Abstract
The increasing global temperature is causing economic losses and animal welfare problems in the poultry industry. Because poultry do not have sweat glands, it is difficult for them to return to their usual body temperature. Heat stress has negative impact on production and health in broilers. Given the effects of chronic stress on broilers, the objective of this study was to identify physiological changes in differentially expressed proteins in broilers with different growth performances using liver tissue from 35-day-old chickens (Ross-308). Changes in protein levels were analyzed with two-dimensional gel electrophoresis (2DE) and mass spectrometry. This study contained 2 groups (control and heat treatment groups) with 8 replicates per group. After d 20, ten birds were assigned to each replicate. On d 35, the heat treatment group was subdivided into 2 groups, a heat stressed high body weight group (HH) and a heat stressed low body weight group (HL). Body weight was lower in the heat treatment group than that in the control group. In the heat treatment group, the HH group had a significantly higher body weight than the HL group. The expression of heat shock protein 70 significantly increased in the HL group. Protein spots with significant differences in 2DE analysis were screened and selected. Thirteen significant spots were excised and analyzed using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF). Among the 13 spots, 8 spots were identified. The identified spots were MRP-126, fatty acid binding protein, ferritin heavy chain, glutathione S-transferase, agmatinase; mitochondrial, alpha-enolase, 60 kDa heat shock protein; mitochondrial, and tubulin beta-7 chain. Our study has showed that high temperature stress aggravated oxidative stress in broilers, which resulted in comparatively slow growth to preserve body homeostasis.
Key words: heat stress, broiler, growth, proteomic analysis
Introduction
Heat stress in poultry has become a major concern due to global warming over the past several decades. High environmental temperatures negatively affect health and even animal welfare (Scanes, 2016; Wang et al., 2018). Moreover, high environmental temperatures have a significant economic impact on the poultry industry. For example, in Korea, livestock mortality due to heat waves and drought is increasing, especially in poultry, which has the highest mortality rate (Kang et al., 2020). Unlike other animals, poultry are more susceptible to high temperatures, because they are covered with heavy feathers and have no sweat glands, not allowing the easy dissipation of heat from bodies to the environment (Loyau et al., 2013). In high-temperature environments, reductions in animal performance are influenced both directly and indirectly by decreased feed intake. Low performance in parameters such as propagative physiology, health and energy has been shown to be a direct effect of high temperatures (Renaudeau et al., 2012). Under heat stress, there is a significant decrease in feed intake and body weight (Sohail et al., 2012). In addition, thyroid activity, which influences metabolic rate, oxygen consumption, feed intake, and growth rate, reduces in high-temperature environments (Mckee, Harrison, & Riskowski, 1997). When broilers are exposed to heat, they undergo negative effects such as immune, behavioral, and physiological responses (Mujahid, Yoshiki, Akiba, & Toyomizu, 2005). For example, they spend more time drinking, resting and panting at high temperatures (Mack, Felver-Gant, Dennis, & Cheng, 2013). Panting is the major mechanism for heat dissipation in poultry, but panting keeps broilers from drinking and feeding, and also increases the risk of acid-base imbalance, and respiratory alkalosis (Marder and Arad, 1989).
The liver is an important organ that maintains nutritional homeostasis and produces circulatory proteins (Jastrebski, Lamont, & Schmidt, 2017; Zhang, Yang, Lu, & Cao, 2021). In addition, the liver, is a major organ that coordinates transitions related to adaptations in carbohydrate, lipid, and protein metabolism (Skibiel, Zachut, Do-Amaral, Levin, & Dahl, 2018). Thus, liver tissue is an optimal materials for investigating adaptation mechanisms under heat stress (Zhang et al., 2021). A previous study discussed how the liver helps maintain the overall metabolism of an organism, so it is appropriate to hypothesize that the liver will observably react to heat stress (Jastrebski et al., 2017).
Many studies have been conducted on growth, feed efficiency and heat shock proteins with the goal of reducing the effects of heat stress (Quinteiro-Filho et al., 2010; Toplu et al., 2014; Yahav & Hurwitz, 1996). In addition, many studies related to heat resistance using different varieties are being conducted (Al-Batshan, 2002; Wang & Edens, 1998; Xu, Lai, Li, Zhang, & Luo, 2018). However, studies on heat resistance in the same breed were limited. Therefore, in this study, broilers with growth differences due to heat stress were selected within the same breed and confirmed which protein difference appears between them using proteomic approach.
Materials and Methods
Animal care and experimental procedures were approved by the Animal Ethics Committee of the Jeonbuk National University (CBNU2018-097), Republic of Korea.
Birds and Experimental Design
In this study, one-day-old Ross-308 chicks (n = 240) were obtained from a commercial hatchery in Iksan, Republic of Korea. Cages (dimensions of each cage: length × width × height, 192 × 120 × 50 cm) were used to raise the broilers in an environmentally controlled farmhouse with fresh water and ad libitum commercial feed (Table 1) until the experiment was completed. The chicks were raised in the abovementioned farmhouse for 20 d in 16 cages (15 chicks/cage). The brooding temperature was 34°C for the first 2 d and was then gradually decreased to 26°C by d 21 (2°C/wk). After d 20, the broilers were separated into 2 temperature groups (control and heat treatment) with eight replicates per group and each replicate containing 10 birds of approximately the same body weight (g). The control group (C) was maintained at normal temperatures without heat exposure. The control temperature was reduced by 2°C until a temperature of 24°C was reached. The temperature was then maintained at 24°C for 35 d. In the heat treatment group, the temperature was maintained at 34°C from d 21 to 35. The relative humidity was kept at 60% during the entire experimental period for both the control and heat treatment groups. On d 35, heat stressed broilers were subdivided into 2 groups, a heat stressed with high body weight group (HH) and a heat stressed low body weight group (HL) based on their body weights.
Table 1.
Composition of the basal diet.
| Chemical composition | Starter (0 to 20 d) | Finisher (21 to 35 d) |
|---|---|---|
| Crude protein | 20.0% | 19.0% |
| Crude fat | 4.0% | 4.0% |
| Calcium | 0.75% | 0.75% |
| Phosphate | 0.70% | 0.70% |
| Crude fiber | 6.0% | 5.50% |
| Crude ash | 8.0% | 8.0% |
| Met + Cys + MHAa | 0.75% | 0.65% |
| MEb | 3.00 Mcal/Kg | 3.05 Mcal/Kg |
Met + Cys + MHA, Methionine + Cysteine + DL-Methionine Hydroxy Analogue.
Abbreviation: ME, metabolizable energy.
Sample Collection
For the control group, 8 birds were randomly selected. For the heat treatment group, the top 8 birds were selected into the HH group and the bottom 8 birds were selected into the HL group. Liver tissue was collected after sacrifice by decapitation, and immediately frozen in liquid nitrogen. Liver tissues were stored at −80°C until analysis.
Western Blot Analysis
Protein expression was measured with the liver tissue using a western blotting. For protein extraction, 100 mg of liver tissue sample was mixed with 1 mL RIPA Buffer (Biosesang, Sungnam, Korea) containing protease inhibitor (Thermo Fisher Scientific, Waltham, MA) and homogenized for 10 s using a homogenizer (IKA, Staufen, Germany). After homogenization, samples were centrifuged (13,475 × g, 15 min, 4°C) and the supernatants were collected. The total protein concentration was quantified using a DC protein assay kit (Bio-Rad, Hercules, CA). The supernatant was mixed with sample buffer (2× Laemmli sample buffer, Bio-Rad, 1:1, vol/vol) and heated at 95°C for 5 min, and then stored at −20°C. The protein samples (30 μg) were separated on a 12% acrylamide gel and transferred to a polyvinylidene fluoride membrane. Each membrane was blocked with 5% skim milk in TBST buffer (20 mM Tris, 137 mM NaCl, 5 mM KCl and 0.05% Tween 20) for 1.5 h at room temperature. After blocking, each membrane was washed with TBST and incubated with a primary antibody at 4°C overnight and secondary antibody for 1.5 h at room temperature. Primary antibodies, including antibodies for heat shock protein 70 (1:1,250, ADI-SPA-820, Enzo, San Diego, CA), heat shock protein 60 (1:2,000, ADI-SPA-806, Enzo, San Diego, CA), FABP7 (1:333, ab32423, Abcam, UK), and GAPDH (1:5,000, MA5-15738, Invitrogen, Waltham, MA) were diluted with 3% skim milk. Secondary antibodies corresponding to the primary antibodies used (the dilution ratio was twice that of each antibody) were diluted with 5% skim milk. The secondary antibodies used were goat anti-mouse IgG (Thermo Fisher Scientific) and goat anti-rabbit IgG (Thermo Fisher Scientific). Protein bands were visualized using SuperSignal Chemiluminescent Substrates (Thermo Fisher Scientific) following the manufacturer's instructions and their density was measured using an iBright Imaging System (Thermo Fisher Scientific). Protein expression levels were normalized with GAPDH.
Two-Dimensional Gel Electrophoresis
The 100 mg powdered liver tissue sample was mixed with 1 mL sample preparation buffer (Urea [FW 60.06] 7 M, Thiourea 2 M, 3-cholamidopropyl dimethylammonio 1-propanesulfonate [CHAPS] 4% (w/v), dithiothreitol (DTT) 40 mM, immobilized pH gradient (IPG) buffer 2% (v/v)) and homogenized for 15 s using a homogenizer, and centrifuged (21,055 × g, 20 min, 4°C) before the supernatant was collected. After extraction, a 2D Clean-up kit (GE80-6484-51, GE Healthcare, Chicago, IL) was used to remove contaminants from the samples following the manufacturer's instructions. Total protein concentration was determined using 2D Quant Kit (GE80-6483-56, GE Healthcare) according to the manufacturer's instructions. Protein samples (800 µg) were mixed in 450 µL of the rehydration buffer (Urea [FW 60.06] 7 M, Thiourea [FW 76.12] 2 M, CHAPS 2% [w/v], IPG buffer 2% [w/v], and 1% bromophenol blue stock solution 0.0002%) and loaded on an IPG strip (24 cm pH 3–10 nonlinear, Bio-Rad, #163-2043). Rehydration was performed using a Protean IEF cell (Bio-Rad) for 16 h. After rehydration, isoelectric focusing (IEF) was performed using an IPGphor3 isoelectric focusing system (GE Healthcare). The program settings were as follows: 100 V for 1 h, 200 V for 1 h, 300 V for 1 h, 500 V for 1 h, 1,000 V for 1 h, 10,000 V for 3 h 45 min, and 10,000 V for 3 h. After the first-dimension electrophoresis, the IPG strips were equilibrated with equilibration buffer (Urea [FW 60.06] 6 M, Tris-HCL, pH8.8 75 mM, glycerol 29.3% [v/v], SDS [FW 288.38] 2% [w/v], 1% bromophenol blue stock solution 0.002% [w/v]) containing 1% DTT for 15 min at room temperature, followed by a second equilibration with 2.5% iodoacetamide (IAA) replacing 1% DTT for 15 min. The 12% separating gels were precast with glass plates using an Ettan DALT 6 six-gel caster, and IPG strips were placed on top of it. Strips were overlaid with Agarose sealing solution (25 mM tris base, 192 mM glycine, 0.2% SDS, 0.5 agarose, 0.002% bromophenol blue). Samples were separated at 120 mA and 200 V until they reached the bottom of the gel. Then, the gels were fixed with fixing solution (MeOH 45%, acetic acid 10%) and stained with Coomassie brilliant blue G-250 solution (Proteome Tech, Seoul, Korea) overnight. For protein spot detection, volume calculation and matching were analyzed using Image Master 2D Platinum 16.0 software (GE Healthcare). The parameter used for the quantifications was the protein volume. Differentially expressed protein spots were considered significant if they showed P < 0.05 (one-way analysis of variance [ANOVA] followed by Duncan's multiple range test).
Protein Identification by Mass Spectrometry
Peptide mass fingerprinting was used for protein identification, and the protein spots from 2DE were excised. To digest the spots, trypsin (Promega, Madison, WI), which also contained α-cyano-4-hydroxycinnamic acid in 50% acetonitrile and 0.1% TFA (trifluoroacetic acid), was used. Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) analysis (Microflex LTF 20, Bruker Daltonics, Billerica, MA) was performed as described in previous study (Fernandez, Gharahdaghi, & Mische, 1998). Spectra were obtained from 300 shots per spectrum over ab m/z range from 700 and 4,000, and trypsin auto-digestion peaks (m/z 842.5099, 2211.1046) were used for adjustment by two-point internal calibration. Flex Analysis 3.0, was performed to generate the peak list. For peak- picking, the threshold used was as follows: The minimum resolution of the monoisotopic mass was 500 and the signal-to-noise ratio (S/N) was 6. For protein identification by peptide mass fingerprinting (PMF), the search program MASCOT, developed by Matrixscience (http://www.matrixscience.com/) was used. For protein identification by peptide mass fingerprinting, the following parameters were used to search the database: trypsin for cleaving enzyme, up to one missing cleavage, IAA (Cys) for complete modification, oxidation (Met) for partial modification, single isotope masses, and mass tolerance of ± 0.2 Da. The PMF acceptance criteria were based on probability scoring.
RNA Extraction and qRT-PCR
Total RNA from liver tissue was extracted using the AccuPrep Universal RNA Extraction Kit (Bioneer, Daejeon, Korea), following the manufacturer's instructions. RNA concentration was quantified using a Nanodrop Plate (Thermo Fisher Scientific). One microgram of total RNA was used to synthesize complementary DNA (cDNA) using the AccuPower RocketScript Cycle RT PreMix (dT20) (Bioneer). The cDNA amplification was performed with AccuPrep 2X GreenStar qPCR Master Mix (Bioneer) using the CFX96 real-time PCR detection system (Bio-Rad) and reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed under the following conditions; pre-denaturation at 95°C for 5 min, followed by denaturation and annealing/extension for 40 cycles each at 95°C for 15 s and 60°C for 30 s. The primers were designed using Primer 3 software (v.0.4.0, https://bioinfo.ut.ee/primer3-0.4.0/) and are listed in Table 2. GAPDH was used to normalize the gene expression. The related gene expression was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
Primer sequences for qRT-PCR.
| Gene name | Primer sequences |
|---|---|
| S100 calcium binding protein A9 (MRP-126) | F:5′- TGACCTCCTTCTGTGCCTCT-3′ R:5′- CCTGGTCAGGGTGTCTTTGT-3′ |
| Fatty acid binding protein 7 (FABP7) | F:5′-CGTGATCAGGACTCAGAGCA-3′ R:5′- TGCCCGAGAGGTCACTTACT -3′ |
| Ferritin heavy chain (FTH1) | F:5′- GTGACCATGTGACCAACCTG -3′ R:5′- GCCTTCAGCTGTCACTTTCC -3′ |
| Agmatinase, Mitochondrial (AGMAT) | F:5′- GGGCAGTGAGCAGAACTAGG -3′ R:5′- GCTGACCTCGCCAGAACTAC -3′ |
| Glutathione S-transferase (GSTA1) | F:5′- CTTCATGTGGTGGGTGACTG-3′ R:5′- AGGAGCACTTGACTCCCTGA -3′ |
| Alpha-enolase (ENO1) | F:5′- GCTGCCTGGAAGAAGTTCAC -3′ R:5′- CCTTAAGGAGCAGGCAGTTG -3′ |
| 60KDa heat shock protein, mitochondrial (HSP60) | F:5′- GCTGCTGTACGTGTGTTCGT -3′ R:5′- ACCCTCCTCTCCAAACACCT -3′ |
| Tubulin beta 7 chain (TUBB) | F:5′- GGTGTCGGAGTACCAGCAGT -3′ R:5′- GCCTTTCTCAGGCCTCTTCT -3′ |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | F:5′- AGAACATCATCCCAGCGT -3′ R:5′-AGCCTTCACTACCCTCTTG -3′ |
Statistical Analysis
The SAS software suite (version 9.4; SAS Institute Inc., Cary, NC) was used for all experimental data. Statistical data were obtained through one-way ANOVA followed by Duncan's multiple range test. All experimental data were expressed as means with error bars as standard error (SE). Statistical significance was set at P < 0.05, P < 0.01, and P < 0.001.
Results
Growth Performance
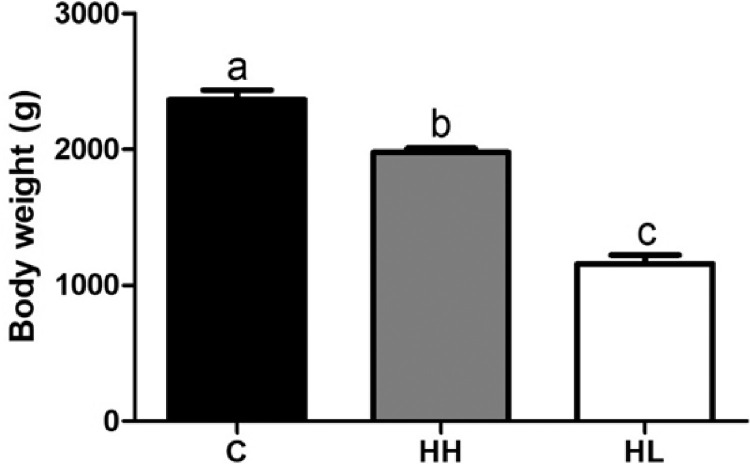
Body weight is shown in Figure 1. The body weight of the chickens in the heat stressed groups were significantly lower (P < 0.001) than that in the control group. In the heat treatment group, HH group was significantly higher (P < 0.001) than HL group.
Figure 1.
Growth performance of broilers. Values are presented as means with error bars as standard error. a-cDifferent superscript letters represent significant differences. (P < 0.001). Abbreviations: C, control; HH, Heat stressed high weight; HL, Heat stressed low weight.
HSP70 Expression
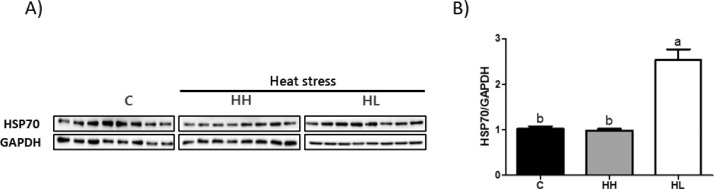
Figure 2 shows HSP70 expression levels. The expression of HSP70 in the HH group was similar to that in the C group. However, the expression of HSP70 was significantly higher (P < 0.001) in the HL group than that in the C and HH groups.
Figure 2.
HSP70 protein expression by western blotting in broiler liver tissue. (A) Protein levels of HSP70 and GAPDH. (B) Protein expressions level calculated by GAPDH. Values are presented as means with error bars as standard error. a-bDifferent superscript letters represent significant differences (P < 0.001). Abbreviations: C, control; HH, Heat stressed high weight; HL, Heat stressed low weight.
Differentially Expressed Proteins Among Three Groups
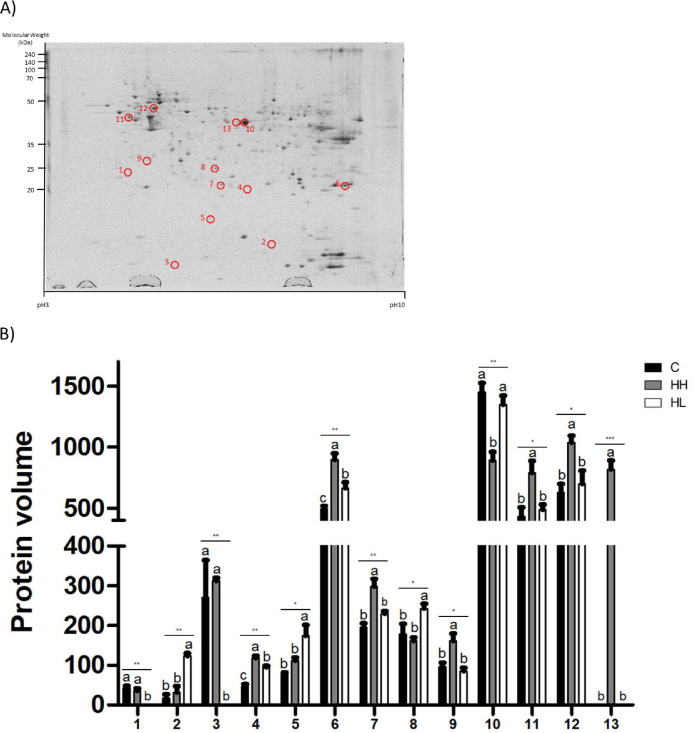
As a result of image analysis for the 3 groups, 13 proteins with significantly different expression levels were selected (Figure 3). Among the selected 13 proteins, 8 proteins were identified by PMF. The names of the identified proteins, their accession numbers, and other information are listed in Table 3.
Figure 3.
Different expression levels in liver tissue of broilers. (A) Representative image of 2DE gel of liver tissue from 35-day-old broilers. (B) Protein volume of each group. Values are presented as means with error bars as standard error. a-cDifferent superscript letters represent significant differences (*P < 0.05, **P < 0.01, ***P < 0.001). Abbreviations: C, control; HH, Heat stressed high weight; HL, Heat stressed low weight.
Table 3.
Features of the differentially expressed proteins identified by mass spectrometry.
| Protein NO. | Protein name | Uniprot ID | MW(KDa) | Mascot Score | P-value | PI | SCB(%) | Protein expression |
||
|---|---|---|---|---|---|---|---|---|---|---|
| C | HH | HL | ||||||||
| 2 | MRP-126A | P28318 | 14.170 | 149 | 1.10E-10 | 6.44 | 75 | 17.29 ± 8.97b | 31.27 ± 15.69b | 122.60 ± 7.13a |
| 3 | Fatty acid-binding protein | Q05423 | 15.031 | 78 | 0.0015 | 5.61 | 78 | 271.38 ± 93.86a | 312.78 ± 7.53a | 0.00 ± 0.00b |
| 5 | Ferritin heavy chain | P08267 | 21.249 | 204 | 3.40E-16 | 5.78 | 68 | 76.79 ± 5.35b | 111.08 ± 8.53b | 174.20 ± 27.35a |
| 6 | Glutathione S-transferase | Q08392 | 25.282 | 153 | 4.30E-11 | 8.86 | 68 | 492.69 ± 24.50c | 894.77 ± 55.12a | 663.90 ± 48.78b |
| 9 | Agmatinase, mitochondrial | Q90XD2 | 37.149 | 185 | 2.70E-14 | 5.98 | 54 | 95.81 ± 10.15b | 160.74 ± 19.26a | 84.87 ± 8.60b |
| 10 | Alpha-enolase | P51913 | 47.617 | 306 | 2.20E-26 | 6.17 | 62 | 1,450.78 ± 73.61a | 890.50 ± 71.32b | 1,346.36 ± 75.94a |
| 11 | Tubulin beta-7 chain | P09244 | 50.095 | 328 | 1.40E-28 | 4.78 | 68 | 428.74 ± 77.45b | 790.71 ± 96.59a | 486.77 ± 44.19b |
| 12 | 60 KDa heat shock protein, mitochondrial | P51913 | 61.105 | 199 | 1.10E-15 | 5.72 | 52 | 627.63 ± 72.13b | 1,035.42±59.96a | 697.65 ± 110.11b |
| 13 | Alpha-enolase | P51913 | 47.617 | 231 | 6.8 | 6.17 | 52 | 0.00 ± 0.00b | 817.81 ± 72.80a | 0.00 ± 0.00b |
Abbreviation: SC, sequence coverage.
MRP- 126: S100 calcium binding protein A9 Values are presented as means with error bars as standard error.
Different superscript letters represent significant differences (P < 0.05).
Validation of Protein Expression From 2DE Using Western Blotting
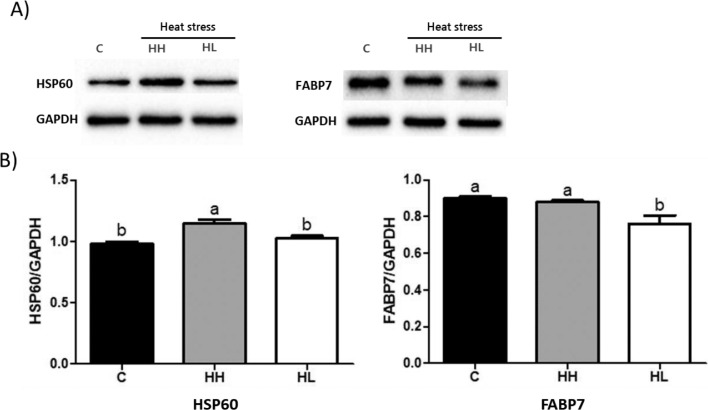
To verify the differential expression of individual proteins among the groups, western blot analysis was performed. As shown in Figure 4, HSP60 expression was significantly higher (P < 0.01) in the HH group than that in the C and HL groups. In addition, the expression of FABP7 in the HL group was significantly lower (P < 0.01) than that in the C and HH groups. There were no significant differences between the C and HH groups. From these results, it can be seen that the protein expression levels from western blotting were the same as those of 2DE.
Figure 4.
Protein expression of HSP60 and FABP7 in broiler liver tissue. (A) Protein levels of HSP60, FABP7 and GAPDH. (B) Protein expressions level calculated by GAPDH. Values are presented as means with error bars as standard error. a-bDifferent superscript letters represent significant differences (P < 0.01). Abbreviations: C, control; HH, Heat stressed high weight; HL, Heat stressed low weight.
Validation of Differentially Expressed Proteins by Their Gene Expression
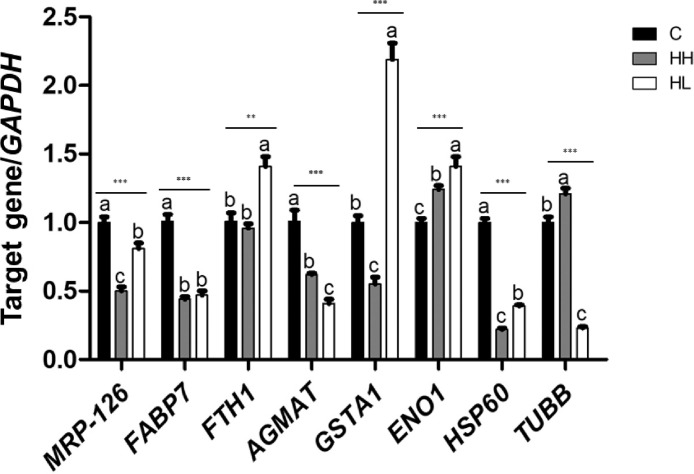
RT-qPCR was performed to compare the mRNA expression levels of the genes corresponding to the 8 differentially expressed proteins among the 3 groups (Figure 5). The expression of MRP-126, FABP7, AGMAT, and HSP60 in C was significantly higher (P < 0.001) than that in the HH and HL groups. In the HH group, TUBB had significantly higher gene expression than that in the C and HL groups (P < 0.001). The gene expression of FTH1, GSTA1, and ENO1 in the HL was significantly higher than that in other groups (P <0.01 or P < 0.001). Only the ferritin heavy chain showed an identical pattern of protein expression, while the other 6 genes showed expression patterns inconsistent with the results of protein expression patterns.
Figure 5.
Quantitative real-time PCR analysis of the differentially expressed proteins in the liver tissue of 35-day-old broilers. Values are presented as means with error bars as standard error. a-cDifferent superscript letters represent significant differences (**P < 0.01, ***P < 0.001). Abbreviations: C, control; HH, Heat stressed high weight; HL, Heat stressed low weight.
Discussion
In an environment with high temperatures, the growth rate and feed consumption of broilers decreases due to changes in digestion (Hai, Rong, & Zhang, 2000). A previous study reported that chronically heat stressed (35 ± 2°C) broilers had significantly lower body weights and body weight gain rates (Al-Fataftah & Abu-Dieyeh, 2007). The decreased body weight gain might be caused by multiple factors, including defective metabolism (Farrell and Swain, 1977), a reduction in thyroid size, and alternations in thyroxine secretion (Keshavarz and Fuller, 1980). The above studies provided results consistent with the present study, indicating that the group exposed to heat treatment showed a significant decrease in body weight compared to the control group.
Heat-shock proteins (HSPs) are present in all organisms and their synthesizes increases dramatically at high temperatures (Lindquist, 1986). Among HSPs, HSP70 is the first to be induced under stress conditions (Gupta, Sharma, Mishra, Mishra, & Chowdhuri, 2010). Therefore, HSP70 was used as an indicator of heat stress. In this study, the expression of HSP70 significantly increased in the HL group. Therefore, the HL group may suffer more stress from exposure to high temperatures than other groups. Another heat shock protein obtained from 2DE was HSP60, which is a mitochondrial stress protein that inhibits protein denaturation (Khadir et al., 2018). Our results showed that the expression of HSP60 was significantly higher in the HH group than that in the C and HL groups. This result suggests that the HH group may have the ability to effectively adapt to chronic heat stress by increasing the production of HSP60
In this study, among eight identified proteins, 5 differentially expressed proteins related to oxidative stress and damage were identified from 2DE. FABP7, also known as brain-FABP7, is also found in other tissues and is expressed abundantly in the liver (Wang et al., 2019). Oxidative stress negatively affects the central nervous system, which regulates the immune response (Lara and Rostagno, 2013; Salim, 2017). Ferritin is related to cytokine signaling, immunity, and liver disease (Gozzelino and Soares, 2014; Li, Luo, Mines, Zhang, & Fan, 2006; Nielsen, Engelhardt, Dullmann, & Fischer, 2002), and the ferritin heavy chain has a major function in protecting cells from oxidative damage (Storr et al., 2009). Glutathione S-transferase alpha is present at high concentrations in centrolobular cells and is sensitive to liver damage (Ozer, Ratner, Shaw, Bailey, & Schomaker, 2008). In addition, GSTA protects against oxidative stress, and enhances adaptation to this stress (Hayes and McLellan, 1999). Agmatinase, also known as agmatine ureohydrolase, catalyzes the hydrolysis of agmatine to form putrescine and urea (Satishchandran and Boyle, 1986). Agmatinase is also essential for the manganese ions needed to produce metal-bound hydroxides (Salas, Lopez, Uribe, & Carvajal, 2004). In the reactive oxygen species (ROS) and antioxidant systems, which are affected by oxidative stress caused by heat stress, manganese inhibits excessive ROS by improving the antioxidant capacity (Zhu et al., 2017). Alpha enolase (ENO1) is related to heat stress because the heat shock resistance gene (HSR1) regulates ENO1 (Iida and Yahara, 1985). Alpha enolase also interacts with HSP70, which protects against oxidative stress (Zeng et al., 2013).
To clarify the proteomic analysis results, qRT-PCR was performed to examine gene expression. Verifying the protein expression obtained from proteomic analysis by analyzing gene expression of their corresponding genes is common method that is widely used in studies (Ma et al., 2019; Pearce, Lonergan, Huff-Lonergan, Baumgard, & Gabler, 2015; Zeng et al., 2013). In this study, the differential expression of eight genes was confirmed, but among these genes, only one gene (FTH1) was consistent with the protein expression shown by proteomic analysis. This is because heat stress affects the initiation of translation, therefore, it affects protein synthesis (Cherkasov et al., 2015; Merret et al., 2015).
In conclusion, we analyzed body weight, protein expression, and 8 differentially expressed proteins, as identified by two-dimensional gel electrophoresis and mass spectrometry in control and heat-stressed broilers. Several differentially expressed proteins were closely related to proteins expressed under oxidative stress. It is likely that high temperature stress exacerbated the oxidative stress on the broilers, which resulted in a relatively slow growth rate because many proteins were involved in metabolism to maintain body homeostasis.
ACKNOWLEDGMENTS
We would like to acknowledge to Ministry of Education for funding of this study. This study was supported by the Basic Science Research Program, through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (Project No. 2020R1I1A3A04038058).
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101794.
Appendix. Supplementary materials
References
- Al-Batshan H. Performance and heat tolerance of broilers as affected by genotype and high ambient temperature. Asian-Australas. J. Anim. Sci. 2002;15:1502–1506. [Google Scholar]
- Al-Fataftah A.R.A., Abu-Dieyeh Z. Effect of chronic heat stress on broiler performance in Jordan. Int. J. Poult. Sci. 2007;6:64–70. [Google Scholar]
- Cherkasov V., Grousl T., Theer P., Vainshtein Y., Glæáer C., Mongis C., Kramer G., Stoecklin G., Knop M., MOGK A. Systemic control of protein synthesis through sequestration of translation and ribosome biogenesis factors during severe heat stress. FEBS Lett. 2015;589:3654–3664. doi: 10.1016/j.febslet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Farrell D.J., Swain S. Effects of temperature treatments on the energy and nitrogen metabolism of fed chickens. Br. Poult. Sci. 1977;18:735–748. doi: 10.1080/00071667708416429. [DOI] [PubMed] [Google Scholar]
- Fernandez J., Gharahdaghi F., Mische S.M. Routine identification of proteins from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels or polyvinyl difluoride membranes using matrix assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) Electrophoresis. 1998;19:1036–1045. doi: 10.1002/elps.1150190619. [DOI] [PubMed] [Google Scholar]
- Gozzelino R., Soares M.P. Coupling heme and iron metabolism via ferritin H chain. Antioxid. Redox Signal. 2014;20:1754–1769. doi: 10.1089/ars.2013.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.C., Sharma A., Mishra M., Mishra R.K., Chowdhuri D.K. Heat shock proteins in toxicology: how close and how far? Life Sci. 2010;86:377–384. doi: 10.1016/j.lfs.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Hai L., Rong D., Zhang Z.Y. The effect of thermal environment on the digestion of broilers. J. Anim. Physiol. Anim. Nutr. 2000;83:57–64. [Google Scholar]
- Hayes J.D., Mclellan L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- Iida H., Yahara I. Yeast heat-shock protein of M r 48,000 is an isoprotein of enolase. Nature. 1985;315:688–690. [Google Scholar]
- Jastrebski S.F., Lamont S.J., Schmidt C.J. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Kim D.H., Lee S., Lee T., Lee K.W., Chang H.H., Moon B., Ayasan T., Choi Y.H. An acute, rather than progressive, increase in temperature-humidity index has severe effects on mortality in laying hens. Front. Vet. Sci. 2020;7:853. doi: 10.3389/fvets.2020.568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarz K., Fuller H.L. The influence of widely fluctuating temperatures on heat production and energetic efficiency of broilers. Poult. Sci. 1980;59:2121–2128. doi: 10.3382/ps.0592121. [DOI] [PubMed] [Google Scholar]
- Khadir A., Kavalakatt S., Cherian P., Warsame S., Abubaker J.A., Dehbi M., Tiss A. Physical exercise enhanced heat shock protein 60 expression and attenuated inflammation in the adipose tissue of human diabetic obese. Front. Endocrinol. 2018;9:16. doi: 10.3389/fendo.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Luo C., Mines M., Zhang J., Fan G.H. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J. Biol. Chem. 2006;281:37616–37627. doi: 10.1074/jbc.M607266200. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu. Rev. Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loyau T., Berri C., Bedrani L., Metayer-Coustard S., Praud C., Duclos M.J., Tesseraud S., Rideau N., Everaert N., Yahav S., Mignon-Grasteau S., Collin A. Thermal manipulation of the embryo modifies the physiology and body composition of broiler chickens reared in floor pens without affecting breast meat processing quality. J. Anim. Sci. 2013;91:3674–3685. doi: 10.2527/jas.2013-6445. [DOI] [PubMed] [Google Scholar]
- Ma D., Liu Q., Zhang M., Feng J., Li X., Zhou Y., Wang X. iTRAQ-based quantitative proteomics analysis of the spleen reveals innate immunity and cell death pathways associated with heat stress in broilers (Gallus gallus) J. Proteomics. 2019;196:11–21. doi: 10.1016/j.jprot.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Mack L.A., Felver-Gant J.N., Dennis R.L., Cheng H.W. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult. Sci. 2013;92:285–294. doi: 10.3382/ps.2012-02589. [DOI] [PubMed] [Google Scholar]
- Marder J., Arad Z. Panting and acid-base regulation in heat stressed birds. Comp. Biochem. Physiol. A Comp. Physiol. 1989;94:395–400. doi: 10.1016/0300-9629(89)90112-6. [DOI] [PubMed] [Google Scholar]
- Mckee J., Harrison P., Riskowski G. Effects of supplemental ascorbic acid on the energy conversion of broiler chicks during heat stress and feed withdrawal. Poult. Sci. 1997;76:1278–1286. doi: 10.1093/ps/76.9.1278. [DOI] [PubMed] [Google Scholar]
- Merret R., Nagarajan V.K., Carpentier M.C., Park S., Favory J.J., Descombin J., Picart C., Charng Y.Y., Green P.J., Deragon J.M. Heat-induced ribosome pausing triggers mRNA co-translational decay in Arabidopsis thaliana. Nucleic Acids Res. 2015;43:4121–4132. doi: 10.1093/nar/gkv234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujahid A., Yoshiki Y., Akiba Y., Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005;84:307–314. doi: 10.1093/ps/84.2.307. [DOI] [PubMed] [Google Scholar]
- Nielsen P., Engelhardt R., Dullmann J., Fischer R. Non-invasive liver iron quantification by SQUID-biosusceptometry and serum ferritin iron as new diagnostic parameters in hereditary hemochromatosis. Blood Cells Mol. Dis. 2002;29:451–458. doi: 10.1006/bcmd.2002.0583. [DOI] [PubMed] [Google Scholar]
- Ozer J., Ratner M., Shaw M., Bailey W., Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Pearce S.C., Lonergan S.M., Huff-Lonergan E., Baumgard L.H., Gabler N.K. Acute heat stress and reduced nutrient intake alter intestinal proteomic profile and gene expression in pigs. PloS one. 2015;10 doi: 10.1371/journal.pone.0143099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteiro-Filho W., Ribeiro A., Ferraz-De-Paula V., Pinheiro M., Sakai M., Sã L., Ferreira A., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., De-Basilio V., Gourkine J.L., Collier R. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal. 2012;6:707–728. doi: 10.1017/S1751731111002448. [DOI] [PubMed] [Google Scholar]
- Salas M., Lopez V., Uribe E., Carvajal N. Studies on the interaction of Escherichia coli agmatinase with manganese ions: structural and kinetic studies of the H126N and H151N variants. J. Inorg. Biochem. 2004;98:1032–1036. doi: 10.1016/j.jinorgbio.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Salim S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017;360:201–205. doi: 10.1124/jpet.116.237503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satishchandran C., Boyle S.M. Purification and properties of agmatine ureohydrolyase, a putrescine biosynthetic enzyme in Escherichia coli. J. Bacteriol. 1986;165:843–848. doi: 10.1128/jb.165.3.843-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanes C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci. 2016;95:2208–2215. doi: 10.3382/ps/pew137. [DOI] [PubMed] [Google Scholar]
- Skibiel A.L., Zachut M., Do-Amaral B.C., Levin Y., Dahl G.E. Liver proteomic analysis of postpartum Holstein cows exposed to heat stress or cooling conditions during the dry period. J. Dairy Sci. 2018;101:705–716. doi: 10.3168/jds.2017-13258. [DOI] [PubMed] [Google Scholar]
- Sohail M.U., Hume M.E., Byrd J.A., Nisbet D.J., Ijaz A., Sohail A., Shabbir M.Z., Rehman H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012;91:2235–2240. doi: 10.3382/ps.2012-02182. [DOI] [PubMed] [Google Scholar]
- Storr H.L., Kind B., Parfitt D.A., Chapple J.P., Lorenz M., Koehler K., Huebner A., Clark A.J.L. Deficiency of ferritin heavy-chain nuclear import in triple a syndrome implies nuclear oxidative damage as the primary disease mechanism. Mol. Endocrinol. 2009;23:2086–2094. doi: 10.1210/me.2009-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplu H.D.O., Tunca R., Aypak S.U., Coven F., Epikmen E.T., Karaarslan S., Yagin O. Effects of heat conditioning and dietary ascorbic acid supplementation on heat shock protein 70 expression, blood parameters and fear-related behavior in broilers subjected to heat stress. Acta Sci. Vet. 2014;42:1–8. [Google Scholar]
- Wang S., Edens F. Heat conditioning induces heat shock proteins in broiler chickens and turkey poults. Poult. Sci. 1998;77:1636–1645. doi: 10.1093/ps/77.11.1636. [DOI] [PubMed] [Google Scholar]
- Wang Y., Saelao P., Chanthavixay K., Gallardo R., Bunn D., Lamont S.J., Dekkers J.M., Kelly T., Zhou H. Physiological responses to heat stress in two genetically distinct chicken inbred lines. Poult. Sci. 2018;97:770–780. doi: 10.3382/ps/pex363. [DOI] [PubMed] [Google Scholar]
- Wang Z., Yue Y.X., Liu Z.M., Yang L.Y., Li H., Li Z.J., Li G.X., Wang Y.B., Tian Y.D., Kang X.T., Liu X.J. Genome-Wide analysis of the FABP gene family in liver of chicken (Gallus gallus): identification, dynamic expression profile, and regulatory mechanism. Int. J. Mol. Sci. 2019;20:5948. doi: 10.3390/ijms20235948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Lai X., Li Z., Zhang X., Luo Q. Effect of chronic heat stress on some physiological and immunological parameters in different breed of broilers. Poult. Sci. 2018;97:4073–4082. doi: 10.3382/ps/pey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav S., Hurwitz S. Induction of thermotolerance in male broiler chickens by temperature conditioning at an early age. Poult. Sci. 1996;75:402–406. doi: 10.3382/ps.0750402. [DOI] [PubMed] [Google Scholar]
- Zeng T., Jiang X., Li J., Wang D., Li G., Lu L., Wang G. Comparative proteomic analysis of the hepatic response to heat stress in Muscovy and Pekin ducks: insight into thermal tolerance related to energy metabolism. PloS one. 2013;8:e76917. doi: 10.1371/journal.pone.0076917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Yang Y., Lu Y., Cao Z. iTRAQ-based quantitative proteomic analyses the cycle chronic heat stress affecting liver proteome in yellow-feather chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Lu L., Liao X., Li W., Zhang L., Ji C., Lin X., Liu H.C., Odle J., Luo X. Maternal dietary manganese protects chick embryos against maternal heat stress via epigenetic-activated antioxidant and anti-apoptotic abilities. Oncotarget. 2017;8:89665–89680. doi: 10.18632/oncotarget.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.