Abstract
Cyclospora cayetanensis is a parasite that causes intestinal disease that can be especially severe in immunocompromised patients. Most cases occur in tropical and subtropical areas, and in industrialized countries their diagnosis is mostly linked to international travel or the ingestion of imported food. We describe this case of severe diarrhoea in a patient with diffuse large B cell lymphoma and no epidemiological risk factors that was successfully treated with trimethoprim–sulfamethoxazole (TMP–STX). C. cayetanensis is a pathogen that should be taken into account in patients with chronic diarrhoea, especially immunocompromised patients, even when no epidemiological risk factors are present.
Keywords: Cyclospora, C. cayetanensis, cyclosporiasis, coccidian, diarrhoea, immunosuppressed
Introduction
Cyclospora cayetanensis is a human coccidian parasite that causes intestinal disease, with the main symptom being watery diarrhoea [1]. In most patients cyclosporiasis is self-limited, but in immunocompromised patients the disease can manifest as severe or chronic diarrhoea. The main transmission mechanism of C. cayetanensis is faecal–oral, with the consumption of contaminated fresh food being the main source of infection [2]. Given that its oocysts are excreted unsporulated and need to sporulate in the environment (7–14 days to mature), direct person-to-person transmission is not likely [1].
Although the infection is distributed worldwide, it is found most frequently in tropical and subtropical areas. This parasite is endemic in Central and South America, several countries of the Middle East and the Indian subcontinent. The cases reported in developed countries are usually related to international travel [3] and outbreaks associated with the ingestion of imported food [1, 4].
We present a case of severe cyclosporiasis in an immunosuppressed patient with no epidemiological factors that was diagnosed via molecular techniques. By reporting this case, we aimed to discuss the importance of molecular techniques in the diagnosis of infrequent parasites.
Case report
A 76-year-old man with diffuse large B cell lymphoma (stage IV) under treatment with six cycles of R-CHOP chemotherapy combination (cyclophosphamide, doxorubicin, vincristine, prednisone plus monoclonal antibody rituximab) presented with severe diarrhoea of 4 weeks’ evolution. A clinical examination carried out in the Emergency Service showed mucocutaneous pallor, blood pressure of 84/64 mmHg, dehydration signs, temperature of 34.6 °C and a descent of the ST segment in all leads. Laboratory reports revealed hypokalaemia (K+ 1.7 mEq) and deterioration of the renal function (creatinine 1.5 mg dl−1). In order to haemodynamically stabilize the patient, 500 ml of physiological saline and 20 mEq of potassium chloride were administered. A stool sample was collected in which the bacterial culture was negative for habitual enteropathogenic bacteria ( Salmonella spp., Shigella spp., Yersinia spp., Campylobacter spp.). As the patient was immunocompromised and the diarrhoea did not cease in a month, 500 mg/12 h of ciprofloxacin was administered. Due to the severe hypokalaemia and the consequent arrhythmia risk, the patient was admitted into the intensive care unit of the hospital.
A new stool sample was sent to the laboratory where a multiplex one-step real-time PCR (Allplex Gastrointestinal, Seegene, Seoul, Republic of Korea) that investigates protozoa, bacteria and viruses was performed. The PCR was positive for Campylobacter spp. (which was not recovered in bacterial culture) and C. cayetanensis. The patient was subjected to a thorough epidemiological survey to determine the probable origin of the infection by C. cayetanensis. Given that the patient did not have any epidemiological risk factor for cyclosporiasis, Campylobacter jejuni was considered to be the aetiological agent of the diarrhoea and 1 g of azithromycin in a unique dose was administered. Because of the improvement of the haemodynamic state and diarrhoea, the patient was discharged from hospital.
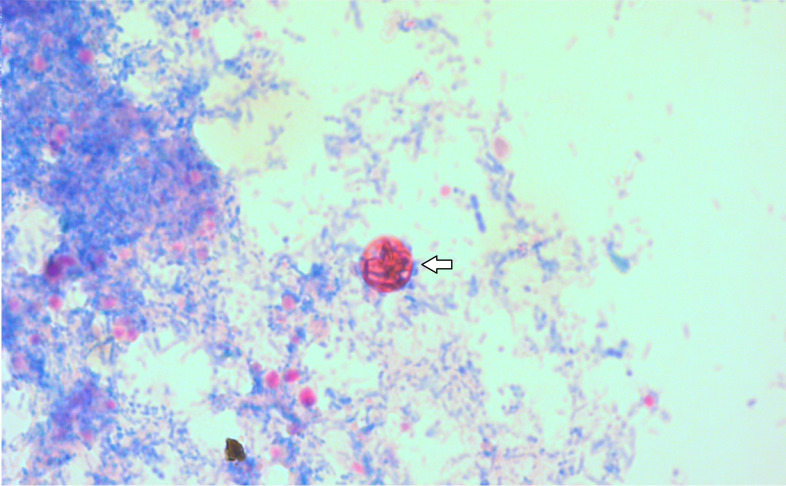
After 15 days the patient relapsed with diarrhoea of eight bowel movements a day. A third stool sample was sent and analysed using the same real-time PCR. This time, only C. cayetanensis was detected. In order to confirm the presence of this parasite, a specific PCR and Kinyoun staining were performed. After cautious examination, a single oocyst 9 µm in diameter was observed in the Kinyoun staining (Fig. 1). In the specific PCR, CCITS2-F and CCITS2-R primers were used to amplify the 116 bp segment of the internal transcribed spacer 2 (ITS-2) region of C. cayetanensis rDNA [5]. This PCR product was sequenced and analysed using the National Center for Biotechnology Information (NCBI) blast platform and showed a 98 % similarity to C. cayetanensis MZ088044.
Fig. 1.
Kinyoun staining of C.cayetanensis oocysts (9 µm) (magnification, ×1000).
With the diagnosis of cyclosporiasis confirmed, the patient was treated with trimethoprim–sulfamethoxazole (TMP–STX). Due to the immunosuppression of the patient, the treatment was prolonged for 31 days. The antibiotic regime was TMP–STX (TMP 160 mg +STX 800 mg)/6 h during 10 days, followed by a single daily dose of the same medication for 3 weeks. The diarrhoea improved as soon as the patient started with the antibiotherapy.
Discussion
There are several species of Cyclospora (C. cercopitheci, C. colobi, C. papionis, C. schneideri), but C. cayetanensis is the only one that infects humans, and humans appear to be the only natural host for this parasite [1]. C. cayetanenesis is the causal agent of cyclosporiasis and its symptoms typically include diarrhoea with anorexia, malaise, nausea, cramping and, less frequently, constipation, vomiting and fever.
Infection occurs after the ingestion of mature oocysts through contaminated food or water. The oocysts contain two ovoid sporocysts, each containing two sporozoites. Once in the jejunum, the oocysts hatch, releasing the sporozoites, which penetrate the enterocyte and evolve into two asexual generations, followed by sexual stages and consequent oocysts. The non-sporulated oocysts are excreted via faecal matter into the environment, where they require a maturation period of 7–14 days before becoming infective [6]. The diagnosis of cyclosporiasis is made by detecting these cysts using different techniques. The diagnosis of cyclosporiasis is made by detecting these cysts using different techniques. Traditionally it is done through visualization with different stainings, but the effectiveness of this is limited in cases with small quantities of parasite and also due to the intermittent excretion of the parasite. Molecular techniques allow the presence of smaller quantities of cysts to be detected.
Cyclosporiasis particularly affects children and is characterized by watery diarrhoea, which is usually self-limited (lasting from 4 to 15 days). However, in immunocompromised patients the diarrhoea can be especially severe, last for several months and have high recurrence rates [3]. There have been many reports of severe diarrhoea, mainly in AIDS/HIV-infected patients, but also in patients with cancer and transplant patients [7–9].
Although it is distributed universally, tropical and subtropical countries have the highest incidence of this parasite. Most cases diagnosed in developed countries are related to international travel, mainly involving Asia, Africa and South America, or consumption of food imported from endemic countries. Fruits (raspberries and blackberries) and vegetables (lettuce and parsley) have been responsible of most of the outbreaks [1]. In the USA, C. cayetanensis is the second most common causal agent in imported food consumption-associated diarrhoea [10]. A prevalence study made in Spain revealed the presence of C. cayetanensis in both drinking and sewage water, findings that support its universal distribution [11]. This means that, even in the absence of epidemiological risk factors, C. cayetanensis has to be taken into consideration, especially in susceptible populations.
Traditionally, the diagnosis of coccidian parasites usually involves microscopic examination by direct visualization of the oocysts, which in the case of C. cayetanensis are acid–alcohol-resistant and measure 8–10 µm in diameter. Wet smears with or without iodine may be used for direct observation, but stainings such as Ziehl–Neelsen, safranin, auramine, rhodamine and Kinyoun or ultraviolet fluorescence observation are used to achieve higher detection sensitivity. It is recommended to examine three stool specimens collected on non-consecutive days, over a period of more than a week, to rule out a C. cayetanensis infection.
Molecular techniques based on PCR have shown an improvement in the diagnosis of enteropathogenic agents due to their high sensitivity. This is especially useful in protozoan parasites, which are often very difficult and arduous to observe directly, and can easily be overlooked as causal agents of diarrhoea. The use of multiplex PCR platforms in the microbiological diagnosis of patients with clinically relevant diarrhoea may help to reveal the role of C. cayetanensis and other protozoa in these situations.
The first-choice treatment for cyclosporiasis is TMP–STX, but the dosage and the duration depend on the patient’s immunological status. In immunocompetent adults the regimen of choice is 160 mg TMP/800 mg STX twice a day for 7–10 days. In children the duration is the same but the dosage changes, being 5 mg TMP/25 mg STX kg−1. In immunosuppressed patients the treatment has to be administrated every 6 h and has to last 10–14 days. Clinical recovery occurs when the parasite is eradicated. In patients who are allergic to sulfamides, the treatment of choice is nitazoxanide 500 mg twice a day for 7 days [12].
Few laboratories have implemented molecular techniques for the diagnosis of enteropathogenic agents, which help to reduce the underdiagnosis of several parasites, including C. cayetanensis. In conclusion, in developed countries, where C. cayetanenesis has a low prevalence, the implementation of molecular techniques is especially useful for its diagnosis. This is very important in immunosuppressed patients, because host susceptibility seems to be the most important factor influencing the course of cyclosporiasis.
Funding information
This work received no specific grant from any funding agency.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
Consent to publish has been obtained.
Footnotes
Abbreviations: AIDS/HIV, Acquired Immunodeficiency Syndrome/Human Immunodeficiency Syndrome; ITS, internal transcribed spacer; NCBI, National Center for Biotechnology Information; PCR, protein chain reaction; R-CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone plus monoclonal antibody rituximab; rDNA, ribosomal deoxyribonucleic acid; TMP-STX, trimethoprim-sulfamethoxazole.
References
- 1.Almeria S, Cinar HN, Dubey JP. Cyclospora cayetanensis and Cyclosporiasis: An Update. Microorganisms. 2019;7:317. doi: 10.3390/microorganisms7090317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortega YR, Sanchez R. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin Microbiol Rev. 2010;23:218–234. doi: 10.1128/CMR.00026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marques DFP, Alexander CL, Chalmers RM, Elson R, Freedman J, et al. Cyclosporiasis in travellers returning to the United Kingdom from Mexico in summer 2017: lessons from the recent past to inform the future. Euro Surveill. 2017;22:30592. doi: 10.2807/1560-7917.ES.2017.22.32.30592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadjilouka A, Tsaltas D. Cyclospora cayetanensis-major outbreaks from ready to eat fresh fruits and vegetables. Foods. 2020;9:1703. doi: 10.3390/foods9111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalonde LF, Gajadhar AA. Highly sensitive and specific PCR assay for reliable detection of Cyclospora cayetanensis oocysts. Appl Environ Microbiol. 2008;74:4354–4358. doi: 10.1128/AEM.00032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortega YR, Nagle R, Gilman RH, Watanabe J, Miyagui J, et al. Pathologic and clinical findings in patients with cyclosporiasis and a description of intracellular parasite life-cycle stages. J Infect Dis. 1997;176:1584–1589. doi: 10.1086/514158. [DOI] [PubMed] [Google Scholar]
- 7.Pape JW, Verdier RI, Boncy M, Boncy J, Johnson WD. Cyclospora infection in adults infected with HIV. Clinical manifestations, treatment, and prophylaxis. Ann Intern Med. 1994;121:654–657. doi: 10.7326/0003-4819-121-9-199411010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lugo R, Angulo-Várguez F, Ávila-Nava A, Gutiérrez-Solis AL, Reyes-Sosa M, et al. Acute kidney injury associated with intestinal infection by Cyclospora cayetanensis in a kidney transplant patient. A case report. Parasitol Int. 2021;80:102212. doi: 10.1016/j.parint.2020.102212. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoudvand H, Sepahvand A, Khatami M, Moayyedkazemi A. Prevalence and associated risk factors of Cystoisospora belli and Cyclospora cayetanensis infection among Iranian patients with colorectal cancer. J Parasit Dis. 2019;43:402–405. doi: 10.1007/s12639-019-01104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon BR. Parasitic illnesses associated with the consumption of fresh produce — an emerging issue in developed countries. Current Opinion in Food Science. 2016;8:104–109. doi: 10.1016/j.cofs.2016.04.009. [DOI] [Google Scholar]
- 11.Galván AL, Magnet A, Izquierdo F, Fenoy S, Rueda C, et al. Molecular characterization of human-pathogenic microsporidia and Cyclospora cayetanensis isolated from various water sources in Spain: a year-long longitudinal study. Appl Environ Microbiol. 2013;79:449–459. doi: 10.1128/AEM.02737-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weller PF, Leder K. In: Basow DS, editor. Waltham, MA: UpToDate; 2019. Cyclospora infection. [Google Scholar]