Background.
Anti-severe acute respiratory syndrome coronavirus 2 mRNA vaccines elicit lower humoral responses in solid-organ transplant recipients. This is the first prospective trial investigating the effect of an inactivated whole-virion vaccine in kidney transplant recipients.
Methods.
Prospective, single-center, phase 4, interventional study. Kidney transplant recipients aged 30–69 y with >30 d of transplantation received two 3 µg intramuscular doses of CoronaVac 28 d apart and are being followed for 6 mo. Primary outcomes: (1) reactogenicity after first dose; (2) antibody responses 28 d after each dose; and (3) incidence/severity of confirmed coronavirus disease 2019 (COVID-19) and 28-d lethality rate. For this analysis, clinical effectiveness was assessed for 3 mo, starting 15 d after the second dose, and compared with 3-mo period before vaccination.
Results.
Of the 3371 individuals who received the first dose, 99% completed vaccination schedule. Mild/local adverse reactions were reported by 33% of the patients. In the immunogenicity cohort (n = 942), the proportion of patients with IgG antibodies to severe acute respiratory syndrome coronavirus 2 increased from 15.2% after first dose to 43% after second dose. Increase in antibody values after second dose was associated with higher proportion of patients with detected neutralizing antibodies. A significant reduction in the incidence of COVID-19 was observed (6.4% versus 4.2%; P < 0.0001), although the 28-d lethality rate remained unchanged (25% versus 22%; P = 0.534). In 45 patients from the immunogenicity cohort who developed COVID-19, all the 6 deaths occurred among those without antibody response (n = 22; 49%).
Conclusions.
CoronaVac vaccine was associated with low reactogenicity, low immunogenicity but reduced incidence of COVID-19 among kidney transplant recipients. The lack of reduction in lethality rates is perhaps associated with the low percentage of patients developing humoral response after the second dose.
INTRODUCTION
Mass vaccination campaigns are expected to change the coronavirus disease 2019 (COVID-19) pandemic’s impact on the society, an unprecedented situation that has already led to the death of >4 million people on all continents by July 2021.1 Vaccines from several platforms have been developed in record time, among them is CoronaVac (Sinovac Life Sciences, Beijing, China), an inactivated whole-virion vaccine approved by the World Health Organization for emergency use that has already had >750 million doses distributed in >40 countries and that will now also be distributed through the COVID-19 vaccines global access initiative, especially to low-income countries.2,3 In addition, a real-life study involving >10 million Chileans4,5 showed effectiveness of 65.9% in preventing COVID-19 in the general population, confirming the previous results of clinical trials.6,7
Kidney transplant recipients constitute a highly vulnerable subgroup of individuals. The accumulation of comorbidities and the continuous and unavoidable state of pharmacological immunosuppression lead to an increased risk of developing severe forms of COVID-19.8,9 Furthermore, to date, available data report that only 34%–48% of patients develop antibody response 4 wk after the traditional 2-dose scheme of mRNA or viral vector vaccines.10-13
The studies already published observed small groups of patients who have been vaccinated as part of a local immunization program, used a variety of different assays to detect anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies, covered a short observation period (maximum 4 wk after the second dose), and did not systematically investigate vaccine breakthrough infection. In this interventional study that recruited a large cohort of patients, we report data on reactogenicity, immunogenicity, and clinical effectiveness over a 3-mo period following the full 2-doses vaccine schedule. Furthermore, we analyzed the clinical effectiveness of the vaccine comparing the COVID-19 incidence and outcomes of fully vaccinated kidney transplant recipients versus a control group of unvaccinated ones.
PATIENTS AND METHODS
Study Design
This is an ongoing prospective, single-center, phase 4, interventional study assessing the reactogenicity, immunogenicity, and clinical effectiveness of a whole-virion inactivated vaccine to prevent SARS-CoV-2 infection and its associated mortality among kidney transplant recipients. All patients will be followed for 6 mo after the administration of the last dose of the vaccine. The study was approved by the local ethics committee, and all enrolled patients provided written informed consent. The study has been conducted following the Declaration of Helsinki and Good Clinical Practices and is registered at ClinicalTrials.gov (NCT04801667).
Inclusion Criteria
Kidney transplant recipients aged 30–69 y, with >30 d of transplantation, living within 200 km from the transplant center, and able to participate in all of the study procedures were eligible for enrollment.
Exclusion Criteria
Patients with a previous confirmed SARS-CoV-2 infection, those who have previously received any vaccine against SARS-CoV-2, or any other vaccine in the previous 4 wk were excluded. Also, individuals who had received blood-derived products or intravenous globulins in the previous 4 wk, those with previous history of angioedema or anaphylactic reactions to vaccines, pregnant women, under current treatment for neoplasia, with a medical history of coagulopathy, and uncontrolled psychiatric illness were not eligible.
Study Procedures
The participants were scheduled to receive the standard 2-dose inactivated whole-virion CoronaVac (3 µg, each dose, intramuscular route) vaccine. The vaccination took place at the transplant center on 4 weekends (first dose on March 20, 21, 27, and 28, 2021; second dose on April 17, 18, 24, and 25, 2021), with approximately 800–900 appointments per day.14
Patients were instructed to seek the Tele-Assistance center, which had been in operation since the beginning of the pandemic,15 in case of any symptoms, any time after vaccination. A dedicated group followed those with the confirmed diagnosis and were referred to local hospitals for COVID-19 treatment when indicated. Confirmed COVID-19 was defined as flu-like symptoms combined with a positive nucleic acid amplification-based or antigen testing obtained during the symptomatic period. For this analysis, the final follow-up date was August 22, 2021 (28 d after the last patient with confirmed COVID-19).
Reactogenicity
Data on the reactogenicity were obtained from all patients who received the first dose of the CoronaVac vaccine using a prespecified questionnaire applied on the day of the second dose administration (D1 study visit).
Immunogenicity
Blood samples were collected from all patients before the first dose (D0) to assess IgG against the SARS-CoV-2 nucleoprotein using the Abbott SARS-CoV-2 IgG assay as a screening test. In a subset of patients labeled as immunogenicity cohort additional samples were collected before the second dose (D1) and at least 28 d after the second dose (D2). This minimum sample size of the immunogenicity cohort (n = 726) was calculated based on the age distribution and seroconversion rate (71%) observed in the phase 3 trial conducted in healthcare professionals,7 with 95% confidence interval (CI) and an absolute error of 10%. Patients with a positive IgG anti-SARS-CoV-2 at D0 were not eligible for this analysis. IgG antibodies to the receptor binding domain of the S1 subunit of the spike protein of SARS-CoV-2 were assessed at D1 and D2 study visits using the AdviseDx SARS-CoV-2 IgG II assay (Abbot Laboratories, IL). Values >50 arbitrary units (AUs)/mL were considered positive.16
Exploratory Analysis of the Neutralization Activity
In a subgroup of patients of the immunogenicity cohort, we explored the association between IgG value and the detection of neutralizing anti-SARS-CoV-2 antibodies (≥30% signal inhibition using the cPass SARS-CoV-2 Neutralization Antibody Detection Kit—GenScript Laboratories).17 The proportion of patients with detected neutralizing anti-SARS-CoV-2 antibodies was analyzed in 2 groups, the first group showing no changes (n = 15) and the second group with the highest changes (n = 15) in IgG values measured after the first and after the second vaccine dose.
Clinical Effectiveness Analysis
The effectiveness of the vaccine was evaluated by comparing the incidence of COVID-19 per 1000 patients at risk 3 mo before the study (unvaccinated control group) with that observed during the following 3 mo, starting 15 d after the second dose, using an interrupted time-series analysis.18 Only patients with at least 28 d of follow-up since the diagnosis were included in this analysis in both periods.
For the unvaccinated control group, the persons at risk were all unvaccinated kidney transplant recipients aged between 30 and 69 y living in the transplant center state, alive, and with functioning grafts on December 20, 2020. Those with symptomatic SARS-CoV-2 infection confirmed by reverse transcription polymerase chain reaction or antigen test between December 20, 2020, and March 19, 2021, were included as COVID-19 cases. For the fully vaccinated group, the persons at risk were all fully vaccinated individuals who were alive, with functioning grafts, and no confirmed COVID-19 up to May 10, 2021 (15 d after the second vaccine dose administration). Those with symptomatic SARS-CoV-2 infection confirmed by reverse transcription polymerase chain reaction or antigen test after May 10, 2021, and until July 24, 2021, were included as COVID-19 cases.
The number of new kidney transplants, deducting loss of follow-up and deaths from other causes, in each week in both periods, was not included in the analysis, as the net number that would be added to the denominator would be quite small and unlikely to interfere with the assessment of the incidence rates.
Clinical Outcomes
The primary outcomes were the incidence of confirmed COVID-19/1000 persons at risk, need for hospitalization and mechanical ventilation, and 28-d lethality rates at least 15 d after the second vaccine dose. Secondary outcomes were the incidence of COVID-19, hospitalization, mechanical ventilation, and 28-d lethality rates after the first dose, <15 d and >15 after the second dose.
Statistical Analysis
Categorical variables were reported as frequencies and percentages. Continuous variables were presented as median and interquartile ranges (IQRs). Multivariable binary logistic regression was used to identify clinical variables independently associated with seroconversion after the second dose of CoronaVac and with 28-d mortality. Variables with P < 0.10 or at the discretion of the investigator in the univariable analysis were included in the multivariable model using a backward conditional selection procedure. Results were expressed as odds ratio (ORs) with their 95% CI. The effectiveness was obtained by comparing the COVID-19 incidence rates before and after the vaccination using the interrupted time-series analysis. The intervention of interest was set at 15 d after second dose of CoronaVac. Differences or effects were considered significant with a P < 0.05. Statistical analysis was performed using the SPSS program v. 22.0 (SPSS Inc, Chicago, IL) and GraphPad Prism program v.9.2.0 (Prisma Inc, CA).
RESULTS
Overall, 3371 patients received at least 1 dose of CoronaVac vaccine; from them, 3340 (99%) completed the vaccination schedule. The disposition of the study cohort is shown in Figure 1, and the baseline demographic characteristics are presented in Table 1. The patients were predominantly male with a median age of 53 (IQR, 45–60) y, and only 10% had a medical history of diabetes. The majority (63%) received a kidney from a deceased donor, and 4% a combined pancreas and kidney transplants. The predominant immunosuppressive regimen included a combination of a calcineurin inhibitor and an antiproliferative agent (84%), and all patients were on a low dose (5 mg/d) of corticosteroid. Finally, 4% of patients had received either high-dose methylprednisolone (defined as a total cumulative dose ≥1.5 g) or antithymocyte globulin within the last 6 mo. At the first vaccine dose, the median follow-up time after transplantation was 7 (IQR, 3–12) y.
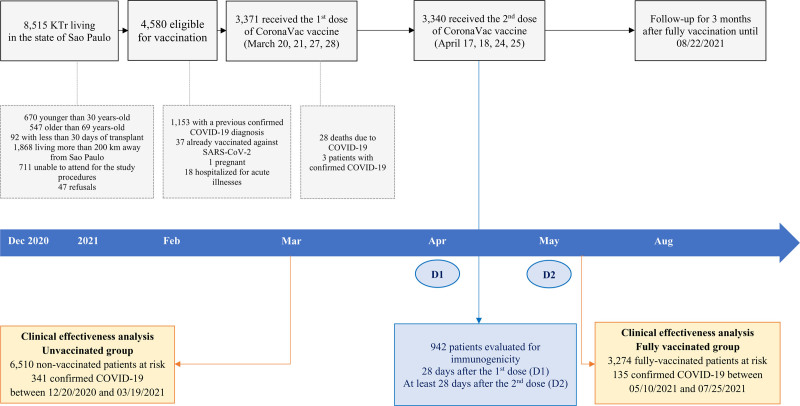
FIGURE 1.
Patient disposition. COVID-19, coronavirus disease 2019; D1, dose 1; D2, dose 2; KTr, kidney transplant recipient; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
TABLE 1.
Baseline demographic characteristics of the study cohort (kidney transplant recipients who received at least 1 dose of coronavac vaccine, n = 3371) and of the immunogenicity cohort (n = 942)
| Parameters | Study cohort | Immunogenicity cohort | P |
|---|---|---|---|
| (n = 3371) | (n = 942) | ||
| Median age (IQR), y | 53 (45–60) | 50 (42–56) | <0.0001 |
| Age category, n (%) | <0.0001 | ||
| 30–60 y | 2517 (75) | 898 (89) | |
| >60 y | 854 (25) | 114 (11) | |
| Male gender, n (%) | 2021 (60) | 592 (59) | 0.259 |
| Diabetes, n (%) | 333 (10) | 100 (10) | 0.997 |
| Deceased donor transplant, n (%) | 2134 (63) | 663 (66) | 0.204 |
| Organ, n (%) | 0.771 | ||
| Kidney | 3230 (96) | 971 (96) | |
| Simultaneous pancreas kidney | 135 (4) | 39 (4) | |
| Median length of transplant (IQR), y | 7 (3–12) | 6 (3–11) | <0.0001 |
| Maintenance immunosuppression, n (%) | |||
| TAC-Pred-AZA | 1021 (30) | 284 (32) | 0.238 |
| TAC-Pred-MPA | 1411 (42) | 403 (43) | 0.582 |
| CSA-Pred-AZA | 397 (12) | 93 (10) | 0.089 |
| TAC-Pred-mTORi | 308 (9) | 103 (11) | 0.063 |
| Other | 234 (7) | 59 (6) | 0.280 |
| High-dose methylprednisolone within the last 6 mo, n (%) | 4 (0.1) | 0 | 0.331 |
| Antithymocyte globulin within the last 6 mo, n (%) | 147 (4) | 27 (3) | 0.154 |
AZA, azathioprine; CSA, cyclosporine; IQR, interquartile range; MPA, mycophenolic acid; mTORi, mechanistic target of rapamycin inhibitor; Pred, prednisone; TAC, tacrolimus.
Reactogenicity
The complete questionnaire on adverse reactions to the first vaccine dose was obtained from 3332 (99%) patients. At least 1 reaction was reported by 33% of the patients. The most frequent were local pain or tenderness, headache, and myalgia. In addition, fever was reported by 1% of the participants (Table 2).
TABLE 2.
Adverse events reported up to 28 d after the first vaccine dose by 3332 kidney transplant recipients who received the second dose
| Adverse event | n (%) |
|---|---|
| Local pain or tenderness | 414 (12) |
| Headache | 210 (6) |
| Myalgia | 159 (5) |
| Runny nose | 106 (3) |
| Diarrhea | 89 (3) |
| Sore throat | 64 (2) |
| Fever | 44 (1) |
| Anosmia/ageusia | 16 (0.5) |
Immunogenicity
The seroprevalence of IgG anti-SARS-CoV-2 nucleoprotein before the first vaccine dose (D0) was 3.6% (n = 122) and 1.5% were undetermined (n = 50). Among the 3199 seronegative patients, 942 randomly selected individuals constituted the immunogenicity cohort and were tested 28 d after the first vaccine dose (D1). Of them, 856 were tested 28 d after the second vaccine dose (D2) (10 had COVID-19, 1 died, and 75 did not show up).
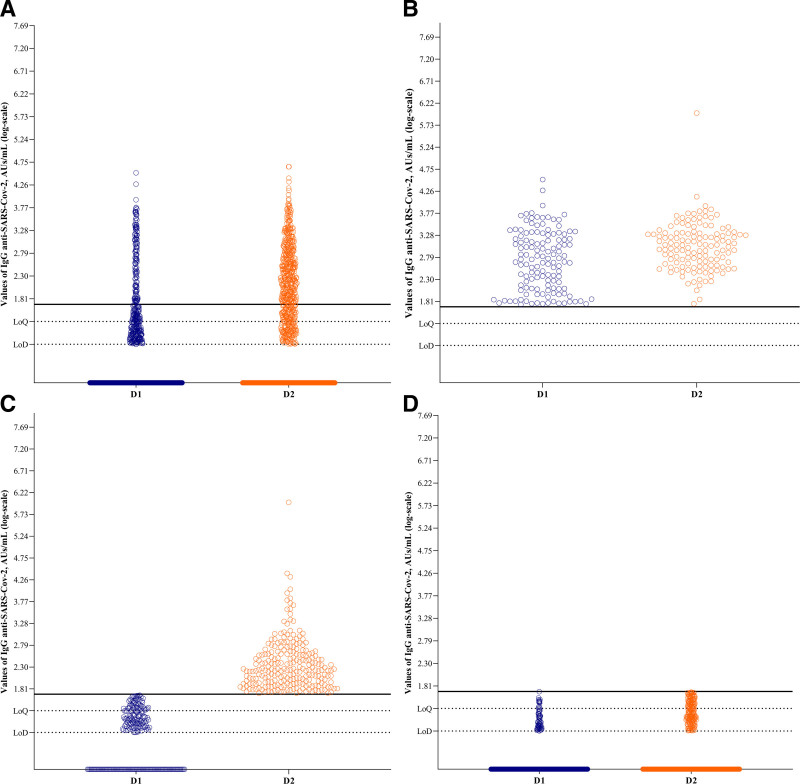
IgG anti-SARS-CoV-2 antibodies were detected in 15.2% of the patients 28 d after the first dose (D1) and in 43% at a median of 40 (IQR, 32–46) d after the second dose (D2). The median antibody levels among the responders were 490 (IQR, 124–1705) AU/mL after the first dose and 319 (IQR, 125–970) AU/mL after the second dose (Figure 2A). Of them, 15% (n = 132) showed antibody response after the first vaccine dose (median = 490 [IQR, 124–1705] AU/mL) and after the second dose (median = 1079 [IQR, 443–2093] AU/mL) (Figure 2B). Another 28% (n = 240) showed seroconversion only after the second dose (median = 169 [IQR, 86–391] AU/mL) (Figure 2C). Finally, 57% (n = 484) did not show any antibody response (Figure 2D). Independent risk factors associated with lack of seroconversion after the second dose were older age (OR, 0.96, 95% CI, 0.94-0.98 for each additional y of age) and recipients of kidneys from deceased donors (OR, 0.57, 95% CI, 0.41-0.79, Table 3).
FIGURE 2.
Abbott AdviseDx severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG II immunoassay for total IgG antibodies against the receptor binding domain of the S1-subunit of the SARS-CoV-2 spike protein, in logarithmic scale. The lowest limit of detection (LoD) by the manufacturer is 6.8 AU/mL (0.83 log), and the analytical measuring interval is 21 (1.32 log, LoQ) to 40 000 (4.60 log) AU/mL. The threshold for considering the test as positive is 50 AU/mL, or 1.69 log (black line). A, Immunogenicity analysis from the 856 patients with 2 sequential measures, showing the 143 seroconverted patients after dose 1 (D1) and 240 after dose 2 (D2) of CoronaVac vaccine. B, Patients with seroconversion both after D1 and D2 (n = 132). C, Patients with seroconversion only after the second dose (n = 240). Of them, 92 had undetectable IgG values at D1, 95 had detectable values under the lowest analytical level, and 53 had values between the lowest analytical level and the threshold for positivity. D, Patients without seroconversion at both D1 and D2 (n = 484). Of them, 74 had detectable IgG values at D1 and 189 at D2. From these 189 individuals, 9 had detectable IgG values only at D1, 125 only at D2, and 64 in both D1 and D2. AU, arbitrary unit.
TABLE 3.
Risk factors associated with seroconversion after the second vaccine dose in the immunogenicity cohort (n = 856)
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |
| Age (each additional y of age), y | <0.0001 | 0.994 (0.991-0.996) | <0.0001 | 0.96 (0.94-0.98) |
| Male gender (vs female gender) | <0.0001 | 0.705 (0.589-0.845) | 0.413 | 0.89 (0.67-1.20) |
| Diabetes (vs no diabetes) | <0.0001 | 0.417 (0.261-0.664) | 0.170 | 0.70 (0.42-1.18) |
| Recipient from deceased donor (vs living donor) | <0.0001 | 0.581 (0.490-0.688) | 0.001 | 0.57 (0.41-0.79) |
| Length of transplant, y | 0.148 | 0.990 (0.976-1.004) | – | – |
| Maintenance regimen | 0.079 | 0.847 | ||
| TAC-Pred-AZA | Ref | Ref | ||
| TAC-Pred-MPA | 0.771 (0.628-0.947) | 1.16 (0.82-1.64) | ||
| CSA-Pred-AZA | 0.909 (0.592-1.395) | 1.32 (0.77-2.26) | ||
| TAC-Pred-mTORi | 0.792 (0.529-1.188) | 1.16 (0.69-1.94) | ||
| Others | 0.778 (0.440-1.376) | 1.05 (0.54-2.03) | ||
AZA, azathioprine; CI, confidence interval; CSA, cyclosporine; MPA, mycophenolic acid; mTORi, mechanistic target of rapamycin inhibitor; OR, odds ratio; Pred, prednisone; ref, reference; TAC, tacrolimus.
Exploratory Analysis of the Neutralization Activity
Among the 15 patients with no changes in IgG values (903 [IQR, 383–1967] AU/mL at D1 versus 888 [IQR, 384–1867] AU/mL at D2), there was no change in the proportion of patients with detected neutralizing activity (80% at D1 versus 80% at D2). Conversely, among the 15 patients with the highest change in IgG values (65 [IQR, 59–113] AU/mL at D1 versus 1005 [IQR, 718–1567] AU/mL at D2), the percentage of patients with detected neutralizing activity increased from 13% at D1 to 100% at D2 (Figure 3).
FIGURE 3.
Exploratory analysis of the value of IgG anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a marker of neutralization activity. A, Antibody values (AU/mL) and (B) percentage of positive neutralization tests of the 15 patients with no changes and of the 15 patients with the highest changes in the antibody values between the first (dose 1 [D1], blue) and second dose of vaccine (dose 2 [D2], orange). AU, arbitrary unit.
Clinical Effectiveness
Clinical effectiveness was determined comparing the incidence of COVID-19 in 6510 unvaccinated kidney transplant recipients between December 20, 2020, and March 19, 2021, with 3274 fully vaccinated patients from May 10, 2021, and until July 24, 2021.
The 6510 patients from the control group had a median age of 45 (IQR, 37–53) y, and 9.4% of them were older than 60 y. Sixty-one percent of them were male, 15.6% had diabetes, 62% were recipients from a deceased donor, and 3% received a simultaneous kidney-pancreas transplant. The median time to transplant was 6.0 (IQR, 2.8–11.1) years, and the 4 main immunosuppressive regimens were tacrolimus, prednisone, and mycophenolate in 42%; tacrolimus, prednisone, and azathioprine in 32%; cyclosporine, prednisone, and azathioprine in 11%; and tacrolimus, prednisone, and mechanistic target of rapamycin inhibitor in 8%.
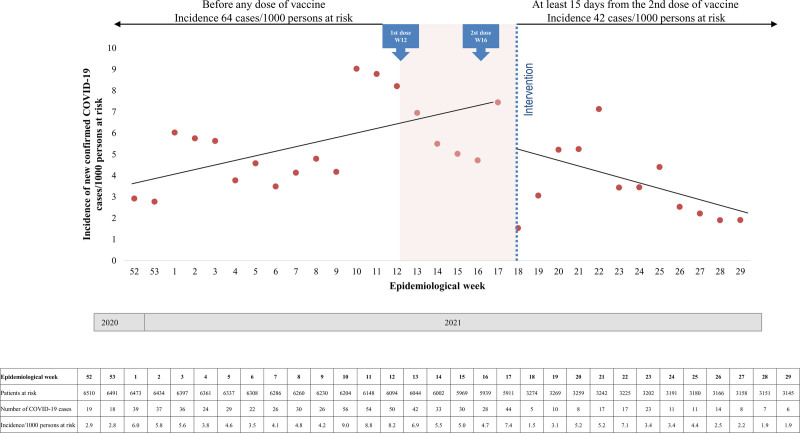
During the 13 epidemiological weeks periods before vaccination, the incidence of new confirmed cases of SARS-CoV-2 infection increased from 2.9 (week 52/2020) to 8.8 (week 11/2021) cases/1000 patients at risk per week. Similarly, during the 12 epidemiological weeks periods 15 d after the second vaccine dose, the incidence of new confirmed cases of SARS-CoV-2 infection increased from 1.5 (week 18/2021) to 7.1 (week 22/2021), decreasing thereafter to 1.9 cases/1000 patients at risk at epidemiological week 29/2021. Through interrupted time-series analysis, before the intervention, there was an upward trend of 0.23 cases/1000 patients at risk per week. After the intervention, there was a downward trend of 0.44 cases/1000 patients at risk per week (95% CI, −0.71 to −0.17; P = 0.0020, Figure 4).
FIGURE 4.
Incidence rates of coronavirus disease 2019 (COVID-19) in the 13 wk before any dose of CoronaVac vaccine and in the 12 wk after 15 d of the second vaccine dose (intervention). Before the intervention, there was an upward trend of 0.23 cases/1000 patients at risk per wk. After the intervention, there was a downward trend of 0.44 cases/1000 patients at risk per wk (95% confidence interval [CI], 0.71-0.17; P = 0.0020).
After the first vaccine dose, 232 (7%) patients developed confirmed SARS-CoV-2 infection, 68 (29%) before the second dose, 29 (12%) <15 d after the second dose, and 135 (59%) >15 d after the second dose. The overall hospitalization rate, need for mechanical ventilation, and 28-d lethality were 62%, 36%, and 30%, respectively. Compared with 341 kidney transplant recipients who developed COVID-19 within 3 mo before vaccination, the 135 fully vaccinated individuals showed a lower proportion of patients with diabetes (30 versus 10%, P < 0.001). There was an increase in the need for hospitalization in the fully vaccinated group (59% versus 49%, P = 0.499). Nevertheless, no significant difference in the need for mechanical ventilation or death at 28 d was observed comparing both groups (Table 4).
TABLE 4.
Comparison of the demographic characteristics and clinical outcomes of all the 232 patients who received at least 1 vaccine dose and developed COVID-19 across the subsequent doses and comparative analysis between the 135 fully vaccinated vs 341 nonvaccinated patients
| Parameters | All COVID-19+ | After first dose | <15 d after the second dose | Fully vaccinated | P a | Nonvaccinated | P b |
|---|---|---|---|---|---|---|---|
| (n = 232) | (n = 68) | (n = 29) | (n = 135) | (n = 341) | |||
| Median age (IQR), y | 53 (45–60) | 52 (47–59) | 56 (48–62) | 52 (44–60) | 0.381 | 50 (42–58) | 0.197 |
| Age category, n (%) | 0.742 | 0.374 | |||||
| 30–60 y | 173 (74) | 52 (76) | 20 (69) | 100 (74) | 268 (79) | ||
| Over 60 y | 61 (26) | 16 (24) | 9 (31) | 35 (26) | 73 (21) | ||
| Male gender, n (%) | 140 (60) | 46 (68) | 17 (59) | 77 (57) | 0.365 | 214 (63) | 0.249 |
| Diabetes, n (%) | 24 (10) | 9 (13) | 2 (7) | 14 (10) | 0.595 | 107 (31) | <0.0001 |
| Deceased donor transplant, n (%) | 157 (67) | 43 (63) | 22 (76) | 93 (68) | 0.104 | 203 (60) | 0.159 |
| Median length of transplant (IQR), y | 7 (3–12) | 7 (3–12) | 5 (1–13) | 7 (4–12) | 0.439 | 7 (3–12) | 0.095 |
| Maintenance immunosuppression, n (%) | 0.237 | 0.044 | |||||
| TAC-Pred-AZA | 68 (29) | 15 (22) | 14 (48) | 39 (29) | 97 (29) | ||
| TAC-Pred-MPA | 90 (38) | 27 (40) | 9 (32) | 53 (40) | 135 (40) | ||
| CSA-Pred-AZA | 38 (16) | 15 (22) | 2 (7) | 21 (15) | 28 (8) | ||
| TAC-Pred-mTORi | 23 (10) | 5 (7) | 3 (10) | 15 (11) | 32 (9) | ||
| Other | 15 (7) | 6 (9) | 1 (3) | 7 (5) | 44 (13) | ||
| Need for hospitalization due to COVID-19, n (%) | 146 (62) | 48 (71) | 18 (62) | 80 (59) | 0.286 | 168 (49) | 0.049 |
| Need for mechanical ventilation, n (%) | 84 (36) | 33 (49) | 11 (38) | 40 (30) | 0.030 | 95 (28) | 0.585 |
| 28-d mortality associated with COVID-19, n (%) | 69 (30) | 30 (44) | 10 (34) | 30 (22) | 0.003 | 85 (25) | 0.534 |
aRefers to comparison between the 3 groups of vaccinated patients who developed COVID-19.
bRefers to the comparison between fully vaccinated vs nonvaccinated patients who developed COVID-19.
AZA, azathioprine; CSA, cyclosporine; COVID-19, coronavirus disease 2019; IQR, interquartile range; MPA, mycophenolic acid; mTORi, mechanistic target of rapamycin inhibitor; Pred, prednisone; TAC, tacrolimus.
Among patients who received at least 1 dose of the vaccine and subsequently developed COVID-19, the independent risk factors associated with 28-d COVID-19-associated lethality were older age (OR, 1.05, 95% CI, 1.02-1.09 for each additional y of age), whereas having received the second dose >15 d before developing COVID-19 was associated with a 68% reduction in the risk of death (OR, 0.32, 95% CI, 0.16-0.63, Table 5).
TABLE 5.
Risk factors associated with 28-d COVID-19-associated lethality among the 232 subjects with confirmed severe acute respiratory syndrome coronavirus 2 infection after the first vaccine dose
| Factors | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |
| Age (each additional y of age), y | 0.002 | 1.05 (1.02-1.09) | 0.004 | 1.05 (1.02-1.09) |
| Male gender (vs female gender) | 0.521 | 1.21 (0.68-2.17) | – | |
| Diabetes (vs no diabetes) | 0.027 | 2.63 (1.11-6.20) | 0.268 | 1.70 (0.66-4.35) |
| Recipient from deceased donor (vs living donor) | 0.041 | 1.96 (1.03-3.75) | 0.081 | 1.86 (0.93-3.72) |
| Length of transplant, y | 0.591 | 0.99 (0.95-1.03) | – | |
| Maintenance regimen | ||||
| TAC-Pred-AZA | Ref | – | ||
| TAC-Pred-MPA | 0.642 | 0.85 (0.43-1.67) | ||
| CSA-Pred-AZA | 0.111 | 0.46 (0.17-1.20) | ||
| TAC-Pred-mTORi | 0.765 | 0.86 (0.31-2.37) | ||
| Others | 0.892 | 1.09 (0.33-3.62) | ||
| COVID-19 after the first dose | Ref | |||
| COVID-19 <15 d after the second dose | 0.379 | 0.67 (0.27-1.64) | 0.223 | 0.55 (0.21-1.44) |
| COVID-19 ≥15 d after the second dose | 0.001 | 0.35 (0.18-0.65) | 0.001 | 0.32 (0.16-0.63) |
AZA, azathioprine; CI, confidence interval; COVID-19, coronavirus disease 2019; CSA, cyclosporine; MPA, mycophenolic acid; mTORi, mechanistic target of rapamycin inhibitor; OR, odds ratio; Pred, prednisone; ref, reference; TAC, tacrolimus.
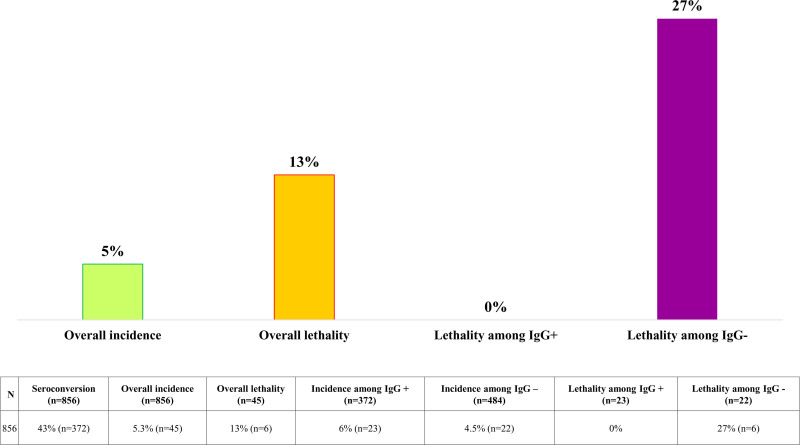
Among the 942 patients of the immunogenicity cohort, 45 developed SARS-CoV-2 infections. Of those 23 who had developed an antibody response to vaccination, there were no COVID-19-related deaths. Among the remaining 22 patients who had not developed an antibody response, 6 COVID-19-related deaths (27%) were recorded (Figure 5).
FIGURE 5.
Coronavirus disease 2019 (COVID-19) incidence and 28-d COVID-19-associated lethality among the 856 patients in the immunogenicity cohort.
DISCUSSION
In this prospective, phase 4, nonrandomized, and single-center study involving 3371 kidney transplant recipients, administration of a 2-dose regimen of CoronaVac vaccine was associated with low reactogenicity and an increase in the seroconversion rate from 15% after the first dose to 43% after the second dose.
The observed low antibody response is congruent with already published data on mRNA and adenovirus vectored vaccines reporting seroconversion between 3% and 59%, depending on the characteristics of the study population and the time of evaluation.10,19 In agreement with this information, a Saxon study involving over 3100 participants between general population, hemodialysis, and transplant patients noted that not only the humoral response but the frequency of SARS-CoV-2 reactive CD4+ T helper cells producing Th1 cytokines is significantly reduced among transplant recipients,20 which reinforces the hypothesis that a high proportion of these individuals remains vulnerable to infection even after vaccination.
Similar to what was found in the phase 3 study involving 9823 healthcare workers vaccinated with CoronaVac,7 older age was the main factor related to the lack of vaccine response, with a 4% reduction in the odds of seroconversion for each additional year. Being a deceased donor transplant recipient was also a risk factor independently associated with a lack of response, and it is plausible to speculate that this variable represents an indirect marker of the recipient’s frailty, including older age, higher comorbidities, and longer time on dialysis.21 Contrary to previously described,11,12 the immunosuppressive regimen was not associated with seroconversion. However, most of our patients were on triple immunosuppression with calcineurin inhibitor, steroids, and antimetabolite, limiting further comparative analyses.
There was a significant reduction in the incidence of COVID-19 starting from 2 wk after the second dose compared to the 3-mo period before the study. Although this finding may have been related to seroconversion, caution should be exercised in attributing a causal relationship, as the period of improvement coincides with adoption of more intense restrictive and physical distancing measures, an increasing percentage of the vaccinated population (reaching 74% of the inhabitants with at least 1 dose and 33% with the complete schedule at August 22, 2021) and a decline in the overall number of new cases of COVID-19 in the state of Sao Paulo. For context, in the period between December 20, 2020, and March 19, 2021, the local incidence of new confirmed cases of SARS-CoV-2 infection increased from 0.09 (week 52/2020) to 0.21 (week 11/2021) cases/1000 patients at risk per week. From May 10, 2021, to July 24, 2021, this rate went up from 0.17 (week 18/2021) to 2.0 (week 22/2021), then declined to 0.13 cases/1000 patients at risk per week in the epidemiological week 29/2021.22
Compared with the unvaccinated control group, there was no reduction in 28-d lethality associated with COVID-19. However, developing COVID-19 15 d after the second vaccine dose was associated with a lower risk of lethality in multivariate analysis. Furthermore, there were no deaths among those who had developed a humoral response in a small subgroup of patients with known postvaccination seroconversion status who subsequently acquired COVID-19.
Limitations of the study include the absence of a randomized control group, potentially receiving another type of vaccine, the underrepresentation of patients receiving alternative immunosuppressive drug combinations, the investigation of neutralizing activity is a small cohort, and the lack of data on cellular immunity. On the other hand, the strengths of the study include the prospective and systematic observation of a large number of patients, the homogeneity of the serological tests, and the extended follow-up time.
In summary, the results also point to low reactivity and immunogenicity of the inactivated whole-virion vaccine among kidney transplant patients. Vaccination was associated with a reduction in the incidence of SARS-CoV-2 infection, possibly related to seroconversion and a lower circulation of the virus in the general population. Nevertheless, overall lethality was unchanged and possibly related to lack of seroconversion. Thus, strategies seeking seroconversion, such as additional doses, modified concentrations, use of other adjuvants, or vaccine combinations, should be urgently pursued in the high-risk population.
ACKNOWLEDGMENTS
This work would not be possible without the provision of the vaccines, coordinated by Ricardo Palacios, MD, PhD, and Roberta Piorelli, MD, from Instituto Butantan, the volunteer work of almost 300 students and professionals of the Hospital do Rim and other medical institutions in São Paulo during vaccination.
Footnotes
The authors declare no funding or conflicts of interest.
The study is registered at ClinicalTrials.gov (NCT04801667).
J.M.-P., D.T.C., M.P.C., L.A.V., H.T.-S., L.R.R.-M., and R.D.F. participated in the research design; M.P.C. and Y.C.D. verified the underlying data; M.P.C., L.A.V., Y.C.D., M.R.N., C.M.C.B.F., E.F.L., and H.T.-S. participated in data analysis; J.M.-P., M.P.C., L.A.V., Y.C.D., M.R.N., L.R.R.-M., and H.T.-S. participated in the writing of the paper.
REFERENCES
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holder J. Coronavirus vaccine tracker. New York Times. 2021. Available at https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html. Accessed August 8, 2021. [Google Scholar]
- 3.Mallapaty S. WHO approval of Chinese coronaVac COVID vaccine will be crucial to curbing pandemic. Nature. 2021;594:161–162. [DOI] [PubMed] [Google Scholar]
- 4.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilder-Smith A, Mulholland K. Effectiveness of an inactivated SARS-CoV-2 vaccine. N Engl J Med. 2021;385:946–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanriover MD, Doğanay HL, Akova M, et al. ; CoronaVac Study Group. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palacios R, Batista AP, Albuquerque CSN, et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: the PROFISCOV study. SSRN. Available at 10.2139/ssrn.3822780. Accessed August 8, 2021. [DOI] [Google Scholar]
- 8.Azzi Y, Bartash R, Scalea J, et al. COVID-19 and solid organ transplantation: a review article. Transplantation. 2021;105:37–55. [DOI] [PubMed] [Google Scholar]
- 9.Cristelli MP, Viana LA, Dantas MTC, et al. The full spectrum of COVID-19 development and recovery among kidney transplant recipients. Transplantation. 2021;105:1433–1444. [DOI] [PubMed] [Google Scholar]
- 10.Boyarsky BJ, Chiang TP, Ou MT, et al. Antibody response to the Janssen COVID-19 vaccine in solid organ transplant recipients. Transplantation. 2021;105:e82–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-Dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marion O, Del Bello A, Abravanel F, et al. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medina-Pestana J, Cristelli MP, Viana LA, et al. Clinical impact, reactogenicity, and immunogenicity after the first CoronaVac dose in kidney transplant recipients. Transplantation. 2022;106:e95–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cristelli MP, Viana LA, Fernandes RA, et al. Kidney transplantation in the time of COVID-19: dilemmas, experiences, and perspectives. Transpl Infect Dis. 2021;23:e13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott Laboratories. AdviseDx SARS-CoV-2 IgG II instructions for use. 2021. Available at https://www.fda.gov/media/146371/download. Accessed August 23, 2021.
- 17.GenScript. cPass™ SARS-CoV-2 surrogate virus neutralization test (sVNT) kit (RUO). 2021. Available at https://www.genscript.com/covid-19-detection-svnt.html. Accessed August 23, 2021.
- 18.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr EJ, Kronbichler A, Graham-Brown M, et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep. 2021;6:2292–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haugen CE, Thomas AG, Chu NM, et al. Prevalence of frailty among kidney transplant candidates and recipients in the United States: Estimates from a National Registry and Multicenter Cohort Study. Am J Transplant. 2020;20:1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sao Paulo State Government. Boletim completo SP contra o coronavirus. 2021. Available at https://www.seade.gov.br/coronavirus/. Accessed August 23, 2021.