Abstract
Engineered molecular circuits that process information in biological systems could address emerging human health and biomanufacturing needs. However, such circuits can be difficult to rationally design and scale. DNA-based strand displacement reactions have demonstrated the largest and most computationally powerful molecular circuits to date but are limited in biological systems due to the difficulty in genetically encoding components. Here, we develop scalable cotranscriptionally encoded RNA strand displacement (ctRSD) circuits that are rationally programmed via base pairing interactions. ctRSD circuits address the limitations of DNA-based strand displacement circuits by isothermally producing circuit components via transcription. We demonstrate circuit programmability in vitro by implementing logic and amplification elements, as well as multilayer cascades. Furthermore, we show that circuit kinetics are accurately predicted by a simple model of coupled transcription and strand displacement, enabling model-driven design. We envision ctRSD circuits will enable the rational design of powerful molecular circuits that operate in biological systems, including living cells.
Self-assembling RNA circuits that execute programmable logic, amplification, and cascades are developed for synthetic biology.
INTRODUCTION
A major goal of synthetic biology is developing programmable molecular circuits that can be rationally engineered to process information in biological systems. Such circuits have the potential to address emerging challenges in human health and disease (1), agriculture (2), and biomanufacturing (3). To meet these diverse needs, molecular circuits must be scalable, modular, and rationally programmable to execute operations like logic, signal amplification, and multilayer cascades. Furthermore, circuits capable of a wide range of computations beyond Boolean logic, such as molecular pattern recognition (4), could greatly expand existing capabilities. A key challenge to developing such circuits is identifying molecular components that not only meet the above criteria but also behave predictably to enable model-driven design.
The predictable and programmable Watson-Crick base pairing interactions of nucleic acids have led to their adoption as versatile components for molecular circuit programming. In particular, in vitro circuits based on toehold-mediated strand displacement (TMSD) reactions have demonstrated sophisticated digital computations and mathematical operations (5), molecular pattern recognition (4, 6), signal cascades (7) and amplifiers (5, 8, 9), and complex dynamics (9, 10). In TMSD reactions, a single-stranded input binds to a double-stranded nucleic acid gate via a single-stranded toehold domain and displaces an output strand with a new exposed toehold that can facilitate further TMSD reactions (Fig. 1A). Interactions between inputs and gates are programmed through sequence complementarity, and the combinatorial nucleic acid sequence space has allowed TMSD reaction networks to be scaled up to >100 components (4). In addition, reaction kinetics can be tuned over six orders of magnitude by simply changing the length of the toehold (11). These properties have enabled predictive models of TMSD circuit behavior that allow circuit design abstraction (12).
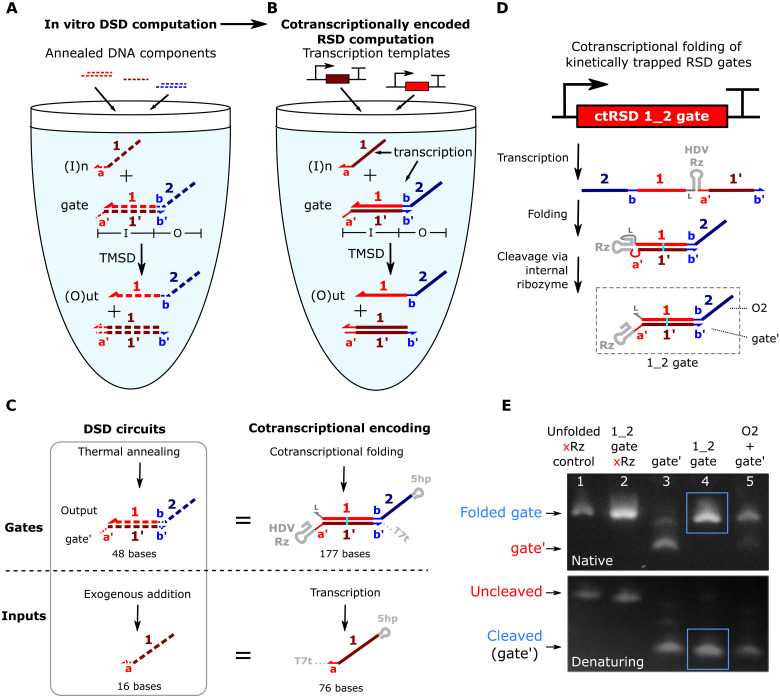
Fig. 1. Cotranscriptionally encoded RNA strand displacement (ctRSD) circuit design.
(A) In DNA strand displacement (DSD) circuits, pre-annealed DNA gates are mixed to build a circuit. Strand exchange between the input and gate releases an output. (B) In ctRSD circuits, designed transcription templates produce the RNA components that make up a circuit. DNA and RNA are represented with dashed and solid lines, respectively. Bold letters and numbers represent sequence identity. A prime (′) denotes complementarity. Only one domain of each complementary pair will be shown in subsequent figures for simplicity. The I and O below the gate represent input and output domains, respectively. (C) Transcriptional encoding of ctRSD components. All RNAs have a 5′ hairpin (5hp) and a 3′ terminator (T7t). For simplicity, these motifs are omitted elsewhere. The cyan line represents a G-U wobble pair. The gate contains a self-cleaving ribozyme (HDV Rz) to enable cotranscriptional folding of kinetically trapped gates (D) (see fig. S1 for schematics with sequences). (D) ctRSD gates fold into RNA hairpins that self-cleave to produce reactive dsRNA products. Input and output domains define gate names (e.g., “1_2 gate”). (E) Gel electrophoresis demonstrating gate folding and cleavage (lane 4, blue box) after 30 min of transcription followed by 30 min of deoxyribonuclease (DNase) degradation. Lane 1: a transcript that is the same length as the gate but does not fold into a hairpin or cleave (xRz). Lane 2: the 1_2 gate without cleavage (xRz). Lane 3: the gate′ strand (Rz, a′-, 1′-, and b′-domains) alone. Lane 5: separate transcription of the output (O2) and gate′ strands. The 46-base single-stranded O2 strand stained poorly for visualization (see fig. S2 for control transcript designs) (52).
Because TMSD circuits are composed of nucleic acids, they have great potential for integration with biological systems. However, these circuits have primarily been implemented in vitro using DNA components that are not easily genetically encoded (13). This restricts their applications in synthetic biology, particularly in vivo (14). A key challenge to operating TMSD circuits in biological systems is developing a method to isothermally prepare all circuit components in a single reaction. Typically, TMSD components are thermally annealed separately to prevent spurious reactions between gates and then mixed to make a circuit (4–6). Thus, these circuits currently cannot be continuously produced in the same place they are operated. Although TMSD circuits can be prepared and then added to biological samples (15) or transfected into cells (16) at fixed concentrations, these implementations are only single use (17, 18), and circuit lifetime is limited by component degradation. A method to continuously produce TMSD circuits in situ could greatly expand their capabilities. Genetically encoded RNA-based circuits that use strand displacement have been developed (19–23), and other transcription-based circuits have achieved some of the capabilities of TMSD circuits (24, 25). However, these systems have yet to demonstrate the predictive design and scale-up seen in state-of-the-art DNA-based circuits.
Here, we develop scalable and programmable cotranscriptionally encoded RNA strand displacement (ctRSD) circuits. In ctRSD circuits, components isothermally self-assemble during transcription and execute programmed computations in the same reaction. We validate ctRSD circuit performance in vitro by building circuits that execute logic, signal amplification, and multilayer cascades. We demonstrate the scalability and modularity of ctRSD circuits by successfully implementing 13 ctRSD gates in eight different circuit topologies. We find ctRSD circuit kinetics are well predicted by a simple model of coupled transcription and strand displacement that assumes uniform kinetic behavior across gates, facilitating predictive circuit engineering. Furthermore, ctRSD circuits are designed so that state-of-the-art DNA-based circuits capable of neural network computations and pattern recognition (4, 6) could be directly adopted. ctRSD circuits should enable the power of TMSD to be realized in biological systems for smart diagnostics or sensors (6, 26, 27). Ultimately, ctRSD circuits could be genetically encoded and continuously operated inside living cells.
RESULTS
Design of ctRSD circuit components
To develop ctRSD circuits, we sought a system in which modular and programmable strand displacement circuit components could be isothermally produced via transcription. In TMSD circuits, modularity is achieved by designing toehold exchange gates that allow any input sequence to be converted into any output sequence through a gate (4, 5, 7, 11). For example, in Fig. 1A, the input domain is composed of the a′-toehold and the 1:1′-domain duplex, both of which are complementary to the input, I1. The output domain is composed of the sequestered b-toehold and the two-domain overhang, neither of which share complementarity with I1. Thus, input and output domain sequences are independent. We adopted an analogous modular gate design for ctRSD circuits (Fig. 1B). In these toehold exchange gates, the b-toehold of the output is sequestered in a duplex, kinetically precluding a reaction downstream unless the gate input is present. In DNA-based circuits, the toehold exchange gates are thermally annealed in separate test tubes to kinetically trap the outputs before circuit components are mixed. In ctRSD circuits, the RNA toehold exchange gates must isothermally assemble into kinetically trapped intermediates in a single pot during transcription. Simply transcribing the two gate strands separately and allowing them to hybridize to form a gate is not a viable option, as the output strand of the gate can also react with downstream gates, introducing prohibitive leak (fig. S7).
To transcriptionally encode kinetically trapped RNA toehold exchange gates, we inserted a self-cleaving RNA ribozyme motif between the two strands of the gate (Fig. 1C). This motif allows us to encode RNA gates as single transcripts that fold into hairpins and then cleave to yield reactive gates (Fig. 1D). Cotranscriptional folding is at least one order of magnitude faster than transcription (28), so the RNA gates should fold before they have time to react downstream. The self-cleaving ribozyme also ensures 1:1 stoichiometry between the gate strands, further reducing the potential for leaks (29). Inclusion of the ribozyme motif is critical, as the cotranscriptionally folded RNA gate exhibited >7-fold lower downstream leak rate than transcribing the two strands of the RNA gate separately (fig. S8). A 5′ hairpin motif and a 3′ hairpin terminator for T7 RNA polymerase (RNAP) were also appended to gates and inputs (Fig. 1C). The 5′ hairpin contains the T7 RNAP consensus initiation sequence to facilitate efficient and uniform transcription across components (30, 31). In addition, the 5′ hairpin ensures that short abortive transcripts produced during transcription initiation will not have sequence overlap with other circuit elements (32). The terminator hairpin reduces unwanted products associated with runoff transcription (33, 34) and enables incorporation into plasmids.
Figure 1C shows the final selection for our ctRSD gate design; however, there are many alternative implementations that would embody the same general features (see section S2). To optimize gate performance, we analyzed four considerations when selecting the final design: (i) directionality of the single-stranded toehold, (ii) domain sequence identity, (iii) domain transcription order, and (iv) self-cleaving ribozyme choice. We designed the ctRSD gates with 5′ toeholds because a 5′ toehold on an RNA gate allows the invading strand to participate in coaxial base stacking, increasing the binding strength compared to a 3′ toehold (35, 36). We restricted the gate output sequences to cytosine (C), adenine (A), or uracil (U) bases. This sequence constraint reduces unwanted secondary structure or dimerization of single-stranded components (4, 5, 7). A G-U wobble pair was also introduced in the middle of the hybridized portion of the gate to reduce DNA template synthesis errors (37) and to drive the forward strand displacement reaction with inputs that convert the G-U wobble pair to a G-C pair (38). The 5′ end of the output strand of the gate was selected as the starting point for transcription so that the first sequence produced would only have C, A, and U bases, preventing cotranscriptional folding into undesired secondary structure. This transcription order ensures that the G, A, and U restricted sequence of the strand that hybridizes to the output strand (i.e., the gate′ strand) is transcribed after its complementary sequence to promote folding of the RNA gate stem over alternative structures with G-U wobble pairs (see section S2B). For the self-cleaving ribozyme, we selected a variant of the hepatitis delta virus (HDV) ribozyme (see section S2C) (39). This ribozyme has no upstream or downstream sequence constraints, has a very stable fold (40), and has been reported to cleave itself with a rate constant of nearly 1 s−1 in vivo (41).
We used native and denaturing agarose gel electrophoresis to confirm the ctRSD gate fold and cleave as designed. On a native gel, the ctRSD gate (lane 4, Fig. 1E) was the same size as a control sample in which the two strands of the gate were transcribed from separate templates (lane 5, Fig. 1E), indicating full-length gate production and folding. On a denaturing gel, the primary product from the ctRSD gate (lane 4, Fig. 1E) migrated faster than the uncleaved control transcript (lane 2, Fig. 1E) and was the same size as the gate′ strand alone (lane 3, Fig. 1E), indicating ribozyme cleavage. The cleavage reaction is efficient and fast; we observed >90% cleavage in less than 15 min with an estimated cleavage rate constant of 0.25 min−1 (fig. S12).
Experimental characterization and modeling of ctRSD circuits
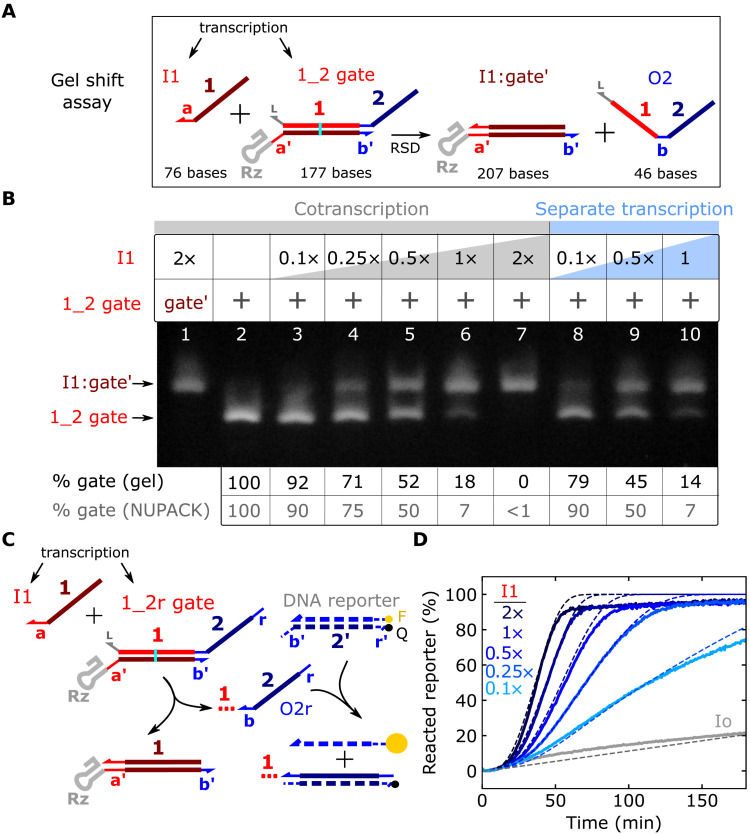
We next sought to characterize the reaction in which a ctRSD gate and its corresponding input are cotranscribed and react via strand displacement to release an output strand (Fig. 1B). The I1:gate′ product of the strand displacement reaction is a higher molecular weight than the unreacted gate, so we first analyzed the reaction with native gel electrophoresis (Fig. 2A). Increasing concentrations of I1 template increased the percentage of I1:gate′ product on the gel, with a 2:1 mixture of the I1 and 1_2 gate templates yielding ≈100% product (lanes 3 to 7, Fig. 2B). Assuming the transcription rates of I1 and 1_2 gate are approximately equal, the fraction of I1:gate′ produced with increasing I1 template concentration provides information about the thermodynamics of the reaction. We found that the percentage of unreacted 1_2 gate across input concentrations in experiments were within ≈12% of the thermodynamic predictions from NUPACK 3.2.2 (42) (Fig. 2B and section S3).
Fig. 2. Characterization of strand displacement in ctRSD circuits.
(A) Strand displacement between an input and a ctRSD gate. The I1:gate′ complex has 30 more bases than the gate. (B) Native RNA gel electrophoresis demonstrating strand displacement in a ctRSD circuit. Lane 1: I1:gate′ complex. Lane 2: 1_2 gate. Lanes 3 to 7: 25 nM 1_2 gate template was cotranscribed with 2.5 nM (0.1×) to 50 nM (2×) I1 template. The 46-base output strand of the gate (O2) was not visible (52). Transcription proceeded for 30 min, and electrophoresis was conducted 2 hours after DNase I addition. Lanes 8 to 10: I1 and 1_2 gate templates were transcribed separately for 30 min and subsequently incubated with DNase I for 30 min. Samples were then mixed in equal volumes and incubated at 37°C for 2 hours before electrophoresis. The table below the gel shows that the percentage of 1_2 gate in each lane agrees with NUPACK predictions (see fig. S14 for additional conditions). (C) Schematic of the fluorescent DNA reporter assay to measure O2r production. The red dotted line trailing O2r represents the upstream portion of the output strand not involved in downstream reactions. F and Q denote fluorophore and quencher modifications, respectively. (D) Experimental (solid lines) and simulated (dashed lines) DNA reporter signal during cotranscription of the 1_2r gate with different I1 template concentrations. The gray lines indicate the 1_2r gate cotranscribed with a randomized input sequence (Io) that does interact with the 1_2r gate. DNA template and T7 RNAP concentrations are tabulated in table S4 (see section S5 for simulation details).
The results in lanes 3 to 7 in Fig. 2B were obtained from simultaneous transcription of I1 and 1_2 gate, so the observed reaction between the two transcripts could result from I1 binding to 1_2 gate before folding, rather than strand displacement. To rule out this potential reaction pathway, we transcribed the I1 and 1_2 gate RNAs separately and then mixed them together after degradation of the DNA templates. Separate transcription followed by mixing yielded similar results to cotranscription (lanes 8 to 10, Fig. 2B and fig. S14), suggesting I1 and 1_2 gate react via the designed strand displacement mechanism. We further confirmed experimentally the correct a-toehold sequence and 1-domain branch migration sequence of the input were required for strand displacement (fig. S15).
To explore ctRSD circuit kinetics, we cotranscribed the input and gate templates alongside a DNA reporter complex designed to release a fluorescent signal upon reaction with the gate output strand (Fig. 2C). We opted to use a DNA-based reporter, rather than an RNA aptamer–based reporter (43), because the DNA reporter is easily calibrated to output concentration for modeling (4, 5, 7). To be stable at 37°C, we designed the reporter with a 16-base pair duplex. The 5′ end of the 1_2 gate was extended to include the full complement of the reporter (1_2r gate) to ensure an irreversible reaction. We fixed the 1_2r gate template concentration and varied the I1 template concentration. To ensure the same transcriptional load for comparison, a template that produced an unreactive input (Io) was added to maintain the same total input template concentration across samples (Materials and Methods). As expected from mass action kinetics, increasing concentrations of the I1 template resulted in faster reaction kinetics (Fig. 2D). Furthermore, separate transcription of the gate and input, followed by mixing and addition of the DNA reporter, exhibited kinetics consistent with strand displacement (fig. S16). A gate with a mutant ribozyme that cannot cleave resulted in >3-fold slower output production (fig. S18). Transcription of the 1_2r gate with only Io resulted in ≈20% of the maximum DNA reporter signal, indicating a slow leak reaction (Fig. 2D). The magnitude of this leak depended on T7 RNAP and total template concentrations (fig. S20).
We next investigated whether a mass action kinetic model of coupled transcription, ribozyme cleavage, and RNA strand displacement (see section S5A) could recapitulate the kinetics observed in ctRSD circuits. For model parameters, we used the ribozyme cleavage rate that we measured (fig. S12) and estimated order of magnitude strand displacement rate constants consistent with previous literature (see section S5B). We calibrated the transcription rate constant for each experiment with a control sample (Materials and Methods). Our initial model did not include any terms to describe the leak observed when the 1_2r gate was transcribed without the correct input and thus could not capture that effect (fig. S21, A and B).
To investigate the source of the leak, we evaluated how well incorporating plausible leak pathways into the model recapitulated the experimental leak kinetics. We first evaluated a leak pathway in which the cleaved 1_2r gate could directly react with the DNA reporter via a 0 base toehold (29). In simulations, this model exhibited a lag time before the leak was observed, inconsistent with experiments (fig. S21, C and D). We next introduced a leak pathway in which the 1_2r gate could react with the DNA reporter before folding. In simulations, this leak pathway closely recapitulated the observed leak kinetics using a folding rate constant consistent with T7 RNAP transcription rate (fig. S21, C and E). To experimentally investigate the presence of this leak pathway, we transcribed the 1_2r gate in the absence of DNA reporter, heat-denatured the T7 RNAP, and then added the DNA reporter to the solution containing the folded 1_2r gate. If the leak pathway involved the unfolded 1_2r gate, no signal should be observed upon reporter addition. We found that reporter addition resulted in a rapid increase and plateau in fluorescence signal, and the magnitude of the plateau increased with increasing 1_2r gate transcription time (fig. S22). From these results, we reasoned the leak is not due to a reaction with the 1_2r gate before folding but rather due to the presence of an unintended 1_2r gate side product that is highly reactive. This could result from premature termination or gate misfolding events that leave the b-toehold of the gate exposed to rapidly react with the DNA reporter. We modeled this leak reaction by assuming the 1_2r gate template produced output at a fraction of the gate transcription rate. In the model, a leak transcription rate of 3% of the gate transcription rate recapitulated the experimental kinetics (fig. S21, C and F). We included this leak in all subsequent simulations. With the inclusion of this leak, the kinetic model exhibited good agreement with experimental ctRSD circuit kinetics (Fig. 2D).
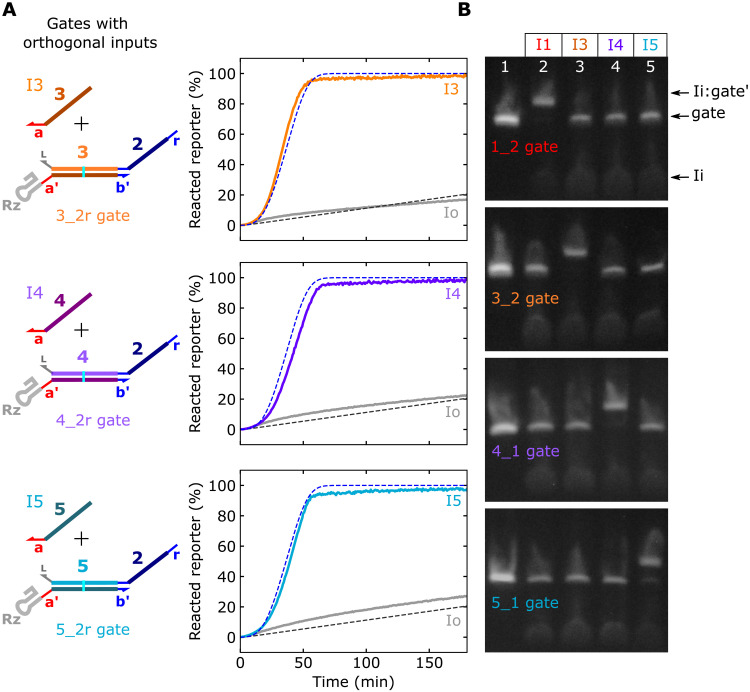
Using the same design as the 1_2r gate, we created three more ctRSD gate sequences with corresponding inputs. We reused the same input toehold sequence across gates to facilitate similar strand displacement kinetics (4, 7). These gate sequences cleaved with similar efficiency to the 1_2r gate (fig. S23) and exhibited nearly identical ctRSD circuit kinetics to the 1_2r gate (Fig. 3A). I1, I3, I4, and I5 only reacted with their designed gate (Fig. 3B), demonstrating orthogonality.
Fig. 3. Orthogonal ctRSD input and gate sequences.
(A) Fluorescent DNA reporter signal during cotranscription of 25 nM gates with orthogonal input domains and 50 nM of the designed input template or 50 nM of the Io template. The dashed lines show the results of the model for the 1_2r gate from Fig. 2D. (B) Native gel electrophoresis results demonstrating orthogonality of the four gate and input sequences. In each gel, 25 nM of a single ctRSD gate was cotranscribed with no input (lane 1) or 50 nM of the (I1, I3, I4, or I5) template. Transcription proceeded for 30 min, and electrophoresis was conducted 2 hours after degradation of DNA templates with DNase I. The 1_2 gate and 3_2 gate samples were analyzed on the same gel. The 4_1 gate and 5_1 gate samples were analyzed on the same gel. Both gel images were taken with the same setting and were otherwise unmodified. See section S1 for schematics with sequences.
ctRSD logic and signal amplification elements
We next investigated whether ctRSD components could be programmed to execute logic (5), signal amplification (4, 5), and multilayer cascades (7). To assess the predictability of ctRSD circuit design, we evaluated how well our kinetic model predicted behavior of each circuit. Our model assumes all ctRSD components are transcribed at the same rate and all gates cleave at the same rate. Furthermore, we assume ctRSD components with the same toehold sequence have the same strand displacement rate constants (see section S5B).
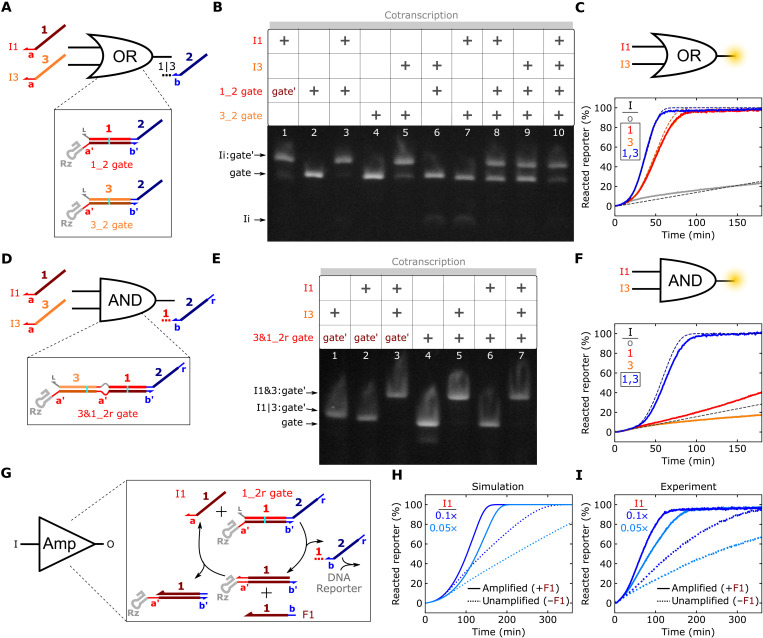
We began by designing OR and AND logic elements. The OR element was composed of two gates that react with different inputs but release the same output (Fig. 4A). We confirmed OR functionality with native gel electrophoresis (Fig. 4B) and the DNA reporter assay (Fig. 4C). OR element kinetics closely matched model predictions (Fig. 4C). The AND element was a gate composed of two input domains separated by an internal loop (Fig. 4D). In this design, I3 reacts with the gate to expose the toehold for I1 in the internal loop. We tested AND gates with internal loops composed of (3, 4, 5, or 6) bases of the a′-toehold. The 5- and 6-base variants resulted in complete gate reaction with 2× input template (fig. S24). To reduce the chance of the gate reacting with I1 alone, we chose the 5-base internal loop design. Native gel electrophoresis confirmed the AND gate reacted with I3 and I3 + I1 but not with I1 alone (Fig. 4E). Similar results were observed with the DNA reporter assay, and the kinetics of output release aligned with model predictions (Fig. 4F). A second AND gate with I4 and I5 as inputs behaved similarly (fig. S25). Our simulations suggested the AND gates exhibited 6% leak transcription compared to 3% for the single input gates. This could arise because AND gates have two input domains in series, which may increase the likelihood of truncated or misfolded transcripts compared to single input gates (see section S5A).
Fig. 4. Characterization of ctRSD logic and catalytic amplification elements.
(A) A ctRSD OR circuit element. (B) Native gel electrophoresis results for the OR element. Transcription proceeded for 30 min, and electrophoresis was conducted 30 min after DNase I addition. The gate′ strand is from the 1_2 gate. (C) Experimental (solid lines) and simulated (dashed lines) reporter signal during cotranscription of the OR element with different inputs. The trajectories for I1 alone and I3 alone overlap. The 1_2r and 3_2r gates were used in this experiment. (D) A ctRSD AND circuit element (see section S1 for schematics with sequences). (E) Native RNA gel electrophoresis results for the AND element. Transcription proceeded for 30 min, and electrophoresis was conducted 1 hour after DNase I addition. The gate′ is from the 3&1_2r gate. (F) Experimental (solid lines) and simulated (dashed lines) DNA reporter signal during cotranscription of the AND element with different inputs. The trajectories for Io alone and I3 alone overlap. (G) ctRSD catalytic amplification element. (H and I) Simulated (H) and experimental (I) DNA reporter signal during cotranscription of the 1_2r gate and I1 with (solid lines) and without (dashed lines) the F1 template (1×). For the gel results, gate and input templates were 25 and 50 nM, respectively. DNA template and T7 RNAP concentrations are tabulated in table S4.
A powerful component in strand displacement circuits is the seesaw element, which facilitates signal amplification in larger circuits (4, 5). In a seesaw element, a single-stranded fuel component reacts with a I:gate′ complex to displace the input, thus allowing multiple rounds of catalytic signal release (Fig. 4G). In DNA-based circuits, which have fixed gate and input concentrations, a seesaw element enables a gate to react completely even when the input is at a lower concentration than the gate. In ctRSD circuits, output release will eventually saturate the DNA reporter signal regardless of the input concentration. However, simulations indicated a seesaw element should decrease the time required to reach reporter saturation for low input template concentrations (Fig. 4H). When the input template was 0.05× or 0.1× the concentration of the gate template, inclusion of the fuel strand template (amplified, Fig. 4I) reduced the time to reach reporter saturation ≈3- and ≈4-fold, respectively, compared to samples without the fuel template (unamplified, Fig. 4I).
Multilayer ctRSD cascades
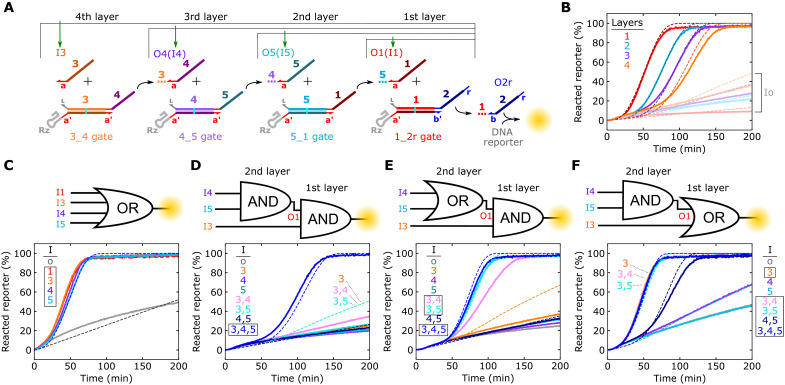
Strand displacement circuits capable of complex digital logic (5), pattern recognition (4), or temporal signal release (7) require cascades of multilayer signal transduction, so we next investigated whether we could program ctRSD cascades. We began by designing circuits with one to four ctRSD reaction layers in which the input and gate of the most upstream layer produce an output that triggers the next layer until the reporting reaction is triggered (Fig. 5A). All four multilayer cascades exhibited kinetics in good agreement with model predictions (Fig. 5B). We next integrated ctRSD logic elements into a four-input OR circuit (Fig. 5C), a cascade of two AND gates (Fig. 5D), and two permutations of AND and OR cascades (Fig. 5, E and F). These cascades successfully executed the designed logic operations, and the experimental kinetics generally agreed with model predictions. However, there were two minor deviations in experimental kinetics compared to model predictions.
Fig. 5. Characterization of ctRSD cascades.
(A) Schematic of one- to four-layer cascades. Green arrows indicate the sole input template included for each cascade layer. The colored dotted lines trailing outputs represent the upstream portion of the output strand not involved in downstream reactions. (B) Experimental (solid lines) and simulated (dashed lines) DNA reporter signal for each layered cascade in (A). Transparent lines represent each cascade with the Io template rather than the correct input template. (C to F) Experimental (solid lines) and simulated (dashed lines) reporter signal for each of the logic circuits depicted above the plots. Boxes in (C) to (F) denote the sets of inputs that should result in output release. Overlapping kinetic trajectories are labeled in the plots. In (F), the simulation results for Io, I4, and I5 all overlap with the experimental results for I4. DNA template and T7 RNAP concentrations are tabulated in table S4.
In the first deviation from the model, the two cascades in which the first layer was the 3&1_2r gate exhibited less leak than predicted when only I3 was present (Fig. 5, D and E). I3 opens the 3&1_2r gate to react with any leak products from the upstream layer in the cascades. Presumably, the 3&1_2r gate and upstream leak products reacted less than anticipated. Our model assumes leak products react with the same rate constant as their corresponding output products, but leak products are likely misfolded gates that are bulkier than single-stranded outputs. For a I3:3&1_2r complex, the region upstream of the toehold that the leak product reacts with is a duplex. Thus, steric hindrance between the I3:3&1_2r complex and a leak product could result in lower leak than predicted in simulations (fig. S26, A to C). Similar steric hindrance between the ctRSD gate ribozyme and an upstream leak product could explain why the observed leak in multilayer ctRSD cascades was less than predicted (fig. S26, A to C, and Fig. 5B). In support of this hypothesis, we found the rate constant for a strand displacement reaction using an input with a hairpin directly adjacent to its toehold was nearly 100-fold lower than with a single-stranded input (fig. S26, D to F).
In the second deviation from the model, the I3, I4 reaction in the OR to AND cascade (Fig. 5E) was slower than predicted. This could be due to a slower strand displacement reaction for the 4_1 gate. The 4_1 gate itself appears to fold, cleave, and react with I4 similarly to other gates (Fig. 3 and fig. S23), so the difference in kinetics is not likely due to the gate misfolding. While all gates reuse the same toehold sequence, the kinetics of the branch migration process can vary over an order of magnitude depending on the sequence (44). The initial branch migration sequence of the 4_1 gate contains a weak UA tract (fig. S3) that could slow strand displacement kinetics (44). This mechanism is consistent with the 4_2r gate reaction being slower than gate reactions with the other three input sequences (Fig. 3A) and the four-layer ctRSD cascade being slower than predicted (Fig. 5B). Consistent with this hypothesis, reducing the I4 RNA strand displacement rate constant 2.5-fold aligned the model predictions more closely to experimental results (fig. S27). Although these hypotheses regarding model deviations are plausible, we present analyses using the model that assumes uniform gate performance.
Varying the toehold lengths in ctRSD circuits
In TMSD, kinetics can be precisely controlled by varying toehold length and sequence (11). Such kinetic control has been demonstrated for both DNA (11) and RNA strand displacement (35, 36). In ctRSD circuits, toehold length could also influence gate folding or ribozyme cleavage kinetics. Furthermore, in our gate designs, the bulky ribozyme is directly adjacent to the toehold and could sterically hinder input binding. Thus, extending the gate toehold alone could influence kinetics by introducing a single-stranded spacer between the ribozyme and the sequence to which the input binds.
To explore the influence of input toehold length on ctRSD circuit performance, we analyzed 1_2r gates with (6, 8, 10, or 12) base toeholds. These gates cleaved to a similar extent (fig. S28) and exhibited similar leak in the DNA reporter assay (fig. S29), indicating proper folding and cleavage. To explore the influence of input toehold and spacer lengths on kinetics, we designed I1 variants with (4, 6, 8, or 10) base toeholds and combinatorially transcribed each input alongside a 1_2r gate with either a (6, 8, 10, or 12) base toehold. Varying both the toehold and spacer lengths allowed us to tune the strand displacement rate constant over four orders of magnitude (fig. S30). Increasing toehold length without spacers increased the strand displacement rate. Inclusion of spacers adjacent to the ribozyme increased strand displacement kinetics for inputs with (4, 6, or 8) base toeholds. With sufficiently long spacers, the reaction rate constants for all input toehold lengths aligned with predictions from DNA-based circuits (11), and (6, 8, or 10) base input toehold rate constants approached the theoretical maximum (see section S8).
DISCUSSION
Here, we developed scalable ctRSD circuits that were rationally programmed to execute logic, signal amplification, and multilayer cascades. Integral to the development of these circuits was encoding RNA gates that cotranscriptionally folded into kinetically trapped intermediates, allowing all circuit components to be produced where they execute computations. We demonstrated the scalability and modularity of ctRSD circuits by implementing 11 single input gates and two AND gates in eight different circuit topologies, all of which exhibited kinetics in agreement with our model that assumed uniform kinetic parameters. Together, these results indicate the robustness of our ctRSD gate design choices. Although other designs were not investigated experimentally, we believe three design choices contributed to the scalability and modularity of ctRSD circuits: (i) selecting the stable and cleavage sequence agnostic HDV ribozyme; (ii) restricting the input and output sequences to C, A, or U bases; and, (iii) transcribing the output strand of the gates first. These choices likely reduced the chances of misfolding during transcription and facilitated proper ribozyme function across gate sequences.
We implemented the ctRSD gates with the same modular toehold exchange design (Fig. 1, A and B) and C, A, and U sequence constraints used in state-of-the-art DNA-based circuits. In DNA computing, these designs have enabled circuits composed of >100 components to be programmed to execute complex pattern recognition tasks (4) and implement arbitrary chemical reaction networks (10, 45), functionalities not accessible with current genetically encodable RNA circuits (19, 20). Thus, ctRSD circuits are poised to achieve the same scalability and functionality as the most advanced DNA-based TMSD circuits, while potentially offering improved component purity and stability at comparable costs (see section S9).
Our design choices also introduce practical limitations. The C, A, and U sequence constraint restricts the use of cellular RNAs composed of all four bases as inputs. Simply redesigning gates with a four-letter code could make it difficult to predictively design sequences that fold correctly in experiments (46). To address this limitation, we envision building upstream ctRSD translation gates that modularly convert RNA inputs with a four-letter code into outputs with a three-letter code that are processed in ctRSD circuits with our prescribed design rules. In this manner, the same robust information processing circuits may be used, and translation gates with four-letter codes that function correctly could be identified by testing sequences spanning a cellular RNA of interest.
Another limitation of our design is the bulky HDV ribozyme left on the gates after cleavage. We found this motif influenced strand displacement kinetics unless a single-stranded spacer between the ribozyme and the toehold binding sequence was inserted (see section S8). Recently, a scheme was reported for transcriptionally encoding strand displacement circuits with a dual hammerhead ribozyme motif that excised itself after folding (25); a similar multi-ribozyme strategy could be applied to ctRSD gates to remove the HDV ribozyme motif during gate production. However, in contrast to the ctRSD circuits presented here, the alternative scheme used a four-letter code and found gate performance varied with sequence. Furthermore, toeholds switched from 5′ to 3′ between circuit layers, reducing modularity and composability. Ultimately, merging ideas from both these implementations offers routes for further optimizing ctRSD circuits.
We envision ctRSD circuits enabling many new applications in nucleic acid computing and synthetic biology. For example, the inclusion of ribonucleases in ctRSD circuits would allow continuous circuit turnover. Circuits could then respond multiple times to changing input signals, overcoming a current challenge in DNA computing (17). Regulating input production with allosteric transcription factors could allow ctRSD circuits to process non-nucleic acid inputs for smart diagnostics (26, 27). The ability to transcriptionally encode strand displacement components on DNA plasmids would allow nucleic acid computing in a number of new environments where DNA computing is limited due to degradation (14), e.g., in blood samples (15), cell-free lysates, or inside living cells (16). In vivo, fluorescent RNA aptamers (43) or RNA regulators that transduce RNA signals into fluorescent protein production (47) could measure ctRSD circuit dynamics. ctRSD circuit outputs could regulate protein expression through existing RNA technologies (19, 20, 23), allowing ctRSD circuits to control cellular function.
Adopting ctRSD circuits for these diverse applications will require overcoming challenges in controlling expression, degradation, and cleavage rates−especially in vivo. These issues could be addressed by optimizing 5′ hairpins to tune expression levels (30) or increase RNA stability (48), as well as exploring HDV ribozyme variants (49). Ultimately, ctRSD circuits are poised to be a versatile, enabling technology across many synthetic biology platforms.
MATERIALS AND METHODS
DNA and materials
DNA transcription templates were ordered as gBlock gene fragments from Integrated DNA Technologies (IDT), amplified via polymerase chain reaction (PCR) with Phusion High-Fidelity PCR Master Mix (catalog no. F531L) from Thermo Fisher Scientific, and purified using Qiagen PCR clean-up kits. All DNA oligo primers were ordered from IDT with standard desalting. For in vitro transcription experiments, T7 RNAP and ribonucleotide triphosphates (NTPs) were ordered from Thermo Fisher Scientific (catalog no. R0481). Deoxyribonuclease (DNase) I (catalog no. M0303S) was purchased from New England Biolabs. Four percent agarose EX E-gels were purchased from Thermo Fisher Scientific (catalog no. G401004). All chemicals were purchased from Sigma-Aldrich.
Transcription template preparation
All transcription templates were prepared by PCR of gBlock DNA (0.2 ng/μl) with Phusion High-Fidelity PCR Master Mix and forward and reverse primers (0.5 μM). PCR was conducted for 30 cycles with a 30-s 98°C denaturing step, a 30-s 60°C primer annealing step, and a 30-s 72°C extension step. A 3-min 72°C final extension step was executed at the end of the program. Following PCR amplification, the samples were purified with Qiagen PCR clean-up kits and eluted in Qiagen Buffer EB [10 mM tris-HCl (pH 8.5)].
RNA agarose gel electrophoresis
Four percent agarose EX E-gels were used for all RNA gel electrophoresis experiments. These gels are prestained with SYBR Gold for fluorescence imaging. Electrophoresis was conducted on an E-gel powerbase, and all E-gels were imaged using the E-gel power snap camera (Thermo Fisher Scientific, catalog no. G8200). Unless otherwise stated, to prepare RNA for gel electrophoresis, DNA templates were transcribed at 37°C for 30 min in transcription conditions (see the next section) with T7 RNAP (0.6 U/μl). To stop transcription, CaCl2 [final concentration (1 to 1.5 mM)] and DNase I [final concentration (0.1 to 0.2 U/μl)] were added to degrade the DNA templates. After DNase I addition, the samples were left at 37°C for (0.5 to 2) hours (see figure legends) and subsequently analyzed with gel electrophoresis. For native gels, the gels were sandwiched between ice packs to keep the gels cool during electrophoresis and were run for (45 to 60) min before imaging. Integrated band intensities were quantified in gel images using the Gel Analysis Tool in ImageJ as previously described (50). For denaturing gels, before electrophoresis, a solution of 100% formamide and 36 mM EDTA was mixed 1:1 by volume with the samples, and the samples were heated to 90°C for 5 min. The samples were then immediately loaded on gels for electrophoresis and run for (20 to 30) min before imaging. Gel images were not postprocessed, and any brightness and contrast adjustments were executed during image acquisition and were thus applied uniformly to the images to aid visualization.
Characterization of RNA strand displacement with a fluorescence DNA reporter
The in vitro transcription reactions with DNA reporter complexes were conducted in transcription buffer prepared in house [40 mM tris-HCl (pH 7.9), 6 mM MgCl2, 10 mM dithiothreitol, 10 mM NaCl, and 2 mM spermidine] supplemented with 2 mM final concentration of each NTP type (adenosine triphosphate, uridine triphosphate, cytidine triphosphate, and guanosine triphosphate). All transcription reactions were conducted at 37°C. Unless otherwise stated, 500 nM DNA reporter was used. For in vitro transcription reactions, all components other than T7 RNAP were mixed and monitored in the plate reader for 15 to 60 min before adding T7 RNAP. The addition of T7 RNAP, followed by mixing, corresponded to t = 0 min in in vitro transcription experiments. The time to mix T7 RNAP into all samples for an experiment was less than 1 min. In our experiments, the T7 RNAP concentration varied depending on the total concentration of DNA templates present. To compare the response of a given ctRSD circuit to different input template concentrations or a different number of input templates, the same total template concentration was used across all reactions to ensure the same transcriptional load across samples. An input template (Io) that produces an RNA that does not interact with the gates was added to maintain the template concentration across samples. Table S4 contains the concentrations of DNA templates (including Io) and T7 RNAP used in each experiment.
Transcription rate calibration and sample variability
In our experiments, the transcription rate depended on the concentration of T7 RNAP and the total concentration of DNA templates (fig. S31). Furthermore, variability of T7 RNAP activity (51) across manufacturer lots was expected to be the primary source of variation in our experiments. To calibrate for these effects, we developed a transcription rate reference sample (fig. S31). This reference sample measured transcription with a template that constitutively expressed the 1_2r strand and contained the same T7 RNAP lot and concentration as the experimental samples on a given day. In addition, the Io template was added so the total template concentration equaled that of the experimental samples. The reference sample calibrated the first-order transcription rate constant chosen for simulations (fig. S32), thus accounting for variation in T7 RNAP activity when assessing how well experimental results agreed with model predictions. To estimate the variability in ctRSD circuit measurements introduced during sample preparation, we conducted reactions between the 1_2r gate and either I1 or Io in triplicate in the DNA reporter assay. Each reaction was prepared independently using the same transcription template, NTP, buffer, and T7 RNAP stocks. These replicates exhibited an SD of <1.5% from the mean value at each time point (fig. S33A). A variability of <5% SD was observed for the AND gate cascade in Fig. 5D (fig. S33B). In addition, reactions between the 1_2r gate and either I1 or Io performed on different days exhibited <3% SD (fig. S34). We therefore assumed a conservative variability of <5% generalized to ctRSD circuits. For the small circuits studied here, we do not expect this level of variability to influence our conclusions. Unless otherwise stated, DNA reporter experiments were conducted with a single experimental replicate.
Fluorescence data acquisition and normalization
BioTek Synergy Neo2 plate readers were used to measure in vitro transcription reactions. Reactions were typically conducted in 70-μl volumes in Greiner μClear 96-well plates (catalog no. 655096) read from the bottom. The DNA reporter complex was labeled with a HEX dye, which was measured with an excitation of 524 nm (20-nm bandwidth), an emission of 565 nm (20-nm bandwidth), and a gain of 85. Fluorescence readings were taken every 46 s. In a typical experiment, fluorescence readings were taken for (25 to 45) min before T7 RNAP was added to initiate the reactions. At the end of most experiments, an excess (2.5 μM) of a DNA version of the O2r strand was added to each sample to obtain an internal maximum DNA reporter fluorescence value. Fluorescence data were then normalized as
If the DNA O2r strand was not added, a control well in which the ctRSD reaction had saturated the reporter signal served as the Max(fluorescence) value for normalization.
Acknowledgments
We thank J. T. Elliot, J. B. Lucks, P. G. Moerman, and M. Rubanov for insightful comments on the manuscript and N. Y. Alperovich, J. K. Jung, A. D. Pressman, D. S. Tack, J. Rammohan, E. F. Romantseva, D. J. Ross, and O. B. Vasilyeva for helpful discussions throughout this work.
Funding: This work was supported by the National Research Council Postdoctoral Fellowship (to S.W.S.).
Author contributions: S.W.S. conceived, designed, and performed research. S.W.S. conducted data analysis, developed simulation code, and executed simulations. E.A.S. supervised the research. S.W.S. and E.A.S. wrote the manuscript.
Competing interests: S.W.S. is an inventor on a provisional patent application related to this work filed at the U.S. Patent and Trademark Office (no. 63/290,457; filed on 16 December 2021). The authors declare no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Fluorescence data and simulation code are available on Zenodo. Fluorescence data: https://doi.org/10.5281/zenodo.5934048. Simulation code: https://doi.org/10.5281/zenodo.6265575. The simulation code is also posted on GitHub at https://github.com/usnistgov/ctRSD-simulator, where new capabilities may be added.
Disclaimer: Certain commercial entities, equipment, or materials may be identified in this document to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. Official contribution of the National Institute of Standards and Technology; not subject to copyright in the United States.
Supplementary Materials
This PDF file includes:
Sections I to X
Figs. S1 to S34
Tables S1 to S4
References
REFERENCES AND NOTES
- 1.Sedlmayer F., Aubel D., Fussenegger M., Synthetic gene circuits for the detection, elimination and prevention of disease. Nat. Biomed. Eng. 2, 399–415 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Ke J., Wang B., Yoshikuni Y., Microbiome engineering: Synthetic biology of plant-associated microbiomes in sustainable agriculture. Trends Biotechnol. 39, 244–261 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Keasling J. D., Manufacturing molecules through metabolic engineering. Science 330, 1355–1358 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Cherry K. M., Qian L., Scaling up molecular pattern recognition with DNA-based winner-take-all neural networks. Nature 559, 370–376 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Qian L., Winfree E., Scaling up digital circuit computation with DNA strand displacement cascades. Science 332, 1196–1201 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Zhang C., Zhao Y., Xu X., Xu R., Li H., Teng X., Du Y., Miao Y., Lin H., Han D., Cancer diagnosis with DNA molecular computation. Nat. Nanotechnol. 15, 709–715 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Scalise D., Rubanov M., Miller K., Potters L., Noble M., Schulman R., Programming the sequential release of DNA. ACS Synth. Biol. 9, 749–755 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Zhang D. Y., Turberfield A. J., Yurke B., Winfree E., Engineering entropy-driven reactions and networks catalyzed by DNA. Science 318, 1121–1125 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Srinivas N., Parkin J., Seelig G., Winfree E., Soloveichik D., Enzyme-free nucleic acid dynamical systems. Science 358, eaal2052 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Chen Y.-J., Dalchau N., Srinivas N., Phillips A., Cardelli L., Soloveichik D., Seelig G., Programmable chemical controllers made from DNA. Nat. Nanotechnol. 8, 755–762 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D. Y., Winfree E., Control of DNA strand displacement kinetics using toehold exchange. J. Am. Chem. Soc. 131, 17303–17314 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Lakin M. R., Youssef S., Polo F., Emmott S., Phillips A., Visual DSD: A design and analysis tool for DNA strand displacement systems. Bioinformatics 27, 3211–3213 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y.-J. A. U., Rao S. D. A. U., Seelig G. A. U., Plasmid-derived DNA strand displacement gates for implementing chemical reaction networks. J. Vis. Exp. 105, e53087 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y.-J., Groves B., Muscat R. A., Seelig G., DNA nanotechnology from the test tube to the cell. Nat. Nanotechnol. 10, 748–760 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Fern J., Schulman R., Design and characterization of DNA strand-displacement circuits in serum-supplemented cell medium. ACS Synth. Biol. 6, 1774–1783 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Groves B., Chen Y.-J., Zurla C., Pochekailov S., Kirschman J. L., Santangelo P. J., Seelig G., Computing in mammalian cells with nucleic acid strand exchange. Nat. Nanotechnol. 11, 287–294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui X., Scalise D., Schulman R., Powering DNA strand-displacement reactions with a continuous flow reactor. Nat. Comput. 20, 821–827 (2020). [Google Scholar]
- 18.Song X., Eshra A., Dwyer C., Reif J., Renewable DNA seesaw logic circuits enabled by photoregulation of toehold-mediated strand displacement. RSC Adv. 7, 28130–28144 (2017). [Google Scholar]
- 19.Green A. A., Kim J., Ma D., Silver P. A., Collins J. J., Yin P., Complex cellular logic computation using ribocomputing devices. Nature 548, 117–121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chappell J., Westbrook A., Verosloff M., Lucks J. B., Computational design of small transcription activating RNAs for versatile and dynamic gene regulation. Nat. Commun. 8, 1051 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren K., Wu R., Karunanayake Mudiyanselage A. P. K. K., Yu Q., Zhao B., Xie Y., Bagheri Y., Tian Q., You M., In Situ Genetically cascaded amplification for imaging RNA subcellular locations. J. Am. Chem. Soc. 142, 2968–2974 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karunanayake Mudiyanselage A. P. K. K., Yu Q., Leon-Duque M. A., Zhao B., Wu R., You M., Genetically encoded catalytic hairpin assembly for sensitive RNA imaging in live cells. J. Am. Chem. Soc. 140, 8739–8745 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oesinghaus L., Simmel F. C., Switching the activity of Cas12a using guide RNA strand displacement circuits. Nat. Commun. 10, 2092 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhadra S., Ellington A. D., Design and application of cotranscriptional non-enzymatic RNA circuits and signal transducers. Nucleic Acids Res. 42, e58 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae W., Stan G.-B. V., Ouldridge T. E., In situ generation of RNA complexes for synthetic molecular strand-displacement circuits in autonomous systems. Nano Lett. 21, 265–271 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Jung J. K., Alam K. K., Verosloff M. S., Capdevila D. A., Desmau M., Clauer P. R., Lee J. W., Nguyen P. Q., Pastén P. A., Matiasek S. J., Gaillard J.-F., Giedroc D. P., Collins J. J., Lucks J. B., Cell-free biosensors for rapid detection of water contaminants. Nat. Biotechnol. 38, 1451–1459 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.J. K. Jung, C. M. Archuleta, K. K. Alam, J. B. Lucks, Programming cell-free biosensors with DNA strand displacement circuits. Nat. Chem. Biol. 10.1038/s41589-021-00962-9 (2022). [DOI] [PMC free article] [PubMed]
- 28.Isambert H., The jerky and knotty dynamics of RNA. Methods 49, 189–196 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Wang B., Thachuk C., Ellington A. D., Winfree E., Soloveichik D., Effective design principles for leakless strand displacement systems. Proc. Natl. Acad. Sci. U.S.A. 115, E12182–E12191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conrad T., Plumbom I., Alcobendas M., Vidal R., Sauer S., Maximizing transcription of nucleic acids with efficient T7 promoters. Commun. Biol. 3, 439 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rong M., He B., McAllister W. T., Durbin R. K., Promoter specificity determinants of T7 RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 95, 515–519 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz G. A., Rong M., McAllister W. T., Durbin R. K., The stability of abortively cycling t7 rna polymerase complexes depends upon template conformation. Biochemistry 35, 10837–10843 (1996). [DOI] [PubMed] [Google Scholar]
- 33.Rong M., Durbin R. K., McAllister W. T., Template strand switching by T7 RNA polymerase. J. Biol. Chem. 273, 10253–10260 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Nacheva G. A., Berzal-Herranz A., Preventing nondesired RNA-primed RNA extension catalyzed by T7 RNA polymerase. Eur. J. Biochem. 270, 1458–1465 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Šulc P., Ouldridge T. E., Romano F., Doye J. P. K., Louis A. A., Modelling toehold-mediated RNA strand displacement. Biophys. J. 108, 1238–1247 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H., Hong F., Smith F., Goertz J., Ouldridge T., Stevens M. M., Yan H., Šulc P., Kinetics of RNA and RNA:DNA hybrid strand displacement. ACS Synth. Biol. 10, 3066–3073 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Geary C., Rothemund P. W. K., Andersen E. S., A single-stranded architecture for cotranscriptional folding of RNA nanostructures. Science 345, 799–804 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Haley N. E. C., Ouldridge T. E., Mullor Ruiz I., Geraldini A., Louis A. A., Bath J., Turberfield A. J., Design of hidden thermodynamic driving for non-equilibrium systems via mismatch elimination during DNA strand displacement. Nat. Commun. 11, 2562 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schürer H., Lang K., Schuster J., Mörl M., A universal method to produce in vitro transcripts with homogeneous 3′ ends. Nucleic Acids Res. 30, e56 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duhamel J., Liu D. M., Evilia C., Fleysh N., Dinter-Gottlieb G., Lu P., Secondary structure content of the HDV ribozyme in 95% formamide. Nucleic Acids Res. 24, 3911–3917 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferré-D’Amaré A. R., Zhou K., Doudna J. A., Crystal structure of a hepatitis delta virus ribozyme. Nature 395, 567–574 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Zadeh J. N., Steenberg C. D., Bois J. S., Wolfe B. R., Pierce M. B., Khan A. R., Dirks R. M., Pierce N. A., NUPACK: Analysis and design of nucleic acid systems. J. Comput. Chem. 32, 170–173 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Alam K. K., Tawiah K. D., Lichte M. F., Porciani D., Burke D. H., A fluorescent split aptamer for visualizing RNA-RNA assembly in vivo. ACS Synth. Biol. 6, 1710–1721 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broadwater Jr. D. W. B., Cook A. W., Kim H. D., First passage time study of DNA strand displacement. Biophys. J. 120, 2400–2412 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soloveichik D., Seelig G., Winfree E., DNA as a universal substrate for chemical kinetics. Proc. Natl. Acad. Sci. U.S.A. 107, 5393–5398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson-Lee J., Fisker E., Kosaraju V., Wu M., Kong J., Lee J., Lee M., Zada M., Treuille A., Das R., Principles for predicting RNA secondary structure design difficulty. J. Mol. Biol. 428, 748–757 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ameruoso A., Gambill L., Liu B., Villegas Kcam M. C., Chappell J., Brave new ‘RNA’ world—Advances in RNA tools and their application for understanding and engineering biological systems. Curr. Opin. Syst. Biol. 14, 32–40 (2019). [Google Scholar]
- 48.Zhang Q., Ma D., Wu F., Standage-Beier K., Chen X., Wu K., Green A. A., Wang X., Predictable control of RNA lifetime using engineered degradation-tuning RNAs. Nat. Chem. Biol. 17, 828–836 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.N. Riccitelli, A. Lupták, in Progress in Molecular Biology and Translational Science, G. A. Soukup, Ed. (Elsevier, 2013), vol. 120, pp. 123–171. [DOI] [PubMed] [Google Scholar]
- 50.K. Ohgane, H. Yoshioka, Quantification of gel bands by an Image J macro, band/peak quantification tool. protocols.io (2019).
- 51.M. Schwarz-Schilling, J. Kim, C. Cuba, M. Weitz, E. Franco, F. C. Simmel, Building a synthetic transcriptional oscillator, in Cell Cycle Oscillators. Methods in Molecular Biology, A. S. Coutts, L. Weston, Eds. (Humana Press, 2016), pp. 185–199. [DOI] [PubMed] [Google Scholar]
- 52.Han X., Wang E., Cui Y., Lin Y., Chen H., An R., Liang X., Komiyama M., The staining efficiency of cyanine dyes for single-stranded DNA is enormously dependent on nucleotide composition. Electrophoresis 40, 1708–1714 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Chen J. L., Dishler A. L., Kennedy S. D., Yildirim I., Liu B., Turner D. H., Serra M. J., Testing the nearest neighbor model for canonical RNA base pairs: Revision of GU parameters. Biochemistry 51, 3508–3522 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chowrira B. M., Pavco P. A., McSwiggen J. A., In vitro and in vivo comparison of hammerhead, hairpin, and hepatitis delta virus self-processing ribozyme cassettes. J. Biol. Chem. 269, 25856–25864 (1994). [PubMed] [Google Scholar]
- 55.Fedor M. J., Structure and function of the hairpin ribozyme. J. Mol. Biol. 297, 269–291 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Scott W. G., Horan L. H., Martick M., The hammerhead ribozyme: Structure, catalysis, and gene regulation. Prog. Mol. Biol. Transl. Sci. 120, 1–23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim J., White K. S., Winfree E., Construction of an in vitro bistable circuit from synthetic transcriptional switches. Mol. Syst. Biol. 2, 68 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaffter S. W., Schulman R., Building in vitro transcriptional regulatory networks by successively integrating multiple functional circuit modules. Nat. Chem. 11, 829–838 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Srinivas N., Ouldridge T. E., Sulc P., Schaeffer J. M., Yurke B., Louis A. A., Doye J. P. K., Winfree E., On the biophysics and kinetics of toehold-mediated DNA strand displacement. Nucleic Acids Res. 41, 10641–10658 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng Y., Li X., Yuan R., Xiang Y., Steric hindrance inhibition of strand displacement for homogeneous and signal-on fluorescence detection of human serum antibodies. Chem. Commun. 52, 12586–12589 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Skinner G. M., Baumann C. G., Quinn D. M., Molloy J. E., Hoggett J. G., Promoter binding, initiation, and elongation by bacteriophage T7 RNA polymerase: A single-molecule view of the transcription cycle. J. Biol. Chem. 279, 3239–3244 (2004). [DOI] [PubMed] [Google Scholar]
- 62.H. Packer, The gene construction revolution; www.idtdna.com/pages/education/decoded/article/the-gene-construction-revolution.
- 63.PCR fidelity calculator (ThermoFisher Scientific; www.thermofisher.com/us/en/home/brands/thermo-scientific/molecular-biology/molecular-biology-learning-center/molecular-biology-resource-library/thermo-scientific-web-tools/pcr-fidelity-calculator.html).
- 64.Brakmann S., Grzeszik S., An error-prone T7 RNA polymerase mutant generated by directed evolution. Chembiochem 2, 212–219 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Pardee K., Green A. A., Ferrante T., Cameron D. E., DaleyKeyser A., Yin P., Collins J. J., Paper-based synthetic gene networks. Cell 159, 940–954 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sections I to X
Figs. S1 to S34
Tables S1 to S4
References