Abstract
The global tuberculosis burden remains substantial, with more than 10 million people newly ill per year. Nevertheless, tuberculosis incidence has slowly declined over the past decade, and mortality has decreased by almost a third in tandem. This positive trend was abruptly reversed by the COVID-19 pandemic, which in many parts of the world has resulted in a substantial reduction in tuberculosis testing and case notifications, with an associated increase in mortality, taking global tuberculosis control back by roughly 10 years. Here, we consider points of intersection between the tuberculosis and COVID-19 pandemics, identifying wide-ranging approaches that could be taken to reverse the devastating effects of COVID-19 on tuberculosis control. We review the impact of COVID-19 at the population level on tuberculosis case detection, morbidity and mortality, and the patient-level impact, including susceptibility to disease, clinical presentation, diagnosis, management, and prognosis. We propose strategies to reverse or mitigate the deleterious effects of COVID-19 and restore tuberculosis services. Finally, we highlight research priorities and major challenges and controversies that need to be addressed to restore and advance the global response to tuberculosis.
This is the first in a Series of three papers about tuberculosis in the time of COVID-19, published in conjunction with eBioMedicine
Introduction
Historically, tuberculosis has arguably been the biggest killer of humans and it remains one of the foremost global infectious causes of death.1 The incidence of tuberculosis has been slowly declining over the past decade, and mortality had decreased by almost a third, although the global burden remains substantial at more than 10 million people per year newly ill with the disease.1 Although the declining trajectory would have fallen far short of milestones outlined in the UN Sustainable Development Goals and the WHO End TB Strategy targets, there was encouraging movement in the right direction. This positive trend has been abruptly and dramatically reversed by the COVID-19 pandemic, which in many parts of the world has resulted in a substantial reduction in tuberculosis testing and access to tuberculosis health services. Data from the latest global tuberculosis report by WHO shows an 18% reduction in the number of tuberculosis cases notified in 2020 compared with 2019.2 These losses have overshadowed the potential reductions in Mycobacterium tuberculosis transmission because of mask use and physical distancing, and have increased the global tuberculosis burden and associated mortality, taking tuberculosis control efforts backwards by approximately a decade.2, 3, 4 With the current delta and omicron variant-driven surges in many low-income and middle-income countries (LMICs), and with the challenges of SARS-CoV-2 vaccine access for most LMICs, it is likely that the negative impact of the COVID-19 pandemic on efforts to control tuberculosis (as well as other major infectious diseases, such as HIV and malaria)5, 6 will continue well into 2022, especially with 3 billion people still waiting to receive their first dose of SARS-CoV-2 vaccine.
The aim of this Series paper is to review the interactions between COVID-19 and tuberculosis, including the population-level impact of COVID-19 on tuberculosis outcomes, the clinical presentation and diagnosis of tuberculosis–COVID-19 co-infection, the patient-level impact of COVID-19 on the management and prognosis of tuberculosis, and interventional strategies that could be used to mitigate the devastating effects of COVID-19 on the global burden of tuberculosis, including lessons learned from responses to the COVID-19 pandemic. We emphasise the ways in which tuberculosis care and management have been neglected compared with COVID-19, and how low SARS-CoV-2 vaccine coverage in tuberculosis-endemic countries, despite the high rates of infection and emergence of new variants, will continue to fuel the global tuberculosis pandemic. Priorities for the rapid restoration of tuberculosis care and prevention—and progress towards End TB Strategy targets—in the era of COVID-19 are presented in panel 1 .
Panel 1. Priorities for rapid restoration of tuberculosis care and prevention in the era of COVID-19.
Priorities for resource allocation, case finding and prevention, and treatment for tuberculosis in the time of COVID-19 are listed below. Communication with the public, training of health-care workers, ongoing monitoring and evaluation, and tuberculosis advocacy are cross-cutting themes across these domains.
Resource allocation
-
•
Increase budget and human resources for existing tuberculosis and other health infrastructures and for operational research, with a view to reducing the fragility of health systems, including through public–private partnerships
-
•
Mobilise community-based organisations and partners to advocate for more resources for care and prevention (eg, personnel, equipment, consumables)
-
•
Develop digital platforms for training and health education
-
•
Develop public-facing dashboards for tuberculosis surveillance data
-
•
Ensure availability and supply of high-quality personal protective equipment for health-care workers and other highly exposed individuals, including N95 (or equivalent) respirators
-
•
Improve and broaden social protection for individuals who develop tuberculosis, COVID-19, or both
-
•
Actively identify and remove sources of structural violence, systemic racism, and discrimination, especially those affecting access to essential services and equitable, high-quality health care
-
•
Integrate tuberculosis and COVID-19 responses at multiple levels, including screening and testing
Case finding and prevention
-
•
Target high-risk groups for consent-based universal testing
-
•
Strengthen community-based active case finding and tuberculosis diagnostic services (including in shelters for people who are homeless)
-
•
Enhance screening through the use of mHealth and self-screening apps
-
•
Develop telemedicine with the use of digital platforms for consultation
-
•
Strengthen availability, speed, and reliability of specimen transportation
-
•
Use rapid turnaround molecular tests with high sensitivity and specificity for dual diagnostic testing for tuberculosis and COVID-19
-
•
Strengthen contact tracing, investigation, and the provision of tuberculosis preventive therapy
-
•
Broaden use of chest radiography with or without computer-aided detection for the tuberculosis community, and improve facility-based screening
-
•
Enhance screening and case-finding activities at health facilities, including targeting high-risk groups (eg, urine lipoarabinomannan and C-reactive protein as a screening tool in people with HIV)
-
•
Strengthen provision of Bacillus Calmette-Guérin vaccination and invest in new vaccine development
-
•
Ensure separation and prompt treatment of those with disease to reduce Mycobacterium tuberculosis and SARS-CoV-2 transmission in health-care settings
Treatment
-
•
Strengthen community-based tuberculosis treatment services
-
•
Scale up virtual care, digital health, and community-monitoring solutions to provide remote support such as video-supported therapy
-
•
Scale up SMS-based communication to improve treatment adherence and patient-centred care and support
-
•
Use bulk dispensing, open-air dispensing, or both to reduce barriers to care and minimise risk to patients and health-care workers
Population-level impact of COVID-19 on tuberculosis
Tuberculosis case detection
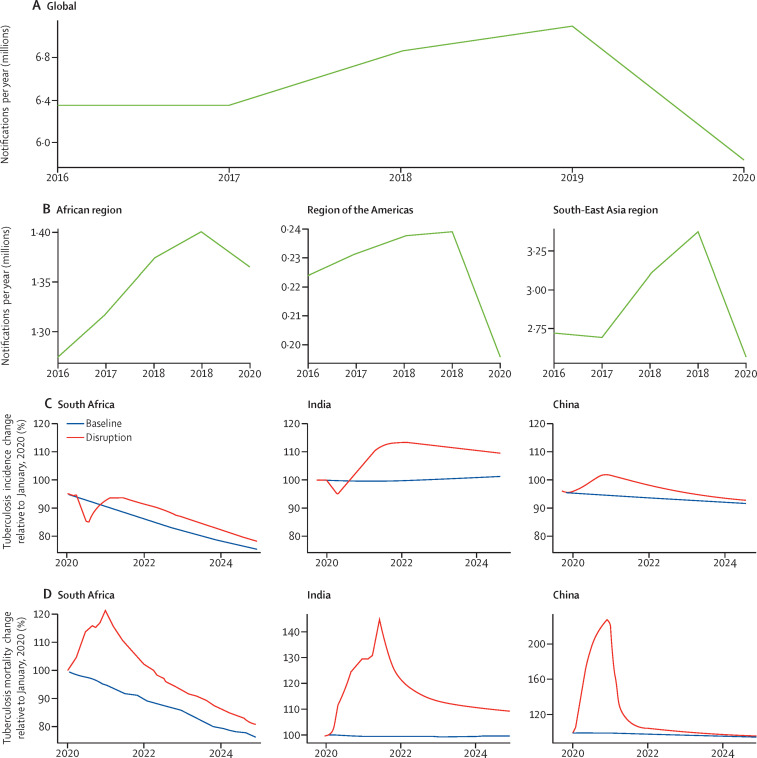
Compared with 2019, tuberculosis case detection in 2020 was reduced by 18% globally (a decrease from 7·1 million to 5·8 million cases) and by up to 24% in the ten worst-affected countries with high tuberculosis burden.2, 7 India, Indonesia, the Philippines, and China account for 1·3 million cases (93%) in the global decline in tuberculosis case detection (figure 1 ). Major reductions in notified cases have been seen in the Philippines (37%), Indonesia (31%), South Africa (26%), and India (25%; table 1 and figure 1).
Figure 1.
Impact of the COVID-19 pandemic on global, regional, and national tuberculosis detection and mortality
There has been a substantial decline in tuberculosis case detection globally of about 18% in newly ill people reported with tuberculosis at the global (A), regional (B), and national (C) levels, across key regions. Modelling has predicted that this acute drop will probably be followed by a rebound increase in tuberculosis incidence (C); data from three exemplar countries are shown relative to baseline trajectories before COVID-19. The decline in case detection is also estimated to have resulted in an acute increase in mortality in 2020 and is anticipated to take the next few years to reach prepandemic baselines (D); data from three exemplar countries are shown relative to baseline trajectories before COVID-19. Images reproduced by permission of WHO.2
Table 1.
Tuberculosis case detection in ten selected countries with high tuberculosis burden in 2019 and 2020
|
Number of tuberculosis cases notified to WHO |
Reduction (shortfall) in tuberculosis case detection from 2019 to 2020 | ||
|---|---|---|---|
| 2019 | 2020 | ||
| Philippines | 409 167 | 256 541 | 37·3% |
| Indonesia | 559 847 | 384 025 | 31·4% |
| South Africa | 209 545 | 154 344 | 26·3% |
| India | 2 176 677 | 1 629 301 | 25·1% |
| Bangladesh | 291 595 | 230 081 | 21·1% |
| Russia | 73 328 | 58 723 | 19·9% |
| Pakistan | 328 312 | 272 990 | 16·9% |
| Kenya | 84 345 | 71 646 | 15·1% |
| Angola | 74 105 | 63 147 | 14·7% |
| China | 728 265 | 624 715 | 14·2% |
| All countries reporting quarterly and monthly data (n=84) | 5 058 801 | 3 833 148 | 24·3% |
Countries providing quarterly and monthly data are listed in descending order of shortfall (for the full list of countries, see appendix p 14). Reproduced and adapted by permission of WHO.2
Previously unpublished data from South Africa's National Institute for Communicable Diseases (NICD) show substantial reductions in tuberculosis testing and case detection nationwide, coinciding with each wave of COVID-19 and the subsequent national lockdown (appendix p 4). In our analysis of NICD data, predictions for 2020 were based on national data from 2018 and 2019, and adjusted for seasonality; detailed methods are available in the appendix (p 1). By May, 2020, tuberculosis testing in South Africa had decreased by more than 50% compared with the previous year: of 193 067 (95% CI 181 119–205 014) expected Xpert MTB/RIF nucleic acid amplification tests (NAATs) for the detection of M tuberculosis for the month, only 99 513 were actually done. The microbiological confirmation of drug-sensitive tuberculosis also declined by 40%, and numbers of rifampicin-resistant tuberculosis cases fell by about 50% (appendix p 5). By the end of 2020, South Africa saw an overall decrease in tuberculosis testing of 22% (95% CI 15–28), and rates of confirmed active tuberculosis cases fell by 15% (9–20%) in the public sector. In addition, there was an 18% reduction in attendance at primary health-care facilities. These trends are substantial given that the highest population-wide limitations to public activities and social interaction occurred for only 6 months of the year.8 These patterns extended to other endemic countries such as India and China (Panel 2, Panel 3 ; detailed versions are available in the appendix, pp 1–2).9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 The full impact of the current omicron wave in South Africa and other tuberculosis-endemic countries is unknown, but likely to be negative for tuberculosis services. The 2021 WHO global tuberculosis report estimated about a 15% reduction in the number of people treated for drug-resistant tuberculosis, and a 21% decrease in people receiving preventive therapy for latent tuberculosis infection globally.2
Panel 2. Perspective from India.
At least 30 million people have developed COVID-19 in India, and a devastating second wave has left many individuals with severe acute respiratory distress syndrome, a proportion of whom might develop post-COVID fibrotic sequelae.9 India also has the largest number of people living with chronic fibrotic lung scarring caused by tuberculosis.10 Post-COVID sequelae superimposed on post-tuberculosis fibrosis is likely to result in considerable disability in many of these individuals.
In March, 2020, the prime minister, Narendra Modi, announced a prolonged and stringent lockdown11 that had catastrophic effects on health-care provision and severely restricted access to tuberculosis diagnosis and treatment. Staff and resources from tuberculosis programmes and health facilities were diverted to COVID-19 efforts, and drug stockouts were common.12 Bacillus Calmette-Guérin vaccination rates also plummeted; in April, 2020, 1 million fewer children were vaccinated than in the same month in 2019.6 Tuberculosis notification rates decreased by 50% in private and public sectors.3 Treatment completion rates also decreased, as patients delayed diagnosis and were often unable to reach clinics. For details of the potential impact of COVID-19 on tuberculosis indices in India, see appendix p 12.
A modelling study in 2020 estimated that each month of lockdown would result in 40 685 additional people developing tuberculosis that year and an additional 151 120 tuberculosis deaths over the next 5 years.13 In 2020, the number of tuberculosis cases from India notified to WHO was 1 629 301, a reduction of 25·1% from the 2019 number.1 Further details about the perspective from India are provided in the appendix (p 1).
Panel 3. Perspective from China.
COVID-19 prevalence peaked in China in February, 2020 (n=68 033 confirmed cases), but by May, 2020, numbers had fallen by 99·8% to 143 confirmed cases.14 Emergency measures deployed to control the virus included cancellation of public transport, prohibition of public gatherings, closing of schools and public amenities, enhanced disinfection, social distancing, stay-at-home orders, and home quarantine for individuals from epidemic areas.14, 15, 16 Moreover, major tuberculosis hospitals were redesignated as COVID-19 hospitals;16, 17 staff from tuberculosis programmes, laboratories, and wards were diverted to the COVID-19 response,14, 15, 16, 17, 18, 19 and there have been substantial reductions in the number of tuberculosis hospital beds and outpatient visits.19
Across the country, tuberculosis notifications were substantially lower in the first 5 months of 2020, decreasing by 24%, 39%, 25%, 15%, and 13% per month, compared with the same months in 2019.14, 15, 16, 17, 18, 19 Several studies found reductions in tuberculosis notifications at provincial levels,17, 19, 20, 21, 22 with missed or delayed sputum and follow-up examinations;22 increased patient delays,21 increased incidence of positive sputum smears and cavities in baseline chest radiographs,21 and reduced treatment success and multidrug-resistant tuberculosis screening were also reported.20
Since the national lockdown was lifted, tuberculosis notifications have gradually approached pre-lockdown levels.19, 21, 22, 23, 24 The observed effect on tuberculosis control has been attributed to traffic restrictions, disrupted tuberculosis services, and the fear of catching COVID-19 among members of the population.15, 17, 19, 22 Further details about the perspective from China are provided in the appendix (pp 1–2).
However, it remains unclear to what extent diminished case detection can be attributed to reduced access to care versus reduction in M tuberculosis transmission from non-pharmaceutical interventions (eg, mask wearing or lockdowns), like those seen for other respiratory infections such as influenza, respiratory syncytial virus, Streptococcus pneumoniae, and Haemophilus influenzae.25, 26, 27 It is widely assumed that reduced health-care access dominates and offsets any transmission reduction; this assumption is supported by the acute nature of the reductions in case detection, as the effects of decreased transmission would probably only be seen months later. Thus, the acute reduction in detection supports reduced access but does not exclude or confirm the effect of reduced transmission.
Tuberculosis mortality and other metrics
Several modelling studies suggested that COVID-19 and the pandemic response might lead to an increase in tuberculosis mortality, driven primarily by reduced access to health services. Cilloni and colleagues13 forecast in 2020 that global deaths would return to those seen in 2013, undoing almost a decade of work, whereas Hogan and colleagues28 estimated that tuberculosis mortality could increase by up to 20% between 2020 and 2025, and stressed the importance of maintaining tuberculosis service provision despite pandemic-related disruption. Analyses from WHO and the Stop TB Partnership predicted, respectively, 190 000 additional tuberculosis deaths in 2020 and about 1·4 million additional tuberculosis deaths between 2020 and 2025, worsened for every month taken to achieve restoration of services.3, 13, 29
The latest WHO global tuberculosis report provides data to substantiate the models. In 2020, there were roughly 1·32 million tuberculosis deaths worldwide.2 These numbers represent the first year-over-year increase in tuberculosis deaths since 2005. The impact of the pandemic on tuberculosis deaths during 2021 is unclear, but probably substantially worse following the large delta-driven surges in many tuberculosis-endemic countries and the current wave of the omicron variant globally. The issue is complicated by the methods used to estimate tuberculosis mortality, which rely heavily on indirect estimates and extrapolation,30 in part because civil registration and vital statistics systems are weak in many countries with high tuberculosis burden.31 In addition, given the frequent underdiagnosis of active tuberculosis among people who die, even outside of a pandemic,32, 33 the disruptions to services and reductions in tuberculosis testing are likely to result in under-reporting of tuberculosis deaths. The expected underdetection yet predicted increase in mortality represents an epidemiological and surveillance conundrum. Preliminary reports provide insight into some of these aspects. A small Italian study reported significantly higher loss to follow-up (p=0·03) and mortality (p=0·04) during the pandemic than in a control period the year before.34 Similarly, brief reports from Pakistan,35 China,20 and Ethiopia36 document deteriorations in treatment outcomes and patient support.
The COVID-19 pandemic has also affected other tuberculosis outcomes and preventive strategies. COVID-19 has affected tuberculosis vaccination, including a reduction in Bacillus Calmette-Guérin (BCG) vaccination of up to 60% in some parts of the world (figure 2 ).6 According to a modelling study,37 the consequences of delayed and missed BCG vaccination might account for up to 33 074 additional paediatric deaths related to tuberculosis. Apart from tuberculosis mortality, other unfavourable outcomes such as treatment failure and loss to follow-up have been negatively affected and are discussed hereafter (figure 2), as is the occurrence of persistent lung disease after active tuberculosis.
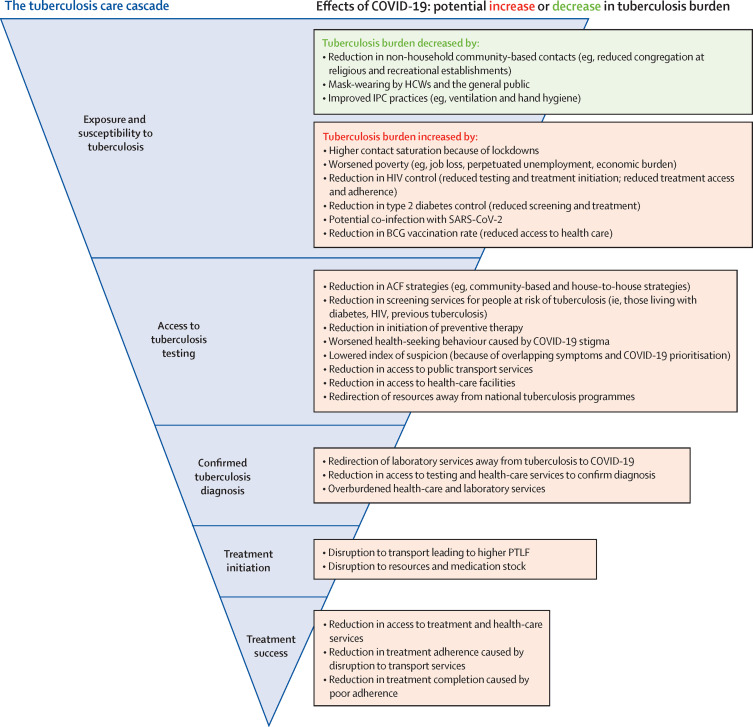
Figure 2.
Tuberculosis cascade of care and the potential effects of the COVID-19 pandemic
A detailed look at the potential effects of the COVID-19 pandemic at each step of the typical tuberculosis care cascade: exposure and susceptibility to Mycobacterium tuberculosis; access to preventive services; testing, diagnosis, and treatment initiation; and completion. The effects of COVID-19 at each step of the tuberculosis care cascade (blue) are detailed in the adjacent boxes, with an increase in the burden of tuberculosis denoted by red text or a decrease denoted by green text. ACF=active case finding. BCG=Bacillus Calmette-Guérin. HCW=health-care worker. IPC=infection prevention and control. PTLF=pretreatment loss to follow-up.
Economic impact
The COVID-19 pandemic has had staggering consequences for the global economy. Economic losses in 2020 were 4% (about US$3 trillion) of the global gross domestic product (GDP),38 with LMICs disproportionately affected.
Several modelling estimates indicate that the tuberculosis case burden will increase by up to 15% (>1 million cases in total) by 2025.3, 13, 28, 29, 39, 40 From a societal perspective, rising unemployment and money required for transport, medication, and food are expected to increase the catastrophic costs substantially (>20% of household income), particularly in tuberculosis-endemic countries where these costs were already high in the prepandemic era.41
These costs have affected the global tuberculosis response at multiple levels and led to worsening poverty, and hence increased susceptibility to tuberculosis mortality, thus exacerbating the vicious cycle of susceptibility, death, and poverty (figure 3 ). Notably, tuberculosis itself has historically caused economic losses, estimated at 2–3% of GDP in tuberculosis-endemic countries.42, 43 Reduced funding of tuberculosis programmes has already been reported in several countries because of the diversion of resources to the COVID-19 response,1, 44 including fewer health-care workers (HCWs) for tuberculosis services, reduced supply of personal protective equipment (PPE) for tuberculosis exposure, diversion of diagnostic testing services to COVID-19, and reductions in support services for patients with tuberculosis (eg, nutrition and mental health; figure 3). The Global Fund estimates that approximately an additional $30 billion will be required to bolster tuberculosis, HIV, and malaria programmes in the wake of COVID-19.45
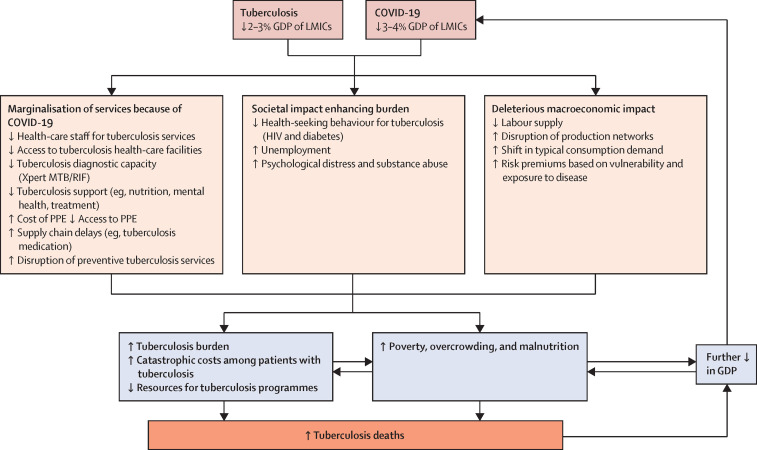
Figure 3.
Catastrophic effects of the COVID-19 pandemic on global tuberculosis response and the global economy
An overview of how the COVID-19 pandemic has affected the resources available for tuberculosis control efforts and its effect on individual households leading to a cycle of increased susceptibility, death, and poverty. Poverty is driven by tuberculosis-related and COVID-19-related deterioration in macroeconomic conditions, leading to catastrophic costs at the patient level, and is a proxy for increased mortality and burden of tuberculosis. GDP=gross domestic product. LMICs=low-income and middle-income countries. PPE=personal protective equipment.
Reality in resource-poor settings
Few of the models discussed accounted for the brutal reality of 2021, during which we witnessed devastating COVID-19 surges in many countries with a high tuberculosis burden, driven by the delta variant from April, 2021, and the omicron variant since November, 2021. Large new delta variant waves in countries such as India, Bangladesh, Nepal, Indonesia, Myanmar, Uganda, and South Africa again forced these countries into new lockdowns and further disruptions of routine tuberculosis and all other essential health services. Many South American countries with high tuberculosis burdens (eg, Brazil and Peru) have also seen massive COVID-19 surges. Since November, 2021, South Africa has been dealing with a large omicron wave, and this new variant is now sweeping the world and keeping the pandemic alive during 2022.
Given the emergence of a highly transmissible omicron variant, and the pre-existing and growing inequity in SARS-CoV-2 vaccine access, in which less than 15% of people in low-income countries have received even one dose of the vaccine (as of March, 2022), additional new waves are highly likely in 2022 and beyond. Simply put, we do not see any easy way out for LMICs to end this pandemic in the near future, unless we vaccinate the whole world.46 This means that the impact of the COVID-19 pandemic on tuberculosis will probably be worse than any mathematical modelling prediction thus far. This scenario and combination of events perpetuate the pervading global apathy and neglect of tuberculosis. None of the End TB Strategy targets for tuberculosis are likely to be achieved by 2030.
Patient-level clinical impact of COVID-19
Susceptibility to tuberculosis and COVID-19
Does tuberculosis increase susceptibility to COVID-19, and does COVID-19 increase susceptibility to tuberculosis? There are no published data around these questions yet. However, we know—based on a meta-analysis incorporating 19 studies—that active tuberculosis increases susceptibility to influenza virus47 and is a risk factor for subsequent influenza hospitalisation48 and increased mortality.49 The reverse has also proved to be true: influenza is associated with increased susceptibility to pulmonary tuberculosis disease (and disease severity). This relationship was also reported anecdotally during the 1918 influenza pandemic. It is speculated that this might be through antagonism of the interferon-γ pathway (by type-1 interferons such as α and β), with excessive production of interleukin-10 and increased apoptosis.50 It is possible that a similar interaction (increased disease susceptibility and severity) could be occurring between COVID-19 and active tuberculosis, although this remains to be proven. A study showed that individuals with COVID-19 had a reduced frequency of M tuberculosis-specific CD4 T cells in the peripheral blood compartment, supporting the hypothesis that COVID-19 might increase susceptibility to and progression to active tuberculosis.51
Transmission and infection control
The mode of transmission of SARS-CoV-2 was initially thought to be limited to large respiratory droplets (>5–10 μm)—as it used to be for tuberculosis—a conclusion biased by the decades-old assumption that most respiratory viruses are not transmitted by the airborne route. Much of this bias was caused by the so-called proximity pitfall, the fallacious logic that if there is evidence of transmission of respiratory pathogens at close contact (eg, 1 m), then there is no transmission at a distance.52 The 1 m rule is also now known to be false. Large droplets of up to 100 μm have been shown to be carried in turbulent gas clouds for 7–8 m.53 We have also learned that human coughs and sneezes create plumes of both small and large particles—ie, both aerosol (≤5 μm) and droplets (>5 μm) that are highest in concentration close to the source case and dissipate with distance. This is the principle underlying the benefit of physical distancing. However, patients with either COVID-19 or active tuberculosis, or both, often need medical, nursing, and other care requiring close contact. This can result in exposure to infectious aerosols that include both larger droplets and smaller aerosol particles. In the case of tuberculosis, only the small particles are likely to transmit disease. In the case of COVID-19, it appears that both the droplets and aerosol particles transmit disease. Fortunately, all infection prevention and control (IPC) practices that prevent transmission of aerosols also prevent transmission of droplets.
The clinical presentations of COVID-19 and active tuberculosis, either alone or as co-infection, can be similar, as discussed hereafter. We are not aware of published data on the transmission of either SARS-CoV-2 or M tuberculosis during co-infection, but both can probably be transmitted if there is evidence of both pathogens in respiratory secretions. As the transmission of these diseases shares many common elements (table 2 ), methods to prevent transmission are nearly identical. A lesson learned from the outbreaks of multidrug-resistant tuberculosis in the 1980s and 1990s was that the airborne transmission of M tuberculosis was preventable by the implementation of administrative, engineering, and personal respiratory-protection control measures.54 In tuberculosis-endemic settings, almost all the spread of rifampicin-resistant and fluoroquinolone-resistant tuberculosis is by primary rather than acquired transmission.55 The only major difference now is that vaccination is the most important administrative control measure for COVID-19, markedly reducing the susceptibility of exposed HCWs.56 The most important administrative control measure for active tuberculosis remains rapid diagnosis and treatment, rendering the patient non-infectious. These findings, apart from their applicability to frontline HCWs caring for patients with possible active tuberculosis or COVID-19, or both, and the use of N95-equivalent respirators,57 have several implications for COVID-19 control, including improved ventilation in congregate settings (eg, schools, buses, taxis, places of worship, restaurants, and shopping malls) and wider use of masking to prevent transmission and superspreader events. Improved COVID-19 control would be beneficial for tuberculosis management at the patient and HCW level.
Table 2.
Evidence for factors related to transmission by Mycobacterium tuberculosis and SARS-CoV-2
| Mycobacterium tuberculosis | SARS-CoV-2 | |
|---|---|---|
| Transmission by small aerosol particles | Strong | Strong |
| Transmission by large aerosol particles (droplets) | Rare | Strong |
| Transmission by fomites or contact | No | Weak |
| Isolation of viable pathogen in room air | No | Yes |
| Identification of pathogen by PCR in room air | Yes | Yes |
| Isolation (culture) of pathogen from cough aerosols | Yes | No |
| Isolation of pathogen from exhaled breath | Yes | Yes |
| Risk to household contacts | High | High |
| Risk to health-care providers | High | High |
| Risk with proximity to index case | High | High |
| Risk of transmission from asymptomatic case | Probable | High |
| Risk of transmission outdoors | Low | Low |
| Risk of transmission indoors | High | High |
| Reduction of risk by patient mask use | Yes | Yes |
| Susceptibility of pathogen to ultraviolet light | High | Probably high |
| Superspreading epidemiology | Yes | Yes |
Clinical presentation
Few comprehensive studies document the prevalence of tuberculosis in patients with COVID-19. Tuberculosis prevalence (past, current, or past and current) is estimated to vary by between about 2% and 8% in hospitalised COVID-19 cohorts from tuberculosis-endemic countries; for example, an analysis of surveillance data from South Africa found that among 3217 individuals hospitalised with COVID-19, 5·5% had current tuberculosis and 4·0% had previous tuberculosis (data from the South African NICD). In a population cohort study of about 3·5 million patients from the Western Cape province of South Africa (22 308 of whom were diagnosed with COVID-19), about 10% of those with COVID-19 had a diagnosis of previous tuberculosis or current active tuberculosis, with about 2% having concurrent active tuberculosis and COVID-19 in an inpatient setting.58 In another study of 219 265 individuals who were hospitalised with COVID-19, 5·0% of those aged 20–39 years had concurrent active tuberculosis (figure 4A ),59 although this is probably an underestimate given the scarcity of comprehensive testing for tuberculosis and because of other reasons, such as lack of clinical suspicion. In a limited (minimally invasive tissue sampling) post-mortem biopsy study undertaken in Johannesburg, South Africa (n=150), microbiologically proven tuberculosis was detected in 3% of COVID-19-positive biopsies and 13% of COVID-19-negative biopsies.61 For comparative purposes, the prevalence of tuberculosis in the same general population, including those with subclinical or undiagnosed tuberculosis in the community, is about 0·5–1%.62
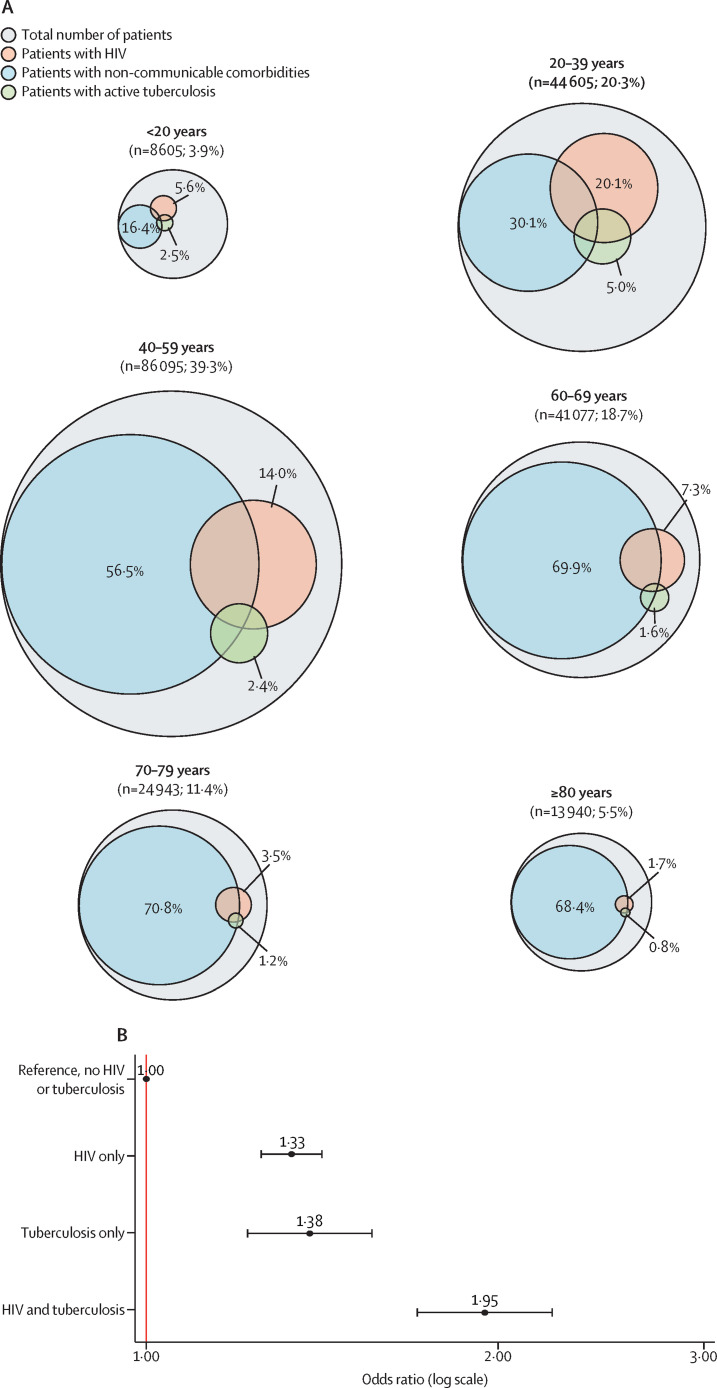
Figure 4.
Co-prevalence of COVID-19, tuberculosis, HIV, and non-communicable diseases
(A) Euler diagram of the estimated prevalence of HIV, current tuberculosis, and non-communicable comorbid conditions in patients admitted to hospital with SARS-CoV-2 infection by age group. (B) Forest plot of the effect of HIV and active tuberculosis on the risk of mortality associated with COVID-19. Multivariable analysis of factors associated with COVID-19 in-hospital mortality from published South African data is shown.59, 60 The model adjusted for age, sex, race, other comorbid conditions, health sector, province, and month of admission. Adjusted odds ratios and 95% CIs are shown for HIV only, current tuberculosis only, and HIV and tuberculosis co-infection. Values that overlap between comorbidities are noted in the appendix (p 13).
Active tuberculosis is often not considered by clinicians in the setting of acute lower respiratory tract infection (LRTI) or acute community-acquired pneumonia. However, this often reflects a misunderstanding and unfamiliarity with the concept of tuberculosis-associated community-acquired pneumonia. Active tuberculosis presenting as an acute LRTI is well documented, but a poorly appreciated and inadequately studied entity.63 A systematic review across several countries in Africa and Asia (including China, India, Malaysia, Cambodia, and Vietnam) showed that 10–15% of community-acquired pneumonia cases or LRTIs in these settings are caused by M tuberculosis. 64 Duration of symptoms is a poor guide to the likelihood of tuberculosis; a study of almost 2500 individuals with respiratory tract infection admitted to two hospitals in South Africa between 2012 and 2014 showed that when the duration of symptoms was less than 14 days, a staggering 18% of participants still had positive microbiological tests for tuberculosis.65 The key message is that in certain high tuberculosis-burden settings, symptom duration of less than 14 days and acute presentation of LRTIs does not exclude the possibility of active tuberculosis. Collectively, these data suggest that the burden of active tuberculosis in people hospitalised with COVID-19 (as aforementioned) is probably underestimated. A patient perspective provided by AvD and DvD is outlined in panel 4 ; a more detailed version is available in the appendix (p 2).
Panel 4. Patient perspective.
Social stigma kills. Like tuberculosis, COVID-19 has fuelled social stigma because it is easy for societies to associate the fear of the unknown with others;66 we have seen people afraid of testing and disclosing test results, and not adhering to guidelines to prevent the spread of disease.
Afraid of being labelled as weak, scared, or sickly, individuals might hide their disease status or struggle with the mental and social challenges of existential stigma.67 To ask those who must battle an airborne infectious disease in isolation to simultaneously fight social stigma and question their worth is unacceptable. To find meaning in the devastation of tuberculosis and COVID-19, and to mitigate the effects of future pandemics, we need a shift in the way in which society views infectious diseases and health.
Like tuberculosis, COVID-19 can cause death in one person but no symptoms in another; we know that some conditions predispose individuals to infection or more severe disease, but we do not always know why some apparently healthy individuals are vulnerable and become so ill. These unknowns can drive stigma.
Understanding the responses of members of society—individuals and communities—to the threats posed by an infectious disease outbreak is crucial to implementing effective strategies for infection prevention and control, and to minimising the negative health impacts of mitigation strategies.
Patient groups have raised concerns about the regrettable inevitability of the destruction caused to affected communities by tuberculosis because of the supposedly insurmountable shortage of resources. Yet US$9 billion were invested in research and development in the first 9 months of the COVID-19 pandemic,68 which claimed 1 million lives in that same time. This is the same amount invested in tuberculosis in the preceding 14 years,69 at the cost of 25 million lives.1 The COVID-19 response has been lauded for its unprecedented global solidarity. Similar shared promises have been made for tuberculosis at the highest levels, but these have repeatedly failed to result in desperately needed actions and investments. Are the lives lost to tuberculosis really worth so much less than those lost to COVID-19?
Diagnosis of tuberculosis in the context of COVID-19
Although night sweats, haemoptysis, loss of weight, and isolated upper-zone pulmonary infiltrates are more suggestive of active tuberculosis, symptoms of active tuberculosis and COVID-19 often overlap.70 Furthermore, although isolated upper-lobe pulmonary infiltrates might indicate active tuberculosis and lower infiltrates might be suggestive of other bacterial infection, the radiology of active tuberculosis and COVID-19 are often similar (appendix p 7). Indeed, tuberculosis-associated community-acquired pneumonia can present as bilateral nodular-to-confluent shadowing, which is often also seen with COVID-19.70 A study indicated that in almost half the cases of active tuberculosis and COVID-19 co-infection, radiological features were unhelpful diagnostically, and in 40% of cases, CT findings were consistent with COVID-19.71, 72 Furthermore, COVID-19 might also occur during tuberculosis treatment, and SARS-CoV-2 infection might unmask a diagnosis of subclinical tuberculosis. In tuberculosis-endemic countries, such an occurrence is not unusual, given the very high incidence of tuberculosis. Indeed, in the South African 2018 national tuberculosis prevalence survey (which estimated an annual incidence of 737 cases per 100 000 people), 58% of patients with culture-proven active tuberculosis did not report any tuberculosis-related symptoms (and thus tuberculosis would have remained undetected were it not for COVID-19-related hospitalisation or presentation).62
Biomarkers are often unhelpful in distinguishing tuberculosis from COVID-19. Both diseases can cause lymphopenia and raise inflammatory markers, including C-reactive protein (CRP). HIV co-infection can further complicate the picture, especially in African settings, because HIV can suppress typical symptoms of active tuberculosis, modulate radiological findings, and influence biomarker profiles.
To further complicate matters, tuberculosis and COVID-19-specific NAATs have a false-negative rate of 20–30% because of the sampling error and variability in pathogen load,70 patients with tuberculosis are not uncommonly sputum scarce, sputum induction is unavailable or inappropriate in a COVID-19 setting, and tuberculosis-specific NAATs might give false-positives in cases of previous tuberculosis.73, 74 Thus, alternative non-respiratory tract-based tests for active tuberculosis and COVID-19 are urgently required. Repeat COVID-19 testing is often required, and tuberculosis culture positivity can only be detected several weeks down the line, which can further complicate the diagnosis. In people who are HIV positive with advanced immunosuppression, testing for urine lipoarabinomannan (LAM)75 and using abdominal ultrasound to detect lymphadenopathy and liver and splenic abscesses could be useful.76, 77
In summary, reliance on typical patterns of presentation of active tuberculosis and COVID-19 (including in tuberculosis-endemic settings) to ascertain a clinicoradiological diagnosis is unreliable, and one should test for both diseases (separate samples are required), or at least have a low index for also testing for tuberculosis when testing for COVID-19.78
Implications for HCWs and clinical practice
The ambiguous presentation of tuberculosis in the context of COVID-19 has implications for HCWs and clinical practice. It is well recognised that HCWs are at increased risk (compared with the general population) of both COVID-19 and tuberculosis.79, 80, 81 In many settings, including in Africa, HCWs might have HIV, placing them at even higher risk of both diseases. Because active tuberculosis and COVID-19 might have similar presentations, present simultaneously, often have overlapping risk factors and imaging characteristics, and take time (usually 24–72 h) to diagnose (especially during surges), and because active tuberculosis might present as an acute LRTI,63 HCWs might be exposed to infectious aerosols of SARS-CoV-2, M tuberculosis, or both for a prolonged period of time. Effectively, it is impossible to clinically tell the difference between active tuberculosis and COVID-19. Thus, airborne IPC packages must be prioritised and implemented,82 particularly in tuberculosis-endemic settings. Such packages will include administrative, environmental, and personal protection measures, including access to particulate filtering facepiece (N95, FFP2, or equivalent) respirators to ensure HCW safety, as well as adequate ventilation. As aerosol transmission of SARS-CoV-2 has been more widely recognised, several international bodies (including WHO, the US Centers for Disease Control and Prevention, and the European Centre for Disease Prevention and Control) have updated their guidelines to recommend the use of respirators for routine care of individuals thought or known to have COVID-19.83, 84, 85 It is already established best practice to protect against M tuberculosis in high tuberculosis-burden settings, but this is rarely implemented in LMICs.82 This is a complex topic with many challenges, including the global shortage of PPE, and several relevant aspects have been reviewed in detail elsewhere as part of South African guidance on protecting HCWs.86
Management and prognosis of co-infected patients
Management of COVID-19 is similar in patients with both active tuberculosis and COVID-19. The need for steroids and respiratory support is dictated by indices of oxygenation (eg, oxygen saturation and partial pressure of oxygen) and clinical presentation. If steroids are indicated for the treatment of COVID-19 in patients with active tuberculosis, then the dose is generally doubled because of the liver enzyme-inducing effect of rifampicin. Similar considerations might apply to some antiviral therapies; for instance, rifampicin is predicted to reduce exposure to remdesivir, although the clinical importance of this exposure remains unclarified.87, 88 The pharmacokinetic effect of rifampicin on newer antivirals such as molnupiravir and paxlovid remains to be clarified. In patients who require mechanical ventilation, tuberculosis treatment might need to be given intravenously or via a nasogastric tube.89 Administration via nasogastric tube might necessitate therapeutic drug monitoring.89 The management of people with rifampicin-resistant active tuberculosis and COVID-19 co-infection is similar to that of people who do not have a co-infection. Given the growing recognition of fungal infections (eg, mucormycoses) among people who survive COVID-19, potentially driven by diabetes and prolonged steroid use, it is important to achieve good glycaemic control and avoid steroid overuse, especially in people with concurrent active tuberculosis.
Data from South Africa59 and elsewhere58 suggest that, similar to co-infection with influenza and active tuberculosis,47 and bacterial co-infection with active tuberculosis,90 patients with COVID-19 and concurrent or past history of tuberculosis have an approximately two-times greater risk of hospital-based mortality than do those without tuberculosis. HIV and COVID-19 co-infection alone is also associated with a similar increased risk of mortality,58, 60 and HIV, active tuberculosis, and COVID-19 co-infection increases this mortality risk even further (figure 4B), with the highest mortality risk in those with advanced immunosuppression and not on antiretroviral therapy.
These risk factors have implications for triaging patients for more advanced respiratory support in tuberculosis-endemic settings and, given the higher mortality in patients co-infected with active tuberculosis and COVID-19, supports a role for giving patients with active tuberculosis and survivors of tuberculosis priority for earlier SARS-CoV-2 vaccination, testing for tuberculosis in all people with COVID-19, and testing for COVID-19 in all people with newly diagnosed active tuberculosis when there is still substantial community transmission of SARS-CoV-2. Moreover, people co-infected with active tuberculosis and COVID-19 should be monitored more closely with a lower threshold for referral and intervention.
In addition to affecting mortality risk in patients with tuberculosis, COVID-19 has also been shown to negatively affect other tuberculosis outcomes, including treatment failure and loss to follow-up rates through several mechanisms (figure 2).
Lung disease after tuberculosis and COVID-19
Persistent pulmonary impairment, disability, or both after active tuberculosis (due to pulmonary remodelling associated with cavitation, fibrosis, and bronchiectasis) is present in about 50% of tuberculosis survivors (a higher proportion in the severe disease group) despite successful microbiological treatment.10 This impairment is one reason why tuberculosis co-infection might be associated with increased susceptibility to COVID-19 and a higher risk of mortality in people with COVID-19.58
Patients with lung disease after tuberculosis might be at greater risk of developing COVID-19 pneumonia, and hence lung disease after COVID-19, and are at increased risk of death when hospitalised with COVID-19 pneumonia, as discussed in the previous subsection. On the basis of animal studies and data obtained from patients with active tuberculosis, dysregulated immunity in patients with lung disease after tuberculosis is probably characterised by the unchecked activity of matrix metalloproteinases, a proinflammatory and fibrogenic cytokine profile, an abundance of neutrophil extracellular traps, and uncontrolled CD4 T-cell activation; these factors might contribute to a heightened risk of developing lung disease after COVID-19 in these patients.10, 91 Genetic polymorphisms in the MMP1 (matrix metalloproteinase 1) and MCP1 (CCL2 or C-C motif chemokine ligand 2) promoter regions have been shown to portend an increased risk for fibrosis and bronchiectasis after tuberculosis. Given the high prevalence of lung disease after tuberculosis, high tuberculosis-burden settings should be prepared for a substantial burden of lung disease after COVID-19 and resulting disability following the COVID-19 pandemic (appendix pp 8–9).92, 93 The convergence of lung disease after tuberculosis and lung disease after COVID-19 necessitates the follow-up of patients with post-tuberculosis lung disease who had COVID-19 pneumonia and the prioritisation of their linkage to respiratory services for optimal care.94, 95 We would advocate for joint or combined services for lung disease after tuberculosis and after COVID-19 in appropriate settings.
Interventions to mitigate the impact of COVID-19
Tuberculosis screening and active case finding
Almost one in three (about 3 million) people with active tuberculosis, even in the pre-COVID-19 era, remained undiagnosed or unreported globally.1 These individuals act as a potential reservoir for transmission. The majority of these so-called missing individuals often reside in peri-urban informal settlements of large cities in Africa and Asia.96, 97 Thus, to address the COVID-19-related reduction in case detection and to reduce transmission, disease burden, and mortality, community-based enhanced case detection is required. We need to take tuberculosis testing closer to where people live and work. Active case-finding strategies have always had important implications for tuberculosis prevention and reduced amplification of the epidemic even before the advent of COVID-19.
Real-time data reporting
Instead of annual tuberculosis reports, global and national public-facing dashboards and trackers reporting real-time numbers of tuberculosis cases and deaths, including monitoring of trends over time, would be a useful addition to tuberculosis monitoring. They would keep the public informed and hold services accountable at national, regional, and global levels. Such dashboards have been used very effectively to monitor the COVID-19 pandemic and to evaluate the effect of interventions. It is not widely appreciated by the lay public that although the direct death toll from COVID-19 is estimated to be about 4·5 million people over an approximately 18-month period,98 the sustained death toll from tuberculosis over the past decade has been close to about 20 million people.1 A public-facing dashboard for tuberculosis has now been instituted in the Western Cape province of South Africa.99
Screening apps and mHealth
Digital tools such as mobile phone-based screening and tracing apps have been used with great effect to manage the COVID-19 pandemic.100 The same methodologies could easily be applied to tuberculosis. In South Africa, the Department of Health has recently introduced a WhatsApp and SMS-based tuberculosis screening app—the TB Health Check app—that requires little cell phone memory, uses a few simple screening questions, and links individuals to tuberculosis testing services. At the time of writing, more than 30 000 screenings had been done, and a staggering 12·8% of those with symptoms who subsequently tested for tuberculosis were positive. Work is underway to integrate the COVID-19 and tuberculosis screening apps. A more sophisticated version of this strategy could link individuals to tuberculosis testing booths in the way individuals have been linked to COVID-19 testing centres. App-based HIV self-testing programmes have already shown great promise,101, 102 and their potential could be expanded to include tuberculosis testing.
Intensified screening of high-risk groups
COVID-19 has increased acceptance of the value of universal testing for infectious diseases irrespective of symptoms. WHO recently updated recommendations for systematic screening of people in high-risk tuberculosis groups (defined as using tests, examinations, or other procedures to identify those most likely to have tuberculosis disease).96 In most settings, symptom screening fulfils this role for tuberculosis, but there is increasing evidence for the use of WHO-recommended molecular assays, chest radiography, and, for HIV-positive inpatients, CRP and urine LAM assays, either as a standalone or in combination with symptom screening.
In the setting of high tuberculosis burden, people living with HIV are at high risk of tuberculosis, especially those with low CD4 counts103, 104 and those not receiving suppressive antiretroviral therapy and tuberculosis preventive treatment.105 Women who are pregnant and HIV positive bear a dual risk for active tuberculosis,106 given that pregnancy confers additional risk for tuberculosis disease when compared with non-pregnant women.107, 108 Patients who have previously had an episode of tuberculosis are at a markedly higher risk of a subsequent episode and warrant additional screening for recurrence.109, 110, 111, 112 Finally, incarcerated adults in virtually every setting have a higher incidence of active tuberculosis than the populations that surround their incarcerating facility.113, 114, 115
All these populations have a reported tuberculosis prevalence that is markedly higher than the threshold recommended by WHO for systematic screening, and they should be targeted for consent-based universal testing with rapid turnaround tests that have high sensitivity and specificity. Better integration of tuberculosis and COVID-19 molecular testing and optimisation of multidisease testing platforms will greatly help to expand access to testing for both infections. Some countries, such as South Africa, are adopting a targeted universal testing strategy that will test (regardless of symptoms) all those attending health-care facilities who have HIV, previous tuberculosis, previous tuberculosis contact, or are pregnant. This is an emergency response to the profound reduction in testing since the start of the pandemic and should be considered by other countries.
Screening in health-care facilities
Symptom screening is often (and preferably routinely) done for all primary clinic attendees as part of screening for COVID-19. This screening offers an opportunity for systematic tuberculosis screening. There is considerable overlap between symptoms of mild COVID-19 and active tuberculosis, which suggests that dual testing for both pathogens should be done at least in attendees at high risk for tuberculosis, possibly using a single specimen to avoid creating an additional hazard for HCWs or other patients. However, because of safety concerns, there might be a reluctance to collect sputum, and alternative specimens that are safer to collect, such as tongue swabs116 or saliva, should be explored for SARS-CoV-2 and M tuberculosis testing.117, 118 Non-sputum-based tuberculosis diagnosis, however, remains a research challenge.
Door-to-door screening versus scalable mobile clinics
There are several approaches to community-based active case finding in high-prevalence settings.96 As noted by WHO, implementation of a population-wide screening programme requires a substantial investment of resources and needs to be judged against the potential for long-term reduction in future tuberculosis incidence. A door-to-door active case-finding or screening strategy has the potential to detect more people with active tuberculosis, but it is more costly and labour intensive than a mobile clinic approach. The door-to-door case-finding strategy, using laboratory-based molecular tools such as Xpert MTB/RIF, has been shown to reduce adult tuberculosis prevalence and have a favourable effect on disease burden in the wider community.119 Similarly, reductions in the prevalence of latent tuberculosis infection have been demonstrated among children in clusters in which door-to-door tuberculosis screening was done.119, 120 Compared with door-to-door strategies, a mobile clinic-based approach has been reported to be more effective for case detection.121 Mobile clinics equipped with point-of-care (POC) molecular tools provide additional opportunities for community-based active case finding. Indeed, a mobile clinic-based strategy has previously been shown to be feasible and highly effective in detecting people in the community with culture-positive tuberculosis,122, 123, 124 although this approach was not readily scalable because of the logistical challenges of running an Xpert platform in the community. Recent advances in technology, however, have enabled the incorporation of portable and battery-operated molecular tools like the GeneXpert systems into active case-finding algorithms. These portable systems enhance the scalability of the mobile clinic-based approaches. A recently completed randomised controlled trial evaluating the effect of a scalable intervention package, including a POC molecular diagnostic tool, on community-based tuberculosis case finding showed the feasibility of such a strategy and its potential to detect the majority of community-based infectious tuberculosis cases (appendix p 10).125 Such models can also be used to screen at-risk individuals (eg, close contacts who are HIV negative) for latent tuberculosis infection and link them to preventive therapy.126
Chest radiography in screening
Chest radiography is one of the most sensitive tests for detecting active tuberculosis, particularly in high-burden countries, and is an important inclusion in tuberculosis active case-finding programmes.127, 128 Results from a national survey in South Africa showed that more than half of bacteriologically confirmed tuberculosis survey cases had chest radiography abnormalities without reported symptoms.62 This finding suggests a potential role for screening chest radiography in detecting presymptomatic or minimally symptomatic disease in the community. A major pitfall of using screening chest radiography is its low specificity (about 50%) in detecting active tuberculosis, especially in a setting of high tuberculosis prevalence in which the prevalence of previous tuberculosis disease is high. Thus, cases that are flagged as active tuberculosis still need to undergo confirmatory bacteriological testing. Furthermore, the use of chest radiography is often hindered by scarcity of resources and trained personnel, technical limitations, high cost of hardware, and intra-reader and inter-reader variability.129, 130, 131 Potential technological answers to these challenges are ultraportable chest radiography machines and artificial intelligence-driven computer-aided detection (CAD) software packages.132 On the basis of available evidence, the WHO recently recommended the use of chest radiography and CAD for pulmonary tuberculosis screening, which has the potential to increase equity in the reach of tuberculosis screening interventions.96
Reprioritising WHO guidance
The WHO consolidated guidelines on screening recommend systematic (untargeted) tuberculosis screening for people in the general population when the background tuberculosis prevalence is greater than 500 per 100 000 people, and (targeted) screening for those with a risk factor for tuberculosis when prevalence is greater than 100 per 100 000 people.96 Chest radiography was endorsed as a sensitive but non-specific screening tool for the early detection of tuberculosis. CAD software packages had similar performance to human readers but varied across contexts, and WHO's recommendation was thus conditional and based on a low certainty of evidence. CRP testing has been recommended as a screening tool in people who are HIV positive. Molecular rapid diagnostic tests for tuberculosis (eg, Xpert Ultra) have been recommended by WHO as tuberculosis screening tools for populations in which screening is recommended (conditional recommendation with a low certainty of evidence). However, how these methods should be deployed remains unclear. We strongly endorse more aggressive tuberculosis screening practices, including the use of chest radiography and molecular tests for people at risk of tuberculosis, as outlined in the guidance. Because of the COVID-19 pandemic, every country has an expanded molecular-testing capacity. This capacity should be leveraged for tuberculosis molecular testing.
Reducing pretreatment loss to follow-up
It is estimated that between 15% and 40% of patients with active tuberculosis in endemic countries do not initiate treatment after a microbiologically confirmed diagnosis (known as pretreatment loss to follow-up or PTLF).133 We assume that COVID-19 would affect people returning to health facilities and exacerbate PTLF (especially among patients diagnosed at hospital, where mortality is higher because of loss to follow-up).134, 135 Two studies reported an increase in sputum-smear positivity and more severe clinical and radiological findings as a consequence of diagnostic and pretreatment delays during the COVID-19 pandemic.21, 136 By contrast, a Chinese study documented no increase in pretreatment delay.21 Data-integration systems connecting different levels of the health-care system are important tools to link patients to care and prevent loss to follow-up.
Facilitating treatment adherence
Some developments in health-care system strengthening and person-centred strategies, originally developed for COVID-19, have been integrated into tuberculosis programmes to mitigate the impact of COVID-19 on tuberculosis treatment outcomes as well as knock-on effects, such drug stockouts.137, 138 The growing use of digital health, telemedicine, digital adherence technologies (eg, video-assisted treatment and smart pill boxes), and clinical management with bulk dispensing from decentralised pick-up points or home delivery of medicines are minimising facility visits, limiting nosocomial M tuberculosis and SARS-CoV-2 transmission, and protecting patients with tuberculosis from COVID-19. However, these strategies have been inconsistently implemented in tuberculosis programmes, patients have reported feeling insufficiently supported, and the value of these strategies has not been prospectively evaluated.44, 134 Augmentation of other interventions shown to improve treatment adherence—such as SMS-based reminders, incentives, and enablers (eg, social grants and incentives with financial value), patient education, counselling, and psychological interventions—will help to facilitate treatment adherence and has become even more relevant in the COVID-19 era.139
SARS-CoV-2 vaccination
The past 18 months have showcased the crucial role of vaccines in COVID-19 control. Although non-pharmaceutical interventions and other public health measures have helped to mitigate the effects of COVID-19 in many LMICs, vaccine nationalism and vaccine inequity mean that COVID-19 is likely to remain a problem in many of these countries for some time, thus exacerbating suboptimal tuberculosis control. Vaccinating the world, therefore, is not only crucial for ending the pandemic, but also for restoring tuberculosis services. Thus, as a tuberculosis intervention strategy, there must be improved access to SARS-CoV-2 vaccines in LMICs; in many settings, vaccine resistance and hesitancy also have to be overcome.140 Lessons learned in design, technological application, testing and fast-tracking of clinical trials, funding, development, and distribution of the SARS-CoV-2 vaccines could also be applied to accelerate tuberculosis vaccine development.
BCG vaccination
The BCG vaccine was first used about 100 years ago and is now given at birth in several tuberculosis-endemic settings to prevent tuberculous meningitis.141 The impact of COVID-19 in reducing BCG vaccination rates has already been highlighted (panel 2 and figure 2). However, epidemiological and other studies have shown several non-tuberculosis disease-specific effects of BCG, including protection from respiratory tract infections other than tuberculosis142 and enhancement of vaccine responses to yellow fever143 and influenza.144 Mechanistic studies suggest that these vaccines induce trained innate immunity (appendix pp 2–3).145 A stronger evidence base to support the clinical application of the heterologous effects of BCG has more recently emerged. The double-blind, placebo-controlled ACTIVATE (A randomized Clinical trial for enhanced Trained Immune responses through Bacillus Calmette-Guérin VAccination to prevenT infections of the Elderly) trial among 202 European older patients showed a 45% reduction in time to first infection and a 42% reduction in all infections attributed to BCG. The biggest effect was in reducing viral respiratory tract infections;146 findings were consistent with the earlier smaller studies in Indonesia147 and Japan.148
This growing evidence-based and biological understanding of the protective effects of BCG vaccination on respiratory viral infections provides a valid justification for clinical studies to establish the effect, if any, of BCG vaccination on SARS-CoV-2 infections, severe COVID-19, or death.149 Although early observational, ecological studies suggested a benefit of BCG, more rigorous and updated analyses have shown no effect of BCG on COVID-19.150 Given the limitations of observational research, as of July 5, 2021, there were more than 20 ongoing clinical trials registered in ClinicalTrials.gov that are evaluating the effects of BCG on SARS-CoV-2 infection (appendix pp 2–3).151, 152 Although the BCG-PRIME (Prevention of Respiratory Tract Infection and COVID-19 through BCG Vaccination in Vulnerable Older Adults) trial153 has shown no benefit, other trials are likely to provide a clear answer about the benefits of BCG vaccination against COVID-19 and other respiratory tract infections in adults.
Lessons learned from COVID-19
The COVID-19 pandemic has taught the world that epidemics can only be controlled by several interventions, some personal (eg, masking and vaccinations) and others societal (eg, social protection and lockdowns). The Swiss cheese respiratory pandemic defence model created by Mackay154 outlines the multiple layers of these personal and shared responsibilities that are needed to prevent the spread of SARS-CoV-2. The model has several layers of Swiss cheese to illustrate the fact that a single layer of protection will not be enough to stop COVID-19. Because of the holes in each slice, the coronavirus will always get through; therefore, multiple layers of protection are needed to halt the pandemic. We need a similar, comprehensive approach for tuberculosis, captured in the tuberculosis Swiss cheese model, with three broad levels: societal, personal, and person-centric health-care systems interventions (appendix p 11).155 By addressing the multiple layers, including improving the glacial pace of tuberculosis research, correcting suboptimal and deficient research funding, and treating tuberculosis as the global emergency that it is, we could end tuberculosis (panel 5 ).156
Panel 5. Research priorities and major challenges that need to be addressed.
A range of research priorities need to be addressed to restore control efforts and reduce the global burden of tuberculosis. Some of the goals listed here are long-standing priorities; others have become relevant in the time of COVID-19.
Diagnosis and care
-
•
Improve tuberculosis (and COVID-19) case finding; for example, through the development of e-health approaches to enable mass triage and targeted screening (including self-screening) of people with risk factors, with linkage to further investigation and care
-
•
Optimise systematic tuberculosis (and COVID-19) active case finding in high-risk populations; establish how active case finding should be done (mobile clinics vs door-to-door visits) and how samples should be processed (on site vs in centralised laboratories)
-
•
Develop sensitive and specific point-of-care diagnostics and cost-effective joint screening strategies for tuberculosis and COVID-19
-
•
Refine diagnostic algorithms for tuberculosis and COVID-19 that optimise the use of available technologies (eg, chest radiography with or without computer-aided detection) and are calibrated to the risk profiles of individuals (eg, previous COVID-19, additional tests for people who are HIV positive, or more frequent testing for people who are incarcerated)
-
•
Explore implementational approaches to integrate patient-centred innovations (such as decentralised dispensing, home delivery, and holistic forms of adherence support) into routine practice, including in resource-constrained settings
-
•
Develop methods and algorithms to integrate screening for latent tuberculosis infection (and COVID-19) and linkage to preventive therapy to all significantly exposed individuals regardless of HIV status
Surveillance and outcomes
-
•
Estimate the prevalence and effects of SARS-CoV-2 infection in individuals with active tuberculosis, including on tuberculosis treatment outcomes and long-term health
-
•
Characterise impairment in individuals with lung disease after tuberculosis and COVID-19
-
•
Establish the prevalence and effects of long COVID in individuals with active tuberculosis
-
•
Better monitor adequacy of response to tuberculosis treatment regimens, including those used for preventive therapy
-
•
Improve and broaden the use of rapid, direct methods to estimate the impact of COVID-19 on cause-specific mortality (eg, minimally invasive autopsy)
Systems strengthening and pandemic preparedness
-
•
Realise a more comprehensive tuberculosis response that simultaneously targets the societal, personal, and health system levels, recognising that many tuberculosis drivers also influence COVID-19
-
•
Explore strategies to protect health-care workers against tuberculosis and COVID-19 without compromising on quality of care (eg, introducing respiratory protection programmes, strengthening occupational health infrastructure, and maintaining reserves of personal protective equipment reserves)
-
•
Explore long-term solutions to vaccine hesitancy, recognising that SARS-CoV-2 vaccination will reduce the tuberculosis burden and a viable tuberculosis vaccine is likely to face similar challenges with uptake
Major challenges
-
•
Improve understanding and effectively communicate the cost of tuberculosis, COVID-19, and their interaction, to the economies of tuberculosis-endemic countries in terms of effect on gross domestic product and other metrics of economic development
-
•
Substantially increase research funding for translational and operational research, and for innovation and commercialisation activities for tuberculosis interventions (as has been done for COVID-19)
-
•
Improve systems and processes to enable rapid review of proposals and to improve regulatory harmonisation (thus reducing massive delays in the completion of clinical trials)
-
•
Treat tuberculosis (like COVID-19) as an important entity in any national or global pandemic response strategy
-
•
Effectively tackle COVID-19 in countries with high tuberculosis and COVID-19 burden (predominantly low-income and middle-income countries) without diverting resources and personnel from tuberculosis programmes
-
•
Develop strategies to address the effect of COVID-19 on tuberculosis treatment outcomes
-
•
Accelerate the development of tuberculosis vaccines through harnessing what has been learned from COVID-19
-
•
Increase the level of ambition in terms of what tuberculosis control metrics should be achieved within specific timelines (using COVID-19 as a benchmark)
-
•
Identify and remove sources of structural violence, systemic racism, and discrimination, especially those affecting access to essential services and equitable, high-quality health care
Conclusion
COVID-19 has set tuberculosis control efforts back by about a decade. This setback will probably translate into long-term increases in tuberculosis-related deaths and structural lung disease. With low rates of SARS-CoV-2 vaccination in tuberculosis-endemic countries and the emergence of new variants, this trend is likely to continue. New strategies involving triage tools and innovative active case-finding interventions require urgent implementation to reverse these alarming trends. We have provided several examples of the successful use of such interventions in different parts of the world. A more cohesive global approach is now urgently required.
Active tuberculosis and COVID-19 screening should be combined in tuberculosis-endemic settings. False-negative results might occur with both tuberculosis and COVID-19 testing, and better diagnostics, independent of samples obtained from the respiratory tract, are needed for both diseases. The inability to distinguish between acute tuberculosis disease and COVID-19 has implications for strategies of IPC in tuberculosis-endemic settings, including the provision of N95 (or equivalent) respirators to HCWs. Individuals with active tuberculosis and COVID-19 should be monitored more closely with a lower threshold for referral and intervention.
The vicious cycle of poverty and death is exacerbated by the twin epidemics of tuberculosis and COVID-19 (with HIV making up a triad of infectious disease epidemics in some settings, and a non-communicable disease epidemic expanding in tandem).157 In some settings, disruption of HIV diagnosis and treatment programmes might fuel the tuberculosis pandemic. It is therefore crucial to support and revitalise both tuberculosis and HIV programmes. That airborne and aerosol-based spread is the dominant form of SARS-CoV-2 and M tuberculosis transmission has important implications for interrupting the spread of both diseases and for protecting HCWs. COVID-19 will exacerbate the situation in endemic settings, in which there is already an appreciable burden of pulmonary disability and lung damage caused by tuberculosis. Addressing the mortality and morbidity associated with tuberculosis and COVID-19 will require global unity, political will—addressing global inequalities and power monopolies—improved funding for vaccine development, and enhanced support for preventive and interventional strategies for both diseases.
Search strategy and selection criteria
We did a literature search of PubMed, MEDLINE, and Google Scholar for papers published in the English language from Jan 1, 2000, to Feb 1, 2022, using the search terms “COVID-19 OR SARS-CoV-2” and “tuberculosis OR TB”, and “case detection” or “outcomes” or “diagnosis” or “screening” or “impact”. Articles were selected—on the basis of a critical review of the title, abstract and, when appropriate, full text—if they were regarded as pertinent to our review of the effect of COVID-19 on the global burden of tuberculosis, including patient-level or clinical-level impact, or our discussion of interventions to reverse the deleterious impact of COVID-19. Preprints were considered as applicable. A selection of citations within the original search documents were reviewed and included if relevant. References were also identified through the authors' own searches online (up to March 1, 2022) and through searches of the authors' personal files and the reference lists of landmark papers and selected publications.
Data sharing
Requests for aggregated laboratory testing data for South Africa should be made in writing by qualifying researchers with valuable research questions to the National Institute for Communicable Diseases, directed to Harry Moultrie (harrym@nicd.ac.za). Further information can be obtained from the corresponding author.
Declaration of interests
NM reports grants from Pfizer and Roche to his institution, outside the submitted work. CC reports research funding from Sanofi Pasteur, awarded to her institution; she is a member of the scientific advisory committee for the BCHW: Burden of COVID-19 Among Health Care Workers project. The other authors declare no competing interests.
Acknowledgments
Acknowledgments
KD acknowledges funding from the South African Medical Research Council (SAMRC; RFA-EMU-02-2017), the European & Developing Countries Clinical Trials Partnership (EDCTP; TMA-2015SF-1043, TMA-1051-TESAII, TMA-CDF2015, RIA2018D-2505, and RIA2020S-3295), the UK Medical Research Council (MR/S03563X/1), and the Wellcome Trust (MR/S027777/1). AP was supported by the EDCTP (TMA2019CDF-2780). CC acknowledges funding from the Wellcome Trust (grant number 221003/Z/20/Z), the SAMRC, and the US Centers for Disease Control and Prevention (co-operative agreement number 6 U01IP001048-04-02), awarded to her institution. KF is supported by the Division of Intramural Research of the National Heart, Lung, and Blood Institute of the National Institutes of Health, Bethesda, MD, USA. MP holds a Canada Research Chair award from the Canadian Institutes of Health Research. We are grateful to Aaron Karat (independent researcher), who contributed to editing of the manuscript.
Contributors
KD, TP, RP, AE, KF, and MP contributed to conceptualisation of this Series paper. KD and TP contributed to formal analysis of the National Institute for Communicable Diseases data, methodology, project administration, resources, visualisation (panels, tables, and figures), and writing of the original draft, review, and editing of the manuscript. HM contributed to data collection, curation, and formal analysis. RP contributed to writing of the original draft, review, and editing. AE contributed to visualisation (supplementary figure 5), writing of the original draft, review, and editing. AJS, JP, AvD, DvD, NM, ML, and SC contributed to writing of the original draft, review, and editing. ZU contributed to writing of the original draft. K-CC contributed to visualisation (supplementary figures), writing of the original draft, review, and editing. AP contributed to visualisation, writing of the original draft, review, and editing. EK contributed to data curation and formal analysis. WJ contributed to data curation, formal analysis, visualisation (figure 4), and review and editing. CC contributed to data curation, formal analysis, methodology, and review and editing. ST contributed to formal analysis, and review and editing. KF and MP contributed to visualisation, writing of the original draft, review, and editing.
Supplementary Material
References
- 1.WHO . World Health Organization; Geneva: 2020. Global tuberculosis report 2020. [Google Scholar]
- 2.WHO . World Health Organization; Geneva: 2021. Global tuberculosis report 2021. [Google Scholar]
- 3.Glaziou P. Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. medRxiv. 2020 doi: 10.1101/2020.04.28.20079582. published online May 4. (preprint). [DOI] [Google Scholar]
- 4.The Stop TB Partnership 12 months of COVID-19 eliminated 12 years of progress in the global fight against tuberculosis. 2021. http://www.stoptb.org/news/stories/2021/ns21_011.html
- 5.Roberts L. How COVID is derailing the fight against HIV, TB and malaria. Sept 10, 2021. https://www.nature.com/articles/d41586-021-02469-8 [DOI] [PubMed]
- 6.Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592:502–504. doi: 10.1038/d41586-021-01022-x. [DOI] [PubMed] [Google Scholar]
- 7.WHO . World Health Organization; Geneva: 2021. Impact of the COVID-19 pandemic on TB detection and mortality in 2020. [Google Scholar]
- 8.Pillay Y, Pienaar S, Barron P, Zondi T. Impact of COVID-19 on routine primary healthcare services in South Africa. SA Med J. 2021;111:714. doi: 10.7196/SAMJ.2021.v111i8.15786. [DOI] [PubMed] [Google Scholar]
- 9.Udwadia ZF, Koul PA, Richeldi L. Post-COVID lung fibrosis: the tsunami that will follow the earthquake. Lung India. 2021;38(suppl):S41–S47. doi: 10.4103/lungindia.lungindia_818_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev. 2018;27 doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raman R, Rajalakshmi R, Surya J, et al. Impact on health and provision of healthcare services during the COVID-19 lockdown in India: a multicentre cross-sectional study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-043590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrinivasan R, Rane S, Pai M. India's syndemic of tuberculosis and COVID-19. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cilloni L, Fu H, Vesga JF, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Zhang K. Insight into impact of COVID-19 epidemic on tuberculosis burden in China. Eur Respir J. 2020;56 doi: 10.1183/13993003.02710-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang C-Y, Islam T, Xu C, et al. The impact of COVID-19 and the restoration of tuberculosis services in the Western Pacific region. Eur Respir J. 2020;56 doi: 10.1183/13993003.03054-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang Y, Liu Y, Du J, Gao J, Li L. Impact of COVID-19 on tuberculosis control in China. Int J Tuberc Lung Dis. 2020;24:545–547. doi: 10.5588/ijtld.20.0127. [DOI] [PubMed] [Google Scholar]
- 17.Shen X, Sha W, Yang C, et al. Continuity of TB services during the COVID-19 pandemic in China. Int J Tuberc Lung Dis. 2021;25:81–83. doi: 10.5588/ijtld.20.0632. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Wang Y, Fleming J, et al. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. medRxiv. 2020 doi: 10.1101/2020.03.10.20033795. published online March 16. (preprint). [DOI] [Google Scholar]
- 19.Wu Z, Chen J, Xia Z, et al. Impact of the COVID-19 pandemic on the detection of TB in Shanghai, China. Int J Tuberc Lung Dis. 2020;24:1122–1124. doi: 10.5588/ijtld.20.0539. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Lu P, Shen Y, et al. Collateral impact of the coronavirus disease 2019 (COVID-19) pandemic on tuberculosis control in Jiangsu Province, China. Clin Infect Dis. 2021;73:542–544. doi: 10.1093/cid/ciaa1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, He W, Lei J, Liu G, Huang F, Zhao Y. Impact of COVID-19 pandemic on pre-treatment delays, detection, and clinical characteristics of tuberculosis patients in Ningxia Hui autonomous region, China. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.644536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fei H, Yinyin X, Hui C, et al. The impact of the COVID-19 epidemic on tuberculosis control in China. Lancet Reg Health West Pac. 2020;3 doi: 10.1016/j.lanwpc.2020.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centre for Health Protection Department of Health Number of notifiable infectious diseases by month in 2019. Feb 10, 2021. https://www.chp.gov.hk/en/statistics/data/10/26/43/6830.html
- 24.Centre for Health Protection Department of Health Number of notifiable infectious diseases by month in 2020. July 12, 2021. https://www.chp.gov.hk/en/statistics/data/10/26/43/6896.html
- 25.Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased influenza activity during the COVID-19 pandemic—United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Week Rep. 2020;69:1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tempia S, Walaza S, Bhiman JN, et al. Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Euro Surveill. 2021;26:1. doi: 10.2807/1560-7917.ES.2021.26.29.2001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brueggemann AB, Jansen Van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;3:e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e1132–e1141. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McQuaid CF, McCreesh N, Read JM, et al. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur Respir J. 2020;56 doi: 10.1183/13993003.01718-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glaziou P, Dodd P, Dean A, Floyd K. Methods used by WHO to estimate the global burden of TB disease. 2020. https://www.who.int/tb/publications/global_report/TB20_Technical_Appendix_20201014.pdf
- 31.Mikkelsen L, Phillips DE, Abouzahr C, et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet. 2015;386:1395–1406. doi: 10.1016/S0140-6736(15)60171-4. [DOI] [PubMed] [Google Scholar]
- 32.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings. AIDS. 2015;29:1987–2002. doi: 10.1097/QAD.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Basteiro AL, Hurtado JC, Castillo P, et al. Unmasking the hidden tuberculosis mortality burden in a large post mortem study in Maputo Central Hospital, Mozambique. Eur Respir J. 2019;54 doi: 10.1183/13993003.00312-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magro P, Formenti B, Marchese V, et al. Impact of the SARS-CoV-2 epidemic on tuberculosis treatment outcome in Northern Italy. Eur Respir J. 2020;56 doi: 10.1183/13993003.02665-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamal WZ, Habib S, Khowaja S, Safdar N, Zaidi SMA. COVID-19: ensuring continuity of TB services in the private sector. Int J Tuberc Lung Dis. 2020;24:870–872. doi: 10.5588/ijtld.20.0400. [DOI] [PubMed] [Google Scholar]
- 36.Mohammed H, Oljira L, Roba KT, Yimer G, Fekadu A, Manyazewal T. Containment of COVID-19 in Ethiopia and implications for tuberculosis care and research. Infect Dis Povert. 2020;9:131. doi: 10.1186/s40249-020-00753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaikh N, Pelzer PT, Thysen SM, Roy P, Harris RC, White RG. Impact of COVID-19 disruptions on global BCG coverage and paediatric TB mortality: a modelling study. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United Nations World economic situation and prospects as of mid-2021. 2021. https://www.un.org/development/desa/dpad/publication/world-economic-situation-and-prospects-as-of-mid-2021/#:~:text=According%20to%20the%20World%20Economic,UN%20forecasts%20released%20in%20January
- 39.Harimurti P, Pradhan E, Cheikh N, et al. The World Bank Group; Washington, DC: 2020. Tuberculosis in Indonesia: epidemic projections and opportunities to accelerate control: findings from an optima TB analysis. [Google Scholar]
- 40.Bhargava A, Shewade HD. The potential impact of the COVID-19 response related lockdown on TB incidence and mortality in India. Indian J Tuberc. 2020;67(suppl 4):S139–S146. doi: 10.1016/j.ijtb.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuady A, Houweling TAJ, Richardus JH. COVID-19 and tuberculosis-related catastrophic costs. Am J Trop Med Hyg. 2020;104:436–440. doi: 10.4269/ajtmh.20-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.KPMG Global economic impact of tuberculosis. 2017. https://mv.ecuo.org/wp-content/uploads/sites/4/2017/12/Global-Economic-Impacts-of-TB.pdf
- 43.The Economist Intelligence Unit A call to action: it's time to end drug-resistant tuberculosis. 2021. https://www.eiu.com/graphics/marketing/pdf/its-time-to-end-drug-resistant-tuberculosis-summary-report.pdf
- 44.The Stop TB Partnership The impact of COVID-19 on the TB epidemic: a community perspective. 2021. http://www.stoptb.org/assets/documents/resources/publications/acsm/Civil%20Society%20Report%20on%20TB%20and%20COVID.df?fbclid=IwAR3SOY4kyBs5a_35HIeUhcvwRIWspePA4vVHESqcQxio7G4irivJ90cSU8k
- 45.The Global Fund . Global Health Campus; Geneva: 2020. Mitigating the impact of COVID-19 on countries affected by HIV, tuberculosis, and malaria. [Google Scholar]
- 46.Pai M, Olatunbosun-Alakija A. Vax the world. Science. 2021;374 doi: 10.1126/science.abn3081. [DOI] [PubMed] [Google Scholar]
- 47.Walaza S, Cohen C, Tempia S, et al. Influenza and tuberculosis co-infection: a systematic review. Influenza Other Respir Vir. 2020;14:77–91. doi: 10.1111/irv.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abadom TR, Smith AD, Tempia S, Madhi SA, Cohen C, Cohen AL. Risk factors associated with hospitalisation for influenza-associated severe acute respiratory illness in South Africa: a case-population study. Vaccine. 2016;34:5649–5655. doi: 10.1016/j.vaccine.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen C, Moyes J, Tempia S, et al. Mortality amongst patients with influenza-associated severe acute respiratory illness, South Africa, 2009–2013. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riou C, Du Bruyn E, Stek C, et al. Relationship of SARS-CoV-2–specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J Clin Invest. 2021;131 doi: 10.1172/JCI149125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020;8:914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions. JAMA. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 54.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 55.Dheda K, Gumbo T, Maartens G, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir Med. 2019;7:820–826. doi: 10.1016/S2213-2600(19)30263-2. [DOI] [PubMed] [Google Scholar]
- 56.Cabezas C, Coma E, Mora-Fernandez N, et al. Associations of BNT162b2 vaccination with SARS-CoV-2 infection and hospital admission and death with covid-19 in nursing homes and healthcare workers in Catalonia: prospective cohort study. BMJ. 2021;374 doi: 10.1136/bmj.n1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mottay L, le Roux J, Perumal R, et al. KN95 filtering facepiece respirators distributed in South Africa fail safety testing protocols. S Afr Med J. 2020;111:234–239. doi: 10.7196/SAMJ.2021.v111i3.15381. [DOI] [PubMed] [Google Scholar]
- 58.Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2021;73:e2005–e2015. doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jassat W, Mudara C, Ozougwu L, et al. Difference in mortality among individuals admitted to hospital with COVID-19 during the first and second waves in South Africa: a cohort study. Lancet Glob Health. 2021;9:e1216–e1225. doi: 10.1016/S2214-109X(21)00289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jassat W, Cohen C, Tempia S, et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. Lancet HIV. 2021;8:e554–e567. doi: 10.1016/S2352-3018(21)00151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nunes MC, Hale MJ, Mahtab S, et al. Clinical characteristics and histopathology of COVID-19 related deaths in South African adults. PLoS One. 2022;17 doi: 10.1371/journal.pone.0262179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.South African Medical Research Council The first national TB prevalence survey South Africa 2018. June 10, 2020. https://www.knowledgehub.org.za/elibrary/first-national-tb-prevalence-survey-south-africa-2018
- 63.Dheda K, Makambwa E, Esmail A. The Great masquerader: tuberculosis presenting as community-acquired pneumonia. Semin Respir Crit Care Med. 2020;41:592–604. doi: 10.1055/s-0040-1710583. [DOI] [PubMed] [Google Scholar]
- 64.Wei M, Yongjie Z, Zhuoyu Q, et al. Pneumonia caused by Mycobacterium tuberculosis. Microb Infect. 2020;22:278–284. doi: 10.1016/j.micinf.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walaza S, Tempia S, Dreyer A, et al. The burden and clinical presentation of pulmonary tuberculosis in adults with severe respiratory illness in a high human immunodeficiency virus prevalence setting, 2012–2014. Open Forum Infectious Diseases. 2017;4 doi: 10.1093/ofid/ofx116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.WHO . World Health Organization; Geneva: 2020. Social stigma associated with COVID-19, 2020. [Google Scholar]
- 67.Flak G. Prometheus Books; New York: 2001. STIGMA: how we treat outsiders. [Google Scholar]
- 68.Policy Cures Research COVID-19 R&D tracker. 2020. https://www.policycuresresearch.org/covid-19-r-d-tracker
- 69.Treatment Action Group . The Stop TB Partnership; New York: 2020. Tuberculosis research funding trends, 2005–2019. [Google Scholar]
- 70.Dheda K, Jaumdally S, Davids M, et al. Diagnosis of COVID-19: Considerations, controversies and challenges. Afr J Thorac Crit Care Med. 2020;26:36. doi: 10.7196/AJTCCM.2020.v26i2.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Motta I, Centis R, D'Ambrosio L, et al. Tuberculosis, COVID-19 and migrants: preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. 2020;26:233–240. doi: 10.1016/j.pulmoe.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tadolini M, Codecasa LR, García-García J-M, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020;56 doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Theron G, Venter R, Smith L, et al. False-positive Xpert MTB/RIF results in retested patients with previous tuberculosis: frequency, profile, and prospective clinical outcomes. J Clin Microbiol. 2018;56:e01696–e01717. doi: 10.1128/JCM.01696-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Theron G, Venter R, Calligaro G, et al. Xpert MTB/RIF results in patients with previous tuberculosis: can we distinguish true from false positive results? Clin Infect Dis. 2016;62:995–1001. doi: 10.1093/cid/civ1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peter JG, Theron G, Dheda K. Can point-of-care urine LAM strip testing for tuberculosis add value to clinical decision making in hospitalised hiv-infected persons? PLoS One. 2013;8 doi: 10.1371/journal.pone.0054875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heller T, Mtemang'Ombe EA, Huson MAM, et al. Ultrasound for patients in a high HIV/tuberculosis prevalence setting: a needs assessment and review of focused applications for Sub-Saharan Africa. Int J Infect Dis. 2017;56:229–236. doi: 10.1016/j.ijid.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 77.WHO . World Health Organization; Geneva: 2019. Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosisi of active tuberculosis in people living with HIV. [Google Scholar]
- 78.Song W-M, Zhao J-Y, Zhang Q-Y, et al. COVID-19 and tuberculosis coinfection: an overview of case reports/case series and meta-znalysis. Front Med (Lausanne) 2021;8:48–50. doi: 10.3389/fmed.2021.657006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uden L, Barber E, Ford N, Cooke GS. Risk of tuberculosis infection and disease for health care workers: an updated meta-analysis. Open Forum Infect Dis. 2017;4 doi: 10.1093/ofid/ofx137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17:488–494. doi: 10.3201/eid1703.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bandyopadhyay S, Baticulon RE, Kadhum M, et al. Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.WHO . World Health Organization; Geneva: 2019. WHO guidelines on tuberculosis infection, prevention and control. [PubMed] [Google Scholar]
- 83.WHO . World Health Organization; Geneva: 2021. COVID-19: occupational health and safety for health workers: interim guidance. [Google Scholar]
- 84.European Centre for Disease Prevention and Control . European Centre for Disease Prevention and Control; Stockholm: 2021. Infection prevention and control and preparedness for COVID-19 in healthcare settings. [Google Scholar]
- 85.Centers for Disease Control and Prevention Using personal protective equipment (PPE) Aug 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html
- 86.Dheda K, Charalambous S, Karat AS, et al. A position statement and practical guide to the use of particulate filtering facepiece respirators (N95, FFP2, or equivalent) for South African health workers exposed to respiratory pathogens including Mycobacterium tuberculosis and SARS-CoV-2. Afr J Thorac Crit Care Med. 2021;26:10. doi: 10.7196/AJTCCM.2021.v27i4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang K. What do we know about remdesivir drug interactions? Clin Translat Sci. 2020;13:842–844. doi: 10.1111/cts.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liverpool Drug Interactions Group University of Liverpool COVID-19 drug interactions. 2021. https://www.covid19-druginteractions.org/
- 89.Esmail A, Sabur NF, Okpechi I, Dheda K. Management of drug-resistant tuberculosis in special sub-populations including those with HIV co-infection, pregnancy, diabetes, organ-specific dysfunction, and in the critically ill. J Thorac Dis. 2018;10:3102–3118. doi: 10.21037/jtd.2018.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moore DP, Klugman KP, Madhi SA. Role of Streptococcus pneumoniae in hospitalization for acute community-acquired pneumonia associated with culture-confirmed Mycobacterium tuberculosis in children: a pneumococcal conjugate vaccine probe study. Pediatr Infect Dis J. 2010;29:1099–1104. doi: 10.1097/inf.0b013e3181eaefff. [DOI] [PubMed] [Google Scholar]
- 91.Dheda K, Booth H, Jim, Margaret, Zumla A, Graham Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005;192:1201–1209. doi: 10.1086/444545. [DOI] [PubMed] [Google Scholar]
- 92.Meghji J, Simpson H, Squire SB, Mortimer K. A systematic review of the prevalence and pattern of imaging defined post-TB lung disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pasipanodya JG, McNabb SJ, Hilsenrath P, et al. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health. 2010;10:259. doi: 10.1186/1471-2458-10-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang CH, Lin HC, Lin SM, et al. MMP-1(-1607G) polymorphism as a risk factor for fibrosis after pulmonary tuberculosis in Taiwan. Int J Tuberc Lung Dis. 2010;14:627–634. [PubMed] [Google Scholar]
- 95.Harries AD, Ade S, Burney P, Hoa NB, Schluger NW, Castro JL. Successfully treated but not fit for purpose: paying attention to chronic lung impairment after TB treatment. Int J Tuberc Lung Dis. 2016;20:1010–1014. doi: 10.5588/ijtld.16.0277. [DOI] [PubMed] [Google Scholar]
- 96.WHO . World Health Organization; Geneva: 2021. WHO consolidated guidelines on tuberculosis module 2: systematic screening for tuberculosis disease. [PubMed] [Google Scholar]
- 97.Dheda K, Gumbo T, Maartens G, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;5:291–360. doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 98.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jeranji T. Western Cape to launch Covid-style TB dashboard. May 20, 2021. https://www.news24.com/citypress/news/western-cape-to-launch-covid-style-tb-dashboard-20210520
- 100.Ruhwald M, Carmona S, Pai M. Learning from COVID-19 to reimagine tuberculosis diagnosis. Lancet Microbe. 2021;2:e169–e170. doi: 10.1016/S2666-5247(21)00057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pai N, Esmail A, Saha Chaudhuri P, et al. Impact of a personalised, digital, HIV self-testing app-based program on linkages and new infections in the township populations of South Africa. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diandreth L, Jarrett BA, Elf JL, et al. Secure delivery of HIV-related and tuberculosis laboratory results to patient cell phones: a pilot comparative study. AIDS Behav. 2020;24:3511–3521. doi: 10.1007/s10461-020-02912-3. [DOI] [PubMed] [Google Scholar]
- 103.Ellis P, Martin W, Dodd P. CD4 count and tuberculosis risk in HIV-positive adults not on ART: a systematic review and meta-analysis. PeerJ. 2017;5 doi: 10.7717/peerj.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Badje A, Moh R, Gabillard D, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health. 2017;5:e1080–e1089. doi: 10.1016/S2214-109X(17)30372-8. [DOI] [PubMed] [Google Scholar]
- 106.Salazar-Austin N, Cohn S, Lala S, et al. Isoniazid preventive therapy and pregnancy outcomes in women living with human immunodeficiency virus in the tshepiso cohort. Clin Infect Dis. 2020;71:1419–1426. doi: 10.1093/cid/ciz1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jonsson J, Kühlmann-Berenzon S, Berggren I, Bruchfeld J. Increased risk of active tuberculosis during pregnancy and postpartum: a register-based cohort study in Sweden. Eur Respir J. 2020;55 doi: 10.1183/13993003.01886-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Odayar J, Rangaka MX, Zerbe A, et al. Burden of tuberculosis in HIV-positive pregnant women in Cape Town, South Africa. Int J Tuberc Lung Dis. 2018;22:760–765. doi: 10.5588/ijtld.17.0448. [DOI] [PubMed] [Google Scholar]
- 109.Reichler MR, Khan A, Sterling TR, et al. Risk and timing of tuberculosis among close contacts of persons with infectious tuberculosis. J Infect Dis. 2018;218:1000–1008. doi: 10.1093/infdis/jiy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hill PC, Jackson-Sillah DJ, Fox A, et al. Incidence of tuberculosis and the predictive value of ELISPOT and Mantoux tests in gambian case contacts. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Osman M, Welte A, Dunbar R, et al. Morbidity and mortality up to 5 years post tuberculosis treatment in South Africa: a pilot study. Int J Infect Dis. 2019;85:57–63. doi: 10.1016/j.ijid.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 113.De Vries G, Commandeur S, Erkens C, et al. Towards selective tuberculosis screening of people in prison in a low-incidence country. Eur Respir J. 2020;55 doi: 10.1183/13993003.02209-2019. [DOI] [PubMed] [Google Scholar]
- 114.Macedo LR, Maciel ELN, Struchiner CJ. Tuberculosis in the Brazilian imprisoned population, 2007–2013. Epidemiol Serv Saude. 2017;26:783–794. doi: 10.5123/S1679-49742017000400010. [DOI] [PubMed] [Google Scholar]
- 115.Cords O, Martinez L, Warren JL, et al. Incidence and prevalence of tuberculosis in incarcerated populations: a systematic review and meta-analysis. Lancet Public Health. 2021;6:e300–e308. doi: 10.1016/S2468-2667(21)00025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luabeya AK, Wood RC, Shenje J, et al. Noninvasive detection of tuberculosis by oral swab analysis. J Clin Microbiol. 2019;57:e01847–e01918. doi: 10.1128/JCM.01847-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Namuganga AR, Chegou NN, Mubiri P, Walzl G, Mayanja-Kizza H. Suitability of saliva for Tuberculosis diagnosis: comparing with serum. BMC Infect Dis. 2017;17:600. doi: 10.1186/s12879-017-2687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nagura-Ikeda M, Imai K, Tabata S, et al. Clinical evaluation of self-collected saliva by quantitative reverse transcription-PCR (RT-qPCR), direct RT-qPCR, reverse transcription-loop-mediated isothermal amplification, and a rapid antigen test to diagnose COVID-19. J Clin Microbiol. 2020;58:e01438–e01520. doi: 10.1128/JCM.01438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marks GB, Nguyen NV, Nguyen PTB, et al. Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med. 2019;381:1347–1357. doi: 10.1056/NEJMoa1902129. [DOI] [PubMed] [Google Scholar]
- 120.Ayles H, Muyoyeta M, Du Toit E, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382:1183–1194. doi: 10.1016/S0140-6736(13)61131-9. [DOI] [PubMed] [Google Scholar]
- 121.Corbett EL, Bandason T, Duong T, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376:1244–1253. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Calligaro GL, Zijenah LS, Peter JG, et al. Effect of new tuberculosis diagnostic technologies on community-based intensified case finding: a multicentre randomised controlled trial. Lancet Infect Dis. 2017;17:441–450. doi: 10.1016/S1473-3099(16)30384-X. [DOI] [PubMed] [Google Scholar]
- 123.Morishita F, Garfin AMCG, Lew W, et al. Bringing state-of-the-art diagnostics to vulnerable populations: The use of a mobile screening unit in active case finding for tuberculosis in Palawan, the Philippines. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khanal S, Baral S, Shrestha P, et al. Yield of intensified tuberculosis case-finding activities using Xpert(®) MTB/RIF among risk groups in Nepal. Public Health Action. 2016;6:136–141. doi: 10.5588/pha.16.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Esmail A, Randall P, Oelofse S, et al. Impact of a scalable intervention package, including a point-of-care molecular diagnostic tool, on community-based active case finding for tuberculosis: a pragmatic randomised controlled trial. Am J Respir Crit Care Med. 2022 (in press). [Google Scholar]
- 126.WHO . World Health Organization; Geneva: 2020. WHO consolidated guidelines on tuberculosis. Module 1: Prevention–tuberculosis preventive treatment. [PubMed] [Google Scholar]
- 127.Madhani F, Maniar RA, Burfat A, et al. Automated chest radiography and mass systematic screening for tuberculosis. Int J Tuberc Lung Dis. 2020;24:665–673. doi: 10.5588/ijtld.19.0501. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen TBP, Nguyen TA, Luu BK, et al. A comparison of digital chest radiography and Xpert(®) MTB/RIF in active case finding for tuberculosis. Int J Tuberc Lung Dis. 2020;24:934–940. doi: 10.5588/ijtld.19.0764. [DOI] [PubMed] [Google Scholar]
- 129.Piccazzo R, Paparo F, Garlaschi G. Diagnostic accuracy of chest radiography for the diagnosis of tuberculosis (TB) and its role in the detection of latent TB infection: a systematic review. J Rheumatol Suppl. 2014;91:32–40. doi: 10.3899/jrheum.140100. [DOI] [PubMed] [Google Scholar]
- 130.van't Hoog A, Langendam M, Mitchell E, et al. A systematic review of the sensitivity and specificity of symptom- and chest-radiography screening for active pulmonary tuberculosis in HIV-negative persons and persons with unknown HIV status. 2013. https://www.who.int/tb/Review2Accuracyofscreeningtests.pdf [DOI] [PMC free article] [PubMed]
- 131.Pinto LM, Pai M, Dheda K, Schwartzman K, Menzies D, Steingart KR. Scoring systems using chest radiographic features for the diagnosis of pulmonary tuberculosis in adults: a systematic review. Eur Respir J. 2013;42:480–494. doi: 10.1183/09031936.00107412. [DOI] [PubMed] [Google Scholar]
- 132.Tavaziva G, Harris M, Abidi SK, et al. Chest X-ray analysis with deep learning-based software as a triage test for pulmonary tuberculosis: an individual patient data meta-analysis of diagnostic accuracy. Clin Infect Dis. 2021;1 doi: 10.1093/cid/ciab639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.MacPherson P, Houben RM, Glynn JR, Corbett EL, Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ. 2014;92:126–138. doi: 10.2471/BLT.13.124800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McQuaid CF, Vassall A, Cohen T, et al. The impact of COVID-19 on TB: a review of the data. Int J Tuberc Lung Dis. 2021;25:436–446. doi: 10.5588/ijtld.21.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Osman M, Meehan S-A, Von Delft A, et al. Early mortality in tuberculosis patients initially lost to follow up following diagnosis in provincial hospitals and primary health care facilities in Western Cape, South Africa. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Di Gennaro F, Gualano G, Timelli L, et al. Increase in tuberculosis diagnostic delay during first wave of the COVID-19 pandemic: data from an italian infectious disease referral hospital. Antibiotics. 2021;10:272. doi: 10.3390/antibiotics10030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loveday M, Cox H, Evans D, et al. Opportunities from a new disease for an old threat: extending COVID-19 efforts to address tuberculosis in South Africa. S Afr Med J. 2020;110 doi: 10.7196/SAMJ.2020.v110i12.15126. [DOI] [PubMed] [Google Scholar]
- 138.Zumla A, Marais BJ, McHugh TD, et al. COVID-19 and tuberculosis—threats and opportunities. Int J Tuberc Lung Dis. 2020;24:757–760. doi: 10.5588/ijtld.20.0387. [DOI] [PubMed] [Google Scholar]
- 139.Alipanah N, Jarlsberg L, Miller C, et al. Adherence interventions and outcomes of tuberculosis treatment: a systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cooper S, Van Rooyen H, Wiysonge CS. COVID-19 vaccine hesitancy in South Africa: a complex social phenomenon. S Afr Med J. 2021;111:702. doi: 10.7196/SAMJ.2021.v111i8.15800. [DOI] [PubMed] [Google Scholar]
- 141.Singh AK, Netea MG, Bishai WR. BCG turns 100: its nontraditional uses against viruses, cancer, and immunologic diseases. J Clin Invest. 2021;131:11. doi: 10.1172/JCI148291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moorlag S, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 143.Arts RJW, Moorlag S, Novakovic B, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 144.Leentjens J, Kox M, Stokman R, et al. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J Infect Dis. 2015;212:1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 145.Arts RJW, Carvalho A, La Rocca C, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016;17:2562–2571. doi: 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183:315–323. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wardhana, Datau EA, Sultana A, Mandang VV, Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43:185–190. [PubMed] [Google Scholar]
- 148.Ohrui T, Nakayama K, Fukushima T, Chiba H, Sasaki H. Prevention of elderly pneumonia by pneumococcal, influenza and BCG vaccinations. Nihon Ronen Igakkai Zasshi. 2005;42:34–36. doi: 10.3143/geriatrics.42.34. [DOI] [PubMed] [Google Scholar]
- 149.O'Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chimoyi L, Velen K, Churchyard GJ, Wallis R, Lewis JJ, Charalambous S. An ecological study to evaluate the association of Bacillus Calmette-Guerin (BCG) vaccination on cases of SARS-CoV2 infection and mortality from COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gonzalez-Perez M, Sanchez-Tarjuelo R, Shor B, Nistal-Villan E, Ochando J. The BCG vaccine for COVID-19: first verdict and future directions. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.632478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bagheri N, Montazeri H. On BCG vaccine protection from COVID-19: a review. SN Compr Clin Med. 2021;15:1–11. doi: 10.1007/s42399-021-00835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Universitair Medisch Centrum Utrecht Tuberculosis vaccine does not protect vulnerable elderly people against COVID-19. Jan 18, 2021. https://www.umcutrecht.nl/en/about-us/news/details/jan-18-tuberculosis-vaccine-does-not-protect-vulnerable-elderly-against-covid-19#:~:text=Monday%2018%20Jan%202021,-Listen&text=The%20BCG%20vaccine%20does%20not,patients%20aged%2060%20and%20over
- 154.Mackay IM. Virology Down Under: The Swiss cheese infographic that went viral. Dec 26, 2020. https://virologydownunder.com/the-swiss-cheese-infographic-that-went-viral/
- 155.Zimmer A, Klinton J, Omenka C, et al. Tuberculosis in times of COVID-19. J Epidemiol Community Health. 2022;76:310–316. doi: 10.1136/jech-2021-217529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chaisson RE, Frick M, Nahid P. The scientific response to TB – the other deadly global health emergency. Int J Tuberc Lung Dis. 2022;26:186–189. doi: 10.5588/ijtld.21.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wong EB, Olivier S, Gunda R, et al. Convergence of infectious and non-communicable disease epidemics in rural South Africa: a cross-sectional, population-based multimorbidity study. Lancet Glob Health. 2021;9:e967–e976. doi: 10.1016/S2214-109X(21)00176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for aggregated laboratory testing data for South Africa should be made in writing by qualifying researchers with valuable research questions to the National Institute for Communicable Diseases, directed to Harry Moultrie (harrym@nicd.ac.za). Further information can be obtained from the corresponding author.