Abstract
Background
Cancer-related fatigue is one of the most important issues for patients, but research on this topic is sparse. This study aimed to determine the prevalence of fatigue in postoperative patients with papillary thyroid carcinoma (PTC) and to identify the clinical features associated with fatigue.
Methods
We conducted a cross-sectional study on 292 thyroid cancer survivors. Fatigue and quality of life were the study outcomes, measured using the Cancer Fatigue Scale (CFS) and the SF-36 version 2.0. Furthermore, correlations of demographic characteristics and hormonal data with the CFS scores were assessed by univariable and multivariable analyses.
Results
The prevalence of fatigue was 41.8% (95% CI: 36.1, 47.5). The CFS score was significantly correlated with the free T3 level (Pearson’s r = −0.123, 95% CI: −0.234, −0.008). Multiple regression analysis revealed that the free T3 level and having a job were significant predictors of the CFS score, with unstandardized regression coefficients of −2.52 (95% CI: −4.94, −0.09) and 2.85 (95% CI: 0.49, 5.20), respectively. The median Z-scores were negative for General Health (−0.28) and Vitality (−0.15) subscales of the SF-36. The CFS score was a significant predictor of summary scores of the SF-36. The free T3 level was significantly associated with the physical component summary score with an unstandardized coefficient of 3.20 (95% CI: 0.77, 5.63).
Conclusions
Fatigue was prevalent and associated with poor quality of life among PTC survivors. Thyroid functional status, particularly the level of free T3, may be worth to be considered in alleviating the burden.
Keywords: papillary thyroid cancer, fatigue, quality of life, thyroid hormone
Introduction
Differentiated thyroid carcinoma is the most common malignancy of the endocrine organs and is increasing in incidence (1). In the United States, the age-adjusted incidence of thyroid cancer increased from 3.6 per 100,000 in 1973 to 14.3 per 100,000 in 2009, while the age-adjusted mortality from differentiated thyroid carcinoma has remained unchanged (2, 3). The disease burden has followed the same trend in Japan. In 2017, the number of patients newly diagnosed with thyroid cancer was estimated to be 18,090, and its corresponding age-adjusted incidence was 10.8 per 100,000 (https://ganjoho.jp/reg_stat/index.html).
Since papillary thyroid carcinoma (PTC) accounts for >80% of all thyroid cancer cases, the disease burden poses paramount importance for society and individuals. Most patients with PTC have a good prognosis, whereas a few may experience recurrence or even death (4, 5). In addition to the oncologic events, subjective sequelae following diagnosis and treatment, such as quality of life or psychological burden, are essential concerns for cancer survivors (6, 7). Besides, labelling people with diagnoses may itself could be harmful to psychological well-being (8, 9). Some thoughtful clinicians may take these issues into consideration in daily practice, but few clinical researchers have addressed such ‘soft’ outcomes in patients with PTC.
The National Comprehensive Cancer Network defines cancer-related fatigue as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer and/or cancer treatment that is not proportional to recent activity and interferes with normal functioning (10, 11). Cancer-related fatigue is one of the most common adverse consequences of cancer diagnosis and treatment, with the estimated prevalence ranging from 17 to 99% depending on the definition and measurement of cancer-related fatigue, the mode and timing of cancer treatment, and the population (i.e. specific disease) (12, 13). Sawka et al. stated in their scoping literature review that long-term fatigue is a common problem among thyroid cancer survivors, with the prevalence ranging from 28 to 62% (14). They argued a need for more research on persistent post-treatment fatigue in thyroid cancer survivors, including studies examining the severity, prevalence, modifying factors, natural history, and associated impact on quality of life. One unresolved question is whether the fatigue is due to thyroid dysfunction. Patients with fatigue often ask, ‘Does this fatigue have something to do with my thyroid?’ Few studies have specifically addressed the relationship between biochemical data and fatigue in thyroid cancer survivors. A randomized controlled trial revealed that restoration of euthyroidism from thyroid-stimulating hormone (TSH) suppression therapy did not affect patient-reported outcomes, as measured by the Multidimensional Fatigue Index-20 (15). A cross-sectional study showed that the levels of free triiodothyronine (fT3) and free thyroxine (fT4) were not significantly associated with the Brief Fatigue Inventory score among thyroid cancer survivors (16). A retrospective longitudinal study found that the complaints, including fatigue or tiredness, of levothyroxine-treated patients were significantly related to a low concentration of fT3 (17).
The objectives of the present study were to determine the prevalence of fatigue among postoperative patients with PTC and to identify the clinical features associated with fatigue. Specifically, our four research questions are as follows: (i) What is the prevalence of fatigue among postoperative PTC patients without recurrence? (ii) Is there any relationship between fatigue and clinical characteristics, including thyroid function? (iii) Do PTC patients feel impaired in health-related quality of life (HR-QOL) compared with the general population? (iv) What is the magnitude of the association between fatigue and HR-QOL after controlling for other clinical parameters?
Materials and methods
Design and patients
We conducted a cross-sectional study of patients with PTC who visited the outpatient clinic for postoperative follow-up between June 2018 and June 2019. Inclusion criteria were patients who underwent initial treatment at our hospital, had no signs of persistent or recurrent disease, and were 16 years or older at the time of consent. Patients who had persistent/recurrent PTC or a history of other malignant diseases and those deemed unsuitable as study subjects were excluded.
Observations and measurements
At the time of the outpatient follow-up, we asked the participants to fill out two questionnaires, the Cancer Fatigue Scale (CFS) and SF-36 version 2.0, to assess clinical fatigue and HR-QOL, respectively.
The CFS is a 15-item scale used to assess fatigue in cancer patients at the time of the survey (‘right now’), with total scores ranging from 0 (no fatigue) to 60 (maximum fatigue). It has three subscales, physical, affective, and cognitive, with maximum scores of 28, 16, and 16, respectively. Okuyama et al. demonstrated the validity and reliability of the CFS (18). They set a cut-off value of 19, which had a sensitivity of 71% and specificity of 74%, for detecting clinical fatigue (19). The CFS showed good reproducibility (test–retest reliability r = 0.69, P < 0.001) and good internal consistency (Cronbach’s α for all 15 items = 0.88) (18).
The SF-36 is a questionnaire to measure generic HR-QOL consisting of eight subscales translated into three component summary scales. Its Japanese version was developed and validated (20, 21). Fukuhara et al. conducted two surveys to establish the national norm, the first using version 1.2 in 1995 and the second using version 2.0 in 2002 (22, 23, 24).
We also obtained the following information from each participant on the day of the survey: age, sex, time since initial treatment, TNM class, surgical procedure, use of levothyroxine, use of radioiodine therapy, comorbidities, work status, marital status, cohabitation status, and serum levels of TSH, fT3, and fT4.
Serum levels of TSH, fT3, and fT4 were measured by means of the electrochemiluminescence immunoassay (Elecsys, Roche Diagnostics KK). The intra-assay coefficient variations were 0.49–0.99% for TSH assay, 0.97–2.58% for fT3 assay, and 1.24–1.71% for fT4 assay. The reference values were 0.38–4.30 µIU/mL for TSH, 2.40–4.00 pg/mL for fT3, and 0.94–1.60 ng/dL for fT4.
Statistical analysis
The sample size was determined to make the width of a 95% CI of the prevalence to be 10%. Based on the normal approximation method, the width of the expected CI was 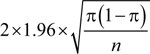 , where n denotes the required sample size and π denotes the expected prevalence (25). Assuming a 30% prevalence of fatigue based on a literature review, the required sample size was calculated as 323.
, where n denotes the required sample size and π denotes the expected prevalence (25). Assuming a 30% prevalence of fatigue based on a literature review, the required sample size was calculated as 323.
We summarized the patient characteristics and scores on the questionnaires using descriptive statistics. For each patient, we calculated the Z score for each subscale of the SF-36 by referring to the national norms stratified by sex and age (<29, 30–39, 40–49, 50–59, 60–69, and 70–79 years). We defined a significantly impaired SF-36 domain as a subscale Z score ≤−1.96. To explore the relationships between clinical fatigue and other variables including HR-QOL, we employed the chi-square test (or Fisher’s exact test) for categorical variables and unpaired t-test or correlation analysis using Pearson’s r for numerical variables. A multiple regression model using the CFS score as a dependent variable was used to examine the relationships between clinical fatigue and potential effect modifiers. We further explored the effects of clinical fatigue on the summary scores of the SF-36 using multiple regression analyses. An iterative approach was adopted to select appropriate covariates to maximize adjusted R2 values or to minimize the root mean square error of the models. We used jamovi 1.6.23, which is built on top of the R statistical package, for the statistical computations (https://www.jamovi.org/). We set a two-sided P-value of <0.05 to indicate the statistical significance of type I error.
Ethical considerations
The Ethics Committee of Tokyo Women’s Medical University approved this study (no. 4791). Each patient provided their written informed consent before participating in the study.
Results
Patient and disease characteristics
Of 321 patients who consented to participate in the study, 29 were excluded for the following reasons: extremely high TSH level (n = 2) and extremely high fT3 level (n = 1) who did not take levothyroxine as prescribed, no measurement of the TSH, fT3, or fT4 serum level (n = 20), and aged >80 years (n = 6; national norm data for HR-QOL measurements are not available for this age group). The remaining 292 patients were included in the analysis.
Table 1 shows the clinicopathological characteristics of the study participants. The mean age was 57.0 years (range 23–79 years), and the median time since the initial treatment was 6.3 years (interquartile range: 3.0–12.8). Of the 292 respondents, more than 80% were women, and 79.8% were married; 256 (87.7%) patients were living with family members, 162 (55.5%) had a job, and 172 (58.9%) had comorbidities. More than half of the participants had stage I (59.9%) or pT1/pT2 (64.4%) disease, and 185 (63.4%) patients had pathological lymph node metastasis. Whereas 157 (53.8%) patients underwent less than total thyroidectomy, 206 (70.5%) were on levothyroxine replacement therapy. Approximately 20% of patients received radioactive iodine treatment. Over half (53.1%) of the patients maintained their TSH level within the reference range. The mean levels of fT3 and fT4 were 2.81 pg/mL and 1.47 ng/dL, respectively.
Table 1.
Patient and disease characteristics.
| Characteristics | Mean (s.d.) or n (%) |
|---|---|
| Age (years) | 57.0 (13.7) |
| Years since initial treatment | 8.7 (7.4) |
| Sex | |
| Female | 236 (80.8%) |
| Male | 56 (19.2%) |
| Marital status | |
| Married | 233 (79.8%) |
| Single | 59 (20.2%) |
| Living with a cohabitant | |
| Yes | 256 (87.7%) |
| No | 36 (12.3%) |
| Having a job | |
| Yes | 162 (55.5%) |
| No | 130 (44.5%) |
| Comorbidities | |
| None | 120 (41.1%) |
| ≥1 | 172 (58.9%) |
| Stage | |
| I | 175 (59.9%) |
| II | 99 (33.9%) |
| III | 18 (6.2%) |
| TMN pT | |
| pT1 | 132 (45.2%) |
| pT2 | 56 (19.2%) |
| pT3 | 76 (26.0%) |
| pT4 | 28 (9.6%) |
| TMN pN | |
| pN0 | 107 (36.6%) |
| pN1 | 185 (63.4%) |
| Extent of thyroidectomy | |
| Less than total | 157 (53.8%) |
| Total | 135 (46.2%) |
| Levothyroxine replacement | |
| Yes | 206 (70.5%) |
| No | 86 (29.5%) |
| Radioactive iodine use | |
| Never | 232 (79.5%) |
| Ever | 60 (20.5%) |
| TSH | |
| Suppressed (<0.380 µIU/L) | 102 (34.9%) |
| Normal (0.380–4.300 µIU/L) | 155 (53.1%) |
| Elevated (>4.300 µIU/L) | 35 (12.0%) |
| fT3 | |
| (reference: 2.40–4.00 pg/mL) | 2.81 (0.439) |
| fT4 | |
| (reference: 0.94–1.60 ng/dL) | 1.47 (0.337) |
Prevalence of fatigue
Figure 1 shows the distribution of the CFS scores. The mean (s.d.) and median CFS scores were 18.0 (9.3) and 17.0, respectively. A score ≥19 was observed in 122 patients; thus, the prevalence of clinical fatigue was 41.8% (95% CI: 36.1, 47.5). The responses to the first question ‘Do you become tired easily?’ were as follows: ‘no’ by 68 (23.3%) patients, ‘a little’ by 80 (27.4%), ‘somewhat’ by 93 (31.8%), ‘considerably’ by 40 (13.7%), and ‘very much’ by 11 (3.8%).
Figure 1.
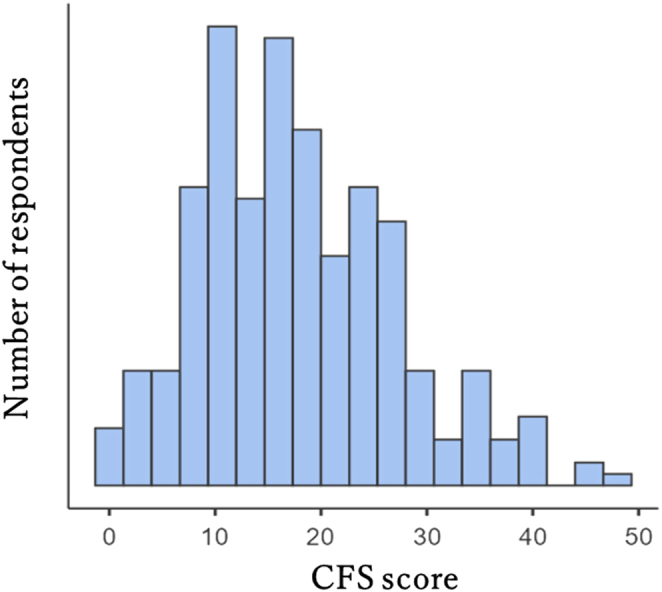
Distribution of the CFS score. CFS, Cancer Fatigue Scale.
Factors associated with fatigue
None of the following variables showed a statistically significant difference between the patients with clinical fatigue (n= 122) and those without (n = 171): age, time since initial treatment, sex, postoperative stage, TNM pT or TMN pN, the extent of thyroidectomy, use of levothyroxine, use of radioiodine therapy, comorbidities, employment status, marital status, cohabitation status, and serum levels of fT3, fT4, and TSH.
Analyses of the correlations between the CFS score and other numerical variables showed that the CFS score was significantly correlated with the fT3 level only (Pearson’s r = −0.123, 95% CI:−0.234, −0.008). Multiple regression analysis of the potential factors associated with the CFS score revealed unstandardized regression coefficients of −2.52 (95% CI: −4.94, −0.09) and 2.85 (95% CI: 0.49, 5.20) for the fT3 level and having a job, respectively (Table 2).
Table 2.
Multiple regression analysis of potential factors associated with the CFS score. Model fit measures: R2 = 0.039, adjusted R2 = 0.029, overall F = 3.89 (P= 0.009).
| Variable | Unstandardized regression coefficient (95% CI) | P-value | Standardized regression coefficient (95% CI) |
|---|---|---|---|
| fT3 | −2.52 (−4.94, −0.09) | 0.042 | −0.23 (−0.23, −0.004) |
| Having a job | 2.85 (0.49, 5.20) | 0.018 | 0.26 (0.03, 0.28) |
| Age | 0.09 (0.007, 0.18) | 0.034 | 0.11 (0.01, 0.26) |
CFS, Cancer Fatigue Scale.
HR-QOL and its associations with clinical fatigue
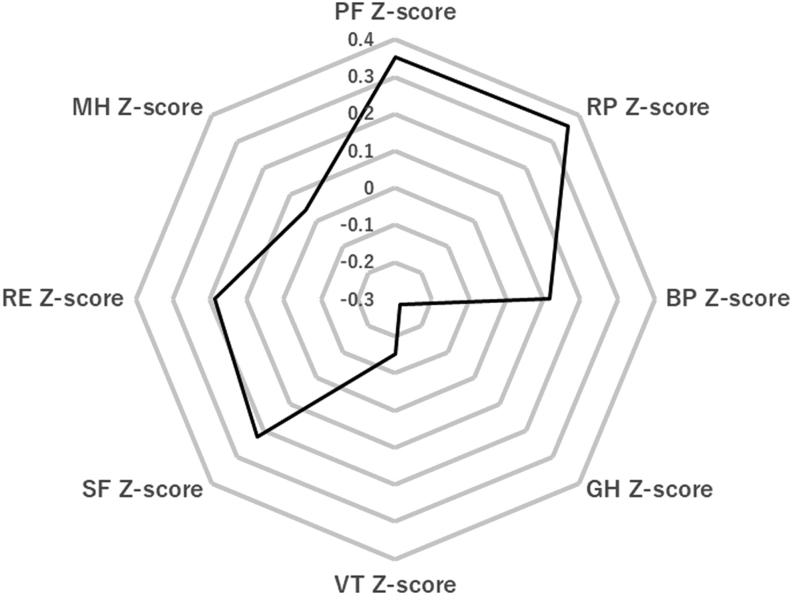
The median Z-scores for each subscale of the SF-36 were as follows: 0.35 for physical functioning (PF), 0.36 for role physical (RP), 0.12 for bodily pain (BP), −0.28 for general health (GH), −0.15 for vitality (VT), 0.23 for social functioning (SF), 0.18 for role emotional (RE), and 0.04 for mental health (MH) (Fig. 2). The numbers (%) of patients with a Z-score ≤−1.96 were 11 (3.8%) for PF, 19 (6.5%) for RP, 18 (6.2%) for BP, 10 (3.4%) for GH, 14 (4.8%) for VT, 18 (6.1%) for SF, 21 (7.2%) for RE, and 12 (4.1%) for MH. Clinical fatigue was associated with impaired HR-QOL in the RP, BP, GH, VT, RE, and MH domains (P < 0.05) (Table 3).
Figure 2.
Median Z-scores of the eight domains. BP, bodily pain; GH, general health; MH, mental health; PF, physical functioning; RE, role emotional; RP, role physical; SF, social functioning; VT, vitality.
Table 3.
Associations between clinical fatigue and each domain of the SF-36.
| Domain | Patients with clinical fatigue (%) | P-value |
|---|---|---|
| PF | ||
| Z score >−1.96 | 116/281 (41.3%) | 0.383 |
| Z score ≤−1.96 | 6/11 (54.5%) | |
| RP | ||
| Z score >−1.96 | 108/273 (39.6%) | 0.003a |
| Z score ≤−1.96 | 14/19 (73.7%) | |
| BP | ||
| Z score >−1.96 | 108/274 (39.4%) | 0.001a |
| Z score ≤−1.96 | 14/18 (77.8%) | |
| GH | ||
| Z score >−1.96 | 112/282 (39.7%) | 0.0001a |
| Z score ≤−1.96 | 10/10 (100%) | |
| VT | ||
| Z score >−1.96 | 110/278 (39.6%) | 0.0006a |
| Z score ≤−1.96 | 12/14 (85.7%) | |
| SF | ||
| Z score >−1.96 | 111/274 (40.5%) | 0.0866 |
| Z score≤−1.96 | 11/18 (61.1%) | |
| RE | ||
| Z score >−1.96 | 105/271 (38.7%) | 0.0001a |
| Z score ≤−1.96 | 17/21 (81.0%) | |
| MH | ||
| Z score >−1.96 | 114/281 (40.6%) | 0.034 |
| Z score ≤−1.96 | 8/11 (72.7%) |
a P < 0.05.
BP, bodily pain; GH, general health; MH, mental health; PF, physical functioning; RE, role emotional; RP, role physical; SF, social functioning; VT, vitality.
The median component summary score was 49.8 for the physical component summary (PCS) score, 49.8 for the mental component summary (MCS) score, and 51.2 for the role component summary (RCS) score. The total CFS score was significantly associated with all summary scores, with a Pearson’s r of −0.255 for the PCS (95% CI:−0.360, −0.145), −0.574 for the MCS (95% CI:−0.646, −0.492), and −0.325 for the RCS (95% CI:−0.424, −0.218) (Table 4).
Table 4.
Correlations between the SF-36 summary scores and CFS scores.
| PCS | MCS | RCS | |
|---|---|---|---|
| CFS total | −0.255a | −0.574a | −0.325a |
| CFS physical | −0.235a | −0.586a | −0.274a |
| CFS affective | −0.123a | −0.288a | −0.234a |
| CFS cognitive | −0.208a | −0.368a | −0.237a |
a P < 0.05.
CFS, Cancer Fatigue Scale; MCS, mental component summary; PCS, physical component summary; RCS, role component summary.
Table 5 shows the results of multiple regression analyses examining the relationship between the CFS score and the summary scores. The CFS score was a significant predictor of PCS, MCS, and RCS after controlling for the fT3 level, age, and sex. The unstandardized regression coefficient of the CFS score was largest for the MCS (−0.57, 95% CI: −0.67, −0.48), followed by the RCS (−0.41, 95% CI: −0.54, −0.27) and PCS (−0.26, 95% CI: −0.38, −0.15). The fT3 level was significantly correlated with the PCS score, with an unstandardized coefficient of 3.20 (95% CI: 0.77, 5.63).
Table 5.
Multiple regression analysis using the PCS, MCS, and PCS as a dependent variable. Model fit measures (PCS): R2 = 0.107, adjusted R2 = 0.0947, overall F = 8.61 (P< 0.01); Model fit measures (MCS): R2 = 0.331, adjusted R2 = 0.322, overall F = 35.5 (P < 0.01); Model fit measures (RCS): R2 = 0.107, adjusted R2 = 0.094, overall F = 8.59 (P < 0.01).
| Dependent variable | Explanatory variable | Unstandardized regression coefficient (95% CI) | P-value | Standardized regression coefficient (95% CI) |
|---|---|---|---|---|
| PCS | CFS score | −0.26 (−0.38, −0.15) | <0.001 | −26 (−0.37, −0.14) |
| fT3 level | 3.20 (0.77, 5.63) | 0.001 | 0.15 (0.019, 0.24) | |
| Age | 0.09 (0.015, 0.17) | 0.020 | 0.13 (0.021, 0.24) | |
| Female | −2.12 (−0.48, 0.57) | 0.122 | −0.087 (−0.2, 0.023) | |
| MCS | CFS score | −0.57 (−0.67, −0.48) | < 0.001 | −0.58 (−0.67, −0.48) |
| fT3 level | −0.024 (−2.04, 1.99) | 0.981 | −0.001 (−0.097, 0.095) | |
| Age | 0.005 (−0.059, 0.07) | 0.87 | 0.0078 (−0.09, 0.10) | |
| Female | −0.96 (−3.19, 1.26) | 0.395 | −0.041 (−0.14, 0.05) | |
| RCS | CFS score | −0.41 (−0.54, −0.27) | <0.001 | −0.33 (−0.44, −0.22) |
| fT3 | −0.12 (−3.04, 2.80) | 0.938 | −0.004 (−0.12, 0.011) | |
| Age | −0.011 (−0.12, 0.082) | 0.812 | −0.013 (−0.12, 0.097) | |
| Female | −0.92 (−4.14, 2.31) | 0.577 | −0.031 (−0.14, 0.079) |
CFS, Cancer Fatigue Scale; MCS, mental component summary; PCS, physical component summary; RCS, role component summary.
Discussion
Cancer-related fatigue is a distressing and persistent subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer diagnosis and treatment (26). The concept of cancer-related fatigue is multidimensional, consisting of physical (e.g. diminished energy, need to rest), cognitive (e.g. diminished concentration or attention), and affective (e.g. decreased motivation or interest) components (27, 28). In a qualitative study, Glaus et al. found that cancer patients perceived fatigue differently from healthy individuals. Cancer survivors felt chronic, unpleasant, distressing, life- and activity-limiting tiredness throughout the day, while healthy individuals reported a pleasant, acute, normal, regulating phenomenon that disappeared after a good night sleep (27). Cancer-related fatigue can be a significant burden for both patients and caregivers. The Fatigue Coalition in the US reported that 91% of cancer patients with fatigue felt impairment of their ‘normal’ life, and 88% experienced altered daily routines (28). Jones et al. reported that 91% of post-treatment cancer survivors with significant fatigue had moderate to severe disability, compared with 30% of those without significant fatigue (29). This issue is not novel (30), but it may not be appropriately recognized by physicians or other medical staff in daily practice because fatigue is a subjective feeling perceived only by the patient (26, 31).
The mechanism of fatigue in cancer survivors is multifactorial. Piper et al. proposed a framework for conceptualizing fatigue in which physiological, biochemical, and psychological factors play key roles in the development of symptoms (30). As pathophysiological mechanisms of cancer-related fatigue, Morrow et al. proposed the following hypotheses: anaemia, ATP dysregulation, vagal afferent nerve activation, and interaction of the hypothalamic–pituitary–adrenal axis/cytokines with serotonin dysregulation (32, 33). Of these, Bower focused on the cytokine hypothesis and proposed a model for the release of pro-inflammatory cytokines from tumour cells or surrounding stromal and immune cells induced by cancer diagnosis or treatment, as the critical pathway causing cancer-related fatigue (26). While Bower reviewed evidence corroborating the association between inflammation and fatigue, she also noted tremendous variation in the experience of fatigue at different stages of the cancer continuum, which is not captured by focusing on the mean fatigue level (26). She proposed a model based on predisposing (vulnerability of the individual), precipitating (situational conditions), and perpetuating (sustaining symptoms over time) factors that may contribute to modifying the key pathway. Of these, predisposing factors include childhood adversity, history of depression, trait anxiety, and loneliness. The perpetuating factors involve a cognitive process characterized by a lack of confidence with an expectation of negative outcomes, reductions in physical activity, and sleep disturbance (26).
Cancer-related fatigue is common among thyroid cancer survivors, as evidenced by the present study and previous reports (34, 35). Aside from the proposed mechanisms mentioned above, endocrinological alterations specific to thyroidectomized patients may be relevant as causes of post-treatment fatigue. Alhashemi et al. estimated Spearman’s correlation coefficient between the fT4 level and the Brief Fatigue Inventory score to be 0.132 (P = 0.060), but the biochemical data were not measured on the day of the survey (16). Hughes et al. reported that TSH suppression was associated with substantial fatigue (odds ratio = 1.63) in patients with differentiated thyroid cancer, but the use of TSH suppression was assessed by mail responses, not by actual data (35). A unique feature of the present study was that we obtained thyroid hormone levels on the day of administering the questionnaires to the patients, which allowed us to examine the relationship between thyroid hormone levels and fatigue more accurately. We found a statistically significant relationship between the fT3 level and CFS score. The association between a low fT3 level and fatigue has also been reported in other populations. Some hypothyroid patients on levothyroxine monotherapy have a low serum fT3 level, and those with a normal serum TSH level were not necessarily euthyroid in terms of energy expenditure, lipid metabolism, dissatisfaction, or quality of life, including fatigue (36, 37, 38). A case–control study indicated that the prevalence of a low fT3 level was more frequent in patients with chronic fatigue syndrome (16%) compared with controls (7%) (39).
A few studies revealed a significantly lower serum fT3 level postoperatively than preoperatively in athyreotic patients on levothyroxine monotherapy (40, 41). This is because there is no production of fT3 from the thyroid gland following total thyroidectomy (40, 41). Further, a genetic alteration in the type 2 deiodinase pathway and/or thyroid autoimmunity may be involved in the development of perceived symptoms in patients with levothyroxine therapy (36). Ito et al. observed that athyreotic patients with mildly suppressed TSH maintained their levels of fT3, metabolic markers, and subjective symptoms closest to the preoperative levels (41, 42, 43). Two crossover trials comparing thyroxine and thyroxine plus triiodothyronine therapies in patients with hypothyroidism showed statistically significant improvements in mood and neuropsychological function during the combination therapy (44, 45). However, adequate comparative studies examining the role of adding triiodothyronine to levothyroxine in thyroid cancer survivors are lacking.
Strategies to alleviate fatigue have been explored. A systematic review on the management of fatigue in thyroid cancer survivors included four randomized controlled trials involving the following experimental and control interventions (46): triiodothyronine plus levothyroxine vs levothyroxine alone (45), euthyroidism vs TSH suppression (15), predefined exercises vs physical inactivity (46, 47). The randomized controlled trials that compared thyroid hormone therapies showed mixed outcomes depending on the subscales of the specific instruments used (15, 45). Other treatments for cancer-related fatigue, in general, include physical exercise, psychosocial interventions, mind–body interventions, pharmacological interventions, nutritional therapies, integrative therapies, and acupuncture (10, 11, 48, 49, 50, 51, 52). In addition, observational studies indicated that unemployment or inability to work was associated with fatigue in thyroid cancer patients (16) and other populations (53, 54), although fatigue might be a cause rather than a result of being jobless. However, these correlational findings may have limited value in fatigue management because patients’ experiences are quite variable (26). In practice, regular screening, assessment, education, and appropriate medical support are essential for managing cancer-related fatigue (10). To this end, good communication with a patient will facilitate understanding of her/his experience. The American Society of Clinical Oncology Clinical Practice Guideline recommends using a quantitative assessment involving an 11-point numerical rating scale from 0 (no fatigue) to 10 (worst fatigue ever) in clinical practice when a patient scores ≥4 on this scale.
We are aware of several limitations of the present study, particularly a causal role of a low fT3 level for cancer-related fatigue. Firstly, as this was a cross-sectional investigation, we could not confirm temporal relationships between the test results and the outcome. Second, although the correlational analysis indicated an inverse association between the fT3 level and CFS score, this association was not very strong.
Finally, our multiple regression analysis of selected explanatory variables predicting the CFS score showed an unstandardized regression coefficient for fT3 of −2.52 (95% CI: −4.94, −0.09) and an adjusted R2 of 0.029, which was small. This observation was in agreement with that of Alhashemi et al., who reported an R2 of 0.177 in their multivariable linear regression model (16). This may indicate that mathematical formulas derived from quantitative research capture only a very small part of the overall picture of cancer-related fatigue.
In conclusion, fatigue was prevalent and associated with poor quality of life among PTC survivors. Since the mechanism of fatigue development seems to highly depend on the individual, an ‘on average’ approach may fail to alleviate symptoms in some patients. Clinicians need to understand the patient’s view as well as her/his specific circumstances. Thyroid functional status, particularly the level of free T3, may be worth to be considered in alleviating the burden.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statement
Yukari Maki: substantial contributions to the conception and design of the work, interpretation of data, statistical analyses, writing, and final approval of the version to be published. Kiyomi Horiuchi: substantial contributions to the conception and design of the work, interpretation of data, statistical analyses, writing, and final approval of the version to be published. Takahiro Okamoto: substantial contributions to the conception and design of the work, interpretation of data, statistical analyses, writing, and final approval of the version to be published.
Acknowledgements
The authors are grateful to Yasuko Nozoe for her managerial work. The authors also thank all participants in this study. It is the patients’ views that provide us with deep insights into their experiences.
References
- 1.Welch HG.Overdiagnosed: Making People Sick in the Pursuit of Health, 228p. Boston, MA, USA: Beacon Press, 2011. [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 20062952164–2167. ( 10.1001/jama.295.18.2164) [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngology: Head and Neck Surgery 2014140317–322. ( 10.1001/jamaoto.2014.1) [DOI] [PubMed] [Google Scholar]
- 4.Adam MA, Pura J, Gu L, Dinan MA, Tyler DS, Reed SD, Scheri R, Roman SA, Sosa JA. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61 775 patients. Annals of Surgery 2014260601–605; discussion 605. ( 10.1097/SLA.0000000000000925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong W, Horiuchi K, Tokumitsu H, Sakamoto A, Noguchi E, Ueda Y, Okamoto T. Time-varying pattern of mortality and recurrence from papillary thyroid cancer: lessons from a long-term follow-up. Thyroid 201929802–808. ( 10.1089/thy.2018.0128) [DOI] [PubMed] [Google Scholar]
- 6.Holland JC, Andersen B, Breitbart WS, Compas B, Dudley MM, Fleishman S, Fulcher CD, Greenberg DB, Greiner CB, Handzo GFet al. Distress management. Journal of the National Comprehensive Cancer Network 20108448–485. ( 10.6004/jnccn.2010.0034) [DOI] [PubMed] [Google Scholar]
- 7.Harms CA, Cohen L, Pooley JA, Chambers SK, Galvão DA, Newton RU. Quality of life and psychological distress in cancer survivors: the role of psycho‐social resources for resilience. Psychooncology 201928271–277. ( 10.1002/pon.4934) [DOI] [PubMed] [Google Scholar]
- 8.MacDonald LA, Sackett DL, Haynes RB, Taylor DW. Labelling in hypertension: a review of the behavioural and psychological consequences. Journal of Chronic Diseases 198437933–942. ( 10.1016/0021-9681(84)90070-5) [DOI] [PubMed] [Google Scholar]
- 9.Li R, Li G, Wang Y, Bao T, Lei Y, Tian L, Li Z, Zhu J, Lei J, Tang H. Psychological distress and sleep disturbance throughout thyroid nodule screening, diagnosis, and treatment. Journal of Clinical Endocrinology and Metabolism 2021106e4221–e4230. ( 10.1210/clinem/dgab224) [DOI] [PubMed] [Google Scholar]
- 10.Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, Schnipper HH, Lacchetti C, Ligibel JA, Lyman GHet al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology Clinical Practice Guideline Adaptation. Journal of Clinical Oncology 2014321840–1850. ( 10.1200/JCO.2013.53.4495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bower JE.Cancer-related fatigue-mechanisms, risk factors, and treatments. Nature Reviews: Clinical Oncology 201411597–609. ( 10.1038/nrclinonc.2014.127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. European Journal of Cancer 20023827–43. ( 10.1016/s0959-8049(01)00332-x) [DOI] [PubMed] [Google Scholar]
- 13.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. Journal of the National Cancer Institute: Monographs 20043240–50. ( 10.1093/jncimonographs/lgh027) [DOI] [PubMed] [Google Scholar]
- 14.Sawka AM, Naeem A, Jones J, Lowe J, Segal P, Goguen J, Gilbert J, Zahedi A, Kelly C, Ezzat S. Persistent posttreatment fatigue in thyroid cancer survivors: a scoping review. Endocrinology and Metabolism Clinics of North America 201443475–494. ( 10.1016/j.ecl.2014.02.007) [DOI] [PubMed] [Google Scholar]
- 15.Eustatia-Rutten CFA, Corssmit EPM, Pereira AM, Frölich M, Bax JJ, Romijn JA, Smit JWA. Quality of life in longterm exogenous subclinical hyperthyroidism and the effects of restoration of euthyroidism, a randomized controlled trial. Clinical Endocrinology 200664284–291. ( 10.1111/j.1365-2265.2006.02458.x) [DOI] [PubMed] [Google Scholar]
- 16.Alhashemi A, Jones JM, Goldstein DP, Mina DS, Thabane L, Sabiston CM, Chang EK, Brierley JD, Sawka AM. An exploratory study of fatigue and physical activity in Canadian thyroid cancer patients. Thyroid 2017271156–1163. ( 10.1089/thy.2016.0541) [DOI] [PubMed] [Google Scholar]
- 17.Larisch R, Midgley JEM, Dietrich JW, Hoermann R. Symptomatic relief is related to serum free triiodothyronine concentrations during follow-up in levothyroxine-treated patients with differentiated thyroid cancer. Experimental and Clinical Endocrinology and Diabetes 2018126546–552. ( 10.1055/s-0043-125064) [DOI] [PubMed] [Google Scholar]
- 18.Okuyama T, Akechi T, Kugaya A, Okamura H, Shima Y, Maruguchi M, Hosaka T, Uchitomi Y. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. Journal of Pain and Symptom Management 2000195–14. ( 10.1016/s0885-3924(99)00138-4) [DOI] [PubMed] [Google Scholar]
- 19.Okuyama T, Tanaka K, Akechi T, Kugaya A, Okamura H, Nishiwaki Y, Hosaka T, Uchitomi Y. Fatigue in ambulatory patients with advanced lung cancer: prevalence, correlated factors, and screening. Journal of Pain and Symptom Management 200122554–564. ( 10.1016/s0885-3924(01)00305-0) [DOI] [PubMed] [Google Scholar]
- 20.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 health survey for use in Japan. Journal of Clinical Epidemiology 1998511037–1044. ( 10.1016/s0895-4356(98)00095-x) [DOI] [PubMed] [Google Scholar]
- 21.Fukuhara S, Ware Jr JE, Kosinski M, Wada S, Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. Journal of Clinical Epidemiology 1998511045–1053. ( 10.1016/s0895-4356(98)00096-1) [DOI] [PubMed] [Google Scholar]
- 22.Fukuhara S, Suzukamo Y. Manual of SF-36v2 Japanese Version. Kyoto, Japan: iHope International Inc, 2015. [Google Scholar]
- 23.Ware JE, Kosinski MA, Keller SD. Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA, USA: The Health Institute, 1994. [Google Scholar]
- 24.Suzukamo Y, Fukuhara S, Green J, Koshinski M, Gandek B, Ware JE. Validation testing of a three-component model of short form-36 scores. Journal of Clinical Epidemiology 201164301–308. ( 10.1016/j.jclinepi.2010.04.017) [DOI] [PubMed] [Google Scholar]
- 25.Colton T.Statistics in Medicine. Boston, MA, USA: Little Brown & Co, 1974. [Google Scholar]
- 26.Bower JE.The role of neuro-immune interactions in cancer-related fatigue: biobehavioral risk factors and mechanisms. Cancer 2019125353–364. ( 10.1002/cncr.31790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. European Journal of Cancer Care 19965 (Supplement) 8–23. ( 10.1111/j.1365-2354.1996.tb00247.x) [DOI] [PubMed] [Google Scholar]
- 28.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RKet al. Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist 20005353–360. ( 10.1634/theoncologist.5-5-353) [DOI] [PubMed] [Google Scholar]
- 29.Jones JM, Olson K, Catton P, Catton CN, Fleshner NE, Krzyzanowska MK, McCready DR, Wong RKS, Jiang H, Howell D. Cancer-related fatigue and associated disability in post-treatment cancer survivors. Journal of Cancer Survivorship: Research and Practice 20161051–61. ( 10.1007/s11764-015-0450-2) [DOI] [PubMed] [Google Scholar]
- 30.Piper BF, Lindsey AM, Dodd MJ. Fatigue mechanisms in cancer patients: developing nursing theory. Oncology Nursing Forum 19871417–23. [PubMed] [Google Scholar]
- 31.Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, Itri LM, Johnson DH, Scherr SL, Portenoy RK. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Seminars in Hematology 199734 (Supplement 2) 4–12. [PubMed] [Google Scholar]
- 32.Morrow GR, Andrews PLR, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Supportive Care in Cancer 200210389–398. ( 10.1007/s005200100293) [DOI] [PubMed] [Google Scholar]
- 33.Ebede CC, Jang Y, Escalante CP. Cancer-related fatigue in cancer survivorship. Medical Clinics of North America 20171011085–1097. ( 10.1016/j.mcna.2017.06.007) [DOI] [PubMed] [Google Scholar]
- 34.Husson O, Nieuwlaat WA, Oranje WA, Haak HR, van de Poll-Franse LV, Mols F. Fatigue among short- and long-term thyroid cancer survivors: results from the population-based PROFILES registry. Thyroid 2013231247–1255. ( 10.1089/thy.2013.0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes DT, Reyes-Gastelum D, Kovatch KJ, Hamilton AS, Ward KC, Haymart MR. Energy level and fatigue after surgery for thyroid cancer: a population-based study of patient-reported outcomes. Surgery 2020167102–109. ( 10.1016/j.surg.2019.04.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? Journal of Clinical Endocrinology and Metabolism 2020105e3090–e3104. ( 10.1210/clinem/dgaa430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clinical Endocrinology 200257577–585. ( 10.1046/j.1365-2265.2002.01654.x) [DOI] [PubMed] [Google Scholar]
- 38.Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, Kopp PA, Ross DS, Samuels MH, Sawka AMet al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid 201828707–721. ( 10.1089/thy.2017.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Núñez B, Tarasse R, Vogelaar EF, Dijck-Brouwer DAJ, Muskiet FAJ. Higher prevalence of ‘low T3 syndrome’ in patients with chronic fatigue syndrome: a case-control study. Frontiers in Endocrinology 20189 97. ( 10.3389/fendo.2018.00097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gullo D, Latina A, Frasca F, Moli RL, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS ONE 20116 e22552. ( 10.1371/journal.pone.0022552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito M, Miyauchi A, Morita S, Kudo T, Nishihara E, Kihara M, Takamura Y, Ito Y, Kobayashi K, Miya Aet al. TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy. European Journal of Endocrinology 2012167373–378. ( 10.1530/EJE-11-1029) [DOI] [PubMed] [Google Scholar]
- 42.Ito M, Miyauchi A, Hisakado M, Yoshioka W, Ide A, Kudo T, Nishihara E, Kihara M, Ito Y, Kobayashi Ket al. Biochemical markers reflecting thyroid function in athyreotic patients on levothyroxine monotherapy. Thyroid 201727484–490. ( 10.1089/thy.2016.0426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito M, Miyauchi A, Hisakado M, Yoshioka W, Kudo T, Nishihara E, Kihara M, Ito Y, Miya A, Fukata Set al. Thyroid function related symptoms during levothyroxine monotherapy in athyreotic patients. Endocrine Journal 201966953–960. ( 10.1507/endocrj.EJ19-0094) [DOI] [PubMed] [Google Scholar]
- 44.Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. New England Journal of Medicine 1999340424–429. ( 10.1056/NEJM199902113400603) [DOI] [PubMed] [Google Scholar]
- 45.Bunevicius R, Prange AJ. Mental improvement after replacement therapy with thyroxine plus triiodothyronine: relationship to cause of hypothyroidism. International Journal of Neuropsychopharmacology 20003167–174. ( 10.1017/S1461145700001826) [DOI] [PubMed] [Google Scholar]
- 46.To J, Goldberg AS, Jones J, Zhang J, Lowe J, Ezzat S, Gilbert J, Zahedi A, Segal P, Sawka AM. A systematic review of randomized controlled trials for management of persistent post-treatment fatigue in thyroid cancer survivors. Thyroid 201525198–210. ( 10.1089/thy.2014.0418) [DOI] [PubMed] [Google Scholar]
- 47.Vigário Pdos S, Chachamovitz DS, Cordeiro MF, Teixeira Pde F, de Castro CL, de Oliveira FP, Vaisman M. Effects of physical activity on body composition and fatigue perception in patients on thyrotropin-suppressive therapy for differentiated thyroid carcinoma. Thyroid 201121695–700. ( 10.1089/thy.2010.0052) [DOI] [PubMed] [Google Scholar]
- 48.Vigário Pdos S, Chachamovitz DS, Teixeira Pde F, Rocque Mde L, Santos ML, Vaisman M. Exercise is associated with better quality of life in patients on TSH-suppressive therapy with levothyroxine for differentiated thyroid carcinoma. Arquivos Brasileiros de Endocrinologia e Metabologia 201458274–281. ( 10.1590/0004-2730000002968) [DOI] [PubMed] [Google Scholar]
- 49.Arring NM, Barton DL, Brooks T, Zick SM. Integrative therapies for cancer-related fatigue. Cancer Journal 201925349–356. ( 10.1097/PPO.0000000000000396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hilfiker R, Meichtry A, Eicher M, Balfe LN, Knols RH, Verra ML, Taeymans J. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. British Journal of Sports Medicine 201852651–658. ( 10.1136/bjsports-2016-096422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Mohr D, Palesh OG, Peppone LJ, Piper BFet al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncology 20173961–968. ( 10.1001/jamaoncol.2016.6914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baguley BJ, Bolam KA, Wright ORL, Skinner TL. The effect of nutrition therapy and exercise on cancer-related fatigue and quality of life in men with prostate cancer: a systematic review. Nutrients 20179 1003. ( 10.3390/nu9091003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen LM, Yang QL, Duan YY, Huan XZ, He Y, Wang C, Fan YY, Cai YC, Li JM, Chen LPet al. Multidimensional fatigue in patients with nasopharyngeal carcinoma receiving concurrent chemoradiotherapy: incidence, severity, and risk factors. Supportive Care in Cancer 2021295009–5019. ( 10.1007/s00520-021-06054-7) [DOI] [PubMed] [Google Scholar]
- 54.Castro-Marrero J, Faro M, Zaragozá MC, Aliste L, de Sevilla TF, Alegre J. Unemployment and work disability in individuals with chronic fatigue syndrome/myalgic encephalomyelitis: a community-based cross-sectional study from Spain. BMC Public Health 201919 840. ( 10.1186/s12889-019-7225-z). [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a