ABSTRACT
We previously found that activated CD8+ T-cells increase expression of PD-1, which can be attenuated in the presence of specific Toll-like receptor (TLR) agonists, mediated by IL-12 secreted by professional antigen-presenting cells. While these CD8+ T-cells had greater anti-tumor activity, T-cells stimulated by different TLR had different gene expression profiles. Consequently, we sought to determine whether combinations of TLR agonists might further affect the expression of T-cell checkpoint receptors and improve T-cell anti-tumor immunity. Activation of CD8+ T-cells in the presence of specific TLR ligands resulted in decreased expression of PD-1, LAG-3, and CD160, notably with combinations of TLR1/2, TLR3, and TLR9 agonists. Immunization of E.G7-OVA or TRAMP-C1 tumor-bearing mice with peptide or DNA vaccines, co-administered with combination of TLR3 and TLR9 agonists, showed greater suppression of tumor growth. The anti-tumor effect of TLR1/2 and/or TLR9, but not TLR3, was abrogated in IL-12KO mice. RNA sequencing of TLR-conditioned CD8+ T-cells revealed IL-12 pathway activation, and type 1 IFN pathway activation following TLR3 stimulation. Our results provide a mechanistic rationale for the choice of optimal combinations of TLR ligands to use as adjuvants to improve the efficacy of anti-tumor vaccines.
KEYWORDS: Toll-like receptor, tumor vaccine, adjuvant, IL-12, type 1 interferon
Introduction
T-cell checkpoint receptors, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1), lymphocyte activation gene 3 (LAG-3), and T-cell immunoglobulin and mucin-domain containing 3 (TIM-3), were first discovered on T-cells and associated with reduced functional phenotype.1,2 More recent studies have demonstrated that multiple other T-cell checkpoint receptors exist, each associated with reduced T-cell functional phenotype, including CD160,3 V-domain Ig suppressor of T-cell activation (VISTA),4 T-cell immunoreceptor with Ig and ITIM domains (TIGIT),5 and CD244.6 This has led to the general acceptance of checkpoint receptors as indicative of T-cell exhaustion.7,8 However, the expression of T-cell checkpoint receptors occurs naturally when T-cells are activated and binding of their ligands leads to reduced effector function.9,10 Moreover, the timing and expression of T-cell checkpoint receptors can be permanently modulated by events during T-cell activation. For example, OT-1 T-cells activated by a high-affinity epitope have increased PD-1 expression, which can persist over time, while OT-1 T-cells stimulated with a lower affinity variant of the same antigen become activated with lower, transient PD-1 expression.10 This altered expression of PD-1, in particular, has consequences for anti-tumor immunity; lower expression of PD-1, or blockade of PD-1/PD-L1, was also demonstrated to lead to greater anti-tumor efficacy in which CD8+ T-cells were activated with vaccination.10,11 These findings suggest that efforts to lower or block the expression of multiple checkpoint receptors could have profound effects on the efficacy of activated CD8+ T-cells used as anti-tumor therapies.
Certain pathogens can induce robust immune responses, which serves as the basis for using them as either delivery vehicles for vaccine antigens, or as vaccine adjuvants. The recent adenovirus vaccine developed by Janssen for SARS-CoV-2 serves as an example.12 This vaccine leads to a rapid innate immune response that is related to the activation of pattern recognition receptors (PRRs), such as toll-like receptors (TLRs). TLRs are transmembrane receptor proteins, which activate the innate immune system by sensing pathogen associated molecules.13 Ten different TLRs have been identified in humans and mice (TLR1-10 for humans, TLR1-9, and 13 for mice). They are expressed on macrophages, dendritic cells, T-cells, and B cells, as well as nonimmune cells, such as epithelial cells or fibroblasts.14–17 Each TLR recognizes a different type of biomolecule, and stimulation leads to the activation of innate and adaptive immune responses. Like other agents, chemical agonists for TLRs have been widely explored as adjuvants for traditional vaccines.18 Many investigators have also evaluated TLR agonists as adjuvants for anti-cancer vaccines. For example, HPV E7 oncoprotein-derived peptide vaccines showed antigen-specific T-cell activation and subsequent regression of HPV-driven tumors in mice when the vaccines were combined with TLR3 or TLR9 agonists.19 Combination of cancer-specific DNA vaccines targeting HPV E7 with either TLR3 or TLR7 ligands, or TLR4 agonists, similarly demonstrated greater antitumor responses in murine tumors.20,21
In previous studies, we found that professional antigen presenting cells (APCs), when stimulated with certain TLR agonists (notably for TLR1/2, TLR 7/8, and TLR9) caused a decrease in PD-1 expression on mouse CD8+ T-cells, conferred by IL-12 released from the APC.22 We also found that using these TLR agonists as vaccine adjuvants for peptide or DNA vaccines decreased PD-1 expression on vaccine-activated mouse T-cells, and this resulted in greater anti-tumor activity in vivo. While the effects of different TLR-stimulated DC on T-cells were similar, they were not identical (e.g., CTLA-4 expression was different depending on the TLR agonists used) and transcriptional profiles showed distinct differences depending on the TLR used. These findings suggested that combinations of different TLR agonists could have different effects on the effector and memory phenotype of T-cells, and more profound effects on the expression of T-cell checkpoint receptors. In fact, several TLR agonists have been administered concurrently as vaccine adjuvants both in pre-clinical23 or clinical studies.24 However, the choice of TLR combinations, or the mechanisms by which they act in combination, have not been well studied.
Given the findings above, we hypothesized that specific combinations of TLR agonists, when used at the time of T-cell activation with vaccines, may suppress the expression of multiple T-cell checkpoint receptors, affect CD8+ T-cell function, and elicit greater antitumor activity. Using OT-1 mice, we assessed CD8+ T-cell checkpoint expression following stimulation with the antigen alone or in combinations with different TLR agonists. Combinations that down-regulated the expression of T-cell checkpoint receptors while maintaining an activated phenotype were prioritized for investigations into the mechanism of activity in vitro and anti-tumor activity in murine tumor models when used with peptide or DNA vaccines.
Materials and methods
Mice
OT-1 (C57BL/6-Tg(TcraTcrb)1100Mjb/J, Stock No: 003831), C57BL/6 J (Stock No: 000664), and IL-12p40 KO (Stock No: 002693) mice were purchased from The Jackson Laboratory (Jax, Bar Harbor, MA). Mice were maintained in microisolator cages under aseptic conditions. All experiments were conducted under an IACUC-approved protocol that conforms to the NIH guide for the care and use of laboratory animals.
Cell lines
E.G7-OVA (derivative of EL4) and TRAMP-C1 cells were obtained from ATCC (Manassas, VA, Cat. # CRL-2113, #CRL-2730) and maintained via the ATCC-recommended culture methods. E.G7-OVA cells were lentivirally transduced to constitutively express PD-L1, as previously described.10
Peptides
Peptides for the H-2b-restricted epitope from chicken ovalbumin (SIINFEKL), or the dominant HLA-A2-restricted epitope from SSX2 (FLQGISPKI) used as a nonspecific peptide control, were synthesized, and the purity and identity were confirmed by mass spectrometry and gas chromatography (LifeTein, LLC., Hillsborough, NJ). Peptides were reconstituted in DMSO (2 mg/ml) and stored at −80°C until use.
In vitro assays
OT-1 splenocyte stimulation
Splenocytes were isolated from OT-1 mice, disaggregated using a mesh screen, and then treated to osmotically lyse red blood cells with an ammonium chloride/potassium chloride lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). Splenocytes were then cultured in RPMI 1640 medium supplemented with L-glutamine, 10% fetal calf serum (FCS), 200 U/mL penicillin/streptomycin, 1% sodium pyruvate, 1% HEPES, and 50 μM β-MeOH with 2 µg/mL SIINFEKL peptide. TLR agonists were purchased from InvivoGen (San Diego, CA) and added 1 hour before stimulating with peptide at the following concentrations: 300 ng/mL Pam3CSK4, 10 mg/mL Poly(I:C) HMW, 10 mg/mL MPLAs, 100 ng/mL FLA-ST, 100 ng/mL FSL-1, 3 mg/mL Gardiquimod, 10 mg/mL R848, 5 mmol/L ODN 1826, 2 μg/mL ORN Sa19. In some studies rIL-12 (50 ng/mL, R&D systems 419-ML-010) or IFNβ (25 ng/mL, R&D systems 8234-MB-010) were added to cultures with peptide. In other studies, anti-IL-12Rβ2 (100 ng/mL, R&D systems MAB8650) or anti-IFNAR1 (100 ng/mL, R&D systems AF3039) were added. At the time points indicated, cells were analyzed via flow cytometry (BD LSRFortessa) with the following antibodies: CD3-FITC (BD 555274), CD4-BUV395 (BD 563790), CD8-BV786 (BD 563332), LAG-3-BV711 (BD 563179), PD1-PECF594 (BD 562523), TIM3-APC (eBioscience 17–5871-82), CTLA4–PE-Cy7 (Tonbo 60–1522-U100), CD160-PE (eBioscience 12–1601-81), TIGIT-BV605 (BD 744212), CD244.2-BV510 (740115), VISTA-BV421 (BD 150212), 41BB–PerCPeF710 (eBioscience 46–1371–82), and Live/Dead Ghost dye 780 (Tonbo, San Diego, CA 13–0865–T100). To compare the samples from multiple time points, each laser voltage was standardized using SPHERO Rainbow Fluorescent Particles (Spherotech, RFP-30-5). Four-day median fluorescence intensity (MFI) values of immune checkpoint receptor expression from flow cytometry were measured in linear scale and computed as an Area Under the Curve (AUC). AUC was calculated with the trapezoid rule, using GraphPad Prism (version 8). Each TLR agonist or agonist combination was evaluated as an AUC ratio compared to OVA alone stimulation group, with the following equation: (B-A)/A, A: AUC of receptor expression following OVA stimulation without TLR, B: AUC of receptor expression with TLR agonist(s) stimulation. Heat-maps of AUC ratios were generated by R 3.3.1.
IL-12p70 and IFNβ ELISA
Dendritic cells (DCs) were enriched from splenocytes of C57BL/6 mice inoculated with Flt3 ligand-expressing B16 tumor cells using PE-labeled antibodies specific for CD11c (StemCell, Vancouver, Canada, Cat.# 17684) as previously described.25 B cells were enriched from splenocytes of six- to ten-week-old C57BL/6 mice, using a mouse B cell isolation kit (Akadeum, Ann Arbor, MI, Cat.# 12210–110). Cells were stimulated with TLR agonists as above for 24 hours, and culture supernatant was evaluated for the presence of IL-12 p70 or IFNβ by ELISA (R&D systems, Minneapolis, MN, Cat.# DY419-05, DY8234-05), according to the manufacturer’s instructions.
RNA preparation and sequencing
OT-1 splenocytes were stimulated in vitro as above, and CD8+ T-cells were isolated via immunomagnetic negative selection (Stemcell Technologies; 19853). RNA was purified according to the manufacturer’s instruction (Direct-zol RNA MiniPrep Plus w/TRI Reagent, Zymo Research), and stored at −80°C until analysis. Total RNA was submitted to the University of Wisconsin-Madison Biotechnology Center, and verified for purity and integrity via the NanoDropOne Spectrophotometer and Agilent 2100 BioAnalyzer, respectively. Samples were then prepared according the TruSeq® Stranded mRNA Sample Preparation Guide (Rev. E) using the Illumina® TruSeq® Stranded mRNA Sample Preparation kit (Illumina Inc., San Diego, CA). For each library preparation, mRNA was purified from 1 μg total RNA using poly-T oligo-attached magnetic beads. The mRNA fragments were converted to double-stranded cDNA (ds cDNA) using SuperScript II (Invitrogen, Carlsbad, California, USA), RNaseH and DNA Pol I, primed by random primers. The ds cDNA was purified with AMPure XP beads (Agencourt, Beckman Coulter), and products were incubated with Klenow DNA Polymerase to add an ‘A’ base (Adenine) to the 3’ end of the blunt DNA fragments. DNA fragments were ligated to Illumina unique dual adapters, and the adapter-ligated DNA products were purified with AMPure XP beads. Adapter ligated DNA was amplified in a Linker Mediated PCR reaction (LM-PCR) for 10 cycles using PhusionTM DNA Polymerase and Illumina’s PE genomic DNA primer set followed by purification with AMPure XP beads. Finally, the quality and quantity of the finished libraries were assessed using an Agilent Tapestation 4200 DNA1000 kit (Agilent Technologies, Inc., Santa Clara, CA, USA) and Qubit® dsDNA HS Assay Kit (Invitrogen, Carlsbad, California, USA), respectively. Libraries were standardized to 2 nM, and sequenced on a NovaSeq 6000 (Illumina Inc.). Paired-end, 250 bp sequencing was performed. Data was processed with bcl2fastq (Illumina Inc.).
RNA-seq data analysis
Data quality was examined by FastQC26 with per-base sequence quality scores. Data that passed the quality control were aligned to the mouse reference genome using RNA STAR.27 The expression level of each gene was calculated by FeatureCounts,28 and heat-maps for genes of interest were generated using R 3.3.1. Gene expression profiles were subsequently used for differential gene expression analysis using DESeq2.29 The false discovery rate was controlled using the Benjamini–Hochberg procedure. Rank lists for Gene Set Enrichment Analysis (GSEA) were generated from DESeq2 results, with the following formula: ‘Sign(log2FoldChange) X -log10(p-value)’. Pre-ranked GSEA was performed with the Molecular Signatures Database (MSigDB) immunologic signature gene sets.30 RNAseq data (BioProject ID PRJNA792998) is publicly available at http://www.ncbi.nlm.nih.gov/bioproject/792998.
Tumor treatment studies
Tumor studies with E.G7-OVA-PD-L1high tumors
E.G7-OVA-PD-L1high cells were injected subcutaneously (in PBS without matrigel) into 6-week old female C57BL/6 mice or 6- to 7-week-old female IL-12p40 KO mice. When tumors were palpable and similarly sized (0.1 cm3), 2 × 106 naïve OT-1 splenocytes were adoptively transferred to each mouse intraperitoneally. The following day, mice were immunized subcutaneously with 100 μg SIINFEKL peptide in PBS, with or without TLR agonist(s) at the following concentration: TLR1/2 (Pam3CSK4, 20 μg/mouse), TLR3 (Poly(I:C) HMW, 100 μg/mouse), TLR9 (ODN 1826, 50 μg/mouse). Doses were chosen based on prior studies.31–33 Tumor volume was measured using calipers and calculated in cubic centimeters according to the formula: (п/6) × (long axis) × (short axis)2. Tumors were collected and digested in collagenase, DNAse I, and protease inhibitors for 1 hour at 37°C, passed through a 100-mm mesh screen, and analyzed by flow cytometry. Animals for which tumors completely regressed were rechallenged with E.G7-OVA-PD-L1high tumor cells 28 days after the date of regression.
Tumor study with TRAMP-C1 tumors
Six- to ten-week-old male C57BL/6 mice were inoculated with 1 × 106 TRAMP-C1 cells, administered subcutaneously with matrigel (Corning Inc., Corning, NY). Each mouse was then immunized intradermally with 100 μg DNA vaccine (pTVG-AR) (or vector control) weekly, beginning 1 day after tumor implantation. TLR agonist(s) were co-administered with the vaccine intradermally at the following concentration: TLR1/2 (Pam3CSK4, 20 μg/mouse), TLR3 (Poly(I:C) HMW, 100 μg/mouse), TLR9 (ODN 1826, 50 μg/mouse). Tumor volumes were measured as described above. Tumors obtained at necropsy were digested in collagenase and DNAse I for 1 hour at 37°C, passed through a 100-mm screen, and analyzed by flow cytometry.
Statistical analysis
Group mean comparisons were performed using GraphPad Prism software, v8.4.3. Analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test was used to compare individual group means. For samples for which ANOVA was not applicable, the linear mixed-effects model with Geisser-Greenhouse correction was used to compare group means among treatment groups. Survival analysis was conducted using a Mantel-Cox log-rank test. For all comparisons, P values ≤ .05 were considered statistically significant.
Results
Combination of TLR agonists can alter the expression of immune checkpoint receptors
We previously demonstrated that certain TLR agonists, acting on APC at the time of T-cell activation, can lower the expression of PD-1.22 To evaluate whether combinations of TLR agonists administered at the time of T-cell activation further affects expression of T-cell checkpoint receptors, we used OT-1 CD8+ T-cells, in which all CD8+ T-cells express a T-cell receptor (TCR) specific for the H-2b-restricted epitope from ovalbumin, SIINFEKL. OT-1 T-cells were activated in vitro with 2 µg/mL SIINFEKL peptide in the presence or absence of TLR agonists, alone or in combination, and assessed daily over four days for the expression of 4–1BB (as a marker of activation), and multiple-checkpoint receptors (PD-1, LAG-3, CD160, VISTA, CTLA-4, TIM3, TIGIT, and CD244). The area under the curve (AUC) was calculated for each checkpoint and expressed as a ratio of the level observed when SIINFEKL alone was used (Figure 1a). As shown in Figure 1b, several pairwise combinations led to reduced expression of PD-1, LAG-3, and CD160, and several combinations led to increases in VISTA, CTLA-4, and TIGIT. In particular, combinations with TLR4 agonist led to increases in VISTA and TIGIT expression. Combinations with TLR3 agonists led to increases in CTLA-4 expression. The greatest reductions in PD-1, LAG-3, and CD160 expression were observed with combinations of TLR1/2, TLR3, and TLR9 (Figure 1c and Supplemental Figure S1). Given the greatest decreases in PD-1 expression with these TLR agonists, combinations of TLR1/2, TLR3, and TLR9 agonists were prioritized for further studies.
Figure 1.
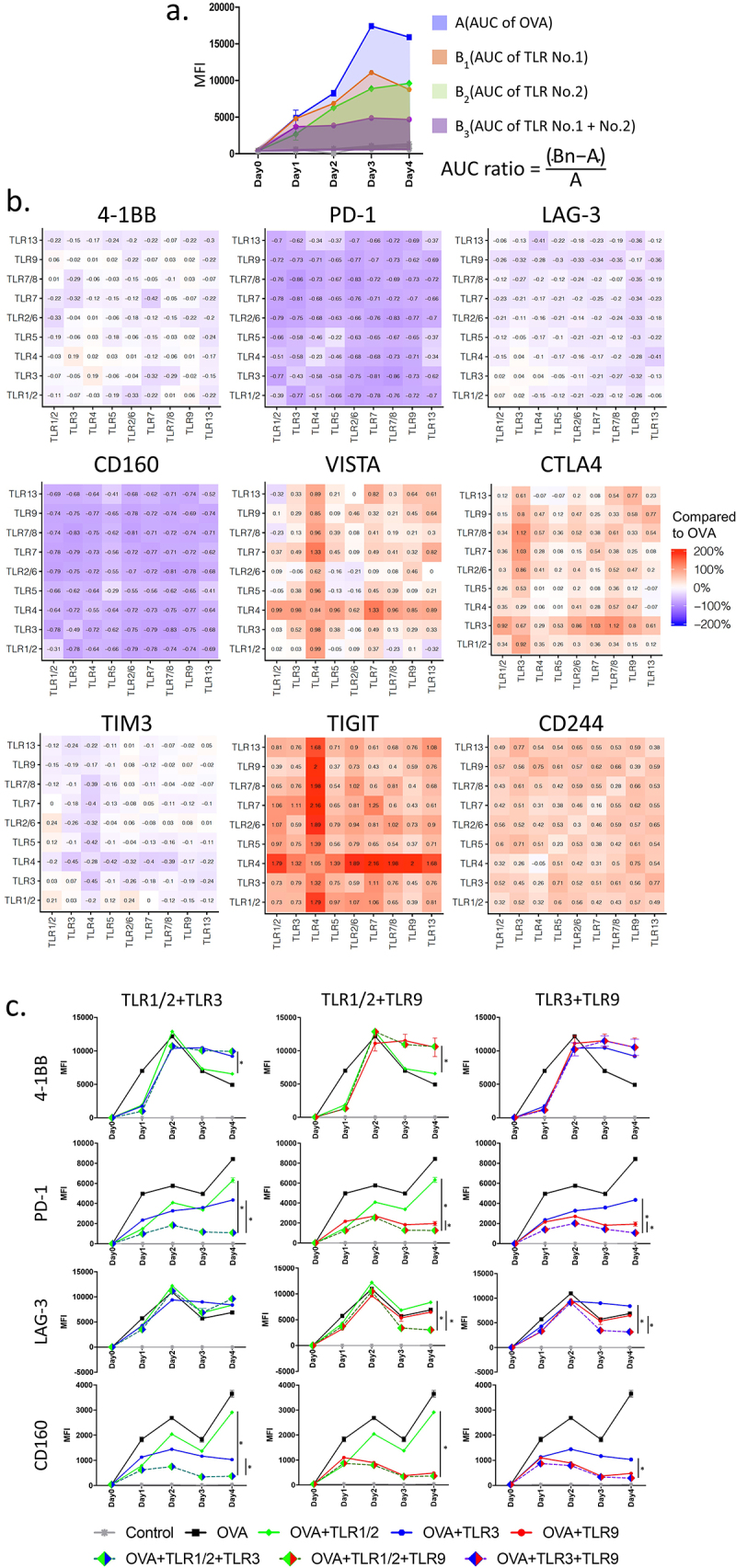
Combinations of TLR agonists at the time of T-cell activation in vitro affect expression of T-cell checkpoint receptors: Splenocytes were prepared from the spleens of OT-1 mice and stimulated in vitro with the high-affinity SIINFEKL (OVA) peptide in the presence or absence of TLR agonists [TLR 1/2 (Pam3CSK4), TLR 3 (Poly I:C), TLR 4 (MPLAs), TLR 5 (FLA-ST), TLR 2/6 (FSL-1), TLR 7 (Gardiquimod), TLR 7/8 (R848), TLR 9 (ODN1826), or TLR 13 (ORN Sa19)] or their pairwise combinations. The median fluorescence intensity (MFI) of 4–1BB and T-cell checkpoint receptor expression on CD8+ T-cells were determined by flow cytometry, collected daily for 4 days, and computed as Area Under the Curve (AUC) using the trapezoid rule. (a) Calculation of AUC ratio to compare AUC of each receptor expression for each pairwise combination with that obtained following OVA stimulation alone, without TLR activation. (b) Heat-map demonstrating AUC ratio of each pairwise combination for 4–1BB and multiple T-cell checkpoint receptors. (c) Representative MFI plots showing 4–1BB, PD-1, LAG-3, and CD160 expression with the combinations of TLR1/2, TLR3, and TLR9 agonists. Results are from one experiment, with samples assessed in triplicate, and are representative of three similar, independent experiments. Asterisks represent significant (p < .05) differences as assessed by ANOVA.
CD8+ T-cells activated in the presence of TLR combinations have improved anti-tumor activity
To determine the effect of TLR combinations on the anti-tumor activity of activated CD8+ T-cells, E.G7 tumor cells (expressing ovalbumin and PD-L1) were implanted in C57BL/6 mice and permitted to grow until palpable (~10 days). OT-1 splenocytes were then adoptively transferred, and the following day mice were immunized with 100 µg SIINFEKL (OVA) peptide alone, peptide and a single TLR1/2, TLR3, or TLR9 agonist, or peptide with combinations of TLR agonists as indicated in Figure 2. Immunization with OVA peptide in the presence of two different TLR agonists produced a greater antitumor effect than peptide alone or when used with a single TLR agonist (Figure 2a). Notably, 1 out of 6 mice receiving the TLR3+ TLR9 combination showed complete regression of the tumor (Supplemental Figure S2), and acquired immune memory, as re-challenge with EG.7 tumor cells did not lead to tumor growth (data not shown). As shown in Figure 2b,c, immunization with combined TLR agonists led to an increase in tumor-infiltrating antigen-specific CD8+ T-cells with lower PD-1 expression.
Figure 2.
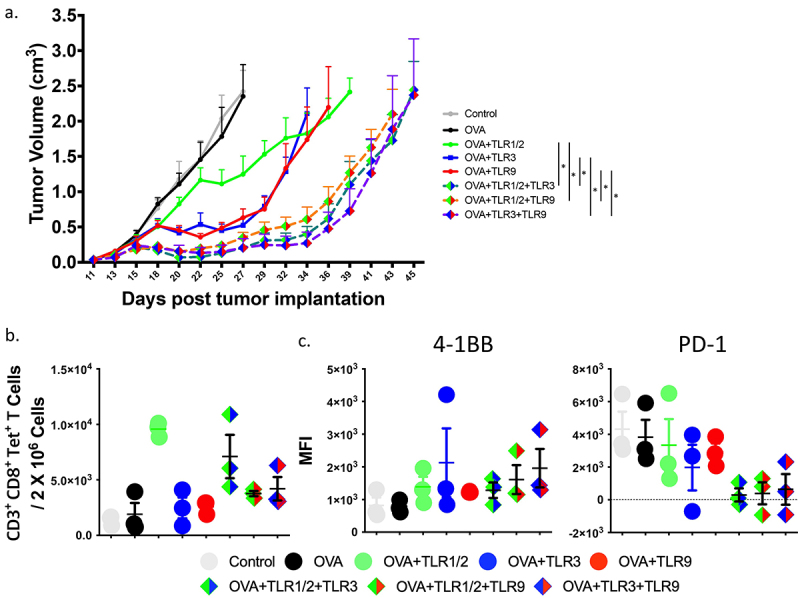
Combination of TLR agonists elicits greater antitumor immunity in vivo. Ovalbumin-expressing E.G7 cells were implanted in C57BL/6 mice and permitted to grow until tumors were palpable (10 days). OT-1 splenocytes were then adoptively transferred and mice were immunized subcutaneously the following day with SIINFEKL (OVA) peptide alone, or in combination with TLR agonists, the day following immunization. (a) Shown are the tumor growth curves (median + standard error, n = 6 to 7 animals per group). Asterisks represent significant (p < .05) differences as assessed by linear mixed effects model with Geisser-Greenhouse correction. (b) Animals were treated as in panel A but tumors were collected at day 15 and evaluated for the frequency of infiltrating CD3+, CD8+, tetramer+ T-cells among all cells. (c) Tumor-infiltrating CD8+, tetramer+ T-cells were further evaluated for 4–1BB and PD-1 expression by flow cytometry. Results are from one experiment and are representative of two independent experiments.
Improved anti-tumor immunity with combinations of TLR agonists is not solely dependent on IL-12
We have previously shown that the effect of TLR agonists on PD-1 expression is due, at least in part, to IL-12 secretion from professional APC.22 Given this, we tested whether combination of TLR agonists affected the secretion of IL-12 from APCs. As shown in Figure 3a (and Supplemental Figure S3), treatment of DCs with two different TLR agonists did not significantly increase IL-12 secretion over the use of a single TLR agonist alone, except for the combination of TLR3 and TLR9. In order to determine whether the anti-tumor efficacy of CD8+ T-cells observed following vaccination with TLR agonist was dependent on IL-12, similar murine tumor studies were conducted using IL-12-deficient mice. Specifically, E.G7 cells were implanted in IL-12p40 deficient mice and permitted to grow until palpable (12 days). OT-1 CD8+ T-cells were then adoptively transferred, and the following day mice were immunized with SIINFEKL (OVA) peptide alone or with TLR1/2, TLR3, or TLR9 agonists. Interestingly, the anti-tumor effect of TLR1/2 and/or TLR9 agonist treatment was abrogated in IL-12-deficient mice, while TLR3 agonist demonstrated improved anti-tumor activity when co-administered with the SIINFEKL peptide (Figure 3b, Supplemental Figure S4). These results suggested that the effect of TLR combinations may not be solely due to IL-12 secretion from APC, and that TLR3 agonists may have entirely different effects on APCs compared with TLR1/2 or TLR9 agonists.
Figure 3.
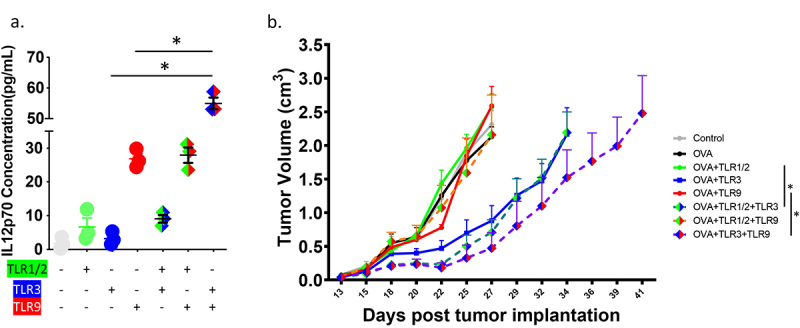
Improved anti-tumor immunity with combinations of TLR agonists is not solely dependent on IL-12. (a) Purified DCs were stimulated in the presence of TLR1/2 agonist (Pam3CSK4), TLR3 agonist (HMW PolyI:C), TLR9 agonist (ODN 1826), pairwise combinations of these TLR agonists, or media only (no TLRs) for 24 hours. Culture supernatant was then evaluated for the presence of IL-12 p70 by ELISA. Asterisks represent significant (p < .05) differences as assessed by t-test. (b) Ovalbumin-expressing E.G7 cells were implanted in IL-12p40 deficient mice and permitted to grow until tumors were palpable (12 days). OT-1 CD8+ T-cells were then adoptively transferred, and mice were immunized subcutaneously the following day with SIINFEKL (OVA) peptide alone, or in combination with TLR agonists. Shown are the tumor growth curves (median + standard error, n = 5 animals per group). Asterisks represent significant (p < .05) differences as assessed by linear mixed effects model with Geisser-Greenhouse correction.
Combination of TLR agonists affects CD8+ T-cell effector and memory functions via IL-12 and type-1 interferon
To identify mechanisms of action for the effect of TLR combinations on CD8+ T-cell function, OT-1 CD8+ T-cells were activated with SIINFEKL peptide-loaded DC that were stimulated with TLRs and evaluated for gene-expression changes by mRNA sequencing (Figure 4a). OT-1 CD8+ T-cells activated in the presence of either TLR1/2, TLR3, or TLR9 agonists showed distinct phenotypes in a principle component analysis (PCA) compared to the cells activated by SIINFEKL peptide alone (Figure 4b). Compared to TLR1 or TLR3 groups, T-cells activated with TLR1+ TLR3 combination also clustered distinctly. However, TLR9, or TLR combinations including TLR9, clustered similarly, suggesting that global gene expression pattern might be similar when TLR9 agonists were combined with other TLR agonists. As shown in Figure 4c, TLR combinations up-regulated genes related to T-cell effector function (Id2, Il2ra, Klrg1, Tnfrsf8, and Tnfrsf9), memory function (CCR4, CCR7, Ly6c1, and Sell) and cytokines (Granzyme A, IL2, IL6, and TGFβ2). PD-1 expression was suppressed in TLR1+ TLR3 and TLR1+ TLR9 combinations, consistent with flow cytometry studies (Figure 1c). The expression of VISTA, TIGIT, and CD160 were also consistent with protein expression observed by flow cytometry. TIM-3 gene (Havcr2) expression, however, was reduced with combinations more markedly than observed by changes in cell surface expression. We further analyzed RNA-seq data from each treatment group using Gene Set Enrichment Analysis (GSEA) and identified IL-12 and type-1 interferon as key signaling pathways representing differences between TLR combinations and each TLR pair (Figure 4d, Supplemental Table S1). In comparison of CD8+ T-cells stimulated with TLR1/2 or TLR9 versus SIINFEKL peptide alone, IL-12 was identified as a key pathway (Figure 4d, column a, c). However, CD8+ T-cells stimulated in the presence of TLR3 had type-1 interferon as a key signaling pathway (Figure 4d, column b). Comparison between TLR1 and TLR1/2+ TLR3 combinations similarly identified type-1 interferon signaling as a main difference between these two groups (Figure 4d, column d).
Figure 4.
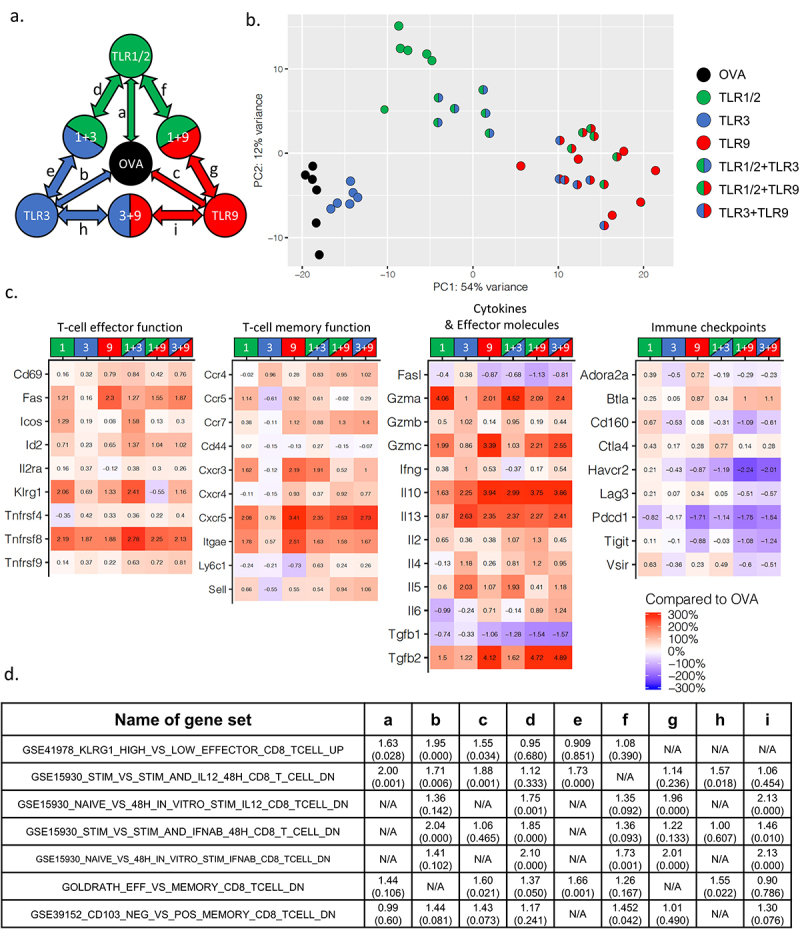
CD8± T-cells activated in the presence of TLR1/2, TLR3, TLR9 agonists exhibit distinct gene expression profiles with differences in effector and memory function. OT-1 splenocytes were activated for 72 hours in the presence of SIINFEKL peptide (OVA) alone, or with TLR1/2, TLR3, and/or TLR9 agonists. CD8+ OT-1 T-cells were then purified and evaluated for gene-expression changes by RNA-seq. All samples were evaluated in six replicates from a single experiment. (a) Representative color codes for each treatment group for subsequent analysis (a: TLR1/2 vs. OVA, b: TLR3 vs. OVA, c: TLR9 vs. OVA, d: TLR1/2+ TLR3 vs. TLR1, e: TLR1.2+ TLR3 vs. TLR1, f: TLR1/2+ TLR9 vs. TLR1, g: TLR1/2+ TLR9 vs. TLR9, h: TLR3+ TLR9 vs. TLR3, i: TLR3+ TLR9 vs. TLR9) (b) Principle-Component Analysis plot for each treatment group. (c) Heat-map of gene expression changes for specific genes associated with CD8+ T-cell differentiation and function for CD8+ T-cells stimulated with OVA in the presence of TLR agonists versus CD8+ T-cells stimulated with OVA alone (red = increased expression, blue = reduced expression, with fold-change indicated by the numbers). (d) Table showing enriched gene sets from pre-ranked GSEA between each treatment group. Gene sets identified in at least 5 of the pairwise comparisons were selected. Numbers indicate normalized enrichment score and (FDR q-value) for each of the 9 pairwise comparisons.
Effects of TLR agonists on CD8+ T-cell immune checkpoint receptor expression are dependent on both IL-12 and IFNβ
We next wished to confirm the gene expression studies and determine whether the effects of TLR agonists on CD8+ T-cells relied on the action of these agonists on APC via IL-12 and IFNβ secretion. It has been demonstrated that CD8 + T-cells also express TLRs,15,17 therefore we first examined the effect of direct stimulation of CD8+ T-cells with TLR agonists on T-cell checkpoint expression. Pre-treatment of dendritic cells, but not CD8 + T-cells, with TLR agonists led to decreased PD-1 expression during co-culture with SIINFEKL peptide, demonstrating the effect of TLR ligands was on APC (Supplemental Figure S5A). We then wished to determine whether treatment of APCs with multiple TLR agonists elicited production of type-1 interferon. As shown in Figure 5a, treatment of DCs with TLR3+ TLR1/2, or TLR3+ TLR9, led to increased secretion of IFNβ. Lower expression was detected from B cells, and primarily following treatment with TLR1/2+ TLR3. No significant secretion of IFNβ was detected following treatment with TLR1/2 or peptide antigen itself. As shown in Figure 5b, OT-1 T-cells activated in the presence of SIINFEKL peptide and IL-12 had decreased PD-1 expression, and this expression was further reduced in the presence of IFNß. CD160 expression was similarly reduced in the presence of IL-12 and IFNß, whereas the expression of LAG-3 was not affected by these cytokines (Figure 5b). OT-1 CD8+ T-cells, activated by SIINFEKL peptide-loaded DC that were stimulated with TLR agonists, had increased expression of PD-1 in the presence of IL-12 receptor blockade (Figure 5c). When DC were stimulated with TLR3 or combinations with TLR3, PD-1 expression was further restored in the presence of type 1 interferon receptor blockade (Figure 5c, Supplemental Figure S5B, C).
Figure 5.
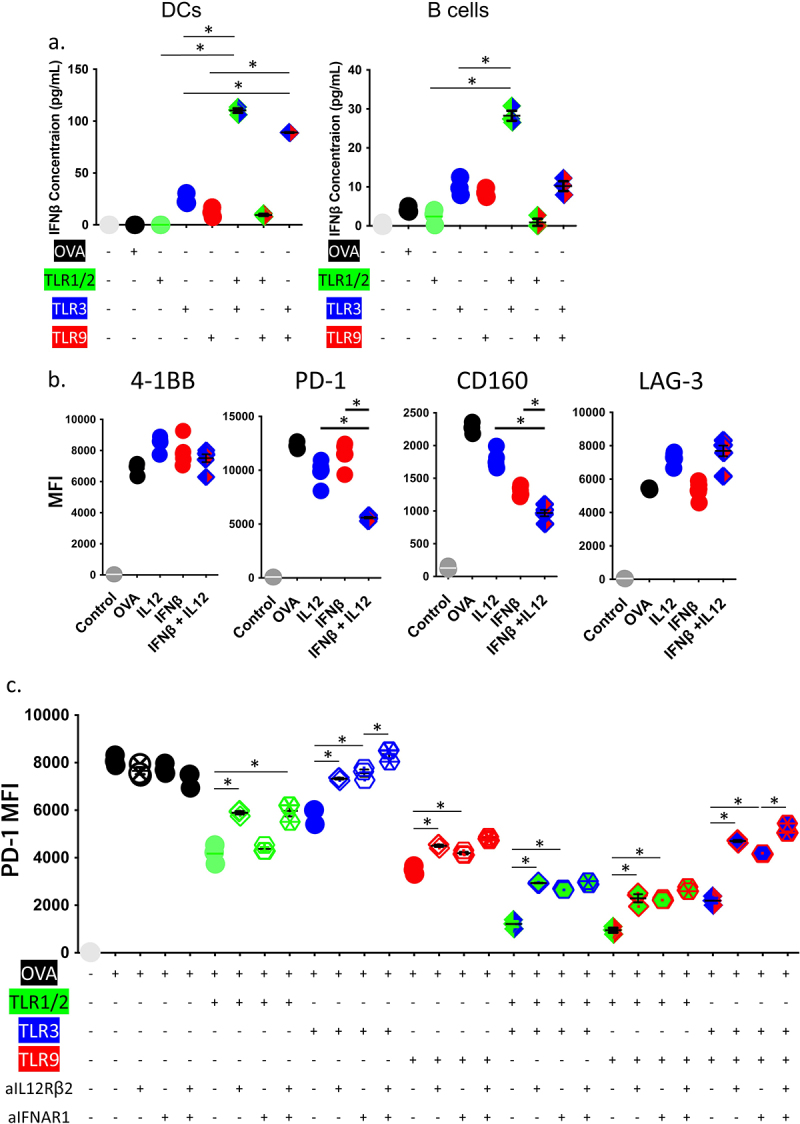
Changes in CD8± T-cell expression of PD-1 following TLR stimulation depends on both IL-12 and type-1 interferons. (a) Purified DCs or B cells were stimulated in the presence of TLR1/2 agonist (Pam3CSK4), TLR3 agonist (HMW PolyI:C), TLR9 agonist (ODN 1826), or media only (Control) for 24 hours. Culture supernatants were then evaluated for the presence of IFNβ by ELISA. (b) OT-1 splenocytes were stimulated in vitro with the high-affinity SIINFEKL peptide in the presence of recombinant IL-12 and/or IFNβ. The median fluorescence intensity (MFI) of 4–1BB, PD-1, CD160, LAG-3 expression on CD8+ T-cells were assessed after 4 days, by flow cytometry. (c) OT-1 splenocytes were stimulated in vitro with the high-affinity SIINFEKL peptide in the presence of specific TLR agonists, anti-IFNAR1 and/or anti-IL-12Rβ2. The median fluorescence intensity (MFI) of PD-1 expression on CD8+ T-cells was assessed after 3 days, by flow cytometry. Asterisks demonstrate significant (p < .05) differences as assessed by ANOVA. Results are from one experiment and are representative of two independent experiments.
Combination of TLR agonists used as adjuvants improves the anti-tumor vaccine efficacy in a prostate cancer model
The studies above used ovalbumin as a model tumor antigen and suggested that combinations of TLR agonists might effectively serve as vaccine adjuvants to improve the function of CD8+ T-cells activated by vaccination. Consequently, we wished to evaluate this in a more relevant tumor model targeting a native antigen. We have previously reported that a DNA vaccine encoding the ligand-binding domain of the androgen receptor (pTVG-AR) can elicit antigen-specific CD8+ T-cells with cytolytic function.34 For these studies, TRAMP-C1 prostate tumor cells were implanted in male C57BL/6 mice. Mice were then immunized intradermally weekly, beginning the day after tumor implantation, with control vector (pTVG4) or pTVG-AR, and co-administered with TLR1/2, TLR3, or TLR9 agonists. As shown in Figure 6a (and Supplemental Figure S6), addition of single TLR agonists showed modest effects on tumor growth, but the combinations of TLR1/2+ TLR3 and TLR3+ TLR9 further suppressed tumor growth and prolonged survival (Figure 6b). As shown in Figure 6c, immunization with two different TLR agonists tended to increase the number of immune-infiltrating CD8+ T-cells, and lower the number of infiltrating CD4+ Treg. These tumor-infiltrating CD8+ T-cells had similar expression of 4–1BB, but reduced expression of PD-1 (Figure 6d).
Figure 6.
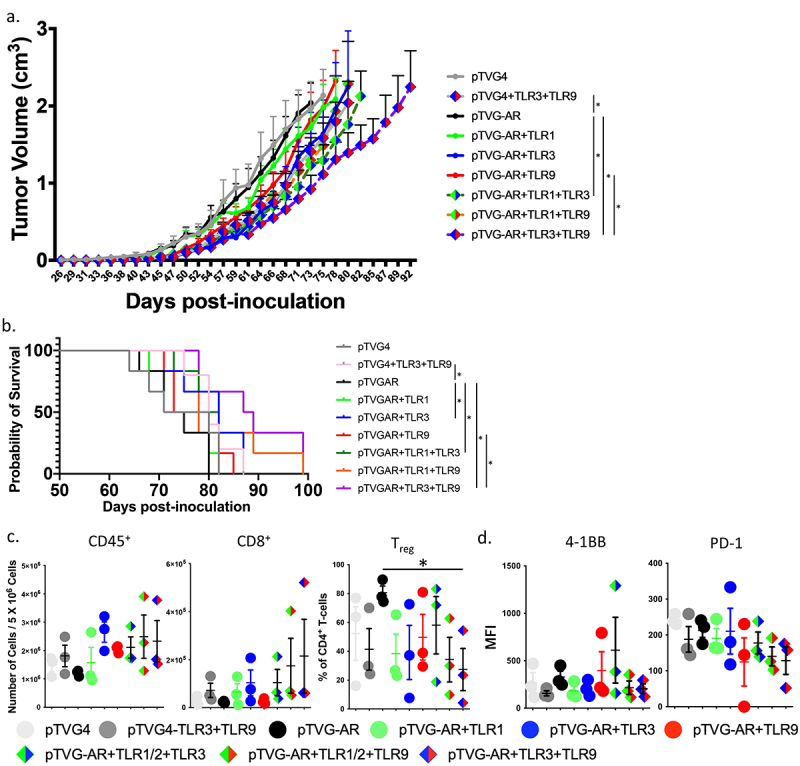
Combinations of TLR agonists improve the anti-tumor efficacy of a DNA vaccine in a prostate cancer tumor model. C57BL/6 mice were implanted subcutaneously with TRAMP-C1 tumor cells. Mice were immunized intradermally weekly with control vector (pTVG4) or DNA encoding AR ligand-binding domain (pTVG-AR) and delivered alone or co-delivered with TLR1/2, TLR3 and/or TLR9 agonists. (a) shown are the growth curves for each group (n = 6 animals per group. Asterisks demonstrate significant (p < .05) differences as assessed by linear mixed effects model with Geisser-Greenhouse correction. (b) Survival plots using the time to death or when tumors reached 2 cm3 in size, whichever occurred first, are shown. Asterisks indicate p < .05 as assessed by log-rank test. (c) Animals were treated as in panel A, and tumors were obtained at day 64. Tumors were evaluated by flow cytometry for the number of infiltrating CD45+ cells, CD8+ T-cells among all live cells, and percentage of Treg cells (CD25+Foxp3+) among CD45+CD3+CD4+ cells. Asterisk demonstrates significant (p < .05) differences as assessed by ANOVA. (d) Tumor-infiltrating CD8+ T-cells were evaluated for 4–1BB and PD-1 expression by flow cytometry. Median Fluorescence Intensity (MFI) for PD-1 was normalized to the lowest value.
Discussion
We have previously found that T-cell activation in the presence of TLR agonists resulted in suppression of PD-1 expression and greater T-cell anti-tumor function.22 TLR agonists have been widely investigated not only as adjuvants for traditional vaccines, but also as reagents that facilitate anti-tumor function of immune cells.18–21 Moreover, there are reports that used multiple TLR agonists, delivered at the same time, to augment the overall immune response.23,35 However, there is little understanding of their mechanism in terms of how combinations of TLR agonists might affect T-cell function. Consequently, in this report, we investigated whether co-administration of different TLR agonists affect the expression of multiple T-cell checkpoint receptors and improve the anti-tumor function of these activated T-cells. We found that specific combinations of TLR1/2, TLR3, and TLR9 agonists led to lower expression of PD-1, CD160, and LAG-3 on activated CD8+ T-cells. Although none of these combinations showed synergistic effect, we found that combinations of TLR agonists showed additive effects in terms of downregulating PD-1 expression on CD8+ T-cells. Combinations of TLR agonists further reduced expression of PD-1 in vivo, and immunization in the presence of two different TLR agonists improved the anti-tumor activity of CD8+ T-cells. We also identified secretion of IL-12 and type-1 IFN from professional APC as contributing mechanisms by which TLR agonists downregulate T-cell checkpoint expression.
In this study, OT-1 models were utilized for both in vitro and in vivo experiment. Since OT-1 mice contain transgenic Tcra-V2 and Tcrb-V5 inserts, all CD8+ T-cells recognize a defined ovalbumin H-2b-restricted epitope, residues 257–264.36 This enabled our studies evaluating antigen-specific CD8+ T-cell activation and response, and the comparison of TLR agonists used during TCR activation. However, the OT-1/ova model is artificial and has several limitations. First, the antigen itself is a non-self antigen to murine immune system, and is a high-affinity epitope, which may not be representative of many autologous tumor-presented epitopes. In addition, given that these cells use a single high-affinity TCR, these may not represent the complexity and diversity of antigen-specific CD8 + T-cells activated by vaccination. Given our previous report that OT-1 T-cells stimulated with lower affinity antigens, including SIINTEKL or SIINFEKP, become activated with lower, transient PD-1 expression, it is possible that the effect of TLR agonists on CD8+ T-cells might vary depending on their affinity. Future studies will be necessary to determine whether CD8+ T-cells with weaker affinity for their target, when stimulated with TLR agonists during T-cell activation, have similar altered expression of T-cell checkpoint receptors.
The blockade of T-cell immune checkpoint receptors, notably antibodies blocking PD-1/PD-L1 or CTLA-4, has revolutionized the treatment of cancer.7,37,38 Subsequently, other T-cell receptors, such as LAG-3, have also become recognized as important immune checkpoint targets. Our group and others have demonstrated that blocking multiple T-cell checkpoint receptors simultaneously, such as combining PD-1 and LAG-3 blockade with anti-tumor vaccination, can result in greater anti-tumor effects.39 In the current report, we found that specific combinations of TLR1/2, TLR3, and TLR9 agonists led to lower expression of PD-1, CD160, and LAG-3 on activated CD8+ T-cells. TLR agonist stimulation could potentially accomplish the same effect as T-cell checkpoint blockade by lowering expression of these molecules, and hence it will be interesting to see whether there is any advantage to combining TLR agonist treatment with PD-1, CD160 and/or LAG3 blockade. On the other hand, receptors including CTLA-4, VISTA, and CD244 were upregulated following TLR stimulation, suggesting that TLR agonists might specifically be combined with the blockade of these T-cell checkpoint receptors. Strategic combinations of TLR agonists and checkpoint blockade could potentially further improve the anti-tumor efficacy of therapies using activated T-cells, and might also lower the cost and toxicity associated with multiple T-cell checkpoint blockade therapies.
We identified IL-12 and type-1 IFN, secreted by professional APC after TLR activation, as part of the mechanism by which TLR agonists downregulate T-cell checkpoint receptor expression. IL-12 is a pro-inflammatory cytokine produced by macrophages or dendritic cells and known to induce T-cell activation and differentiate CD4+ T-cells toward a Th1 phenotype.40–42 Type-1 IFNs are composed of IFNα and IFNß, and are involved in anti-viral responses by activating NK cells.43,44 However, their role in T-cell activation is controversial: Type-1 interferons can facilitate antigen presentation to T-cells and maximize T-cell mediated antitumor immunity,45,46 however chronic type-1 interferon signals also recruit MDSC and Treg, and induce expression of inhibitory molecules, such as PD-L1 or IDO.47,48 Our RNA sequencing studies identified that stimulation with single versus a combination of TLR agonists affected the activation of both of these different cytokine pathways. In direct treatment studies, combinations of TLR3 and TLR9 agonists significantly upregulated secretion of IL-12 by DCs, and combinations with TLR3 increased IFNß secretion by APCs. Activating T-cells with the antigen along with recombinant IL-12 and/or IFNß suppressed PD-1 and CD160 expression on CD8+ T-cells, implicating both IL-12 and IFNß as contributing mechanisms by which TLR agonists suppress the expression of these receptors. However, inhibition of downstream signaling of IL-12 or IFNß only partially restored the expression of PD-1, and did not affect CD160 or LAG-3 expression, suggesting that there could be additional mechanisms by which TLR agonists regulate the expression of T-cell checkpoint receptors. Future studies will further explore the means by which TLR agonists affect the expression of these other T-cell checkpoint receptors.
In our tumor studies, combinations of TLR agonists improved the T-cell mediated anti-tumor immunity when they were co-administered as vaccine adjuvants. Even though the ovalbumin-expressing tumor-bearing mice were immunized with only a single dose of SIINFEKL and TLR agonists, all combinations of TLR1/2, TLR3, and TLR9 agonists improved the anti-tumor response. Notably, one mouse treated with TLR3 and TLR9 agonists developed complete regression of tumor as well as immunological memory, as this mouse was resistant to re-challenge with tumor. However, only TLR3 and TLR9 agonists improved the anti-tumor function of a DNA vaccine in a murine prostate tumor model targeting a native, tolerant antigen. The more modest anti-tumor response in this latter tumor model is likely due to the lower frequency of tumor-specific CD8 + T-cells. That is, for the murine E.G7-OVA model, OT-1 CD8 + T-cells were provided before immunization with the peptide, while treatment of the mice with prostate tumors relied upon the augmentation of tumor-specific T-cells targeting the self antigen using repetitive vaccination. Nevertheless, these findings indicate that the TLR3 and TLR9 combination, in particular, can improve vaccine efficacy by augmenting both effector and memory functions of T-cells, and in our studies this combination was the optimal combination to further pursue as a cancer vaccine adjuvant.
Interestingly, TLR3 and TLR9 agonist treatment alone was able to suppress tumor growth, without vaccination, in the TRAMP-C1 tumor model. This could be due to the fact that TLR agonists can also affect tumor cells directly. TLR3 has been shown to induce tumor cell death,49–51 and TLR9 induces apoptosis of tumor cells and improves the survival of mice.52 Hence, TLR agonists used as vaccine adjuvant might directly affect different types of tumors. However, the fact that we delivered low doses of TLR agonists at infrequent intervals suggests that the effect of TLR agonists observed in the current study is more likely immune-mediated, likely by activating other tumor-specific CD8+ T-cells than those activated by the antigen-specific vaccine.
TLR agonists have been used as vaccine adjuvants for several human vaccines. For example, Shingrix, a vaccine for herpes zoster approved by FDA in 2017, uses a TLR4 agonist.53 Recently developed mRNA-based vaccines for COVID-19 have used TLR agonism for robust immune responses.54 However, there has been little exploration of the mechanism of action of multiple TLR stimulation in humans. In fact, while human TLRs share common characteristics with murine TLRs, they have different tissue expression patterns and signal intensity,55,56 suggesting that dosing of TLR agonists for similar effects on T-cell activation might be different in humans. Furthermore, TLR10 is only expressed in humans, and hence could not be studied in our murine models.57 Future studies are needed to determine whether human TLR agonists, including TLR10, can similarly affect the expression of IL-12 and IFNß and suppress T-cell checkpoint expression on activated CD8+ T-cells. Nevertheless, our findings suggest that specific TLR agonists, notably TLR3 and TLR9 agonists, might be specifically evaluated with anti-tumor vaccines to determine if they modulate the effector and memory function, and expression of T-cell checkpoint receptors, of human CD8+ T-cells.
Supplementary Material
Acknowledgments
We thank the UW Biotechnology Center and UWCCC Flow Cytometry Core staff for support and technical assistance. We also thank Ms. Jena Moseman and Drs. Chris Zahm and Laura Johnson for critical review of the manuscript.
Funding Statement
Grant support was provided by NIH [P30 CA014520 and R01 CA129154].
Disclosure statement
DGM has ownership interest, has received research support, and serves as consultant to Madison Vaccines, Inc., which has licensed material described in this manuscript. The other author has no relevant potential conflicts of interest.
Availability of data and material
The data generated and/or analyzed during this study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Abbreviations
| 4-1BB APC AUC CTLA4 E.G7 E.G7-OVA-PDL1high ELISA Flt3 Flt3L GSEA HPV IFN IL-12 LAG-3 MFI OT-1 OVA PD-1 PD-L1 PRR T-cell TCR TIGIT TIM-3 TLR TRAMP VISTA |
CD137; tumor necrosis factor receptor superfamily member 9 Antigen-presenting cell Area under the curve Cytotoxic T lymphocyte-associated protein 4 Tumor cell line derived from T-cell lymphoma EL-4 E.G7 cell line expressing Ovalbumin and PD-L1 Enzyme-linked immunosorbent assay Fms-like tyrosine kinase 3 Ligand of Flt3 Gene set enrichment analysis Human papillomavirus Interferon Interleukin-12 Lymphocyte activating gene 3 Median fluorescence intensity Transgenic mice expressing TCR specific for ovalbumin SIINFEKL epitope Ovalbumin Programmed cell death protein 1 PD-1 ligand Pattern recognition receptor Thymic lymphocyte T-cell receptor T-cell immunoreceptor with Ig and ITIM domains T-cell immunoglobulin and mucin domain-containing protein 3 Toll-like receptor Transgenic adenocarcinoma of the murine prostate V-domain Ig suppressor of T-cell activation |
References
- 1.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–13. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 2.Tian X, Zhang A, Qiu C, Wang W, Yang Y, Qiu C, Liu A, Zhu L, Yuan S, Hu H, et al. The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. J Immunol. 2015;194(8):3873–3882. doi: 10.4049/jimmunol.1402176. [DOI] [PubMed] [Google Scholar]
- 3.Vigano S, Banga R, Bellanger F, Pellaton C, Farina A, Comte D, Harari A, Perreau M.. CD160-associated CD8 T-cell functional impairment is independent of PD-1 expression. PLoS Pathog. 2014;10:e1004380. doi: 10.1371/journal.ppat.1004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ElTanbouly MA, Croteau W, Noelle RJ, Lines JL. Vista: a novel immunotherapy target for normalizing innate and adaptive immunity. Semin Immunol. 2019;42:101308. doi: 10.1016/j.smim.2019.101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harjunpaa H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020;200:108–119. doi: 10.1111/cei.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agresta L, Hoebe KHN, Janssen EM. The emerging role of CD244 signaling in immune cells of the tumor microenvironment. Front Immunol. 2018;9:2809. doi: 10.3389/fimmu.2018.02809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang F, Du X, Liu M, Zheng P, Liu Y. Anti-CTLA-4 antibodies in cancer immunotherapy: selective depletion of intratumoral regulatory T cells or checkpoint blockade? Cell Biosci. 2018;8:30. doi: 10.1186/s13578-018-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris RL, Lu B, Kane LP. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J Immunol. 2014;193:1525–1530. doi: 10.4049/jimmunol.1400557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zahm CD, Colluru VT, McNeel DG. Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8(+) T cells. Cancer Immunol Res. 2017;5:630–641. doi: 10.1158/2326-6066.CIR-16-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 epitope-modified DNA vaccine immunization. Cancer Immunol Res. 2015;3:946–955. doi: 10.1158/2326-6066.CIR-14-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos R, Rutten L, van der Lubbe JEM, Bakkers MJG, Hardenberg G, Wegmann F, Zuijdgeest D, de Wilde AH, Koornneef A, Verwilligen A, et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5:91. doi: 10.1038/s41541-020-00243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol 2007. Chapter 14:Unit 14.2. doi: 10.1002/0471142735.im1412s77. [DOI] [PubMed] [Google Scholar]
- 14.Delneste Y, Beauvillain C, Jeannin P. Innate immunity: structure and function of TLRs. Med Sci (Paris). 2007;23:67–73. doi: 10.1051/medsci/200723167. [DOI] [PubMed] [Google Scholar]
- 15.Zhang E, Ma Z, Li Q, Yan H, Liu J, Wu W, Guo J, Zhang X, Kirschning CJ, Xu H, et al. TLR2 stimulation increases cellular metabolism in CD8(+) T cells and thereby enhances CD8(+) T cell activation, function, and antiviral activity. J Immunol. 2019;203(11):2872–2886. doi: 10.4049/jimmunol.1900065. [DOI] [PubMed] [Google Scholar]
- 16.Smith AS, Knochelmann HM, Wyatt MM, Rangel Rivera GO, Rivera-Reyes AM, Dwyer CJ, Ware MB, Cole AC, Neskey DM, Rubinstein MP, et al. B cells imprint adoptively transferred CD8 + T cells with enhanced tumor immunity. J Immunother Cancer. 2022;10(1):e003078. doi: 10.1136/jitc-2021-003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cottalorda A, Verschelde C, Marçais A, Tomkowiak M, Musette P, Uematsu S, Akira S, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol. 2006;36(7):1684–1693. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 18.Hammerich L, Bhardwaj N, Kohrt HE, Brody JD. In situ vaccination for the treatment of cancer. Immunotherapy. 2016;8:315–330. doi: 10.2217/imt.15.120. [DOI] [PubMed] [Google Scholar]
- 19.Domingos-Pereira S, Decrausaz L, Derré L, Bobst M, Romero P, Schiller JT, Jichlinski P, Nardelli-Haefliger D. Intravaginal TLR agonists increase local vaccine-specific CD8 T cells and human papillomavirus-associated genital-tumor regression in mice. Mucosal Immunol. 2013;6:393–404. doi: 10.1038/mi.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gableh F, Saeidi M, Hemati S, Hamdi K, Soleimanjahi H, Gorji A, Ghaemi A. Combination of the toll like receptor agonist and α-Galactosylceramide as an efficient adjuvant for cancer vaccine. J Biomed Sci. 2016;23(1):16. doi: 10.1186/s12929-016-0238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sajadian A, Tabarraei A, Soleimanjahi H, Fotouhi F, Gorji A, Ghaemi A. Comparing the effect of Toll-like receptor agonist adjuvants on the efficiency of a DNA vaccine. Arch Virol. 2014;159:1951–1960. doi: 10.1007/s00705-014-2024-4. [DOI] [PubMed] [Google Scholar]
- 22.Zahm CD, Colluru VT, McIlwain SJ, Ong IM, McNeel DG. TLR Stimulation during T-cell activation lowers PD-1 expression on CD8(+) T cells. Cancer Immunol Res. 2018;6:1364–1374. doi: 10.1158/2326-6066.CIR-18-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva A, Mount A, Krstevska K, Pejoski D, Hardy MP, Owczarek C, Scotney P, Maraskovsky E, Baz Morelli A. The combination of ISCOMATRIX adjuvant and TLR agonists induces regression of established solid tumors in vivo. J Immunol. 2015;194:2199–2207. doi: 10.4049/jimmunol.1402228. [DOI] [PubMed] [Google Scholar]
- 24.Chung YH, Beiss V, Fiering SN, Steinmetz NF. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14:12522–12537. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]
- 25.Kapadia D, Sadikovic A, Vanloubbeeck Y, Brockstedt D, Fong L. Interplay between CD8α+ dendritic cells and monocytes in response to Listeria monocytogenes infection attenuates T cell responses. PLoS One. 2011;6:e19376. doi: 10.1371/journal.pone.0019376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrews S. FastQC: a quality control tool for high throughput sequence data. Version 0.11. 2. https://www.bioinformatics.babraham.ac.uk/projects/fastqc. Accessed 19 March 2022.
- 27.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 29.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:1–21. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Davidson TS, Huter EN, Shevach EM. Engagement of TLR2 does not reverse the suppressor function of mouse regulatory T cells, but promotes their survival. J Immunol. 2009;183:4458–4466. doi: 10.4049/jimmunol.0901465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y-P, Wang Y-F, Wang J-R, Huang S-W, C-k Y. Enterovirus 71 blocks selectively type I interferon production through the 3C viral protein in mice. J Med Virol. 2012;84:1779–1789. doi: 10.1002/jmv.23377. [DOI] [PubMed] [Google Scholar]
- 33.Amendt T, Jumaa H. Memory IgM protects endogenous insulin from autoimmune destruction. EMBO J. 2021;40:e107621. doi: 10.15252/embj.2020107621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson BM, Johnson LE, McNeel DG. The androgen receptor: a biologically relevant vaccine target for the treatment of prostate cancer. Cancer Immunol Immunother. 2013;62:585–596. doi: 10.1007/s00262-012-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uher O, Huynh -T-T, Zhu B, Horn LA, Caisova V, Hadrava Vanova K, Medina R, Wang H, Palena C, Chmelar J, et al. Identification of immune cell infiltration in murine pheochromocytoma during combined mannan-BAM, TLR ligand, and anti-CD40 antibody-based immunotherapy. Cancers. 2021;13:3942. doi: 10.3390/cancers13163942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 37.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zahm CD, Moseman JE, Delmastro LE, GM D. PD-1 and LAG-3 blockade improve anti-tumor vaccine efficacy. Oncoimmunology. 2021;10:1912892. doi: 10.1080/2162402X.2021.1912892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick RM, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 41.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE Jr., Murphy KM. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trinchieri G, Rengaraju M, D’Andrea A, Valiante NM, Kubin M, Aste M, Chehimi J. Producer cells of interleukin 12. Parasitol Today. 1993;9:97. doi: 10.1016/0169-4758(93)90215-2. [DOI] [PubMed] [Google Scholar]
- 43.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 44.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc London Ser B Containing Papers Biol Charact R Soc. 1957;147:258–267. [Google Scholar]
- 45.Sikora AG, Jaffarzad N, Hailemichael Y, Gelbard A, Stonier SW, Schluns KS, Frasca L, Lou Y, Liu C, Andersson HA, et al. IFN-alpha enhances peptide vaccine-induced CD8+ CD8 + T cell numbers, effector function, and antitumor activity. J Immunol. 2009;182:7398–7407. doi: 10.4049/jimmunol.0802982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, et al. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci U S A. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, Munn D, Mellor AL. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 2016;76:2076–2081. doi: 10.1158/0008-5472.CAN-15-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacquelot N, Yamazaki T, Roberti MP, Duong CPM, Andrews MC, Verlingue L, Ferrere G, Becharef S, Vétizou M, Daillère R, et al. Sustained type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res. 2019;29(10):846–861. doi: 10.1038/s41422-019-0224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takemura R, Takaki H, Okada S, Shime H, Akazawa T, Oshiumi H, Matsumoto MP-I, Teshima T, Seya T. TLR3/RIP3-dependent necroptosis backs up immune effector-mediated tumor elimination in vivo. Cancer Immunol Res. 2015;3:902–914. doi: 10.1158/2326-6066.CIR-14-0219. [DOI] [PubMed] [Google Scholar]
- 50.Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P. Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin Cancer Res. 2007;13:4565–4574. doi: 10.1158/1078-0432.CCR-07-0274. [DOI] [PubMed] [Google Scholar]
- 51.Nomi N, Kodama S, Suzuki M. Toll-like receptor 3 signaling induces apoptosis in human head and neck cancer via survivin associated pathway. Oncol Rep. 2010;24:225–231. doi: 10.3892/or_00000850. [DOI] [PubMed] [Google Scholar]
- 52.Brignole C, Marimpietri D, Di Paolo D, Perri P, Morandi F, Pastorino F, Zorzoli A, Pagnan G, Loi M, Caffa I, et al. Therapeutic targeting of TLR9 inhibits cell growth and induces apoptosis in neuroblastoma. Cancer Res. 2010;70:9816–9826. doi: 10.1158/0008-5472.CAN-10-1251. [DOI] [PubMed] [Google Scholar]
- 53.Tricco AC, Zarin W, Cardoso R, Veroniki AA, Khan PA, Nincic V, Ghassemi M, Warren R, Sharpe JP, Page AV, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis. Bmj. 2018;363:k4029. doi: 10.1136/bmj.k4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sprent J, King C. COVID-19 vaccine side effects: the positives about feeling bad. Sci Immunol. 2021;6(60):eabj9256. doi: 10.1126/sciimmunol.abj9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huhta H, Helminen O, Kauppila JH, Salo T, Porvari K, Saarnio J, Lehenkari PP, Karttunen TJ. The expression of toll-like receptors in normal human and murine gastrointestinal organs and the effect of microbiome and cancer. J Histochem Cytochem. 2016;64(8):470–482. doi: 10.1369/0022155416656154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perkins DJ, Vogel SN. Inflammation: species-specific TLR signalling – insight into human disease. Nat Rev Rheumatol. 2016;12:198–200. doi: 10.1038/nrrheum.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bryant CE, Monie TP. Mice, men and the relatives: cross-species studies underpin innate immunity. Open Biol. 2012;2:120015. doi: 10.1098/rsob.120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and/or analyzed during this study are available from the corresponding author on reasonable request.


