Abstract
Multiple endocrine neoplasia type 1 (MEN1) is the most common cause of hereditary primary hyperparathyroidism (PHPT). Bone disorders are considered one of the key symptoms in PHPT present with the significant reduction in bone mineral density and low-energy fractures. Previously, these bone disorders were believed to be caused solely by the increase in the level of parathyroid hormone and its subsequent effect on bone resorption. The current paradigm, however, states that the mutations in the menin gene, which cause the development of MEN1, can also affect the metabolism of the cells of the osteoid lineage. This review analyzes both the proven and the potential intracellular mechanisms through which menin can affect bone metabolism.
Keywords: menin, men 1 protein, osteoporosis, multiple endocrine neoplasia type 1
Introduction
Menin is a highly conserved protein encoded by the ubiquitously expressed MEN1gene. It partakes in the regulation of various intracellular processes, including transcription, maintaining genome stability, proliferation and intracellular signaling. Mutations in the menin gene cause the development of multiple endocrine neoplasia type 1 (MEN1) syndrome. This autosomal dominant disease is characterized by the formation of multiple tumors, predominantly located in the endocrine glands. In MEN1, lesions of parathyroid glands have the highest penetrance; they manifest with primary hyperparathyroidism (PHPT). Conversely, PHPT is a powerful etiological factor that can lead to bone disorders including the significant decrease in bone mineral density (BMD) followed by multiple low-energy fractures. In contrast to sporadic PHPT, bone disorders in patients with PHPT in multiple endocrine neoplasias (PHPT/MEN1) are more severe. The higher severity of bone and mineral disorders in patients with menin gene mutations has been clinically proven (1): bone mineral densities of the lumbar spine and at the femoral neck observed in PHPT/MEN1 patients are significantly lower compared to those of sporadic PHPT patients. Bone demineralization is observed in up to 77.8% o
reaches osteoporotic levels (1, 2, 3). Additionally, the bone formation rate in PHPT/MEN1 patients after parathyroidectomy is significantly lower, than for sporadic PHPT (4).
Several differences exist in the structure of the changes as well. In PHPT/MEN1, both cortical and trabecular bone tissues are demineralized, while in sporadic PHPT, primarily cortical bone tissue is affected (5).
At the same time, the quality of the clinical studies that have been published to date is lacking, mainly due to the orphan nature of the disease and the variety of possible comorbidities. An unambiguous interpretation of these studies is not possible, which necessitates the generation of a suitable experimental model.
In the absence of human cell-based models, mice were used to study the role of menin in bone metabolism in vitro and in vivo. A number of recent works have shown that menin is directly involved in the regulation of osteoblastogenesis, differentiation and functioning of osteoblasts, and in the osteocyte-osteoclast interaction. It has been shown that the effects of menin can be mediated by several potential mechanisms, such as the interactions with the Runx2 transcription factor, molecules involved in the β-catenin pathway, BMP, and Smad3, Smad1/5 and JunD signaling molecules (6, 7, 8). Through these, menin affects osteoclastogenesis, as well as the formation and functioning of osteoblasts.
Bone remodeling is a process that takes place throughout a person’s life. It involves the destruction of old bone tissue and its replacement with a newly formed bone matrix. The main cells involved in bone remodeling are osteoblasts (bone-forming cells) and osteoclasts (cells that degrade bone tissue). The combined action of these cells leads to the successive resorption of the old bone and the formation of a new one (9). The existing findings point to the important role of menin in bone remodeling, which is a continuous dynamic interaction of the bone-forming cells (osteoblasts) and the bone-resorbing cells (osteoclasts). However, models that use transformed cell lines and/or MEN1-knockout animals cannot fully reproduce the extremely variable clinical presentation of MEN1 in humans and cannot account for the possible influence of individual genetic and epigenetic changes on the course of bone disorders. An in vitro isogenic model based on MEN1 patients’ cells, generated using cell reprogramming and genome editing, would present a promising approach to uncover the mechanisms underlying the role of menin in bone metabolism considering individual genetic and epigenetic features. In addition to the benefits provided by personalization, this model can be used to identify specific therapeutic targets and develop modern effective therapeutic algorithms for MEN1. However, differentiation of induced pluripotent stem cells, mesenchymal stem cells (MSCs) or fibroblasts into osteoblasts is also a non-trivial task. In particular, stem cells can form teratomas, their collection is a traumatic procedure, and they also lose their ability to differentiate after five passages. Dermal fibroblasts are capable to undergo 15 passages, but they are differentiated cells, and it is challenging to redirect them toward specific lineages (10).
Structure of menin
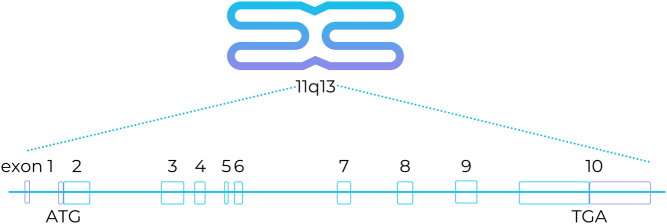
Menin is a highly conserved protein encoded by the MEN1 gene, which is located on chromosome 11 and consists of 10 exons that encode 610 amino acid residues.
The schematic structure of the coding regions of MEN1 is shown in Fig. 1.
Figure 1.
Schematic chromosomal localization and structure of MEN1. Blue and violet boxes indicate the coding and non‐coding regions of exons, respectively (11).
It has no homology domains with known consensus sequences, which makes the study of its intracellular interactions problematic. Some insights were gained after the structure of menin was determined.
It was established that the C-terminus of menin has two functionally independent nuclear localization signals (NLS) which provide its predominant nuclear localization. Additionally, C-terminus allows menin to bind to the dsDNA directly, with no specific binding site required. NLS is necessary to form such a bond. It has also been suggested that menin may specifically bind to some DNA regions via two leucine zipper motifs (12).
The N-terminus of menin contains five motifs usually found in GTPases and GTP-binding proteins. At the same time, the affinity of menin for GTP is relatively low, and it displays GTPase activity only in presence of nm23 nucleoside diphosphate kinase (12).
Menin is expressed in many tissues; however, its function in an adult organism is tissue-specific. In fact, the effects of menin in different organs can be directly opposite. For example, it acts as a tumor suppressor in endocrine tissues but induces proliferation in leukopoiesis. The reasons for this are ambiguous, as to date there is no clear understanding of the various intracellular cascades menin participates in (12).
Menin in the development of bone disorders in MEN1 patients
Parathyroid lesions in MEN1 manifest with PHPT, usually followed by hypersecretion of parathyroid hormone and hypercalcemia. Development of complications in key target organs is characteristic both for sporadic and hereditary PHPT. That includes the reduction of the BMD up to the development of osteoporosis and osteitis fibrosa cystica (1).
A study performed in the Russian population shows that skeletal complications arise in 87% of patients with sporadic hyperparathyroidism (13). In PHPT/MEN1, BMD is decreased in 58–72% of the cases (5, 14).
The clinical course of bone disease in PHPT/MEN1 patients differs from that in sporadic PHPT/MEN1. For instance, BMD at the time of diagnosis is usually lower in PHPT/MEN1 (2, 15). Additionally, in osteoporosis caused by sporadic hyperparathyroidism, the parathyroidectomy is followed by a relatively rapid gain in bone mass; for that reason, anti-osteoporotic drugs are not prescribed to these patients for the first few years after the surgery (16). Parathyroidectomy in PHPT/MEN1 patients leads to a significantly slower bone mass gain (4).
There is no definite explanation for these differences. In part, they may be caused by the poor methodology of research (e.g. the use of surrogate endpoints instead of the immediate BMD values) (4). Additional BMD loss in PHPT/MEN1 may be caused by the components associated with the syndrome (i.e. hormone-producing neuroendocrine tumors of the gastrointestinal tract) (4, 17). Slower bone mass gain after surgery may be caused by a greater extent of surgery and a high incidence of postoperative hypoparathyroidism (4).
The results of fundamental and clinical research suggest that heterozygous deletions of MEN1 affect bone metabolism. The review of the main intracellular pathways in which menin participates is presented below. Disruption of these pathways can result in bone metabolism disorders in MEN1 patients.
According to the Knudson two-hit hypothesis, inactivation of both alleles of the gene is required for carcinogenesis: the first mutation is inherited, the second is acquired during lifetime (18). Haploinsufficiency is not necessarily accompanied by disruption of cellular functions. For instance, the expression of menin and its messenger molecules (mixed lineage leukemia (MLL), p27 and p18) in the pancreas and pituitary gland does not differ in MEN1+/− and WT mice (19).
At the same time, in 2001 it was already shown by Crabtree et al. (20) that homozygotic inactivation of menin in mice results in embryo loss, with some fetuses developing gross malformations of the skull and facial bones. MEN1 deletion can result in perinatal death, development of cleft lip or other cranial malformations associated with disorders of bone mineralization; additionally, menin deficiency leads to anomalies of rib development (20, 21, 22). Since the skull and facial bones are formed by intramembranous ossification, further questions arose regarding the role of menin in the differentiation of multipotent MSCs into osteoblast lineage cells and in the differentiation of osteoblasts.
Later experiments used mice with MEN1 and have repeatedly confirmed that menin participates in the regulation of normal osteogenesis and bone mass gain (7). Trabecular bone volume and cortical bone thickness are significantly lower in osteoblast Men1 knockout mice (Men1−/−) compared to menin overexpression mice (7). Men1−/− mice have fewer osteoblasts and osteoclasts, lower mineral apposition rate, and a higher number of osteocytes (7). At the same time, transgenic mice with hyperexpression of menin display the opposite phenotype with an increased number of osteoblasts, mineral apposition rate and bone mass in comparison with WT mice (7).
In agreement with a study by Kanazava et al., Liuet al. observed a decrease in bone formation in 12-month-old female mice. However, in middle-aged mice, the authors show that selective Men1 deletion in the osteoblastic lineage in three distinct mouse lines does not alter bone formation. In contrast to Kanazawa et al., in primary uncommitted mesenchymal stromal cells of young mice with an efficient deletion of Men1, there were no differences in osteoblast proliferation and their differentiation potential. No major difference was evident in either Smad1/5/8 phosphorylation or Smad3 phosphorylation upon BMP2 and TGFβ stimulation, respectively. These differences may be related to different methodologies in these studies as well as that primary cells analyzed by authors were received from the different aged mice (8).
This may be in part due to the disruption of intracellular interactions between osteocytes and osteoblasts. Changes characteristic for menin haploinsufficiency manifest in the bone tissue even if the mutation only affects osteocytes without disrupting the function of menin in osteoblasts. Presumably, this is due to a change in the osteocytic expression of the C-X-C motif chemokine 10, a soluble mediator which promotes osteoclastogenesis (23).
Menin and transcription factors
Menin can interact with multiple regulatory molecules, including JunD, NF-κB, Smad3 and MLL (12, 24, 25). It is involved in various cellular processes such as transcriptional regulation, DNA replication and repair and cell cycle (26). Although much is known about the effects of menin in different tissues, the data on the role of menin in osteogenic cells are limited.
Menin and NFκB
NFκB is a transcription factor that functions as an important regulator of apoptosis and proto-oncogene. In intact cells, NFκB is predominantly localized in the cytoplasm in an inactive form, bound to the inhibitor protein IkB. In response to certain stimuli (ionizing radiation, mitogens, growth factors, cytokines and others) IkB is phosphorylated, and NFκB is translocated into the nucleus (26, 27).
NFκB complexes are represented by homo- and heterodimers of transcription factors p50, p52, p65 (RelA), c-Rel and RelB. p50, p52 and p65 directly interact with menin in vivo and in vitro. p65 activates transcription in NFκB-associated sites, while p50 and p52 suppress transcription by competitively binding to these respective sites (27). Menin also suppresses p65-mediated transcriptional activation of NF-κB sites in a dose-dependent manner; in this case, menin insufficiency can lead to oncogenesis (27).
Receptor activator of NFκB and its ligand (RANK/RANKL) system presents a major regulatory pathway of bone metabolism. RANK/RANKL are responsible for the differentiation and maturation of osteoclasts, which are required for the bone resorption phase of bone remodeling (28). Thus, mutations in the menin gene may lead to transcriptional activation of NFκB-associated sites; that can, presumably, mimic the intensive functioning of the RANK/RANKL system, increasing bone resorption.
Menin and JunD
JunD is a transcription factor from the activator protein 1 family. It is involved in the suppression of cell proliferation (29, 30). Menin and JunD interact via the C-terminus of menin and the transactivation domain located at the N-terminus of JunD.
Menin inhibits JunD-induced transcription suppression in two ways. First, it interacts with histone deacetylases, mSin3A in particular (30, 31). Secondly, it inhibits c-Jun N-terminal kinase, which activates JunD by phosphorylation in the S90, S100 and T117 positions (32, 33).
The fact that menin, an oncosuppressor, inhibits the activity of another proliferation inhibitor – JunD – may seem puzzling. However, JunD acts as a proliferation inhibitor only in complex with menin; without it, JunD activates proliferation instead. That being said, JunD mostly exists in the form of a JunD-menin complex (34, 35).
Two isoforms of JunD are currently known: the full-length JunD-FL and the truncated ΔJunD. Transcription of these isoforms begins at two different start codons of the same mRNA. Menin suppresses the transcriptional activity of JunD-FL, but not ΔJunD, so only the full-length isoform is a functional partner of menin (35).
Little is known about the specific endogenous targets affected during the suppression of cell proliferation by the menin/JunD complex. In bone metabolism, JunD activates osteoblast differentiation and their subsequent mineralization. Excessive expression of menin suppresses these processes (and inhibits alkaline phosphatase (ALP) activity) (36). Naito et al. studied the menin-JunD interactions in regard to osteoblast differentiation using the MC3T3-E1 mouse osteoblastic cell line. Immunoblotting was used to measure JunD expression, which gradually increased during osteoblast differentiation. Stable expression of JunD enhanced the expression of differentiation markers, Runx2, type 1 collagen, and increased osteocalcin and alkaline phosphatase activity and mineralization. In MC3T3-E1 cells with artificially reduced menin expression, JunD levels were significantly increased. JunD overexpression increased the transcriptional activity of AP-1, while inhibition of menin expression reduced it. In turn, menin suppressed JunD-induced transcription activity and inhibited ALP activity in the MC3T3-E1 osteoblasts (36). Kaji et al., who also showed that menin suppresses the maturation of osteoblasts (6), obtained similar data. This is confirmed by the fact that JunD-knockout mice have higher bone mass and trabecular number, and display higher osteoblast activity (37). Thus, according to the literature data, menin suppresses osteoblast maturation, in part, by inhibiting the anabolic effect of JunD on the bone tissue.
Menin and Runx2
Runx2 is a key transcription factor for osteoid cells, expressed in MSCs, osteoblasts and chondrocytes. Runx2 is required for osteoblast differentiation and proliferation of osteoprogenitor cells (where it can work in conjunction with the Wnt signaling pathway). In addition, Runx2 induces the proliferation of MSCs and their transition into osteoid cells (37, 38).
Bone morphogenetic protein 2 (BMP-2) belongs to the transforming growth factor β (TGF-β) superfamily, which plays a key role in osteoblast differentiation. Inactivation of menin by antisense oligonucleotide sequences suppresses the BMP-induced ALP activity and the expression of type 1 collagen, Runx2 and osteocalcin. Adipogenesis and chondrogenesis, in which BMP-2 is also involved, remain unaffected (39).
Menin/Runx2 are important not only during the early phases of osteoblast differentiation; they participate in the inhibition of late stages of the process as well. Menin interacts with Runx2 in MSCs, but not in differentiated osteoblasts. Menin suppression of Runx2-induced transcription leads to the inhibition of late stages of osteoblast differentiation (40).
Menin and histone modifiers
Menin is predominantly a nuclear protein. It can act as a scaffold protein, regulating gene transcription by coordinating various chromatin-associated proteins. For example, menin can interact with MLL (41) and enhancer of zeste homolog 2 (EZH2) (42) histone methyltransferases and histone deacetylases (43).
Menin and cyclin-dependent kinase inhibitors
Menin can directly regulate the expression of the cyclin-dependent kinase inhibitor (CDKI) genes Cdkn1b and Cdnk2c,which encode p27Kip1 and p18Ink4c, respectively (43, 44, 45, 46). P27Kip1 and p18Ink4c, in turn, play a key role in suppressing proliferation (47). To achieve this effect, menin attracts MLL to the promoter and coding regions of Cdkn1b and Cdkn2c, where MLL catalyzes histone H3 lysine 4 (H3K4) methylation, stimulating the transcription of these genes (43, 44, 45, 46). In mouse embryonic fibroblasts, loss of both MLL and menin leads to reduced expression of p27Kip1 and p18Ink4c and to reduced proliferative activity (43, 44, 45). Here, the role of tumor suppressor is played by CDKI.
Cdkn1b−/− mice display an increase in proliferative activity of osteoprogenitor cells with the formation of numerous large colonies of osteoblasts. At the same time, intensive proliferation is not followed by differentiation and tumor growth (47, 48). This suggests that menin mutations can also lead to increased proliferation of osteoid cells, but the experiments proving that Cdkn1b is indeed involved in menin function in bone are missing.
Menin and homeobox genes
The nature of interaction between menin and homeobox genes (Hox genes, highly concerned DNA sequences required during prenatal development) is ambiguous.
On the one hand, menin acts as an oncogenic transcription coregulator of Hox genes in leukemia, binding to various homeobox genes during hematopoiesis and myeloid transformation (49). On the other hand, menin can also interact with Hox genes as a tumor suppressor, for example, in parathyroid tumors (49, 50). Therefore, the direction of this interaction depends on the specific cell type in question.
Hox genes control the spatial patterning of the developing embryo through multicomponent transcription factors. These are not only limited to transcriptases but also include transcription coregulators. Since coregulators can inhibit transcription as well as promote it, Hox genes simultaneously inhibit the expression of ‘old’ morphogenetic genes and promote the expression of the ‘new’ ones. Hox genes are important for the development of the head-tail axis in animals with bilateral symmetry, including humans, and skeleton development as a part of it. These genes retain their function in the adult organism. For instance, Hox11 is expressed in the MSCs and in the areas where bone healing occurs (i.e. at fracture sites) (51). In osteoblast culture, upregulation of Msh homeobox 2 corresponds to an increased cell proliferation, migration and osteogenic differentiation (52). To date, no studies on the interaction between menin and these homeobox genes have been published.
Menin, pleiotrophin and Polycomb group proteins
Pleiotrophin (PTN) is a growth factor, which is richly expressed in many solid carcinomas (53). In lung epithelial cells, menin inhibits the transcription of the PTN gene by recruiting Polycomb group proteins (PcG) and inducing histone H3 lysine 27 (H3K27) methylation (53, 54). In this case, loss of menin increases PTN expression (53, 54, 55).
PcG genes encode a family of proteins responsible for chromatin remodeling, in particular, the polycomb repressive complex 2, which contains the EZH2 methyltransferase and its regulatory protein, SUZ12 (56, 57). EZH2 is a chromatin-associated protein with a conserved SET domain, which performs H3K27 methylation. This, in turn, leads to chromatin condensation and inhibition of transcription (58).
Menin, therefore, binds to the promoter region of the PTN gene and, together with EZH2, performs H3K27 methylation, suppresses PTN and inhibits proliferation (54). Without menin, EZH2 and SUZ12 cannot bind to PTN, and the intensity of H3K27 methylation is reduced.
In osteogenesis, EZH2 plays an ambiguous role: it inhibits the differentiation of MSCs into the osteogenic lineage, and at the same time enhances the proliferation of osteoprogenitor cells (59, 60). Given that menin regulates EZH2, it can be assumed that this methyltransferase is one of the molecules responsible for the development of bone disease in MEN1 mutation cases.
Menin and HDAC3
Cyclin B2 is one of the molecules that control the G2/M cell cycle transition. It is encoded by the Ccnb2 gene. By binding to the promoter region of Ccnb2, menin inhibits its transcription. Additionally, it interacts with histone deacetylase 3 (HDAC3), which is found in the same locus; that reduces the intensity of H3 acetylation. Cultured cells with menin mutations analogous to those found in MEN1 patients display no inhibition of histone H3 acetylation of the Ccnb2 locus and, therefore, no inhibition of Ccnb2 transcription. Since G2/M transition is facilitated by cyclin B2, it occurs faster in the cells with menin mutations (43).
Apparently, Ccnb2 also participates in bone metabolism: it has been shown to have a richer expression in bone calluses of osteoporotic mice compared to the WT mice (60). It can be assumed that patients with osteoporosis in MEN1 will display similar features; perhaps, they would be even more pronounced due to the lack of functional menin.
Menin and nuclear receptors
The nuclear receptor PPARγ is predominantly expressed in adipose tissue, the large intestine and macrophages, but can be found in other tissues as well. Regulation of carbohydrate and lipid metabolism and activation of M2 macrophages are considered its classical effects. Menin interacts with the activation domain of PPARγ in a ligand-independent manner and uses the H3K4 methyltransferase to increase the expression of the receptor. PPARγ inhibition is believed to trigger the development of lipomas in MEN1 patients (61).
PPARγ is also expressed in bone tissue (62), where it is responsible for the conversion of osteoblasts into adipocyte-like cells (63, 64) and their differentiation (65). Different PPARγ ligands can affect bone metabolism; for example, rosiglitazone inhibits the expression of Runx2/Cba1, the transcription factor required for osteoblast differentiation and synthesis of osteocalcin (66). In animals, this leads to loss of BMD in various parts of the skeleton (67). Endogenous activators of PPARγ display similar properties regarding bone formation (68). Also, according to some research, PPARγ is able to induce apoptosis in cells of the osteogenic line (69). Moreover, PPARγ activation inhibits bone resorption: as such, ciglitazone inhibits osteoclastogenesis in a dose-dependent manner, apparently due to the effect on osteoprotegerin (70). Therefore, it can be argued that MEN1 mutations may lead to increased osteoclastogenic activity through PPARγ inhibition.
WT menin also directly promotes the transcription of vitamin D receptor (VDR) and estrogen receptor alpha (ERα), two other steroid receptors crucial for bone formation and remodeling (71). VDR, in turn, regulates the activity of p27Kip1 and p18Ink4c (their potential role in bone metabolism has been discussed earlier) (72, 73). However, despite the undoubted importance of vitamin D in bone metabolism, no data have been published regarding the effect of MEN1 mutations on the implementation of VDR-mediated and ERα-mediated effects.
Menin and intracellular signaling pathways
Menin can inhibit cell proliferation through several intracellular signaling pathways.
Menin and the transforming growth factor β signaling pathway
TGF-β is richly expressed in the bone matrix; there, it is stored in an inactive form and can be activated by the cells of the bone microenvironment (74). The TGF-β-mediated pathway inhibits the proliferative and transcriptional activity of the cell through specific Smad signal transductors, such as Smad2, Smad3 and others. Receptor-mediated phosphorylation of Smad2 or Smad3 allows them to bind to Smad4. The resulting complex translocates into the nucleus, where it activates the transcription of target genes (75). Here, menin activates TGF-β and Smad3-induced transcription by inhibiting the binding of Smad3 to the transcription regulatory binding sites of the DNA (76). Menin inactivation inhibits the TGF-β-dependent signaling pathway, which, in turn, leads to excessive cell proliferation and tumor growth. Smad3 appears to be important for osteoclast formation; its inactivation inhibits osteoclastogenesis (77).
Mice with menin deletions in MSCs or osteoblast progenitor cells develop ossifying fibromas of the jaw. These tumors are characterized by lower BMD, higher bone resorption and a more pronounced stromal component. The stromal cells isolated from these masses display higher TGF-β expression (78).
Interestingly, TGF-β increased ALP expression and osteoblast mineralization in a study by Sowa et al., even though it typically inhibits osteoblast differentiation (79).
Additionally, it is through Smad that menin drives MSC differentiation toward the osteogenic or myogenic lineage. BMP-2 induces the ectopic formation of bone and cartilage in nonskeletal tissues. It is crucial for bone formation and osteoblast differentiation (74). In response to BMP-2, menin increases Smad1/5 transcriptional activity. This directs the differentiation of MSCs into the osteoblast lineage. Moreover, when influenced by TGF-β1, menin increases the inhibition of Smad3/TGF-β1-mediated myogenic differentiation of MSCs. In case of menin deficiency, MSCs primarily differentiate into the myogenic lineage (80). Menin is also a positive regulator of miR-26a, a miRNA that inhibits SMAD1 expression during the osteoblastic differentiation of MSCs (81).
Menin and Wnt/β-catenin signaling pathway
The classic Wnt/β-catenin signaling pathway plays a crucial role in bone formation. It mediates the antiapoptotic effect of parathyroid hormone on osteoblasts and participates in their BMP-induced differentiation (82, 83).
The C-terminus of menin directly interacts with β-catenin and activates its ubiquitin-mediated degradation. Additionally, menin suppresses the expression of β-catenin and its target genes, thus displaying an antiproliferative effect (84).
Administration of antisense nucleotides can inhibit menin, which prevents the BMP-2- and β-catenin-mediated increase in Runx2 and ALP expression. Thus, menin can affect BMP-induced osteoblast differentiation (85).
Conclusion perspectives of studying the function of menin in bone tissue
The reviewed limited experimental data obtained in transformed mammalian cells suggest a general increase in osteoclast activity following MEN1 mutations. Briefly, available information on the effect of menin on bone metabolism is shown in Figs 2 and 3. However, specific molecular mechanisms underlying the more severe PHPT-associated osteoporosis in MEN1 patients have not yet been studied, despite the fact that this research could potentially introduce a dramatic change to the existing therapeutic strategies.
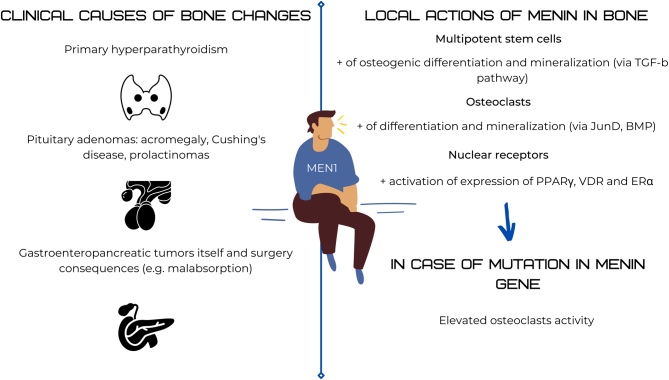
Figure 2.
Schematic overview of menin and bone interactions.
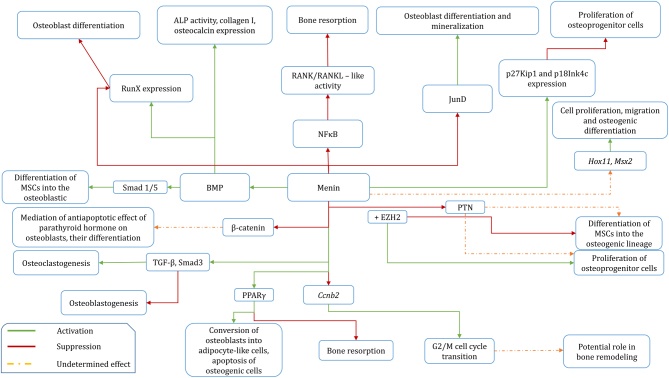
Figure 3.
Schematic overview of molecular interaction of menin in the aspect of bone metabolism. ALP, alkaline phosphatase; BMP, bone morphogenic protein; Ccnb2, cyclin B2 gene; EZH2, enhancer of zeste homolog 2; MSCs, multipotent stem cells; PPARγ, peroxisome proliferator-activated receptor γ; PTN, pleiotrophin; TGF-β, transforming growth factor β.
The lack of sufficient knowledge in this area is explained by the difficulties in the generation of adequate in vitro models based on patients’ cells. Manifestation age of MEN1 is highly variable, as are the spectrum and severity of clinical signs and symptoms. This is likely due to a combination of individual genetic and epigenetic factors. Additionally, the genetic reasons for such a variety of possible clinical manifestations are not limited to MEN1 mutations but are dictated by the genotype as a whole: mutated MEN1 is inherited along with a plethora of other gene combinations that can affect its role in different tissues.
Animal models are not sufficiently pure and detailed to study the role of menin in bone metabolism due to their multifactorial nature. A model based on human cells with MEN1mutation seems to present an optimal solution for the development of new effective therapeutic approaches. Such a model should account for the individual features of the genome and would allow to pinpoint the changes introduced to osteogenic cell formation and functioning by MEN1 mutations.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work was supported by Russian Science Foundation, agreement № 20-75-00077.
Author contribution statement
Gorbacheva A M, Eremkina A K, Goliusova D V, Krupinova J A and Mokrysheva N G contributed to the analysis of the literature data and to the writing of the manuscript.
References
- 1.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi MLet al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). Journal of Clinical Endocrinology and Metabolism 2012972990–3011. ( 10.1210/jc.2012-1230) [DOI] [PubMed] [Google Scholar]
- 2.Eller-Vainicher C, Chiodini I, Battista C, Viti R, Mascia ML, Massironi S, Peracchi M, D’Agruma L, Minisola S, Corbetta Set al. Sporadic and MEN1-related primary hyperparathyroidism: differences in clinical expression and severity. Journal of Bone and Mineral Research 2009241404–1410. ( 10.1359/jbmr.090304) [DOI] [PubMed] [Google Scholar]
- 3.Lourenco DMJ, Coutinho FL, Toledo RA, Montenegro FLM, Correia-Deur JEM, Toledo SPA. Early-onset, progressive, frequent, extensive, and severe bone mineral and renal complications in multiple endocrine neoplasia type 1-associated primary hyperparathyroidism. Journal of Bone and Mineral Research 2010252382–2391. ( 10.1002/jbmr.125) [DOI] [PubMed] [Google Scholar]
- 4.Silva AM, Vodopivec D, Christakis I, Lyons G, Wei Q, Waguespack SG, Petak SM, Grubbs E, Lee JE, Perrier N. Operative intervention for primary hyperparathyroidism offers greater bone recovery in patients with sporadic disease than in those with multiple endocrine neoplasia type 1-related hyperparathyroidism. Surgery 2017161107–115. ( 10.1016/j.surg.2016.06.065) [DOI] [PubMed] [Google Scholar]
- 5.Lourenco DMJ, Toledo RA, Mackowiak II, Coutinho FL, Cavalcanti MG, Correia-Deur JEM, Montenegro F, Siqueira SAC, Margarido LC, Machado MCet al. Multiple endocrine neoplasia type 1 in Brazil: MEN1 founding mutation, clinical features, and bone mineral density profile. European Journal of Endocrinology 2008159259–274. ( 10.1530/EJE-08-0153) [DOI] [PubMed] [Google Scholar]
- 6.Kaji H.Menin and bone metabolism. Journal of Bone and Mineral Metabolism 201230381–387. ( 10.1007/s00774-012-0355-3) [DOI] [PubMed] [Google Scholar]
- 7.Kanazawa I, Canaff L, Abi Rafeh J, Angrula A, Li J, Riddle RC, Boraschi-Diaz I, Komarova SV, Clemens TL, Murshed Met al. Osteoblast menin regulates bone mass in vivo. Journal of Biological Chemistry 20152903910–3924. ( 10.1074/jbc.M114.629899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu P, Lee S, Knoll J, Rauch A, Ostermay S, Luther J, Malkusch N, Lerner UH, Zaiss MM, Neven Met al. Loss of menin in osteoblast lineage affects osteocyte-osteoclast crosstalk causing osteoporosis. Cell Death and Differentiation 201724672–682. ( 10.1038/cdd.2016.165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsimbri P.The biology of normal bone remodelling. European Journal of Cancer Care 201726 e12740. ( 10.1111/ecc.12740) [DOI] [PubMed] [Google Scholar]
- 10.Lu Z, Chiu J, Lee LR, Schindeler A, Jackson M, Ramaswamy Y, Dunstan CR, Hogg PJ, Zreiqat H. Reprogramming of human fibroblasts into osteoblasts by insulin-like growth factor-binding protein 7. Stem Cells Translational Medicine 20209403–415. ( 10.1002/sctm.19-0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukada T, Nagamura Y, Ohkura N. MEN1 gene and its mutations: basic and clinical implications. Cancer Science 2009100209–215. ( 10.1111/j.1349-7006.2008.01034.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendy GN, Kaji H, Canaff L. Cellular functions of menin. Advances in Experimental Medicine and Biology 200966837–50. ( 10.1007/978-1-4419-1664-8_4) [DOI] [PubMed] [Google Scholar]
- 13.Mokrysheva NG, Mirnaya SS, Dobreva EA, Maganeva IS, Kovaleva EV, Krupinova JA, Kryukova IV, Tevosyan LK, Lukyanov SV, Markina NVet al. Primary hyperparathyroidism in Russia according to the registry. Problemy Endokrinologii 201965300–310. ( 10.14341/probl10126) [DOI] [PubMed] [Google Scholar]
- 14.Lamas C, Navarro E, Casteras A, Portillo P, Alcazar V, Calatayud M, Alvarez-Escola C, Sastre J, Boix E, Forga Let al. MEN1-associated primary hyperparathyroidism in the Spanish registry: clinical characteristics and surgical outcomes. Endocrine Connections 201981416–1424. ( 10.1530/EC-19-0321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong J, Wang O, Nie M, Shi J, Hu Y, Jiang Y, Li M, Xia W, Meng X, Xing X. Clinical and genetic analysis of multiple endocrine neoplasia type 1-related primary hyperparathyroidism in Chinese. PLoS ONE 201611 e0166634. ( 10.1371/journal.pone.0166634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witteveen JE, van Thiel S, Romijn JA, Hamdy NAT. Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. European Journal of Endocrinology 2013168R45–R53. ( 10.1530/EJE-12-0528) [DOI] [PubMed] [Google Scholar]
- 17.Norton JA, Venzon DJ, Berna MJ, Alexander HR, Fraker DL, Libutti SK, Marx SJ, Gibril F, Jensen RT. Prospective study of surgery for primary hyperparathyroidism (HPT) in multiple endocrine neoplasia-type 1 and Zollinger-Ellison syndrome: long-term outcome of a more virulent form of HPT. Annals of Surgery 2008247501–510. ( 10.1097/SLA.0b013e31815efda5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knudson AGJ.Mutation and cancer: statistical study of retinoblastoma. PNAS 197168820–823. ( 10.1073/pnas.68.4.820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taguchi R, Yamada M, Horiguchi K, Tomaru T, Ozawa A, Shibusawa N, Hashimoto K, Okada S, Satoh T, Mori M. Haploinsufficient and predominant expression of multiple endocrine neoplasia type 1 (MEN1)-related genes, MLL, p27Kip1 and p18Ink4C in endocrine organs. Biochemical and Biophysical Research Communications 2011415378–383. ( 10.1016/j.bbrc.2011.10.077) [DOI] [PubMed] [Google Scholar]
- 20.Crabtree JS, Scacheri PC, Ward JM, Garrett-Beal L, Emmert-Buck MR, Edgemon KA, Lorang D, Libutti SK, Chandrasekharappa SC, Marx SJet al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. PNAS 2001981118–1123. ( 10.1073/pnas.98.3.1118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engleka KA, Wu M, Zhang M, Antonucci NB, Epstein JA. Menin is required in cranial neural crest for palatogenesis and perinatal viability. Developmental Biology 2007311524–537. ( 10.1016/j.ydbio.2007.08.057) [DOI] [PubMed] [Google Scholar]
- 22.Maraghelli D, Giusti F, Marini F, Brandi ML. Bone tissue and mineral metabolism in hereditary endocrine tumors: clinical manifestations and genetic bases. Orphanet Journal of Rare Diseases 202015 102. ( 10.1186/s13023-020-01380-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan AA, Hanley DA, Rizzoli R, Bollerslev J, Young JEM, Rejnmark L, Thakker R, D’Amour P, Paul T, Van Uum Set al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and International Consensus. Osteoporosis International 2017281–19. ( 10.1007/s00198-016-3716-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendy GN, Kaji H, Sowa H, Lebrun JJ, Canaff L. Menin and TGF-beta superfamily member signaling via the Smad pathway in pituitary, parathyroid and osteoblast. Hormone and Metabolic Research 200537375–379. ( 10.1055/s-2005-870152) [DOI] [PubMed] [Google Scholar]
- 25.Kaji H, Canaff L, Hendy GN. Role of menin in bone development. Advances in Experimental Medicine and Biology 200966859–67. ( 10.1007/978-1-4419-1664-8_6) [DOI] [PubMed] [Google Scholar]
- 26.Wu T, Hua X. Menin represses tumorigenesis via repressing cell proliferation. American Journal of Cancer Research 20111726–739. [PMC free article] [PubMed] [Google Scholar]
- 27.Heppner C, Bilimoria KY, Agarwal SK, Kester M, Whitty LJ, Guru SC, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJet al. The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation. Oncogene 2001204917–4925. ( 10.1038/sj.onc.1204529) [DOI] [PubMed] [Google Scholar]
- 28.Kenkre JS, Bassett J. The bone remodelling cycle. Annals of Clinical Biochemistry 201855308–327. ( 10.1177/0004563218759371) [DOI] [PubMed] [Google Scholar]
- 29.Karin M, Liu Zg, Zandi E. AP-1 function and regulation. Current Opinion in Cell Biology 19979240–246. ( 10.1016/S0955-0674(9780068-3) [DOI] [PubMed] [Google Scholar]
- 30.Agarwal SK, Guru SC, Heppner C, Erdos MR, Collins RM, Park SY, Saggar S, Chandrasekharappa SC, Collins FS, Spiegel AMet al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 199996143–152. ( 10.1016/s0092-8674(0080967-8) [DOI] [PubMed] [Google Scholar]
- 31.Kim H, Lee JE, Cho EJ, Liu JO, Youn HD. Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Research 2003636135–6139. [PubMed] [Google Scholar]
- 32.Huang J, Gurung B, Wan B, Matkar S, Veniaminova NA, Wan K, Merchant JL, Hua X, Lei M. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature 2012482542–546. ( 10.1038/nature10806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng Z, Ma J, Hua X. Epigenetic regulation by the menin pathway. Endocrine-Related Cancer 201724T147–T159. ( 10.1530/ERC-17-0298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal SK, Novotny EA, Crabtree JS, Weitzman JB, Yaniv M, Burns AL, Chandracekharappa SC, Collins FS, Spiegel AM, Marx SJ. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. PNAS 200310010770–10775. ( 10.1073/pnas.1834524100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yazgan O, Pfarr CM. Differential binding of the Menin tumor suppressor protein to JunD isoforms. Cancer Research 200161916–920. [PubMed] [Google Scholar]
- 36.Naito J, Kaji H, Sowa H, Hendy GN, Sugimoto T, Chihara K. Menin suppresses osteoblast differentiation by antagonizing the AP-1 factor, JunD. Journal of Biological Chemistry 20052804785–4791. ( 10.1074/jbc.M408143200) [DOI] [PubMed] [Google Scholar]
- 37.Kawamata A, Izu Y, Yokoyama H, Amagasa T, Wagner EF, Nakashima K, Ezura Y, Hayata T, Noda M. JunD suppresses bone formation and contributes to low bone mass induced by estrogen depletion. Journal of Cellular Biochemistry 20081031037–1045. ( 10.1002/jcb.21660) [DOI] [PubMed] [Google Scholar]
- 38.Komori T.Molecular mechanism of Runx2-dependent bone development. Molecules and Cells 202043168–175. ( 10.14348/molcells.2019.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sowa H, Kaji H, Canaff L, Hendy GN, Tsukamoto T, Yamaguchi T, Miyazono K, Sugimoto T, Chihara K. Inactivation of menin, the product of the multiple endocrine neoplasia type 1 gene, inhibits the commitment of multipotential mesenchymal stem cells into the osteoblast lineage. Journal of Biological Chemistry 200327821058–21069. ( 10.1074/jbc.M302044200) [DOI] [PubMed] [Google Scholar]
- 40.Sowa H, Kaji H, Hendy GN, Canaff L, Komori T, Sugimoto T, Chihara K. Menin is required for bone morphogenetic protein 2- and transforming growth factor beta-regulated osteoblastic differentiation through interaction with Smads and Runx2. Journal of Biological Chemistry 200427940267–40275. ( 10.1074/jbc.M401312200) [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Doepner M, Hojnacki T, Feng Z, Katona BW, He X, Ma J, Cao Y, Busino L, Zhou Fet al. Disruption of the menin-MLL interaction triggers menin protein degradation via ubiquitin-proteasome pathway. American Journal of Cancer Research 201991682–1694. [PMC free article] [PubMed] [Google Scholar]
- 42.Gherardi S, Ripoche D, Mikaelian I, Chanal M, Teinturier R, Goehrig D, Cordier-Bussat M, Zhang CX, Hennino A, Bertolino P. Menin regulates Inhbb expression through an Akt/Ezh2-mediated H3K27 histone modification. Biochimica et Biophysica Acta: Gene Regulatory Mechanisms 20171860427–437. ( 10.1016/j.bbagrm.2017.02.003) [DOI] [PubMed] [Google Scholar]
- 43.Wu T, Zhang X, Huang X, Yang Y, Hua X. Regulation of cyclin B2 expression and cell cycle G2/m transition by menin. Journal of Biological Chemistry 201028518291–18300. ( 10.1074/jbc.M110.106575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. PNAS 200510214659–14664. ( 10.1073/pnas.0503484102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, Schnepp RW, Krankel C, Livolsi VA, Gibbs Det al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. PNAS 2005102749–754. ( 10.1073/pnas.0408836102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnepp RW, Chen YX, Wang H, Cash T, Silva A, Diehl JA, Brown E, Hua X. Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Research 2006665707–5715. ( 10.1158/0008-5472.CAN-05-4518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zindy F, Nilsson LM, Nguyen L, Meunier C, Smeyne RJ, Rehg JE, Eberhart C, Sherr CJ, Roussel MF. Hemangiosarcomas, medulloblastomas, and other tumors in Ink4c/p53-null mice. Cancer Research 2003635420–5427. [PubMed] [Google Scholar]
- 48.Drissi H, Hushka D, Aslam F, Nguyen Q, Buffone E, Koff A, Wijnen A, Lian JB, Stein JL, Stein GS. The cell cycle regulator p27Kip1 contributes to growth and differentiation of osteoblasts. Cancer Research 1999593705–3711. [PubMed] [Google Scholar]
- 49.Chen YX, Yan J, Keeshan K, Tubbs AT, Wang H, Silva A, Brown EJ, Hess JL, Pear WS, Hua X. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. PNAS 20061031018–1023. ( 10.1073/pnas.0510347103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen HC, Rosen JE, Yang LM, Savage SA, Burns AL, Mateo CM, Agarwal SK, Chandrasekharappa SC, Spiegel AM, Collins FSet al. Parathyroid tumor development involves deregulation of homeobox genes. Endocrine-Related Cancer 200815267–275. ( 10.1677/ERC-07-0191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rux DR, Wellik DM. Hox genes in the adult skeleton: novel functions beyond embryonic development. Developmental Dynamics 2017246310–317. ( 10.1002/dvdy.24482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Chen B, Li Y. microRNA-203 promotes proliferation, differentiation, and migration of osteoblasts by upregulation of Msh homeobox 2. Journal of Cellular Physiology 201923417639–17648. ( 10.1002/jcp.28387) [DOI] [PubMed] [Google Scholar]
- 53.Perez-Pinera P, Chang Y, Deuel TF. Pleiotrophin, a multifunctional tumor promoter through induction of tumor angiogenesis, remodeling of the tumor microenvironment, and activation of stromal fibroblasts. Cell Cycle 200762877–2883. ( 10.4161/cc.6.23.5090) [DOI] [PubMed] [Google Scholar]
- 54.Gao SB, Feng ZJ, Xu B, Wu Y, Yin P, Yang Y, Hua X, Jin GH. Suppression of lung adenocarcinoma through menin and polycomb gene-mediated repression of growth factor pleiotrophin. Oncogene 2009284095–4104. ( 10.1038/onc.2009.273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.La P, Schnepp RW, D Petersen C, C Silva A, Hua X. Tumor suppressor menin regulates expression of insulin-like growth factor binding protein 2. Endocrinology 20041453443–3450. ( 10.1210/en.2004-0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ringrose L, Paro R. Polycomb/trithorax response elements and epigenetic memory of cell identity. Development 2007134223–232. ( 10.1242/dev.02723) [DOI] [PubMed] [Google Scholar]
- 57.Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell 2007128735–745. ( 10.1016/j.cell.2007.02.009) [DOI] [PubMed] [Google Scholar]
- 58.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science 20022981039–1043. ( 10.1126/science.1076997) [DOI] [PubMed] [Google Scholar]
- 59.Dudakovic A, Camilleri ET, Paradise CR, Samsonraj RM, Gluscevic M, Paggi CA, Begun DL, Khani F, Pichurin O, Ahmed FSet al. Enhancer of zeste homolog 2 (Ezh2) controls bone formation and cell cycle progression during osteogenesis in mice. Journal of Biological Chemistry 201829312894–12907. ( 10.1074/jbc.RA118.002983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C, Yu T, Tan L, Cheng J. Bioinformatics analysis of gene expression profile in callus tissues of osteoporotic phenotype mice induced by osteoblast-specific Krm2 overexpression. International Journal of Rheumatic Diseases 2016191263–1271. ( 10.1111/1756-185X.12840) [DOI] [PubMed] [Google Scholar]
- 61.Dreijerink KMA, Varier RA, van Beekum O, Jeninga EH, Höppener JWM, Lips CJM, Kummer JA, Kalkhoven E, Timmers HTM. The multiple endocrine neoplasia type 1 (MEN1) tumor suppressor regulates peroxisome proliferator-activated receptor gamma-dependent adipocyte differentiation. Molecular and Cellular Biology 2009295060–5069. ( 10.1128/MCB.01001-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 1996137354–366. ( 10.1210/endo.137.1.8536636) [DOI] [PubMed] [Google Scholar]
- 63.Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 20051461226–1235. ( 10.1210/en.2004-0735) [DOI] [PubMed] [Google Scholar]
- 64.Kawaguchi H, Akune T, Yamaguchi M, Ohba S, Ogata N, Chung UI, Kubota N, Terauchi Y, Kadowaki T, Nakamura K. Distinct effects of PPARgamma insufficiency on bone marrow cells, osteoblasts, and osteoclastic cells. Journal of Bone and Mineral Metabolism 200523275–279. ( 10.1007/s00774-005-0599-2) [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Jin D, Xie W, Wen L, Chen W, Xu J, Ding J, Ren D. PPAR-γ and Wnt regulate the differentiation of MSCs into adipocytes and osteoblasts respectively. Current Stem Cell Research and Therapy 201813185–192. ( 10.2174/1574888X12666171012141908) [DOI] [PubMed] [Google Scholar]
- 66.Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. Journal of Cellular Biochemistry 199974357–371. () [DOI] [PubMed] [Google Scholar]
- 67.Sottile V, Seuwen K, Kneissel M. Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone) Calcified Tissue International 200475329–337. ( 10.1007/s00223-004-0224-8) [DOI] [PubMed] [Google Scholar]
- 68.Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Peroxisome proliferator-activated receptor-gamma ligands as bone turnover modulators. Expert Opinion on Investigational Drugs 200716195–207. ( 10.1517/13543784.16.2.195) [DOI] [PubMed] [Google Scholar]
- 69.Shan ZZ, Masuko-Hongo K, Dai SM, Nakamura H, Kato T, Nishioka K. A potential role of 15-deoxy-delta(12,14)-prostaglandin J2 for induction of human articular chondrocyte apoptosis in arthritis. Journal of Biological Chemistry 200427937939–37950. ( 10.1074/jbc.M402424200) [DOI] [PubMed] [Google Scholar]
- 70.Bendixen AC, Shevde NK, Dienger KM, Willson TM, Funk CD, Pike JW. IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor gamma 1. PNAS 2001982443–2448. ( 10.1073/pnas.041493198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dreijerink KMA, Varier RA, van Nuland R, Broekhuizen R, Valk GD, van der Wal JE, Lips CJM, Kummer JA, Timmers HTM. Regulation of vitamin D receptor function in MEN1-related parathyroid adenomas. Molecular and Cellular Endocrinology 20093131–8. ( 10.1016/j.mce.2009.08.020) [DOI] [PubMed] [Google Scholar]
- 72.Cheng HT, Chen JY, Huang YC, Chang HC, Hung WC. Functional role of VDR in the activation of p27Kip1 by the VDR/Sp1 complex. Journal of Cellular Biochemistry 2006981450–1456. ( 10.1002/jcb.20780) [DOI] [PubMed] [Google Scholar]
- 73.Saramäki A, Banwell CM, Campbell MJ, Carlberg C. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Research 200634543–554. ( 10.1093/nar/gkj460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning Det al. Regulation of osteogenic differentiation during skeletal development. Frontiers in Bioscience 2008132001–2021. ( 10.2741/2819) [DOI] [PubMed] [Google Scholar]
- 75.Massagué J.TGF-beta signal transduction. Annual Review of Biochemistry 199867753–791. ( 10.1146/annurev.biochem.67.1.753) [DOI] [PubMed] [Google Scholar]
- 76.Kaji H, Canaff L, Lebrun JJ, Goltzman D, Hendy GN. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. PNAS 2001983837–3842. ( 10.1073/pnas.061358098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gruber R, Roos G, Caballé-Serrano J, Miron R, Bosshardt DD, Sculean A. TGF-βRI kinase activity mediates Emdogain-stimulated in vitro osteoclastogenesis. Clinical Oral Investigations 2014181639–1646. ( 10.1007/s00784-013-1129-6) [DOI] [PubMed] [Google Scholar]
- 78.Lee S, Liu P, Tschaffon M, Tasdogan A, Wittig R, Frappart L, Knoll J, Bertolino P, Zhang C, Tuckermann J. Menin is a tumor suppressor in bone – a novel benign jaw tumor mouse model. Bone Abstracts 20165 118. ( 10.1530/boneabs.5.P118) [DOI] [Google Scholar]
- 79.Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K. Smad3 promotes alkaline phosphatase activity and mineralization of osteoblastic MC3T3-E1 cells. Journal of Bone and Mineral Research 2002171190–1199. ( 10.1359/jbmr.2002.17.7.1190) [DOI] [PubMed] [Google Scholar]
- 80.Aziz A, Miyake T, Engleka KA, Epstein JA, McDermott JC. Menin expression modulates mesenchymal cell commitment to the myogenic and osteogenic lineages. Developmental Biology 2009332116–130. ( 10.1016/j.ydbio.2009.05.555) [DOI] [PubMed] [Google Scholar]
- 81.Luzi E, Marini F, Tognarini I, Galli G, Falchetti A, Brandi ML. The regulatory network menin-microRNA 26a as a possible target for RNA-based therapy of bone diseases. Nucleic Acid Therapeutics 201222103–108. ( 10.1089/nat.2012.0344) [DOI] [PubMed] [Google Scholar]
- 82.Hartmann C.A Wnt canon orchestrating osteoblastogenesis. Trends in Cell Biology 200616151–158. ( 10.1016/j.tcb.2006.01.001) [DOI] [PubMed] [Google Scholar]
- 83.Kokabu S, Rosen V. BMP3 expression by osteoblast lineage cells is regulated by canonical Wnt signaling. FEBS Open Bio 20188168–176. ( 10.1002/2211-5463.12347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim B, Song TY, Jung KY, Kim SG, Cho EJ. Direct interaction of menin leads to ubiquitin-proteasomal degradation of β-catenin. Biochemical and Biophysical Research Communications 2017492128–134. ( 10.1016/j.bbrc.2017.08.011) [DOI] [PubMed] [Google Scholar]
- 85.Inoue Y, Hendy GN, Canaff L, Seino S, Kaji H. Menin interacts with β-catenin in osteoblast differentiation. Hormone and Metabolic Research 201143183–187. ( 10.1055/s-0030-1270527) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a