Abstract
Objective
COVID-19 is a multisystem disease that causes endothelial dysfunction and organ damage. Aim of the study was to evaluate the microvascular status in COVID-19 survivors with past different disease severity, in comparison with age and sex-matched primary Raynaud's phenomenon (PRP) patients and control subjects (CNT), including possible effects of concomitant therapies.
Methods
Sixty-one COVID-19 survivors (mean age 58 ± 13 years, mean days from disease onset 126 ± 53 and mean days from recovery 104 ± 53), thirty-one PRP patients (mean age 59 ± 15 years, mean disease duration 11 ± 10 years) and thirty CNT (mean age 58 ± 13 years) underwent nailfold videocapillaroscopy (NVC) examination. The following capillaroscopic parameters were searched and scored (0–3): dilated capillaries, giant capillaries, isolated microhemorrhages, capillary ramifications (angiogenesis) and capillary number, including absolute capillary number per linear millimeter at the nailfold bed.
Results
The mean nailfold capillary number per linear millimeter was significantly lower in COVID-19 survivors when compared with PRP patients and CNT (univariate and multivariate analysis p < 0.001). On the contrary, COVID-19 survivors showed significantly less isolated microhemorrhages than PRP patients and CNT (univariate and multivariate analysis, p = 0.005 and p = 0.012, respectively). No statistically significant difference was observed between COVID-19 survivors and control groups concerning the frequency of dilated capillaries and capillary ramifications. COVID-19 selective therapies showed a promising trend on preserving capillary loss and deserving further investigations.
Conclusions
SARS-CoV-2 seems to mainly induce a significant loss of capillaries in COVID-19 survivors at detailed NVC analysis in comparison to controls. The presence of a significant reduced score for isolated microhaemorrhages in COVID-19 survivors deserves further analysis.
Abbreviations: BMI, Body Mass Index; COVID-19, Coronavirus disease-19; CNT, control subjects; FiO2, fractional inspired oxygen; NVC, Nailfold videocapillaroscopy; PaO2, arterial oxygen partial pressure; PRP, primary Raynaud's phenomenon
Keywords: COVID-19, Nailfold capillaroscopy, Microcirculation, Capillaries, Raynaud's phenomenon, SARS-CoV-2, Glucocorticoids, Endothelial cells
1. Introduction
The human SARS-CoV-2 infection resulting in the Coronavirus disease-19 (COVID-19) is characterized by a wide range of clinical manifestations, including microvascular dysfunction and organ damage (Nägele et al., 2020).
In particular, SARS-CoV-2 directly binds to the angiotensin-converting enzyme 2 receptor, largely expressed on the endothelial cells, thanks to S1 subunit of viral receptor-binding domain. The S2 subunit allows the virus to adhere and enter the endothelium, disrupting the integrity of the endothelial barrier and driving a cascade of pro-inflammatory and pro-thrombotic events, that together with other risk factors, may aggravate the severity of the disease (Raghavan et al., 2021; Beyerstedt et al., 2021; Cutolo et al., 2020; Sulli et al., 2021). A recent autopsy-based study demonstrated the presence of endothelitis of small and medium-sized vessels in more than 90% of cases and thrombotic events in 30–60% of subjects depending on the organ involved (Haberecker et al., 2022).
Nailfold videocapillaroscopy (NVC) is the best non invasive tool to identify microvascular status in a large spectrum of rheumatic and non-rheumatic diseases (Cutolo and Smith, 2021). NVC has been recently performed both in children and adult COVID-19 patients and several nonspecific abnormalities have been reported (Çakmak et al., 2021; Natalello et al., 2021). However, a healthy population of comparison was taken into account only in the study involving paediatric patients (Çakmak et al., 2021).
As a consequence, the aim of this study was the comparison of NVC status between adult COVID-19 survivors and both primary Raynaud's phenomenon (PRP) patients and control subjects (CNT), looking for statistically significant differences in microvascular architecture, considering also the possible impact of COVID-19 pharmacological treatments.
2. Materials and methods
2.1. Study population
Sixty-one COVID-19 survivors, thirty-one PRP patients and thirty age and sex-matched CNT underwent NVC examination at the Department of Rheumatology of the University of Genoa, Italy. Patients with functional PRP were considered as controls since potential carriers of benign nonspecific microvascular abnormalities at NVC (Cutolo and Smith, 2021).
COVID-19 survivors entered the University Hospital between March and November 2020 (first and second Italian pandemic wave). COVID-19 clinical and/or radiographic diagnosis was confirmed by the detection of SARS-CoV-2 RNA on nasopharyngeal swab. Demographic data of all COVID-19 survivors were collected with attention to body mass index (BMI), smoking habit, number and type of comorbidities, concomitant drug therapies. Pulmonary involvement (monolateral/bilateral opacities, indirect signs of interstitial fibrosis) was evaluated by conventional chest X-rays. D-dimer and C-reactive protein were assessed at admission by standard methods. Arterial oxygen partial pressure (PaO2) and ratio of PaO2 to fractional inspired oxygen (PaO2/FiO2) were measured by arterial blood gas analysis. COVID-19 therapies included antivirals (antiretrovirals, nucleoside analog), antibiotics (beta-lactams, macrolides), anticoagulants (low molecular weight heparin) and immunomodulant drugs (glucocorticoids, hydroxychloroquine, IL-6 receptor antagonist-tocilizumab – single infusion, 8 mg/kg).
COVID-19 survivors were divided in two subgroups according to the severity of the active infection: thirty-four survivors with past mild-moderate disease (not needing oxygen supplementation, or requiring Venturi mask) and twenty-seven survivors with past severe disease (need for Continuous Positive Airways Pressure – CPAP – and/or mechanical ventilation).
PRP was diagnosed according to LeRoy and Medsger criteria (LeRoy et al., 1992). Concomitant therapies of both PRP and CNT were also recorded.
Exclusion criteria for CNT were to be affected by connective tissue diseases or other pathological conditions and therapy possibly influencing the microcirculation, including the secondary Raynaud's phenomenon (the subjects used as controls were affected by osteoporosis, osteoarthritis or fibromyalgia).
COVID-19 survivors, PRP and CNT with recent traumatic events to their hands, due to physical trauma, jobs and/or hobbits were not taken into account for NVC examination, and excluded from the study. COVID-19 survivors that developed chilblain manifestations were also excluded from the study.
2.2. Nailfold videocapillaroscopy examination
The same trained Rheumatologist performed the NVC evaluation in all patients and controls, using an optical probe, equipped with a 200× magnification lens and connected to a picture analysis software (Videocap, DS Medica, Milan, Italy). All the participants underwent NVC examination after acclimatization in the test room for 15 min at a temperature of 22 °C (Cutolo et al., 2008). Two pictures of the middle area of the nailfold bed of the fingers (thumbs excluded) were collected for each subject, together with the following NVC parameters: dilated capillaries (increase of capillary diameter between 20 and 50 μm), giant capillaries (homogeneously dilated capillary with a diameter ≥ 50 μm), microhemorrhages (dark masses due to deposits of hemosiderin), capillary ramifications (branching or bushy capillaries, direct sign of neoangiogenesis) and absolute capillary number per linear millimeter (normal density ≥ 7 capillaries) (Smith et al., 2020). A semiquantitative rating was also used to score the NVC abnormalities as follows: no changes (normality) = 0; <33% of capillary alterations/reduction = 1; 33–66% of capillary alterations/reduction = 2, >66% of capillary alterations/reduction per linear millimeter = 3) (Sulli et al., 2008).
2.3. Statistical analysis
Descriptive data are reported as mean with standard deviation and median with range (minimum-maximum) for continuous variables, or with frequency and percentage for categorical data.
Any relationship between groups and capillary characteristics was preliminary explored with appropriate univariate analysis and then investigated with a subsequent multinomial logistic regression model with control group as reference category. All analysis were adjusted for age, sex, BMI, habits, comorbidities and presence of concomitant drugs.
Supplementary subgroup univariate analysis on COVID-19 patients with severe disease for evaluating the impact of different treatment on the capillary number per linear millimeter was assessed by performing the independent sample t-test or the Mann-Whitney U test for independent samples.
Any p value ≤0.05 was considered statistically significant. IBM SPSS version 24 was used for all analyses.
2.4. Ethics
The study was conducted in accordance with the principles of the Helsinki Declaration of 1975/83 and Good Clinical Practice guidelines and was approved by Regional Ethics Committee (ID 10851, 459/2020). All COVID-19 survivors and controls voluntarily signed the informed consent for the management of their clinical data and for the NVC analysis, as per University Hospital rules.
3. Results
3.1. Clinical features of enrolled subjects
A total number of one hundred twenty-two subjects were enrolled. Baseline features, comorbidities and concomitant therapies at NVC time are respectively reported in Table 1, Table 2 and Supplementary Table 1. Among COVID-19 survivors none of them suffered from Raynaud's phenomenon prior to the viral infection nor developed it after recovery. Disease features and COVID-19 treatments of all survivors are reported in Table 3.
Table 1.
Baseline characteristics of COVID-19 survivors, primary Raynaud's phenomenon patients and control subjects at the time of nailfold videocapillaroscopy.
| Controls (N = 30) |
Primary Raynaud's phenomenon (N = 31) |
Mild/moderate COVID-19 (N = 34) |
Severe COVID-19 (N = 27) |
||
|---|---|---|---|---|---|
| Sex | Males | 10 (33.3%) | 7 (22.6%) | 11 (32.4%) | 22 (81.5%) |
| Females | 20 (66.7%) | 24 (77.4%) | 23 (67.6%) | 5 (18.5%) | |
| Age (years) | 58.1 ± 13.36 59.0 (31.0–80.0) |
59.7 ± 14.60 58.0 (35.0–88.0) |
59.4 ± 14.13 59.5 (23.0–88.0) |
57.1 ± 11.96 61.0 (32.0–75.0) |
|
| BMI (kg/m2) | Normal | 27 (90.0%) | 27 (87.1%) | 31 (91.2%) | 20 (74.1%) |
| Obesity | 3 (10.0%) | 4 (12.9%) | 3 (8.8%) | 7 (25.9%) | |
| Smoking habit | No | 28 (93.3%) | 29 (93.5%) | 33 (97.1%) | 26 (96.3%) |
| Yes | 2 (6.7%) | 2 (6.5%) | 1 (2.9%) | 1 (3.7%) | |
| Primary Raynaud's phenomenon |
No | 30 (100.0%) | 0 (0.0%) | 34 (100.0%) | 27 (100.0%) |
| Yes | 0 (0.0%) | 31 (100.0%) | 0 (0.0%) | 0 (0.0%) | |
| Primary Raynaud's phenomenon duration (years) |
/ | 10.4 ± 8.60 10.0 (1.0–37.0) |
/ | / | |
| Presence of at least one comorbidity | No | 11 (36.7%) | 11 (35.5%) | 8 (23.5%) | 8 (29.6%) |
| Yes | 19 (63.3%) | 20 (64.5%) | 26 (76.5%) | 19 (70.4%) | |
| Concomitant drugs | No | 11 (36.7%) | 9 (29.0%) | 13 (38.2%) | 6 (22.2%) |
| Yes | 19 (63.3%) | 22 (71.0%) | 21 (61.8%) | 21 (77.8%) | |
Results are express as: mean with standard deviation and median with range (minimum-maximum), or with frequency and percentage, as appropriate. BMI: Body Mass Index (“Normal”: between 16 and 30 kg/m2; “Obesity”: more than 30 kg/m2).
Table 2.
Comorbidities of COVID-19 survivors, primary Raynaud's phenomenon patients and control subjects at the time of nailfold videocapillaroscopy.
| Controls (N = 30) |
Primary Raynaud's phenomenon (N = 31) |
Mild/moderate COVID-19 (N = 34) |
Severe COVID-19 (N = 27) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Arterial Hypertension | No | 21 | (70.0%) | 19 | (61.3%) | 20 | (58.8%) | 13 | (48.1%) |
| Yes | 9 | (30.0%) | 12 | (38.7%) | 14 | (41.2%) | 14 | (51.9%) | |
| Type II diabetes | No | 29 | (96.7%) | 29 | (93.5%) | 32 | (94.1%) | 23 | (85.2%) |
| Yes | 1 | (3.3%) | 2 | (6.5%) | 2 | (5.9%) | 4 | (14.8%) | |
| Chronic atrial fibrillation | No | 28 | (93.3%) | 27 | (87.1%) | 32 | (94.1%) | 26 | (96.3%) |
| Yes | 2 | (6.7%) | 4 | (12.9%) | 2 | (5.9%) | 1 | (3.7%) | |
| CHD | No | 29 | (96.7%) | 31 | (100%) | 33 | (97.1%) | 25 | (92.6%) |
| Yes | 1 | (3.3%) | 0 | (0.0%) | 1 | (2.9%) | 2 | (7.4%) | |
| COPD | No | 27 | (90.0%) | 30 | (96.8%) | 33 | (97.1%) | 23 | (85.2%) |
| Yes | 3 | (10.0%) | 1 | (3.2%) | 1 | (2.9%) | 4 | (14.8%) | |
| Asthma | No | 25 | (83.3%) | 29 | (93.5%) | 26 | (76.5%) | 24 | (88.9%) |
| Yes | 5 | (16.7%) | 2 | (6.5%) | 8 | (23.5%) | 3 | (11.1%) | |
| Neurological disorders | No | 27 | (90.0%) | 29 | (93.5%) | 32 | (94.1%) | 27 | (100%) |
| Yes | 3 | (10.0%) | 2 | (6.5%) | 2 | (5.9%) | 0 | (0.0%) | |
| Dyslipidemia | No | 27 | (90.0%) | 26 | (83.9%) | 31 | (91.2%) | 24 | (88.9%) |
| Yes | 3 | (10.0%) | 5 | (16.1%) | 3 | (8.8%) | 3 | (11.1%) | |
| Cancer | No | 28 | (93.3%) | 28 | (90.3%) | 33 | (97.1%) | 24 | (88.9%) |
| Yes | 2 | (6.7%) | 3 | (9.7%) | 1 | (2.9%) | 3 | (11.1%) | |
| Chronic arthritis | No | 30 | (100%) | 31 | (100%) | 28 | (82.4%) | 26 | (96.3%) |
| Yes | 0 | (0.0%) | 0 | (0.0%) | 6 | (17.6%) | 1 | (3.7%) | |
| Thyroid disease | No | 26 | (86.7%) | 24 | (77.4%) | 27 | (79.4%) | 26 | (96.3%) |
| Yes | 4 | (13.3%) | 7 | (22.6%) | 7 | (20.6%) | 1 | (3.7%) | |
Results are express with frequency and percentage, as appropriate. CHD: Coronary Heart Disease; COPD: Chronic Obstructive Pulmonary Disease.
Table 3.
Disease features and treatments of COVID-19 survivors.
| Total COVID-19 | 61 |
| Severe COVID-19 | 27 (44.3%) |
| Mild-moderate COVID-19 | 34 (55.7%) |
| Days between NVC and onset of COVID-19 symptoms | 125.6 ± 53.38 111.0 (60.0–317.0) |
| Days between NVC and hospital discharge | 103.8 ± 52.71 90.0 (40.0–309.0) |
| Days between hospitalization and discharge | 15.4 ± 11.14 14.0 (0.0–57.0) |
| Days between onset symptoms and hospitalization | 7.5 ± 4.28 7.0 (0.0–19.0) |
| Active disease duration (days) | 23.2 ± 9.46 22.0 (8.0–58.0) |
| D-dimer (mcg/l) | 2602.1 ± 4046.75 1282.0 (193.0–23,653.0) |
| C-reactive protein (mg/l) | 97.1 ± 70.42 85.0 (3.0–274.0) |
| PaO2 (mmHg) | 63.8 ± 12.66 64.5 (40.0–99.0) |
| No need of oxygen supplementation | 20 (32.8%) |
| Venturi Mask | 14 (23.0%) |
| CPAP | 21 (34.4%) |
| Mechanical ventilation | 6 (9.8%) |
| P/F ratio | 178.2 ± 87.72 152.0 (50.0–351.0) |
| Lung thromboembolism | 6 (9.8%) |
| Normal X-ray chest | 10 (16.4%) |
| Monolateral opacity X-ray chest | 7 (11.5%) |
| Bilateral opacities X-ray chest | 34 (55.7%) |
| Interstitial involvement plus opacities X-ray chest | 10 (16.4%) |
| Glucocorticoids | 48 (78.7%) |
| Dose of glucocorticoids (mg/day) | 47.5 ± 23.78 40.0 (5.0–100.0) |
| Duration of glucocorticoids therapy | 13.8 ± 8.10 14.0 (2.0–35.0) |
| Hydroxychloroquine | 29 (47.5%) |
| Anticoagulants | 51 (83.6%) |
| Antibiotics | 51 (83.6%) |
| Antivirals | 23 (37.7%) |
| Tocilizumab | 17 (27.9%) |
Results are express as: mean with SD and median with range (min-max), or with frequency and percentage, as appropriate. D-dimer normal range: 0–500 μg/l. C-reactive protein: 0–5 mg/l. PaO2 and P: arterial partial pressure of oxygen, F: fractioned inspired oxygen, CPAP: continuous positive airway pressure.
3.2. Nailfold videocapillaroscopy findings
COVID-19 survivors underwent NVC examination after a mean period of 126 ± 53 days from the disease onset. NVC abnormalities and related statistical analysis are reported in Table 4 and Table 5 .
Table 4.
Nailfold videocapillaroscopy alterations in COVID-19 survivors, primary Raynaud's phenomenon patients and control subjects. The absolute capillary number per linear millimeter is also reported. A semiquantitative rating was used to score the NVC abnormalities as follows: no changes = 0; <33% of capillary alterations/reduction = 1; 33–66% of capillary alterations/reduction = 2, >66% of capillary alterations/reduction per linear millimeter = 3.
| Controls (N = 30) |
Primary Raynaud's phenomenon (N = 31) |
Mild/moderate COVID-19 (N = 34) |
Severe COVID-19 (N = 27) |
||
|---|---|---|---|---|---|
| Dilated capillaries | Normal | 0 (0.0%) | 0 (0.0%) | 1 (2.9%) | 0 (0.0%) |
| < 33% | 19 (63.3%) | 13 (41.9%) | 23 (67.6%) | 14 (51.9%) | |
| 34%–66% | 11 (36.7%) | 18 (58.1%) | 10 (29.4%) | 13 (48.1%) | |
| >66% | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Microhemorrhages | Normal | 16 (53.3%) | 11 (35.5%) | 23 (67.6%) | 21 (77.8%) |
| < 33% | 13 (43.3%) | 19 (61.3%) | 11 (32.4%) | 6 (22.2%) | |
| 34%–66% | 1 (3.3%) | 1 (3.2%) | 0 (0.0%) | 0 (0.0%) | |
| >66% | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Giant capillaries | Normal | 30 (100.0%) | 31 (100.0%) | 34 (100.0%) | 27 (100.0%) |
| Capillary number | Normal | 30 (100.0%) | 31 (100.0%) | 33 (97.1%) | 22 (81.5%) |
| < 33% | 0 (0.0%) | 0 (0.0%) | 1 (2.9%) | 5 (18.5%) | |
| 34%–66% | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| >66% | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Capillary ramifications | Normal | 23 (76.7%) | 27 (87.1%) | 29 (85.3%) | 19 (70.4%) |
| < 33% | 7 (23.3%) | 3 (9.7%) | 4 (11.8%) | 8 (29.6%) | |
| 34%–66% | 0 (0.0%) | 1 (3.2%) | 1 (2.9%) | 0 (0.0%) | |
| >66% | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Absolute capillary number per linear mm |
9.3 ± 0.53 9.0 (9.0–11.0) |
8.7 ± 0.68 9.0 (8.0–10.0) |
8.4 ± 0.75 8.0 (6.0–10.0) |
8.2 ± 1.15 8.0 (5.0–10.0) |
|
Results are express as: mean with standard deviation and median with range (minimum-maximum), or with frequency and percentage, as appropriate.
Table 5.
Statistical analysis of nailfold videocapillaroscopy abnormalities in COVID-19 survivors, primary Raynaud's phenomenon patients, and control subjects.
| Mild - Moderate COVID-19 |
Severe COVID-19 |
PRP | Controls | P univariate | P multivariatea | p-valueb | RRb | p-valuec | RRc | p-valued | RRd | p-valuee | RRe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 34 | 27 | 31 | 30 | ||||||||||
| NVC SCORES | ||||||||||||||
| Dilated capillaries | ||||||||||||||
| Normal | 1 (2.9%) |
0 (0%) |
0 (0%) |
0 (0%) |
0.46 | 0.73 | n.s. | – | n.s. | – | n.s. | – | n.s. | – |
| Abnormal | 33 (97.1%) |
27 (100%) |
31 (100%) |
30 (100%) |
||||||||||
| Microhemorrhages | ||||||||||||||
| Normal | 23 (67.6%) |
21 (77.8%) |
11 (35.5%) |
16 (53.3%) |
0.005 | 0.012 | 0.02 | 0.27 | 0.004 | 0.12 | n.s. | – | 0.06 | 0.23 |
| Abnormal | 11 (32.4%) |
6 (22.2%) |
20 (64.5%) |
14 (46.7%) |
||||||||||
| Giant capillaries | ||||||||||||||
| Normal | 34 (100%) |
27 (100%) |
31 (100%) |
30 (100%) |
– | – | – | – | – | – | – | – | – | – |
| Abnormal | 0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
||||||||||
| Capillary ramifications | ||||||||||||||
| Normal | 29 (85.3%) |
19 (70.4%) |
27 (87.1%) |
23 (76.7%) |
0.34 | 0.40 | n.s. | – | n.s. | – | n.s. | – | n.s. | – |
| Abnormal | 5 (14.7%) |
8 (29.6%) |
4 (12.9%) |
7 (23.3%) |
||||||||||
| Capillary number | ||||||||||||||
| Normal | 33 (97.1%) |
22 (81.5%) |
31 (100%) |
30 (100%) |
0.005 | – | – | – | – | – | – | – | – | – |
| Abnormal | 1 (2.9%) |
5 (18.5%) |
0 (0%) |
0 (0%) |
||||||||||
| ABSOLUTE CAPILLARY NUMBER PER LINEAR MILLIMETER |
8.44 ± 0.75 |
8.22 ± 1.15 |
8.74 ± 0.68 |
9.30 ± 0.53 |
<0.001 |
<0.001 |
n.s. |
- |
0.038 |
2.75 |
<0.001 |
7.76 |
<0.001 |
11.18 |
P-values equal or lower than 0.05 were considered statistically significant.
Analysis adjusted for age, sex, body mass index, habits, comorbidities and concomitant drugs. NVC: nailfold videocapillaroscopy. PRP: primary Raynaud's phenomenon. RR: risk ratio. Normal: NVC semiquantitative rating of abnormalities = 0. Abnormal: NVC semiquantitative rating of abnormalities between 1 and 3.
P-values comparisons between mild-moderate COVID-19 survivors and PRP patients.
P-values comparisons between severe COVID-19 survivors and PRP patients.
P-values comparisons between mild-moderate COVID-19 survivors and controls.
P-values comparisons between severe COVID-19 survivors and controls.
Multivariate analysis showed differences in absolute capillary number per linear millimeter (p < 0.001) and in the frequency of abnormal cases of microhemorrhages (p = 0.006) after adjusting for age, sex, BMI, habits, comorbidities and concomitant drugs.
The mean (± standard deviation) absolute nailfold capillary number per linear millimeter was significantly lower in severe (8.2 ± 1.15) and mild-moderate (8.4 ± 0.75) COVID-19 survivors than in both PRP (8.7 ± 0.68) and CNT subjects (9.3 ± 0.53) (p < 0.001) (Fig. 1 ). Risk ratios of capillary reduction are reported in Table 5.
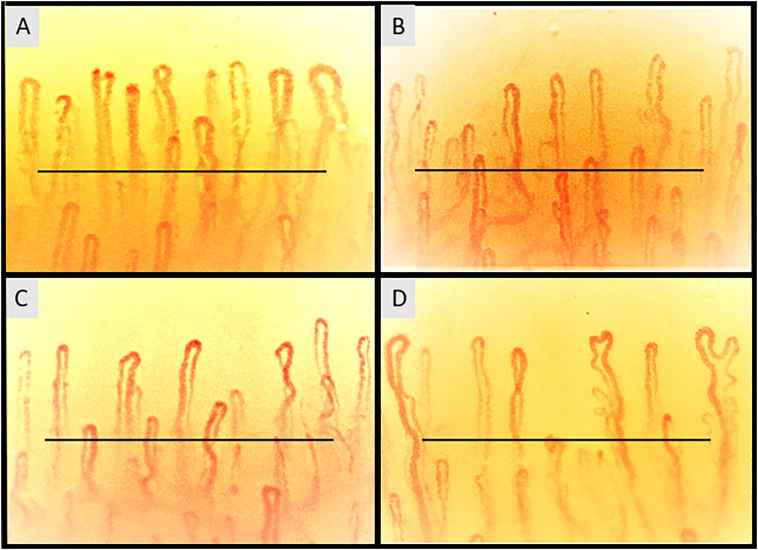
Fig. 1.
Nailfold videocapillaroscopic images of: (A) control (S.C.), showing a regular capillary array and density (mean capillaries 9.30 ± 0.53 per linear millimeter), with a majority of stereotype hairpin shaped capillaries and occasional crossing capillaries (B) primary Raynaud's phenomenon patient (E.S.), some nonspecific capillary dilations and crossing shapes are evident (mean capillaries 8.74 ± 0.68 per linear millimeter), (C) mild-moderate COVID-19 survivor (M.C.) with reduced number of capillaries and nonspecific abnormalities (tortuous and crossing capillaries) (mean capillaries 8.44 ± 0.75 per linear millimeter) and (D) severe COVID-19 survivor (G.G.) showing reduced number of capillaries, capillary ramifications and abnormal shaped capillaries (non-convex head) (mean capillaries 8.22 ± 1.15 per linear millimeter) (200× magnification). Horizontal bar is one millimeter long.
No other significant NVC differences were observed in COVID-19 survivors in comparison with PRP and CNT subjects, with the exception of isolated microhemorrhages that, on the contrary, were significantly more frequent in PRP patients than in COVID-19 survivors (p univariate = 0.005 and p multivariate = 0.012); the difference between COVID-19 survivors and CNT was not statistically significant.
COVID-19 survivors showed other nonspecific NVC abnormalities, such as dilated capillaries and isolated capillary ramifications, but none of these was statistically more frequent than in PRP patients and CNT. Giant capillaries were not found in any group (Table 4 and Table 5).
When we analyzed the impact of treatments (CPAP or mechanical ventilation, glucocorticoids, anticoagulants, antibiotics, antivirals and IL-6 receptor antagonist-tocilizumab) on capillary density in the severe COVID-19 patients (Supplementary Table 2), a positive trend (preservation of the capillary number) was observed with antivirals and anti-IL-6 receptor antagonist administration, while none of the other drugs was shown to be effective (Supplementary Table 2).
4. Discussion
Endothelial dysfunction and vasculopathy represent a well characterized pathophysiological process in course of SARS-CoV-2 infection (Wagner et al., 2021; McGonagle et al., 2021). As previously mentioned, NVC has been recently used to investigate microvascular involvement in active and recovered COVID-19 patients (Çakmak et al., 2021; Natalello et al., 2021). Concerning these two studies, in child COVID-19 patients, Authors reported statistically significant differences in capillary density, capillary ramifications, microhemorrhages and neoangiogenesis compared to a control group (Çakmak et al., 2021). In adult COVID-19 subjects, a comparison between acute and recovered patients was carried out, however without the inclusion of a control group of healthy subjects (Natalello et al., 2021). Indeed, in recovered patients, a higher prevalence of enlarged capillaries, meandering capillaries and lower capillary density than in acute patients was reported (Natalello et al., 2021).
In our study we examined only the most objective NVC parameters reported in the literature, without taking into account questionable items such as pericapillary edema, capillary meandering, microvascular derangement, sludge flow, and similar.
We report that the reduction of the absolute capillary number is the only significantly altered parameter in adult COVID-19 survivors at the level of the nailfold bed, while the number of isolated microhemorrhages was found significantly lower in COVID-19 with respect to PRP patients.
This last observation might be explained in part by the increased tendency of COVID-19 patients to develop thrombotic rather than haemorrhagic events (Bonaventura et al., 2021), and in part by the fact that PRP patients may develop alterations of the microcirculation attributable to underlying connective tissue diseases (Bernero et al., 2013).
Other nonspecific NVC abnormalities observed in all COVID-19 survivors were not significantly more frequent than in both PRP and CNT subjects; on the other hand, the absence of giant capillaries and avascular areas in COVID-19 survivors excluded the presence of a scleroderma-like pattern (Smith et al., 2019).
These data partially contrast with the previous observation in the paediatric COVID-19 population, where Authors reported significantly higher frequency of microhemorrhages and neoangiogenesis (Çakmak et al., 2021). These discrepancies could be explained by the different age of the enrolled subjects (median age 60 vs 12 years) and by the different time of NVC examinations after COVID-19 diagnosis: NVC was performed 111 median days after COVID-19 diagnosis in our cohort of patients and within a median 73 days after diagnosis in the study of Çakmak, probably reflecting still active disease sequelae in the cohort of young subjects. Furthermore, microvasculature may differently answer to damage in young than adult subjects.
Even if the reduction of capillary number reflects neither a clinical peripheral vascular alteration in COVID-19 survivors neither a real pathological relief (in rheumatic diseases a normal density is considered ≥7 capillaries per linear mm), the data is of real interest from a pathophysiological point of view and reinforce the current literature that reports a lower microvessels density in different peripheral body districts (Kanoore Edul et al., 2021; Rovas et al., 2021; Abrishami et al., 2021; Cennamo et al., 2021; Rodman et al., 2021; Cetinkaya et al., 2021).
A report including twenty-seven mechanically ventilated patients with COVID-19-related acute respiratory distress syndrome and an observational study regarding twenty-three hospitalized COVID-19 patients, remarked significant alterations of tissue perfusion at sublingual level with reduction of capillary density as evaluated by video-microscopy (Kanoore Edul et al., 2021; Rovas et al., 2021).
A reduction of microvascular density has also been described in two independent study groups of thirty-one and forty COVID-19 survivors respectively, using optical coherence tomography angiography, in the retinal superficial capillary plexus, deep capillary plexus and radial peripapillary capillaries (Abrishami et al., 2021; Cennamo et al., 2021; Rodman et al., 2021; Cetinkaya et al., 2021).
Moreover, a functional laser Doppler perfusion imaging investigation on thirty-two COVID-19 patients showed a marked reduction of microvascular flow in the skin of the forearm, in comparison with healthy controls (Sabioni et al., 2021).
The most dramatic consequence of this pathological process in course of COVID-19 is the rare “systemic capillary leak syndrome” or Clarkson's disease, a life-threatening condition characterized by an increased capillary permeability, with massive extravasation of plasma, hypoalbuminemia, hypotension, edema and subsequent organs failure due to shock (Case et al., 2020; Lacout et al., 2021).
In our study we also tried to assess for the first time the correlation between COVID-19 therapies and capillary parameters as analyzed at NVC, including capillary density. No drug was found to induce statistically significant effects in reducing the capillary loss in COVID-19 survivors, although very interesting and protective trends on capillary loss were suggested and deserve further investigations.
However, such results might be explained by a direct viral damage on endothelial cells rather than a less specific effect secondary to the pro-inflammatory cytokine storm (Vanderbeke et al., 2021; Castro et al., 2021; Landewé et al., 2021; Alunno et al., 2020).
Our study has some limitations. First of all, it is a monocentric study with a numerically limited cohort of subjects; by considering this limitation, the evaluation of a larger number of subjects might show statistically significant results also for other capillaroscopic parameters, including microhemorrhages and ramifications. Secondly, NVC examinations at baseline and in course of active COVID-19 disease are lacking: it was not possible to perform the NVC analysis in Hospital COVID-19 wards or intensive care units for safety and ethical reasons, so conferring a cross-sectional nature to our study. Furthermore, COVID-19 variant was not defined in our cohort of patients, therefore no data are evaluable on possible different effect of alpha or delta variants on nailfold microcirculation (omicron variant was not yet present between March and November 2020). Finally, no further evaluations over time were performed in our cohort of COVID-19 survivors, therefore prospective data concerning the behaviour of capillary number are not available.
Despite these limitations, it should be considered the relevance of these pilot results emerging from the short-term report. The collection of NVC data is ongoing as a longer follow-up needs to better detect and analyze the full NVC microvascular changes related to COVID-19.
5. Conclusions
In conclusion, SARS-CoV-2 infection doesn't seem to induce in survivors a peculiar “COVID-19 pattern” at NVC examination, rather it is associated to a significant capillary loss as distinctive feature.
Studies including the comparison of capillary density pre-COVID-19 and post-COVID-19 are desirable to reinforce the strength of the current observations. The positive trend in saving the number of capillaries induced by aggressive anti-inflammatory therapies in COVID-19 survivors needs larger cohorts of patients to confirm this important hypothesis.
The following are the supplementary data related to this article.
Concomitant drugs of COVID-19 survivors, primary Raynaud's phenomenon patients and control subjects at the time of nailfold videocapillaroscopy.
Statistical analysis of the impact of different treatments in COVID-19 survivors on saving capillary number per linear millimeter.
CRediT authorship contribution statement
AS, EG and MC were involved in the conception and design of the study, analysis and interpretation of data, drafting of the manuscript, and revising it critically for important intellectual content. CP performed NVC examinations. IS performed statistical analysis. EG, PFB, CP, TA, MG, EB and SP were involved in the acquisition, analysis and interpretation of data, drafting of the manuscript, and revising it critically for important intellectual content. VS critically revised interpretation of NVC pictures and discussion. All Authors have read the manuscript and agreed to its content and they are accountable for all aspects of the accuracy and integrity.
Declaration of competing interest
All Authors declare no competing interests concerning this manuscript.
Acknowledgments
Acknowledgments
We thank Dr. Sara De Gregorio for the graphical support.
AS, EG, CP, VS and MC are members of the European Alliance of Associations for Rheumatology (EULAR) Study Group on Microcirculation in Rheumatic Diseases.
VS is a Senior Clinical Investigator of the Research Foundation – Flanders (Belgium) (FWO) [1.8.029.20 N].
Funding
None.
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available for ethical and privacy reasons, but are available from the corresponding author on reasonable request.
Author statement
The study was conducted in accordance with the principles of the Helsinki Declaration of 1975/83 and Good Clinical Practice guidelines and was approved by Regional Ethics Committee (ID 10851, 459/2020). All COVID-19 survivors and controls voluntarily signed the informed consent for the management of their clinical data and for the nailfold videocapillaroscopic analysis, as per University Hospital rules.
References
- Abrishami Mojtaba, et al. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can. J. Ophthalmol. 2021;56:24–30. doi: 10.1016/j.jcjo.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alunno Alessia, et al. Storm, typhoon, cyclone or hurricane in patients with COVID-19? Beware of the same storm that has a different origin. RMD Open. 2020;6 doi: 10.1136/rmdopen-2020-001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernero Elena, et al. Prospective capillaroscopy-based study on transition from primary to secondary Raynaud's phenomenon: preliminary results. Reumatismo. 2013;65(4):186–191. doi: 10.4081/reumatismo.2013.186. [DOI] [PubMed] [Google Scholar]
- Beyerstedt Stephany, et al. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(5):905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura Aldo, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakmak Figen, et al. Nailfold capillaroscopy: a sensitive method for evaluating microvascular involvement in children with SARS-CoV-2 infection. Microvasc. Res. 2021;138 doi: 10.1016/j.mvr.2021.104196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case Robert, et al. Systemic capillary leak syndrome secondary to coronavirus disease 2019. Chest. 2020;158(6):e267–e268. doi: 10.1016/j.chest.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro Pedro, et al. Is the endothelium the missing link in the pathophysiology and treatment of COVID-19 complications? Cardiovasc. Drugs Ther. 2021;1–14 doi: 10.1007/s10557-021-07207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cennamo Gilda, et al. Optical coherence tomography angiography features in post-COVID-19 pneumonia patients: a pilot study. Am. J. Ophthalmol. 2021;227:182–190. doi: 10.1016/j.ajo.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetinkaya Tugba, et al. Analysis of swept-source optical coherence tomography angiography measurement alterations in adult patients recovered from COVID-19. Clin. Exp. Optom. 2021 doi: 10.1080/08164622.2021.1999770. [DOI] [PubMed] [Google Scholar]
- Cutolo Maurizio, Smith Vanessa. Detection of microvascular changes in systemic sclerosis and other rheumatic diseases. Nat. Rev. Rheumatol. 2021;17(11):665–677. doi: 10.1038/s41584-021-00685-0. [DOI] [PubMed] [Google Scholar]
- Cutolo Maurizio, et al. Capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2008;22(6):1093–1108. doi: 10.1016/j.berh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Cutolo Maurizio, et al. Evidences for a protective role of vitamin D in COVID-19. RMD Open. 2020;6(3) doi: 10.1136/rmdopen-2020-001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberecker Martina, et al. Autopsy-based pulmonary and vascular pathology: pulmonary endotheliitis and multi-organ involvement in COVID-19 associated deaths. Respiration. 2022;101(2):155–165. doi: 10.1159/000518914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoore Edul Vanina Siham, et al. Microcirculation alterations in severe COVID-19 pneumonia. J. Crit. Care. 2021;61:73–75. doi: 10.1016/j.jcrc.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacout Carole, et al. A new diagnosis of systemic capillary leak syndrome in a patient with COVID-19. Rheumatology (Oxford) 2021;60(1):e19–e20. doi: 10.1093/rheumatology/keaa606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landewé Robert B.M., et al. COVID-19-induced hyperinflammation, immunosuppression, recovery and survival: how causal inference may help draw robust conclusions. RMD Open. 2021;7 doi: 10.1136/rmdopen-2021-001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy, Carwile E., Medsger Thomas A. Raynaud's phenomenon: a proposal for classification. Clin. Exp. Rheumatol. 1992;10(5):485–488. [PubMed] [Google Scholar]
- McGonagle Dennis, et al. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021;3(3):e224–e233. doi: 10.1016/S2665-9913(20)30420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägele Matthias P., et al. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natalello Gerlando, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement. Microvasc. Res. 2021;133 doi: 10.1016/j.mvr.2020.104071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan Somasundaram, et al. SARS-CoV-2 spike protein induces degradation of junctional proteins that maintain endothelial barrier integrity. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.687783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman Julie, et al. Optical coherence tomography angiography of retinal vasculature in recovered COVID-19 patients compared to age and ethnic matched controls. Clin Exp Optom. 2021 doi: 10.1080/08164622.2021.1978817. [DOI] [PubMed] [Google Scholar]
- Rovas Alexandros, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24:145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabioni Letícia, et al. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc. Res. 2021;134 doi: 10.1016/j.mvr.2020.104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Vanessa, et al. Fast track algorithm: how to differentiate a "scleroderma pattern" from a "non-scleroderma pattern". Autoimmun. Rev. 2019;18(11) doi: 10.1016/j.autrev.2019.102394. [DOI] [PubMed] [Google Scholar]
- Smith Vanessa, et al. EULAR study group on microcirculation in rheumatic diseases and the scleroderma clinical trials consortium group on capillaroscopy. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud's phenomenon and systemic sclerosis. Autoimmun. Rev. 2020;19(3) doi: 10.1016/j.autrev.2020.102458. [DOI] [PubMed] [Google Scholar]
- Sulli Alberto, et al. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann. Rheum. Dis. 2008;67(6):885–887. doi: 10.1136/ard.2007.079756. [DOI] [PubMed] [Google Scholar]
- Sulli Alberto, et al. Vitamin D and lung outcomes in elderly COVID-19 patients. Nutrients. 2021;13(3):717. doi: 10.3390/nu13030717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderbeke Lore, et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat. Commun. 2021;12:4117. doi: 10.1038/s41467-021-24360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner Julian U.G., et al. Increased susceptibility of human endothelial cells to infections by SARS-CoV-2 variants. Basic Res. Cardiol. 2021;116(1):42. doi: 10.1007/s00395-021-00882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Concomitant drugs of COVID-19 survivors, primary Raynaud's phenomenon patients and control subjects at the time of nailfold videocapillaroscopy.
Statistical analysis of the impact of different treatments in COVID-19 survivors on saving capillary number per linear millimeter.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available for ethical and privacy reasons, but are available from the corresponding author on reasonable request.