Figure 1.
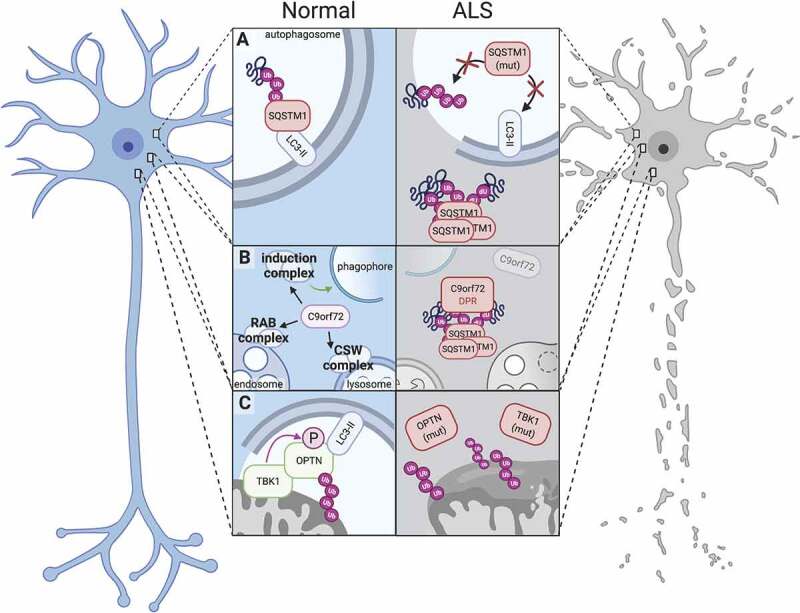
Dysfunction of autophagy-related proteins impairs proteostasis and leads to neurotoxicity in ALS. (A) Under normal conditions, SQSTM1 serves as a receptor protein in selective autophagy and binds both LC3-II and polyubiquitinated proteins, thereby targeting ubiquitinated substrates to phagophores (left); Mutations in SQSTM1 abrogate SQSTM1’s binding activities (right top) or result in the aggregation of SQSTM1 into ubiquitin-positive inclusions (right bottom). (B) The C9orf72 protein participates in several autophagy-related complexes, including the autophagy induction complex (ULK1-RAB1A) that promotes autophagosome biogenesis, the RAB7-RAB11 complex (RAB complex) that regulates endosome maturation, and the C9orf72-SMCR8-WDR41 (CSW) complex that regulates lysosomal dynamics and autophagic flux (left). Disease-associated C9orf72 mutations reduce C9orf72 protein levels (right), while dipeptide repeat proteins generated from the C9orf72 expansion localize to SQSTM1- and ubiquitin-positive inclusions (right). (C) In normal mitophagy, TBK1 binds and phosphorylates OPTN, enhancing its affinity for polyubiquitinated mitochondria and LC3-II (left). TBK1 and OPTN mutations perturb these functions and compromise efficient mitophagy, leading to failed mitochondrial clearance and dysfunctional mitochondria.
