Abstract
Background
People living with HIV might have a poor or delayed response to vaccines, mainly when CD4 cell counts are low, and data concerning COVID-19 vaccines in this population are scarce. This prospective cohort study assessed the safety and immunogenicity of the inactivated SARS-CoV-2 vaccine CoronaVac in people with HIV compared with people with no known immunosuppression.
Methods
In this prospective cohort study, adults (aged ≥18 years) living with HIV who were regularly followed up at the University of Sao Paulo HIV/AIDS outpatient clinic in Sao Paulo, Brazil, were included in the study. Eligibility for people with HIV was independent of antiretroviral use, HIV viral load, or CD4 cell count. Adults with no known immunosuppression with CoronaVac vaccination history were included as a control group. CoronaVac was given intramuscularly in a two-dose regimen, 28 days apart. Blood was collected before vaccine administration and 6 weeks after the second dose (day 69). Immunogenicity was assessed at baseline (day 0), before second vaccine (day 28), and 6 weeks after second vaccine dose (day 69) through SARS-CoV-2 IgG titre and seroconversion, neutralising antibody (NAb) positivity and percentage activity, and factor increase in IgG geometric mean titres (FI-GMT). We investigated whether HIV status and CD4 count (<500 or ≥500 cells per μL) were associated with CoronaVac immunogenicity by use of multivariable models adjusted for age and sex.
Findings
Between Feb 9, 2021, and March 4, 2021, 776 participants were recruited. Of 511 participants included, 215 (42%) were people with HIV and 296 (58%) were people with no known immunosuppression. At 6 weeks after the second vaccine dose (day 69), 185 (91%) of 204 participants with HIV and 265 (97%) of 274 participants with no known immunosuppression had seroconversion (p=0·0055). 143 (71%) of 202 participants with HIV were NAb positive compared with 229 (84%) of 274 participants with no known immunosuppression (p=0·0008). Median IgG titres were 48·7 AU/mL (IQR 26·6–88·2) in people with HIV compared with 75·2 AU/mL (50·3–112·0) in people with no known immunosuppression (p<0·0001); and median NAb activity was 46·2% (26·9–69·7) compared with 60·8% (39·8–79·9; p<0·0001). In people with HIV who had CD4 counts less than 500 cells per μL seroconversion rates, NAb positivity, and NAb activity were lower than in those with CD4 counts of at least 500 cells per μL. In multivariable models for seroconversion, NAb positivity, IgG concentration, and NAb activity after a complete two-dose regimen, adjusted for age and sex, people with HIV who had CD4 counts of at least 500 cells per μL and people with no known immunosuppression had higher immunogenicity than did people with HIV with CD4 counts less than 500 cells per μL. No serious adverse reactions were reported during the study.
Interpretation
Immunogenicity following CoronaVac in people with HIV seems strong but reduced compared with people with no known immunosuppression. Our findings highlight the need for strategies to improve vaccine immunogenicity in people with HIV.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and B3—Bolsa de Valores do Brasil.
Introduction
Several vaccines have been implemented to prevent severe COVID-19 cases and related deaths. Up to January 2022, four vaccines have been implemented in Brazil. ChAdOx1 (AstraZeneca) and CoronaVac (Sinovac and Butantan Institute) have been the most frequently used, followed by Ad26.COV2.S (Janssen) and BNT162b2 (Pfizer and BioNTech). Mass vaccination campaigns with CoronaVac have occurred since early 2021 in Brazil, Chile, Indonesia, and Turkey with approval for emergency use in more than 20 low-income and middle-income countries.1, 2
Numerous risk factors have been associated with poor outcomes in COVID-19, including pulmonary, cardiac, and chronic renal conditions, old age, obesity, and immunosuppression, including HIV infection. Although some studies showed an increased risk of COVID-19-associated death among people living with HIV compared with individuals with no known immunosuppression,3 other studies suggested this association did not exist, especially among people with well controlled HIV infection.4 However, previous data demonstrate that people with HIV might have poor or delayed response to vaccines, or reduced duration of immunogenicity after vaccination against pneumococcus, influenza, hepatitis A and B,5, 6, 7 and yellow fever.8
Research in context.
Evidence before this study
Several studies have shown that people living with HIV might have a poor or delayed response to vaccines, or even reduced duration of immunogenicity after vaccination. Data are scarce concerning safety and immunogenicity of COVID-19 vaccines in people with HIV. We searched PubMed on Jan 2, 2022, for English, Portuguese, and Spanish language publications with no date restrictions using the terms “COVID-19 vaccine” and “HIV”. We found few studies addressing COVID-19 vaccines in people living with HIV, with reassuring safety information but conflicting data regarding the effect of HIV and CD4 cell counts on vaccine immunogenicity.
Added value of this study
This is the first large cohort study addressing the safety and immunogenicity of the inactivate SARS-CoV-2 vaccine CoronaVac in people with HIV compared with people with no known immunosuppression. 6 weeks after the second vaccine dose, the proportion of participants with seroconversion and neutralising antibody positivity was high in both groups. However, immunogenicity was significantly lower among people with HIV than in people with no known immunosuppression. Moreover, people living with HIV with CD4 counts of less than 500 cells per μL had lower SARS-CoV-2 immunogenicity than did people with HIV with CD4 counts of at least 500 cells per μL.
Implications of all the available evidence
Strategies to improve COVID-19 vaccine-induced immunogenicity might be needed for people living with HIV, which could differ according to vaccine platform and CD4 cell count. Data on clinical efficacy and real-life effectiveness studies are still insufficient for this population.
Few studies have explored safety and immunogenicity of SARS-CoV-2 vaccines in people with HIV. In a small cohort of people with HIV vaccinated with an mRNA vaccine, individuals with low CD4 cell counts had lower immunogenicity than did those with CD4 cell counts greater than 200 cells per μL.9 A study on safety and immunogenicity of BNT162b2 in 143 people with HIV and 261 controls showed a favourable safety profile and similar immunogenicity (IgG and neutralising antibodies [NAb]) in both groups.10 Data on ChAdOx1 use in a South African cohort (52 people with HIV, 28 controls)11 and a subgroup analysis of a phase 2/3 study in England (54 people with HIV, 50 controls)12 suggested no significant differences in immunogenicity. One study describing immune responses after vaccination with BNT161b2, ChAdOx1, or heterologous regimens in 100 people with HIV and 152 controls found that HIV status was not associated with significant differences in IgG or NAb against SARS-CoV-2.13 However, data on safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people with HIV are scarce and studies suggest a potential role of immune recovery on SARS-CoV-2-specific antibody responses. A study including 42 people with HIV and 28 controls vaccinated with BBIBP-CorV (Sinopharm) showed no solicited adverse reactions, and both groups had similar IgG, NAb, and antigen-specific T cell responses. However, people with HIV with low CD4 to CD8 ratios had lower antibody responses than did those with CD4 to CD8 ratios within normal range.14 A study including 55 people with HIV and 21 age-matched and sex-matched controls vaccinated with CoronaVac found similar IgG concentrations, although people with HIV with CD4 counts less than 350 cells per μL had lower SARS-CoV-2-specific IgG titres than did those with CD4 counts 350 cells per μL or more.15
Clinical studies in the general population showed that CoronaVac is highly immunogenic, with 92% positivity of NAb for adults vaccinated with a two-dose regimen with a 14-day interval, and 97% positivity of NAb for a 28-day interval.16 CoronaVac efficacy to prevent symptomatic infections varied between 51% and 84% in the first 3–4 months after vaccination, with higher efficacy to prevent hospitalisations and deaths.1, 17 Studies on the effectiveness of CoronaVac showed that this vaccine prevents 47–66% of COVID-19 cases, with higher effectiveness to prevent hospitalisations and deaths, and declining effectiveness with increasing age.2, 18
This cohort study assessed the safety and immunogenicity of CoronaVac in people with HIV compared with people with no known immunosuppression. We hypothesised that immunogenicity after vaccination with CoronaVac would be negatively affected by HIV and low CD4 cell count.
Methods
Study design and participants
All participants in this prospective cohort study were included in a large phase 4 protocol (clinicaltrials.gov, NCT04754698). Consecutive people with HIV aged 18 years or older vaccinated with CoronaVac and regularly followed up at the HIV/AIDS outpatient clinic at University of Sao Paulo, Sao Paulo, Brazil were invited to participate. Eligibility for people with HIV was independent of antiretroviral use, HIV viral load, or CD4 cell count. We also included adults with no known immunosuppression who received CoronaVac as controls. Controls had been frequency-matched on age and sex with the subgroup of participants with autoimmune rheumatic diseases, but no matching was done with people with HIV. We excluded potential participants with a history of anaphylactic reaction to vaccine components, acute febrile illness, a history of Guillain-Barré syndrome or demyelinating disease, vaccination history with any SARS-CoV-2 vaccine, vaccination history with a live virus vaccine 4 weeks or less before enrolment or an inactivated vaccine 2 weeks or less before enrolment, a history of blood product transfusion 6 months or less before enrolment, and participants who were admitted to hospital. Participants with well controlled comorbidities were included, but individuals reporting other types of immunosuppression or COVID-19 symptoms at enrolment were excluded. Participants with missing or positive results at baseline assessment of SARS-CoV-2 IgG or NAb were excluded from the analysis.
The national (Comissao Nacional de Etica em Pesquisa, CONEP) and local (Comissao de Etica para Analise de Projetos de Pesquisa, CAPPesq) ethics committees approved the protocol. Each participant provided written informed consent in Portuguese before enrolment. Participant identifiable data remained confidential throughout the study.
Procedures
We collected demographic and clinical characteristics of participants at baseline and extracted laboratory variables from medical charts. CoronaVac was given in a two-dose regimen with doses 28 days apart, according to the manufacturer's recommendations.19 CoronaVac (Sinovac Life Sciences, Beijing, China, batch 20200412) contains 3 μg in 0·5 mL of beta-propiolactone inactivated SARS-CoV-2 derived from SARS-CoV-2 CN02 strain grown in African green monkey kidney cells—Vero 25 cells—with aluminium hydroxide as an adjuvant. Single-use CoronaVac syringes containing 0·5 mL were administered intramuscularly in the deltoid area. Blood was collected from participants immediately before each vaccine administration and 6 weeks after the second dose (day 69). Serum samples were stored at −70°C. In case of incident COVID-19 during the study, the second vaccination was delayed by 4 weeks.
Immunogenicity tests were done with samples collected at baseline (day 0), immediately before the second vaccine dose (day 28, intermediary assessment), and 6 weeks after the second vaccine dose (day 69, final assessment). The immunogenicity evaluation comprised two serological tests: the chemiluminescent immunoassay that measured IgG antibodies targeting spike 1 and 2 proteins in the receptor binding domain (Indirect ELISA, LIAISON SARS-CoV-2 S1/S2 IgG, DiaSorin, Italy), and the virus NAb detection assay SARS-CoV-2 sVNT RBD-HRP Kit (GenScript, Piscataway, NJ, USA), a validated surrogate neutralisation test. Seroconversion was defined as a positive (≥15·0 AU/μL) IgG test. NAb activity was reported as percentages and categorised as positive when at least 30%.20 We also calculated IgG geometric mean titres (GMTs) at all timepoints and factor increase in GMT (FI-GMT) as the ratio of the GMT after vaccination to the GMT before vaccination.
We monitored local and systemic side-effects using standardised forms and clinical evaluations at each study visit up to day 69. Participants received standardised adverse events diaries on days 0 and 28 and completed forms with solicited adverse reactions after each vaccine dose. Solicited local adverse reactions included pain, erythema, swelling, bruise, pruritus, and induration at the injection site. Systemic reactions included fever, malaise, somnolence, lack of appetite, sweating, nausea, vomit, diarrhoea, abdominal pain, vertigo, tremor, headache, fatigue, myalgia, muscle weakness, arthralgia, back pain, cough, sneezing, coryza, runny nose, sore throat, shortness of breath, conjunctivitis, pruritus, and skin rash. Moderate and severe adverse events were recorded on days 0–69 and classified as vaccine-related and vaccine-unrelated. Severity of adverse events was defined according to WHO definition.21 Participants with COVID-19 symptoms during the study period underwent a SARS-CoV-2 reverse transcriptase polymerase-chain-reaction test.
Statistical analysis
We present the characteristics of study participants using descriptive statistics. Comparisons between people living with HIV and people with no known immunosuppression were made using Mann-Whitney Wilcoxon rank-sum tests for numeric variables and χ2 or Fisher's exact tests for categorical variables. We generated categorical variables for age (<40 years, 40–49 years, 50–59 years, ≥60 years), and CD4 count (<500 cells per μL, ≥500 cells per μL). We used multivariable Poisson regression models with robust variance estimations to assess risk ratios associated with HIV infection and CD4 cell counts on the positivity of IgG and NAb at day 69, adjusted for age and sex. We fit multivariable linear regression models with robust variance estimations to assess the effect of HIV infection and CD4 cell counts on log-transformed concentrations of IgG and NAb activity at day 69, adjusted for age and sex. Given the longitudinal nature of immunogenicity assessments, we also did a sensitivity analysis using generalised estimating equations with robust variance estimations to assess the effect of HIV infection and CD4 cell counts on mean log-transformed concentrations of IgG and NAb activity at days 28 and 69, adjusted for age and sex. We used STATA, version 15.1, in all analyses, with a two-tailed significance level of 0·05. Missing data were handled as missing completely at random. We present study results in accordance with STROBE guidelines.
Role of the funding source
The funding sources had no role in study design, data collection, analysis, interpretation, writing of the report, or in the decision to submit the paper for publication.
Results
Between Feb 9 and March 4, 2021, 776 participants were recruited, of whom 282 (29%) were people living with HIV and 494 (64%) were people with no known immunosuppression (appendix p 1). Two people with no known immunosuppression were lost to follow-up after the first vaccine dose. Additionally, 244 individuals were excluded from this analysis due to a positive IgG test, NAb test, or both at baseline (53 people living with HIV, 191 people with no known immunosuppression), and 19 individuals were excluded due to missing baseline results of IgG or NAb tests. The remaining 511 individuals comprised the study sample for the immunogenicity analysis: 215 people living with HIV and 296 with no known immunosuppression. For the safety analysis, 465 participants completed the first solicited adverse events form and 454 completed the second solicited adverse events form, totalling 485 participants with at least one completed form (appendix p 1). Compared with controls, people with HIV had a higher proportion of males and were older (table 1 ). The proportion of comorbidities was similar between groups, except for a higher proportion of dyslipidaemia, previous stroke, and chronic kidney disease among people with HIV.
Table 1.
Demographic and clinical characteristics of participants eligible for immunogenicity analysis
| HIV-positive (n=215) | HIV-negative (n=296) | p value | ||
|---|---|---|---|---|
| Age | .. | 64 (21·6) | <0·0001 | |
| <40 years | 34 (16) | 88 (30) | .. | |
| 40–49 years | 45 (21) | 75 (25) | .. | |
| 50–59 years | 68 (32) | 69 (23) | .. | |
| >60 years | 68 (32) | 64 (22) | .. | |
| Median age | 54 (45–60) | 48 (37–58) | <0·0001 | |
| Female sex | 85 (40%) | 187 (63%) | <0·0001 | |
| CD4 cell count | ||||
| <200 cells per μL | 9 (4) | .. | .. | |
| 200–349 cells per μL | 24 (11) | .. | .. | |
| 350–499 cells per μL | 31 (14) | .. | .. | |
| ≥500 cells per μL | 151 (70) | .. | .. | |
| Median CD4 cell count | 655 (458–900) | .. | .. | |
| Viral suppression (<50 copies per mL) | 191 (89%) | .. | .. | |
| Median months between last CD4 cell count and inclusion | 21 (10–33) | .. | .. | |
| Comorbidities | ||||
| Any comorbidity | 172 (80%) | 174 (59%) | <0·0001 | |
| Smoking | 28 (13%) | 33 (11%) | 0·52 | |
| Hypertension | 52 (24%) | 71 (24%) | 0·96 | |
| Diabetes | 27 (13%) | 37 (13%) | 0·98 | |
| Cardiopathy | 5 (2%) | 4 (1%) | 0·50 | |
| Dyslipidaemia | 37 (17%) | 15 (5%) | <0·0001 | |
| COPD | 0 | 3 (1%) | 0·27 | |
| Asthma | 5 (2%) | 10 (3%) | 0·60 | |
| Chronic kidney disease | 5 (2%) | 0 | 0·013 | |
| Chronic liver disease | 4 (2%) | 1 (<1%) | 0·17 | |
| Neoplasia | 2 (1%) | 0 | 0·18 | |
| Previous stroke | 5 (2%) | 0 | 0·013 | |
| Active tuberculosis | 2 (1%) | 0 | 0·18 | |
Data are mean (SD), median (IQR), or n (%), unless otherwise specified. COPD=chronic obstructive pulmonary disease.
All except one person with HIV received antiretroviral therapy (one person withdrew from treatment for personal reasons); median time of antiretroviral treatment was 243 months (IQR 8–343). The most used regimen was tenofovir, lamivudine, and dolutegravir, used by 63 (29%) of 215 people; 35 (16%) used tenofovir, lamivudine, and efavirenz. We had CD4 cell counts of 215 people with HIV, with a median of 22 months (IQR 11–33) from the last measurement and study enrolment. Of 215 people with HIV, 64 (30%) had CD4 counts lower than 500 cells per μL (table 1). Overall, 191 (89%) of 215 people with HIV had undetectable viral load (<50 copies per mL) in at least three measurements before inclusion and were considered for viral suppression. Median time between the last HIV viral load assessment and study enrolment was 2 months (IQR 1–3).
In unadjusted analysis at day 69, IgG concentrations were significantly lower in people with HIV than in those with no known immunosuppression (table 2 ). At day 69, rates of seroconversion of SARS-CoV-2 IgG and NAb positivity were high in both groups, but were significantly lower in people with HIV than in people with no known immunosuppression. FI-GMT and NAb activity were moderate and lower in people with HIV than in people with no known immunosuppression. At 28 days, people with HIV had lower rates of seroconversion and NAb positivity, levels of FI-GMT, and percentage of NAb activity than people with no known immunosuppression.
Table 2.
Immunogenicity among participants with negative baseline IgG and NAb after one dose (day 28) and two doses (day 69) for people with HIV, according to CD4 cell count categories, and controls
| HIV-positive (n=215) | HIV-negative (n=296) | p value | CD4 count <500 cells per μL (n=64)* | CD4 count ≥500 cells per μL (n=151)* | p value | |
|---|---|---|---|---|---|---|
| Day 69 | ||||||
| IgG titre (AU/mL) | 48·7 (26·6–88·2) | 75·2 (50·3–112.0) | <0·0001 | 42·6 (22·9–68·9) | 53·3 (30·2–92·4) | 0·053 |
| Seroconversion | 185/204 (91%) | 265/274 (97%) | 0·0055 | 51/62 (82%) | 134/142 (94%) | 0·0062 |
| FI-GMT | 22·6 (10·9–41·2) | 31·9 (16·7–53·2) | 0·0003 | 19·4 (7·6–33·5) | 23·1 (11·0–45·0) | 0·12 |
| NAb positivity | 143/202 (71%) | 229/274 (84%) | 0·0008 | 36/61 (59%) | 107/141 (76%) | 0·015 |
| NAb activity (%) | 46·2 (26·9–69·7) | 60·8 (39·8–79·9) | <0·0001 | 41·6 (20·8–64·6) | 49·9 (30·6–73·1) | 0·031 |
| Day 28 | ||||||
| IgG titre (AU/mL) | 5·2 (0·0–11·3) | 10·4 (4·7–30·5) | <0·0001 | 5·2 (0·0–7·9) | 5·1 (0·0–12·3) | 0·45 |
| Seroconversion | 41/214 (19%) | 114/295 (39%) | <0·0001 | 10/64 (16%) | 31/150 (21%) | 0·39 |
| FI-GMT | 2·4 (1·0–5·3) | 4·7 (2·3–10·4) | <0·0001 | 2·3 (1·0–3·9) | 2·5 (1·0–6·0) | 0·34 |
| NAb positivity | 40/211 (19%) | 112/289 (39%) | <0·0001 | 7/64 (11%) | 33/147 (22%) | 0·050 |
| NAb activity (%) | 0·0 (0·0–27·3) | 23·7 (0–39·6) | <0·0001 | 0·0 (0·0–0·0) | 0·0 (0·0–29·4) | 0·0025 |
Data are median (IQR) or n/N (%). Seroconversion is when positive IgG is 15AU/mL or more. NAb test is positive when 30% or greater. FI-GMT=factor of increase-geometric mean titre. NAb=neutralising antibody test.
People with HIV.
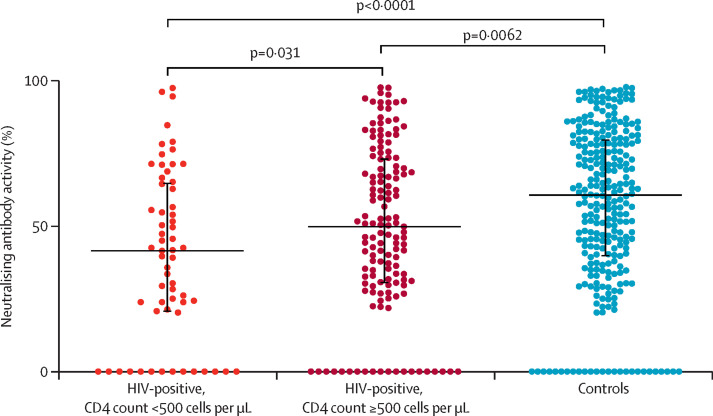
At day 69, people with HIV with CD4 counts less than 500 cells per μL had lower rates of seroconversion and NAb positivity, and lower median NAb activity than participants with CD4 counts of at least 500 cells per μL. We also observed a trend toward lower median IgG concentration among people with HIV with CD4 counts less than 500 cells per μL. At day 28, people with HIV with CD4 counts less than 500 per μL or at least 500 cells per μL had similar IgG concentrations, rates of IgG seroconversion, and FI-GMT; however, lower CD4 cell counts were associated with lower NAb activity and rates of NAb positivity (table 2). At day 69, median final rates of SARS-CoV-2 NAb activity were 42% (IQR 21–65) in people with HIV with CD4 counts less than 500 cells per μL, 50% (31–73) in people with HIV with CD4 counts at least 500 cells per μL, and 61% (40–80) in people with no known immunosuppression (figure 1 ).
Figure 1.
Neutralising antibody activity at day 69
Controls are people with HIV and no known immunosuppression. Dots represent people vaccinated. Whiskers represent IQR.
Multivariable Poisson models showed that, compared with people with HIV with CD4 counts less than 500 cells per μL, those with counts of at least 500 cells per μL and participants with no known immunosuppression were more likely to be positive for IgG (table 3 ). In the model addressing NAb positivity at day 69 as an outcome, people with lower CD4 cell counts had worse outcomes (table 3). Female sex and age categories were not significantly associated with risk of having positive IgG or NAb (table 3).
Table 3.
Multivariable regression models for IgG and neutralising antibody positivity after vaccination according to HIV status and CD4 cell counts
| aRR (95% CI) | p value | ||
|---|---|---|---|
| IgG positivity | |||
| HIV-positive, CD4 count <500 cells per μL | 1 (ref) | .. | |
| HIV-positive, CD4 count ≥500 cells per μL | 1·15 (1·02–1·30) | 0·026 | |
| HIV-negative | 1·17 (1·04–1·32) | 0·010 | |
| Female sex | 1·01 (0·96–1·06) | 0·75 | |
| Age | |||
| <40 years | 1 (ref) | .. | |
| 40–49 years | 1·02 (0·96–1·07) | 0·53 | |
| 50–59 years | 1·00 (0·94–1·06) | 0·98 | |
| >60 years | 0·98 (0·91–1·04) | 0·47 | |
| Neutralising antibody positivity | |||
| HIV-positive, CD4 count <500 cells per μL | 1 (ref) | .. | |
| HIV-positive, CD4 count ≥500 cells per μL | 1·29 (1·03–1·63) | 0·027 | |
| HIV-negative | 1·38 (1·12–1·72) | 0·0032 | |
| Female sex | 1·03 (0·94–1·14) | 0·50 | |
| Age | |||
| <40 years | 1 (ref) | .. | |
| 40–49 years | 1·01 (0·90–1·14) | 0·83 | |
| 50–59 years | 0·96 (0·85–1·09) | 0·53 | |
| >60 years | 0·88 (0·76–1·01) | 0·076 | |
aRR=adjusted risk ratio.
Multivariable linear models also showed that people with HIV with low CD4 cell counts had worse outcomes than those with CD4 counts of at least 500 cells per μL and those with no known immunosuppression (appendix p 2). Sensitivity analyses using generalised estimating equations adjusted for age and sex showed higher mean concentrations of IgG and NAb activity across both post-vaccination study visits for people living with HIV with CD4 counts of at least 500 cells per μL and for people with no known immunosuppression than for people living with HIV with CD4 counts less than 500 cells per μL (appendix p 2).
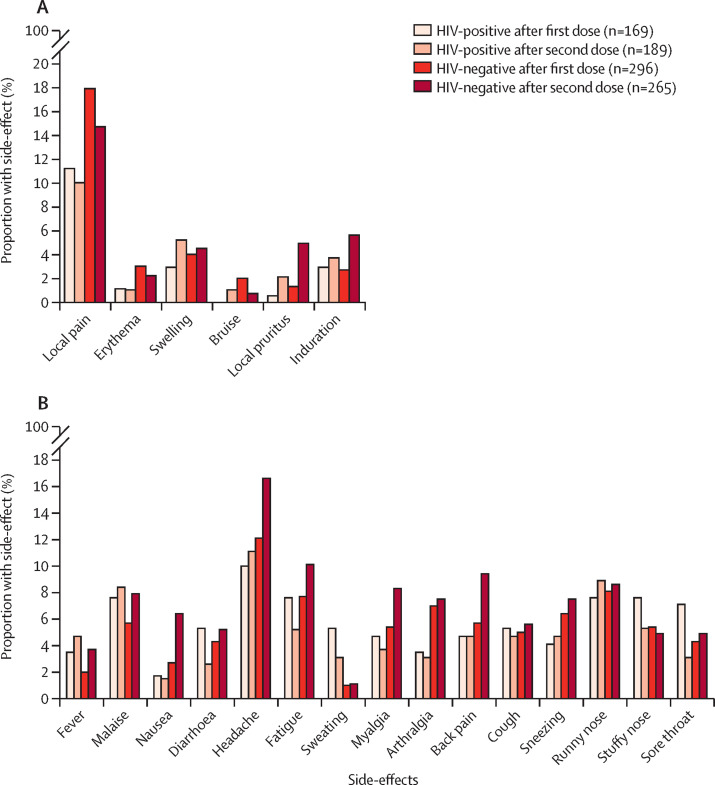
Information regarding adverse reactions was available for 169 people living with HIV and 296 people with no known immunosuppression after the first dose, and 189 people living with HIV and 265 people with no known immunosuppression after the second vaccine dose (appendix p 3; figure 2 ). Most participants were asymptomatic after vaccination with the first dose (appendix p 3). Only mild adverse events were reported during the study. No significant differences were observed in the overall rate of vaccine adverse events after the first (p=0·23) and second dose (p=0·24) between groups. Differences after the first dose were not clinically significant in people with HIV and people with no known immunosuppression for any local reactions (12% vs 21%) and sweating (5% vs 1%). Similarly, more mild and clinically non-significant adverse reactions occurred among people with no known immunosuppression than in people with HIV after the second dose, including nausea (2% vs 6%), myalgia (4% vs 8%), arthralgia (3% vs 8%), shortness of breath (0% vs 3%), and pruritus (0% vs 3%).
Figure 2.
Adverse events after vaccination
Local (A). Systemic (B).
Discussion
We present the first large cohort study addressing the safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 among people living with HIV compared with people with no known immunosuppression. No serious adverse reactions were reported during the study; we found a few clinically non-significant differences with higher rates of adverse reactions in people with no known immunosuppression. At day 69, rates of seroconversion and NAb positivity were high in both groups. However, overall immunogenicity was lower in people with HIV in the unadjusted analysis. At day 69, people living with HIV with CD4 counts less than 500 cells per μL had lower SARS-CoV-2 immunogenicity than did those with higher CD4 cell counts.
Differences occurred between groups in baseline demographics and clinical characteristics. Female sex was more frequent among people with no known immunosuppression, and people with HIV were older. Both factors have been adjusted for in the multivariable model based on a priori hypothesis of a potential confounding effect. Regarding comorbidities, the only significant differences were higher proportions of dyslipidaemia, previous stroke, and chronic kidney diseases among people with HIV. The higher rate of chronic non-communicable diseases in people with HIV than people without HIV is a documented phenomenon.22 Because of low overall frequency, we did not include these variables in the multivariable models addressing immunogenicity. Our multivariable models for IgG concentrations and positivity, and for NAb percentage activity and positivity at day 69 adjusted for age and sex showed that people with no known immunosuppression and people with HIV with CD4 counts of at least 500 cells per μL had significantly higher humoral immunogenicity than did people with HIV with CD4 counts less than 500 cells per μL. These findings were confirmed in the sensitivity analysis using generalised estimating equations for mean values of IgG and NAb activity at days 28 and 69.
Our results are consistent with previous findings on immunogenicity elicited by vaccines among people with HIV and people with lower than average CD4 cell counts.5, 6, 7 HIV infection impairs the immune system beyond reducing CD4 cell counts,23 which affects various immunological pathways causing immune activation, impaired humoral and cellular responses, and clinical outcomes, including decreased immunogenicity to several vaccines. Vaccines such as live attenuated yellow fever, inactivated influenza, hepatitis A and B, pneumococcal (polysaccharide and conjugated formulations), and conjugated Haemophilus influenzae type B elicit less robust immune responses in people with HIV than in people with no known immunosuppression.5, 6, 8 Vaccine-induced immune responses seem to be particularly impaired in advanced or uncontrolled HIV infection, with low CD4 cell counts and detectable HIV viral load.6 Studies also suggest that vaccine-induced immunogenicity might wane more rapidly for people with HIV than people with no known immunosuppression.7
Studies on the immunogenicity of COVID-19 vaccines in immunosuppressed patients suggest that the antibody response might be impaired in these populations. Medeiros-Ribeiro and colleagues24 investigated immunogenicity following CoronaVac in patients with autoimmune rheumatological diseases and found 56% NAb positivity compared with 79% among controls. Additional studies evaluating other COVID-19 vaccines, such as BNT162b2, also found a reduced antibody response in immunosuppressed patients, such as chronic corticosteroid users,25 patients taking immunosuppressive drugs,26 and solid-organ transplant recipients.27
Few studies have addressed the safety and immunogenicity against SARS-CoV-2 after vaccination with COVID-19 vaccines among people with HIV. One study15 assessed people with HIV and controls vaccinated with CoronaVac in China and found no significant differences in IgG titres comparing 55 people living with HIV and 21 controls. However, IgG titres were lower in individuals with CD4 counts less than 350 cells per μL. One study comparing 42 people with HIV and 28 healthy individuals vaccinated with two doses of another inactivated COVID-19 vaccine (BBIBP-CorV, Sinopharm) showed similar IgG, NAb, and antigen-specific T cell responses in both groups; however, people with HIV with low CD4 to CD8 ratios had lower antibody responses than those with medium or high CD4 to CD8 ratios.14 Similar humoral responses after vaccination with BNT162b2 were observed in a study including 261 immunocompetent controls and 143 people with HIV with a mean CD4 count of 700 cells per μL, 95% of whom had undetectable HIV viral loads.10 In a study in South Africa, 52 people living with HIV were randomly assigned to receive ChAdOx1, of whom 32 were followed up to 14 days after their booster doses. The vaccine was well tolerated and immunogenicity was similar among people with HIV and those with no known immunosuppression; however, there was a trend to lower neutralisation responses in people with HIV.11 In a study comparing 54 people with HIV and 50 people with no known immunosuppression vaccinated with ChAdOx1 in the UK, adverse events were mild or moderate and occurred at similar rates in both groups. Immune responses using SARS-CoV-2 humoral and cellular assays were similar regardless of HIV status and CD4 cell counts. In this study, all people with HIV had antiretroviral treatment, undetectable HIV viral load, and CD4 counts of more than 350 cells per μL, with median CD4 counts of 694 cells per μL at enrolment.12 In our study, although median CD4 cell counts were similar between participants, 15 (7%) of 215 participants had CD4 counts less than 350 cells per μL and 11% had detectable HIV viral load. Differences in clinical characteristics of study populations and variations in mechanisms and immunogenicity of mRNA, vector-based, and inactivated vaccines likely account for the difference between our findings and other studies'.
Studies comparing the efficacy and effectiveness of different COVID-19 vaccines in the general population have shown that mRNA vaccines and vector-based Gam-COVID-Vax had the highest efficacy in preventing symptomatic cases in clinical trials,28 with a decline in infection protection observed for BNT161b2, ChAdOx1, and CoronaVac from 3–6 months after immunisation.29 It is still unclear if clinical efficacy and duration of protection following COVID-19 vaccination is the same in people with HIV.
Our study has a few limitations. As seen in any observational study, groups were subject to imbalances in demographic and clinical characteristics. Older age and fewer women among people with HIV than in people with no known immunosuppression could partly explain the lower immune response to vaccination, as old age has been associated with reduced immunogenicity30 and female sex was associated with increased vaccine immunogenicity and reactogenicity.31 These differences could also partly explain the higher rates of some adverse reactions among people with no known immunosuppression. We fit multivariable regression models, including sex and age categories, to adjust for these imbalances. In this model, sex and age had no significant effect on final NAb positivity, whereas HIV status and categories of CD4 cell count remained associated with NAb positivity. Another limitation was use of broad categories of CD4 cell count due to the low number of participants with CD4 counts less than 350 cells per μL. Therefore, we were unable to explore the effect of lower CD4 cell count on vaccine immunogenicity. Other potential problems include short follow-up for immunogenicity analysis, risk of residual confounding by unadjusted variables, and absence of recent CD4 cell count measurements for some people living with HIV.
External validity should not be directly presumed from our results. We recruited participants from a single clinic; most people with HIV in our study had high CD4 cell counts and undetectable viral load, and all participants received a two-dose regimen of CoronaVac. Populations with different genetic backgrounds, different clinical profiles, and exposure to other COVID-19 vaccines and regimens might behave differently regarding immunogenicity measurements.
Despite some limitations, our study had several strengths. We prospectively followed up more than 200 people living with HIV and almost 300 people with no known immunosuppression with negative baseline IgG and NAb after a two-dose regimen of CoronaVac and measured humoral immune responses at days 28 and 69. The longitudinal nature of data allowed for sensitivity analyses that confirmed findings of higher immunogenicity in controls and people with HIV with high CD4 cell counts. The sample size also allowed use of multivariable models adjusted for age and sex.
Although people with HIV have been acknowledged as vulnerable to complications of common viral respiratory diseases, such as influenza,32 the interaction between HIV and SARS-CoV-2 is still unclear. Some epidemiological data suggest no greater risk of detrimental outcomes of COVID-19 among people with HIV, especially those with well controlled HIV infection;4 however, some studies show greater mortality in people with HIV than in people with no known immunosuppression.3
Our results showed that CoronaVac has robust immunogenicity in people living with HIV after a two-dose regimen, but antibody responses in this population are slightly lower than in controls. Strategies should be developed to improve vaccine-induced immunogenicity in people living with HIV, especially in the subgroup with low CD4 cell counts. Possible approaches include booster doses or administration of higher antigen titres per vaccine dose. In September, 2021, the Brazilian Immunisation Programme implemented booster doses for COVID-19 vaccination regimens, prioritising adults aged 60 years and older and those with immunosuppressive conditions, including people with HIV.33 This strategy should partly counterbalance differences in immunogenicity in this population. Further studies could help to define the best timing for booster doses, as well as subgroups of people with HIV who will greatly benefit from this intervention.
Although this is the first large, controlled study analysing inactivated SARS-CoV-2 vaccine-induced immunogenicity among people living with HIV, data on clinical efficacy and real-life effectiveness are still scarce for this population. More than 38 million people are estimated to be living with HIV worldwide, with almost 1 million cases living in Brazil. With the overlap of these two pandemics, it is essential to reinforce strategies to mitigate the damage caused by the SARS-CoV-2 pandemic in an already vulnerable HIV population.
Data sharing
De-identified, individual participant data, a data dictionary defining each field in the dataset, study protocol, and statistical analysis plan will be made available to others after publication of this manuscript, following approval of a proposal. Proposals should be directed to esper.kallas@usp.br. To gain access, data requestors will need to sign a data access agreement.
Declaration of interests
EGK is the Principal Investigator for the CoronaVac phase 3 clinical trial at the University of Sao Paulo. VIA-S is the Principal Investigator for the Janssen COVID-19 vaccine phase 3 clinical trial at University of Sao Paulo. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was sponsored by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (NEA, SGP, CAS, EB, number 2015/03756–4; TP, number 2017/14352-7), from Conselho Nacional de Desenvolvimento Científico e Tecnológico (EB, number 305242/2019-9), and from B3—Bolsa de Valores do Brasil. Instituto Butantan supplied the study product and had no other role in the trial. This study was not funded by the NIH, nor are any authors employed by the NIH; none of the authors receive an NIH grant. We thank the study participants for their voluntary contribution.
Contributors
EB, ACM-R, and EGK conceptualised the study. KYI, CMP, APPSA, EVA, MRS, PSSP, and ANL contributed to data collection and follow-up visits. NEA, ACM-R, SGP, EFNY, CGSS, TP, and CC contributed to data collection and follow-up visits for controls. VIA-S performed statistical analysis. LCN, VIA-S, EGK, and EB wrote the manuscript. VIA-S and LCN have access to and verified the underlying data. All authors revised and approved the final version of the manuscript.
Supplementary Material
References
- 1.Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jara A, Undurraga EA, Gonzalez C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrosioni J, Blanco JL, Reyes-Uruena JM, et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV. 2021;8:e294–e305. doi: 10.1016/S2352-3018(21)00070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madhi SA, Maskew M, Koen A, et al. Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis. 2011;52:128–137. doi: 10.1093/cid/ciq004. [DOI] [PubMed] [Google Scholar]
- 6.Garrido HMG, Schnyder JL, Tanck MWT, et al. Immunogenicity of pneumococcal vaccination in HIV infected individuals: a systematic review and meta-analysis. EClinicalMedicine. 2020;29–30 doi: 10.1016/j.eclinm.2020.100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerneis S, Launay O, Turbelin C, Batteux F, Hanslik T, Boelle PY. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin Infect Dis. 2014;58:1130–1139. doi: 10.1093/cid/cit937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avelino-Silva VI, Miyaji KT, Hunt PW, et al. CD4/CD8 ratio and KT ratio predict yellow fever vaccine immunogenicity in HIV-infected patients. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruddy JA, Boyarsky BJ, Werbel WA, et al. Safety and antibody response to the first dose of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine in persons with HIV. AIDS. 2021;35:1872–1874. doi: 10.1097/QAD.0000000000002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy I, Wieder-Finesod A, Litchevsky V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect. 2021;27:1851–1855. doi: 10.1016/j.cmi.2021.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhi SA, Koen AL, Izu A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV. 2021;8:e568–e580. doi: 10.1016/S2352-3018(21)00157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;8:e474–e485. doi: 10.1016/S2352-3018(21)00103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brumme ZL, Mwimanzi F, Lapointe HR, et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. medRxiv. 2021 doi: 10.1101/2021.10.03.21264320. published online Oct 15. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Zhang Y, He Z, et al. Immunogenicity of an inactivated SARS-CoV-2 vaccine in people living with HIV-1: a non-randomized cohort study. EClinicalMedicine. 2022;43 doi: 10.1016/j.eclinm.2021.101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Han J, Li X, et al. COVID-19 vaccination in people living with HIV (PLWH) in China: a cross sectional study of vaccine hesitancy, safety, and immunogenicity. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadlyana E, Rusmil K, Tarigan R, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: an interim analysis in Indonesia. Vaccine. 2021;39:6520–6528. doi: 10.1016/j.vaccine.2021.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranzani OT, Hitchings MDT, Dorion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;374 doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Instituto Butantan Vacina adsorvida covid-19 (inativada) 2021. https://vacinacovid.butantan.gov.br/assets/arquivos/Bulas_Anvisa/Bula_PS_vacina%20adsorvida%20covid-19%20(inativada).pdf
- 20.Taylor SC, Hurst B, Charlton CL, et al. A new SARS-CoV-2 dual-purpose serology test: highly accurate infection tracing and neutralizing antibody response detection. J Clin Microbiol. 2021;59:e02438–e02440. doi: 10.1128/JCM.02438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO World alliance for patient safety: WHO draft guidelines for adverse event reporting and learning systems: from information to action. 2005. https://apps.who.int/iris/handle/10665/69797(WHO/EIP/SPO/QPS/05.3
- 22.Ghosn J, Taiwo B, Seedat S, Autran B, Katlama C. HIV. Lancet. 2018;392:685–697. doi: 10.1016/S0140-6736(18)31311-4. [DOI] [PubMed] [Google Scholar]
- 23.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 24.Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744–1751. doi: 10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- 25.Deepak P, Kim W, Paley MA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. medRxiv. 2021 doi: 10.1101/2021.04.05.21254656. published online April 9. (preprint). [DOI] [Google Scholar]
- 26.Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotshild V, Hirsh-Raccah B, Miskin I, Muszkat M, Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-02321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hitchings MDT, Ranzani OT, Lind ML, et al. Change in COVID-19 risk over time following vaccination with CoronaVac: a test-negative case-control study. medRxiv. 2021 doi: 10.1101/2021.12.23.21268335. published online Dec 24. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyaji KT, Avelino-Silva VI, Simoes M, et al. Prevalence and titers of yellow fever virus neutralizing antibodies in previously vaccinated adults. Rev Inst Med Trop Sao Paulo. 2017;59:e2. doi: 10.1590/S1678-9946201759002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheth AN, Patel P, Peters PJ. Influenza and HIV: lessons from the 2009 H1N1 influenza pandemic. Curr HIV/AIDS Rep. 2011;8:181–191. doi: 10.1007/s11904-011-0086-4. [DOI] [PubMed] [Google Scholar]
- 33.ANVISA Anvisa faz recomendações sobre doses de reforço de vacinas contra Covid-19. Nov 24, 2021. https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/anvisa-faz-recomendacoes-sobre-doses-de-reforco-de-vacinas-contra-covid-19
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified, individual participant data, a data dictionary defining each field in the dataset, study protocol, and statistical analysis plan will be made available to others after publication of this manuscript, following approval of a proposal. Proposals should be directed to esper.kallas@usp.br. To gain access, data requestors will need to sign a data access agreement.