Abstract
Objective
Despite the known benefit of vitamin D in reducing sarcopenia risk in older adults, its effect against muscle loss in the young population is unknown. We aimed to examine the association of serum 25-hydroxy vitamin D [25(OH)D] level and its changes over time with the risk of incident low muscle mass (LMM) in young and middle-aged adults.
Design
This study is a cohort study.
Methods
The study included Korean adults (median age: 36.9 years) without LMM at baseline followed up for a median of 3.9 years (maximum: 7.3 years). LMM was defined as the appendicular skeletal muscle (ASM) mass by body weight (ASM/weight) of 1 s.d. below the sex-specific mean for the young reference group. Cox proportional hazard models were used to estimate hazard ratios (HRs) with 95% CIs.
Results
Of the 192,908 individuals without LMM at baseline, 19,526 developed LMM. After adjusting for potential confounders, the multivariable-adjusted HRs (95% CIs) for incident LMM comparing 25(OH)D levels of 25–<50, 50–<75, and ≥75 nmol/L to 25(OH)D <25 nmol/L were 0.93 (0.90–0.97), 0.85 (0.81–0.89), and 0.77 (0.71–0.83), respectively. The inverse association of 25(OH)D with incident LMM was consistently observed in young (aged <40 years) and older individuals (aged ≥40 years). Individuals with increased 25(OH)D levels (<50–≥50 nmol/L) or persistently adequate 25(OH)D levels (≥50 nmol/L) between baseline and follow-up visit had a lower risk of incident LMM than those with persistently low 25(OH)D levels.
Conclusions
Maintaining sufficient serum 25(OH)D could prevent unfavourable changes in muscle mass in both young and middle-aged Korean adults.
Introduction
Sarcopenia is characterized by a progressive decline in skeletal muscle mass and muscle strength (1) and represents a major public health concern in older adults. Sarcopenia can lead to serious health consequences that impair the quality of life and pose a considerable burden on healthcare systems (1, 2). Although sarcopenia is more commonly associated with older ages, there is growing recognition that sarcopenia also occurs early in life, partly due to increased sedentary lifestyle and physical inactivity in the modern young population (1, 3, 4). However, risk or protective factors associated with sarcopenia or muscle loss in younger individuals have not been adequately addressed and remain largely unknown.
Beyond its widely recognized effects on bone health, vitamin D is known to affect skeletal muscle via vitamin D receptors (VDRs) (5). The link between low serum 25-hydroxyvitamin D [25(OH)D] levels, a reliable marker of vitamin D status, and the risk of sarcopenia in older individuals is well established (6, 7). Several cross-sectional studies explored the relationship between vitamin D deficiency and muscle mass, but the results were conflicting (8, 9, 10, 11). Also, findings from small randomized–controlled trials (RCTs) showed no benefit of vitamin D supplementation on muscle mass gain in young individuals (12, 13). With the lack of large and high-quality studies, it remains unclear whether vitamin D has any protective effect against low muscle mass (LMM) development in young people. In addition, no studies have yet to evaluate the effect of changes in serum 25(OH)D levels overtime on the risk of developing LMM.
Thus, we examined the association of serum 25(OH)D level and its changes over time with the risk of incident LMM in young and middle-aged adults without LMM at baseline.
Subjects and methods
Study participants
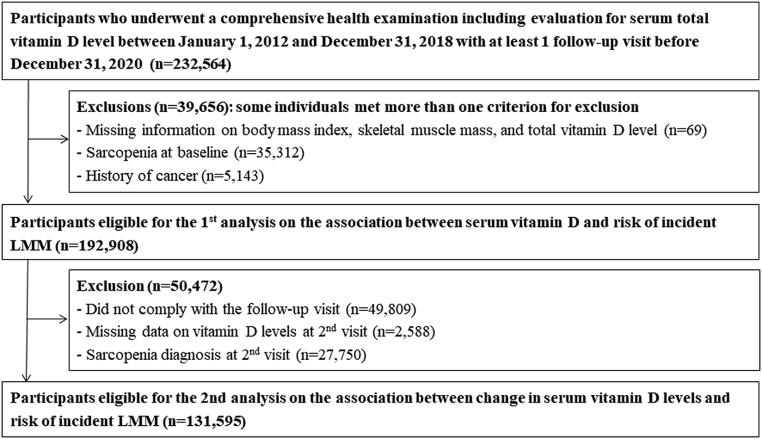
The Kangbuk Samsung Health Study is a cohort study of Korean men and women aged ≥18 years who participated in comprehensive health examinations every 1–2 years at Kangbuk Samsung Hospital Total Healthcare Center in Seoul and Suwon, South Korea, as previously described (14). The present cohort study included participants who underwent comprehensive health examinations between January 2012 and December 2018. From 2012, data on appendicular skeletal muscle mass and serum 25(OH)D levels were available, and all participants had at least one follow-up visit between recruitment and 31 December 2020 (n = 232,564 participants). A total of 39,656 participants were excluded in a two-step selection process (Fig. 1) (see Supplementary Material for detailed exclusion criteria, see section on supplementary materials given at the end of this article).
Figure 1.
Selection of study participants.
This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (IRB no. KBSMC 2021-09-032), which waived the requirement for informed consent because de-identified retrospective data routinely collected during health screenings were used.
Measurements
At baseline and follow-up visits, information on demographic factors, lifestyle factors such as physical activity, medical history, and medication use was obtained using standardized, self-administered questionnaires (14).
Physical activity was assessed using the short form of the validated Korean version of the International Physical Activity Questionnaire (15). According to this questionnaire’s results, the participants were categorized as being inactive, being minimally active, or engaging in health-enhancing physical activity (HEPA). HEPA was defined as follows: (i) vigorous activity for ≥3 days/week with ≥1500 accumulated metabolic equivalent (MET)-min/week or (ii) a combination of walking and moderate- or vigorous-intensity activities for 7 days totalling to ≥3000 MET-min/week.
Blood pressure, height, weight, and body composition measurements were performed by trained nurses. A multi-frequency bio-impedance analyzer (BIA) with eight-point tactile electrodes (InBody 720; Biospace Inc., Seoul, Korea) was used to measure body composition including lean body mass of individuals’ limbs, appendicular skeletal muscle mass (ASM), and fat mass. The body composition using the BIA (InBody720) was reliable in men and women as indicated by high intraclass correlation coefficient for measures of body composition of ≥0.98 including skeletal muscle mass (16, 17). The BIA technique was validated for the assessment of body composition, showing a good correlation with dual-energy X-ray absorptiometry (DEXA), and applied to estimate ASM in various populations (18, 19, 20, 21, 22, 23). The InBody720 demonstrated a strong correlation with DXA in ASM (Pearson correlation coefficients 0.944 and 0.903, and s.e. of estimate 1.051 kg and 0.927 kg in men and women, respectively) (24). Obesity was defined as BMI ≥ 25 kg/m2, which is the cut-off value for diagnosing obesity in Asians (25).
Blood specimens were collected after a fasting period of at least 10 h, and fasting blood tests evaluated glycemic parameters, lipid profiles, liver enzyme levels, and high-sensitivity C-reactive protein (hsCRP) levels (26). Insulin resistance was estimated using the homeostatic model assessment–insulin resistance (HOMA-IR) equation as follows: fasting blood insulin (uU/mL) × fasting blood glucose (mmol/L)/22.5; the cut-off value of 2.5 was used (27). Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or current use of blood pressure-lowering medication. Diabetes mellitus was defined as a fasting serum glucose level ≥ 126 mg/dL, haemoglobin A1c ≥ 6.5%, or current use of anti-diabetic medications or insulin.
Skeletal muscle mass index (SMI) was calculated using BIA as SMI (%) = appendicular skeletal muscle mass (kg)/body weight (kg) × 100, according to the methods by Janssen et al (28). Normal SMI was defined as an SMI higher than −1 s.d. below the sex-specific mean of young reference adults (aged 20–39 years) (28). Among young adults (20–39 years old) in this study population, the mean (s.d.) of SMI was 32.9 % (2.7) for men and 28.6 % (2.5) for women. Class I LMM was defined as an SMI within −1 to −2 s.d. below the mean values of young adults, and class II LMM was defined as SMI below −2 s.d. below the mean values of young adults (28). Because early detection of muscle mass loss in young adults is important, incident LMM was defined according to class I LMM development.
To assess serum 25(OH)D status, total 25(OH)D levels, including 25(OH)D2 and 25(OH)D3, were measured with a competitive immunoassay using an Elecsys Vitamin D Total assay on the Modular E170 (Roche Diagnostics) until April 2015 and Cobas e801 (Roche Diagnostics) thereafter. Total 25(OH)D measurement using the Elecsys vitamin D total assay demonstrated acceptable performance compared to using liquid chromatography-tandem mass spectrometry, the reference standard for 25(OH)D measurement (29, 30). When the analytical performance for precision was evaluated according to CLSI-EP15-A2 guidelines, the inter-assay coefficients of variation for quality control specimens of lower and higher levels of total 25(OH)D were 2.01–5.94 and 2.69–5.03%, respectively, during the study period. The detection limit was determined according to the CLSI EP17-A2 guidelines and was reported to be <3 ng/mL (<7.5 nmol/L). Serum 25(OH)D levels were categorized as <10, 10–<20, 20–<30, and ≥30 ng/mL (for conversion to SI units: ng/mL × 2.5 = nmol/L; e.g. <25, 25–<50, 50–<75, and ≥75 nmol/L) (31, 32). Despite some controversy, serum 25(OH)D level >20 ng/mL (>50 nmol/L) is considered sufficient for skeletal health in the healthy general population (33, 34). Therefore, the change in 25(OH)D status from baseline to the second visit was analysed in the following four groups based on the presence/absence of insufficient serum 25(OH)D (defined as serum 25(OH)D level <20 ng/mL (50 nmol/L)): (i) insufficient 25(OH)D level at baseline and follow-up (persistently low); (ii) insufficient 25(OH)D level at baseline but no insufficiency at follow-up (increased); (iii) no insufficiency at baseline but insufficiency at follow-up (decreased); and (iv) no 25(OH)D insufficiency at baseline and follow-up (persistently adequate).
Statistical analysies
Baseline characteristics of the study participants are presented according to the 25(OH)D categories mentioned above. To determine linear trends, the median values of each category were included in each model.
The primary outcome was the development of incident LMM. Each participant was followed from the baseline visit until either the occurrence of incident LMM or the last health examination conducted through the end of 2020, whichever occurred first. The incidence rates were calculated as the number of incident cases divided by person-years of follow-up. Cox proportional hazard models were used to estimate the hazard ratios (HRs) with 95% CIs for incident LMM in each 25(OH)D category compared with the reference category.
We used three models with progressive adjustment to control for potential confounders. The first model (Model 1) was adjusted for age, sex, centre, year of screening, alcohol consumption, smoking, physical activity, total energy intake, education level, medication for hypertension, medication for diabetes, multivitamin supplementation, calcium supplementation, and season. Given the potential impact of obesity on the relationship between serum 25(OH)D levels and sarcopenia (10), the model was additionally adjusted for BMI (Model 2). Alternatively, analyses were performed with adjustment for waist circumference instead of BMI. To evaluate the effects of changes in serum 25(OH)D levels and other covariates during the follow-up period, we performed additional analyses by introducing serum 25(OH)D levels and other factors as time-varying covariates in the models.
We assessed the proportional hazards assumption by examining graphs of estimated log (−log) survival. Pre-defined subgroup analyses were performed after stratifying by age (<40 vs ≥40 years), current smoking status (no vs yes), alcohol intake (<20 vs ≥20 g/day), HEPA (no vs yes), obesity defined using the specific criteria for Asians (BMI<25 kg/m2 vs ≥25 kg/m2 (25, 35)), hypertension (no vs yes), diabetes (no vs yes), HOMA-IR (<2.5 vs ≥2.5), and hsCRP (<1.0 mg/L vs ≥1.0 mg/L). The interactions according to subgroup characteristics were tested using likelihood ratio tests that compared models with and without multiplicative interaction terms. As a sensitivity analysis, the association between serum 25(OH)D levels and incident LMM was tested using LMM defined as SMI less than −2 s.d. below the mean values of young adults.
STATA version 16.0 (Stata Corp.) was used for data analysis. All P-values were two-tailed, and P-values <0.05 were considered statistically significant.
Results
The median age of the participants was 36.9 years (interquartile range, 32.4–41.8 years), and 44.5% of patients were females. At baseline, the proportions of participants with 25(OH)D levels <25, 25–<50, 50–<75, and ≥75 nmol/L were 16.2, 56.6, 21.9, and 5.3%, respectively (Table 1). Serum 25(OH)D levels were positively associated with age, alcohol intake, physical activity, education level, medication use for hyperlipidaemia, and use of multivitamin, vitamin D, and/or calcium supplements (Table 1). Baseline characteristics of the study participants are also presented according to 25(OH)D levels at baseline and subsequent visits (Supplementary Table 1).
Table 1.
Estimateda mean and adjusteda proportions of baseline characteristics by serum 25(OH)D levels among participants (n = 192,908).
| Characteristics | Serum 25(OH)D levels (nmol/L) | P | |||
|---|---|---|---|---|---|
| <25 | 25–<50 | 50–<75 | ≥75 | ||
| Participants, n | 31,224 | 109,197 | 42,200 | 10,287 | |
| Age (years) | 37.3 (37.2–37.4) | 37.5 (37.5–37.6) | 38.6 (38.5–38.6) | 40.0 (39.8–40.1) | <0.001 |
| Male (%) | 35.27 (34.74–35.80) | 56.68 (56.38–56.97) | 66.27 (65.81–66.72) | 59.94 (58.99–60.89) | <0.001 |
| Alcohol intake (%)b | 14.50 (14.05–14.94) | 18.78 (18.55–19.00) | 23.31 (22.94–23.68) | 25.69 (24.89–26.49) | <0.001 |
| Current smoker (%) | 15.10 (14.63–15.57) | 16.75 (16.54–16.95) | 19.28 (18.96–19.61) | 20.09 (19.39–20.78) | <0.001 |
| HEPA (%) | 11.06 (10.70–11.42) | 13.58 (13.38–13.79) | 17.00 (16.64–17.36) | 19.73 (18.96–20.50) | <0.001 |
| Education level (%)c | 82.92 (82.51–83.33) | 84.95 (84.74–85.16) | 85.33 (84.98–85.67) | 85.16 (84.48–85.84) | <0.001 |
| History of diabetes (%) | 1.61 (1.44–1.77) | 1.83 (1.75–1.91) | 1.83 (1.71–1.94) | 1.76 (1.55–1.96) | 0.336 |
| History of hypertension (%) | 5.79 (5.49–6.10) | 6.02 (5.88–6.16) | 6.19 (5.99–6.40) | 6.40 (5.99–6.81) | 0.006 |
| History of CVD (%) | 0.81 (0.70–0.93) | 0.84 (0.78–0.89) | 0.80 (0.72–0.88) | 0.86 (0.71–1.01) | 0.986 |
| Anti-lipid medication use (%) | 1.98 (1.80–2.16) | 1.80 (1.72–1.88) | 1.73 (1.62–1.84) | 1.97 (1.76–2.18) | 0.507 |
| Multivitamin supplement (%) | 3.49 (3.29–3.69) | 6.05 (5.91–6.19) | 10.42 (10.13–10.72) | 14.95 (14.28–15.62) | <0.001 |
| Vitamin D supplement (%) | 0.21 (0.17–0.26) | 0.62 (0.57–0.66) | 1.74 (1.61–1.87) | 4.79 (4.38–5.19) | <0.001 |
| Calcium supplement (%) | 0.20 (0.16–0.25) | 0.38 (0.35–0.42) | 0.98 (0.87–1.08) | 2.12 (1.85–2.39) | <0.001 |
| Obesity (%)d | 19.42 (18.94–19.91) | 21.49 (21.26–21.72) | 22.49 (22.14–22.84) | 19.22 (18.53–19.92) | <0.001 |
| BMI (kg/m2) | 22.5 (22.5–22.6) | 22.7 (22.7–22.7) | 22.8 (22.8–22.8) | 22.5 (22.4–22.5) | <0.001 |
| SBP (mmHg) | 107.0 (106.9–107.1) | 107.5 (107.5–107.6) | 107.8 (107.7–107.9) | 108.0 (107.8–108.2) | <0.001 |
| DBP (mmHg) | 68.8 (68.7–68.9) | 69.3 (69.2–69.3) | 69.5 (69.5–69.6) | 69.2 (69.1–69.4) | <0.001 |
| Glucose (mg/dL) | 93.2 (93.1–93.4) | 93.5 (93.5–93.6) | 93.6 (93.5–93.7) | 93.1 (92.9–93.3) | 0.191 |
| Total cholesterol (mg/dL) | 188.0 (187.6–188.3) | 191.1 (190.9–191.3) | 191.8 (191.5–192.1) | 190.6 (190.0–191.3) | <0.001 |
| GGT (U/L) | 26.4 (26.0–26.8) | 28.1 (27.9–28.3) | 30.3 (30.0–30.6) | 29.9 (29.3–30.6) | <0.001 |
| ALT (U/L) | 21.5 (21.3–21.7) | 22.0 (21.9–22.1) | 22.5 (22.3–22.6) | 22.6 (22.3–23.0) | <0.001 |
| HOMA-IR | 1.45 (1.43–1.46) | 1.44 (1.44–1.45) | 1.43 (1.42–1.44) | 1.33 (1.32–1.35) | <0.001 |
| hsCRP (mg/L) | 0.90 (0.87–0.93) | 0.91 (0.89–0.93) | 0.94 (0.91–0.97) | 0.94 (0.88–1.00) | <0.001 |
| Total energy intake (kcal/d)e, f | 1,450.6 (1,442.2–1,458.9) | 1,446.5 (1,442.2–1,450.9) | 1,434.7 (1,427.6–1,441.7) | 1,411.7 (1,397.4–1,426.1) | <0.001 |
aAdjusted for age and sex; b≥20 g/day; c≥College graduate; dBMI ≥ 25 kg/m2; eAmong 132,466 participants with plausible estimated energy intake levels (within 3 s.d. from the log-transformed mean energy intake); f1 kcal equals to 4185.8 J.
ALT, alanine aminotransferase; CVD, cardiovascular disease; DBP, diastolic blood pressure; GGT, gamma-glutamyltransferase; HEPA, health-enhancing physically active; hsCRP, high-sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance; SBP, systolic blood pressure
Within 720,713.2 person-years of follow-up (median, 3.9 years; interquartile range, 2.1–5.0 years; maximum, 7.3 years), 19,526 participants developed LMM (incidence rate, 27.1 per 1000 person-years) (Table 2). Overall, baseline 25(OH)D levels were inversely associated with the risk of incident LMM. After adjusting for age, sex, physical activity, and other potential confounders (model 1), HRs (95% CI) for incident LMM at baseline 25(OH)D levels of 25–<50, 50–<75, and ≥75 nmol/L (compared to the reference, <25 nmol/L) were 0.93 (0.90–0.97), 0.83 (0.79–0.88), and 0.67 (0.62–0.73), respectively. Further adjustment for either BMI or waist circumference attenuated the association, which remained significant (Table 2, model 2, and Supplementary Table 2). The inverse association was consistently observed in men and women but with a slightly stronger effect in men than in women (P for interaction = 0.025). The association between 25(OH) level and incident LMM became stronger in time-dependent analyses than in the original analyses. Corresponding HRs (95% CI) comparing 25(OH)D levels of 25–<50, 50–<75, and ≥75–<25 nmol/L were 0.79 (0.76–0.83), 0.65 (0.62–0.68), and 0.52 (0.48–0.56), respectively. In spline regression models, the LMM risk decreased across the range of the 25(OH) level in both men and women (Fig. 2). Similar results were observed in a sensitivity analysis using LMM defined as SMI less than −2 s.d. below the mean values of young adults (Supplementary Table 3).
Table 2.
Development of low muscle mass according to serum 25(OH)D levels among participants at baseline (n = 192,908). P = 0.025 for the overall interaction between sex and serum 25(OH)D levels for incident low muscle mass (multivariable-adjusted model 2).
| 25(OH)D levels (nmol/L) | Person-years | Incident cases | Incidence density (/103 PY) | Age-adjusted HR (95% CI) | Multivariable-adjusted HRa (95% CI) | HR (95% CI)b in a model with time-dependent variables | |
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||
| Total (n = 192,908) | |||||||
| <25 | 120,174.0 | 3539 | 29.4 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25–<50 | 412,350.5 | 11,324 | 27.5 | 0.92 (0.89–0.96) | 0.93 (0.90–0.97) | 0.93 (0.90–0.97) | 0.79 (0.76–0.83) |
| 50–<75 | 154,073.9 | 3914 | 25.4 | 0.83 (0.79–0.87) | 0.83 (0.79–0.88) | 0.85 (0.81–0.89) | 0.65 (0.62–0.68) |
| ≥75 | 34,114.8 | 749 | 22.0 | 0.68 (0.63–0.74) | 0.67 (0.62–0.73) | 0.77 (0.71–0.83) | 0.52 (0.48–0.56) |
| P | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Women (n = 85,898) | |||||||
| <25 | 78,608.8 | 2499 | 31.8 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25–<50 | 175,005.9 | 5428 | 31.0 | 0.97 (0.92–1.01) | 0.94 (0.90–0.99) | 0.96 (0.91–1.00) | 0.81 (0.77–0.86) |
| 50–<75 | 47,255.6 | 1359 | 28.8 | 0.87 (0.81–0.92) | 0.84 (0.78–0.90) | 0.90 (0.84–0.97) | 0.67 (0.63–0.72) |
| ≥75 | 11,863.1 | 291 | 24.5 | 0.69 (0.61–0.78) | 0.67 (0.59–0.76) | 0.82 (0.72–0.92) | 0.56 (0.50–0.62) |
| P | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Men (n = 107,010) | |||||||
| <25 | 41,565.2 | 1040 | 25.0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25–<50 | 237,344.6 | 5896 | 24.8 | 0.98 (0.92–1.05) | 0.92 (0.86–0.98) | 0.88 (0.82–0.94) | 0.74 (0.69–0.80) |
| 50–<75 | 106,818.3 | 2555 | 23.9 | 0.92 (0.86–0.99) | 0.82 (0.77–0.89) | 0.78 (0.72–0.84) | 0.61 (0.56–0.65) |
| ≥75 | 22,251.7 | 458 | 20.6 | 0.76 (0.68–0.84) | 0.67 (0.60–0.75) | 0.71 (0.63–0.79) | 0.48 (0.43–0.53) |
| P | <0.001 | <0.001 | <0.001 | <0.001 | |||
aEstimated using Cox proportional hazard models. Multivariable model 1 was adjusted for age, sex (only for total), centre, year of screening examination, alcohol consumption, smoking, physical activity, total energy intake, education level, medication for hypertension, medication for diabetes, multivitamin supplement use, calcium supplement use, and season; model 2: model 1 plus adjustment for BMI; bEstimated using Cox proportional hazard models with categories of serum 25(OH)D levels, smoking, alcohol consumption, physical activity, total energy intake, medication for hypertension, medication for diabetes, multivitamin supplement use, calcium supplement use, season, and BMI as time-dependent variables and baseline age, sex (only for total), centre, year of screening examination, and education level as time-fixed variables.
HR, hazards ratio; PY, person-year.
Figure 2.
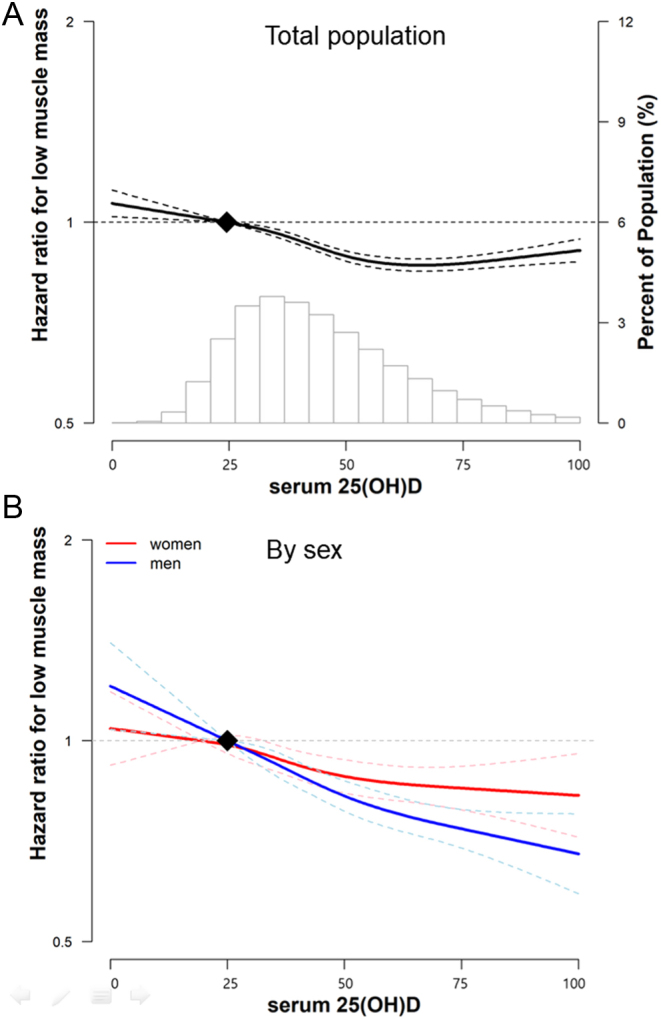
Multivariable-adjusted hazard ratios for the development of low muscle mass in the total population (A) and sex-specific (B). Curves represent adjusted hazard ratios for low muscle mass based on restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles of serum 25(OH)D distribution. Models were adjusted for age, sex (only for total), centre, year of screening examination, alcohol consumption, smoking, physical activity, total energy intake, education level, ongoing medication for hypertension and/or diabetes, multivitamin and/or calcium supplementation, season, and BMI. A full colour version of this figure is available at https://doi.org/10.1530/EJE-21-1229.
Changes in 25(OH)D levels from baseline to follow-up were significantly associated with the risk of incident LMM without any significant interaction by sex (P for interaction = 0.326) (Table 3). The multivariable-adjusted HRs (95% CI) for the ‘decreased’, ‘increased’, and ‘persistently adequate’ groups vs the ‘persistently low’ group for LMM development were 0.84 (0.77–0.92), 0.85 (0.79–0.91), and 0.81 (0.75–0.87), respectively (model 2). The significant associations persisted after serum 25(OH)D levels and other confounders were considered time-varying variables.
Table 3.
LMM development by the changes in serum 25(OH)D level from baseline to subsequent visit (n = 131,595). P = 0.326 for the overall interaction between sex and sex and serum 25(OH)D levels for incident low muscle mass (multivariable-adjusted model 2).
| 25(OH)D levels (nmol/L) | Person-years | Incident cases | Incidence density (/103 PY) | Age- and sex-adjusted HR (95% CI) | Multivariable-adjusted HRa (95% CI) | HR (95% CI)b in a model with time-dependent variables | ||
|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Model 1 | Model 2 | |||||
| Total | ||||||||
| <50 | <50 | 212,785.0 | 4157 | 19.5 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥50 | <50 | 33,319.2 | 556 | 16.7 | 0.85 (0.78–0.93) | 0.85 (0.78–0.93) | 0.84 (0.77–0.92) | 0.86 (0.78–0.94) |
| <50 | ≥50 | 63,741.6 | 1001 | 15.7 | 0.80 (0.75–0.86) | 0.85 (0.80–0.92) | 0.85 (0.79–0.91) | 0.87 (0.81–0.93) |
| ≥50 | ≥50 | 60,559.7 | 956 | 15.8 | 0.79 (0.74–0.85) | 0.81 (0.75–0.87) | 0.81 (0.75–0.87) | 0.84 (0.78–0.90) |
| Women | ||||||||
| <50 | <50 | 101,903.1 | 2262 | 22.2 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥50 | <50 | 10,964.2 | 201 | 18.3 | 0.83 (0.72–0.95) | 0.81 (0.70–0.93) | 0.88 (0.76–1.02) | 0.87 (0.75–1.01) |
| <50 | ≥50 | 25,365.8 | 410 | 16.2 | 0.73 (0.66–0.81) | 0.76 (0.69–0.85) | 0.80 (0.72–0.89) | 0.84 (0.75–0.93) |
| ≥50 | ≥50 | 15,201.1 | 257 | 16.9 | 0.75 (0.66–0.85) | 0.75 (0.66–0.86) | 0.83 (0.72–0.94) | 0.89 (0.78–1.01) |
| Men | ||||||||
| <50 | <50 | 110,881.9 | 1895 | 17.1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥50 | <50 | 22,355.0 | 355 | 15.9 | 0.93 (0.83–1.04) | 0.84 (0.75–0.94) | 0.83 (0.74–0.93) | 0.85 (0.76–0.95) |
| <50 | ≥50 | 38,375.8 | 591 | 15.4 | 0.90 (0.82–0.99) | 0.93 (0.85–1.03) | 0.90 (0.82–0.98) | 0.89 (0.81–0.97) |
| ≥50 | ≥50 | 45,358.6 | 699 | 15.4 | 0.88 (0.81–0.97) | 0.82 (0.75–0.90) | 0.80 (0.74–0.88) | 0.82 (0.76–0.90) |
aEstimated using Cox proportional hazard models. The multivariable model 1 was adjusted for age, sex, centre, year of screening examination, alcohol consumption, smoking, physical activity, total energy intake, education level, and BMI; model 2: model 1 plus adjustment for medication for hyperlipidaemia, medication for diabetes, multivitamin supplement, vitamin D supplement, and calcium supplement. bEstimated using Cox proportional hazard models with categories of serum 25(OH)D levels, smoking, alcohol consumption, physical activity, total energy intake, BMI, medication for hyperlipidaemia, medication for hyperlipidaemia, medication for diabetes, multivitamin supplement, vitamin D supplement, and calcium supplement as time-dependent variables and baseline age, centre, year of screening examination, and education level as time-fixed variables.
HR, hazards ratio; LMM, low muscle mass; PY, person-years.
In subgroup analyses (Supplementary Table 4), the association between 25(OH)D level and incident LMM differed with respect to hypertension, insulin resistance, and inflammation status; the association was evident in participants without either homeostasis model assessment of insulin resistance (HOMA-IR) of ≥2.5 or hypertension but was attenuated in those with either insulin resistance or hypertension (P for interaction <0.001 and 0.002, respectively). The graded dose-response association between 25(OH)D levels and incident LMM was slightly stronger in those with hsCRP < 1.0 mg/L than in those with hsCRP ≥ 1.0 mg/L (P for interaction = 0.018). Otherwise, there were no other significant interactions by subgroup, including the age group (<40 vs ≥40 years). Participants taking vitamin D supplements tended to engage in a healthier lifestyle including physical activity and less smoking (Supplementary Table 5); however, after adjustments for physical activity and smoking status, there was an independent and inverse association between serum 25(OH)D levels and incident LMM. Additionally, in subgroup analyses, these associations were similarly observed, and there was no significant interaction, by smoking status, alcohol intake, and physical activity.
Discussion
In this large cohort study of young Korean adults without LMM at baseline, serum 25(OH)D levels were inversely associated with LMM development in a dose-response manner. The protective association between higher serum 25(OH)D levels and decreased LMM incidence was consistently observed irrespective of sex and age. Furthermore, increases in 25(OH)D levels from insufficient levels at baseline to 50 nmol/L at follow-up and adequate 25(OH)D levels over time were associated with lower risk of incident LMM; these associations were independent of factors such as vitamin D supplementations, exercise, BMI, or season of the blood draw.
It has been well documented that vitamin D insufficiency/deficiency is frequently observed in older people with sarcopenia (6, 7). However, as most studies exploring the link between vitamin D and sarcopenia/LMM by far were almost exclusively focused on older adults, the effect of vitamin D on the risk of LMM among younger adults is unknown. There are few cross-sectional studies that have explored the effects of serum vitamin D levels in muscle mass in younger individuals. A study of 667 community-dwelling adults aged 21–97 years showed significant associations between 25(OH)D levels and muscle mass only in participants younger than 65 years (9). Some other studies have also reported the potential benefit of serum vitamin D on muscle mass; however, these studies were undertaken in specific population subgroups (e.g. obese men) (10, 11). To our knowledge, our study is the first cohort study showing that adequate serum 25(OH)D levels confer decreased risk of incident LMM in young and middle-aged individuals without comorbidities. Also, while there is scarce data on the prevalence of sarcopenia or LMM in young populations, a previous report has estimated that, among adults aged 21–59 years, up to 32% have LMM and 7% have sarcopenia, suggesting that it is already prevalent among younger adults (4). Likewise, our findings on the incident rate of LMM (27.1 per 1,000 person-years) further supports the notion that LMM in young adults is no longer an uncommon condition.
In our study, persistently adequate serum 25(OH)D levels over time and increases in serum 25(OH)D levels from being insufficient to sufficient were significantly associated with decreased LMM risk. The effect of time-dependent changes of serum 25(OH)D levels on preserving muscle mass has been uncertain, with a lack of comparable data. Two previous RCTs evaluated the benefit of vitamin D supplementation and changes in vitamin D levels on muscle mass and strength in young and middle-aged individuals, but neither found any significant benefits of vitamin D in improving muscle mass (12, 13). However, it is difficult to directly compare these study results with ours because these trials were underpowered with a sample size <40 and had a very short-term follow-up (12 weeks) in a setting of resistance training, wherein vitamin D was supplemented only as an adjunct intervention. In our large sample of 192,908 healthy participants free of LMM at baseline, we could account for various known confounders, as well as time-dependent variables. Also, the extended follow-up duration of approximately 4 years allowed the extended time frame for us to better observe the development of LMM over time. Although the possibility of residual confounding remains due to unmeasured factors including sun exposure or outdoor physical activity, our findings suggest that improved or persistently adequate serum 25(OH)D status over time may have benefit in reducing the risk of incident LMM.
The present study has several important clinical implications. Earlier onset of sarcopenia has constantly been increasing, especially in developed countries, possibly owing to changes in lifestyle and diets (4), and there is an emerging need for taking a life-course approach to sarcopenia prevention during early years (4, 36). Given the progressive nature of and the seriousness of disability and complications associated with sarcopenia (1), preventing mild LMM may in turn delay further loss of muscle mass, consequently lowering the risk of sarcopenia as well as sarcopenia-related health consequences in later life. In light of this, our findings suggest that the prevention of early unfavourable changes in muscle mass and mild LMM may be achievable in young individuals by maintaining sufficient serum 25(OH)D levels. In addition, the proportion of our study participants with sub-optimal 25(OH)D levels (<50 nmol/L) at baseline (approximately 74%) is considerably higher than that in the United States and Europe (24–40%) (37), although it is comparable to the previously reported national prevalence in the Korean population (38). We assume that a high proportion of white-collar workers in our population who are likely to have less sun exposure may have contributed to the relatively high prevalence of sub-optimal serum 25(OH)D levels. Our findings thus highlight the importance of maintaining adequate serum 25(OH)D levels to reduce the risk of LMM in populations with a high prevalence of low vitamin D status. Large and well-designed intervention trials are necessary to confirm our findings.
The mechanism by which serum 25(OH)D reduces LMM risk is not completely understood, but recent studies confirm that VDR is expressed in skeletal muscle and that a substantial level of signalling via VDR is required for normal muscle growth and muscle mass maintenance (39). In animal studies, VDR knockout mice had small and variable muscle fibres (40); vitamin D deficiency in rats inhibited mammalian target of rapamycin complex 1 (mTORC1) signalling and contributed to decreased protein synthesis in skeletal muscles (41), while VDR overexpression induced muscle hypertrophy (5). In human muscle tissue, VDR expression levels, which decline with age, can be altered using vitamin D supplementation (42), indicating that maintaining adequate 25(OH)D levels could reduce LMM risk. 25(OH)D may also stimulate protein synthesis through mTORC1 signalling; this mechanism may play an important role in muscle hypertrophy and muscle loss prevention (39).
According to our subgroup analyses, the association between serum 25(OH)D and LMM was attenuated in participants with insulin resistance defined as HOMA-IR ≥ 2.5 and/or hypertension. Skeletal muscle is the key tissue responsible for insulin-stimulated glucose disposal and is the major site of peripheral insulin resistance (43). Muscle mass is determined by the balance between protein synthesis and breakdown in the tissue, and particularly in younger people, insulin has a predominant role in inhibiting protein catabolism, thereby preventing muscle atrophy (44). Insulin resistance thus may represent a state of ‘anabolic resistance’ in skeletal muscle, wherein the insulin-mediated suppression of muscle breakdown is inhibited, potentially leading to increased proteolysis that may eventually result in sarcopenia (45). VDRs are also involved in the pathogenesis of insulin resistance (46); a recent report showed that decreased glucose uptake reduced VDR expression in a diabetic mouse model (47). Therefore, a series of these interactive processes may act synergistically to attenuate the effect of vitamin D. The reason for the null association observed in the presence of hypertension is unclear. Hypertension is pathologically related to hyperactivity of the renin-angiotensin system (RAS), and animal studies show the involvement of VDR activation in downregulating RAS (48). High circulating levels of angiotensin II decrease muscle protein homeostasis and accelerate proteolysis, thereby promoting skeletal muscle fibre atrophy (49). Thus, in hypertension, vitamin D metabolism may not compensate for the effects of RAS overactivation. Future studies are warranted to better elucidate the role of insulin resistance and hypertension in the association between 25(OH)D and LMM.
The strengths of our study include the large sample size, carefully standardized clinical examination, imaging, and laboratory procedures and include the assessment of physical activity and other lifestyle factors. Also, the longitudinal cohort study design with repeated measurements of 25(OH)D levels and confounders enabled us to examine temporal and independent associations of serum 25(OH)D status and changes in serum 25(OH)D status over time with LMM risk. We also considered the changes of covariates during follow-up in our time-dependent model, wherein 25(OH)D levels and other covariates were considered time-varying variables.
This study had some limitations. First, we used bioimpedance analysis instead of DEXA, which is the gold standard body composition measurement for assessing muscle mass. DEXA, however, may expose participants to low-level ionizing radiation and is expensive to perform in large cohort studies. Secondly, we did not collect information on variables that could influence the serum 25(OH)D levels such as vitamin D intake via food consumption, details on the amount and frequency of vitamin D supplementation (e.g. dose, frequency, and duration), outdoor activities, or sunlight exposure, or presence of genetic polymorphism. Therefore, the potential for residual confounding remains. Thirdly, the reference values used in our study in defining LMM were derived from the young adults in this study population since there is no available value derived from the representative sample of the Korean population based on bioimpedance analysis. According to the fourth and fifth KNHANES, the cut-off values of 1 s.d. below the mean for DEXA-based SMI of young adults were 32.2 and 29.9%, respectively, for men and 25.6 and 23.5%, respectively, for women (50, 51), which were similar to the cut-off values used in our study (30.2% for men and 26.1% for women). Finally, our study participants represented a relatively young and healthy Korean working population. Although this could be perceived as a limitation, it also represented a strength of our study as relatively few study participants had existing comorbidities that are associated with low serum 25(OH)D levels. Nevertheless, the generalizability of our findings to other populations with comorbidities or different sociodemographic characteristics may be limited.
In conclusion, we demonstrated that serum 25(OH)D levels are inversely associated with LMM risk in young adults. Favourable changes in serum 25(OH)D levels from insufficient to sufficient were associated with reduced LMM risk. Considering the importance of attaining high peak muscle mass during adulthood for sarcopenia prevention, maintaining sufficient serum 25(OH)D levels, which may be easily achieved by sun exposure or vitamin D supplementation, could be an effective primary prevention strategy to slow muscle loss and its associated consequences in later years.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this study.
Funding
This work was supported by SKKU Excellence in Research Award Research Fund, Sungkyunkwan University, 2020. C D B is supported in part by the Southampton NIHR Biomedical Research Centre (IS-BRC-20004).
Author contribution statement
Yejin Kim: interpretation of data, drafting and critical revision of the manuscript. Yoosoo Chang: study concept and design, acquisition of data, interpretation of data, and drafting and critical revision of the manuscript. Seungho Ryu: study concept and design, acquisition of data, analysis and interpretation of data, and critical revision of the manuscript. In Young Cho: interpretation of data and critical revision of the manuscript. Min-Jung Kwon: acquisition of data and critical revision of the manuscript. Sarah H Wild: interpretation of data and critical revision of the manuscript. Christopher D Byrne: interpretation of data and critical revision of the manuscript.
References
- 1.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AAet al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing 20194816–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roubenoff R, Parise H, Payette HA, Abad LW, D’Agostino R, Jacques PF, Wilson PW, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. American Journal of Medicine 2003115429–435. ( 10.1016/j.amjmed.2003.05.001). [DOI] [PubMed] [Google Scholar]
- 3.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. Journal of Nutrition, Health and Aging 200812427–432. ( 10.1007/BF02982703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang BWJ, Wee S-L, Lau LK, Jabbar KA, Seah WT, Ng DHM, Ling Tan QL, Chen KK, Jagadish MU, Ng TP. Prevalence and associated factors of sarcopenia in Singaporean adults – the Yishun study. Journal of the American Medical Directors Association 202122885.e1–885.e10. ( 10.1016/j.jamda.2020.05.029) [DOI] [PubMed] [Google Scholar]
- 5.Bass JJ, Nakhuda A, Deane CS, Brook MS, Wilkinson DJ, Phillips BE, Philp A, Tarum J, Kadi F, Andersen Det al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Molecular Metabolism 202042101059. ( 10.1016/j.molmet.2020.101059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirani V, Cumming RG, Naganathan V, Blyth F, Le Couteur DG, Hsu B, Handelsman DJ, Waite LM, Seibel MJ. Longitudinal associations between vitamin d metabolites and sarcopenia in older australian men: the concord health and aging in men project. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences 201773131–138. ( 10.1093/gerona/glx086) [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Jang G, Park JW, Lee YK, Koo KH. Vitamin D deficiency and sarcopenia in hip fracture patients. Journal of Bone Metabolism 20212879–83. ( 10.11005/jbm.2021.28.1.79) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. Journal of Clinical Endocrinology and Metabolism 2010951595–1601. ( 10.1210/jc.2009-2309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marantes I, Achenbach SJ, Atkinson EJ, Khosla S, Melton 3rd LJ, Amin S. Is vitamin D a determinant of muscle mass and strength? Journal of Bone and Mineral Research 2011262860–2871. ( 10.1002/jbmr.510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shantavasinkul PC, Phanachet P, Puchaiwattananon O, Chailurkit LO, Lepananon T, Chanprasertyotin S, Ongphiphadhanakul B, Warodomwichit D. Vitamin D status is a determinant of skeletal muscle mass in obesity according to body fat percentage. Nutrition 201531801–806. ( 10.1016/j.nut.2014.11.011) [DOI] [PubMed] [Google Scholar]
- 11.Yakout SM, Al-Daghri NM, Bukhari I, Khattak MNK, Sabico S, Alokail MS, Al-Attas OS. Vitamin D level and its relation to muscle and fat mass in adult male Arabs. Saudi Journal of Biological Sciences 2020272452–2456. ( 10.1016/j.sjbs.2020.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrillo AE, Flynn MG, Pinkston C, Markofski MM, Jiang Y, Donkin SS, Teegarden D. Impact of vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clinical Nutrition 201332375–381. ( 10.1016/j.clnu.2012.08.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savolainen L, Timpmann S, Mooses M, Maestu E, Medijainen L, Tonutare L, Ross F, Lellsaar M, Unt E, Oopik V. Vitamin D supplementation does not enhance resistance training-induced gains in muscle strength and lean body mass in vitamin D deficient young men. European Journal of Applied Physiology 20211212077–2090. ( 10.1007/s00421-021-04674-9) [DOI] [PubMed] [Google Scholar]
- 14.Chang Y, Ryu S, Sung KC, Cho YK, Sung E, Kim HN, Jung HS, Yun KE, Ahn J, Shin Het al. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: evidence from the Kangbuk Samsung Health Study. Gut 2019681667–1675. ( 10.1136/gutjnl-2018-317666) [DOI] [PubMed] [Google Scholar]
- 15.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JFet al. International physical activity questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise 2003351381–1395. ( 10.1249/01.MSS.0000078924.61453.FB) [DOI] [PubMed] [Google Scholar]
- 16.McLester CN, Nickerson BS, Kliszczewicz BM, McLester JR. Reliability and agreement of various in body body composition analyzers as compared to dual-energy X-ray absorptiometry in healthy men and women. Journal of Clinical Densitometry 202023443–450. ( 10.1016/j.jocd.2018.10.008) [DOI] [PubMed] [Google Scholar]
- 17.Sergi G, De Rui M, Stubbs B, Veronese N, Manzato E. Measurement of lean body mass using bioelectrical impedance analysis: a consideration of the pros and cons. Aging Clinical and Experimental Research 201729591–597. ( 10.1007/s40520-016-0622-6) [DOI] [PubMed] [Google Scholar]
- 18.Petta S, Ciminnisi S, Di Marco V, Cabibi D, Camma C, Licata A, Marchesini G, Craxi A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Alimentary Pharmacology and Therapeutics 201745510–518. ( 10.1111/apt.13889) [DOI] [PubMed] [Google Scholar]
- 19.Rangel Peniche DB, Raya Giorguli G, Aleman-Mateo H. Accuracy of a predictive bioelectrical impedance analysis equation for estimating appendicular skeletal muscle mass in a non-Caucasian sample of older people. Archives of Gerontology and Geriatrics 20156139–43. ( 10.1016/j.archger.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 20.Kim M, Shinkai S, Murayama H, Mori S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatrics and Gerontology International 2015151013–1022. ( 10.1111/ggi.12384) [DOI] [PubMed] [Google Scholar]
- 21.Ling CH, de Craen AJ, Slagboom PE, Gunn DA, Stokkel MP, Westendorp RG, Maier AB. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clinical Nutrition 201130610–615. ( 10.1016/j.clnu.2011.04.001) [DOI] [PubMed] [Google Scholar]
- 22.Kim M, Kim H. Accuracy of segmental multi-frequency bioelectrical impedance analysis for assessing whole-body and appendicular fat mass and lean soft tissue mass in frail women aged 75 years and older. European Journal of Clinical Nutrition 201367395–400. ( 10.1038/ejcn.2013.9) [DOI] [PubMed] [Google Scholar]
- 23.Malavolti M, Mussi C, Poli M, Fantuzzi AL, Salvioli G, Battistini N, Bedogni G. Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Annals of Human Biology 200330380–391. ( 10.1080/0301446031000095211) [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Hai S, Cao L, Zhou J, Liu P, Dong BR. Estimation of prevalence of sarcopenia by using a new bioelectrical impedance analysis in Chinese community-dwelling elderly people. BMC Geriatrics 201616 216. ( 10.1186/s12877-016-0386-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization & Regional Office for the Western. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications Australia, 2000. [Google Scholar]
- 26.Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, Zhao D, Jung HS, Choi Y, Ahn Jet al. Metabolically-healthy obesity and coronary artery calcification. Journal of the American College of Cardiology 2014632679–2686. ( 10.1016/j.jacc.2014.03.042) [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 198528412–419. ( 10.1007/BF00280883) [DOI] [PubMed] [Google Scholar]
- 28.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society 200250889–896. ( 10.1046/j.1532-5415.2002.50216.x) [DOI] [PubMed] [Google Scholar]
- 29.Elsenberg EHAM, Ten Boekel E, Huijgen H, Heijboer AC. Standardization of automated 25-hydroxyvitamin D assays: how successful is it? Clinical Biochemistry 2017501126–1130. ( 10.1016/j.clinbiochem.2017.06.011) [DOI] [PubMed] [Google Scholar]
- 30.Heijboer AC, Blankenstein MA, Kema IP, Buijs MM. Accuracy of 6 routine 25-hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clinical Chemistry 201258543–548. ( 10.1373/clinchem.2011.176545) [DOI] [PubMed] [Google Scholar]
- 31.Cashman KD.Vitamin D deficiency: defining, prevalence, causes, and strategies of addressing. Calcified Tissue International 202010614–29. ( 10.1007/s00223-019-00559-4) [DOI] [PubMed] [Google Scholar]
- 32.Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National Academies Collection: Reports funded by National Institutes of Health. In: Eds Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Washington (DC), 2011. ( 10.17226/13050) [DOI] [Google Scholar]
- 33.Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie Oet al. Global consensus recommendations on prevention and management of nutritional rickets. Journal of Clinical Endocrinology and Metabolism 2016101394–415. ( 10.1210/jc.2015-2175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giustina A, Adler RA, Binkley N, Bouillon R, Ebeling PR, Lazaretti-Castro M, Marcocci C, Rizzoli R, Sempos CT, Bilezikian JP. Controversies in vitamin D: summary statement from an International Conference. Journal of Clinical Endocrinology and Metabolism 2019104234–240. ( 10.1210/jc.2018-01414) [DOI] [PubMed] [Google Scholar]
- 35.Wen CP, David Cheng TY, Tsai SP, Chan HT, Hsu HL, Hsu CC, Eriksen MP. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutrition 200912497–506. ( 10.1017/S1368980008002802) [DOI] [PubMed] [Google Scholar]
- 36.Azzolino D, Spolidoro GCI, Saporiti E, Luchetti C, Agostoni C, Cesari M. Musculoskeletal changes across the lifespan: nutrition and the life-course approach to prevention. Frontiers in Medicine 20218697954. ( 10.3389/fmed.2021.697954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Kostenberger M, Tmava Berisha A, Martucci G, Pilz S, Malle O. Vitamin D deficiency 2.0: an update on the current status worldwide. European Journal of Clinical Nutrition 2020741498–1513. ( 10.1038/s41430-020-0558-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SJ, Ryu SY, Park J, Choi SW. Association of sarcopenia with metabolic syndrome in Korean population using 2009–2010 Korea national health and nutrition examination survey. Metabolic Syndrome and Related Disorders 201917494–499. ( 10.1089/met.2019.0059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagatsuma A, Sakuma K. Vitamin D signaling in myogenesis: potential for treatment of sarcopenia. BioMed Research International 20142014121254. ( 10.1155/2014/121254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srikuea R, Zhang X, Park-Sarge OK, Esser KA. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. American Journal of Physiology: Cell Physiology 2012303C396–C405. ( 10.1152/ajpcell.00014.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gogulothu R, Nagar D, Gopalakrishnan S, Garlapati VR, Kallamadi PR, Ismail A. Disrupted expression of genes essential for skeletal muscle fibre integrity and energy metabolism in vitamin D deficient rats. Journal of Steroid Biochemistry and Molecular Biology 2020197105525. ( 10.1016/j.jsbmb.2019.105525) [DOI] [PubMed] [Google Scholar]
- 42.Pojednic RM, Ceglia L, Olsson K, Gustafsson T, Lichtenstein AH, Dawson-Hughes B, Fielding RA. Effects of 1,25-dihydroxyvitamin D3 and vitamin D3 on the expression of the vitamin D receptor in human skeletal muscle cells. Calcified Tissue International 201596256–263. ( 10.1007/s00223-014-9932-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocrine Reviews 20133433–83. ( 10.1210/er.2012-1012) [DOI] [PubMed] [Google Scholar]
- 44.Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia 20165944–55. ( 10.1007/s00125-015-3751-0) [DOI] [PubMed] [Google Scholar]
- 45.Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia 2009521889–1898. ( 10.1007/s00125-009-1430-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen S, Villalta SA, Agrawal DK. FOXO1 mediates vitamin D deficiency-induced insulin resistance in skeletal muscle. Journal of Bone and Mineral Research 201631585–595. ( 10.1002/jbmr.2729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morro M, Vila L, Franckhauser S, Mallol C, Elias G, Ferre T, Molas M, Casana E, Rodo J, Pujol Aet al. Vitamin D receptor overexpression in beta-cells ameliorates diabetes in mice. Diabetes 202069927–939. ( 10.2337/db19-0757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekiz T, Kara M, Ata AM, Ricci V, Kara Ö, Ozcan F, Ozcakar L. Rewinding sarcopenia: a narrative review on the renin-angiotensin system. Aging Clinical and Experimental Research 2021332379–2392. ( 10.1007/s40520-020-01761-3) [DOI] [PubMed] [Google Scholar]
- 49.Yoshida T, Tabony AM, Galvez S, Mitch WE, Higashi Y, Sukhanov S, Delafontaine P. Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: potential therapeutic targets for cardiac cachexia. International Journal of Biochemistry and Cell Biology 2013452322–2332. ( 10.1016/j.biocel.2013.05.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, Song Ge, Kim HJ, Choi YJ, Kim KM. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the fourth Korean National Health and Nutritional Examination Surveys. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences 2012671107–1113. ( 10.1093/gerona/gls071) [DOI] [PubMed] [Google Scholar]
- 51.Moon JJ, Park SG, Ryu SM, Park CH. New skeletal muscle mass index in diagnosis of sarcopenia. Journal of Bone Metabolism 20182515–21. ( 10.11005/jbm.2018.25.1.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a