Abstract
Circadian rhythms in mammals are coordinated by the central clock in the brain, located in the suprachiasmatic nucleus (SCN). Multiple molecular and cellular signals display a circadian variation within SCN neurons, including intracellular Ca2+, but the mechanisms are not definitively established. SCN cytosolic Ca2+ levels exhibit a peak during the day, when both action potential firing and Ca2+ channel activity are increased, and are decreased at night, correlating with a reduction in firing rate. In this study, we employ a single-color fluorescence anisotropy reporter (FLARE), Venus FLARE-Cameleon, and polarization inverted selective-plane illumination microscopy to measure rhythmic changes in cytosolic Ca2+ in SCN neurons. Using this technique, the Ca2+ channel subtypes contributing to intracellular Ca2+ at the peak and trough of the circadian cycle were assessed using a pharmacological approach with Ca2+ channel inhibitors. Peak (218 ± 16 nM) and trough (172 ± 13 nM) Ca2+ levels were quantified, indicating a 1.3-fold circadian variance in Ca2+ concentration. Inhibition of ryanodine-receptor-mediated Ca2+ release produced a larger relative decrease in cytosolic Ca2+ at both time points compared to voltage-gated Ca2+channels. These results support the hypothesis that circadian Ca2+ rhythms in SCN neurons are predominantly driven by intracellular Ca2+ channels, although not exclusively so. The study provides a foundation for future experiments to probe Ca2+ signaling in a dynamic biological context using FLAREs.
Introduction
Ca2+ signaling is essential for the production of time-keeping signals in the mammalian circadian clock, which is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Ca2+ is required for SCN neurons to maintain 24-h rhythms in clock gene expression (1,2) and action potential activity (3, 4, 5, 6). These main SCN output signals ultimately control the timing of downstream physiological processes (7, 8, 9) and behaviors (10, 11, 12). Ca2+ signaling is also required for SCN neurons to respond to external inputs (13, 14, 15, 16, 17, 18, 19, 20) that lead to shifts in molecular (21), cellular (14,22), and behavioral rhythms (23).
A circadian pattern in intracellular free Ca2+ has been identified in both SCN neurons and glia (24, 25, 26, 27, 28, 29). SCN neurons exhibit a circadian rise in cytosolic Ca2+ concentration that typically reaches a peak during the day, ∼0–5 h before the peak in action potential firing, and is reduced at night (24,27,30, 31, 32). These rhythmic Ca2+ signals can be observed in single SCN neurons as well as from whole SCN slices (19,24,28,30,32, 33, 34, 35). However, the major Ca2+ channels that drive these Ca2+ rhythms are still under investigation.
Previous studies have implicated multiple Ca2+ channel subtypes that contribute to Ca2+ signaling in SCN neurons (27). Membrane depolarization stimulates Ca2+ influx by activating voltage-gated Ca2+ channels (VGCCs) including L-, N-, P/Q-, R-, and T-type channels (2, 3, 4, 5). Ca2+ release from intracellular stores in the endoplasmic reticulum (ER) is mediated by ryanodine receptors (RyR2 and RyR3) (24,36, 37, 38). IP3 signaling stimulates Ca2+ release from the ER by activating inositol 1,4,5-trisphosphate receptors (IP3Rs) (2,39). Prior studies have shown that cytosolic Ca2+ levels may be mediated in part by Ca2+ release from intracellular ER stores, as pharmacological inhibition of RyRs produces a large decrease in cytoplasmic Ca2+ (24,40,41). However, Ca2+ influx through the plasma membrane from voltage-gated Ca2+ channels may also contribute. Inhibition of L-type voltage-gated Ca2+ channels with nimodipine or action potential firing with the Na+ channel blocker tetrodotoxin partially reduce cytoplasmic Ca2+ (40). To date, no single study has directly compared the contributions of the main plasma membrane and intracellular Ca2+ channels at both the peak and trough of the circadian cycle from intact SCN slices.
This study utilizes a newly developed fluorescent biosensor to provide a quantifiable and direct comparison for the contributions of voltage-gated and intracellular Ca2+ channels to daytime (peak) and nighttime (trough) Ca2+ levels in SCN neurons from intact brain slice cultures. Polarization inverted selective-plane illumination microscopy (piSPIM) was used to measure Ca2+ concentration within SCN using a ratiometric, neuronally expressed Ca2+ sensor, Venus FLARE-Cameleon (Venus-cp172Venus FLARE-Cameleon) (42). The Venus FLARE-Cameleon sensor is a fluorescence resonance energy transfer (FRET)-based fluorescence anisotropy reporter (FLARE) (42). Ca2+ concentrations were estimated from in situ calibration of Venus FLARE-Cameleon fluorescence anisotropy signals in SCN slices. Pharmacological inhibitors targeting the major Ca2+ channel subtypes were applied during the peak and trough phases of the diurnal cycle to evaluate the impact of different Ca2+ sources on Ca2+ levels. These data revealed a peak-to-trough difference in cytosolic Ca2+ concentration that was higher during the day, with ryanodine receptors providing the largest contribution at both times of the diurnal cycle.
Materials and methods
Animals and ethical approval
Wild-type C57BL/6J mice were bred in a standard 12:12-h light-dark cycle. Male and female mice were killed for experiments via decapitation at postnatal day 4. All procedures involving mice were conducted in accordance with the University of Maryland School of Medicine Animal Care and Use Guidelines and approved by the Institutional Animal Care and Use Committee.
Organotypic slice culture and viral transduction
Brains were dissected during the light cycle as described previously (3). Coronal sections of the hypothalamus (300 μm) were made on a manual tissue chopper (Stoelting, Wood Dale, IL) in ice-cold dissection medium containing bicarbonate-free Dulbecco’s modified Eagle’s medium (12100-046; Gibco, Gaithersburg, MD), 10 mM HEPES (pH 7.3), 100 U/mL penicillin/streptomycin (30-002-CI; MediaTech, Manassas, VA), and 2 mM L-glutamine (25-005-CI; MediaTech). Slices containing the SCN (one per animal) were cultured as organotypic interface explants (43).
For piSPIM experiments, SCN slices were plated onto filter membranes (PICM0RG50; Millipore Sigma, Burlington, MA) in 35-mm culture dishes (353001; Corning, Corning, NY) with 1.2 mL culture medium containing minimal essential medium (11095-080; Gibco), 25 mM HEPES (pH 7.3), 25% horse serum (16050-130; Gibco), 28 mM D-glucose (G8270; Millipore Sigma), 10 U/mL penicillin/streptomycin (30-002-CI; MediaTech), and 2 mM L-glutamine (25-005-CI; MediaTech). Cytosine β-D-arabinofuranoside (Ara-C, 20 μM; C6645; Millipore Sigma) was added to culture medium starting on culture day 2 to inhibit glial cell growth. Immediately after plating, slices were transduced with 1 μL of adeno-associated viral vector (AAV, serotype 1) containing Venus-cp172Venus FLARE-Cameleon biosensor DNA (42) (AAV1.hSyn1.Vencp172Ven Cameleon; stock 2.18 × 1012 vg/mL; plasmid #pOTTC1612; Genetic Engineering and Viral Vector Core, National Institute on Drug Abuse, Baltimore, MD). Neuron-specific expression was driven by the human synapsin 1 (hSyn1) promoter (44). Slices were maintained for 14–21 days in a humidified incubator at 37°C (5% CO2) with 100% of the culture medium exchanged every ∼72 h.
A subset of slices transduced with Venus FLARE-Cameleon AAVs were plated onto multielectrode arrays on culture day 10 as described previously (43,45). SCN slices cultured on filters were excised from the surrounding filter and flipped (SCN side down) onto multielectrode arrays pretreated overnight with 500 μL of 0.1 mg/mL collagen (C8919; Sigma-Aldrich, St. Louis, MO) and maintained in culture medium as described in Supporting materials and methods.
piSPIM imaging of fluorescence anisotropy
Imaging experiments were conducted in 6-h time windows using the peak and trough of firing rhythms as the reference point, in which images were obtained between 5 h before to 1 h after the time of the peak or the trough in action potential firing. Filter sections with SCN slices were excised, rinsed in phosphate-buffered saline, transferred to the microscope chamber, and equilibrated for 20–30 min in 6 mL of prewarmed imaging solution containing 125 mM NaCl, 8 mM NaOH, 5 mM KCl, 1 mM MgCl2, 20 mM HEPES, 5 mM D-glucose, and 2.5 mM CaCl2 (pH 7.20 ± 0.01 at 35°C). Fluorescence anisotropy imaging was performed on a polarization inverted selective-plane illumination microscopy (piSPIM) microscope with stage-scanning capability assembled and aligned as described previously (46, 47, 48). The collection arms of the microscope were fitted with filter wheels containing emission filters and an image splitting device, OptoSplit II (Cairn, Faversham, UK), to separate parallel (P) and perpendicular (S) polarizations. The microscope was housed in an environmentally controlled incubator (Okolab, Ambridge, PA) maintained at 37°C. Automated stage and piezo focus control hardware elements were purchased from Applied Scientific Instruments (Eugene, OR). Camera and piezo electronics were controlled using Micromanager software (available at https://micro-manager.org/) (49) on a Z840 workstation (Hewlett Packard, Palo Alta, CA). Volumetric images (16-bit grayscale) were collected on a Nikon Eclipse TE2000-U microscope with water-dipping objectives (MRD07420, 40×, 0.8 NA; Nikon, Tokyo, Japan) and a digital camera (Flashv4 Orca, C13440; Hamamatsu, Hamamatsu, Japan) as stack files with 20 image slices per volume (1-μm spacing, 512 × 1024 pixels per image slice, 332-nm pixel width and height, 2 × 2 binning). Samples were excited in 10-s (KCl experiments) or 30-s (Ca2+ inhibitor experiments) intervals with a 488-nm laser. Images were collected from a ∼170 × 340 × 20 μm area within the center of the SCN, which was visually identified under brightfield illumination at 4× magnification using the optic chiasm and third ventricle as reference landmarks. After baseline control images were acquired, imaging solution (100–200 μL) was removed from the bath, mixed with the appropriate amount of drug stocks or dimethyl sulfoxide (DMSO) (<0.01%, D2650; Sigma-Aldrich), and reapplied to the bath chamber. The temperature of the bath solution was 35 ± 0.1°C.
piSPIM image processing and data analysis
Images were processed and analyzed according to Ross et al. (42,50) with some modifications using ImageJ (FIJI) macros and script executed in Python (v3.7). Volumetric image stacks were split to separate P and S channels. Corresponding P and S image stacks (512 × 1024 pixels) were aligned using a Python script and separated into individual images. The median grayscale value of the background intensities for each image was calculated and subtracted. An adaptive local thresholding method was used to obtain a binary clipping mask to separate cell signals from image background. The local threshold value for each pixel was calculated using the Gaussian-weighted sum of the neighborhood pixel intensities (51,52). Anisotropies (r) were calculated using pixel intensities above the threshold value from the corresponding background-subtracted P and S images using the equation (53)
The g-factor constant (g) was measured using an isotropic fluorescein solution and calculated to account for the difference between P and S channel transmission efficiencies as previously described (50). The r-values for each image were summed across all images in each stack and plotted as a histogram distribution. A single mean r-value for each image stack was calculated with a Gaussian fit of the r histogram distribution in Prism v8.4 (GraphPad Software, San Diego, CA). Scripts for automated image alignment, background subtraction, pixel thresholding, and r-value calculations were executed in Python.
In situ calcium calibration
Ca2+ buffering solutions were prepared using the method described in McGuigan et al. (54). To ensure EGTA concentrations in Ca2+-EGTA and EGTA solutions were identical, a 2× EGTA stock solution containing all ingredients (except for NaOH and CaCl2) was prepared and split into two volumes. CaCl2 and NaOH were added to one volume and diluted to obtain a 1× Ca2+-EGTA solution containing 125 mM NaCl, 44 mM NaOH, 5 mM KCl, 2 mM KOH, 1 mM MgCl2, 20 mM HEPES, 1.8 mM 2-deoxy-D-glucose, 5 mM EGTA, 5 mM CaCl2, 0.01 mM rotenone, 0.01 mM ionomycin, and 0.01 mM cyclopiazonic acid (CPA) (pH 7.20 ± 0.01 at 35°C). NaOH and HCl were added to the second volume to produce a final 1× EGTA (zero free Ca2+) solution containing 125 mM NaCl, 44 mM NaOH, 5 mM KCl, 2 mM KOH, 1 mM MgCl2, 20 mM HEPES, 1.8 mM 2-deoxy-D-glucose, 5 mM EGTA, 0.01 mM rotenone, 0.01 mM ionomycin, and 0.01 mM CPA (pH 7.20 ± 0.01 at 35°C). The appropriate quantities of Ca2+-EGTA and EGTA solutions were mixed to obtain solutions with known free Ca2+ concentrations calculated with WebMaxC standard (available online at https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/webmaxc/webmaxcS.htm). SCN slices were equilibrated in Ca2+ buffer solutions at least 20 min before imaging. All imaging solutions were prepared with Ca2+-free liquid-chromatography mass-spectrometry (LC-MS)-grade water (WX0001-6; Sigma-Aldrich). The dissociation constant (Kd) and Hill coefficient (n) were determined by fitting a plot of the r vs. Ca2+ concentration data in Prism (GraphPad Software) with the equation
Statistics
Statistical tests were performed in Prism v8.4 (Graphpad Software). Changes in anisotropy values across baseline time points were tested with a two-way analysis of variance (ANOVA) with repeated measures. Student’s t-tests (two tailed) were used to determine significant differences in anisotropy values and Ca2+ concentrations between peak and trough time points. One-way ANOVA with Bonferroni’s post hoc tests were used to determine significant differences in ΔCa2+ between conditions at each time point. Paired t-tests (two tailed) were used to test for changes in anisotropy and Ca2+ concentration between baseline and drug conditions for individual SCN slices at each condition. Significant differences in GCaMP6f fluorescence across multiple peak and trough time points were tested with a two-way, repeated-measures ANOVA and Bonferroni’s post hoc test using the F/Fmax-values from individual cells for all slices across time points.
Pharmacology
Pharmacological reagents were used at final concentrations of 10 μM nimodipine (Nim; N150; Alomone Labs, Jerusalem, Israel), 10 μM dantrolene (Dan; D9175; Sigma-Aldrich), 30 μM CPA (C-750; Alomone Labs), 3 μM ω-conotoxin GVIA (ConoGVIA; C-300; Alomone Labs), 200 nM ω-agatoxin IVA (AgaIVA; STA-500; Alomone Labs), 30 μM NiCl2 (Ni2+; 22387; Sigma-Aldrich), 10 μM ionomycin (407951; Sigma-Aldrich), 10 μM rotenone (R8875; Sigma-Aldrich), and 50 mM KCl (P9333; Sigma-Aldrich). Reagent stocks (1000×) were prepared in DMSO (Nim, Dan, CPA, ionomycin, rotenone) or water (ConoGVIA, AgaIVA, Ni2+) and stored at −20°C. KCl was prepared as a 4 M HEPES-buffered stock solution.
Results
In the ex vivo organotypic slice preparation, the isolated SCN exhibits intrinsic circadian rhythmicity. First, rhythms in long-term spontaneous action potential activity were recorded by multielectrode array (Supporting materials and methods) to verify robust intrinsic circadian rhythms in cultured SCN slices before imaging. After establishing the diurnal phase using action potential firing, standard confocal imaging was used to verify intracellular Ca2+ was also rhythmic under these experimental conditions using the Ca2+ sensor GCaMP6f (Fig. S1). These data were then used to determine the time windows for quantitative Ca2+ imaging using piSPIM.
To measure intracellular Ca2+ using Venus FLARE-Cameleon (42), SCN slices were cultured on filter membranes (Fig. 1 A i) and transduced with AAVs containing Venus FLARE-Cameleon cDNAs (42) expressed under the neuron-specific hSyn1 promoter (44). Ca2+ binding to the Venus FLARE-Cameleon protein induces FRET between the two Venus fluorophores (55, 56, 57, 58, 59), which is detected as a decrease in the polarization (anisotropy) of emitted light from the sensor (42,53,59, 60, 61). FRET-based measurements from Venus FLARE-Cameleon provide a ratiometric quantification of Ca2+ concentration that is insensitive to variation in expression levels, cell morphology, illumination, or experimental preparations. Thus, this biosensor circumvents the variability in measurements that are based on fluorescence intensity (42,53,55,56,59,62,63,64), enabling quantitative measurements of Ca2+ that are comparable across experimental time points and different SCN slices.
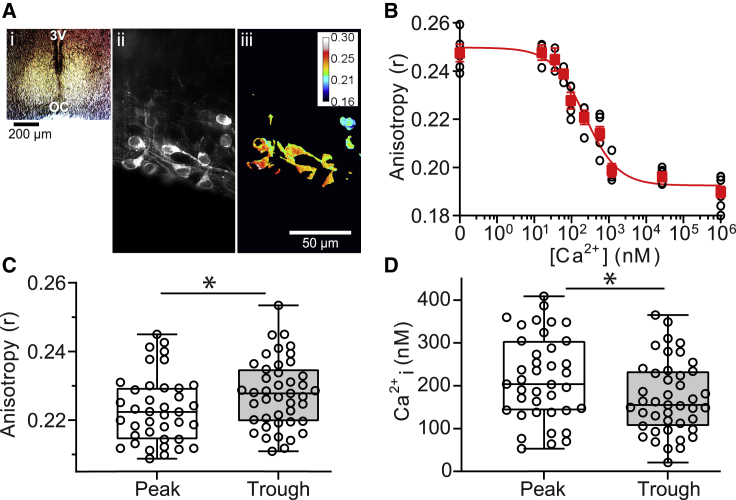
Figure 1.
Neuronal Ca2+ concentrations at peak and trough in SCN. (A i) Brightfield image of SCN at 4× magnification. (A ii) piSPIM image of polarized fluorescence signals from SCN neurons expressing Venus FLARE-Cameleon at 40× magnification. (A iii) Pseudocolor map of anisotropy values from the image in (A ii) calculated from parallel and perpendicular polarized fluorescence signals after image thresholding and background subtraction. (B) In situ calibration of the Venus FLARE-Cameleon sensor in SCN neurons. Anisotropy values from regions of SCN slices incubated in solutions with known free Ca2+ (0–1 mM) are plotted against Ca2+ concentration. Data points (open black circles) representing anisotropy measurements from separate imaging regions were fitted with a nonlinear regression (red line) overlayed with the mean ± SEM of the anisotropy values from all slices in each Ca2+ condition (closed red squares). N = 2–4 slices per Ca2+ condition with one to three imaging regions per slice. (C) Plots of median, 25th and 75th percentile (boxes), and minimal and maximal (whiskers) anisotropy values at peak and trough time points. (D) Box plots of estimated peak and trough Ca2+ concentrations calculated from anisotropy values in (C). Compared to the trough, peak anisotropy was significantly decreased (p = 0.04) and peak Ca2+ concentration was significantly increased (p = 0.02). ∗p < 0.05, unpaired Student’s t-test. Data points represent measurements from individual SCN slices (one imaging region per slice). N = 39 slices for peak, N = 43 slices for trough.
Fluorescent signals from neurons expressing the Venus FLARE-Cameleon biosensor were clearly detectable within the SCN (Fig. 1 A ii). Volumetric images of the polarized fluorescence signals were collected from a cubic area of the SCN (Fig. S2). A local threshold, calculated based on the sum from a Gaussian window, was applied to each image to delineate cell signals (above threshold) from background (below threshold). Anisotropies were calculated from integrating the signal from all pixels above the threshold in each image, which were summed across all images (20 images per stack) to provide a single anisotropy distribution per image stack (Fig. 1 A iii). Anisotropy histograms were fitted with a Gaussian distribution to calculate a single mean anisotropy value encompassing signals from all cells within the imaging region.
Because the relationship between anisotropy values and Ca2+ concentration can be sensitive to variations in temperature and pH (65), the Ca2+ concentration and fluorescence anisotropy for Venus FLARE-Cameleon was calibrated in situ from SCN slices incubated in buffered standards of known Ca2+ concentration at 35°C (Fig. 1 B). For in situ calibration experiments, ionomycin, which permeabilizes the cell membrane to Ca2+; rotenone, an ATP inhibitor; and CPA, a SERCA-ATPase inhibitor, were added to the bath solution to limit homeostatic compensation and promote the clamping of intracellular Ca2+ concentration (66). We found that the dissociation constant (Kd = 230 nM) and hillslope (n = −1.0) values obtained from this in situ Ca2+ calibration curve were similar to those previously reported for this sensor in vitro (42) (Fig. 1 B). The maximal (Rmax) and minimal (Rmin) anisotropy values were 0.259 and 0.184 in 0 and 1 mM Ca2+, respectively. These results indicated that the Venus FLARE-Cameleon reporter was functionally expressed and responsive to changes in clamped Ca2+ concentration.
The in situ calibration values were next used to calculate Ca2+ concentrations from images obtained during peak and trough of the circadian cycle. Anisotropy values were calculated from all pixels, in all cells within the imaging region. Each image typically contained between two and five neurons expressing Venus FLARE-Cameleon after image thresholding. Average anisotropies obtained during the peak (0.223 ± 0.002) were significantly lower compared to anisotropies obtained during the trough (0.228 ± 0.002), indicating that more Ca2+ was bound to the sensor during the peak of the circadian cycle (Fig. 1 C). This corresponded to an estimated Ca2+ concentration that was 1.3-fold higher at the peak (218 ± 16 nM; range 53–408 nM) compared to the trough (172 ± 13 nM; range 21–365 nM) (Fig. 1 D). These data show that a circadian rhythm in intracellular Ca2+ can be detected from SCN neurons with the Venus FLARE-Cameleon sensor, and to our knowledge, provides a new method to track circadian changes in cytosolic Ca2+.
To define the response of the biosensor to acute changes in Ca2+ signaling under these in situ conditions, 50 mM KCl was applied to a subset of SCN slices at the peak and trough. Volumetric images were acquired in 10-s intervals for a 2-min baseline period before KCl was applied to the bath chamber and up to 10 min after KCl application. Consistent with prior studies (67), SCN slices responded to KCl treatment with a transient decrease in anisotropy values (peak: −0.024 ± 0.005; trough: −0.018 ± 0.003), corresponding to a transient increase in neuronal Ca2+ (peak: +271 ± 77 nM; trough: +524 ± 212 nM). The maximal KCl-evoked responses were compared with the average baseline anisotropies and Ca2+ concentrations at the circadian peak and trough. KCl produced a transient increase in Ca2+ levels of 3.66-fold during the peak (baseline: 102 ± 25 nM, KCl: 373 ± 78 nM; n = 5) and 3.52-fold during the trough (baseline: 208 ± 20 nM, KCl: 732 ± 228 nM; n = 8). Thus, Venus FLARE-Cameleon detects changes in Ca2+ evoked during maximal Ca2+ signaling at both the peak and trough.
Next, to test the contributions of the different voltage-gated and intracellular Ca2+ channel subtypes to neuronal Ca2+ in the SCN, we measured the effects of Ca2+ channel inhibitors on anisotropy (Fig. S3, A–D) and estimated Ca2+ concentration (Fig. 2, A–D) during the peak and trough of the circadian cycle. For Ca2+ channel pharmacology experiments, volumetric images were captured in 30-s intervals for 2 min to obtain baseline anisotropy values before drugs or vehicle controls were applied. The effects of each drug were analyzed in slices imaged in 30-s intervals for 2 min of baseline and for 10 min after the application of a drug or vehicle control (Veh, <0.1% DMSO), which were added to the bath solution just before time 0. The average change in anisotropy values (Fig. S3, A and B) and corresponding change in Ca2+ concentrations (Fig. 2, A and B) relative to the baseline average of each slice were plotted as a function of time. Anisotropy values were stable, with no significant change for the duration of the 2-min baseline recordings in each condition (Fig. S3, A and B) (p = 0.3, two-way repeated-measures ANOVA). As a control for neuronal health after drug treatments, 50 mM KCl was applied to some slices after drug effects were obtained. KCl produced decreases in anisotropy values corresponding to increases in Ca2+ concentration that were 2–20 times higher than baseline Ca2+ levels (data not shown). The duration and magnitudes of these transient KCl-evoked responses were similar to those observed for slices in control conditions. These KCl responses obtained at the end of the experimental protocol verify that SCN slices are able respond to stimuli after Ca2+ channel inhibitors were applied, demonstrating that drug exposure did not affect slice viability.
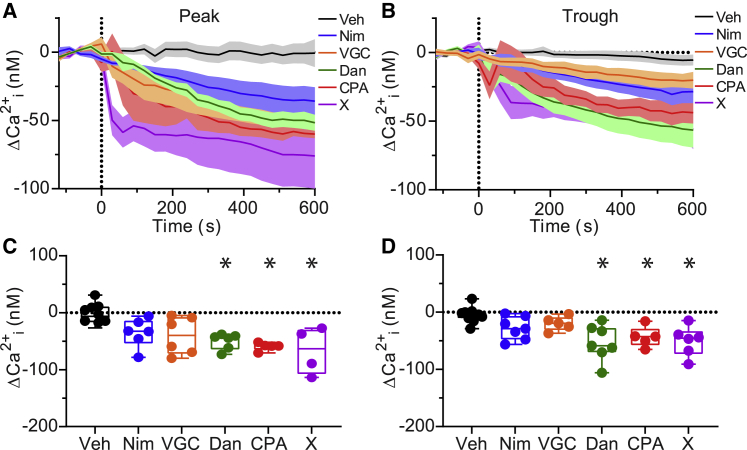
Figure 2.
Effects of Ca2+ channel inhibitors on peak and trough Ca2+ concentration. (A and B) Time course of the change in Ca2+ concentration (ΔCa2+ = Ca2+ − average Ca2+ from 2-min baseline) before and after the application of vehicle control (Veh) or Ca2+ channel inhibitors at the peak (A) and trough (B). Data are mean ± SEM. (C and D) Plots of median, 25th and 75th percentile (boxes), and minimal and maximal (whiskers) changes in Ca2+ concentration for individual slices quantified from 9 to 10 min after drugs were applied at the peak (C) and trough (D). Inhibition of L-type Ca2+ channels with nimodipine (Nim, 10 μM) or inhibition of N/P/Q/R/T-type Ca2+ channels with VGC (a mixture of 3 μM ConoGVIA, 200 nM AgaIVA, 30 μM nickel, and 1 μM TTA-P2) did not significantly affect peak or trough Ca2+ levels compared to Veh. Inhibition of ryanodine receptors with dantrolene (Dan, 10 μM), inhibition of SERCA-ATPase with cyclopiazonic acid (CPA, 10 μM) and combined inhibition of voltage-gated Ca2+ channels and ryanodine receptors with a cocktail containing Dan, Nim, and VGC (cocktail X) significantly decreased Ca2+ at peak and trough. ∗p < 0.05, one-way ANOVA and Bonferroni post hoc test between drug and vehicle control conditions at peak (Nim, p = 0.3; VGC, p = 0.1; Dan, p = 0.02; CPA, p = 0.005; X, p = 0.003) and trough (Nim, p = 0.4; VGC, p = 1; Dan, p = 0.0004; CPA, p = 0.02; X, p = 0.002). Post hoc values were p > 0.05 for all other comparisons. Data points represent average measurements from individual SCN slices (one imaging region per slice). N = 4–11 slices per condition.
First, to probe the contributions of voltage-gated channels, we used 10 μM Nim to target L-type Ca2+ channels and a cocktail containing voltage-gated channel inhibitors (VGCs) targeting N-type (3 μM ConoGVIA), P/Q-type (200 nM AgaIVA), R-type (30 μM nickel), and T-type (1 μM TTA-P2) Ca2+ channels. For each slice, paired comparisons were made between baseline, and the Ca2+ concentration averaged from 9 to 10 min after drugs were applied (Fig. S3, C–F). Application of vehicle control (Veh, <0.1% DMSO) during the peak or the trough did not significantly affect anisotropy values (Fig. S3, C and D) or Ca2+ concentrations (Fig. S3, E and F) compared to baseline. In contrast, voltage-gated Ca2+ channel inhibitors produced an increase in anisotropy values (Fig. S3, C and D), which corresponded to a reduction in the Ca2+ levels in paired comparison to baseline values (Fig. S3, E and F). These data implicate voltage-gated Ca2+ channels as contributors to the cytosolic Ca2+ levels during the peak and the trough.
We then tested the contributions of intracellular Ca2+ channels. Previous studies have shown that inhibiting RyR-mediated Ca2+ release from the ER produced a decrease in cytosolic Ca2+ levels (24,41), but the effect of inhibiting intracellular Ca2+ channels at both the peak and trough has not been systematically tested. 10 μM Dan was used to inhibit RyRs, and 10 μM CPA was used to target the SERCA-ATPase, which inhibits refilling of ER Ca2+ stores. CPA produces ER store depletion and subsequent inhibition of both RyR-and IP3R-mediated Ca2+ release (68). In a third condition, a cocktail (X) containing a combination of the VGCs, plus nimodipine and dantrolene to collectively inhibit RyRs along with the voltage-gated channels, was applied. Application of cocktail X thus inhibits other channels without blocking IP3Rs. We found that each inhibitor of intracellular Ca2+ channels produced a significant decrease in Ca2+ levels at the peak and the trough in a paired comparison to the baseline values for each slice (Fig. S3, E and F). These results suggest that intracellular channels also contribute to cytosolic Ca2+ levels at both the peak and the trough.
Determination of the relative contributions for VGCCs and intracellular Ca2+ channels across experiments requires accounting for the variation in baseline Ca2+ levels in each SCN slice. To make this comparison, the average Ca2+ concentration from 2 min of baseline was subtracted from the average Ca2+ levels 9–10 min after drug application to obtain the change in Ca2+ (ΔCa2+) within each slice. First, we focused on the ΔCa2+ produced by each drug during the peak (Fig. 2, A and C). Starting with the vehicle controls, the ΔCa2+ was negligible at −2 ± 7 nM (−2 ± 5% change) (Fig. 2 C). The voltage-gated channel inhibitors produced a ΔCa2+ of −35 ± 10 nM (−20 ± 4% change) (Nim) and −40 ± 13 nM (−19 ± 4%) (VGC); however, these decreases were not statistically different than the vehicle control. This may be partly explained by the variability in the ΔCa2+ responses of individual slices, which ranged from −6 to −78 nM (Nim) and −5 to −79 nM (VGC) (Fig. 2 C). In contrast, intracellular Ca2+ channel inhibitors produced decreases in peak Ca2+ that were significantly larger than the vehicle controls. Dantrolene produced the largest decrease, with a ΔCa2+ of −50 ± 6 nM (−36 ± 6% change). Similarly, the SERCA inhibitor CPA produced a ΔCa2+ of −59 ± 3 nM (−24 ± 5%), and the mixture of VGC inhibitors along with nimodipine and dantrolene added together (X cocktail) produced a ΔCa2+ of −67 ± 21 nM (−22 ± 6%) (Fig. 2 C). The responses of individual slices to intracellular Ca2+ channel inhibitors were less variable, with the range of −38 to −73 nM for dantrolene, −52 to −71 nM for CPA, and −27 to −114 nM for cocktail X. Taken together, these results suggest that peak intracellular Ca2+ is predominantly set by RyR channel contribution.
These inhibitors had similar effects on Ca2+ during the trough of the circadian cycle (Fig. 2 D). Vehicle control had little effect, −5 ± 4 nM (−7 ± 5% change). Nimodipine decreased Ca2+ by −28 ± 8 nM (−17 ± 5%) overall with a range of responses between −2 and −56 nM. Similarly, VGC decreased Ca2+ by −20 ± 6 nM (−16 ± 3%), with changes in Ca2+ ranging from −3.5 to −37 nM. The decreases in trough Ca2+ were significantly larger for dantrolene, −53 ± 12 nM (−28 ± 6%); CPA, −43 ± 8 nM (−32 ± 7%); and cocktail X, −51 ± 11 nM (−23 ± 5%). These overall decreases were accompanied by a larger range in ΔCa2+ responses, which were −14 to −106 nM for dantrolene, −16 to −65 nM for CPA, and −15 to −91 nM for cocktail X (Fig. 2 D). Thus, at both peak and trough time points, the largest decrease in Ca2+ was produced by dantrolene. In contrast, the contribution of voltage-gated channels appears less significant and in some cases has more variability.
Discussion
A primary advance of this study, to our knowledge, is the novel application of Venus-cp172Venus FLARE-Cameleon sensor (42) with piSPIM (59) to measure biologically controlled changes in Ca2+ in live organotypic brain tissue. The Venus FLARE-Cameleon Ca2+ sensor captured the collective signal in basal cytosolic Ca2+ averaged from multiple neurons within a region of the SCN without integrating shorter-timescale Ca2+ signals, such as action-potential-evoked Ca2+ transients. Using these techniques, Ca2+ concentrations (218 ± 16 and 172 ± 13 nM, respectively) were refined over prior absolute and relativistic estimates (27). These SCN concentrations are consistent with basal Ca2+ levels typically measured in other neuronal cell types, ∼40–190 nM (69), and the values reported using other ratiometric Ca2+ sensors in the SCN. In prior studies, peak values ranged between 50 and 440 nM, and trough values ranged between 50 and 150 nM (27). However, prior measurements using Fura-2 (13,29,41,70) or genetically encoded sensors such as Yellow Cameleons (24,25,33,71) did not utilize in situ calibration of the Ca2+ sensor. These measurements relied on cell-free in vitro calibrations, which does not account for factors in the intracellular environment that could affect the Ca2+ estimates (65). In this study, Ca2+ measurements were obtained using a Ca2+ sensor that was calibrated in SCN slices under the same experimental conditions at baseline and across different Ca2+ inhibitor experiments. Venus FLARE-Cameleon also has the added advantage of only occupying a single-color channel, which will allow imaging of multiple biosensors expressed in the same neuron. Thus, this study provides a foundation for future experiments to investigate the cross talk between Ca2+ and other cellular signaling components toward piecing together how the ensemble circadian clock mechanism functions at a cellular level.
A central feature of SCN neurons is that they express different properties depending on the time of the circadian cycle. SCN neurons exhibit a state of increased excitability and increased activation of voltage-gated Ca2+ channels during the day (peak of the cycle) and a state of decreased excitability at night (trough of the cycle), during which voltage-gated Ca2+ channel activity is reduced (3,4,38,72). Yet, it has remained unclear whether this daily increase in voltage-gated Ca2+ channel activity is involved in maintaining the circadian pattern in cytosolic Ca2+, which is also highest during the circadian peak. Furthermore, no single study has directly compared the contributions for these different Ca2+ channel types to cytosolic Ca2+ levels at both peak and trough of the circadian cycle in intact SCN slices. Prior studies measured the effects of inhibitors on Ca2+ levels only at a single time point or employed only a single Ca2+ channel inhibitor (24,28,30,41,67). As a result of these methodological discrepancies, the relative contributions of the Ca2+ channel subtypes at both times of the circadian cycle have been less than fully conclusive. For example, in studies measuring the Ca2+ rhythms from the whole SCN, inhibition of L-type voltage-gated Ca2+ channels with nimodipine reduced the magnitude of the day-night difference in Ca2+ levels (28,30). However, other studies found no effect of nimodipine on Ca2+ levels (24,73).
With direct comparison of the relative contributions for each Ca2+ source under equivalent experimental conditions, the results in this study support the current view that intracellular RyR Ca2+ channels are major contributors to the Ca2+ levels during both the peak and trough of the circadian cycle. Because the combined inhibition of voltage-gated channels and RyRs did not significantly decrease Ca2+ levels further compared to inhibiting RyRs alone, it suggests that RyR inhibition produced the majority of the decrease in Ca2+. Inhibiting the SERCA-ATPase, which prevents Ca2+ reuptake into the ER and leads to a depletion of ER stores (68), also did not produce a decrease in Ca2+ that was larger in magnitude than the decrease observed when inhibiting RyRs alone. This further suggests that IP3Rs, which also can mediate ER Ca2+ release, may have a lesser contribution to cytosolic Ca2+ compared to RyRs, although this was not tested directly because of a lack of selective IP3R inhibitors (74). This study corroborates prior reports of decreased peak Ca2+ with RyR inhibition (6,24,41). RyR2 messenger RNA (mRNA) and protein also exhibit a daytime peak in expression (38,75). However, it is unlikely that an expression-based mechanism would fully account for the circadian oscillation in Ca2+ levels, as RyR activity is regulated by increases in intracellular Ca2+ (27). Although it remains to be determined whether other VGCCs contribute to Ca2+-induced Ca2+ release in SCN neurons, the lesser effect of inhibiting these channels suggests they do not serve as the primary sensors for RyR-mediated Ca2+ release. Other sources of calcium in the SCN not tested here include ionotropic glutamate receptors, including N-methyl-D-aspartate receptors and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (20). Ca2+ homeostasis is also maintained by the activity of Na+-Ca2+ exchanger types 1 and 2 (NCX1 and NCX2) (38,75) and endoplasmic reticulum Ca2+-ATPases (SERCA) (70), which mediate Ca2+ efflux or uptake into ER stores, whereas uptake of Ca2+ via mitochondrial NCX (76) and Ca2+-binding proteins buffer cytosolic Ca2+ (77, 78, 79). There is also potential involvement of store-operated Ca2+ entry channels (80). Another mechanism that could be involved in regulating basal Ca2+ over the circadian cycle, in conjunction with the activity of ion channels, is Ca2+ buffering by Ca2+-binding proteins (69), which can alter basal Ca2+ as well as influence the amplitude and decay of stimulus-evoked Ca2+ transients (81,82), leading to changes in firing properties of neurons (69). SCN neurons express calbindin D28K and calretinin (77,83). In SCN, levels of cytosolic calbindin protein have been observed to change over the course of the circadian cycle (77). This evidence suggests that these Ca2+ binding proteins can be regulated based on the daily requirements of SCN neurons and could play an important role in circadian Ca2+ rhythms. Further studies will be required to investigate their contributions.
This study did not focus on defining the Ca2+ regulatory mechanisms by subregion, but the phase of the Ca2+ rhythm exhibits regional differences. It has not yet been addressed whether the Ca2+ channels themselves differ by subregion as the basis. The shell region of the SCN, defined by expression of the neuropeptide arginine vasopressin, exhibits a rhythmic Ca2+ peak 3–5 h before the core, defined by vasoactive intestinal polypeptide (VIP) expression (28,30,33). Shell Ca2+ rhythms also had higher amplitudes. These regional phase and amplitude differences could contribute to the wide variation in Ca2+ values observed in the baseline measurements of this study, as the ROIs were located in the center of the SCN and undefined with respect to the core and shell boundaries. In addition, sequential application of inhibitors could reveal whether the relative contribution of Ca2+ channel types differs between subregions. However, in previous studies, at least one inhibitor (nimodipine) failed to show a regional difference in its effects on Ca2+ levels (30), leaving open the question of which Ca2+ channels produce these phase differences. Regional differences are also present in Drosophila clock neurons (84,85), in which basal Ca2+ levels were sensitive to RNA interference (RNAi) knockdown of intracellular Ca2+ release via IP3Rs or SERCA, but only one set of neurons was sensitive to VGCC knockdown (86).
Author contributions
A.E.P. and A.L.M. designed the experiments and wrote the manuscript. A.E.P. performed the experiments and analyzed the data, V.P.R. wrote the computer code and assisted with image analysis, and M.A.R. designed and built the piSPIM microscope.
Acknowledgments
We thank Joe Mauban, the University of Maryland Confocal Core Facility, and members of the Blanpied lab for assistance with confocal imaging. We thank members of the Meredith lab for comments on the manuscript.
This work was supported by grants from NHLBI R01-HL102758 (A.L.M.), the Training Program in Integrative Membrane Biology NHLBI T32-GM008181 (A.L.M., V.P.R., and A.E.P.), NIMH R01-MH111527 (A.L.M. and M.A.R.), NIH Fellowship Grant F30DK124986 (V.P.R.), NIDDK R01DK077140 (M.A.R.), and NHLBI R01HL122827 (M.A.R.).
Declaration of interests
The authors declare no competing interests.
Editor: Shi-Wei Chu.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.bpr.2021.100005.
Supporting citations
References (87, 88, 89) appear in the Supporting material.
Supporting material
References
- 1.Lundkvist G.B., Kwak Y., et al. Block G.D. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J. Neurosci. 2005;25:7682–7686. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nahm S.S., Farnell Y.Z., et al. Earnest D.J. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J. Neurosci. 2005;25:9304–9308. doi: 10.1523/JNEUROSCI.2733-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNally B.A., Plante A.E., Meredith A.L. Diurnal properties of voltage-gated Ca2+ currents in suprachiasmatic nucleus and roles in action potential firing. J. Physiol. 2020;598:1775–1790. doi: 10.1113/JP278327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson A.C., Yao G.L., Bean B.P. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J. Neurosci. 2004;24:7985–7998. doi: 10.1523/JNEUROSCI.2146-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennartz C.M., de Jeu M.T., et al. Geurtsen A.M. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar-Roblero R., Mercado C., et al. Díaz-Muñoz M. Ryanodine receptor Ca2+-release channels are an output pathway for the circadian clock in the rat suprachiasmatic nuclei. Eur. J. Neurosci. 2007;26:575–582. doi: 10.1111/j.1460-9568.2007.05679.x. [DOI] [PubMed] [Google Scholar]
- 7.Moore R.Y., Eichler V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 8.Silver R., LeSauter J., et al. Lehman M.N. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Bernstein E.L., Jetton A.E., et al. Bittman E.L. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz W.J., Gross R.A., Morton M.T. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc. Natl. Acad. Sci. USA. 1987;84:1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ralph M.R., Foster R.G., et al. Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 12.Stephan F.K., Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irwin R.P., Allen C.N. Calcium response to retinohypothalamic tract synaptic transmission in suprachiasmatic nucleus neurons. J. Neurosci. 2007;27:11748–11757. doi: 10.1523/JNEUROSCI.1840-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim D.Y., Choi H.J., et al. Kim Y.I. Voltage-gated calcium channels play crucial roles in the glutamate-induced phase shifts of the rat suprachiasmatic circadian clock. Eur. J. Neurosci. 2005;21:1215–1222. doi: 10.1111/j.1460-9568.2005.03950.x. [DOI] [PubMed] [Google Scholar]
- 15.Hirota T., Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zool. Sci. 2004;21:359–368. doi: 10.2108/zsj.21.359. [DOI] [PubMed] [Google Scholar]
- 16.Meijer J.H., Schwartz W.J. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J. Biol. Rhythms. 2003;18:235–249. doi: 10.1177/0748730403018003006. [DOI] [PubMed] [Google Scholar]
- 17.van den Pol A.N., Finkbeiner S.M., Cornell-Bell A.H. Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J. Neurosci. 1992;12:2648–2664. doi: 10.1523/JNEUROSCI.12-07-02648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Pol A.N. Glutamate and GABA presence and action in the suprachiasmatic nucleus. J. Biol. Rhythms. 1993;8(Suppl):S11–S15. [PubMed] [Google Scholar]
- 19.Jones J.R., Simon T., et al. Herzog E.D. SCN VIP neurons are essential for normal light-mediated resetting of the circadian system. J. Neurosci. 2018;38:7986–7995. doi: 10.1523/JNEUROSCI.1322-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tominaga K., Geusz M.E., et al. Inouye S.T. Calcium imaging in organotypic cultures of the rat suprachiasmatic nucleus. Neuroreport. 1994;5:1901–1905. doi: 10.1097/00001756-199410000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Tischkau S.A., Mitchell J.W., et al. Gillette M.U. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J. Biol. Chem. 2003;278:718–723. doi: 10.1074/jbc.M209241200. [DOI] [PubMed] [Google Scholar]
- 22.Ding J.M., Buchanan G.F., et al. Gillette M.U. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama M., Kouzu Y., et al. Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J. Neurosci. 1999;19:1115–1121. doi: 10.1523/JNEUROSCI.19-03-01115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeda M., Sugiyama T., et al. Allen C.N. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003;38:253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 25.Enoki R., Ono D., et al. Honma K. Single-cell resolution fluorescence imaging of circadian rhythms detected with a Nipkow spinning disk confocal system. J. Neurosci. Methods. 2012;207:72–79. doi: 10.1016/j.jneumeth.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Brancaccio M., Patton A.P., et al. Hastings M.H. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron. 2017;93:1420–1435.e5. doi: 10.1016/j.neuron.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvey J.R.M., Plante A.E., Meredith A.L. Ion channels controlling circadian rhythms in suprachiasmatic nucleus excitability. Physiol. Rev. 2020;100:1415–1454. doi: 10.1152/physrev.00027.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noguchi T., Leise T.L., et al. Welsh D.K. Calcium circadian rhythmicity in the suprachiasmatic nucleus: cell autonomy and network modulation. eNeuro. 2017;4 doi: 10.1523/ENEURO.0160-17.2017. ENEURO.0160-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colwell C.S. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur. J. Neurosci. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enoki R., Oda Y., et al. Honma K.I. Synchronous circadian voltage rhythms with asynchronous calcium rhythms in the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA. 2017;114:E2476–E2485. doi: 10.1073/pnas.1616815114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ono D., Honma S., et al. Honma K.I. Dissociation of Per1 and Bmal1 circadian rhythms in the suprachiasmatic nucleus in parallel with behavioral outputs. Proc. Natl. Acad. Sci. USA. 2017;114:E3699–E3708. doi: 10.1073/pnas.1613374114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patton A.P., Edwards M.D., et al. Hastings M.H. The VIP-VPAC2 neuropeptidergic axis is a cellular pacemaking hub of the suprachiasmatic nucleus circadian circuit. Nat. Commun. 2020;11:3394. doi: 10.1038/s41467-020-17110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enoki R., Kuroda S., et al. Honma K. Topological specificity and hierarchical network of the circadian calcium rhythm in the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA. 2012;109:21498–21503. doi: 10.1073/pnas.1214415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong J.H., Jeong B., et al. Lee K.J. Circadian waves of cytosolic calcium concentration and long-range network connections in rat suprachiasmatic nucleus. Eur. J. Neurosci. 2012;35:1417–1425. doi: 10.1111/j.1460-9568.2012.08069.x. [DOI] [PubMed] [Google Scholar]
- 35.Brancaccio M., Maywood E.S., et al. Hastings M.H. A Gq-Ca2+ axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron. 2013;78:714–728. doi: 10.1016/j.neuron.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding J.M., Chen D., et al. Gillette M.U. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- 37.Schurov I.L., McNulty S., et al. Hastings M.H. Glutamatergic induction of CREB phosphorylation and Fos expression in primary cultures of the suprachiasmatic hypothalamus in vitro is mediated by co-ordinate activity of NMDA and non-NMDA receptors. J. Neuroendocrinol. 1999;11:43–51. doi: 10.1046/j.1365-2826.1999.00289.x. [DOI] [PubMed] [Google Scholar]
- 38.Díaz-Muñoz M., Dent M.A., et al. Aguilar-Roblero R. Circadian modulation of the ryanodine receptor type 2 in the SCN of rodents. Neuroreport. 1999;10:481–486. doi: 10.1097/00001756-199902250-00007. [DOI] [PubMed] [Google Scholar]
- 39.Hamada T., Liou S.Y., et al. Ishida N. The role of inositol trisphosphate-induced Ca2+ release from IP3-receptor in the rat suprachiasmatic nucleus on circadian entrainment mechanism. Neurosci. Lett. 1999;263:125–128. doi: 10.1016/s0304-3940(99)00111-1. [DOI] [PubMed] [Google Scholar]
- 40.Cheng P.C., Wang Y.C., et al. Huang R.C. Differential regulation of nimodipine-sensitive and -insensitive Ca2+ influx by the Na+/Ca2+ exchanger and mitochondria in the rat suprachiasmatic nucleus neurons. J. Biomed. Sci. 2018;25:44. doi: 10.1186/s12929-018-0447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguilar-Roblero R., Quinto D., et al. Lundkvist G. Ryanodine-sensitive intracellular Ca2+ channels are involved in the output from the SCN circadian clock. Eur. J. Neurosci. 2016;44:2504–2514. doi: 10.1111/ejn.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross B.L., Tenner B., et al. Zhang J. Single-color, ratiometric biosensors for detecting signaling activities in live cells. eLife. 2018;7:e35458. doi: 10.7554/eLife.35458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montgomery J.R., Whitt J.P., et al. Meredith A.L. Mis-expression of the BK K(+) channel disrupts suprachiasmatic nucleus circuit rhythmicity and alters clock-controlled behavior. Am. J. Physiol. Cell Physiol. 2013;304:C299–C311. doi: 10.1152/ajpcell.00302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kügler S., Kilic E., Bähr M. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 2003;10:337–347. doi: 10.1038/sj.gt.3301905. [DOI] [PubMed] [Google Scholar]
- 45.Whitt J.P., Montgomery J.R., Meredith A.L. BK channel inactivation gates daytime excitability in the circadian clock. Nat. Commun. 2016;7:10837. doi: 10.1038/ncomms10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar A., Wu Y., et al. Shroff H. Dual-view plane illumination microscopy for rapid and spatially isotropic imaging. Nat. Protoc. 2014;9:2555–2573. doi: 10.1038/nprot.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar A., Christensen R., et al. Shroff H. Using stage- and slit-scanning to improve contrast and optical sectioning in dual-view inverted light sheet microscopy (diSPIM) Biol. Bull. 2016;231:26–39. doi: 10.1086/689589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y., Wawrzusin P., et al. Shroff H. Spatially isotropic four-dimensional imaging with dual-view plane illumination microscopy. Nat. Biotechnol. 2013;31:1032–1038. doi: 10.1038/nbt.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edelstein A.D., Tsuchida M.A., et al. Stuurman N. Advanced methods of microscope control using μManager software. J. Biol. Methods. 2014;1:e10. doi: 10.14440/jbm.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross B., Wong S.H., et al. Rizzo M.A. Triple fluorescence anisotropy reporter imaging in living cells. Bio Protoc. 2019;9:ew3226. doi: 10.21769/BioProtoc.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez G. In: Proceedings of the BMVC 2000. Mirmehdi M., Thomas B., editors. BMVA Press; 2000. Local smoothness in terms of variance: the adaptive Gaussian filter; pp. 815–824. [Google Scholar]
- 52.Sezgin M., Sankur B. Survey over image thresholding techniques and quantitative performance evaluation. J. Electron. Imaging. 2004;13:146–166. [Google Scholar]
- 53.Rizzo M.A., Piston D.W. High-contrast imaging of fluorescent protein FRET by fluorescence polarization microscopy. Biophys. J. 2005;88:L14–L16. doi: 10.1529/biophysj.104.055442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGuigan J.A., Lüthi D., Buri A. Calcium buffer solutions and how to make them: a do it yourself guide. Can. J. Physiol. Pharmacol. 1991;69:1733–1749. doi: 10.1139/y91-257. [DOI] [PubMed] [Google Scholar]
- 55.Miyawaki A., Llopis J., et al. Tsien R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 56.Miyawaki A., Griesbeck O., et al. Tsien R.Y. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc. Natl. Acad. Sci. USA. 1999;96:2135–2140. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heim N., Garaschuk O., et al. Griesbeck O. Improved calcium imaging in transgenic mice expressing a troponin C-based biosensor. Nat. Methods. 2007;4:127–129. doi: 10.1038/nmeth1009. [DOI] [PubMed] [Google Scholar]
- 58.Nagai T., Yamada S., et al. Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Markwardt M.L., Snell N.E., et al. Rizzo M.A. A genetically encoded biosensor strategy for quantifying non-muscle myosin II phosphorylation dynamics in living cells and organisms. Cell Rep. 2018;24:1060–1070.e4. doi: 10.1016/j.celrep.2018.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jares-Erijman E.A., Jovin T.M. FRET imaging. Nat. Biotechnol. 2003;21:1387–1395. doi: 10.1038/nbt896. [DOI] [PubMed] [Google Scholar]
- 61.Mattheyses A.L., Hoppe A.D., Axelrod D. Polarized fluorescence resonance energy transfer microscopy. Biophys. J. 2004;87:2787–2797. doi: 10.1529/biophysj.103.036194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian L., Hires S.A., et al. Looger L.L. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rizzo M.A., Springer G., et al. Piston D.W. Optimization of pairings and detection conditions for measurement of FRET between cyan and yellow fluorescent proteins. Microsc. Microanal. 2006;12:238–254. doi: 10.1017/S1431927606060235. [DOI] [PubMed] [Google Scholar]
- 64.Rizzo M.A., Piston D.W. High-contrast imaging of fluorescent protein FRET by fluorescence polarization microscopy. Biophys J. 2005;88(2):14–16. doi: 10.1529/biophysj.104.055442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glaum S.R., Alford S., et al. Slater N.T. In: Methods in Neurosciences. Narahashi T., editor. Elsevier; 1994. Whole-cell patch recording with simultaneous measurement of intracellular calcium concentration in mammalian brain slices in vitro; pp. 340–358. [Google Scholar]
- 66.Thomas D., Tovey S.C., et al. Lipp P. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 2000;28:213–223. doi: 10.1054/ceca.2000.0152. [DOI] [PubMed] [Google Scholar]
- 67.Cheng R.C., Cheng P.C., et al. Huang R.C. Role of intracellular Na+ in the regulation of [Ca2+]i in the rat suprachiasmatic nucleus neurons. Int. J. Mol. Sci. 2019;20:E4868. doi: 10.3390/ijms20194868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garaschuk O., Yaari Y., Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J. Physiol. 1997;502:13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwaller B. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2010;2:a004051. doi: 10.1101/cshperspect.a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burkeen J.F., Womac A.D., et al. Zoran M.J. Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J. Neurosci. 2011;31:8432–8440. doi: 10.1523/JNEUROSCI.6576-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Enoki R., Ono D., et al. Honma K.I. Dual origins of the intracellular circadian calcium rhythm in the suprachiasmatic nucleus. Sci. Rep. 2017;7:41733. doi: 10.1038/srep41733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Jeu M., Hermes M., Pennartz C. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. Neuroreport. 1998;9:3725–3729. doi: 10.1097/00001756-199811160-00028. [DOI] [PubMed] [Google Scholar]
- 73.Ikeda M., Ikeda M. Bmal1 is an essential regulator for circadian cytosolic Ca2+ rhythms in suprachiasmatic nucleus neurons. J. Neurosci. 2014;34:12029–12038. doi: 10.1523/JNEUROSCI.5158-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bootman M.D., Collins T.J., et al. Peppiatt C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 75.Aguilar-Roblero R., Díaz-Muñoz M., et al. Michel S. In: Mechanisms of Circadian Systems in Animals and Their Clinical Relevance. Aguilar-Roblero R., Díaz-Muñoz M., Fanjul-Moles M., editors. Springer; 2015. Intracellular calcium as a clock output from SCN neurons; pp. 115–132. [Google Scholar]
- 76.Wang Y.C., Chen Y.S., et al. Huang R.C. Role of Na+/Ca2+ exchanger in Ca2+ homeostasis in rat suprachiasmatic nucleus neurons. J. Neurophysiol. 2015;113:2114–2126. doi: 10.1152/jn.00404.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamada T., LeSauter J., et al. Silver R. Calbindin influences response to photic input in suprachiasmatic nucleus. J. Neurosci. 2003;23:8820–8826. doi: 10.1523/JNEUROSCI.23-26-08820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carafoli E. Calcium signaling: a tale for all seasons. Proc. Natl. Acad. Sci. USA. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bryant D.N., LeSauter J., et al. Romero M.T. Retinal innervation of calbindin-D28K cells in the hamster suprachiasmatic nucleus: ultrastructural characterization. J. Biol. Rhythms. 2000;15:103–111. doi: 10.1177/074873040001500204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kononenko N.I., Medina I., Dudek F.E. Persistent subthreshold voltage-dependent cation single channels in suprachiasmatic nucleus neurons. Neuroscience. 2004;129:85–92. doi: 10.1016/j.neuroscience.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 81.Chard P.S., Bleakman D., et al. Miller R.J. Calcium buffering properties of calbindin D28k and parvalbumin in rat sensory neurones. J. Physiol. 1993;472:341–357. doi: 10.1113/jphysiol.1993.sp019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matthews E.A., Dietrich D. Buffer mobility and the regulation of neuronal calcium domains. Front. Cell. Neurosci. 2015;9:48. doi: 10.3389/fncel.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silver R., Romero M.T., et al. LeSauter J. Calbindin-D28K cells in the hamster SCN express light-induced Fos. Neuroreport. 1996;7:1224–1228. doi: 10.1097/00001756-199604260-00026. [DOI] [PubMed] [Google Scholar]
- 84.Liang X., Holy T.E., Taghert P.H. Synchronous Drosophila circadian pacemakers display nonsynchronous Ca2+ rhythms in vivo. Science. 2016;351:976–981. doi: 10.1126/science.aad3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang X., Holy T.E., Taghert P.H. A series of suppressive signals within the Drosophila circadian neural circuit generates sequential daily outputs. Neuron. 2017;94:1173–1189.e4. doi: 10.1016/j.neuron.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang X., Holy T.E., Taghert P.H. Circadian pacemaker neurons display co-phasic rhythms in basal calcium level and in fast calcium fluctuations. bioRxiv. 2021 doi: 10.1101/2021.05.03.442479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schindelin J., Arganda-Carreras I., et al. Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thévenaz P., Ruttimann U.E., Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- 89.Chen T.W., Wardill T.J., et al. Kim D.S. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.