Abstract
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread into more than 200 countries and infected approximately 203 million people globally. COVID-19 is associated with high mortality and morbidity in some patients, and this disease still does not have effective treatments with reproducibly appreciable outcomes. One of the leading complications associated with COVID-19 is acute respiratory distress syndrome (ARDS); this is an anti-viral host inflammatory response, and it is usually caused by a cytokine storm syndrome which may lead to multi-organ failure and death. Currently, COVID-19 patients are treated with approaches that mostly fall into two major categories: immunomodulators, which promote the body’s fight against viruses efficiently, and antivirals, which slow or stop viruses from multiplying. These treatments include a variety of novel therapies that are currently being tested in clinical trials, including serum, IL-6 antibody, and remdesivir; however, the outcomes of these therapies are not consistently appreciable and remain a subject of debate. Mesenchymal stem/stromal cells (MSCs), the multipotent stem cells that have previously been used to treat viral infections and various respiratory diseases such as ARDS exhibit immunomodulatory properties and can ameliorate tissue damage. Given that SARS-CoV-2 targets the immune system and causes tissue damage, it is presumable that MSCs are being explored to treat COVID-19 patients. This review summarizes the potential mechanisms of action of MSC therapy, progress of MSC, and its related products in clinical trials for COVID-19 therapy based on the outcomes of these clinical studies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-022-02810-6.
Keywords: MSC, Cell therapy, COVID-19
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1] has caused a global pandemic of coronavirus disease 2019 (COVID-19) which has an R0 value that is similar to Spanish influenza but higher than the Middle East respiratory syndrome (MERS) and H1N1 influenza [2]. Recently, several SARS-CoV-2 variants, especially the highly transmissible delta (B.1.617.2) and the immuno-evasive lambda (B.37) have caused a second wave of pandemics in many countries [3, 4]. Until August 31, 2021, more than 202,608,306 COVID-19 cases had been confirmed globally with a mortality rate of 2.1% based on a report by the World Health Organization (WHO) (www.WHO.int). COVID-19 has a variety of symptoms, ranging from the mild, moderate, severe, and critically severe [5]. According to the WHO report, the vast majority of COVID-19-positive people developed only mild (40%) or moderate (40%) symptoms; approximately 15% of COVID-19 patients developed severe symptoms that needed oxygen support, and 5% of patients developed critically severe symptoms with complications such as acute respiratory distress syndrome (ARDS), respiratory failure, sepsis and septic shock, and/or multiorgan failure (including cardiac and acute kidney injury). ARDS is an anti-viral host inflammatory response that is usually caused by cytokine storm syndrome (CSS) which may lead to multi-organ failure and has become the leading cause of death in severe and critically severe COVID-19 patients. CSS, which is triggered by SARS-CoV-2, involves a variety of inflammatory cytokines such as tumor necrosis factor (TNF), IL-2, IL-6, IL-7, MIP1A, granulocyte colony-stimulating factor, interferon gamma-induced protein 10, and chemokine (CC motif) ligand (CCL) family members [6, 7]; CSS is closely linked to COVID specific ARDS, multi-organ failure, and eventual death [8–10].
Currently, there is no cure for COVID-19. According to WHO guidelines, severe and critically severe COVID-19 patients should be administered anti-inflammatory and anti-viral drugs in addition to supportive therapies such as invasive and non-invasive mechanical ventilation [11]. To date, various drugs have been tested in clinical trials for their safety and efficacy, including in two major categories: (1) anti-viral drugs such as remdesivir [12], lopinavir-ritonavir [13], favipiravir chloroquine, and hydroxychloroquine [14]; and (2) immune-modulators such as anakinra, an IL-1 receptor antagonist [15], tocilizumab and sarilumab, both of which are IL-6 receptor antagonists [16], and ruxolitinib and baricitinib, which are Janus kinase signal inducer pathway inhibitors [17]. In addition to the aforementioned two major categories of treatments, other methods, such as neutralizing antibodies [18, 19] and convalescent plasma therapy [20, 21], are also used in the clinical fight against COVID-19. However, none of the above-mentioned drugs/treatments can significantly and reproducibly improve the symptoms of COVID-19 patients and are thus not recommended by the WHO for COVID-19 treatment. Recently, the emergence of several COVID-19 variants, especially the delta variant that has caused rapid infection in India and other countries, provides an urgent impetus to find an effective therapy for COVID-19, especially for severe and critically severe patients [22, 23]. More recently, mesenchymal stem/stromal cells (MSCs), the multipotent stem cells that exhibit both virus-resistant and immunomodulatory activity, and that can differentiate into a variety of cell types (as well as its derivatives), have been used to treat COVID-19 patients in the clinic owing to their immunomodulatory and tissue repair functions [24, 25]. This review focuses on the potential mechanisms of actions of MSC therapy, progress of MSC, and its related products in clinical trials for COVID-19 therapy based on outcomes of clinical studies.
MSCs as a potential therapy against COVID-19
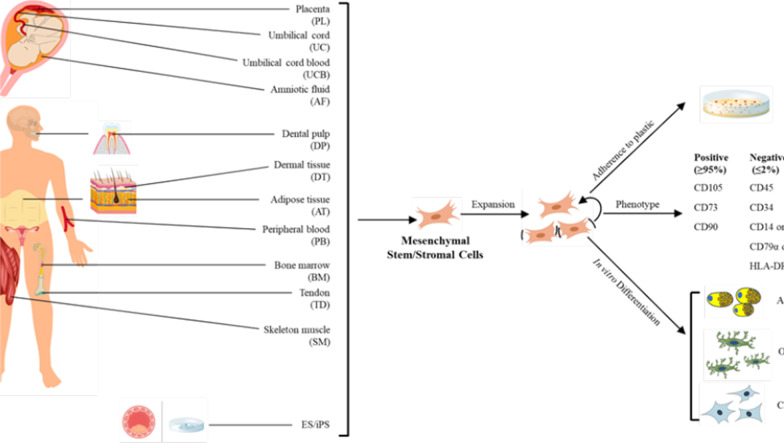
MSCs are multipotent stem cells that may be isolated from several adult tissues including adipose tissue (AT-MSCs), bone marrow (BM-MSCs), skin, dental pulp (DP-MSCs), foreskin, salivary gland, and fetal tissues including amniotic fluid, umbilical cord (UC-MSCs), placenta, Wharton’s jelly, and cord blood (Fig. 1) [26, 27]. MSCs are characterized by: (1) their capacity to adhere to plastic surfaces, (2) their expression of CD105, CD73, and CD90, and their lack of expression of CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR surface molecules, and (3) their capacity to differentiate into multiple cell lineages including adipocytes, osteoblasts, and chondroblasts in vitro differentiating conditions [28], all of which fit the definition of stem cells as suggested by the International Society for Cell & Gene Therapy (ISCT). MSCs have been widely used for improving immune dysfunction and for facilitating damaged tissue regeneration [29]. Both autologous and allogeneic BM-MSCs, UC-MSCs, and AT-MSCs [30] are applied in the clinic due to the low expression levels of major histocompatibility complex class I and the near absence of major histocompatibility complex class II on their surface [31]. Given the current extensive application of MSCs in the clinic, it is presumable that MSCs may be used to treat COVID-19 patients with a compromised immune system, damage of organs such as the lung, and ARDS.
Fig. 1.
Sources and minimal characteristic criteria of MSCs
Possible mechanisms of MSCs underlying COVID-19 treatment
In advanced stages of COVID-19 infection, CSS can induce ARDS, pulmonary edema, dysfunctional air exchange, cardiac injury, and even death. CSS also occurs in graft-versus-host disease during graft failure and leukemia or lymphoma in response to CD19 CAR-T therapy [32]. CSS in COVID-19 often occurs with a median time of 8–14 days in an average of 15% of the infected patients [33]. Stimulated by the pro-inflammatory factor such as TNF-α [34], MSCs secrete trophic and immunomodulatory factors as mentioned above, MSCs express their functions mainly through paracrine effects, i.e., secreting immunomodulatory cytokines such as indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), IL-6, and IL-10 to balance pro-and anti-inflammatory responses [35–38], as well as growth factors such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), insulin-like growth factor 1, and fibroblast growth factor 2 (FGF2) which promote cell regeneration and angiogenesis following tissue injury [39, 40]. These mechanisms support the notion that MSCs may reduce or even eliminate CSS among COVID-19 patients. On the other hand, MSCs can also be regulated by cell-to-cell contact, and they can secrete extracellular vesicles [41]. These mechanisms support the notion that MSCs may reduce or even eliminate CSS of COVID-19 patients. The detailed possible mechanisms of action of MSCs are described below (Fig. 2).
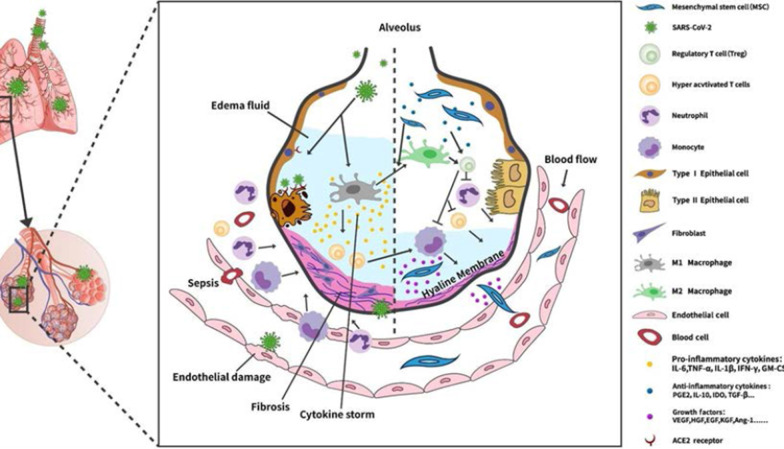
Fig. 2.
Potential mechanisms of action of MSCs in the treatment of COVID-19 pneumonia. MSCs regulate the COVID-19-triggered cytokine storm and lung damage through its immunomodulatory and trophic functions. MSCs can secrete various anti-inflammatory cytokines, e.g., PGE2, TGF-β, IDO, and IL-10, to promote the differentiation of macrophages from the pro-inflammatory type M1 to the anti-inflammatory type M2, to reduce the neutrophil infiltration, and to regulate hyper-activated T cells. On the other hand, MSCs can secrete growth factors to inhibit fibrosis and to suppress epithelial/endothelial cell apoptosis and influx of alveolar fluid
Anti-inflammation
During inflammation, impaired epithelial cells, the major barrier component of blood vessels and tissues, increase the permeability of lung tissues [42]. Previously, intratracheal administration of MSCs in lipopolysaccharide-induced inflammatory conditions in a mouse model has been shown to reduce inflammation and injury-increased permeability of the lung tissues by inducing IL-10 through secreting PGE2, granulocyte–macrophage colony-stimulating factor (GM-CSF), and granulocyte colony stimulating factor (G-CSF) [31]. Additionally, MSCs release anti-inflammatory factors IL-10 and IL-4 to repress the activation of lymphocytes and inflammatory cytokines such as IL-1-α-β,, -6, -17, and TNF-α [43]. On the other hand, MSCs prevent infection-induced damage to lung tissues by decreasing the excessive secretion of neutrophil extracellular traps at the infectious site [44]. During bacterial infections, MSCs reduces inflammation and ameliorates tissue injury through at least the following mechanisms: (1) by diminishing the excessive production of neutrophils and enhancing neutrophil-mediated phagocytosis [45], (2) promoting macrophages to differentiate into M1, which induces phagocytosis and promotes bacterial clearance, and M2, which benefits tissue repair by attenuating inflammation at the infection site [46, 47], and (3) promoting the proliferation of regulatory T cells and inhibiting the proliferation of effector T cells, thereby diminishing the immune response and ameliorating lung damage in ARDS [48].
MSCs can also attenuate inflammation-linked tissue injury through the regulation of transcriptional responses and protein–protein interaction. In a septic mouse model, MSCs were able to induce transcriptional responses via upregulating the nuclear factor of activated T cells calcineurin gene family members that mediate the expression of cytokine genes and downregulating Toll-like receptor-mediated nuclear factor kappa light chain enhancer of activated B cells [49]. In an acute lung injury mouse model induced by lipopolysaccharide, BM-MSCs were able to generate a physical contact with connexin 43, a gap junction protein, through which MSCs released mitochondria-containing micro-vesicles into alveolar epithelial cells. This transfer increased the amount of ATP in epithelial cells, thereby promoting the repair of alveolar endothelial and epithelial barriers in acute lung injury [50]. In addition, a study on an Escherichia coli-induced pneumonia model showed that mitochondrial transfer from MSCs to macrophages, which occurs in part through nanotube-like structures, enhances phagocytic activity and establishes a mechanism for anti-microbial effect through cell-to-cell contact [51].
As mentioned above, MSCs can perform functions through a paracrine activity. In a rat lung injury model that was generated by a ventilator, the conditioned medium collected from an MSC culture reversed the lung injury via keratinocyte growth factor (KGF), which ameliorates the epithelial cell injury by potentiating the activity of Na-KATPase, anti-inflammatory cytokine (matrix metallopeptidase 9, IL-1α), and GM-CSF [52, 53]. Overexpression of some factors released by MSCs, such as EGF, PDGF-b, angiogenin 1, and basic FGF, increases cell proliferation and facilitates lung repair [54]. Studies from different research groups also suggested that the overexpression CXCR4, angiogenin 1, ACE-2, KGF, and HGF attenuates endotoxin-triggered lung injury, collagen deposition, and fibrosis and edema formation, in part by enhancing the anti-inflammatory and chemotactic properties of MSCs [55–58].
Immunomodulation
It has been shown that MSCs modulate the immune system during lung injury that is induced by respiratory viruses, which may also be one of the mechanisms underlying the COVID-19 treatment by MSCs. For example, in a mouse model of avian influenza virus (H5N1)-induced lung injury, UC-MSCs recovered the function of alveolar epithelial cells, as evidenced by reduced permeability and elevated alveolar fluid clearance [59]. In addition, the function of MSCs is not significantly affected by viral infections, which may be partially attributed to the fact that intrinsically expressed interferon-stimulated genes (ISGs) prevent viruses from “attacking” MSCs because the induction of intrinsic ISGs in human MSCs triggers the expression of anti-viral factors including SAT1, PMAIP1, ISG15, IF16, CCL2, and interferon-induced transmembrane protein 1 (IFITM1) [60].
The IFITM family members are important protectors and prevent several viruses, including Rift Valley fever virus, Ebola virus, influenza A virus, dengue virus, reovirus, and SARS-CoV from entering cells through the lipid bilayer [61, 62]. Interestingly, the host cell receptor ACE-2 in IFITM-expressing cells prevents the internalization of SARS-CoV viruses [61]. In the lungs, the ACE-2 receptor is expressed in endothelial and alveolar type II cells, and these cells contribute to blocking virus entry and diminishing fibrosis and thus exhibiting endothelial-protective and anti-inflammatory functions [63–65]. The potential benefits of overexpression of ACE-2 receptors in MSCs in COVID-19 treatment warrants further exploration.
Cell death prevention
Regulated cell death (RCD), such as apoptosis, pyroptosis, necroptosis, and autophagic cell death, plays an important role in injury of tissues and organs, including lung [66]. While appropriate levels of regulated cell death may help tissue regeneration by removing damaged cells and reduce the accumulation of toxins released by injured cells and thus are appreciated, exacerbated regulated cell death may heighten inflammatory response and promote tissue injury [67]. Histological examination of postmorten lungs of COVID-19 patients uncovered the presence of apoptosis and necroptosis [68], suggesting the contribution of regulated cell death to lung injury in COVID-19 patients. Interestingly, one of the major functions of MSCs is to prevent RCD [69]. For example, ARDS patients who received MSCs treatment and had significant improvements also had significant decrease in the levels of cell death [70]. However, the direct evidence that links MSCs treatment and decrease in cell death in COVID-19 patients remains to be provided, although MSCs may counter regulated cell death through anti-inflammatory and immunomodulatory actions as mentioned above.
While it is well known that MSCs possess multiple biological functions, exact mechanisms behind the treatment of COVID-19 patients by MSCs remain to be elucidated.
Clinical experience and outcomes of treatment of viral infections and respiratory diseases with MSCs: lessons from the past
MSCs have been extensively applied to treat infectious and non-infectious diseases because of their regenerative and immunomodulatory activity.
Hepatitis B virus (HBV)
Chronic HBV infection is a primary cause of severe liver diseases, especially in East Asian populations, and liver transplantation is the sole cure for the end-stage liver diseases such as acute liver failure and decompensated liver cirrhosis [71]. BM-MSCs was shown to be resistant to HBV infection [72], and the administration of both autologous BM-MSCs and allogenic UC-MSCs in liver failure patients caused by HBV significantly increased the survival rates, elevated the circulating levels of serum cholinesterase, albumin, platelet, prothrombin, and decreased serum levels of alanine aminotransferase and total bilirubin [73, 74]. Consistent with the above observations, Wang et al. also reported that UC-MSCs were well-tolerant and increased the survival rate of patients with chronic HBV induced liver failure and cirrhosis [75]. Recently, after the long-term follow-up of 75 months, the MSC-treated group showed significantly improved liver function and a higher overall survival rate than the control group while there was no significant difference in the hepatocellular carcinoma event-free survival rate between these two groups [75][75]. However, to further clarify the safety and efficacy of MSCs in treatment of HBV-induced severe liver disease patients, double-blind, placebo control, multi-center randomized clinical trials with a long-term follow-up period are needed in the future.
Avian influenza virus (AIV)
AIV, such as H7N9, is another potential threat in terms of a global pandemic of a respiratory tract infectious disease. H7N9-infected patients usually develop ARDS, acute pneumonia, and lung failure, which are similar to the complications of COVID-19 patients. Little is known from a clinical perspective regarding whether MSCs can be safe and effective for treatment among H7N9 patients. In a recent open-label clinical trial at a single center, Chen et al. transplanted allogeneic MB-MSCs into 17 patients with H7N9-induced ARDS and 44 patients with H7N9-induced ARDS were included as a control group. Notably, the MSCs treatment group had a significantly lower mortality rate than the control group. It is noteworthy that MSCs transplantation did not cause any harm to patients during the five-year follow-up period [77].
Human immunodeficiency virus (HIV)
Very few clinical studies were performed that use MSCs to treat HIV patients. In an open-label study, Zhang et al.[78]. treated seven non-immune responders (NIRs) patients with three doses of UC-MSCs, and the control group had six NIRs. They found increased levels of naive and central memory CD4+ T-cell as well as elevated production of IL-2 and HIV-1-specific interferon. Recently, Wang et al. reported that hUC-MSC treatment for NIRs with chronic HIV-1 infection was safe and well-tolerated [79]. In addition, this study revealed significantly increased CD4+ T counts in the low-and high-MSC dose groups after a 48-week treatment, compared with no change observed in the control group. Moreover, the cumulative probability of achieving an immunological response was higher than the control. However, there were no significant differences in the CD4/CD8+ T counts and CD4/CD8 ratio between these two groups [79].
ARDS
ARDS exhibits high morbidity and mortality and lacks effective treatments. In a phase I clinical trial, MSCs were administered to patients with acute lung injury (ALI) and ARDS, and this trial demonstrated that MSCs from different sources were safe and well-tolerated in ARDS patients [80] with some patients showing improvement in the respiratory and hemodynamic function as well as outcomes regarding multiorgan failure [81]. A phase II randomized controlled trial (RCT) conducted in the United States demonstrated that a high dose (10 × 106 cells/kg) of allogeneic BM-MSCs infusion did not cause any significant respiratory AEs [82]. Moreover, this high dose of BM-MSCs infusion improved the oxygenation index and reduced the circulating levels of ANG-2 in these patients, which suggests ameliorating endothelial injury [82].
Current clinical trials of MSCs and its products in treatment of COVID-19
To date, more than 90 clinical trials have been registered at ClinicalTrials.gov that use MSCs to treat COVID-19. Among these MSCs used, UC-MSCs accounted for 35%, followed by BM-MSCs (20%), AD-MACs (15%), and DP-MSCs (7.5%) [83–85]. MSCs are usually administrated intravenously a single time or multiple times, and the doses range from 0.5 × 106 to 5 × 106 cells/kg per injection. Currently, more than 65% of these trials are in phase I/II or II, and approximately, 22% are in the early phase or phase I; only a few (less than 5%) are in phase II/III or III. The detailed information of clinical trials is listed in Additional file 1: Table S1.
Several MSC products have also been used in the above-mentioned clinical trials, and some of them are in the phase II or III stage. Mesoblast in collaboration with Novartis initiated a double-blind, randomized, and placebo-controlled phase III trial with 300 patients using an intravenous infusion of 2 × 106 cells/kg BM-MSCs after encouraging results had been obtained from 12 ventilator-dependent ARDS patients [86–88]. A completed phase I/II clinical trial of intravenous injection of MultiStem in COVID-19 patients has shown appreciable findings: a phase I study confirmed safety with a small starting dose, and a phase II study on 36 patients was a randomized, double-blind, and placebo-controlled trial. Compared to the placebo group, the treatment group had lower mortality and a shorter stay in the intensive care unit without any adverse effects [89, 90]. Athersys is currently carrying out a phase II/III clinical trial to examine the safety and efficacy of the BM-MSC product MultiStem in 400 COVID-19 patients with ARDS. Hope Biosciences is currently performing three clinical trials using autologous and allogenic AD-MSCs to evaluate its safety and efficacy in COVID-19 patients [91]. Pluristem has conducted a phase II double-blind, placebo-controlled, multi-center RCT study with 140 patients using intramuscular injection (300 × 106 cells) of placenta-derived mesenchymal-like cells after a previous finding of the full recovery of six severely ill COVID-19 patients [92]. Cynata has initiated an open-label, RCT with an iPSC-derived MSC product Cymerus in 24 patients in the intensive care unit. MSCs are derived from iPSC-differentiated mesenchymal angioblasts, which were generated by transgene-, viral-and feeder-free techniques with the reprograming of donated blood cells [93]. Novellus and Citius proposed a placebo-controlled randomized dose escalation trial to assess the safety and efficacy of NoveCite in COVID-19 patients with ARDS. NoveCite is a product of MSCs derived from induced pluripotent stem cells, which are reprogrammed from fibroblasts with messenger RNA [94]. The detailed information of clinical trials with MSC products is listed in Table 1.
Table 1.
MSC products in COVID-19 treatment
| Product | Company | Source | Dose | Phase | Country | Trial ID NO | |
|---|---|---|---|---|---|---|---|
| 1 | VUM02 | VCANBIO | Allogenic UC-MSCs | 4E7 cells, i.v. at days 0, 3, and 6 | I II | China | NCT04252118 NCT04288102 |
| 2 | RYONCIL™(remestemcel-L) | Mesoblast, Ltd./Novartis | Allogenic BM-MSCs | 2E6 cells/kg, 2 times/week | III | USA | NCT04371393 |
| 3 | Multistem | Athersys, Inc | Allogenic BM-MSCs | – | II/III | USA | NCT04367077 |
| 4 | HB-adMSCs | Hope Biosciences | Autologous AT-MSCs | 5 times, i.v | II | USA | NCT04349631 |
| 5 | HB-adMSCs | Hope Biosciences | Allogenic AT-MSCs | 2E8 cells, i.v. at weeks 0, 2, 6, 10, and 14 | II | USA | NCT04348435 |
| 1E6 cells, at days 0, 3, 7, and 10 | II | USA | NCT04362189 | ||||
| 6 | PLX-PAD | Pluristem Ltd | Allogenic placenta MSCs | 3E8, i.v | II | USA, Germany, & Israel | NCT04389450 NCT04614025 |
| 7 | JadiCell™ | Therapeutic Solutions International | Allogenic UC-MSCs | (10 ± 2) E7 cells; at days 0 and 3 | I/II | USA | NCT04355728 |
| 8 | CYP-001 (Cymerus MSCs) | Cynata Therapeutics Ltd | iPSC-MSCs | 2E6 cells/kg, i.v | I/II | Australia | NCT04537351 |
| 9 | NestaCell | Cellavita | iPSC-MSCs | 2E7 cells i.v. at days 1, 3, 5, and 7 | II | Brazil | NCT04315987 |
| 10 | HCLM051 | Healios/Athersys, Inc | Allogenic BM-MSCs | 9E8 (± 20%) cells | II | Japan | NCT03807804 |
| 11 | itMSCs | Stemedica Cell Technologies, Inc | Allogenic BM-MSCs | – | II | USA | NCT04780685 |
| 12 | ACT-20 | Aspire Health Science | Allogenic UC-MSCs | 1E6 cells/kg, i.v | I/II | USA | NCT04398303 |
| 13 | i-MSC | Citius Pharma/Novellus | iPSC-MSCs | – | II | USA | – |
| 14 | CAStem | Zephyrm Biotech | ESC-MSCs | 3, 5 or 10 E6 cells/kg, i.v | I/II | China | NCT04331613 |
| 15 | ULSC-CV-01 | Restem, LLC | Allogenic UC-MSCs | 1E8 cells, i.v | I/IIa | USA | NCT04494386 |
| 16 | COVI-MSC | Sorrento Therapeutics, Inc | Allogeneic AT-MSCs | 1E6 cells/kg or 1.5E6 cells/kg, depending on CRP level | II | USA | NCT04728698 |
| 3E7 cells at days 0, 2, and 4 | II | USA | NCT04903327 NCT04905836 | ||||
| 1.85E7 cells at days 0, 2, and 4;3.7E7 cells at days 0, 2, and 4 | Ib | USA | NCT04909892 | ||||
| 17 | BX-U001 | Baylx Inc | Allogenic UC-MSCs | Low dose: 0.5E6 cells/kg; Middle dose: 1.0E6 cells/kg; High dose: 1.5E6 cells/kg | I/IIa | China | NCT04452097 |
| 18 | PSC-04 | Sorrento Therapeutics, Inc | Allogenic AT-MSCs | – | I | USA | NCT04486001 |
| 19 | BM-Allo.MSC | ImmunityBio, Inc./NantKwest | Allogenic BM-MSCs | – | Ib | USA | NCT04397796 |
| 20 | MB-MSC injection | IPM Biotech | Allogenic MB-MSCs | 9E7 cells, i.v. at days 1, 3, and 5 | I | China | ChiCTR2000029606 |
| 21 | LMSCs | Longeveron Inc | – | 1E8 cells, i.v. at days 0, 3, and 6 | I | USA | NCT04629105 |
| 22 | Descartes-30 | Cartesian Therapeutics | Allogenic UC-MSCs | – | I/II | USA | NCT04524962 |
| 23 | SB1-101 | Sentien Biotechnologies, Inc | Allogenic MB-MSCs with an FDA-approved plasmapheresis device | High dose: 7.5E8; Low dose: 2.5E8 | I/II | USA | NCT04445220 |
| 24 | AlloRx™ | Vitro Biopharma/GIOSTAR | Allogenic MSC engineered to secrete human DNases | – | I | USA | – |
| 25 | DW-MSC | Daewoong Pharmaceutical | Allogenic UC-MSCs | 5E7 cells, i.v | I | Indonesia | NCT04535856 |
| 26 | hDP-MSC injection | SH Bio-Tech | Allogenic DP-MSCs | 3E7 stem cells at days 1, 4, and 7 | I | China | NCT04336254 |
i.v., intravenous injection
Current outcomes of clinical trials of MSC in COVID-19 treatment
Clinical experience with MSC treatment for COVID-19 is still limited. Although many clinical trials have been registered in clinicaltrials.gov (Additional file 1: Table S1), only a few have reported their findings (Additional file 1: Table S2). In China, MSCs have also been initiated as a therapeutic strategy for COVID-19, which was shown in several case reports. For example, the injection of human UC-MSCs into a 65-year-old woman on ventilation decreased the circulating levels of C-reactive protein (CRP), serum bilirubin, and liver function enzymes, and increased the circulating levels of CD8+ T, CD3+ T, and CD4+ T cells to the normal level [95]. This patient eventually recovered and tested negative for the virus [95].
As mentioned above, most MSCs used in the reported clinical research were derived from the umbilical cord. In one clinical trial [96], UC-MSCs were injected intravenously into seven COVID-19 patients (two mild, four severe, and one critically severe) with a dose of 1 × 106 MSCs/kg, and three severe COVID-19 patients administered with the placebo served as controls. This trial was followed up for 14 days. The treatment group significantly improved lung function, which was accompanied by a decrease in the levels of serum CXCR3+ CD4+ T cells, CRP, CXCR3+ CD8+ T cells, CXCR3, and NK cells, and an increase in the levels of regulatory dendritic cells. Furthermore, ACE-2 or transmembrane protease serine 2 was not expressed in MSCs. Shu et al. conducted a clinical trial in which 12 COVID-19 patients received hUC-MSCs treatment, and 29 patients were in the control group [97]. This study showed that the hUC-MSC group had no patients with a progression from severe to critically ill and zero deaths during the 28-day study period, while the control group had four patients with a 10.34% mortality rate. Also, the treatment group had a shorter time for clinical improvement and lung inflammation absorption as revealed by CT imaging. In addition, the treatment group had significantly lower levels of CRP and IL-6 after three days of infusion and faster time for the lymphocyte count to return to a normal level [97]. Guo et al. demonstrated that MSC therapy restored oxygenation, elevated PaO2/FiO2 and lymphocyte count, downregulated cytokine storms, decreased levels of serum CRP, procalcitonin, D-dimer and IL-6, without AEs after infusion of UC-MSCs into 31 severe COVID-19 patients [98]. Feng et al. revealed that intravenous infusion of UC-MSCs partially recovered pulmonary function through forced expiratory volumes in 1 s, and it improved the quality of life as revealed by the St. George's Respiratory Questionnaire, which indicates relatively long-term safety and preliminary efficacy for severe COVID-19 patients after a 3-month follow-up [99]. Recently, a Turkish group reported that UC-MSC treatment was safe in 210 severe/critically severe COVID-19 patients with 1–2 × 106/kg infusion. The treated group had a significantly higher survival rate; they were accompanied by restored oxygenation such as SaO2 parameters and downregulated cytokine storm [100].
MSCs from other sources also exhibited a similar benefit to treating COVID-19 patients. Spanish researchers used AT-MSCs to treat 13 COVID-19 patients, with two receiving a single dose, 10 receiving two doses, and one receiving three doses. Median number of cells per dose was 0.98 × 106 AT-MSC/kg body weight. These researchers showed that the treatment improved immune cell profiling with no adverse effects observed [101]. Menstrual blood-derived MSCs were infused into 26 patients with a total of 9 × 107 cells per infusion every other day for three times a day, and 18 patients received only concomitant medications as control. While there was no significant difference in the incidence of most AEs between these groups, the MSC group showed a significantly lower mortality rate, significant improvement in dyspnea, SpO2, and chest imaging results [102]. In another clinical trial, 27 COVID-19 patients who received an infusion of MSCs derived from hESCs had normal levels of all hematological and clinical parameters and tumor markers; none of these treated patients developed any abnormal responses/AEs that are associated with hESC-MSC therapy. Clinical improvements were also—observed in patients within 84 days after treatment with hESC-MSC therapy [103].
In addition to these case reports and clinical studies, three research groups have demonstrated outcomes from a more strictly designed phase I/II double-blind RCT. Giacomo et al. tested a single-center, double-blind, phase 1/2a, RCT of UC-MSC infusions in treatment of 12 COVID-19 ARDS patients compared with 12 patients who received two infusions of vehicle. Patients in these two groups received comparable standard care. There was no significant difference in infusion-associated AEs between these two groups, and no serious AEs that are linked to UC-MSC infusion were observed; this indicates the safety of UC-MSC infusions. UC-MSC treatment was associated with significantly improved patient survival, SAEs (SAE)-free survival, and time to recovery [104]. Ismail et. al. conducted a double-blind, multicentered RCT with 40 critically ill COVID-19 patients. Among these patients, 20 received an intravenous infusion of UC-MSCs with 1 × 106/kg body weight, and 20 received 100 ml saline (0.9%) solution as the control. The UC-MSC group had a survival rate 2.5 times higher than the control group, and even 4.5 times compared to patients with comorbidities in the control group. The UC-MSCs infusion remarkably reduced the circulating levels of IL6 in the recovered patients, with no ADs observed [105]. Wang’s group reported two consistent studies: a phase I exploratory study with 18 COVID-19 patients and a phase II double-blind, placebo control, multi-centric randomized clinical study with 100 severe patients. In the phase I study, zero patients developed serious infusion-associated ADs in nine treated patients (four of them were severe, five were moderate). Two patients in the treatment group developed transient facial flushing and fever, and one had transient hypoxia. One patient in the treatment group needed mechanical ventilation compared with four in the control group [106]. In the phase II study, compared with the placebo control group which contained 35 patients, UC-MSCs administration in 65 patients significantly improved the whole lung lesion volume from baseline to day 28, decreased the proportion of solid component lesion volume, and increased 6-MWT [107]. There were no differences in AEs and serum levels of tumor markers at 12 follow-ups between these two groups (unpublished data). In line with the above findings, several clinical trials have suggested that MSC treatment greatly improved solid component lesion volume, reduced sleep difficulties, and improved daily activity compared to the placebo treatment [108–117]. In addition, the MSC treatment group had a significantly higher ratio of patients who had normal CT images at month 12 follow-up than the placebo group. Neutralizing antibodies were all positive with a similar median inhibition rate in both groups during 12 month follow-up [108–117]. The detailed outcomes of all these published clinical trials are listed in Additional file 1: Table S2.
Perspectives
The persistent COVID-19 pandemic has prompted scientists and clinicians to explore effective treatments since a clear unmet medical need still exists for severe and critically severe patients. Although anti-viral molecules, immunomodulators, neutralizing antibodies, and plasma have been administered for COVID-19 patients with ARDS and other life-threatening conditions as treatments, very few of these approaches showed reproducibly appreciable efficacy. MSCs are considered to be a candidate for treating CSS and repairing damaged lung tissues due to their multiple potent activities, including anti-inflammation, immunomodulation, and ability to secrete soluble vesicles and multiple growth factors. Promising outcomes have been reported from ongoing clinical trials involving MSC treatment for COVID-19: (1) patients were safe and well-tolerated after treatment with MSCs that were generated from various sources with a wide range of doses, (2) improvements were observed in patients after MSCs treatment, such as through decreasing circulating levels of pro-inflammatory cytokines and laboratory parameters, better lung inflammation absorption, and (3) MSCs-treated patients had faster increase in SpO2, a shorter hospital stay, and a higher survival rate. However, some scientific and clinical questions remain to be addressed. For example, what are the exact mechanisms underlying the MSCs’ treatment of COVID-19 patients? Which source of MSCs is the best for this treatment? At which stage will COVID-19 patients have the best outcomes as a result of MSCs treatment? Are there any COVID-19 patients who should not receive MSCs treatment? Can MSCs treatment reduce the long-term residual adverse effects associated with COVID-19 infection? In addition, will the combinatory therapy of MSCs with other supportive drugs work better than the single use of each individual treatment? Apart from cell-based therapy, exosome vesicles, and secretome of MSCs can be considered as an alternative. However, limitations of these closed trials were also acknowledged. For example, most of these trials had a small sample size and were single-arm or parallel control; they lacked a strict design such as the double-blind, randomized, placebo control with multiple centers. Also, the main and secondary evaluation criteria were not uniform, and the long-term follow-up was not carried out. Currently, consensus and guidelines require doctors to better manage severely ill COVID-19 patients by using MSCs treatment [118]. For example, for subject selection, it is recommended to choose the patients with the most appropriate risk–benefit balance. In addition, the heterogeneity of subjects’ baseline conditions is also an important factor in determining the success or failure of clinical trials. For safety and efficacy evaluation, the first clinical trial is expected to explore the safety and tolerability of MSCs in patients, including exploring the maximum tolerated dose, observing the incidence, timing, and severity of expected or unexpected AEs that are correlated with cell infusion. It is not recommended to include patients with large differences in baseline and prognostic prediction in the same clinical trial. For risk control, the tumorigenicity should be continuously monitored t hrough long-term follow-up.
Conclusions
The general mechanisms of action of MSCs include immunomodulation and tissue repair capability (antifibrosis and angiogenesis), and current preliminary clinical results of MSC-based therapies have shown some favorable outcomes for severe and critically severe COVID-19 patients, thus making it a promising therapy. However, double blind RCTs with large sample sizes are still required to thoroughly examine the safety and efficacy of MSCs and each specific MSC product. Nevertheless, MSC-based therapies during a global pandemic brings hope to combating COVID-19 and in meeting urgent medical needs, although a variety of challenges still lay ahead.
Supplementary Information
Additional file 1. Table S1. Clinical trials of cell-based therapies for COVID-19 patients registered in clinicaltrials.gov/. Table S2. Current outcomes of clinical trials of MSCs in COVID-19 treatment.
Acknowledgements
We are grateful to Ji Qi and Lingling Cui for the help in this work. We thank Medjaden Inc. for scientific editing of this manuscript.
Abbreviations
- ADs
AEs
- AT
Adipose tissues
- ALI
Acute lung injury
- BM
Bone marrow
- ESC
Embryonic stem cells
- GI
Gingival tissue
- IDO
Indoleamine 2, 3-Dioxygenase
- IL-6
Interleukin-6
- i.v.
Intravenous injection
- MD
Mean difference
- MOA
Mechanism of action
- MSC
Mesenchymal stem/stromal cells
- PBS
Phosphate buffered saline
- SADs
SAEs
- TGF
Transforming growth factors
- TNF
Tumor necrosis factor
- Treg
Regulatory T cells
- UC
Umbilical cord
- UCB
Umbilical cord blood
- hES-M
MSC-like cells derived from human embryonic stem cells
- P-MSCs
Placenta-derived MSCs
- DP-MSC
Dental pulp-derived MSCs
- MSC-EV
Extracellular vesicles derived from MSCs
- POM-MSC
Pooled olfactory mucosa MSCs
- MB-MSCs
Menstrual blood-derived MSCs
- ARDS
Acute respiratory distress syndrome
- PI
Pulmonary interstitial fibrosis
- NR
Not reported
Authors' contributions
WY and LS drafted the manuscript. Yu Z was responsible for conception and design of the study, revised the manuscript and supervised overall project. Yun Z and HD was responsible for data curation and writing—original draft. All authors approved the final version to be published.
Funding
This work is supported by VCANBIO Cell & Gene Engineering Corporation Ltd.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Yu Zhang has financial relationships with VCANBIO Cell & Gene Engineering Corporation Ltd. that develops MSC-based products. Other authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weiqi Yao and Lei Shi contributed equally to this work
References
- 1.Becker SJ, Taylor J, Sharfstein JM. Identifying and tracking SARS-CoV-2 variants—A challenge and an opportunity. N Engl J Med. 2021;385:389–391. doi: 10.1056/NEJMp2103859. [DOI] [PubMed] [Google Scholar]
- 2.Cevik M, Mishra S. SARS-CoV-2 variants and considerations of inferring causality on disease severity. Lancet Infect Dis. 2021;21:1472–1474. doi: 10.1016/S1473-3099(21)00338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scudellari M. How the coronavirus infects cells - and why Delta is so dangerous. Nature. 2021;595:640–644. doi: 10.1038/d41586-021-02039-y. [DOI] [PubMed] [Google Scholar]
- 4.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEnery T, Gough C, Costello RW. COVID-19: respiratory support outside the intensive care unit. Lancet Respir Med. 2020;8:538–539. doi: 10.1016/S2213-2600(20)30176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gattinoni L, Coppola S, Cressoni M. COVID-19 Does not lead to a "Typical" acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: A Randomized Clinical Trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 15.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaspari V, Zengarini C, Greco S, Vangeli V, Mastroianni A. Side effects of ruxolitinib in patients with SARS-CoV-2 infection: two case reports. Int J Antimicrob Agents. 2020;56:106023. doi: 10.1016/j.ijantimicag.2020.106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, et al. AZD1222-induced neutralising antibody activity against SARS-CoV-2 Delta VOC. Lancet. 2021;398:207–209. doi: 10.1016/S0140-6736(21)01462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batista CM, Foti L. Anti-SARS-CoV-2 and anti-cytokine storm neutralizing antibody therapies against COVID-19: update, challenges, and perspectives. Int Immunopharmacol. 2021;99:108036. doi: 10.1016/j.intimp.2021.108036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy M, Estcourt L, Grant-Casey J, Dzik S. International survey of trials of convalescent plasma to treat COVID-19 infection. Transfus Med Rev. 2020;34:151–157. doi: 10.1016/j.tmrv.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yiğenoğlu TN, Hacıbekiroğlu T, Berber İ, Dal MS, Baştürk A, Namdaroğlu S, et al. Convalescent plasma therapy in patients with COVID-19. J Clin Apher. 2020;35:367–373. doi: 10.1002/jca.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020;80:1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baden LR, Rubin EJ. Covid-19 - The search for effective therapy. N Engl J Med. 2020;382:1851–1852. doi: 10.1056/NEJMe2005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev Rep. 2020;16:427–433. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Silva KN, Gobatto ALN, Costa-Ferro ZSM, Cavalcante BRR, Caria ACI, de Aragão França LS, et al. Is there a place for mesenchymal stromal cell-based therapies in the therapeutic armamentarium against COVID-19? Stem Cell Res Ther. 2021;12:425. doi: 10.1186/s13287-021-02502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Khan D, Delling J, Tobiasch E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. ScientificWorldJournal. 2012;2012:793823. doi: 10.1100/2012/793823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 29.Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu H, Xiong Y, Xia Y, Zhang R, Tian D, Wang T, et al. Therapeutic effects of human umbilical cord-derived mesenchymal stem cells in acute lung injury mice. Sci Rep. 2017;7:39889. doi: 10.1038/srep39889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cancio M, Ciccocioppo R, Rocco PRM, Levine BL, Bronte V, Bollard CM, et al. Emerging trends in COVID-19 treatment: learning from inflammatory conditions associated with cellular therapies. Cytotherapy. 2020;22:474–481. doi: 10.1016/j.jcyt.2020.04.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 35.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35. [DOI] [PMC free article] [PubMed]
- 36.Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 37.Alcayaga-Miranda F, Cuenca J, Khoury M. Antimicrobial activity of mesenchymal stem cells: current status and new perspectives of antimicrobial peptide-based therapies. Front Immunol. 2017;8:339. doi: 10.3389/fimmu.2017.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Babczyk P, Pansky A, Kassack MU, Tobiasch E. P2 receptors influence hMSCs differentiation towards endothelial cell and smooth muscle cell lineages. Int J Mol Sci. 2020;21:6210. doi: 10.3390/ijms21176210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Gentile P, Sterodimas A, Pizzicannella J, Dionisi L, De Fazio D, Calabrese C, et al. Systematic review: allogenic use of stromal vascular fraction (SVF) and decellularized extracellular matrices (ECM) as advanced therapy medicinal products (ATMP) in tissue regeneration. Int J Mol Sci. 2020;21:4982. doi: 10.3390/ijms21144982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas R, Verin AD, Black SM, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol. 2009;77:1763–1772. doi: 10.1016/j.bcp.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 44.Pedrazza L, Cunha AA, Luft C, Nunes NK, Schimitz F, Gassen RB, et al. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J Cell Physiol. 2017;232:3552–3564. doi: 10.1002/jcp.25816. [DOI] [PubMed] [Google Scholar]
- 45.Hall SR, Tsoyi K, Ith B, Padera RF, Jr, Lederer JA, Wang Z, et al. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem Cells. 2013;31:397–407. doi: 10.1002/stem.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 47.Ye LZ, Zhang AD, Shi HP, Zhao SM, Luo HY, Yuan LF, et al. Analysis of 4628 cases in the genetic counselling clinic of PUMC Hospital. Proc Chin Acad Med Sci Peking Union Med Coll. 1989;4:126–130. [PubMed] [Google Scholar]
- 48.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 49.dos Santos CC, Murthy S, Hu P, Shan Y, Haitsma JJ, Mei SH, et al. Network analysis of transcriptional responses induced by mesenchymal stem cell treatment of experimental sepsis. Am J Pathol. 2012;181:1681–1692. doi: 10.1016/j.ajpath.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guery BP, Mason CM, Dobard EP, Beaucaire G, Summer WR, Nelson S. Keratinocyte growth factor increases transalveolar sodium reabsorption in normal and injured rat lungs. Am J Respir Crit Care Med. 1997;155:1777–1784. doi: 10.1164/ajrccm.155.5.9154891. [DOI] [PubMed] [Google Scholar]
- 53.Shyamsundar M, McAuley DF, Ingram RJ, Gibson DS, O'Kane D, McKeown ST, et al. Keratinocyte growth factor promotes epithelial survival and resolution in a human model of lung injury. Am J Respir Crit Care Med. 2014;189:1520–1529. doi: 10.1164/rccm.201310-1892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S, Mo M, Wang J, Sadia S, Shi B, Fu X, et al. Platelet-derived growth factor receptor beta identifies mesenchymal stem cells with enhanced engraftment to tissue injury and pro-angiogenic property. Cell Mol Life Sci. 2018;75:547–561. doi: 10.1007/s00018-017-2641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He H, Liu L, Chen Q, Liu A, Cai S, Yang Y, et al. Mesenchymal stem cells overexpressing angiotensin-converting enzyme 2 rescue lipopolysaccharide-induced lung injury. Cell Transplant. 2015;24:1699–1715. doi: 10.3727/096368914X685087. [DOI] [PubMed] [Google Scholar]
- 57.Yang JX, Zhang N, Wang HW, Gao P, Yang QP, Wen QP. CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J Biol Chem. 2015;290:1994–2006. doi: 10.1074/jbc.M114.605063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, Yang YF, Zhao L, Xiao FJ, Zhang QW, Wen ML, et al. Hepatocyte growth factor gene-modified mesenchymal stem cells reduce radiation-induced lung injury. Hum Gene Ther. 2013;24:343–353. doi: 10.1089/hum.2012.177. [DOI] [PubMed] [Google Scholar]
- 59.Loy H, Kuok DIT, Hui KPY, Choi MHL, Yuen W, Nicholls JM, et al. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating Influenza A(H5N1) virus-associated acute lung injury. J Infect Dis. 2019;219:186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X, Dao Thi VL, Huang Y, Billerbeck E, Saha D, Hoffmann HH, et al. Intrinsic immunity shapes viral resistance of stem cells. Cell. 2018;172:423–38.e25. doi: 10.1016/j.cell.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang IC, Bailey CC, Weyer JL, Radoshitzky SR, Becker MM, Chiang JJ, et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7:e1001258. doi: 10.1371/journal.ppat.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bailey CC, Zhong G, Huang IC, Farzan M. IFITM-family proteins: the cell's first line of antiviral defense. Annu Rev Virol. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–35.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Molina-Molina M, Abdul-Hafez A, Uhal V, Xaubet A, Uhal BD. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L178–L185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He HL, Liu L, Chen QH, Cai SX, Han JB, Hu SL, et al. MSCs modified with ACE2 restore endothelial function following LPS challenge by inhibiting the activation of RAS. J Cell Physiol. 2015;230:691–701. doi: 10.1002/jcp.24794. [DOI] [PubMed] [Google Scholar]
- 66.Sauler M, Bazan IS, Lee PJ. Cell death in the lung: the apoptosis-necroptosis axis. Annu Rev Physiol. 2019;81:375–402. doi: 10.1146/annurev-physiol-020518-114320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naji A, Suganuma N, Espagnolle N, Yagyu KI, Baba N, Sensebé L, et al. Rationale for determining the functional potency of mesenchymal stem cells in preventing regulated cell death for therapeutic use. Stem Cells Transl Med. 2017;6:713–719. doi: 10.5966/sctm.2016-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S, Zhang Y, Guan Z, Li H, Ye M, Chen X, et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct Target Ther. 2020;5:235. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naji A, Favier B, Deschaseaux F, Rouas-Freiss N, Eitoku M, Suganuma N. Mesenchymal stem/stromal cell function in modulating cell death. Stem Cell Res Ther. 2019;10:56. doi: 10.1186/s13287-019-1158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, Dalén M, et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl Med. 2015;4:1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang K, Ji F, Xie Z, Wu D, Xu X, Gao H, et al. Artificial liver support system therapy in acute-on-chronic hepatitis B liver failure: classification and regression tree analysis. Sci Rep. 2019;9:16462. doi: 10.1038/s41598-019-53029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma R, Xing Q, Shao L, Wang D, Hao Q, Li X, et al. Hepatitis B virus infection and replication in human bone marrow mesenchymal stem cells. Virol J. 2011;8:486. doi: 10.1186/1743-422X-8-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 74.Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725–731. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi M, Li Y-Y, Xu R-N, Meng F-P, Yu S-J, Fu J-L, et al. Mesenchymal stem cell therapy in decompensated liver cirrhosis: a long-term follow-up analysis of the randomized controlled clinical trial. 2021. [DOI] [PMC free article] [PubMed]
- 76.Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(Suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 77.Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection: A Hint for COVID-19 Treatment. Engineering (Beijing) 2020;6:1153–1161. doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Z, Fu J, Xu X, Wang S, Xu R, Zhao M, et al. Safety and immunological responses to human mesenchymal stem cell therapy in difficult-to-treat HIV-1-infected patients. AIDS. 2013;27:1283–1293. doi: 10.1097/QAD.0b013e32835fab77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang L, Zhang Z, Xu R, Wang X, Shu Z, Chen X, et al. Human umbilical cord mesenchymal stem cell transfusion in immune non-responders with AIDS: a multicenter randomized controlled trial. Signal Transduct Target Ther. 2021;6:217. doi: 10.1038/s41392-021-00607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rogers CJ, Harman RJ, Bunnell BA, Schreiber MA, Xiang C, Wang FS, et al. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J Transl Med. 2020;18:203. doi: 10.1186/s12967-020-02380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gentile P, Sterodimas A, Pizzicannella J, Calabrese C, Garcovich S. Research progress on mesenchymal stem cells (MSCs), adipose-derived mesenchymal stem cells (AD-MSCs), drugs, and vaccines in inhibiting COVID-19 disease. Aging Dis. 2020;11:1191–1201. doi: 10.14336/AD.2020.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zumla A, Wang FS, Ippolito G, Petrosillo N, Agrati C, Azhar EI, et al. Reducing mortality and morbidity in patients with severe COVID-19 disease by advancing ongoing trials of Mesenchymal Stromal (stem) Cell (MSC) therapy - Achieving global consensus and visibility for cellular host-directed therapies. Int J Infect Dis. 2020;96:431–439. doi: 10.1016/j.ijid.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Disptach B. First patients dosed in Mesoblast COVID-19 trial, 2020. https://biotechdispatch.com.au/news/first-patients-dosed-in-mesoblast-covid-19-trial. (Accessed 23 May 2020).
- 87.Meldrum J, Bothwell K. First Patients Dosed in Phase 2/3 Randomized Controlled Trial of Mesoblast’s Remestemcel-l for COVID-19 Acute Respiratory Distress Syndrome, 2020. https://www.globenewswire.com/news-release/2020/05/06/2028234/0/en/First-Patients-Dosed-in-Phase-2–3-Randomized-Controlled-Trial-of-Mesoblast-s-Remestemcel-l-for-COVID-19-Acute-Respiratory-Distress-Syndrome.html. (Accessed 23 May 2020).
- 88.Mesoblast COVID ARDS Trial 90-Day Survival Outcomes Presented at ISCT, 2021. http://investorsmedia.mesoblast.com/static-files/876a2e51-6238-4172-9504-fa246f303c1c. (Accessed 19 July 2021).
- 89.Weermeijer R. Athersys and UH Cleveland trial stem cell therapy for Covid-19, 2020 https://www.clinicaltrialsarenacom/news/athersys-stem-cell-therapycovid-19/ (Accessed 25 May 2020).
- 90.Staff A. COVID-19 and other Viral Induced ARDS, 2020. https://www.athersys.com/clinical-trials/ards/default.aspx. (Accessed 27 July 2020).
- 91.Hope Biosciences Announces Third FDA-approved Clinical Trial for COVID-19. https://www.hope.bio/post/hope-biosciences-announces-third-fda-approved-clinical-trial-for-covid-19. (Accessed 21 April 2020).
- 92.Rubin D. U.S. FDA Clears Pluristem’s IND Application for Phase II COVID-19 Study, 2020. https://www.globenewswire.com/news-release/2020/05/08/2030212/0/en/U-S-FDA-Clears-Pluristem-s-IND-Application-for-Phase-IICOVID-19-Study.html. (Accessed 25 May 2020).
- 93.Macdonald R, LaCagnina C. Cynata Receives Ethics Approval to Commence Clinical Trial in COVID-19and Clinical DevelopmentUpdate,2020. https://www.globenewswire.com/news-release/2020/05/08/2030332/0/en/Cynata-Receives-Ethics-Approval-to-Commence-Clinical-Trial-in-COVID-19-and-Clinical- Development-Update.html. (Accessed 27 May 2020).
- 94.Staff R. Novel Stem Cell Therapy for COVID-19-related ARDS in Development, 2020. https://www.rtmagazine.com/products-treatment/pharmaceuticals/uspharmaceuticals/stem-cells-covid-19-ards.(Accessed 26 May 2020).
- 95.Liang B, Chen J, Li T, Wu H, Yang W, Li Y, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine (Baltimore) 2020;99:e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo Z, Chen Y, Luo X, He X, Zhang Y, Wang J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit Care. 2020;24:420. doi: 10.1186/s13054-020-03142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng G, Shi L, Huang T, Ji N, Zheng Y, Lin H, et al. Human umbilical cord mesenchymal stromal cell treatment of severe COVID-19 patients: a 3-month follow-up study following hospital discharge. Stem Cells Dev. 2021;30:773–781. doi: 10.1089/scd.2021.0015. [DOI] [PubMed] [Google Scholar]
- 100.Ercelen NO, Pekkoc-Uyanik KC, Alpaydin N, Gulay GR, Simsek M. Clinical experience on umbilical cord mesenchymal stem cell treatment in 210 severe and critical COVID-19 cases in Turkey. Stem Cell Rev Rep. 2021;17:1917–1925. doi: 10.1007/s12015-021-10214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sánchez-Guijo F, García-Arranz M, López-Parra M, Monedero P, Mata-Martínez C, Santos A, et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25:100454. doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu X, Jiang W, Chen L, Xu Z, Zhang Q, Zhu M, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clin Transl Med. 2021;11:e297. doi: 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu J, Zhou X, Tan Y, Wang L, Li T, Li Z, et al. Phase 1 trial for treatment of COVID-19 patients with pulmonary fibrosis using hESC-IMRCs. Cell Prolif. 2020;53:e12944. doi: 10.1111/cpr.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med. 2021;10:1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5:172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6:58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adas G, Cukurova Z, Yasar KK, Yilmaz R, Isiksacan N, Kasapoglu P, et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant. 2021;30:9636897211024942. doi: 10.1177/09636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen X, Shan Y, Wen Y, Sun J, Du H. Mesenchymal stem cell therapy in severe COVID-19: a retrospective study of short-term treatment efficacy and side effects. J Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feng Y, Huang J, Wu J, Xu Y, Chen B, Jiang L, et al. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: A pilot study. Cell Prolif. 2020;53:e12947. doi: 10.1111/cpr.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hashemian SR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini SE, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12:91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mazzeo A, Santos EJC. Mesenchymal stem cells in the treatment of coronavirus-induced pneumonia (COVID-19). Einstein (Sao Paulo). 2020;18:eCE5802. [DOI] [PMC free article] [PubMed]
- 113.Saleh M, Vaezi AA, Aliannejad R, Sohrabpour AA, Kiaei SZF, Shadnoush M, et al. Cell therapy in patients with COVID-19 using Wharton's jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res Ther. 2021;12:410. doi: 10.1186/s13287-021-02483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang L, Jiang Y, Zhu M, Chen L, Zhou X, Zhou C, et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020;14:664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peng H, Gong T, Huang X, Sun X, Luo H, Wang W, et al. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Res Ther. 2020;11:291. doi: 10.1186/s13287-020-01802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liang S, Jiao HL, Chi LK, Shi XY, Liang AM, Tian Y, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. Chin J Tissue Eng Res. 2020;16:9179–9185. [Google Scholar]
- 117.Zhang Y, Ding J, Ren S, Wang W, Yang Y, Li S, et al. Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther. 2020;11:207. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu RN, Shi L, Xie WF, Xu Z, Meng FP, Fu JL, et al. Diagnosis and Treatment Guidelines for Mesenchymal Stem Cell Therapy for Coronavirus Disease 2019 (Beijing, 2021) Infect Diseases Immunity. 2021;1:68–73. [Google Scholar]
- 119.Kesari S, Kasper GC, Verkh L, Hammond TC, Matal ML, Hammerling JW, et al. Mesenchymal stem cells in the treatment of severe COVID-19. Transl Med Commun. 2021;6:16. doi: 10.1186/s41231-021-00095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Clinical trials of cell-based therapies for COVID-19 patients registered in clinicaltrials.gov/. Table S2. Current outcomes of clinical trials of MSCs in COVID-19 treatment.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.