Abstract
Staphylococcus aureus is a major etiological agent of clinical and subclinical bovine mastitis. The versatile and adaptative evolutionary strategies of this bacterium have challenged mastitis control and prevention globally, and the high incidence of S. aureus mastitis increases concerns about antimicrobial resistance (AMR) and zoonosis. This study aims to describe the evolutionary relationship between bovine intramammary infection (IMI)-associated S. aureus and human pathogenic S. aureus and further elucidate the specific genetic composition that leads to the emergence of successful bovine IMI-associated S. aureus lineages. We performed a phylogenomic analysis of 187 S . aureus isolates that originated from either dairy cattle or humans. Our results revealed that bovine IMI-associated S. aureus isolates showed distinct clades compared to human-originated S. aureus isolates. From a pan-genome analysis, 2070 core genes were identified. Host-specific genes and clonal complex (CC)-specific genes were also identified in bovine S. aureus isolates, mostly located in mobile genetic elements (MGEs). Additionally, the genome sequences of three apparent human-adapted isolates (two from CC97 and one from CC8), isolated from bovine mastitis samples, may provide an snapshot of the genomic characteristics in early host spillover events. Virulence and AMR genes were not conserved among bovine IMI-associated S. aureus isolates. Restriction-modification (R-M) genes in bovine IMI-associated S. aureus demonstrated that the Type I R-M system was lineage-specific and Type II R-M system was sequence type (ST)-specific. The distribution of exclusive, virulence, and AMR genes were closely correlated with the presence of R-M systems in S. aureus , suggesting that R-M systems may contribute to shaping clonal diversification by providing a genetic barrier to the horizontal gene transfer (HGT). Our findings indicate that the CC or ST lineage-specific R-M systems may limit genetic exchange between bovine-adapted S. aureus isolates from different lineages.
Keywords: Staphylococcus aureus, bovine mastitis, comparative genomics, restriction-modification system
Data Summary
Short read data for bovine IMI-associated S. aureus isolates are available at NCBI-SRA under BioProject numbers PRJNA609123 and PRJNA622791.
Highly assembled human-originated S. aureus genomes used in this study are available at NCBI under the accession numbers listed in Additional file S1 (available in the online version of this article).
Metadata including source, collection year, geographical area, associated disease, and ST/CC, is summarized in Additional files S1 and S2.
Impact Statement.
S. aureus bovine mastitis is a costly disease in dairy cattle. Despite its clinical importance and overall burden in the dairy industry, studies of this bacterium isolated from cows have suffered due to a limited number of whole-genome sequences being available and a further limited subset of strains that can be genetically manipulated. Fully understanding S. aureus R-M systems may help explain HGT in this species and understand the dissemination of MGEs containing important virulence or AMR genes. Moreover, an understanding of S. aureus R-M systems may aid in designing strategies to bypass genetic barriers to make hyper recipients in a lineage of interest for genetic engineering applications. This approach may help facilitate studies on S. aureus , providing an improved understanding of its pathogenicity in a specific host.
Introduction
Staphylococcus aureus is an opportunistic pathogen that can infect humans as well as economically important livestock such as cows, sheep, and goats. Among livestock, cows are a common reservoir of S. aureus, and dairy cattle frequently experience clinical and subclinical mastitis due to S. aureus intramammary infections (IMIs) [1]. S. aureus produces biofilms, survives in non-phagocytic and phagocytic host cells, and dynamically switches its phenotypes between wild-type and small colony variants [2–4]. Each of these characteristics results in the persistence of S. aureus colonization in intramammary environments. This persistence within the mammary glands typically leads to treatment failure and recurrent bovine mastitis. Bovine mastitis control programmes have been developed with the intention of infection prevention; however, knowledge gaps in clonal diversity, host immune response, and other elements that affect S. aureus IMIs hinder the development of effective prevention strategies [5]. Moreover, robust genetic defence mechanisms owing to restriction-modification (R-M) systems in S. aureus impede genetic manipulations, limiting researchers from understanding bovine IMI-associated S. aureus physiology, metabolism, and pathogenesis [6]. Thus, S. aureus remains a primary etiological agent of bovine IMIs, causing significant challenges for researchers, dairy farmers, and veterinarians.
On a short evolutionary time-scale, S. aureus lineages are host-specific; although, on a longer time-scale, lineages often undergo zoonosis and zooanthroponosis. The word lineage is used in this study to refer to a group of isolates that have a commonality either through being from the same sequence type (ST) or clonal complex (CC). Bovine-adapted S. aureus lineages include CC97, CC133, and CC151 and human-adapted lineages include CC1, CC5, CC8, CC30, and CC45 [7]. S. aureus host spillover, followed by adaptation to a new host, is generally accompanied by loss of virulence and immune evasion genes from the previous host and acquisition of a new set of genes specific for survival in the new host [8]. Whole-genome sequencing and a subsequent comparative genomic analysis can be used to understand the movement of virulence and other host adaptation genes between S. aureus isolates from different host species. Recent comparative genomic studies have shown that bovine-adapted strains rapidly lose genes involved in human infections, which likely increases their fitness in bovine hosts [8, 9]. For instance, bovine-specific mobile genetic elements (MGEs) found in bovine adapted S. aureus lineages include the temperate phages φSaBov and φPV83, as well as pathogenicity islands SaPlbov1, SaPlbov2, and SaPlbov3 [7, 9, 10]. These MGEs carry bovine-specific virulence factors such as LukMF’ and vWbp [11, 12].
Cows are a source of antimicrobial-resistant (AMR) S. aureus , which may be transferred to persist in humans [8]. Specifically, livestock-associated methicillin-resistant S. aureus (LA-MRSA) is believed, at least partially, to be responsible for community-associated MRSA due to the use of antimicrobials in veterinary medicine and modern agriculture [13]. Interestingly, the prevalence of AMR S. aureus among dairy cows differs significantly based on geography. In North America, for example, a low prevalence (less than 10%) of blaZ positive S. aureus has been reported, while China, Finland, Sweden, Iran, and Brazil have noted that 50–94 % of bovine S. aureus isolates are penicillin-resistant [14–19]. Most studies investigate only common STs or the prevalence of AMR genes, making it challenging to elucidate the links between genetic lineage, virulence, AMR, and adaptations to the bovine niche. Very few studies have attempted to correlate lineage and AMR to gain insight into this relationship. Among S. aureus CC97 isolated from cows, which includes several STs (ST97, ST115, and ST352), only a few STs are reported to be positive for blaZ or mecA [20–22]. Some human-adapted lineages, such as ST5 (CC5), ST8 (CC8), and their variants have been isolated from cows and were positive for blaZ and mecA [20–22]. These studies support the idea that certain S. aureus lineages may be more prone to obtaining specific AMR genes, suggesting that lineage-specific factors might be involved in horizontal gene transfer (HGT). The existence of S. aureus lineage-specific R-M systems is a possible explanation for the unequal distribution of virulence/AMR genes in different lineages from the bovine niche.
Acquisition of foreign DNA is important in terms of bacterial evolution, fitness, adaptation, and clonal diversification. Many virulence, host-specific, and AMR genes are carried by S. aureus MGEs, and the movement of these MGEs contributes to strain differentiation. Indeed, 15 % of any S. aureus genome consists of MGEs which play a prominent role in host adaptation and pathogenicity [23, 24]. However, acquiring foreign DNA is not always advantageous due to the possibility of obtaining harmful, lethal, or superfluous genes. To control the retention of foreign DNA some bacteria including S. aureus have developed R-M systems. R-M systems are grouped into four types based on subunit architecture, the requirement for ATP/GTP, the level of sequence specificity, and DNA cleavage mechanism [25]. Lineage-specific R-M systems with the combination of Type I, II, III, and IV R-M genes control the spread of clinically important genes between S. aureus CCs, with Type I and II systems being the most common [23, 26, 27]. Type I R-M systems are multi-subunit complexes that consist of two M subunits, two R subunits, and one S subunit, encoded by hsdM, hsdR, and hsdS, respectively [25]. The S subunit is responsible for recognizing a specific DNA sequence, the R subunit cleaves DNA, and the M subunit catalyses the methylation reaction [25]. Type I R-M systems are located in νSaα and νSaβ as a part of the core genome in S. aureus . The Type I R-M system is the primary R-M system in S. aureus , the alleles present are lineage-specific, and it constitutes a significant barrier to the movement of MGEs and intentional genetic manipulation [28]. Type II R-M systems consist of a restriction endonuclease (res), that recognizes a specific DNA sequence and then introduces double-strand DNA breaks, and a methyltransferase that recognizes the same DNA sequence and methylates it (mod) [29]. Methylation modifies and thus protects target DNA from cleavage by hiding it from the restriction endonuclease [29]. Type II R-M systems are widely used in recombinant DNA technology and because of this application more than 3500 have been discovered and characterized [30]. Type III R-M systems are also composed of two genes, mod and res that also function in DNA modification or restriction, respectively [25]. Type IV R-M systems are less well characterized, but are composed of one or two genes that encode proteins that cleave only modified sequences [25]. Understanding S. aureus lineage-specific genetic barriers can outline the HGT network and potential evolutionary directions, which would aid in understanding S. aureus and ultimately preventing S. aureus infections and dissemination of AMR genes. Furthermore, this knowledge enables us to improve our ability to manipulate non-transformable S. aureus for the purposes of future S. aureus studies.
In this study, we used a comparative genomics approach to investigate several aspects of bovine IMI-associated S. aureus . We attempted to further understand the evolutionary relationships between S. aureus isolated from humans and cows by identifying unequally distributed genes among the two hosts, as well as correlations between the presence of mastitis-associated virulence factors, AMR genes, and R-M system genes in bovine IMI-associated S. aureus .
Methods
Sequence genomes, assembly, and gene annotation
We previously reported whole-genome sequencing on bovine IMI-associated S. aureus isolates obtained from the Mastitis Pathogen Culture Collection (Additional file S1) [31–33]. Each of these isolates was obtained from the cows in different health status (Additional file S2). The raw DNA sequences of bovine isolates (n=63) were assembled following the same pipeline as previously described using the software pipeline ProkaryoteAssembly (v. 0.1.6) (https://pypi.org/project/ProkaryoteAssembly/) [31, 34]. The quality of the genome assemblies (draft genomes) was evaluated using Qualimap (v. 2.2.2) [35]. Complete genomes of human S. aureus isolates (n=122) and two reference genomes, RF122 and Newbould 305 isolated from a bovine milk samples, were obtained from the National Centre for Biotechnology Information (NCBI) database (Additional file S1). All S. aureus genomes (n=187) were then run through the annotation pipeline via Prokka (v. 1.14.5) with the genus/species option [36]. All draft and complete genomes were verified as S. aureus by confirming the presence of crtOPQMN operon and the binding site of unc universal primers [37, 38].
Pan-genome and phylogenomic tree
A pan-genome of 187 S . aureus genomes was created using Roary (v. 3.13.0) with no paralog splitting and R plots options [39]. The pan-genome analysis was restricted to identifying the presence and absence of orthologs only; thus, paralogs copies were not taken into account during the analysis. The gene_presence_absence.csv file generated by Roary was used for pan-genome analysis. Core genes (core and soft core genes) and accessory genes (shell and cloud) were also identified using Roary.
The core gene alignment with 187 S . aureus genomes established by Roary was used to obtain phylogenetic estimates using IQ-TREE ModelFinder with 1000 replicate bootstraps [40]. The best model was found to be GTR+F+R2, which was then used to construct a phylogenomic tree and later displayed by iTOL (https://itol.embl.de/) [41]. We analysed seven housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, and yqiL) from each S. aureus genome to determine ST using mlst (https://github.com/tseemann/mlst) against the PubMLST database [42].
Exclusive gene analysis
The genes from bovine and human S. aureus genomes were compared using Venny (v. 2.1.0) using the list of genes from the gene_presence_absence.csv file generated by Roary [43]. The genes only present in either bovine or human S. aureus were classified as exclusive genes. Within bovine S. aureus , lineage-specific genes were examined by comparing the genes present in each CC. The relative location of each exclusive gene or gene cluster was determined by aligning the draft genomes to the complete/reference genome of S. aureus RF122 (ET3-1) isolated from bovine using IslandViewer 4 [9, 44]. All exclusive or lineage-specific genes annotated as hypothetical or unknown functions from Roary were also searched on NCBI BLASTP using their amino acid sequences to identify potential functions [45].
Identification of virulence/antimicrobial resistance/R-M genes
Virulence factors were analysed using VFanalyzer (http://www.mgc.ac.cn/VFs/main.htm) and confirmed with gene_presence_absence.csv file produced by Roary [46]. Antimicrobial resistance genes were analysed using ABRicate (https://github.com/tseemann/abricate) through MEGARes database and Roary [39, 47]. R-M genes were searched on Restriction-ModificationFinder 1.1 (https://cge.cbs.dtu.dk/services/Restriction-ModificationFinder/) and then analysed with REBASE searching through amino acid sequences and then grouped with >95 % amino acid sequence homology [48]. We classified agr types based on the conserved regions of amino acid sequences in AgrD (AIP precursor) [49]. The presence of the partially assembled or non-assembled genes was confirmed by Sanger sequencing followed by PCR with the target gene-specific primers (Additional file S3).
Mobile genetic elements (MGEs) identification
Plasmids were analysed using NCBI blast initially with circular contigs from assembled sequences of bovine IMI-associated S. aureus isolates and then using ABRicate through the PlasmidFinder database [50]. The verified plasmids were run through a local blast with the parameter of 97 % identity and coverage against 65 bovine IMI-associated S. aureus genomes. Prophage and genomic islands sequences were identified using PHASTER and IslandViewer 4, respectively [44, 51]. All bovine IMI-associated S. aureus draft genomes were aligned against the bovine-adapted S. aureus RF122 (ET3-1) genome in IslandViewer 4 [9, 44].
Results
Phylogenomic tree and S. aureus pan-genome
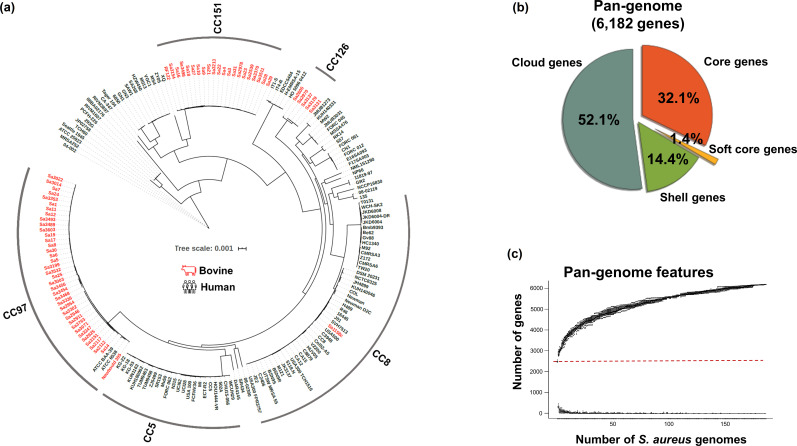
Phylogenomic analysis was conducted using a core gene alignment and revealed that bovine IMI-associated S. aureus lineages included CC151, CC8, CC126, and CC97, and the majority of human isolates belonged to CC8 and CC5 (Fig. 1a). Bovine IMI-associated S. aureus isolates were clustered into three clades and distinct from human isolates. Three suspicious isolates that show evidence of having recently jumped betwen host species were CC8 (Sa1158c) and CC97 (ATCC BAA-39 and ATCC 6538).
Fig. 1.
Phylogenomic tree and pan-genome of 187 S . aureus from human and bovine origins. (a) All bovine IMI-associated S. aureus isolates except Sa1158c (CC8) were clustered into three main clades: CC151, CC126, and CC97. (b) The pan-genome of 187 S . aureus isolates were subdivided into four groups: core (genes present in 99 % ≤isolates ≤100 %), soft core (95 % ≤isolates <99 %), shell (15 % ≤isolates <95 %), and cloud genes (0 % ≤isolates <15 %). (c) The red dotted line divides the graph into two features: total pan-genome in size (top) and the number of new genomes added to the total pan-genome (bottom) as new S. aureus genomes are added. The graph indicates an open state, and new genes are likely to be discovered continually as new genomes are added to the analysis.
All 489 086 coding sequences (CDS) from 187 S . aureus genomes were grouped into 6182 gene clusters. Grouping of the CDS revealed that the core genome to be 2070 genes (33.5 %) shared by more than 95 % of isolates and the accessory genome to be composed of 4112 genes (66.5 %) (Fig. 1b). The pan-genome increased in size upon the addition of new genomes suggesting an open pan-genome (Fig. 1c).
Unequally distributed genes between clonal complexes
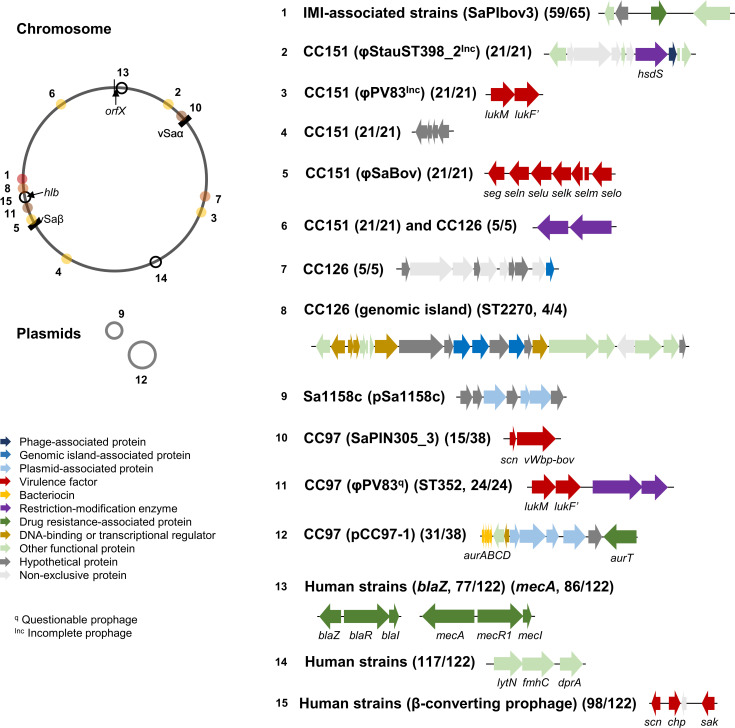
Unique and exclusive genes were examined, with a concentration on identifying genes that were unique to bovine IMI-associated S. aureus isolates. From the total pan-genome, 326 and 2653 elements were exclusive in either bovine IMI-associated or human S. aureus isolates, respectively. Most exclusive genes were either isolate-specific or CC lineage-specific. The most variable loci were found near orfX – the location of SCCmec integration in human isolates – and hlb (β-hemolysin) due to β-converting prophages and adjacent MGEs (Fig. 2).
Fig. 2.
Distribution of lineage-specific genes in S. aureus . This figure illustrates the genes present mainly in bovine IMI-associated S. aureus isolates. The unequally distributed genes were shown with their associated lineages, MGEs, and frequency. A reference genome, S. aureus RF122, was used to identify the relative location of these genes in the genome as indicated by the corresponding numbers (1-15).
Among bovine IMI-associated S. aureus , >90 % (59/65) of isolates contained a pathogenicity island known as SaPIbov3, which encodes four common proteins: a class I SAM-dependent methyltransferase, a hypothetical protein, a multidrug transporter, and a CAAX protease immunity-related protein. These unique genes found in bovine IMI-associated isolates were absent in five CC126 isolates and in one CC8 isolate (Sa1158c). We also found lukMF’, bovine-specific virulence genes, in CC151 and ST352 (CC97). All isolates in CC151 encoded common lineage-specific genes at five different loci: an incomplete prophage close to φStauST398, lukMF’ genes carried by φPV83, four genes in a non-MGE region, an enterotoxin gene cluster located in φSaBov, and two genes encoding Type II R-M subunits in a putative genomic island. The same Type II R-M genes in CC151 were found in CC126. CC126 also had five unique genes, the functions of which were unclear. All ST2270 (CC126) isolates (n=4) contained 20 unique genes within the lineage located in a putative genomic island near hlb. Sa1158c (ST8/CC8) possessed its own plasmid that encoded seven open reading frames (ORFs) with unknown functions. Among the CC97 isolates, 15 isolates mainly ST2187 (n=13) carried bovine variants vWbp and scn located in a pathogenicity island. The vWbp and scn genes, associated with bovine immune evasion and commonly found in S. aureus isolated from cows, were rarely found in human isolates – only two human isolates (PCFH-226 and S58) encoded them. Additionally, 81.5 % (31/38) of CC97 isolates carried the pCC97-1 plasmid, which encodes the aurABCD, aurI, aurR, and aurT genes for aureocin synthesis and transportation.
Additionally, exclusive genes were also found in human-originated S. aureus isolates. Three genes: lytN, fmhC, and dprA associated with cell division, cell wall protection, and DNA processing, respectively, were present in most human isolates (95.9 %, 117/122) as a part of the core genome near sucCD, and these genes were found in only one bovine isolate Sa1158c (ST8), which was phylogenetically related to the human-adapted lineage. The majority of human isolates encoded an immune evasion cluster (80.3 %, 98/122) at the hlb locus: scn, chp, and sak. The enterotoxin gene cluster found in CC151 was also present among human isolates (n=45).
Genetic elements suggesting recent host spillover
Three isolates which might be part of recent host spillover events (ATCC 6538, ATCC BAA-39, and Sa1158c) were identified in the phylogenomic tree (Fig. 1a). To identify potential genetic elements that might help S. aureus to enhance fitness or adaptation in a new host species, we further examined the presence of host-specific genes in these three isolates. One caveat to this analysis is that human and bovine isolates were derived from different geographic regions, which may confound the analysis.
ATCC 6538 and ATCC BAA-39 were isolated from humans and belong to ST464 (CC97). The four genes commonly found in bovine isolates (n=59) were absent in these two isolates. However, two bovine specific prophages similar to φPT1028 (incomplete) and φJS01 (intact) were found in these two isolates. A partial φPT1028 prophage that is frequently found in bovine IMI-associated S. aureus isolates (64.6 %, 42/65) was found; however, the version of φPT1028 found in the ATCC6538 and ATCC BAA-39 human isolates contained an additional pathogenicity island encoding enterotoxin genes (entK and entQ) that is not present in the version of φPT1028 that is associated with bovine isolates. These two enterotoxin genes; however, were also present in 34.4 % (42/122) of human-associated S. aureus isolates. Another prophage common to bovine isolates, φJS01, carried scn and sak and was inserted into hlb locus. The isolates ATCC 6538 and ATCC BAA-39 shared one hsdS with the bovine CC97 isolates, and showed defective Type I R-M genes, indicating a possible diverged evolutionary path via HGT (Additional file S5, Fig. S1).
Sa1158c (ST8), which was isolated from a bovine sample, encoded neither bovine-specific virulence genes nor the human immune evasion cluster carried by β-converting prophages. Sa1158c contained pSa1158c, a plasmid that encoded seven ORFs, but no known host adaptation genes. The pSa1158c plasmid was only found in the Sa1158c isolate, and did not have high sequence homology to any other plasmid. Type I R-M system genes were present, as with other ST8 isolates, although the presence of one of the hsdM genes was not confirmed due to incomplete genome assembly.
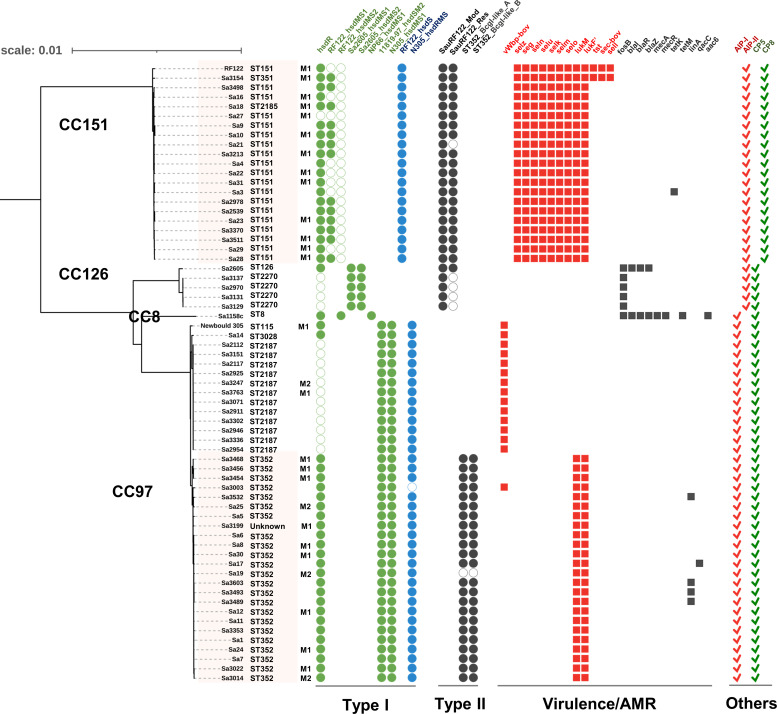
Distribution of R-M systems and clonal diversification
Using RESBASE database, four R-M systems (Type I, II, III and IV) were found among the 187 S . aureus genomes. Both human and bovine IMI-associated S. aureus isolates mainly possessed Type I and/or II R-M systems and only few human isolates carried Type III/IV R-M genes (Additional file S5, Fig. S1). As a part of the core genome, the Type I R-M system is a primary barrier to free HGT, and the combination of two hsdS gene copies was found to be lineage-specific. In bovine IMI-associated S. aureus isolates, Type II R-M system seemed to be more ST-specific (Fig. 3). In human S. aureus isolates, R-M systems in MGEs were either isolate-specific or ST-specific (Additional file S5, Fig. S1).
Fig. 3.
Distribution of restriction-modification genes and virulence/AMR genes in bovine IMI-associated S. aureus . The phylogenomic tree of 65 bovine IMI-associated S. aureus and relevant genetic content show lineage-specific R-M genes. Virulence and AMR genes are either ST-specific or isolate-specific. In the Type I R-M system, the green circular boxes indicate the Type I R-M system genes (hsdR and hsdMS) that are located in νSaα and νSaβ and are part of the core genome. The blue circular boxes show additional Type I R-M hsdRMS genes which are not a part of the S. aureus core genome. The hsdR and hsdM genes are highly conserved within the S. aureus species; however, several hsdS alleles exist. While each of the hsdMS genes shown in green are interchangeable, part of the same R-M system, and should, in combination with the hsdR, form a functional complex, the Type I R-M system genes shown in blue would not be expected to be interchangeable, and would instead form a separate, and independent Type I R-M system. The black circular boxes represent Type II R-M genes that encode a pair of enzymes: a methyltransferase and a restriction endonuclease. The red and the black square boxes represent virulence and AMR genes, respectively. The open circular boxes in all shapes and colours indicate the presence of a pseudogene. The red and green checkmarks indicate signal molecule (AIP-I and AIP-II) and capsular polysaccharide (CP5 and CP8). The highlighted isolates were associated with clinical mastitis. M1 is used to indicate that the milk sample was collected on the day clinical mastitis was diagnosed, and M2 indicates the sample was collected 14 days after a diagnosis of clinical mastitis was made. An extended report of each of the R-M genes identified in all isolates included in this study can be found in Fig. S1.
Bovine isolates had unique hsdS genes in two genomic islands (νSaα and vSaβ) and HsdS with >95 % amino acid sequence homology was only found in two ST80 human isolates (11819–97 and GR2). However, the combination of two Type 1 R-M system hsdS genes was unique in bovine-adapted STs. The two major bovine CCs (CC151 and CC97) carried unique hsdS alleles, which provide specificity to the Type I R-M enzymes. In CC151, the hsdS gene in vSaβ was truncated and additional hsdS genes were found in a prophage, likely to aid the primary Type I R-M system owing to high amino acid homology of this additional hsdS with the primary Type I hsdS found in other STs . All bovine CC97 isolates carried two sets of Type I R-M systems. Unlike the original Type I R-M genes, an additional hsdRMS locus was located near orfX (Additional file S5, Fig. S1). In ST2187 (CC97), due to the mutation in hsdR the primary Type I R-M was inactivated, yet the presence of additional hsdRMS suggested it replaced the original Type I R-M system in this ST.
All bovine isolates, except Sa1158c, possessed Type II R-M genes encoding two subunits (Mod and Res) in putative genomic islands. Although CC151 and CC126 shared the same Type II R-M genes, defective genes were found in SauRF122 Res subunit in all ST2270 isolates. Interestingly, Type II R-M genes in ST352 (CC97) were found right beside bovine-specific virulence genes lukMF’, suggesting that dissemination of these virulence genes is likely to be limited within ST352 and closely related STs.
Bovine IMI-associated S. aureus virulence factors and AMR genes in MGEs
To better understand the correlation between the distribution of virulence/AMR genes and S. aureus R-M systems, we examined virulence/AMR genes (Fig. 3). Due to the lack of information regarding the chronological acquisition order of virulence/AMR and R-M genes, we only investigated their distribution. Their causal relationships were not taken into account in this analysis.
We identified a total of 103 virulence genes from 65 bovine IMI-associated S. aureus isolates, which included adhesions (n=17), enzymes (n=15), immune evasion elements (n=23), secretion system (n=12), and toxins (n=36) (Additional file S4). Most virulence genes were conserved in all bovine isolates as a part of the core genome. It is noteworthy that the ST2187 isolate (CC97), in which the hsdR was inactivated, carried a bovine variant gene encoding for a von Willebrand factor-binding protein (vWbp). Several toxin genes (n=12) were located in MGEs and showed uneven distribution between STs. All CC151 isolates contained selz near orfX and an enterotoxin gene cluster (seg, seln, selu, selk, selm, and selo) in an intact prophage φSaBov closest to φIpla88. On the contrary, isolates from CC97 which had two hsdRMS sets were found to carry no enterotoxin gene cluster. We also found Sa3154 contained a pathogenicity island encoding three virulence factors (tst, sec, and sell) that was integrated adjacent to νSaα. Among CC97 isolates, only ST352 carried lukMF’ in a putative prophage closest to φPV83, which contained Type II R-M genes for BcgI-like alpha/beta subunits serving as a self-restricted MGE. Interestingly, no CC lineage-specific virulence gene was found in CC126 although their Type I and II R-M genes were inactivated.
A total of 25 AMR genes were found using MEGARes database and Roary from 65 S . aureus isolated from bovine sources (Additional file S4). Of the 25 AMR genes, majority encoded efflux pumps (n=10) and regulators (n=7). A total of 14 genes were found in all bovine IMI-associated S. aureus isolates examined in this study: aac(3), aph(3), arlR, arlS, pbuE, lmrS, mepA, mepB, slyA, mgrA, norA, norB, rlmH and tet(38). The remaining AMR genes (n=11) in bovine isolates were located in MGEs, with the exception of fosB. Unlike the virulence genes identified, no AMR genes exclusive to bovine isolates were found. All CC126 isolates (n=5) had fosB, and among them, only Sa2605 (CC126) carried blaI, blaR, and blaZ. In addition to the 14 core AMR genes, Sa1158c (CC8) contained eight more AMR genes: fosB in non-MGE, mecR and mecA at orfX, blaI and blaZ at the downstream of SCCmec, aacA in a genomic island, and tetM in another genomic island. Only Sa3 (CC151) among bovine IMI-associated isolates carried tetK in pSX10B1. Four isolates in ST352 (CC97) were found with linA carried by plasmids (Fig. 3). The plasmids with linA were rarely present in human isolates (2.5 %, 3/122). Instead of carrying bovine-specific virulence genes, CC126 and CC8 isolated from bovine niche carried AMR genes which were highly restrained within the STs and not found in other STs. Unlike virulence genes, correlations between S. aureus R-M system and AMR genes were not found.
Gene content involved in bovine mastitis in non-MGEs
We additionally investigated agr genes and capsular biosynthesis genes that are associated with S. aureus quorum-sensing and pathogenesis (Fig. 3). We investigated the autoinducing peptide (AIP) type in STs. Only AIP-I and II were found in bovine isolates, while all four known AIP types were found in human isolates; although only a minority of isolates (13.1%, 16/122) encoded either the AIP-III or AIP-IV precursor (Additional file S5, Fig. S2). All CC151 isolates encoded the AIP-I precursor, and others had agrD for the AIP-II precursor. The capsular biosynthesis gene cluster capABCDEFGHIJKLMNOP in CC151 was different from the other bovine isolates, specifically in four genes (capHIJK). CC151 encoded cap8HIJK while others carried cap5HIJK, as known that two capsular types are produced by bovine IMI-associated S. aureus isolates.
Discussion
In this study, we highlighted the pan-genome of 187 S . aureus isolates obtained from bovine and humans and compared their gene contents. Human S. aureus isolates (n=122) contributed significantly to the pan-genome, which was mainly from the accessory genes. The origin of the isolates, collection period and area, and quality of the assembled genomes likely influence the pan-genome size [52, 53]. The accessory genomes of bovine and human isolates were composed of 1375 and 3771 genes, respectively. S. aureus genomes originated from humans used in this study were from various geographical areas over a broad range of collection years, while bovine S. aureus isolates were mainly collected for 2 years in Canada (Additional file S1). Moreover, the completeness of S. aureus genomes from humans was much better than bovine IMI-associated S. aureus genomes.
We examined host-specific genes of S. aureus isolated from both humans and cows. S. aureus host-specifity is closely correlated to genetic lineage – CC97, CC126, CC133, and CC151 are bovine adapted lineages, while human-adapted lineages include CC1, CC5, CC8, CC30, and CC45 [7]. The pathogenicity island known as SaPIbov3 carries four bovine-specific genes and has been reported to be exclusively found in bovine isolates [54]. In this study, SaPIbov3 was not found in bovine isolates from either the CC126 or CC8 lineages. Similarly, human-specific genes such as lytN, fmhC, dprA, scn, chp, and sak were encoded within the majority of human STs, yet not all human isolates. The distribution of these host-specific genes suggests that there is no absolute or universal host-specific element, although they can increase an opportunity for the successful adaptation in a new host niche. Alternatively, losing human-specific MGEs and acquiring a single mutation may also confer a fitness advantage and alter host tropism [55, 56].
From the phylogenomic tree, we found three suspicious host jumping isolates: ATCC 6538 and ATCC BAA-39 in CC97 and Sa1158c in CC8. Zoonotic and zooanthroponotic transfers of S. aureus between humans and cows in both CC97 and CC8 have been reported in other studies [8, 21, 57]. It is still speculative whether these three S. aureus isolates truly result from the host-switching between humans and cows. However, the genetic elements in these isolates could provide an insight into the potential host jumping route or mechanism of other isolates in the same CC. The two human isolates (CC97), ATCC 6538 and ATCC BAA-39, carried two enterotoxin genes in a pathogenicity island and the immune evasion cluster located in β-converting prophage φJS01, which are highly associated with virulence and host-adaptation [58, 59]. Thus, these two CC97 isolates seem to be successfully adapted to the new host by acquiring this human immune evasion cluster. We previously observed that plasmid transformation from RN4220 (CC8) to CC97 more efficient than those from CC8 to CC151 under laboratory conditions [60, 61]. This indicates CC97 is more prone to be accept genes from human-originated S. aureus than CC151, increasing its chance to survive in human niche. On the contrary, no host-specific gene was found in bovine-isolated Sa1158c (CC8). However, the loss of a beta-converting prophage has shown to be associated with human-to-bovine jump of S. aureus . The possibility of spillover and transmission of CC8 from human to bovine is still valid in this specific isolate and more zooanthroponotic transfer is possible via the same manner under the right selection pressure while repeated exposure of CC8 to bovine occurs.
Most genes exclusive to bovine-associated S. aureus were located within MGEs. These MGEs explain that clonal diversification of S. aureus may occur via HGT during the adaptation to the bovine niche. The differences between CC97 and CC151, two major bovine lineages, in MGEs were enterotoxin gene cluster in CC151 and vWbp for bovine-specific coagulase in CC97. Although CC151 encoded more toxin genes than CC97, we found tst located in SaPIbov in RF122 and Sa3154, probably due to ST bias to ST151 that was previously reported not to carry SaPIbov [62]. We showed that all bovine-associated S. aureus had Type I R-M genes with a unique combination of hsdS genes in different lineages, and many bovine isolates also carried Type II R-M genes. This lineage- or ST-specific genetic barrier suggests that R-M systems in S. aureus are, at least in part, responsible for shaping clonal diversification. The original and additional Type I R-M systems are unlikely to form an interchangeable functional complex due to the low amino acid identity between the various subunits. This additional HsdRMS may play a critical role in ST2187 (CC97) due to a defective hsdR in the original complex and an overall enhanced genetic barrier in CC97. ST2270 (CC126) may be a restriction-defective ST with no known functional R-M system due to the inactivated HsdR (Type I R-M) and Res subunit (Type II R-M). However, restriction endonuclease deficient S. aureus strains are not necessarily hypersusceptible to gene transfer. We observed that ST2270 isolates did not carry more MGEs than other STs. It was previously demonstrated that the inactivation of Type I R-M system was insufficient to construct S. aureus mutants capable of efficiently accepting foreign DNA [63]. S. aureus may naturally develop another barrier for gene transfer because lacking R-M system is more vulnerable to bacteriophage suggesting its detrimental effect over beneficial effect [64].
Of note, S. aureus R-M systems are not an absolute barrier for gene transfers. Under certain environmental pressure, increased dissemination of MGEs can occur within a lineage or across different lineages. Exposure to antibiotic pressure is one stimulator of genetic dissemination since antibiotic-induced SOS response promotes HGT of pathogenicity islands [65]. This HGT network raises concerns regarding the dissemination of AMR genes from human to bovine hosts via host transmission we described in this study. Sa1158c (ST8) carrying blaZ and mecA is a potential donor of AMR genes to S. aureus in the bovine niche. AMR genes (mecA and blaZ) have been identified in ST97 (CC97) and ST126 (CC126) in Brazil, suggesting that right selective pressure may overcome or bypass the genetic barrier to disseminate AMR genes in bovine-adapted lineages [66]. S. aureus ST97 is a MRSA lineage extensively found in pigs and dairy cattle in Italy [67]. Although ST97 shares the same Type I R-M genes with other STs of CC97 [68], it does not carry Type II R-M genes that are present in ST352, making HGT from ST97 to ST352 more challenging than other STs of CC97. However, the HGT network of AMR genes in bovine-adapted lineage CC97 is already open under the right selective pressure.
Interestingly, we also observed antagonistic characteristics of CC151 and CC97 against each other. CC151 and CC97 encoded agrD for different AIP precursors involved in S. aureus quorum-sensing activity. AIP-I and II produced by S. aureus are known to exhibit cross-inhibition [49]. Indeed, we previously confirmed that CC151 (agr type II) inhibited the quorum-sensing of CC97 (agr type I) in co-culture conditions [60]. The pCC97-1 plasmid, which is most homologous to pRJ80 plasmid with 99.78 % identity, was found in CC97 isolates and encoded genes for aureocin 4181 products: aureocin peptides, known as heat-stable bacteriocins, bacteriocin immunity protein, bacteriocin regulatory protein, and bacteriocin exporter protein [69]. Aureocin 4181 is known to exhibit strong antimicrobial activity against isolates of Micrococcus luteus , Streptococcus agalactiase, S. aureus , and other staphylococci [69]. This bacteriocin may modify the microbial composition of the udder skin and teat canal thus disturbing the native microbiome. We also observed that CC97 carrying pCC97-1 plasmid inhibited the growth of CC151 in vitro [60]. The antagonistic relationship within bovine-adapted S. aureus lineages (CC97 and CC151) suggests that they have evolved independently and are unlikely to dominate the same host at the same time point.
The main limitation of this study was the lack of information on the chronological order of the acquisition of MGEs and R-M genes in S. aureus , leading to a failure to elucidate the causal relationships between them. Also, sequence recognition sites of each R-M enzyme commonly found in bovine IMI-associated S. aureus are unknown, so the presence of cognition sequences in MGEs could not be determined. An additional limitation of this study was from the incomplete draft genomes of bovine IMI-associated S. aureus . It is often recommended to use a draft genome with a ‘near finished’ status (less than 1 % missing fraction) in pan-genome computations [52]. Apart from common criteria such as GC%, N50, and a number of contigs, the genome size and exclusion of draft genomes smaller than 2.65 Mb, corresponding to <97 % of S. aureus RF122 genome size, was performed in this study. Some important genes in a few isolates were not assembled, yet the genes were still present. To confirm the presence of target genes, such as hsdMS, PCR amplification and Sanger sequencing needed to be performed. The presence of plasmids was also confirmed by plasmid DNA extraction and mapping to verify the size of the predicted plasmids. Lastly, 63 bovine IMI-associated S. aureus isolates used in this study give a bias to Canada while human isolates were from various continents. This strong geographical bias may result in misleading the data interpretation such as CC lineage-specific exclusive genes.
Conclusion
The genetic differences among bovine IMI-associated S. aureus lineages reveal that S. aureus in the bovine niche has evolved in multiple directions. Our results suggest that bovine-specific and exclusive genes, which are mainly located in MGEs, play an important role in clonal diversification and host adaptation. Moreover, R-M systems in S. aureus shape S. aureus clonal diversification and pathogenicity by discriminating MGEs. We highlight that S. aureus ST identification in dairy herds is important to assess the risk of transmission and intervention strategies due to the various potential impacts of certain STs on dairy cows. We also bring attention to the possible MRSA transmission from humans to cows, suggesting the continued importance of farm biosecurity.
Supplementary Data
Funding information
Funding for this project was provided by an Op+lait Subvention Nouvelles Initiatives 2018/2019 grant awarded to J.Ronholm and S.Dufour. S.P., D.J., A.D.D., and E.D. are each supported by scholarships from the NSERC CREATE in Milk Quality and the Op +lait Complements de Bourse program. J.Ruffini received summer funding from the Op+lait program. Additional funding support was obtained from NSERC (grant to F. M. No. 2015-05916). We are grateful to Calcul Québec, Compute Canada and the Centre de Calcul Scientifique of the Université de Sherbrooke for access and technical support while using the Mammouthmp2 supercomputer.
Author contributions
The responsibilities for conceptualization, supervision, project administration and funding for this paper were provided by J.R. and S.D. F.D., provided the assembly software ProkaryoteAssembly. S.P., D.J., J.R., A.D., E.D. and J-F.L., performed whole-genome sequencing. The analysis tasks and tools were shared between S.P. and D.J. For data analysis, S.P., performed the formal analysis, investigation, data curation and visualization and B.O., participated in R-M gene and MGEs analysis. The original draft of the manuscript was prepared by S.P. All authors were responsible for the review and editing of the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: AMR, antimicrobial resistant; CC, clonal complex; IMI, intramammary infection; LA-MRSA, livestock-associated methicillin-resistant Staphylococcus aureus; MGE, mobile genetic element; res, restriction endonuclease; R-M, restriction-modification; SCC, somatic cell count; ST, sequence type.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Supplementary material is available with the online version of this article.
References
- 1.Watts JL. Etiological agents of bovine mastitis. Vet Microbiol. 1988;16:41–66. doi: 10.1016/0378-1135(88)90126-5. [DOI] [PubMed] [Google Scholar]
- 2.Fox LK, Zadoks RN, Gaskins CT. Biofilm production by Staphylococcus aureus associated with intramammary infection. Vet Microbiol. 2005;107:295–299. doi: 10.1016/j.vetmic.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Hébert A, Sayasith K, Sénéchal S, Dubreuil P, Lagacé J. Demonstration of intracellular Staphylococcus aureus in bovine mastitis alveolar cells and macrophages isolated from naturally infected cow milk. FEMS Microbiol Lett. 2000;193:57–62. doi: 10.1111/j.1574-6968.2000.tb09402.x. [DOI] [PubMed] [Google Scholar]
- 4.Atalla H, Gyles C, Mallard B. Persistence of a Staphylococcus aureus small colony variants (S. aureus SCV) within bovine mammary epithelial cells. Vet Microbiol. 2010;143:319–328. doi: 10.1016/j.vetmic.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Rainard P, Foucras G, Fitzgerald JR, Watts JL, Koop G, et al. Knowledge gaps and research priorities in Staphylococcus aureus mastitis control. Transbound Emerg Dis. 2018;65 Suppl 1:149–165. doi: 10.1111/tbed.12698. [DOI] [PubMed] [Google Scholar]
- 6.Sadykov MR. Restriction-modification systems as a barrier for genetic manipulation of Staphylococcus aureus . Methods Mol Biol. 2016;1373:9–23. doi: 10.1007/978-1-4939-3158-3. [DOI] [PubMed] [Google Scholar]
- 7.Park S, Ronholm J. Staphylococcus aureus in agriculture: lessons in evolution from a multispecies pathogen. Clin Microbiol Rev. 2021;34:e00182-20. doi: 10.1128/CMR.00182-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matuszewska M, Murray GGR, Harrison EM, Holmes MA, Weinert LA. The evolutionary genomics of host specificity in Staphylococcus aureus . Trends Microbiol. 2020;28:465–477. doi: 10.1016/j.tim.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Herron-Olson L, Fitzgerald JR, Musser JM, Kapur V, Ahmed N. Molecular correlates of host specialization in Staphylococcus aureus . PLoS ONE. 2007;2:e1120. doi: 10.1371/journal.pone.0001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naushad S, Nobrega DB, Naqvi SA, Barkema HW, De Buck J, et al. Genomic analysis of bovine Staphylococcus aureus isolates from milk to elucidate diversity and determine the distributions of antimicrobial and virulence genes and their association with mastitis. mSystems. 2020;5:e00063-20. doi: 10.1128/mSystems.00063-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vrieling M, Koymans KJ, Heesterbeek DAC, Aerts PC, Rutten VPMG, et al. Bovine Staphylococcus aureus Secretes the Leukocidin LukMF’ To Kill Migrating Neutrophils through CCR1. mBio. 2015;6:e00335. doi: 10.1128/mBio.00335-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viana D, Blanco J, Tormo-Más MA, Selva L, Guinane CM, et al. Adaptation of Staphylococcus aureus to ruminant and equine hosts involves SaPI-carried variants of von Willebrand factor-binding protein. Mol Microbiol. 2010;77:1583–1594. doi: 10.1111/j.1365-2958.2010.07312.x. [DOI] [PubMed] [Google Scholar]
- 13.Cuny C, Wieler LH, Witte W. Livestock-Associated MRSA: the impact on humans. Antibiotics (Basel) 2015;4:521–543. doi: 10.3390/antibiotics4040521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saini V, McClure JT, Scholl DT, DeVries TJ, Barkema HW. Herd-level association between antimicrobial use and antimicrobial resistance in bovine mastitis Staphylococcus aureus isolates on Canadian dairy farms. J Dairy Sci. 2012;95:1921–1929. doi: 10.3168/jds.2011-5065. [DOI] [PubMed] [Google Scholar]
- 15.Haran KP, Godden SM, Boxrud D, Jawahir S, Bender JB, et al. Prevalence and characterization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, isolated from bulk tank milk from Minnesota dairy farms. J Clin Microbiol. 2012;50:688–695. doi: 10.1128/JCM.05214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang F, Wang Q, Wang X, Wang L, Xiao M, et al. Prevalence of blaZ gene and other virulence genes in penicillin-resistant Staphylococcus aureus isolated from bovine mastitis cases in Gansu, China. Turk J Vet Anim Sci. 2015;39:634–636. doi: 10.3906/vet-1504-81. [DOI] [Google Scholar]
- 17.Bagcigil AF, Taponen S, Koort J, Bengtsson B, Myllyniemi A-L, et al. Genetic basis of penicillin resistance of S. aureus isolated in bovine mastitis. Acta Vet Scand. 2012;54:69. doi: 10.1186/1751-0147-54-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamali H, Radmehr B, Ismail S. Short communication: prevalence and antibiotic resistance of Staphylococcus aureus isolated from bovine clinical mastitis. J Dairy Sci. 2014;97:2226–2230. doi: 10.3168/jds.2013-7509. [DOI] [PubMed] [Google Scholar]
- 19.Marques VF, Motta CC, Soares BS, de Melo DA, Coelho SMO, et al. Biofilm production and beta-lactamic resistance in Brazilian Staphylococcus aureus isolates from bovine mastitis. Braz J Microbiol. 2017;48:118–124. doi: 10.1016/j.bjm.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klibi A, Jouini A, Gómez P, Slimene K, Ceballos S, et al. Molecular characterization and clonal diversity of methicillin-resistant and -susceptible Staphylococcus aureus isolates of milk of cows with clinical mastitis in Tunisia. Microb Drug Resist. 2018;24:1210–1216. doi: 10.1089/mdr.2017.0278. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt T, Kock MM, Ehlers MM. Molecular characterization of Staphylococcus aureus isolated from bovine mastitis and close human contacts in South African dairy herds: genetic diversity and inter-species host transmission. Front Microbiol. 2017;8:511. doi: 10.3389/fmicb.2017.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Käppeli N, Morach M, Corti S, Eicher C, Stephan R, et al. Staphylococcus aureus related to bovine mastitis in Switzerland: Clonal diversity, virulence gene profiles, and antimicrobial resistance of isolates collected throughout 2017. J Dairy Sci. 2019;102:3274–3281. doi: 10.3168/jds.2018-15317. [DOI] [PubMed] [Google Scholar]
- 23.Waldron DE, Lindsay JA. Sau1: a novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineages. J Bacteriol. 2006;188:5578–5585. doi: 10.1128/JB.00418-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay JA, Holden MTG. Staphylococcus aureus: superbug, super genome? Trends Microbiol. 2004;12:378–385. doi: 10.1016/j.tim.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Roberts RJ, Belfort M, Bestor T, Bhagwat AS, Bickle TA, et al. A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res. 2003;31:1805–1812. doi: 10.1093/nar/gkg274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts GA, Houston PJ, White JH, Chen K, Stephanou AS, et al. Impact of target site distribution for Type I restriction enzymes on the evolution of methicillin-resistant Staphylococcus aureus (MRSA) populations. Nucleic Acids Res. 2013;41:7472–7484. doi: 10.1093/nar/gkt535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corvaglia AR, François P, Hernandez D, Perron K, Linder P, et al. A type III-like restriction endonuclease functions as a major barrier to horizontal gene transfer in clinical Staphylococcus aureus strains. Proc Natl Acad Sci U S A. 2010;107:11954–11958. doi: 10.1073/pnas.1000489107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JYH, Carter GP, Pidot SJ, Guérillot R, Seemann T, et al. Mining the methylome reveals extensive diversity in Staphylococcus epidermidis restriction modification. mBio. 2019;10:e02451-19. doi: 10.1128/mBio.02451-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogdanova E, Djordjevic M, Papapanagiotou I, Heyduk T, Kneale G, et al. Transcription regulation of the type II restriction-modification system AhdI. Nucleic Acids Res. 2008;36:1429–1442. doi: 10.1093/nar/gkm1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts RJ, Vincze T, Posfai J, Macelis D. REBASE: restriction enzymes and methyltransferases. Nucleic Acids Res. 2003;31:418–420. doi: 10.1093/nar/gkg069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S, Jung D, Dufour S, Ronholm J, Dunning Hotopp JC. Draft genome sequences of 27 Staphylococcus aureus strains and 3 Staphylococcus species strains isolated from bovine intramammary infections. Microbiol Resour Announc. 2020;9:19. doi: 10.1128/MRA.00300-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dufour S, Labrie J, Jacques M, Rasko D. The mastitis pathogens culture collection. Microbiol Resour Announc. 2019;8:15. doi: 10.1128/MRA.00133-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demontier E, Dubé-Duquette A, Brouillette E, Larose A, Ster C, et al. Relative virulence of Staphylococcus aureus bovine mastitis strains representing the main Canadian spa types and clonal complexes as determined using in vitro and in vivo mastitis models. J Dairy Sci. 2021;104:11904–11921. doi: 10.3168/jds.2020-19904. [DOI] [PubMed] [Google Scholar]
- 34.Jung D, Park S, Ruffini J, Dussault F, Dufour S, et al. Comparative genomic analysis of Escherichia coli isolates from cases of bovine clinical mastitis identifies nine specific pathotype marker genes. Microb Genom. 2021;7 doi: 10.1099/mgen.0.000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okonechnikov K, Conesa A, García-Alcalde F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics. 2016;32:292–294. doi: 10.1093/bioinformatics/btv566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 37.Tong SYC, Schaumburg F, Ellington MJ, Corander J, Pichon B, et al. Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: the non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. nov. Int J Syst Evol Microbiol. 2015;65:15–22. doi: 10.1099/ijs.0.062752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker K, Schaumburg F, Kearns A, Larsen AR, Lindsay JA, et al. Implications of identifying the recently defined members of the Staphylococcus aureus complex S. argenteus and S. schweitzeri: a position paper of members of the ESCMID Study Group for Staphylococci and Staphylococcal Diseases (ESGS) Clin Microbiol Infect. 2019;25:1064–1070. doi: 10.1016/j.cmi.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 39.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–5. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jolley KA, Maiden MCJ. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliveros J. 2007-2015. Venny. An interactive tool for comparing lists with Venn’s diagrams. 2017 https://bioinfogp.cnb.csic.es/tools/venny/index.html
- 44.Bertelli C, Laird MR, Williams KP, Simon Fraser University Research Computing Group. Lau BY, et al. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017;45:W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, et al. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36:W5–9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B, Zheng D, Jin Q, Chen L, Yang J. VFDB 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019;47:D687–D692. doi: 10.1093/nar/gky1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lakin SM, Dean C, Noyes NR, Dettenwanger A, Ross AS, et al. MEGARes: an antimicrobial resistance database for high throughput sequencing. Nucleic Acids Res. 2017;45:D574–D580. doi: 10.1093/nar/gkw1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts RJ, Vincze T, Posfai J, Macelis D. REBASE--a database for DNA restriction and modification: enzymes, genes and genomes. Nucleic Acids Res. 2015;43:D298–9. doi: 10.1093/nar/gku1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang B, Muir TW. Regulation of virulence in Staphylococcus aureus: molecular mechanisms and remaining puzzles. Cell Chem Biol. 2016;23:214–224. doi: 10.1016/j.chembiol.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arndt D, Grant JR, Marcu A, Sajed T, Pon A, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Setubal JC, Almeida NF, Wattam AR. Comparative genomics for prokaryotes. Methods Mol Biol. 2018;1704:55–78. doi: 10.1007/978-1-4939-7463-4_3. [DOI] [PubMed] [Google Scholar]
- 53.Bosi E, Monk JM, Aziz RK, Fondi M, Nizet V, et al. Comparative genome-scale modelling of Staphylococcus aureus strains identifies strain-specific metabolic capabilities linked to pathogenicity. Proc Natl Acad Sci U S A. 2016;113:E3801–9. doi: 10.1073/pnas.1523199113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozytska S, Stauss D, Pawlik M-C, Hensen S, Eckart M, et al. Identification of specific genes in Staphylococcus aureus strains associated with bovine mastitis. Vet Microbiol. 2010;145:360–365. doi: 10.1016/j.vetmic.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 55.Viana D, Comos M, McAdam PR, Ward MJ, Selva L, et al. A single natural nucleotide mutation alters bacterial pathogen host tropism. Nat Genet. 2015;47:361–366. doi: 10.1038/ng.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Resch G, François P, Morisset D, Stojanov M, Bonetti EJ, et al. Human-to-bovine jump of Staphylococcus aureus CC8 is associated with the loss of a β-hemolysin converting prophage and the acquisition of a new staphylococcal cassette chromosome. PLoS One. 2013;8:e58187. doi: 10.1371/journal.pone.0058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spoor LE, McAdam PR, Weinert LA, Rambaut A, Hasman H, et al. Livestock origin for a human pandemic clone of community-associated methicillin-resistant Staphylococcus aureus . mBio. 2013;4:e00356-13. doi: 10.1128/mBio.00356-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia H, Dong W, Yuan L, Ma J, Bai Q, et al. Characterization and complete genome sequence analysis of Staphylococcus aureus bacteriophage JS01. Virus Genes. 2015;50:345–348. doi: 10.1007/s11262-015-1168-y. [DOI] [PubMed] [Google Scholar]
- 59.McClure J-AM, Lakhundi S, Kashif A, Conly JM, Zhang K. Genomic comparison of highly virulent, moderately virulent, and avirulent strains from a genetically closely-related MRSA ST239 sub-lineage provides insights into pathogenesis. Front Microbiol. 2018;9:1531. doi: 10.3389/fmicb.2018.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S, Classen A, Gohou HM, Maldonado R, Kretschmann E, et al. A new, reliable, and high-throughput strategy to screen bacteria for antagonistic activity against Staphylococcus aureus . BMC Microbiol. 2021;21:189. doi: 10.1186/s12866-021-02265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair D, Memmi G, Hernandez D, Bard J, Beaume M, et al. Whole-genome sequencing of Staphylococcus aureus strain RN4220, a key laboratory strain used in virulence research, identifies mutations that affect not only virulence factors but also the fitness of the strain. J Bacteriol. 2011;193:2332–2335. doi: 10.1128/JB.00027-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson GJ, Tuffs SW, Wee BA, Seo KS, Park N, et al. Bovine Staphylococcus aureus superantigens stimulate the entire T cell repertoire of cattle. Infect Immun. 2018;86:11. doi: 10.1128/IAI.00505-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veiga H, Pinho MG. Inactivation of the SauI type I restriction-modification system is not sufficient to generate Staphylococcus aureus strains capable of efficiently accepting foreign DNA. Appl Environ Microbiol. 2009;75:3034–3038. doi: 10.1128/AEM.01862-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moller AG, Lindsay JA, Read TD. Determinants of phage host range in Staphylococcus Species. Appl Environ Microbiol. 2019;85:11. doi: 10.1128/AEM.00209-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ubeda C, Maiques E, Knecht E, Lasa I, Novick RP, et al. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol. 2005;56:836–844. doi: 10.1111/j.1365-2958.2005.04584.x. [DOI] [PubMed] [Google Scholar]
- 66.Oliveira CJB, Tiao N, de Sousa FGC, de Moura JFP, Santos Filho L, et al. Methicillin-resistant Staphylococcus aureus from Brazilian dairy farms and identification of novel sequence types. Zoonoses Public Health. 2016;63:97–105. doi: 10.1111/zph.12209. [DOI] [PubMed] [Google Scholar]
- 67.Feltrin F, Alba P, Kraushaar B, Ianzano A, Argudín MA, et al. A livestock-associated, multidrug-resistant, methicillin-resistant Staphylococcus aureus clonal complex 97 lineage spreading in dairy cattle and pigs in Italy. Appl Environ Microbiol. 2016;82:816–821. doi: 10.1128/AEM.02854-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cormican P, Keane OM. Complete genome sequences of sequence type 71 (ST71) and ST97 Staphylococcus aureus isolates from bovine milk. Microbiol Resour Announc. 2018;7:e00954-18. doi: 10.1128/MRA.00954-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salustiano Marques-Bastos SL, Varella Coelho ML, Ceotto-Vigoder H, Carlin Fagundes P, Silva Almeida G, et al. Molecular characterization of aureocin 4181: a natural N-formylated aureocin A70 variant with a broad spectrum of activity. Braz J Microbiol. 2020;51:1527–1538. doi: 10.1007/s42770-020-00315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.