Abstract
Background and aims
Consumption of ultra-processed foods is negatively associated with health outcomes, however, the contribution to sleep quality is limited. Therefore, the objective of this study was to evaluate the association between food intake by frequency and degree of processing and sleep quality in adults during the covid-19 pandemic.
Methods
Population-based survey of adults from October to December 2020 in the Iron Quadrangle region, Brazil. The exposure variable was a food intake score that considered the frequency of consumption and food processing degree. The total score ranged from 0 (best) to 48 points (worst food quality), categorized into quartiles. Furthermore, we also evaluated whether individuals replaced their lunch and/or dinner based mostly on fresh/minimally processed foods for ultra-processed foods, for five or more days in the week. The outcome variable was sleep quality assessed with the Pittsburgh Sleep Quality Index. We constructed a contrasting directed acyclic graph (DAG) model to estimate the adjusted odds ratio of the association between score eating and sleep, by logistic regression.
Results
Most of the 1762 individuals evaluated had poor sleep quality (52.5%). The minimum and maximum food scores were 0 and 30 points (mean 9.16; 95% CI 8.50, 9.81). The higher values of the score corresponded to lower consumption of fresh and minimally processed foods and higher consumption of ultraprocessed foods. In multivariate analysis, individuals in the third food consumption score had 71% greater odds of poor sleep quality (OR = 1.71; 95% CI: 1.03, 2.85) and in the fourth quartile 144% greater odds (OR = 2.44; 95% CI: 1.32, 2.44). Besides, replacing the dinner meal with ultra-processed foods five days or more in the week was also associated with poor sleep quality (OR = 2.01; 95%CI: 1.14, 3.57).
Conclusion
Higher consumption of ultra-processed foods concomitant with lower consumption of fresh and minimally processed foods is associated with a higher chance of poor sleep quality.
Keywords: Food consumption, Ultra-processed foods, Sleep, SARS-CoV-2
Abbreviations: BMI, Body mass index; CI, Confidence interval; DAG, Directed acyclic graph; DQI-I, Diet Quality Index-International; FFQ, Food frequency questionnaire; GAD-7, Generalized Anxiety Disorder Scale; HEI, Healthy Eating Index; MW, Minimum wage; OR, Odds ratio; PSQI, Pittsburgh Sleep Quality Index; UK, United Kingdom; UPF, Ultra-processed foods
1. Introduction
Sleep is vital to the maintenance of physiological parameters and plays an important role in hormone release, cardiovascular activity, and glucose regulation [1]. Several longitudinal studies point to adverse health effects related to a sleep time restriction, including obesity, metabolic disorders, and chronic non-communicable disease risk [2]. Furthermore, sleep is a very critical component in times of pandemic, helping the immune system, and also interfering with the production of antibodies after vaccination or previous contact with the viral agent [3,4].
Sleep is considered one of the three main components of lifestyle, along with diet and physical activity, and is closely linked to health maintenance [5]. Poor sleep quality is often associated with unhealthy habits and lifestyle modifications, such as decreased physical activity and the consumption of high-calorie foods and beverages, and greater fat intake [6,7].
Currently, another important point to consider in dietary practices refers to the degree of food processing. Ultra-processed foods (UPF) are quickly replacing fresh or minimally processed and the traditional cuisine from most countries [[8], [9], [10], [11]].
UPF is related to worse nutritional intake, with high energy density, added sugars, sodium, saturated and trans fats, and refined carbohydrates [9,12,13] encouraging excessive consumption based on characteristics such as convenience and hyper palatability [14] in addition to containing industrial additives linked to inflammation and harmful to health [11,13,[15], [16], [17], [18]].
UPF is defined as industrially manufactured, ready-to-eat, or ready-to-heat formulations made mostly from substances that are derived from foods with little if any, whole foods. Such foods are characterized by the addition of synthetic substances, sugar, salt, fat, and stabilizers [12].
The study of this food group was first documented by Monteiro et al., in 2010, along with the NOVA classification [19], which is an important tool to describe the degree of food processing and its implication on food systems and dietary patterns, and how these may affect health and well-being patterns, and how these may affect health and the risk of disease [20,21]. Now, this classification is considered as the one being most valued in nutritional epidemiology and recommended by the Food and Agriculture Organization of the United Nations [11].
Changes in lifestyle and food consumption patterns have been exacerbated by the covid-19 pandemic, as shown by González-Monroy et al. in a systematic review with only longitudinal studies. González-Monroy noted from the studies that, there was a modification of eating behaviors, characterized by an increased snack frequency and a preference for sweets and ultra-processed food rather than fruits, vegetables, and fresh food after the beginning of the pandemic of covid-19 [22].
High consumption of ultra-processed foods is negatively associated with several health outcomes, including chronic non-communicable diseases, cardiovascular disease, and depression [15,18,[23], [24], [25]]. However, data on the contribution of UPF consumption on sleep quality is still limited in the literature.
Various foods and nutritional supplements have been used to try to benefit sleep [26]. However, the relationship between nutritional components and sleep is complicated. Nutritional factors change significantly with different dietary patterns and depend significantly on the digestive and metabolic functions of each [26,27]. In this regard, it is necessary to assess not only the intake of specific nutrients but also the dietary pattern of consumption.
Therefore, the central hypothesis of this study is that a dietary pattern characterized by high consumption of ultra-processed foods and low in natural/minimally processed foods is associated with poor sleep quality. To this end, we aimed to determine if the co-occurrence of the food consumption according to the degree of processing was associated with poor sleep quality in adults during the covid-19 pandemic.
2. Methods
2.1. Study design
A population-based serological cross-sectional study was conducted between October and December 2020 in two medium-sized cities in the south-central region of Minas Gerais, known as Iron Quadrangle, one of the largest iron ore producing areas in Brazil. The survey was carried out in three surveys with intervals of 21 days, considering the incubation period of the SARS-CoV-2 virus.
All procedures were adopted according to the Brazilian guidelines and norms for research involving human beings of the Declaration of Helsinki. The study was approved by the Research Ethics Committee (Ethics Submission Certificate No. 32815620.0.1001.5149).
The sample size was calculated using the OpenEpi tool, with the 2019 population estimate by the demographic census 29 for the urban areas, 95% confidence level, design effect equal to 1.5, the estimated proportion of infection, and precision. In addition, it was considered a 20% re-composition percentage for any losses.
The sample was selected in three stages: census sector (probability proportional to the number of households), household (systematic sampling), and resident (randomly). Therefore, for each city, four strata were defined according to the average monthly incomes available in the 2010 demographic census 29, to ensure representativeness of different socioeconomic strata in the sample [28,29]. Based on the sample calculation, 1464 individuals should have been interviewed in the two cities evaluated.
2.2. Data collection
Data collection was performed face-to-face, using an electronic questionnaire with sociodemographic, health status, pandemic, and sleep information by trained interviewers. The health monitoring of the interviewers was conducted through periodic evaluation, before the beginning of each stage of the survey, using health questionnaires associated with the results of serological testing (immunochromatography reaction).
2.3. Sleep quality
The Pittsburgh Sleep Quality Index (PSQI) questionnaire was used to measure the subjects' sleep quality in this study. The Brazilian version of the Pittsburgh Sleep Quality Index had an overall reliability coefficient (Cronbach's α) of 0.82, indicating a high degree of internal consistency [30]. This instrument is composed of 19 questions categorized into seven components, each component scoring from 0 to 3: subjective sleep quality (C1), sleep latency (C2), sleep duration (C3), habitual sleep efficiency (C4), sleep disturbances (C5), use of sleep medication (C6), and daytime dysfunction (C7). The sum of the scores produces an overall score, ranging from 0 to 21, where the highest score indicates the worst sleep quality [31]. In this study, sleep quality was classified as good quality (PSQI score ≤5) and poor quality (PSQI >5). A moderate to severe difficulty in a sleep-specific domain (C1 to C7) was defined as a score of ≥2 31. Cut-off point also used by Wang et al., 2020 [32].
2.4. Food consumption
Food consumption was assessed using a qualitative food frequency questionnaire (FFQ), referring to consumption for the last three months. The frequency of food consumption was reported on weekdays, considering the answers: i) Never; ii) 1–2 days/per week; iii) 3–4 days/per week; iv) 5–6 days/per week v) Every day including Saturday and Sunday. Participants described the frequency of consumption of 20 food items. However, to meet the objective of this study, we used only 10 food items. The FFQ was analyzed according to the degree of processing [19], and it was assessed by two explanatory variables: fresh/minimally processed foods and ultra-processed food classified according to the Food Guide for the Brazilian Population and the NOVA classification [12,19].
The group of fresh/minimally processed foods was composed of fruits, vegetables, leafy vegetables, milk, and beans, and the ultra-processed group by processed meats, sweets, instant noodles, soft drinks, and snack chips.
To assess the co-occurrence of the consumption of the two groups, a score was calculated according to the degree of processing of each food group as proposed by Francisco et al., 2019 [33]. The score ranged from zero to four points, depending on the food and weekly frequency of consumption. The score was calculated inversely: fresh/minimally processed foods consumed daily, and ultra-processed foods rarely consumed or never received the minimum score (zero). The highest score (four points) was received by the unprocessed and minimally processed foods rarely consumed or never, and by the ultra-processed foods consumed daily (Supplementary Table 1). The total score included the sum of the food items, ranging from 0 (best diet quality) to 40 points (worst diet quality). The total score was categorized into quartiles of the distribution.
Furthermore, we also evaluated whether individuals replaced their lunch and/or dinner, a home-cooked meal, mostly prepared with fresh/minimally processed foods, with ultra-processed foods, for five or more days in the week.
2.5. Co-variates
The questionnaire also included related variables to describe the sample, and for possible confounding controls in the analysis of the association between eating by NOVA classification and sleep quality. The variables evaluated are sex, age (18–34, 35–59 or > 60 years), marital status (married or unmarried), education (0–8, 9–11 or > 12 years), family income (≤2, 2–4 or > 4 minimum wage), and worker (yes or no).
Health variables evaluated were self-reported chronic diseases (high blood pressure, diabetes, asthma, lung disease, chronic kidney disease, depression, anxiety disorder, cancer, heart or thyroid disease), which were dichotomized into morbidity (reporting at least one disease) and without morbidity (no disease).
Anxiety symptoms were assessed by the Generalized Anxiety Disorder Scale (GAD-7), and a cut-off score of 10 was considered to determine symptoms of a generalized anxiety disorder [34]. Alcohol consumption and smoking were evaluated by self-report (yes or no). Nutritional status was evaluated by the body mass index (BMI) from self-reported weight and height, and classified as underweight, eutrophic, or excess weight [35,36].
2.6. Statistical analysis
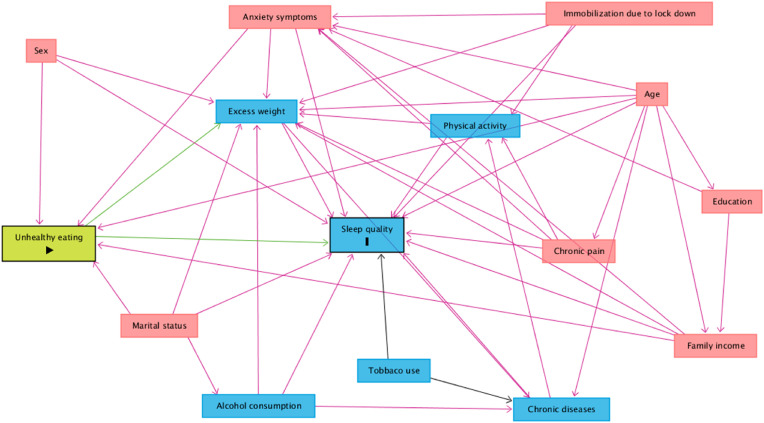
A theoretical causality model based on a directed acyclic graph (DAG) was elaborated with exposure (food consumption score), outcome (sleep quality), and confounding variables, using the online software Dagitty, version 3.2 [37]. Causal connections represented by arrows were established among the variables (Supplementary Figure 2). Each variable in the DAG was represented by a rectangle and the colors had different meanings: green was the exposure variable; blue circled by black was the response variable; variables considered to be potential confounders were included, in blue are the antecedents of the outcome variable, and those in red are antecedents of the outcome and exposure variables. To avoid unnecessary adjustments, spurious associations, and estimation errors, the backdoor criterion was used to select a minimum set of confounding variables to fit the analyses [38]. The model was adjusted by the following minimum set of variables: age, sex, family income, marital status, and anxiety symptoms.
Unadjusted and adjusted logistic regression for the variables indicated by the DAG were conducted. Furthermore, linear trend tests were performed for each logistic regression model. Categorical variables were described as relative frequencies and 95% confidence interval (CI), and continuous variables were described as means and standard deviations (SD). Statistical analyses were performed considering the weighting factors of sampling using the svy command of Stata® software, version 15.0. The level of significance was set at 0.05.
3. Results
3.1. Characteristics of study participants
Table 1 shows the sociodemographic characteristics and health conditions of the study participants. Of the participants, 51.9% were women, the most prevalent age group was 35–59 years (45.6%), most were married (53.2%), had from 9 to 11 years of education (39.7%), and had a family income equal or less than two minimum wage (41.1%). About work-related variables, 52.5% of the evaluated individuals were active workers.
Table 1.
Sociodemographic and health conditions in adults during the covid-19 pandemic.
| Characteristics | Total % (95%CI) |
PSQI ≤5 % (95%CI) |
PSQI >5 % (95%CI) |
p-value∗ |
|---|---|---|---|---|
| Sociodemographic | ||||
| Sex | ||||
| Male | 48.1 (41.0–55.2) | 52.9 (44.7–60.9) | 43.9 (36.3–51.8) | 0.011 |
| Female | 51.9 (44.7–59.0) | 47.1 (39.0–55.3) | 56.1 (48.2–63.6) | |
| Age | ||||
| 18–34 years | 35.6 (31.1–40.3) | 39.3 (30.8–48.4) | 32.4 (26.2–39.2) | 0.405 |
| 35–59 years | 45.6 (41.1–50.2) | 43.6 (36.0–51.6) | 47.3 (39.7–55.0) | |
| ≥ 60 years | 18.8 (15.5–22.7) | 17.1 (12.6–22.6) | 20.3 (16.1–25.3) | |
| Marital statusa | ||||
| Married | 53.2 (47.2–59.2) | 57.5 (50.3–64.4) | 49.6 (41.5–57.6) | 0.105 |
| Not married | 46.8 (40.8–52.8) | 42.5 (35.6–49.7) | 50.4 (42.4–58.5) | |
| Education | ||||
| 0–8 years | 31.2 (26.7–36.0) | 76.5 (69.7–82.2) | 62.2 (54.1–69.6) | 0.009 |
| 9–11 years | 39.7 (35.6–43.9) | 23.0 (17.3–29.8) | 35.0 (27.4–43.5) | |
| ≥ 12 years | 29.1 (23.8–35.1) | 0.5 (0.2–1.3) | 2.8 (1.2–6.1) | |
| Family Incomeb | ||||
| ≤ 2 MW | 41.1 (35.6–46.8) | 37.9 (29.3–47.2) | 43.8 (34.9–53.1) | 0.450 |
| > 2 to ≤ 4 MW | 32.0 (26.9–37.5) | 31.6 (24.7–39.5) | 32.3 (26.1–39.1) | |
| > 4 MW | 26.9 (22.0–32.5) | 30.5 (22.0–40.6) | 23.9 (17.8–31.3) | |
| Workingc | ||||
| Not workers | 47.5 (42.7–52.3) | 43.8 (36.1–51.9) | 50.7 (43.8–57.7) | 0.233 |
| Active workers | 52.5 (47.7–57.3) | 56.2 (48.1–63.9) | 49.3 (42.3–56.2) | |
| Health conditions | ||||
| Chronic diseases | ||||
| No | 47.7 (41.3–54.2) | 53.8 (44.7–62.7) | 42.4 (35.4–49.8) | 0.020 |
| Yes | 52.3 (45.8–58.7) | 46.2 (37.3–55.2) | 57.6 (50.2–64.6) | |
| Chronic pain | ||||
| No | 65.7 (61.4–69.7) | 74.6 (66.3–81.4) | 58.0 (50.4–65.3) | 0.013 |
| Yes | 34.3 (30.3–38.6) | 25.4 (18.6–33.7) | 42.0 (34.7–49.6) | |
| Anxiety symptomsd | ||||
| Minimal | 47.6 (43.6–51.7) | 63.8 (56.0–70.9) | 33.7 (26.6–41.6) | <0.001 |
| Mild | 29.0 (23.9–34.7) | 22.3 (17.4–28.2) | 34.8 (28.2–42.0) | |
| Moderate | 15.6 (12.1–19.8) | 11.4 (5.3–22.8) | 19.2 (14.7–24.7) | |
| Severe | 7.8 (6.2–9.8) | 2.5 (1.5–4.2) | 12.3 (9.6–15.7) | |
| Depression symptomse | ||||
| Minimal | 61.1 (55.9–66.1) | 83.1 (77.2–87.6) | 61.1 (55.9–66.1) | <0.001 |
| Mild | 23.1 (19.0–27.9) | 14.2 (9.9–20.0) | 23.1 (19.0–27.9) | |
| Moderate | 9.7 (7.2–12.8) | 1.9 (1.1–3.3) | 9.7 (7.2–12.8) | |
| Moderate to severe | 4.3 (2.6–6.9) | 0.7 (0.1–1.4) | 4.3 (2.6–6.9) | |
| Severe | 1.8 (1.2–2.8) | 0.1 (0.1–0.2) | 1.8 (1.2–2.8) | |
| Smoking | ||||
| No | 83.0 (78.6–86.7) | 81.6 (73.8–87.4) | 84.3 (78.7–88.6) | 0.530 |
| Yes | 17.0 (13.3–21.4) | 18.4 (12.6–26.2) | 15.7 (11.4–21.3) | |
| Alcohol consumption | ||||
| No | 41.8 (36.0–47.9) | 36.8 (31.1–42.9) | 46.1 (38.4–53.9) | 0.016 |
| Yes | 58.2 (52.1–64.0) | 63.2 (57.0–68.9) | 53.9 (46.1–61.6) | |
| Nutritional status | ||||
| Eutrofic | 36.0 (30.7–41.7) | 39.6 (32.0–47.6) | 39.7 (32.2–47.7) | 0.899 |
| Underweight | 2.6 (1.8–3.6) | 2.2 (1.3–3.7) | 2.7 (1.5–4.7) | |
| Excess weight | 61.4 (55.6–66.9) | 58.2 (50.1–65.9) | 57.6 (49.6–65.3) | |
MW: Minimum wage.
∗p-value of Pearson's chi-square test.
Not married: Widowed, divorced, single.
Minimum wage value: BRL 1045.00 ≈ USD 194.25 (1 USD = 5.3797 BRL).
Not workers: Unemployed, pensioner, retiree.
Anxiety symptoms evaluated by Generalized Anxiety Disorder Scale-7.
Depression symptoms evaluated by Patient Health Questionnaire-9.
Concerning health conditions, more than half of the individuals had at least one chronic disease (52.3%), consumed alcohol at least once a month (58.2%), and were overweight (61.4%). Furthermore, 20.6% of the subjects had symptoms of an anxiety disorder (Table 1).
3.2. Distribution of sleep and food consumption score
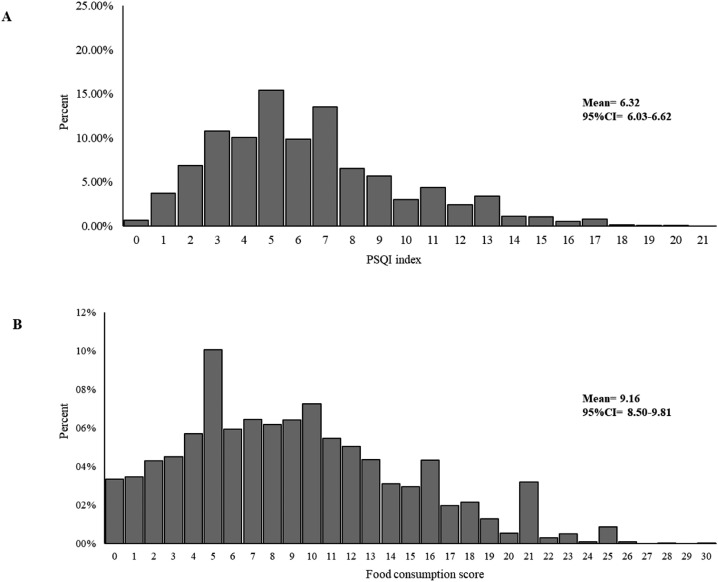
The distribution of the PSQI and food consumption score is shown in Fig. 1 . The PSQI had a mean score of 6.32 (95% CI 6.03–6.62) (Fig. 1), and the prevalence of poor sleep quality was 52.5% (Table 1). Evaluating the food consumption score, the mean score obtained was 9.16 (95% CI 8.50–9.81), with a minimum value of 0 and a maximum value of 30 (see Fig. 2).
Fig. 1.
Frequency distribution of PSQI score (A) and food consumption score (B).
Fig. 2.
Directed acyclic graph (DAG) on unhealthy eating score according to food processing level and sleep quality in adults during the covid-19 pandemic. Legend: The variable in green and with the “►” symbol inside the rectangle was the exposure variable; those in blue and with the letter “I” inside the rectangle were the response variables; variables in blue are the antecedents of the outcome variable; and those in red are antecedents of the outcome and exposure variables.
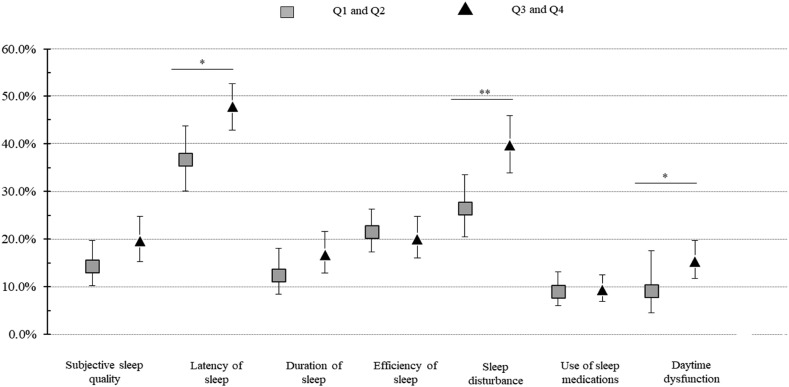
Besides, Fig. 3 shows the distribution of the food consumption score by moderate to difficult sleep subdomains, by PSQI. Individuals in the third and fourth quartile of the food consumption score had a 30% higher prevalence of moderate to difficult sleep latency (36.7% vs. 47.8%; p = 0.003), 28% higher moderate to difficulties sleep disturbances (26.4% vs. 33.8%; p < 0.001), and 68% higher moderate to difficulties daytime dysfunction (9.1% vs. 15.3%; p = 0.001) when compared with individuals in the first and second quartile (Fig. 3).
Fig. 3.
Prevalence of moderate to difficulties Pittsburgh Sleep Quality Index subdomainsa by quartiles of food consumption score distribution. Legend: aScore for each domain ranges from 0 to 3 (no difficulty to severe difficulty), and a domain score ≥2 indicates moderate to difficulties sleep in the domain. Performed chi-squared analysis; ∗ p-value < 0.05; ∗∗ p-value < 0.001.
3.3. Food consumption score and poor sleep quality
Table 2 shows the association between sleep quality and diet quality according to the food consumption score. Individuals in the first quartile had the lowest frequency of ultra-processed food consumption and the highest frequency of fresh/minimally processed food consumption. And individuals in the fourth quartile represent the highest frequency of consumption of ultra-processed foods and the lowest frequency of consumption of fresh/minimally processed foods. In adjusted analysis, the individuals in the third quartile were 71% more likely to have poor sleep quality (OR = 1.71; 95%CI: 1.03–2.85), and those in the fourth quartile, had a 144% greater chance of having poor sleep quality (OR = 2.44; 95%CI: 1.32–4.50).
Table 2.
Association between unhealthy eating by NOVA system and sleep quality in adults during the covid-19 pandemic.
| Food consumption score | Min. and max. values | Frequency (95% CI) | Unadjusted analysis |
Adjusted analysisc |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-trendd | OR | 95% CI | p-trendd | |||
| Quartile scorea | ||||||||
| Q1 | 0–4 | 21.4 (17.7–25.5) | 1.00 | – | 0.056 | 1.00 | - | 0.013 |
| Q2 | 5–8 | 28.6 (23.8–33.9) | 1.43 | 0.82–2.49 | 1.54 | 0.82–2.87 | ||
| Q3 | 9–12 | 24.1 (20.2–28.6) | 1.44 | 0.92–2.24 | 1.71 | 1.03–2.85 | ||
| Q4 | 13–30 | 25.9 (21.3–31.1) | 2.01 | 1.09–3.73 | 2.44 | 1.32–4.50 | ||
| Replaced lunch or dinner for UPFb | ||||||||
| Not replace lunch | 98.9 (98.1–99.4) | 1.00 | - | 1.00 | – | |||
| Replace lunch with UPF | 1.1 (0.6–1.9) | 2.07 | 0.72–5.92 | 1.45 | 0.42–5.01 | |||
| Not replace dinner | 85.4 (81.9–88.4) | 1.00 | - | 1.00 | – | |||
| Replace dinner with UPF | 14.6 (11.6–18.2) | 2.15 | 1.27–3.46 | 2.01 | 1.14–3.57 | |||
UPF: Ultra-processed foods.
Quartiles of food consumption score distribution. Q1 are the lowest values of the score, characterized by the highest consumption of fresh and minimally processed foods and highest consumption of ultra-processed foods. Q4 are the highest values of the score, characterized by the lowest consumption of fresh and minimally processed foods and the highest consumption of ultra-processed foods.
Replaced lunch and/or dinner, a home cooked meal, mostly prepared with fresh/minimally processed foods, to ultra-processed foods, for five or more days in the week.
Adjusted analysis by the following minimum set of variables: age, sex, family income, marital status, and anxiety symptoms.
Linear trend tests.
Furthermore, 10.8% and 14.6% of the individuals replaced their lunch and dinner, a home-cooked meal mostly prepared with fresh/minimally with ultra-processed foods, for five or more days of the week, respectively (Table 2). In adjusted analysis, the individuals who reported replacing dinner in the evening period with UPF were 101% more likely to have poor sleep quality (OR = 2.01; 95%CI: 1.14–3.57) (Table 2).
4. Discussion
To our knowledge, this is the first study that evaluated the association between the frequency of food consumption, according to the NOVA classification, and sleep quality in adults. Our main findings were that the higher frequency of consumption of ultra-processed foods and the lower frequency of consumption of fresh and minimally processed foods were associated with a higher chance of poor sleep quality. Furthermore, replacing a home-cooked meal prepared mostly of fresh/minimally processed foods with ultra-processed foods frequently was associated with poor sleep quality. The results of this study can contribute to the development of other studies that seek to define the effects of food consumption, according to the degree of processing and human health, as well as helping to understand how sleep quality is multifactorial and is influenced by various aspects of human behavior.
UPF consumption represents an important and growing part of the world food supply. Recent studies have reported that these foods represent a significant percentage of about 50–60% of the energy content in the usual diet of the average consumer in the United States, Canada, or the United Kingdom [13,39]. Over the past 30 years, Brazilian society has experienced major social transformations that have resulted in changes in its pattern of food consumption, nutrition, and health [40]. In this sense, we have observed increased access to ultra-processed foods and the beginning of substitution of fresh/minimally processed foods by ready-to-eat foods. In Brazil, the National Food Purchasing Survey shows that the contribution of UPF in total calories increased by 58.7% between the years 2002–2020 [41]. The increase of UPF in the global food supply has coincided with an increase in the prevalence of obesity and non-communicable diseases in many countries [20,21]. The relationship between high UPF consumption and obesity is well described in literature [21]. However, there are no studies in the literature evaluating food consumption by NOVA classification with sleep quality in adults.
We found relevant and alarming results, in which individuals with the highest consumption of ultra-processed foods and the lowest consumption of fresh foods, according to the food consumption score, may be more than twice as likely to have poor sleep quality.
The relationship between food consumption and sleep is still recent in the scientific literature, but some experimental studies have demonstrated the role of some nutrients in the improvement or worsening of sleep [27,42]. The high consumption of saturated fats has been considered the most harmful to sleep, and some nutrients such as zinc, vitamin B, polyphenols, and tryptophan have been associated with better sleep quality [27], but the studies are highly heterogeneous and still incipient.
The role of some specific UPFs was evaluated, such as processed meats and dairy products, showing negative associations with sleep quality [42]. Consistent with our findings, previous studies have reported an inverse association between diet quality and sleep [26,27,42,44,45]. A recent study of diabetic women assessed the association of sleep quality with two indicators of healthy eating, the Healthy Eating Index (HEI) and Diet Quality Index-International (DQI-I) emphasize increased consumption of fruits, vegetables, and whole grains, concomitant with reduced intake of refined grains, empty calories, added sugar, sodium, and saturated fatty acids. They found that the risk of sleeping poorly decreased by 88% and 92% of participants in the upper tertile of DQI-I and HEI, respectively [46].
Furthermore, the largest investigation of diet, sleep, and mental health by utilizing the UK Biobank with 502,494 middle-aged adults, observed a significant main effect of healthy diet score on healthy sleep score in the unadjusted and adjusted analyses. To examine the specific contributions of food groups and items, healthy sleep scores are associated with increased intake of vegetables, fruit, and fish, but a lower intake of processed meat [43]. A similar result is reported in the review by St-Onge et al. (2016) in which dairy products, fish, fruit, and vegetables, are described with sleep-promoting effects [27].
The mechanism by which sleep quality is determined by food consumption is multifactorial. First, UPFs are high in fat, calories, sugars, and low in micronutrients and fiber [47]. As shown in a systematic review of 19 studies with 86,961 individuals, where the highest caloric contribution of carbohydrates and fats in the diet, had the worst night's sleep [48]. A review study suggested that hyperglycemia-induced after a high sugar diet and the resulting compensatory hyperinsulinemia could induce the release of autonomic counterregulatory hormones, including adrenaline, cortisol, glucagon, and growth hormone [26], leading to shorter time in deep sleep, and therefore non-restorative sleep [49]. Furthermore, high sugar consumption may also contribute to a higher inflammatory profile [50], which may negatively impact sleep [51].
Another hypothesis is that the consumption of these foods is higher during the nighttime period, which may further worsen sleep quality [[52], [53], [54]]. Although we did not assess at what point in time UPF consumption was highest, we observed that individuals who reported replacing a home-cooked meal prepared primarily from fresh/minimally processed foods with ultra-processed foods were frequently associated with poor sleep quality. Since the circadian system is signaled in part by dietary intake, eating at consistent times is important for robust circadian rhythms. Thus, consuming UPF in the evening, because it contains high levels of sugars and saturated fats, can compromise sleep physiology, similar to what happens with disrupted sleep patterns after an abrupt change in time zone, called jet lag [53]. In addition, a high-carbohydrate meal in the evening can interfere with the circadian rhythm, altering body temperature and consequently reducing nocturnal melatonin secretion, determining factors for good sleep induction and quality [55].
The consumption of some nutrients from fresh or minimally processed foods can alleviate the symptoms of poor sleep quality. Some of these nutrients are calcium, magnesium, vitamins A, C, D, E, and K, tryptophan, and isoflavones [42]. Among the potential mechanisms for the impact of these nutrients on sleep, it is highlighted that they may be related to the synthesis of serotonin and melatonin. These neurotransmitters are known to be involved in the sleep and wake cycle [27].
In addition to poor sleep quality, high UPF consumption can worsen health status as they contribute to overweight and obesity, as well as cause hormonal, metabolic, and physiological changes [56]. Since psychological dysfunctions are linked to poor sleep quality, it is of utmost importance to study these relationships. However, as this is a cross-sectional study, it is not possible to assess whether the consumption of UPF causes bad sleep. Therefore, a clear understanding of what matters and how various aspects contribute to the effects is highly necessary.
Our study has some strengths. To our knowledge, this is the first study to assess the consumption of UPF in association with sleep quality. We use the NOVA classification, which classifies groups of foods according to their nature, purpose, and extent of industrial processing and not merely by nutrients and type of food. Furthermore, the sample design brings robustness to the study: i) representative random sample of the resident population from different socioeconomic strata; ii) assessment by household survey iii) face-to-face study during the pandemic of covid-19. Also, DAG was used to make explicit assumptions about the relationship between food consumption and sleep; used to guide analysis plans and decisions about possible confounders.
The main limitations of this study are the variables obtained by self-report, which can lead to underestimation of risk behaviors or overestimation of protective behaviors. However, the assessment of sleep quality needs to be performed subjectively, since it considers intrinsic factors to the individuals' perception of their sleep. However, potential confounding may still be possible. Furthermore, the assessment of ultraprocessed food consumption was performed with the use of an FFQ that was not specifically designed to collect data regarding this new classification of foods. Therefore, there is the potential for some degree of misclassification of ultraprocessed food consumption inherent in our methodology. However, the FFQ was created using the foods most consumed by the Brazilian population and according to the most important national surveys [57,58]. Although we adjusted for poor sleep quality factors and other potential confounders to account for the fact that those participants who reported higher consumption of UPF also had an unhealthier lifestyle in general and the sleep quality can be worse in immobilization due to lockdown, anxiety about becoming ill, loss of relatives due to covid-19 and depressed mood, residual confounding cannot be ruled out in our study. Not performing an interrater reliability analysis of the interview participants may also be a potential limitation. To reduce any biases related to the application of the questionnaire, the interview was conducted face-to-face, the interviewers were extensively trained before the data collection, and also, instruments validated in previous studies.
There are significant public health implications of our study. To date, prevention and control of non-communicable diseases are becoming a growing concern. UPFs are beginning to be recognized as an emerging health risk as they are related to a worse nutritional pattern, characterized by high energy density, added sugars, sodium, saturated and trans fats, and refined carbohydrates. The positive association between UPFs consumption and poor sleep quality may increase the chances of understanding adverse health events. Encouraging reduced consumption of UPFs and increasing the proportion of unprocessed or minimally processed foods is a direct way to address the problem [12]. Macro interventions and state regulations play a vital role, such as taxation of food and enforcement of food marketing.
5. Conclusion
Most of the individuals studied had poor sleep quality, and the higher frequency of ultra-processed foods consumption concomitant with lower consumption of fresh and minimally processed foods during the pandemic is a factor associated with a higher chance of poor sleep quality. Furthermore, replacing a home-cooked meal prepared mostly of fresh/minimally processed foods with ultra-processed foods frequently was associated with poor sleep quality. The results of this study can contribute to the development of other studies that assess causality between food consumption, according to the degree of processing and sleep quality.
Grants and funding
This study was supported by the Brazilian Council for Scientific and Technological Development (CNPq, Distrito Federal, Brazil) and Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES) [nº 88887.504994/2020–00] and finance code 001 for Ph.D. student scholarship.
Authors' contributions
ACSA, RDM, MCM GLLMC, and ALM contributed to the conception and design of the work, to the acquisition, analysis, and interpretation of data, and to the draft of the manuscript. LAAMJ, ACSA, and HNC contributed to the analysis, interpretation of data, and the draft of the manuscript. All authors revised it critically for important intellectual content and approved the submitted version.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors acknowledge the Federal University of Ouro Preto (UFOP) and the Research and Education Group in Nutrition and Collective Health (GPENSC) for their support and incentive. And also, the support of the Municipal Health Secretariats of the municipalities evaluated in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnesp.2022.03.023.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Irwin M.R. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015 Jan 3;66(1):143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cauter E., Spiegel K., Tasali E., Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008 Sep;9(SUPPL. 1) doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedict C., Cedernaes J. Could a good night's sleep improve covid-19 vaccine efficacy? Lancet Respir Med. 2021 May 1;2600(21):12–13. doi: 10.1016/S2213-2600(21)00126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel K. Effect of sleep deprivation on response to immunizaton. JAMA: JAMA, J Am Med Assoc. 2002 Sep 25;288(12):1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 5.Abe M., Abe H. Lifestyle medicine – an evidence based approach to nutrition, sleep, physical activity, and stress management on health and chronic illness. Personalized Medicine Universe. 2019 Jul 1;8:3–9. [Google Scholar]
- 6.Zuraikat F.M., Makarem N., Liao M., St-Onge M.P., Aggarwal B. Measures of poor sleep quality are associated with higher energy intake and poor diet quality in a diverse sample of women from the go red for women strategically focused research network. J Am Heart Assoc. 2020 Feb 18;9(4) doi: 10.1161/JAHA.119.014587. https://pubmed.ncbi.nlm.nih.gov/32063123/ Internet. [cited 2022 Jan 19] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godos J., Grosso G., Castellano S., Galvano F., Caraci F., Ferri R. Association between diet and sleep quality: a systematic review. Sleep Med Rev. 2021 Jun 1;57:101430. doi: 10.1016/j.smrv.2021.101430. [DOI] [PubMed] [Google Scholar]
- 8.Julia C., Martinez L., Allès B., Touvier M., Hercberg S., Méjean C., et al. Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Santé study. Publ Health Nutr. 2018 Jan 13;21(1):27–37. doi: 10.1017/S1368980017001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luiten C.M., Steenhuis I.H., Eyles H., Ni Mhurchu C., Waterlander W.E. Ultra-processed foods have the worst nutrient profile, yet they are the most available packaged products in a sample of New Zealand supermarkets. Publ Health Nutr. 2016 Feb 29;19(3):530–538. doi: 10.1017/S1368980015002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer K.A., Taillie L.S. Intake of ultraprocessed foods among US youths. JAMA. 2021 Aug 10;326(6):485. doi: 10.1001/jama.2021.9845. [DOI] [PubMed] [Google Scholar]
- 11.Monteiro C.A., Cannon G., Moubarac J.-C., Levy R.B., Louzada M.L.C., Jaime P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Publ Health Nutr. 2018 Jan;21(1):5–17. doi: 10.1017/S1368980017000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brasil . Brasília; 2014. Dietary guidelines for the Brazilian population. [Google Scholar]
- 13.Rauber F., da Costa Louzada M.L., Steele E., Millett C., Monteiro C.A., Levy R.B. Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008–2014) Nutrients. 2018 May 9;10(5):587. doi: 10.3390/nu10050587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passos CM dos, Maia E.G., Levy R.B., Martins A.P.B., Claro R.M. Association between the price of ultra-processed foods and obesity in Brazil. Nutrition. Metabolism and Cardiovascular Diseases. 2020 Apr;30(4):589–598. doi: 10.1016/j.numecd.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Fiolet T., Srour B., Sellem L., Kesse-Guyot E., Allès B., Méjean C., et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ. 2018 Feb 14:k322. doi: 10.1136/bmj.k322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez-Donoso C., Sánchez-Villegas A., Martínez-González M.A., Gea A., Mendonça R. de D., Lahortiga-Ramos F., et al. Ultra-processed food consumption and the incidence of depression in a Mediterranean cohort: the SUN Project. Eur J Nutr. 2020 Apr 4;59(3):1093–1103. doi: 10.1007/s00394-019-01970-1. [DOI] [PubMed] [Google Scholar]
- 17.Jacka F.N., Cherbuin N., Anstey K.J., Sachdev P., Butterworth P. Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Med. 2015 Dec 8;13(1):215. doi: 10.1186/s12916-015-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juul F., Martinez-Steele E., Parekh N., Monteiro C.A., Chang V.W. Ultra-processed food consumption and excess weight among US adults. Br J Nutr. 2018 Jul 14;120(1):90–100. doi: 10.1017/S0007114518001046. [DOI] [PubMed] [Google Scholar]
- 19.Monteiro C.A., Levy R.B., Claro R.M., de Castro I.R.R., Cannon G. Uma nova classificação de alimentos baseada na extensão e propósito do seu processamento. Cad Saúde Pública. 2010;26(11):2039–2049. doi: 10.1590/s0102-311x2010001100005. [DOI] [PubMed] [Google Scholar]
- 20.Mm L, Ja D, S B, C G-D, A L, A O, et al. Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta-analysis of 43 observational studies. Obes Rev. 2021 Mar 9;22(3) doi: 10.1111/obr.13146. [DOI] [PubMed] [Google Scholar]
- 21.Pagliai G., Dinu M., Madarena M.P., Bonaccio M., Iacoviello L., Sofi F. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr. 2021 Feb 14;125(3):308–318. doi: 10.1017/S0007114520002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Monroy C., Gómez-Gómez I., Olarte-Sánchez C.M., Motrico E. Eating behaviour changes during the covid-19 pandemic: a systematic review of longitudinal studies. Int J Environ Res Publ Health. 2021;18(21) doi: 10.3390/ijerph182111130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bentham J., Di Cesare M., Bilano V., Bixby H., Zhou B., Stevens G.A., et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017 Dec 16;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendonça R. de D., Lopes A.C.S., Pimenta A.M., Gea A., Martinez-Gonzalez M.A., Bes-Rastrollo M. Ultra-processed food consumption and the incidence of hypertension in a mediterranean cohort: the seguimiento universidad de Navarra project. Am J Hypertens. 2016 Dec 7:hpw137. doi: 10.1093/ajh/hpw137. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T., Gan S., Ye M., Meng G., Zhang Q., Liu L., et al. Association between consumption of ultra-processed foods and hyperuricemia: TCLSIH prospective cohort study. Nutr Metabol Cardiovasc Dis. 2021;31(7):1993–2003. doi: 10.1016/j.numecd.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Zhao M., Tuo H., Wang S., Zhao L. The effects of dietary nutrition on sleep and sleep disorders. Mediat Inflamm. 2020:2020. doi: 10.1155/2020/3142874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St-Onge M.-P., Mikic A., Pietrolungo C.E. Effects of diet on sleep quality. Advances in Nutrition. 2016 Sep 1;7(5):938–949. doi: 10.3945/an.116.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meireles A.L., Machado-Coelho G.L.L., Lourenção L.G., Júnior Laa de M., Coletro H.N. COVID-Inconfidentes - SARS-CoV-2 seroprevalence in two Brazilian urban areas during the pandemic first wave: study protocol and initial results. SciELO Preprints. 2021 doi: 10.1590/SciELOPreprints.2720. Submitted for publication. [DOI] [Google Scholar]
- 29.Menezes-Junior L.A.A., Lourencao L.G., Andrade AC. de S., Carraro J.C.C., Machado-Coelho G.L.L., Meireles A.L. Determinants of sleep quality in adults during the covid-19 pandemic: COVID-Inconfidentes, a population-based study. medRxiv. 2021 Sep 30 doi: 10.1101/2021.09.29.21264305. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertolazi A.N., Fagondes S.C., Hoff L.S., Dartora E.G., da Silva Miozzo I.C., de Barba M.E.F., et al. Validation of the Brazilian Portuguese version of the Pittsburgh sleep quality index. Sleep Med. 2011 Jan 1;12(1):70–75. doi: 10.1016/j.sleep.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. https://linkinghub.elsevier.com/retrieve/pii/0165178189900474 [Internet] [DOI] [PubMed] [Google Scholar]
- 32.Wang P., Song L., Wang K., Han X., Cong L., Wang Y., et al. Prevalence and associated factors of poor sleep quality among Chinese older adults living in a rural area: a population-based study. Aging Clin Exp Res. 2020;32:125–131. doi: 10.1007/s40520-019-01171-0. [Internet]. Springer International Publishing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francisco P.M.S.B., Assumpção D de, Borim F.S.A., Senicato C., Malta D.C. Prevalência e coocorrência de fatores de risco modificáveis em adultos e idosos. Rev Saude Publica. 2019 Oct 21;53:86. doi: 10.11606/s1518-8787.2019053001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno A.L., DeSousa D.A., Souza A.M.F.L.P., Manfro G.G., Salum G.A., Koller S.H., et al. Factor structure, reliability, and item parameters of the Brazilian-Portuguese version of the GAD-7 questionnaire. Temas em Psicologia. 2016;24(1):367–376. [Google Scholar]
- 35.Organização Pan-Americana de Saúde O . 2001. XXXVI Reunión del Comitê Asesor de Investigaciones en Salud. Washington. [Google Scholar]
- 36.Who . 1995. Physical status: the use and interpretation of anthropometry. Technical report series no 854. [PubMed] [Google Scholar]
- 37.Textor J., DAGitty Hardt J. A graphical tool for analyzing causal diagrams. Epidemiology. 2011 Sep;22(5):745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 38.Cortes T.R., Faerstein E., Struchiner C.J. Use of causal diagrams in epidemiology: application to a situation with confounding. Cad Saúde Pública. 2016;32(8) doi: 10.1590/0102-311X00103115. [DOI] [PubMed] [Google Scholar]
- 39.Martínez Steele E., Baraldi L.G., Louzada ML. da C., Moubarac J.-C., Mozaffarian D., Monteiro C.A., et al. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. 2016 Jan 9;6(3) doi: 10.1136/bmjopen-2015-009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaime P.C., Delmuè D.C.C., Campello T., Silva DO e, Santos L.M.P. Um olhar sobre a agenda de alimentação e nutrição nos trinta anos do Sistema Único de Saúde. Ciência Saúde Coletiva. 2018 Jun;23(6):1829–1836. doi: 10.1590/1413-81232018236.05392018. [DOI] [PubMed] [Google Scholar]
- 41.Costa C. dos S., Sattamini I.F., Steele E.M., Louzada ML. da C., Claro R.M., Monteiro C.A. Consumo de alimentos ultraprocessados e associação com fatores sociodemográficos na população adulta das 27 capitais brasileiras (2019) Rev Saude Publica. 2021;55:47. doi: 10.11606/s1518-8787.2021055002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikonte C.J., Mun J.G., Reider C.A., Grant R.W., Mitmesser S.H. Micronutrient inadequacy in short sleep: analysis of the NHANES 2005-2016. Nutrients. 2019 Oct 1;11(10) doi: 10.3390/nu11102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hepsomali P., Groeger J.A. Diet, sleep, and mental health: insights from the UK biobank study. Nutrients. 2021 Jul 27;13(8):2573. doi: 10.3390/nu13082573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du C., Almotawa J., Feldpausch C.E., Folk S.Y.L., Parag H., Tucker R.M. Effects of macronutrient intake on sleep duration and quality: a systematic review. Nutr Diet. 2021 Apr 19;79(1):1–17. doi: 10.1111/1747-0080.12671. 1747-0080.12671. [DOI] [PubMed] [Google Scholar]
- 45.Hur S., Oh B., Kim H., Kwon O. Associations of diet quality and sleep quality with obesity. Nutrients. 2021;13(9):1–9. doi: 10.3390/nu13093181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daneshzad E., Mansordehghan M., Larijani B., Heshmati J., Rouzitalab T., Pizarro A.B., et al. Diet quality indices are associated with sleep and mental health status among diabetic women: a cross-sectional study. Eat Weight Disord. 2021 doi: 10.1007/s40519-021-01294-2. [DOI] [PubMed] [Google Scholar]
- 47.Louzada ML. da C., Martins A.P.B., Canella D.S., Baraldi L.G., Levy R.B., Claro R.M., et al. Ultra-processed foods and the nutritional dietary profile in Brazil. Rev Saude Publica. 2015;49:38–49. doi: 10.1590/S0034-8910.2015049006132. www.scielo.br/rsp Internet. [cited 2019 Dec 10] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutanto C.N., Wang M.X., Tan D., Kim J.E. Association of sleep quality and macronutrient distribution: a systematic review and meta-regression. Nutrients. 2020;12(1):1–21. doi: 10.3390/nu12010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlahoyiannis A., Giannaki C.D., Sakkas G.K., Aphamis G., Andreou E. A systematic review, meta-analysis and meta-regression on the effects of carbohydrates on sleep. Nutrients. 2021 Apr 14;13(4):1283. doi: 10.3390/nu13041283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freeman C.R., Zehra A., Ramirez V., Wiers C.E., Volkow N.D., Wang G.J. Impact of sugar on the body, brain, and behavior. Front Biosci. 2018 Jun 1;23(12):2255–2266. doi: 10.2741/4704. [DOI] [PubMed] [Google Scholar]
- 51.Irwin M.R., Olmstead R., Carroll J.E. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatr. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gill S., Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can Be modulated for health benefits. Cell Metabol. 2015;22(5):789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manoogian E.N.C., Chaix A., Panda S. When to eat: the importance of eating patterns in health and disease. J Biol Rhythm. 2019;34(6):579–581. doi: 10.1177/0748730419892105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marot L.P., Rosa D.E., Lopes T do V.C., Moreno CR. de C., Crispim C.A. Eating duration throughout a rotating shift schedule: a case study. J Am Coll Nutr. 2021;40(7):624–631. doi: 10.1080/07315724.2020.1814899. [DOI] [PubMed] [Google Scholar]
- 55.St-Onge M.P., Roberts A., Shechter A., Choudhury A.R. Fiber and saturated fat are associated with sleep arousals and slow wave sleep. J Clin Sleep Med. 2016;12(1):19–24. doi: 10.5664/jcsm.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawrence M.A., Baker P.I. Ultra-processed food and adverse health outcomes. BMJ. 2019 May 29:l2289. doi: 10.1136/bmj.l2289. [DOI] [PubMed] [Google Scholar]
- 57.Ibge C de T e R. Instituto Brasileiro de Geografia e Estatística; Rio de Janeiro: 2020. Pesquisa de Orçamentos Familiares 2017-2018: análise de consumo alimentar pessoal no Brasil. [Google Scholar]
- 58.Brasil M.D.S. Brasília; 2020. Vigitel Brasil 2019 : vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico : estimativas sobre frequência e distribuição sociodemográfica de fatores de risco e proteção para doenças crônicas nas capitais dos 26 estados. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.