ABSTRACT
Mammalian oocytes undergo two rounds of developmental arrest during maturation: at the diplotene of the first meiotic prophase and metaphase of the second meiosis. These arrests are strictly regulated by follicular cells temporally producing the secondary messengers, cAMP and cGMP, and other factors to regulate maturation promoting factor (composed of cyclin B1 and cyclin-dependent kinase 1) levels in the oocytes. Out of these normally appearing developmental arrests, permanent arrests may occur in the oocytes at germinal vesicle (GV), metaphase I (MI), or metaphase II (MII) stage. This issue may arise from absence or altered expression of the oocyte-related genes playing key roles in nuclear and cytoplasmic maturation. Additionally, the assisted reproductive technology (ART) applications such as ovarian stimulation and in vitro culture conditions both of which harbor various types of chemical agents may contribute to forming the permanent arrests. In this review, the molecular determinants of developmental and permanent arrests occurring in the mammalian oocytes are comprehensively evaluated in the light of current knowledge. As number of permanently arrested oocytes at different stages is increasing in ART centers, potential approaches for inducing permanent arrests to obtain competent oocytes are discussed.
KEYWORDS: Oocyte arrest, permanent arrest, meiotic maturation, gene expression, female infertility, developmental arrest
Introduction
A group of primordial germ cells (PGCs) initially appears in the endoderm of dorsal wall of yolk sac at the time periods of 3th-4th weeks of prenatal development in humans [1]. Then, PGCs migrate from primitive streak between yolk sac and developing allantois to the genital ridges by the way of hindgut. Meanwhile, these cells undergo many consecutive mitotic divisions during migration and colonization to the genital ridges as well 2. Following colonization, female PGCs differentiate into oogonial stem cells (OSCs) that participate in forming germ cell nests. The germ cell nests basically consist of a cluster of interconnected OSCs surrounded by pre-granulosa cells 3. Herein, OSCs experience a series of mitotic divisions in the developing ovaries and subsequently enter into meiotic division after that time they are defined as oocytes, arrested at the diplotene stage of the first meiotic prophase.
Before birth, most primordial follicles composed of the primordial oocyte arrested at the first meiotic division and surrounded by squamous pre-granulosa cells are formed, and this formation is completed at the early postnatal period [4]. In the process of folliculogenesis [5], a subset of primordial follicles is induced under control of gonadotropin-independent mechanism to generate primary follicles that have a layer of cuboidal follicular cells enclosing the primary oocyte. Following sequential mitotic divisions in the follicular cells, secondary follicles whose primary oocyte is covered by multiple layers of granulosa cells occur. Under control of gonadotropin-dependent mechanism [6], small spaces among granulosa cells, which are filled with follicular fluid, can be monitored in the preantral follicles. The preantral follicles include a germinal vesicle (GV) oocyte still arrested at the first meiosis, as former ones. Subsequently, these small spaces bring together to form a single antrum during antral follicles formation. The antral follicles are briefly characterized by having a big antrum and eccentrically localized GV oocyte, enclosed by specified granulosa cells described as cumulus cells [7]. Notably, the layers of granulosa cells surrounding the antral wall are specially defined as membrana granulosa or mural granulosa. By the process of ovulation taken place following luteinizing hormone (LH) surge, cumulus oocyte complexes (COCs) including the oocyte arrested at the metaphase II (MII) stage of second meiotic division (also known as secondary oocyte) and surrounded by cumulus cells are ovulated [8].
In more detail, meiotic maturation in oocytes from GV to MII stage includes many correctly programmed molecular intrinsic developmental programs and the gonadotropins secreted from pituitary [9]. Basically, there are two main events in the process of meiotic maturation: nuclear and cytoplasmic molecular maturation. Resumption of the first meiotic arrest with germinal vesicle breakdown (GVBD), chromatin condensation, extrusion of first polar body, and progressing to metaphase II stage of second meiosis is the main events of nuclear maturation [10]. Dynamic chromatin modifications in oocyte nucleus modulated by intracellular cascades and molecules are required for meiotic resumption [11]. During cytoplasmic maturation, the following events such as attaining fertilization features, reorganization of organelles, storages of mRNAs and transcription factors are taken place throughout oocyte growing [12]. Understanding the pathways and molecules implicating to the nuclear and cytoplasmic maturation of oocytes will contribute to defining the oocytes having high quality and developmental competence. Otherwise, the underlying molecular defects of the meiotic maturation-related imperfections that lead to producing incompetent oocytes and early embryos would not be defined.
Developmental arrest at the first meiotic division in oocytes
Developmental arrest in oocytes at the diplotene stage of the first meiotic prophase is initiated at embryonic day (E) 17.5 and completed until postnatal day 5 in the developing mouse ovaries [13]. In humans, this phenomenon occurs in the ovaries at the period from 8- to 28-week of fetal development [5]. In other words, oocytes localizing in the follicles from primordial to antral stages are maintained at an arrested state until LH surge in mammals. During folliculogenesis, the arrested oocytes sustain their developmental progression in three phases: (i) growing oocytes in the follicles from primordial to secondary stages cannot resume meiotic maturation when extruded from the follicles and cultured in vitro, (ii) medium-sized oocytes in preantral follicles, when undergone maturation in vitro, they commonly remain an arrested state at metaphase I (MI) stage, and (iii) fully grown oocytes in antral follicles are able to progress to MII stage through responding to gonadotropins upon cultured in vitro [14]. Indeed, only fully grown oocytes whose nucleoli are surrounded with a ring of condensed chromatin exhibit better maturation ability and development potential to reach blastocyst stage after fertilization [15]. In contrast, the oocytes with non-surrounded nucleoulus (NSN) can develop only to 2-4-cell embryonic stages [15]. Of note, if a condensed chromatin ring encloses the nucleolus-like body of fully grown oocytes, it is defined as surrounded nucleoulus (SN), displaying transcriptional repression [16]. On the other hand, nuclei of some oocytes are surrounded with a less condensed rim, which is described as NSN, showing transcriptional activation. It is known that transcriptional activity is ceased in the fully grown oocytes of antral follicles based on gonadotropin stimulation for successful early embryonic development [17].
In mammals, maturation-promoting factor (MPF, also known as cytoplasmic maturation-promoting factor) regulates the timing of meiotic progression from the first meiotic prophase to metaphase stage of the second meiosis [18]. MPF is composed of two heterodimeric proteins: the catalytic subunit, cyclin-dependent kinase 1 (CDK1) and the regulatory subunit, cyclin B1 (CCNB1) [19]. As is known, CDK1 has a kinase activity, thereby enables to phosphorylate serine and threonine residues of target proteins after binding to CCNB1 [20]. For maintaining the first meiotic arrest in GV oocytes, cyclic adenosine monophosphate (cAMP) continuously stimulates protein kinase A (PKA) that activates the nuclear kinases, G2 checkpoint kinase (WEE1), and myelin transcription factor 1 (MYT1) through phosphorylating them [21]. Then, both WEE1 and MYT1 phosphorylate CDK1 at threonine 14 and tyrosine 15 residues to inhibit its enzymatic activity. On the other hand, anaphase-promoting complex/cyclosome (APC/C) decreases the CCNB1 levels in GV oocytes (Figure 1).
Figure 1.
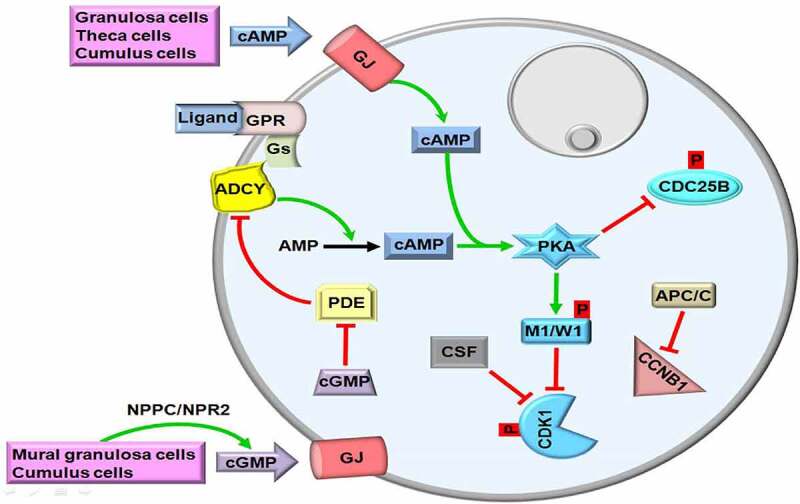
The first meiotic arrest in germinal vesicle (GV) oocytes. Production of cyclic adenosine monophosphate (cAMP) with adenylyl cyclase (ADCY) by granulosa, theca and cumulus cells activates protein kinase A (PKA) that phosphorylates nuclear kinases, myelin transcription factor 1 (MYT1, abbreviated as M1) and G2 checkpoint kinase (WEE1; abbreviated as W1). Thus, cyclin dependent kinase 1 (CDK1) is remained in a phosphorylated state, and maturation promoting factor (MPF) composed of CDK1 and Cyclin B1 (CCNB1) is repressed. To ensure continuity of meiotic arrest, cAMP in the GV oocytes is holded at high levels via inhibiting phosphodiesterase (PDE) activity with the action of cyclic guanosine monophosphate (cGMP). cGMP is generated by mural granulosa and cumulus cells and then transferred though gap junctions (GJs). GPR, G protein-coupled receptor; Gs, G proteins; CSF, Cytostatic factor; CDC25B, Cell division cycle 25B; APC/C, Anaphase promoting complex/cyclosome; NPPC/NPR2, Natriuretic peptide precursor type C/Natriuretic peptide receptor 2; p, Phosphorylation. The GV is depicted by a gray circle at the upper-right site. The green and red arrows represent activation and repression, respectively.
The oocyte in Figure 1 is appearing in an eliptic format. Its original format is in a round format. Please, provide its appearance in a round format as in the word document i have sent a few days ago with e-mail.
To keep cAMP at high levels in GV oocytes, the constitutively active G protein-coupled receptor 3 (GPR3) bound by a relative ligand interacts with G proteins (Gs) [22]. Subsequently, Gs activate adenylyl cyclase (ADCY) that converts AMP into cAMP. In other words, the ligands such as ions, peptides, hormones, and growth factors interact with GPR3 to stimulate the GPR3-Gs-ADCY cascade [23,24] (Figure [1]. As expected, lack of the Gpr3 gene leads to infertility in female mice because of its pivotal role in maintaining meiotic arrest in GV oocytes [25]. In addition, mouse oocytes without ADCY3 isoform [also known as adenylate cyclase 3 (AC3)] are largely unable to remain at the first meiotic arrest in vivo and exhibit accelerated spontaneous maturation in vitro [26]. In the Adcy3-null mice, the researchers also observed no GV oocytes in most early antral follicles, but some follicles included multinucleated oocytes and more than %50 of the oocytes resumed meiosis in vivo [26]. However, the subcellular distributions and potential roles of ADCYs during oocyte maturing remain still unknown, further studies are required on this subject.
In addition to being produced by oocytes, cAMP is generated by theca cells, mural granulosa cells, and cumulus cells and then transferred into oocytes to sustain MPF inactivation [reviewed in Ref. [24]]. For this purpose, follicle-stimulating hormone (FSH) increases the permeability of gap junctions to cAMP through changing distribution of connexin 43 among granulosa cells [27]. As a result, the oocytes either in the growing or antral follicles are remained at the diplotene stage of the first meiotic prophase up to LH surge [28]. Another mechanism for maintaining the first meiotic arrest in the oocytes, protein kinase A (PKA) inactivates the phosphatase, cell division cycle 25B (CDC25B) via phosphorylating at Ser321 [29]. The basic function of CDC25B is to make dephosphorylation of CDK1 for its activation [30]. Moreover, the anaphase-promoting complex/cyclosome (APC/C) mediates degradation of CCNB1 to prevent formation of CDK1-CCNB1 complex so that first meiotic arrest continues during follicular development [31] (Figure 1). As an ubiquitin E3 ligase, APC/C performs CCNB1 ubiquitination for its degradation by the proteasome pathway [32].
The third way of holding cAMP at high levels in growing oocytes is the inhibition of phosphodiesterases (PDEs) by cyclic guanosine monophosphate (cGMP) action. For this purpose, cGMP is synthesized from guanosine triphosphate (GTP) by guanylyl cyclase in the mural granulosa and cumulus cells of antral follicles, and it inhibits the hydrolytic activity of PDE3 on cAMP; thereby, meiotic arrest is maintained [33]. There is a close cooperation for cGMP generation: while mural granulosa cells synthesize the ligand, natriuretic peptide precursor type C (NPPC), the granulosa and cumulus cells generate its receptor, natriuretic peptide receptor 2 (NPR2; also known as NPR-B) [34]. Activation of NPPC/NPR2 in a way of ligand-receptor interaction stimulates cGMP production within granulosa cells and transfer of these cGMP molecules into oocytes through gap junctions contributes to blocking PDEs [34,35] (Figure 1). It is worth noting that eleven different PDE isoenzymes (PDE1-11) were defined in mammals [36]. Among them, PDE3 is exclusively present in the mouse [37], bovine [38], and porcine [39] oocytes, but it is absent in the somatic cells [37]. Expectedly, lack of the Pde3 gene ceases meiotic resumption in the mouse oocytes obtained in vitro or in vivo way [25]. It remains elusive that whether other PDE isoforms play any roles in meiotic repression during oogenesis.
Overall, there are three main mechanisms maintaining first meiotic arrest in oocytes until LH surge: (i) continuously producing cAMP molecules with the GPR3-Gs-ADCY pathway, (ii) prevention of cAMP degradation by inhibiting PDEs activity, and (iii) inactivation of CDK1 by phosphorylation, and degradation of CCNB1. Thus, MPF is kept at low levels.
Progression from first to second meiotic arrest
Estradiol secretion by fully grown follicles before ovulation contributes to producing LH hormone at high levels from the pituitary gland in mammals [40]. The LH surge leads to a predominant decrease in the permeability of the gap junctions between granulosa cells and oocytes to molecular transition so that cAMP is reduced to minimal levels in the GV oocytes of antral follicles [41]. It is worth noting that LH binds to own receptors on the outer granulosa cells of antral follicles [42] to promote epiregulin and amphiregulin releases [43]. Indeed, both factors activate epidermal growth factor receptors located on the mural granulosa cells in order to decrease the gap junction numbers by the way of mitogen-activated protein kinase (MAPK) signaling [44]. As is well-known, MAPK is a family of Ser/Thr protein kinases and contributes to meiotic cell cycle progression in oocytes via implicating in activating GVBD, microtubule organization, MPF stabilization, and meiotic spindle assembly as well as maintaining MII arrest [45–47].
The level of intracellular cGMP involving in blocking meiotic progression in GV oocytes is rapidly reduced by three main mechanisms [41]: (i) repression of NPR2 guanylyl cyclase: the LH signaling in the granulosa cells results in inactivating NPR2 guanylyl cyclase activity by rapid dephosphorylation of its regulatory regions, and thereby cGMP is decreased to low levels in GV oocytes [33]. It is noteworthy that dephosphorylation of the NPR2 guanylyl cyclase is carried out by the activity of phosphoprotein phosphatase (PPP)-family members, stimulated through LH signaling [48]; (ii) activation of the phosphodiesterase, PDE5, performing cGMP degradation [49]; and (iii) as gap junction numbers between oocytes and granulosa cells are predominantly reduced following LH surge [50], PDEs can no longer be repressed in the oocytes by cGMP [49]. Thus, transforming cAMP into AMP by PDEs does not only drop intracellular cAMP to minimal levels but also leads to loss of PKA activity. Decreases of cGMP and cAMP levels through these three mechanisms in GV oocytes promote meiotic resumption [24] (Figure 2).
Figure 2.
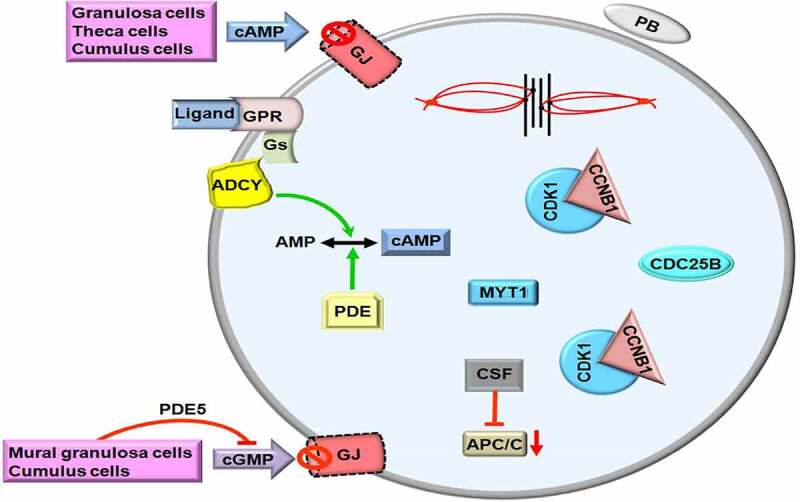
The second meiotic arrest in metaphase II (MII) oocytes. Loss of the gap junctions (GJs) on the oolemma due to luteinizing hormone (LH) surge prevents entrance of cyclic adenosine monophosphate (cAMP) into ooplasm. Meanwhile, intracellular cAMP is converted to AMP by the enzyme phosphodiesterase (PDE). The phosphatase cell division cycle 25B (CDC25B) dephosphorylates cyclin dependent kinase 1 (CDK1). Thus, maturation promoting factor (MPF) consisting of CDK1 and Cyclin B1 (CCNB1) passes through the nuclear region. GPR, G protein-coupled receptor; Gs, G proteins; CSF, Cytostatic factor; MYT1, Myelin transcription factor 1; cGMP, Cyclic guanosine monophosphate; PB, First polar body. The metaphase plate is depicted at the upper site. The green and red arrows represent activation and repression, respectively.
The oocyte in this figure should also be in a round format as in the word document i have sent in the last e-mail. Please, provide the oocyte in a round format, not in an eliptic format.
The CDC25B protein that normally resides in GV oocyte cytoplasm translocates into nucleus shortly before GVBD [51]. This translocation is associated with reduced PKA activity because lack of CDC25B phosphorylation at serine 321 results in decreased cytoplasmic maintenance [52]. Meanwhile, as a phosphatase, CDC25B makes dephosphorylation of CDK1 for facilitating meiotic progression (Figure 2). As a result of CDC25B action, MPF at low levels in GV oocytes is increased, and remains at high levels during progression from MI to telophase I stages and after that it is decreased again and kept at low levels in MII oocytes dependent on the functional interaction between CSF and APC/C [53]. As is known, CSF contributes maintenance of meiotic arrest at MII stage by the way of blocking formation of CDK1-CCNB1 complex or stabilizing MPF directly [54]. In case of fertilization, parthenogenetic activation, or intracytoplasmic sperm injection (ICSI), CSF is inactivated by a rapid increase of intracellular Ca+2 amounts to complete the second meiotic division [55–58].
Critical roles of SAC and APP/C in meiotic progression
The SAC components including BUBR1, MAD1 and 2, BUB1 and 3, MPS1, and Aurora B/C kinase provide correct binding between kinetochores and bipolar spindles in mouse oocytes so that chromosome mis-segregation is prevented [59]. After binding of the spindles to sister kinetochores, the SAC system is switched off. Thus, the inhibitory effect of SAC on the APCCdc20 disappears, and oocytes proceeds to anaphase. It is important to note that if there are unattached kinetochores that form a platform, the SAC activation occurs [59]. As compared to somatic cells, SAC is unable to detect a single unattached kinetochore [59] that may be an explanation for the aneuploidies emerging in some oocytes.
The loss of the SAC components, BUB1 [60] and MPS1 [61], leads to an accelerated meiotic progression in oocytes. Upon lacking of BUBR1, MAD2 cannot interact with unattached kinetochores that result in producing aneuploidy [62]. The Bubr1 gene knocked out mouse models revealed that BUBR1 provides persistent SAC activity, duration of the first meiosis, and establishment of kinetochore-microtubule interactions; however, there is no effect on the first meiotic arrest in the oocytes [63]. Taken together, functional loss of SAC system causes chromosome missegregation, aneuploidy, changed first meiotic duration, and ultimately infertility [64].
Another meiotic progression controller, APC/C, is a multi-subunit E3 ubiquitin ligase complex and marks some proteins that will undergo degradation so that chromatids/chromosomes readily separate from each other [65]. APC/C also has CDC20 and CDH1 coactivators involved in the continual degradation of CCNB1 during first meiotic arrest [66]. Along with holding cAMP at a low concentration, CCNB1 degradation keeps MPF activity at low levels to maintain the first meiotic arrest [67]. As expected, loss of the Chd1 gene accelerates the first meiotic progression that causes missegregation of chromosomes and nondisjunction [68]. In the second arrest of meiosis, APC/C is repressed by CSF-EMI2, which undergoes inactivation dependent on intracellular Ca+2 surge upon fertilization [69].
Overall, the SAC and APC/C complexes work in a coordination to prevent improper meiotic progression before establishing successful spindle-kinetochore interactions. Once an error is detected during meiotic division, both mechanisms become activated. To elucidate the functional importance and mechanistic roles of each component of these complexes in mammalian oocytes, further knockout or knockdown studies are required.
Molecular determinants of permanent arrest at GV stage in the first meiotic division
Functional loss of genes
It has been reported that some GV oocytes obtained from preantral or antral follicles of women undergoing assisted reproductive technology (ART) are unable to resume meiotic maturation even following IVM [70]. The same situation can also be observed in vivo because mouse oocytes collected following ovarian stimulation were not at the same developmental stages [71]. Although molecular background of this challenge is not yet fully understood, the most possible reasons of permanent arrests occurring during the first meiotic division either in vivo or in vitro are loss or altered expression of the genes playing crucial roles in meiotic maturation (Table 1). For example, the female mice lacking the Cdc25b gene can produce only GV oocytes, unable to achieve GVBD [30]. As CDC25B is required for MPF activation, the GV-arrested oocytes cannot succeed in nuclear maturation upon in vitro culturing. Similarly, lack of the Pde3a gene (encoding an inhibitor of ADCY) leads to an arrest at GV stage in the mouse oocytes [72]. Moreover, demonstrated that deficiency of ubiquitin B (UBB) in mice results in developmental arrest at GV or MI stage owing to defective meiotic progression [73]. These defects may arise from loss of the basic functions of UBB in the cellular processes including ubiquitin-proteasome pathway [74] and CDK1 inactivation for polar body extrusion [75].
Table 1.
The potential factors leading to permanent meiotic arrests in mammalian germinal vesicle (GV) and metaphase II (MII) oocytes. ND, Not detected. The full names of all abbreviations were given in the text
| Arrest stage | Lack of gene | Gene mutation | Down-regulation | Up-regulation | Inhibition | ART application | Chemical | Other factors | |
|---|---|---|---|---|---|---|---|---|---|
| GV oocyte |
|
|
|
ND |
|
|
|
|
|
| MI oocyte |
|
|
|
|
|
|
|
|
|
| MII oocyte | ND | ND |
|
|
ND | ND |
|
ND | |
Meiosis arrest female 1 (MARF1) plays key roles in regulating the first meiotic progression, cytoplasmic maturation, and genomic integrity in oocytes [76]. Expectedly, loss of the Marf1 gene results in permanent GV-stage arrest in the mouse oocytes [77]. Similarly, double knock out of the Ccnb1 and Ccnb2 genes in mouse oocytes causes permanent arrest at GV stage due to failure in elevating MPF activity [78]. It is noteworthy that Ccnb1-null oocytes enable to complete the first meiosis and extrude the first polar body by the way of increasing CCNB2 levels to activate CDK1. However, these oocytes exhibit interphase-like appearance in the second meiotic division and can not achieve metaphase II arrest. Thus, CCNB1 deficiency results in female infertility as is observed in the double knocked out mouse models [78]. In contrast, Brandeis ., (1998) revealed that Ccnb2-null mice are fertile and show a normal prenatal development [79]. Overall, lack of these genes implicating in nuclear maturation of oocytes is closely associated with permanent GV-stage arrest. More genes having this kind of effects upon their absence would be defined in the future studies because meiotic maturation is a complex process that requires many proteins working together.
Genetic mutations
Different gene mutations in women are found to be associated with permanent arrests in oocytes. For instance, homozygous nonsense or compound-heterozygous mutation in the PAT1 homolog 2 (PATL2) gene (encoding an RNA-binding protein that involves in translational repression) was detected in a consanguineous family, and these mutations lead to an arrest at GV stage [80]. The novel point mutations (c.1127 G > A; c.1225–2A > G; c.1282 G > T; c.1300 C > T; c.865delA) and one recurrent splicing mutation (c.223–14_223-2delCCCTCCTGTTCCA) in the PATL2 gene also cause an arrest in the GV oocytes [81].
The point mutations (c.G1249A, p.D417N; c.T1088C, p.M363T; c.G5A, p.R2K; c.G900A, p.M300I; c.G785A, p.R262Q; c.C527T, p.S176L) in the tubulin beta 8 class VIII (TUBB8, expressing β-tubulin) gene leads to MI-stage arrest [82]. Indeed, these mutations disrupt the formation of α/β-tubulin heterodimer and microtubule organization in the mouse and human oocytes [82]. A novel missense mutation (c.1054 G > T, p.A352S) was additionally characterized in the TUBB8 gene in a patient and her elder sister, who had first meiotic arrest in their oocytes [83]. In the recently published study, homozygous and compound heterozygous missense pathogenic variants (c.77A > G, p.His26Arg; c.518 G > A, p.Arg173Gln; c.907 G > A, p.Glu303Lys; c.592A > G, p.Ile198Val; c.739 G > A, p.Val247Met) in the thyroid receptor interacting protein 13 (TRIP13) gene were reported to be associated with female infertility arising from meiotic arrest at MI stage [84]. Notably, TRIP13 encodes an AAA-ATPase, which is a key component of spindle assembly checkpoint. Based on literature analysis, there are a limited number of studies established a close relationship between genetic mutations and meiotic arrest. Therefore, further studies utilizing whole genome and exome sequencing technologies in the infertile women producing arrested oocytes would contribute to define novel variants.
Change of gene expression
Down- or up-regulation of some genes by using RNA interfering technology or other ways causes permanent arrests in the oocytes. Down-regulation of cyclin O (Ccno; implicating in meiotic resumption) by using short interfering RNA (siRNA) arrests mouse oocytes at GV stage because of repressing dephosphorylation of CDK1 (also known as CDC2) at Tyr15 [85]. However, it is worth noting that CCNB1 overexpression rescues this arrest by a yet undefined mechanism [85]. The polo-like kinase 4 (PLK4) is a Ser/Thr protein kinase and participates in centriole biogenesis during cell division [86]. Its depletion by employing siRNA in the mouse oocytes leads to a meiotic arrest at GV stage [87]. This arrest most likely derives from inhibited CCNB1-CDK1 activity as well as reduced CDC25C levels. In line with the previous studies, down-regulation of the Orf1p mRNA levels entails an arrest at GV stage in the mouse oocytes due to reduced levels of the MPF components, CCNB1 and CDK1 [88]. As is known, the Orf1p gene encodes a LINE-1 protein which is required for LINE-1 retrotransposition [89].
The growth and differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) are oocyte-specific factors, both of which function in normal follicle and oocyte development [90]. Significant decreases of GDF9 and BMP15 levels in the canine GV oocytes result in an arrest at GVBD stage higher than that in controls [91]. This arrest may originate from their roles during folliculogenesis [90]. On the other hand, a previous study also demonstrated that GDF9 and BMP15 contribute to the transition from primordial to primary follicle stages and oocyte maturation process as well as modulating the levels of steroidogenic acute regulatory protein (StAR), plasminogen activator, and luteinizing hormone receptor (LHR) in the follicles [92]. As meiotic progression from GV to MII stages requires temporal regulation of gene expression, analyzing these genes at mRNA and protein levels by using high throughput technologies such as next generation sequencing and proteomic analysis may allow for elucidating the molecular background of permanent arrest.
Use of inhibitors
In human oocytes, inhibition of PDE3 by utilizing Org9935 leads to an enhanced rate of meiotic arrest at GV stage, and this arrest treatment contributes to increasing the rates of oocyte maturation and fertilization after removing the inhibitor [93,94]. In a similar manner, CDK1 inhibition with RO-3306 causes meiotic arrest at GV stage in the porcine oocytes so that temporal usage of this inhibitor provides synchronized cytoplasmic and nuclear maturation for all oocytes, some of which have developmental delay [95]. Additionally, transcriptional repression in bovine oocytes [cultured with 5,6-dichloro-1-beta-d-ribofuranosylbenzimidazole (DRB)] leads to meiotic arrest at GV stage [96]. Treating ovine oocytes with cycloheximide (an inhibitor of protein synthesis) also results in meiotic arrest at GV stage, and GVBD occurs after removing this inhibitor [97].
Roscovitine was also used as a CDK inhibitor to extend GV arrest timing and thereby improved the IVM success rates in the sheep COCs [98]. The polo-like kinase 1 (PLK1) involves in modulating cell division as a serine/threonine protein kinase [99]. A recent study by Liao et al., (2018) revealed that inhibition of PLK1 by its specific inhibitor (GSK461364) in the porcine oocytes elevates the GVBD arrest rate when compared to the control [100]. Majority of the oocytes exhibited abnormally aligned chromosomes and spindle defects during the first meiotic division most likely due to loss of the kinase activity of PLK1.
The use of 6-diazo-5-oxo-l-norleucine (DON, an inhibitor of hyaluronan synthase) during IVM also gives rise to a transient meiotic arrest in the porcine GV oocytes; therefore, nuclear and cytoplasmic maturation of these oocytes can be enhanced by incubating with this inhibitor for ensuring synchronization [101]. In addition to these inhibitors, Soto-Heras et al., (2019) reported that cattle GV oocytes treated with C-type natriuretic peptide [CNP, functioning as an oocyte maturation inhibitor (OMI)] plus 3-isobutyl-1-methylxanthine (IBMX, inhibiting meiotic progression via increasing cAMP level) experience GV arrest [102]. It is worth noting that this culturing system before IVM not only improves cumulus-oocyte communications but also increases blastocyst formation rates.
In addition to GV arrest, some chemicals such as nocodazole at a low dose (400 nM for 3 h at 6 h after GVBD) arrest mouse oocytes transiently at MI stage and decrease polar body extrusion [103]. Nocodazole can inhibit the spindle formation via leading to spindle depolymerization and thereby prevents bipolar spindle formation [104]. Moreover, treating mouse oocytes with cytochalasin D (at a concentration of 10 μg/mL), a potent actin polymerization inhibitor, causes a large number of arrest at MI stage [105]. The same effect of cytochalasin D [106] and cytochalasin B [107] was also demonstrated in the porcine oocytes. Overall, the arrest of GV or MI oocytes in a short-term program by using the chemicals such as IBMX, hypoxanthine, dibutyryl cyclic AMP (dbcAMP), milrinone, 8-bromo-cGMP (8-Br-cGMP), cytochalasin, and nocodazole [108] may increase the rates of MII oocytes and eventually early embryos in women producing a limited number of oocytes. On the other hand, it is important to note that more studies related to evaluating the short- and long-term of effects of these chemicals on the prenatal development processes are warranted before initiating use of them in routine ART applications.
Culture conditions
In a few studies, the potential effects of culture conditions on nuclear maturation of oocytes were evaluated. Crocomo ., (2015) demonstrated that overlaying GV oocytes with mineral oil during culturing is associated with arrest at GV stage in the sheep oocytes [109]. Moreover, exposure of bovine oocytes to high concentrations of gonadotropins (FSH and LH) leads to maturation arrest at GV stage [110]. As culture conditions are known to change the epigenetic status of oocytes [111], these GV arrests may derive from abnormally established epigenetic mechanisms such as DNA methylation [112] and histone modifications [113,114], both of which participate in regulating gene expression during oocyte maturation. Thus, more studies related to determining potential impacts of the culture conditions including oxygen tension, IVM, and ovarian stimulation protocols being used in ART centers on the meiotic processes are required.
Other factors
A various types of factors including beverages, drugs, and environmental pollutants can also affect meiotic progression in the oocytes. An interesting work by Kren ., (2004) reported that treating porcine oocytes with caffeine causes arrest at GV stage in 95.5% of the treated oocytes after 24 h of culture, possibly due to enhanced intracellular cAMP levels and inhibition of both CDC2 and MAPK activities [115]. Withdrawal of caffeine results in progression of the GV-arrested oocytes to MII stage following 48 h culture; however, blastocyst formation rate of these oocytes is lower than control group [115].
On the other hand, exposure of bovine oocytes to metformin [an activator of 5′ AMP-activated protein kinase (AMPK), and being used for treating PCOS] ensues an arrest at GV stage [116]. This issue may originate from increased phosphorylation of PRKAA (protein kinase AMP-activated catalytic subunit alpha) at Thr172 and reduced MAPK3/1 (mitogen-activated protein kinase 3/1) levels in both oocytes and cumulus cells. The decreased AURKA (aurora kinase A) and CCNB1 levels in the oocytes, which adversely influences nuclear maturation, are other potential factors of this arrest [116].
Benzo[a]pyrene (BaP) is a polycyclic aromatic hydrocarbon, which is emitted from solid fuel combustion. BaP exposure caused meiotic arrest in the mouse MI oocytes and generated from GV oocytes after culturing 8 h [117,118]. This arrest likely originated from impaired meiosis-linked processes such as spindle assembly, chromosome alignment, and kinetochore-microtubule attachments [117,118]. Furthermore, thiamethoxam (TMX, an insecticide, detected on a variety of crops) ceased meiotic maturation in the cattle GV and MI oocytes via reducing CDC25 and CDK1 activities, and holding CCNB1 in the cytoplasm [119]. These findings suggest that externally exposing to these chemicals can influence the chance of obtaining highly competent oocytes.
In addition to the exogenous factors, several internal factors involving signaling molecules such as Ca+2 and DNA damages can affect the meiotic progression. Lee ., (2004) revealed that most GV-arrested oocytes in mice do not have the Ca+2 channels including P/Q-, N-, and L-type as not detected in the GVBD-arrested oocytes [120]. It is noteworthy that there is a uniform and localized distribution of these channel proteins in the GV and GVBD oocytes [120]. One of the commonly occurring DNA damages, double-strand breaks (DSBs) also lead to GV arrest in the human oocytes during nuclear maturation [121]. The potential effects of other DNA damages and inefficiency of the DNA repair mechanisms involving DSB repair on developing first meiotic arrest remain elusive.
As a result, current studies show that intracellular and extracellular factors to which oocytes are exposing during culturing are capable of impacting first meiotic progression. Most likely, new molecules associating with GV arrest will be defined in the future studies. These studies would increase the chance of producing high quality oocytes via removing or adjusting concentrations of those molecules. Also, potential biomarkers showing the oocytes having higher developmental capability may be characterized in these investigations. One of these investigations performed by Hemmings ., (2013) found that amino acid profiling contributes to predicting the developmental potential of human oocytes, in which arrested-GV oocytes use significantly more valine and isoleucine amino acids when compared to the GV oocytes progressed to MI or MII stage [122].
Molecular background of the permanent arrest at MI stage in the first meiotic division
Some MI oocytes undergo an arrest before extruding first polar body and display typical morphologic appearances such as homogenous cytoplasm and no visible nucleus. This arrest may occur due to intracellular defects (arising from endogenous and/or exogenous factors) in the oocytes and/or surrounding granulosa cells during folliculogenesis [105,123]. Loss or altered gene expression, impaired signal transduction pathways mediating meiotic progression, and abnormality in the meiotic spindles activating the SAC are frequently encountered intracellular defects [124].
Loss of genes
It has been reported that loss of certain genes involved in meiotic progression causes an arrest in oocytes at MI stage (Table 1). For example, lack of the MutL homolog 1 (Mlh1) [125] or 3 (Mlh3) [126] gene results in remarkably reduced meiotic recombination rates and increased maturation arrest at MI-like stage. These effects may give rise from functional loss of the MutL homologs, MLH1 and MLH3, in the formation and repair of the DSBs during crossing over [127]. As is known, there is a close relationship between unrepaired DSBs and permanent cell cycle arrest, apoptosis, or cell death [128].
As is known, CDC28 protein kinase regulatory subunit 2 (CKS2) involves in regulating CDK and MPF activation, GVBD, APC/C induction, and meiotic spindle assembly so that it contributes to meiotic maturation [129]. Consistently, Cks2−/− mouse oocytes arrest at MI stage [130]. Additionally, lack of O-fucosylpeptide 3-beta-N-acetylglucosaminyltransferase (Lfng) gene (implicating in oocyte meiotic maturation) leads to first meiotic arrest in the mouse oocytes [131]. This outcome seems to derive from dysregulation of LFNG in the Notch signaling having crucial roles during folliculogenesis [132]. It is noteworthy that the Lfng gene is expressed in the granulosa and theca cells of the growing follicles from primary to preovulatory stages [131].
The MI-stage arrest also occurs in the absence of meiosis defective 1 (Mei1) gene in the mouse oocytes possibly due to loss of its functions in forming correct synapses as well as paring and proper organization of the chromosomes at metaphase plate [133]. Analogously, the lack of Fmn2 (Formin-2, implicating in chromosome organization as MEI1 does) gene results in arresting at MI stage [134]. This arrest originates from lacking fundamental role of FMN2, as an actin nucleation factor, in regulating asymmetric division via promoting formation and modulation of new actin filaments during nuclear maturation [135]. Expectedly, when Fmn2−/− oocytes are fertilized with normal spermatozoa, the polyploid embryos are produced [134]. Knocking out of the Dcaf13 (DDB1 and CUL4 associated factor 13) gene contributing to chromosome organization causes an arrest at prometaphase MI (pro-MI) stage in most mouse oocytes based on misaligned chromosomes at the equatorial plate and the SAC activation [136]. Taken together, as loss of these genes impedes successful meiotic progression that results in MI arrest, mutation analysis on these genes may help to address the molecular background of female infertility deriving from this arrest.
Genetic mutations
Some genetic mutations and down-regulation of several genes exhibit a close relationship with meiotic arrest at MI stage. The point mutations (two missense mutations: c.1528 C > A and c.1376 C > A) in the PAT1 homolog 2 (PATL2) gene lead to MI arrest as well as other phenotypes including morphological abnormalities [137]. In more detail, these mutations detected in the two consanguineous families entail a significant decrease in RNA binding ability of PATL2; therefore, it is unable to regulate posttranscriptional translation in a time-dependent manner.
Change of gene expression
Altered expression of the genes playing roles in intracellular signaling in oocytes was found be associated with meiotic arrest at MI stage. One of these genes, the Erk3 (extracellular signal-regulated kinase 3) gene is an atypical member of MAPK family and encodes ERK3 localizing to the spindles of mouse oocytes at MI or MII stage [138]. Depletion of Erk3 gene expression through morpholino injection results in MI stage-arrest in the mouse oocytes owing to abnormal spindles formation and impaired chromosome alignment [138]. The oocyte-specific homeobox 4 (Obox4) implicates in cAMP-dependent intracellular signaling cascades. Its overexpression by microinjecting dsRNA into GV oocytes results in MI arrest in 78% of the oocytes, and also there is MII arrest in the remained ones [139].
The ras-related dexamethasone induced 1 (RASD1) implicates in signal transduction pathways through both G proteins and G protein-coupled receptors. Once the Rasd1 gene expression was down-regulated in mouse GV oocytes, maturation arrest occurs at MI stage based on the defects both in meiotic spindles and chromosome alignment [140]. Another intracellular signaling-linked protein, cyclic adenosine monophosphate (cAMP)-dependent type 2 regulatory subunit beta (PRKAR2B) contributes to modulating cAMP-dependent PKA activity. Similarly, its down-regulation leads to MI-stage arrest in the mouse oocytes, which likely results from abnormal spindle formation, chromosome aggregation, and impaired pentose phosphate metabolism [141]. Depletion of the f-box protein 30 (Fbxo30; participating in phosphorylation-dependent ubiquitination) gene expression also causes MI-stage arrest in the mouse oocytes, failing to achieve chromosome segregation, but there is no SAC over-activation [142]. It is important to note that in normal meiotic progression, Fbxo30 mRNA levels decrease predominantly following MI stage in mouse oocytes [142]. Overall, as there are many intracellular signaling pathways working during early phase of meiotic division in oocytes, much more genes whose expressional changes are related to meiotic arrest would be defined in the future studies.
Some studies have analyzed the potential effects of spindle-related proteins on the onset of MI arrest. One of them performed by Wassmann ., (2003) documented that overexpression of mitotic arrest deficient 2 (MAD2, a member of SAC complex) protein during early meiosis causes MI arrest, and dominant negative mutation on its gene impairs normally appearing spindle check point arrest [103]. Another study revealed that depletion of the Spindly (localizing to kinetochores where spindles bind) gene in the mouse oocytes leads to an arrest at pro-MI or MI stage due to chromosome-alignment defects and abnormal spindle morphology as wells as failure in entering anaphase stage and extruding first polar body [143]. As is known, BUBR1 is a member of SAC family and functions in kinetochore localization of other SAC components during cell division. Its up-regulation causes arrest of the mouse oocytes at MI or earlier stage, displaying chromosome alignment defects [144]. On the other hand, dominant-negative mutation or expressional depletion of the BubR1 gene enhances meiotic progression [144]. This issue may derive from stimulating meiotic process without undergoing spindle check point control in the absence of BUBR1.
A previous study also documented that SPC25 component of NDC80 kinetochore complex (SPC25) implicates in modulating kinetochore-microtubule attachments and the SAC process during cell division [145]. Its overexpression associates with meiotic arrest at pro-MI or MI stage in most mouse oocytes due to abnormal segregation of homologous chromosomes (Table 1). As is known, centrosomal protein 55 (CEP55) is a member of centrosomal protein family, and is detected in the oocytes from GV to MII stages [146]. Knockdown of the Cep55 gene expression in GV oocytes causes meiotic arrest at MI stage, most likely dependent on abnormal spindle formation and defective chromosome alignment as well as abundance of CCNB and SAC activation [146]. Moreover, a predominant decrease of the Syt1 (Synaptotagmin 1) gene expression results in pro-MI or MI arrest in the majority of mouse oocytes, that may originates from disrupted spindles, activation of the SAC component, BUB3, failure in homologous chromosome segregation, and abnormal chromosome alignment as well as from aberrant MTOC protein localization, and impaired γ-tubulin distribution in the cytoplasm [147]. It is noteworthy that SYT1 is known as a calcium sensor for exocytosis and contributes to localization and fusion of the vesicles to plasma membrane [148]. These findings indicate that changed spindle-related gene expression initially disrupts spindles function and their regulation that may lead to abnormal chromosome organization and eventually impaired progression of cell division.
As is acceptable, meiotic arrest largely depends on strictly controlling spatiotemporal distribution of the proteins directly or indirectly functioning in the cell cycle process. Therefore, abnormal expression of these genes may lead to permanent arrests. For instance, overexpression of the Ccnb1 gene in the bovine oocytes causes a meiotic arrest at MI or MII stage owing to non-disjunction of homologous chromosomes and defect in the first polar body extrusion [149], as is observed in the mouse oocytes [150]. In a recent study, Yi ., (2019) demonstrated that down-regulation of the protein kinase C βI (PkcβI, participating in regulation of cell cycle transition) gene expression results in an arrest at MI stage due to the SAC activation and failure of first polar body extrusion. On the other hand, its up-regulation delays G2/M transition in the mouse oocytes [151].
Another cyclin type, cyclin B3 (CCNB3), functions in inhibiting premature metaphase-anaphase transition, its down-regulation entails MI-arrest in the mouse oocytes [152]. It was also determined here that CCNB3 stimulates APC/C to facilitate metaphase-anaphase progression. Expectedly, lack of CCNB3 in female mice results in sterility due to blocking the transition from metaphase to anaphase stages in the first meiotic division [153,154]. The checkpoint 1 (CHK1) synthesized in GV and MII oocytes serves in DNA replication checkpoint and DNA damage response as well [155]. Its down-regulation results in meiotic arrest at MI stage in the porcine oocytes based on the chromosome misalignment, undegradation of CCNB1, and decrease in the mitotic arrest deficient 2-like 1 (MAD2L1; one of the SAC proteins) levels [156]. As revealed by Chen ., (2012), CHK1 localizes to the meiotic spindles of the mouse oocytes from pro-MI to MII stages [157]. Its overexpression after GVBD leads to the arrests at pro-MI or MI stage owing to the SAC activation and suppressed homologous chromosome segregation [157,158]. The Survivin protein [a component of the chromosomal passenger complex (CPC) and inhibitor of apoptosis proteins (IAPs) [159]] generated in the porcine oocytes at GV or GVBD stage [160] plays a role in cell division. A remarkable reduction in the Survivin gene expression leads to an arrest at MI stage in these oocytes most likely due to chromosome alignment abnormalities [160]. In parallel with its reduced expression, overexpression of the Survivin gene results in pro-MI or MI stage-arrest in the majority of mouse oocytes, having impaired chromosome alignment and the SAC activation [161]. Although a limited number of genes have been evaluated to determine their potential relationship with MI arrest in mammalian oocytes, the remained genes [162] implicating in meiotic cell cycle progression must also be analyzed.
The expressional changes in the genes playing roles in RNA binding, linker DNA interaction, cell proliferation, and S-adenosylmethionine biosynthesis were found to associate with developing MI arrest. The staufen 2 [STAU2) protein is a double-stranded RNA-binding protein and involves in mRNA metabolism and translation process. Cao ., (2016) reported that down-regulation of the Stau2 gene expression using a knockdown technology in the mouse oocytes leads to MI arrest in most of them [163]. This arrest seems to arise from the SAC activation, chromosome misalignment, and abnormalities in both spindle formation and microtubule-kinetochore interactions [163]. The spindlin 1 (SPIN1) also contributes regulation of maternal transcripts via interacting with RNA binding proteins. Its overexpression entails an arrest at MI stage probably because of impaired modulation of the CCNB1 levels [164]. The Orf1p gene participates in cell proliferation and cell death, and its enhanced expression by the way of mRNA microinjection ceases the meiotic progression at MI stage in the mouse oocytes [88]. As is known, H1FOO (H1 histone family member o oocyte specific) is a linker histone protein and acts in modulating gene expression and chromatin modification during oogenesis and early embryogenesis [165]. Although the precise cause is unknown, its down-regulation results in MI stage-meiotic arrest [165,166]. In a recent study, it was demonstrated that silenced expression of the methionine adenosyltransferase 2b (Mat2b) gene, catalyzing biosynthesis of S-adenosylmethionine, causes MI arrest in the mouse oocytes, possibly arising from the microtubule assembly defects [167].
All findings on this subject indicate that successfully passing from MI stage requires temporal and strict regulation of gene expression. Otherwise, many abnormalities such as chromosome misalignment, SAC activation, and dysfunctional spindle formation in the oocytes arrested at MI stage may occur upon altering expression of the genes playing roles in cell cycle, intracellular signaling, and other cellular processes.
Use of inhibitors
In addition to down- or up-regulation of gene expression, there is a close relationship between functional inhibition of target proteins serving in cell cycle and meiotic arrest at MI stage. For instance, checkpoint kinase 2 (CHK2) playing roles in cell cycle regulation, replication checkpoint, and DNA damage repair localizes in the nucleus of GV oocytes and spindle poles of MI oocytes in mice [168]. Inhibition of the CHK2 activity in the mouse oocytes results in arresting at GV or MI stage or at the first cleavage of early embryonic development possibly due to disrupting cell cycle progression [169]. Additionally, Liu et al., (2017) reported that inhibition of phospholipase D2 (PLD2) in the mouse oocytes causes MI-stage arrest deriving from the spindle abnormalities [170]. It is worth noting that these abnormalities may originate from the fundamental roles of PLD2 in the cell cycle, cytoskeletal reorganization, transcriptional control, and cell migration [171].
Inhibiting phosphorylation of p38 MAPK (implicating in cell cycle regulation) with SB203580 compound in the cumulus cells of porcine oocytes leads to an increased rate of meiotic arrest at MI stage, but do not influence GVBD [172]. This arrest may derive from impairment in the well-known missions of p38 MAPK pathway in the cellular events such as cell cycle progression, gene expression, and apoptosis [173].
Some studies were additionally evaluated the potential effects of inhibiting other proteins having roles in several cellular events. One of those studies performed by Qu ., (2016) reported that ectopic expression of enhancer of zeste homolog 2 (EZH2) associates with meiotic maturation arrest at MI stage in the mouse oocytes, displaying chromosomal alignment defect and aneuploidy [174]. Of note, EZH2 is involved in the trimethylation of H3 lysine 27 and stability of the SAC protein, BUBR1. Inhibition of platelet activating factor acetylhydrolase 1b catalytic subunit 3 (PAFAH1B3; making platelet-activating factor hydrolysis) by using a selective inhibitor or a specific antibody causes MI arrest, showing abnormal spindle morphology, and these cattle oocytes are also unable to extrude the first polar body [175] (Table 1). Although a limited number of studies have examined functional loss of some proteins implicating in various types of cellular processes, more studies on this subject would define further proteins whose functional loss is related to meiotic arrest at MI stage.
Other factors
A recently published case report demonstrated that ovarian stimulation with gonadotrophin-releasing hormone (GnRH) agonist and recombinant human chorionic gonadotropin (rhCG) enhanced the rates of permanent arrest at GV or MI stage when compared to the stimulation only with rhCG [176]. Similar results were also observed in the cancer patients who underwent ovarian stimulation had more arrested immature oocytes than in the non-stimulated group [177]. To the best of my knowledge, there are a limited number of investigations analyzed the potential effects of ART applications on MI-stage arrest. As many molecules are being used in ART centers, further studies are required for evaluating whether these molecules have any adverse effects on nuclear maturation of oocytes, especially on weak ones.
Some oocytes at MI stage are unable to reach MII stage based on negative effects of several environmental chemicals. One of these chemicals, bisphenol A (BPA; used in producing polycarbonate plastics), predominantly decreases meiotic progression from GV to MII stages and increases the degeneration and spontaneous activation rates in the exposed human oocytes [178]. In the mice, bisphenol AF (BPAF; an analogue of BPA) inhibits meiotic maturation from GV to MII stages and causes an arrest after GVBD through triggering the SAC [179]. Moreover, Nie ., (2019) revealed that thiamethoxam (TMX; being utilized as an insecticide) blocks meiotic progression at MI stage and also provokes chromosome abnormality by unknown mechanisms [119].
Doxorubicin (DOX) is employed to cure patients with malignancies, it leads to meiotic arrest at MI stage in the mouse oocytes because of enhanced DNA damages and the SAC activation [180]. It is well-known that use of the synthetic estrogen, diethylstilbestrol [DES), utilizing for triggering animal growing, can increase environmental estrogen levels. A recent study by Ding ., (2020) demonstrated that DES exposure of mouse oocytes during maturation resulted in meiotic arrest largely at MI stage possibly due to disturbed spindle assembly and chromosome misalignment [181]. Taken together, these findings suggest that women intending to conceive must be protected from the environmental agents during reproductive ages in order to preserve their oocytes from undergoing developmental arrest.
DNA damage is another factor closely related to developmental arrest appearing during oocyte maturation. Once mouse oocytes are treated with etoposide (a DNA damage-inducer agent), meiotic arrest occurs at MI stage due to the SAC activation [182]. Consistently, induction of DSBs in the porcine GV oocytes using the same agent results in meiotic arrest at MI stage, displaying abnormal spindle formation and chromosome misalignments [183]. It is noteworthy that the SAC activation based on DNA damage takes places independent of action of the DNA damage response kinase, ATM or ATR [184]. In contrast to the former studies, a recently published investigation documented that DNA damage in the human GV oocytes did not exhibit an arrest at MI or other stages, suggesting that meiotic progression to MII stage can be achieved despite of present DNA damage in humans [185]. This issue means that oocytes experiencing successful meiotic maturation may not include completely intact genomic DNA.
In addition to DNA damage, an appropriate amount of zinc in culture environment is essential for normal oocyte maturation and early embryo development as well [186]. As is known, the trace element zinc functions as a cofactor for enzyme activation and contributes to maintaining structural integrity of some proteins as well. Indeed, zinc deficiency during IVM leads to MI-stage arrest in the porcine oocytes [187]. Although these oocytes have a normal microtubule distribution, there is an abnormality in the microfilament formation, which may underlie this arrest [187].
In summary, the possible causes of MI stage-arrest in mammalian oocytes can be (i) incompetency of achieving meiotic maturation due to lack or altered gene expression directly or indirectly implicating meiotic progression, (ii) unsuccessful meiotic recombination normally appearing at pachytene stage. (iii) inability of GV oocytes in generating essential cell cycle regulators required to progress from G2 to M phases [14], (iv) failure in temporally degrading the p34cdc2 and CCNB proteins as revealed in the oocytes from LT/Sv mice [188], (v) exposure to various types of endogenous or exogenous factors adversely affecting meiotic process. Despite all these causes, it is possible to conceive in a limited number of MI-arrested oocytes after applying a modified IVM process [189].
The potential factors leading to permanent arrest in the second meiotic division
Some MII oocytes undergo a permanent arrest in the second meiotic division, which are unable to produce zygotes with two pronuclei, and these oocytes cannot achieve decondensation of sperm chromatin following fertilization [24]. Therefore, it should be kept in mind that if further development does not occur following fertilization, there may be an arrest in the late meiotic stage [190,191].
Altered gene expression
Changes in expression of the genes playing crucial roles during meiotic maturation can cease developmental progression in the oocytes. For example, down-regulation of the early mitotic inhibitor 2 (Emi2) gene in mouse GV oocytes causes defects in establishing meiotic spindles and chromosome decondensation after anaphase I stage, eventually enter an interphase-like phase [192]. Notably, GVBD and polar body extrusion events take place normally in these oocytes. On the other hand, overexpression of the EMI2 gene but not EMI1 leads to meiotic arrest at MII stage in the porcine oocytes [193]. As is known, EMI2 implicates in inhibiting APC/C (functioning in the degradation of CCNB) to resume meiosis [194]. For keeping EMI2 at the required levels, the mitogen- and stress-activated kinase 1 (MSK1), a downstream kinase of MOS-MAPK pathway, phosphorylates four Ser/Thr residues of the EMI2 protein in the mouse oocytes [195]. As a result, holding EMI2 in a stable level is important for successfully passing the MII stage. Otherwise, several defects related to formation of meiotic spindles and chromosome organization, and ultimately MII arrest occurs. While down-regulation of the GPR3 gene expression activates meiotic resumption, its overexpression enhances cGMP and cAMP levels and thus represses the meiotic maturation in the porcine oocytes [196]. Additionally, overexpression of another cell cycle-regulator gene, Cdc2b (cell division control protein 2 homolog b), inhibits Ca2+-induced exit from MII arrest so that permanent arrest appears in these oocytes [197] (Table 1).
The expressional changes in several genes functioning in various cellular events involving proliferation and differentiation, meiotic resumption, and ubiquitin-based protein degradation were also found to be associated with meiotic arrest. Repressing the growth arrest-specific gene 6 [Gas6; participating cellular proliferation and differentiation [198]] expression in the mouse GV oocytes by using RNAi technology entails MII arrest at 90% of the oocytes [199]. The down-regulation of SPIN1 gene (involving in meiotic resumption) in the pig MII oocytes decreased the MPF levels and MAPK activity which resulted in pronucleus formation independent of calcium activation [200]. In contrast, its overexpression inhibited the transition from metaphase to anaphase stages in the oocytes and early embryos possibly due to induced DNA damage response activation [200]. On the other hand, overexpression of the ubiquitin conjugating enzyme E2 S (UBE2S) gene caused MII-stage arrest and even pronucleus formation in some oocytes at the second day of culturing porcine oocytes [201]. It is worth noting that UBE2S is a member of ubiquitin-conjugating enzyme family and implicates in poly-ubiquitination-based protein degradation in oocytes [202]. Taken together, down- or upregulation of certain genes regulating meiotic maturation results in permanent arrest at MII stage in mammalian oocytes (Table 1). In addition to down-regulation and overexpression analyses, knockout mouse models were created to understand the outcomes of functional loss of few genes in meiotic progression. The lack of extracellular signal-regulated kinases 1 and 2 (Erk1 and 2) genes in the mouse oocytes caused abnormal spindle assembly and spontaneous extrusion of second polar body in the MII oocytes and eventually results in developmental arrest at metaphase III stage (MIII) owing to these defects [203]. The MIII stage is briefly defined as an arrest at metaphase phase following second polar body emission.
According to the literature analysis, there are a limited number of studies addressing the molecular background of permanent MII arrest formation. For instance, there is only one study examined the effect of ART application on developing MII arrest. Supplementation of vitrification media of mouse MII oocytes with 1 μM of paclitaxel (a microtubule stabilizer) increased the rate of MII arrest when compared to the control and 0.5 μM of paclitaxel-treated group [204]. Most studies have investigated the molecular determinants playing roles in normally appearing arrest at MII stage. Further studies in the aspects of alterated gene expression, ART conditions, and environmental factors are needed to ascertain the potential reasons for permanent MII arrest.
Rescuing oocytes from arrested state
Although molecular determinants underlying permanent arrests in mammalian oocytes are not fully elucidated, yet there are a few treatment options for rescuing these arrests. Meiotic spindle transfer (referred to GV or cytoplasmic replacement), increase in certain factor levels or addition of some chemical molecules to in vitro culture environment are possible interventions for inducing meiotic progression [205]. As revealed in the study by Zhang and Liu (2015), cytoplasm replacement of an arrested mouse or human GV oocyte, known as GV transfer, resumes meiosis and provides extrusion of the first polar body; thereby, mature MII oocytes are generated [206].
Increasing the AMPK levels through microinjecting its active form facilitates meiotic resumption in the mouse GV oocytes, arrested by treating with dibutyryl cAMP (dbcAMP), and following that these oocytes accomplish GVBD [207]. Moreover, overexpression of the exportin 1 (XPO1) gene by mRNA injection stimulates meiotic resumption in the porcine fully grown GV oocytes by the way of predominantly decreasing nuclear localization of WEE1B [208]. Notably, as an oocyte-specific kinase at the downstream of PKA, WEE1B plays a key role in maintaining meiotic arrest in mouse oocytes [209]. In the future studies, it should be considered that long term effects of these treatments especially on the early embryo development and onward need to be examined before routinely using them in ART centers.
The chemicals such as methylene blue, phenazine ethosulfate, and pyrroline-5-carboxylate (P5C) perform oxidization of NADPH to NADP and induce NADP-dependent enzymes so that meiotic resumption in the cumulus cell-enclosed oocytes arrested by hypoxanthine exposure takes place following treated with either of these chemicals [210]. Once ribose-5-phosphate metabolizing to phosphoribosyl-pyrophosphate is added to glucose-free medium, the arrested oocytes with hypoxanthine treatment are triggered for meiotic maturation regardless of presence of FSH [210]. Additionally, the PKC activator, phorbol-12-myristate 13-acetate (PMA), stimulates GVBD in the cumulus cell-enclosed mouse oocytes treated with isobutylmethylxanthine or guanosine for maintaining in an arrested state [211, 212 further reported that addition of sterol and 4,4-dimethyl-5 alpha-cholesta-8,14, 24-trien-3 beta-ol (FF-MAS) to culture media of mouse denuded oocytes arrested with hypoxanthine ensures meiotic activation [213]. The 5-aminoimidazole-4-carboxamide 1-beta-d-ribofuranoside (AICA riboside; inducing AMPK) was found as a potent factor for meiotic resumption of denuded or cumulus cell-enclosed mouse GV oocytes arrested by dbcAMP or hypoxanthine treatment [214]. In the same investigation, it was also demonstrated that AMP enables to stimulate GVBD in the denuded GV oocytes, arrested through treating with dbcAMP or hypoxanthine.
The halogenated adenosine nucleosides but not native ones are capable of inducing meiosis when the mouse cumulus cell-enclosed oocytes arrested by hypoxanthine treatment are cultured [215]. Meiotic progression of denuded oocytes can also be carried out by treating with 8-bromo-adenosine (8-Br-Ado) or the adenosine analog, methylmercaptopurine riboside, independent of initially used inhibitor for maintaining meiotic arrest [215]. Moreover, as ionomycin is able to increase intracellular calcium levels, treatment of MI-arrested human oocytes with this chemical contributes to extruding the first polar body [216]. However, the MII oocytes produced by this way can not be fertilized or do not accomplish cleavage divisions following ICSI application. In a study by Yoon ., (2017), reported that the MI-arrested oocytes are matured into MII stage after providing intracellular Ca+2 oscillations [217]. Although blastocysts could be produced from these MII oocytes, no pregnancy was reported possibly due to incompetent cytoplasmic maturation required for achieving a normal prenatal development.
An interesting study by Chen ., (2009) revealed that pulsatile production of cAMP with forskolin stimulates meiotic maturation in the denuded mouse oocytes by activating AMPK [218]. The potential factors such as forskolin [as an adenyl cyclase (AC) activator] [218] and metal zinc (mediating synchronization of the first meiotic arrest in cooperation with the MOS-MAPK pathway) [219] may be used in obtaining MII oocytes at a high rate. Inhibition of acetyl CoA carboxylase by CP-640186 or soraphen A in the mouse cumulus cell-enclosed, denuded and follicle-enclosed oocytes, maintained at an arrested state resumes meiosis possibly by the way of stimulating fatty acid oxidation for activating PRKA [220]. Moreover, acute heat pulsing can trigger mouse GV oocytes arrested with dibutyryl cAMP, and subsequently GVBD occurs [221]. This meiotic progression may be carried out by activating PRKA since it functions as a stress response kinase [222].
As a result, all these studies contribute to creating a future perspective on inducing arrested oocytes by several activators or synchronizing these oocytes developmentally to increase the rate of meiotic progression. However, it should be kept in mind that there may be potential adverse effects of these inventions on oocyte aging and the later developmental terms. Therefore, after performing more studies to understand their biological reliability, embryologists can start to use them in the regular ART applications.
Conclusion
Based on literature analysis, there are many factors such as altered gene expression, genetic mutations, ART applications, and various types of chemicals that affect meiotic progression in the mammalian oocytes. Most probably, novel molecular determinants associating with permanent arrests in the oocytes at different developmental stages would be defined in the future studies. After defining these determinants, further studies will also be required to elucidate the molecular signaling mechanisms, directly or indirectly leading to meiotic arrests. Following characterization of these mechanisms, most effective treatment applications for meiotic resumption in the human oocytes should be determined for increasing the rate of early embryo production.
Acknowledgments
The author thanks Robert Glen (PhD) for helpful comments and corrections on the manuscript.
Funding Statement
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- [1].Mamsen L Salto, Brchner C Beltoft, Byskov A Grete and Mllgard K. (2012). The migration and loss of human primordial germ stem cells from the hind gut epithelium towards the gonadal ridge. Int. J. Dev. Biol., 2012;56(10–11–12):771–778. doi: 10.1387/ijdb.120202lm [DOI] [PubMed] [Google Scholar]
- [2].Nikolic A, Volarevic V, Armstrong L, Lako M, Stojkovic M.. Primordial Germ Cells: Current Knowledge and Perspectives. Stem Cells Int, 2016;1741072. doi: 10.1155/2016/1741072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wear H M, McPike M J, Watanabe K H. From primordial germ cells to primordial follicles: a review and visual representation of early ovarian development in mice. J Ovarian Res, 2016;9(1):36. doi: 10.1186/s13048-016-0246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142:2554–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25:2944–2954. [DOI] [PubMed] [Google Scholar]
- [6].Hsueh AJ, Kawamura K, Cheng Y, et al. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. [DOI] [PubMed] [Google Scholar]
- [8].Arroyo A, Kim B, Yeh J. Luteinizing hormone action in human oocyte maturation and quality: signaling pathways, regulation, and clinical impact. Reprod Sci. 2020;27:1223–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sanchez F, Smitz J. Molecular control of oogenesis. Biochim Biophys Acta. 2012;1822:1896–1912. [DOI] [PubMed] [Google Scholar]
- [10].Fulka J Jr., First NL, Moor RM. Nuclear and cytoplasmic determinants involved in the regulation of mammalian oocyte maturation. Mol Hum Reprod. 1998;4:41–49. [DOI] [PubMed] [Google Scholar]
- [11].He M, Zhang T, Yang Y, et al. Mechanisms of oocyte maturation and related epigenetic regulation. Front Cell Dev Biol. 2021;9:654028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reader KL, Stanton JL, Juengel JL. The role of oocyte organelles in determining developmental competence. Biology (Basel). 2017;6(3): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang X, Pepling ME. Regulation of meiotic prophase one in mammalian oocytes. Front Cell Dev Biol. 2021;9:667306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mrazek M, Fulka J Jr. Failure of oocyte maturation: possible mechanisms for oocyte maturation arrest. Hum Reprod. 2003;18:2249–2252. [DOI] [PubMed] [Google Scholar]
- [15].Wang T, Na J. Fibrillarin-GFP facilitates the identification of meiotic competent oocytes. Front Cell Dev Biol. 2021;9:648331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bouniol-Baly C, Hamraoui L, Guibert J, et al. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod. 1999;60:580–587. [DOI] [PubMed] [Google Scholar]
- [17].Tan JH, Wang HL, Sun XS, et al. Chromatin configurations in the germinal vesicle of mammalian oocytes. Mol Hum Reprod. 2009;15:1–9. [DOI] [PubMed] [Google Scholar]
- [18].Jones KT. Turning it on and off: m-phase promoting factor during meiotic maturation and fertilization. Mol Hum Reprod. 2004;10:1–5. [DOI] [PubMed] [Google Scholar]
- [19].Li J, Qian WP, Sun QY. Cyclins regulating oocyte meiotic cell cycle progression. Biol Reprod. 2019a;101:878–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hashimoto N, Kishimoto T. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Dev Biol. 1988;126:242–252. [DOI] [PubMed] [Google Scholar]
- [21].Oh JS, Han SJ, Conti M. Wee1B, Myt1, and CDC25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J Cell Biol. 2010;188:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mehlmann LM, Saeki Y, Tanaka S, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. [DOI] [PubMed] [Google Scholar]
- [23].Ignatov A, Lintzel J, Hermans-Borgmeyer I, et al. Role of the G-protein-coupled receptor GPR12 as high-affinity receptor for sphingosylphosphorylcholine and its expression and function in brain development. J Neurosci. 2003;23:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pan B, Li J. The art of oocyte meiotic arrest regulation. Reprod Biol Endocrinol. 2019;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vaccari S, Horner K, Mehlmann LM, et al. Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev Biol. 2008;316:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Horner K, Livera G, Hinckley M, et al. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258:385–396. [DOI] [PubMed] [Google Scholar]
- [27].Burghardt RC, Barhoumi R, Sewall TC, et al. Cyclic AMP induces rapid increases in gap junction permeability and changes in the cellular distribution of connexin43. J Membr Biol. 1995;148:243–253. [DOI] [PubMed] [Google Scholar]
- [28].Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. [DOI] [PubMed] [Google Scholar]
- [29].Pirino G, Wescott MP, Donovan PJ. Protein kinase A regulates resumption of meiosis by phosphorylation of CDC25B in mammalian oocytes. Cell Cycle. 2009;8:665–670. [DOI] [PubMed] [Google Scholar]
- [30].Lincoln AJ, Wickramasinghe D, Stein P, et al. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet. 2002;30:446–449. [DOI] [PubMed] [Google Scholar]
- [31].Reis A, Chang HY, Levasseur M, et al. APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat Cell Biol. 2006;8:539–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sun J, Guo Y, Zhang Q, et al. Chronic restraint stress disturbs meiotic resumption through APC/C-mediated cyclin B1 excessive degradation in mouse oocytes. Cell Cycle. 2018;17:1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shuhaibar LC, Egbert JR, Norris RP, et al. Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc Natl Acad Sci U S A. 2015;112:5527–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kiyosu C, Tsuji T, Yamada K, et al. NPPC/NPR2 signaling is essential for oocyte meiotic arrest and cumulus oophorus formation during follicular development in the mouse ovary. Reproduction. 2012;144:187–193. [DOI] [PubMed] [Google Scholar]
- [35].Kawamura K, Cheng Y, Kawamura N, et al. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum Reprod. 2011;26:3094–3101. [DOI] [PubMed] [Google Scholar]
- [36].Conti M, Jin SL. The molecular biology of cyclic nucleotide phosphodiesterases. Prog Nucleic Acid Res Mol Biol. 1999;63:1–38. [DOI] [PubMed] [Google Scholar]
- [37].Shitsukawa K, Andersen CB, Richard FJ, et al. Cloning and characterization of the cyclic guanosine monophosphate-inhibited phosphodiesterase PDE3A expressed in mouse oocyte. Biol Reprod. 2001;65:188–196. [DOI] [PubMed] [Google Scholar]
- [38].Mayes MA, Sirard MA. Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol Reprod. 2002;66:180–184. [DOI] [PubMed] [Google Scholar]
- [39].Sasseville M, Cote N, Guillemette C, et al. New insight into the role of phosphodiesterase 3A in porcine oocyte maturation. BMC Dev Biol. 2006;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jaffe LA, Egbert JR. Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian follicle. Annu Rev Physiol. 2017;79:237–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Norris RP, Freudzon M, Nikolaev VO, et al. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Park JY, Su YQ, Ariga M, et al. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. [DOI] [PubMed] [Google Scholar]
- [44].Panigone S, Hsieh M, Fu M, et al. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22:924–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liang CG, Su YQ, Fan HY, et al. Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol. 2007;21:2037–2055. [DOI] [PubMed] [Google Scholar]
- [46].Su YQ, Denegre JM, Wigglesworth K, et al. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol. 2003;263:126–138. [DOI] [PubMed] [Google Scholar]
- [47].Tiwari M, Gupta A, Sharma A, et al. Role of mitogen activated protein kinase and maturation promoting factor during the achievement of meiotic competency in mammalian oocytes. J Cell Biochem. 2018;119:123–129. [DOI] [PubMed] [Google Scholar]
- [48].Egbert JR, Shuhaibar LC, Edmund AB, et al. Dephosphorylation and inactivation of NPR2 guanylyl cyclase in granulosa cells contributes to the LH-induced decrease in cGMP that causes resumption of meiosis in rat oocytes. Development. 2014;141:3594–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vaccari S, Weeks JL 2nd, Hsieh M, et al. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Norris RP, Freudzon M, Mehlmann LM, et al. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Solc P, Saskova A, Baran V, et al. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev Biol. 2008;317:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang Y, Zhang Z, Xu XY, et al. Protein kinase A modulates CDC25B activity during meiotic resumption of mouse oocytes. Dev Dyn. 2008;237:3777–3786. [DOI] [PubMed] [Google Scholar]
- [53].Russell JB. In In vitro oocyte maturation. Totowa: Humana Press; 2001. p. 67–79. [Google Scholar]
- [54].Schmidt A, Rauh NR, Nigg EA, et al. Cytostatic factor: an activity that puts the cell cycle on hold. J Cell Sci. 2006;119:1213–1218. [DOI] [PubMed] [Google Scholar]
- [55].Tunquist BJ, Maller JL. Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev. 2003;17:683–710. [DOI] [PubMed] [Google Scholar]
- [56].Adhikari D, Zheng W, Shen Y, et al. CDK1, but not CDK2, is the sole CDK that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Hum Mol Genet. 2012;21(11):2476–2484. [DOI] [PubMed] [Google Scholar]
- [57].Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. [DOI] [PubMed] [Google Scholar]
- [58].Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several CDKS, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28:2925–2939. [DOI] [PubMed] [Google Scholar]
- [59].Jones KT, Lane SI. Molecular causes of aneuploidy in mammalian eggs. Development. 2013;140:3719–3730. [DOI] [PubMed] [Google Scholar]
- [60].McGuinness BE, Anger M, Kouznetsova A, et al. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr Biol. 2009;19:369–380. [DOI] [PubMed] [Google Scholar]
- [61].Hached K, Xie SZ, Buffin E, et al. Mps1 at kinetochores is essential for female mouse meiosis I. Development. 2011;138:2261–2271. [DOI] [PubMed] [Google Scholar]
- [62].Ricke RM, van Deursen JM. Aneuploidy in health, disease, and aging. J Cell Biol. 2013;201:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Touati SA, Buffin E, Cladiere D, et al. Mouse oocytes depend on BubR1 for proper chromosome segregation but not for prophase I arrest. Nat Commun. 2015;6:6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lagirand-Cantaloube J, Ciabrini C, Charrasse S, et al. Loss of centromere cohesion in aneuploid human oocytes correlates with decreased Kinetochore Localization of the Sac Proteins BUB1 and BUBR1. Sci Rep. 2017;7:44001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yamano H. APC/C: current understanding and future perspectives. F1000Res. 2019;23(8):F1000 Faculty Rev–725. [Google Scholar]
- [66].Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. [DOI] [PubMed] [Google Scholar]
- [67].Yamamuro T, Kano K, Naito K. Functions of FZR1 and CDC20, activators of the anaphase-promoting complex, during meiotic maturation of swine oocytes. Biol Reprod. 2008;79:1202–1209. [DOI] [PubMed] [Google Scholar]
- [68].Reis A, Madgwick S, Chang HY, et al. Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes. Nat Cell Biol. 2007;9:1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shoji S, Yoshida N, Amanai M, et al. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 2006;25:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Borsuk E, Kubiak JZ. In vitro culture of mouse oocytes for meiotic maturation. Methods Mol Biol. 2018;1818:13–21. [DOI] [PubMed] [Google Scholar]
- [71].Taiyeb AM, Muhsen-Alanssari SA, Dees WL, et al. Improvement in in vitro fertilization outcome following in vivo synchronization of oocyte maturation in mice. Exp Biol Med (Maywood). 2015;240:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Masciarelli S, Horner K, Liu C, et al. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ryu KY, Sinnar SA, Reinholdt LG, et al. The mouse polyubiquitin gene Ubb is essential for meiotic progression. Mol Cell Biol. 2008;28:1136–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Huo LJ, Fan HY, Zhong ZS, et al. Ubiquitin-proteasome pathway modulates mouse oocyte meiotic maturation and fertilization via regulation of MAPK cascade and cyclin B1 degradation. Mech Dev. 2004;121:1275–1287. [DOI] [PubMed] [Google Scholar]
- [75].Pomerantz Y, Elbaz J, Ben-Eliezer I, et al. From ubiquitin-proteasomal degradation to CDK1 inactivation: requirements for the first polar body extrusion in mouse oocytes. FASEB J. 2012;26:4495–4505. [DOI] [PubMed] [Google Scholar]
- [76].Su YQ, Sun F, Handel MA, et al. Meiosis arrest female 1 (MARF1) has nuage-like function in mammalian oocytes. Proc Natl Acad Sci U S A. 2012b;109:18653–18660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Su YQ, Sugiura K, Sun F, et al. MARF1 regulates essential oogenic processes in mice. Science. 2012a;335:1496–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li J, Tang JX, Cheng JM, et al. Cyclin B2 can compensate for Cyclin B1 in oocyte meiosis I. J Cell Biol. 2018;217:3901–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Brandeis M, Rosewell I, Carrington M, et al. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc Natl Acad Sci U S A. 1998;95:4344–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen B, Zhang Z, Sun X, et al. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet. 2017;101:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Huang L, Tong X, Wang F, et al. Novel mutations in PATL2 cause female infertility with oocyte germinal vesicle arrest. Hum Reprod. 2018;33:1183–1190. [DOI] [PubMed] [Google Scholar]
- [82].Feng R, Sang Q, Kuang Y, et al. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Xiang J, Wang W, Qian C, et al. Human oocyte maturation arrest caused by a novel missense mutation in TUBB8. J Int Med Res. 2018;46:3759–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhang Z, Li B, Fu J, et al. Bi-allelic Missense Pathogenic Variants in TRIP13 Cause Female Infertility Characterized by Oocyte Maturation Arrest. Am J Hum Genet. 2020c;107:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ma JY, Ou-Yang YC, Luo YB, et al. Cyclin O regulates germinal vesicle breakdown in mouse oocytes. Biol Reprod. 2013;88:110. [DOI] [PubMed] [Google Scholar]
- [86].Denu RA, Sass MM, Johnson JM, et al. Polo-like kinase 4 maintains centriolar satellite integrity by phosphorylation of centrosomal protein 131 (CEP131). J Biol Chem. 2019;294:6531–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Luo YB, Kim NH. PLK4 is essential for meiotic resumption in mouse oocytes. Biol Reprod. 2015;92:101. [DOI] [PubMed] [Google Scholar]
- [88].Luo YB, Zhang L, Lin ZL, et al. Distinct subcellular localization and potential role of LINE1-ORF1P in meiotic oocytes. Histochem Cell Biol. 2016;145:93–104. [DOI] [PubMed] [Google Scholar]
- [89].Zhang X, Zhang R, Yu J. New Understanding of the Relevant Role of LINE-1 Retrotransposition in Human Disease and Immune Modulation. Front Cell Dev Biol. 2020b;8:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet. 2018;35:1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Garcia P, Aspee K, Ramirez G, et al. Influence of growth differentiation factor 9 and bone morphogenetic protein 15 on in vitro maturation of canine oocytes. Reprod Domest Anim. 2019;54:373–380. [DOI] [PubMed] [Google Scholar]
- [92].de Castro FC, Cruz MH, Leal CL. Role of Growth Differentiation Factor 9 and Bone Morphogenetic Protein 15 in Ovarian Function and Their Importance in Mammalian Female Fertility - A Review. Asian-Australas J Anim Sci. 2016;29:1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nogueira D, Ron-El R, Friedler S, et al. Meiotic arrest in vitro by phosphodiesterase 3-inhibitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biol Reprod. 2006;74:177–184. [DOI] [PubMed] [Google Scholar]
- [94].Vanhoutte L, Nogueira D, De Sutter P. Prematuration of human denuded oocytes in a three-dimensional co-culture system: effects on meiosis progression and developmental competence. Hum Reprod. 2009;24:658–669. [DOI] [PubMed] [Google Scholar]
- [95].Jang WI, Lin ZL, Lee SH, et al. A specific inhibitor of CDK1, RO-3306, reversibly arrests meiosis during in vitro maturation of porcine oocytes. Anim Reprod Sci. 2014;144:102–108. [DOI] [PubMed] [Google Scholar]
- [96].Rodriguez KF, Farin CE. Developmental capacity of bovine cumulus oocyte complexes after transcriptional inhibition of germinal vesicle breakdown. Theriogenology. 2004;61:1499–1511. [DOI] [PubMed] [Google Scholar]
- [97].German SD, Lee JH, Campbell KH, et al. Actin depolymerization is associated with meiotic acceleration in cycloheximide-treated ovine oocytes. Biol Reprod. 2015;92:103. [DOI] [PubMed] [Google Scholar]
- [98].Crocomo LF, Marques Filho WC, Ackermann CL, et al. Time course of the meiotic arrest in sheep cumulus-oocyte complexes treated with roscovitine. Zygote. 2016;24:310–318. [DOI] [PubMed] [Google Scholar]
- [99].Golsteyn RM, Schultz SJ, Bartek J, et al. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci. 1994;107(Pt 6):1509–1517. [DOI] [PubMed] [Google Scholar]
- [100].Liao Y, Lin D, Cui P, et al. Polo-like kinase 1 inhibition results in misaligned chromosomes and aberrant spindles in porcine oocytes during the first meiotic division. Reprod Domest Anim. 2018;53:256–265. [DOI] [PubMed] [Google Scholar]
- [101].Yang HJ, Lee S, Sim BW, et al. Transient meiotic arrest maintained by DON (6-diazo-5-oxo-l-norleucine) enhances nuclear/cytoplasmic maturation of porcine oocytes. Reproduction. 2019;158:543–554. [DOI] [PubMed] [Google Scholar]
- [102].Soto-Heras S, Paramio MT, Thompson JG. Effect of pre-maturation with C-type natriuretic peptide and 3-isobutyl-1-methylxanthine on cumulus-oocyte communication and oocyte developmental competence in cattle. Anim Reprod Sci. 2019;202:49–57. [DOI] [PubMed] [Google Scholar]
- [103].Wassmann K, Niault T, Maro B. Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Curr Biol. 2003;13:1596–1608. [DOI] [PubMed] [Google Scholar]
- [104].Brunet S, Maria AS, Guillaud P, et al. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J Cell Biol. 1999;146:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Soewarto D, Schmiady H, Eichenlaub-Ritter U. Consequences of non-extrusion of the first polar body and control of the sequential segregation of homologues and chromatids in mammalian oocytes. Hum Reprod. 1995;10:2350–2360. [DOI] [PubMed] [Google Scholar]
- [106].Wang WH, Abeydeera LR, Prather RS, et al. Polymerization of nonfilamentous actin into microfilaments is an important process for porcine oocyte maturation and early embryo development. Biol Reprod. 2000;62:1177–1183. [DOI] [PubMed] [Google Scholar]
- [107].Sun QY, Lai L, Park KW, et al. Dynamic events are differently mediated by microfilaments, microtubules, and mitogen-activated protein kinase during porcine oocyte maturation and fertilization in vitro. Biol Reprod. 2001;64:879–889. [DOI] [PubMed] [Google Scholar]
- [108].Downs SM. Mouse versus rat: profound differences in meiotic regulation at the level of the isolated oocyte. Mol Reprod Dev. 2011;78:778–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Crocomo LF, Marques Filho WC, Ulian CM, et al. Effect of oil overlay on inhibition potential of roscovitine in sheep cumulus-oocyte complexes. Reprod Domest Anim. 2015;50:410–416. [DOI] [PubMed] [Google Scholar]
- [110].Wang X, Tsai T, Qiao J, et al. Impact of gonadotropins on oocyte maturation, fertilisation and developmental competence in vitro. Reprod Fertil Dev. 2014;26:752–757. [DOI] [PubMed] [Google Scholar]
- [111].Anckaert E, De Rycke M, Smitz J. Culture of oocytes and risk of imprinting defects. Hum Reprod Update. 2013;19:52–66. [DOI] [PubMed] [Google Scholar]
- [112].Uysal F, Akkoyunlu G, Ozturk S. Dynamic expression of DNA methyltransferases (DNMTs) in oocytes and early embryos. Biochimie. 2015;116:103–113. [DOI] [PubMed] [Google Scholar]
- [113].Bilmez Y, Talibova G, Ozturk S,et al. Dynamic changes of histone methylation in mammalian oocytes and early embryos .Histochem Cell Biol.2021; Online ahead of print . [DOI] [PubMed] [Google Scholar]
- [114].Gu L, Wang Q, Sun QY. Histone modifications during mammalian oocyte maturation: dynamics, regulation and functions. Cell Cycle. 2010;9:1942–1950. [DOI] [PubMed] [Google Scholar]
- [115].Kren R, Ogushi S, Miyano T. Effect of caffeine on meiotic maturation of porcine oocytes. Zygote. 2004;12:31–38. [DOI] [PubMed] [Google Scholar]
- [116].Tosca L, Uzbekova S, Chabrolle C, et al. Possible role of 5ʹAMP-activated protein kinase in the metformin-mediated arrest of bovine oocytes at the germinal vesicle stage during in vitro maturation. Biol Reprod. 2007;77:452–465. [DOI] [PubMed] [Google Scholar]
- [117].Sui L, Nie J, Xiao P, et al. Maternal benzo[a]pyrene exposure is correlated with the meiotic arrest and quality deterioration of offspring oocytes in mice. Reprod Toxicol. 2019;93:10–18. [DOI] [PubMed] [Google Scholar]
- [118].Zhang M, Miao Y, Chen Q, et al. BaP exposure causes oocyte meiotic arrest and fertilization failure to weaken female fertility. FASEB J. 2018;32:342–352. [DOI] [PubMed] [Google Scholar]
- [119].Nie ZW, Niu YJ, Zhou W, et al. Thiamethoxam induces meiotic arrest and reduces the quality of oocytes in cattle. Toxicol In Vitro. 2019;61:104635. [DOI] [PubMed] [Google Scholar]
- [120].Lee JH, Yoon SY, Bae IH. Studies on Ca2+-channel distribution in maturation arrested mouse oocyte. Mol Reprod Dev. 2004;69:174–185. [DOI] [PubMed] [Google Scholar]
- [121].Coticchio G, Dal Canto M, Guglielmo MC, et al. Double-strand DNA breaks and repair response in human immature oocytes and their relevance to meiotic resumption. J Assist Reprod Genet. 2015;32:1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Hemmings KE, Maruthini D, Vyjayanthi S, et al. Amino acid turnover by human oocytes is influenced by gamete developmental competence, patient characteristics and gonadotrophin treatment. Hum Reprod. 2013;28:1031–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Eppig JJ, Wigglesworth K, Hirao Y. Metaphase I arrest and spontaneous parthenogenetic activation of strain LTXBO oocytes: chimeric reaggregated ovaries establish primary lesion in oocytes. Dev Biol. 2000;224:60–68. [DOI] [PubMed] [Google Scholar]
- [124].Beall S, Brenner C, Segars J. Oocyte maturation failure: a syndrome of bad eggs. Fertil Steril. 2010;94:2507–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Woods LM, Hodges CA, Baart E, et al. Chromosomal influence on meiotic spindle assembly: abnormal meiosis I in female Mlh1 mutant mice. J Cell Biol. 1999;145:1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Lipkin SM, Moens PB, Wang V, et al. Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat Genet. 2002;31:385–390. [DOI] [PubMed] [Google Scholar]
- [127].Manhart CM, Alani E. Roles for mismatch repair family proteins in promoting meiotic crossing over. DNA Repair (Amst). 2016;38:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Rothkamm K, Kruger I, Thompson LH, et al. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23:5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ellederova Z, Del Rincon S, Koncicka M, et al. CKS1 Germ Line Exclusion Is Essential for the Transition from Meiosis to Early Embryonic Development. Mol Cell Biol. 2019;39(13):e00590–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Spruck CH, de Miguel MP, Smith AP, et al. Requirement of Cks2 for the first metaphase/anaphase transition of mammalian meiosis. Science. 2003;300:647–650. [DOI] [PubMed] [Google Scholar]
- [131].Hahn KL, Johnson J, Beres BJ, et al. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development. 2005;132:817–828. [DOI] [PubMed] [Google Scholar]
- [132].Zhang CP, Yang JL, Zhang J, et al. Notch signaling is involved in ovarian follicle development by regulating granulosa cell proliferation. Endocrinology. 2011;152:2437–2447. [DOI] [PubMed] [Google Scholar]
- [133].Libby BJ, De La Fuente R, O’Brien MJ, et al. The mouse meiotic mutation mei1 disrupts chromosome synapsis with sexually dimorphic consequences for meiotic progression. Dev Biol. 2002;242:174–187. [DOI] [PubMed] [Google Scholar]
- [134].Leader B, Lim H, Carabatsos MJ, et al. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol. 2002;4:921–928. [DOI] [PubMed] [Google Scholar]
- [135].Namgoong S, Kim NH. Roles of actin binding proteins in mammalian oocyte maturation and beyond. Cell Cycle. 2016;15:1830–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zhang J, Zhang YL, Zhao LW, et al. The CRL4-DCAF13 ubiquitin E3 ligase supports oocyte meiotic resumption by targeting PTEN degradation. Cell Mol Life Sci. 2020a;77:2181–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Liu Z, Zhu L, Wang J, et al. Novel homozygous mutations in PATL2 lead to female infertility with oocyte maturation arrest. J Assist Reprod Genet. 2020;37:841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Li S, Ou XH, Wang ZB, et al. ERK3 is required for metaphase-anaphase transition in mouse oocyte meiosis. PLoS One. 2010;5(9): e13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lee HS, Kim EY, Kim KH, et al. Obox4 critically regulates cAMP-dependent meiotic arrest and MI-MII transition in oocytes. FASEB J. 2010;24:2314–2324. [DOI] [PubMed] [Google Scholar]
- [140].Lee Y, Kim KH, Yoon H, et al. RASD1 Knockdown Results in Failure of Oocyte Maturation. Cell Physiol Biochem. 2016;40:1289–1302. [DOI] [PubMed] [Google Scholar]
- [141].Yoon H, Jang H, Kim EY, et al. Knockdown of PRKAR2B Results in the Failure of Oocyte Maturation. Cell Physiol Biochem. 2018;45:2009–2020. [DOI] [PubMed] [Google Scholar]
- [142].Jin Y, Yang M, Gao C, et al. Fbxo30 regulates chromosome segregation of oocyte meiosis. Cell Mol Life Sci. 2019;76:2217–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhang QH, Wei L, Tong JS, et al. Localization and function of mSpindly during mouse oocyte meiotic maturation. Cell Cycle. 2010;9:2230–2236. [DOI] [PubMed] [Google Scholar]
- [144].Wei L, Liang XW, Zhang QH, et al. BubR1 is a spindle assembly checkpoint protein regulating meiotic cell cycle progression of mouse oocyte. Cell Cycle. 2010;9:1112–1121. [DOI] [PubMed] [Google Scholar]
- [145].Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol. 2013;14:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Xu ZY, Ma XS, Qi ST, et al. Cep55 regulates spindle organization and cell cycle progression in meiotic oocyte. Sci Rep. 2015;5:16978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Zhu XL, Qi ST, Liu J, et al. Synaptotagmin 1 is required for spindle stability and metaphase-to-anaphase transition in mouse oocytes. Cell Cycle. 2012;11:818–826. [DOI] [PubMed] [Google Scholar]
- [148].Courtney NA, Bao H, Briguglio JS, et al. Synaptotagmin 1 clamps synaptic vesicle fusion in mammalian neurons independent of complexin. Nat Commun. 2019;10:4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Liu W, Yin J, Zhao G, et al. Differential regulation of cyclin B1 degradation between the first and second meiotic divisions of bovine oocytes. Theriogenology. 2012;78:1171–1181. e1171. [DOI] [PubMed] [Google Scholar]
- [150].Ledan E, Polanski Z, Terret ME, et al. Meiotic maturation of the mouse oocyte requires an equilibrium between cyclin B synthesis and degradation. Dev Biol. 2001;232:400–413. [DOI] [PubMed] [Google Scholar]
- [151].Yi ZY, Liang QX, Meng TG, et al. PKCbeta1 regulates meiotic cell cycle in mouse oocyte. Cell Cycle. 2019;18:395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhang T, Qi ST, Huang L, et al. Cyclin B3 controls anaphase onset independent of spindle assembly checkpoint in meiotic oocytes. Cell Cycle. 2015a;14:2648–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Karasu ME, Keeney S. Cyclin B3 is dispensable for mouse spermatogenesis. Chromosoma. 2019;128:473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Li Y, Wang L, Zhang L, et al. Cyclin B3 is required for metaphase to anaphase transition in oocyte meiosis I. J Cell Biol. 2019b;218:1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Smits VAJ, Cabrera E, Freire R, et al. Claspin - checkpoint adaptor and DNA replication factor. FEBS J. 2019;286:441–455. [DOI] [PubMed] [Google Scholar]
- [156].Nie ZW, Chen L, Jin QS, et al. Function and regulation mechanism of CHK1 during meiotic maturation in porcine oocytes. Cell Cycle. 2017;16:2220–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Chen L, Chao SB, Wang ZB, et al. Checkpoint kinase 1 is essential for meiotic cell cycle regulation in mouse oocytes. Cell Cycle. 2012;11:1948–1955. [DOI] [PubMed] [Google Scholar]
- [158].McDougall A, Levasseur M. Sperm-triggered calcium oscillations during meiosis in ascidian oocytes first pause, restart, then stop: correlations with cell cycle kinase activity. Development. 1998;125:4451–4459. [DOI] [PubMed] [Google Scholar]
- [159].Wheatley SP, Altieri DC. Survivin at a glance. J Cell Sci. 2019;132(7): jcs223826 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Chen L, Yin T, Nie ZW, et al. Survivin regulates chromosome segregation by modulating the phosphorylation of Aurora B during porcine oocyte meiosis. Cell Cycle. 2018;17:2436–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Sun SC, Wei L, Li M, et al. Perturbation of survivin expression affects chromosome alignment and spindle checkpoint in mouse oocyte meiotic maturation. Cell Cycle. 2009;8:3365–3372. [DOI] [PubMed] [Google Scholar]
- [162].Nebreda AR, Ferby I. Regulation of the meiotic cell cycle in oocytes. Curr Opin Cell Biol. 2000;12:666–675. [DOI] [PubMed] [Google Scholar]
- [163].Cao Y, Du J, Chen D, et al. RNA- binding protein STAU2 is important for spindle integrity and meiosis progression in mouse oocytes. Cell Cycle. 2016;15:2608–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Choi JW, Zhou W, Nie ZW, et al. Spindlin1 alters the metaphase to anaphase transition in meiosis I through regulation of BUB3 expression in porcine oocytes. J Cell Physiol. 2019;234:8963–8974. [DOI] [PubMed] [Google Scholar]
- [165].Hayakawa K, Ohgane J, Tanaka S, et al. Oocyte-specific linker histone H1FOO is an epigenomic modulator that decondenses chromatin and impairs pluripotency. Epigenetics. 2012;7:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Furuya M, Tanaka M, Teranishi T, et al. H1FOO is indispensable for meiotic maturation of the mouse oocyte. J Reprod Dev. 2007;53:895–902. [DOI] [PubMed] [Google Scholar]
- [167].An Q, Sun H, Zhang J, et al. Methionine adenosyltransferase 2beta participates in mouse oocyte maturation by regulating the MAPK pathway. Reprod Sci. 2020;27:163–171. [DOI] [PubMed] [Google Scholar]
- [168].Ahn J, Urist M, Prives C. The Chk2 protein kinase. DNA Repair (Amst). 2004;3:1039–1047. [DOI] [PubMed] [Google Scholar]
- [169].Dai XX, Duan X, Liu HL, et al. Chk2 regulates cell cycle progression during mouse oocyte maturation and early embryo development. Mol Cells. 2014;37:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Liu X, Liu X, Chen D, et al. PLD2 regulates microtubule stability and spindle migration in mouse oocytes during meiotic division. PeerJ. 2017;5:e3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].McDermott M, Wakelam MJ, Morris AJ. Phospholipase D. Biochem Cell Biol. 2004;82:225–253. [DOI] [PubMed] [Google Scholar]
- [172].Villa-Diaz LG, Miyano T. Activation of p38 MAPK during porcine oocyte maturation. Biol Reprod. 2004;71:691–696. [DOI] [PubMed] [Google Scholar]
- [173].Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. [DOI] [PubMed] [Google Scholar]
- [174].Qu Y, Lu D, Jiang H, et al. EZH2 is required for mouse oocyte meiotic maturation by interacting with and stabilizing spindle assembly checkpoint protein BubRI. Nucleic Acids Res. 2016;44:7659–7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Vandenberghe LTM, Heindryckx B, Smits K, et al. Platelet-activating factor acetylhydrolase 1B3 (PAFAH1B3) is required for the formation of the meiotic spindle during in vitro oocyte maturation. Reprod Fertil Dev. 2018;30:1739–1750. [DOI] [PubMed] [Google Scholar]
- [176].Mishra V, Chirumamilla L. Unexpected repeat immature oocyte response after IVF stimulation: a case report. Gynecol Endocrinol. 2018;34:100–102. [DOI] [PubMed] [Google Scholar]
- [177].Michaan N, Ben-David G, Ben-Yosef D, et al. Ovarian stimulation and emergency in vitro fertilization for fertility preservation in cancer patients. Eur J Obstet Gynecol Reprod Biol. 2010;149:175–177. [DOI] [PubMed] [Google Scholar]
- [178].Machtinger R, Combelles CM, Missmer SA, et al. Bisphenol-A and human oocyte maturation in vitro. Hum Reprod. 2013;28:2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Nakano K, Nishio M, Kobayashi N, et al. Comparison of the effects of BPA and BPAF on oocyte spindle assembly and polar body release in mice. Zygote. 2016;24:172–180. [DOI] [PubMed] [Google Scholar]
- [180].Ding ZM, Zhang SX, Jiao XF,et al. Doxorubicin exposure affects oocyte meiotic maturation through DNA damage induced meiotic arrest . 2019;171(2): 359–368. [DOI] [PubMed] [Google Scholar]
- [181].Ding ZM, Hua LP, Ahmad MJ, et al. Diethylstilbestrol exposure disrupts mouse oocyte meiotic maturation in vitro through affecting spindle assembly and chromosome alignment. Chemosphere. 2020;249:126182. [DOI] [PubMed] [Google Scholar]
- [182].Marangos P, Stevense M, Niaka K, et al. DNA damage-induced metaphase I arrest is mediated by the spindle assembly checkpoint and maternal age. Nat Commun. 2015;6:8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Wang H, Luo Y, Zhao MH, et al. DNA double-strand breaks disrupted the spindle assembly in porcine oocytes. Mol Reprod Dev. 2016;83:132–143. [DOI] [PubMed] [Google Scholar]
- [184].Lane SIR, Morgan SL, Wu T, et al. DNA damage induces a kinetochore-based ATM/ATR-independent SAC arrest unique to the first meiotic division in mouse oocytes. Development. 2017;144:3475–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Remillard-Labrosse G, Dean NL, Allais A, et al. Human oocytes harboring damaged DNA can complete meiosis I. Fertil Steril. 2020;113:1080–1089. e1082 [DOI] [PubMed] [Google Scholar]
- [186].Kim AM, Vogt S, O’Halloran TV, et al. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Jeon Y, Yoon JD, Cai L, et al. Zinc deficiency during in vitro maturation of porcine oocytes causes meiotic block and developmental failure. Mol Med Rep. 2015;12:5973–5982. [DOI] [PubMed] [Google Scholar]
- [188].Hampl A, Eppig JJ. Analysis of the mechanism(s) of metaphase I arrest in maturing mouse oocytes. Development. 1995;121:925–933. [DOI] [PubMed] [Google Scholar]
- [189].Alvarez C, Garcia-Garrido C, Taronger R, et al. In vitro maturation, fertilization, embryo development & clinical outcome of human metaphase-I oocytes retrieved from stimulated intracytoplasmic sperm injection cycles. Indian J Med Res. 2013;137:331–338. [PMC free article] [PubMed] [Google Scholar]
- [190].Balczon R, Simerly C, Takahashi D, et al. Arrest of cell cycle progression during first interphase in murine zygotes microinjected with anti-PCM-1 antibodies. Cell Motil Cytoskeleton. 2002;52:183–192. [DOI] [PubMed] [Google Scholar]
- [191].Wu X, Viveiros MM, Eppig JJ, et al. Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat Genet. 2003;33:187–191. [DOI] [PubMed] [Google Scholar]
- [192].Madgwick S, Hansen DV, Levasseur M, et al. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol. 2006;174:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Fujioka YA, Onuma A, Fujii W, et al. Analyses of EMI functions on meiotic maturation of porcine oocytes. Mol Reprod Dev. 2016;83:983–992. [DOI] [PubMed] [Google Scholar]
- [194].Schmidt A, Duncan PI, Rauh NR, et al. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 2005;19:502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Miyagaki Y, Kanemori Y, Baba T. Possible involvement of mitogen- and stress-activated protein kinase 1, MSK1, in metaphase-II arrest through phosphorylation of EMI2 in mouse oocytes. Dev Biol. 2011;359:73–81. [DOI] [PubMed] [Google Scholar]
- [196].Yang CR, Wei Y, Qi ST, et al. The G protein coupled receptor 3 is involved in cAMP and cGMP signaling and maintenance of meiotic arrest in porcine oocytes. PLoS One. 2012;7:e38807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Kang H, Hwang SC, Park YS, et al. Cdc25B phosphatase participates in maintaining metaphase II arrest in mouse oocytes. Mol Cells. 2013;35:514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Laurance S, Lemarie CA, Blostein MD. Growth arrest-specific gene 6 (gas6) and vascular hemostasis. Adv Nutr. 2012;3:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Kim KH, Kim EY, Kim Y, et al. Gas6 downregulation impaired cytoplasmic maturation and pronuclear formation independent to the MPF activity. PLoS One. 2011;6:e23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Choi JW, Zhao MH, Liang S, et al. Spindlin 1 is essential for metaphase II stage maintenance and chromosomal stability in porcine oocytes. Mol Hum Reprod. 2017;23:166–176. [DOI] [PubMed] [Google Scholar]
- [201].Fujioka YA, Onuma A, Fujii W, et al. Contributions of UBE2C and UBE2S to meiotic progression of porcine oocytes. J Reprod Dev. 2018;64:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Ben-Eliezer I, Pomerantz Y, Galiani D, et al. Appropriate expression of UBE2C and UBE2S controls the progression of the first meiotic division. FASEB J. 2015;29:4670–4681. [DOI] [PubMed] [Google Scholar]
- [203].Zhang YL, Liu XM, Ji SY, et al. ERK1/2 activities are dispensable for oocyte growth but are required for meiotic maturation and pronuclear formation in mouse. J Genet Genomics. 2015b;42:477–485. [DOI] [PubMed] [Google Scholar]
- [204].Fesahat F, Faramarzi A, Khoradmehr A, et al. Vitrification of mouse MII oocytes: developmental competency using paclitaxel. Taiwan J Obstet Gynecol. 2016;55:796–800. [DOI] [PubMed] [Google Scholar]
- [205].Fulka J Jr., Mrazek M, Fulka H, et al. Mammalian oocyte therapies. Cloning Stem Cells. 2005;7:183–188. [DOI] [PubMed] [Google Scholar]
- [206].Zhang J, Liu H. Cytoplasm replacement following germinal vesicle transfer restores meiotic maturation and spindle assembly in meiotically arrested oocytes. Reprod Biomed Online. 2015;31:71–78. [DOI] [PubMed] [Google Scholar]
- [207].Chen J, Hudson E, Chi MM, et al. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev Biol. 2006;291:227–238. [DOI] [PubMed] [Google Scholar]
- [208].Onuma A, Fujioka YA, Fujii W, et al. Effects of exportin 1 on nuclear transport and meiotic resumption in porcine full-grown and growing oocytes. Biol Reprod. 2018;98:501–509. [DOI] [PubMed] [Google Scholar]
- [209].Han SJ, Chen R, Paronetto MP, et al. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol. 2005;15:1670–1676. [DOI] [PubMed] [Google Scholar]
- [210].Downs SM, Humpherson PG, Leese HJ. Meiotic induction in cumulus cell-enclosed mouse oocytes: involvement of the pentose phosphate pathway. Biol Reprod. 1998;58:1084–1094. [DOI] [PubMed] [Google Scholar]
- [211].Downs SM, Cottom J, Hunzicker-Dunn M. Protein kinase C and meiotic regulation in isolated mouse oocytes. Mol Reprod Dev. 2001a;58:101–115. [DOI] [PubMed] [Google Scholar]
- [212].Downs S M, Ruan B and Schroepfer G J. Meiosis-Activating Sterol and the Maturation of Isolated Mouse Oocytes1. Biology of Reproduction, 2001;64(1):80–89. doi: 10.1095/biolreprod64.1.80 [DOI] [PubMed] [Google Scholar]
- [213].Downs SM, Ruan B, Schroepfer GJ Jr. Meiosis-activating sterol and the maturation of isolated mouse oocytes. Biol Reprod. 2001b;64:80–89. [DOI] [PubMed] [Google Scholar]
- [214].Downs SM, Hudson ER, Hardie DG. A potential role for AMP-activated protein kinase in meiotic induction in mouse oocytes. Dev Biol. 2002;245:200–212. [DOI] [PubMed] [Google Scholar]
- [215].Downs SM, Chen J. Induction of meiotic maturation in mouse oocytes by adenosine analogs. Mol Reprod Dev. 2006;73:1159–1168. [DOI] [PubMed] [Google Scholar]
- [216].Heindryckx B, Lierman S, Combelles CM, et al. Aberrant spindle structures responsible for recurrent human metaphase I oocyte arrest with attempts to induce meiosis artificially. Hum Reprod. 2011;26:791–800. [DOI] [PubMed] [Google Scholar]
- [217].Yoon J, Juhn KM, Yoon SH, et al. Effects of sperm insemination on the final meiotic maturation of mouse oocytes arrested at metaphase I after in vitro maturation. Clin Exp Reprod Med. 2017;44:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Chen J, Chi MM, Moley KH, et al. cAMP pulsing of denuded mouse oocytes increases meiotic resumption via activation of AMP-activated protein kinase. Reproduction. 2009;138:759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Kong BY, Bernhardt ML, Kim AM, et al. Zinc maintains prophase I arrest in mouse oocytes through regulation of the MOS-MAPK pathway. Biol Reprod. 2012;87(11):11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Valsangkar DS, Downs SM. Acetyl CoA carboxylase inactivation and meiotic maturation in mouse oocytes. Mol Reprod Dev. 2015;82:679–693. [DOI] [PubMed] [Google Scholar]
- [221].LaRosa C, Downs SM. Meiotic induction by heat stress in mouse oocytes: involvement of AMP-activated protein kinase and MAPK family members. Biol Reprod. 2007;76:476–486. [DOI] [PubMed] [Google Scholar]
- [222].Puscheck EE, Bolnick A, Awonuga A, et al. Why AMPK agonists not known to be stressors may surprisingly contribute to miscarriage or hinder IVF/ART. J Assist Reprod Genet. 2018;35:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]


