Background.
There is a paucity of data on the prevalence, adequate timing, and outcome of solid organ transplantation after severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and the kinetics of immunoglobulin G (IgG) antibodies in these patients.
Methods.
SARS-CoV-2 antinucleocapsid (N) IgG and polymerase chain reaction via a nasopharyngeal swab were analyzed in all patients within 24 h before liver or kidney transplantation. Kinetics of IgG antibodies were analyzed and compared with an immunocompetent cohort.
Results.
Between May 1, 2020, and March 18, 2021, 168 patients underwent liver or kidney transplantation in our center, of which 11 (6.54%) patients with a previous SARS-CoV-2 infection were identified. The median interval between SARS-CoV-2 infection and transplantation was 4.5 mo (range, 0.9–11). After a median posttransplant follow-up of 4.9 mo, 10 out of 11 patients were alive without clinical signs of viral shedding or recurrent or active infection. One patient without symptom resolution at time of transplantation died after combined liver-kidney transplantation. In 9 out of 11 patients with previously polymerase chain reaction-confirmed infection, SARS-CoV-2 anti-N and antispike (S) IgG were detectable at day of transplantation. Absolute levels of anti-N and anti-S IgG were positively correlated, declined over time in all patients, and were significantly lower compared with immunocompetent individuals. All patients remained anti-S IgG positive until the last posttransplant follow-up, whereas 3 patients became anti-N negative.
Conclusions.
We observed an uncomplicated course of liver or kidney transplantation after SARS-CoV-2 infection in selected patients. Although having lower absolute IgG antibody levels than immunocompetent individuals, all seroconverted patients remained anti-S IgG positive. These encouraging data need validation in larger studies.
INTRODUCTION
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is associated with a worse outcome in patients with end-stage organ damage1,2 and in patients on the waiting list for solid organ transplantation.3 The prevalence of previous SARS-CoV-2 infection in patients undergoing liver or kidney transplantation is unknown. Moreover, uncertainty remains on whether and when it is safe to perform solid organ transplantation in transplant candidates with previous SARS-CoV-2 infection and/or coronavirus disease 2019 (COVID-19). There might be a significant increase in postoperative morbidity and mortality related to previous SARS-CoV-2 infection.4,5 Concerns of prolonged viral RNA shedding, viral reactivation after introduction of immunosuppressive regimens, and patient fitness after COVID-19 should be weighed against the risk of delaying the transplantation and staying on the waiting list.6 Although most societies recommend applying a time interval between SARS-CoV-2 infection and transplantation,4,7,8 there is a paucity of real-life data on the adequate timing and outcome of solid organ transplantation after SARS-CoV-2 infection.9,10
Additionally, the posttransplant kinetics of immunoglobulin G (IgG) antibodies against the SARS-CoV-2 nucleocapsid (anti-N) and spike (anti-S) proteins in patients with a pretransplant SARS-CoV-2 infection are unknown. In immunocompetent patients, seroconversion for anti-N IgG antibodies occurs on average 1 to 2 d before anti-S IgG antibodies, and antibody levels start to decrease 2 to 4 mo after seroconversion.2 However, these kinetics might be altered in patients under immunosuppressive therapy after solid organ transplantation. This could impact the risk of (reinfection because anti-S antibodies can block the binding of the virus to the human angiotensin-converting enzyme 2 receptor.
Here, we assessed the prevalence of previous SARS-CoV-2 infection in patients who underwent a liver or kidney transplantation in our center and provided data on their short-term posttransplant outcome. Finally, we analyzed the kinetics of SARS-CoV-2 anti-N and anti-S IgG antibodies in these patients and assessed whether these differ from immunocompetent individuals with a previous infection.
MATERIALS AND METHODS
Patient Selection
All patients receiving a liver or kidney transplantation at University Hospitals Leuven between May 1, 2020, and March 18, 2021, were included in this study. From May 1, 2020, onward, a SARS-CoV-2 polymerase chain reaction (PCR) analysis via a nasopharyngeal swab in combination with an assessment of SARS-CoV-2 anti-N IgG antibodies were performed in all patients undergoing liver or kidney transplantation (Figure S1, SDC, http://links.lww.com/TP/C289). Both analyses were performed within 24 h before transplantation. Patients were transplanted in the case of a negative SARS-CoV-2 PCR analysis and no other clinical symptoms (ie, fever, acute dyspnea, and oxygen dependency) suggestive for ongoing SARS-CoV-2 infection. Regarding the latter, 1 exception was made for a patient (PCR negative at day of transplantation) who was still oxygen dependent after a critical COVID-19 with intensive care unit (ICU) cholangiopathy and kidney failure and who, therefore, received a combined liver-kidney transplantation. Furthermore, all donors had a negative nasopharyngeal SARS-CoV-2 PCR and a computed tomography thorax unsuspicious of COVID-19.
Previous SARS-CoV-2 infection in the recipient was defined as previous positive SARS-CoV-2 PCR via nasopharyngeal swabbing or the presence of SARS-CoV-2 anti-N IgG antibodies. Patient characteristics at the time of transplantation were prospectively collected. Data on prior SARS-CoV-2 infection were retrospectively collected from the electronic patient records. In all patients with SARS-CoV-2 infection before transplantation, a serial assessment of SARS-CoV-2 anti-N IgG antibodies was performed after transplantation. Additionally, we measured SARS-CoV-2 IgG anti-S in all patients with a confirmed previous infection on all included time points before and after transplantation. None of the recipients received a SARS-CoV-2 vaccine in the study period. The study was approved by the local ethical committee of UZ Leuven (S64036).
Immunocompetent Control Cohort
The immunocompetent patients with PCR-proven SARS-CoV-2 infection were included in a retrospective study on the longitudinal follow-up of SARS-CoV-2 anti-N and anti-S IgG antibodies (local ethics committee approval S63897). Our control cohort consisted of, in total, 231 patients, of which 116 patients had a nonsevere (asymptomatic, mild to moderate) SARS-CoV-2 infection and 115 patients had a severe to critical SARS-CoV-2 infection. The results of anti-N IgG of a subset of the patients were included in a published multicenter study.11 The presence of hypoxemia (Spo2 ≤93% or Pao2 <60 mm Hg combined with Paco2 >50 mm Hg) was applied to discriminate between nonsevere and severe disease. The median age of the applied control cohort was 60 y (range, 23–91) for the nonsevere group and 62 y (range, 28–92) for the severe group with 47.4% and 73.9% males, respectively.
PCR and Antibody Assays
SARS-CoV-2 PCR analyses on nasopharyngeal swabs were performed using either the Aptima SARS-CoV-2 assay (Hologic, San Diego, CA) on the Panther system, the Alinity m SARS-COV-2 assay (Abbott, Lake Forest, IL) on the Alinity m system, the TaqPath COVID-19 kit (ThermoFisher Scientific, Waltham, MA) on the Kingfisher high throughout the platform, or the Xpert Xpress SARS-CoV-2 assay (Cepheid, Sunnyvale, CA) on the GeneXpert System.
SARS-CoV-2 anti-N and anti-S IgG antibodies in our case series and in the immunocompetent control cohort were measured using Abbott’s SARS-COV-2 IgG and IgG II Quant chemiluminescent immunoassays on the Architect I2000SR (Abbott, Lake Forest, IL). A signal/cutoff value ≥1.40 was considered positive for anti-N IgG, and a value ≥50 arbitrary units per mL (AU/mL) was considered positive for anti-S IgG.
Statistics
Data are expressed as the median (range) or percentage of the total group. Correlations between variables were assessed using Spearman’s r. Antibody levels were compared between the transplant cohort and the immunocompetent controls using a nonparametric rank sum test (Mann-Whitney-Wilcoxon). P values <0.05 were considered statistically significant. Statistical analysis was performed using SPSS V.23 (IBM, Armonk, NY). Figures were generated using Graphpad Prism V.9 (GraphPad Software, La Jolla, CA).
RESULTS
Prevalence of Prior SARS-CoV-2 in Patients Who Underwent Transplantation
From May 1, 2020, until March 18, 2021, a total of 168 liver or kidney transplantation procedures were performed, including 62 single liver transplantations, 101 single kidney transplantations, and 5 combined liver-kidney transplantations. Out of these 168 patients, 11 (6.54%) patients had a previous SARS-CoV-2 infection (defined as a previous positive SARS-CoV-2 PCR via a nasopharyngeal swab and/or the presence of SARS-CoV-2 anti-N IgG antibodies) (Figure S1, SDC, http://links.lww.com/TP/C289). Five out of the 62 (8.06%) single liver recipients, 3 out of the 101 (2.97%) single kidney recipients, and 3 out of 5 patients who underwent combined liver-kidney transplantation had a previous SARS-CoV-2 infection (Table 1). All 11 patients identified with previous infection were SARS-CoV-2 PCR positive (nasopharyngeal swab) at the moment of infection, and in 9 of them (except patients 6 and 10), we could document the presence of both SARS-CoV-2 anti-N and anti-S IgG.
TABLE 1.
Patient characteristics and outcomes
| Patient | Tx organ | Age (y)/gender | BMI | Transplant indication | MELD/eGFR on day Tx | COVID-19 severity | Interval positive PCR and Tx (d) | Follow-up after Tx (d) | Post-Tximmunosuppression | Alive/functioning graft at last follow-up | Allograft rejection/reinfection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Liver | 67/M | 21 | ICU cholangiopathy | 20/92 | Critical: ARDS for which ICU admission with invasive ventilation, ECMO, and dialysis | 167 | 226 | <3 m: BM + Tac + MMF>3 m: Tac + MMF | Yes/yes | No/no |
| 2 | Liver | 68/M | 29 | NASH and HCC | 10/93 | Asymptomatic | 169 | 148 | <3 m: BM + Tac + MMF + MP>3 m: Tac + MMF | Yes/yes | No/no |
| 3 | Liver | 50/F | 27 | AIH and PBC | 25/116 | Critical: ARDS for which ICU admission with invasive ventilation | 195 | 156 | <3 m: BM + Tac + MMF + MP>3 m: Tac + MMF | Yes/yes | No/no |
| 4 | Liver | 57/F | 36 | ALD and HCC | 17/87 | Mild: anosmia and loss of taste, no hospitalization | 26 | 95 | <3 m: BM + Tac + MMF + MP>3 m: Tac + MMF | Yes/yes | No/no |
| 5 | Liver | 52/F | 26 | Decompensated ALD | 31/123 | Mild: cough and myalgia, no hospitalization | 60 | 65 | <3 m: BM + Tac + MMF + MP | Yes/yes | No/no |
| 6 | Liver + kidney | 72/F | 22 | ADPKD and ESKD | 23/9 | Asymptomatic | 56 | 233 | <3 m: BM + Tac + MMF + MP>3 m: Tac + MP | Yes/yes | No/no |
| 7 | Liver + kidney | 69/M | 25 | ICU cholangiopathy and kidney failure | 19/52 | Critical: ARDS for which ICU admission with invasive ventilation, ECMO, dialysis, and MARS | 148 | 38 | <3 m: BM + Tac + MMF | No/no | No/no |
| 8 | Liver + kidney | 63/M | 28 | NASH and hepatorenal syndrome | 45/17 | Asymptomatic | 47 | 30 | <3 m: BM + Tac + MMF + MP | Yes/yes | No/no |
| 9 | Kidney | 69/M | 30 | Dent’s disease | –/6 | Severe: loss of taste, anorexia, nausea, dyspnea with hospitalization, and noninvasive O2 supply | 246 | 151 | <3 m: Tac + MMF + MP>3 m: Tac + MMF + MP | Yes/yes | No/no |
| 10 | Kidney | 62/M | 35 | Nephroangiosclerosis | –/6 | Asymptomatic | 49 | 270 | <3 m: Tac + MMF + MP>3 m: Tac + MMF | Yes/yes | No/no |
| 11 | Kidney | 61/M | 30 | Hypertensive nephropathy | –/16 | Critical: ARDS for which ICU admission with invasive ventilation | 339 | 10 | <3 m: Tac + MMF + MP | Yes/yes | No/no |
ADPKD, autosomal dominant polycystic kidney disease; AIH, autoimmune hepatitis; ALD, alcohol-related liver disease; ARDS, acute respiratory distress syndrome; BM, basiliximab; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate in mL/min/1.73 m2 (Chronic Kidney Disease Epidemiology Collaboration); ESKD, end-stage kidney disease; HCC, hepatocellular carcinoma; ICU, intensive care unit; MARS, molecular adsorbent recirculating system; MELD, model of end-stage liver disease; MMF, mycophenolate mofetil; MP, methylprednisolone; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cell; Tac, tacrolimus; Tx, transplantation; <3 m, within first 3 mo after Tx; >3 m, beyond 3 mo after Tx.
In addition to these 168 procedures, 2 combined liver-pancreas-intestine, 1 liver-kidney-lung, 2 combined kidney-heart, and 2 combined kidney-pancreas procedures were performed during the study period. None of these patients were identified with a previous SARS-CoV-2 infection.
Patient Characteristics, Disease Severity of SARS-CoV-2 Infection, and Interval Between Infection and Transplantation
Characteristics of the patients with a pretransplant SARS-CoV-2 infection are shown in Table 1. Sixty-four percent of patients were male. In 6 patients, the disease course ranged from asymptomatic (n = 4) to mild (n = 2) without need for hospitalization. In 5 patients, the disease course was severe or critical with need for hospitalization, of which 4 were admitted to the ICU with need for mechanical ventilation. Patient 1 received hydroxychloroquine, patient 3 received methylprednisolone, and patients 7 and 11 received the combination of hydroxychloroquine and methylprednisolone, whereas the other patients did not receive specific pharmacological treatment against SARS-CoV-2/COVID-19. The median interval between the SARS-CoV-2 infection (based on day of SARS-CoV-2 PCR positivity) and transplantation was 4.5 mo (range, 0.9–11).
Short-term Outcome After Transplantation
The median follow-up after transplantation was 4.9 mo (range, 0.3–8.9). The immunosuppressive regimen per patient can be found in Table 1. No modifications of immunosuppressive therapy were made because of pretransplant SARS-CoV-2 infection, and no graft rejections were observed.
At the last posttransplant follow-up visit, 10 out of 11 patients were alive with adequate graft function and without need for renal replacement therapy (Table 1). One patient died from septic shock 5 wk after a combined liver-kidney transplantation. Preoperatively, this patient had severe COVID-19 pneumonitis, for which he was admitted to the ICU and received extracorporeal membrane oxygenation. He subsequently developed ICU cholangiopathy and kidney failure, for which he received molecular adsorbent recirculating system therapy and dialysis, respectively. Although no active SARS-CoV-2 infection was detected at the time of transplantation (PCR negative and presence of both anti-N and anti-S IgG), he was still oxygen dependent and in an extremely frail condition. In total, 4 patients experienced a non–SARS-CoV-2 infection in the posttransplantation period (Table S1, SDC, http://links.lww.com/TP/C289).
No clinical signs of active or recurrent SARS-CoV-2 infection were detected in any of the recipients. In 9 out of 11 patients, nasopharyngeal SARS-CoV-2 PCR was repeated at least once postoperatively in the context of surveillance within standard care, and all remained negative, confirming the absence of viral shedding (Figure S2, SDC, http://links.lww.com/TP/C289). The median time between transplantation and the last postoperative PCR analysis was 97 d (range, 4–156).
Kinetics of SARS-CoV-2 Anti-N and Anti-S IgG Antibodies and Comparison With Immunocompetent Cohort
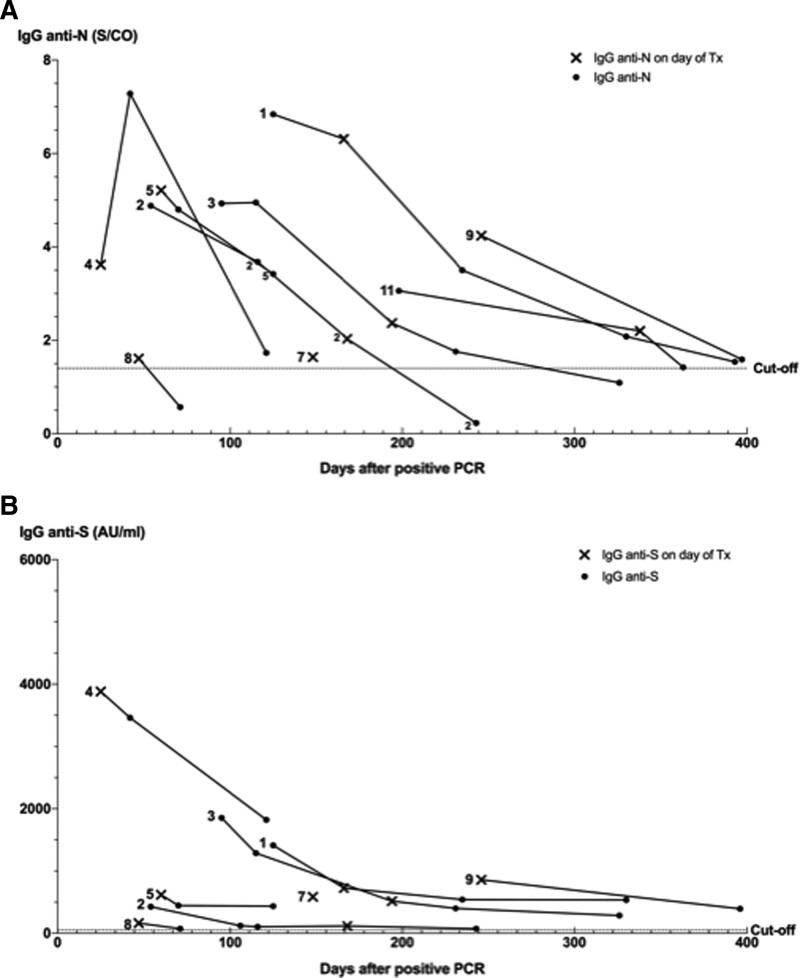
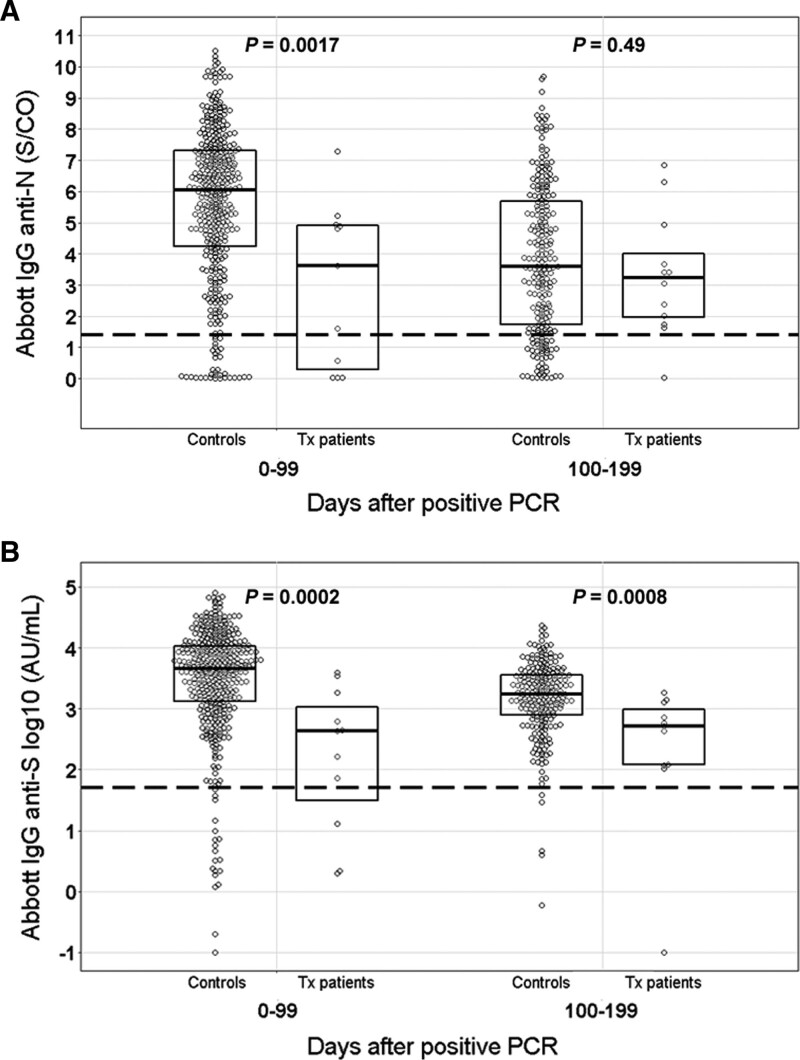
The kinetics of SARS-CoV-2 anti-N and anti-S IgG antibodies are shown in Figures 1A and B, respectively. The median documented duration of IgG seropositivity was 168 d (range, 47–397) for anti-N and 196 d (range, 71–397) for anti-S IgG after positive PCR. In 3 patients, anti-N IgG disappeared after a median of 243 d (range, 71–326) after positive PCR. In contrast, all patients with documented seroconversion remained anti-S IgG positive until the last posttransplant follow-up visit. Absolute anti-N and anti-S IgG levels were positively correlated (r = 0.58, P < 0.001), and both declined over time in all patients. In 2 out of 11 patients with pretransplant PCR-confirmed SARS-CoV-2 infection (patients 6 and 10), anti-N IgG and anti-S IgG were negative at the day of transplantation and remained negative after transplantation. Absolute anti-S IgG levels were significantly lower in both the first 100 d (P = 0.0002) and 100 to 200 d (P = 0.0008) time periods after PCR positivity in our seroconverted transplant patients compared with immunocompetent individuals (Figure 2B), whereas anti-N IgG antibodies were only significantly lower in the first 100-d period (P = 0.0017) (Figure 2A).
FIGURE 1.
SARS-CoV-2 IgG kinetics. A, Kinetics SARS-CoV-2 IgG anti-N antibody in relation to positive nasopharyngeal PCR. B, Kinetics SARS-CoV-2 IgG anti-S antibody in relation to positive nasopharyngeal PCR. Anti-N and anti-S IgG for patients 6 and 10 (not shown in figure) were seronegative at d of transplantation and remained seronegative after transplantation (at 136 d of follow-up). Patient 7 died during follow-up, and, consequently, only a single measurement was performed. Anti-S IgG was not available for patient 11. A S/CO value of ≥1.40 for anti-N IgG and a value of ≥50 arbitrary units per milliliter (AU/mL) for anti-S IgG were considered positive. Anti-N, antinucleocapsid; anti-S, antispike; IgG, immunoglobulin G; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; S/CO, single cutoff; Tx, transplantation.
FIGURE 2.
Comparison of SARS-CoV-2 IgG antibodies with immunocompetent cohort. A, SARS-CoV-2 anti-N IgG antibodies. B, SARS-CoV-2 anti-S IgG antibodies. Antibody levels were compared using a nonparametric rank sum test (Mann-Whitney-Wilcoxon). P values < 0.05 were considered statistically significant. Anti-N, antinucleocapsid; anti-S, antispike; IgG, immunoglobulin G; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; Tx, transplantation.
DISCUSSION
Here, we present, to our knowledge, the largest case series of patients that received a liver or kidney transplantation after SARS-CoV-2 infection to date. In 10 out of 11 patients with a previous SARS-CoV-2 infection, the short-term clinical outcome after transplantation was uncomplicated.
The overall prevalence of prior SARS-CoV-2 infection among our 168 patients undergoing liver or kidney transplantation was 6.54% (based on PCR testing and/or antibody positivity) over the entire 10-mo study period. We only tested the prevalence of prior infection in patients who ultimately were transplanted. However, we are not aware of documented SARS-CoV-2 infections among our patients on the liver and/or kidney organ waiting list within the study period other than presented in this report. No patients on these waiting lists were permanently delisted or died because of COVID-19.
The recommended time interval between SARS-CoV-2 symptom resolution and transplantation varies between transplant societies from 10 to 28 d or is not specified.4,7,8 The median time between positive SARS-CoV-2 PCR and the day of transplantation (all patients PCR negative at day of procedure) in our case series was 4.9 mo, and all but 1 patient had complete respiratory symptom resolution. No clinical signs of viral shedding (based on PCR analysis after transplantation), reactivation, or reinfection were observed in any of the transplanted patients with a prior SARS-CoV-2 infection. We applied no specific measures concerning immunosuppression because of pretransplant infection. Of note, reactivation of SARS-CoV-2 after solid organ transplantation has not been reported to date.4 The 1 patient who died (patient 7 in Table 1) received a combined liver-kidney transplantation with an interval of 148 d after a severe SARS-CoV-2 infection. However, this patient was still oxygen dependent and in an extremely frail condition due to his multiorgan failure and long stay in the ICU until the transplantation procedure. He died from a bacterial and fungal infection with septic shock, pointing to the potential additional risk of bacterial and fungal infections after transplantation, especially in patients who received corticosteroids as targeted therapy for COVID-19. The shortest interval between SARS-COV-2 PCR positivity and transplantation in our study was 26 d in a patient with mild disease (anosmia and loss of taste) who had an uncomplicated postoperative course. Only a few cases of uncomplicated liver or kidney transplantation within a shorter time interval after PCR positivity or even in a patient with an active infection have been reported to date.10,12-15 Taken together, it appears that respiratory symptom resolution and a lack of fever, patient fitness, and PCR negativity (whether or not combined with positive IgG antibodies) are the crucial factors in assessing liver and/or kidney transplant eligibility rather than applying an arbitrary time interval after PCR positivity or symptom resolution. In our opinion, solid organ transplantation in patients with an active infection and respiratory symptoms should be avoided when possible because of the risk of the progression of COVID-19 with subsequent organ failure and death and the risk of viral exposure of the transplant personnel.
Most of our patients had a positive anti-N IgG and anti-S IgG at the day of transplantation, and we observed a positive correlation between anti-N IgG and anti-S IgG antibody levels. However, SARS-CoV-2 anti-N IgG dropped below the cutoff level in 3 seropositive patients after a median time of 243 d after PCR positivity, whereas anti-S remained positive in all initially seropositive patients at the last follow-up. This is in line with our recent findings suggesting that anti-S IgG antibodies persist longer than anti-N IgG antibodies after SARS-CoV-2 infection. We showed in immunocompetent, nontransplanted patients that at 1 to 3 mo after PCR positivity, 98.3% were positive for anti-S compared with 85.6% for anti-N, whereas at 7 to 10 mo, 92.4% of patients were still positive for anti-S compared with only 17.8% for anti-N.16 Analyzing SARS-CoV-2 anti-S IgG might, thus, be superior to detect a prior SARS-CoV-2 infection and determine the duration of protective immunity. However, the universal SARS-CoV-2 vaccination (based on the endogenous synthesis of antiviral spike protein antibody) will impede the value of anti-S IgG to detect prior infection. Interestingly, we found lower absolute levels of SARS-CoV-2 anti-N and, in particular, anti-S IgG in our transplant patients compared with immunocompetent individuals. The questions on whether this finding does imply a reduced protective immunity and on whether this finding differs from transplant recipients who had a SARS-CoV-2 infection after solid organ transplantation need further study. Overall, more data on the diagnostic accuracy, neutralizing capacities, and the kinetics of the SARS-CoV-2 anti-N and anti-S IgG antibodies before and especially after solid organ transplantation are needed, which is the subject of ongoing research in our center. This knowledge will further clarify the clinic utility of measuring SARS-CoV-2 antibodies in this specific patient population.
The strength of this single-center study is the systematic screening by both PCR and antibody testing in all of our patients who received a liver or kidney transplantation in a consecutive time window of 10 mo in the current COVID-19 pandemic. Although the absolute number of positive cases is rather low, it is the largest series to date, and the postoperative outcome is in line with the few individual cases that are reported to date.6,10,12-14,17-19
In conclusion, we report an uncomplicated outcome of liver or kidney transplantation in selected patients with prior SARS-CoV-2 infection who are fit, have no residual pulmonary symptoms or fever, and are PCR negative at the moment of transplantation. Although we observed significantly lower absolute levels of SARS-CoV-2 IgG antibodies in our cohort compared with immunocompetent individuals, all our patients remained anti-S IgG positive during the study period. Our results are encouraging for the transplant community, but more data are needed to firmly establish the minimal infection-to-transplantation time interval and the short- and long-term safety of liver or kidney transplantation in patients with a previous SARS-CoV-2 infection.
Supplementary Material
Footnotes
J.V. participated in conceptualization, data analysis, revising of article, and supervision. C.V. participated in data acquisition, data analysis, and drafting article. P.V., J.V.E., and K.L. participated in data acquisition, data analysis, and revising article. S.V. participated in data acquisition and data analysis. R.V., J.V.C., I.J., D.M., J.P., D.K., and F.N. participated in revising article.
The authors declare no conflicts of interest.
The study was funded by the Research Council (KOOR) UZ Leuven. R.V. is a senior clinical research fellow of the Research Foundation Flanders (FWO).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Iavarone M, D’Ambrosio R, Soria A, et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Elslande J, Decru B, Jonckheere S, et al. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin Microbiol Infect. 2020;26:1557.e1–1557.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravanan R, Callaghan CJ, Mumford L, et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020;20:3008–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Association for the Study of Liver Diseases. COVID-19 and the liver. Available at https://www.aasld.org/about-aasld/covid-19-and-liver. Accessed April 17, 2021.
- 5.Khonsari RH, Bernaux M, Vie JJ, et al. ; AP-HP/Universities/INSERM COVID-19 research collaboration, AP-HP COVID Clinical Data Warehouse initiative. Risks of early mortality and pulmonary complications following surgery in patients with COVID-19. Br J Surg. 2021;108:e158–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niess H, Börner N, Muenchhoff M, et al. Liver transplantation in a patient after COVID-19—rapid loss of antibodies and prolonged viral RNA shedding. Am J Transplant. 2021;21:1629–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Society of Transplantation. FAQs for organ transplantation. Available at https://www.myast.org/faqs-organ-transplantation. Accessed March 28, 2021.
- 8.The Transplantation Society. Guidance on coronavirus disease 2019 (COVID-19) for transplant clinicians. Available at https://tts.org/tid-about/tid-presidents-message/23-tid/tid-news/657-tid-update-and-guidance-on-2019-novel-coronavirus-2019-ncov-for-transplant-id-clinicians. Accessed April 27, 2021.
- 9.Di Maira T, Berenguer M. COVID-19 and liver transplantation. Nat Rev Gastroenterol Hepatol. 2020;17:526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni AV, Parthasarathy K, Kumar P, et al. Early liver transplantation after COVID-19 infection: the first report. Am J Transplant. 2021;21:2279–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Elslande J, Oyaert M, Ailliet S, et al. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol. 2021;136:104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouphael C, D’Amico G, Ricci K, et al. Successful orthotopic liver transplantation in a patient with a positive SARS-CoV2 test and acute liver failure secondary to acetaminophen overdose. Am J Transplant. 2021;21:1312–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martini S, Patrono D, Pittaluga F, et al. Urgent liver transplantation soon after recovery from COVID-19 in a patient with decompensated liver cirrhosis. Hepatol Commun. 2020;5:144–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murad H, Dubberke E, Mattu M, et al. Repeat SARS-CoV-2 testing after recovery. Is a pretransplant PCR necessary? Am J Transplant. 2021;21:3206–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raveh Y, Simkins J, Nicolau-Raducu R. Liver transplantation in COVID-19 positive patients. Am J Transplant. 2021;21:1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Elslande J, Gruwier L, Godderis L, et al. Estimated half-life of SARS-CoV-2 anti-spike antibodies more than double the half-life of anti-nucleocapsid antibodies in healthcare workers. Clin Infect Dis. 2021;73:2366–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhand A, Bodin R, Wolf DC, et al. Successful liver transplantation in a patient recovered from COVID-19. Transpl Infect Dis. 2021;23:e13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raut V, Sonavane A, Shah K, et al. Successful liver transplantation immediately after recovery from COVID-19 in a highly endemic area. Transpl Int. 2021;34:376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varotti G, Dodi F, Garibotto G, et al. Successful kidney transplantation after COVID-19. Transpl Int. 2020;33:1333–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]