Abstract
Trypanothione synthetase (TryS) catalyses the synthesis of N1,N8-bis(glutathionyl)spermidine (trypanothione), which is the main low molecular mass thiol supporting several redox functions in trypanosomatids. TryS attracts attention as molecular target for drug development against pathogens causing severe and fatal diseases in mammals. A drug discovery campaign aimed to identify and characterise new inhibitors of TryS with promising biological activity was conducted. A large compound library (n = 51,624), most of them bearing drug-like properties, was primarily screened against TryS from Trypanosoma brucei (TbTryS). With a true-hit rate of 0.056%, several of the TbTryS hits (IC50 from 1.2 to 36 µM) also targeted the homologue enzyme from Leishmania infantum and Trypanosoma cruzi (IC50 values from 2.6 to 40 µM). Calmidazolium chloride and Ebselen stand out for their multi-species anti-TryS activity at low µM concentrations (IC50 from 2.6 to 13.8 µM). The moieties carboxy piperidine amide and amide methyl thiazole phenyl were identified as novel TbTryS inhibitor scaffolds. Several of the TryS hits presented one-digit µM EC50 against T. cruzi and L. donovani amastigotes but proved cytotoxic against the human osteosarcoma and macrophage host cells (selectivity index ≤ 3). In contrast, seven hits showed a significantly higher selectivity against T. b. brucei (selectivity index from 11 to 182). Non-invasive redox assays confirmed that Ebselen, a multi-TryS inhibitor, induces an intracellular oxidative milieu in bloodstream T. b. brucei. Kinetic and mass spectrometry analysis revealed that Ebselen is a slow-binding inhibitor that modifies irreversible a highly conserved cysteine residue from the TryS’s synthetase domain. The most potent TbTryS inhibitor (a singleton containing an adamantine moiety) exerted a non-covalent, non-competitive (with any of the substrates) inhibition of the enzyme. These data feed the drug discovery pipeline for trypanosomatids with novel and valuable information on chemical entities with drug potential.
Keywords: Trypanothione synthetase, Leishmania, Trypanosoma, covalent inhibitor, non-competitive inhibition
Introduction
Diseases caused by trypanosomatids (e.g. Leishmaniasis, Chagas disease and African sleeping sickness) represent a major health problem in endemic regions and an emerging threat in industrialised countries due to global migration. To date, the therapeutic arsenal to combat human Trypanosomiasis and Leishmaniasis is rather limited in quantity and quality (low efficacy, safety, high cost and difficult administration) as it is also the number of backup drugs that await (pre)clinical assessment1.
Devoid of catalase and canonical thioredoxin- and glutaredoxin-redox systems, the thiol-redox homeostasis of trypanosomatids depends exclusively on a molecule absent in mammals: the polyamine dithiol N1,N8-bis(glutathionyl)spermidine or trypanothione2 (T(SH)2, Figure 1). Trypanothione biosynthesis is carried out by a bifunctional enzyme, namely trypanothione synthetase (TryS), harbouring an N-terminal amidase domain homologous to the catalytic domain of papain-like cysteine peptidases3 that is fused to an ATP-dependent carbon-nitrogen ligase domain homologous to the ATP-grasp superfamily of enzymes4. A paralog enzyme, namely monoglutathionylspermidine amidase/synthetase (GspS), with capacity to synthesise the conjugation of only one molecule of glutathione (GSH) to the polyamine moiety, is expressed by some trypanosomatids5. TryS is indispensable for parasite survival since GSH cannot take over T(SH)2 functions5–9, in part, because many enzymes with thiol-active groups evolved specificity for using bis-glutathionyl spermidine as redox substrate10. In line with experimental evidences from dsRNAi5,11 and/or chemical inhibition approaches8,9,11–13 that lead to partial suppression of TryS activity, this enzyme has been shown to be one of the bottle-necks for the metabolic output of the trypanothione pathway14. Thus, at variance with other components of this pathway, even partial inhibition of TryS impairs parasite survival in vivo11 and increase pathogen’s susceptibility to most clinical drugs7,9.
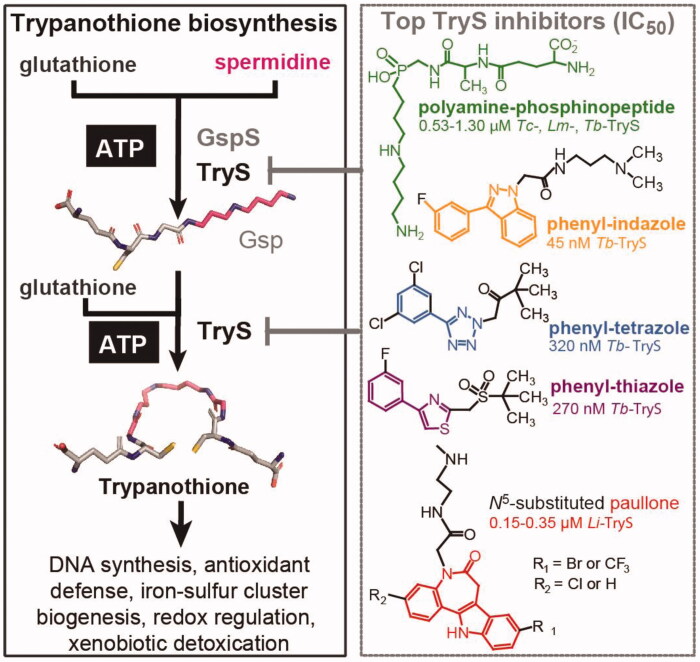
Figure 1.
Trypanothione biosynthesis/functions and inhibitors. Trypanothione synthetase (TryS) catalyses the stepwise addition of one or two glutathione molecules to spermidine or monoglutathionylspermidine (Gsp) to form trypanothione. In contrast, monoglutathionylspermidine synthetase (GspS) is able to add a single glutathione molecule to spermidine to form monoglutathionylspermidine (Gsp). The biosynthetic reactions are ATP-dependent. The structure and IC50 value of the most potent TryS inhibitors (Tc: T. cruzi, Lm: L. major, Tb: T. brucei and Li: L. infantum) emerging from a large (phenyl-indazole, -tetrazole and -thiazole) or drug-focused (polyamine-phophinopeptide and paullone) screenings are shown.
The search and development of TryS inhibitors was initially biased towards substrate-like and mechanism-based molecules15–22. Despite their reasonable anti-TryS activity in vitro (e.g. nanomolar IC50), the majority of the compounds failed to exert anti-proliferative activity against pathogenic trypanosomatids, probably due to their peptidic nature that make them prone to hydrolysis by esterases and amidases23.
Up to date, there is a single report for a large HTS against TryS12. The study involved a library of nearly 62,000 compounds and TbTryS as molecular target. Pharmacophore structures (Figure 1) with nM potency against the enzyme (IC50 95–317 nM) and 36- to 99-fold higher EC50 against bloodstream T. brucei (EC50 5–10 μM) were identified and the on-target effect was confirmed for the compound with highest anti-T. brucei activity (phenyl-indazole, Figure 1).
Although some physicochemical properties (low MW, Log P < 5 and low polar surface area) of the candidate scaffolds were improved by a hit-to-lead optimisation campaign, none of the new molecules superseded the anti-T. brucei or anti-TryS activity of the original ones12,24. Furthermore, none of these compounds were tested for their potential broad range activity against other TryS and trypanosomatid species neither for their pharmacological performance in animal infection models.
With the aim to identify multi-TryS inhibitors, we recently reported the establishment of a 96-well plate HTS assay against TryS from the three major trypanosomatid species (Trypanosoma cruzi: Tc, Leishmania infantum: Li, and Trypanosoma brucei: Tb)25. From a small but chemically diverse compound library (n = 144), several hits with TryS species-specific activity were identified. The most potent compounds targeted LiTryS and belonged to the scaffold N5-acetamide substituted, 3-chlorokenpaullone (Figure 1). A series of analogues containing different substituents at position N5 of the 3-chlorokenpaullone were synthetised and yielded TryS hits that gained activity against the T. cruzi enzyme and infective stage of the parasite9,26 or showed improved selectivity for the intracellular form of L. infantum13. In both cases, the new analogues retained their on-target effect against the pathogen as confirmed by genetic9 or metabolic approaches13. Kinetic, thermodynamic and computational approaches suggested that paullones occupy the polyamine and second glutathione binding site of TryS13. Yet, the identification of multi-spectrum TryS inhibitors with reasonable biological activity and selectivity proved unsuccessful.
One of the major factors contributing to failure during the early phase of the drug discovery process is ascribed to the poor drug-like properties of the hits or to the impossibility to attain it during optimisation. This is particularly challenging for diseases caused by trypanosomatids since each species show different tropism and clinical manifestations (i.e. haemolymphatic, meningoencephalic, muco-cutaneous, visceral), which may demand disease-specific drugs/formulations to achieve a proper biodistribution.
In order to pave the way towards the identification of new molecules inhibiting TryS and/or the proliferation of the infective stages of biomedically relevant trypanosomatids, we performed a target-based HTS of a large and diverse chemolibrary.
TbTryS was selected as molecular target because it has the lowest KM values for substrates25 and, therefore, represents a challenging molecular target for finding potent and selective inhibitors with wide-spectrum activity. The screening assay was adapted to favour the detection of non-competitive and slow-binding inhibitors, in addition to potent competitive ones. The TbTryS hits were further tested for their capacity to inhibit TryS from T. cruzi and L. infantum, and those displaying satisfactory inhibition against the molecular target were screened for their anti-trypanosomatid and host-cell cytotoxicity. The inhibition mechanism for non-competitive TryS inhibitors was elucidated. In conclusion, this study disclosed a set of novel compounds with potential to be optimised as TryS or anti-trypanosomal inhibitors.
Materials and methods
Reagents
Unless otherwise stated all chemical reagents were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO). The libraries or single compounds employed in the pilot and large HTS, or subsequent studies, respectively, were purchased to LOPAC® (SIGMA), Selleck Chemicals, NIH clinical, TOCRIS and ENAMINE. Human acute monocytic leukaemia cell line THP-1 (TIB-20TM), human osteosarcoma cell line U-2 OS (HTB-96™) and rhesus monkey kidney epithelial cell LLC-MK2 (CCL-7™) were purchased from the ATCC®.
Heterologous expression and purification of recombinant TryS
Expression and purification of His-tagged TcTryS, LiTryS and TbTryS were performed according to protocols previously reported25 except that the size exclusion chromatography (SEC) was performed with a HiLoad®16/60 Superdex®200 pg column (GE Healthcare, Chicago, IL). This column was equilibrated in running buffer (10 mM MgSO4, 0.5 mM EDTA, 100 mM HEPES pH 7.4, containing DTT 1 mM and 150 mM NaCl) and injected with 2 ml of a TryS sample (≤60 mg/mL protein) from the peak of the preceding chromatographic step. SEC was performed at a flow rate of 0.75 ml/min, at 4 °C using an Äkta-FPLC device (GE Healthcare). About 6 mg and 30 mg TryS per litre of culture medium were obtained for TcTryS and LiTryS or TbTryS, respectively. The purity and specific activity of the recombinant TryS, assessed by SDS-PAGE and enzymatic assays, resembled those reported earlier25. The recombinant enzymes (6–10 mg/mL TryS in running buffer containing 40% v/v glycerol) were stored at −20 °C until use.
High-throughput TryS assay
General considerations
The TryS assay consists in quantifying the inorganic phosphate (Pi), released from ATP during catalysis, which is detected upon complexation to the colorimetric reagent BIOMOL GREENTM (Enzo Life Sciences, Farmingdale, NY). The original screening assay (96-well plate and reaction volume = 50 µL)25 was miniaturised to a 384-well plate and a total reaction volume of 16 µL. The assay conditions were adjusted to yield optimal signal (absorbance ≤ 0.6) and to increase the chances to detect slow-binding as well as potent competitive inhibitors. Hence, substrates were added at near-physiological concentrations (≥ KM values) that do not interfere with the colorimetric reaction [ATP 150 µM, spermidine (SP) 2 mM and glutathione (GSH) 150 µM] and compounds were pre-incubated for 1 h with TryS before starting the reaction. Under these conditions, the limit of detection (LOD; calculated as the Pi concentration corresponding to three SD of the blank mean A620 nm) and the sensitivity (slope for A620 nm versus Pi concentration) of the screening assay was ∼0.9 µM Pi and ∼0.008 (R2 ∼0.98), respectively (Figure S1(A)). The reaction was stopped by chelating Mg++ with EDTA (end concentration 50 mM). The concentration-dependent effect of EDTA in assay sensitivity and signal stability is shown in Figure S1(B,C).
The compounds were tested at a final concentration of 25 µM and, whenever possible, from freshly prepared solutions. All procedures were performed at room temperature (RT, 23–26 °C) and false positive hits were discriminated in subsequent tests (Dose response curves, DRC and interference assays). Assay automation was achieved using a Personal Pipettor liquid handler (Apricot Designs, Covinba, CA), a Matrix Wellmate Microplate Dispenser (Thermo Fisher Scientific, Waltham, MA), a Multidrop™ Combi Reagent Dispenser (Thermo Fisher Scientific), a robotic arm Plate Handler II and an Envision multilabel reader (both from Perkin Elmer, Waltham, MA). Full details of the standard operating procedure (SOP) used for the HTS assay are provided as Supplemental materials.
TryS inhibition is expressed as follows: % Inhibition = {[(A620 nm C- – A620 nm C+) – (A620 nm Cx – A620 nm C+)/(A620 nm C- – A620 nm C+)] × 100}, where A620 nm Cx is the mean absorbance at 620 nm for the reaction test with compound x at 25 µM, A620 nm C + is the mean absorbance at 620 nm for the “inhibition control” with DMSO and reaction buffer, A620 nm C- is the mean absorbance at 620 nm for the “activity control” with DMSO and enzyme. Error values, which never exceeded 10%, are expressed as percentage calculated as follows: (σn−1/C-) × 100%., where σn−1 refers to one standard deviation (SD).
Hits’ validation
All TbTryS hits were repurchased and retested at 25 µM against the T. brucei enzyme, as well as again TcTryS and LiTryS. Enzyme concentration was adjusted for each TryS (TbTryS = 6.1 × 10−6 µmol/min mL, TcTryS = 2.1 × 10−6 µmol/min mL and LiTryS = 6.5 × 10−6 µmol/min mL) to obtain a satisfactory linear regression for A620 nm versus time under the assay conditions (Figure S1(D–F)). For each TryS, the substrates’ concentrations used in the assay are the same reported previously25.
The screening at 25 µM was performed in a 384-well plate, essentially as described in the previous section, except for: (a) the compounds were evaluated by quadruplicate, (b) the reagents were dispensed manually using a 16-multichannel pipette (Thermo Fisher Scientific Finnpipette), (c) the reaction was performed at controlled temperature (28 °C) and stopped by addition of the BIOMOL GREENTM reagent, and (d) absorbance at 620 nm was measured with a MultiScan FC plate reader (Thermo Fisher Scientific).
The compounds showing TryS inhibition ≥ 50% at 25 µM were subjected to DRC studies, performed at eight-point concentrations starting from 125 µM and 1:3 serial dilutions. In all assays, the DMSO concentration was 1.25% (v/v).
Interference of the compounds with the colorimetric reaction was corrected for each DRC point by preparing replicate wells containing 50 µM K2HPO4, dissolved in the corresponding TryS master mix, and the corresponding concentrations of compounds.
IC50 values were obtained from DRC (% TryS inhibition ± SD versus Log [compound] µM) fitted to a four-parameter sigmoidal equation (Boltzmann model) and expressed as IC50 ± SD.
Substructure search analysis and drug-likeness analysis
The search for substructures and the drug-likeness analysis (metrics measured: FSP3, XLogP and TryS % inhibition values) for the CPA, AMTPh and AMPh scaffolds were done using the program Vortex (Dotmatics, San Diego, CA). This program was also used to search for substructures similar to compound 17 devoid of the adamantine moiety.
Cell culture and cytotoxicity assays
Bloodstream T. brucei
The bloodstream form of T. brucei (strain 427, cell line 449: encoding one copy of the tetracycline-repressor protein)27 expressing the redox biosensor roGFP2 fused to human glutaredoxin-1 (hGrx1-roGFP2)28 was cultivated aerobically in HMI-9 medium29 supplemented with 10% (v/v) foetal bovine serum (FBS) (Gibco®, Carlsbad, CA), 1% (v/v) penicillin–streptomycin (10,000 U/mL, Gibco®), 0.2 µg/mL phleomycin (Gibco®) and 5 µg/mL hygromycin (InvitrogenTM), inside a humidified incubator (Thermo Fisher Scientific) with controlled temperature (37 °C) and CO2 (5%). Phleomycin and hygromycin were added to keep the constitutive expression of the tetracycline repressor protein and the tet-inducible hGrx1-roGFP2 gene, respectively.
The cytotoxicity of the compounds was tested according to the protocol described previously30. Briefly, mid-exponential phase trypanosomes were harvested by centrifugation at 2000 g for 10 min at RT and resuspended at a density of 5 × 105 cells/mL in fresh culture medium. Two hundred µL of this cell suspension were seeded per well in a 96-well culture plate (Corning 3599) containing the compound of interest at a final concentration of 25 µM and 1% (v/v) DMSO. For compounds showing more than 90% parasite growth inhibition at 25 µM, the EC50 values were determined from at least 7-point concentrations. After 24 h incubation at 37 °C and CO2 (5%), 100 µL from each well was transferred to a 2 mL tube (DELTALAB 408002) or to a 96 U bottom well plate (DELTALAB 900010.1) containing 200 µL of sterile phosphate-buffered saline (PBS)-glucose 1% (w/v) (pH 7.4), 2 µg/mL propidum iodide (PI). The samples were immediately acquired with an AccuriTM C6 (BD) device equipped with the following laser/filter pairs: λex 488 nm/λem 613/30 nm and the data analysed with the Accuri C6 software (BD, Franklin Lakes, NJ).
The controls included, a positive control with Nifurtimox (Lampit® from Bayer) tested at its EC50 (15 µM) and a proliferation control with DMSO 1% (v/v). All conditions were tested by triplicate. The relative percentage of viable parasites is expressed as: viability (%) = [(number of PI negative parasites for compound X at concentration Y)/(number of PI negative parasites in the proliferation control) × 100%. EC50 values with errors expressed as one SD were obtained from DRC fitted to a dose–response (variable slope) equation using GraphPad software (GraphPad Software, La Jolla, CA).
Intracellular L. donovani
THP-1 (TIB-20TM) cells and parasites, were cultured in Roswell Park Memorial Institute 1640 Medium (Gibco®) supplemented with 10% FBS (Gibco®), 1% (v/v) penicillin–streptomycin (10,000 U/mL, Gibco®) at 37 °C in 5% CO2. For the assay, THP-1 cells were washed with media once and plated directly to 384-well µCLEAR® plate (Greiner Bio-One 781091) containing 50 ng/mL phorbol 12-myristate 13-acetate at 1.0 × 104 cells per well and incubated for 48 h. For the infection, the metacyclic form of L. donovani MHOM/ET/67/HU3 was purified from stationary phase cultures (∼3 × 107 parasites/mL, day 4 after inoculation) by incubating the cells with Lectin from Arachis hypogaea (peanut) at 50 µg/mL for 30 min and 28 °C in a shaker. Then parasites were washed twice with Dulbecco's phosphate-buffered saline (Gibco®) with intermediate centrifugations at 40 g for 5 min and the parasite density was quantified prior to cell infection. Next, THP-1 cells were infected with metacyclic L. donovani at a multiplicity of infection 1:20. Twenty-four hours after infection, test compounds were added and the plates further incubated for 96 h. Thereafter, all wells were treated with 5 µM of DRAQ5TM (Biostatus) and 4% (w/v) paraformaldehyde. For DRC studies, compounds were tested in duplicate at 10-point concentrations (1:2 serial dilutions of the highest concentration tested: 100 µM). Amphotericin B deoxycholate (Sigma-Aldrich, A9528) was used as reference drug tested in triplicate from 10-point concentrations. DMSO at 0.5% (v/v) was included as proliferation control. See below section Image-based analysis for further details on assay read-out and analysis.
Intracellular T. cruzi
Cells U-2 OS and LLC-MK2 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with high-glucose (Gibco®) supplemented with 10% FBS (Gibco®), 1% (v/v) penicillin–streptomycin (10,000 U/mL, Gibco®) at 37 °C in 5% CO2. LLC-MK2 was used to produce the highly infective trypomastigote forms of T. cruzi (strain Y) and the U-2 OS cells were used as host cells for the screening assays. Tissue culture trypomastigotes (TCTs) of T. cruzi were harvested from culture supernatant of LLC-MK2 infected cells (on day 7 post-infection) by centrifugation at 2000 g for 5 min. A cell suspension containing U-2 OS cells and TCTs at a multiplicity of infection 1:12.5 was incubated with gentle swirling for 5 min. The cell mixture was then seeded (50 µL) into a 384-well µCLEAR® plate (Greiner Bio-One 781091) containing test compounds. After 72 h, the cells and parasites were stained using 5 µM of DRAQ5TM (Biostatus, Loughborough, UK) and fixed with 4% (w/v) paraformaldehyde.
For DRC studies, compounds were tested in duplicate at 10-point concentrations (1:2 serial dilutions of the highest concentration tested: 100 µM). Benznidazole (ChunyangTech, FB64954) was used as reference drug at its EC100 400 µM. See below section Image-based analysis for further details on assay read-out and analysis.
Image-based analysis
For the L. donovani and T. cruzi bioassays, images of 4 fields/well, which correspond to a 45% coverage of the well, were acquired with the Operetta CLS confocal microscope (Perkin Elmer) at 20× magnification. The images were analysed with the ColumbusTM software (Perkin Elmer) and the number of mammalian cells and intracellular parasites (recognized by the DRAQ5TM nuclei staining) were quantified to estimate EC50 and CC50 values (expressed as mean ± one SD), respectively, from DRC plots fitted to a dose-response (variable slope) equation. GraphPad Prism6 (GraphPad Software, San Diego, CA) was used for the analysis of quantitative data.
Redox assays for bloodstream T. b. brucei
Bloodstream T. brucei brucei (strain 427, cell line 449) expressing the redox biosensor hGrx1-roGFP2 was cultivated as indicated above and 24 h prior to the redox assays, the expression of the reporter gene was induced by adding 1 µg/mL oxytetracycline to the culture medium. Induced parasites in exponential growth phase were harvested by centrifugation and resuspended in fresh medium at a density of 2 × 106 cells/mL. Two hundred µL of this cell suspension were seeded per well in a 96-well plate and incubated (5% CO2 and 37 °C for 4 h) with the compound of interest added a 1× and 2× its EC50. Control conditions included parasites treated with DMSO 1% (v/v) for 4 h, and DTT (1 mM), menadione (250 µM) or diamide (250 µM) for 20 min. All conditions/compounds were tested in triplicates. Next, 100 µL from each well were transferred to a tube containing 200 µL of sterile PBS with glucose 1% (w/v) and 2 µg/mL PI. All samples were analysed with an AccuriTM C6 flow cytometer and the following laser/filter pairs: λex 488 nm/λem 613/30 nm for PI and λex 488 nm/λem 530/40 nm for GFP. A maximum of 10,000 events was acquired per sample. The mean fluorescence intensity (MFI; PI and GFP) of the samples was normalised to the corresponding fluorescence reference values of parasites incubated in the presence of DMSO 1% (v/v) and DTT. The level of roGFP2 mean fluorescence intensity was analysed only for samples presenting > 40% viable cells (PI negative). The relative level of biosensor reduction was calculated as follows: % biosensor reduction = [(MFI hGrx1-roGFP2 for compound X at concentration Y)/(MFI hGrx1-roGFP2 for DMSO 1% (v/v) with DTT) – (MFI hGrx1-roGFP2 for menadione)] × 100%.
For compounds inducing changes in roGFP2 fluorescence, DTT (1 mM) or menadione (250 µM) was added to the sample and, after 20 min incubation at RT, re-analysed by flow cytometry as indicated above.
The data were processed and analysed with the Accuri C6 software. Plots were prepared with the GraphPad software and errors expressed as one SD.
Inhibition mode of candidate hits against TbTryS
Time-dependent assays
Under identical assay conditions described in section High-throughput TryS assay, 5 µM Ebselen (IC50 for TbTryS) (stocks prepared at 80 µM in water with DMSO 20% v/v) was pre-incubated for 1 h at 28 °C with: TbTryS (6.1 × 10−6 µmol/min mL; Sample A) or MM (Sample B). Next, MM and TbTryS were added to sample A and B, respectively, and a reaction (Sample C) containing Ebselen (5 µM), TbTryS (6.1 × 10−6 µmol/min mL) and MM was prepared. All samples were incubated for 1 h at 28 °C and then BIOMOL GREENTM reagent was added to detect Pi formation. A620 nm was measured with a MultiScan FC plate reader (Thermo Fisher Scientific). Control samples (TbTryS + MM) lacking Ebselen were run in parallel. All conditions were tested in 6 replicates and data analysed as described in the aforementioned section.
Dilution and reduction-based assays
TbTryS (1.44 µM, 2.4 × 10−5 µmol/min.mL) and 10 µM Ebselen (stock prepared at 100 µM in water with DMSO 20% v/v) were mixed and incubated during 1 h at 20–23 °C. Thereafter, the excess compound was removed with a Zeba Spin Desalting Column 7 kDa Molecular Weight Cut-Off (Thermo Fisher Scientific) previously equilibrated with reaction buffer. Next, samples (10 µL) were incubated for 30 min in the absence or presence of DTT 5 mM for 30 min at 28 °C. TryS activity was measured in a 384-plate upon diluting the samples in reaction buffer (1:2 dilution) and incubating for 1 h at 28 °C with MM added or not of DTT 5 mM (stock prepared at 80 mM in reaction buffer), respectively. Controls with and without enzyme along with DMSO at 2% (v/v) were included. The reaction was stopped with the addition of the BIOMOL GREENTM reagent. The absorbance was measured at 620 nm with a MultiScan FC plate reader (Thermo Fisher Scientific). Six replicates were tested per sample and the % TryS inhibition calculated as indicated in the section High-throughput TryS assay.
Mass spectrometry assays
In order to identify the residue of TbTryS that is target of covalent modification by Ebselen, 100 µL samples containing 1.44 µM recombinant enzyme (2.4 × 10−5 µmol/min mL) in TryS reaction buffer were prepared and incubated for 1 h with: (A) Ebselen 20 or 50 µM (stocks prepared at 200 or 500 µM in water with DMSO 20% v/v), (B) 15 mM iodoacetamide (IAM, 500 mM stock solution prepared in distilled water) or (C) DMSO 2% v/v. Next, samples (A) and (B) were desalted using a Zeba Spin Desalting Column 7 kDa Molecular Weight Cut-Off (Thermo Fisher Scientific) pre-equilibrated in distilled water and then treated for 1 h with 15 mM IAM. All steps were performed at 20–23 °C.
TryS activity (8 replicates) was determined in all samples after the first hour of incubation as described in the previous section. Equal amounts of protein (∼0.011 mg) were adjusted to pH 8.0 with 50 mM ammonium bicarbonate and digested overnight at 37 °C using sequencing grade trypsin (Promega, Madison, WI) at a final protease:protein ratio of 1:20 (w/w). The digestions were then acidified with 10% trifluoracetic acid up to a final concentration of 1%. Peptide samples were concentrated under vacuum in a CentriVap concentrator (LabconoTM) and desalted using ZipTip® C18 tips (Merck Millipore, Kenilworth, NJ). Eluted peptides were finally resuspended with 0.1% formic acid in liquid chromatography/mass quality water (LiChrosolv®, Merck KGaA).
Liquid chromatography-MS/MS analysis was performed with an UltiMate 3000 liquid chromatography system (Thermo Fisher Scientific) coupled to a Q Exactive Plus mass spectrometer equipped with an Easy-SprayTM source (Thermo Fisher Scientific). Samples were loaded into a precolumn (AcclaimTM PepMapTM 100, C18, 75 µm × 2 cm, 3 µm particle size, Thermo Fisher Scientific) and separated with an Easy-SprayTM analytical column (PepMapTM RSLC, C18, 75 µm × 50 cm, 2 µm particle size. Thermo Fisher Scientific) at 40 °C using a two-solvent system: (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. Column was equilibrated at 1% B followed by a gradient elution performed as follows: 1–50% B for 115 min, 50–99% B for 10 min, 99% B for 5 min and 1% A for 10 min, with a constant flow rate of 200 nL/min. The mass spectrometer was operated in a positive mode. Ion spray voltage was set at 2.5 kV; capillary temperature at 250 °C and S-lens radio frequency level 50. A top-12 data-dependent method was used for mass data acquisition. Full mass scans were acquired in a range of 200–2000 m/z with a resolution of 70,000 at 200 m/z, automatic gain control target value of 1 × 106 and a maximum ion injection time of 100 ms. Precursor fragmentation occurred in a high-energy collision dissociation cell with a resolution of 17,500 at 200 m/z, automatic gain control target value of 1 × 105 and a maximum ion injection time of 50 ms. Normalised collision energy was used in a stepped mode (25, 30 and 35). Precursor ions with single, unassigned or eight and higher charge states were excluded. A dynamic exclusion time was set to 30 s.
Computational analysis
PatternLab for Proteomics 4.0 (PatternLab) software was used to process raw data files31. The sequence of recombinant TbTryS was added to the Escherichia coli proteome downloaded from Uniprot on July 2020 (http://www.uniprot.org/) and a target-reverse database was generated using PatternLab. The most common contaminants in Proteomics experiments were also included in the database. Search parameters were set as follows: methionine oxidation, cysteine di and tri oxidation, cysteine carbamidomethylation, and Ebselen covalent modification (+274.985 Da) in cysteine/lysine were defined as variable modifications. A maximum of 2 missed cleavages and 3 variable modifications per peptide were allowed. Search results were filtered by PatternLab Search Engine Processor algorithm with a maximum false discovery rate value ≤ 1% at protein level. The inspection of the MS/MS spectra of Ebselen-modified peptides identified by Patternlab allowed us to recognise a very characteristic fragmentation pattern dominated by the release of Ebselen modification and the presence of low intensity sequence ions. This fragmentation behaviour might result in low confidence assignment of spectrum to peptide sequences. To cover possible modification sites not identified by the search engine, we rely on the presence of the Ebselen modification reporter ions (an intense signal of m/z = 275.9938 corresponding to protonated Ebselen plus 2 fragmentation products of m/z = 196.0751 and 183.9417) (https://mona.fiehnlab.ucdavis.edu/spectra/display/FiehnHILIC001906). Raw data were mass filtered using Thermo Scientific XcaliburTM 4.0 software to identify MS/MS spectra containing these ions, and the putative modification by Ebselen was further manually validated. Mass spectrum were plotted using OriginPro 8 software. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE32 partner repository with the dataset identifier PXD027729.
Kinetic mechanism of inhibition
Assays were conducted at 28 °C, using 384-well plates and a total reaction volume of 16 µL, 1 µL of compound 17 or DMSO 20% and 5 µL of MM. Compound and TbTryS were incubated for 60 min, then the reaction was started by adding 5 µL MM and stopped after 60 min with 60 µL BIOMOL GREENTM reagent. The colorimetric reaction was allowed to develop for 20 min and then absorbance at 620 nm was measured with a MultiScan FC plate reader (Thermo Fisher Scientific).
Before running the kinetic studies, the enzyme concentration was adjusted in order to obtain a linear relationship at optimal substrates concentration for TbTryS (4.5 mM SP or 100 µM Gsp, 150 µM GSH and 200 µM ATP; max. A620 nm ∼0.5; ∼75 µM Pi), which corresponded to an enzyme activity of 3.3 × 10−5 µmol/min.mL with SP or 2.6 × 10−5 µmol/min.mL with Gsp.
Compound 17 was tested at the following concentrations: 0, 0.625-, 1.25-, 2.5-, 5-, 10- and/or 20-folds its IC50. Due to substrate inhibition by GSH25 the assays were performed at 150 µM for GSH (∼KM value), whereas SP, Gsp and ATP were added at 4.5 mM (∼19-fold the KM value), 100 µM (∼42-fold the KM value)33 and 200 µM (∼10-fold the KM value), respectively. When GSH was the variable substrate, the concentrations ranged from 600 to 75 µM. When ATP was the variable substrate, the concentrations tested ranged from 200 to 75 µM. For SP, the concentrations were varied from 4.5 to 0.167 mM. Gsp was tested at a fixed concentration of 100 µM.
All tests were performed in seven replicates for each concentration of compound evaluated. A single blank per condition was prepared by adding 10 µL of screening reaction buffer instead of enzyme.
The assays were run manually and yielded an intra-assay coefficient variation ≤10%. Lineweaver-Burk plots (1/[Abs] versus 1/[substrate]) were prepared and analysed using the OriginPro 8 software. An R-square (R2) ≥0.96 was used as criteria to eliminate outliers for fitting plots. For ATP, GSH and SP the fitting was performed using a linear, an exponential (y = y0 + A × eR×X) and a sigmoideal [y = (Vmax×Xn)/(Kn + Xn)] equation, respectively.
Results
Identification of TryS inhibitors
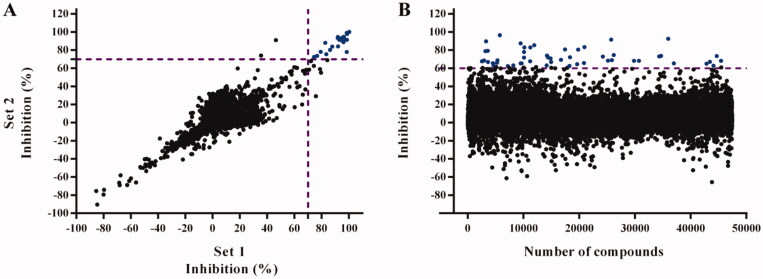
A pilot screening with 4210 compounds from the LOPAC® (SIGMA), Selleck Chemicals, NIH Clinical and TOCRIS libraries (from now on named: in-house library), all of them with well-known biochemical and physiological activities, was performed in duplicate to assess the robustness and reproducibility of the 384-well plate screening assay. The Z’ factor34 for the set 1 and set 2 was 0.78 and 0.79, respectively, and the R2 value obtained for the plot from both assays sets was 0.96. These results confirmed the suitability of the HTS assay for running a screening with a larger number of compounds. For this preliminary screening, a hit was defined as a compound exerting ≥ 70% TbTryS inhibition at 25 µM. Twenty compounds fulfilled this criterion, which yielded a hit ratio of 0.47% (Figure 2(A)).
Figure 2.
HTS screening against Trypanosoma brucei Trypanothione synthetase (TbTryS). (A) Pilot screening. An in-house library of 4210 compounds was tested at 25 μM, and in duplicate in two independent experiments (indicated as Set 1 and Set 2), for their inhibitory activity against TbTryS. Lineal regression fitting for TryS inhibition (%) from both sets yielded a R2 value of 0.96. Twenty compounds displayed ≥ 70% enzyme inhibition (blue dots), which corresponds to a hit ratio of 0.47%. (B) Large screening. A commercial library of 47,414 small compounds (ENAMINE) was tested at 25 μM for their inhibitory activity against TbTryS. Sixty-two compounds displayed ≥ 60% enzyme inhibition (blue dots), which corresponds to a hit ratio of 0.13%.
Based on the robustness of the miniaturised screening assay, next, a large HTS was performed with a commercial library (ENAMINE) consisting of 47,414 drug-like compounds of wide chemical diversity. The compounds were tested once at a single point concentration of 25 µM. For practical reasons, the screening was divided into three runs that overall rendered an average Z’ factor of 0.83 and a signal-to-background value of 8.07. For this assay, the cut-off for a hit was set at ≥60% TbTryS inhibition, which yielded 62 compounds and a hit ratio of 0.13% (Figure 2(B)).
Thus, a total of 82 hit candidates from the in-house and the ENAMINE libraries were re-purchased and, with the aim to identify multi-species TryS inhibitors, tested at 25 µM against TryS from T. brucei, T. cruzi and L. infantum (Tri-Tryp TryS; Table S1). Twenty-five out of the 82 potential hits were confirmed as true inhibitors of TbTryS with IC50 values ranging from 1.2 to 36.2 µM (Table 1).
Table 1.
Hit compounds targeting trypanothione synthetase (TryS) inhibition.
| Compound |
% TryS inhibition at 25 μMa or IC50 (μM)b |
|||||
|---|---|---|---|---|---|---|
| #c | Chemical name/library code | Core scaffoldd | T. brucei | T. cruzi | L. infantum | |
| 1 | Aurintricarboxylic acid | Singleton | 8.2 ± 0.6 | 5.5 ± 2.8 | 4.6 ± 0.7 | |
| 2 | 4-Chloromercuribenzoic acid | Singleton | 2.20 ± 0.05 | 32.0 ± 9.7 | NA | |
| 3 | 6-Hydroxy-DL-DOPA | Singleton | 13.0 ± 3.5 | 16.8 ± 2.8 | 4.0 ± 1.4 | |
| 4 | NH125 | Singleton | 12.0 ± 2.5 | 15.5 ± 2.9 | 25.7 ± 2.6 | |
| 5 | Ebselen | Singleton | 5.3 ± 0.2 | 13.8 ± 1.5 | 2.6 ± 0.2 | |
| 6 | Sanguinarine chloride | Singleton | 3.3 ± 0.4 | 40.0 ± 1.2 | 11.6 ± 0.4 | |
| 7 | Calmidazolium chloride | Singleton | 9.5 ± 2.5 | 2.7 ± 0.5 | 1.6 ± 0.8 | |
| 8 | SCH 202676 hydrobromide | Singleton | 3.7 ± 0.5 | 43.4 ± 5.7 | 3.0 ± 0.8 | |
| 9 | PD 404,182 | Singleton | 15.3 ± 1.1 | 10.9 ± 5.6 | 8.9 ± 5.9 | |
| 10 | Demethylasterriquinone B1 | Singleton | 31.0 ± 7.0 | 27.7 ± 7.6 | 69.3 ± 3.5 | |
| 11 | CGP 71683 hydrochloride | Singleton | 14.4 ± 8.2 | 34.7 ± 7.6 | 13.9 ± 1.8 | |
| 12 | PQ 401 | Singleton | 1.5 ± 0.4 | 6.2 ± 5.6 | NA | |
| 13 | WIN 64338 hydrochloride | Singleton | 9.2 ± 7.4 | 25.9 ± 6.5 | 70.9 ± 6.8 | |
| 14 | Z109494586 | Singleton | 8.0 ± 1.8 | 7.1 ± 4.0 | 16.8 ± 17.9 | |
| 15 | Z1188133056 | Singleton | ∼25 c | NA | NA | |
| 16 | Z13601063 | Singleton | 28.0 ± 4.5 | NA | NA | |
| 17 | Z147103388 | Singleton | 1.2 ± 0.2 | NA | NA | |
| 18 | Z1547375509 | Singleton | 15.2 ± 3.7 | NA | 28.5 ± 11.1 | |
| 19 | Z21459859 | Singleton | 36.2 ± 2.6 | 13.3 ± 3.3 | NA | |
| 20 | Z225233562 | AMTPh | 20.3 ± 4.6 | NA | NA | |
| 21 | Z227072034 | AMTPh | 8.4 ± 0.9 | NA | NA | |
| 22 | Z227978766 | Singleton | NA | 12.9 ± 6.1 | 8 | |
| 23 | Z244772346 | AMPh | 28.2 ± 1.3 | NA | NA | |
| 24 | Z318180488 | AMPh | 13.1 ± 0.7 | NA | NA | |
| 25 | Z339866610 | CPA | 26.4 ± 0.9 | NA | NA | |
| 26 | Z363062290 | CPA | 18.4 ± 2.5 | 8.8 ± 7.3 | NA | |
| 27 | Z51971326 | AMTPh | 19.9 ± 2.7 | NA | NA | |
| 28 | Z572699208 | CPA | ∼ 25 c | NA | NA | |
| 29 | Z95976280 | CPA | 12.0 ± 2.6 | NA | NA | |
a% TryS inhibition is expressed as the mean ± standard deviation, where NA, denotes “not active” (i.e. TryS inhibition ≤ 0.0 ± 5.0% at 25 µM).
bValues in italic and bold refers to IC50. For compounds showing TryS inhibition of 45–55% at 25 μM, the IC50 was approached to ∼25 µM.
cCompounds 1–13 (grey background) and 14–29 correspond to hits from the in-house and ENAMINE library, respectively.
dCPA: Carboxy piperidine amide; AMTPh: amide methylene thiazole phenyl; AMPh: amide methylene phenyl. Structural information of molecules belonging to the ENAMINE library can be retrieved from the company website (https://www.enaminestore.com/search) using the corresponding compound code.
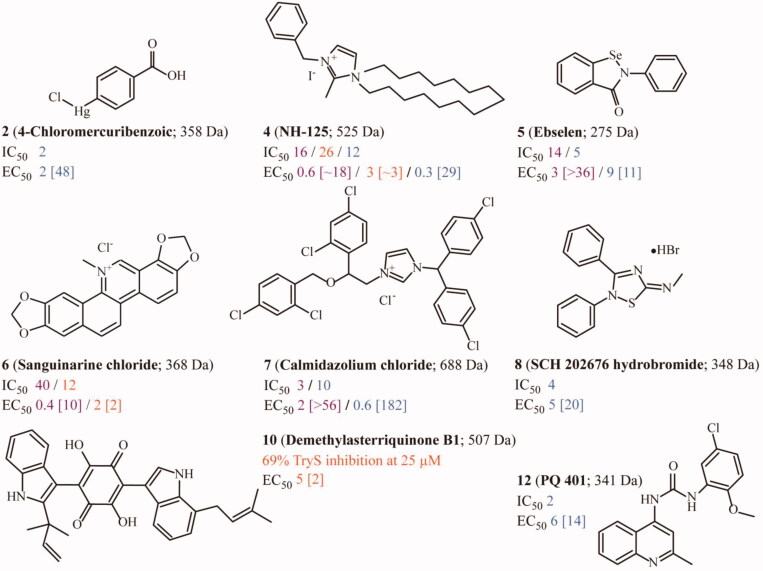
Interestingly, the hit confirmation ratio for TbTryS was higher for compounds belonging to the in-house library (10 out of 20 hit candidates: 50% hit ratio) than for those from the ENAMINE library (15 out 62 hit candidates: 24% hit ratio). This difference can be ascribed to the fact that compounds from the pilot-screening were tested in duplicates whereas those from the ENAMINE library were assayed in uniplicates. Interestingly, extending the screening of the 82 hits TbTryS hit candidates to the related enzymes from T. cruzi and L. infantum led to the identification of several compounds with multi-TryS inhibitory activity: 13 belonging to the in-house library and 16 from the ENAMINE library (Table 1). Strikingly, the selected hit candidates from the ENAMINE library showed a high selectivity for TbTryS (Table 1). The exception were the singletons 22 (IC50 8 µM, Table 1), 46 and 82 (74% and 43% TryS inhibition; Table S1) that inhibited LiTryS and proved inactive or poorly active against TbTryS or TcTryS. In contrast, the in-house library yielded a high number of compounds with wide-spectrum inhibitory activity against Tri-Tryp TryS with IC50 values ranging from 1.6 to 40 µM (i.e. 3, 4, 5, 6 and 7). A few compounds displayed species-specific activity against two (1 and 8 inhibited LiTryS and TbTryS) or a single TryS (i.e. 12 versus TbTryS and 13 versus LiTryS). Interestingly, three singletons (10, 11 and 13) that were poorly active against TbTryS showed higher inhibitory activity towards LiTryS. Overall, TcTryS resulted the most refractory enzyme to inhibition.
Strikingly, the remarkable differences in hits’ ratio and species-specificity for TryS correlated inversely with the library size and its chemical diversity. The in-house library consisted only of singletons (4210) with proven biochemical/biological activity and led to the identification of wide-spectrum TryS inhibitors. In contrast, the larger and more diverse ENAMINE library, consisting of 3753 compounds’ clusters and 4332 singletons (all together totalising 47,414 compounds), led to the identification of a few and TryS species-specific inhibitor’ scaffolds.
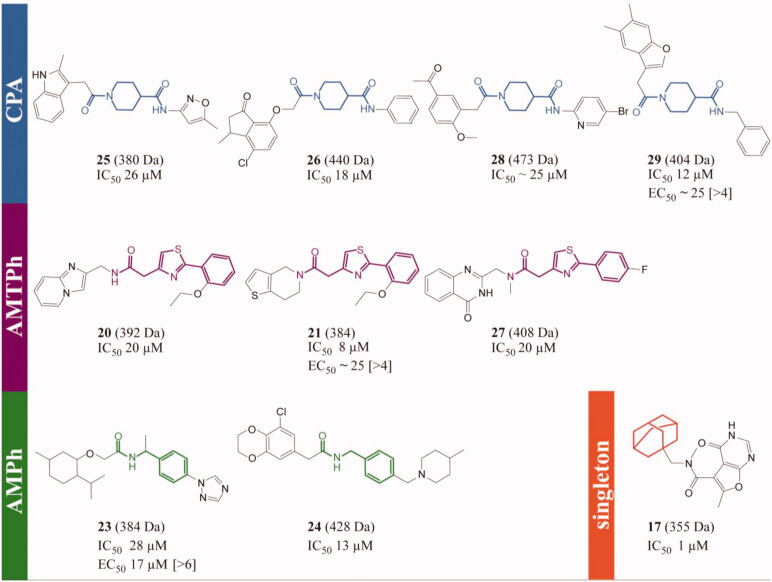
The TbTryS hits from the ENAMINE library can be clustered into three major scaffolds: (A) carbonyl piperidine amide (CPA) (4 active compounds, with 7 out of 62 exerting an inhibition ≥60%), (B) amide methylene thiazole phenyl (AMTPh) (3 active compounds, with 4 out of 62 compounds exerting an inhibition ≥60%) and (C) amide methylene phenyl (AMPh) (2 active compounds, with 10 out of 62 exerting inhibition ≥60%) (Tables 1 and S1 and Figure 3). A substructure mining of the ENAMINE library delivered the following number of analogues: 255 for CPA, 47 for AMTPh and 1547 for AMPh, one being common to the last two scaffolds. Based on this information, the AMPh can be dismissed as a real active scaffold given that only 2 out of 1547 analogues (0.001%) proved active against TryS. However, the AMTPh (hit ratio = 6.4%) and the CPA (hit ratio = 2.7%) series may be considered as real active scaffolds.
Figure 3.
T. brucei TryS hit compounds from the ENAMINE library. CPA: carboxy piperidine amide derivatives, AMTPh: amide methylene thiazole phenyl derivatives, AMPh: amide methylene phenyl derivatives and singleton: 17, the most potent singleton with its adamantine moiety highlighted in orange. The scaffolds or moiety of interest are highlighted in different bold colours. The EC50 (bloodstream T. brucei) and selectivity index (value in brackets, reference cell line: human macrophages, THP-1) are shown only for compounds with anti-proliferative action against T. brucei at concentrations ≤25 µM. All the errors are expressed as one SD and the compounds’ molecular mass in Daltons.
The drug-likeness of the scaffolds was analysed considering the following parameters: the fraction of sp3 hybrized or saturated carbons (FSP3) and the lipophilicity (XLogP). The CPA compounds showed better drug-like properties than the other scaffolds since the FSP3 score was >0.3 (pointing to a higher molecular flexibility, as commented above) and the XLogP <5 (Figure S2). Regarding the hit singletons from the ENAMINE library, the most potent was 17 with an IC50 = 1.2 µM (Tables 1 and S1 and Figure 3). A search for related compounds revealed 24 analogues lacking the adamantine moiety (data not showed). Interestingly, none of them was active against TbTryS suggesting that the lipophilic adamantine moiety is relevant for anchoring the inhibitor to the enzyme active site.
Phenotypic-based screening
The most active inhibitors of Tri-Tryp TryS were tested for their biological activity against the clinically relevant forms of the corresponding trypanosomatid species: T. brucei, L. donovani and T. cruzi (Table 2 and Figure 3 for hits from ENAMINE library and Figure 4 for hits from the in-house library).
Table 2.
Biological activity of TryS inhibitors against the infective stage of different trypanosomatids (bloodstream T. brucei and intracellular amastigotes of T. cruzi and L. donovani) and mammalian (host) cells.
| Cytotoxicity (EC50 μM)a and selectivity index [SI]b |
|||
|---|---|---|---|
| Compoundc |
T. bruceid [SI vs. THP-1] |
T. cruzid [SI vs. U-2 OS/THP-1 ] |
L. donovanid [SI vs. THP-1] |
| 1 | >25 | ND | >100 |
| 2 | 2.1 ± 0.2 [48] | ND | ND |
| 3 | >25 | ∼34 [2.7 / >2.7] | >100 |
| 4 | 0.34 ± 0.01 [29] | 0.56 ± 0.01 [2.6 /∼18] | 3.2 ± 0.6 [∼3] |
| 5 | 8.9 ± 0.7 [11] | 2.8 ± 0.1 [2.4 / >36] | >100 |
| 6 | ND | 0.37 ± 0.11 [1.3 / 9.5] | 1.7 ± 0.2 [2] |
| 7 | 0.55 ± 0.03 [182] | 1.6 ± 0.1 [1.1 / >55.6] | >100 |
| 8 | 4.6 ± 0.5 [20] | ND | ∼ 56 [∼1.4] |
| 9 | >25 | ND | ND |
| 10 | ND | ND | 4.5 ± 0.7 [1.6] |
| 11 | ND | ND | 26.0 ± 8.1 [1.1] |
| 12 | 5.9 ± 0.5 [14] | ND | ND |
| 13 | ND | ND | 31 ± 5 [1.9] |
| 14 | 5.2 ± 3.2 [11] | ND | ND |
| 15 | >25 | ND | ND |
| 16 | >25 | ND | ND |
| 17 | >25 | ND | ND |
| 18 | >25 | ND | ND |
| 19 | ∼20 [>4] | ND | ND |
| 20 | >25 | ND | ND |
| 21 | ∼25 [>4] | ND | ND |
| 22 | ND | ND | >100 |
| 23 | 17.1 ± 4.4 [>6] | ND | ND |
| 24 | >25 | ND | ND |
| 25 | >25 | ND | ND |
| 26 | >25 | ND | ND |
| 27 | >25 | ND | ND |
| 28 | >25 | ND | ND |
| 29 | ∼25 [>4] | ND | ND |
| Nifurtimox | 15.0 ± 2.5 | ND | ND |
| Benznidazole | ND | 2.5 ± 0.2 [>40] | ND |
| Amphotericin B | ND | ND | 0.18 ± 0.02 [>22] |
aThe effect of the compounds in trypanosomatids viability is expressed as EC50 (half-maximum effective concentration). The values correspond to the mean ± SD (n = 3). For some compounds the EC50 reported corresponds to the closest concentration reducing cell viability to 45–55% and is indicated with the symbol “∼”.
bThe selectivity index is calculated as the quotient CC50 (half-maximum mammalian cytotoxic concentration)/EC50 for the indicated host cell/pathogen pair.
cCompounds 1–13 (grey background) and 14–29 correspond to hits from the in-house and ENAMINE library, respectively.
dND: not determined.
Figure 4.
Most prominent TryS hits from the in-house library tested against the clinically relevant forms of major trypanosomatid species. Compound’s structure, molecular weight (in Daltons) and activity (IC50 and EC50 are in µM units, and the selectivity index is referred to human macrophages and shown within brackets). The biodata is highlighted in blue for T. brucei, violet for T. cruzi and orange for Li-TryS or L. donovani.
Twenty-four compounds with IC50 ≤36 µM against TbTryS (Table 1) and CC50 against THP-1 ≥ 10 µM were evaluated against the bloodstream form of T. brucei (Table 2). Six (2, 4, 5, 7, 8 and 12) out of nine compounds from the in-house library showed good potency (EC50 0.34–8.9 µM) and remarkable cytotoxic selectivity against the pathogen (SI 11–182). Among them, 4 (EC50 0.34, SI 29) and 7 (EC50 0.55, SI 182) resulted the top biological hits (Table 2). In contrast, five (two singletons: 14 and 19, and one from each main inhibitor scaffold AMPh: 23, AMTPh: 21 and CPA: 29) out of fifteen compounds from the ENAMINE library showed moderate activity (EC50 5–25 µM) and poor selectivity (SI > 4 to 11) against T. brucei (Table 1 and Figure 3).
Compounds with an IC50 ≤40 µM against TcTryS (3, 4, 5, 6 and 7) were evaluated against the intracellular form of T. cruzi infecting human osteosarcoma cells (cell line U-2 OS). Except for 3 (EC50 34 µM), the other compounds showed low (sub)µM potency (EC50 0.4–2.8 µM). The four most active compounds against T. cruzi displayed a higher toxicity towards the osteosarcoma cells (selectivity index = SI 1.1–2.6) than against human macrophages (SI 9.5 to >56), which are also physiological hosts for this pathogen.
Finally, 11 compounds showing IC50 <26 µM against LiTryS (1, 3, 4, 5, 6, 7, 8, 10, 11, 13 and 22) (Table 1) were evaluated against the intracellular form of L. donovani hosted by human macrophages (cell line THP-1). Three of these compounds (4, 6 and 10) displayed activity against the pathogen at low µM concentrations (EC50 1.7–4.5 µM) and with a remarkable poor selectivity (SI 1.6–3). Three additional compounds (8, 11 and 13) were one order of magnitude less potent (EC50 26–56 µM) and with almost negligible selectivity (SI 1.1–1.9) (Table 2).
Correlation between on-target (in vitro TryS inhibition) and anti-trypanosomatid activity of selected TryS hits
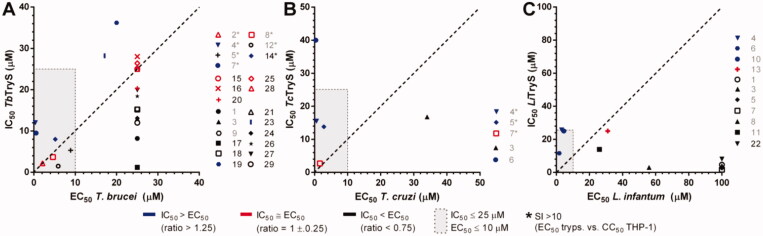
The compounds tested in our study have drug-like properties (low molecular weight, cell permeability and/or, some of them, proven bioactivity), which allow to comparatively analyse their IC50 and EC50 to establish a potential correlation in bioactivity (Figure 5). Twelve of the TbTryS hits presented higher inhibitory activity towards the molecular target than against the parasite (Figure 5(A), black symbols), seven showed a good correlation between target inhibition and anti-T. brucei activity (2, 8, 15, 16, 20, 25 and 28, red symbols), whereas five compounds proved more active in killing the pathogen (4, 7, 14, 19 and 23, blue symbols). In contrast, only two TryS hits, one per parasite species, showed a good correlation between IC50 and EC50 for the intracellular pathogens T. cruzi (7, Figure 5(B)) and L. infantum (13, Figure 5(C)). Three of the TcTryS hits tested proved more active in killing the parasite than in inhibiting the enzyme (4, 5 and 6), and only one (3) showed the opposite behaviour. Most of the LiTryS hits proved to be far more active against the enzyme than towards the pathogen (1, 3, 5, 7, 8, 11 and 22) and three showed a comparatively higher potency against the parasite (4, 6 and 10). Interestingly, the linear correlation between IC50 versus EC50 exhibited by some compounds was species specific. This does not necessarily suggest a lack of conserved on-target effect of the hits but may, at least in part, be attributed to differences in the capacity of the compounds to achieve effective intracellular concentrations in pathogens that dwell in different environments (T. brucei: extracellular, T. cruzi: host cell cytosol, and L. infantum: host cell endolysosome).
Figure 5.
Correlation between on-target (in vitro) and anti-trypanosomatid (in vivo) activity for selected TryS hits. For the most potent inhibitors of TryS from different trypanosomatid species, the IC50 and EC50 values are plotted. Compounds showing different correlations for the quotient IC50/EC50 are shown in different colours (blue: IC50 > EC50, red: IC50 ≈ EC50 and black: IC50 < EC50). The dashed line denotes a correlation for IC50/EC50 = 1. Compounds inside the grey box are hits against the corresponding TryS (IC50 ≤ 25 μM) and parasite species (EC50 ≤ 10 μM). Those hits that, in addition, present a selectivity index > 10 are highlighted with * in the plot’s legend. The results are shown for (A) Trypanosoma brucei TryS and bloodstream parasites, (B) Trypanosoma cruzi TryS and amastigote stage and (C) Leishmania infantum TryS and amastigote stage.
Notably, against trypanosomes, seven and five of the TbTryS and TcTryS hits, respectively, qualified also as biological hits according to official criteria (EC50 ≤ 10 µM and SI > 10)35.
Assays with redox-reporter cell line of bloodstream T. brucei
Inhibition of trypanothione synthesis either by genetic6,7 or chemical9,12,13 approaches leads to an intracellular oxidative milieu due to exhaustion of the antioxidant system to cope with the elimination of endogenous peroxides caused by trypanothione depletion.
In order to provide preliminary evidence on the on-target effect of the TryS’s hit displaying anti-proliferative activity against T. brucei (EC50 ≤ 25 µM and IC50 ≤ 25 µM), their capacity to alter the intracellular redox state of the parasites was addressed using a redox-reporter cell line of bloodstream T. b. brucei. The transgenic cell line expresses a GFP-based redox biosensor (roGFP2)28 that allows monitoring non-invasively, and in a dynamic fashion, perturbations in the pool of reduced and oxidised low molecular weight thiols (i.e. GSH/GSSG and T(SH)2/TS2)36. For instance, a high fluorescence intensity of roGFP2 excited at 488 nm indicates a reducing intracellular milieu (i.e. high GSH/GSSG or T(SH)2/TS2 ratio) whereas a decrease in roGFP2 fluorescence intensity (Ex 488 nm) points to an oxidising intracellular milieu (i.e. low GSH/GSSG or T(SH)2/TS2 ratio). This redox reporter cell line was successfully used to study the anti-trypanosomal mode of action of different families of compounds37–42.
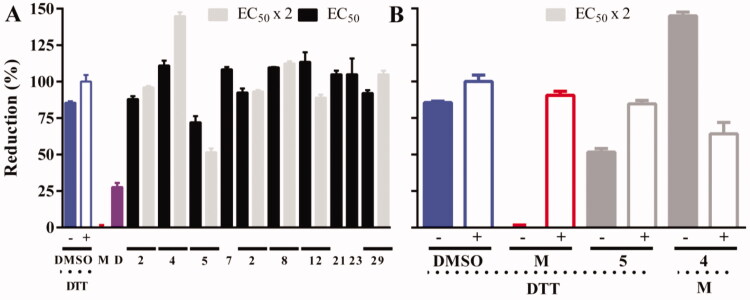
Bloodstream T. b. brucei expressing the redox biosensor was treated with the most potent compounds targeting TryS and T. brucei (EC50 ≤ 25 µM and IC50 ≤ 25 µM: 2, 4, 5, 7, 8, 12, 14, 21, 23 and 29) added at concentrations corresponding to 1× or 2× their EC50 (Table 2). After 4 h exposure, the fluorescence intensity of roGFP2 was analysed by flow cytometry for parasites exhibiting membrane integrity (PI negative cells). The redox analysis was not performed for 7, 21 and 23 at 2× their EC50 because of the massive cell death they caused. As shown in Figure 6(A), and compared to the vehicle treated control (1% v/v DMSO), only compound 5 (Ebselen) added at 18 µM (2-fold its EC50) induced a 48% oxidation of the biosensor upon 4 h treatment that was reverted by exposing the parasites to the membrane permeant reducing agent DTT (1 mM for 20 min, Figure 6(B)).
Figure 6.
Intracellular redox status of bloodstream T. b. brucei treated with TryS’s inhibitors. (A) The bloodstream form of a redox reporter cell line of T. brucei was treated for 4 h with the most potent compounds targeting TryS and T. brucei added at concentrations corresponding to 1× or 2× their EC50 (Table 2). The thiol-specific oxidant Diamide (D, 250 µM for 20 min) was included as control. (B) Samples treated with compounds exerting significant changes in biosensor fluorescence, namely 4 and 5 both at 2 × EC50, were treated for 20 min with menadione (M, 250 µM) and DTT (1 mM) to confirm the redox basis of changes observed, respectively. In both plots, the values corresponding to the % reduction of the biosensor are normalised against conditions yielding full biosensor reduction (DTT 1 mM, 20 min) and oxidation (menadione 250 µM for 20 min) in parasites grown in medium containing DMSO 1% v/v.
Strikingly, at 2-fold its EC50, compound 4 increased significantly biosensor fluorescence at a value 45% above that obtained under fully reducing conditions (1 mM DTT). In order to verify the redox basis of this phenomena, a fraction of this sample was treated with the oxidising agent menadione under conditions that in vehicle alone treated parasites cause full biosensor oxidation (250 µM menadione for 20 min; Figure 6(A)). This treatment caused an oxidation of the biosensor (i.e. from 145% to 62% biosensor reduction, drop of 83%) of similar magnitude to that induced in vehicle-alone treated cells (i.e. from 82% to 0% biosensor reduction, drop of 82%). This clearly indicates that compound NH125 is not reducing the biosensor at intracellular level but increasing the background level of (auto)fluorescence. Altogether, these data suggest that Ebselen is the only TryS’ hit whose mechanism of parasite killing involves the generation of a decrease in the ratio of reduced vs. oxidised low molecular weight thiols upon a short time exposure.
Mode of inhibition of TbTryS by Ebselen
Ebselen was chosen for further mechanistic studies because it was one of the few compounds displaying multi-species TryS inhibitory activity (Table 1) and anti-proliferative action against the infective stages of Trypanosoma (Table 2) by rapidly inducing the generation of an intracellular oxidative millieu (Figure 6).
Inhibition of TbTryS by Ebselen was observed as long as the compound (5 µM) was pre-incubated for 1 h with the enzyme prior to assaying its activity (sample A; Table 3), which is suggestive of a slow-binding inhibition mechanism. A pre-incubation of Ebselen with the substrates (sample B), including the thiol-containing molecule glutathione, has not impact on enzyme activity (0% TryS inhibition) and, hence, rules out the possibility of a covalent complex between glutathione and Ebselen acting as enzyme inhibitor. Notably, when the pre-incubation enzyme-inhibitor is omitted (sample C), there is a minor effect of Ebselen on enzyme activity (1.8% inhibition), supporting the slow-binding inhibition mechanism and suggesting that the substrates may induce conformational changes in the enzyme that lower the accessibility to the solvent of the site targeted by Ebselen.
Table 3.
Mode of inhibition of TbTryS by Ebselen.
| Time-dependent inhibition assaya | Sample |
||
|---|---|---|---|
| A | B | C | |
| Pre-incubation (1 h) | Ebselen + TbTryS | Ebselen + MM | No |
| Reaction (1 h) started with | MM | TbTryS | Ebselen + TbTryS + MM |
| TryS inhibition ± SD (%, n = 6) | 43.6 ± 4.5 | −0.7 ± 2.4 | 1.8 ± 4.2 |
| Irreversible inhibition assayb | |||
| Pre-incubation Ebselen + TbTryS (1 h) | Yes | Yes | Yes |
| Buffer exchanged | Yes | Yes | No |
| Incubation with DTT (5 mM, 30 min) | Yes | No | No |
| TryS inhibition ± SD (%, n = 6) | 89 ± 8.5 | 97.0 ± 5.0 | 100 ± 9 |
| Mass spectrometry analysisc | |||
| 1. Pre-incubation with Ebselen (1 h) | Yes | No | No |
| 2. Pre-incubation IAMd (15 mM, 1 h) | No | Yes | No |
| 3. Desalting and IAMd treatment (15 mM, 1 h) | Yes | Yes | No |
| TryS inhibition ± SD (%, n = 8) | 100 ± 7 (96 ± 8)e | 20 ± 9 | |
TryS activity data (% inhibition) here reported was determined according to the assay described in section Inhibition mode of candidate hits against TbTryS.
aEbselen tested at its IC50 against TbTryS (5 µM), MM: master mix.
bEbselen added at two-fold its IC50 against TbTryS (10 µM).
cThe data reported correspond to two independent experiments.
dIAM, iodoacetamide.
eEbselen added at 4- (20 µM) or 10-folds (50 µM, value in parenthesis) its IC50 against TbTryS.
Next, a fraction of a sample corresponding to TbTryS incubated with the compound (10 µM) for 1 h was buffer exchanged to remove the excess of inhibitor and further treated or not with an excess DTT to evaluate the reversibility of a potential covalent modification of enzyme’s cysteine residues. TbTryS remained almost fully inhibited upon removal of the inhibitor (97%) and following treatment with an excess DTT (5 mM, 30 min) only a minor fraction of the enzyme recovered activity (89% TbTryS inhibition). Overall, these results point to an irreversible modification of the enzyme by Ebselen that may involve the formation of a thiol-selenide bond.
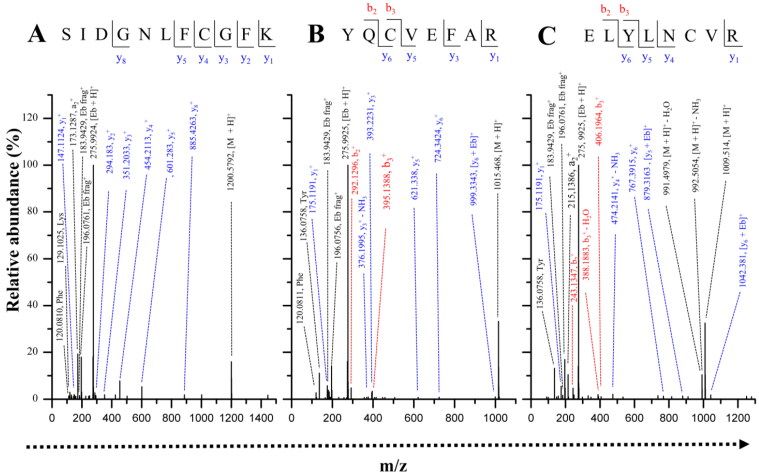
In order to identify a potential covalent modification of TbTryS by Ebselen, samples from inhibitor-treated enzyme (sample A, >96% TryS inhibition; Table 3) were desalted and, to avoid non-specific disulphide cross-linking during trypsin digestion, free-thiols alkylated with an excess IAM prior to mass spectrometry analysis. A sample of TbTryS untreated with Ebselen (Sample B, Mass spectrometry analysis in Table 3) was used as control. Computational analysis using PatternLab allowed a confident identification of TbTryS with a sequence coverage ranging from 88.3% to 95.2%. On average, 643 ± 148 spectra could be assigned to peptides from TbTryS, covering all peptides containing cysteine residues. Three tryptic and Cys-containing peptides with a mass increment of 274.985 Da (which is compatible with the incorporation of a single Ebselen molecule per peptide) were detected in TbTryS treated with EbSelen (Figure 7(A–C)) but not in the control sample. Given the labile nature of the selenide-sulphide bond, during MS/MS analysis the predominant fragmentation is the loss of Ebselen rendering native ion series. Thus, not always was possible to confidently identify the Ebselen-modified residue. However, the detection of the Ebselen modification reporter ions (an intense signal of m/z = 275.9938 corresponding to protonated Ebselen plus 2 signals of its most intense fragmentation product of m/z = 196.0751 and 183.9417) confirmed the presence of the inhibitor molecule in the peptides of interest. Two of the peptides belong to the papain-like amidase domain (peptide sequences: S44IDGNLFCGFK54 and Y55QCVEFAR62) (Figure 7(A,B)) where Cys51 is located close to the catalytic site and Cys57 along with His128 form the catalytic dyad that hydrolyses the C–N bonds of Gsp and T(SH)2 (Figure 7(A)). Cys57 is strictly conserved in Tri-Tryp TryS (Figure 7(A)) whereas Cys51 is replaced by a phenylalanine residue in the leishmanial homologue. Although the modification of these cysteines will impair the hydrolytic activity of TryS, it is very unlikely that it will affect the synthetase activity. Therefore, the modification of these peptides has been dismissed as effector of TryS inhibition by Ebselen. The third peptide modified by Ebselen belongs to the synthetase domain (peptide sequence: E265LYLNCVR272) (Figure 7(C)) and is located at the interphase between the amidase and synthetase domain in a region accessible to the solvent (Figure 8(A,B)).
Figure 7.
MS/MS spectra of peptides modified by Ebselen. Representative MS/MS spectra for the 3 peptide sequences covalently modified by Ebselen (A) SIDGNLFCGFK (m/z = 738.2831, z = 2), (B) YQCVEFAR (m/z = 645.7306, z = 2) and (C) ELYLNCVR (m/z = 642.7532, z = 2). The Ebselen modification reporter ions (an intense signal of m/z = 275.9938 corresponding to protonated Ebselen ([Eb + H]+) and the signal of the two most intense fragmentation products of m/z = 196.0751 and 183.9417) are present in all spectra (Eb frag+). Peaks are assigned to the y and b series of native sequence; +Eb indicates those fragment ions containing the +274.985 modification. Phe, Lys and Tyr indicate the immoniun ions of the respective amino acids. Eb: Ebselen.
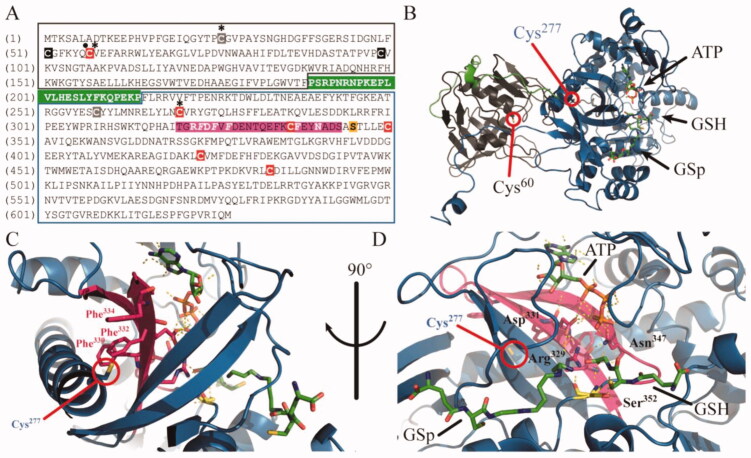
Figure 8.
Molecular model for allosteric inhibition of TryS by Ebselen. (A) Amino acid sequence of TbTryS highlighting the N-terminal amidase domain (black box), the C-terminal synthetase domain (blue box) and the linker region (white bold letters on green background). The cysteine residues (red background: conserved in Tc- and Li-TryS, black background: conserved in TcTryS, grey background: unique to TbTryS) that were found modified by Ebselen (asterisk) and fulfils a role in amidase catalytic activity (dot) are highlighted. The region harbouring structural elements with highly-conserved residues (in white bold letters) relevant for the catalytic activity of the synthetase domain and forming a hydrophobic pocket (italics) in the vicinity of Cys277 is highlighted in magenta background. The strictly conserved serine residue acting as nucleophilic attack facilitator for conjugation of the polyamine moiety (SP or Gsp) to GSH is shown in yellow background. (B) Cartoon representation of L. major TryS 3D structure (PDB 2VOB) showing the conserved cysteine residues from the amidase (grey cartoon, Cys60) and the synthetase (blue cartoon, Cys277) domain that bind Ebselen covalently in TbTryS. The linker region is shown in green and the substrates bound to the synthetase active site are shown in sticks. (C) Location of the cysteine from the synthetase domain modified by Ebselen (Cys 277 in LiTryS and Cys270 in TbTryS). Hydrophobic residues (Phe330, Phe332 and Phe334) in the vicinity of Cys277 and forming part of structural elements harbouring several residues relevant for catalytic activity are coloured magenta. (D) Synthetase active site with bound substrates and strictly conserved residues relevant for catalysis are shown in magenta (Arg329, Asp331 and Asn347) and yellow (Ser352) coloured sticks. The network of hydrogen bonds and electrostatic interactions between these residues and with substrates is shown with dashed lines.
Interestingly, although the sequence of this peptide varies among Tri-Tryp TryS, its cysteine residue (Cys270) is strictly conserved. Cys270 is far from the synthetase active site but is embedded in a highly hydrophobic region formed by three strictly conserved, and consecutive, phenylalanine residues (Phe323, Phe325 and Phe327) (Figure 8(A,C)). Such hydrophobic pocket likely favours the anchoring and stabilisation of the Ebselen’s benzyl rings. The β-strand containing these residues (β-20) appears to be tightly packed along with the contiguous β-21, both shaping the ATP-binding site (Asp331 and Phe344, respectively) and harbouring amino acids that place substrates’s reactive groups in the right orientation and proximity of the nucleophilic facilitator residue Ser352 (i.e. Arg329 and Asn347 are hydrogen bonded to γ-phosphate of ATP and glycyl carboxylate of GSH) (Figure 8(D)). Thus, it is tempting to speculate that Ebselen binding to Cys270 has a negative allosteric effect on enzyme active site by destabilising the conformation of strand β-20 (and the vicinal bonds network with strand β-21) and, consequently, by disrupting interactions that might be critical for orienting the substrates in catalytically active conformations.
Mode of inhibition of TbTryS by 17
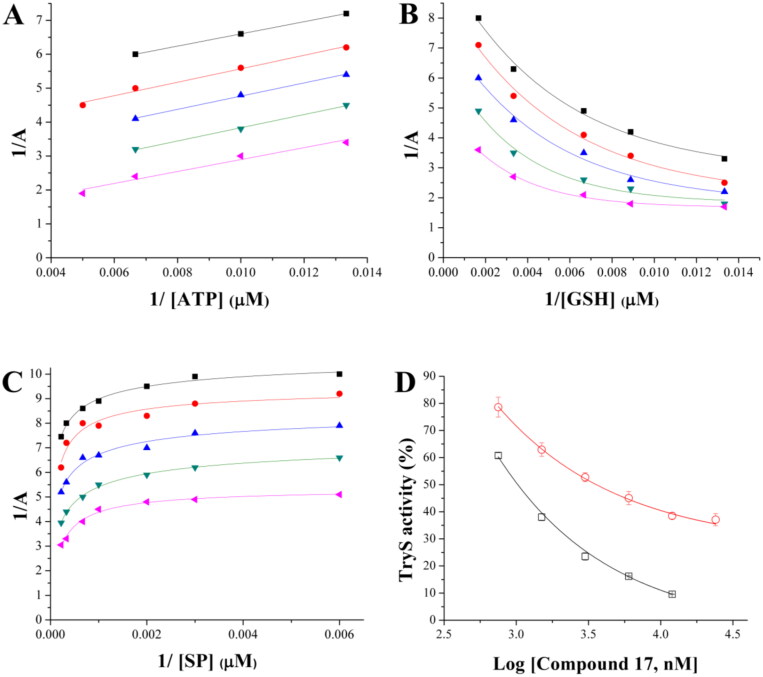
The mode of TryS inhibition was also investigated for 17, which represented the most potent and selective singleton from the ENAMINE library targeting the T. brucei enzyme. At variance with Ebselen, TbTryS inhibition by 17 did not require a pre-incubation of the enzyme with the inhibitor in the absence of substrates (not shown).
Kinetic analysis revealed a complex and substrate-dependent behaviour for the inhibitor. When ATP was the varying substrate, increasing inhibitor concentrations correlated with an increase in enzyme’s Vmax and KM that rendered a parallel pattern of the double reciprocal substrate versus velocity plots (Figure 9(A)). This behaviour is typical of an uncompetitive inhibition and indicates that the inhibitor does not interfere with ATP binding but, instead, formation of the TbTryS-ATP complex favours the interaction with (inhibition by) 17. For GSH as varying substrate, the Lineweaver-Burk plots were fitted to an exponential equation (R2 ≥ 0.98) because inhibition by substrate is observed at >250 µM GSH (Figure 9(B)). The plots’ pattern shows dependency on GSH concentration, with converging curves at low GSH concentrations (<33 µM) and parallel curves at high GSH concentrations (>33 µM). This behaviour suggests that 17 does not compete with GSH but exert an allosteric inhibition of TryS (see Equations for substrate inhibition in Supplemental material). With respect to SP, the reciprocal plots displayed a non-linear and parallel pattern, which is compatible with an uncompetitive inhibition of TbTryS (Figure 9(C)). Due to the low KM value of TbTryS for Gsp (∼2.4 µM)33, and the low sensitivity of the method used to monitor enzyme activity (Figure S1), it was not possible to perform reactions at sub-KM concentrations of this substrate. Thus, enzyme inhibition was tested at a single and saturating concentration of Gsp (100 µM) and the inhibition profile compared to that obtained for SP, also tested at a saturating concentration (4.5 mM). As shown in Figure 9(D), 17 exerts a similar exponential decay in TbTryS activity under saturating concentrations of both polyamine substrates. However, the lower degree of TbTryS inhibition achieved by 17 in the presence of Gsp suggests that the last displaces the inhibitor from the polyamine binding-site better than SP.
Figure 9.
Inhibition mechanism of 17 towards TbTryS. Lineweaver–Burk plots are shown for the varying substrate: (A) ATP, (B) glutathione (GSH) and (C) spermidine (SP). The kinetic analysis was performed at different inhibitors (black square: 12 µM, red circle: 6 µM, blue triangle: 3 µM, green inverted triangle: 1.5 µM, and pink triangle: 0.75 µM) and single substrate concentrations while maintaining fixed the concentration of the co-substrates (4.5 mM for SP, 200 µM for ATP and 150 µM for GSH). Enzyme velocity was measured using an end-point assay (see the section 1.3 Materials and methods for details). (D) TbTryS activity determined at different inhibitor concentrations and saturating concentrations of all substrates (SP: black empty square and Gsp: red empty circle).
Discussion
For its multiple and essential roles in parasite biology and drug resistance, the trypanothione-dependent metabolism of trypanosomatids has attracted attention for the development of specific and effective drugs against these pathogens43–45.
Although TryS proved druggable, the identification and design of inhibitors is challenging due to some peculiar structural and mechanistic features of this enzyme. TryS’ active site accommodates four substrates and contains several regions involved in substrate binding that are highly dynamic46,47. During catalysis, the protein adopts different conformations and establishes kinetic complexes with substrates and their intermediate reactants48. Such conformational plasticity give rise to large variations in the substrates KM values among TryS and may explain the reported species-specific inhibition by compounds25. Except for Gsp, the other substrates of TryS (i.e. GSH, ATP and SP) are ubiquitous to and found at high intracellular concentrations in the parasite and host. Thus, the identification of non-competitive inhibitors is highly desirable to overcome competition by a high physiological concentration of substrates and to avoid potential off-target effects. Our screening assay was performed at near-physiological concentrations (≥KM values) of substrates and, hence, highly biased towards the detection of non-competitive or high-affinity competitive ligands. This is in line with the low hit ratio against the molecular target of our screening approach (0.05%) and the good correlation between in vitro TbTryS inhibition (IC50 1–36 µM) and the selective anti-proliferative activity against bloodstream T. brucei (EC50 5–25 µM, seven hits with SI >10). In contrast, a previous screening campaign against TbTryS conducted at sub-KM concentrations of SP (25 µM) and GSH (20 µM) reported a 24-fold higher hit ratio (1.14%), albeit the hits displayed a 10- to 100-fold lower biological activity (i.e. IC50 ≪ EC50)12. Although several biological arguments were raised by the authors to explain this difference, the more reasonable explanation is that compounds' potency towards TryS was overestimated under sub-KM assay conditions.
Most of the broad spectrum TryS inhibitors here identified belong to a library containing molecules with known biochemical and physiological activities, although not all of them with desirable properties as drug candidates. For instance, 6-hydroxy-dl-DOPA (3) is a precursor of the catecholaminergic neurotoxin 6-hydroxydopamine used as drug model to induce neurotoxicity in animal models49. 6-Hydroxy-dl-DOPA has been reported as an allosteric inhibitor of proteins involved DNA repair (APE1 and RAD52) by interfering with the formation of protein complexes50,51. TryS are monomeric enzymes and structurally unrelated to APE1 and RAD52, moreover 6-hydroxy-dl-DOPA lacks structural similarity with any of the TryS’ substrates. Thus, the remarkable capacity of the compound to target Tri-Tryp TryS suggests a common binding mode, likely at an allosteric site, which deserves further investigation. In this respect and given the lack of pharmacological profile of 6-hydroxy-dl-DOPA and its weak anti-trypanosomatid activity, this scaffold may nevertheless serve as building block for a fragment-based design of multi-species TryS inhibitors.
NH125 (4) is an imidazolium N-alkylated derivative and a non-specific colloidal aggregator that, as such, inhibits non-specifically and only in vitro different kinases (eukaryotic elongation factor 2 (eEF-2) kinase, protein kinase A and C, histidine kinases)52. Therefore, this compound can be disregarded as a true active site inhibitor and its potent anti-trypanosomatid activity is likely associated to its capacity to disrupt cell membranes in a detergent-like fashion52.
Sanguinarine chloride (6) is a polycyclic alkaloid from plants with broad spectrum microbial and cytotoxic activity by targeting multiple molecules and processes53. In eukaryotic cells, it induces an early apoptotic response linked to a substantial depletion of intracellular GSH54. Although the compound has no prospect for further clinical applications, biochemically guided molecular downsizing may open the possibility for the development of TryS inhibitors with improved biological activity and selectivity.
Calmidazolium chloride (7) is a positively charged and hydrophobic molecule that has been suggested to exert its biological effect by destabilising membrane phospholipids55 rather than by being a calmodulin antagonist56. This compound was one of the most potent and selective anti-Trypanosoma molecule identified in the biological screening. Interestingly, trypanosomal calmodulin has been shown less sensitive than its vertebrate homologue to inhibition by calmidazolium57, which all together suggests that calmidazolium has a different molecular target in the pathogens.
Ebselen (5) is an organoselenium compound reported as cysteine oxidant or modifier58–60 that inhibits Tri-Tryp TryS within a narrow range of concentrations (IC50 = 3–14 µM). Despite Ebselen may show some promiscuity for modifying thiol groups, not all cysteine residues are capable to react and generate stable complexes with it. This is largely determined by solvent accessibility, polarity of the surrounding medium/residues and thiol pKa. In fact, 6 out of 10 cysteine residues conserved among TryS are accessible to the solvent, likely a few of them deprotonated (to favour the nucleophilic attack on the β-lactam group of Ebselen) and immersed in a non-polar environment to facilitate interactions with the aromatic rings of the ligand. Such criteria were apparently met by two residues from the amidase active site (Cys51 and Cys57 ) and one from the synthetase domain (Cys270), as suggested by mass spectrometry analysis of Ebselen-Cys protein covalent complexes. Cys270 is at the N-terminus of the longest and highly conserved α-helix (26-residues long: His271-Glu297 in LiTryS) of TryS. In this position, thiol deprotonation of Cys270 is favoured by the α-helix dipole moment. Although helix α5 is not part of the synthetase active site, it likely serves as structural scaffold for stabilising the nearby strands β-20 and β-21 that shape the nucleotide binding site. It is then tempting to speculate that the incorporation of a bulky Ebselen moiety in helix α5 will exert a conformational change on the vicinal β-20 and -21 leading to the allosteric inhibition of the enzyme. Supporting this hypothesis, alkylation of cysteines residues with the small molecule iodoacetamide had negligible effects on TryS activity. Worth noting, Cys270 as well as other residues and structural elements targeted by this inhibition mechanism are highly conserved in TryS, which agrees with the wide-spectrum inhibitory activity of Ebselen towards Tri-Tryp TryS.
Ebselen was the only hit for which there was a clear correlation between anti-trypanosomal activity and on-target effect in T. brucei (intracellular oxidative milieu). This compound has been shown to inhibit trypanothione reductase by covalent formation of seleno-sulphide bonds with enzyme's cysteines60. Thus, the strong oxidant effect exerted by Ebselen against bloodstream T. brucei is likely consequence of the simultaneous inhibition of two major enzymes from the parasite thiol-redox metabolism: trypanothione reductase and TryS. Ebselen represents an interesting multi-target compound (the essential Cys327 from T. brucei hexokinase has also been shown to be irreversibly oxidised and inactivated by Ebselen)61,62 that has been explored in different clinical trials including acute ischaemic stroke63, bipolar disorders in substitution of lithium-therapy64,65 and prevention of noise-induced hearing loss66. The promiscuity of Ebselen to react with cysteine residues may hamper its clinical efficacy (i.e. sequestration by the cysteine residue from the abundant serum albumin) and repositioning. However, our findings open the possibility for designing new derivatives with improved target selectivity.
Notably, and with the exception of 6-hydroxy-dl-DOPA, a common feature of the multi-TryS inhibitors described above is their remarkable hydrophobic nature, which suggests the presence of druggable hydrophobic pockets in the enzyme.
In contrast to the pan-inhibitory activity of the hits from the in-house library, screening of the ENAMINE library yielded inhibitors (scaffolds and singletons) highly specific for TbTryS. Among them, the AMPTPh scaffold shares a common pharmacophore structure (with two hydrogen bond atoms in a 1.5 relationship and one of them located in the thiazole ring) with the phenyl thiazole-based compounds (Figure 1) previously identified as TbTryS hits12. Similar to the hits from the in-house library, compound’s aromaticity seems to be an important determinant of activity that is conserved in the scaffolds CPA, AMTPh and AMPh, as well as in several singletons. As example, analogues of 17 (potent inhibitor of TbTryS) devoid of the adamantine moiety were inactive against TbTryS. Lipophilic substitutions in non-competitive, albeit structurally unrelated, TryS hits also proved relevant for inhibitory activity24. Worth noting, both scaffolds consist of a central heterocyclic ring (thiazole for AMTPh and piperidine for CPA) that links aromatic rings of different chemical nature. For the CPA analogues, the aromatic moieties are linked to the heterocycle by acetamide bonds, which suggest that both substituents may freely adopt different spatial conformations. A similar flexibility is expected for one of the substituents of the AMTPh analogues whereas the benzene ring of the second substituent may adopt a planar orientation with the thiazole ring. Probably, this conformational flexibility favour accommodation into the enzyme active site that is structurally complex35.
Our biological data also show an overall higher selectivity index towards T. brucei for the hits from the in-house library than for those from the ENAMINE library, and highlights the difficulties in identifying bioactive and selective compounds acting on the intracellular form of pathogenic trypanosomatids (e.g. T. cruzi and L. infantum). Differences in the proteome of the infective stage of trypanosomatid species and/or in the molecular targets of the hits may in part account for the low attrition rate. On the other hand, compound metabolisation, sequestration, efflux or modification by the host cell are additional factors that may affect the bioactivity against intracellular pathogens and represent major challenges for drug development. Worth noting, at least three hits (4, 5 and 7) showed a killing potency against T. cruzi amastigotes in the same order of magnitude than that of the clinical drug benznidazole. Although several hits from the in-house library were active against L. donovani amastigotes, selectivity was highly compromised. Leishmania amastigotes reside in the parasitophorous vacuole of macrophages, a hybrid compartment derived from the host endocytic- and containing elements from the secretory-pathway67. In addition, to its extreme low pH (4.7–5.3)68, the parasitophorous vacuole contains several hydrolytic, proteolytic and efflux activities that may eventually reduce compounds bioavailability and activity. Importantly, our study identified seven new biological hits against bloodstream T. brucei.
Our drug discovery campaign disclosed new chemical structures targeting a key enzyme of the parasite redox metabolism and the infective stage of pathogenic trypanosomatids. The hits represent good candidates to undertake optimisation strategies aimed to enhance their inhibitory activity against TryS and pharmacological properties. Last but least, the full set of data (positive and negative results) collected in the TryS-screening deems valuable for feeding artificial intelligence drug discovery approaches69, which have the potential for screening larger virtual chemolibraries (including drug repositioning libraries).
Supplementary Material
Acknowledgements
The authors thank Dr Alan Fairlamb (Dundee University, Scotland) for providing expression plasmid for TryS from T. brucei. D. B. acknowledges Dr Hyu Ho Paul Park (Dengue Research Laboratory, Institut Pasteur Korea, South Korea) for assistance during protein purification. The authors thank Fanny Lenglart (Université de Caen Normandie, France) for her assistance to FS during her short-internship at Institut Pasteur of Montevideo. DB thanks Institut Pasteur Korea staff, in particular its former Chief Executive Officer Dr Hakim Djaballah, for his kind help during D. B.’s internship in South Korea.
Funding Statement
This work was supported by the “Le Réseau International des Instituts Pasteur (RIIP)”, "Actions Concertees Internacionales Pasteuriennes" [Grant nos. 17–2015]; and FOCEM (Fondo para la Convergencia Estructural del Mercosur) [Grant no. COF 03/11].
Disclosure statement
The authors report no conflict of interest.
References
- 1.WHO. Research priorities for Chagas disease, Human African Trypanosomiasis and Leishmaniasis. Technical report of the TDR disease reference group on Chagas disease, Human African Trypanosomiasis and Leishmaniasis. Technical Report 2012. https://apps.who.int/iris/handle/10665/77472 [PubMed]
- 2.Comini MA, Flohé L.. The trypanothione-based redox metabolism of trypanosomatids. In: T Jäger, O Koch, L Flohé, eds. Trypanosomatids diseases: molecular routes to drug discovery (drug discovery in infectious diseases). Oxford: Wiley-Blackwell; 2013:167–199. [Google Scholar]
- 3.Anantharaman V, Aravind L.. Evolutionary history, structural features and biochemical diversity of the NlpC/P60 superfamily of enzymes. Genome Biol 2003;4:R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fawaz MV, Topper ME, Firestine SM.. The ATP-grasp enzymes. Bioorg Chem 2011;39:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comini MA, Biosynthesis of polyamine–glutathione derivatives in Enterobacteria and Kinetoplastida. In: Flohé L, ed. Glutathione. London: CRC Press; 2018:285–305. [Google Scholar]
- 6.Comini MA, Guerrero SA, Haile S, et al. Validation of Trypanosoma brucei trypanothione synthetase as drug target. Free Radic Biol Med 2004;36:1289–302. [DOI] [PubMed] [Google Scholar]
- 7.Ariyanayagam MR, Oza SL, Guther ML, Fairlamb AH.. Phenotypic analysis of trypanothione synthetase knockdown in the African trypanosome. Biochem J 2005;391:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sousa AF, Gomes-Alves AG, Benítez D, et al. Genetic and chemical analyses reveal that trypanothione synthetase but not glutathionylspermidine synthetase is essential for Leishmania infantum. Free Radic Biol Med 2014;73:229–38. [DOI] [PubMed] [Google Scholar]
- 9.Mesías AC, Sasoni N, Arias DG, et al. Trypanothione synthetase confers growth, survival advantage and resistance to anti-protozoal drugs in Trypanosoma cruzi. Free Radic Biol Med 2019;130:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manta B, Bonilla M, Fiestas L, et al. Polyamine-based thiols in trypanosomatids: evolution, protein structural adaptations, and biological functions. Antioxid Redox Signal 2018;28:463–86. [DOI] [PubMed] [Google Scholar]
- 11.Wyllie S, Oza SL, Patterson S, et al. Dissecting the essentiality of the bifunctional trypanothione synthetase-amidase in Trypanosoma brucei using chemical and genetic methods. Mol Microbiol 2009;74:529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torrie LS, Wyllie S, Spinks D, et al. Chemical validation of trypanothione synthetase: a potential drug target for human trypanosomiasis. J Biol Chem 2009;284:36137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medeiros A, Benítez D, Korn RS, et al. Mechanistic and biological characterisation of novel N5-substituted paullones targeting the biosynthesis of trypanothione in Leishmania. J Enzyme Inhib Med Chem 2020;35:1345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olin-Sandoval V, González-Chávez Z, Berzunza-Cruz M, et al. Drug target validation of the trypanothione pathway enzymes through metabolic modelling. Febs J 2012;279:1811–33. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Lin CH, Walsh CT, Coward JK.. Novel inhibitors of trypanothione biosynthesis: synthesis and evaluation of a phosphinate analog of glutathionyl spermidine (GSP), a potent, slow binding inhibitor of GSP synthetase. Bioorg Med Chem Lett 1997;7:505–10. [Google Scholar]
- 16.Chen S, Lin CH, Kwon DS, et al. Design, synthesis, and biochemical evaluation of phosphonate and phosphonamidate analogs of glutathionylspermidine as inhibitors of glutathionylspermidine synthetase/amidase from Escherichia coli. J Med Chem 1997;40:3842–50. [DOI] [PubMed] [Google Scholar]
- 17.Verbruggen C, De Craecker S, Rajan PK, et al. Phosphonic acid and phosphinic acid tripeptides as inhibitors of glutathionylspermidine synthetase. Bioorg Med Chem Lett 1996;6:253–8. [Google Scholar]
- 18.Kwon DS, Lin CH, Chen S, et al. Dissection of glutathionylspermidine synthetase/amidase from Escherichia coli into autonomously folding and functional synthetase and amidase domains. J Biol Chem 1997;272:2429–36. [DOI] [PubMed] [Google Scholar]
- 19.Lin CH, Chen S, Kwon DS, et al. Aldehyde and phosphinate analogs of glutathione and glutathionylspermidine: potent, selective binding inhibitors of the E. coli bifunctional glutathionylspermidine synthetase/amidase. Chem Biol 1997;4:859–66. [DOI] [PubMed] [Google Scholar]
- 20.Amssoms K, Oza SL, Ravaschino E, et al. Glutathione-like tripeptides as inhibitors of glutathionylspermidine synthetase. Part 1: substitution of the glycine carboxylic acid group. Bioorg Med Chem Lett 2002;12:2553–6. [DOI] [PubMed] [Google Scholar]
- 21.Amssoms K, Oza SL, Augustyns K, et al. Glutathione-like tripeptides as inhibitors of glutathionylspermidine synthetase. Part 2: substitution of the glycine part. Bioorg Med Chem Lett 2002;12:2703–5. [DOI] [PubMed] [Google Scholar]
- 22.Oza SL, Chen S, Wyllie S, et al. ATP-dependent ligases in trypanothione biosynthesis-kinetics of catalysis and inhibition by phosphinic acid pseudopeptides. Febs J 2008;275:5408–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Silva C, Daunes S.. Structure-activity study on the in vitro antiprotozoal activity of glutathione derivatives. J Med Chem 2000;43:2072–8. [DOI] [PubMed] [Google Scholar]
- 24.Spinks D, Torrie LS, Thompson S, et al. Design, synthesis and biological evaluation of Trypanosoma brucei trypanothione synthetase inhibitors. ChemMedChem 2012;7:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benítez D, Medeiros A, Fiestas L, et al. Identification of novel chemical scaffolds inhibiting trypanothione synthetase from pathogenic trypanosomatids. PLoS Negl Trop Dis 2016;10:e0004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orban OC, Korn RS, Benítez D, et al. 5-Substituted 3-chlorokenpaullone derivatives are potent inhibitors of Trypanosoma brucei bloodstream forms. Bioorg Med Chem 2016;24:3790–800. [DOI] [PubMed] [Google Scholar]
- 27.Biebinger S, Wirtz LE, Lorenz P, Clayton C.. Vectors for inducible expression of toxic gene products in bloodstream and procyclic Trypanosoma brucei. Mol Biochem Parasitol 1997;85:99–112. [DOI] [PubMed] [Google Scholar]
- 28.Gutscher M, Pauleau AL, Marty L, et al. Real-time imaging of the intracellular glutathione redox potential. Nat Methods 2008;5:553–9. [DOI] [PubMed] [Google Scholar]
- 29.Hirumi H, Hirumi K.. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol 1989;75:985–9. [PubMed] [Google Scholar]
- 30.Maiwald F, Benítez D, Charquero D, et al. 9- and 11-Substituted 4-azapaullones are potent and selective inhibitors of African trypanosoma. Eur J Med Chem 2014;83:274–83. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho PC, Lima DB, Leprevost FV, et al. Integrated analysis of shotgun proteomic data with PatternLab for proteomics 4.0. Nat Protoc 2016;11:102–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Riverol Y, Csordas A, Bai J, et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 2019;47:D442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oza SL, Ariyanayagam MR, Aitcheson N, Fairlamb AH.. Properties of trypanothione synthetase from Trypanosoma brucei. Mol Biochem Parasitol 2003;131:25–33. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JH, Chung TD, Oldenburg KR.. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 1999;4:67–73. [DOI] [PubMed] [Google Scholar]
- 35.Don R, Ioset JR.. Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitology 2014;141:140–6. [DOI] [PubMed] [Google Scholar]
- 36.Ebersoll S, Bogacz M, Günter LM, et al. A tryparedoxin-coupled biosensor reveals a mitochondrial trypanothione metabolism in trypanosomes. Elife 2020;9:e53227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franco J, Sardi F, Szilágyi L, et al. Diglycosyl diselenides alter redox homeostasis and glucose consumption of infective African trypanosomes. Int J Parasitol Drugs Drug Resist 2017;7:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco J, Medeiros A, Benítez D, et al. In vitro activity and mode of action of distamycin analogues against African trypanosomes. Eur J Med Chem 2017;126:776–88. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez Arce E, Putzu E, Lapier M, et al. New heterobimetallic ferrocenyl derivatives are promising antitrypanosomal agents. Dalton Trans 2019;48:7644–58. [DOI] [PubMed] [Google Scholar]
- 40.Franco J, Scarone L, Comini MA.. Novel distamycin analogues that block the cell cycle of African trypanosomes with high selectivity and potency. Eur J Med Chem 2020;189:112043. [DOI] [PubMed] [Google Scholar]
- 41.Rivas F, Medeiros A, Quiroga C, et al. New Pd-Fe ferrocenyl antiparasitic compounds with bioactive 8-hydroxyquinoline ligands: a comparative study with their Pt-Fe analogues. Dalton Trans 2021;50:1651–65. [DOI] [PubMed] [Google Scholar]
- 42.Ortíz C, Moraca F, Laverriere M, et al. Glucose 6-phosphate dehydrogenase from trypanosomes: selectivity for steroids and chemical validation in bloodstream Trypanosoma brucei. Molecules 2021;26:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franco J, Scarone L, Comini MA, Drugs and drug resistance in African and American Trypanosomiasis. In: Botta M, ed. Annual reports in medicinal chemistry: neglected diseases: extensive space for modern drug discovery. New York (NY): Academic Press, Elsevier Inc.; 2018:97–133. [Google Scholar]
- 44.Talevi A, Carrillo C, Comini M.. The thiol-polyamine metabolism of Trypanosoma cruzi: molecular targets and drug repurposing strategies. Curr Med Chem 2019;26:6614–35. [DOI] [PubMed] [Google Scholar]
- 45.Saccoliti F, Di Santo R, Costi R.. Recent advancement in the search of innovative antiprotozoal agents targeting trypanothione metabolism. ChemMedChem 2020;15:2420–35. [DOI] [PubMed] [Google Scholar]
- 46.Fyfe PK, Oza SL, Fairlamb AH, Hunter WN.. Leishmania trypanothione synthetase-amidase structure reveals a basis for regulation of conflicting synthetic and hydrolytic activities. J Biol Chem 2008;283:17672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koch O, Cappel D, Nocker M, et al. Molecular dynamics reveal binding mode of glutathionylspermidine by trypanothione synthetase. PLoS One 2013;8:e56788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leroux AE, Haanstra JR, Bakker BM, Krauth-Siegel RL.. Dissecting the catalytic mechanism of Trypanosoma brucei trypanothione synthetase by kinetic analysis and computational modeling. J Biol Chem 2013;288:23751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duty S, Jenner P.. Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol 2011;164:1357–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simeonov A, Kulkarni A, Dorjsuren D, et al. Identification and characterization of inhibitors of human apurinic/apyrimidinic endonuclease APE1. PLoS One 2009;4:e57403rd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandramouly G, McDevitt S, Sullivan K, et al. Small-molecule disruption of RAD52 rings as a mechanism for precision medicine in BRCA-deficient cancers. Chem Biol 2015;22:1491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devkota AK, Tavares CD, Warthaka M, et al. Investigating the kinetic mechanism of inhibition of elongation factor 2 kinase by NH125: evidence of a common in vitro artifact. Biochemistry 2012;51:2100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim W, Fricke N, Conery AL, et al. NH125 kills methicillin-resistant Staphylococcus aureus persisters by lipid bilayer disruption. Future Med Chem 2016;8:257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vlachojannis C, Magora F, Chrubasik S.. Rise and fall of oral health products with Canadian bloodroot extract. Phytother Res 2012;26:1423–6. [DOI] [PubMed] [Google Scholar]
- 55.Debiton E, Madelmont JC, Legault J, Barthomeuf C.. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol 2003;51:474–82. [DOI] [PubMed] [Google Scholar]
- 56.Anderson KW, Coll RJ, Murphy AJ.. Inhibition of skeletal muscle sarcoplasmic reticulum CaATPase activity by calmidazolium. J Biol Chem 1984;259:11487–90. [PubMed] [Google Scholar]
- 57.Gietzen K. Comparison of the calmodulin antagonists compound 48/80 and calmidazolium. Biochem J 1983;216:611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Marchan Y, Sojo F, Rodriguez E, et al. Trypanosoma cruzi calmodulin: cloning, expression and characterization. Exp Parasitol 2009;123:326–33. [DOI] [PubMed] [Google Scholar]
- 59.Terentis AC, Freewan M, Sempértegui Plaza TS, et al. The selenazal drug Ebselen potently inhibits indoleamine 2,3-dioxygenase by targeting enzyme cysteine residues. Biochemistry 2010;49:591–600. [DOI] [PubMed] [Google Scholar]
- 60.Lu J, Vodnala SK, Gustavsson AL, et al. Ebsulfur is a benzisothiazolone cytocidal inhibitor targeting the trypanothione reductase of Trypanosoma brucei. J Biol Chem 2013;288:27456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joice AC, Harris MT, Kahney EW, et al. Exploring the mode of action of Ebselen in Trypanosoma brucei hexokinase inhibition. Int J Parasitol Drugs Drug Resist 2013;3:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharlow ER, Lyda TA, Dodson HC, et al. A target-based high throughput screen yields Trypanosoma brucei hexokinase small molecule inhibitors with antiparasitic activity. PLoS Negl Trop Dis 2010;4:e659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaguchi T, Sano K, Takakura K, et al. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen study group. Stroke 1998;29:12–7. [DOI] [PubMed] [Google Scholar]
- 64.Masaki C, Sharpley AL, Cooper CM, et al. Effects of the potential lithium-mimetic, Ebselen, on impulsivity and emotional processing. Psychopharmacology (Berl) 2016;233:2655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh N, Sharpley AL, Emir UE, et al. Effect of the putative lithium mimetic Ebselen on brain myo-inositol, sleep, and emotional processing in humans. Neuropsychopharmacology 2016;41:1768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kil J, Lobarinas E, Spankovich C, et al. Safety and efficacy of Ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017;390:969–79. [DOI] [PubMed] [Google Scholar]
- 67.Young J, Kima PE.. The Leishmania parasitophorous vacuole membrane at the parasite–host interface. Yale J Biol Med 2019;92:511–21. [PMC free article] [PubMed] [Google Scholar]
- 68.Antoine JC, Prina E, Jouanne C, Bongrand P.. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect Immun 1990;58:779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alice JI, Bellera CL, Benítez D, et al. Ensemble learning application to discover new trypanothione synthetase inhibitors. Mol Divers 2021;25:1361–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.