Abstract
Aim
To investigate the effects of cleansing Fleet’s™ enema (FE) on rectal distention and image quality of diffusion-weighted imaging (DWI) in prostate magnetic resonance imaging (MRI).
Methods
This study included 117 prospectively accrued active surveillance patients who underwent prostate MRI both without (prep−) and with bowel preparation consisting of FE (prep+) obtained within 12 months of each other. The anterior–posterior (AP) diameter of the rectum, degree of perceived distention in the rectum and image quality scores were assessed by two independent readers for both (prep− and prep+) scans. DWI distortion was assessed quantitatively using the degree of anatomic mismatches between images obtained at different b values and the T2-weighted MRI. DWI artifact was qualitatively scored based on the presence of blurring, poor signal-to-noise, and artifact lines. The difference in rectal AP diameters between the two methods was tested by the paired Wilcoxon rank test. Stuart Maxell test was used in compar-ing rectal distention, DWI distortion, and artifact. Reader agreement was estimated by kappa statistics. p values < 0.05 were considered statistically significant.
Results
Mean rectal AP diameter was significantly larger in prep− compared with prep+ scans (p = 0.002). Subjective scores demonstrated inter-reader variability. For instance, the rectal distention score was significantly lower in prep+ for reader 2 (p < 0.001) whereas it was not significant for reader 1 (p = 0.09). Reader 2 also found significant improvement in DWI distortion (p = 0.02) in prep+ scans. There was no significant difference between prep− and prep+ in DWI distortion and artifacts for reader 1 (p = 0.17 and p = 0.49, respectively), or DWI artifacts for reader 2 (p = 0.55). Kappa scores were moderate for rectal distension, but weak for DWI distortion, and artifacts.
Conclusion
Bowel preparation with enema prior to prostate MRI may diminish rectal gas but has modest effects on DWI distortion and overall image quality. The value of bowel prep is not conclusively validated in this study.
Keywords: Prostate MRI, Bowel preparation, MRI quality, Enema, Artifact
Introduction
Multiparametric magnetic resonance imaging (mpMRI) improves prostate cancer diagnosis in at-risk patients [1, 2]. MpMRI consists of anatomic (T2 weighed imaging (T2WI)) and functional sequences (diffusion-weighted (DWI) MRI and dynamic contrast enhancement (DCE)) [3]. Among these pulse sequences, DWI MRI plays the key role in detection and categorization of prostate lesions [4, 5]. DWI MRI is commonly acquired using a spin-echo echo-planar imaging sequence which is more prone to magnetic field inhomogeneities, susceptibility related artifacts including ghosting and geometric distortions [6, 7]. Susceptibility artifacts often occur near air-tissue interfaces such as between the prostate and rectum [7]. Susceptibility artifacts are more prominent at 3 Tesla (T) and increases with field strength and therefore, gradient strength [5, 8, 9]. Thus, rectal distention secondary to gas and motion are among the major factors affecting prostate MRI quality [10, 11].
The success of mpMRI depends on consistent, high-quality imaging [12, 13]. The prostate imaging-reporting and data system version 2 (PI-RADSv2) guidelines proposed several strategies but reached no consensus on the value of patient preparation. Among the methods suggested in PI-RADSv2 are antispasmodic agents (glucagon, scopolamine or hyoscine butylbromide etc.) to reduce motion artifact from bowel peristalsis and enema administration or decompression of the rectum with a catheter to reduce air in the rectum [5]. Enema application had been documented to reduce rectal gas [14] but the impact on image quality is still unknown since this requires an intra-patient comparison approach. In this retrospective study, the effects of cleansing enema prior to mpMRI on rectal distention, distortion, and artifact were investigated using an intra-patient comparison design.
Materials and methods
Study population
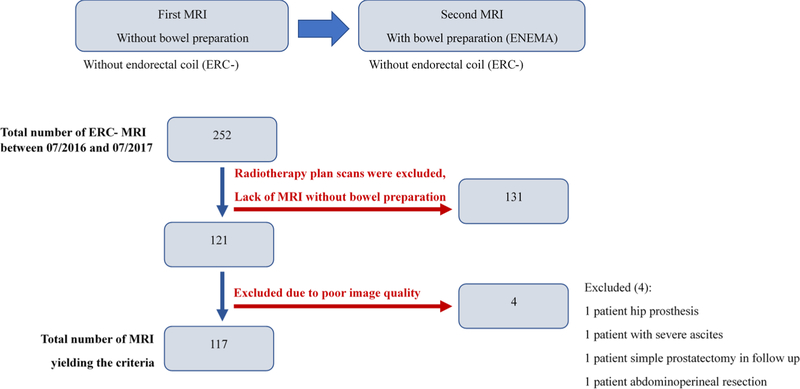
This retrospective single institution study was approved by the local institutional review board and was compliant with the Health Insurance Portability and Accountability Act of 1996. The study population included patients who underwent two mpMRIs within 12 months of each other. The patient population included patients between July 2016 and July 2017 who were on active surveillance for low risk prostate cancer who underwent annual MRIs at our institution. The first mpMRI scan was done without bowel preparation (prep−), whereas the 12 months follow-up mpMRI was performed after a bowel preparation (prep+) consisting of a Fleet’s™ enema. The flow chart for patient inclusion in this study is presented in Fig. 1.
Fig. 1.
Flow chart in patient selection
Multiparametric prostate MRI acquisition and bowel preparation
Multiparametric prostate MRIs were acquired with a 3T MRI scanner (Achieva 3 T-Tx, Philips Healthcare) using a 32-channel cardiac coil (Invivo, Philips Healthcare) without endorectal coil. MpMRIs included T1WI, three plane T2WI, DWI MRI with high b value (b1500) and ADC maps, DCE and post-contrast T1WI with fat suppression. Slice thickness and locations were kept constant for axial T2WI, DWI MRI and DCE images without gap. All parameters were compatible with the PI-RADSv2 minimum technical standards (Table 1). For bowel preparation, the Fleet’s™ enema was administered by the patients themselves approximately 12 h before mpMRI.
Table 1.
Multiparametric prostate MRI acquisition parameters at 3T
| Parameters | Axial T2 Weighted | DWIa | DCEb |
|---|---|---|---|
|
| |||
| Field of view (mm) | 140 × 140 | 140 × 140 | 262 × 262 |
| Acquisition matrix | 304 × 234 | 112 × 108 | 256 × 186 |
| Repetition time (ms) | 8869 | 3709 | 3.7 |
| Echo time (ms) | 120 | 52 | 2.2 |
| Flip angle (°) | 90 | 90 | 8.5 |
| Section thickness/gap (mm) | 3/0 | 3/0 | 3/0 |
| Reconstruction pixel size (mm) | 0.27 × 0.27 × 3 | 1.02 × 1.02 × 3 | 1.5 × 1.5 × 3 |
| Acquisition time (min:sec) | 5:37 | 4:46 | 5:12 |
DWI diffusion-weighted imaging, DCE dynamic contrast enhancement
Highest b value is obtained at b = 1500 s/mm2
Temporal resolution is 5.6 s
Evaluation of rectum distention
Rectal distention was scored by two independent readers (Reader 1 [R1]: A urologist with fellowship training in prostate MRI with a cumulative experience of 2 years (≥ 500 prostate MRI evaluations per year); Reader 2 [R2]: A diagnostic radiologist with a cumulative experience of 3 years (≥ 1000 prostate MRI evaluations per year), using a subjective Likert scale 1 to 5 (collapsed rectum: 1, distended rectum: 5). The readers were blinded to clinical information (use of bowel preparation), MRI reports. Evaluations were performed in 2 different rounds with a 4 week wash out period.
Rectal anterior–posterior (AP) diameter was measured from the mid-sagittal T2W image at the level of the base of the seminal vesicles by R2.
Assessment of prostate MRI
Quantitative DWI distortion and qualitative DWI artifact scores were evaluated independently by two readers.
In quantitative DWI distortion scoring, b1500 and ADC were spatially compared with axial T2WI using a 3D registration cursor available on the picture archiving and communication systems (Carestream, Rochester NY). This cursor was placed at 20 different points (apex, apical-mid, mid, mid-base, base level; right, left, anterior, posterior directions) on the prostate capsule on the b1500 DWI and ADC maps to evaluate the spatial mismatches with axial T2WI, which was used as a reference location. The maximal displacement was recorded and used for scoring on the following 4-point scale: displacement between 0 and 1.9 mm, score 1 (best quality); 2–3.9 mm, score 2; 4–5.9 mm, score 3; 6 mm or more, score 4 (lowest quality).
Qualitative DWI artifacts were scored using b1500 DWI and ADC maps using a quantitative scale 1 to 4 (best quality 1, lowest quality 4). Readers were asked to evaluate presence of blurring or subtle artifact lines (+ 1 point), poor signal-to-noise ratio (SNR) (+ 1 point), or prominent artifact lines (+ 2 points) within the prostate on b1500 DWI MRI or ADC maps and the final score was determined by summing the points.
Statistical analysis
Difference in rectal AP diameter in prep− and prep+ scans was tested by the paired Wilcoxon rank test for R2. Impact of bowel preparation on image quality of DWI MRI was tested by comparing each of the 3 quality parameters (Rectal distention, DWI distortion, and DWI artifact) in prep− and prep+ scans evaluated by each reader using the Stuart Maxell test [15]. Reader agreement on those measures in prep− and prep+ scans was estimated separately by both proportion of agreement (PA) and the kappa statistic with linear weight. All p values were two-sided and p values < 0.05 were considered statistically significant. The analyses were performed using R version 3.4.0.
Results
The median age of the 117 patients was 67 (range 46–83, SD 7). The mean PSA of the patients was 8.63 ng/ml (median 7.25 ng/ml; range 0.5–83.98 ng/ml). The mean interval between prep− and prep+ scans was 367 days (range 192–380).
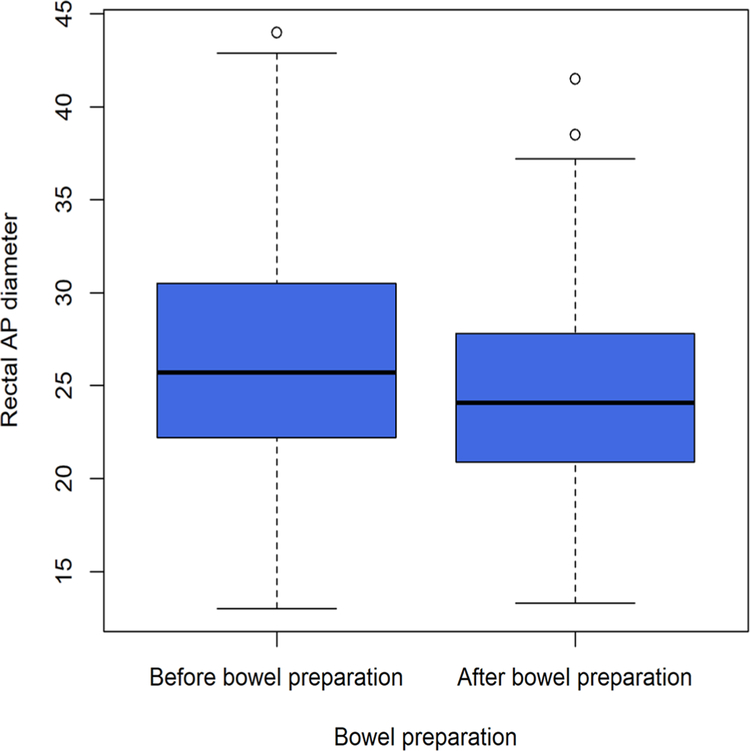
Mean rectal AP diameter was significantly larger in prep− (26.7 mm ± 6.4 mm) than prep+ scans (24.8 mm ± 5.5 mm) (p=0.002) (Fig. 2). Rectal distension scored as 4–5 occurred in 37 and 53 prep− scans according to R1 and R2 readings, respectively; this was reduced to 18 and 25 for R1 and R2, respectively, on prep+ scans (Table 2). Rectal distention score was significantly lower in prep+ scans for R2 (p < 0.001) whereas it was not for R1 (p = 0.09) (Fig. 3).
Fig. 2.
Rectum AP diameter without and with bowel preparation
Table 2.
Rectal distention scores of the readers without and with bowel preparation
| Reader 1 | With bowel preparation |
|||||
| Score | 1 | 2 | 3 | 4 | 5 | Total |
|
| ||||||
| Without bowel preparation | ||||||
| 1 | 19 | 1 | 0 | 0 | 1 | 21 |
| 2 | 8 | 23 | 2 | 1 | 1 | 35 |
| 3 | 5 | 5 | 11 | 0 | 3 | 24 |
| 4 | 2 | 3 | 6 | 5 | 1 | 17 |
| 5 | 2 | 1 | 5 | 5 | 7 | 20 |
| Total | 36 | 33 | 24 | 11 | 13 | 117 |
|
| ||||||
| Reader 2 | With bowel preparation |
|||||
| Score | 1 | 2 | 3 | 4 | 5 | Total |
|
| ||||||
| Without bowel preparation | ||||||
| 1 | 1 | 5 | 0 | 2 | 0 | 0 |
| 2 | 2 | 12 | 7 | 10 | 2 | 1 |
| 3 | 3 | 4 | 6 | 6 | 7 | 2 |
| 4 | 4 | 4 | 8 | 6 | 6 | 4 |
| 5 | 5 | 0 | 5 | 5 | 5 | 10 |
| Total | Total | 25 | 26 | 29 | 20 | 17 |
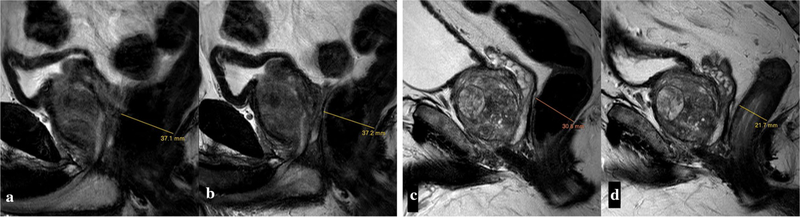
Fig. 3.
Effect of bowel preparation on rectal gas. Sagittal T2W images of patient 1 (a, b) and 2 (c, d) without (a, c) and with (b, d) bowel preparation. Enema did not reduce bowel loading in patient 1, while it decreased rectal distension in patient 2. Rectal distention scores were 5, 5, 4, 1 for reader 1 and 5, 5, 5, 2 for reader 2, respectively (a–d)
There were 17 and 57 cases rated by R1 and R2, respectively, with a distortion score of 3–4 in prep− scans; this was reduced to 7 and 31 cases, respectively, on prep+ scans (Tables 3, 4). However, there was no significant difference between prep− and prep+ scans in the assessment of rectal distention, DWI distortion, or artifact for R1 (p = 0.09, p = 0.17, and p = 0.49, respectively), whereas R2 reported significant improvement in rectal distension (p < 0.001) and DWI distortion (p = 0.02) with bowel preparation. The proportion of patients achieving category 1 (best quality) results was increased for both readers. R2 also did not report a significant difference between prep− and prep+ scans in DWI artifact (p = 0.55). Total number of score 3 and 4 (poor quality) DWI decreased from 17 to 16 for R1 and from 57 to 41 for R2 with bowel preparation (Fig. 4).
Table 3.
DWI distortion scores of the readers without and with bowel preparation
| Reader 1 | With bowel preparation |
||||
| Score | 1 | 2 | 3 | 4 | Total |
|
| |||||
| Without bowel preparation | |||||
| 1 | 11 | 5 | 0 | 0 | 16 |
| 2 | 0 | 75 | 8 | 1 | 84 |
| 3 | 0 | 9 | 5 | 0 | 14 |
| 4 | 0 | 1 | 1 | 1 | 3 |
| Total | 11 | 90 | 14 | 2 | 117 |
|
| |||||
| Reader 2 | With bowel preparation |
||||
| Score | 1 | 2 | 3 | 4 | Total |
|
| |||||
| Without bowel preparation | |||||
| 1 | 5 | 5 | 0 | 0 | 10 |
| 2 | 9 | 31 | 9 | 1 | 50 |
| 3 | 5 | 19 | 16 | 3 | 43 |
| 4 | 1 | 1 | 5 | 7 | 14 |
| Total | 20 | 56 | 30 | 11 | 117 |
Table 4.
DWI artifact scores of the readers without and with bowel preparation
| Reader 1 | With bowel preparation |
||||
| Score | 1 | 2 | 3 | 4 | Total |
|
| |||||
| Without bowel preparation | |||||
| 1 | 2 | 0 | 0 | 0 | 2 |
| 2 | 1 | 100 | 6 | 0 | 107 |
| 3 | 0 | 4 | 3 | 0 | 7 |
| 4 | 0 | 1 | 0 | 0 | 1 |
| Total | 3 | 105 | 9 | 0 | 117 |
|
| |||||
| Reader 2 | With bowel preparation |
||||
| Score | 1 | 2 | 3 | 4 | Total |
|
| |||||
| Without bowel preparation | |||||
| 1 | 10 | 6 | 0 | 0 | 16 |
| 2 | 6 | 36 | 6 | 3 | 51 |
| 3 | 2 | 10 | 24 | 3 | 39 |
| 4 | 0 | 2 | 4 | 5 | 11 |
| Total | 18 | 54 | 34 | 11 | 117 |
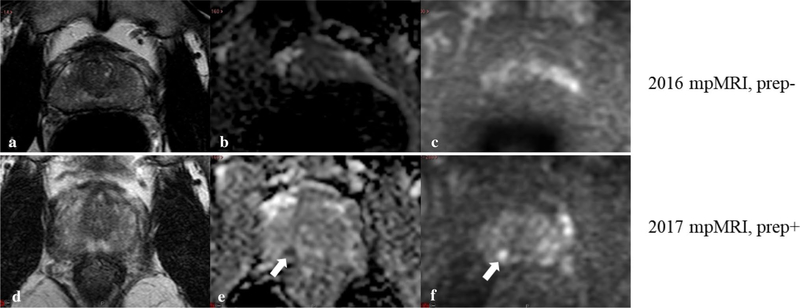
Fig. 4.
Effect of bowel preparation on lesion detection. T2W (a, b), ADC (b, e) and b1500 DWI images (c, f) of a patient on active surveillance. The unprepped scan was scored as PI-RADS 1 in 2016 (a-c). However considerable artifact is present on the ADC (b) and high b value image (c). A hypointense nodular lesion 5 mm diameter was described as PI-RADS score 4 on the prep+ scan in 2017 (d–f), and a targeted biopsy revealed a Gleason 4 + 3 tumor. In comparison to the prep− scan, the rectum is collapsed, and the peripheral zone was more clearly depicted on the prep+ scan
Inter-reader agreement was similar for prep− and prep+ scans. Kappa was moderate for rectal distension and weak for DWI distortion and artifact (Table 5).
Table 5.
Inter-reader agreement on rectal distension and MRI quality
| Without bowel preparation |
With bowel preparation |
|||||||
|---|---|---|---|---|---|---|---|---|
| PA | 95% CI | Kappa | 95% CI | PA | 95% CI | Kappa | 95% CI | |
|
| ||||||||
| Rectal distension | 0.45 | 0.37–0.54 | 0.53 | 0.42–0.65 | 0.39 | 0.31–0.48 | 0.45 | 0.33–0.57 |
| DWI distortion | 0.38 | 0.29–0.47 | 0.1 | 0.01–0.2 | 0.49 | 0.4–0.58 | 0.24 | 0.14–0.34 |
| DWI artifact | 0.44 | 0.36–0.53 | 0.08 | 0.01–0.15 | 0.47 | 0.38–0.56 | 0.1 | 0.03–0.18 |
Discussion
The results of our study showed that the impact of bowel preparation with enema on the image quality of mpMRI was not convincing. Only one of the two readers consistently evaluated the prep+ scans as superior to prep− scans. Objective finding in prep+ patients was a decrease in rectal AP diameter. Qualitative rectal distention and DWI distortion significantly improved with bowel prep but only for one reader. Traditionally, rectal distension secondary to air and stool is believed to cause susceptibility and motion artifacts which diminish prostate MRI quality [7, 10]. For instance, Caglic et al. evaluated the effect of rectal distension on image quality of prostate MRI and reported that rectal distention decreased DWI quality and this correlated with increased distortion and artifact in DWI and motion in T2WI [16]. In our study, an enema bowel preparation decreased rectal AP diameter significantly; however, this did not translate into improvement in image quality. Nonetheless, both readers reported less distention and distortion on prep+ scans. These results suggest that image quality is more complex than simply assessing the degree of rectal distention.
To our knowledge there is only one previous study addressing the effect of enema on the prostate MRI quality. Lim et al. also demonstrated less rectal gas but no difference in image quality on T2WI, b1000 DWI, and ADC. They did not find significant effects on blur, distortion or artifact. These results are concordant with our own where no definitive advantage was seen with bowel prep. The prior study was limited by a small population (n = 32 with non-enema, n = 28 with enema), and unmatched patients [14]. In our study, patients were matched for prep− and prep+ cases albeit obtained within a year of each of other due to the active surveillance population we used. It would be difficult to accrue to a study in which the patient had to undergo two MRIs in the same time frame, and this would also raise ethical questions. The intra-patient comparison of enema effect on image quality normalizes for anatomic differences among patients. This study also employed two readers which showed differences in perception for the value of bowel prep. For instance, the more experienced radiologist reader found significant improvement in DWI distortion with bowel preparation, whereas the less experienced urologist reader could not show a benefit for bowel prep. It is possible that the experienced reader had higher expectations of image quality and perceived more subtle improvements due to the enema. However, one would expect if the results were truly striking in the two studies that both readers would have reported a marked improvement on prep+ scans, which was not the case.
Although the PI-RADS document did not formally recommend a specific patient preparation, several studies have investigated patient preps outside bowel prep [17]. Ullrich et al., underwent mpMRI pre and post intravenous injection of 40 mg hyoscine butylbromide (HBB). They found significant improvement in anatomic quality score and reductions in artifacts after HBB [18]. Slough et al. showed similar results and, further showed that T2W image quality was significantly higher with less motion and blur in the HBB group. However, they found no improvement in DWI or ADC image quality or artifacts in the HBB group [19]. Thus, HBB does not appear to be a solution to the problem of DWI-related artifacts.
Our study has several limitations. Subjective assessments, such as rectal distention and DWI artifact score are notoriously difficult to reproduce among readers. To overcome this problem, we measured rectum diameter and defined criteria for DWI artifact scoring. Also, DWI distortion criteria were quantitative. The two readers in this study had unequal experience with mpMRI. It is important to use readers with variable experience as that better replicates practice settings. Specifically, the urologist reader although very familiar in using mpMRI data for clinical practice, was asked to evaluate the quality of these MRIs from their perspective. This reader was not as experienced in assessing image quality as the radiologist reader. This might introduce a bias into the results as these preliminary results suggest experience plays a role in the ability to evaluate the value of a bowel preparation prior to mpMRI The two scans in this study, although obtained in the same patients, were obtained at a mean of 367 days apart. Some changes may have occurred in the interval that might have affected results, but we think this population of low risk patients is most likely stable in the study interval.
In conclusion, bowel preparation objectively diminishes rectal gas and may reduce DWI distortion during prostate MRI acquisition. However, the effects of bowel prep on image quality are inconsistent among readers and therefore, cannot be definitively recommended.
Funding
This research is funded by intramural research program of NIH,
Footnotes
Compliance with ethical standards
Conflict of interest Author BJW is supported by the Intramural Research Program of the NIH and the NIH Center for Interventional Oncology and NIH Grant # Z1A CL040015–08. NIH and Philips/InVivo Inc have a cooperative Research and Development Agreement. NIH and Philips/InVivo Inc have a patent license agreement and NIH and BJW, BT, PAP, PLC may receive royalties. The remaining authors have no disclosures.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015;313(4):390–7. Epub 2015/01/28. 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. The New England journal of medicine. 2018;378(19):1767–77. Epub 2018/03/20. 10.1056/nejmoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turkbey B, Brown AM, Sankineni S, Wood BJ, Pinto PA, Choyke PL. Multiparametric prostate magnetic resonance imaging in the evaluation of prostate cancer. CA Cancer J Clin. 2016;66(4):326–36. Epub 2015/11/26. 10.3322/caac.21333. Pub Med PMID: 26594835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–57. Epub 2012/02/11. 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. European urology. 2016;69(1):16–40. Epub 2015/10/03. 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jambor I. Optimization of prostate MRI acquisition and post-processing protocol: a pictorial review with access to acquisition protocols. Acta Radiol Open. 2017;6(12):2058460117745574. Epub 2017/12/16. 10.1177/2058460117745574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazaheri Y, Vargas HA, Nyman G, Akin O, Hricak H. Image artifacts on prostate diffusion-weighted magnetic resonance imaging: trade-offs at 1.5 Tesla and 3.0 Tesla. Acad Radiol. 2013;20(8):1041–7. Epub 2013/07/09. 10.1016/j.acra.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillard JH, Papadakis NG, Martin K, Price CJ, Warburton EA, Antoun NM, et al. MR diffusion tensor imaging of white matter tract disruption in stroke at 3 T. Br J Radiol. 2001;74(883):642–7. Epub 2001/08/18. 10.1259/bjr.74.883.740642. [DOI] [PubMed] [Google Scholar]
- 9.Kuhl CK, Gieseke J, von Falkenhausen M, Textor J, Gernert S, Sonntag C, et al. Sensitivity encoding for diffusion-weighted MR imaging at 3.0 T: intraindividual comparative study. Radiology. 2005;234(2):517–26. Epub 2005/01/27. 10.1148/radiol.2342031626. [DOI] [PubMed] [Google Scholar]
- 10.Ogino I, Kaneko T, Suzuki R, Matsui T, Takebayashi S, Inoue T, et al. Rectal content and intrafractional prostate gland motion assessed by magnetic resonance imaging. J Radiat Res. 2011;52(2):199–207. Epub 2011/03/26. 10.1269/jrr.10126. [DOI] [PubMed] [Google Scholar]
- 11.Padhani AR, Khoo VS, Suckling J, Husband JE, Leach MO, Dearnaley DP. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys. 1999;44(3):525–33. Epub 1999/05/29. 10.1016/s0360-3016(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 12.Brizmohun Appayya M, Adshead J, Ahmed HU, Allen C, Bainbridge A, Barrett T, et al. National implementation of multi-parametric magnetic resonance imaging for prostate cancer detection - recommendations from a UK consensus meeting. BJU Int. 2018;122(1):13–25. Epub 2018/04/27. 10.1111/bju.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turkbey B, Choyke PL. Future Perspectives and Challenges of Prostate MR Imaging. Radiol Clin North Am. 2018;56(2):327–37. Epub 2018/02/09. 10.1016/j.rcl.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim C, Quon J, McInnes M, Shabana WM, El-Khodary M, Schieda N. Does a cleansing enema improve image quality of 3T surface coil multiparametric prostate MRI? J Magn Reson Imaging. 2015;42(3):689–97. Epub 2015/01/06. 10.1002/jmri.24833. [DOI] [PubMed] [Google Scholar]
- 15.Agresti A. Categorical Data Analysis. John Wiley & Sons. Inc, Publication. 2002. [Google Scholar]
- 16.Caglic I, Hansen NL, Slough RA, Patterson AJ, Barrett T. Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur J Radiol. 2017;90:174–80. Epub 2017/06/07. 10.1016/j.ejrad.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Caglic I, Barrett T. Optimising prostate mpMRI: prepare for success. Clinical radiology. 2019;74(11):831–40. Epub 2019/01/07. 10.1016/j.crad.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Ullrich T, Quentin M, Schmaltz AK, Arsov C, Rubbert C, Blondin D, et al. Hyoscine butylbromide significantly decreases motion artefacts and allows better delineation of anatomic structures in mp-MRI of the prostate. Eur Radiol. 2018;28(1):17–23. Epub 2017/07/09. 10.1007/s00330-017-4940-7. [DOI] [PubMed] [Google Scholar]
- 19.Slough RA, Caglic I, Hansen NL, Patterson AJ, Barrett T. Effect of hyoscine butylbromide on prostate multiparametric MRI anatomical and functional image quality. Clin Radiol. 2018;73(2):216.e9–.e14. Epub 2017/08/15. 10.1016/j.crad.2017.07.013. [DOI] [PubMed] [Google Scholar]