Abstract
Background
Liver transplant, the definitive treatment of decompensated cirrhosis (DC), is constrained by donor shortage and long-term complications. Granulocyte colony-stimulating factor (G-CSF) has been explored as an alternative option in open-label studies. This double-blind, randomized, placebo-controlled trial was designed to elucidate the efficacy of G-CSF in DC.
Methods
Seventy patients were randomized to either G-CSF plus standard medical therapy (group A, n = 35) or placebo plus standard medical therapy (group B, n = 35). Primary outcome was 12-month overall survival in patients who received at least one cycle of intervention. Secondary outcomes were mobilization of CD34+ cells at day 6, improvement in Child–Turcotte–Pugh (CTP), and model for end-stage liver disease (MELD), liver stiffness measurement, quality of life, nutrition, hepatic decompensation, infection, hospitalization, and acute kidney injury.
Results
Survival in group A was higher than that in Group B although the difference was not statistically significant (87.9% vs 66.7%; p = 0.053). CD34+ cells at day 6 were significantly higher in group A as compared to baseline (p < 0.001). Ascites control (p = 0.03) and CTP score improvement (p = 0.02) were better in group A at 12-months. Encephalopathy episodes (p = 0.005), infections (p = 0.005) were fewer in group A than group B at 12 months. Other secondary outcomes did not improve post-therapy. There were no treatment-related discontinuations or severe adverse events.
Conclusions
G-CSF therapy is safe. The improvement in survival at 12 months is not statistically significant. Better control of ascites, improvement of CTP score, fewer encephalopathy episodes and decreased rate of infections were observed with G-CSF therapy (NCT03911037).
Trials Registration NCT03911037
Supplementary Information
The online version contains supplementary material available at 10.1007/s12072-022-10314-x.
Keywords: Chronic liver disease, End-stage liver disease, G-CSF, Growth factors, Liver regeneration, Portal hypertension, Hematopoietic stem cells, Cirrhosis, Ascites, Hepatic encephalopathy, Variceal bleed
Introduction
Decompensated cirrhosis (DC) is riddled with complications like refractory ascites, variceal bleeding, hepatic encephalopathy, and recurrent infections [1]. High mortality, morbidity, and complications leave the patients struggling with a poor quality of life [2]. Once decompensation occurs, the median survival is only around 2 years [1, 3]. The definitive management of DC is liver transplantation (LT). However, LT has its demerits like high cost, organ shortage, and a need for long-term immunosuppression. It is important to find alternative therapeutic modalities, as LT does not satisfy the huge demand–supply mismatch of donor organs [4].
Multiple studies have reported improved survival and disease severity scores using single and multiple cycles of granulocyte colony-stimulating factor (G-CSF) [5–8]. However, Newsome et al. reported no improvement in model for end-stage liver disease (MELD) score after a single session of G-CSF therapy [9]. The open-label nature of these previous studies prevents us from drawing firm conclusions. To resolve these discrepancies, a double-blind, randomized, placebo-controlled, adequately powered trial was conducted to evaluate the efficacy of multiple cycles of G-CSF in DC.
Materials and methods
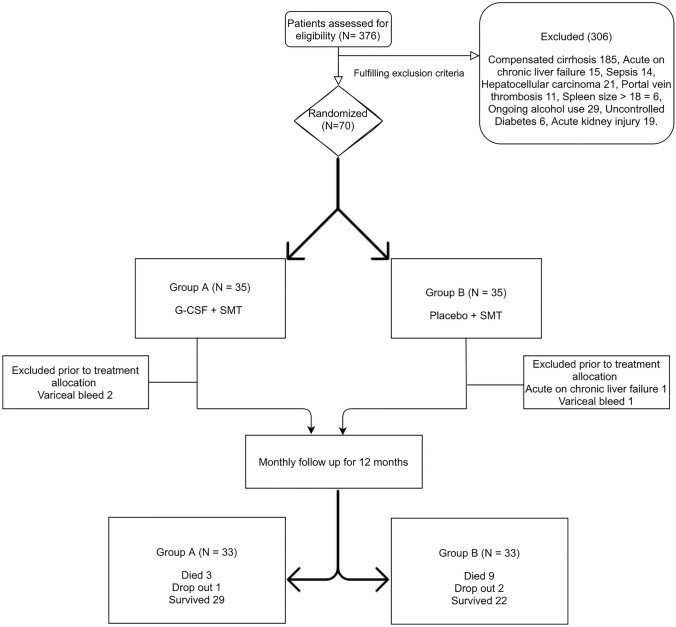
This single-center, double-blind, randomized, placebo-controlled trial was conducted from May 2019 to June 2020. Institutional Ethical approval was obtained (INT/IEC/2019/000727) and the trial was registered at clinicaltrials.gov (NCT03911037). The study was compliant with the Declaration of Helsinki and has been reported according to CONSORT guidelines. Written informed consent was obtained from all patients. Consort diagram of the study is shown in Fig. 1.
Fig. 1.
Consort diagram of the study
Eligibility criteria
Adults between 18 and 80 years of age with DC were included, irrespective of etiology. Decompensations included ascites, hepatic encephalopathy, and variceal bleeding. Cirrhosis was diagnosed by history, clinical examination, laboratory investigations, radiology, and endoscopy. Patients with acute-on-chronic liver failure (APASL or CANONIC criteria), the diameter of spleen > 18 cm, co-existent hepatocellular carcinoma or any other malignant tumor, variceal bleed in the last seven days, portal vein thrombosis, uncontrolled diabetes (HbA1c ≥ 9 or retinopathy), renal dysfunction (serum creatinine > 1.5 mg/dl), severe cardio-pulmonary derangement, infection, disseminated intravascular coagulation, alcohol use in the previous 3 months, prior hypersensitivity episode to G-CSF, HIV coinfection and pregnancy were excluded. Eligibility in the trial was evaluated by 2 independent clinicians. All patients with chronic viral hepatitis had undetectable viral load at inclusion.
Groups and therapy
Study participants were randomized into group A (G-CSF group) or Group B (placebo group) by computer-generated random number tables. Allocation concealment was done by serially numbered opaque sealed envelopes. Dispensing of drugs according to code generated was done by an independent research fellow (BL), who was not involved in patient care. The specific intervention was initiated within one week of randomization.
Group A received G-CSF (5 μg/kg subcutaneously Q 12 hourly for 5 consecutive days, a total of 4 cycles, once every 3 months) and standard medical treatment (SMT). Group B received normal saline in vials identical to G-CSF and SMT. All patients were administered the interventional drug under supervision in the hospital. SMT included nutrition (salt restriction, calories 35–40 kcal/kg/day, protein 1.5 g/kg/day, and alcohol abstinence), rifaximin and lactulose for hepatic encephalopathy, diuretics and large-volume paracentesis for ascites, prophylaxis with norfloxacin for past spontaneous bacterial peritonitis (SBP) and beta-blockers for variceal bleed prophylaxis. All patients with Hepatitis B were on treatment with tenofovir disoproxil fumarate.
Patient monitoring
Patients were admitted for G-CSF or placebo injection. Complete hemogram, liver, and renal function tests, serum electrolytes, and prothrombin time were assessed at baseline and monthly intervals. CD 34+ cells were measured on day 0 and day 6 of G-CSF administration by flow cytometry. Total leukocyte counts and splenic size on ultrasound were also measured on day 6 of each G-CSF cycle. Reports of day 6 blood counts, splenic size, and CD34+ cell count were placed in concealed envelopes by an independent research fellow (BL), such that the treating physicians were blinded. These concealed envelopes were only opened at the end of the trial.
Lipid profile, HbA1c, and alpha-fetoprotein levels were assessed at baseline and 3-monthly intervals. Computed tomography abdomen was done at the baseline and after every 3 months. Liver Stiffness Measurement (LSM) was done by transient elastography using FibroScan (Echosens, Paris) at 0 and 12 months. Nutritional status[measurement of body mass index (BMI), handgrip strength (HGS), and skeletal muscle index (SMI)] and quality of life (QOL) using SF-36v2 questionnaire were assessed at baseline and 12 months. A careful watch was kept for any possible adverse effects which were graded according to Common Terminology Criteria for Adverse Events version 5. Data were entered into a pre-specified proforma.
End points
The primary endpoint was overall survival at 1 year from the beginning of therapy. Secondary endpoints included mobilization of CD34+ cells in peripheral blood at day 6, improvement in MELD and Child–Turcotte–Pugh (CTP) scores, ascites control at the end of therapy, effect on other decompensation events like hepatic encephalopathy and variceal bleeding, improvement in nutritional status (BMI, SMI, and HGS), quality of life, number of acute kidney injury episodes, hospitalizations, infections, change in LSM and adverse events. Complete control of ascites was defined as the absence of ascites while partial control was defined as persistent ascites not requiring large-volume paracentesis. Acute kidney injury was defined as increase in serum creatinine by ≥ 0.3 mg/dl within 48 h or increase in serum creatinine to ≥ 1.5 × baseline, which is known or presumed to have occurred within the prior 7 days or urine volume < 0.5 ml/kg/h for 6 h.
Sample size calculation
One-year survival in DC is estimated to be around 60% [3]. Verma et al. previously reported 1-year survival of 91.3% in DC patients treated with G-CSF [6]. To assess similar survival proportions in the two groups with a power of 80%, α error of 0.05, and beta error of 0.2, the minimum required sample size in each group was calculated as 29. Keeping 20% as dropouts, a total of 35 patients were planned for inclusion in each group.
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences (IBM SPSS Statistics for windows, version 22.0). Quantitative variables were expressed as mean ± standard deviation or median (range), whereas qualitative variables were shown as number (frequency or proportion). Kolmogorov–Smirnov and Shapiro–Wilk tests were used to check normality. To compare between 2 groups, student t-test was used for normally distributed data, Mann–Whitney U test for unpaired skewed data, and Wilcoxon signed-rank tests for paired skewed data. For categorical data, chi-square test and McNemar tests were used for unpaired and paired data, respectively. The analysis was originally planned on the intention-to-treat principle. As 4 patients did not receive the allocated intervention, a modified intention-to-treat analysis was performed including patients who received at least one cycle of intervention. We used the last available parameters for secondary outcome analysis especially when a particular patient’s data were missing due to death or dropout. Kaplan–Meier method with cox-proportional hazard analysis was performed for survival analysis with inter-group comparisons using log-rank test. Patients lost to follow-up were considered as events assuming worst case scenario. Patients undergoing LT were censored. Sensitivity analysis was also performed by evaluating survival using a right-hand censoring approach where in patients lost to follow-up were censored. All statistical analyses were performed as two sided and p < 0.05 was considered statistically significant.
Results
Out of the 70 patients who were initially included in the study, 3 patients (2 in group A and 1 in group B) developed variceal bleed and one patient in group B developed acute-on-chronic liver failure, after randomization but before treatment initiation. These 4 patients (2 each in groups A and B) were neither given G-CSF nor placebo. They were excluded in the modified intention-to-treat analysis which was performed in the study.
Thirty-three patients each (total of 66), were included for analysis in groups A and group B. These patients were followed up for 1 year or till their death. All except one patient in group A were abstinent from alcohol intake during the follow-up. Both groups were well matched for their baseline characteristics. Alcoholic liver disease was the most common etiology of cirrhosis in these patients followed by non-alcoholic steatohepatitis. Baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline characteristics of the study population
| Baseline characteristics | Group A (n = 35) | Group B (n = 35) | p value |
|---|---|---|---|
| Age (years) | 49 (25–74) | 48 (32–79) | 0.85 |
| Gender | |||
| Male | 29 (82.9%) | 28 (80%) | 0.76 |
| Female | 6 (17.1%) | 7 (20%) | |
| Etiology | |||
| Autoimmune hepatitis | 1 (3.03%) | 0 | |
| Hepatitis B | 1 (3.03%) | 2 (3.03%) | |
| Hepatitis C | 3 (9.09%) | 1 (3.03%) | 0.49 |
| NASH | 7 (18.18%) | 11 (30.3%) | |
| Alcohol | 23 (63.63%) | 20 (60.6%) | |
| Alcohol + hepatitis B | 0 | 1 (3.03%) | |
| Ascites | 31 (88.6%) | 33 (94.28%) | 0.39 |
| Duration (Months) | 3 (1–8) | 3 (1–6) | 0.43 |
| Grade of ascites | |||
| Grade 1 | 6 (17.1%) | 5 (14.3%) | |
| Grade 2 | 13 (37.1%) | 17 (48.6%) | 0.74 |
| Grade 3 | 12 (34.3%) | 11 (31.4%) | |
| Need for LVP | 12 (34.3%) | 11 (31.4%) | 0.79 |
| Past SBP | 12 (34.3%) | 15 (42.9%) | 0.46 |
| Hepatic Encephalopathy | 9 (25.7%) | 9 (25.7%) | > 0.9 |
| Variceal Bleed | 13 (37.1%) | 14 (40%) | 0.8 |
| Receiving Betablockers | 18 (51.4%) | 16 (45.7%) | 0.63 |
| Receiving Norfloxacin | 12 (34.3%) | 15 (42.9%) | 0.46 |
| Receiving Rifaximin | 9 (25.7%) | 9 (25.7%) | > 0.9 |
| Hemoglobin (g/dl) | 10.21 (2.05) | 10.25(1.67) | 0.92 |
| Platelet, × 10/L | 72 (21–198) | 82(29–217) | 0.59 |
| TLC | 4.5 (1.5–9.8) | 4.1(1.9–9.9) | 0.33 |
| Neutrophils | 75 (40–82) | 72 (43–82) | .64 |
| Lymphocytes | 29.5 (20–40) | 30.5 (20–40) | > .99 |
| Monocytes | 4 (2–8) | 4 (2–10) | 0.06 |
| Eosinophils | 2 (1–6) | 2 (1–6) | 0.24 |
| Basophils | 1 (0–2) | 1 (1–2) | 0.22 |
| Na (mEq/L) | 138 (129–150) | 138(128–144) | 0.87 |
| Serum creatinine (mg/dl) | 0.88 (0.5–1.34) | 0.8(0.56–1.3) | 0.72 |
| Bilirubin (mg/dl) | 1.7 (0.67–4.76) | 1.7(0.5–3.2) | 0.39 |
| Albumin (g/dl) | 3.1 (2.36–4.7) | 3.14(2.34–3.85) | 0.67 |
| INR | 1.34 (1–2.27) | 1.3(1.1–2) | 0.89 |
| MELD | 12 (6–22) | 13(8–23) | 0.14 |
| CTP | 7 (5–11) | 7 (5–12) | 0.35 |
| Body mass index (kg/m2) | 24.21 (20.57- 34.97) | 24.25 (18.75- 32.44) | 0.69 |
| LSM (kPa) | 56 (15–75) | 60 (22–75) | 0.87 |
| Hand-grip strength (Kg) | 32 (24.5–42.6) | 30 (25.4–45.6) | 0.12 |
| Skeletal muscle index (cm2/m2) | 39.9 (29.5–55.6) | 39.2 (29.2–45.2) | 0.99 |
| Physical Component Summary score | 42.47 (6.43) | 44.26 (6.47) | 0.21 |
| Mental Component Summary score | 41.55 (7.75) | 42.85 (8.47) | 0.70 |
INR, International Normalized Ratio; MELD, Model for End-Stage Liver Disease; CTP, Child–Turcotte–Pugh; LSM, liver stiffness measurement
Endpoints
Primary endpoint
In group A, out of the 33 patients who were followed up for 12 months, 29 patients survived, three patients died, and 1 patient dropped out of therapy due to COVID-19 related mobility restrictions. All deaths were due to sepsis and related multi-organ dysfunction. In group B, out of the 33 patients who were followed up for 12 months, 22 patients survived, 9 patients died, and 2 patients dropped out of therapy due to COVID-19 related mobility restrictions. One patient died of intracranial hemorrhage and raised intracranial pressure, whereas the rest of the patients died of sepsis and multi-organ dysfunction. None of the patients underwent LT.
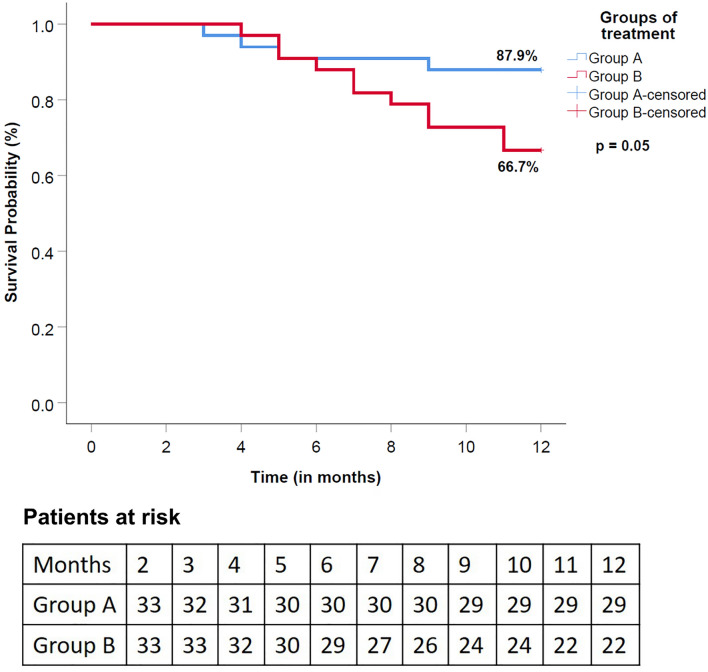
Overall survival at 12 months using worst case scenario approach in group A was better (87.9%) as compared to group B (66.7%) with a hazard ratio of 0.34 (95% CI 0.11–1.08). However, the difference in survival was not statistically significant (p = 0.053). Kaplan–Meier curve for survival is shown in Fig. 2. On sensitivity analysis using a right-hand censoring approach too, the difference in survival was not significant (p = 0.071).
Fig. 2.
Kaplan–Meier curve showing overall survival at 12 months in groups A and B
Secondary endpoints
After G-CSF therapy, there was a marked increase in total leukocyte counts, CD34+ cell count, and spleen size on day 6 as compared to day 0 in Group A patients. Similar changes were not seen in Group B patients at day 6 (Supplementary table 1).
The CTP scores were significantly reduced in group A [baseline vs 12 months: 8 (5–10) vs. 6 (5–12), p = 0.02], while they worsened in group B [baseline vs 12 months: 8 (5–12) vs. 8(6–14), p = 0.03]. Also, the percentage change in CTP at the end of therapy was significantly higher in group A as compared to group B [%ΔCTP: − 12.5 (− 33.3 to 40) vs. 0 (− 20 to 44.4), p = 0.002] (Table 2). Overall, CTP improved in 18 (54.5%) patients of group A and 9 (27.3%) patients in group B, p = 0.04.
Table 2.
Disease severity scores of the study population
| Outcome | Group A | Group B | p value between the groups at 12 months | ||||
|---|---|---|---|---|---|---|---|
| Baseline (n = 33) | 12 months (n = 33) | p value within the group | Baseline (n = 33) | 12 months (n = 33) | p value within the group | ||
| CTP | 8 (5–10) | 6 (5–12) | 0.020 | 8 (5–12) | 8(6–14) | 0.03 | 0.002 |
| Median % Δ change in CTP (0–12) | −12.5 (−33.3 to 40) | – | 0 (−20 to 44.4) | – | 0.002 | ||
| MELD | 12(6–18) | 12(7–22) | 0.47 | 13(8–23) | 13(7–22) | 0.73 | 0.33 |
| Median % Δ change in MELD (0–12) | 0 (−35.3 to 90.9) | – | 0 (−37.50 to 58.3) | – | 0.54 | ||
MELD, Model for End-Stage Liver Disease; CTP, Child–Turcotte–Pugh
There were no significant changes in median MELD scores in both groups at 12 months, as compared to baseline (p > 0.05) (Table 2). Median percentage change in MELD at the end of therapy was not statistically significant between groups A and B [%ΔMELD:0 (− 35.3 to 90.9) vs. 0 (− 37.50 to 58.3), p = 0.54]. MELD score improvement was seen in 13 (39.4%) patients of group A and 14 (42.4%) patients of group B (p = 0.80).
Ascites control was better in group A as compared to group B (p = 0.03) as shown in Table 3. No patient developed new-onset ascites in either group. During follow-up, 1 (3.4%) patient in group A and 4 (18.2%) patients in group B had variceal bleed (p = 0.08). Gastric variceal bleed occurred in 1 patient each of groups A and B. Three patients in group B had an esophageal variceal bleed. Five patients (17.2%) in group A and 12 (54.5%) patients in group B had hepatic encephalopathy during the 12 months of follow-up (p = 0.005).
Table 3.
Ascites control, infections, hospitalizations and acute kidney injury in the study population
| Outcomes | Group A (n = 33) | Group B (n = 33) | p value between the groups |
|---|---|---|---|
| No. of patients with infections | 6 (18.2%) | 17 (51.5%) | 0.005 |
| Types of infections | |||
| Acute gastroenteritis | 1 | 0 | |
| SBP + Blood Stream Infection | 1 | 4 | |
| Cellulitis | 0 | 1 | |
| Pneumonia | 0 | 1 | |
| Cellulitis + Blood Stream | 0 | 1 | |
| Infection + SBP | 3 | 7 | |
| SBP | 0 | 1 | |
| SBP + SBE | 1 | 2 | |
| Urinary tract infection | |||
| No. of patients requiring hospitalization | 8 (24.24%) | 15 (45.5%) | 0.07 |
| No. of patients who developed AKI | 8 (24.24%) | 11 (33.3%) | 0.42 |
| Ascites control | n = 29 | n = 31 | |
| Complete | 18 (62.1%) | 9 (29%) | |
| Partial | 4 (13.8%) | 10 (32.3%) | 0.03 |
| None | 7 (24.1%) | 12 (38.7%) | |
SBP, Spontaneous Bacterial peritonitis; SBE, Spontaneous Bacterial Empyema; AKI, Acute Kidney Injury
The number of patients with infections was significantly lower in group A (n = 6, 18.2%) as compared to group B (n = 17, 51.5%), with a relative risk of 0.35 (p = 0.005) with a number needed to treat of 3. SBP episodes were significantly higher in group B (n = 13, 39.4%) as compared to group A (n = 4, 12.1%) (p = 0.01). However, non-SBP infections were not significantly different between the groups (p = 0.23) (Table 3).
Although, there was a decreased need for hospitalization in group A (24.24%) as compared to group B (45.5%), this difference was not statistically significant (p = 0.07). There was no difference in the number of acute kidney injury episodes (group A vs B: 24.24% vs 33.3%, p = 0.42) between the groups during follow-up (Table 3).
There was no improvement in median LSM scores in either group at 12 months as compared to baseline (p > 0.05, Table 4). QOL assessment revealed no significant improvement in the physical or mental component summary scores at 12 months in either group (p > 0.05).Similarly, we did not observe any significant improvement in nutritional parameters (BMI, HGS, and SMI) in both groups A and B at 12 months, as compared to baseline (p > 0.05, Table 4). However, it is pertinent to note that the analysis of LSM, QOL, and nutritional parameters was restricted only to survivors, as the data for non-survivors and dropouts were not available.
Table 4.
Quality of life, nutritional parameters, and liver stiffness in the study population
| Outcome | Group A | p value within the group | Group B | p value within the group | p value between the groups at 12 months | ||
|---|---|---|---|---|---|---|---|
| Baseline (n = 33) | 12 months (n = 29) | Baseline (n = 33) | 12 months (n = 22) | ||||
| Physical component summary of QOL score | 42.78 (27.84–59.49) | 47.50 (25.71–59.84) | 0.11 | 43.75 (35.19–57.53) | 46.22 (31.79–55.48) | 0.69 | 0.49 |
| Mental component summary of QOL score | 41.89 (19.71–59.52) | 52.17 (17.85–63.87) | 0.23 | 42.06 (29.55–62.34) | 41.17 (25.76–57.25) | 0.63 | 0.15 |
| Body mass index (kg/m2) | 24.79 (20.57–34.97) | 24.21 (21.48–37.00) | 0.92 | 24.07 (18.75–32.44) | 24.95 (19.50–33.58) | 0.24 | 0.64 |
| Skeletal muscle index (cm2/m2) | 40.2 (29.5–55.6) | 40.5 (27–53.5) | 0.08 | 39.2 (29.2–45.2) | 38.1 (27.2–56.6) | 0.72 | 0.52 |
| Hand-grip strength (Kg) | 32 (24.5–42.6) | 34 (24–42) | 0.92 | 30 (25.4–38) | 32 (25–40) | 0.19 | 0.71 |
| LSM (kPa) | 56 (15–75) | 60 (17–75) | 0.65 | 60 (22–75) | 55 (22–75) | 0.96 | 0.74 |
QOL, quality of life (as assessed by SF36v2); LSM, Liver Stiffness Measurement
Safety of G-CSF therapy
Treatment-related adverse events were seen in 23 (69.7%) patients of group A (Supplementary table 2). All adverse effects were mild (grade I and II) and transient. No grade III or IV adverse effects were noted in the study population. The most frequent adverse effects noted were body ache (24.2%), backache (45.5%), headache (15.2%), fever (15.2%), and pain abdomen (6%). Vomiting (3%), loose stools (3%), and pain in the sole of the foot (3%) were seen less frequently. Overall, G-CSF was well tolerated and there were no adverse effect-related treatment discontinuations.
Discussion
There has been much controversy about the use of G-CSF as an alternative therapy in patients with DC. We evaluated the use of multiple sessions of G-CSF in DC in a double-blind randomized controlled trial. One-year survival in the G-CSF group was 87.9% as compared to 66.7% in the placebo group. Although, a statistical significance was not reached, the difference in survival was clinically meaningful suggestive of a plausible biological effect. Indeed, this is supported by the fact that G-CSF led to statistically significant improvement in CTP score, ascites control, reduction in infections and HE, which are all factors that are predictive of improved survival. Survival benefit with G-CSF in DC has been documented in previous open-label studies [5–8]. Our observed survival of 87.9% in patients treated with G-CSF is comparable to that reported in previous studies that have used multiple cycles of G-CSF [5–7]. However, survival in the control group (66.7%) was substantially higher than what was observed by Verma et al. and De et al. [5, 6] The patients in our study had lower CTP and MELD scores as compared to these prior studies which may explain a higher survival in the control group. On post-hoc analysis, the power of our study was 53.9% which was mainly due to the mortality being unexpectedly lower in the control arm than what was anticipated during calculation of sample size. It is possible that a larger sample size may have resulted in translation of this perceived clinical benefit into statistically significant differences in survival.
G-CSF administration mobilizes hematopoietic stem cells from the bone marrow along a gradient of stromal-derived factor 1 (SDF-1) and its receptor CXCR-4 [10]. Consistent mobilization of these stem cells has been demonstrated in severe alcoholic hepatitis [11, 12] and DC [13] post-G-CSF administration. Similarly, we too observed an increase in CD34+ cells at day 6 in peripheral blood. Our regimen of multiple cycles of G-CSF was designed with the purpose of attaining sustained homing of CD34+ cells to the liver. An intriguing observation in our study was that there is a delay of a few months before the survival curves begin to separate out, similar to what has previously been reported with multiple cycles of G-CSF [5, 6]. This suggests that a single cycle of G-CSF may be inadequate.
Improvement in disease severity scores (CTP and MELD), improved control of ascites, decreased incidence of other decompensations and a decreased need for hospitalization have been reported in previous studies with the use of G-CSF [5, 6, 8, 14]. Intriguingly, while CTP scores significantly improved in the G-CSF group in the current study, MELD scores did not. It seems that the improvement in CTP was predominantly due to improvement in ascites control and encephalopathy episodes which were significantly better in G-CSF arm. The median MELD score in patients of this study was lower than that in the studies by De et al. and Verma et al. which may also have accounted for the discrepancy [5, 6]. Although the need for hospitalization was less in patients treated with G-CSF, the difference was not significant which might have been due to the small sample size of the current study.
Cirrhosis is associated with innate and acquired immune dysfunction which is termed cirrhosis-associated immune dysfunction [15]. Theoretically, G-CSF may be beneficial in countering this immunoparetic state by improving neutrophil oxidative burst, intensifying dendritic cell multiplication, antigen presentation, and modulating T-cells [16–18]. Previous studies showed a decreased rate of infections in patients receiving G-CSF [5, 6, 8]. G-CSF has also been reported to have an antifibrotic effect over a period [19]. In our study, the number of overall infections, as well as, SBP was lower among those who received G-CSF. Verma et al. had previously reported improved nutrition using subjective global assessment and anthropometric measurements (often unreliable in DC) with the use of G-CSF [6]. However, in the larger study by De et al., where nutritional assessment was done using hand grip strength and the gold standard of skeletal muscle index, we did not observe any improvement in nutrition which was corroborated by the findings of this study [5]. Unlike previous studies [5, 6], we did not observe any improvement in QOL after G-CSF therapy. However, this analysis was restricted only to survivors. We also acknowledge that the previous trials were open label and a placebo effect cannot be ruled out.
Our study has strengths worth mentioning. This was the first study using G-CSF in DC in a double-blind, randomized, placebo-controlled manner. Both groups were well matched at baseline. The main limitation of our study was the lack of a histologic endpoint. Further, stem cell fate tracking would have helped in confirming homing of CD34+ cells to the liver. COVID-19-imposed restrictions led to the premature loss to follow-up of 3 patients. Mortality was unexpectedly lower in the control arm than what was anticipated. Finally, this was a single-center study and further multi-centric, multi-ethnic, double-blind, placebo-controlled studies are needed.
Conclusion
G-CSF therapy is safe in DC, mobilizes CD 34+ cells, and is associated with a reduction in 1-year mortality that did not reach statistical significance. There were better ascites control, improvement in CTP score, and fewer episodes of encephalopathy and infection in patients treated with G-CSF.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
VS: conceptualization and design of the study, patient recruitment, data analysis and interpretation, manuscript writing, approval of final manuscript and study coordinator; AV: design of the study protocol, patient enrollment, data collection and analysis, statistical calculations, and manuscript writing; AD: patient enrollment, data collection, and analysis, manuscript writing; BL: randomization and dispensation of drugs; SK: data collection and analysis; NV: patient enrollment, data collection, and analysis; RS: estimation of CD34+ cells; SG: assessment of quality of life; NK: sarcopenia assessment and data collection.
Funding
Partially funded by Society for the Study of Liver Diseases (SSLD).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
Aswath Venkitaraman, Arka De, Nipun Verma, Sunita Kumari, Bidyalaxmi Leishangthem, Ratti Ram Sharma, Naveen Kalra, Sandeep Grover, Virendra Singh declare no conflicting interests and have no financial disclosures.
Ethics approval
Institutional Ethical approval was obtained (INT/ IEC/ 2019/ 000727).
Consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent was obtained from all patients for being included in the study.
Consent to publish
Not applicable as this is not a case report; no individual patient identifiers, images, etc. have been used.
Plant reproducibility
Not applicable.
Research involving human and animal participants
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aswath Venkitaraman and Virendra Singh are joint first authors
References
- 1.Angeli P, Bernardi M, Villanueva C, Francoz C, Mookerjee RP, Trebicka J, et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Loria A, Escheik C, Gerber NL, Younossi ZM. Quality of life in cirrhosis. CurrGastroenterol Rep. 2013;15(1):301. doi: 10.1007/s11894-012-0301-5. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 4.de I’Hortet AC, Takeishi K, Guzman-Lepe J, Handa K, Matsubara K, Fukumitsu K, et al. Liver-regenerative transplantation: regrow and reset. Am J Transplant. 2016;16(6):1688–96. doi: 10.1111/ajt.13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De A, Kumari S, Singh A, Kaur A, Sharma R, Bhalla A, et al. Multiple cycles of granulocyte colony-stimulating factor increase survival times of patients with decompensated cirrhosis in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):375–383.e5. doi: 10.1016/j.cgh.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Verma N, Kaur A, Sharma R, Bhalla A, Sharma N, De A, et al. Outcomes after multiple courses of granulocyte colony-stimulating factor and growth hormone in decompensated cirrhosis: a randomized trial. Hepatology. 2018;68(4):1559–1573. doi: 10.1002/hep.29763. [DOI] [PubMed] [Google Scholar]
- 7.Prajapati R, Arora A, Sharma P, Bansal N, Singla V, Kumar A. Granulocyte colony-stimulating factor improves survival of patients with decompensated cirrhosis. Eur J GastroenterolHepatol. 2017;29:448–455. doi: 10.1097/MEG.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 8.Kedarisetty CK, Anand L, Bhardwaj A, Bhadoria AS, Kumar G, Vyas AK, et al. Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology. 2015;148(7):1362–1370.e7. doi: 10.1053/j.gastro.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 9.Newsome PN, Fox R, King AL, Barton D, Than N-N, Moore J, et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3(1):25–36. doi: 10.1016/S2468-1253(17)30326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 11.Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109(9):1417–1423. doi: 10.1038/ajg.2014.154. [DOI] [PubMed] [Google Scholar]
- 12.Singh V, Keisham A, Bhalla A, et al. Efficacy of granulocyte colony-stimulating factor and N-acetylcysteine therapies in patients with severe alcoholic hepatitis. Clin Gastroenterol Hepatol. 2018;16:1650–1656.e2. doi: 10.1016/j.cgh.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Gaia S, Olivero A, Smedile A, Ruella M, Abate ML, Fadda M, et al. Multiple courses of G-CSF in patients with decompensated cirrhosis: consistent mobilization of immature cells expressing hepatocyte markers and exploratory clinical evaluation. Hepatol Int. 2013;7(4):1075–1083. doi: 10.1007/s12072-013-9473-9. [DOI] [PubMed] [Google Scholar]
- 14.Anand L, Bihari C, Kedarisetty CK, et al. Early cirrhosis and a preserved bone marrow niche favour regenerative response to growth factors in decompensated cirrhosis. Liver Int. 2019;39:115–126. doi: 10.1111/liv.13923. [DOI] [PubMed] [Google Scholar]
- 15.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(9):727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Rathi S, Hussaini T, Yoshida EM. Granulocyte colony stimulating factor: a potential therapeutic rescue in severe alcoholic hepatitis and decompensated cirrhosis. Ann Hepatol. 2020 doi: 10.1016/j.aohep.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors. 2005;23(1):33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 18.Khanam A, Trehanpati N, Garg V, Kumar C, Garg H, Sharma BC, et al. Altered frequencies of dendritic cells and IFN-gamma-secreting T cells with granulocyte colony-stimulating factor (G-CSF) therapy in acute-on- chronic liver failure. Liver Int. 2014;34(4):505–513. doi: 10.1111/liv.12415. [DOI] [PubMed] [Google Scholar]
- 19.Tsolaki E, Athanasiou E, Gounari E, Zogas N, Siotou E, Yiangou M, et al. Hematopoietic stem cells and liver regeneration: differentially acting hematopoietic stem cell mobilization agents reverse induced chronic liver injury. Blood Cells Mol Dis. 2014;53(3):124–132. doi: 10.1016/j.bcmd.2014.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.