Abstract
The most important autonomic function test—the autonomic medical history—is the patient’s account, interpreted by a clinician skilled in asking the right questions, of symptoms during daily activities that relate to the autonomic nervous system. The chronology and pattern of these symptoms combine to create a portrait of autonomic function or dysfunction. We summarize the steps in obtaining a comprehensive autonomic medical history
Keywords: Autonomic, Sympathetic, Parasympathetic, Noradrenergic, Cholinergic
INTRODUCTION
This review serves as guidance for the proper evaluation of patients with symptoms suggesting autonomic dysfunction. We summarize the steps in obtaining a comprehensive autonomic medical history, the most reliably informative autonomic function test. The autonomic medical history comprises the patient’s account of symptoms during daily activities that relate to the autonomic nervous system, guided and interpreted by a clinician skilled in asking the right questions.
The description of symptoms may be quite subjective and may be influenced by educational, ethnic, cultural, and language backgrounds, as well as text posted on social media. The clinician should focus on symptoms related to autonomic functions, since patients often present with many other non-associated issues and incorrect understanding of the connection between these and the autonomic disorders.
In the United States, reimbursement by third party payers for management of patients with dysautonomias is based mainly on procedures, even though skillful elicitation and interpretation of the autonomic history may provide the greatest diagnostic value. Autonomic history-taking cannot be done well in a brief clinic visit. Reduced visit time is a growing problem for community-based physicians seeking to diagnose dysautonomias.
The medical history consists of several parts. These include the Chief Complaint, the History of the Present Illness (HPI), the Past History, the Family History, the Personal and Social History, and the Review of Systems (ROS). Each of these parts is important for diagnosing and managing dysautonomias, but the key component is the HPI. Figure 1 shows a synopsis of the key elements.
Figure 1: Key elements of the autonomic medical history.

These include the Chief Complaint, the History of the Present Illness, and questions related to components of the autonomic nervous system.
ORGANIZATION OF THE AUTONOMIC NERVOUS SYSTEM
In taking the autonomic history, the clinician must keep in mind the different components of the autonomic nervous system (Figure 2) and the general organization of autonomic innervation in the body (Figure 3). John Newport Langley, who coined the term, “autonomic nervous system,” partitioned the autonomic nervous system into three components—the sympathetic nervous system, parasympathetic nervous system (PNS), and enteric nervous system (ENS) [25].
Figure 2: Components of the autonomic nervous system (ANS).
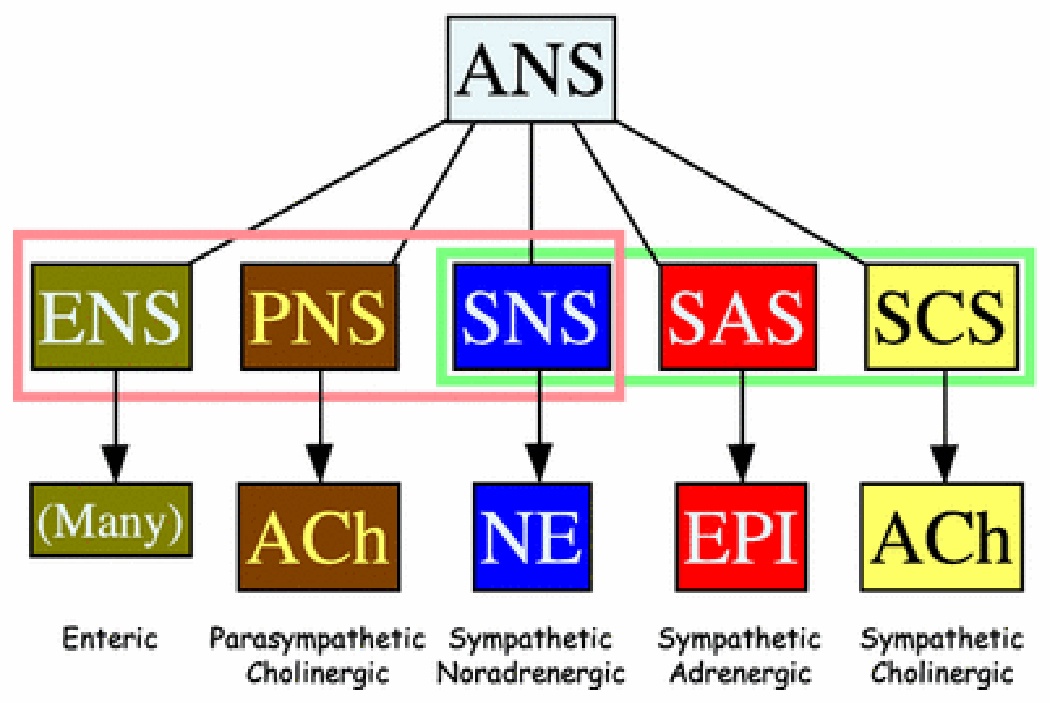
Langley’s ANS consists of the enteric nervous system (ENS), parasympathetic nervous system (PNS), and sympathetic nervous system. The sympathetic nervous system can be sub-classified based on the main chemical messenger, into the sympathetic noradrenergic system (SNS), the sympathetic adrenergic system (SAS), and the sympathetic cholinergic system (SCS). The SNS chemical messenger is norepinephrine (NE), the SAS messenger epinephrine (EPI), and the SCS messenger acetylcholine (ACh).
Figure 3: Overview of the organization of the ANS.
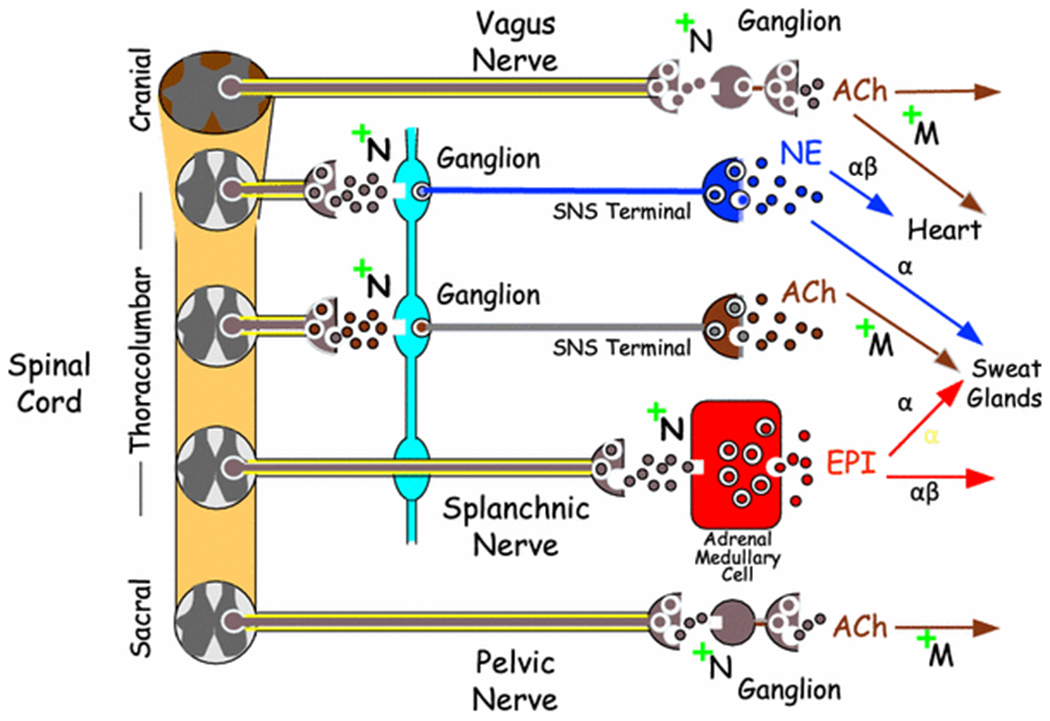
At all ganglia and adrenomedullary chromaffin cells ACh mediates signaling via neuronal nicotinic receptors (+N). Post-ganglionic ACh receptors are muscarinic (+M). Parasympathetic nerves include the vagus nerve (the tenth cranial nerve) and pelvic nerves (although this has been disputed recently). Sympathetic nerves mainly consist of post-ganglionic, non-myelinated fibers, whereas parasympathetic nerves mainly consist of pre-ganglionic, myelinated fibers.
The sympathetic nervous system has three sub-systems based on the main chemical messenger—the sympathetic noradrenergic system (SNS), sympathetic adrenergic system (SAS), and sympathetic cholinergic system (SCS) [12]. Nerves of the SNS and SCS are derived from thoracolumbar ganglia; the fibers are slow-conducting and non-myelinated. Nerves of the SAS that innervate the adrenomedullary chromaffin cells pass through ganglia without synapsing, are rapidly conducting, and are myelinated. PNS innervation is derived from the brainstem or sacral spinal cord (this classic view has been challenged recently [10]) and involves long, rapidly conducting, myelinated fibers, with the ganglia close to or embedded in the target organs. Ganglionic neurotransmission occurs via acetylcholine binding to neuronal nicotinic receptors.
THE SYNDROMIC NATURE OF DYSAUTONOMIAS
Dysautonomias occur in syndromes depending on the involved ANS component (Tables 1–3). For instance, in a patient with orthostatic symptoms, one should ask not only about symptoms that occur during standing, but also about post-prandial hypotension (often presenting as fatigue or orthostatic syncope following a large meal), heat and exercise intolerance, and “coat hanger” pain (Table 1) that improve when the patient sits or lies down. In a patient with sicca syndrome (dry mouth and dry eyes), one should ask also about urinary retention and constipation (Table 2).
Table 1:
Symptoms & signs of sympathetic noradrenergic system (SNS) failure or hyperactivity
| SNS failure |
|---|
| Orthostatic intolerance & hypotension |
| Post-prandial lightheadedness & hypotension. |
| Heat intolerance & hypotension. |
| Fatigue |
| Tendency to slow pulse rate during exercise. |
| “Coat hanger” pain. |
| Droopy eyelids (ptosis). |
| Decreased ability to ejaculate. |
| Tendency to constricted pupils. |
| No goosebumps |
| SNS Hyperactivity |
| Pallor |
| Tendency to high blood pressure |
| Tendency to fast pulse rate |
| Trembling |
| Bristling hair (piloerection) |
| Increased production of thick saliva |
| Tendency to dilated pupils (mydriasis) |
| Sweating |
Table 3:
Symptoms and signs of sympathetic adrenergic system (SAS) hyperactivity
| Pallor |
| Increased sweating |
| Increased blood glucose (hyperglycemia) |
| Dilated pupils (mydriasis) |
| Decreased gastrointestinal transit (functional ileus) |
| Increased heart rate & contractility |
| Increased systolic & pulse pressures |
| Tendency to decreased serum potassium (hypokalemia) |
| Tendency to decreased bleeding time |
| Tendency to increased core temperature |
Table 2:
Symptoms & signs of parasympathetic nervous system (PNS) failure or hyperactivity
| PNS Failure |
|---|
| Dry mouth (decreased watery saliva) |
| Constipation |
| Dry eyes |
| Urinary retention |
| Tendency to fast pulse rate with low variability |
| Slow gastrointestinal transit |
| Erectile failure |
| PNS Hyperactivity |
| Increased salivation |
| Tendency to slow pulse rate or heart block |
| Nausea & vomiting |
| Diarrhea or fast gastrointestinal transit |
| Tendency to constricted pupils (miosis) |
| Urge to urinate (increased bladder motility) |
| Increased stomach acid secretion |
It is important for the diagnostician to keep in mind the many possible causes of these syndromes (Table 4–7). Some are common (e.g., drugs) and some rare (e.g., autoimmune autonomic ganglionopathy, or AAG).
Table 4:
Some Causes of Sympathetic Noradrenergic System (SNS) Underactivity or Hyperactivity
| SNS Underactivity |
|---|
| Drugs |
| Diabetes |
| Cancer (paraneoplastic) |
| Parkinson’s disease (PD) |
| Multiple system atrophy (MSA) |
| Spinal cord injury |
| Pure autonomic failure (PAF) |
| Amyloidosis |
| Familial dysautonomia |
| Dopamine-beta-hydroxylase deficiency |
| Acquired sensory and autonomic neuropathy |
| Autoimmune autonomic ganglionopathy |
| SNS Hyperactivity |
| Drugs |
| Dehydration |
| Congestive heart failure |
| Blood volume depletion |
| Hypernoradrenergic hypertension |
| Hypothyroidism |
| Postural tachycardia syndrome (POTS) |
| Pseudopheochromocytoma |
| Status post adrenalectomies |
| Baroreflex failure |
| Hypoadrenalism |
| Guillain-Barre syndrome |
Table 7:
Some causes of sympathetic cholinergic system (SCS) hyperactivity
| Drugs |
| Gustatory stimulation (chili peppers) |
| Emotion |
| Menopause |
| Idiopathic hyperhidrosis |
Drugs can alter functions of any component of the ANS, and so a rigorous review of medications and dietary supplements is mandatory.
THE CHIEF COMPLAINT
The Chief Complaint is a single phrase or sentence that describes in the patient’s own words what has been bothering the patient that has led the patient to come in for evaluation.
The Chief Complaint can be surprisingly informative. For example, an elderly woman was referred for pure autonomic failure (PAF) because of persistent, consistent orthostatic hypotension (OH). When queried about her Chief Complaint, the expectation was that she would report dizziness or lightheadedness when she was upright or perhaps fainting episodes while standing on line. Instead, her Chief Complaint was that she couldn’t make spit and was constipated. Dry mouth and constipation are symptoms of parasympathetic nervous system failure, not sympathetic noradrenergic system failure, which manifests with neurogenic orthostatic hypotension (OH). When asked if she sweated like other people, she said no, she couldn’t sweat at all. Sweating is largely a sympathetic cholinergic function. In other words, the patient had symptoms that seemed to involve all the components of the autonomic nervous system—a pandysautonomia. Eventually she was found not to have PAF but to have a previously undescribed condition that has come to be called autoimmune autonomic ganglionopathy (AAG), from a circulating antibody to the neuronal nicotinic receptor [14]. The first clue to the diagnosis was her unexpected Chief Complaint.
Another patient was a young man whose Chief Complaint was “gravity tripled.” He was experiencing episodes of neurally mediated syncope during prolonged standing in warm environments. His perception that the force of gravity seemed stronger at those times was an indication of orthostatic intolerance. Normally, standing redistributes 500-800 mL of blood downward into the splanchnic, pelvic, and lower extremity circulatory beds. In healthy persons the combined action of sympathetic noradrenergic peripheral vasoconstriction with muscular compression of vascular beds is sufficient to maintain arterial pressure and cerebral perfusion. In this case, local vasodilation and withdrawal of sympathetic noradrenergic tone overwhelmed the ability to sustain arterial pressure. The patient felt as if the force of gravity had suddenly increased and was pulling more of his blood downward. The problem was not, however, an aberration in the forces of nature, but rather in regulation of his autonomic nervous system [3].
HISTORY OF THE PRESENT ILLNESS (HPI)
The History of the Present Illness (HPI) is a narrative history of the condition. The chronology and pattern of the HPI paint a valuable portrait of autonomic functions and dysfunctions.
It is best to obtain the HPI directly from the patient. Records from previous hospitalizations, test results, and previously transcribed accounts of the medical history and physical examination can be subject to mistakes and redundancies that often are uninformative or misleading. On the other hand, the patient’s own story of the chronology of his or her symptoms (with assistance from family or significant others as appropriate) is likely to be both correct and informative. Supplementary historical information from witnesses is essential when evaluating transient loss of consciousness.
Obtaining the medical history—especially the HPI—is a skill that must be honed by learning and experience, ideally under the supervision of a mentor. The patient knows best how he or she feels, but intelligent questioning is required to draw meaningful inferences.
Unfortunately, this key aspect of the medical encounter is not reimbursed adequately, considering its importance to reaching an accurate diagnosis and the time and intellectual effort involved.
Timing is Everything
In obtaining the HPI, one of the most important skills a clinician can acquire is the ability to get the sequence straight. One may start by asking the patient, “When was the last time you felt completely well?” The answers can range from “I’ve always been sick,” to “I was fine until…” a particular date, to “It was such a gradual thing, I don’t know.”
Some dysautonomias develop in a rather stereotyped sequence. An example is the cerebellar form of multiple system atrophy (MSA-C) in a man. Men with MSA-C typically relate that the first thing to go wrong, in retrospect, was erectile failure. The erectile failure is typically followed by urinary problems—especially urinary retention, eventually to the point of requiring self-catheterization. Then come slurred speech, a wide-based, unsteady gait “like a drunken sailor,” and lightheadedness when standing. In our opinion, in a man with symptoms or signs of central neurodegeneration and OH, the absence of erectile failure as an early finding rules out MSA.
In postural tachycardia syndrome (POTS) a viral illness, surgical procedure, or physical or psychological trauma may precede the onset of the symptom complex and signs that constitute POTS. In patients with possible POTS the sequence of events can be very informative. Subacute development of orthostatic intolerance (OI) after a flu-like illness suggests either an autoimmune component of the pathophysiology or decompensation due to dehydration or deconditioning, whereas a history of frequent fainting or “seizures” since childhood points more to a congenital or genetic component [31]. Lyme disease is another potential cause of autonomic dysfunction [22].
In the evaluation of a patient with POTS, which occurs mainly in relatively young women, it is important to ask, in a private setting, about emotional, physical, or sexual abuse in childhood. These can have long-term consequences in terms of chronic fatigue, altered memory or concentration, panic or anxiety, or suicidal ideation [27]. The issue of physical deconditioning often arises in patients with chronic orthostatic intolerance. This can be a sensitive topic, because the patient might gain the impression that the entire problem reflects simply being out of shape.
In a patient with labile blood pressure and OI, a remote history of irradiation of the neck brings up the possibility of arterial baroreflex failure due to accelerated arteriosclerosis in the carotid sinus area [32]. Carotid endarterectomy, other surgical manipulations involving the region of the carotid bifurcation, or carotid atherosclerosis may also interfere with baroreflex function, although it is uncommon for these to cause labile hypertension [18, 36].
In obtaining the details about symptoms of dysautonomias, it is also important to determine which situations make things worse and which make them better. It is important to ask about the positional nature of symptoms (supine, standing, sitting). One should attempt to distinguish the “dizziness” of orthostatic intolerance from positional vertigo. Patients with neurogenic OH often relate that their symptoms are worst in the morning, upon heat exposure, after eating a large meal, or after exercise. Patients with POTS may report being unable to stand up to take a warm shower, so that they bathe seated in a chair or tub. The clinician should ask about shortness of breath (dyspnea), at rest or during activities of daily living. Dysautonomias symptoms can vary from day to day, without a clear explanation. POTS and related disorders often are multi-system, multi-disciplinary, and complex.
Because of associations of autonomic failure with non-motor aspects of Lewy body diseases in Parkinson’s disease (PD) and PAF, it is important to ask whether the patient is able to smell things like other people do, whether the patient sees things like other people do, and whether the patient has any problems with sleep. The clinician is probing for tell-tale evidence of olfactory dysfunction, visual hallucinations (a feature of dementia with Lewy bodies), and dream enactment behavior (a feature of rapid eye movement behavior disorder).
A patient with previously diagnosed PAF reported at follow-up that he was having visual hallucinations. This symptom, which occurred concurrently with neuroimaging evidence of a striatal dopaminergic lesion, was the first clue to evolution of PAF to dementia with Lewy bodies [15]. Another PAF patient had a normal sense of smell throughout the disease course. The patient died suddenly, and at autopsy he was found to have no Lewy bodies or alpha-synuclein deposition anywhere in the brain, sympathetic ganglion tissue, or heart [19]. This case highlighted the heterogeneity of PAF and challenged the definition of PAF as a form of autonomic synucleinopathy [23].
Current Medications, Dietary Supplements, and Herbal Remedies
A complete listing of all prescribed drugs, over-the-counter medications, herbal remedies, and dietary supplements is a key part of the autonomic medical history, not only because these can affect autonomic function but also because they can interact to produce unexpected, serious adverse events.
A patient with MSA first came to medical attention because of paroxysmal high blood pressure after taking ma huang tea. He had thought this would alleviate his sense of fatigue and lack of energy. The active ingredient in ma huang tea is ephedrine, an amphetamine. The drug increased delivery of norepinephrine to its receptors, which caused the blood pressure to increase, and because of arterial baroreflex failure as part of the patient’s disease, the increase in blood pressure was not buffered by the baroreflex. The patient developed a severe headache and went to the emergency room. The headache and paroxysmal hypertension led to an initial diagnosis of stroke from subarachnoid hemorrhage.
Ma huang is no longer sold as a dietary supplement in the United States, but yohimbe bark still is. Yohimbine, a drug derived from yohimbe bark, increases norepinephrine release. Theoretically, in a patient with baroreflex failure taking this dietary supplement could result in severe hypertension in a manner analogous to our MSA patient.
Another patient was a middle-aged woman thought to have POTS on the basis of palpitations and tachycardia; however, the symptoms did not improve with recumbency. Her heart rate was 98 beats/min supine and 130 beats/min upright on the tilt table, without OH. On further review of her medications, she was taking phentermine for weight loss. Phentermine, a stimulant closely related structure to dextroamphetamine, releases norepinephrine into the synapse and increases heart rate. The patient’s heart rate normalized once phentermine was discontinued [4].
Another patient was an adolescent girl taking topiramate for migraine prevention who presented with heat intolerance. When questioned about how much she sweated when exercising outdoors in hot weather, she replied that she no longer was able to sweat. Topiramate inhibits carbonic anhydrase, which is an enzyme involved in sweat gland secretion, and can cause reversible anhidrosis, especially in children [5].
A patient had physiological evidence of neurogenic OH and neurochemical and neuroimaging evidence of cardiac and generalized sympathetic noradrenergic denervation without evidence of central neurodegeneration and was diagnosed with PAF. At follow-up 18 months later, he had resolution of all these abnormalities, despite the fact that PAF is a progressive disease. When queried about hospitalizations and operations, the patient reported that, in the interim, he had undergone successful Nissen fundoplication for gastroesophageal reflux, and he no longer needed to take a prescription proton pump inhibitor. Catecholamine uptake in vesicles in sympathetic nerves requires a proton pump known as the V-ATPase [8]. We speculated that the reversible physiological, biochemical, and neuroimaging abnormalities could have been the result of proton pump inhibition and not PAF.
SYMPTOMS OF DYSAUTONOMIAS DEPEND ON THE INVOLVED COMPONENT
In constructing the HPI narrative, it is helpful to keep in mind the syndromic nature of altered activities of components of the autonomic nervous system.
Sympathetic noradrenergic system
Failure of the sympathetic noradrenergic system (SNS) manifests as OH. OH can produce symptoms such as lightheadedness, dizziness, faintness, visual changes, and muscle weakness but can also occur without producing symptoms. Some patients may not recognize specific symptoms but when hypotensive will report a strong urge to sit down.
OH often is accompanied by post-prandial lightheadedness and hypotension. In patients with SNS failure, heat exposure also can decrease the blood pressure. SNS failure can also manifest with fatigue, low pulse rate at rest or during exercise, exercise intolerance, ptosis, inability to ejaculate in men, or even failure to experience goose bumps (piloerection). SNS hyperactivity produces pallor due to constriction of blood vessels in the skin. Blood pressure and heart rate tend to increase. The heart may pound (palpitations). The hair may bristle due to activation of noradrenergic nerves supplying arrector pili (pilomotor) muscles. The pupils may be dilated, and there may be increased salivation.
Parasympathetic nervous system
Probably the most prominent symptoms of parasympathetic nervous system (PNS) failure are dry mouth and constipation. Other manifestations include a tendency to urinary retention, slowed gastrointestinal transit, and erectile failure in men. PNS hyperactivity results in increased salivation, a tendency to slow heart rate, nausea, gastrointestinal upset, and urinary frequency or urgency.
Sympathetic cholinergic system
The sympathetic cholinergic system (SCS) is the main part of the autonomic nervous system mediating sweating. SCS failure manifests as decreased sweating, and SCS overactivity manifests as excessive sweating.
Sympathetic adrenergic system
Adrenaline (synonymous with epinephrine), the main chemical messenger of the sympathetic adrenergic system (SAS), is a hormone. As such, adrenaline is distributed by the bloodstream to all the organs (with the exception of most of the central nervous system). Adrenaline injection produces characteristic symptoms, including pallor, a forceful heartbeat, and dilated pupils. Adrenaline also exerts well-known anti-fatigue effects [2] and tends to increase the intensity of emotional experiences [30]. SAS failure, on the other hand, produces relatively few symptoms or signs—perhaps a tendency to fatigue or to hypoglycemia. In patients with diabetes, repeated episodes of insulin-induced hypoglycemia can produce a form of SAS failure, resulting in hypoglycemia unawareness [7].
FAMILY HISTORY
In obtaining the family history it can be informative to ask about the ethnic origins of the patient. The most well known form of inherited autonomic failure is familial dysautonomia, also known as Riley-Day syndrome and type III hereditary sensory and autonomic neuropathy (HSAN), The disease is transmitted as an autosomal recessive trait. Virtually all patients with familial dysautonomia are of Ashkenazic extraction.
Genetic studies of a large Greek-Italian-American kindred in which PD was transmitted as an autonomic dominant trait provided the first demonstration that PD can result from mutation of the gene encoding α-synuclein. An affected family member had neurogenic orthostatic hypotension and cardiac sympathetic denervation [16]. It is now well accepted that inherited synucleinopathy can manifest with symptoms or signs of sympathetic neurocirculatory failure [33].
In the evaluation of patients with chronic orthostatic intolerance (COI) one should query about a family history of syncope or “double-jointedness,” as there is an association between Ehlers-Danlos syndrome and POTS [17, 29].
PERSONAL AND SOCIAL HISTORY
One should inquire about the patient’s living situation, including the local climate and housing. A POTS patient was so intolerant of heat that she moved from Florida to Minnesota. A patient with Raynaud’s phenomenon was so intolerant of cold that she moved from Wisconsin to Florida. A PAF patient cloistered herself in an upstairs bedroom in her house because of lightheadedness when going down and up the staircase.
Dysautonomias often are disabling. One should ask if the patient is working or has had to go on disability, and if so, when.
Addictive drugs, whether legal (caffeine, nicotine) or illegal (cocaine, amphetamine), can result in particular autonomic abnormalities. The country music singer Johnny Cash admitted many years of amphetamine abuse before quitting in the late 1960s. In 1997 he became dizzy during a performance after bending down to retrieve a guitar pick. He was diagnosed with the Shy-Drager syndrome (MSA with OH), but he fared better than is typical of patients with MSA. Because amphetamines are toxic to central monoaminergic neurons, one wonders whether chronic neurotoxic effects of amphetamines might have led to a disorder that mimicked MSA. According to Wikipedia when visited May 13, 2017, Cash died of complications of diabetes (https://en.wikipedia.org/wiki/Johnny_Cash).
PAST HISTORY
The patient’s birthplace, where the patient spent childhood, childhood illnesses, and whether the patient was out of school for prolonged periods should be noted.
Environmental exposures are potentially relevant to some autonomic failure syndromes. For instance, a patient with PAF had a history of having grown up on a farm with multiple, repeated pesticide exposures, drinking well water, and having been a crew member in an attack submarine. One may speculate that PAF in this case might have resulted from one or more environmental agents that produced oxidative stress in catecholaminergic neurons [19].
Injuries such as automobile accidents involving concussive head trauma have been linked to subsequent PD [20] or possibly POTS [21].
Hospitalizations and operations should be considered. A patient underwent neurosurgery for symptomatic Chiari malformation. This not only eliminated neck pain but also OI and sympathoadrenal imbalance. Similar cases have been reported [26, 28]; however, it should be noted that whether Chiari malformation can cause POTS has been disputed [11].
Transfusion or blood donation history may reveal a history of fainting, so that the patient cannot give blood [9].
Chemotherapy or radiation therapy can result in a variety of forms of dysautonomia, even many years after successful eradication of the cancer [32].
REVIEW OF SYSTEMS
Screening Questions
As part of the review of systems, each of the components of the autonomic nervous system should be queried.
The questions should not be leading. About sympathetic cholinergic function, ask, “Do you sweat like other people?” or “How much do you sweat when it is hot or when you are exercising?” rather than “Do you have decreased sweating?” About sympathetic noradrenergic function, ask, “Do you have any issues standing still?” or “For how long can you stand in one place?” rather than “Do you have decreased ability to tolerate prolonged standing?” About parasympathetic cholinergic function, ask, “Are you able to make spit and tears like other people?” rather than “Do you have dry mouth or dry eyes?” Also ask, “Have you noticed anything different about how your GI system is working?” rather than “Do you have constipation?” Similarly, ask, “Have you noticed anything different about your urination?” If the patient is a man ask, “Any issues with sexual functions?”
A Pain in the Neck
In patients with OI or OH, standing upright can result in an annoying pain in the back of the neck and along the shoulders. Because of the distribution of the discomfort, this is sometimes referred to as the “coat hanger” phenomenon [24].
In a PAF patient, the initial manifestation of OH was the development of progressive orthostatic neck and shoulder aches over about a year.
The mechanism of the coat hanger phenomenon is poorly understood. We think of it as a kind of cramp, when the anti-gravity muscles holding up the head receive too little blood flow. These muscles are active all the time, which means that they are continuously using up the oxygen that is delivered to them via the arterial blood. If the blood flow falls to below a certain rate, then metabolic waste products that cause pain build up. Muscle ischemic pain does not appear to be sufficiently explained by the release of lactic acid but may involve an interplay of ATP and acid-sensing ion channels on sensory neurons [1].
Who Does Your Shopping?
Most patients with COI are women. At the risk of seeming chauvinistic, a screening question for a woman referred for OI is, “Who does your shopping?”
If the answer is, “I do. I love to shop,” then that is the end of this line of questioning, unless the shopping is done online. A positive answer is something like, “Well not me.” When asked, “Why not?” the answer sought is, “Because I can’t tolerate standing still in line. I start to feel faint or lightheaded or weak, or I have to twist my legs like a pretzel, or I have to sit down.”
For nonshoppers, whether male or female, other daily activities involving prolonged standing are important to ask about. How well the patient is able to stand at a golf tee, while holding a fishing line, while watching a parade, or when taking a shower can provide essential clinical clues to OI.
Pretzel Legs
A patient was sitting in a chair in the examining room with her legs twisted around each other like a pretzel. She had learned from experience that doing this delayed the onset of feeling lightheaded when she was sitting up. By working the muscles of the legs against each other and tightening her buttocks, she was squeezing blood upward in her body toward the heart. When there is deficient reflexive sympathetically-mediated vasoconstriction during orthostasis, “pretzel legs” help maintain venous return to the heart. Pretzel legs in this patient was a sign of PAF.
Adopting the same posture is a countermeasure in patients with autonomically mediated presyncope [37]. Similarly, shifting the weight back and forth while standing, or irresistibly fidgeting the legs while seated, are learned behaviors that activate the lower extremity “muscle pump” that helps to restore venous return to the heart to compensate for OH or OI [6].
The Water Bottle Sign
It is common for a patient with COI to bring a bottle of water to the clinical encounter and sip from it periodically as the history is taken. This has been called the “water bottle sign.” The patients often report that, although continuously drinking water doesn’t eliminate the symptoms, not drinking water rapidly makes the symptoms worse.
The water bottle sign might be a clue as to the pathophysiology of COI. Perhaps the kidneys are less efficient in reabsorbing filtered water, and the water bottle sign is part of a behavioral compensation. The kidneys filter about 100 mL of plasma per minute. Since there are 1440 minutes in a day, this means the kidneys filter about 144 liters per day. Normal urine output is about 1.5 liters per day, meaning that the kidneys are roughly 99% efficient in reabsorbing water. One might expect that even the slightest decrease in efficiency of water reabsorption would result in a tendency to dehydration.
The water bottle sign can also be a response to dry mouth as part of sicca syndrome.
A Bit of a Stretch
Joint hypermobility (“double jointedness”) seems to occur rather frequently among patients with POTS. When obtaining the medical history in a patient with COI, it is worthwhile to ask whether the patient is double jointed and if so to ask what sorts of things involving limb mobility the patient can do that other people cannot. Although joint laxity is common in the general population of teenage girls, we do not think that this explanation is sufficient to account for the frequent association of POTS with joint hypermobility. For instance, when groups of patients with POTS or neurocardiogenic syncope were compared, where the groups had similar mean ages and a similar preponderance of women, joint hypermobility was reported only in the POTS group [13].
Conversely, POTS occurs frequently in EDS. One possible explanation for this association is that a problem with collagen in blood vessel walls makes them more stretchy or compliant, so that blood tends to pool in the abdomen or pelvis during prolonged standing.
The Beighton score is used to gauge the severity of joint hypermobility, based on 5 tests. The Beighton score is calculated as follows:
One point for each little finger that you can bend backwards by more than 90 degrees
One point for each thumb that you can touch to your forearm when bent backwards
One point for each elbow that you can bend backwards
One point for each knee that you can bend backwards
One point if while standing you can bend forward and place your palms on the ground with your legs straight
Out of a possible 9 points, a score of 0-4 is considered normal, 5-6 increased in range of motion, and 7-9 hypermobile [35].
The Composite Autonomic Symptom Score (COMPASS): A Research Tool
Over the years, progressively more refined “composite” autonomic symptom scores (COMPASS) have been introduced [34]. The “COMPASS 31” scale contains a total of 31 questions in 6 domains, yielding an overall autonomic symptom score from 0 to 100. The domains are OI (4 questions), vasomotor (3 questions), secretomotor (4 questions), pupillomotor (5 questions), bladder (3 questions), and gastrointestinal (including diarrhea, constipation, and gastroparesis, 12 questions). Erectile dysfunction is not included, since this is gender specific. For each question there is a numeric rating based on factors such as site, consistency, severity, frequency, or trends.
Here are the topics and simplified questions of the COMPASS 31:
Orthostatic intolerance: In the past year, have you ever felt faint, dizzy, “goofy”, or had difficulty thinking soon after standing up from a sitting or lying position? If so, how frequently? How severe are these feelings or symptoms? Have they changed?
Vasomotor: In the past year, have you ever noticed color changes in your skin, such as red, white, or purple? If so, which body parts are affected? Have these symptoms changed?
Secretomotor: In the past 5 years, what changes, if any, have occurred in your general body sweating? Do your eyes feel excessively dry? Does your mouth feel excessively dry? For the symptom of dry eyes or dry mouth that you have had for the longest period of time, has this symptom changed over time?
Gastrointestinal: In the past year, have you noticed any changes in how quickly you get full when eating a meal? Have you felt excessively full or persistently full (bloated feeling) after a meal? Vomited after a meal? Had cramping or colicky abdominal pain? Bouts of diarrhea? If so, how frequently? How severe are the episodes? Have they changed? In the past year, have you been constipated? If so, how frequently? How severe are the episodes? Have they changed?
Bladder: In the past year, have you ever lost control of your bladder function? If so, how frequently? Have you had trouble completely emptying your bladder? If so, how frequently?
Pupillomotor: In the past year, without sunglasses or tinted glasses, has bright light bothered your eyes? If so, how frequently? How severe is this sensitivity to bright light? Have you had trouble focusing your eyes? How frequent is the problem? How severe is the problem? Is the problem with light sensitivity or focusing changing?
While internally consistent statistically and useful for research purposes, particularly in patients already diagnosed with autonomic disorders, composite scoring of autonomic symptoms is inadequate from the point of view of the diagnostic interview as applied to dysautonomias.
For instance, within the “orthostatic intolerance” domain, time of day, relationships with meals, exercise, and heat exposure, associated symptoms such as the coat hanger phenomenon, chronic fatigue, chronic pain, and “brain” fog all should be considered. Some patients who are somatically focused may respond affirmatively to leading questions. In a diagnostic interview, false positive answers can be distinguished by exploring the context, consistency, and potential physiologic factors.
Within the “bladder” domain, a report of urinary retention and the need for self-catheterization is important for differentiating the parkinsonian form of MSA from PD with OH. Urinary retention strongly favors MSA-P over PD+OH. The lack of inclusion of erectile dysfunction in men is a glaring omission that is understandable given the scientific purposes of the scoring.
The COMPASS approach does not take into account the syndromic nature of particular forms of dysautonomia. For instance, in an elderly patient with parkinsonism, it is highly relevant to ask about olfactory dysfunction, since anosmia (lack of sense of smell) is common in PD+OH; about cognitive function, since dementia is more commonly associated with PD+OH than with MSA-P; about speech, since slurred speech favors MSA-P over PD+OH; and about breathing, since stridor favors MSA-P over PD+OH. In a young woman with COI, asking about double-jointedness and stretchy skin may reveal Ehlers-Danlos syndrome. In a patient with labile hypertension, the past history may disclose a remote history of neck irradiation, raising the possibility of arterial baroreflex failure from carotid sinus denervation.
Because the COMPASS has been validated in patients with autonomic failure, the usefulness of this inventory as a research tool in the area of chronic orthostatic intolerance may be limited.
Perhaps most importantly, the COMPASS approach does not take into account the sequence of symptoms, the chronology that is the essence of the HPI. For instance, in a man with central neurodegeneration, the lack of early erectile function excludes MSA. The checklist concerns only events within the past year (except for 5 years for secretomotor). In contrast, the non-directed approach to the HPI starts with an open-ended question like, “What was the first thing you noticed that went wrong?”
SUMMARY AND CONCLUSIONS
The most important autonomic function test is the autonomic medical history. Obtaining this autonomic history reliably is a finely-honed and continuously refined skill. One must keep in mind the Chief Complaint, the chronology of the narrative in the History of the Present Illness, the syndromes produced by hyperfunction or hypofunction of components of the autonomic nervous system, the site of the lesion, effects of medications or dietary supplements on autonomic functions, and the non-autonomic aspects of different forms of dysautonomia.
Table 5:
Some causes of parasympathetic nervous system (PNS) underactivity hyperactivity
| PNS Underactivity |
|---|
| Drugs |
| Distress |
| Congestive heart failure |
| Vagotomy |
| Sjogren’s syndrome |
| Autoimmune autonomic ganglionopathy |
| PNS Hyperactivity |
| Drugs |
| Autonomically mediated syncope (fainting) |
| Startle |
| Carotid sinus syncope |
| Athleticism |
| Vagal nerve stimulation |
| Carotid sinus stimulation |
Table 6:
Some causes of sympathetic adrenergic system (SAS) hyperactivity
| Drugs |
| Distress |
| Autonomically mediated syncope (fainting) |
| Hypoglycemia |
| Panic/terror |
| Shock |
| Postural tachycardia syndrome (POTS) |
| Stress cardiopathy |
| Asphyxia |
| Hypothermia/hyperthermia |
| Adrenomedullary hyperplasia |
SYNOPSIS.
The Autonomic Medical History
The most important autonomic function test is the autonomic medical history. Obtaining this history is a skill. One must keep in mind the Chief Complaint, the chronology of the narrative in the History of the Present Illness, the syndromes produced by hyperfunction or hypofunction of components of the autonomic nervous system, the site of the lesion, effects of medications or dietary supplements on autonomic functions, and the non-autonomic aspects of different forms of dysautonomia.
Financial support:
The research reported here was supported by the Division of Intramural Research, NINDS, NIH.
Abbreviations:
- AAG
autoimmune autonomic ganglionopathy
- ANS
autonomic nervous system
- COI
chronic orthostatic intolerance
- EDS
Ehlers-Danlos syndrome
- ENS
enteric nervous system
- EPI
epinephrine
- NE
norepinephrine
- HPI
history of the present illness
- HSAN
hereditary sensory and autonomic neuropathy
- MSA
multiple system atrophy
- OH
orthostatic hypotension
- OI
orthostatic intolerance
- PAF
pure autonomic failure
- PNS
parasympathetic nervous system
- POTS
postural tachycardia syndrome
- ROS
review of systems
- SAS
sympathetic adrenergic system
- SCS
sympathetic cholinergic system
- SNS
sympathetic noradrenergic system
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors state that there is no conflict of interest.
REFERENCES
- 1.Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW (2010) Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron 68:739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon WB (1919) Bodily Changes in Pain, Hunger, Fear and Rage. D. Appleton & Co., New York [Google Scholar]
- 3.Cheshire WP Syncope. Continuum; (in press) [DOI] [PubMed] [Google Scholar]
- 4.Cheshire WP (2016) Stimulant medication and postural orthostatic tachycardia syndrome: a tale of two cases. Clin. Auton. Res 26:229–233 [DOI] [PubMed] [Google Scholar]
- 5.Cheshire WP, Fealey RD (2008) Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 31:109–126 [DOI] [PubMed] [Google Scholar]
- 6.Cheshire WP Jr. (2000) Hypotensive akathisia: autonomic failure associated with leg fidgeting while sitting. Neurology 55:1923–1926 [DOI] [PubMed] [Google Scholar]
- 7.Cryer PE (2006) Hypoglycemia in diabetes: pathophysiological mechanisms and diurnal variation. Prog. Brain Res 153:361–365 [DOI] [PubMed] [Google Scholar]
- 8.Dean GE, Nelson PJ, Rudnick G (1986) Characterization of native and reconstituted hydrogen ion pumping adenosinetriphosphatase of chromaffin granules. Biochemistry 25:4918–4925 [DOI] [PubMed] [Google Scholar]
- 9.Eder AF (2012) Current efforts to reduce the risk of syncope among young blood donors. Curr Opin Hematol 19:480–485 [DOI] [PubMed] [Google Scholar]
- 10.Espinosa-Medina I, Saha O, Boismoreau F, Chettouh Z, Rossi F, Richardson WD, Brunet JF (2016) The sacral autonomic outflow is sympathetic. Science 354:893–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland EM, Robertson D (2001) Chiari I malformation as a cause of orthostatic intolerance symptoms: a media myth? Am. J. Med 111:546–552. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS (2017) Principles of Autonomic Medicine. https://neuroscience.nih.gov/publications/Principles%20of%20Autonomic%20Medicine%20v.%202.1.pdf
- 13.Goldstein DS, Eldadah B, Holmes C, Pechnik S, Moak J, Sharabi Y (2005) Neurocirculatory abnormalities in chronic orthostatic intolerance. Circulation 111:839–845 [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DS, Holmes C, Dendi R, Li ST, Brentzel S, Vernino S (2002) Pandysautonomia associated with impaired ganglionic neurotransmission and circulating antibody to the neuronal nicotinic receptor. Clin. Auton. Res 12:281–285 [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DS, Holmes C, Sewell L, Park MY, Sharabi Y (2012) Sympathetic noradrenergic before striatal dopaminergic denervation: relevance to Braak staging of synucleinopathy. Clin. Auton. Res 22:57–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein DS, Li ST, Kopin IJ (2001) Sympathetic neurocirculatory failure in Parkinson disease: Evidence for an etiologic role of alpha-synuclein. Ann. Intern. Med 135:1010–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakim A, O’Callaghan C, De Wandele I, Stiles L, Pocinki A, Rowe P (2017) Cardiovascular autonomic dysfunction in Ehlers-Danlos syndrome-hypermobile type. Am. J. Med. Genet. C Semin. Med. Genet [DOI] [PubMed] [Google Scholar]
- 18.Hayat A, Whittam D (2014) Baroreceptor failure related to bilateral carotid artery disease: an uncommon cause of labile hypertension. Intern Med J 44:105–106 [DOI] [PubMed] [Google Scholar]
- 19.Isonaka R, Holmes C, Cook GA, Sullivan P, Sharabi Y, Goldstein DS (2017) Pure autonomic failure without synucleinopathy. Clin. Auton. Res [DOI] [PubMed] [Google Scholar]
- 20.Jafari S, Etminan M, Aminzadeh F, Samii A (2013) Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov. Disord 28:1222–1229 [DOI] [PubMed] [Google Scholar]
- 21.Kanjwal K, Karabin B, Kanjwal Y, Grubb BP (2010) Autonomic dysfunction presenting as postural tachycardia syndrome following traumatic brain injury. Cardiol. J 17:482–487 [PubMed] [Google Scholar]
- 22.Kanjwal K, Karabin B, Kanjwal Y, Grubb BP (2011) Postural orthostatic tachycardia syndrome following Lyme disease. Cardiol J 18:63–66 [PubMed] [Google Scholar]
- 23.Kaufmann H, Goldstein DS (2010) Pure autonomic failure: a restricted Lewy body synucleinopathy or early Parkinson disease? Neurology 74:536–537 [DOI] [PubMed] [Google Scholar]
- 24.Khurana RK (2012) Coat-hanger ache in orthostatic hypotension. Cephalalgia 32:731–737 [DOI] [PubMed] [Google Scholar]
- 25.Langley JN (1921) The Autonomic Nervous System. W. Heffer and Sons, Ltd., Cambridge, England [Google Scholar]
- 26.Nogues M, Delorme R, Saadia D, Heidel K, Benarroch E (2001) Postural tachycardia syndrome in syringomyelia: response to fludrocortisone and beta-blockers. Clin Auton Res 11:265–267 [DOI] [PubMed] [Google Scholar]
- 27.Pederson CL, Brook JB (2017) Health-related quality of life and suicide risk in postural tachycardia syndrome. Clin. Auton. Res 27:75–81 [DOI] [PubMed] [Google Scholar]
- 28.Prilipko O, Dehdashti AR, Zaim S, Seeck M (2005) Orthostatic intolerance and syncope associated with Chiari type I malformation. J Neurol Neurosurg Psychiatry 76:1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe PC, Barron DF, Calkins H, Maumenee IH, Tong PY, Geraghty MT (1999) Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. J. Pediatr 135:494–499 [DOI] [PubMed] [Google Scholar]
- 30.Schachter S, Singer J (1962) Cognitive, social, and physiological determinants of emotional state. Psychol. Rev 69:379–399 [DOI] [PubMed] [Google Scholar]
- 31.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D (2000) Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N. Engl. J. Med 342:541–549 [DOI] [PubMed] [Google Scholar]
- 32.Sharabi Y, Dendi R, Holmes C, Goldstein DS (2003) Baroreflex failure as a late sequela of neck irradiation. Hypertension 42:110–116 [DOI] [PubMed] [Google Scholar]
- 33.Singleton A, Gwinn-Hardy K, Sharabi Y, Li ST, Holmes C, Dendi R, Hardy J, Crawley A, Goldstein DS (2004) Association between cardiac denervation and parkinsonism caused by alpha-synuclein gene triplication. Brain 127:768–772 [DOI] [PubMed] [Google Scholar]
- 34.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W (2012) COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc 87:1196–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smits-Engelsman B, Klerks M, Kirby A (2011) Beighton score: a valid measure for generalized hypermobility in children. J. Pediatr 158:119–123, 123, e111–114 [DOI] [PubMed] [Google Scholar]
- 36.Timmers HJ, Buskens FG, Wieling W, Karemaker JM, Lenders JW (2004) Long-term effects of unilateral carotid endarterectomy on arterial baroreflex function. Clin Auton Res 14:72–79 [DOI] [PubMed] [Google Scholar]
- 37.Wieling W, van Dijk N, Thijs RD, de Lange FJ, Krediet CT, Halliwill JR (2015) Physical countermeasures to increase orthostatic tolerance. J. Intern. Med 277:69–82 [DOI] [PubMed] [Google Scholar]


