Figure 3.
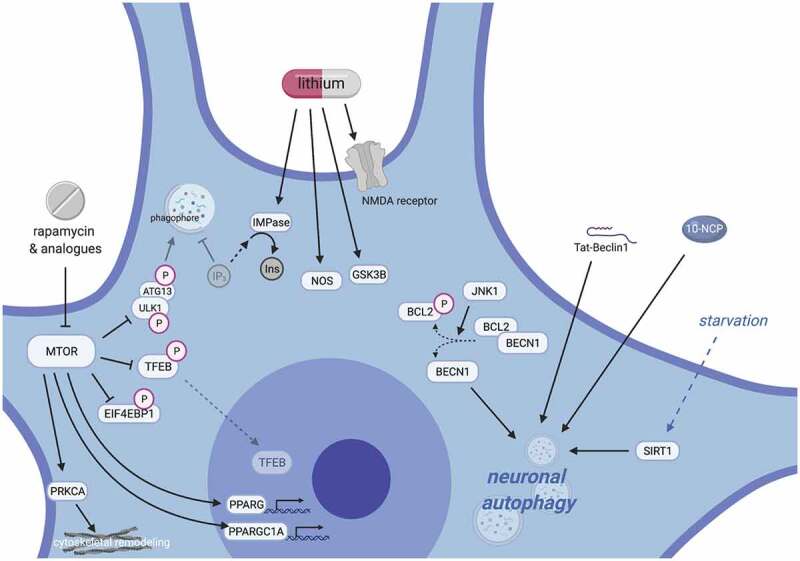
Mechanisms of neuronal autophagy and their impact for therapeutic design in ALS. Pharmacodynamic considerations limit the use of currently available drugs for modulating autophagy. Inhibitors of MTOR inhibitors (rapalogs) only weakly activate autophagy in neurons, and their wide-ranging effects target myriad cellular pathways, including growth signaling, translation, stress responses, transcriptional regulation, and cytoskeletal remodeling (left). Such pleiotropy leads to well-documented and multi-systemic toxicities, especially with long-term use; however, newer rapalogs may enable more specific targeting and selective autophagy modulation. Similarly, lithium has numerous multi-target effects, including depletion of IP3 through IMPase, activating nitric oxide synthase, inhibiting GSK3B, stimulating NMDA receptors and enhancing glutamatergic tone, among many others (middle). This results in a narrow therapeutic index for lithium, potentially explaining its apparent lack of neuroprotective effects in human trials for neurodegenerative disease to date. To reduce off-target effects, recent efforts have focused on MTOR-independent strategies for stimulating autophagy, including modulation of SIRT1, phenoxazine compounds such as 10-NCP, or synthetic peptides such as Tat-Beclin 1 (right).
