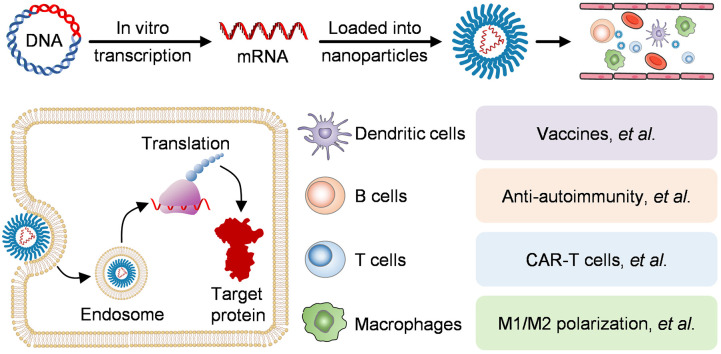
Abstract
Abnormal immune cell functions are commonly related to various diseases, including cancer, autoimmune diseases, and infectious diseases. Messenger RNA (mRNA)-based therapy can regulate the functions of immune cells or assign new functions to immune cells, thereby generating therapeutic immune responses to treat these diseases. However, mRNA is unstable in physiological environments and can hardly enter the cytoplasm of target cells; thus, effective mRNA delivery systems are critical for developing mRNA therapy. The two mRNA vaccines of Pfizer-BioNTech and Moderna have demonstrated that lipid nanoparticles (LNPs) can deliver mRNA into dendritic cells (DCs) to induce immunization against severe acute respiratory syndrome coronavirus 2, which opened the floodgates to the development of mRNA therapy. Apart from DCs, other immune cells are promising targets for mRNA therapy. This review summarized the barriers to mRNA delivery and advances in mRNA delivery for regulating the functions of different immune cells.
Keywords: mRNA, Drug delivery, Immune cells, Vaccine, Immunotherapy
Graphical abstract
Delivery of mRNA to regulate the functions of immune cells holds great promises in the prevention or treatment of various diseases.
1. Introduction
Messenger RNA (mRNA) vaccines are among the first vaccines to be approved for protection against coronavirus disease 2019 (COVID-19) [1]. Clinical trials have demonstrated that the COVID-19 mRNA vaccines of Pfizer-BioNTech and Moderna are approximately 90% effective [2,3]. COIVD-19 mRNA vaccines work by delivering severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral antigen-encoding mRNA into dendritic cells (DCs), in which the mRNA is translated into the viral antigen to activate T cells and B cells [4,5]. When the virus enters the body again, antigen-specific T cells or antibodies produced by B cells can rapidly destroy the virus. Owing to the breakthroughs in COVID-19 mRNA vaccines, mRNA-based therapy has become an interesting topic in drug development. Current applications of mRNA-based therapy mainly include 1) mRNA vaccines for infectious disease prevention and cancer treatment, which account for most mRNA-based therapies in clinical trials. 2) Protein replacement therapy, a method for expressing cytokines, antibodies, and other proteins by mRNA, is also an attractive approach for mRNA-based therapy. 3) Expression of cell reprogramming factors. 4) Expression of Cas nucleases and the TALENs (transcription activator-like effector nucleases) for gene editing (Table 1 ) [[5], [6], [7], [8]].
Table 1.
Therapeutic applications of mRNA therapies
| Vaccines | |
|---|---|
| COVID-19 vaccines | mRNA-1237 (EUA and CMA-NCT04860297) |
| BNT162b2 (EUA and CMA-NCT04368728) | |
| CMV vaccine | mRNA-1647 (Phase II-NCT04232280) |
| Personalized cancer vaccine | mRNA-4157 (Phase II-NCT03897881) |
| Protein replacement therapy | |
|---|---|
| Cytokines | SAR441000 (mRNA mixture encoding IL-12, IFN-α2b, GM-CSF, and IL-15, phase I-NCT03871348) |
| Antibodies | mRNA-6981 (mRNA encoding PD-L1) |
| Other self-deficient proteins | ARCT-810 (mRNA encoding OTC, phase I-NCT04442347) |
| Cell engineering | |
|---|---|
| IRF5/IKKβ | mRNA encoding IRF5 and IKKβ to reprogram macrophages from an M2 to an M1 phenotype [9] |
| CAR | mRNA encoding CAR structure [10,11] |
| Gene editing | |
|---|---|
| Cas9 | mRNA encoding Cas9 [[12], [13], [14]] |
| TALEN | mRNA encoding TALENs to knock out endogenous TCR of T cells for preventing mispairing of endogenous with transgenic TCR chains [15] |
EUA, emergency use authorization; CMA, conditional marketing authorization; CMV, cytomegalovirus; IL-12, interleukin 12; GM-CSF, granulocyte macrophage colony-stimulating factor; IL-15, interleukin 15; PD-L1, programmed death ligand 1; OTC, ornithine transcarbamylase; IRF5, interferon regulatory factor 5; IKKβ, IκB kinase β; CAR, chimeric antigen receptor; Cas9, CRISPR-associated protein 9; TALEN, transcription activator-like effector nuclease.
mRNA has many excellent features for drug development [16]. First, mRNA is a long linear RNA which can be easily designed according to the genomic sequences and can be rapidly synthesized by in vitro transcription technology. Second, mRNAs can transiently express therapeutic proteins without the risk of genomic integration. In addition, compared to DNA vectors that must be transported into the nucleus, mRNA can achieve protein expression as soon as being transfected into the cytoplasm [17]. Furthermore, once a mature mRNA drug pipeline is set up, later mRNA drugs can be rapidly developed based on a well-defined workflow by changing its sequences only. However, some critical aspects should be considered when developing mRNA drugs: 1) how to effectively deliver mRNA into target cells, 2) how to ensure mRNA is effectively transcribed into a protein, and 3) how to reduce or regulate the mRNA immunogenicity. To overcome the susceptibility to enzymatic degradation and the inability to get into the cytoplasm of mRNA, numerous non-viral delivery systems have been reported for mRNA delivery [[18], [19], [20], [21], [22]]. In addition, many mRNA modification strategies, such as 7-methylguanosine 5′-Cap (m7G 5′-Cap), poly(A) tails, introducing untranslated regions (UTRs), and inserting modified nucleotides [18,[22], [23], [24]], have been developed to regulate the immunogenicity and stability of mRNA.
Currently, the major research direction of mRNA therapy is to develop mRNA vaccines, and many comprehensive reviews have summarized the progress of delivering mRNA into DCs to stimulate antiviral and antitumor immune responses [22,[25], [26], [27]]. Apart from DCs, other immune cells also play important roles in the protection of the body and the progression of various diseases [28]. For example, B and T cells are the main adaptive immune cells. B cells are mainly responsible for producing antibodies and can also act as antigen-presenting cells (APCs) [[29], [30], [31]]. T cells can differentiate into effector cells to exert cell-mediated immunity, produce cytokines, and assist humoral immunity [[32], [33], [34]]. Macrophages are critical innate immune cells for the immune surveillance and clearance of pathogens and abnormal cells [35,36]. Regulating the differentiation and functions of these immune cells is promising for treating cancers, autoimmune diseases, cardiovascular diseases, and immune deficiencies. Here, we reviewed the progress of mRNA delivery for regulating the functions of different immune cells and treating immune-related diseases. We analyzed the advantages and potential applications of mRNA for regulating the functions of immune cells, discussed the barriers of delivering mRNA into these immune cells, and highlighted the recent research on immune cell-targeted mRNA delivery. We also proposed an outlook on new opportunities for mRNA-based therapy.
2. mRNA: an excellent method to regulate the functions of immune cells
The immune system is the defensive mechanism to protect human against threats. Immune cells are consisted of innate immune cells, including DCs, macrophages, and NK cells, and adaptive immune cells, including T and B cells. Each type of immune cell holds different pivotal immune functions.
DCs, the most professional APCs, have excellent antigen-presenting ability. Antigens expressed by mRNA can be processed by DCs and presented onto the major histocompatibility complex (MHC) molecules (known as peptide-MHC complex, pMHC) [37]. In addition, proinflammatory mRNA modification can promote the maturation of DCs, which empowers the high vaccination efficacy of the COVID-19 mRNA vaccines [38,39]. Moreover, the costimulatory molecules CD40, CD80 and CD86 are crucial for efficiently activating T cells by DCs. Using mRNA to express these costimulatory molecules or Cas9 nuclease to disrupt these costimulatory molecules is supposed to be effective in regulating the functions of DCs. For instance, our group inhibit the T cell responses by delivering Cas9 mRNA and CD40 gRNA to interrupt the CD40 expression on DCs [12].
Macrophages can eliminate pathogens and abnormal cells through phagocytosis. Using mRNA to intervene the phagocytosis is a promising strategy for regulating the functions of macrophages. For example, mRNA encoding a specific ligand or a single-chain variable fragment (scFv) that can block the “do not eat me” signal (CD47-SIRPα) was supposed to be a potential strategy for cancer immunotherapy [40]. In addition, macrophages are highly heterogeneous and are usually categorized into classically activated proinflammatory M1 macrophages and alternatively activated anti-inflammatory M2 macrophages. For instance, delivering mRNAs encoding interferon regulatory factor 5 (IRF5) and inhibitor kappa B kinase β (IKKβ) to M2 macrophages was able to reprogram immunosuppressive M2 macrophages into antitumor M1 macrophages [9]. On the contrary, delivering mRNA encoding anti-inflammatory glucocorticoid-induced leucine zipper to macrophages could alleviate autoimmune diseases [41].
NK cells are cytotoxic lymphocytes of the innate immune system with ability of eliminating malignant cancer cells. However, the immunosuppressive tumor microenvironment inhibits their cytotoxic function, limiting the clinical application of NK cell therapy. Delivering Cas9-encoding mRNA to knock out inhibitory receptors of NK cells, such as programmed cell death 1 (PD1) [42], transforming growth factor beta receptor 2 (TGFBR2) [43], is a promising solution for enhancing the antitumor functions of NK cells. In addition, CAR-NK cell therapy has shown to efficiently eliminate cancer cells with minimal side effects by using CAR structures to redirect NK cells to specifically recognize and attack cancer cells [44]. Delivering mRNA encoding CAR molecules to engineer NK cells into CAR-NK cells is a promising cancer therapy [45].
T cells can recognize pMHC I and pMHC II of DCs, and develop into different subtypes, including effector or regulatory T cells. CAR structures equip T cells with cytotoxic function without the help of MHC molecules [46]. Using mRNA to express CAR molecules on T cells have been proven to be a promising technology for generating CAR-T cells [[47], [48], [49]]. In addition, delivering Cas9-encoding mRNA to knock out inhibitory immune checkpoint PD1, or delivering PD1-scFv-encoding mRNA to block PD1 are promising solutions for enhancing the antitumor functions of CAR-T cells [50,51].
B cells can recognize antigens and develop into plasma cells to produce antigen-specific antibodies, which is also an important target cells for mRNA vaccines [52]. Delivering mRNA to express the transcriptional repressors B cell lymphoma 6 and B-lymphocyte induced maturation protein-1, or transcription factors paired box 5 and X-box-binding protein-1 is supposed to regulate the formation of plasma cells [53]. In addition, B cells participate in many autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis [31,54]. Using Cas9-encoding mRNA to knock out B-cell activating factor receptor (BAFFR), CD20 or CD19 is a promising strategy for treating these autoimmune diseases by interfering the survival of B cells [55,56].
3. Challenges and potential solutions of delivering mRNA into immune cells
Carrier-encapsulated mRNA should be efficiently delivered to target tissues and endocytosed by target cells, escape from endosomes, and ultimately be translated into proteins [18]. These requirements have been extensively discussed in previous studies [1,16,57]. However, some specific barriers to delivering mRNA into immune cells should also be considered.
3.1. The immunogenicity of mRNA and nanoparticles
When mRNA enters the endosome and the cytoplasm, it can be detected by pattern recognition receptors (PRRs), such as Toll-like receptor 3 (TLR3), TLR7, TLR8, and retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs). The recognition of mRNA by PRRs will stimulate downstream signaling pathways to produce type I interferon (IFN-I) and proinflammatory cytokines [38,39]. The carriers used for the delivery of mRNA can also evoke immune activation [58]. In addition, the secondary structure of mRNA, such as the form of dsRNA, can activate 2′-5′-oligoadenylate synthetases (OASs) to produce 2′-5′-oligoadenylates (2-5A) for inducing RNase L to degrade mRNA [59].
The immunogenicity of mRNA and nanoparticles is a double-edged sword, posing the challenge of balancing the immune-stimulus responses and mRNA translation efficiency. For vaccination, the immune stimulating property of mRNA vaccines can be used as an adjuvant to induce DC maturation and T cell activation [20]. The common strategy used for controlling the immunogenicity of mRNA involves modifying the structure of mRNA, such as introducing m7G 5′-Cap, poly(A) tails, UTRs, and modified nucleotides, which have been extensively discussed [18,[22], [23], [24]]. Another strategy for controlling the immunogenicity of mRNA is co-delivery of vaccines with unmodified mRNA and modified mRNA by TLR4 ligand-modified nanoparticles. The unmodified mRNA serves as an adjuvant, and the modified mRNA ensures high efficiency of protein expression [60].
For immunosuppressive applications, the immunogenicity of mRNA can be reduced by the chemical modification of mRNA. For instance, a lipoplex carrier-delivered mRNA was modified by N1-methylpseudouridine (m1φ), a modification known to reduce the immunogenicity of mRNA, to express myelin oligodendrocyte glycoprotein (MOG) in DCs, which results in the proliferation of MOG-specific regulatory T cells and dampened autoimmunity in multiple sclerosis mice [61].
3.2. The differences between target tissues
Immune cells are widely distributed in human body, and each type of immune cells has different tissue distributions. The physiological structure of different tissues varies significantly, which may heavily affect the efficiency of delivering mRNA to the immune cells residing in these tissues. For example, owing to the unique physiological characteristics of the liver, the blood velocity of the liver is reduced up to 1000-fold, which increases the interaction between nanoparticles and hepatocytes, hepatic endothelial cells and Kupffer cells, leading to low transfection efficacy of other immune cells, such as DCs and T cells, in other tissues [62]. The blood enters the spleen through the afferent splenic artery, branching into central arterioles surrounded by white pulp, and then the blood leaves the arterioles into the red pulp where the blood flow rate slows down [62,63]. Nanoparticles larger than 200 nm are easily trapped and internalized by macrophages of the red pulp [64]. The blood–brain barrier (BBB), which is composed of highly connected vascular endothelial cells, is also a specific structure that impedes drug delivery into the brain. Current methods to cross the BBB mainly focus on adsorption- and receptor-mediated delivery, such as using cationic nanoparticles to enhance adsorption or ligand-modified nanoparticles to induce receptor-mediated endocytosis to cross the vascular endothelial cells [65].
Recently, Dilliard et al. reported selective organ targeting nanoparticles (SORT nanoparticles) for tissue-specific mRNA delivery and provided a paradigm for optimizing the design of tissue-specific delivery system [66]. They synthesized the tissue-targeting SORT LNPs by formulating the additional fifth component (termed a SORT molecule) into the conventional LNPs, which are composed of four components: ionizable cationic lipids, amphipathic phospholipids, cholesterol, and PEG-lipids. The incorporation of SORT molecules alters the apparent pKa of nanoparticles and further affects the properties of nanoparticles to adsorb plasma proteins. Briefly, the liver-targeting SORT LNPs had an apparent pKa within the 6-7 range, while spleen-targeting LNPs had a lower pKa between 2 and 6. Finally, surface-adsorbed proteins interact with cognate receptors expressed by cells in the target organs to facilitate functional mRNA delivery to those tissues.
3.3. The obstacles for mRNA delivery to DCs and macrophages
DCs and macrophages are mainly differentiated from monocytes formed in the bone marrow [67]. DCs are widely distributed in different tissues, such as secondary lymphoid organs, epithelial tissues of the skin and gastrointestinal tract, and connective tissues of the heart, lung, liver, kidney, and lymphatic vessels [68]. Macrophages are highly heterogeneous, including microglial cells in the brain, Kupffer cells in the liver, and alveolar macrophages in the lungs. Their functions vary a lot according to the tissues they reside, the extent of differentiation, and the stimulus they have encountered [69].
The delivery efficacy of nanoparticles mainly relies on the phagocytic ability of different immune cells. The nanoparticles circulated in the blood absorb abundant of serum proteins to form a protein corona, which makes the nanoparticles to be easily endocytosed by the mononuclear phagocytic system (MPS), including monocytes, macrophages and DCs [70].
The endosomal escape of nanoparticles is the key for mRNA translation into proteins. It is reported that less than 1% of the mRNA delivered into human epithelial (HTB-177) cells by LNPs was detected in the cytoplasm [71]. Ensuring the mRNA-loaded nanoparticles to efficiently escape from the endosome is important for mRNA therapy. Ionizable lipids are proven to be a key component in the formulation of mRNA vaccines in clinical trials to increase endosomal escape [72]. The pH-sensitive ionizable lipids are neutral under physiological conditions and are protonated at low pH in endosomes. Positively charged ionizable lipids in endosomes can assist the mRNA in escaping into the cytoplasm.
The specific biology of DCs and macrophages makes the escape from the endosome a little different. The average pH value in phagosomes of DCs ranges from 7.4 to 7.8 in the first 4 h after phagocytosis, in contrast to that in macrophages reaching below pH 6 in 30 min and maintaining for 4 h after phagocytosis [[73], [74], [75]]. Thus, the contents endocytosed by DCs were more stable than those in macrophages. mRNA delivered to macrophages needs to escape from endosomes more quickly than that delivered to DCs. Furthermore, the weakly alkaline environment of the phagosomes of DCs may interfere with the process by which nanoparticles formulated with pH-sensitive ionizable lipids escape from endosomes.
3.4. The obstacles for mRNA delivery to T, B and NK cells
NK cells mainly develop in the bone marrow and are widely distributed in blood, liver, spleen, lung, intestine and lymph nodes [76]. T cells migrate into lymphoid organs after development in the thymus [77]. B cells mainly develop in the bone marrow, and reside in secondary lymphoid organs, and abdominal cavity [78]. When delivering mRNA to NK, T or B cells with weak phagocytosis, it is of great importance to enhance the interaction between nanoparticles and target cells while reducing the cellular uptake by the phagocytes of the MPS.
Polyethylene glycol (PEG) modification is a good strategy for avoiding the capture of MPS by reducing protein absorption [1]. In addition, natural-cell-membrane modification to imitate “self” materials is also utilized to reduce the uptake by MPS. Cell membrane of red blood cells, leukocytes, and platelet have been widely applied in research to modify nanoparticles [79,80]. CD47 presented on the cell provides a “do not eat me” signal, reducing endocytosis by the MPS [81]. Ligand modification is a commonly used strategy to enhance the interaction between nanoparticles and target cells. For example, CD7 in human T cells and CD3 in mouse T cells are often used as target molecules [82,83]. Transferrin is decorated as a targeting ligand for activated T cells that overexpress the transferrin receptor (CD71) [84].
4. mRNA-based regulation of DCs
As professional APCs, DCs play major roles in the initiation of adaptive immune responses [85]. The antigen protein translated by mRNA vaccines delivered to DCs can be processed into a peptide and be further displayed by MHC I or II on DCs for the activation of CD8+ T and CD4+ T cells respectively [37], triggering cellular immunity against infectious diseases and cancers to achieve preventive and therapeutic effects [86,87]. The previous mRNA delivery strategy is to transfect mRNA into DCs isolated autologously from the blood samples of patients [[88], [89], [90]]. Despite the high in vitro transfection efficiency and survival rate of DCs, the purification process of DCs is complicated and expensive, and the purity of the products is mostly insufficient, which restricted their clinical use [91]. In 1993, Martinon et al. first used non-viral carriers to directly deliver antigen-encoding mRNA into DCs in vivo. They demonstrated that liposomes could deliver mRNA encoding the nucleoprotein of influenza virus to elicit virus-specific cytotoxic T lymphocytes (CTLs) [92]. Currently, mRNA vaccines have opened a new era, and a lot of promising candidates are in clinical trials (Table 2 ).
Table 2.
mRNA drugs for different diseases in clinical trials.
| Conditions | Name | Immunogen [delivery system] | Phase | Manufacturer | Dose (μg) | Schedule | NCT Number |
|---|---|---|---|---|---|---|---|
| Infectious diseases | |||||||
| COVID-19 | BNT162b2 [93] [i.m.] | Full-length prefusion spike protein [LNP] | EUA and CMA | BioNTech /Pfizer (US) | 30 | 2 doses 21 d apart | NCT04816669 |
| mRNA-1273 [94] [i.m.] | Full-length prefusion spike protein [LNP] | EUA and CMA | Moderna (US) | 100 | 2 doses 28 d apart | NCT04860297 | |
| CVnCoV [95] [i.m.] | Unmodified full-length prefusion spike protein [LNP] | III | CureVac (Germany) | 12 | 2 doses 28 d apart | NCT04860258 | |
| ARCoV [96] [i.m.] | Modified RBD [LNP] | III | Abogen Biosciences (China) | 15 | 2 doses 14 d apart or 2 doses 28 d apart | NCT04847102 | |
| LUNAR-COV19/ARCT-021 [97] [i.m.] | Unmodified full-length prefusion spike protein [LNP] | II | Arcturus Therapeutics (US) | 5 or 7.5 | Prime: 2 doses 28 d apart Boost: 180 d after 2nd vaccination | NCT04668339 | |
| CoV2 SAM [i.m.] | Self-amplifying mRNA encoding spike protein [LNP] | I | GlaxoSmithKline (UK) | 1 | 2 doses 30 d apart | NCT04758962 | |
| nCoVsaRNA [98] [i.m.] | Self-amplifying mRNA encoding spike protein [LNP] | I | Imperial College London (UK) | NA | 2 doses | ISRCTN17072692 | |
| ChulaCov19 [i.m.] | Spike protein [LNP] | I | Chulalongkorn University (Thailand) | NA | 2 doses 21 d apart | NCT04566276 | |
| PTX-COVID19-B [99] [i.m.] | Full-length spike protein [LNP] | I | Providence Therapeutics (Canada) | NA | 2 doses 28 d apart | NCT04765436 | |
| DS-5670a [i.m.] | / | I/II | Daiichi Sankyo (Japan) | NA | 2 doses | NCT04821674 | |
| HDT-301 [i.m.] | Self-amplifying mRNA encoding spike protein [Lipid-Inorganic Nanoparticle] | I | Senai Cimatec (Brazil) | NA | 2 doses 28 d apart or 2 doses 56 d apart | NCT04844268 | |
| EXG-5003 [i.d.] | A temperature-controllable, self-replicating RNA encoding RBD protein | I/II | Elixirgen Therapeutics (US) | NA | NA | NCT04863131 | |
| VAW00001 /MRT5500 [100] [i.m.] | Spike protein [LNP] | I/II | Sanofi Pasteur-Translate Bio (US) | NA | 2 doses 21 d apart | NCT04798027 | |
| mRNA-1283 [i.m.] | Spike protein (a potential refrigerator stable mRNA vaccine) [LNP] | I | Moderna (US) | NA | 2 doses 28 d apart | NCT04813796 | |
| mRNA -1273.351 [i.m.] | Full-length spike protein of the SARS-CoV-2 B.1.351 variant [LNP] | I | Moderna (US) | NA | 3 doses 28 d apart | NCT04785144 | |
| Respiratory syncytial virus (RSV) | mRNA-1345 | mRNA encoding a prefusion F glycoprotein [LNP] | I | Moderna (US) | NA | 3 doses 56 d apart | NCT04528719 |
| Cytomegalovirus (CMV) | mRNA-1647 [i.d.] | CMV glycoprotein H (gH) pentamer complex and gB protein [LNP] | II | Moderna (US) | NA | Day 1, Day 56, Day 168 | NCT04232280 |
| Human metapneumovirus (hMPV) and parainfluenza infection (PIV3) | mRNA-1653 [i.d.] | Fusion proteins of hMPV and PIV3 [LNP] | I | Moderna (US) | NA | 2 doses 57 d apart | NCT04144348 |
| Zika virus | mRNA-1893 | mRNA encoding the structure proteins of Zika virus | II | Moderna (US) Biomedical Advanced Research and Development Authority | NA | 2 doses 28 d apart | NCT04917861 |
| mRNA-1325 [i.d.] | mRNA encoding the Zika virus antigens (prM-E) [LNP] | I | NA | NA | NCT03014089 | ||
| Rabies | CV7201 [i.m.] | Rabies virus glycoprotein (RABV-G) [RNActive®] | I | CureVac (Germany) | NA | NA | NCT02241135 |
| CV7202 [i.m.] | Rabies virus glycoprotein (RABV-G) [LNP] | I | CureVac (Germany) | NA | 2 doses 28 d apart | NCT03713086 | |
| Influenza | VAL-506440/mRNA-1440 [101] [i.m. or i.d.] | H10N8 Hemagglutinin (HA) A/Jiangxi Donghu/346/2013 [LNP] | I | Moderna (US) | NA | 2 doses 3 w apart | NCT03076385 |
| mRNA-1851 [101] [i.m.] | H7N9 HA A/Anhui/1/2013 [LNP] | I | Moderna (US) | NA | NCT03345043 | ||
| AVX502 [i.m. or s.c.] | H3N2 HA A/Wyoming/03/2003 | I/II | AlphaVax (US) | 2 doses 4 w apart | NCT00706732 | ||
| Tuberculosis | GSK 692342 [i.m.] | mRNA encoding fusion protein (M72) [LNP] | II | GlaxoSmithKline (UK) | NA | 2 doses 30 d apart | NCT01669096 |
| Chikungunya virus | mRNA-1944 | mRNA encoding Chikungunya antibody [LNP] | I | Moderna (US) | NA | NA | NCT03829384 |
| HIV | iHIVARNA-01 | mRNA encoding CD40L and the HIV target antigens contained in HIVACAT [DC] | II | Rob Gruters | 1200 | 3 doses 2 w apart | NCT02888756 |
| AGS-004 [102] [i.d.] | mRNA encoding HIV antigens (Gap, Nef, Rev, Vpr) [DC] | II | Argos Therapeutics (US) | At least 1×107 DCs in each injection | 4 doses 4 w apart 1 dose 4 w apart at week 16 | NCT00672191 | |
| Cancers | |||||||
| HPV16+ head and neck cancer | BNT113 [i.d.] | mRNA encoding HPV16-derived oncoproteins E6 and E7 [lipoplex, LPX] | I/II | BioNTech (Germany) | NA | NA | NCT03418480 |
| Glioblastoma | Human CMV pp65-LAMP mRNA [i.d.] | [autologous DCs] | II | Duke University (US) | A total of 20 DC vaccines | 2×107 human CMV pp65-LAMP mRNA-pulsed autologous DCs | NCT03688178 |
| Human CMV pp65-LAMP mRNA [i.d.], GM-CSF | [autologous DCs] | II | A total of 10 DC vaccines | NCT03927222 | |||
| Ovarian cancer | W_ova1 Vaccine/BNT115 | Ovarian TAAs [lipoplex, LPX] | I | BioNTech (Germany) | NA | 8 doses | NCT04163094 |
| Triple negative breast cancer | IVAC_W_bre1_uID/BNT114 [i.v.] | TAAs, p53 and neoantigens [liposome] | I | BioNTech (Germany) | NA | NA | NCT02316457 |
| Melanoma | mRNA-4157 [i.m.] | Neoantigens | II | Moderna (US) Merck Sharp & Dohme Corp (US) |
NA | 9 doses 21 d apart | NCT03897881 |
| RO7198457/BNT122 [i.v.] | Individualized neoantigen [LPX] | II | BioNTech (Germany) | NA | a 3-week cycle | NCT03815058 | |
| Lipo-MERIT/BNT111 [i.v.] | mRNA encoding NY-ESO-1, MAGE-A3, tyrosinase, and TPTE (TAAs) [LPX] | I | BioNTech (Germany) | NA | 7 doses | NCT02410733 | |
| Metastatic non-small cell lung cancer (NSCLC) | CV9202/BI 1361849 [i.d.] | mRNA encoding NY-ESO-1, MAGE-C1, MAGE-C2, TPBG, survivin, MUC1 (TAAs) [RNActive®] | I/II | CureVac (Germany) | NA | NA | NCT03164772 |
| KRAS-mutant NSCLC, colorectal cancer, pancreatic adenocarcinoma | mRNA-5671/V941 [i.m.] | KRAS mutations [LNP] | I | Merck Sharp & Dohme Corp (US) | NA | Once every 3 weeks for nine 3-week cycles | NCT03948763 |
| Pancreatic cancer | MVT-5873 /BNT321 [i.v.] | mRNA encoding a fully human IgG1 monoclonal antibody targeting sialyl Lewis A | I | BioNTech (Germany) | NA | NA | NCT02672917 |
| Relapsed/refractory solid tumor malignancies or lymphoma | mRNA-2416 i.t.] | mRNA encoding human OX40L [LNP] | I/II | Moderna (US) | NA | Day 1, 15 for six 28-day cycles | NCT03323398 |
| Myelodysplastic syndromes, acute myeloid leukemia | WT1 mRNA | mRNA encoding Wilm tumor gene1 (WT1) [Autologous DCs] | I/II | University of Campinas (Brazil) | NA | 4 doses 2 w apart | NCT03083054 |
| Advanced malignancies | mRNA-2752 [i.t.] | mRNA encoding human OX40L, IL-23, and IL-36γ [LNP] | I | Moderna (US) | NA | Days 1, 15 of Cycle 1 and days 1, 6 of Cycle 2, each cycle is 28 d | NCT03739931 |
| Solid tumors | MEDI1191 [103] [i.t.] | mRNA encoding IL-12 [LNP] | I | MedImmune LLC (US) | NA | NA | NCT03946800 |
| Metastatic neoplasm | SAR441000/BNT131 [i.t.] | mRNA encoding IL-12sc, IL-15sushi, IFNα, and GM-CSF | I | BioNTech (Germany) | NA | a 28-day cycle | NCT03871348 |
| Rare diseases | |||||||
| Methylmalonic Acidemia | mRNA-3705 [i.v.] | Modified mRNA encoding methylmalonyl-coenzyme A (CoA) mutase [LNP] | I/II | Moderna (US) | NA | NA | NCT04899310 |
| Propionic acidemia (PA) | mRNA-3927 [104] [i.v.] | Dual mRNAs encoding propionyl-CoA carboxylase (PCC) A and B [LNP] | I/II | Moderna (US) | NA | NA | NCT04159103 |
| Isolated methylmalonic acidemia (MMA) | mRNA-3704 [i.v.] | mRNA encoding methylmalonyl-coenzyme A mutase [LNP] | I/II | Moderna (US) | NA | NA | NCT03810690 |
| Cystic fibrosis (CF) | MRT5005 [Nebulization] | mRNA encoding fully functional CF transmembrane conductance regulator (CFTR) protein | I/II | Translate Bio (US) | 4,000 | 5 consecutive days | NCT03375047 |
| Ornithine transcarbamylase (OTC) deficiency | ARCT-810 [i.v.] | mRNA encoding OTC [LNP] | Ib | Arcturus Therapeutics (US) | NA | NA | NCT04442347 |
| MRT5201 [i.v.] | mRNA encoding OTC [LNP] | I/II | Translate Bio (US) | NA | NA | NCT03767270 | |
| Hereditary transthyretin amyloidosis with polyneuropathy | NTLA-2001 [i.v.] | CRISPR/Cas9 gene editing system [LNP] | I | Intellia Therapeutics (US) | NA | NA | NCT04601051 |
4.1. LNPs for mRNA delivery to DCs
As Table 3 listed, LNPs are formulated with ionizable lipids (e.g., 1,2-dilinoleyloxy-3-dimethylaminopropane, also known as DLinDMA), PEG-modified lipids (PEG-lipids), neutral lipids, and cholesterol. The diameter of LNPs is about 80 nm, and their surface charge is usually neutral in the physiological environment [22]. As the key element of LNPs, ionizable lipids are used to stabilize nucleic acids during the preparation of nanoparticles [105]. They also mediate the fusion of LNPs with the membrane of endosome, enabling the release of nucleic acids into the cytoplasm. The PEG-lipids stabilize the nanoparticles [22] and hinder the plasma proteins from binding to the nanoparticles and prolong the blood circulation [106]. Neutral lipids and cholesterol are important for the structural integrity of nanoparticles and the escape from endosome and promote the membrane fusion of nanoparticles [107,108].
Table 3.
Carrier characteristics of mRNA vaccines.
| Manufacturer | Name | Composition | Disease | Phase | Reference |
|---|---|---|---|---|---|
| Lipid nanoparticle (LNP) | |||||
| Moderna | mRNA-1273 (LNP) [1] | Ionizable lipid SM-102 (heptadecan-9-yl 8-((2-hydroxyethyl) (6-oxo-6-(undecyloxy) hexyl) amino) octanoate); DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine); PEG2000-DMG ((R)-2,3-bis(myristoyloxy)propyl-1-(methoxy poly(ethylene glycol)2000) carbamate); Cholesterol | COVID-19 | III (EUA and CMA) | NCT04860297 |
| BioNTech | BNT162b2 (LNP) [109] | ALC-0315 (((4-hydroxybutyl) azanediyl) bis (hexane-6,1-diyl) bis (2-hexyldecanoate)); ALC-0159 (2- [(polyethylene glycol)-2000]-N, N-ditetradecylacetamide); DSPC; Cholesterol | COVID-19 | III (EUA and CMA) | NCT04816669 |
| CureVac AG | CVnCoV (LNP) [95] | Ionizable amino lipid, phospholipid, cholesterol, and a PEGylated lipid (Acuitas Therapeutics) | COVID-19 | III | NCT04860258 |
| Abogen Biosciences | ARCoV (LNP) [96] | Ionizable lipid; DSPC; PEG-lipid; Cholesterol | COVID-19 | III | NCT04847102 |
| Arcturus Therapeutics | LUNAR-COV19/ARCT-021 (LNP) [97] | Ionizable lipid; DSPC; PEG2000-DMG; Cholesterol | COVID-19 | II | NCT04758962 |
| BioNTech | BNT111 (LPX) [110] | R-DOTMA (Cationic synthetic lipid (R)-N, N, N trimethyl-2-3-dioleyloxy-1-propanaminium chloride); DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine phospholipid) | Melanoma | I | NCT02410733 |
| Cationic peptide nanoparticle | |||||
| CureVac AG | CV7202 [111,112] | A protamine/mRNA complex | Rabies vaccine | I | NCT03713086 |
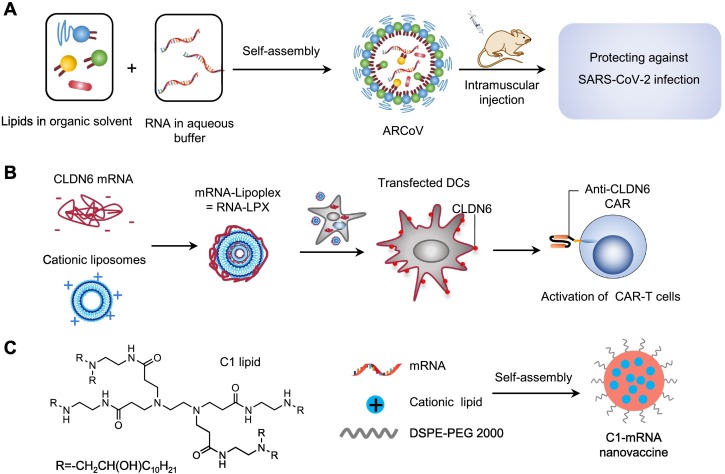
However, mRNA vaccines delivered by LNPs must be maintained at an ultra-low temperature for shipping and long-term storage. To design a more stable LNP-based mRNA vaccine, Qin et al. encapsulated mRNA encoding the receptor-binding domain (RBD) of SARS-CoV-2 into a liquid formulation of LNPs (called ARCoV) [96] (Fig. 1A), which can be stored at room temperature for at least 1 week and has more advantages than mRNA-1273 and BNT162b2 in transportation and preservation. ARCoV is being evaluated in phase 3 clinical trials (NCT04847102) since April 2021.
Fig. 1.
LNPs for mRNA delivery to DCs. (A) LNP used for delivering mRNA to prevent SARS-CoV-2 infection. Adapted with permission from [96]. Copyright (2020) Elsevier. (B) Delivery of antigen-encoding mRNA to DCs with LPX for the activation of CAR-T cells. Adapted with permission from [113]. Copyright (2020) The American Association for the Advancement of Science. (C) The structure of C1-lipid and self-assembly of C1-mRNA nanovaccine for OVA mRNA delivery [114].
Sahin et al. developed a liposomal RNA (RNA-LPX) vaccine that encoded four non-mutated tumor-associated antigens (TAAs) of melanoma to target DCs in lymph nodes [110]. The RNA-LPX was prepared by complexing mRNA with cationic liposomes manufactured with the cationic synthetic lipid R-DOTMA and the phospholipid DOPE using the ethanol injection technique. The data of clinical trials (NCT02410733) demonstrated that intravenous injection of RNA-LPX alone or in combination with anti-PD-1 antibody could induce a durable objective response in melanoma patients by activating strong antigen-specific immune responses of CD4+ T and CD8+ T cells. In addition, this platform can be used as a CAR-T cell-amplifying RNA vaccine (Fig. 1B). They proved that mRNA encoding the tight junction protein claudin 6 (CLDN6), a new targeting molecule of CAR-T cells, can be effectively delivered into DCs by LPX after intravenous injection, thereby activating the proliferation and anti-tumor effects of anti-CLDN6 CAR-T cells [113]. Xia et al. reported an LNP nanovaccine based on C1 lipid with a 12-carbon tail to deliver mRNA into DCs (Fig. 1C) [114]. The C1 lipid-based LNP were synthesized by self-assembly method using DSPE-PEG 2000 (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000]) and a cationic lipid-like material synthesized from PAMAM dendrimer G0 (C1 lipid). They demonstrated that the C1-mRNA nanovaccine could activate TLR4 signaling to promote strong antigen presentation and induce robust T cell responses. Mice immunized subcutaneously with the C1-mRNA nanovaccine encoding ovalbumin (OVA) antigen can activate robust OVA-specific CD8+ T cell responses for the effective inhibition of MC38-OVA and B16-OVA tumor growth.
4.2. Ligand-modified LNPs for mRNA delivery to DCs
To improve the selective distribution of nanoparticles, antibodies or other targeting modules can be incorporated into nanoparticles. For example, Palliser et al. decorated LNPs with a scFv specific to murine DEC205, a marker highly expressed on CD8α+ DCs. They firstly synthesized the LNPs by extrusion using DSPC, DLinDMA, DSPE-PEG-MAL (1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-(maleimide-(polyethylene glycol)-2000)) and cholesterol. Then, the reduced anti-DEC205 scFv was conjugated to the maleimide group of the DSPE-PEG-MAL of the LNPs by simply mixing. They demonstrated that anti-DEC205 scFv-modified LNPs preferentially targeted DEC205+ DCs [115]. In addition, Adamo et al. incorporated a mannose-cholesterol into LNPs composed of DLinDMA, DSPC, cholesterol and PEG-DMG-2000 to prepare mannosylated nanoparticles (MLNPs) for the delivery of self-amplifying mRNA. MLNPs carrying self-amplifying mRNA encoding hemagglutinin of influenza were administered intramuscularly or intradermally and were shown to enhance antigen-specific immune responses [116].
However, the introduction of targeting modules to LNPs mostly requires multiple steps of synthesis, purification, and characterization, which significantly increase the complexity, cost, and regulatory barriers of production [117]. Furthermore, the targeting ability of functionalized nanoparticles may be reduced or disappear in the biological environment because of the effect of “protein corona” [118]. Therefore, the utilization of targeting molecules to functionalize LNP-mRNA needs to be carefully considered.
4.3. Protamine-based nanoparticles for mRNA delivery to DCs
Immunization with protamine-based mRNA vaccines, such as the RNActive® technology (developed by CureVac), can also induced effective protection to infectious diseases. RNActive®, which is consist of two components, the free and protamine-complex mRNA, was shown to effectively support antigen expression and innate immune responses mediated by TLR7 [119]. Based on the RNActive® technology, Penjamin et al. developed an mRNA vaccine (CV7201) encoding the rabies virus glycoprotein (RABV-G) [120]. They demonstrated that CV7201 can be lyophilized for long time preservation and be reconstituted using specific buffer before injection, which is a breakthrough when compared with the existing mRNA vaccines. Specifically, CV7201 remained immunogenicity and protective effects (100%) after being exposed to 70 °C and long-term storage for 90 days. The result of phase I clinical trial of CV7201 demonstrated that 71% (32/45) of the subjects administered 80 or 160 μg intradermally and 46% (6/13) of the subjects administered 200 or 400 μg intramuscularly via needle-free device injection induced rabies virus-neutralizing antibody titers comparable to or higher than the standard titer of WHO (0.5 IU/mL) [121]. In addition, several vaccines based on the RNActive® technology have now been tested in clinical trials, such as rabies vaccine (CV7201, NCT02241135), non-small cell lung cancer vaccine (CV9201, NCT00923312), and prostate carcinoma vaccine (CV9104, NCT02140138).
4.4. Polymer nanoparticles for mRNA delivery to DCs
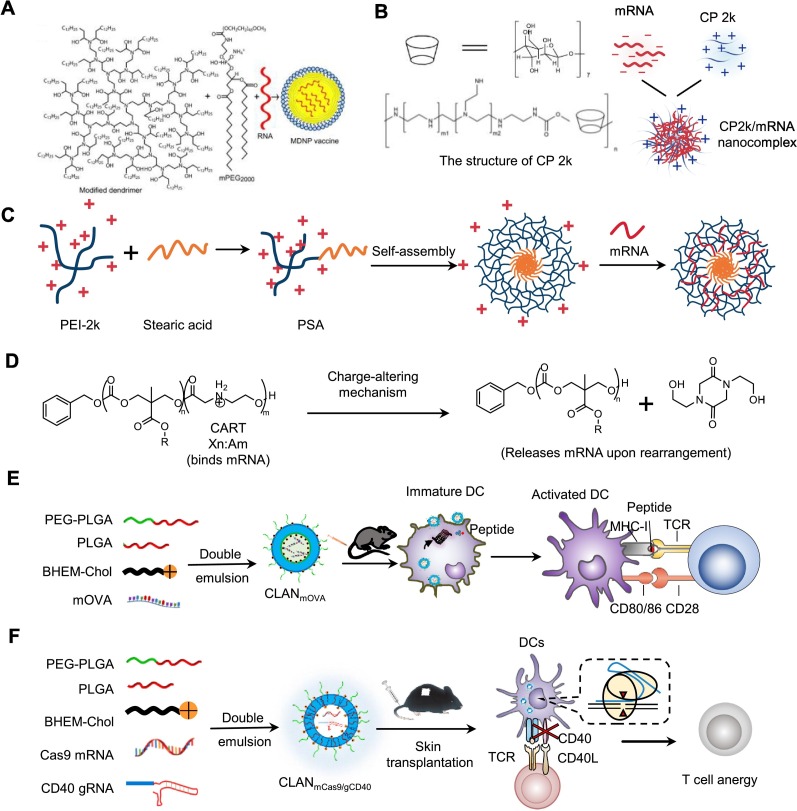
Polymer nanoparticles have also been demonstrated to be effective in delivering mRNA. For example, Anderson et al. synthesized a single-dose, adjuvant-free dendrimer nanoparticle vaccine platform by designing mRNA replicons to realize long-term expression of the antigens [122] (Fig. 2A). The dendrimer-based RNA vaccine were prepared by self-assembly method with an ionizable dendrimer-based nanomaterial, DMPE-PEG 2000 (1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]), and antigen-encoding RNA. They demonstrated that dendrimer-based RNA vaccines could effectively express the antigen in several cell types, such as the mouse DC2.4 cell line. In addition, they demonstrated that immunizing mice by the dendrimer-based RNA vaccines stimulated antigen-specific T cells and antibodies, which protected against H1N1 influenza, Toxoplasma godii, and Ebola virus. Furthermore, they indicated that encapsulating mRNA by nanoparticles is sequence-independent, and various replicons encoding multiple distinct antigens can be co-encapsulated into a single formulation to protect against several deadly pathogens.
Fig. 2.
Polymer-based nanoparticles for mRNA delivery to DCs. (A) Adjuvant-free dendrimer nanoparticle vaccine platform for mRNA delivery into several cell types, such as DC2.4. Adapted with permission from [122]. Copyright (2016) National Academy of Sciences. (B) CP 2k for the transfection of gp120-encoding mRNA. Adapted with permission from [123]. Copyright (2016) Elsevier. (C) PSA copolymer for delivering mRNA encoding HIV-1 gag to DCs. Adapted with permission from [124]. Copyright (2016) Taylor & Francis. (D) The mechanism of charge-altering releasable transporters (CARTs) for binding and releasing mRNA. Adapted with permission from [125]. Copyright (2018) National Academy of Sciences. (E) Delivery of mOVA by CLANmOVA to DCs for the treatment of E·G7-OVA lymphoma. Adapted with permission from [126]. Copyright (2018) Royal Society of Chemistry. (F) CLANmCas9/gCD40 delivered mCas9 and gCD40 to DCs for inducing transplant tolerance. Adapted with permission from [12]. Copyright (2019) Elsevier.
Sun et al. synthesized an intranasal mRNA vaccine (CP 2k/mRNA complexes) for the treatment of HIV-1. They demonstrated that CP 2k, a cationic cyclodextrin-polyethylenimine 2k (PEI-2k) conjugate, was more capable of delivering mRNA encoding the glycoprotein 120 (gp120) of HIV than PEI, enhancing gp120-specific humoral immunity and cell-mediated immune responses (Fig. 2B) [123]. In addition, they reported a self-assemble cationic micelle formulated with stearic acid and branched PEI-2k conjugates (PSA) and mRNA encoding the gag (mGag) of HIV-1, denoted as PSA/mGag. PSA/mGag was proved to be effective in delivering the mGag to DC2.4 and elicited gag-specific CD4+ T and CD8+ T cell responses after being subcutaneously injected to BALB/c mice (Fig. 2C) [124].
In addition, Waymouth et al. reported a series of oligo(carbonate-b-α-amino ester)s called charge-altering releasable transporters (CARTs) for the delivery of mRNA [125] (Fig. 2D). They demonstrated that CARTs could complex mRNA through electrostatic interactions and release mRNA into the cytoplasm via charge alteration. OVA-mRNA-CART complex has been demonstrated to effectively deliver OVA-mRNA to the DC2.4 cell line, and the OVA peptide was effectively presented on MHC-I molecules [127]. In addition, CART formulated with mRNA encoding OVA and short oligo anionic nucleic acid adjuvant CpG was demonstrated to effectively induce OVA-specific responses of T cells to treat A20-OVA tumors.
Our group developed cationic lipid-assisted nanoparticles (CLAN) composed of a block copolymer poly (ethylene glycol)-block-poly (lactic-co-glycolic acid) (PEG-b-PLGA) and a cationic lipid for the delivery of nucleic acids. for delivery to DCs. We demonstrated that CLAN encapsulated mRNA encoding OVA (mOVA), called CLANmOVA, could effectively deliver mOVA into DCs for activating OVA-specific T cells. In E·G7-OVA lymphoma mouse model, CLANmOVA treatment provoked a strong OVA-specific T-cell response and inhibited the growth of tumor (Fig. 2E) [126]. Furthermore, to address the allograft rejection caused by DCs-activated T cell responses in organ transplantation, our group encapsulated Cas9 mRNA (mCas9) and a guide RNA targeting costimulatory molecule CD40 (gCD40) into CLANmCas9/gCD40 for inducing the immune tolerance of transplant. We demonstrated that CLANmCas9/gCD40 effectively delivered mCas9 and gCD40 to DCs and disrupted the expression of CD40 in DCs. In the acute graft rejection mouse model, CLANmCas9/gCD40 treatment effectively inhibited the activation of T cells, induced the immune tolerance of transplant, and dramatically prolonged the survival of graft (Fig. 2F) [12].
5. mRNA-based regulation of macrophages
Macrophages are critical innate immune cells, providing immune regulation and immune surveillance, including the secretion of abundant cytokines and phagocytosis of pathogen-infected cells, apoptotic cells, cell debris, and tumor cells. They also have important functions in maintaining homeostasis in healthy tissues and in repairing tissue damage. Macrophages are highly heterogeneous and can be categorized into classically activated M1 macrophages and alternatively activated M2 macrophages [128]. M1 macrophages are proinflammatory and can secrete multiple proinflammatory cytokines, including TNF-α, IL-1β, and IL-12. M2 macrophages are anti-inflammatory and can secrete anti-inflammatory cytokines, including IL-4, IL-10, or IL-13 [129]. Macrophages that are enriched in tumors are usually denoted as tumor-associated macrophages (TAMs) with M2 phenotype which highly express CD206 [130,131].
5.1. LNPs for mRNA delivery to Kupffer cells
Kupffer cells that reside within the liver sinusoids are the largest cell population of the MPS and have important roles in immune surveillance and clearance [132]. Intravenously injected NPs are usually trapped in the liver owing to the special structure of hepatic sinusoids, resulting in high endocytosis by hepatocytes, hepatic endothelial cells, and Kupffer cells [62,133]. For example, LNPs were first opsonized by apolipoprotein E (ApoE) in the blood circulation after intravenous injection and entered the hepatocytes by binding to the ApoE receptor on the surfaces of hepatocytes [134,135]. Holmes et al. demonstrated that intravenous injection of ionizable lipid-containing LNPs delivered mRNA of zinc-finger nuclease (ZFN) that induced the highest gene knockout efficiency in hepatocytes (approximately 80%), whereas the gene knockout efficiency in Kupffer cells and endothelial cells was approximately 60% [136]. Thus, achieving high transfection efficiency in Kupffer cells while reducing the transfection efficiency to other liver cells remains a challenge.
The structure of lipids has significant impacts on the delivery efficiency of LNPs in vivo. For example, the structure of cholesterol, which is an important component for stabilizing the LNPs, may affect the efficiency of LNPs for delivering mRNA to Kupffer cells in vivo [137]. Paunovska et al. established a library of cholesterol variants and evaluated the efficiency of LNPs for mRNA delivery. Their results demonstrated that the LNPs formulated using C18-PEG2K, DOPE, cKK-E12 ionizable lipid, and 20α-hydroxycholesterol (20α-OH)-modified cholesterol had the highest efficiency for delivering mRNA-encoding Cre recombinase into Kupffer cells (approximately 50% transfection efficiency), which was significantly higher than that of endothelial cells (approximately 10%–15%) and hepatocytes (approximately 15%–20%) [138]. In addition, they found that an LNP consisting of a lipid with adamantane group could preferentially deliver Cre mRNA to Kupffer cells and achieve over 80% gene editing efficiency [139].
5.2. Polymer nanoparticles for mRNA delivery to macrophages
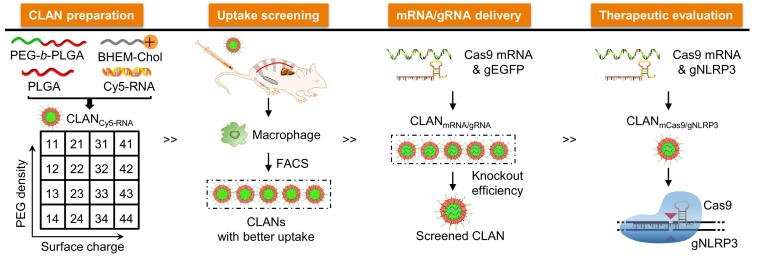
To screen for an optimal CLAN for delivering mCas9 and gRNA into macrophages, our group created a library of CLANs in which each CLAN has different surface PEG density and Zeta potential, and screened the optimal formulation of CLAN for delivering mCas9 and a guide RNA targeting NLRP3 (gNLRP3) for the prevention or treatment of inflammatory diseases (Fig. 3 ). We demonstrated that CLANmCas9/gNLRP3 effectively delivered mCas9/gNLRP3 into macrophages, which disrupted NLRP3 and inhibited the activation of NLRP3 inflammasome in macrophages. Furthermore, we proved that CLANmCas9/gNLRP3-induced NLRP3 knockout inhibited the inflammation of lipopolysaccharide-induced septic shock, monosodium urate crystal-induced peritonitis, and high-fat diet-induced type II diabetes [13].
Fig. 3.
CLANmCas9/gNLRP3 delivered mCas9/gNLRP3 into macrophages to reduce inflammation [13]. Copyright (2018) Springer Nature.
5.3. Ligand-modified nanoparticles for mRNA delivery to macrophages
CD206 is an endocytic receptor expressed by macrophages and DCs. The major role of CD206 is to mediate endocytosis of glycoproteins and pathogenic microbes coated with mannose-containing structures [69]. Using mannose or anti-CD206 antibody to modify the delivery system is a common strategy for targeted delivery to M2 macrophages. For example, Zhang et al. developed a TAM-targeting poly (β-amino ester) (PBAE) NPs by coating Di-mannose-PGA on the surface and encapsulated the mRNAs encoding IRF5 and IKKβ into PBAE NPs, denoted as IRF5/IKKβ NPs. They demonstrated that the IRF5/IKKβ NPs exhibited high TAM-targeting ability due to the high expression of CD206 on TAMs and significantly reduced the population of TAMs in mice bearing ovarian cancer by regulating the IRF5 and IKKβ signaling pathways [9].
6. mRNA-based regulation of T cells
T cells play pivotal roles in cellular immunity and humoral immunity and have been reported with great potentials in treating diseases, including cancers, infectious diseases, and autoimmune diseases. T cells develop in the thymus and are found to be distributed in the whole body, especially the spleen and lymph nodes. After recognizing antigens presented by APCs, naïve T cells are activated and will differentiate into antigen-specific effector T cells with cytotoxic or immune regulatory functions.
Tumor cells can escape from the recognition and attack of T cells by downregulating the expression of MHC molecules [140]. To avoid the immune escape of tumors, CAR-T cell therapy has been designed and applied for mediating MHC-independent kill of tumor cells [33]. Current strategies for constructing CAR-T cells are mainly depended on in vitro viral vector-mediated transfection or electroporation of plasmids to integrate the gene sequence of CAR into the genome of T cells for the long-term persistence of CAR-T cells [141]. However, these methods require to isolate T cells from patients through a complicated process and have been reported to increase the side effects, including cytokine storms and neurotoxicity [141]. To address these deficiencies, the delivery of mRNA encoding CAR molecules to construct CAR-T cells has been developed. Electroporating mRNA encoding CAR molecules into T cells to construct CAR T cells has been proved to be feasible [47,48]. However, this approach requires several rounds of CAR T cell infusion, which is time consuming and expensive.
6.1. LNPs for mRNA delivery to T cells
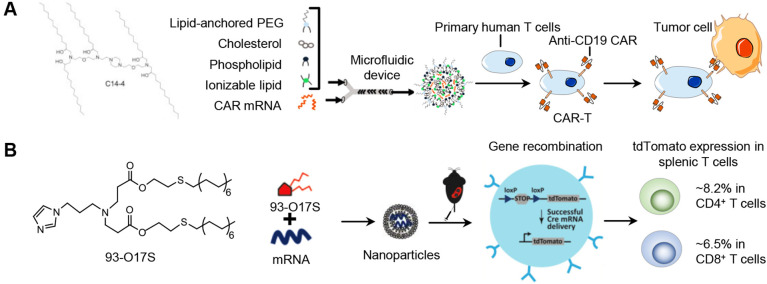
Utilizing nanoparticles (NPs) to deliver mRNA into T cells to directly construct CAR T cells in vivo is expected to simplify the procedures of CAR-T cell therapy and reduce the cost and may significantly expand its clinical use. Billingsley et al. prepared a library of ionizable lipids for the preparation of LNPs and screened an optimal ionizable lipid for delivering CAR-encoding mRNA into T cells (Fig. 4A). The LNPs were prepared with the synthesized ionizable C14-4 lipid, cholesterol, DOPE and C14-PEG using microfluidic device. They demonstrated that transfecting human T cells ex vivo with the optimal LNP-carried mRNA encoding CAR achieved comparable expression of CAR molecules to that of the electroporation method [142].
Fig. 4.
LNPs for mRNA delivery to T cells. (A) Screened ionizable lipid-formulated nanoparticles for the transfection of mRNA to human T cells ex vivo. Adapted with permission from [142]. Copyright (2020) American Chemical Society. (B) LNPs with imidazole head are effective for the in vivo mRNA delivery to T cells. Adapted with permission from [143]. Copyright (2020) American Chemical Society.
Recently, Zhao et al. reported a structure-based screening and found that the imidazole group is a key structure of the synthetic lipid-like molecules for delivering mRNA to T cells (Fig. 4B). The LNPs were prepared with synthetic lipid-like molecule with imidazole head (93-O17S), cholesterol, DOPE and DSPE-PEG using a self-assembly method. After intravenously injecting the LNP-carrying mRNA encoding Cre recombinase into the Cre-loxP tdTomato reporter mice, they detected ~8.2% of gene-editing efficiency in CD4+ T cells and ~6.5% in CD8+ T cells [143].
6.2. Polymer nanoparticles for mRNA delivery to T cells
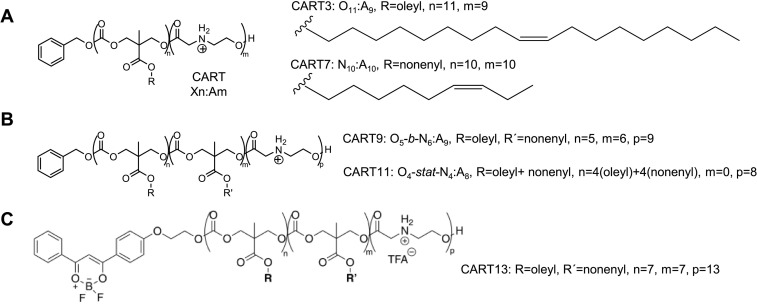
McKinlay et al. designed a library of amphiphilic CARTs, which are polymers with different lipid side chains and a polycationic α-amino ester mRNA-binding block. Through a charge-altering mechanism, the CART polymers can rearrange to be electroneutral for rapidly releasing mRNA (Fig. 2D). After a high-throughput assay of noncovalent binary combinations of eight different side-chain CARTs, McKinlay et al. found that the NPs formulated with the noncovalent combination of CARTs (1:1 mixture of CART3 (3) to CART7 (7), the structures were shown in Fig. 5A) as well as the hybrid triblock CART9 and CART11 (the structures were shown in Fig. 5B) exhibited 77%–81% transfection efficiency in Jurkat cells, a human T cell line. In addition, they demonstrated that the NPs formulated with the covalent hybrid fluorescent CART13 (the structure was shown in Fig. 5C) transfected luciferase mRNA to ~1.6% of CD4+ T cells and 1.5% of CD8+ T cells in the spleen of mice [144].
Fig. 5.
The structure of CARTs [144]. (A) The structure of CAR3 and CART7. (B) The structure of CART9 and CART11. (C) The structure of covalent hybrid fluorescent CART13. Adapted with permission from [144]. Copyright (2018) National Academy of Sciences.
6.3. Ligand-modified nanoparticles for mRNA delivery to T cells
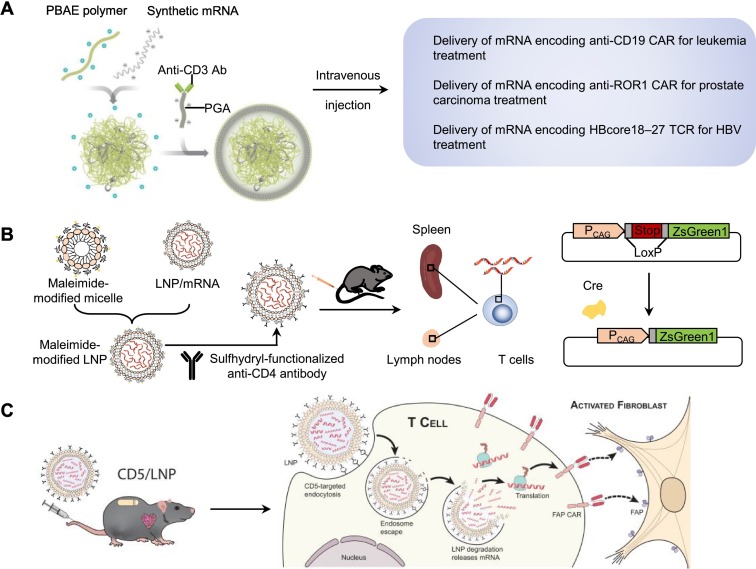
Moffett et al. developed a targeting NP system that could deliver CAR-encoding mRNA to T cells in vivo. The targeting NP system was composed of an anti-CD3 antibody for T cell targeting, a negatively charged coating of polyglutamic acid (PGA) that shielded the NPs to reduce off-target binding, and a PBAE polymer for packing mRNA through its cationic structure (Fig. 6A) [11]. The NPs carrying mRNA encoding tumor-specific CAR or virus-specific TCR were shown to effectively transfect T cells in vivo and induce the regression of tumors and hepatitis B virus (HBV) infection after intravenous injection.
Fig. 6.
Ligand-modified nanoparticles for mRNA delivery to T cells. (A) Anti-CD3 antibody (α-CD3Ab)-modified nanoparticles for the in vivo delivery of mRNA encoding CAR to T cells [11]. Copyright (2020) Springer Nature. (B) Anti-CD4 antibody modified LNP for the in vivo delivery of mRNA to T cells. The delivery efficiency was evaluated in Ai6 mice [145]. Copyright (2021) Elsevier. (C) Anti-CD5 antibody modified LNP encapsulated mRNA encoding FAP-CAR for the treatment of cardiac injury and fibrosis mice [49]. Copyright (2022) The American Association for the Advancement of Science. ROR1, receptor tyrosine kinase-like orphan receptor 1; HBcore18-27, hepatitis B virus (HBV) core antigen, FAPCAR, a CAR designed against fibroblast activation protein (FAP).
Tombácz et al. developed anti-CD4 antibody-modified LNPs (anti-CD4/mRNA-LNPs) for delivering mRNA to CD4+ T cells. Briefly, they firstly prepared the LNPs with ionizable cationic lipid ALC-0307 (proprietary to Acuitas), phosphatidylcholine, cholesterol, and PEG-lipid by a self-assembly process. Then, the LNPs were mixed with micelles composed of DSPE-PEG and DSPE-PEG-maleimide to prepare maleimide-modified LNPs, and the sulfhydryl-functionalized antibody was conjugated to the maleimide-modified LNPs [146]. Due to CD4-mediated endocytosis after antibody binding [145], mice injected with anti-CD4/mRNA-LNPs encapsulated luciferase mRNA demonstrated ~7-fold higher luminescence signal in the spleen when compared to the mice injected with the control IgG-modified LNPs, and the luciferase activity in CD3+ T cells of mice injected with anti-CD4/mRNA-LNPs was 33-fold higher than that of the control IgG-modified LNPs group. In addition, twenty-four hours after intravenously injecting the anti-CD4/mRNA-LNPs carrying mRNA encoding Cre recombinase into the Cre-loxP ZsGreen1 reporter mice, ~50% of CD3+CD8- splenic T cells and 20%-40% of CD3+CD8- T cells in lymph nodes were fluorescent protein ZsGreen1 positive (Fig. 6B).
Recently, Rurik et al. reported anti-CD5 antibody-modified LNPs for the delivery of mRNA encoding a CAR designed against fibroblast activation protein (FAP) (CD5/LNP-FAPCAR) to construct transient antifibrotic CAR-T cells in vivo to eliminate activated fibroblasts for cardiac fibrosis treatment. The anti-CD5 antibody-modified LNPs were prepared by a self-assembly process as the same as work of Tombácz et al. mentioned above. They wisely chose to target CD5 for enhancing the delivery specificity of LNPs to T cells without affecting the T cell effector function. They also evaluated the delivery efficiency and specificity of CD5/LNP encapsulated mRNA encoding Cre recombinase (CD5/LNP-Cre) in the Cre-loxP ZsGreen1 reporter mice. At twenty-four hours after intravenous injection of CD5/LNP-Cre, ZsGreen1 was expressed in 81.1% of splenic CD4+ T cells and in 75.6% of splenic CD8+ T cells, while only 15.0% of CD3– cells expressed ZsGreen1. In addition, 17.5-24.7% FAPCAR+ T cells were found in the spleen of the cardiac injury and fibrosis mice at 48 h after the injection of CD5/LNP-FAPCAR, and the cardiac function of the injured mice was significantly improved at two weeks after the injection of CD5/LNP-FAPCAR (Fig. 6C) [49].
Furthermore, some delivery systems have been reported to deliver siRNA into T cells, though they have not been used for mRNA delivery [147]. These delivery systems are usually modified with T cell-targeting ligands, such as anti-CD7 [83] and anti-CD4 antibodies [82,148]. For example, Keil et al. exploited transferrin as a targeting ligand to deliver GATA3 siRNA to activated T cells by binding to the overexpressed transferrin receptor, which achieved high endocytosis by T cells in a murine asthma model [84].
7. mRNA-based regulation of B cells
B cells play critical roles in the development of many diseases, including multiple sclerosis [149], systemic lupus erythematosus [150], autoimmune rheumatic diseases [54], and central nervous system (CNS) diseases [31] by producing autoantibodies. Thus, regulating the functions of B cells is of great importance for the treatment of these diseases. Delivery of mRNA to B cells can regulate multiple B cells functions, including the antibody production, cytokine secretion, and T cell activation. Researchers have confirmed that B cells can be transfected in vitro and be effectively expanded. However, improving transfection efficiency is usually achieved at the expense of cell viability [91].
7.1. LNPs for mRNA delivery to B cells
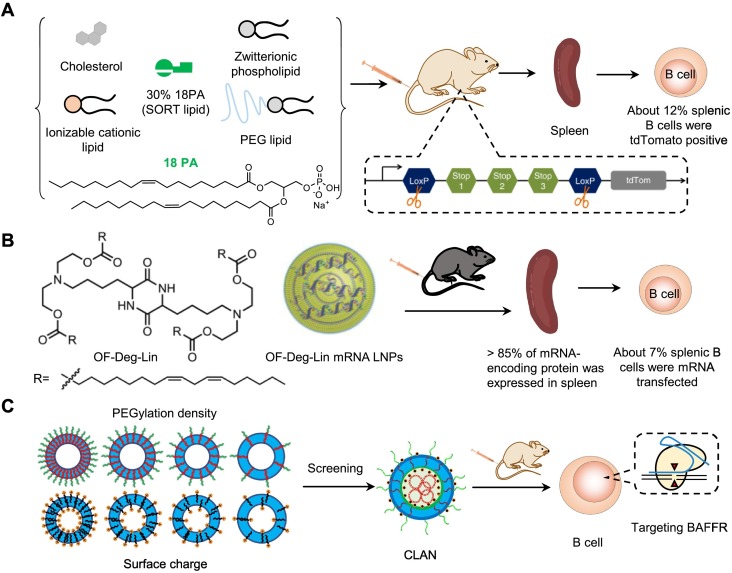
Recently, Siegwart et al. added a supplemental component, negatively charged 1,2-dioleoyl-sn-glycero-3-phosphate (18PA), into traditional LNPs composed of a degradable dendrimer ionizable cationic lipid 5A2-SC8, DOPE, cholesterol and DMG-PEG, and developed selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery [151]. They demonstrated that mixing 30% 18PA into SORT LNPs exhibited excellent spleen-targeting performance (Fig. 7A). Two days after intravenous injection of SORT LNPs formulated with 30% 18PA and Cre-encoding mRNA into the Cre-loxP tdTomato reporter mice at a dose of 0.3 mg kg-1 Cre-encoding mRNA, they detected the tdTomato fluorescent signal predominantly in spleen and liver and the transfection efficiency was found to be ~12% in splenic B cells, ~10% in splenic T cells, and ~20% in splenic macrophages. Their study provided an excellent strategy for improving the selectivity of nanoparticles to the spleen for treating immune disorders. In addition, Anderson et al. developed an ionizable lipid (OF-Deg-Lin) to prepare the LNP delivery system with DOPE, C14-PEG-2000, cholesterol, and mRNA using microfluidics. The surface pKa of OF-Deg-Lin mRNA LNPs is 5.7. Their results proved that the OF-Deg-Lin LNPs could navigate to the spleen and effectively transfect B cells (Fig. 7B) [152]. In C57BL/6 mice injected intravenously with the OF-Deg-Lin LNPs carrying mRNA encoding firefly luciferase (OF-Deg-Lin Fluc-mRNA LNPs) at a 0.75 mg kg-1 dose, more than 85% of the expressed luciferase was measured in the spleen by average radiance at 6 h post-injection. In addition, about 7% of splenic B cells were Cy5 positive at 1 hour after the intravenous injection of OF-Deg-Lin Cy5-mRNA at a 0.75 mg kg-1 dose.
Fig. 7.
Nucleic acid nanocarrier for regulating B cell functions. (A) Screening 18PA (one screened SORT lipids) to prepare LNP for mRNA delivery into B cells. Adapted with permission from [151]. Copyright (2020) Springer Nature. (B) OF-Deg-Lin-based LNPs for delivering mRNA to splenic B cells. Adapted with permission from [152]. Copyright (2017) John Wiley and Sons. (C) Screening the best formulation of CLANs for delivering CRISPR-Cas9 plasmid to B cells [55].
7.2. Polymer nanoparticles for mRNA delivery to B cells
In addition to LNPs, other nucleic acid delivery systems targeting B cells also have the ability for delivering mRNA into B cells. For example, our group developed a PEG-b-PLA or PEG-b-PLGA-based CLAN to deliver nucleic acids, including siRNA and plasmids. To optimize the CLAN for targeted delivery to B cells, we created a library of CLANs in which each CLAN has different surface PEG density and Zeta potential by changing the formulation of PEG-b-PLGA or cationic lipids. Thereafter, we screened an optimal CLAN for the in vivo delivery of the CRISPR-Cas9 plasmid to B cells [55] (Fig. 7C). We proved that the screened CLAN could efficiently deliver the CRISPR-Cas9 plasmid into B cells in vivo, which exerted a therapeutic effect in mouse rheumatoid arthritis by knocking out the BAFFR of B cells.
8. Conclusions and future perspectives
mRNA-based therapy has shown great promises for vaccination and disease treatment. LNP-based mRNA vaccines have demonstrated striking antiviral effects in the prevention of COVID-19 [93,94]. In addition, delivery of antigen-encoding mRNA to DCs has shown to be effective in the prevention of infectious diseases and cancers [102]. mRNA-based therapy is expected to be promising in effectively regulating the functions of immune cells and treating immune-related diseases.
This review discussed the challenges for delivering mRNA into immune cells: 1) how to achieve immune cell-specific delivery and 2) how to realize efficient translation of mRNA into protein. For the challenge of cell-specific delivery, efforts are mainly optimizing the chemical structure of the components of NPs and modifying them with targeting modules. Regarding the translation of mRNA, current methods mainly include: 1) using cationic materials to assist NPs escape from endosomes, and 2) controlling the mRNA immunogenicity through chemical modification and the co-delivery of immunosuppressive agents.
For immune cell-targeted mRNA delivery, both enhancing the on-target efficiency and reducing off-target efficiency are of great importance. Although LNPs have been reported to deliver mRNA to different cells, the properties and composition of these LNPs varies due to different target cells (Table 4 ). For instance, previous works have proved that the targeting ability of LNPs can be affected by the surface charge [153], the structure of lipids [143,144] and the structure of cholesterol [138]. The properties and composition of LNPs can affect their pKa, leading to the difference of serum protein absorption. The proteins absorbed on LNPs increase the interaction with receptors expressed in target tissues, thereby realizing tissue-specific delivery. Using chemically conjugated antibodies on the surface of NPs can also efficiently reduce off-target effects [9,11,115,154]. In addition, engineered cell membranes that express specific targeting ligands can also be used to modify NPs for targeted delivery [155]. For example, ligand-modified engineered cell-membrane-derived vesicles have been used as mRNA delivery systems for targeted delivery to Ly6C+ inflammatory monocytes [156]. Furthermore, barcoded mRNA-based high-throughput in vivo screening platforms can be used for developing mRNA delivery systems [157,158]. For T, B and NK cells with weak phagocytosis, virus-mimicking membrane fusion is a potential strategy for mRNA delivery. Cell membranes engineered with viral fusion protein such as the hemagglutinin protein of influenza A virus can be used to coat mRNA-loaded nanoparticles to facilitate nanoparticles enter target cells via cell membrane fusion [159].
Table 4.
The composition of LNPs for mRNA delivery to different immune cells.
| Target cells | Composition of LNPs | Reference |
|---|---|---|
| B cells | Degradable dendrimer ionizable cationic lipid 5A2-SC8; DOPE; DMG-PEG; Cholesterol; Additional component 1,2-dioleoyl-sn-glycero-3-phosphate (18PA). | [151] |
| Synthetic ionizable lipid (OF-Deg-Lin); DOPE; C14-PEG-2000; Cholesterol. | [152] | |
| T cells | Synthetic ionizable C14-4 lipid; DOPE; C14-PEG; Cholesterol. | [142] |
| Synthetic lipid-like molecule with imidazole head (93-O17S); DOPE; DSPE-PEG; Cholesterol. | [143] | |
| Ionizable cationic lipid ALC-0307 (proprietary to Acuitas); Phosphatidylcholine; PEG-lipid; Cholesterol; DSPE-PEG; DSPE-PEG-maleimide. | [49,145] | |
| Macrophages | Ionizable lipid cKK-E12; DOPE; C18-PEG2K; Synthetic 20α-hydroxycholesterol (20α-OH)-modified cholesterol. | [138] |
| Ionizable lipid (cKK-E15); Synthetic phospholipids with a constrained adamantyl group; Lipid-PEG(C14PEG2000); Cholesterol (cholesterol, 20α-hydroxycholesterol, β-sitosterol). | [139] |
In summary, LNP-based COVID-19 mRNA vaccines has opened the floodgates to develop mRNA therapy for regulating the functions of different immune cells, including DCs, macrophages, T cells, and B cells, which have great potential in preventing or treating infectious diseases, cancers, hereditary diseases, and cardiovascular diseases. Although many efforts have been made, a lot of problems remain to be solved, particularly in enhancing the delivery of mRNA to hard-transfected T and NK cells.
CRediT authorship contribution statement
Jia Shi: Investigation, Writing – original draft, Writing - review & editing. Meng-Wen Huang: Investigation, Writing – original draft. Zi-Dong Lu: Writing – review & editing, Funding acquisition. Xiao-Jiao Du: Investigation, Funding acquisition. Song Shen: Investigation, Funding acquisition. Cong-Fei Xu: Conceptualization, Supervision, Writing – original draft, Writing - review & editing, Funding acquisition. Jun Wang: Conceptualization, Supervision, Writing – original draft, Writing - review & editing, Funding acquisition.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (52130301, 82072048, 81901875, 32071380, and 32071378), Guangdong Provincial Pearl River Talents Program (2017GC010713 and 2017GC010482), the Science and Technology Program of Guangzhou, China (202103030004), and the Fundamental Research Funds for the Central Universities.
References
- 1.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., C.S. Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas S.J., Moreira E.D., Jr., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G. Perez, Polack F.P., Zerbini C., Bailey R., Swanson K.A., Xu X., Roychoudhury S., Koury K., Bouguermouh S., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Tureci O., Nell H., Schaefer A., Unal S., Yang Q., Liberator P., Tresnan D.B., Mather S., Dormitzer P.R., Sahin U., Gruber W.C., Jansen K.U., C.C.T. Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oberhardt V., Luxenburger H., Kemming J., Schulien I., Ciminski K., Giese S., Csernalabics B., Lang-Meli J., Janowska I., Staniek J., Wild K., Basho K., Marinescu M.S., Fuchs J., Topfstedt F., Janda A., Sogukpinar O., Hilger H., Stete K., Emmerich F., Bengsch B., Waller C.F., Sagar S. Rieg, Boettler T., Zoldan K., Kochs G., Schwemmle M., Rizzi M., Thimme R., Neumann-Haefelin C., Hofmann M. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature. 2021;597:268–273. doi: 10.1038/s41586-021-03841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., Pascal K.E., Maurus D., Brachtendorf S., Lorks V., Sikorski J., Koch P., Hilker R., Becker D., Eller A.K., Grutzner J., Tonigold M., Boesler C., Rosenbaum C., Heesen L., Kuhnle M.C., Poran A., Dong J.Z., Luxemburger U., Kemmer-Bruck A., Langer D., Bexon M., Bolte S., Palanche T., Schultz A., Baumann S., Mahiny A.J., Boros G., Reinholz J., Szabo G.T., Kariko K., Shi P.Y., Fontes-Garfias C., Perez J.L., Cutler M., Cooper D., Kyratsous C.A., Dormitzer P.R., Jansen K.U., Tureci O. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary N., Weissman D., Whitehead K.A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021;20:817–838. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck J.D., Reidenbach D., Salomon N., Sahin U., Tureci O., Vormehr M., Kranz L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer. 2021;20:69. doi: 10.1186/s12943-021-01348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F., Parayath N.N., Ene C.I., Stephan S.B., Koehne A.L., Coon M.E., Holland E.C., Stephan M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019;10:3974. doi: 10.1038/s41467-019-11911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moffett H.F., Coon M.E., Radtke S., Stephan S.B., McKnight L., Lambert A., Stoddard B.L., Kiem H.P., Stephan M.T. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nat. Commun. 2017;8:389. doi: 10.1038/s41467-017-00505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parayath N.N., Stephan S.B., Koehne A.L., Nelson P.S., Stephan M.T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 2020;11:6080. doi: 10.1038/s41467-020-19486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Shen S., Zhao G., Xu C.F., Zhang H.B., Luo Y.L., Cao Z.T., Shi J., Zhao Z.B., Lian Z.X., Wang J. In situ repurposing of dendritic cells with CRISPR/Cas9-based nanomedicine to induce transplant tolerance. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119302. [DOI] [PubMed] [Google Scholar]
- 13.Xu C., Lu Z., Luo Y., Liu Y., Cao Z., Shen S., Li H., Liu J., Chen K., Chen Z., Yang X., Gu Z., Wang J. Targeting of NLRP3 inflammasome with gene editing for the amelioration of inflammatory diseases. Nat. Commun. 2018;9:4092. doi: 10.1038/s41467-018-06522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu M., Glass Z., Chen J., Haas M., Jin X., Zhao X., Rui X., Ye Z., Li Y., Zhang F., Xu Q. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2020401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berdien B., Mock U., Atanackovic D., Fehse B. TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther. 2014;21:539–548. doi: 10.1038/gt.2014.26. [DOI] [PubMed] [Google Scholar]
- 16.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12:102. doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai Y., Kan S., Zhou S., Wang Y., Xu J., Cooke J.P., Wen J., Deng H. Enhancement of the in vivo persistence and antitumor efficacy of CD19 chimeric antigen receptor T cells through the delivery of modified TERT mRNA. Cell Discov. 2015;1:15040. doi: 10.1038/celldisc.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchida S., Perche F., Pichon C., Cabral H. Nanomedicine-based approaches for mRNA delivery. Mol. Pharm. 2020;17:3654–3684. doi: 10.1021/acs.molpharmaceut.0c00618. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Yu C. Emerging concepts of nanobiotechnology in mRNA delivery. Angew. Chem. Int. Ed. 2020;59:23374–23385. doi: 10.1002/anie.202003545. [DOI] [PubMed] [Google Scholar]
- 20.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trepotec Z., Lichtenegger E., Plank C., Aneja M.K., Rudolph C. Delivery of mRNA therapeutics for the treatment of hepatic diseases. Mol. Ther. 2019;27:794–802. doi: 10.1016/j.ymthe.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Zhang Z., Luo J., Han X., Wei Y., Wei X. mRNA vaccine: a potential therapeutic strategy. Mol. Cancer. 2021;20:33. doi: 10.1186/s12943-021-01311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granot-Matok Y., Kon E., Dammes N., Mechtinger G., Peer D. Therapeutic mRNA delivery to leukocytes. J. Control. Release. 2019;305:165–175. doi: 10.1016/j.jconrel.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Gao M., Zhang Q., Feng X.H., Liu J. Synthetic modified messenger RNA for therapeutic applications. Acta Biomater. 2021;131:1–15. doi: 10.1016/j.actbio.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kauffman K.J., Webber M.J., Anderson D.G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Control. Release. 2016;240:227–234. doi: 10.1016/j.jconrel.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y., Nakamura T., Yamada Y., Harashima H. The nanomedicine rush: New strategies for unmet medical needs based on innovative nano DDS. J. Control. Release. 2021;330:305–316. doi: 10.1016/j.jconrel.2020.12.032. [DOI] [PubMed] [Google Scholar]
- 27.Park K.S., Sun X., Aikins M.E., Moon J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasgupta S., Dasgupta S., Bandyopadhyay M. Regulatory B cells in infection, inflammation, and autoimmunity. Cell. Immunol. 2020;352 doi: 10.1016/j.cellimm.2020.104076. [DOI] [PubMed] [Google Scholar]
- 30.Rawlings D.J., Metzler G., Wray-Dutra M., Jackson S.W. Altered B cell signalling in autoimmunity. Nat. Rev. Immunol. 2017;17:421–436. doi: 10.1038/nri.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatino J.J., Jr., Probstel A.K., Zamvil S.S. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat. Rev. Neurosci. 2019;20:728–745. doi: 10.1038/s41583-019-0233-2. [DOI] [PubMed] [Google Scholar]
- 32.Kumar B.V., Connors T.J., Farber D.L. Human T cell development, localization, and function throughout life. Immunity. 2018;48:202–213. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong M., Clubb J.D., Chen Y.Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38:473–488. doi: 10.1016/j.ccell.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi S., Mikami N., Wing J.B., Tanaka A., Ichiyama K., Ohkura N. Regulatory T cells and human disease. Annu. Rev. Immunol. 2020;38:541–566. doi: 10.1146/annurev-immunol-042718-041717. [DOI] [PubMed] [Google Scholar]
- 35.DeNardo D.G., Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Annu. Rev. Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia Y., Rao L., Yao H., Wang Z., Ning P., Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv. Mater. 2020;32 doi: 10.1002/adma.202002054. [DOI] [PubMed] [Google Scholar]
- 37.Rittig S.M., Haentschel M., Weimer K.J., Heine A., Muller M.R., Brugger W., Horger M.S., Maksimovic O., Stenzl A., Hoerr I., Rammensee H.G., Holderried T.A., Kanz L., Pascolo S., Brossart P. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol. Ther. 2011;19:990–999. doi: 10.1038/mt.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minnaert A.K., Vanluchene H., Verbeke R., Lentacker I., De Smedt S.C., Raemdonck K., Sanders N.N., Remaut K. Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: Getting the message across. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devoldere J., Dewitte H., De Smedt S.C., Remaut K. Evading innate immunity in nonviral mRNA delivery: don't shoot the messenger. Drug Discov. Today. 2016;21:11–25. doi: 10.1016/j.drudis.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Liu X., Pu Y., Cron K., Deng L., Kline J., Frazier W.A., Xu H., Peng H., Fu Y.X., Xu M.M. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat. Med. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parayath N.N., Hao S., Stephan S.B., Koehne A.L., Watson C.E., Stephan M.T. Genetic in situ engineering of myeloid regulatory cells controls inflammation in autoimmunity. J. Control. Release. 2021;339:553–561. doi: 10.1016/j.jconrel.2021.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pomeroy E.J., Hunzeker J.T., Kluesner M.G., Lahr W.S., Smeester B.A., Crosby M.R., Lonetree C.L., Yamamoto K., Bendzick L., Miller J.S., Geller M.A., Walcheck B., Felices M., Webber B.R., Starr T.K., Moriarity B.S. A genetically engineered primary human natural killer cell platform for cancer immunotherapy. Mol. Ther. 2020;28:52–63. doi: 10.1016/j.ymthe.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang X., Potter J., Kumar S., Zou Y., Quintanilla R., Sridharan M., Carte J., Chen W., Roark N., Ranganathan S., Ravinder N., Chesnut J.D. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J. Biotechnol. 2015;208:44–53. doi: 10.1016/j.jbiotec.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 44.Xie G., Dong H., Liang Y., Ham J.D., Rizwan R., Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daher M., Melo Garcia L., Li Y., Rezvani K. CAR-NK cells: The next wave of cellular therapy for cancer. Clin. Transl. Immunol. 2021;10 doi: 10.1002/cti2.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rafiq S., Hackett C.S., Brentjens R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pohl-Guimaraes F., Yang C., Dyson K.A., Wildes T.J., Drake J., Huang J., Flores C., Sayour E.J., Mitchell D.A. RNA-modified T cells mediate effective delivery of immunomodulatory cytokines to brain tumors. Mol. Ther. 2019;27:837–849. doi: 10.1016/j.ymthe.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beatty G.L., O'Hara M.H., Lacey S.F., Torigian D.A., Nazimuddin F., Chen F., Kulikovskaya I.M., Soulen M.C., McGarvey M., Nelson A.M., Gladney W.L., Levine B.L., Melenhorst J.J., Plesa G., June C.H. Activity of Mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a Phase 1 trial. Gastroenterology. 2018;155:29–32. doi: 10.1053/j.gastro.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rurik J.G., Tombacz I., Yadegari A., Mendez Fernandez P.O., Shewale S.V., Li L., Kimura T., Soliman O.Y., Papp T.E., Tam Y.K., Mui B.L., Albelda S.M., Pure E., June C.H., Aghajanian H., Weissman D., Parhiz H., Epstein J.A. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91–96. doi: 10.1126/science.abm0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stadtmauer E.A., Fraietta J.A., Davis M.M., Cohen A.D., Weber K.L., Lancaster E., Mangan P.A., Kulikovskaya I., Gupta M., Chen F., Tian L., Gonzalez V.E., Xu J., Jung I.Y., Melenhorst J.J., Plesa G., Shea J., Matlawski T., Cervini A., Gaymon A.L., Desjardins S., Lamontagne A., Salas-Mckee J., Fesnak A., Siegel D.L., Levine B.L., Jadlowsky J.K., Young R.M., Chew A., Hwang W.T., Hexner E.O., Carreno B.M., Nobles C.L., Bushman F.D., Parker K.R., Qi Y., Satpathy A.T., Chang H.Y., Zhao Y., Lacey S.F., June C.H. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367:976. doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rafiq S., Yeku O.O., Jackson H.J., Purdon T.J., van Leeuwen D.G., Drakes D.J., Song M., Miele M.M., Li Z., Wang P., Yan S., Xiang J., Ma X., Seshan V.E., Hendrickson R.C., Liu C., Brentjens R.J. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018;36:847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin S.J.S., Bloom M.S., Robinson W.H. B cell checkpoints in autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2019;15:303–315. doi: 10.1038/s41584-019-0211-0. [DOI] [PubMed] [Google Scholar]
- 53.Nutt S.L., Taubenheim N., Hasbold J., Corcoran L.M., Hodgkin P.D. The genetic network controlling plasma cell differentiation. Semin. Immunol. 2011;23:341–349. doi: 10.1016/j.smim.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Sun W., Meednu N., Rosenberg A., Rangel-Moreno J., Wang V., Glanzman J., Owen T., Zhou X., Zhang H., Boyce B.F., Anolik J.H., Xing L. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat. Commun. 2018;9:5127. doi: 10.1038/s41467-018-07626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li M., Fan Y.-N., Chen Z.-Y., Luo Y.-L., Wang Y.-C., Lian Z.-X., Xu C.-F., Wang J. Optimized nanoparticle-mediated delivery of CRISPR-Cas9 system for B cell intervention. Nano Res. 2018;11:6270–6282. [Google Scholar]
- 56.Lee D.S.W., Rojas O.L., Gommerman J.L. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat. Rev. Drug Discov. 2021;20:179–199. doi: 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thapa Magar K., Boafo G.F., Li X., Chen Z., He W. Liposome-based delivery of biological drugs. Chin. Chem. Lett. 2022;33:587–596. [Google Scholar]
- 58.Hassett K.J., Higgins J., Woods A., Levy B., Xia Y., Hsiao C.J., Acosta E., Almarsson O., Moore M.J., Brito L.A. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J. Control. Release. 2021;335:237–246. doi: 10.1016/j.jconrel.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 59.Lokugamage M.P., Gan Z., Zurla C., Levin J., Islam F.Z., Kalathoor S., Sato M., Sago C.D., Santangelo P.J., Dahlman J.E. Mild innate immune activation overrides efficient nanoparticle-mediated RNA delivery. Adv. Mater. 2020;32 doi: 10.1002/adma.201904905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verbeke R., Lentacker I., Wayteck L., Breckpot K., Van Bockstal M., Descamps B., Vanhove C., De Smedt S.C., Dewitte H. Co-delivery of nucleoside-modified mRNA and TLR agonists for cancer immunotherapy: Restoring the immunogenicity of immunosilent mRNA. J. Control. Release. 2017;266:287–300. doi: 10.1016/j.jconrel.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 61.Krienke C., Kolb L., Diken E., Streuber M., Kirchhoff S., Bukur T., Akilli-Öztürk Ö., Kranz L.M., Berger H., Petschenka J., Diken M., Kreiter S., Yogev N., Waisman A., Karikó K., Türeci Ö., Sahin U. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–153. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 62.Tsoi K.M., MacParland S.A., Ma X.Z., Spetzler V.N., Echeverri J., Ouyang B., Fadel S.M., Sykes E.A., Goldaracena N., Kaths J.M., Conneely J.B., Alman B.A., Selzner M., Ostrowski M.A., Adeyi O.A., Zilman A., McGilvray I.D., Chan W.C. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016;15:1212–1221. doi: 10.1038/nmat4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mebius R.E., Kraal G. Structure and function of the spleen. Nat. Rev. Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 64.Petros R.A., DeSimone J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Y., Peng Z., Seven E.S., Leblanc R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release. 2018;270:290–303. doi: 10.1016/j.jconrel.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 66.Dilliard S.A., Cheng Q., Siegwart D.J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2109256118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Worbs T., Hammerschmidt S.I., Forster R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017;17:30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 69.Taylor P.R., Martinez-Pomares L., Stacey M., Lin H.H., Brown G.D., Gordon S. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 70.Pustulka S.M., Ling K., Pish S.L., Champion J.A. Protein nanoparticle charge and hydrophobicity govern protein corona and macrophage uptake. ACS Appl. Mater. Interfaces. 2020;12:48284–48295. doi: 10.1021/acsami.0c12341. [DOI] [PubMed] [Google Scholar]
- 71.Maugeri M., Nawaz M., Papadimitriou A., Angerfors A., Camponeschi A., Na M., Holtta M., Skantze P., Johansson S., Sundqvist M., Lindquist J., Kjellman T., Martensson I.L., Jin T., Sunnerhagen P., Ostman S., Lindfors L., Valadi H. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019;10:4333. doi: 10.1038/s41467-019-12275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Habrant D., Peuziat P., Colombani T., Dallet L., Gehin J., Goudeau E., Evrard B., Lambert O., Haudebourg T., Pitard B. Design of ionizable lipids to overcome the limiting step of endosomal escape: Application in the intracellular delivery of mRNA, DNA, and siRNA. J. Med. Chem. 2016;59:3046–3062. doi: 10.1021/acs.jmedchem.5b01679. [DOI] [PubMed] [Google Scholar]
- 73.Savina A., Jancic C., Hugues S., Guermonprez P., Vargas P., Moura I.C., Lennon-Dumenil A.M., Seabra M.C., Raposo G., Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 74.Mantegazza A.R., Savina A., Vermeulen M., Perez L., Geffner J., Hermine O., Rosenzweig S.D., Faure F., Amigorena S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu M.M., Pu Y., Han D., Shi Y., Cao X., Liang H., Chen X., Li X.D., Deng L., Chen Z.J., Weichselbaum R.R., Fu Y.X. Dendritic cells but not macrophages sense tumor mitochondrial DNA for cross-priming through signal regulatory protein alpha signaling. Immunity. 2017;47:363–373. doi: 10.1016/j.immuni.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020;19:200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 77.Fowell D.J., Kim M. The spatio-temporal control of effector T cell migration. Nat. Rev. Immunol. 2021;21:582–596. doi: 10.1038/s41577-021-00507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allman D., Pillai S. Peripheral B cell subsets. Curr. Opin. Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell membrane coating nanotechnology. Adv. Mater. 2018;30 doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu H., Miao Z., Zha Z. Cell membrane-coated nanoparticles for immunotherapy. Chin. Chem. Lett. 2021 doi: 10.1016/j.cclet.2021.10.057. [DOI] [Google Scholar]
- 81.Yong S.B., Song Y., Kim H.J., Ain Q.U., Kim Y.H. Mononuclear phagocytes as a target, not a barrier, for drug delivery. J. Control. Release. 2017;259:53–61. doi: 10.1016/j.jconrel.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 82.Ramishetti S., Kedmi R., Goldsmith M., Leonard F., Sprague A.G., Godin B., Gozin M., Cullis P.R., Dykxhoorn D.M., Peer D. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano. 2015;9:6706–6716. doi: 10.1021/acsnano.5b02796. [DOI] [PubMed] [Google Scholar]
- 83.Kumar P., Ban H.S., Kim S.S., Wu H., Pearson T., Greiner D.L., Laouar A., Yao J., Haridas V., Habiro K., Yang Y.G., Jeong J.H., Lee K.Y., Kim Y.H., Kim S.W., Peipp M., Fey G.H., Manjunath N., Shultz L.D., Lee S.K., Shankar P. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keil T.W.M., Baldassi D., Merkel O.M. T-cell targeted pulmonary siRNA delivery for the treatment of asthma, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020;12 doi: 10.1002/wnan.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.See P., Dutertre C.A., Chen J., Gunther P., McGovern N., Irac S.E., Gunawan M., Beyer M., Handler K., Duan K., Sumatoh H.R.B., Ruffin N., Jouve M., Gea-Mallorqui E., Hennekam R.C.M., Lim T., Yip C.C., Wen M., Malleret B., Low I., Shadan N.B., Fen C.F.S., Tay A., Lum J., Zolezzi F., Larbi A., Poidinger M., Chan J.K.Y., Chen Q., Renia L., Haniffa M., Benaroch P., Schlitzer A., Schultze J.L., Newell E.W., Ginhoux F. Mapping the human DC lineage through the integration of high-dimensional techniques. Science. 2017;356 doi: 10.1126/science.aag3009. eaag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., Pascal K.E., Maurus D., Brachtendorf S., Lorks V., Sikorski J., Koch P., Hilker R., Becker D., Eller A.K., Grutzner J., Tonigold M., Boesler C., Rosenbaum C., Heesen L., Kuhnle M.C., Poran A., Dong J.Z., Luxemburger U., Kemmer-Bruck A., Langer D., Bexon M., Bolte S., Palanche T., Schultz A., Baumann S., Mahiny A.J., Boros G., Reinholz J., Szabo G.T., Kariko K., Shi P.Y., Fontes-Garfias C., Perez J.L., Cutler M., Cooper D., Kyratsous C.A., Dormitzer P.R., Jansen K.U., Tureci O. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 87.Lazzaro S., Giovani C., Mangiavacchi S., Magini D., Maione D., Baudner B., Geall A.J., De Gregorio E., D'Oro U., Buonsanti C. CD8 T-cell priming upon mRNA vaccination is restricted to bone-marrow-derived antigen-presenting cells and may involve antigen transfer from myocytes. Immunology. 2015;146:312–326. doi: 10.1111/imm.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boczkowski D., Nair S.K., Nam J.H., Lyerly H.K., Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60:1028–1034. [PubMed] [Google Scholar]
- 89.Boczkowski D., Nair S.K., Snyder D., Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dannull J., Nair S., Su Z., Boczkowski D., DeBeck C., Yang B., Gilboa E., Vieweg J. Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand. Blood. 2005;105:3206–3213. doi: 10.1182/blood-2004-10-3944. [DOI] [PubMed] [Google Scholar]
- 91.Mullins C.S., Wegner T., Klar E., Classen C.F., Linnebacher M. Optimizing the process of nucleofection for professional antigen presenting cells. BMC Res. 2015;8:472. doi: 10.1186/s13104-015-1446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinon F., Krishnan S., Lenzenoo G., Mag R., Gomard E., Guillet J.-G., LCvy J.-P., Meulien P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993;23:1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 93.Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M., Kranz L.M., Walzer K.C., Hein S., Guler A., Loschko J., Maddur M.S., Ota-Setlik A., Tompkins K., Cole J., Lui B.G., Ziegenhals T., Plaschke A., Eisel D., Dany S.C., Fesser S., Erbar S., Bates F., Schneider D., Jesionek B., Sanger B., Wallisch A.K., Feuchter Y., Junginger H., Krumm S.A., Heinen A.P., Adams-Quack P., Schlereth J., Schille S., Kroner C., de la Caridad Guimil Garcia R., Hiller T., Fischer L., Sellers R.S., Choudhary S., Gonzalez O., Vascotto F., Gutman M.R., Fontenot J.A., Hall-Ursone S., Brasky K., Griffor M.C., Han S., Su A.A.H., Lees J.A., Nedoma N.L., Mashalidis E.H., Sahasrabudhe P.V., Tan C.Y., Pavliakova D., Singh G., Fontes-Garfias C., Pride M., Scully I.L., Ciolino T., Obregon J., Gazi M., Carrion R., Jr., Alfson K.J., Kalina W.V., Kaushal D., Shi P.Y., Klamp T., Rosenbaum C., Kuhn A.N., Tureci O., Dormitzer P.R., Jansen K.U., Sahin U. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592:283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 94.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schafer A., Ziwawo C.T., DiPiazza A.T., Dinnon K.H., Elbashir S.M., Shaw C.A., Woods A., Fritch E.J., Martinez D.R., Bock K.W., Minai M., Nagata B.M., Hutchinson G.B., Wu K., Henry C., Bahl K., Garcia-Dominguez D., Ma L., Renzi I., Kong W.P., Schmidt S.D., Wang L., Zhang Y., Phung E., Chang L.A., Loomis R.J., Altaras N.E., Narayanan E., Metkar M., Presnyak V., Liu C., Louder M.K., Shi W., Leung K., Yang E.S., West A., Gully K.L., Stevens L.J., Wang N., Wrapp D., Doria-Rose N.A., Stewart-Jones G., Bennett H., Alvarado G.S., Nason M.C., Ruckwardt T.J., McLellan J.S., Denison M.R., Chappell J.D., Moore I.N., Morabito K.M., Mascola J.R., Baric R.S., Carfi A., Graham B.S. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rauch S., Roth N., Schwendt K., Fotin-Mleczek M., Mueller S.O., Petsch B. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. NPJ Vaccines. 2021;6:57. doi: 10.1038/s41541-021-00311-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang N.N., Li X.F., Deng Y.Q., Zhao H., Huang Y.J., Yang G., Huang W.J., Gao P., Zhou C., Zhang R.R., Guo Y., Sun S.H., Fan H., Zu S.L., Chen Q., He Q., Cao T.S., Huang X.Y., Qiu H.Y., Nie J.H., Jiang Y., Yan H.Y., Ye Q., Zhong X., Xue X.L., Zha Z.Y., Zhou D., Yang X., Wang Y.C., Ying B., Qin C.F. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182:1271–1283. doi: 10.1016/j.cell.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Alwis R., Gan E.S., Chen S., Leong Y.S., Tan H.C., Zhang S.L., Yau C., Low J.G.H., Kalimuddin S., Matsuda D., Allen E.C., Hartman P., Park K.J., Alayyoubi M., Bhaskaran H., Dukanovic A., Bao Y., Clemente B., Vega J., Roberts S., Gonzalez J.A., Sablad M., Yelin R., Taylor W., Tachikawa K., Parker S., Karmali P., Davis J., Sullivan B.M., Sullivan S.M., Hughes S.G., Chivukula P., Ooi E.E. A single dose of self-transcribing and replicating RNA-based SARS-CoV-2 vaccine produces protective adaptive immunity in mice. Mol. Ther. 2021;29:1970–1983. doi: 10.1016/j.ymthe.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McKay P.F., Hu K., Blakney A.K., Samnuan K., Brown J.C., Penn R., Zhou J., Bouton C.R., Rogers P., Polra K., Lin P.J.C., Barbosa C., Tam Y.K., Barclay W.S., Shattock R.J. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020;11:3523. doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J., Budylowski P., Samson R., Griffin B.D., Babuadze G., Rathod B., Colwill K., Abioye J.A., Schwartz J.A., Law R., Yip L., Ahn S.K., Chau S., Naghibosadat M., Arita Y., Hu Q., Yue F.Y., Banerjee A., Mossman K., Mubareka S., Kozak R.A., Pollanen M.S., Orozco N.M., Gingras A.-C., Marcusson E.G., Ostrowski M.A. Preclinical evaluation of a SARS-CoV-2 mRNA vaccine PTX-COVID19-B. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abj9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalnin K.V., Plitnik T., Kishko M., Zhang J., Zhang D., Beauvais A., Anosova N.G., Tibbitts T., DiNapoli J., Ulinski G., Piepenhagen P., Cummings S.M., Bangari D.S., Ryan S., Huang P.D., Huleatt J., Vincent D., Fries K., Karve S., Goldman R., Gopani H., Dias A., Tran K., Zacharia M., Gu X., Boeglin L., Abysalh J., Vargas J., Beaulieu A., Shah M., Jeannotte T., Gillis K., Chivukula S., Swearingen R., Landolfi V., Fu T.M., DeRosa F., Casimiro D. Immunogenicity and efficacy of mRNA COVID-19 vaccine MRT5500 in preclinical animal models. NPJ Vaccines. 2021;6:61. doi: 10.1038/s41541-021-00324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feldman R.A., Fuhr R., Smolenov I., Mick Ribeiro A., Panther L., Watson M., Senn J.J., Smith M., Almarsson H.S., Pujar M.E., Laska J., Thompson T., Zaks G. Ciaramella, mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326–3334. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 102.Jacobson J.M., Routy J.-P., Welles S., DeBenedette M., Tcherepanova I., Angel J.B., Asmuth D.M., Stein D.K., Baril J.-G., McKellar M., Margolis D.M., Trottier B., Wood K., Nicolette C. Dendritic cell immunotherapy for HIV-1 infection using autologous HIV-1 RNA. J. Acquir. Immune Defic. Syndr. 2016;72:31–38. doi: 10.1097/QAI.0000000000000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hewitt S.L., Bailey D., Zielinski J., Apte A., Musenge F., Karp R., Burke S., Garcon F., Mishra A., Gurumurthy S., Watkins A., Arnold K., Moynihan J., Clancy-Thompson E., Mulgrew K., Adjei G., Deschler K., Potz D., Moody G., Leinster D.A., Novick S., Sulikowski M., Bagnall C., Martin P., Lapointe J.M., Si H., Morehouse C., Sedic M., Wilkinson R.W., Herbst R., Frederick J.P., Luheshi N. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin. Cancer Res. 2020;26:6284–6298. doi: 10.1158/1078-0432.CCR-20-0472. [DOI] [PubMed] [Google Scholar]
- 104.Jiang L., Park J.-S., Yin L., Laureano R., Jacquinet E., Yang J., Liang S., Frassetto A., Zhuo J., Yan X., Zhu X., Fortucci S., Hoar K., Mihai C., Tunkey C., Presnyak V., Benenato K.E., Lukacs C.M., Martini P.G.V., Guey L.T. Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic acidemia. Nat. Commun. 2020;11:5339. doi: 10.1038/s41467-020-19156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K., Sah D.W., Stebbing D., Crosley E.J., Yaworski E., Hafez I.M., Dorkin J.R., Qin J., Lam K., Rajeev K.G., Wong K.F., Jeffs L.B., Nechev L., Eisenhardt M.L., Jayaraman M., Kazem M., Maier M.A., Srinivasulu M., Weinstein M.J., Chen Q., Alvarez R., Barros S.A., De S., Klimuk S.K., Borland T., Kosovrasti V., Cantley W.L., Tam Y.K., Manoharan M., Ciufolini M.A., Tracy M.A., de Fougerolles A., MacLachlan I., Cullis P.R., Madden T.D., Hope M.J. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 106.Barros S.A., Gollob J.A. Safety profile of RNAi nanomedicines. Adv. Drug Deliv. Rev. 2012;64:1730–1737. doi: 10.1016/j.addr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 107.Samaridou E., Heyes J., Lutwyche P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020;154:37–63. doi: 10.1016/j.addr.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 108.Kauffman K.J., Dorkin J.R., Yang J.H., Heartlein M.W., DeRosa F., Mir F.F., Fenton O.S., Anderson D.G. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15:7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 109.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck, Jr. R.W., Hammitt L.L., Tureci O., Nell H., Schaefer A., Unal S., Tresnan D.B., Mather S., Dormitzer P.R., Sahin U., Jansen K.U., Gruber W.C., Clinical Trial Group C4591001. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sahin U., Oehm P., Derhovanessian E., Jabulowsky R.A., Vormehr M., Gold M., Maurus D., Schwarck-Kokarakis D., Kuhn A.N., Omokoko T., Kranz L.M., Diken M., Kreiter S., Haas H., Attig S., Rae R., Cuk K., Kemmer-Bruck A., Breitkreuz A., Tolliver C., Caspar J., Quinkhardt J., Hebich L., Stein M., Hohberger A., Vogler I., Liebig I., Renken S., Sikorski J., Leierer M., Muller V., Mitzel-Rink H., Miederer M., Huber C., Grabbe S., Utikal J., Pinter A., Kaufmann R., Hassel J.C., Loquai C., Tureci O. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 111.Fotin-Mleczek M., Zanzinger K., Heidenreich R., Lorenz C., Thess A., Duchardt K.M., Kallen K.J. Highly potent mRNA based cancer vaccines represent an attractive platform for combination therapies supporting an improved therapeutic effect. J. Gene Med. 2012;14:428–439. doi: 10.1002/jgm.2605. [DOI] [PubMed] [Google Scholar]
- 112.Aldrich C., Leroux-Roels I., Huang K.B., Bica M.A., Loeliger E., Schoenborn-Kellenberger O., Walz L., Leroux-Roels G., von Sonnenburg F., Oostvogels L. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine. 2021;39:1310–1318. doi: 10.1016/j.vaccine.2020.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reinhard K., Rengstl B., Oehm P., Michel K., Billmeier A., Hayduk N., Klein O., Kuna K., Ouchan Y., Woll S., Christ E., Weber D., Suchan M., Bukur T., Birtel M., Jahndel V., Mroz K., Hobohm K., Kranz L., Diken M., Kuhlcke K., Tureci O., Sahin U. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367:446–453. doi: 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- 114.Zhang H., You X., Wang X., Cui L., Wang Z., Xu F., Li M., Yang Z., Liu J., Huang P., Kang Y., Wu J., Xia X. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2005191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Katakowski J.A., Mukherjee G., Wilner S.E., Maier K.E., Harrison M.T., DiLorenzo T.P., Levy M., Palliser D. Delivery of siRNAs to dendritic cells using DEC205-targeted lipid nanoparticles to inhibit immune responses. Mol. Ther. 2016;24:146–155. doi: 10.1038/mt.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goswami R., Chatzikleanthous D., Lou G., Giusti F., Bonci A., Taccone M., Brazzoli M., Gallorini S., Ferlenghi I., Berti F., O'Hagan D.T., Pergola C., Baudner B.C., Adamo R. Mannosylation of LNP results in improved potency for self-amplifying RNA (SAM) vaccines. ACS Infect. Dis. 2019;5:1546–1558. doi: 10.1021/acsinfecdis.9b00084. [DOI] [PubMed] [Google Scholar]
- 117.Cheng Z., Zaki A.A., Hui J.Z., Muzykantov V.R., Tsourkas A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Salvati A., Pitek A.S., Monopoli M.P., Prapainop K., Bombelli F.B., Hristov D.R., Kelly P.M., Aberg C., Mahon E., Dawson K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 119.Kubler H., Scheel B., Gnad-Vogt U., Miller K., Schultze-Seemann W., Vom Dorp F., Parmiani G., Hampel C., Wedel S., Trojan L., Jocham D., Maurer T., Rippin G., Fotin-Mleczek M., von der Mulbe F., Probst J., Hoerr I., Kallen K.J., Lander T., Stenzl A. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J. Immunother. Cancer. 2015;3:26. doi: 10.1186/s40425-015-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stitz L., Vogel A., Schnee M., Voss D., Rauch S., Mutzke T., Ketterer T., Kramps T., Petsch B. A thermostable messenger RNA based vaccine against rabies. PLoS Neglect. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., Gottardo R., Bica M.A., Garofano A., Koch S.D., Fotin-Mleczek M., Hoerr I., Clemens R., von Sonnenburg F. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 122.Chahala J.S., Khanb O.F., Cooperc C.L., McPartlana J.S., Tsosieb J.K., Tilleya L.D., Sidika S.M., Louridoa S., Langerb R., Bavaric S., Ploegha H.L., Anderson D.G. Correction for Chahal et al., Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. U. S. A. 2016;113:e4133–e4142. doi: 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li M., Zhao M.N., Fu Y., Li Y., Gong T., Zhang Z.R., Sun X. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J. Control. Release. 2016;228:9–19. doi: 10.1016/j.jconrel.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 124.Zhao M.N., Li M., Zhang Z.R., Gong T., Sun X. Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA. Drug Deliv. 2016;23:2596–2607. doi: 10.3109/10717544.2015.1038856. [DOI] [PubMed] [Google Scholar]
- 125.McKinlay C.J., Vargas J.R., Blake T.R., Hardy J.W., Kanada M., Contag C.H., Wender P.A., Waymouth R.M. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc. Natl. Acad. Sci. U. S. A. 2017;114:e448–e456. doi: 10.1073/pnas.1614193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fan Y.N., Li M., Luo Y.L., Chen Q., Wang L., Zhang H.B., Shen S., Gu Z., Wang J. Cationic lipid-assisted nanoparticles for delivery of mRNA cancer vaccine. Biomater. Sci. 2018;6:3009–3018. doi: 10.1039/c8bm00908b. [DOI] [PubMed] [Google Scholar]
- 127.Haabeth O.A.W., Blake T.R., McKinlay C.J., Waymouth R.M., Wender P.A., Levy R. mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice. Proc. Natl. Acad. Sci. U. S. A. 2018;115:e9153–e9161. doi: 10.1073/pnas.1810002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Orecchioni M., Ghosheh Y., Pramod A.B., Ley K. Corrigendum: Macrophage polarization: Different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front. Immunol. 2020;11:234. doi: 10.3389/fimmu.2020.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mantovani A., Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr. Opin. Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 131.Sylvestre M., Crane C.A., Pun S.H. Progress on modulating tumor-associated macrophages with biomaterials. Adv. Mater. 2020;32 doi: 10.1002/adma.201902007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bonnardel J., T’Jonck W., Gaublomme D., Browaeys R., Scott C.L., Martens L., Vanneste B., De Prijck S., Nedospasov S.A., Kremer A., Van Hamme E., Borghgraef P., Toussaint W., De Bleser P., Mannaerts I., Beschin A., van Grunsven L.A., Lambrecht B.N., Taghon T., Lippens S., Elewaut D., Saeys Y., Guilliams M. Stellate cells, hepatocytes, and endothelial cells imprint the kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity. 2019;51:638–654. doi: 10.1016/j.immuni.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tavares A.J., Poon W., Zhang Y.N., Dai Q., Besla R., Ding D., Ouyang B., Li A., Chen J., Zheng G., Robbins C., Chan W.C.W. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc. Natl. Acad. Sci. U. S. A. 2017;114:e10871–e10880. doi: 10.1073/pnas.1713390114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cullis P.R., Hope M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017;25:1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Akinc A., Querbes W., De S., Qin J., Frank-Kamenetsky M., Jayaprakash K.N., Jayaraman M., Rajeev K.G., Cantley W.L., Dorkin J.R., Butler J.S., Qin L., Racie T., Sprague A., Fava E., Zeigerer A., Hope M.J., Zerial M., Sah D.W., Fitzgerald K., Tracy M.A., Manoharan M., Koteliansky V., Fougerolles A., Maier M.A. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Conway A., Mendel M., Kim K., McGovern K., Boyko A., Zhang L., Miller J.C., DeKelver R.C., Paschon D.E., Mui B.L., Lin P.J.C., Tam Y.K., Barbosa C., Redelmeier T., Holmes M.C., Lee G. Non-viral delivery of zinc finger nuclease mRNA enables highly efficient in vivo genome editing of multiple therapeutic gene targets. Mol. Ther. 2019;27:866–877. doi: 10.1016/j.ymthe.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paunovska K., Gil C.J., Lokugamage M.P., Sago C.D., Sato M., Lando G.N., Gamboa Castro M., Bryksin A.V., Dahlman J.E. Analyzing 2000 in vivo drug delivery data points reveals cholesterol structure impacts nanoparticle delivery. ACS Nano. 2018;12:8341–8349. doi: 10.1021/acsnano.8b03640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paunovska K., Da Silva Sanchez A.J., Sago C.D., Gan Z., Lokugamage M.P., Islam F.Z., Kalathoor S., Krupczak B.R., Dahlman J.E. Nanoparticles containing oxidized cholesterol deliver mRNA to the liver microenvironment at clinically relevant doses. Adv. Mater. 2019;31 doi: 10.1002/adma.201807748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gan Z., Lokugamage M.P., Hatit M.Z.C., Loughrey D., Paunovska K., Sato M., Cristian A., Dahlman J.E. Nanoparticles containing constrained phospholipids deliver mRNA to liver immune cells in vivo without targeting ligands. Bioeng. Transl. Med. 2020;5 doi: 10.1002/btm2.10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garrido F., Ruiz-Cabello F., Aptsiauri N. Rejection versus escape: the tumor MHC dilemma. Cancer Immunol. Immunother. 2017;66:259–271. doi: 10.1007/s00262-016-1947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levine B.L., Miskin J., Wonnacott K., Keir C. Global manufacturing of CAR T cell therapy. Mol. Ther. Methods Clin. Dev. 2017;4:92–101. doi: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Billingsley M.M., Singh N., Ravikumar P., Zhang R., June C.H., Mitchell M.J. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–1589. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao X., Chen J., Qiu M., Li Y., Glass Z., Xu Q. Imidazole-based synthetic lipidoids for in vivo mRNA delivery into primary T lymphocytes. Angew. Chem. Int. Ed. 2020;59:20083–20089. doi: 10.1002/anie.202008082. [DOI] [PubMed] [Google Scholar]
- 144.McKinlay C.J., Benner N.L., Haabeth O.A., Waymouth R.M., Wender P.A. Enhzanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc. Natl. Acad. Sci. U. S. A. 2018;115:e5859–e5866. doi: 10.1073/pnas.1805358115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tombacz I., Laczko D., Shahnawaz H., Muramatsu H., Natesan A., Yadegari A., Papp T.E., Alameh M.G., Shuvaev V., Mui B.L., Tam Y.K., Muzykantov V., Pardi N., Weissman D., Parhiz H. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol. Ther. 2021;29:3293–3304. doi: 10.1016/j.ymthe.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ishida T., Iden D.L., Allen T.M. A combinatorial approach to producing sterically stabilized (Stealth) immunoliposomal drugs. FEBS Lett. 1999;460:129–133. doi: 10.1016/s0014-5793(99)01320-4. [DOI] [PubMed] [Google Scholar]
- 147.Li S.Y., Liu Y., Xu C.F., Shen S., Sun R., Du X.J., Xia J.X., Zhu Y.H., Wang J. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J. Control. Release. 2016;231:17–28. doi: 10.1016/j.jconrel.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 148.Kedmi R., Veiga N., Ramishetti S., Goldsmith M., Rosenblum D., Dammes N., Hazan-Halevy I., Nahary L., Leviatan-Ben-Arye S., Harlev M., Behlke M., Benhar I., Lieberman J., Peer D. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 2018;13:214–219. doi: 10.1038/s41565-017-0043-5. [DOI] [PubMed] [Google Scholar]
- 149.Matsushita T., Kobayashi T., Mizumaki K., Kano M., Sawada T., Tennichi M., Okamura A., Hamaguchi Y., Iwakura Y., Hasegawa M., Fujimoto M., Takehara K. BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci. Adv. 2018;4:eaas9944. doi: 10.1126/sciadv.aas9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sciascia S., Rubini E., Radin M., Cecchi I., Rossi D., Roccatello D. Anticardiolipin and anti-beta 2 glycoprotein-I antibodies disappearance in patients with systemic lupus erythematosus and antiphospholipid syndrome while on belimumab. Ann. Rheum. Dis. 2018;77:1694–1695. doi: 10.1136/annrheumdis-2018-213496. [DOI] [PubMed] [Google Scholar]
- 151.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020;15:313. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fenton O.S., Kauffman K.J., Kaczmarek J.C., McClellan R.L., Jhunjhunwala S., Tibbitt M.W., Zeng M.D., Appel E.A., Dorkin J.R., Mir F.F., Yang J.H., Oberli M.A., Heartlein M.W., DeRosa F., Langer R., Anderson D.G. Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv. Mater. 2017;29 doi: 10.1002/adma.201606944. [DOI] [PubMed] [Google Scholar]
- 153.Caffrey L.M., deRonde B.M., Minter L.M., Tew G.N. Mapping optimal charge density and length of ROMP-based PTDMs for siRNA internalization. Biomacromolecules. 2016;17:3205–3212. doi: 10.1021/acs.biomac.6b00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Song Y., Huang Y., Zhou F., Ding J., Zhou W. Macrophage-targeted nanomedicine for chronic diseases immunotherapy. Chin. Chem. Lett. 2022;33:597–612. [Google Scholar]
- 155.Liu X., Zhong X., Li C. Challenges in cell membrane-camouflaged drug delivery systems: Development strategies and future prospects. Chin. Chem. Lett. 2021;32:2347–2358. [Google Scholar]
- 156.Veiga N., Goldsmith M., Granot Y., Rosenblum D., Dammes N., Kedmi R., Ramishetti S., Peer D. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 2018;9:4493. doi: 10.1038/s41467-018-06936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Correction for Sago, et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc. Natl. Acad. Sci. U. S. A. 2018;115 doi: 10.1073/pnas.1811276115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guimaraes P.P.G., Zhang R., Spektor R., Tan M., Chung A., Billingsley M.M., El-Mayta R., Riley R.S., Wang L., Wilson J.M., Mitchell M.J. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J. Control. Release. 2019;316:404–417. doi: 10.1016/j.jconrel.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park J.H., Mohapatra A., Zhou J., Holay M., Krishnan N., Gao W., Fang R.H., Zhang L. Virus-mimicking cell membrane-coated nanoparticles for cytosolic delivery of mRNA. Angew. Chem. Int. Ed. Eng. 2022;61 doi: 10.1002/anie.202113671. [DOI] [PMC free article] [PubMed] [Google Scholar]