Abstract
Animal evidence suggests that regular exposure to phthalates may increase the risk of several disorders, including nonalcoholic fatty liver disease (NAFLD), which is the liver manifestation of metabolic syndrome.1 In particular, di(2-ethylhexyl) phthalate (DEHP), the most commonly used of these chemicals,2 is understood to contribute to NAFLD by disrupting normal lipid metabolism.3,4 Many studies of DEHP in liver tissue have focused on hepatocytes, the organ’s major functional cells.5 However, a new study in Environmental Health Perspectives highlights the role of a second cell type: hepatic macrophages, the most abundant type of liver immune cell.6
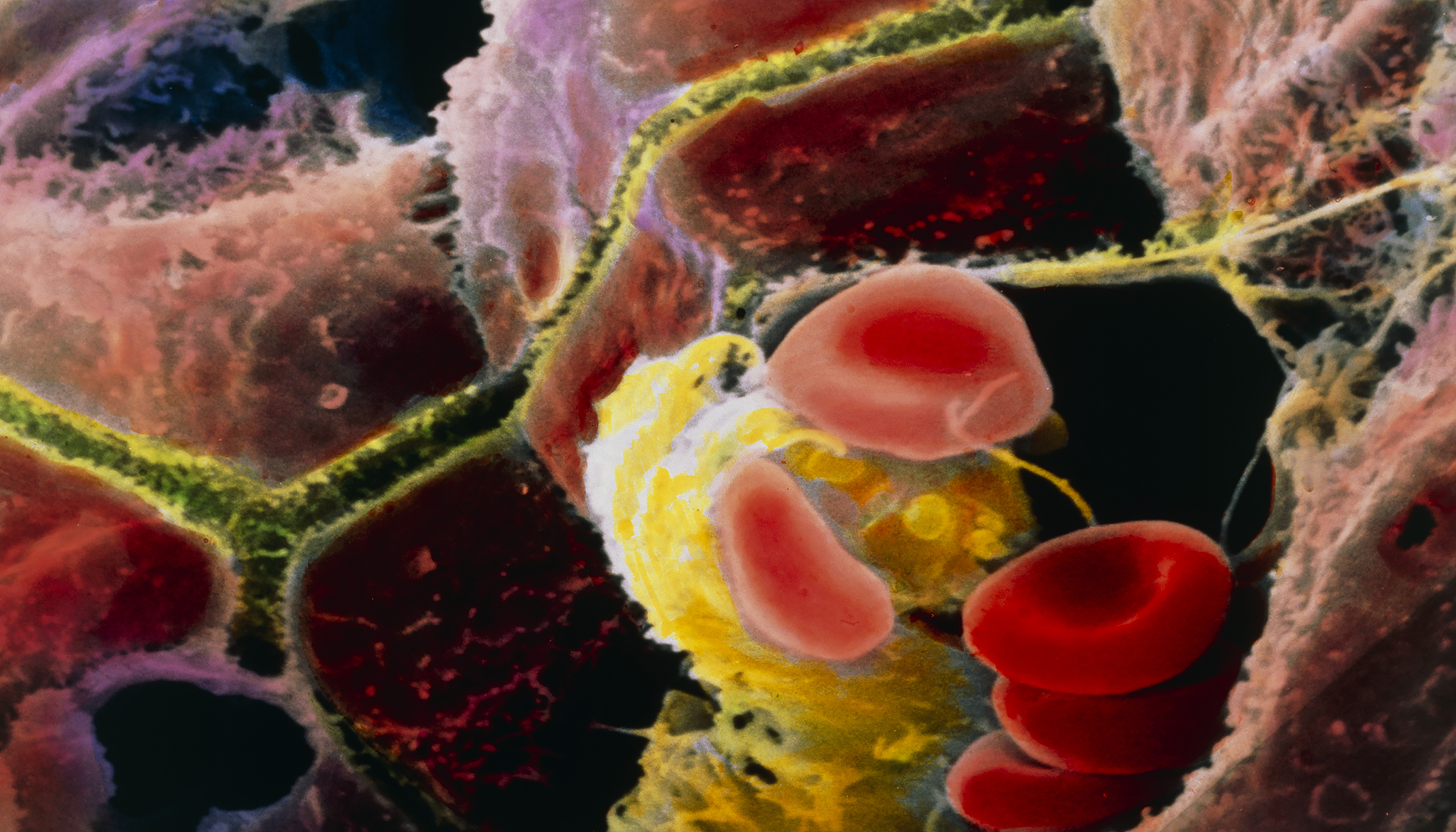
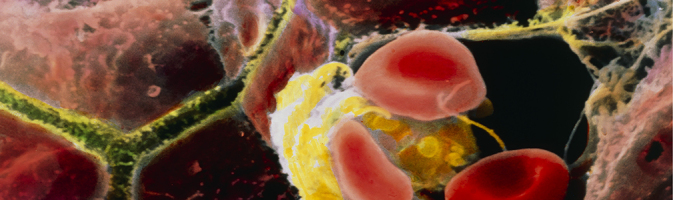
Colored scanning electron micrograph of brown human hepatocytes and a yellow Kupffer cell (a type of liver-specific macrophage) surrounded by red blood cells. Hepatocytes and Kupffer cells line the capillaries of the liver. Liver cells secrete bile, which is carried through the canaliculi (shown in yellowish green) to be stored in the gallbladder. Image: © Prof. P.M. Motta, Sapienza University of Rome/Science Photo Library.
The authors of the new paper suggest that transcription factors called peroxisome proliferator–activated receptors (PPARs) may regulate the joint response of macrophages and hepatocytes to DEHP. PPARs are nuclear receptors with three distinct subtypes: , , and . Activated upon binding dietary fatty acids and other compounds, they help control the expression of genes involved in glucose and lipid metabolism.7,8
PPARs play an important role in several diseases, including NAFLD; is highly expressed in hepatocytes, and is highly expressed in adipose tissue and nonhepatocyte liver cells.8 Recent in vitro studies reported that may modulate the activity of mouse and human macrophages9 and may interact with mono(2-ethylhexyl) phthalate (MEHP), the main DEHP metabolite, in mouse adipocytes.10
The goal of the new study was to test whether and how the cell type–specific presence of PPARs may influence the effect of DEHP on fatty liver development. “In our in silico analysis, we found that MEHP binds most strongly to , less strongly to , and not at all to ,” says Hui Yang, an associate professor at China’s National Center for Food Safety Risk Assessment and one of the study’s senior authors. Because is highly expressed in nonhepatocyte cells in liver tissue, the authors’ in vivo analyses focused on this receptor subtype, adds Yang.
The researchers orally administered DEHP for 28 days to three groups of mice: wild type (WT) mice with normal hepatocytes and macrophages, hepatocyte- knockout mice (Hep-KO), and macrophage- knockout mice (Mac-KO). The team used RNA sequencing and lipid metabolomic analysis to compare the liver’s response to DEHP in each group. Although the DEHP dose was higher than typical human exposure levels, the measured plasma levels of MEHP were similar to those observed in human studies.11,12
The researchers found that DEHP exposure resulted in increased lipid accumulation in the liver of WT mice and of Hep-KO mice. Relative to these two groups, Mac-KO mice had less lipid accumulation. This finding suggests that in macrophages, rather than in hepatocytes, is potentially involved in fatty liver development.
The biological function of mammalian macrophages ranges from proinflammatory (M1) to restorative (M2) activities.13 In their M1 stage, macrophages release cytokines to recruit other types of immune cells to the site of tissue infection or inflammation. After the other immune cells have completed tissue repair activities, macrophages switch to their M2 stage to remove cellular debris and promote healing.13
In the new study, Mac-KO mice exposed to DEHP had significantly more hepatic macrophages in the restorative M2 stage, compared with similarly exposed WT mice. This finding suggests that the binding of DEHP to in the macrophages of WT mice may have prolonged the inflammatory M1 stage. In the absence of , however, more hepatic macrophages switched to the restorative M2 stage.
To further explore the potential mechanism behind their in vivo observations, the researchers exposed mouse- and human-derived macrophages to either DEHP, MEHP, or both compounds combined. They found that both DEHP and MEHP suppressed the switch to the restorative M2 stage in both types of -containing macrophages. This in vitro finding provided further evidence that DEHP may promote inflammation and lipid buildup by prolonging the inflammatory M1 stage.
The researchers combined their data with existing knowledge14 about to propose a new model for fatty liver development in mice, in which MEHP activates both and in a cell type–specific manner. This activation may promote inflammation and lipid accumulation by disrupting normal macrophage function.
For Kari Neier, a postdoctoral fellow at the University of California, Davis, who was not involved in the project, the study is a significant advance in the field. “We may have underestimated the importance of activation in macrophages for DEHP-induced fatty liver development,” says Neier. “This novel finding highlights a critical role of the immune system in metabolic diseases like NAFLD.”
Jose Cordoba-Chacon, an assistant professor of endocrinology at the University of Illinois at Chicago, who also was not involved in the project, agrees that the study adds to the growing evidence for a contribution of macrophages to fatty liver development.15 He notes, however, that the proposed model for NAFLD has not yet been tested in obese mice.
“An effect of DEHP on macrophage-specific processes is plausible, but the role of may be different in obese mice whose livers are much more prone to lipid accumulation,” says Cordoba-Chacon. “More research is warranted to understand how PPARs interact with DEHP in different cell types and what this means for human NAFLD.”
Biography
Silke Schmidt, PhD, writes about science, health, and the environment from Madison, Wisconsin.
References
- 1.Maradonna F, Carnevali O. 2018. Lipid metabolism alteration by endocrine disruptors in animal models: an overview. Front Endocrinol (Lausanne) 9:654, PMID: , 10.3389/fendo.2018.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zota AR, Calafat AM, Woodruff TJ. 2014. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect 122(3):235–241, PMID: , 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tassinari R, Tait S, Busani L, Martinelli A, Narciso L, Valeri M, et al. 2021. Metabolic, reproductive and thyroid effects of bis(2-ethylhexyl) phthalate (DEHP) orally administered to male and female juvenile rats at dose levels derived from children biomonitoring study. Toxicology 449:152653, PMID: , 10.1016/j.tox.2020.152653. [DOI] [PubMed] [Google Scholar]
- 4.Neier K, Montrose L, Chen K, Malloy MA, Jones TR, Svoboda LK, et al. 2020. Short- and long-term effects of perinatal phthalate exposures on metabolic pathways in the mouse liver. Environ Epigenet 6(1):dvaa017, PMID: , 10.1093/eep/dvaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rusyn I, Peters JM, Cunningham ML. 2006. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol 36(5):459–479, PMID: , 10.1080/10408440600779065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu M, Li Y, Wang X, Zhang Q, Wang L, Zhang X, et al. 2022. Role of hepatocyte- and macrophage-specific PPARγ in hepatotoxicity induced by diethylhexyl phthalate in mice. Environ Health Perspect 130(1):17005, PMID: , 10.1289/EHP9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cave MC, Clair HB, Hardesty JE, Falkner KC, Feng W, Clark BJ, et al. 2016. Nuclear receptors and nonalcoholic fatty liver disease. Biochim Biophys Acta 1859(9):1083–1099, PMID: , 10.1016/j.bbagrm.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francque S, Szabo G, Abdelmalek MF, Byrne CD, Cusi K, Dufour J-F, et al. 2021. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol 18(1):24–39, PMID: , 10.1038/s41575-020-00366-5. [DOI] [PubMed] [Google Scholar]
- 9.Xu M, Wang X, Li Y, Geng X, Jia X, Zhang L, et al. 2021. Arachidonic acid metabolism controls macrophage alternative activation through regulating oxidative phosphorylation in PPARγ dependent manner. Front Immunol 12:618501, PMID: , 10.3389/fimmu.2021.618501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manteiga S, Lee K. 2017. Monoethylhexyl phthalate elicits an inflammatory response in adipocytes characterized by alterations in lipid and cytokine pathways. Environ Health Perspect 125(4):615–622, PMID: , 10.1289/EHP464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki A, Mitsui T, Miyashita C, Nakajima T, Naito H, Ito S, et al. 2014. Association between maternal exposure to di(2-ethylhexyl) phthalate and reproductive hormone levels in fetal blood: the Hokkaido Study on Environment and Children’s Health. PLoS One 9(10):e109039, PMID: , 10.1371/journal.pone.0109039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva MJ, Barr DB, Reidy JA, Kato K, Malek NA, Hodge CC, et al. 2003. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch Toxicol 77(10):561–567, PMID: , 10.1007/s00204-003-0486-3. [DOI] [PubMed] [Google Scholar]
- 13.Edholm ES, Rhoo KH, Robert J. 2017. Evolutionary aspects of macrophages polarization. Results Probl Cell Differ 62:3–22, PMID: , 10.1007/978-3-319-54090-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corton JC, Cunningham ML, Hummer BT, Lau C, Meek B, Peters JM, et al. 2014. Mode of action framework analysis for receptor-mediated toxicity: the peroxisome proliferator-activated receptor alpha (PPARα) as a case study. Crit Rev Toxicol 44(1):1–49, PMID: , 10.3109/10408444.2013.835784. [DOI] [PubMed] [Google Scholar]
- 15.Tacke F. 2017. Targeting hepatic macrophages to treat liver diseases. J Hepatol 66(6):1300–1312, PMID: , 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]


