Abstract
Patients exhibit good tolerance to messenger ribonucleic acid (mRNA) vaccines, and the choice of encoded molecules is flexible and diverse. These vaccines can be engineered to express full-length antigens containing multiple epitopes without major histocompatibility complex (MHC) restriction, are relatively easy to control and can be rapidly mass produced. In 2021, the U.S. Food and Drug Administration (FDA) approved the first mRNA-based coronavirus disease 2019 (COVID-19) vaccine produced by Pfizer and BioNTech, which has generated enthusiasm for mRNA vaccine research and development. Based on the above characteristics and the development of mRNA vaccines, mRNA cancer vaccines have become a research hotspot and have undergone rapid development, especially in the last five years. This review analyzes the advances in mRNA cancer vaccines from various perspectives, including the selection and expression of antigens/targets, the application of vectors and adjuvants, different administration routes, and preclinical evaluation, to reflect the trends and challenges associated with these vaccines.
Key words: mRNA, Cancer vaccine, Tumor-associated antigens, Neoantigens, mRNA delivery vectors, Adjuvants, Administration routes
Graphical abstract
This review analyzes the advances in mRNA cancer vaccines from various perspectives, including the selection and expression of antigens/targets, the application of vectors and adjuvants, administration routes, and preclinical evaluation.
1. mRNA vaccines and mRNA cancer vaccines
In 1961, Brenner et al.1 first discovered mRNA, which is a key intermediate molecule necessary for expressing genes as proteins and contains codon information corresponding to amino acids (basic units of proteins)2. In 1990, Wolff et al.3 first showed that a specific protein [e.g., chloramphenicol acetyltransferase and luciferase (Luc)] could be effectively expressed in vivo by intramuscularly injecting pure RNA encoding the corresponding protein into mice; specifically, protein expression from βgLucβgAn RNA occurred in both a dose-dependent and time-dependent manner; this work also proposed the concept of an mRNA vaccine. In 2020, the U.S. Food and Drug Administration (FDA) granted emergency approval to two mRNA-based vaccines produced by Pfizer-BioNTech/BNT162b24,5 and Moderna/mRNA-12736 for the prevention of coronavirus disease 2019 (COVID-19). In 2021, the FDA approved the first COVID-19 vaccine, produced by Pfizer-BioNTech (marketed as Comirnaty), which has stimulated enthusiasm for research and development of mRNA vaccines and created expectations for breakthroughs in mRNA cancer vaccines.
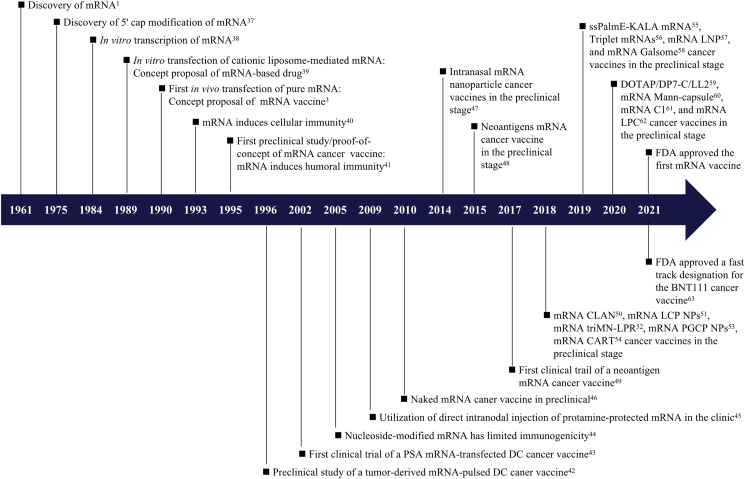
To date, researchers have used mRNA as a vaccine platform [e.g., influenza virus7, 8, 9, human immunodeficiency virus10,11, coronavirus12,13, viral antigens (rabies virus glycoprotein14,15 and proteins from Zika virus16, 17, 18 and Venezuelan equine encephalitis virus19), bacterial pathogens20 (Mycobacterium tuberculosis21) and cancer22,23] and as a protein replacement platform (e.g., factor IX24, follistatin25, ornithine transcarbamylase26 and erythropoietin27) for prevention and treatment of disease. mRNA vaccines have many shared characteristics. Unlike plasmid deoxyribonucleic acid (DNA) and viral vectors, which carry the risk of mutation caused by gene insertion and/or infection, mRNA can be directly translated into proteins after entering the cytoplasm; thus, mRNA vaccines are nonintegrated, noninfectious and well tolerated28,29. mRNA is also briefly expressed in cells, allowing repeated inoculation30. The choice of units encoded in an mRNA transcript is flexible and diverse, allowing encoding of both antigenic and immunomodulatory molecules to induce and regulate both the adaptive and innate immune responses31,32, and an encoded full-length antigen containing multiple epitopes can be presented by MHC class I (MHC-I) and II (MHC-II) molecules without MHC restriction29,33. The production of in vitro-transcribed (IVT) mRNA does not require cells, preventing contamination with proteins or viruses and allowing fast, economical and easy mass production30,33,34. mRNA cancer vaccines use mRNA encoding tumor antigens or immunomodulatory molecules to deliver the corresponding proteins, combined with relevant delivery vectors and adjuvants, to induce antitumor responses35,36. A timeline showing the development of mRNA cancer vaccines is presented in Fig. 11, 3, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63.
Figure 1.
Timeline showing the development of mRNA cancer vaccines1,3,37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63. Abbreviations: CARTs, charge-altering releasable transporters; CLAN, cationic lipid-assisted nanoparticles; DCs, dendritic cells; DOTAP/DP7-C, 1,2-diol-3-trimethylpropane chloride/cholesterol-modified cation peptide DP7; LCP NPs, lipid/calcium/phosphate (LCP) nanoparticles (NPs); LPC, cationic liposome/protamine complex; LNPs, lipid nanoparticles; Mann, mannan; PGCP NPs, poly (lactic-co-glycolic acid) (PLGA)/G0-C14/ceramide-poly (ethylene glycol) (PEG) (PGCP) NPs; PSA, prostate-specific antigen; ssPalmE-KALA, a vitamin E-scaffold (ssPalmE)-lipid nanoparticle and an α-helical cationic peptide “KALA”; triMN-LPR, cationic liposomes (L)-a cationic polymer (P)-mRNA (R) called lipopolyplexes (LPR) functionalized with a tri-antenna of α-d-mannopyranoside (triMN).
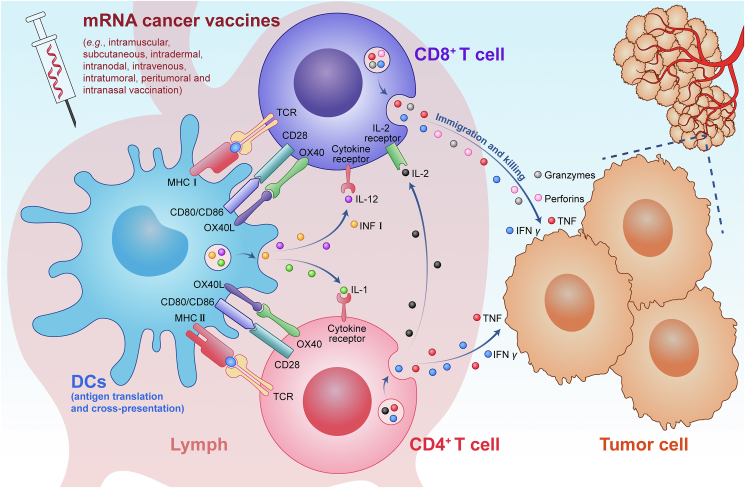
Antitumor T cells are the main expected effector cells that mediate the therapeutic effects of these vaccines, and the mechanism underlying the production and action of antitumor T cells is summarized in Fig. 2. mRNA is taken up by DCs at the vaccine site, translated and processed into antigen-MHC I/II complexes and presented on the cell surface64. Activated DCs travel to the draining lymph nodes, and the presented antigen-MHC I/II complexes bind to T cell receptor (TCR) on the surface of cluster of differentiation 8 (CD8)+/CD4+ T cells (the first signal) in the lymph nodes, resulting in T cell activation and proliferation, with the participation of costimulatory signaling molecules [e.g., CD80/CD86, OX40 ligand (OX40L)] binding to receptors (e.g., CD28, OX40) on the T cells (the second signal) and cytokines [e.g., interferon (IFN) I, interleukin 12 (IL-12), IL-1] binding to cytokine receptors on the T cells (the third signal)65. Additionally, IL-2 secreted by CD4+ T cells can promote amplification of CD8+ T cells65. Activated T cells migrate to and infiltrate tumor tissue under the action of chemokines [e.g., CC-chemokine receptor 7, CC-chemokineligand (CCL) 5, CXC-chemokine ligand 9/10] to maximize the antitumor effect of their secreted effectors [e.g., IFN-γ, tumor necrosis factor (TNF), perforins, granzymes]66,67. Endogenous antigens are mainly presented by MHC-I molecules to activate cytotoxic CD8+ T cells, while exogenous antigens are mainly presented by MHC-II molecules to activate helper CD4+ T cells68. Cytotoxic CD8+ T cells usually have a strong direct killing effect on target cells, and these cells are the main effector cells expected to be induced by cancer vaccines.
Figure 2.
Diagram showing the mechanism of mRNA cancer vaccines.
2. Progress in mRNA cancer vaccines in preclinical and clinical settings
The core role of a vaccine is to deliver antigens that can be recognized by the body's immune cells to trigger immune responses. The selection and expression of antigens/targets, the application of vectors and adjuvants, and administration routes are key factors to be considered in vaccine design. Table 122,40, 41, 42, 43,45,47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62,69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106 summarizes the progress in those factors in the preclinical and clinical settings. Table 2 summarizes clinical trials employing mRNA-based cancer vaccines conducted between 2016 and 2021.
Table 1.
An overview of mRNA cancer vaccines in preclinical and clinical settings.
| Key factor | Stage | mRNA cancer vaccine | |
|---|---|---|---|
| Antigens/targets | Tumor-associated antigens (TAAs) | Preclinical | CEA41, tumor-derived mRNA42, gp10069, MART1/MART1-LAMP170,71, PSMA/STEAP72, TRP2/WT1/P1A73, total tumor RNA74, thymus cell antigen 1a/truncated nerve growth factor receptor/TRP275, truncated nerve growth factor receptor76, TRP-1/gp7022, gp100/TRP277, MUC151, MART152, TRP222,57,61,78,79, cytokeratin 1962, claudin 680 |
| Clinical | PSA43; PSA, prostate stem cell antigen, PSMA, and STEAP1 (NCT00831467); PSA, prostate stem cell antigen, PSMA, STEAP1, prostatic acid phosphatase, and MUC1 (NCT01817738); autologous tumor mRNA81; AML lysate plus mRNA (NCT00514189); tumor stem cell-derived mRNA82; mRNA from primary prostate cancer tissue, hTERT, and surviving (NCT01197625); hTERT and LAMP (NCT00510133); amplified OC stem cell mRNA, hTERT and surviving (NCT01334047); hTERT, surviving and tumor cell-derived mRNA (NCT00961844); Melan-A, MAGE-A1, MAGE-A3, survivin, and gp100, tyrosinase45; gp100 and tyrosinase83; MAGE-C1, MAGE-C2, NY-ESO-1, survivin, and 5T4 (NCT00923312); TRP2 (NCT01456104); MUC1 and survivin84; NY-ESO-1, MAGE-A3, tyrosinase, and TPTE85 (NCT04526899); suppressor of cytokine signaling-1, MUC1 and surviving (NCT02688686); NY-ESO-1, MAGE-C1, MAGE-C2, 5T4, survivin, and MUC1 (NCT03164772); 3 OC TAAs (NCT04163094); 5 PC TAAs (NCT04382898); fixed combination of shared cancer antigens (NCT04534205); WT186,87; WT1, PRAME, and CMV pp6588; cancer-testis antigen 7, MAGE-A3, and WT1 (NCT01995708); WT1 and PRAME (NCT02405338); CEA (NCT00529984, NCT01890213); personalized TAA panels89 | ||
| Tumor-specific antigens (TSAs) | Preclinical | Poly-neo-epitope48, neoantigens22, HPV E6/E722, HPV E752,57,75, HPV E7-TriMix90, human CMV pp6554 | |
| Clinical | CMV pp65-LAMP (NCT02529072); neoantigens91, Kirsten rat sarcoma viral oncogene mutated proteins (NCT03948763) | ||
| Immunomodulatory molecules | Preclinical | TriMix (CD40 ligand, CD70, constitutively active TLR4)73,90,92, mRNA-2752 (OX40L, IL-23 and IL-36γ)56, BNT131 (IL-12, IL-15, GM-CSF, IFN-α)93, BisCCL2/5i94 | |
| Clinical | TriMix95,96, CV8102 (TLR7/8-agonist, RIG-1-agonist) (NCT03291002, NCT03203005), mRNA-2752 (human OX40L, IL-23 and IL-36γ) (NCT02872025, NCT03739931), mRNA-2416 (human OX40L) (NCT03323398), BNT131 (IL-12, IL-15, GM-CSF, IFN-α) (NCT03871348), BNT151/MEDI1191 (IL-12) (NCT03946800, NCT04455620), BNT152/BNT153 (IL-7, IL-12) (NCT04710043) | ||
| Tumor suppressor genes | Preclinical | PTEN53,97, p5398 | |
| Clinical | p53 [two to three shared TAAs plus p53, 20 neoantigens (NCT02316457); survivin, hTERT and p53 (NCT00978913)] | ||
| Vectors | Preclinical | Liposome40,41, DCs42,79, hemagglutinating virus of Japan–liposomes69, cationic liposome-protamine99, cationic liposomes100, histidylated lipopolyplexes70, protamine-complexed72, Man11-LPR10071, nanoparticle47,97,98, protamine-formulated101, cationic lipids48, mannosylated liposomes74, lipid mRNA particles75, lipoplexes22, a cell-penetrating peptide rich in the arginine peptide amphiphile RALA motif102, CLAN50, LCP NPs51, triMN-LPR52, PGCP NPs53, CARTs54, ssPalmE-KALA55, LNPs56,57,77, Mann-capsule60, LPC62, DOTAP/DP7-C59, lipid-like material C161, VLVP103 | |
| Clinical | DCs43,81, 82, 83,86, 87, 88, 89,95,96,104, Langerhans-type DCs (NCT01456104), protamine (RNActive®)45, Lipo-MERIT85, LNP91 | ||
| Adjuvants | Preclinical | GM-CSF100, FLT3105, LPS/poly (I:C)73, TriMix73,90, cholera toxin47, Td106, LPS77, CpG54,103, NKT ligand α-GC58 | |
| Clinical | GM-CSF45, TriMix96,104, CV8102 (NCT03291002, NCT03203005), mRNA-2752 (NCT02872025, NCT03739931), mRNA-2416 (NCT03323398), BNT131 (NCT03871348), MEDI1191 (NCT03946800) | ||
| Administration routes | Preclinical | Intramuscular41,75, intraperitoneal42, injection directly into the spleen69, intravenous22,48,50,53,54,58,70, 71, 72, 73, 74, 75,97, 98, 99, 100, subcutaneous51,54,56,57,59, 60, 61,77, 78, 79,99,100, intradermal52,55,56,73,100,102,106, intranodal73,90,105, nasal47,62, intratumoral56,76, peritumoral56, intrafootpad injection103 | |
| Clinical | Intravenous43,85, intradermal45,81, 82, 83,86, 87, 88, intranodal49,81, intradermal and intravenous89,95,96,104, intramuscular91, subcutaneous (NCT03468244), intratumoral (NCT03788083), intralesional (NCT02872025) |
Abbreviations: AML, acute myelogenous leukemia; α-GC, α-galactosylceramide; BisCCL2/5i, an antibody that bispecifically binds and neutralizes CCL2 and CCL5; CEA, carcinoembryonic antigen; CMV pp65, cytomegalovirus phosphor protein 65; CpG, cytosine–guanine cytosine–phosphate–guanine; FLT3, Fms-like tyrosine kinase 3; GM-CSF, granulocyte-macrophage colony-stimulating factor; gp, glycoprotein; hTERT, human telomerase reverse transcriptase gene; HPV, human papilloma virus; LAMP1, lysosomal-associated membrane protein 1; LPS, lipopolysaccharide; MART1, melanoma antigen recognized by T cells; MAGE, melanoma-associated antigen; MUC1, mucin 1; NY-ESO-1, New York esophageal squamous cell carcinoma 1; NKT, natural killer T cell; OC, ovarian cancer; PC, prostate cancer; poly(I:C), polyinosinic:polycytidylic acid; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PRAME, preferentially expressed antigen in melanoma; PTEN, phosphatase and tensin homolog deleted on chromosome ten; RIG-1, retinoic acid-inducible gene 1 protein; STEAP, six-transmembrane epithelial antigen of prostate; TLR, Toll-like receptor; TRP, tyrosinase-related protein; TPTE, transmembrane phosphatase with tensin homology; Td, tetanus/diphtheria; VLVP, virus-like vaccine particle; WT1, Wilms' tumor 1.
Table 2.
Clinical trials employing mRNA-based cancer vaccines between 2016 and 2021.
| mRNA encoding | Vector | Start Year | NCI Number | Status | Phase | Conditions | Interventions | Route | |
|---|---|---|---|---|---|---|---|---|---|
| TAA | Personalized TAA panels containing 3–13 different TAAs | DC | 2016 | NCT02709616 | Unknown status | 1 | GBM |
|
Intradermal and intravenous |
| Personalized TAA panels containing 3–13 different TAAs | DC | 2016 | NCT02808364 | Unknown status | 1 | GBM |
|
Intradermal and intravenous | |
| Personalized TAA panels containing 3–13 different TAAs | DC | 2016 | NCT02808416 | Unknown status | 1 | Brain cancer, neoplasm metastases |
|
Intradermal and intravenous | |
| Suppressor of cytokine signaling-1, MUC1 and survivin | DC | 2016 | NCT02688686 | Unknown status | 1/2 | NSCLC with bone metastases |
|
Subcutaneous | |
| NY-ESO-1, MAGE-C1, MAGE-C2, 5T4, survivin, MUC1 | Protamine (RNActive®) | 2017 | NCT03164772 | Completed | 1/2 | Metastatic NSCLC, NSCLC |
|
Intradermal | |
| 3 OC TAAs | Lipo-MERIT | 2019 | NCT04163094 | Recruiting | 1 | OC | Drug: W_ova1 Vaccine | Intravenous | |
| 5 PC TAAs | Lipo-MERIT | 2019 | NCT04382898 | Recruiting | 1/2 | PC |
|
Intravenous | |
| NY-ESO-1, MAGE-A3, tyrosinase, and TPTE | Lipo-MERIT | 2021 | NCT04526899 | Recruiting | 2 | Melanoma stage III/IV, unresectable melanoma |
|
Intravenous | |
| Fixed combination of shared cancer antigens | Lipo-MERIT | 2021 | NCT04534205 | Recruiting | 2 | Unresectable HNSCC, metastatic/recurrent HNC |
|
Intravenous | |
| Tumor-specific antigen (TSA)/personalized TAA | CMV pp65-LAMP | DC | 2016 | NCT02529072 | Completed | 1 | Malignant glioma, astrocytoma, GBM |
|
/ |
| Two to three shared TAAs plus p53, 20 neoantigens | / | 2016 | NCT02316457 | Active, not recruiting | 1 | Breast cancer (e.g., TNBC) |
|
Intravenous | |
| 20 neoantigens | Lipo-MERIT | 2017 | NCT03289962 | Recruiting | 1 | Melanoma, NSCLC, bladder cancer, colorectal cancer, TNBC, renal cancer, HNC, other solid cancers |
|
Intravenous | |
| ∼20 neoepitopes | LNP | 2017 | NCT03313778 | Recruiting | 1 | Solid tumors |
|
Intramuscular | |
| Neoantigens | LNP | 2018 | NCT03480152 | Terminated | 1/2 | Melanoma, colon/gastrointestinal/genitourinary/hepatocellular cancer |
|
Intramuscular | |
| Neoantigen | / | 2018 | NCT03468244 | Recruiting | Not applicable | Advanced esophageal squamous carcinoma, gastric adenocarcinoma, pancreatic adenocarcinoma, colorectal adenocarcinoma |
|
Subcutaneous | |
| Neoantigen | / | 2019 | NCT03908671 | Not yet recruiting | Not applicable | Esophageal cancer, NSCLC | • Biological: Personalized mRNA tumor vaccine | Subcutaneous | |
| 20 neoantigens | Lipo-MERIT | 2019 | NCT04161755 | Recruiting | 1 | Pancreatic cancer |
|
/ | |
| 20 neoantigens | Lipo-MERIT | 2019 | NCT03815058 | Recruiting | 2 | Advanced melanoma |
|
Intravenous | |
| 20 neoantigens | Lipo-MERIT | 2021 | NCT04486378 | Recruiting | 2 | Colorectal cancer stage II/III |
|
Intravenous | |
| ∼20 neoepitopes | LNP | 2019 | NCT03897881 | Recruiting | 2 | Melanoma |
|
/ | |
| 4 Kirsten rat sarcoma viral oncogene mutated proteins (G12C, G12D, G12V and G13C) | LNP | 2019 | NCT03948763 | Recruiting | 1 | Neoplasms, carcinoma, non-small-cell lung, pancreatic neoplasms, colorectal neoplasms |
|
Intramuscular | |
| Immunomodulators and cytokines | TLR7/8-agonist, RIG-1-agonist | / | 2017 | NCT03291002 | Active, not recruiting | 1 |
|
|
Intratumoral |
| TLR7/8-agonist, RIG-1-agonist | / | 2017 | NCT03203005 | Completed | 1/2 |
|
|
Intradermal | |
| Human OX40L, IL-23, and IL-36γ | / | 2017 | NCT02872025 | Recruiting | Early1 | Carcinoma, intraductal, noninfiltrating |
|
Intralesional | |
| Human OX40L, IL-23, and IL-36γ | / | 2018 | NCT03739931 | Recruiting | 1 |
|
|
Intratumoral | |
| TriMix | / | 2018 | NCT03788083 | Recruiting | 1 | Breast cancer female, early-stage breast cancer |
|
Intratumoral | |
| Human OX40L | / | Study Completion: 2022 | NCT03323398 | Active, not recruiting | 1/2 | Relapsed/refractory solid tumor malignancies or lymphoma, OC |
|
Intratumoral | |
| IL-12, IL-15, GM-CSF, IFN-α | / | 2019 | NCT03871348 | Recruiting | 1 | Metastatic neoplasm |
|
Intratumoral | |
| IL-12 | / | 2019 | NCT03946800 | Recruiting | 1 | Solid tumors, cancer |
|
Intratumoral | |
| IL-12 | / | 2021 | NCT04455620 | Recruiting | 1/2 | Solid tumor |
|
Intravenous | |
| IL-7, IL-12 | / | 2021 | NCT04710043 | Recruiting | 1 | Solid tumor |
|
Intravenous | |
Abbreviations: GBM, glioblastoma; HNC, head and neck cancer; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small-cell lung cancer; OC, ovarian cancer; PC, prostate cancer; PCV, personalized cellular vaccine; TNBC, triple-negative breast cancer.
2.1. Selected antigens or targets
2.1.1. Tumor-associated antigens
The major first step in developing cancer vaccines is the selection of an antigen, which should have high tumor specificity and induce strong and controllable antitumor T cell responses105. Tumor antigens can be divided into TAAs and TSAs according to their tissue distribution, expression level and central tolerance status106. TAAs are generally overexpressed in tumors and expressed in normal tissues, exhibit weak tumor specificity, strong central tolerance and weak immunogenicity, and these antigens mainly include tissue differentiation antigens and carcinoembryonic antigens65. The central immune tolerance of TAAs is a major challenge in developing cancer vaccines using those antigens. Using combinations of multiple (e.g., 2–6) shared TAAs has become the trend in development of targeted mRNA cancer vaccines in the clinic. The selected TAAs are often widely expressed in related tumors and can induce antitumor immune responses when combined with different vectors or adjuvants.
In 2009, Weide et al.45 conducted a phase 1/2 clinical study on a protamine (RNActive®)-protected mRNA cancer vaccine, adopting GM-CSF as an adjuvant that encoded 6 TAAs (Melan-A, tyrosinase, gp100, MAGE-A1, MAGE-A3, and survivin). The vaccine was administered by intradermal injection. This vaccine significantly reduced immunosuppressive cells [e.g., Foxp3+/CD4+ regulatory T cells (Tregs) in peripheral blood and myeloid suppressor cells] and increased specific T cells in a subset of patients. One treated patient had a complete response, and no adverse reactions greater than grade II occurred (NCT00204607). In 2011, Fotin-Mleczek et al.72 conducted a preclinical study on protamine-complexed mRNA cancer vaccines encoding ovalbumin (OVA)/PSMA/STEAP and showed that the two-component mRNA cancer vaccine could induce self-adjuvant action via TLR7, balanced adaptive immune responses and sustained antitumor effects (NCT00831467, NCT00923312). In 2014, Fotin-Mleczek et al.101 showed the strong synergistic antitumor effect of a combination of a protamine-complexed OVA-encoding mRNA cancer vaccine and preclinical radiation. Protamine-complexed mRNA cancer vaccines encoding 4–5 prostate-specific antigens [e.g., CV9103 (NCT00831467) and CV9104 (NCT01817738)] or 5–6 TAAs for melanoma and NSCLC [e.g., CV9201 (NCT00923312) and CV9202 (NCT03164772)] are in clinical trials. BN111 is an mRNA cancer vaccine candidate encoding a fixed combination of 4 TAAs (NY-ESO-1, MAGE-A3, tyrosinase, and TPTE) that are prevalent in melanoma and delivered as an RNA-lipoplex formulation (Lipo-MERIT). BNT111 alone or in combination with an immune checkpoint PD-1 inhibitor induces persistent and strong antigen-specific CD4+/CD8+ T cell responses and objective responses in patients with unresectable melanoma. Related adverse events occurred in more than 5% of patients, and the majority of adverse reactions were grade 1–2 (NCT02410733)85. Based on these results, BNT111 has received FDA fast track designation for clinical translation to treat advanced melanoma (NCT04526899). Lipo-MERIT mRNA cancer vaccines encoding 3 TAAs for OC (NCT04163094), 5 TAAs for PC [e.g., BNT112 (NCT04382898)], or a fixed combination of shared cancer antigens for HNSCC and HNC [BNT113 (NCT04534205)] are in clinical trials.
The clinical translation of mRNA cancer vaccines for AML and myeloma has also shown a trend from the application of a single TAA [e.g., WT1 (NCT00834002, NCT0096522486, NCT0129142087)] to a combination of multiple TAAs [e.g., WT1, PRAME, CMV pp65, cancer-testis antigen 7, and MAGE-A3 (NCT0173430488, NCT02405338, NCT01995708)]. DCs electroporated with WT1 mRNA were found to prevent or delay relapse in 43% of AML patients in remission after chemotherapy (NCT0096522486), and the improved overall survival (OS) rates or clinical responses were correlated with the induction of a WT1-specific CD8+ T cell response (NCT0096522486, NCT0129142087). TLR7/8-matured DCs transfected with RNA encoding WT1, PRAME, and CMV pp65 prevented relapse in a subset of AML patients in complete remission (NCT0173430488).
mRNA cancer vaccines are also being developed toward individualization and precision, and the preliminary trend is from the application of autologous tumor [e.g., AML (NCT00514189-Terminated), prostate cancer (NCT01197625), NCT0127894081, NCT0084645682, NCT00961844-Terminated] or tumor stem cell [e.g., OC (NCT01334047-Terminated)]-derived mRNAs to adopting personalized TAA panels (NCT01334047, NCT02709616, NCT02808364, NCT02808416). DCs loaded with complete tumor-mRNA can induce T cell responses targeting a wide range of antigens within the tumor, even those unique to the patient (NCT01278940). DCs transfected with cancer stem cell-derived mRNA were found to induce an immune response in patients and showed promising preliminary safety results (NCT00846456). DCs pulsed with personalized TAA panels containing 3–13 different TAA mRNAs were associated with favorable OS, and the treated patients experienced no grade III/IV adverse events (NCT02709616, NCT02808364, NCT02808416)89. mRNA derived from autologous tumor cells or tumor stem cells contains all the proteins in tumor cells. This strategy is simple and feasible; however, its targeting and effectiveness need to be improved, and safety should also be considered.
2.1.2. Tumor-specific antigens
TSAs are usually tumor neoantigens formed by nonsynonymous mutations in the genome of tumor cells; these antigens are not expressed in normal cells and have strong tumor specificity and immunogenicity and weak central tolerance65,66. A correlation between TSAs and antitumor immune responses has been confirmed in several studies. Analysis of thousands of RNA sequences in data for 18 solid tumors from The Cancer Genome Atlas showed a positive correlation between the number of neoantigens in each tumor and the expression of genes related to the cytotoxic activity of T cells107. Analysis of RNA-seq data for 6 sites in 515 patients from The Cancer Genome Atlas showed that a high level of immunogenic mutant epitopes was associated with improved patient survival. Tumors with high levels of immunogenic mutations had much higher levels of CD8A, PD-1 and CTLA4108. Analysis of the whole-exon sequences of 619 colorectal cancer samples showed that a high level of neoantigens in the tumor was correlated with both an increase in tumor-infiltrating lymphocytes and an improvement in survival109. An association between the level of neoantigens and the number of tumor-infiltrating lymphocytes has also been confirmed in endometrial carcinoma110. Furthermore, tumors with a high level of neoantigens were found to be significantly more homogenous than those with a low level of neoantigens111. Tumors with a mutational load greater than 10 somatic cell mutations per million bases (equivalent to 150 nonsynonymous mutations in expressed genes) are more likely to form immunogenic neoantigens, and tumors with a mutational load less than 1 somatic cell mutation per million bases are less likely to form immunogenic neoantigens. Most tumors have a mutational load of 1–10 somatic cell mutations per million bases and can generally form neoantigens recognized by T cells112. Rajasagi et al.113 analyzed the predicted mutant HLA-binding peptides of 13 different tumors (2488 samples) using whole-exon sequencing and an HLA-peptide predictive binding algorithm (i.e., NetMHCpan) and showed that each tumor could produce tens to thousands of neoantigens, indicating that neoantigens are common in most tumors.
The prevailing trend in mRNA cancer vaccines toward individualization and precision aims to develop mRNA cancer vaccines using multiple (e.g., 20) neoantigens [e.g., IVAC MUTANOME (NCT0203595649), IVAC_W_bre1_uID and IVAC_W_bre1_uID/IVAC_M_uID (NCT02316457), RO7198457 (NCT03289962, NCT04161755, NCT03815058, NCT04486378), mRNA-4157 (NCT03313778, NCT03897881), NCI4650/mRNA-4650 (NCT0348015291), NCT03468244, NCT03908671]. In 2015, Kreiter et al.48 analyzed the mutant peptides in murine tumor cells (e.g., the melanoma cell line B16F10, colon cancer cell line CT26 and breast cancer cell line 4T1) via exome sequencing and an MHC-II epitope predictive binding algorithm and prepared RNA vaccines encoding those mutant peptides to evaluate their antitumor effects in a preclinical setting. The poly-neoepitope RNA effectively induced T cell responses in vivo and inhibited the growth and metastasis of the tumors in mice, and the majority of the immunogenic mutanome was recognized by CD4+ T cells; even RNA encoding just one neoepitope (e.g., B16-M30) induced stronger T cell responses and controlled the growth of B16F10 melanoma in mice48. Zhang et al.59 conducted a preclinical study on DOTAP/DP7-C liposomes, as both the carrier and the adjuvant, loaded with mRNA encoding five tumor neoantigens of the mouse LLC cell line LL2 (DOTAP/DP7-C/LL2). DOTAP/DP7-C/LL2 significantly inhibited the growth of in situ and subcutaneous LL2 tumors and stimulated antigen-specific lymphocyte reactions. In 2017, Sahin et al.49 showed that an RNA-based multiple neoepitope vaccine could induce antigen-specific polyclonal T cell immune responses in patients with melanoma; 60% of the selected neoepitopes had immunogenicity, the main T cell responses induced by those neoepitopes were CD4+ T cell responses, tumor metastasis was significantly reduced, and approximately 75% of patients had a progression-free survival of 27 months (NCT0203595649). In 2020, Cafri et al.91 showed that NCI4650/mRNA-4650 encoding 20 neoantigens could induce a neoantigen-specific T cell response in patients with gastrointestinal cancer; 21% of the selected neoantigens were immunogenic, and 59% of neoantigen-specific T cells from patients were CD4+ T cells (NCT0348015291).
Although mRNA preparation is rapid and economical (good manufacturing practice grade RNA can be prepared within 3 weeks48), the screening and identification of tumor neoantigens can take a long time and be expensive, and the patients' condition may change during vaccine preparation, resulting in researchers missing the best treatment opportunity for patients. Based on the parameters for deep sequencing of genes and proteomics analysis of big datasets using high-throughput and bioinformatic techniques, the preparation times of therapeutic tumor neoantigenic peptide vaccines, RNA vaccines and fused DC-tumor cell vaccines are approximately 160 days114, 103 (89–160) days49 and 10 days115, respectively. The speed of screening and identification of neoantigens directly affects the clinical efficacy of mRNA neoantigenic vaccines, which is a major challenge faced by those vaccines. Meanwhile, the accuracy of predicting tumor neoantigens needs to be improved.
2.1.3. Immunomodulatory molecules and tumor suppressor genes
mRNA encoding CD70, CD40 ligand, and constitutively active TLR4 (named TriMix, NCT03788083); mRNA encoding human OX40L, IL-23, and IL-36γ (named mRNA-2752, NCT02872025, NCT03739931); and mRNA encoding IL-12, IL-15, GM-CSF, and IFN-α (named SAR441000/BNT131, NCT0387134893) are three representative mRNA cancer vaccines that encode immunomodulatory molecules, and such vaccines also include mRNA vaccines encoding a TLR7/8 agonist and RIG-1 agonist (CV8102/RNAdjuvant®, NCT03291002, NCT03203005); mRNA encoding OX40L [mRNA-2416 (NCT03323398)]; mRNA encoding IL-12 [MEDI1191 (NCT03946800), BNT151 (NCT04455620)]; mRNA encoding IL12 and IL-7 (BNT152, BNT153, NCT04710043); and mRNA encoding BisCCL2/5i94. Several studies have shown that mRNA cancer vaccines encoding immunomodulatory molecules (e.g., TriMix, mRNA-2752, BNT131, and mRNA encoding BisCCL2/5i) and tumor suppressor genes (e.g., PTEN or p53-encoding mRNA), which also have antitumor effects as a monotherapy, are often used as adjuvant treatment in combination with multiple tumor antigens (e.g., MAGE-A3, MAGE-C2, tyrosinase, gp100, survivin, hTERT, and neoantigens) and immune checkpoint inhibitors (e.g., anti-PD-1, anti-CTLA-4, and anti-PD-L1 antibodies). In 2012, Van Lint et al.73 showed that intranodal injection of TriMix together with TAA (e.g., TRP2/WT1/P1A) mRNA could induce the maturation of DCs and the priming of antigen-specific T cells in situ. Compared with DCs pulsed with firefly luciferase (FLuc) mRNA without the adjuvant, TriMix significantly reduced the expression of FLuc in DCs, and the reduction effect induced by LPS, monophosphoryl lipid A, or poly(I:C) was even stronger; however, TriMix can generate an immunostimulatory environment to improve T cell responses, which was superior to that induced by LPS73. In 2016, Bialkowski et al.90 showed that HPV16-E7-TriMix mRNA could induce CD8+ T lymphocytes to migrate into mucosally located tumors and control tumor growth. When combined with cisplatin, HPV16-E7-TriMix mRNA resisted the immunosuppressive microenvironment by downregulating the number of myeloid-derived suppressor cells (MDSCs) and Tregs, leading to complete regression of tumors in the genital tract90. Intratumoral injection of TriMix has been shown to be taken up by tumor-infiltrating dendritic cells and then presented to T cells in tumor-draining lymph nodes to induce antitumor T cell responses and antitumor effects in a variety of mouse tumor models76. In 2013 and 2016, Wilgenhof et al.95 showed that DCs coelectroporated with TriMix and mRNA encoding one of four melanoma-associated antigens (either MAGE-A3, MAGE-C2, tyrosinase or gp100) linked to an HLA II targeting signal (DC-LAMP) (named TriMixDC-MEL) were well tolerated in pretreated advanced melanoma patients and caused a complete response and a partial response in two patients (NCT0106639095). TriMixDC-MEL combined with an immune checkpoint inhibitor (ipilimumab) was tolerated and induced highly durable tumor responses in pretreated advanced melanoma patients (NCT01302496104). In 2020, De Keersmaecker et al.96 showed that the combination of TriMixDC-MEL and ipilimumab could induce potent CD8+ T cell responses, which were correlated with the clinical responses of patients, in a meaningful portion of advanced melanoma patients (NCT0130249696). In 2019, Hewitt showed that intratumoral injection of triplet mRNAs encoding IL-23, IL-36γ, and OX40L encapsulated in LNPs could activate and recruit multiple immune cells (e.g., DCs and T cells) into the tumor to induce long-lasting antitumor immunity depending on Batf3-dependent cross-presenting DCs and cytotoxic CD8+ T cells. Combination of this vaccine with immune checkpoint inhibitors (e.g., anti-PD-1, anti-CTLA-4, and anti-PD-L1 antibodies) had potent antitumor effects in in vivo models resistant to immune checkpoint inhibitors56. In 2021, Hotz et al.93 showed that BNT131 combined with an anti-PD-1 antibody could significantly improve the survival of tumor-bearing (e.g., B16 and MC38 tumor-bearing) mice93. In 2021, Wang et al.94 showed that mRNA encoding BisCCL2/5i LNPs combined with mRNA encoding PD-1 ligand inhibitor LNPs could significantly prolong the survival of tumor-bearing (e.g., primary liver cancer and liver metastases of colorectal and pancreatic cancers) mice, and BisCCL2/5i could promote the sensitivity of those tumors to PD-1 ligand inhibitor.
Several preclinical studies have shown the feasibility of using mRNAs encoding tumor suppressor genes (e.g., PTEN and p53) to treat tumors. In 2018, Islam et al.53 showed that PEG-coated polymer–lipid hybrid NPs loaded with the tumor suppressor gene PTEN-encoding mRNA (e.g., mRNA-PGCP NPs) could effectively transfect PTEN-deficient prostate cancer cells with PTEN mRNA in vitro and in vivo and significantly inhibit tumor growth by inhibiting the phosphatidylinositol 3-kinase–Akt pathway to promote cancer cell apoptosis53. In 2021, Lin et al.97 showed that tumor suppressor gene PTEN-encoding mRNA NPs could induce autophagy and death of PTEN-mutated melanoma cells and PTEN-deficient prostate cancer cells. The PTEN-mRNA NPs upregulated CD8+ T cells and proinflammatory cytokines (e.g., IL-12, TNF-α and IFN-γ) in the immunosuppressive TME and downregulated Tregs and MDSCs, and combination with an anti-PD-1 antibody produced potent antitumor effects against those tumors97. In 2019, Kong et al.98 showed that tumor suppressor gene p53-encoding mRNA NPs could promote the sensitivity of p53-deficient hepatocellular carcinoma and NSCLC cells to mammalian target of rapamycin inhibitors (e.g., everolimus), and the combination of p53-mRNA NPs and everolimus produced significantly synergistic antitumor effects in in vitro and in vivo models of hepatocellular carcinoma and NSCLC98. mRNA cancer vaccines encoding p53 and tumor antigens (e.g., survivin, hTERT, neoantigens) are currently in clinical trials (NCT00978913, NCT02316457).
2.1.4. Combination of mRNA cancer vaccines and immune checkpoint inhibitors
Central immune tolerance to TAAs and peripheral immune tolerance (e.g., immune checkpoint pathways, TME) during tumor development are two major challenges faced by cancer vaccines. Both can affect the efficacy and duration of cancer vaccines. To target central immune tolerance, a combination of multiple TAAs or multiple TSAs is a major trend in the development of mRNA cancer vaccines. To target peripheral immune tolerance, a combination of mRNA cancer vaccines and immune checkpoint inhibitors (e.g., anti-PD-1, anti-CTLA-4, and anti-PD-L1 antibodies) is another major trend in the application of mRNA cancer vaccines.
In 2018, Liu et al.51 conducted a preclinical evaluation of LCP NPs loaded with MUC1 mRNA in combination with an anti-CTLA-4 antibody to treat TNBC and showed that LCP-mRNA NPs as a monotherapy or part of a combined treatment (LCP-mRNA NPs + anti-CTLA-4) could significantly inhibit tumor growth, and the inhibitory effect of the combined treatment was significantly stronger than that of LCP-mRNA NP monotherapy51. In 2018, Wang et al.78 showed that LCP NPs loaded with both mRNA encoding melanoma-associated antigen TRP2 and small interfering RNA targeting PD-L1 could effectively deliver mRNA into DCs in vitro and in vivo and promote the maturation of DCs. The small interfering RNA targeting PD-L1 downregulated the expression of PD-L1 in DCs to enhance antitumor immunity and antitumor effects, and the vaccine effectively inhibited tumor growth78. In 2019, Verbeke et al.58 showed that the in vivo antitumor effects of Galsomes mRNA alone were modest, and this treatment could increase the number of cytotoxic T lymphocyte (CTL), invariant NKT (iNKT), NK and M1 tumor-associated macrophages (TAMs) in the immune microenvironment; these authors also found that negative regulation of the PD-1/PD-L1 pathway by the treatment might limit its antitumor effects. Compared to the vaccine alone, the combination of OVA mRNA Galsomes and an anti-PD-L1 antibody significantly increased the number of iNKT cells in the spleen, reduced the level of PD-L1 on DCs from the spleen and the level of PD-1 on proliferative iNKT cells from the spleen, and significantly improved the antitumor effect58. Additionally, Oberli et al.77 showed that both LNP-containing mRNA encoding a single tumor antigen (e.g., gp100 or TRP2) and sequential therapy with the two antigens had significant antitumor effects in vivo; however, there was no significant difference between the antitumor effects of the two methods.
2.2. Expression of antigens or targets
2.2.1. Pharmacodynamics of mRNA molecules
The mRNA used to prepare vaccines mainly includes conventional nonreplicating mRNA and virus-derived self-amplifying mRNA. IVT of mRNA is the main technique applied to prepare the molecule and utilizes a bacteriophage RNA polymerase, such as T3, T7 or SP6 RNA polymerase, and a linearized DNA template containing the targeted antigen sequences2,29. The fundamental structure of nonreplicating IVT mRNA includes an open reading frame (ORF) that encodes the protein of interest, flanking five-prime (5′) and three-prime (3′) untranslated regions (UTRs), a 7-methylgaunosine 5′ cap and a 3′ poly(A) tail30,116. The 5′ cap and 3′ poly(A) can be added during IVT or added enzymatically after initial IVT117. Self-amplifying mRNA contains two ORFs, one that encodes the targeted antigen sequences and another that encodes viral replication machinery, which enables durable intracellular RNA amplification118. An mRNA vaccine (called AVX701) consisting of an alphavirus replicon encoding CEA is in clinical trials (NCT00529984, NCT01890213). Unlike protein or peptide vaccines, the first step necessary for an mRNA cancer vaccine to produce its effects is that the sequence information of a coded protein can be translated into the functional protein. The factors affecting the translation process include positive, negative and bidirectional regulating factors.
Positive regulatory factors are summarized as follows: ① The 5′ cap and its modification [e.g., anti-reverse cap analogs119, 120, 121, 122, 123, CleanCap124] can recruit eukaryotic translation initiation factor 4E to facilitate ribosome recognition and translation initiation29 and eliminate free phosphate groups in the mRNA sequence to significantly enhance the stability of mRNA125. ② The poly(A) sequence and its modification (e.g., length126, 127, 128) can slow the process of degradation by RNA exonuclease, which increases stability, extends the in vivo half-life, and enhances the translation efficiency of mRNA129. ③ UTR optimization [e.g., 3′ UTR sequence derived from α-globin and β-globin, AU and GU-enriched sequences130, 131, 132; stable elements in the 3′ UTR130,133; GCC-(A/G)-CCAUGG in the 5′ UTR134; a short and loose 5′ UTR135] and codon optimization of the ORF (e.g., uridine depletion, enrichment of G:C content, synonymous frequent codons, codons with higher transfer RNA abundance) can increase mRNA stability and protein translation136,137. Epitopes in the expression vector are linked by different sequences and signaling peptides (e.g., endosome/lysosome signal sorting fragment and transmembrane-cytoplasmic domain) to increase IVT and improve the targeting of intracellular processing and presentation of antigens79. ④ Nucleoside modification [e.g., pseudouridine (Ψ), 1-methylpseudouridine, 5-methylcytidine (5meC), and posttranscriptional RNA modification with N4-acetylcytidine]138, 139, 140, 141 and purification of IVT-mRNA (e.g., Mg2+31, temperature31, high-pressure liquid chromatography142,143, and fast protein liquid chromatography144) to reduce contamination with double-stranded RNA can decrease innate immune activation of the molecule and increase protein translation. Compared with unmodified mRNA, nucleoside-modified mRNA (5meC, Ψ) was found to significantly promote FLuc expression in mice58. The in vivo antitumor effects of LCP (modified mRNA) have been shown to be significantly stronger than those of LCP (unmodified mRNA)78.
Negative regulatory factors include the following: ① Extracellular RNases can rapidly degrade naked mRNA29. ② double-stranded RNA impurities produced by IVT can bind pattern recognition receptors (PRRs) in the cytoplasm [e.g., RIG-I, melanoma differentiation-associated protein 5 (one type of RIG-I receptor), protein kinase RNA-activated (also known as eukaryotic translation initiation factor 2alpha kinases 2), 2′-5′-oligoadenylate synthetase] and endosomes (e.g., TLR3) to activate specific pathways [e.g., RIG-I/MAD5→mitochondrial antiviral signaling protein→IFN I, (IFN I→) protein kinase RNA-activated→eukaryotic translation initiation factor 2alpha, (IFN I→) 2′-5′-oligoadenylate synthetase→ribonuclease L, TLR3→Toll/IL-1 receptor domain containing adaptor inducing IFN-β→IFN I], which can inhibit mRNA translation and promote mRNA enzymolysis145, 146, 147. Bidirectional regulating factors include unmodified single-stranded RNA as a pathogen-associated molecular pattern (PAMP) that can bind PRRs in endosomes [e.g., TLR7, TLR8]147 to activate specific pathways (TLR8→myeloid differentiation factor 88→proinflammatory cytokines; TLR7→myeloid differentiation factor 88→interferon regulatory factor 7→IFN I)2,31. On the one hand, mRNA can activate innate immune responses (DC maturation and activation), which further activate adaptive immune responses (T and B cell immune responses); on the other hand, premature and overly strong activation of IFN I can inhibit mRNA translation, promote mRNA enzymatic hydrolysis, and promote apoptosis of DCs and T cells146. Overly strong inflammatory reactions can also cause toxic side effects. Activation of type I IFN receptor signaling precedes that of TCR signaling in T cells, which can serve as the true third signal to promote immune responses146. Udhayakumar et al.102 showed that compared with RALA mRNA nanocomplexes containing unmodified mRNA, mRNA nanocomplexes containing Ψ- and 5meC-modified mRNA induced potent antigen-specific cytotoxic T cell responses and had superior efficacy and that the modified (5meC, Ψ) mRNA nanocomplex significantly reduced the inhibitory effect of type I IFNs on CTLs by suppressing IFN-β activation and effectively induced CTLs102. In contrast, Oberli et al.77 showed that unmodified mRNA LNP vaccines induced much stronger CD8 T cell responses in peripheral blood (7.8%) than nucleoside-modified mRNA (5meC, Ψ) LNP vaccines (1.0%) and suggested that type I interferon was necessary for a protective CD8 T cell response. These contradictory results may be related to bidirectional regulatory factors.
2.2.2. Methods of antigen expression for vaccines
According to the expression of antigens, vaccines can be divided into peptide or protein vaccines, cell vaccines (e.g., tumor cell vaccines, DC vaccines and engineered cell vaccines), nucleic acid vaccines (e.g., DNA and RNA vaccines) and viral vector vaccines. Peptide or protein vaccines are widespread vaccine types. The sequence of an antigenic peptide is well defined and easily controlled. Peptide vaccines include short and long peptide vaccines. Short peptide vaccines containing nine amino acids can provide minimal epitopes to induce CD8+ T cell responses68. Short peptide vaccines have some shortcomings, including antigen degradation caused by protein hydrolysis and a weak duration of immune responses148, and short peptides can bind MHC I molecules on the surface of many nuclear cells, which, as nonprofessional antigen-presenting cells (APCs), often do not contain costimulatory signals, leading to antigen tolerance and T cell dysfunction. Long peptide vaccines generally contain 20–30 amino acids and can activate both CD4+ and CD8+ T cells68. Protein vaccines can also induce T cell responses; however, long peptides are generally much more efficiently internalized and processed by APCs than proteins149. The shortcomings of long peptide vaccines include their sensitivity to enzyme degradation, rapid clearance and inadequate uptake at the injection site150,151.
Cell vaccines mainly include cancer cell vaccines and DC vaccines. Cancer cell vaccines immunize the body with autogenous or allogeneic inactivated whole cells and their derivatives (e.g., cell lysates, derivatives of DC fusion, modified whole cells expressing TSAs or immune-enhancing factors, and tumor-derived mRNAs), which often contain all antigens of the cells and do not require laborious identification prior to vaccine design and production, leading to a relatively quick preparation and certain personalized characteristics68. However, the method using cancer cells cannot accurately determine and control the corresponding tumor antigens, quality control is difficult, and cancer cells often contain fewer specific antigens, resulting in weak immunogenicity and potential carcinogenicity152. DCs are the most potent APCs and play a central role linking innate and adaptive immune responses. DC vaccines generally use autologous DCs as carriers to express and present antigens. In 1996, Boczkowski et al.42 showed the feasibility of using DCs pulsed with tumor-derived mRNA cancer cells. Before 2017, in approximately 24 clinical trials, mRNA cancer vaccines used DCs as the carrier. However, the preparation technology for DC vaccines is complicated, production is expensive, and quality control is difficult153,154. Moreover, the patient must have a relatively normal immune function without marrow suppression caused by chemotherapy or other treatments and provide a large number of functional DCs, leading to a limited number of available vaccines147.
Nucleic acid vaccines are prepared using nucleic acids (e.g., DNA, RNA) encoding antigens. The features of mRNA vaccines have been described in part 1 of this paper. In contrast to mRNA, DNA must enter the nucleus to be translated into the corresponding antigens, which has a potential risk caused by insertional mutations and is likely less safe than mRNA. On the whole, nucleic acids are sensitive to degradation155, unstable and have a short half-life, resulting in a poor efficiency of naked nucleic acids taken up by APCs. Novel carriers and administration routes have been developed to improve the uptake and presentation efficiency of nucleic acids (discussed below)156. Limited studies have shown the antitumor advantages of mRNA cancer vaccines compared with peptide or protein cancer vaccines. DCs pulsed with in vitro synthesized chicken OVA RNA were more effective than OVA peptide-pulsed DCs in stimulating primary, OVA-specific CTL responses in vitro42. DOTAP/DP-C/mRNA encoding five neoantigens was significantly stronger than DOTAP/DP-C/mutant peptides in inducing the spleen to produce activated T cells (CD3+ CD8+ IFN-γ+) in an in situ therapeutic tumor model59. The in vivo antitumor effects of LCP (modified mRNA) were significantly stronger than those of LCP (TRP2 peptide/CpG). The ability of triplet mRNAs encoding IL-23, IL-36, and OX40L to improve the survival rate of MC38-S tumor-bearing mice was significantly stronger than that of the corresponding protein treatments78. Peptide antigens often contain only one epitope, while full-length antigens encoded by mRNAs contain multiple epitopes, which can induce T cells to target those epitopes and produce stronger antitumor effects.
Viral vector vaccines are prepared by using a virus as a carrier for expressing or presenting antigens. Currently, viral vectors that have been widely studied include poxvirus, adenovirus and herpesvirus152. For safety reasons, replication-defective viruses or attenuated viruses are adopted65. Poxvirus can contain multiple genes, replication and transcription are limited to the cytoplasm, the risk of insertional mutations is low, and the expressed products can be presented by MHC I and II157. Nonavian poxviruses can induce the host to produce immune responses that can neutralize the viruses, limiting their application to one or at most two vaccines152. Recombinant avipoxviruses can be inoculated multiple times, their viral coat protein cannot be produced in mammalian cells, and the virus cannot induce the host to produce immune responses that neutralize the virus158. Recombinant adenovirus vectors are easy to design and have shown utility as vectors for vaccines and gene therapy drugs; however, their immunogenicity can impact the effects of vaccines159. Herpesvirus has a wide host range; can infect nerve cells, peripheral blood monocytes and DCs; and has a short replication cycle, a large capacity and relatively good safety160, 161, 162. In addition, other vectors, such as bacteria and yeast, have shown potential as vaccine vectors in preclinical studies163, 164, 165. On the whole, immunogenicity, carcinogenicity, infectivity, limited packaging capacities and difficulties in producing viral vectors are challenges to wide application.
2.3. Carriers for mRNA cancer vaccines
Developing mRNA delivery carriers with good safety, targeting, stability, self-adjuvant effects, load capacity and versatility that can efficiently and continuously deliver and present antigens and activate APCs has been a fundamental direction in the field of mRNA cancer vaccines. The carriers adopted for mRNA cancer vaccines in the preclinical setting are summarized in Table 322,41,42,46, 47, 48,50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62,69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79,90,94,97, 98, 99, 100, 101, 102, 103,105,106.
Table 3.
An overview of delivery systems adopted for mRNA cancer vaccines in the preclinical setting.
| Type | Time | Delivery system or adjuvant | Administration | Size (nm) | Charge (mV) |
|---|---|---|---|---|---|
| Liposomes and their derivatives | 199541 | Liposomes | Intramuscular | / | / |
| 199969 | Hemagglutinating virus of Japan–liposomes | Injection directly into the spleen | / | / | |
| 200099 | Cationic liposome-protamine | Intravenous, subcutaneous | / | / | |
| 2006100 | Cationic liposomes; GM-CSF as the adjuvant | Intradermal, intravenous and subcutaneous | / | / | |
| 200970 | Histidylated lipopolyplexes (PEG-HpK/HDHE:cholesterol complexes) | Intravenous | 60–100 | / | |
| 201171 | Mannosylated and histidylated lipopolyplexes (Man11-LPR100) | Intravenous | 162 | 18.3 | |
| 201447 | NPs; cholera toxin as the adjuvant | Nasal | 180–300 | −12–40 | |
| 201548 | Cationic lipids | Intravenous | / | / | |
| 201574 | Mannosylated liposomes | Intravenous | / | / | |
| 201675 | Lipid mRNA particles | Intramuscular or intravenous | ∼242 | −58.9 | |
| 201622 | RNA-lipoplexes (DOTMA/DOPE liposomes) | Intravenous | 200–400 | / | |
| 201777 | LNP (ionizable lipid + phospholipid + cholesterol + lipid-anchored PEG); LPS as the adjuvant | Subcutaneous | 50–150 | −15, −3 | |
| 2017102 | A cell-penetrating peptide rich in the arginine peptide amphiphile RALA motif + DOTAP + DOPE | Intradermal | 90–144 | –7–26 | |
| 201850 | CLAN (PEG5K-b-PLGA11K + PLGA11K + cationic lipid BHEM-Chol) | Intravenous | ∼110 | 25 | |
| 201851,78 | LCP NPs (calcium phosphate cores + DOPA/DOPE + DOTAP + cholesterol + DSPE-PEG-2000+DSPE-PEG-mannose) | Subcutaneous | 58, ∼45 | 38, 0 | |
| 201852 | triMN-LPR (PEG-HpK + triMN-liposome) | Intradermal, intravenous and subcutaneous | / | / | |
| 201853 | PGCP NPs [cationic lipid-like compound (G0-C4)+PLGA + DSPE-PEG] | Intravenous | ∼120 | 6 | |
| 201854 | CARTs; CpG as the adjuvant | Subcutaneous or intravenous | / | / | |
| 201955 | ssPalmE-KALA [a vitaminE-scaffold (ssPalmE)-LNP (ssPalmE + DOPE + cholesterol)+an α-helical cationic peptide “KALA”] | Intradermal | 126 | 29 | |
| 201956,57 | LNPs (ionizable lipid + structural lipid + helper lipid + PEG-lipid) | Intratumoral or peritumoral, subcutaneous or intradermal | 80–100 | / | |
| 201958 | DOTAP-cholesterol LNPs, the NKT ligand α-GC as the adjuvant | Intravenous | 190 | 47 | |
| 201998 | A redox-responsive NP | Intravenous | / | / | |
| 202060 | Mann-capsule (PEI + polysaccharide layer) | Subcutaneous | ∼220 | −10 | |
| 202062 | LPC (DOTAP/cholesterol/DSPE-PEG-2000 cationic liposomes/protamine complex) | Nasal | 170 | 10 | |
| 202059 | DOTAP/DP7-C liposomes (DOTAP modified with cholesterol-modified cationic peptide DP7) | Subcutaneous | 130 | 35 | |
| 202161 | Lipid-like material C1 (cationic lipid)+DSPE-PEG-2000 | Subcutaneous | 150 | 16 | |
| 2021103 | VLVP [CpG-core (protamine + mRNA + CpG)+lipid mixture (cationic lipid EDOPC + helper lipid DOPE + PEGylated lipid DSPE-PEG2k)] | Intrafootpad injection | 80–90 | ∼25 | |
| 202197 | NPs (copolymer of mPEG-PLGA + cationic molecule G0-C14) | Intravenous | / | / | |
| 202194 | LNPs (Dlin-MC3-DMA + DOPE + cholesterol + C14-PEG2000) | Intravenous | / | / | |
| DC | 199642 | DCs | Intraperitoneal | / | / |
| 201273 | DCs; LPS, poly(I:C), or TriMix as the adjuvant | Intravenous | / | / | |
| 2015106 | DCs; Td toxoid as the adjuvant | Intradermal | / | / | |
| 202079 | DC | Subcutaneous | / | / | |
| Protamine | 201172 | Protamine-formulated (RNActive®) | Intravenous | 250–300 | / |
| 2014101 | Protamine-formulated (RNActive®) | Intravenous | / | / | |
| Nake mRNA | 201046 | Naked antigen (e.g., OVA)-encoding RNA | Intranodal, subcutaneous, intradermal | / | / |
| 2011105 | OVA naked mRNA, FLT3 ligand as the adjuvant | Intranodal | / | / | |
| 201690 | HPV16 E7-TriMix naked mRNA | Intranodal | / | / | |
| 201676 | TriMix naked mRNA | Intratumoral | / | / |
Abbreviations: BHEM-Chol, N,N-bis(2-hydroxyethyl)-N-methyl-N-(2-cholesteryloxycarbonyl aminoethyl) ammonium bromide; DOPA, dioleoylphosphatydic acid; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; DOTMA, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DSPE-PEG-2000, 1,2-distearoryl-sn-glycero-3-phosphoethanolamine-N-(methoxy[polyethyleneglycol-2000]); EDOPC, 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine; HDHE, l-histidine-(N,N-di-n-hexadecylamine)ethylamide; HpK, histidylated polylysine; histidylated lipopolyplexes, PEGylated derivative of HpK and HDHE liposomes; PEG5K-b-PLGA11K, poly(ethylene glycol)-block-poly(lactic-co-glycolic acid); PEI, polyethyleneimine.
One of the major vectors used in mRNA cancer vaccines is liposomes and their derivatives. In 1995, Conry et al.41 showed the humoral immunogenicity of liposome mRNA encoding human CEA complexes, which first confirmed the proof-of-concept of mRNA cancer vaccines in preclinical studies. Mannose can bind to mannose receptors expressed on the surface of DCs and facilitate efficient mRNA delivery and transfection using vectors targeting DCs. In 2011, Perche et al.71 showed that mRNA-loaded Man11-LPR100 was 4 times more efficient than sugar-free LPR100 in transfection of DCs and had a better antitumor effect in vivo. Due to the weak binding force between mannose and its receptors, increasing the density of mannose on the carrier surface may be an effective way to improve the delivery efficiency of mannose-modified LPR. In 2018, Le Moignic et al.52 showed that trimannosylated-LPR could more effectively induce transfection with antigens, recruit more DCs into the draining lymph nodes by inducing a local inflammatory response at the injection site, and more effectively induce antigen-specific immune responses than monomannosylated-LPR. In 2018, Wang et al.166 used mannose-cholesterol conjugates (MPn-CHs) to prepare DC-targeted liposomes (MPn-LPs) as mRNA carriers and showed that MP1000-LPs loaded with mRNA (MP1000-LPX) had good transfection efficiency and that MP1000-LPX enhanced mRNA expression mainly by enhancing expression of the mannose receptor (e.g., CD206) on DCs. In 2020, Son et al.60 showed that Mann-capsules, prepared using polysaccharide-coated silica nanoparticles, could activate bone marrow-derived dendritic cells (BMDCs) via Dectin-2 or TLR-4 and that the ability of Mann-capsules to promote BMDC differentiation and maturation was significantly stronger than that of PEI or Lipofectamine; moreover, PEI and Lipofectamine were highly toxic.
LNPs seem to be a promising carrier for delivery of mRNA cancer vaccines. The components of LNPs mainly include ionizable lipids, which facilitate self-assembly and endosomal release of mRNA; phospholipids, which support the lipid bilayer structure; cholesterol, a stabilizing agent; and lipid-anchored PEG, which extends the half-life of formulations. High-throughput techniques for screening and identifying these vectors have also been considered30. In 2017, Oberli et al.77 constructed and optimized an LNP library and showed that LNPs containing mRNAs encoding tumor antigens (e.g., gp100 and TRP2) combined with LPS as the adjuvant could effectively induce antigen-specific CD8+ T cells, inhibit tumor growth and prolong OS in mice. In 2018, McKinlay et al.167 performed a high-throughput screening study on a library of mRNA carriers based on amphiphilic CARTs and showed that dual CARTs could improve the in vitro mRNA transfection efficiency in lymphocytes by 9-fold compared with a single CART or Lipofectamine 2000; the in vitro mRNA transfection efficiency was >80%, and the mRNA transfection efficiency in mouse lymphocytes was >1.5%167. In 2019, Miao et al.57 established a high-throughput technique for ionizable lipidoid construction, which enabled synthesis of thousands of lipid formulations in one day, and used DCs (e.g., HeLa cells, BMDCs or bone marrow-derived macrophages) to evaluate the transfection efficiency of the LNPs with high throughput. The results showed that the mRNA LNPs could induce APC maturation and enhance antitumor efficacy through the intracellular stimulator of interferon genes pathway57. In 2021, Meng et al.103 showed that VLVPs containing CpG cores can promote DC maturation and antigen presentation, antigen-specific CD8+ T cell proliferation in lymphatic organs and T cell infiltration in tumors and decrease immunosuppressive cells (e.g., tumor-associated bone marrow-derived suppressor cells and arginase 1-expressing suppressive DCs). mRNA cancer vaccines using LNPs as the carrier [e.g., mRNA-4157 encoding neoantigens (NCT03313778, NCT03897881) and V941 encoding mutated proteins (NCT03948763)] are currently in clinical trials.
The polycationic peptide protamine and DCs are two other major vectors adopted for use in mRNA cancer vaccines. The characteristics of protamine and DCs and the progress in their use as vectors in mRNA cancer vaccines are summarized in Sections 2.1.1, 2.2.2, respectively. Protamine can protect mRNA from being degraded by serum RNases to promote mRNA delivery. Additionally, protamine can be used in combination with liposomes (e.g., cationic liposome-protamine99, LPC62 and VLVP103). In 2000, Hoerr et al.99 showed that a liposome-encapsulated condensed RNA–peptide complex could induce antigen-specific cellular and humoral immune responses and that both naked and protamine-protected RNA could induce a specific immune response in vivo, while the protected RNA was stable in vitro for a longer period of time. Mai et al.62 showed that intranasal delivery of a LPC loaded with cytokeratin 19-encoding mRNA could induce APC maturation and strong cellular immune responses and decrease the growth of tumors in mice. The carrier has a critical effect on the efficacy of the vaccine. Phua et al.47 showed that intranasal naked mRNA vaccination cannot induce antitumor immune responses in the absence of a NP vectors. However, both preclinical and clinical studies have demonstrated the feasibility and effectiveness of naked mRNA based on comprehensive consideration of selected targets, adjuvants and delivery methods. FLT3 was found to enhance the antitumor effects of intranodal naked RNA105. Both intranodal HPV16-E7-TriMix naked mRNA and intratumoral TriMix naked mRNA have been shown to induce efficient antitumor T cell responses76,90. An intranodal naked RNA-based multiple neoepitope vaccine induced effective antigen-specific T cell immune responses (NCT0203595649).
2.4. Adjuvants
A major class of adjuvants [e.g., LPS73, poly(I:C)73, Td106, CpG54] developed in preclinical studies of mRNA cancer vaccines is PAMPs, which activate DCs through PAMP–PRR pathways and then modulate innate and adaptive immune responses. As a potent recall antigen, Td can promote DC migration and improve antitumor effects through the CCL3106. Adjuvant LPS treatment further improved the level of CD8+ T cells and the antitumor activity of the cells induced by LNP mRNAs77. The in vivo antitumor effects of mRNA-CART combined with CpG were significantly stronger than those of mRNA-CART and the combination of naked mRNA and CpG, which were also stronger than those of the combination of mRNA-CART and TLR7 ligand or CD80/86 mRNA54.
Most novel carriers (e.g., lipid-like material C161, DOTAP/DP7-C59, Mann-capsule60, LNPs57,58) have the characteristics of both carriers and adjuvants, while some of those carriers function as adjuvants and are similar to PAMPs. Mann-capsules activate BMDCs through Dectin-2 or TLR-4, and dextran-capsules activate BMDCs through CD206, CD209, or macrophage-inducible C-type lectin60. DP7-C, DOTAP and mRNA can activate DCs through TLR2, TLR4 and TLR7, respectively. The ability of DOTAP/DP7-C to induce DC maturation and antigen presentation was significantly stronger than that of DOTAP, poly(I:C) and CpG59. C1 or C1 mRNA can promote BMDC activation through the TLR4-dependent nuclear factor κB signaling pathway, and the in vivo antitumor effects of C1-OVA mRNA were TLR4-dependent61. CpG, as the adjuvant contained in the VLVP, can improve the effects of the vaccine and prevent PD-1 expression in T cells103. However, these PAMP-like adjuvants may affect the translation efficiency and degradation of mRNAs and have potential toxicity, as discussed in Section 2.2.1.
Developing novel adjuvants that do not affect the efficiency of mRNA translation and can also positively regulate a variety of innate and adaptive immune responses with relatively mild proinflammatory effects and low toxicity has become an important direction. The immunogenicity of TAA mRNA can be augmented by codelivery of GM-CSF mRNA100. FLT3 ligand can promote the amplification of plasmacytoid DCs, classical DCs and NK cells; induce a T helper 1-type microenvironment; enhance antigen-specific CD8+ T cells in lymph nodes; and enhance T cell infiltration in tumors and the antitumor effects of naked RNA105. mRNA LNPs can induce APC maturation through stimulator of interferon genes-dependent activation of type I IFN, restrict systemic cytokine expression and enhance antitumor efficacy57. The adjuvant α-GC adopted for use in mRNA Galsomes can be presented by DCs to activate iNKT cells. Activated iNKT cells can have bidirectional positive regulatory effects with DCs and can positively regulate NK cells and immunosuppressive cells (e.g., MDSCs and M1 TAMs), which does not affect the efficiency of mRNA translation and promotes direct and indirect antitumor effects58. mRNA encoding immune costimulatory molecules can be used as an adjuvant (discussed in Section 2.1.3). TriMix (NCT01066390, NCT01302496); mRNA-2752 (NCT02872025); mRNA encoding human OX40L (mRNA-2416, NCT03323398); mRNA encoding IL-12, IL-15, GM-CSF and IFN-α (BNT131, NCT03871348); mRNA encoding IL-12 (MEDI1191, NCT03946800); and mRNA encoding a TLR7/8-agonist and RIG-1-agonist [CV8102 (RNAdjuvant®), NCT03291002, NCT03203005] are in clinical trials as adjuvant treatments in combination with immune checkpoint inhibitors.
2.5. Administration routes
The administration route has a direct effect on the efficacy of a vaccine, and the administration routes often adopted for use in mRNA cancer vaccines include intravenous, intradermal, subcutaneous, intramuscular, intranodal, and intratumoral administration. Intravenous administration allows for a larger vaccine volume and direct delivery of the vaccine into the lymphatic organs168 but also carries a greater risk of systemic toxicity169,170. The dermis into which the vaccine is intradermally injected contains APCs (e.g., DCs and macrophages) as well as vascular and lymphatic vessels171,172 but is mainly composed of dense connective tissue, resulting in a small vaccine volume when administered via the intradermal route. Intradermal delivery can also lead to adverse reactions at the injection site (e.g., swelling, pain, erythema and pruritus)173. The subcutis region into which the vaccine is subcutaneously injected contains fewer APCs than the dermis; however, it is mainly composed of a loose network of adipose tissues, permitting a larger injection volume via the subcutaneous route, which induces fewer local side effects (e.g., pain)174,175. The muscle into which a vaccine is intramuscularly injected contains dense blood networks that can help recruit and recirculate different types of immune cells (e.g., infiltrating APCs) to the injection site176. Intramuscular administration permits a relatively larger injection volume than the intradermal route and causes milder local side effects than the intradermal and subcutaneous routes177. Lymph nodes into which a vaccine is intranodally injected contain multiple APCs, and intranodal administration has a high delivery efficiency178, permits a small volume of vaccine, but involves complicated procedures179. Intratumoral administration is mainly used for mRNA vaccines encoding immunocostimulatory molecules (e.g., TriMix, CV8102, mRNA-2752, mRNA-2416, BNT131 and MEDI1191) as immunoadjuvant therapy and also permits a small vaccine volume and involves complicated procedures.
Predicting exactly the best administration route for a particular vaccine is difficult, and direct comparative studies are recommended to select the best administration route for a vaccine. The in vivo transfection efficiency of Luc-RNA injected via intranodal administration was superior to that following intradermal or subcutaneous administration46. The killing effects of CTLs induced by intravenous administration of OVA RNA complexed with DOTAP-DOPE on target cells were superior to those induced by intradermal or subcutaneous administration (intravenous > intradermal > subcutaneous)100. The in vivo cytotoxicity of antigen-specific T cells induced by intranodal delivery of OVA mRNA combined with TriMix was significantly stronger than that induced by intradermal delivery of the vaccine73. The antigen-specific T cell responses induced by intradermal vaccination with E7 mRNA monomannosylated-LPR were significantly stronger than those induced by the subcutaneous route52. mRNA was efficiently delivered into APCs by CARTs (secondary lymphoid APCs were preferentially targeted via intravenous injection, while local APCs were targeted via subcutaneous injection)54. The antitumor effects of intratumorally administered triplet mRNAs encoding IL-23, IL-36, and OX40L at three doses were significantly better than those of the same drug administered intradermally or subcutaneously; however, there was no significant difference in the antitumor effects via these routes at one dose, suggesting that the effect of administration on tumor vaccines is also related to the frequency of administration56. The nasal mucosa is rich in APCs and immune cells, and preclinical studies have demonstrated the preliminary effectiveness of mRNA cancer vaccines injected via intranasal administration. Both intranasal mRNA nanoparticle vaccination47 and intranasal delivery of LPC mRNA62 can induce antitumor immune responses.
3. Challenges and trends in mRNA cancer vaccines
To date, hundreds of cancer vaccines have undergone clinical evaluation180,181, and the U.S. FDA has approved three therapeutic cancer vaccines182 [Bacillus Calmette-Guerin (TheraCys®), a live attenuated strain of Mycobacterium bovis for the treatment of nonmuscle invasive bladder cancer; Sipuleucel-T (Provenge®), a DC vaccine for the treatment of metastatic castration-resistant prostate cancer; and an oncolytic herpes virus vaccine (talimogene laherparepvec, T-VEC) (Imlygic®) for the treatment of advanced melanoma] and two prophylactic cancer vaccines29 [a HPV vaccine and a hepatitis B virus vaccine]. Factors influencing the development of mRNA cancer vaccines mainly include intrinsic factors of the molecule itself and external factors [e.g., the central tolerance to tumor antigens (discussed in Section 2.1.1), the heterogeneity of tumors and HLA183, and the tumor immune microenvironment105,112]. These factors have deeply influenced the development of mRNA cancer vaccines.
In view of intrinsic factors, researchers have improved the effectiveness of mRNA cancer vaccines to a certain extent by improving the mRNA structure and sequences, advancing mRNA preparation and purification technologies (discussed in Section 2.2.1) and developing novel delivery vectors (discussed in Section 2.3). The essence of tumor heterogeneity is the genomic heterogeneity of tumor cells, leading to antigen heterogeneity, which is the key factor affecting the generation of antitumor T cell responses and the main reason for developing personalized cancer vaccines. HLA heterogeneity mainly refers to the different types of HLA molecules among individuals, resulting in differences among individuals in the binding region or binding affinity of those molecules to the tumor antigen, which affects the generation and strength of antitumor T cell responses. HLA heterogeneity is caused by polymorphisms of HLA alleles in different ethnic and regional populations183,184. HLA I alleles include 9–11 common supertypes, of which the coverage rate is 90%185,186. HLA-A loci (HLA-A alleles: A∗0101, A∗0201, A∗0301, A∗1101 and A∗2402) account for 60% of HLA I alleles, and HLA-B loci (HLA-B alleles: B∗0702, B∗0801, B∗2705, B∗3501 and B∗5701) account for more than 35% of those alleles 183.
The TME is composed of immune cells, mesenchymal cells and various cytokines and tissue factors, which play an important role in tumorigenesis and immune escape187,188. Interstitial pressure within a large mass can reduce the diffusion of large molecules (e.g., antibodies) and effector cells (e.g., T cells)67. Most solid tumors also lack T cell costimulatory molecules189. The TME often contains immunosuppressive cells, including CD4+ Tregs, MDSCs, suppressor CD8+ T cells, M2 TAMs and regulatory NK/NKT cells190,191. These immunosuppressive cells and tumor cells in the TME can release a large number of soluble immunosuppressive factors, including transforming growth factor β, IL-10, PD-L1, indoleamine 2,3-dioxygenase and vascular endothelial growth factor, into the microenvironment192,193. With regard to tumor and HLA heterogeneity, the development of tumor neoantigen vaccines, which in theory have much stronger specific antitumor effects and weaker toxic side effects than TAA-directed vaccines, has been a leading hotspot in cancer vaccine research (discussed in Section 2.1.2). In view of the tumor immune microenvironment, the development of immune-based combination therapies (e.g., combination with adjuvants or immune checkpoint inhibitors) has been a key trend in the application of cancer vaccines (discussed in Sections 2.1.4, 2.4).
4. Discussion
Preclinical evaluation of a product is a prerequisite to entering clinical translation, and reasonable evaluation can improve the reliability of predicted clinical outcomes. Moreover, the parameters, technologies and methods adopted in preclinical evaluations are an important part of the quality standard of vaccines. Preclinical evaluation of vaccines should fully elucidate the action and mechanism of vaccines, and the key points in preclinical evaluation of mRNA cancer vaccines are to identify the production (e.g., number and activation of T cells) and the effect (e.g., killing effect and antigen affinity of T cells) of antigen-specific T cell responses. In general, quantitative in vitro and in vivo tests are used to evaluate the effects and mechanisms of mRNA cancer vaccines in preclinical evaluations. According to the current understanding of antigen-specific T cell immune responses, the evaluated parameters mainly include ① identification of the physical and chemical characteristics of the vaccine; ② the efficiency of APC transfection with the mRNA (e.g., binding and uptake, internalization and transport, and expression and distribution); ③ differentiation, maturation and antigen presentation of APCs; ④ immunological stimulation by vaccines; ⑤ cellular immunogenicity (e.g., production, proliferation and target cell killing of CTLs); ⑥ humoral immunogenicity; ⑦ antitumor effect and associated mechanism; and ⑧ preliminary toxicities (e.g., cytotoxicity, visceral toxicity and hemolysis).
Factors such as particle size, charge, the binding capability to mRNA and load rate, stability (e.g., time, temperature, and serum stability), PEGylation, and hardness can affect antigen delivery (e.g., lymphatic drainage). Son et al.60 showed that Mann-capsules ∼220 nm in size had good deformability, and the recovery rate of the capsules after being passed through a 50 nm pore membrane was approximately 30%. Different from protein-based or peptide-based vaccines, the first step in mRNA vaccine development is ensuring that the information encoded in the mRNA sequence can be effectively translated into the corresponding protein or peptide. The in vitro and in vivo transfection efficiencies of mRNA are important parameters in the preclinical evaluation of mRNA vaccines, and the key way to improving the pharmacodynamics of an mRNA vaccine is to improve the mRNA transfection efficiency194. However, the in vitro transfection efficiency can be different in APCs, the in vitro efficiency of transfection with DOTAP/DP7-C-enhanced green fluorescent protein mRNA in APCs, including 293T, JAWSII, DC2.4 and BMDCs, was 84.87 ± 3.21%, 12.23 ± 1.35%, 28.49 ± 2.46% and 14.51 ± 2.35%, respectively59. To verify the mRNA transfection efficiency in vitro and in vivo, a variety of APCs were used as in vitro transfection models, and the mRNA transfection efficiencies for a variety of APCs from different organs was detected in vivo. Enhanced green fluorescent protein or FLuc is often used as the transfection protein in experiments. A dye-tagged mRNA or vaccine vector has been widely used in transfection studies. Confocal microscopy and flow cytometry are often used to evaluate intracellular microtransfection (e.g., delivery, uptake, and translation), confocal microscopy can be used to evaluate the translation efficiency of mRNA more intuitively and accurately, and an interactive video information system is used to evaluate systemic or local macrotransfection (e.g., distribution, lymphatic drainage).
The tumor models used for evaluating in vivo antitumor activity mainly include therapeutic or prophylactic subcutaneous, in situ and lung metastasis tumor models, and the established in vivo tumor models should accurately simulate human pathology. Bialkowski et al.90 showed that the TME of TC-1 tumors can be significantly different depending on the site of tumor inoculation (i.e., subcutaneous, in the lungs and in the genital tract), which directly affected the antitumor effects of the E7-TriMix mRNA vaccine. Species specificity is an important factor to be considered when selecting cell or animal models for preclinical evaluation. To obtain more accurate evaluation information, humanized animal models can be established. Do ASS used humanized mice, which were established via intravenous injection of CD34+ hematopoietic stem cells from human peripheral blood mononuclear cells (HLA-A2 type) into immunodeficient NOD/Shi-scid IL-2Rγnull mice or C57BL/6 mice, to establish in vivo tumor models (e.g., human brain tumor stem cell 5 and murine GL261) and showed that the median survival of the humanized mouse model treated by DCs transfected with modified human CD133 mRNA was more than 60 days and that of the homologous mouse tumor model treated by DCs transfected with modified mouse CD133 mRNA was 38 days79. Cancer vaccines can resist tumor recurrence and metastasis in theory, and multiple in vivo tumor models could be established for evaluation to clarify the advantages of these vaccines related to preventing or treating tumor metastasis or recurrence.
Effective antitumor responses require the synergistic action of multiple immune cells rather than the action of a single cell. At present, most tumor vaccines focus on inducing CD8+ T cells, but CD4+ T cells and other immune cells also play important roles in inducing and maintaining immune memory and enhancing the tumor-killing effect of CTLs67,68,105. mRNA Galsomes adopting an NKT ligand (e.g., α-GC) as the adjuvant induced antitumor effects through iNKT cells58. DCs transfected with CD133 mRNA in an incubation system containing both CD4+ and CD8+ T cells can activate T cells and kill tumor target cells more effectively than DCs in an incubation system containing only CD4+ or CD8+ T cells79. There are also concerns about the safety and side effects of mRNA cancer vaccines. Modified mRNA can combine with serum protein to form a vascular occlusion, which has potential toxicity. DOTAP/DP7-C mRNA shows good serum stability and low cytotoxicity; however, Lipo2000 and PEI25K have strong cytotoxicity59. Finally, we look forward to the successful clinical translation of mRNA cancer vaccines.
Author contributions
Jun-zhi Wang designed the study. Qing He and Heng Zhang collected the data and wrote the manuscript. Hua GAO, Dejiang Tan, and Jun-zhi Wang checked and revised the article. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.BrenneR S., Jacob F., Meselson M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature. 1961;190:576–581. doi: 10.1038/190576a0. [DOI] [PubMed] [Google Scholar]
- 2.Xu S., Yang K., Li R., Zhang L. mRNA vaccine era-mechanisms, drug platform and clinical prospection. Int J Mol Sci. 2020;21:6582. doi: 10.3390/ijms21186582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shroff R.T., Chalasani P., Wei R., Pennington D., Quirk G., Schoenle M.V., et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27:2002–2011. doi: 10.1038/s41591-021-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petsch B., Schnee M., Vogel A.B., Lange E., Hoffmann B., Voss D., et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol. 2012;30:1210–1216. doi: 10.1038/nbt.2436. [DOI] [PubMed] [Google Scholar]
- 8.Pardi N., Parkhouse K., Kirkpatrick E., McMahon M., Zost S.J., Mui B.L., et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat Commun. 2018;9:3361. doi: 10.1038/s41467-018-05482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman R.A., Fuhr R., Smolenov I., Mick Ribeiro A., Panther L., Watson M., et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326–3334. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 10.Guardo A.C., Joe P.T., Miralles L., Bargalló M.E., Mothe B., Krasniqi A., et al. Preclinical evaluation of an mRNA HIV vaccine combining rationally selected antigenic sequences and adjuvant signals (HTI-TriMix) AIDS. 2017;31:321–332. doi: 10.1097/QAD.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 11.Jong W., Leal L., Buyze J., Pannus P., Guardo A., Salgado M., et al. Therapeutic vaccine in chronically HIV-1-infected patients: a randomized, double-blind, placebo-controlled phase IIa trial with HTI-TriMix. Vaccines (Basel) 2019;7:209. doi: 10.3390/vaccines7040209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erasmus J.H., Khandhar A.P., O'Connor M.A., Walls A.C., Hemann E.A., Murapa P., et al. An alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang N.N., Li X.F., Deng Y.Q., Zhao H., Huang Y.J., Yang G., et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182:1271–1283. doi: 10.1016/j.cell.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnee M., Vogel A.B., Voss D., Petsch B., Baumhof P., Kramps T., et al. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Neglected Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 16.Pardi N., Hogan M.J., Pelc R.S., Muramatsu H., Andersen H., DeMaso C.R., et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richner J.M., Himansu S., Dowd K.A., Butler S.L., Salazar V., Fox J.M., et al. Modified mRNA vaccines protect against Zika virus infection. Cell. 2017;168:1114–1125. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Z., Portela Catani J.P., McCafferty S., Couck L., Van Den Broeck W., Gorlé N., et al. Immunogenicity and protection efficacy of a naked self-replicating mRNA-based Zika virus vaccine. Vaccines (Basel) 2019;7:96. doi: 10.3390/vaccines7030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samsa M.M., Dupuy L.C., Beard C.W., Six C.M., Schmaljohn C.S., Mason P.W., et al. Self-amplifying RNA vaccines for Venezuelan equine encephalitis virus induce robust protective immunogenicity in mice. Mol Ther. 2019;27:850–865. doi: 10.1016/j.ymthe.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruggi G., Chiarot E., Giovani C., Buccato S., Bonacci S., Frigimelica E., et al. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine. 2017;35:361–368. doi: 10.1016/j.vaccine.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 21.Xue T., Stavropoulos E., Yang M., Ragno S., Vordermeier M., Chambers M., et al. RNA encoding the MPT83 antigen induces protective immune responses against Mycobacterium tuberculosis infection. Infect Immun. 2004;72:6324–6329. doi: 10.1128/IAI.72.11.6324-6329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 23.Pastor F., Berraondo P., Etxeberria I., Frederick J., Sahin U., Gilboa E., et al. An RNA toolbox for cancer immunotherapy. Nat Rev Drug Discov. 2018;17:751–767. doi: 10.1038/nrd.2018.132. [DOI] [PubMed] [Google Scholar]
- 24.Ramaswamy S., Tonnu N., Tachikawa K., Limphong P., Vega J.B., Karmali P.P., et al. Systemic delivery of factor IX messenger RNA for protein replacement therapy. Proc Natl Acad Sci U S A. 2017;114:E1941–E1950. doi: 10.1073/pnas.1619653114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumann C., Nguyen D.X., Norgard M., Bortnyak Y., Korzun T., Chan S., et al. Increasing lean muscle mass in mice via nanoparticle-mediated hepatic delivery offollistatin mRNA. Theranostics. 2018;8:5276–5288. doi: 10.7150/thno.27847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prieve M.G., Harvie P., Monahan S.D., Roy D., Li A.G., Blevins T.L., et al. Targeted mRNA therapy for ornithine transcarbamylase deficiency. Mol Ther. 2018;26:801–813. doi: 10.1016/j.ymthe.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tombácz I., Weissman D., Pardi N. Vaccination with messenger RNA: a promising alternative to DNA vaccination. Methods Mol Biol. 2021;2197:13–31. doi: 10.1007/978-1-0716-0872-2_2. [DOI] [PubMed] [Google Scholar]
- 29.Miao L., Zhang Y., Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linares-Fernández S., Lacroix C., Exposito J.Y., Verrier B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol Med. 2020;26:311–323. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Faghfuri E., Pourfarzi F., Faghfouri A.H., AbdoliShadbad M., Hajiasgharzadeh K., Baradaran B. Recent developments of RNA-based vaccines in cancer immunotherapy. Expet Opin Biol Ther. 2021;21:201–218. doi: 10.1080/14712598.2020.1815704. [DOI] [PubMed] [Google Scholar]
- 33.Van Nuffel A.M., Wilgenhof S., Thielemans K., Bonehill A. Overcoming HLA restriction in clinical trials: immune monitoring of mRNA-loaded DC therapy. Oncoimmunology. 2012;1:1392–1394. doi: 10.4161/onci.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iavarone C., O'hagan D.T., Yu D., Delahaye N.F., Ulmer J.B. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines. 2017;16:871–881. doi: 10.1080/14760584.2017.1355245. [DOI] [PubMed] [Google Scholar]
- 35.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson N.A.C., Kester K.E., Casimiro D., Gurunathan S., DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furuichi Y., Miura K. A blocked structure at the 5′ terminus of mRNA from cytoplasmic polyhedrosis virus. Nature. 1975;253:374–375. doi: 10.1038/253374a0. [DOI] [PubMed] [Google Scholar]
- 38.Krieg P.A., Melton D.A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984;12:7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malone R.W., Felgner P.L., Verma I.M. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci U S A. 1989;86:6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinon F., Krishnan S., Lenzen G., Magné R., Gomard E., Guillet J.G., et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol. 1993;23:1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 41.Conry R.M., LoBuglio A.F., Wright M., Sumerel L., Pike M.J., Johanning F., et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 1995;55:1397–1400. [PubMed] [Google Scholar]
- 42.Boczkowski D., Nair S.K., Snyder D., Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heiser A., Coleman D., Dannull J., Yancey D., Maurice M.A., Lallas C.D., et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–417. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Weide B., Pascolo S., Scheel B., Derhovanessian E., Pflugfelder A., Eigentler T.K., et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32:498–507. doi: 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]
- 46.Kreiter S., Selmi A., Diken M., Koslowski M., Britten C.M., Huber C., et al. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010;70:9031–9040. doi: 10.1158/0008-5472.CAN-10-0699. [DOI] [PubMed] [Google Scholar]
- 47.Phua K.K., Staats H.F., Leong K.W., Nair S.K. Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Sci Rep. 2014;4:5128. doi: 10.1038/srep05128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kreiter S., Vormehr M., van de Roemer N., Diken M., Löwer M., Diekmann J., et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahin U., Derhovanessian E., Miller M., Kloke B.P., Simon P., Löwer M., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 50.Fan Y.N., Li M., Luo Y.L., Chen Q., Wang L., Zhang H.B., et al. Cationic lipid-assisted nanoparticles for delivery of mRNA cancer vaccine. Biomater Sci. 2018;6:3009–3018. doi: 10.1039/c8bm00908b. [DOI] [PubMed] [Google Scholar]
- 51.Liu L., Wang Y., Miao L., Liu Q., Musetti S., Li J., et al. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther. 2018;26:45–55. doi: 10.1016/j.ymthe.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Moignic A., Malard V., Benvegnu T., Lemiègre L., Berchel M., Jaffrès P.A., et al. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J Control Release. 2018;278:110–121. doi: 10.1016/j.jconrel.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 53.Islam M.A., Xu Y., Tao W., Ubellacker J.M., Lim M., Aum D., et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat Biomed Eng. 2018;2:850–864. doi: 10.1038/s41551-018-0284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haabeth O.A.W., Blake T.R., McKinlay C.J., Waymouth R.M., Wender P.A., Levy R. mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice. Proc Natl Acad Sci U S A. 2018;115:E9153–E9161. doi: 10.1073/pnas.1810002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tateshita N., Miura N., Tanaka H., Masuda T., Ohtsuki S., Tange K., et al. Development of a lipoplex-type mRNA carrier composed of an ionizable lipid with a vitamin E scaffold and the KALA peptide for use as an ex vivo dendritic cell-based cancer vaccine. J Control Release. 2019;310:36–46. doi: 10.1016/j.jconrel.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Hewitt S.L., Bai A., Bailey D., Ichikawa K., Zielinski J., Karp R., et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aat9143. [DOI] [PubMed] [Google Scholar]
- 57.Miao L., Li L., Huang Y., Delcassian D., Chahal J., Han J., et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat Biotechnol. 2019;37:1174–1185. doi: 10.1038/s41587-019-0247-3. [DOI] [PubMed] [Google Scholar]
- 58.Verbeke R., Lentacker I., Breckpot K., Janssens J., Van Calenbergh S., DeSmedt S.C., et al. Broadening the message: a nanovaccine co-loaded with messenger RNA and α-GalCer induces antitumor immunity through conventional and natural killer T cells. ACS Nano. 2019;13:1655–1669. doi: 10.1021/acsnano.8b07660. [DOI] [PubMed] [Google Scholar]
- 59.Zhang R., Tang L., Tian Y., Ji X., Hu Q., Zhou B., et al. DP7-C-modified liposomes enhance immune responses and the antitumor effect of a neoantigen-based mRNA vaccine. J Control Release. 2020;328:210–221. doi: 10.1016/j.jconrel.2020.08.023. [DOI] [PubMed] [Google Scholar]
- 60.Son S., Nam J., Zenkov I., Ochyl L.J., Xu Y., Scheetz L., et al. Sugar-nanocapsules imprinted with microbial molecular patterns for mRNA vaccination. Nano Lett. 2020;20:1499–1509. doi: 10.1021/acs.nanolett.9b03483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H., You X., Wang X., Cui L., Wang Z., Xu F., et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2005191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mai Y., Guo J., Zhao Y., Ma S., Hou Y., Yang J. Intranasal delivery of cationic liposome–protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell Immunol. 2020;354:104143. doi: 10.1016/j.cellimm.2020.104143. [DOI] [PubMed] [Google Scholar]
- 63.BioNTech . November 19, 2021. BioNTech receives FDA fast track designation for its FixVac candidate BNT111 in advanced melanoma.https://investors.biontech.de/news-releases/news-release-details/biontech-receives-fda-fast-track-designation-its-fixvac Available from: [Google Scholar]
- 64.Melief C.J., van Hall T., Arens R., Ossendorp F., van der Burg S.H. Therapeutic cancer vaccines. J Clin Invest. 2015;125:3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollingsworth R.E., Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blass E., Ott P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18:215–229. doi: 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saxena M., van der Burg S.H., Melief C.J.M., Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21:360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 68.Kwak M., Leick K.M., Melssen M.M., Slingluff C.L., Jr. Vaccine strategy in melanoma. Surg Oncol Clin. 2019;28:337–351. doi: 10.1016/j.soc.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou W.Z., Hoon D.S., Huang S.K., Fujii S., Hashimoto K., Morishita R., et al. RNA melanoma vaccine: induction of antitumor immunity by human glycoprotein 100 mRNA immunization. Hum Gene Ther. 1999;10:2719–2724. doi: 10.1089/10430349950016762. [DOI] [PubMed] [Google Scholar]
- 70.Mockey M., Bourseau E., Chandrashekhar V., Chaudhuri A., Lafosse S., Le Cam E., et al. mRNA-based cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexes. Cancer Gene Ther. 2007;14:802–814. doi: 10.1038/sj.cgt.7701072. [DOI] [PubMed] [Google Scholar]
- 71.Perche F., Benvegnu T., Berchel M., Lebegue L., Pichon C., Jaffrès P.A., et al. Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine. 2011;7:445–453. doi: 10.1016/j.nano.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 72.Fotin-Mleczek M., Duchardt K.M., Lorenz C., Pfeiffer R., Ojkić-Zrna S., Probst J., et al. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J Immunother. 2011;34:1–15. doi: 10.1097/CJI.0b013e3181f7dbe8. [DOI] [PubMed] [Google Scholar]
- 73.Van Lint S., Goyvaerts C., Maenhout S., Goethals L., Disy A., Benteyn D., et al. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res. 2012;72:1661–1671. doi: 10.1158/0008-5472.CAN-11-2957. [DOI] [PubMed] [Google Scholar]
- 74.Markov O.V., Mironova N.L., Shmendel E.V., Serikov R.N., Morozova N.G., Maslov M.A., et al. Multicomponent mannose-containing liposomes efficiently deliver RNA in murine immature dendritic cells and provide productive anti-tumour response in murine melanoma model. J Control Release. 2015;213:45–56. doi: 10.1016/j.jconrel.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 75.Broos K., Van der Jeught K., Puttemans J., Goyvaerts C., Heirman C., Dewitte H., et al. Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific T-cell responses despite the induction of type I interferon. Mol Ther Nucleic Acids. 2016;5:e326. doi: 10.1038/mtna.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Lint S., Renmans D., Broos K., Goethals L., Maenhout S., Benteyn D., et al. Intratumoral delivery of TriMix mRNA results in T-cell activation by cross-presenting dendritic cells. Cancer Immunol Res. 2016;4:146–156. doi: 10.1158/2326-6066.CIR-15-0163. [DOI] [PubMed] [Google Scholar]
- 77.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A., et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y., Zhang L., Xu Z., Miao L., Huang L. mRNA vaccine with antigen-specific checkpoint blockade induces an enhanced immune response against established melanoma. Mol Ther. 2018;26:420–434. doi: 10.1016/j.ymthe.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Do A.S.S., Amano T., Edwards L.A., Zhang L., De Peralta-Venturina M., Yu J.S. CD133 mRNA-loaded dendritic cell vaccination abrogates glioma stem cell propagation in humanized glioblastoma mouse model. Mol Ther Oncolytics. 2020;18:295–303. doi: 10.1016/j.omto.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reinhard K., Rengstl B., Oehm P., Michel K., Billmeier A., Hayduk N., et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367:446–453. doi: 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- 81.Kyte J.A., Mu L., Aamdal S., Kvalheim G., Dueland S., Hauser M., et al. Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Ther. 2006;13:905–918. doi: 10.1038/sj.cgt.7700961. [DOI] [PubMed] [Google Scholar]
- 82.Vik-Mo E.O., Nyakas M., Mikkelsen B.V., Moe M.C., Due-Tønnesen P., Suso E.M., et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother. 2013;62:1499–1509. doi: 10.1007/s00262-013-1453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Vries I.J., Bernsen M.R., Lesterhuis W.J., Scharenborg N.M., Strijk S.P., Gerritsen M.J., et al. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23:5779–5787. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]
- 84.Wang D., Zhang B., Gao H., Ding G., Wu Q., Zhang J., et al. Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. BMC Cancer. 2014;14:251. doi: 10.1186/1471-2407-14-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sahin U., Oehm P., Derhovanessian E., Jabulowsky R.A., Vormehr M., Gold M., et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 86.Anguille S., Van de Velde A.L., Smits E.L., Van Tendeloo V.F., Juliusson G., Cools N., et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood. 2017;130:1713–1721. doi: 10.1182/blood-2017-04-780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Tendeloo V.F., Van de Velde A., Van Driessche A., Cools N., Anguille S., Ladell K., et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms' tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A. 2010;107:13824–13829. doi: 10.1073/pnas.1008051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lichtenegger F.S., Schnorfeil F.M., Rothe M., Deiser K., Altmann T., Bücklein V.L., et al. Toll-like receptor 7/8-matured RNA-transduced dendritic cells as post-remission therapy in acute myeloid leukaemia: results of a phase I trial. Clin Transl Immunology. 2020;9:e1117. doi: 10.1002/cti2.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Q.T., Nie Y., Sun S.N., Lin T., Han R.J., Jiang J., et al. Tumor-associated antigen-based personalized dendritic cell vaccine in solid tumor patients. Cancer Immunol Immunother. 2020;69:1375–1387. doi: 10.1007/s00262-020-02496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bialkowski L., van Weijnen A., Van der Jeught K., Renmans D., Daszkiewicz L., Heirman C., et al. Intralymphatic mRNA vaccine induces CD8 T-cell responses that inhibit the growth of mucosally located tumours. Sci Rep. 2016;6:22509. doi: 10.1038/srep22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cafri G., Gartner J.J., Zaks T., Hopson K., Levin N., Paria B.C., et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J Clin Invest. 2020;130:5976–5988. doi: 10.1172/JCI134915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bonehill A., Tuyaerts S., Van Nuffel A.M., Heirman C., Bos T.J., Fostier K., et al. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther. 2008;16:1170–1180. doi: 10.1038/mt.2008.77. [DOI] [PubMed] [Google Scholar]
- 93.Hotz C., Wagenaar T.R., Gieseke F., Bangari D.S., Callahan M., Cao H., et al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abc7804. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y., Tiruthani K., Li S., Hu M., Zhong G., Tang Y., et al. mRNA delivery of a bispecific single-domain antibody to polarize tumor-associated macrophages and synergize immunotherapy against liver malignancies. Adv Mater. 2021;33 doi: 10.1002/adma.202007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilgenhof S., Van Nuffel A.M.T., Benteyn D., Corthals J., Aerts C., Heirman C., et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol. 2013;24:2686–2693. doi: 10.1093/annonc/mdt245. [DOI] [PubMed] [Google Scholar]
- 96.De Keersmaecker B., Claerhout S., Carrasco J., Bar I., Corthals J., Wilgenhof S., et al. TriMix and tumor antigen mRNA electroporated dendritic cell vaccination plus ipilimumab: link between T-cell activation and clinical responses in advanced melanoma. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin Y.X., Wang Y., Ding J., Jiang A., Wang J., Yu M., et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.aba9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kong N., Tao W., Ling X., Wang J., Xiao Y., Shi S., et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aaw1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoerr I., Obst R., Rammensee H.G., Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol. 2000;30:1–7. doi: 10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 100.Hess P.R., Boczkowski D., Nair S.K., Snyder D., Gilboa E. Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol Immunother. 2006;55:672–683. doi: 10.1007/s00262-005-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fotin-Mleczek M., Zanzinger K., Heidenreich R., Lorenz C., Kowalczyk A., Kallen K.J., et al. mRNA-based vaccines synergize with radiation therapy to eradicate established tumors. Radiat Oncol. 2014;9:180. doi: 10.1186/1748-717X-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Udhayakumar V.K., De Beuckelaer A., McCaffrey J., McCrudden C.M., Kirschman J.L., Vanover D., et al. Arginine-rich peptide-based mRNA nanocomplexes efficiently instigate cytotoxic T cell immunity dependent on the amphipathic organization of the peptide. Adv Healthc Mater. 2017;6:1601412. doi: 10.1002/adhm.201601412. [DOI] [PubMed] [Google Scholar]
- 103.Meng C., Chen Z., Mai J., Shi Q., Tian S., Hinkle L., et al. Virus-mimic mRNA vaccine for cancer treatment. Adv Ther (Weinh) 2021;4:2100144. doi: 10.1002/adtp.202100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilgenhof S., Corthals J., Heirman C., van Baren N., Lucas S., Kvistborg P., et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol. 2016;34:1330–1338. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 105.Kreiter S., Diken M., Selmi A., Diekmann J., Attig S., Hüsemann Y., et al. FLT3 ligand enhances the cancer therapeutic potency of naked RNA vaccines. Cancer Res. 2011;71:6132–6142. doi: 10.1158/0008-5472.CAN-11-0291. [DOI] [PubMed] [Google Scholar]
- 106.Mitchell D.A., Batich K.A., Gunn M.D., Huang M.N., Sanchez-Perez L., Nair S.K., et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rooney M.S., Shukla S.A., Wu C.J., Getz G., Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown S.D., Warren R.L., Gibb E.A., Martin S.D., Spinelli J.J., Nelson B.H., et al. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014;24:743–750. doi: 10.1101/gr.165985.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Giannakis M., Mu X.J., Shukla S.A., Qian Z.R., Cohen O., Nishihara R., et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;17:1206. doi: 10.1016/j.celrep.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Howitt B.E., Shukla S.A., Sholl L.M., Ritterhouse L.L., Watkins J.C., Rodig S., et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 111.McGranahan N., Furness A.J., Rosenthal R., Ramskov S., Lyngaa R., Saini S.K., et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 113.Rajasagi M., Shukla S.A., Fritsch E.F., Keskin D.B., DeLuca D., Carmona E., et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124:453–462. doi: 10.1182/blood-2014-04-567933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hilf N., Kuttruff-Coqui S., Frenzel K., Bukur V., Stevanović S., Gouttefangeas C., et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 115.Wei Y., Sticca R.P., Holmes L.M., Burgin K.E., Li J., Williamson J., et al. Dendritoma vaccination combined with low dose interleukin-2 in metastatic melanoma patients induced immunological and clinical responses. Int J Oncol. 2006;28:585–593. [PubMed] [Google Scholar]
- 116.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics—developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 117.Martin S.A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975;250:9322–9329. [PubMed] [Google Scholar]
- 118.Brito L.A., Kommareddy S., Maione D., Uematsu Y., Giovani C., Berlanda Scorza F., et al. Self-amplifying mRNA vaccines. Adv Genet. 2015;89:179–233. doi: 10.1016/bs.adgen.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 119.Stepinski J., Waddell C., Stolarski R., Darzynkiewicz E., Rhoads R.E. Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3′-O-methyl)GpppG and 7-methyl (3′-deoxy)GpppG. RNA. 2001;7:1486–1495. [PMC free article] [PubMed] [Google Scholar]
- 120.Jemielity J., Fowler T., Zuberek J., Stepinski J., Lewdorowicz M., Niedzwiecka A., et al. Novel “anti-reverse” cap analogs with superior translational properties. RNA. 2003;9:1108–1122. doi: 10.1261/rna.5430403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grudzien-Nogalska E., Stepinski J., Jemielity J., Zuberek J., Stolarski R., Rhoads R.E., et al. Synthesis of anti-reverse cap analogs (ARCAs) and their applications in mRNA translation and stability. Methods Enzymol. 2007;431:203–227. doi: 10.1016/S0076-6879(07)31011-2. [DOI] [PubMed] [Google Scholar]
- 122.Kuhn A.N., Diken M., Kreiter S., Selmi A., Kowalska J., Jemielity J., et al. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Ther. 2010;17:961–971. doi: 10.1038/gt.2010.52. [DOI] [PubMed] [Google Scholar]
- 123.Strenkowska M., Grzela R., Majewski M., Wnek K., Kowalska J., Lukaszewicz M., et al. Cap analogs modified with 1,2-dithiodiphosphate moiety protect mRNA from decapping and enhance its translational potential. Nucleic Acids Res. 2016;44:9578–9590. doi: 10.1093/nar/gkw896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vaidyanathan S., Azizian K.T., Haque A.K.M.A., Henderson J.M., Hendel A., Shore S., et al. Uridine depletion and chemical modification increase Cas9 mRNA activity and reduce immunogenicity without HPLC purification. Mol Ther Nucleic Acids. 2018;12:530–542. doi: 10.1016/j.omtn.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weissman D. mRNA transcript therapy. Expert Rev Vaccines. 2015;14:265–281. doi: 10.1586/14760584.2015.973859. [DOI] [PubMed] [Google Scholar]
- 126.Lima S.A., Chipman L.B., Nicholson A.L., Chen Y.H., Yee B.A., Yeo G.W., et al. Short poly(A) tails are a conserved feature of highly expressed genes. Nat Struct Mol Biol. 2017;24:1057–1063. doi: 10.1038/nsmb.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Holtkamp S., Kreiter S., Selmi A., Simon P., Koslowski M., Huber C., et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 128.Park J.E., Yi H., Kim Y., Chang H., Kim V.N. Regulation of poly(A) tail and translation during the somatic cell cycle. Mol Cell. 2016;62:462–471. doi: 10.1016/j.molcel.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 129.Gallie D.R. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 1991;5:2108–2116. doi: 10.1101/gad.5.11.2108. [DOI] [PubMed] [Google Scholar]
- 130.Orlandini von Niessen A.G., Poleganov M.A., Rechner C., Plaschke A., Kranz L.M., Fesser S., et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3′ UTRs identified by cellular library screening. Mol Ther. 2019;27:824–836. doi: 10.1016/j.ymthe.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murray E.L., Schoenberg D.R. A+U-rich instability elements differentially activate 5′–3′ and 3′–5′ mRNA decay. Mol Cell Biol. 2007;27:2791–2799. doi: 10.1128/MCB.01445-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vlasova-St Louis I., Bohjanen P.R. Coordinate regulation of mRNA decay networks by GU-rich elements and CELF1. Curr Opin Genet Dev. 2011;21:444–451. doi: 10.1016/j.gde.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferizi M., Leonhardt C., Meggle C., Aneja M.K., Rudolph C., Plank C., et al. Stability analysis of chemically modified mRNA using micropattern-based single-cell arrays. Lab Chip. 2015;15:3561–3571. doi: 10.1039/c5lc00749f. [DOI] [PubMed] [Google Scholar]
- 134.Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987;196:947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- 135.Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5′ noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985;40:515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- 136.Gustafsson C., Govindarajan S., Minshull J. Codon bias and heterologous protein expression. Trends Biotechnol. 2004;22:346–353. doi: 10.1016/j.tibtech.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 137.Schlake T., Thess A., Fotin-Mleczek M., Kallen K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012;9:1319–1330. doi: 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pardi N., Weissman D. Nucleoside modified mRNA vaccines for infectious diseases. Methods Mol Biol. 2017;1499:109–121. doi: 10.1007/978-1-4939-6481-9_6. [DOI] [PubMed] [Google Scholar]
- 139.Willis E., Pardi N., Parkhouse K., Mui B.L., Tam Y.K., Weissman D., et al. Nucleoside-modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aav5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kariko K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arango D., Sturgill D., Alhusaini N., Dillman A.A., Sweet T.J., Hanson G., et al. Acetylation of cytidine in mRNA promotes translation efficiency. Cell. 2018;175:1872–1886. doi: 10.1016/j.cell.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kariko K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39:e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weissman D., Pardi N., Muramatsu H., Kariko K. HPLC purification of in vitro transcribed long RNA. Methods Mol Biol. 2013;969:43–54. doi: 10.1007/978-1-62703-260-5_3. [DOI] [PubMed] [Google Scholar]
- 144.Baiersdorfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U., et al. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol Ther Nucleic Acids. 2019;15:26–35. doi: 10.1016/j.omtn.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bourquin C., Schmidt L., Hornung V., Wurzenberger C., Anz D., Sandholzer N., et al. Immunostimulatory RNA oligonucleotides trigger an antigen-specific cytotoxic T-cell and IgG2a response. Blood. 2007;109:2953–2960. doi: 10.1182/blood-2006-07-033258. [DOI] [PubMed] [Google Scholar]
- 146.De Beuckelaer A., Grooten J., De Koker S. Type I interferons modulate CD8+ T cell immunity to mRNA vaccines. Trends Mol Med. 2017;23:216–226. doi: 10.1016/j.molmed.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 147.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 148.Knutson K.L., Schiffman K., Cheever M.A., Disis M.L. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369-377, results in short-lived peptide-specific immunity. Clin Cancer Res. 2002;8:1014–1018. [PubMed] [Google Scholar]
- 149.Bijker M.S., van den Eeden S.J., Franken K.L., Melief C.J., Offringa R., van der Burg S.H. CD8+ CTL priming by exact peptide epitopes in incomplete Freund's adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179:5033–5040. doi: 10.4049/jimmunol.179.8.5033. [DOI] [PubMed] [Google Scholar]
- 150.Melief C.J., van der Burg S.H. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 151.Li W., Joshi M.D., Singhania S., Ramsey K.H., Murthy A.K. Peptide vaccine: progress and challenges. Vaccines (Basel) 2014;2:515–536. doi: 10.3390/vaccines2030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ye Z., Li Z., Jin H., Qian Q. Therapeutic cancer vaccines. Adv Exp Med Biol. 2016;909:139–167. doi: 10.1007/978-94-017-7555-7_3. [DOI] [PubMed] [Google Scholar]
- 153.Qin H., Sheng J., Zhang D., Zhang X., Liu L., Li B., et al. New strategies for therapeutic cancer vaccines. Anti Cancer Agents Med Chem. 2019;19:213–221. doi: 10.2174/1871520618666181109151835. [DOI] [PubMed] [Google Scholar]
- 154.Le Gall C.M., Weiden J., Eggermont L.J., Figdor C.G. Dendritic cells in cancer immunotherapy. Nat Mater. 2018;17:474–475. doi: 10.1038/s41563-018-0093-6. [DOI] [PubMed] [Google Scholar]
- 155.Yan S., Zhao P., Yu T., Gu N. Current applications and future prospects of nanotechnology in cancer immunotherapy. Cancer Biol Med. 2019;16:486–497. doi: 10.20892/j.issn.2095-3941.2018.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aurisicchio L., Ciliberto G. Emerging cancer vaccines: the promise of genetic vectors. Cancers (Basel) 2011;3:3687–3713. doi: 10.3390/cancers3033687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci U S A. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marshall J.L., Hoyer R.J., Toomey M.A., Faraguna K., Chang P., Richmond E., et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 160.Okur F.V., Yvon E., Biagi E., Dotti G., Carrum G., Heslop H., et al. Comparison of two CD40-ligand/interleukin-2 vaccines in patients with chronic lymphocytic leukemia. Cytotherapy. 2011;13:1128–1139. doi: 10.3109/14653249.2011.592523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kleinschmidt-DeMasters B.K., Gilden D.H. The expanding spectrum of herpesvirus infections of the nervous system. Brain Pathol. 2001;11:440–451. doi: 10.1111/j.1750-3639.2001.tb00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Warden C., Tang Q., Zhu H. Herpesvirus BACs: past, present, and future. J Biomed Biotechnol. 2011;2011:124595. doi: 10.1155/2011/124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Singh R., Paterson Y. Listeria monocytogenes as a vector for tumor-associated antigens for cancer immunotherapy. Expert Rev Vaccines. 2006;5:541–552. doi: 10.1586/14760584.5.4.541. [DOI] [PubMed] [Google Scholar]
- 164.Wansley E.K., Chakraborty M., Hance K.W., Bernstein M.B., Boehm A.L., Guo Z., et al. Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res. 2008;14:4316–4325. doi: 10.1158/1078-0432.CCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Remondo C., Cereda V., Mostböck S., Sabzevari H., Franzusoff A., Schlom J., et al. Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine. 2009;27:987–994. doi: 10.1016/j.vaccine.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang F., Xiao W., Elbahnasawy M.A., Bao X., Zheng Q., Gong L., et al. Optimization of the linker length of mannose-cholesterol conjugates for enhanced mRNA delivery to dendritic cells by liposomes. Front Pharmacol. 2018;9:980. doi: 10.3389/fphar.2018.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McKinlay C.J., Benner N.L., Haabeth O.A., Waymouth R.M., Wender P.A. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc Natl Acad Sci U S A. 2018;115:E5859–E5866. doi: 10.1073/pnas.1805358115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Diehl K.H., Hull R., Morton D., Pfister R., Rabemampianina Y., Smith D., et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- 169.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sedic M., Senn J.J., Lynn A., Laska M., Smith M., Platz S.J., et al. Safety evaluation of lipid nanoparticle-formulated modified mRNA in the Sprague–Dawley rat and cynomolgus monkey. Vet Pathol. 2018;55:341–354. doi: 10.1177/0300985817738095. [DOI] [PubMed] [Google Scholar]
- 171.Kashem S.W., Haniffa M., Kaplan D.H. Antigen-presenting cells in the skin. Annu Rev Immunol. 2017;35:469–499. doi: 10.1146/annurev-immunol-051116-052215. [DOI] [PubMed] [Google Scholar]
- 172.Melo M., Porter E., Zhang Y., Silva M., Li N., Dobosh B., et al. Immunogenicity of RNA replicons encoding HIV env immunogens designed for self-assembly into nanoparticles. Mol Ther. 2019;27:2080–2090. doi: 10.1016/j.ymthe.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Engmann L., Shaker A., White E., Bekir J.S., Jacobs H.S., Tan S.L. Local side effects of subcutaneous and intramuscular urinary gonadotropins for ovarian stimulation in in vitro fertilization: a prospective, randomized study. Fertil Steril. 1998;69:836–840. doi: 10.1016/s0015-0282(98)00043-0. [DOI] [PubMed] [Google Scholar]
- 174.Ibrahim M.M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 175.Zeng C., Zhang C., Walker P.G., Dong Y. Springer; Heidelberg: 2020. Formulation and delivery technologies for mRNA vaccines; pp. 1–40. (Current Topics in Microbiology and Immunology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liang F., Lindgren G., Lin A., Thompson E.A., Ols S., Röhss J., et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol Ther. 2017;25:2635–2647. doi: 10.1016/j.ymthe.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moyer T.J., Zmolek A.C., Irvine D.J. Beyond antigens and adjuvants: formulating future vaccines. J Clin Invest. 2016;126:799–808. doi: 10.1172/JCI81083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Joe P.T., Christopoulou I., van Hoecke L., Schepens B., Ysenbaert T., Heirman C., et al. Intranodal administration of mRNA encoding nucleoprotein provides cross-strain immunity against influenza in mice. J Transl Med. 2019;17:242. doi: 10.1186/s12967-019-1991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Johansen P., Kündig T.M. Intralymphatic immunotherapy and vaccination in mice. JoVE. 2014;84 doi: 10.3791/51031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guo C., Manjili M.H., Subjeck J.R., Sarkar D., Fisher P.B., Wang X.Y. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Grunwitz C., Kranz L.M. mRNA cancer vaccines-messages that prevail. Curr Top Microbiol Immunol. 2017;405:145–164. doi: 10.1007/82_2017_509. [DOI] [PubMed] [Google Scholar]
- 182.Gatti-Mays M.E., Redman J.M., Collins J.M., Bilusic M. Cancer vaccines: enhanced immunogenic modulation through therapeutic combinations. Hum Vaccines Immunother. 2017;13:2561–2574. doi: 10.1080/21645515.2017.1364322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Krishna S., Anderson K.S. T-Cell epitope discovery for therapeutic cancer vaccines. Methods Mol Biol. 2016;1403:779–796. doi: 10.1007/978-1-4939-3387-7_45. [DOI] [PubMed] [Google Scholar]
- 184.Purcell A.W., McCluskey J., Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. 2007;6:404–414. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- 185.Lund O., Nielsen M., Kesmir C., Petersen A.G., Lundegaard C., Worning P., et al. Definition of supertypes for HLA molecules using clustering of specificity matrices. Immunogenetics. 2004;55:797–810. doi: 10.1007/s00251-004-0647-4. [DOI] [PubMed] [Google Scholar]
- 186.Sette A., Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics. 1999;50:201–212. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 187.Li J., Burgess D.J. Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharm Sin B. 2020;10:2110–2124. doi: 10.1016/j.apsb.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 189.Kim P.S., Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cesana G.C., DeRaffele G., Cohen S., Moroziewicz D., Mitcham J., Stoutenburg J., et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 191.Vergati M., Cereda V., Madan R.A., Gulley J.L., Huen N.Y., Rogers C.J., et al. Analysis of circulating regulatory T cells in patients with metastatic prostate cancer pre- versus post-vaccination. Cancer Immunol Immunother. 2011;60:197–206. doi: 10.1007/s00262-010-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vesely M.D., Kershaw M.H., Schreiber R.D., Smyth M.J. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 193.Lizée G., Cantu M.A., Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin Cancer Res. 2007;13:5250–5255. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- 194.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]