Abstract
The in vitro activities of magainin II, nisin, and ranalexin alone and in combination with other antimicrobial agents against six clinical isolates of Rhodococcus equi were investigated by MIC and time-kill studies. All isolates were more susceptible to nisin. A positive interaction was observed when the peptides were combined with ampicillin, ceftriaxone, rifabutin, rifampin, azithromycin, clarithromycin, and vancomycin.
Rhodococcus equi infections have been described with increasing frequency, especially in immunocompromised hosts; pulmonary infections are the most commonly described form of disease (4, 17). The rate of mortality is high in human immunodeficiency virus-infected patients, ranging from 20 to 50% (7, 15). R. equi is a facultatively intracellular pathogen that infects both macrophages and neutrophils, and its persistence in phagocytes may allow the organism to escape the defense mechanisms of the host (20). Clinical reports have shown that, despite antibiotic therapy, frequent relapses occur during the course of the disease, especially in individuals with AIDS (15, 17). For these reasons, the optimal treatment of R. equi infection remains to be determined. In recent years many positively charged polypeptides have been isolated from a wide range of animal, plant, and bacterial species (2, 3, 9, 11, 12, 14). In mammals, including humans, they have been found on the surfaces of the tongue, trachea, lungs, and upper intestine and are thought to be a major antibacterial defense on mucosal surfaces (5, 6). It has been suggested that the mode of action of these compounds on the membranes of bacteria, fungi, and protozoa involves the formation of ion-channel pores that span the membranes without the requirement of a specific target receptor. Recent reports demonstrated that the site for the antibacterial action of the peptides is the cytoplasmic membrane; the lethal event which occurs at the cytoplasmic membrane is not fully understood (2, 11, 16). The peptides may act by inserting into the cytoplasmic membrane and triggering the activity of bacterial murein hydrolases, resulting in damage or degradation of the peptidoglycan and lysis of the cell (4, 9, 11, 12).
The magainins and ranalexin are polycationic peptides that have been isolated from amphibian skin. Magainin II is a small peptide (23 residues) present in the skin of Xenopus laevis, while ranalexin is a 20-amino-acid peptide that was recently isolated from the skin of the American bullfrog (Rana catesbiana) (3, 19). Magainins and ranalexin appear to be responsible for the extraordinary freedom from infection characteristic of wound healing in laboratory animals, despite the use of nonsterile surgical procedures. Magainins form an amphipathic helical structure; this geometry is considered the key factor for the formation of transmembrane pores, which leads to cell death (19). Ranalexin shares remarkable structural similarity with the polymyxins, a class of membrane-active antibiotics (6, 16, 18). Nisin is a cationic peptide produced by Streptococcus lactis subsp. lactis; it has activity against a wide range of gram-positive bacteria including several important, contemporary multidrug-resistant gram-positive pathogens (14). In this study we investigated the in vitro activities of magainin II, nisin, and ranalexin against R. equi.
Six distinct clinical isolates of R. equi were tested. They were isolated over a 10-year period from distinct patients with unrelated sources of infection. When a strain was repeatedly isolated from the same individual, only the first isolate was tested. The strains were identified according to the following characteristics or criteria: gram-positive, coccobacillary, non-spore-forming, nonmotile organisms; weak or partial acid fastness; mucoid colonies; salmon pink- or coral-colored colonies; inability to ferment carbohydrates; inability to hydrolyze casein, hypoxanthine, xanthine, tyrosine, and gelatin; catalase positivity; urease positivity; oxidase negativity; nitrate reduction positivity; and alkaline phosphatase positivity (1).
Magainin II, nisin, and ranalexin were obtained from Sigma-Aldrich (Milan, Italy). They were solubilized in phosphate-buffered saline (pH 7.2), yielding a stock solution of 1,000 mg/liter. Other agents evaluated were ampicillin, ceftriaxone, doxycycline, netilmicin, ofloxacin, rifampin, and vancomycin (all from Sigma-Aldrich), azithromycin (Pfizer/Roerig, Rome, Italy), clarithromycin (Abbott, Rome, Italy), and rifabutin (Pharmacia & Upjohn, Milan, Italy). Laboratory standard powders were diluted in accordance with the manufacturer’s recommendations, yielding a stock solution of 1 mg/ml. The stock solutions of these antimicrobial drugs were stored at −80°C until they were used. Each antibiotic was assayed over a concentration range of 0.06 to 256 μg/ml.
The MIC was determined in triplicate by a microbroth dilution method with Mueller-Hinton (MH) broth (Becton Dickinson Italia, Milan, Italy) and an initial inoculum of 5 × 105 CFU/ml. Ninety-six well polystyrene plates (Becton Dickinson and Co., Franklin Lakes, N.J.) were incubated for 18 h at 37°C in air. Since the peptides have a tendency to precipitate, the plates were shaken throughout the study. The MIC was the lowest drug concentration at which observable growth was inhibited.
To study the in vitro killing effects of the peptides, the six isolates were grown at 37°C in MH broth. Aliquots of exponentially growing bacteria were resuspended in fresh MH broth at approximately 107 cells/ml and were exposed to each peptide (final concentration, 32 μg/ml) for 0, 10, 20, 30, 40, 50, and 60 min at 37°C. After these times the samples (0.1 ml) were serially diluted by preparing consecutive 1:10 (vol/vol) dilutions in MH broth, and the dilutions were plated onto MH agar plates to obtain viable colonies. The experiments were performed in triplicate. Antibiotic carryover was not a problem in these experiments (8, 13), although preliminary studies showed that it occurs at higher concentrations (>128 μg/ml).
In interaction studies, the antibiotics that are used clinically were tested at the following concentrations: rifampin and rifabutin, 1 mg/liter; doxycycline, ofloxacin, azithromycin, and clarithromycin, 2 mg/liter; ampicillin and vancomycin, 4 mg/liter; and netilmicin and ceftriaxone, 8 mg/liter. Each drug was combined with each peptide at 0.5 and 1× the MIC. In all tubes a logarithmic-phase inoculum of 5 × 105 CFU/ml was added along with MH broth to give a final volume of 10 ml. All tubes were incubated overnight at 37°C and the bacterial growth in each tube was determined by preparing consecutive 1:10 (vol/vol) dilutions of a 0.1-ml aliquot of each tube in MH broth and by plating a 0.1-ml volume of each dilution onto MH agar plates. The experiments were performed in triplicate. This procedure avoided any antimicrobial carryover effect. If a combination of the peptide with other drugs caused a decrease in the viable cell count of ≥2 log10 compared with that obtained with the most active single agent, the effect of the combination was considered a positive interaction. If the decrease in viable cell count was 1 to 2 log10, the effect of the combination was considered additive (8, 13).
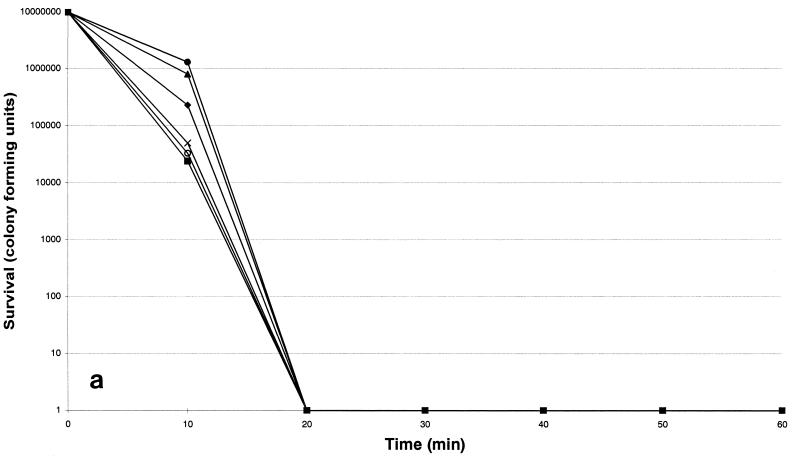
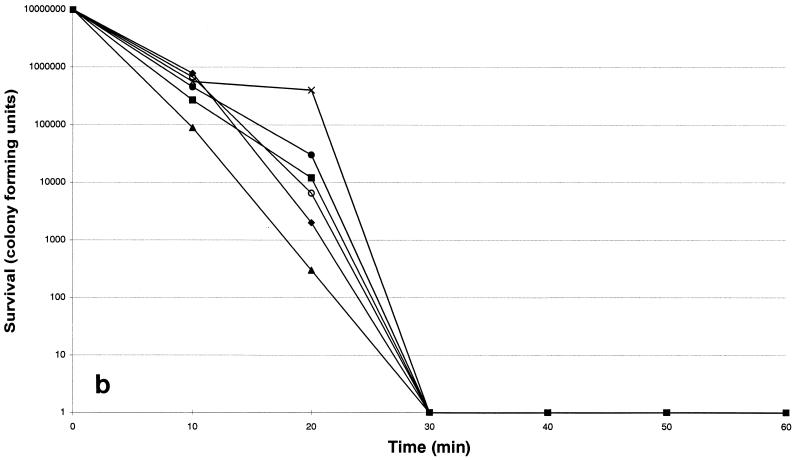
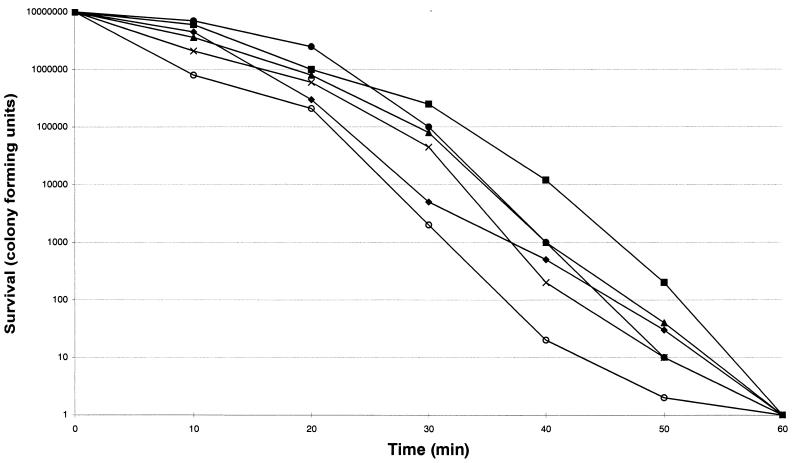
The susceptibilities of each strain are listed in Table 1. The six isolates were more susceptible to nisin and ranalexin, while magainin II was less potent. Killing by nisin was shown to be the most rapid (Fig. 1a): its activity was complete after a 20-min exposure period. Killing by ranalexin was complete after a 30-min exposure period (Fig. 1b), while killing by magainin II was complete after a 60-min exposure (Fig. 1c). Overall, an increase in killing at 24 h of greater than 100-fold was observed when the peptides were combined with ampicillin, ceftriaxone, rifabutin, rifampin, azithromycin, clarithromycin, and vancomycin, while additive effects were observed with doxycycline and ofloxacin. In particular, a ≥4 log10 decrease in viable cell counts was found when nisin and ranalexin at concentrations of 0.5 and 1× the MICs were combined with azithromycin, clarithromycin, rifampin, and rifabutin. A 3-log10 decrease in viable cell counts was found when nisin and ranalexin at concentrations of 0.5 and 1× the MICs were combined with beta-lactams and vancomycin. Finally, a 3-log10 decrease in viable cell counts was found when magainin II at a concentration of 1× the MIC was combined with ampicillin, ceftriaxone, rifabutin, rifampin, azithromycin, clarithromycin, and vancomycin.
TABLE 1.
In vitro activities of polycationic peptides and other antimicrobial agents against R. equi
| Strain no. | MIC (μg/ml)a
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MGII | NSN | RNL | AM | AZI | CLR | CRO | D | NET | OFX | RFB | RA | VA | |
| 1 | 50.8 | 1.6 | 3.2 | 4.0 | <0.1 | <0.1 | 0.3 | 0.1 | 1.0 | 1.3 | <0.1 | <0.1 | <0.1 |
| 2 | 25.4 | 1.0 | 4.0 | 6.3 | 0.1 | <0.1 | 0.1 | 0.5 | 1.6 | 1.0 | <0.1 | <0.1 | <0.1 |
| 3 | 64.0 | 16.0 | 10.1 | 8.0 | <0.1 | <0.1 | 0.2 | 0.1 | 0.5 | 2.0 | 0.1 | 0.1 | <0.1 |
| 4 | 25.4 | 5.0 | 4.0 | 5.0 | 0.1 | 0.1 | <0.1 | 0.6 | 0.4 | 0.5 | <0.1 | <0.1 | <0.1 |
| 5 | 101.6 | 10.1 | 12.7 | 16.0 | <0.1 | <0.1 | 0.8 | 1.0 | 2.0 | 2.5 | 0.1 | 0.2 | <0.1 |
| 6 | 32.0 | 5.0 | 4.0 | 10.1 | <0.1 | <0.1 | <0.1 | 0.5 | 0.1 | 0.6 | 0.1 | 0.2 | <0.1 |
MICs are geometric means of independent MIC determinations conducted in triplicate. Abbreviations: MGII, magainin II; NSN, nisin; RNL, ranalexin; AM, ampicillin; AZI, azithromycin; CLR, clarithromycin; CRO, ceftriaxone; D, doxycycline; NET, netilmicin; OFX, ofloxacin; RFB, rifabutin; RA, rifampin; VA, vancomycin.
FIG. 1.
Time-kill kinetics of nisin (a), ranalexin (b), and magainin II (c) against six strains of R. equi. The peptides were each tested at a concentration of 32 μg/ml. ⧫, strain 1; ■, strain 2; ▴, strain 3; ×, strain 4; ○, strain 5; ●, strain 6.
Our data demonstrate that nisin and ranalexin are active against R. equi and show rapid bactericidal effects. On the contrary, magainin II demonstrated less potency. There are few data on the concentration- or time-dependent killing kinetics of bacteria by polycationic peptides; nevertheless, our observations are in agreement with those in recent reports of studies which showed that killing by peptides is very rapid and results in log orders of cell death within minutes of peptide addition (11, 14, 19). Studies with combinations of drugs and peptides showed that each peptide exhibited a positive interaction with several antibiotics. Previous reports demonstrated that combinations of polycationic peptides, especially the polymyxin-like peptides, and lipophilic and amphiphilic agents such as rifampin, the macrolides, fusidic acid, and novobiocin have synergistic properties. Actually, polymyxin-like peptides allow maximal entry of several hydrophobic substrates inside the cell (6, 10, 16, 18).
In conclusion, our data suggest that the polycationic peptides may be valuable as adjuvants for antimicrobial chemotherapy. Their positive in vitro interaction with agents that are used clinically also suggest that the peptides should be considered for testing in vivo for use as part of antimicrobial combination therapy.
REFERENCES
- 1.Beaman B L, Saubolle M A, Wallace R J. Nocardia, Rhodococcus, Streptomyces, Oerskovia, and other aerobic actinomycetes of medical importance. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 379–399. [Google Scholar]
- 2.Bevins C L, Zasloff M. Peptides from frog skin. Annu Rev Biochem. 1990;59:395–414. doi: 10.1146/annurev.bi.59.070190.002143. [DOI] [PubMed] [Google Scholar]
- 3.Clark D P, Durell S, Maloy W L, Zasloff M. Ranalexin. A novel antimicrobial peptide from bullfrog (Rana catasbeiana) skin, structurally related to the bacterial antibiotic polymyxin. J Biol Chem. 1994;269:10849–10855. [PubMed] [Google Scholar]
- 4.Emmons W, Reichwein B, Winslow D L. Rhodococcus equi infection in the patient with AIDS: literature review and report of an unusual case. Rev Infect Dis. 1991;13:91–96. doi: 10.1093/clinids/13.1.91. [DOI] [PubMed] [Google Scholar]
- 5.Falla T J, Hancock R E W. Improved activity of a synthetic indolicidin analog. Antimicrob Agents Chemother. 1997;41:771–775. doi: 10.1128/aac.41.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock R E W. Antibacterial peptides and the outer membranes of gram-negative bacilli. J Med Microbiol. 1997;46:1–3. doi: 10.1099/00222615-46-1-1. [DOI] [PubMed] [Google Scholar]
- 7.Harvey R L, Sunstrum J C. Rhodococcus equi infection in patients with and without human immunodeficiency virus infection. Rev Infect Dis. 1991;13:139–145. doi: 10.1093/clinids/13.1.139. [DOI] [PubMed] [Google Scholar]
- 8.Hindler J. Tests to assess bactericidal activity. In: Eisenberg H D, editor. Clinical microbiology procedures handbook. Washington, D.C: American Society for Microbiology; 1992. pp. 5.16.14–5.16.24. [Google Scholar]
- 9.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunin C M. Antimicrobial activity of rifabutin. Clin Infect Dis. 1996;22:S3–S14. doi: 10.1093/clinids/22.supplement_1.s3. [DOI] [PubMed] [Google Scholar]
- 11.Moore A J, Beazley W D, Bibby M C, Devine D A. Antimicrobial activity of cecropins. J Antimicrob Chemother. 1996;37:1077–1089. doi: 10.1093/jac/37.6.1077. [DOI] [PubMed] [Google Scholar]
- 12.Sahl H-G, Jack R W, Bierbaum G. Biosynthesis and biological activities of antibiotics with unique post-translational modifications. Eur J Biochem. 1995;230:827–853. doi: 10.1111/j.1432-1033.1995.tb20627.x. [DOI] [PubMed] [Google Scholar]
- 13.Sambatakou H, Giamarellos-Bourboulis E J, Grecka P, Chryssouli Z, Giamarellou H. In-vitro activity and killing effect of quinupristin/dalfopristin (RP59500) on nosocomial Staphylococcus aureus and interactions with rifampicin and ciprofloxacin against methicillin-resistant isolates. J Antimicrobial Chemother. 1998;41:349–355. doi: 10.1093/jac/41.3.349. [DOI] [PubMed] [Google Scholar]
- 14.Severina E, Severin A, Tomasz A. Antibacterial efficacy of nisin against multidrug-resistant gram-positive pathogens. J Antimicrob Chemother. 1998;41:341–347. doi: 10.1093/jac/41.3.341. [DOI] [PubMed] [Google Scholar]
- 15.Sirera G, Romeu J, Clotet B, Velasco P, Arnal J, Rius F, Foz M. Relapsing systemic infection due to Rhodococcus equi in a drug abuser seropositive for human immunodeficiency virus. Rev Infect Dis. 1991;13:509–510. doi: 10.1093/clinids/13.3.509. [DOI] [PubMed] [Google Scholar]
- 16.Vaara M, Porro M. Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria. Antimicrob Agents Chemother. 1996;40:1801–1805. doi: 10.1128/aac.40.8.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vernille T D, Huycke M M, Greenfield R A, Fine D P, Kuhls T L, Slater L N. Rhodococcus equi infections of humans. Medicine (Baltimore) 1994;73:119–132. doi: 10.1097/00005792-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Viljanen P, Matsunaga H, Kimura Y, Vaara M. The outer membrane permeability-increasing action of deacylpolymyxins. J Antibiot. 1991;44:517–523. doi: 10.7164/antibiotics.44.517. [DOI] [PubMed] [Google Scholar]
- 19.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zink M C, Yager J A, Prescott J F, Fernando M A. Electron microscopic investigation of intracellular events after ingestion of Rhodococcus equi by foal alveolar macrophages. Vet Microbiol. 1987;14:295–305. doi: 10.1016/0378-1135(87)90117-9. [DOI] [PubMed] [Google Scholar]