M, Matsumoto N, Hasegawa Y, Yamano Y. 2018. Mogamulizumab (anti-CCR4) in HTLV-1-associated myelopathy. N Engl J Med 378:529–538. 10.1056/NEJMoa1704827. [DOI] [PubMed] [Google Scholar]
- 418.Lee H, Swanson P, Shorty VS, Zack JA, Rosenblatt JD, Chen IS. 1989. High rate of HTLV-II infection in seropositive i.v. drug abusers in New Orleans. Science 244:471–475. 10.1126/science.2655084. [DOI] [PubMed] [Google Scholar]
- 419.Khabbaz RF, Hartel D, Lairmore M, Horsburgh CR, Schoenbaum EE, Roberts B, Hartley TM, Friedland G. 1991. Human T lymphotropic virus type II (HTLV-II) infection in a cohort of New York intravenous drug users: an old infection? J Infect Dis 163:252–256. 10.1093/infdis/163.2.252. [DOI] [PubMed] [Google Scholar]
- 420.Zanetti AR, Zehender G, Tanzi E, Galli C, Rezza G, Cargnel A, Boschini A, Mari D, Pizzocolo G, Mazzotta F, Canavaggio M, Lee H. 1992. HTLV-II among Italian intravenous-drug users and hemophiliacs. Eur J Epidemiol 8:702–707. 10.1007/BF00145387. [DOI] [PubMed] [Google Scholar]
- 421.Krook A, Albert J, Andersson S, Biberfeld G, Blomberg J, Eklund I, Engstrom A, Julander I, Kall K, Martin C, Stendahl P, Struve J, Sonnerborg A. 1997. Prevalence and risk factors for HTLV-II infection in 913 injecting drug users in Stockholm, 1994. J Acquir Immune Defic Syndr Hum Retrovirol 15:381–386. 10.1097/00042560-199708150-00009. [DOI] [PubMed] [Google Scholar]
- 422.Henrard DR, Soriano V, Robertson E, Gutierrez M, Stephens J, Dronda F, Miles F, Pujol E, Buytendorp M, Castro A, Chan E, Vallejo A, Llibre J, Motley C, Prillaman J. 1995. Prevalence of human T-cell lymphotropic virus type I (HTLV-I) and HTLV-II infection among Spanish drug users measured by HTLV-I assay and HTLV-I and -II assay. J Clin Microbiol 33:1735–1738. 10.1128/jcm.33.7.1735-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Hall WW, Ishak R, Zhu SW, Novoa P, Eiraku N, Takahashi H, Ferreira Mda C, Azevedo V, Ishak MO, Ferreira Oda C, Monken C, Kurata T. 1996. Human T lymphotropic virus type II (HTLV-II): epidemiology, molecular properties, and clinical features of infection. J Acquir Immune Defic Syndr Hum Retrovirol 13(Suppl 1):S204–S214. 10.1097/00042560-199600001-00031. [DOI] [PubMed] [Google Scholar]
- 424.Murphy EL, Wang B, Sacher RA, Fridey J, Smith JW, Nass CC, Newman B, Ownby HE, Garratty G, Hutching ST, Schreiber GB. 2004. Respiratory and urinary tract infections, arthritis, and asthma associated with HTLV-I and HTLV-II infection. Emerg Infect Dis 10:109–116. 10.3201/eid1001.020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Murphy EL, Glynn SA, Fridey J, Smith JW, Sacher RA, Nass CC, Ownby HE, Wright DJ, Nemo GJ, Retrovirus Epidemiology Donor Study. 1999. Increased incidence of infectious diseases during prospective follow-up of human T-lymphotropic virus type II- and I-infected blood donors. Arch Intern Med 159:1485–1491. 10.1001/archinte.159.13.1485. [DOI] [PubMed] [Google Scholar]
- 426.Murphy EL, Ownby HE, Smith JW, Garratty G, Hutching ST, Wu Y, Ameti DI. 2003. Pulmonary function testing in HTLV-I- and HTLV-II-infected humans: a cohort study. BMC Pulm Med 3. 10.1186/1471-2466-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.World Health Organization. 1992. Weekly Epidemiological Record, number 29. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 428.Sawada T, Iwahara Y, Ishii K, Taguchi H, Hoshino H, Miyoshi I. 1991. Immunoglobulin prophylaxis against milkborne transmission of human T cell leukemia virus type I in rabbits. J Infect Dis 164:1193–1196. 10.1093/infdis/164.6.1193. [DOI] [PubMed] [Google Scholar]
- 429.Murata N, Hakoda E, Machida H, Ikezoe T, Sawada T, Hoshino H, Miyoshi I. 1996. Prevention of human T cell lymphotropic virus type I infection in Japanese macaques by passive immunization. Leukemia 10:1971–1974. [PubMed] [Google Scholar]
- 430.Franchini G, Tartaglia J, Markham P, Benson J, Fullen J, Wills M, Arp J, Dekaban G, Paoletti E, Gallo RC. 1995. Highly attenuated HTLV type I lenv poxvirus vaccines induce protection against a cell-associated HTLV type I challenge in rabbits. AIDS Res Hum Retroviruses 11:307–313. 10.1089/aid.1995.11.307. [DOI] [PubMed] [Google Scholar]
- 431.Dezzutti CS, Frazier DE, Huff LY, Stromberg PC, Olsen RG. 1990. Subunit vaccine protects Macaca nemestrina (pig-tailed macaque) against simian T-cell lymphotropic virus type I challenge. Cancer Res 50:5687S–5691S. [PubMed] [Google Scholar]
- 432.Rodriguez SM, Florins A, Gillet N, de Brogniez A, Sanchez-Alcaraz MT, Boxus M, Boulanger F, Gutierrez G, Trono K, Alvarez I, Vagnoni L, Willems L. 2011. Preventive and therapeutic strategies for bovine leukemia virus: lessons for HTLV. Viruses 3:1210–1248. 10.3390/v3071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Ando Y, Saito K, Nakano S, Kakimoto K, Furuki K, Tanigawa T, Hashimoto H, Moriyama I, Ichijo M, Toyama T. 1989. Bottle-feeding can prevent transmission of HTLV-I from mothers to their babies. J Infections 19:25–29. 10.1016/S0163-4453(89)94772-5. [DOI] [PubMed] [Google Scholar]
- 434.Lawrence RM, Lawrence RA. 2004. Breast milk and infection. Clin Perinatol 31:501–528. 10.1016/j.clp.2004.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Ando Y, Nakano S, Saito K, Shimamoto I, Ichijo M, Toyama T, Hinuma Y. 1986. Prevention of HTLV-I transmission through the breast milk by a freeze-thawing process. Jpn J Cancer Res 77:974–977. [PubMed] [Google Scholar]
- 436.Ando Y, Kakimoto K, Tanigawa T, Furuki K, Saito K, Nakano S, Hashimoto H, Moriyama I, Ichijo M, Toyama T. 1989. Effect of freeze-thawing breast milk on vertical HTLV-I transmission from seropositive mothers to children. Jpn J Cancer Res 80:405–407. 10.1111/j.1349-7006.1989.tb02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Ministry of Health Labour and Welfare of Japan. 2010. Revision of the announcement on screening of pregnant women for HTLV-1. (In Japanese.) https://www.mhlw.go.jp/stf/houdou/2r9852000000thw2.html. Accessed 3 September 2021.
- 438.Carneiro-Proietti AB, Catalan-Soares BC, Castro-Costa CM, Murphy EL, Sabino EC, Hisada M, Galvao-Castro B, Alcantara LC, Remondegui C, Verdonck K, Proietti FA. 2006. HTLV in the Americas: challenges and perspectives. Rev Panam Salud Publica 19:44–53. 10.1590/S1020-49892006000100007. [DOI] [PubMed] [Google Scholar]
- 439.Director General of Health (J. Ménard). 1996. Circular DGS/SP 2 97-785 of 16 December 1997 on the personalized donation of milk from a mother to her hospitalized child and a reminder of the provisions in force concerning breastfeeding. (In French.) Health Branch Population Health Branch Offices of the Ages of Life and Population, Paris, France. https://solidarites-sante.gouv.fr/fichiers/bo/1998/98-03/a0030114.htm. Accessed 3 November 2021. [Google Scholar]
- 440.Centre for Clinical Practice at NICE. 2010. National Institute for Health and Clinical Excellence: guidance. National Institute for Health and Clinical Excellence, London, United Kingdom. [Google Scholar]
- 441.Rosadas C, Malik B, Taylor GP, Puccioni-Sohler M. 2018. Estimation of HTLV-1 vertical transmission cases in Brazil per annum. PLoS Negl Trop Dis 12:e0006913. 10.1371/journal.pntd.0006913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Kamihira S, Nakasima S, Oyakawa Y, Moriuti Y, Ichimaru M, Okuda H, Kanamura M, Oota T. 1987. Transmission of human T cell lymphotropic virus type I by blood transfusion before and after mass screening of sera from seropositive donors. Vox Sang 52:43–44. 10.1111/j.1423-0410.1987.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 443.Nelson KE, Donahue JG, Munoz A, Cohen ND, Ness PM, Teague A, Stambolis VA, Yawn DH, Callicott B, McAllister H, Reitz BA, Lee H, Farzadegan H, Hollingsworth CG. 1992. Transmission of retroviruses from seronegative donors by transfusion during cardiac surgery. A multicenter study of HIV-1 and HTLV-I/II infections. Ann Intern Med 117:554–559. 10.7326/0003-4819-117-7-554. [DOI] [PubMed] [Google Scholar]
- 444.Inaba S, Okochi K, Sato H, Fukada K, Kinukawa N, Nakata H, Kinjyo K, Fujii F, Maeda Y. 1999. Efficacy of donor screening for HTLV-I and the natural history of transfusion-transmitted infection. Transfusion 39:1104–1110. 10.1046/j.1537-2995.1999.39101104.x. [DOI] [PubMed] [Google Scholar]
- 445.Prinsze FJ, Zaaijer HL. 2012. The outcome of donor screening for Human T-Cell Lymphotropic Virus infection in the Netherlands. Vox Sang 102:198–203. 10.1111/j.1423-0410.2011.01538.x. [DOI] [PubMed] [Google Scholar]
- 446.Hewitt PE, Davison K, Howell DR, Taylor GP. 2013. Human T-lymphotropic virus lookback in NHS Blood and Transplant (England) reveals the efficacy of leukoreduction. Transfusion 53:2168–2175. [DOI] [PubMed] [Google Scholar]
- 447.Japanese Red Cross Society. 2017. Blood services. JRC Society, Tokyo, Japan. [Google Scholar]
- 448.Laperche S, Worms B, Pillonel J, for the European Network of Transfusion Medecine Societies and the Steering Committee of the Epidemiological Surveillance of Blood Donors in France. 2009. Blood safety strategies for human T-cell lymphotropic virus in Europe. Vox Sang 96:104–110. 10.1111/j.1423-0410.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 449.Kaul DR, Davis JA, Practice ASTIDCo. 2013. Human T cell lymphotrophic virus 1/2 in solid organ transplantation. Am J Transplant 13(Suppl 4):355–360. 10.1111/ajt.12127. [DOI] [PubMed] [Google Scholar]
- 450.U.S. Department of Health and Human Services. 2014. Guidance for HTLV-1 screening and confirmation in potential donors and reporting potential HTLV-1 infection. DHHS, Washington, DC. https://optn.transplant.hrsa.gov/resources/guidance/guidance-for-htlv-1-screening-and-confirmation-in-potential-donors-and-reporting-potential-htlv-1-infection/. Accessed 3 November 2021. [Google Scholar]
- 451.European Centre for Disease Prevention and Control. 2015. Geographical distribution of areas with a high prevalence of HTLV-1 infection. ECDC, Stockholm, Sweden. [Google Scholar]
- 452.Gallo RC, Willems L, Hasegawa H, the Global Virus Network’s Task Force on HTLV-1. 2016. Screening transplant donors for HTLV-1 and -2. Blood 128:3029–3031. 10.1182/blood-2016-09-739433. [DOI] [PubMed] [Google Scholar]
- 453.Martin F, Tagaya Y, Gallo R. 2018. Time to eradicate HTLV-1: an open letter to WHO. Lancet 391:1893–1894. 10.1016/S0140-6736(18)30974-7. [DOI] [PubMed] [Google Scholar]
- 454.Public Health England. 2021. Surveillance methods: HTLV. Public Health England, London, United Kingdom. https://webarchive.nationalarchives.gov.uk/20140714093033/http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/HTLV/SurveillanceMethods/. Accessed 3 September 2021. [Google Scholar]
- 455.Northern Territory Government of Australia. 2019. Human T lymphotropic virus: HTLV-I. https://nt.gov.au/wellbeing/health-conditions-treatments/viral/htlv. Accessed 3 November 2021.
- 456.Kashiwagi K, Furusyo N, Nakashima H, Kubo N, Kinukawa N, Kashiwagi S, Hayashi J. 2004. A decrease in mother-to-child transmission of human T lymphotropic virus type I (HTLV-I) in Okinawa, Japan. Am J Trop Med Hyg 70:158–163. 10.4269/ajtmh.2004.70.158. [DOI] [PubMed] [Google Scholar]
- 457.Kusuhara K, Sonoda S, Takahashi K, Tokugawa K, Fukushige J, Ueda K. 1987. Mother-to-child transmission of human T-cell leukemia virus type I (HTLV-1): a 15-year follow-up study in Okinawa. Int J Cancer 40:755–757. 10.1002/ijc.2910400607. [DOI] [PubMed] [Google Scholar]
- 458.Ando Y, Nakano S, Saito K, Shimamoto I, Ichijo M, Toyama T, Hinuma Y. 1987. Transmission of adult T-cell leukemia retrovirus (HTLV-I) from mother to child: comparison of bottle- with breast-fed babies. Jpn J Cancer Res 78:322–324. [PubMed] [Google Scholar]
- 459.Monplaisir N, Neisson-Vernant C, Bouillot M, Duc-Dodon M, Ugarte E, Valette I, Dezaphy Y, Ouka M, Eudaric MG, Gazzolo L. 1993. HTLV-I maternal transmission in Martinique, using serology and polymerase chain reaction. AIDS Res Hum Retroviruses 9:869–874. 10.1089/aid.1993.9.869. [DOI] [PubMed] [Google Scholar]
- 460.Gotuzzo E, Moody J, Verdonck K, Cabada MM, González E, Van Dooren S, Vandamme A-M, Terashima A, Vermund SH. 2007. Frequent HTLV-1 infection in the offspring of Peruvian women with HTLV-1-associated myelopathy/tropical spastic paraparesis or strongyloidiasis. Rev Panam Salud Publica 22:223–230. 10.1590/s1020-49892007000900001. [DOI] [PubMed] [Google Scholar]
- 461.Paiva A, Smid J, Haziot MEJ, Assone T, Pinheiro S, Fonseca LAM, de Oliveira ACP, Casseb J. 2017. High risk of heterosexual transmission of human T-cell lymphotropic virus type 1 infection in Brazil. J Med Virol 89:1287–1294. 10.1002/jmv.24745. [DOI] [PubMed] [Google Scholar]
- 462.Okochi K, Sato H, Hinuma Y. 1984. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang 46:245–253. 10.1111/j.1423-0410.1984.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 463.Etzel A, Shibata GY, Rozman M, Jorge ML, Damas CD, Segurado AA. 2001. HTLV-1 and HTLV-2 infections in HIV-infected individuals from Santos, Brazil: seroprevalence and risk factors. J Acquir Immune Defic Syndr 26:185–190. 10.1097/00126334-200102010-00015. [DOI] [PubMed] [Google Scholar]
- 464.Khabbaz RF, Onorato IM, Cannon RO, Hartley TM, Roberts B, Hosein B, Kaplan JE. 1992. Seroprevalence of HTLV-1 and HTLV-2 among intravenous drug users and persons in clinics for sexually transmitted diseases. N Engl J Med 326:375–380. 10.1056/NEJM199202063260604. [DOI] [PubMed] [Google Scholar]
- 465.Khabbaz RF, Darrow WW, Hartley TM, Witte J, Cohen JB, French J, Gill PS, Potterat J, Sikes RK, Reich R. 1990. Seroprevalence and risk factors for HTLV-I/II infection among female prostitutes in the United States. JAMA 263:60–64. 10.1001/jama.1990.03440010058030. [DOI] [PubMed] [Google Scholar]
- 466.Tang AR, Taylor GP, Dhasmana D. 2019. Self-flagellation as possible route of human t-cell lymphotropic virus type 1 transmission. Emerg Infect Dis 25:811–813. 10.3201/eid2504.180984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Styles CE, Hoad VC, Denham-Ricks P, Brown D, Seed CR. 2019. Self-flagellation as possible route of human T-cell lymphotropic virus type 1 transmission. Emerg Infect Dis 25:1996–1997. 10.3201/eid2510.190484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Rouet F, Herrmann-Storck C, Courouble G, Deloumeaux J, Madani D, Strobel M. 2002. A case-control study of risk factors associated with human T-cell lymphotrophic virus type-I seropositivity in blood donors from Guadeloupe, French West Indies. Vox Sang 82:61–66. 10.1046/j.0042-9007.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- 469.Siddiqui A. 2002. Essential safety requirements (ESR): standards for Saudi CBAHI accreditation in hospitals and PHC’s. Saudi Central Board for Accreditation of Health Care Institutions (CBAHI), Riyadh, Saudi Arabia. [Google Scholar]
- 470.Abolghasemi H, Maghsudlu M, Amini Kafi-Abad S, Cheraghali A. 2009. Introduction to iranian blood transfusion organization and blood safety in Iran. Iranian J Publ Health 38:82–87. [Google Scholar]
- 471.Ireland G, Croxford S, Tosswill J, Raghu R, Davison K, Hewitt P, Simmons R, Taylor G. 2017. Human T-lymphotropic viruses (HTLV) in England and Wales, 2004 to 2013: testing and diagnoses. Euro Surveill 22:30539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Legifrance le Service Public de la Diffusion du Droit. 2018. Public health code: article D1221-6. Legifrance le Service Public de la Diffusion du Droit, Paris, France. https://www.legifrance.gouv.fr/affichCodeArticle.do?idArticle=LEGIARTI000037924559&cidTexte=LEGITEXT000006072665&dateTexte=20181227. Accessed 11 October. [Google Scholar]
- 473.de Mendoza C, Caballero E, Aguilera A, Requena S, de Lejarazu RO, Piron M, Gonzalez R, Jimenez A, Roc L, Trevino A, Benito R, Fernandez-Alonso M, Aguinaga A, Rodriguez C, Garcia-Costa J, Blanco L, Ramos JM, Calderon E, Eiros JM, Sauleda S, Barreiro P, Soriano V, Spanish HTLV Network. 2017. Human T-lymphotropic virus type 1 infection and disease in Spain. AIDS 31:1653–1663. 10.1097/QAD.0000000000001527. [DOI] [PubMed] [Google Scholar]
- 474.Government of Spain. 2014. Boletin Oficial Del Estado. Government of Spain, Madrid, Spain. https://www.mscbs.gob.es/profesionales/saludPublica/sanidadExterior/MB/rd_9_2014.pdf. Accessed 3 November 2021. [Google Scholar]
- 475.Ministry of Health Canada. 2014. Guidance document: blood regulations. Ministry of Health Canada, Ottowa, Ontario, Canada. https://www.canada.ca/en/health-canada/services/drugs-health-products/biologics-radiopharmaceuticals-genetic-therapies/applications-submissions/guidance-documents/blood-regulations/guidance-document-blood-regulations-1.html. Accessed 3 November 2021. [Google Scholar]
- 476.Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Piccinini V, Liumbruno GM, Grazzini G. 2016. Human T-lymphotropic virus and transfusion safety: does one size fit all? Transfusion 56:249–260. 10.1111/trf.13329. [DOI] [PubMed] [Google Scholar]
- 477.Pan American Health Organization. 2012. Caribbean regional standards for blood banks and transfusion services, 2nd ed. Pan American Health Organization, Washington, DC. https://iris.paho.org/handle/10665.2/3196. Accessed 3 September 2020. [Google Scholar]
- 478.Mexico Ministry of Health. 2012. Official Mexican standard NOM-253-SSA1-2012 for the disposal of human blood and its components for therapeutic purposes. Center NBt, Mexico City, Mexico. [Google Scholar]
- 479.Quispe NCS, Feria EB, Santos-Fortuna E.dl, Caterino-de-Araujo A. 2009. Confirming the presence of HTLV-1 infection and the absence of HTLV-2 in blood donors from Arequipa. Rev Inst Med Trop Sao Paulo 51:25–29. 10.1590/S0036-46652009000100005. [DOI] [PubMed] [Google Scholar]
- 480.Kaul DR, Davis JA, AST Infectious Diseases Community of Practice. 2013. Human T cell lymphotrophic virus 1/2 in solid organ transplantation. In Special Issue: the American Society of Transplantation infectious diseases guidelines, 3rd ed. Am J Transpl 13:355–360. 10.1111/ajt.12127. [DOI] [PubMed] [Google Scholar]
- 481.Khabbaz RF, Fukuda K, Kaplan JE, Bianco C, Blattner W, Busch M, Dodd R, Epstein J, Gilcher R, Jackson C, Katz L, Kleinman S, Murphy EL, Nemo G, Poiesz BJ, Rios M, Sloand E, Sullivan M, Williams AE. 1993. Guidelines for counseling persons infected with human T-lymphotropic virus type I (HTLV-I) and type-II (HTLV-II). Ann Intern Med 118:448–454. [DOI] [PubMed] [Google Scholar]
- 482.Ministry of Health and Social Protection. 2014. Resolución número 000437 de 2014 [Resolution no. 000437 of 2014]. Ministry of Health and Social Protection, Bogota, Columbia. https://www.minsalud.gov.co/Normatividad_Nuevo/Resoluci%C3%B3n%200437%20de%202014.pdf. Accessed 3 November 2021. [Google Scholar]
- 483.Mirna M, Biglione CAB. 2013. Contributions and considerations on infection with human T-lymphotropic viruses type 1 and 2 in Argentina. Updates AIDS Infectol 21:84–94. [Google Scholar]
- 484.Du J, Chen C, Gao J, Xie J, Rong X, Xu X, Wang Y, Wang F, Li J, Lu Z, Guo W, Li G, Wang Z, Xu D, Weng J, Zhao Z, Weng W, Li H, Du Y, Li S, Zhen C, Liu B, Guo T. 2014. History and update of HTLV infection in China. Virus Res 191:134–137. [DOI] [PubMed] [Google Scholar]