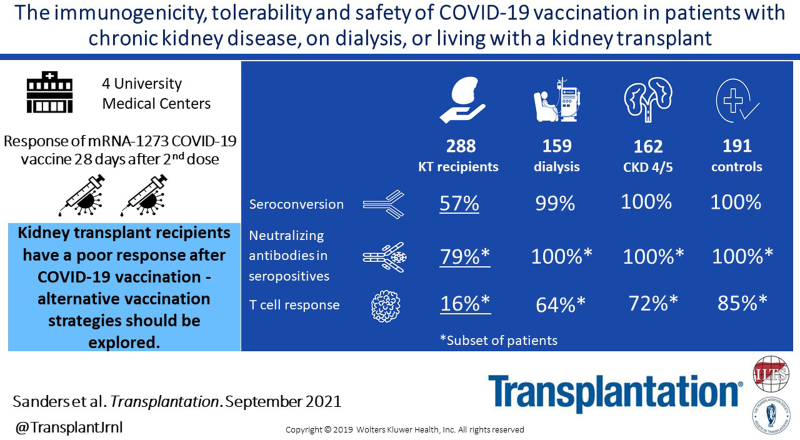
Background.
In kidney patients COVID-19 is associated with severely increased morbidity and mortality. A comprehensive comparison of the immunogenicity, tolerability, and safety of COVID-19 vaccination in different cohorts of kidney patients and a control cohort is lacking.
Methods.
This investigator driven, prospective, controlled multicenter study included 162 participants with chronic kidney disease (CKD) stages G4/5 (eGFR < 30 mL/min/1.73m2), 159 participants on dialysis, 288 kidney transplant recipients, and 191 controls. Participants received 2 doses of the mRNA-1273 COVID-19 vaccine (Moderna). The primary endpoint was seroconversion.
Results.
Transplant recipients had a significantly lower seroconversion rate when compared with controls (56.9% versus 100%, P < 0.001), with especially mycophenolic acid, but also, higher age, lower lymphocyte concentration, lower eGFR, and shorter time after transplantation being associated with nonresponder state. Transplant recipients also showed significantly lower titers of neutralizing antibodies and T-cell responses when compared with controls. Although a high seroconversion rate was observed for participants with CKD G4/5 (100%) and on dialysis (99.4%), mean antibody concentrations in the CKD G4/5 cohort and dialysis cohort were lower than in controls (2405 [interquartile interval 1287–4524] and 1650 [698–3024] versus 3186 [1896–4911] BAU/mL, P = 0.06 and P < 0.001, respectively). Dialysis patients and especially kidney transplant recipients experienced less systemic vaccination related adverse events. No specific safety issues were noted.
Conclusions.
The immune response following vaccination in patients with CKD G4/5 and on dialysis is almost comparable to controls. In contrast, kidney transplant recipients have a poor response. In this latter, patient group development of alternative vaccination strategies are warranted.
INTRODUCTION
In patients with severely impaired kidney function, patients on dialysis and kidney transplant recipients coronavirus disease 2019 (COVID-19)-associated mortality risk has been reported to be three- to fourfold higher than in the general population.1 Therefore, the availability of effective and safe COVID-19 vaccines is of great importance for these patients.2 Two mRNA vaccines have been widely used in Europe and the United States, and pivotal trials reported an efficacy of 95% for the BNT162b2 BioNTech/Pfizer vaccine and 94% for the mRNA-1273 Moderna COVID-19 vaccine.3,4 Response to vaccination can be considerably lower in patients with severely impaired kidney function due to the immunosuppressive effect of uremia and specific medications. This has been shown for vaccination against hepatitis B, influenza, and Streptococcus pneumonia.5 Unfortunately, the large phase 3 registration trials with mRNA vaccines excluded high risk groups, among which kidney transplant recipients. Patients, with severely impaired kidney function and patients on dialysis, were highly underrepresented in these studies. A number of investigator-initiated studies recently suggested a lower vaccine-specific antibody response in patients on kidney function replacement treatment after 2 doses of mRNA vaccines.6–9 Most of these studies, however, were small-scale single center studies, included only 1 subgroup of kidney patients or were uncontrolled. In addition, prevaccination samples and combined assessment of vaccine induced antibody formation (including virus neutralizing capacity) and cellular immune responses were not included in most studies.
As Dutch REnal patients COVID-19 VACcination (RECOVAC) consortium, we therefore performed a multicenter investigator-initiated study to assess the immunogenicity at the humoral as well as cellular level, tolerability, and safety of the mRNA-1273 COVID-19 vaccine (Moderna Biotech Spain, S.L.) in patients with severely impaired kidney function, patients on dialysis, kidney transplant recipients, and control subjects without known kidney disease.
MATERIALS AND METHODS
The design of the RECOVAC Immune Response study has been published previously in detail.10 The study was performed between February 1 and May 31, 2021, at the outpatient clinics of 4 university medical centers in the Netherlands (Amsterdam UMC, UMC Groningen, Radboudumc Nijmegen and Erasmus MC Rotterdam). Approval was obtained from the Dutch Central Committee on Research Involving Human Subjects (CCMO, NL76215.042.21) and the local ethics committees of the participating centers. Funding was supplied by The Netherlands Organization for Health Research and Development (ZonMW, project number: 10430072010002). The study is registered in www.ClinicalTrials.gov (NCT04741386).
Study Participants and COVID-19 Vaccination
Four different cohorts were included in the study. Cohort A, the control group, consisted of subjects without kidney disease (eGFR >45 mL/min/1.73m2), cohort B of patients with severely impaired kidney function (eGFR <30 mL/min/1.73m2 or chronic kidney disease (CKD) stages G4/5); cohort C of patients on hemodialysis or peritoneal dialysis; and cohort D of kidney transplant recipients. The control cohort included partners, siblings, or household members of participants in cohorts B, C, and D. The numbers of participants in each cohort were equally divided over the 4 participating centers. Further inclusion and exclusion criteria are reported in Table S1, SDC, http://links.lww.com/TP/C306. All participants received 2 mRNA-1273 COVID-19 vaccinations (Moderna Biotech Spain, S.L.) with an interval of 28 d according to the manufacturer’s instructions. To assess immunogenicity and safety, blood samples were collected at baseline (ie, before first vaccination), immediately before the second vaccination, and 28 d after the second vaccination. To evaluate tolerability and possible vaccination related adverse events (AEs), patients were asked to report solicited local and systemic AEs for 7 d after each vaccination using a standardized questionnaire. The number and proportion of AEs and serious AEs until day 28 after the second vaccination were reported for each study group. Table S2 (SDC, http://links.lww.com/TP/C306) provides a detailed overview of study visits and assessments.
SARS-CoV-2 Spike S1-specific IgG Antibody Response
SARS-CoV-2 Spike S1-specific IgG antibodies were measured in serum samples by a validated fluorescent bead-based multiplex-immunoassay with a specificity and sensitivity of 99.7% and 91.6%, respectively, as previously described.11,12 Concentrations were interpolated from a reference consisting of pooled sera using a 5-parameter logistic fit and NIBSC/WHO COVID-19 reference serum 20/136, and expressed as international binding antibody units per mL (BAU/mL). SARS-CoV-2 Spike S1-specific IgG antibodies were measured at baseline for exclusion of subjects who had a previous SARS-CoV-2 infection before vaccination, and at second vaccination to assess the immune-response after the first vaccination. Primary outcome was defined as seroconversion rate at 28 d after the second vaccination. Participants were classified as responder or nonresponder based on seroconversion with a threshold for seropositivity based on receiver operator curve analysis set at S1-specific IgG antibody concentration ≥10 BAU/mL.12,13
Virus Neutralizing Antibodies
The presence of antibodies with neutralizing capacity against SARS-CoV-2 was tested by a plaque reduction neutralization test (PRNT50) as previously described.13,14 Since there is a strong correlation between titers of neutralizing antibodies and the concentration of SARS-CoV-2 Spike S1 IgG antibodies, the presence of neutralizing antibodies15 was only tested in sera of patients that had a measurable S1-specific IgG concentration (ie, >0.1 BAU/mL) at 28 d after the second vaccination. In patients with an immeasurable S1-specific IgG concentration, we assumed that virus neutralizing antibodies would be immeasurable. For reasons of feasibility, it was a priori decided to measure virus neutralizing antibodies only in a random sample of patients included in 1 of the participating centers (Erasmus MC Rotterdam). This concerned 17 control subjects, 18 CKD G4/5 patients, 20 dialysis patients, and 20 kidney transplant recipients. Because during the study kidney transplant recipients proved to have a low S1-specific IgG seroconversion rate, additionally all samples of kidney transplant recipients in the aforementioned center were tested. A titer of virus neutralizing antibodies >20 was considered to be positive based on assay validation.
SARS-CoV-2-specific T-cell Response
The SARS-CoV-2-specific T-cell response was measured in all subjects participating in the same aforementioned center (Erasmus MC Rotterdam). This concerned 46 control subjects, 39 CKD G4/5 patients, 42 dialysis patients, and 68 kidney transplant recipients. Measurement was performed by a commercially available Interferon-gamma (IFN-γ) release assay (IGRA) according to the manufacturer’s instructions (Qiagen).16 In short, heparinized whole blood obtained prevaccination and at 28 d after the second vaccination was incubated with 2 different SARS-CoV-2 antigens for 24 h using a combination of S peptides stimulating both CD4+ and CD8+ T cells. After incubation, plasma was obtained and IFN-γ production in response to the antigens was measured by ELISA. Results are expressed in IU/mL after subtraction of the negative control values as interpolated from a standard calibration curve.
Statistical Analysis
Continuous data are presented as mean with SD or as median and interquartile interval (IQI) in case of nonnormal distribution. Categorical data are presented as percentages. Differences between cohorts were tested using ANOVA, Kruskal Wallis test, or Pearson Chi-square test depending on data distribution. Primary immunogenicity outcome was response to vaccination defined as reaching a level of SARS-CoV-2 Spike S1-specific IgG antibodies ≥10 BAU/mL on day 28 after the second vaccination. The study was powered to show noninferiority versus controls as described previously in detail.10 The noninferiority margin for the difference in response between the control group and CKD G4-5 and dialysis patients was 20%, and for transplant recipients 25%. The power analysis indicated that 175 subjects had to be included in the CKD G4/5 and dialysis cohorts, 300 in the kidney transplant recipient cohort, and 200 in the control cohort. The percentage difference in seroconversion rate after vaccination between the patient and control cohorts with corresponding 95% confidence interval (CI) was calculated. CI limits were checked against the noninferiority margin to assess whether noninferiority was demonstrated. Levels of SARS-CoV-2 Spike S1-specific IgG antibodies level (continuous) were compared between groups. In a sensitivity analysis, we adjusted for possible differences in age and sex between the patient and control cohorts in multivariable regression analyses. To identify independent risk factors for being a nonresponder, the associations of patient characteristics with nonresponder status were examined using logistic regression analysis. Odds ratios, corresponding 95% CIs, and P were reported. Variables with a P of <0.1 in univariable analysis were considered as candidate predictors. These candidate predictors were introduced in a multivariable logistic regression model. Using a backward elimination procedure, the least significant variables were removed in a stepwise manner, until none met the criterion of P > 0.1. Missing data were handled on list-wise deletion bases. The proportions of (serious) AEs between study groups were compared using chi-square tests.
All analyses were performed with the statistical software Stata version 17.0 (Stata Corp., Texas). A 2-sided P < 0.05 was adopted to denote statistical significance, and corrected in case of multiple testing using Bonferroni correction unless stated otherwise.
RESULTS
Baseline Characteristics
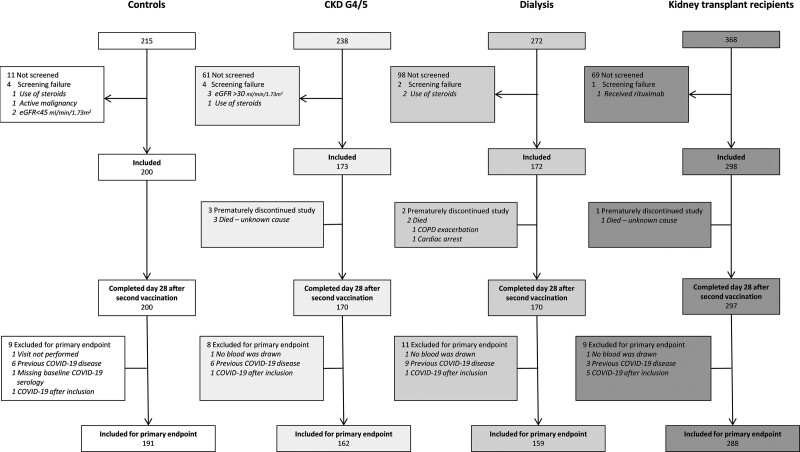
Study enrollment is depicted in Figure 1. In total 843 subjects participated in the study, of whom 800 were included for analysis of the primary endpoint. Per protocol 43 patients were excluded, 24 because they were seropositive for SARS-CoV-2 Spike S1-specific IgG antibodies at baseline, 8 who developed COVID-19 between the first vaccination and 28 d after the second vaccination, 6 who ended the study prematurely, and 5 because of missing serum samples. This left for analysis of the primary endpoint 191 controls, 162 patients with CKD G4/5, 159 dialysis patients, and 288 kidney transplant recipients. Baseline characteristics of these participants are shown in Table 1. In the CKD G4/5 cohort mean eGFR was 17.7 ± 6.1 mL/min/1.73m2, dialysis modality was hemodialysis in 73% and peritoneal dialysis in 27%, and in the kidney transplant recipients, median time after transplantation was 6.9 (2.6–3.3) y.
FIGURE 1.
Subject enrollment and outcomes. CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate.
TABLE 1.
Baseline characteristics per study cohort
| Control (n = 191) | CKD G4/5 (n = 162) | Dialysis (n = 159) | KTR (n = 288) | |
|---|---|---|---|---|
| Female, n (%) | 115 (60.2) | 60 (37.0) | 53 (33.3) | 128 (44.4) |
| Caucasian, n (%) | 176 (94.1) | 140 (86.4) | 134 (85.4) | 263 (91.6) |
| Age, y | 58.5 ± 13.0 | 60.6 ± 13.4 | 59.8 ± 14.3 | 56.1 ± 14.0 |
| BMI, kg/m2 | 27.6 ± 5.3 | 27.9 ± 5.0 | 26.8 ± 5.6 | 26.9 ± 4.6 |
| SBP, mm Hg | 146.7 ± 22.7 | 150.6 ± 23.9 | 139.1 ± 25.2 | 146.6 ± 21.1 |
| DBP, mm Hg | 84.6 ± 11.7 | 83.9 ± 11.7 | 78.0 ± 16.4 | 84.7 ± 10.9 |
| Current smoking, n (%) | 32 (16.9) | 25 (15.4) | 39 (24.5) | 29 (10.1) |
| Current alcohol consumption, n (%) | 111 (59.0) | 63 (39.1) | 34 (21.5) | 117 (40.9) |
| Number of comorbidities | 0 (0–1) | 1 (1–2) | 1 (1–2) | 1 (1–2) |
| Comorbidities, n (%) | ||||
| Hypertension | 52 (27.2) | 132 (81.5) | 106 (67.1) | 233 (80.9) |
| Diabetes mellitus | 18 (9.4) | 45 (27.8) | 39 (24.5) | 61 (21.2) |
| History of coronary artery disease | 9 (4.7) | 34 (21.0) | 36 (22.8) | 38 (13.2) |
| Heart failure | 3 (1.6) | 12 (7.4) | 11 (7.0) | 13 (4.5) |
| Chronic lung disease | 16 (8.4) | 16 (9.9) | 17 (10.8) | 15 (5.2) |
| History of malignancya | 11 (5.8) | 20 (12.3) | 38 (24.1) | 44 (15.3) |
| Autoimmune disease | 4 (2.1) | 3 (1.9) | 5 (3.2) | 15 (5.2) |
| Lymphocytes, 109/L | 2.0 (1.6–2.5) | 1.6 (1.2–2.0) | 1.2 (0.9–1.6) | 1.3 (0.9–1.9) |
| eGFR, mL/min/1.73m2 | 82.1 ± 18.9 | 17.7 ± 6.1 | – | 49.3 ± 18.8 |
| Primary renal diagnosis, n (%) | ||||
| Primary glomerulonephritis | – | 18 (12.6) | 17 (12.5) | 57 (22.0) |
| Pyelonephritis | – | 2 (1.4) | 1 (0.7) | 5 (1.9) |
| Interstitial nephritis | – | 7 (4.9) | 5 (3.7) | 9 (3.5) |
| Familial/hereditary renal diseases | – | 26 (18.2) | 19 (14.0) | 57 (22.0) |
| Congenital diseases | – | 6 (4.2) | 5 (3.7) | 19 (7.3) |
| Vascular diseases | – | 34 (23.8) | 30 (22.1) | 29 (11.2) |
| Secondary glomerular/systemic disease | – | 4 (2.8) | 8 (5.9) | 14 (5.4) |
| Diabetic kidney disease | – | 12 (8.4) | 22 (16.2) | 10 (3.9) |
| Other | – | 29 (20.3) | 25 (18.4) | 41 (15.8) |
| Unknown | – | 19 (11.7) | 22 (13.9) | 29 (10.1) |
| Dialysis characteristics, n (%) | ||||
| Hemodialysis | – | – | 116 (73.0) | – |
| Peritoneal dialysis | – | – | 43 (27.0) | – |
| Time on dialysis, mo | – | – | 30.8 (12.9–75.0) | – |
| Transplant characteristics | ||||
| First kidney transplant, n (%) | – | – | – | 227 (78.8) |
| Time after last transplantation, y | – | – | – | 6.9 (2.6–13.3) |
| Last transplant | ||||
| Living, n (%) | – | – | – | 200 (69.4) |
| Preemptive, n (%) | – | – | – | 107 (37.1) |
| Number of immunosuppressive agents | – | – | – | 3 (2–3) |
| Immunosuppressive treatment, n (%) | ||||
| Steroids | – | – | – | 219 (76.0) |
| Azathioprine | – | – | – | 34 (11.8) |
| Mycophenolate mofetil | – | – | – | 197 (68.4) |
| Calcineurin inhibitor | – | – | – | 236 (81.9) |
| mTOR inhibitor | – | – | – | 17 (5.9) |
| Other | – | – | – | 5 (1.7) |
| Induction with rituximab last year | 2 (0.7) |
Variables are presented as mean ± SD, or as median (interquartile interval) in case of nonnormal distribution.
aIncluding melanomas, excluding all other skin malignancies.
BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; KTR, kidney transplant recipient; mTOR, mammalian target of rapamycin; SBP, systolic blood pressure.
SARS-CoV-2 Spike S1-specific IgG Antibody Response
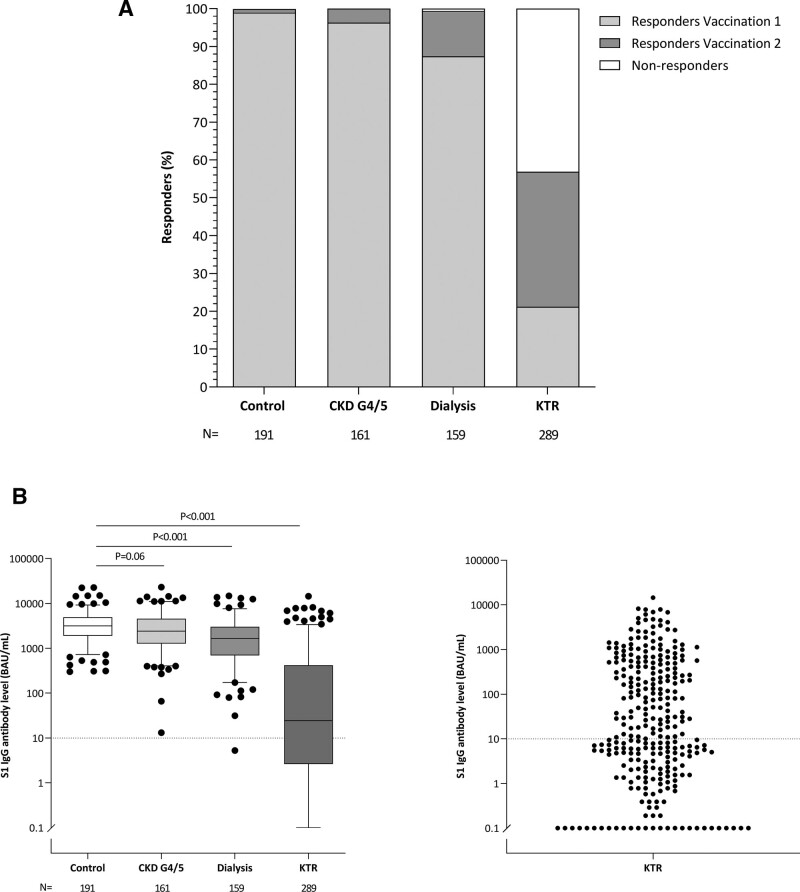
After the first vaccination, the seroconversion rate was 99.0% in controls, whereas in the CKD G4/5 cohort, it was 96.3%. In the dialysis cohort (87.4%) as well as in kidney transplant recipients (21.2%), it was significantly lower than in controls (both P < 0.001) (Table 2; Figure 2A; Figure S1, SDC, http://links.lww.com/TP/C306).
TABLE 2.
SARS-CoV-2 spike S1-specific IgG antibody level and percentage responders 28 d after first vaccination (upper panel) and 28 d after second vaccination (lower panel)
| Control (N = 191) | CKD G4/5 (N = 162) | P a | Dialysis (N = 159) | P a | KTR (N = 288) | P a | |
|---|---|---|---|---|---|---|---|
| Vaccination 1 | |||||||
| Responder,b n (%) | 189 (99.0) | 156 (96.3) | 0.28 | 139 (87.4) | <0.001 | 61 (21.2) | <0.001 |
| Difference in response (%) | Ref. | 2.7% (−0.6 to 5.9) | 11.5% (6.2–16.9) | 77.8% (72.8–82.7) | |||
| S1 IgG antibody level (BAU/mL) | 435 (220–813) | 236 (104–430) | <0.001 | 105 (22–285) | <0.001 | 0.87 (0.29–6.82) | <0.001 |
| Vaccination 2 | |||||||
| Responder,b n (%) | 191 (100.0) | 162 (100.0) | – | 158 (99.4) | 0.81 | 164 (56.9) | <0.001 |
| Difference in response (%) | Ref. | – | 0.6% (−0.6 to 1.9) | 43.1% (37.3–48.8) | |||
| S1 IgG antibody level (BAU/mL) | 3186 (1896–4911) | 2405 (1287–4524) | 0.06 | 1650 (698–3024) | <0.001 | 25 (3–416) | <0.001 |
Variables are presented as mean ± SD, or as median (interquartile interval) in case of nonnormal distribution. P values are calculated using independent sample t test in case of normal distribution, Mann-Whitney U in case of nonnormal distribution and chi-squared in case of proportion.
aP values are given for the comparison between patient groups and controls and corrected for multiple testing by Bonferroni.
bSubjects were defined as responder with a level of SARS-CoV-2 Spike S1-specific IgG antibodies of ≥10 BAU/mL.
BAU, binding antibody unit; CKD, chronic kidney disease; IgG, immunoglobulin G; KTR, kidney transplant recipient; Ref., reference group; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
FIGURE 2.
Proportion of responders (A) and SARS-CoV-2 Spike S1-specific IgG antibody levels (B) per study cohort at 28 d after second vaccination. A, Responders were defined as subjects with a S1-specific IgG antibody level ≥10 BAU/mL after first vaccination or second vaccination. B (left panel), Depicted are box and whisker plots together with outliers, with the box representing median and interquartile range, whiskers representing the 95% CI; (right panel) dot plot of S1-specific IgG antibody level of kidney transplant recipients only. Dotted horizontal line indicates threshold for definition of responder at ≥10 BAU/; P were calculated using Mann-Whitney U test and corrected for multiple testing by Bonferroni. BAU, binding antibody unit; CKD, chronic kidney disease; IgG, immunoglobulin G; KTR, kidney transplant recipient; N, number; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The seroconversion rate at day 28 after the second vaccination (the primary outcome of the study) was 56.9% in the kidney transplant recipients, which was significantly lower than the 100% seroconversion rate in the control cohort (P < 0.001). Moreover, the lower limit of the 95% CI for the difference in response compared with controls (37.3%–48.8%) remained outside the predefined noninferiority limit (Table 2; Figure 2A). In patients with CKD G4/5 and dialysis patients, the seroconversion rates were 100% and 99.4%, respectively, which the latter not statistically different from that in controls (P = 0.81) and did not exceed the predefined noninferiority margins (Table 2). The seroconversion rate was as high in hemodialysis as in peritoneal dialysis patients, with 99.1% and 100%, respectively.
The median concentration of SARS-CoV-2 Spike S1-specific IgG antibodies in patients with CKD G4-5, dialysis patients, kidney transplant recipients, and controls were 2405 (1287–4524), 1650 (698–3024), 25 (3–416), and 3186 (1896–4911) BAU/mL, respectively, with concentrations in dialysis patients and kidney transplant recipients being significantly lower than in controls (both P < 0.001, Figure 2B). When adjusted for age and sex, P for the difference in median SARS-CoV-2 Spike S1-specific IgG antibodies level was 0.11 for CKD G4-5, and <0.001 for dialysis and transplant patients compared with controls. Median antibody concentrations were 1758 (702–3179) in hemodialysis patients and 1425 (667–2385) in peritoneal dialysis patients (P = 0.40).
When plotting the concentrations of serum anti-S1 IgG after the first and second vaccination significant correlations were found for the overall group of subjects (R = 0.86, P < 0.001), as well as for each cohort separately (Figure S2, SDC, http://links.lww.com/TP/C306). Nearly all patients were situated above the line of identity, indicating that in all these subjects, the seroconversion rate increased after the second vaccination. The only exception to this rule were kidney transplant recipients, with several subjects showing no seroresponse after the first and second vaccination.
Virus Neutralizing Antibodies
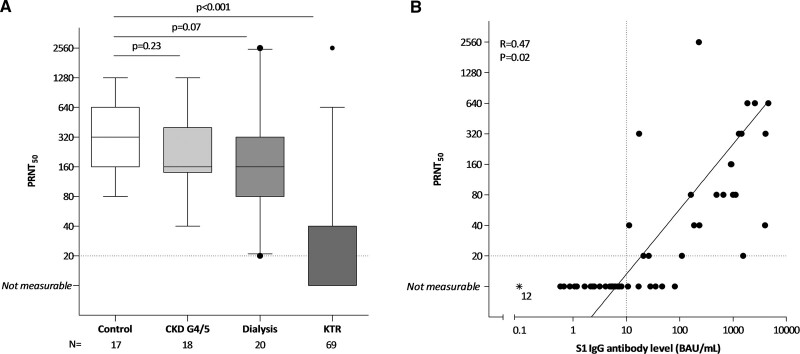
In kidney transplant recipients with a seroconversion at day 28 after the second vaccination (S1 IgG ≥10 BAU/mL), neutralizing antibody titers were significantly lower as compared to controls (P < 0.001, Figure 3A). In these responding kidney transplant recipients (N = 29), 23 (79.3%) had a measurable level of neutralizing antibodies (PRNT50 ≥20), with a significant correlation between S1-specific IgG antibody level and the titer of virus neutralizing antibodies (R = 0.47, P = 0.02, Figure 3B). Compared with controls the titers of neutralizing antibodies were slightly reduced in dialysis patients, and not different in patients with CKD G4/5 (Figure 3A).
FIGURE 3.
SARS-CoV-2 neutralizing antibody titer (PRNT50). A, Data of a random subset of subjects included in 1 of the participating centers. Data are shown as box and whisker plots together with outliers, with the box representing median and interquartile range, whiskers representing the 95% confidence interval. P were calculated using Mann-Whitney U. B, S1 specific IgG antibody levels for all kidney transplant recipients included at that center (N = 69) and correlation with PRNT50. Dotted vertical line indicates threshold for seroresponse, and horizontal line indicates the threshold for neutralizing capacity. Asterisks represent samples assumed not measurable. The solid line represents the regression line calculated for only subjects with seroconversion (S1-specific IgG response ≥10 BAU/mL) and a measurable T-cell response (PRNT50 ≥20), with corresponding R and P calculated using Spearman correlation. CKD, chronic kidney disease; IgG, immunoglobulin G; KTR, kidney transplant recipient; N, number; PRNT50, 50% plaque reduction neutralization test; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SARS-CoV-2-specific T-cell Response
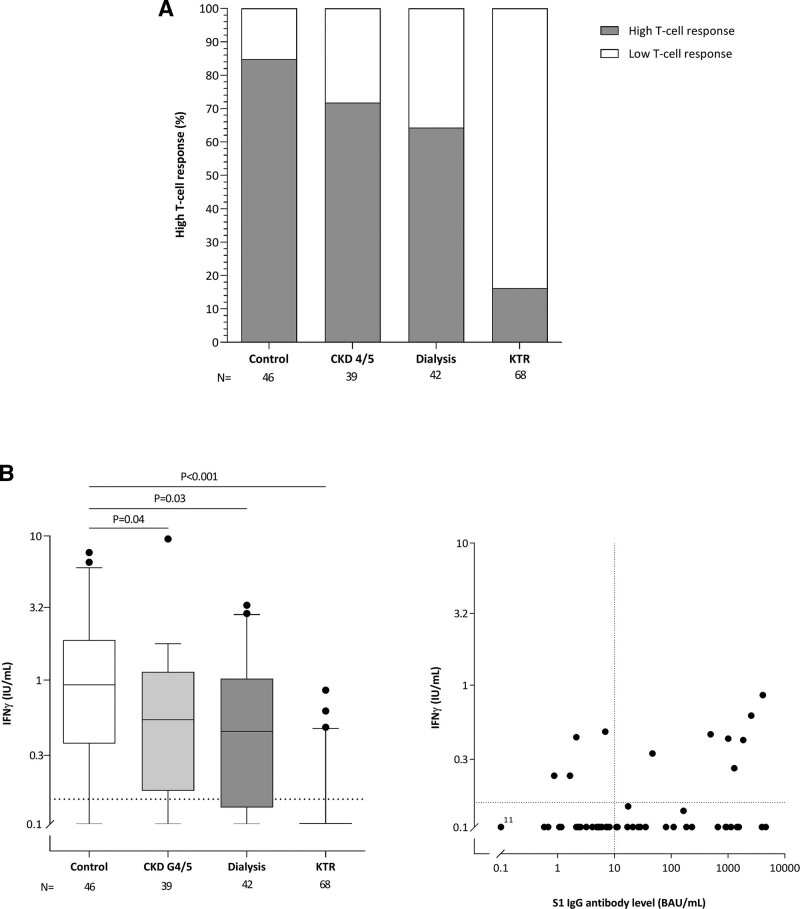
In the patients in whom the SARS-CoV-2-specific T-cell response was measured, a high response (defined as an IFN-γ production of ≥0.149 IU/mL) was found in 71.8% of CKD G4/5 patients, 64.3% of dialysis patients, and 16.2% of kidney transplant recipients compared with 84.8% in controls (P = 0.14, P = 0.03, and P < 0.001, respectively; Figure 4A). Median IFN- γ production was significantly lower in CKD G4/5 patients, dialysis patients and kidney transplant recipients when compared with controls (P = 0.04, P = 0.03, and P < 0.001, respectively; Figure 4B), indicating impaired induction of T-cell responses after vaccination in all 3 patients groups. Seven out of 29 (24.1%) kidney transplant recipients showing a response to vaccination (S1-specific IgG ≥10 BAU/mL) had a T-cell response (IFN-γ ≥0.149 IU/mL), whereas this was 4 out of 39 (10.3%) in nonresponding kidney transplant recipients (P = 0.12).
FIGURE 4.
SARS-CoV-2-specific T-cell response in all subjects in 1 of the participating centers. A, Percentage of high T-cell response per group after vaccination (defined as antigen 1 and antigen 2 ≥0.149 IU/mL, ie, 3 times the background). B, Left panel shows individual IFNγ levels per group. Depicted are box and whisker plots together with outliers, with the box representing median and interquartile range, whiskers representing the 95% confidence interval. P were calculated using Mann-Whitney U. B, Right panel shows S1 specific IgG antibody levels vs T-cell response kidney transplant recipients (n = 68). Dotted vertical line indicates threshold for seroresponse and dotted horizontal line the threshold for cellular response. BAU, binding antibody unit; CKD, chronic kidney disease; IFNγ, interferon-gamma; IgG, immunoglobulin G; KTR, kidney transplant recipient; N, number; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Predictors of an Antibody Response in Kidney Transplant Recipients
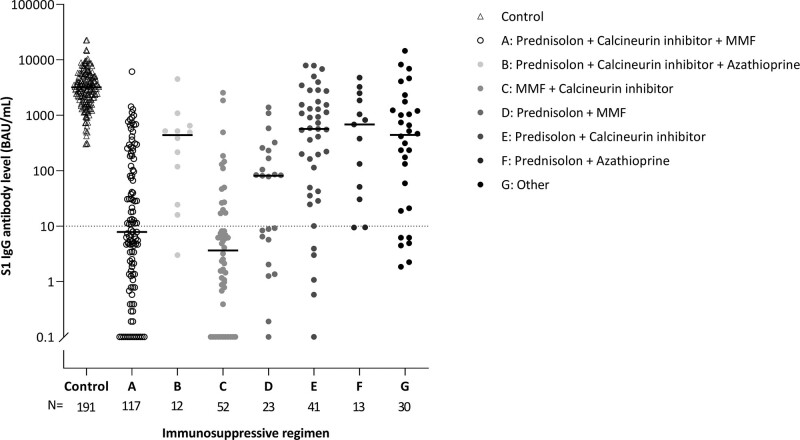
Because there was a large proportion of nonresponders in the cohort of kidney transplant recipients, we tried to identify baseline predictors of the seroresponse in these patients. Several subject characteristics differed significantly between responders and nonresponders (Table 3). These variables were included in a multivariate stepwise backward logistic regression analysis that showed that higher age, lower lymphocyte count, lower eGFR, not using steroids, shorter time after transplantation, and use of mycophenolate mofetil or mycophenolic acid (MMF/MPA) were significantly associated with the risk of being a nonresponder (Table 4). Missingness for all univariable and multivariable analyses was <10%. The impact of the type of immunosuppressive drug agents on the chance of responding to vaccination is also illustrated in Figure 5, that shows that especially treatment regimens including MMF/MPA are associated with a low seroconversion rate after vaccination. The number of kidney transplant recipients showing a T-cell response was too low to allow investigating predictors in a multivariate regression analysis.
TABLE 3.
Differences in subject characteristics between responders vs nonresponders in kidney transplant recipients (N = 288)
| Responder a (N = 164) | Nonresponder (N = 124) | P | |
|---|---|---|---|
| Female, n (%) | 68 (41.5) | 60 (48.4) | 0.24 |
| Caucasian, n (%) | 149 (91.4) | 114 (91.9) | 0.87 |
| Age, y | 53.5 ± 14.0 | 59.5 ± 13.2 | <0.001 |
| BMI, kg/m2 | 27.0 (4.6) | 26.8 ± 4.7 | 0.83 |
| SBP, mm Hg | 145.5 ± 22.1 | 148.0 ± 19.6 | 0.32 |
| DBP, mm Hg | 85.8 ± 11.0 | 83.2 ± 10.6 | 0.05 |
| Current smoking, n (%) | 20 (12.3) | 9 (7.3) | 0.16 |
| Current alcohol consumption, n (%) | 71 (43.8) | 46 (37.1) | 0.25 |
| Number of comorbidities | 1 (1–2) | 1 (1–2) | 0.07 |
| Comorbidities, n (%) | |||
| Hypertension | 128 (78.0) | 105 (84.7) | 0.16 |
| Diabetes mellitus | 30 (18.3) | 31 (25.0) | 0.17 |
| History of coronary artery disease | 18 (11.0) | 20 (16.1) | 0.20 |
| Heart failure | 5 (3.0) | 8 (6.5) | 0.17 |
| Chronic lung disease | 7 (4.3) | 8 (6.5) | 0.41 |
| History of malignancyb | 25 (15.2) | 19 (15.3) | 0.98 |
| Autoimmune disease | 10 (6.1) | 5 (4.0) | 0.43 |
| Lymphocytes, 109/L | 1.3 (1.0–1.9) | 1.1 (0.8–1.7) | 0.006 |
| eGFR, mL/min/1.73m2 | 52.3 ± 19.9 | 45.4 ± 16.5 | 0.002 |
| Primary diagnosis, n (%) | |||
| Primary glomerulonephritis | 36 (24.5) | 21 (18.8) | 0.27 |
| Pyelonephritis | 3 (2.0) | 2 (1.8) | 0.88 |
| Interstitial nephritis | 6 (4.1) | 3 (2.7) | 0.54 |
| Familial/hereditary renal diseases | 34 (23.1) | 23 (20.5) | 0.62 |
| Congenital diseases | 11 (7.5) | 8 (7.1) | 0.92 |
| Vascular diseases | 14 (9.5) | 15 (13.4) | 0.33 |
| Secondary glomerular/systemic disease | 8 (5.4) | 6 (5.4) | 0.98 |
| Diabetic kidney disease | 4 (2.7) | 6 (5.4) | 0.27 |
| Other | 22 (15.0) | 19 (17.0) | 0.66 |
| Unknown | 17 (10.4) | 12 (9.7) | 0.85 |
| Transplant characteristics | |||
| First kidney transplant, n (%) | 133 (81.1) | 94 (75.8) | 0.28 |
| Time after last transplantation, y | 8.9 (4.2–15.7) | 5.0 (1.4–10.0) | <0.001 |
| Last transplant | |||
| Living, n (%) | 117 (71.3) | 83 (66.9) | 0.42 |
| Preemptive, n (%) | 56 (34.1) | 51 (41.1) | 0.22 |
| Number of immunosuppressive agents | 2 (2–3) | 3 (2–3) | 0.001 |
| Immunosuppressive treatment, n (%) | |||
| Steroids | 137 (83.5) | 82 (66.1) | 0.001 |
| Azathioprine | 31 (18.9) | 3 (2.4) | <0.001 |
| Mycophenolate mofetil | 85 (51.8) | 112 (90.3) | <0.001 |
| Calcineurin inhibitor | 127 (77.4) | 109 (87.9) | 0.02 |
| mTOR inhibitor | 14 (8.5) | 3 (2.4) | 0.03 |
| Induction with rituximab last year, n (%) | 1 (0.6) | 1 (0.8) | 0.84 |
Variables are presented as mean ± SD, or as median (interquartile range) in case of nonnormal distribution. P values are calculated using independent sample t test in case of normal distribution, Mann-Whitney U in case of nonnormal distribution and chi-square in case of proportion.
aSubjects were defined as responder with a level of SARS-CoV-2 spike S1-specific IgG antibodies of ≥10 BAU/mL, 28 d after the second vaccination.
bIncluding melanomas, excluding all other skin malignancies.
BAU, binding antibody unit; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; KTR, kidney transplant recipient; mTOR, mammalian target of rapamycin; SBP, systolic blood pressure; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
TABLE 4.
Associations of patient characteristics with being a responder vs nonresponder in kidney transplant recipients (N = 288)
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | |
| Age, y | 0.97 (0.95-0.98) | <0.001 | 0.97 (0.95-0.99) | 0.003 |
| Diastolic blood pressure, mm Hg | 1.02 (1.00-1.05) | 0.04 | ||
| Number of comorbidities (n) | 0.76 (0.60-0.98) | 0.03 | ||
| Log lymphocytes | 1.60 (1.04-2.47) | 0.03 | 2.21 (1.29-3.78) | 0.004 |
| eGFR, mL/min/1.7m2 | 1.02 (1.01-1.03) | 0.002 | 1.03 (1.01-1.05) | 0.004 |
| Time after last transplantation, y | 1.09 (1.05-1.13) | <0.001 | 1.05 (1.01-1.10) | 0.020 |
| Number of immunosuppressants (n) | 0.58 (0.41-0.82) | 0.002 | ||
| Steroids, yes vs no | 2.55 (1.46-4.44) | 0.001 | 2.30 (1.16-4.53) | 0.020 |
| Azathioprine, yes vs no | 9.55 (2.85-32.04) | <0.001 | ||
| Mycophenolate mofetil, yes vs no | 0.13 (0.07-0.24) | <0.001 | 0.10 (0.05-0.22) | <0.001 |
| Calcineurin inhibitor, yes vs no | 0.46 (0.24-0.89) | 0.02 | ||
| mTOR, yes vs no | 3.82 (1.07-13.60) | 0.04 | ||
(a)OR’s (adjusted odds ratio) and P values were calculated using logistic regression analysis. Dependent variable is responder vs nonresponder (S1 IgG antibody level 28 d after second vaccination ≥10 BAU/mL vs <10 BAU/mL) independent variables are variables from Table 3 with a P < 0.10.
Univariable: Showing only variables from Table 3 with a P < 0.10.
Multivariable: All variables from univariable analysis that remain significantly associated in a stepwise backward analysis.
aOR, adjusted odds ratio; BAU, binding antibody unit; CI, confidence interval; eGFR, estimated glomerular filtration rate; mTOR, mammalian target of rapamycin; OR, odds ratio.
FIGURE 5.
Impact of the various immunosuppressive regimens on S1-specific IgG antibody level 28 d after the second vaccination. Regimens with <10 subjects were categorized as “Other.” BAU, binding antibody unit; IgG, immunoglobulin G; MMF, mycophenolate mofetil; N, number.
Adverse Events
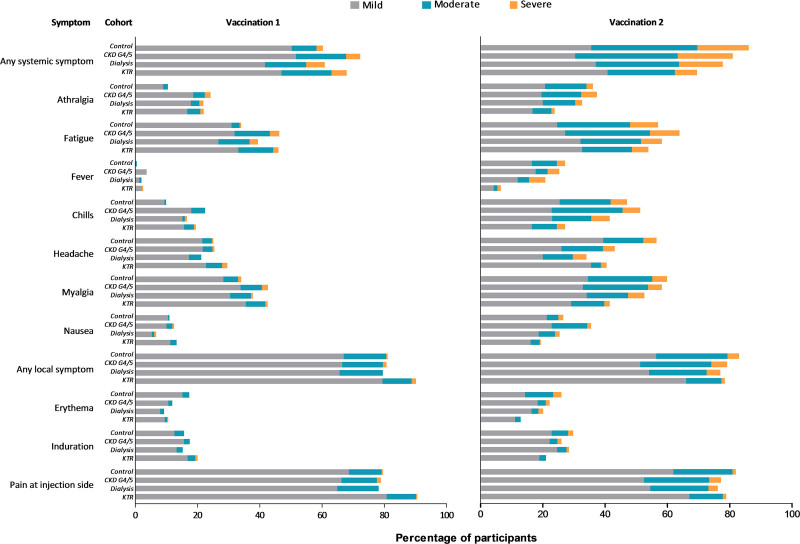
The percentage of participants who reported any solicited AE after first or second vaccination was significantly lower in the dialysis cohort than in controls (88.0% versus 96.9%, P = 0.001). For both the dialysis and kidney transplant cohort, the frequency of reported systemic AEs was lower than in controls (76.1% and 80.2% versus 92.1%, both P < 0.001) (Table 5). In general, more AEs, especially systemic symptoms, were observed after the second vaccination. At that time point, all patient cohorts reported significantly less frequently headache as systemic symptom and the kidney transplant recipients also reported less arthralgia, fatigue, fever, chills and myalgia, as systemic symptoms, and erythema and induration as local symptom when compared with controls (Figure 6; Tables S3 and S4, SDC, http://links.lww.com/TP/C306).
TABLE 5.
Listing of incidence solicited adverse events (for first and second vaccination combined) and serious adverse at least possibly related and not related to vaccination until 28 d after second vaccination
| Control (N = 191) | CKD G4/5 (N = 162) | P a | Dialysis (N = 159) | P a | KTR (N = 288) | P a | |
|---|---|---|---|---|---|---|---|
| Adverse events | |||||||
| Any adverse event, n (%) | 185 (96.9) | 156 (96.3) | 0.77 | 140 (88.0) | 0.001 | 280 (97.2) | 0.82 |
| Any systemic symptom, n (%) | 176 (92.1) | 146 (90.1) | 0.50 | 121 (76.1) | <0.001 | 231 (80.2) | <0.001 |
| Arthralgia, n (%) | 76 (39.8) | 72 (44.4) | 0.38 | 59 (37.1) | 0.61 | 99 (34.4) | 0.23 |
| Fatigue, n (%) | 136 (71.2) | 113 (69.7) | 0.77 | 93 (58.5) | 0.01 | 184 (63.9) | 0.10 |
| Fever, n (%) | 52 (27.2) | 44 (27.2) | 0.99 | 30 (18.9) | 0.07 | 24 (8.3) | <0.001 |
| Chills, n (%) | 97 (50.8) | 95 (58.6) | 0.14 | 65 (40.9) | 0.06 | 102 (35.4) | <0.001 |
| Headache, n (%) | 121 (63.3) | 80 (49.4) | 0.008 | 61 (38.4) | <0.001 | 147 (51.0) | 0.008 |
| Myalgia, n (%) | 130 (68.1) | 108 (66.7) | 0.78 | 89 (56.0) | 0.02 | 157 (54.5) | 0.003 |
| Nausea, n (%) | 60 (31.4) | 67 (41.4) | 0.05 | 39 (24.5) | 0.15 | 80 (27.8) | 0.39 |
| Any local symptom, n (%) | 172 (90.0) | 148 (91.4) | 0.67 | 132 (83.0) | 0.05 | 269 (93.4) | 0.18 |
| Erythema, n (%) | 67 (35.1) | 41 (25.3) | 0.05 | 36 (22.6) | 0.01 | 53 (18.4) | <0.001 |
| Induration, n (%) | 70 (36.6) | 54 (33.3) | 0.51 | 47 (29.6) | 0.16 | 85 (29.5) | 0.10 |
| Pain at injection side, n (%) | 170 (89.0) | 142 (87.6) | 0.69 | 130 (81.8) | 0.05 | 269 (93.4) | 0.09 |
| Serious adverse events | |||||||
| Any serious adverse event, n (%) | 0 | 6 (3.7) | 0.007 | 9 (5.7) | 0.001 | 12 (4.2) | 0.004 |
| Related to vaccination, n (%) | |||||||
| Exacerbation lichen sclerosis | – | 1 (0.6) | – | – | – | – | – |
| Cellulitis | – | – | – | – | – | 1 (0.3) | – |
| Malaise | – | 1 (0.6) | – | – | – | 1 (0.3) | – |
| Not related to vaccination, n (%) | |||||||
| Abdominal pain | – | – | – | 1 (0.6) | – | – | – |
| Cellulitis | – | 1 (0.6) | – | – | – | – | – |
| Chest pain | – | – | – | – | – | 1 (0.3) | – |
| Ischemic CVA | – | – | – | 1 (0.6) | – | 1 (0.3) | – |
| Cardiac decompensation | – | – | – | 1 (0.6) | – | 1 (0.3) | – |
| Fever | – | – | – | 1 (0.6) | – | 1 (0.3) | – |
| Bone fracture | – | 1 (0.6) | – | – | – | – | – |
| Hypomagnesemia | – | 1 (0.6) | – | – | – | – | – |
| Infection | – | – | – | 1 (0.6) | – | – | – |
| Kidney transplant rejection | – | – | – | 1 (0.6)b | – | – | – |
| Peritonitis | – | – | – | 2 (1.3) | – | – | – |
| Pneumonia | – | – | – | – | – | 1 (0.3) | – |
| Trauma | – | – | – | 1 (0.6) | – | – | – |
| Urinary tract infection | – | 1 (0.6) | – | – | – | 3 (1.2) | – |
| Enteritis | – | – | – | – | – | 1 (0.3) | – |
| Reanimation | – | – | – | – | – | 1 (0.3) | – |
Variables are given as number and percentage. P Value were calculated using chi-squared test.
aP values are given for the comparison between patient groups and controls.
bSubject received a kidney transplant after baseline visit.
CKD, chronic kidney disease; CVA, cerebrovascular accident; KTR, kidney transplant recipient.
FIGURE 6.
Solicited adverse events after first and second vaccination per study cohort. CKD, chronic kidney disease; KTR, kidney transplant recipient.
Serious Adverse Events
There were more serious AEs in the CKD G4/5, dialysis, and kidney transplant recipient cohorts than in controls (3.7%, 5.7%, and 4.2% versus 0%, P = 0.007, P = 0.001, and P = 0.004, respectively). Most were not related to vaccination. This concerned 16 serious AEs, that occurred in 23 subjects (Table 5). Three types of serious AEs, occurring in 4 subjects were deemed possibly related to vaccination (ie, malaise (twice), cellulitis, and exacerbation of lichen sclerosis). Overall 2 patients died after the first vaccination (1 patient with CKD G4/5 and 1 KTR patient) and 4 after the second vaccination (2 patients with CKD G4/5 and 2 patients on dialysis) (Figure 1). In none of these patients, a relationship between vaccination and dead could be established.
DISCUSSION
In this study, we show that the seroresponse after vaccination with the mRNA-1273 COVID-19 vaccine (Moderna Biotech Spain, S.L.) in CKD G4/5 and dialysis patients is not inferior to that of controls at 28 d after the second vaccination, whereas the percentage of seroresponders in kidney transplant recipients is significantly lower.
The strongly reduced response to vaccination in our cohort of kidney transplant recipients confirms findings from mostly smaller, single center studies in solid organ transplant recipients that showed seroconversion rates after 2 vaccinations varying from 22% to 48%.7,9,17–20 The seroconversion rate of 57% in the current study appears relatively high as compared to previous data, which might be explained by the use of the mRNA-1273 vaccine (Moderna), whereas nearly all other studies in transplant patients were performed with the mRNA vaccine BNT162b2 (Pfizer-BioNTech). Notably, in the 2 previous studies with the highest rate of responders, the same mRNA-1273 vaccine was used in all patients in 1 study,20 and in 47% of patients in the other study.9 In the latter study, the seroconversion rate to vaccination was higher with the mRNA-1273 vaccine (Moderna) as compared to the mRNA vaccine BNT162b2 (Pfizer-BioNTech).9 Comparisons between mRNA vaccines and other types of vaccines are not available in this specific population.
Even if vaccination was followed by a seroconversion, the anti-S1 antibody concentration was significantly lower in kidney transplant recipients than in control subjects. This was accompanied by a lower titer of virus neutralizing antibodies suggesting a lower level of protection against COVID-19.21 Furthermore, kidney transplant recipients also showed a reduced T-cell response after vaccination, as measured by IFN-γ release after stimulation of whole blood samples with SARS-CoV-2-specific peptides in a commercial IGRA assay. How this relates to the response of different subsets of T cells and the induction of T-cell memory will be the subject of in-depth analysis of peripheral blood mononuclear cells collected in the present study. Limited data from literature indicate that kidney transplant recipients have significantly reduced T helper cell responses, an impaired memory T-cell differentiation, and nearly absent CD8+ T-cell responses after vaccination.22
The variation in immunosuppressive treatment regimes in our cohort of kidney transplant recipients allowed to identify the effect of different immunosuppressants on the response to vaccination. A multivariable regression analysis showed that especially the use of MMF/MPA, next to higher age, lower lymphocyte count, lower eGFR, not using steroids and shorter time after transplantation, was significantly associated with the risk of being a nonresponder. The negative impact of the use of MMF/MPA on COVID-19 vaccination was also observed by others in kidney transplant recipients and patients with autoimmune diseases.9,23,24 Moreover, the use of MMF as well as lower graft function have been shown to reduce the seroresponse to influenza vaccination in kidney transplant recipients.25 Notably, we found no strong effect of the use of the antiproliferative agent azathioprine on antibody formation.
Nearly all hemodialysis and peritoneal dialysis patients showed a seroconversion upon vaccination with the mRNA-1273 COVID-19 vaccine, and the seroconversion rate in these patient groups was not inferior to that in controls. While antibody concentrations and T-cell responses were significantly reduced in these patients, this was not as severe as in kidney transplant recipients. Again, the seroconversion rate of 99.4% obtained with the mRNA-1273 vaccine (Moderna) was relatively high as compared to reported seroconversion rates of 80%–96% in other cohorts of dialysis patients in which the mRNA vaccine BNT162b2 (Pfizer-BioNTech) was administered.7,26–30
Patients with CKD G4/5 had only a marginally reduced antibody and T-cell response, despite their average kidney function being much lower than that of kidney transplant recipients. This indicates that the use of immunosuppressive drugs is a stronger determinant of the response to vaccination than the uremic state. To our knowledge, no study has previously reported the preserved response to COVID-19 vaccination in this important cohort of patients that represents approximately 0.5% of the general population in high-income countries and that has a very high COVID-19 related mortality risk.1,31
The rate and type of AEs in control subjects of this study were similar to those described in phase 3 studies with COVID-19 mRNA vaccines.3,4,32 The mRNA-1273 vaccine showed a good tolerability with no unexpected AEs or serious safety issues in the 3 cohorts of kidney patients, although data from large scale registry studies are necessary to draw definitive conclusions about the safety of COVID-19 vaccination in these specific subgroups of patients. Interestingly, we observed a significantly lower incidence of several solicited AEs in kidney transplant recipients as compared to controls, especially after the second vaccination and especially systemic AEs, which might be related to the reduced immunogenicity of the vaccine in this cohort.
What do these findings mean for our patients with kidney failure and kidney function replacement therapy? While the immunologic correlate of protection against COVID-19 is not completely clear yet, it has been demonstrated that higher anti-S1 IgG antibody concentrations, and especially higher concentrations of virus neutralizing antibodies, are associated with al lower risk of disease.21,33,34 It is therefore likely that kidney transplant recipients, and to a lesser degree also dialysis patients, remain at risk for COVID-19 and a more severe course of the disease after a complete vaccination schedule. This implies that these vulnerable patients have to adhere to strict safety measures as long as SARS-CoV-2 circulates in the population. Furthermore, based on the better response to vaccination in CKD G4/5 and dialysis patients as compared to kidney transplant recipients, it should be recommended that kidney transplant candidates are vaccinated before transplantation whenever possible.
Finally, our data suggest that it should be considered to administer a third dose to patients with an insufficient immune response after 2 vaccine administrations. Recently, studies have reported on a third dose in organ transplant recipients after mRNA vaccination. Werbel et al reported that of 24 patients with negative antibody titers after initial vaccination with 2 doses of BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) only 6 had high-positive titers after a third vaccination, whereas of the 6 patients with low-positive antibody titers after initial vaccination all had high-positive antibody titers after the third vaccination.35 Kamar et al reported that from the 59 patients who were seronegative after initial vaccination with 2 doses of BNT162b2 (Pfizer-BioNTech), 26 became seropositive after a third dose.36 Hall et al performed a randomized trial of a third dose of mRNA-1273 in 120 transplant recipients, and demonstrated a significant increase in serologic response after the third dose compared with placebo.37 These studies support the use of a third vaccination in kidney transplant recipients, especially in low responders. How to define low responders has not been established yet. The data of Werbel et al and Kamar et al also indicate that in patients who remain nonresponder after 2 mRNA vaccine administrations, a third vaccination is unsuccessful in the majority of cases. For these patients, additional strategies have to be investigated to make additional vaccination strategies effective. Firstly, a higher dose of the vaccines could be appropriate, because using higher doses of other vaccines has led to stronger immunogenicity in kidney transplant recipients.38,39 Second, as the intensity of immunosuppression and especially the use of MMF/MPA was identified as a potential modifiable factor influencing immunogenicity of vaccination, temporarily withdrawing MMF/MPA, when deemed safe and with adequate allograft function monitoring, may represent an interesting option to increase the response to additional vaccination in nonresponders. Finally, it could be considered to use a different type of vaccine for heterologous vaccination, because it has been suggested that after vaccination with viral vector vaccines a better T-cell response can be achieved.40 Before implementing such options in clinical care, however, adequately powered, randomized controlled studies should be performed to identify the most efficacious vaccination strategy and its safety.
The main strength of our study is the prospective design with the inclusion of different cohorts of kidney patients as well as a control cohort. The study was sufficiently powered to show relevant differences in humoral as well as cellular responses that were assessed on predefined fixed time points using standardized assays. The study has also limitations. First, all patients received the same mRNA vaccine, which precludes conclusions about the response to other types of vaccines. In general, the mRNA-1273 (Moderna) and the BNT162b2 (Pfizer-BioNTech) vaccines seem comparable in efficacy, tolerability, and safety, although, as discussed earlier, our data suggest a slightly higher seroreponse in dialysis patients and kidney transplant recipients with the mRNA-1273 vaccine (Moderna). A second limitation is that patients using immunosuppressive therapy were excluded from the CKD G4/5 as well as dialysis cohorts, which could have affected the seroconversion rate in these patients. On the other hand, this enabled specific evaluation of the role of impaired kidney function and kidney function replacement treatment. Third, we excluded patients who previously experienced COVID-19 to have a uniform, immunologically naïve patient group. Since humoral and cellular responses have been observed after COVID-19 in dialysis patients and kidney transplant recipients,41,42 higher seroconversion rates after additional vaccination might be obtained in these cases.43 Finally, we measured the immune response at day 28 after the second vaccination and have currently no information on the durability of the response. Long-term follow-up of our study cohorts, with sample collection at 6 and 12 mo after the second vaccination and with in-depth analysis of B- and T-cell responses at several time points, will yield important additional data.
In conclusion, in patients with CKD G4/5 and dialysis patients, the seroconversion rates after 2 doses of the mRNA-1273 COVID-19 vaccine are almost comparable to that in controls. In contrast, kidney transplant recipients have a markedly lower immune response, especially when they use MMF/MPA, are older, have a lower lymphocyte count or kidney function, do not use steroids, or when the transplantation was more recent. These patients should be informed about this result, and be advised that even after COVID-19 vaccination, they should maintain the general hygienic measures such as social distancing until effective vaccination strategies are developed. Moreover, in this group of transplant recipients alternative vaccination strategies to reach a higher seroconversion rate should be considered.
ACKNOWLEDGMENTS
We would like to thank the RECOVAC collaborators: Alferso C. Abrahams, MD, PhD, Department of Nephrology and Hypertension, University Medical Center Utrecht, Utrecht, the Netherlands; Marije C. Baas, MD, PhD, Department of Nephrology, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands; Pim Bouwmans, MD, Division of Nephrology, Department of Internal Medicine, Maastricht University Medical Center and CARIM School for Cardiovascular Disease, University of Maastricht, Maastricht, the Netherlands; Marc A. G. J. ten Dam, MD, PhD, Dutch Registry RENINE, Nefrovisie, Utrecht, the Netherlands; Lennert Gommers, BSc, Department of Viroscience, Erasmus Medical Center, Rotterdam, the Netherlands; Marc H. Hemmelder, MD, PhD, Division of Nephrology, Department of Internal Medicine, Maastricht University Medical Center and CARIM School for Cardiovascular Disease, University of Maastricht, Maastricht, the Netherlands; and Aiko P. J. de Vries, MD, PhD, Department of Internal Medicine, Nephrology, and Transplantation, Erasmus MC Transplant Institute, Erasmus Medical Center, Rotterdam, the Netherlands.
Supplementary Material
Footnotes
A list of RECOVAC Collaborators is added in the Acknowledgments.
This study is funded by The Netherlands Organization for Health Research and Development (ZonMw), project number: 10430072010002. This organization had no role in the design of the study, data interpretation, writing of the article, nor in the decision to submit the article.
The authors declare no conflicts of interest
Trial registration number: NCT04741386 (ClinicalTrials.gov)
R.T.G. and J.-S.F.S. designed the study protocol. M.M.L.K., M.E.J.R., C.C.B., D.v.B., F.J.B., R.M., R.v.d.M., E.B.M.R., R.D.d.V., and L.B.H. contributed to the protocol design. D.A.D., F.K., M.P.G.K., A.L.M., and P.V. provided intellectual content of critical importance to the study. A.L.M., C.C.B., R.v.B., D.A.D., S.C.F., D.G., C.H.G.v.K., G.d.H., M.v.d.H., C.I., S.R.K.M., W.B.M., R.M., D.v.M., and R.D.d.V. were involved with data acquisition. F.K. and N.R. provided the COVID-19 vaccine. M.M.L.K., M.E.J.R., C.C.B., D.v.B., F.J.B., D.A.D., R.T.G., A.L.M., M.H., R.v.d.M., E.B.M.R., R.D.d.V., L.B.H., and J.-S.F.S. participated in the study, analysis of the data, preparation of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ERA-EDTA Council; ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy S, Chitturi C, Yee J. Vaccination in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:72–78. [DOI] [PubMed] [Google Scholar]
- 6.Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21:2913–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grupper A, Sharon N, Finn T, et al. Humoral response to the pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kho MML, Reinders MEJ, Baan CC, et al. ; RECOVAC Collaborators. The RECOVAC IR study: the immune response and safety of the mRNA-1273 COVID-19 vaccine in patients with chronic kidney disease, on dialysis or living with a kidney transplant. Nephrol Dial Transplant. 2021;36:1761–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Hartog G, Schepp RM, Kuijer M, et al. SARS-CoV-2-specific antibody detection for seroepidemiology: a multiplex analysis approach accounting for accurate seroprevalence. J Infect Dis. 2020;222:1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.den Hartog G, Vos ERA, van den Hoogen LL, et al. Persistence of antibodies to SARS-CoV-2 in relation to symptoms in a nationwide prospective study. Clin Infect Dis. 2021;73:2155–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geers D, Shamier MC, Bogers S, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6:eabj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okba NMA, Müller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Praet JT, Vandecasteele S, De Roo A, et al. Humoral and cellular immunogenicity of the BNT162b2 mRNA Covid-19 vaccine in nursing home residents. Clin Infect Dis. 2021;73:2145–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavarot N, Ouedrani A, Marion O, et al. Poor anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105:e94–e95. [DOI] [PubMed] [Google Scholar]
- 18.Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech). Viruses. 2021;13:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27:1173.e1–1173.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. [DOI] [PubMed] [Google Scholar]
- 22.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131:150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. [DOI] [PubMed] [Google Scholar]
- 25.Mulley WR, Visvanathan K, Hurt AC, et al. Mycophenolate and lower graft function reduce the seroresponse of kidney transplant recipients to pandemic H1N1 vaccination. Kidney Int. 2012;82:212–219. [DOI] [PubMed] [Google Scholar]
- 26.Agur T, Ben-Dor N, Goldman S, et al. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients—a prospective cohort study. Nephrol Dial Transplant. 2021;36:1347–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frantzen L, Cavaillé G, Thibeaut S, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in a haemodialysis cohort. Nephrol Dial Transplant. 2021;36:1756–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanay NB, Freiman S, Shapira M, et al. Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 2021;99:1496–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attias P, Sakhi H, Rieu P, et al. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021;99:1490–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol Dial Transplant. 2021;36:1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.United States Renal Data System. USRDS annual data report: epidemiology of kidney disease in the United States. 2020. Available at https://www.usrds.org/annual-data-report/. Accessed July 23, 2021.
- 32.Anderson EJ, Rouphael NG, Widge AT, et al. ; mRNA-1273 Study Group. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng S, Phillips DJ, White T, et al. ; Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv. [Epub ahead of print. June 24, 2021]. doi: 10.1101/2021.06.21.21258528. [DOI] [PMC free article] [PubMed]
- 34.Gilbert PB, Montefiori DC, McDermott A, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. medRxiv. [Epub ahead of print. August 15, 2021]. doi: 10.1101/2021.08.09.21261290 [DOI] [PMC free article] [PubMed]
- 35.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mombelli M, Rettby N, Perreau M, et al. Immunogenicity and safety of double versus standard dose of the seasonal influenza vaccine in solid-organ transplant recipients: a randomized controlled trial. Vaccine. 2018;36:6163–6169. [DOI] [PubMed] [Google Scholar]
- 39.Bel’eed K, Wright M, Eadington D, et al. Vaccination against hepatitis B infection in patients with end stage renal disease. Postgrad Med J. 2002;78:538–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stock PG, Henrich TJ, Segev DL, et al. Interpreting and addressing suboptimal immune responses after COVID-19 vaccination in solid-organ transplant recipients. J Clin Invest. 2021;131:151178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke CL, Prendecki M, Dhutia A, et al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int. 2021;99:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thieme CJ, Anft M, Paniskaki K, et al. The magnitude and functionality of SARS-CoV-2 reactive cellular and humoral immunity in transplant population is similar to the general population despite immunosuppression. Transplantation. 2021;105:2156–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Firket L, Descy J, Seidel L, et al. Serological response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients depends on prior exposure to SARS-CoV-2. Am J Transplant. 2021;21:3806–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]