ABSTRACT
Vitamins (Vit) C and D are widely used as immunogenic supplements among severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected patients. The SAR-CoV-2 virus enters into the pulmonary endothelial cells through attachment to angiotensin converting enzyme 2 receptor (Ace2) and the proteolytic activity of Cathepsin L (Ctsl) and transmembrane serine protease 2 (Tmprss2) enzymes. This study aimed to determine the influence of Vit C and D on the mRNA expression of Ace2, Tmprss2, and Ctsl genes in the mouse lungs. Vitamins C and D were administrated to different groups of mice through intra-peritoneal route in doses equivalent to human for 30 days. Then, the mRNA expression of SARS-CoV-2 entry gene was analyzed using qRT-PCR. It is found that Vit D, but not C, upregulated significantly (P < 0.05) the mRNA expression of Ace2 by more than six folds, while downregulated the expression of Ctsl and Tmprss2 genes by 2.8 and 2.2 folds, respectively. It can be concluded from this study that Vit D alters the mRNA expression of Ace2, Tmprss, and Ctsl genes in the mouse lungs. This finding can help us in understanding, at least in part, the molecular influence of Vit D on genes involved in the entry of SARS-CoV-2 into the cells.
KEYWORDS: Ace2, COVID-19, lungs, SARS-CoV-2, vitamin C, vitamin D
1. Introduction
COVID-19 infection is a global endemic disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2]. This virus infects the epithelial cells in the lung through interaction between the viral spike and certain proteins in the host cells [3]. SARS-CoV-2 attaches with the angiotensin converting enzyme type 2 (ACE2) receptor on the host cells [4,5]. Cathepsin isoform L (Ctsl) is a protease enzyme which cuts the S-glycoprotein in SARS-CoV-2, which facilitates the attachment of the virus to ACE2 receptor on the host cells. Furthermore, the transmembrane protease serine 2 (TMPRSS2) enhances the entrance of SARS-CoV-2 to the host cells through splitting certain parts in the virus [6]. It was pointed out that alterations in the expression of Ace2, Tmprss, and Ctsl proteins in the host cells affect the risk and severity of COVID-19 infection [7,8].
Immunogenic agents are widely prescribed for COVID-19 patients [9]. Vitamin (Vit) D affects the immune system and modulates both the innate and adaptive responses. Low plasma level of Vit D was associated with increased susceptibility to COVID-19 infection [10]. Jain et al., 2020, showed that Vit D level was low among critically ill COVID-19 patients [11]. Accordingly, they recommended to prescribe Vit D to high risk and COVID-19 infected patients.
Vitamin C plays a major role in immune defense through enhancing of the innate and adaptive immune system [12]. Chiscano-Camón et al., 2020, found that low level of plasma Vit C was associated with acute respiratory distress syndrome among COVID-19 patients [13]. However, the mechanism of how Vit C protects against acute respiratory distress syndrome is still unknown. Malla et al., 2021, reported that Vit C has a capability for direct inhibition of protease enzymes, which are responsible for SARS-CoV-2 entry [14]. The researchers used in silico method and showed that Vit C can inhibit the 3-chymotrypsin protease. Additionally, they reported that Vit C itself can inhibit other protease enzymes expressed in other viruses rather than SAR-CoV-2 [13].
Vitamins C and D are widely used for SARS-CoV-2 infected patients. However, their influences on SARS-CoV-2 entry receptors and enzymes are still not investigated. We hypothesized that Vit C and D decrease the mRNA expression of the SARS-CoV-2 entry genes. The results of the present study can increase the understanding of the influence of Vit C and D on the entrance of SARS-CoV-2 into the host cells.
2. Materials and methods
2.1. Chemicals
Vitamins C and D were bought from Sigma-Aldrich (St. Louis, USA). The Trizol solution was purchased from Thermo Fisher Scientific (Massachusetts, USA). The cDNA synthesis kit was obtained from Applied Biosystems (Massachusetts, USA). The SYBER GREEN master mixture for real-time PCR was bought from Takara (Tokyo, Japan). The oligo DNA primers were purchased from Integrated DNA technologies (Coralville, Iowa, USA).
2.2. Experimental animals
Twenty-eight male Balb/c mice (Mus musculus), with an average weight between 24 and 28 g, were obtained from the animal house of Al-Zaytoonah University (Amman, Jordan). The mice were handled according to the guide of Canadian Council on Animal Care [15] and the ethical committee at Al-Zaytoonah University approved the protocol of this study. The animals were housed at 23 ± 1°C, and a 12 hours light/12 hour dark cycle. All mice were fed with standard animal pellets ad libitum.
2.3. Experimental protocol
The mice were grouped according to the Vit administration, as follows:
Negative control group: the mice were administrated the drug vehicle 10% DMSO through the intraperitoneal route once daily.
Vitamin C group: the mice were administrated 179 mg/kg vit C, dissolved in 10% DMSO, through a single intraperitoneal dose once daily.
Vitamin D group: the mice were administrated 357 units/kg vit D, dissolved in 10% DMSO, through a single intraperitoneal dose once daily.
Each group contained seven mice. The Vit C and D were administrated to the animals for 1 month. The used doses of Vits, in this study, were equivalent to the daily dose of humans, depending on the surface area of the mouse’s body [16].
2.4. RNA extraction and cDNA synthesis
The mice were sacrificed at the last day of drug administration. Then, around 100 mg of lung was isolated from each mouse. The RNA was isolated from the mouse lungs using Trizol solution, as described by the instructions of the factory. After that, the isolated mRNA was converted to cDNA as following: 1 µg of total RNA was reconstituted in a reaction mixture containing 100 pmol oligo deoxythymine, 2.5 mM deoxy-nucleic triphosphate mixture, 0.1 M dithiothreitol, 1X reverse transcriptase buffer, and 10 units of reverse transcriptase isolated from Moloney Murine Leukemia Virus. This reaction mixture was incubated at 37°C for 50 minutes.
2.5. Gene expression analysis
The mRNA expression of Ace2, Ctsl, Tmprss 2 genes was analyzed in this research using quantitative real-time polymerase chain reaction (qRT-PCR). The primer sequence, amplicon size, and the annealing temperature for each amplified gene are shown in Table 1. Briefly, 10 ng of the synthesized cDNA was added to a reaction mixture containing 1X of SYPER GREEN master mix and 10 pmoles of sense and antisense primers. The PCR reaction consisted of 40 cycles. Each cycle was as the followings: denaturation step at 95°C for 12 sec, annealing step at 53−58°C for 30 sec, and lastly the extension step at 72°C for 25 sec. β-Actin gene was used as a housekeeping gene. The relative mRNA expression of the targeted genes was calculated using Δ ΔCT method [17].
Table 1.
The primer sequence, amplicon size, and annealing temperature of Ace2, Ctsl, and Tmprss genes
| Gene name | Forward | Reverse | Size | Annealing temp |
|---|---|---|---|---|
| Ace2 | ATTCACCCAACACTTGAGCC | TGTCCATCGAGTCATAAGGGT | 213 | 55 |
| Ctsl | AGGAAAATGGAGGTCTGGACT | GCAACAGAAATAGGCCCCAC | 205 | 58 |
| Tmprss 2 | CGTTCCCGTATACTCCAGGT | CGTTCCCGTATACTCCAGGT | 221 | 58 |
| β-Actin | CCCCTGAGGAGCACCGTGTG | ATGGCTGGGGTGTTGAAGGT | 106 | 53 |
Histological analysis
The lungs of the mice were isolated and washed with 0.9% normal saline, then fixed in 10% formalin. After that, dehydration of the lung samples was done through passing the samples through a graded series of alcohol followed by xylene. Then, the samples were embedded in paraffin wax. Lastly, hematoxylin and eosin were used to stain the sample sections.
2.6. Statistical analysis
The relative mRNA expression was expressed as a fold change in comparison with the negative control group. One-way ANOVA with Tukey’s post-test was used as a statistical tool for comparison between the mRNA expression of the tested genes of the control and Vit C and D treated groups. P value was considered significant when it was less than 0.05.
3. Results
3.1. mRNA levels of Ace2 gene after administration of Vit C and D
Figure 1 shows the effect of Vit C and D on the mRNA levels in the mouse lungs. It is found that Vit D upregulated significantly (P value <0.05) the mRNA expression of Ace2 in the lungs by six folds. The level of Ace2 mRNA was increased slightly (1.7 folds) after Vit C administration, however it failed to reach the statistical difference (P value >0.05).
Figure 1.
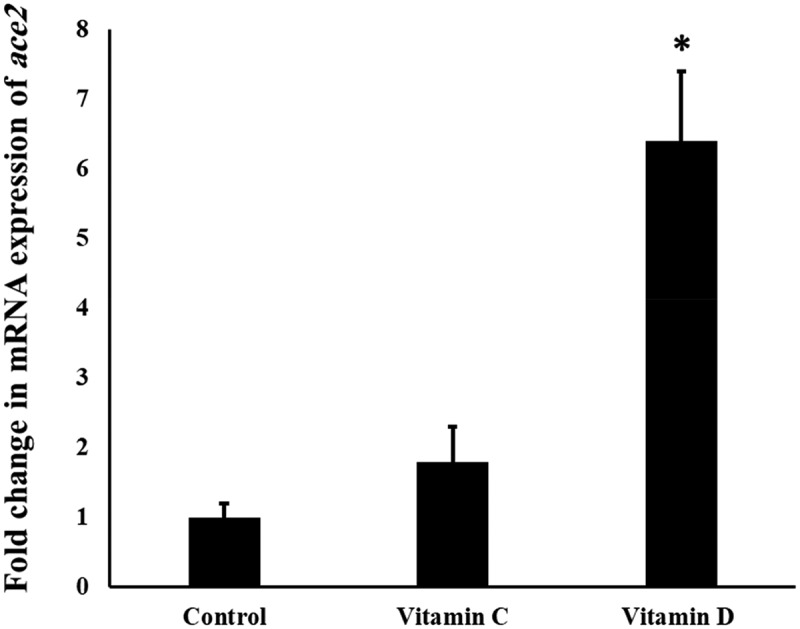
The influence of Vit C and D on the mRNA expression of Ace2 gene in the mouse lungs.
‘*’ indicates statistical significance (P value <0.05) using one-way ANOVA test.
3.2. mRNA levels of Tmprss gene after administration of Vit C and D
It was found in this study that Vit D downregulated significantly (P value <0.05) the mRNA expression of Tmprss2 gene in the mouse lungs by 2.8 folds, while Vit C did not show any significant influence on the expression of Tmprss2 gene, as represented by Figure 2.
Figure 2.
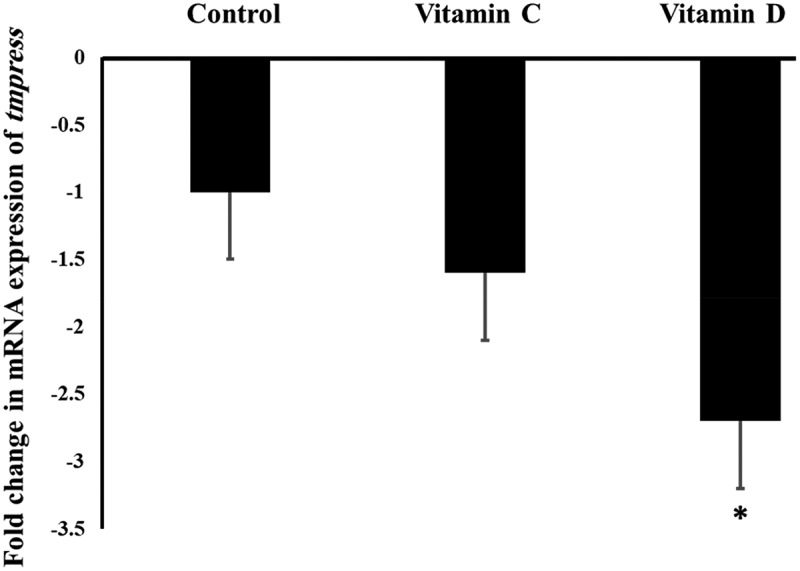
The influence of Vit C and D on the mRNA expression of Tmprss2 gene in the mouse lungs.
‘*’ indicates statistical significance (P value <0.05) using one-way ANOVA test.
4. mRNA levels of Ctsl gene after administration of Vit C and D
As shown in Figure 3, the expression of Ctsl gene in the mouse lungs was downregulated significantly (P value <0.05) after Vit D treatment by 2.2 folds, while Vit C did not show any significant (P value >0.05) influence on the expression of Ctsl gene.
Figure 3.
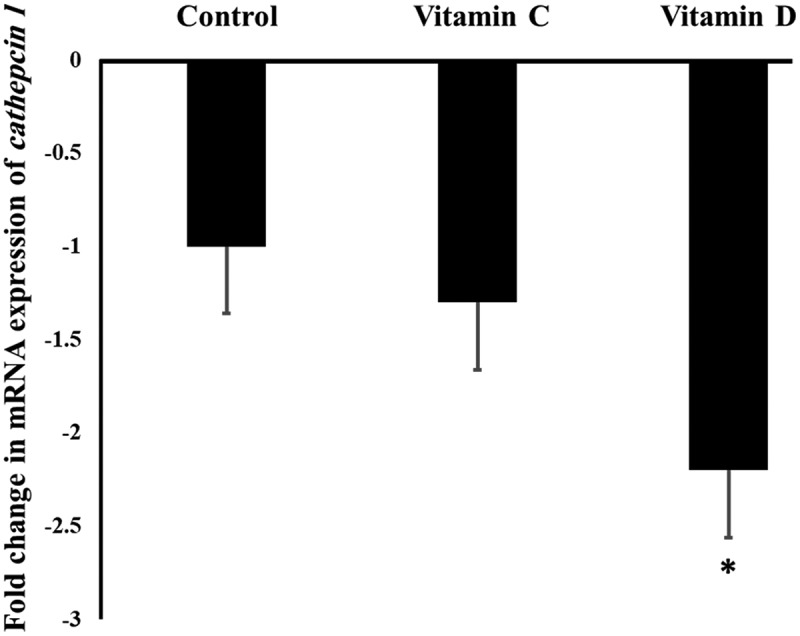
The influence of Vit C and D on the mRNA expression of Ctsl gene in the mouse lungs.
‘*’ indicates statistical significance (P value <0.05) using one-way ANOVA test.
Histological examination
Figure 4 represents the histological analysis of the lung sections after administration of Vit C and D to the mice for 1 month. The results showed that administration of Vit C and D did not induce pathohistological alterations in the mouse lungs.
Figure 4.
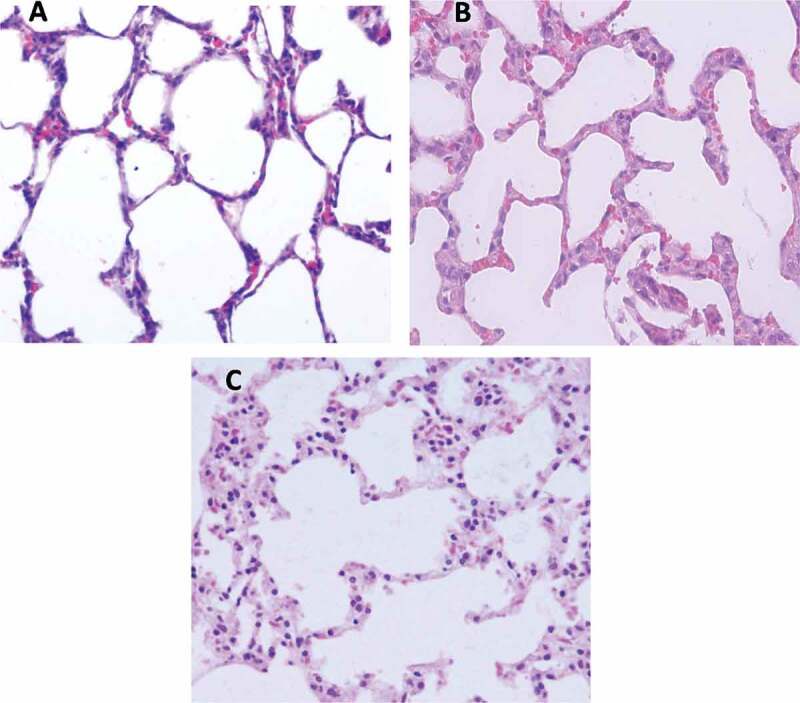
Light micrograph in the lung sections of mice received (A) the vehicle 10% DMSO, (B) Vit C dissolved in 10% DMSO, and (C) Vit D dissolved in 10% DMSO.
5. Discussion
The entry of SARS-CoV-2 virus in pulmonary cells requires the host Ace2, Tmprss, and Ctsl [18]. It is hypothesized that alterations in the functionality and number of Ace2, Tmprss, and Ctsl in the lung affect the severity and the spread of SARS-CoV-2 infection [19]. Vitamins C and D are widely administrated to SARS-CoV-2 infected patients, and as prophylaxis against COVID-19 infection [20]. Some clinical studies found that these Vits have favorable effects among SARS-CoV-2 infected patients [21]. Therefore, this study investigated the influence of Vit C and D on the mRNA expression of SARS-CoV-2 entry genes. It is found in this study that Vit D altered the mRNA expression of SARS-CoV-2 entry genes, while Vit C did not show a significant influence. Vitamin D upregulated (P < 0.05) the mRNA expression of Ace2, while downregulated significantly (P < 0.05) the mRNA expression of Tmprss2 and Ctsl genes in the treated mice. These results suggest that Vit D has a molecular influence on SARS-CoV-2 entry genes that can prevent or reduce the transmission of SARS-CoV-2 virus within the epithelial cells in the lung. Further investigations are needed to determine the role of Vit D in the prevention of the entry of SARS-CoV-2 into the epithelial cells.
It was found that Vit D levels were negatively correlated with the severity of COVID-19 infection symptoms [22]. Accordingly, it was suggested that Vit D plays a major role in reducing the severity of the disease symptoms, since Vit D has a boosting immunogenic effect [23]. The present study added another possible mechanism regarding the favorable effect of Vit D against COVID-19 infection that Vit D downregulated the mRNA expression of Ctsl and Tmprss2 while upregulated Ace2 that may affect the entry of SARS-CoV-2 into the epithelial cells.
Tmprss2 is considered as a target for the treatment of viral infections, including COVID-19 [24]. It was found that mucolytic ambroxol decreased influenza infection through inhibition of Tmprss2 protein [25]. Furthermore, Vit D has an anti-influenza effect [26]. In the current study, it was found that Vit D reduced the mRNA levels of Tmprss2, in addition to Ctsl. Therefore, it can be speculated that Vit D reduces the number of functional Tmprss2 and Ctsl and hence reduces the transmission of viral diseases.
The role of Ace2 in the severity of COVID-19 infection is still not fully understood. It was found that Ace2 expression level is negatively associated with the severity of COVID-19 infection [27]. It was reported that upregulation of Ace2 receptor decreases the activation of the renin-angiotensin-aldosterone system and hence reduces the possible harmful effects on the hearts and kidneys [28]. The results of this study showed that Ace2 was sharply upregulated by more than 6 folds in the mouse lungs after 30 days administration of Vit D. Interestingly, administration of Vit D can protect against cardiovascular diseases [29,30].
It was reported previously that Vit C has a potentiality to alter the expression of SARS-CoV-2 entry genes [31]. However, the present study did not find any significant influence of Vit C on the mRNA expression of SARS-CoV-2 entry genes in the mouse lungs. The difference between Vit C and D regarding their influence on SARS-CoV-2 entry genes might be due to the different physio-chemical properties of both Vits, where Vit D is more lipophilic than Vit C. Different water solubility can affect the distribution of Vits to specific organs, such as the lung. It was found that more lipophilic drugs can be widely distributed within the body [32]. In addition, more lipophilic drugs can inter the cells and activate transcriptional factors, while more water-soluble compounds have lower capacity to inter the cells [33].
There are some limitations in this study. First: the protein expressions of the tested genes were not analyzed. Second: this study evaluated only a single time point of 1-month administration of Vits. Further studies are needed to find out the influence of chronic administration of these Vits on the expression of Ace2, Tmprss2, and Ctsl.
6. Conclusion
The present study investigated the influence of the most commonly used Vits among humans during COVID-19 pandemic on the mRNA expression of SARS2-CoV-2 entry genes and found that Vit D induced the expression of Ace2 and reduced the expression of Tmprss2 and Cstl genes in the mouse lungs. These findings may increase our understanding of the molecular regulation of SARS2-CoV-2 entry genes by Vits.
Acknowledgments
The authors would like to thank Jouf University (Skaka, Saudi Arabia) and Al-Zaytoonah University (Amman, Jordan) for supporting this research.
Funding Statement
The authors extend their appreciation to the deanship of scientific research at Jouf University for funding this work through research grant no (DSR-2021-01-0215).
Ethics approval and consent to participate
The ethical committee at Al-Zaytoonah University approved the protocol of this study.
Human and animal rights
The mice were handled according to the guide of Canadian Council on Animal Care.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Almomani EY, Qablan AM, Atrooz FY, et al. The influence of coronavirus diseases 2019 (COVID-19) pandemic and the quarantine practices on university students’ beliefs about the online learning experience in Jordan. Front Public Health. 2020;8:595874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cui J, Li F, Shi Z-L.. Origin and evolution of pathogenic coronaviruses. Nature Rev Microbiol. 2019;17:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Glowacka I, Bertram S, Müller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sungnak W, Huang N, Becavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sabbah DA, Hajjo R, Bardaweel SK, et al. An updated review on betacoronavirus viral entry inhibitors: learning from past discoveries to advance COVID-19 drug discovery. Curr Top Med Chem. 2021;21:571–596. [DOI] [PubMed] [Google Scholar]
- [9].Hafeez A, Ahmad S, Siddqui SA, et al. A review of COVID-19 (Coronavirus Disease-2019) diagnosis. Treatments Prev. 2019. [Google Scholar]
- [10].Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jain A, Chaurasia R, Sengar NS, et al. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10(1):20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chiscano-Camon L, Ruiz-Rodriguez JC, Ruiz-Sanmartin A, et al. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care. 2020;24(1):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jr. TNMSPIPLADFMNGNP . Vitamin C inhibits SARS coronavirus-2 main protease essential for viral replication. biorxiv 2020.
- [15].Olfert ED, Cross BM, McWilliam AA. Guide to the care and use of experimental animals. Ottawa: Canadian Council on Animal Care; 1993. [Google Scholar]
- [16].Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hou F, Li S, Wang J, et al. Identification and validation of reference genes for quantitative real-time PCR studies in long yellow daylily, Hemerocallis citrina Borani. PloS one. 2017;12:e0174933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mahmoud IS, Jarrar YB, Alshaer W, et al. SARS-CoV-2 entry in host cells-multiple targets for treatment and prevention. Biochimie. 2020;175:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gkogkou E, Barnasas G, Vougas K, et al. Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol. 2020;36:101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Name JJ, Souza ACR, Vasconcelos AR, et al. Vitamin D and vitamin C: perspectives for COVID-19 with a focus on physical tissue barrier integrity. Front Nutr. 2020;7:606398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Speakman LL, Michienzi SM, Badowski ME. Vitamins, supplements and COVID-19: a review of currently available evidence. Drugs Context. 2021;10. DOI: 10.7573/dic.2021-6-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Teshome A, Adane A, Girma B, et al. The impact of vitamin D level on COVID-19 Infection: systematic review and meta-analysis. Front Public Health. 2021;9:624559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12:2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mahmoud IS, Jarrar YB. Targeting the intestinal TMPRSS2 protease to prevent SARS-CoV-2 entry into enterocytes-prospects and challenges. Mol Biol Rep. 2021;48:4667–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Depfenhart M, de Villiers D, Lemperle G, et al. Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy? Intern Emerg Med. 2020;15:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhou J, Du J, Huang L, et al. Preventive effects of vitamin D on seasonal influenza a in infants: a multicenter, randomized, open, controlled clinical trial. Pediatr Infect Dis J. 2018;37:749–754. [DOI] [PubMed] [Google Scholar]
- [27].Chaudhry F, Lavandero S, Xie X, et al. Manipulation of ACE2 expression in COVID-19. Open Heart. 2020;7(2):e001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dettlaff-Pokora A, Swierczynski J, Bagnato VS. Dysregulation of the Renin-Angiotensin-Aldosterone System (RAA) in Patients Infected with SARS-CoV-2-Possible Clinical Consequences. Int J Mol Sci. 2021;23(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Michos ED, Cainzos-Achirica M, Heravi AS, et al. Vitamin D, calcium supplements, and implications for cardiovascular health: JACC focus seminar. J Am Coll Cardiol. 2021;77:437–449. [DOI] [PubMed] [Google Scholar]
- [30].de la Guia-galipienso F, Martinez-Ferran M, Vallecillo N, et al. Vitamin D and cardiovascular health. Clin Nutr. 2021;40:2946–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ivanov V, Goc A, Ivanova S, et al. Inhibition of ACE2 expression by ascorbic acid alone and its combinations with other natural compounds. Infect Dis (Auckl). 2021;14:1178633721994605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Alavijeh MS, Chishty M, Qaiser MZ, et al. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx. 2005;2:554–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang Y, Luo XY, Wu DH, et al. ROR nuclear receptors: structures, related diseases, and drug discovery. Acta Pharmacol Sin. 2015;36:71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


