Figure 1. Physical and chemical characterization of MN patches.
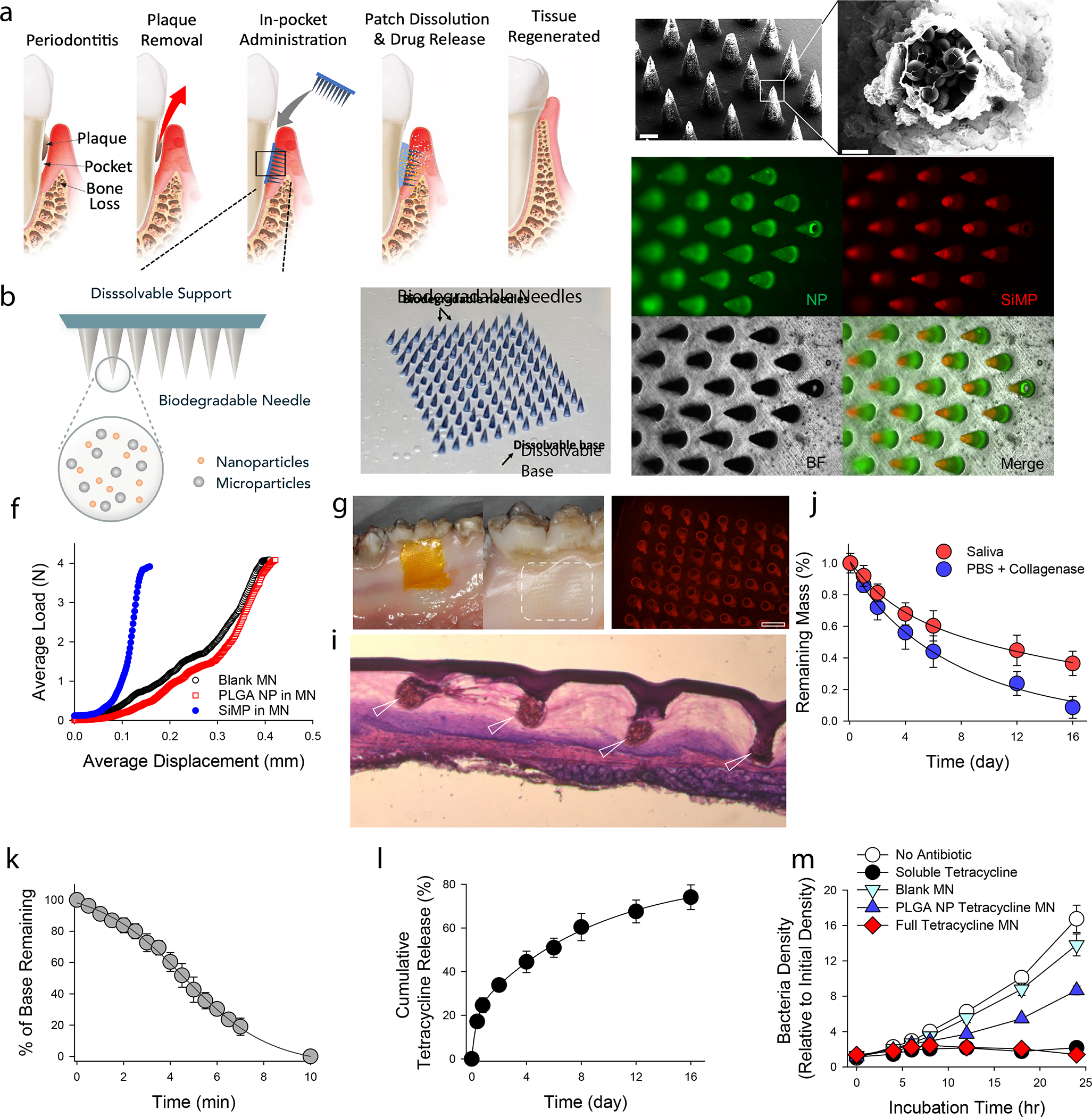
a, Proposed working mechanism of the designed immunotherapeutic periodontal MN patch. MN patches are designed to provide transgingival administration of antibiotics and anti-inflammatory cytokines. Following administration, MNs will detach from the supporting membrane upon gelatin dissolution and stay in gingival tissue, release their cargos, and degrade. b, Illustration of the multifunctional MN patch design. The image on the right shows an example of fabricated MN patch. MNs were visualized by encapsulating Trypan Blue dye. c, Scanning electron microscopy (SEM) image of fabricated MNs. d, Encapsulated SiMPs can be seen inside truncated needles. See also Figure S2. e, Fluorescent imaging demonstrates the distribution of fluorophore-conjugated PLGA NPs (in Green) and SiMPs (in Red) inside GelMA-based MNs. f, Mechanical strength of MNs measured by Instron compression test. g, MN patch penetrated on freshly harvested porcine gingiva. Following the administration, the tissue was kept in 37°C incubator for 30 minutes to monitor gelatin dissolution. Pig’s gingival surface is showing insertion of MNs. h, MN-treated pig gingival tissue was imaged to demonstrate the presence of fluorophore-loaded MNs (in Red). Scale bar: 400 μm. i, Penetration of MN into fresh rat gingival tissue was visualized by hematoxylin and eosin (H&E) staining. j, In vitro degradation of MNs over time in PBS containing collagenase enzyme or human saliva at 37°C under gentle shaking. k, FITC-conjugated gelatin was used as the support layer to measure the rate of gelatin base dissolution. l, Cumulative in vitro release of tetracycline was assessed from the dissolution of gelatin base (burst release) and from GelMA MNs loaded with PLGA-tetracycline NPs (sustained release) at 37°C and pH 7.4. m, Antibacterial effect of designed patches against P.g. Full tetracycline MN includes tetracycline-loaded gelatin base and tetracycline-loaded PLGA NPs in MNs. The data in j-m are presented as mean ± standard deviation (SD) (n = 5).
